Abstract
Faster rates of age-related cognitive decline might result in early onset of cognitive impairment and dementia. The relationship between ethanol intake and cognitive decline, although studied extensively, remains poorly understood. Previous studies used single measurements of ethanol, and few were conducted in diverse populations. We assessed the association of 9-year trajectories of ethanol intake (1987–1998) with 15-year rate of decline in cognitive performance from mid- to late life (1996–2013) among 2,169 Black and 8,707 White participants of the US Atherosclerosis Risk in Communities study using multivariable linear regression models. We hypothesized that stable, low to moderate drinking would be associated with lesser 15-year cognitive decline, and stable, heavy drinking with greater 15-year cognitive decline. Stable, low to moderate drinking (for Blacks, adjusted mean difference (MD) = 0.03 (95% confidence interval (CI): −0.13, 0.19); for Whites, adjusted MD = 0.02 (95% CI: −0.05, 0.08)) and stable, heavy drinking (for Blacks, adjusted MD = 0.08 (95% CI: −0.34, 0.50); for Whites, adjusted MD = −0.03 (95% CI: −0.18, 0.11)) in midlife compared with stable never-drinking were not associated with 15-year decline in general cognitive function from mid- to late life. No association was observed for the stable former and “mostly” drinking trajectories with 15-year cognitive decline. Stable low, low to moderate, and stable heavy drinking in midlife are not associated with lesser and greater cognitive decline, respectively, from mid- to late life among Black and White adults.
Keywords: African-Americans, aging, cognitive decline, ethanol, middle-aged, multivariable regression models, trajectories
Abbreviations
-
APOE
 4
4 apolipoprotein E
 4 allele
4 allele- ARIC
Atherosclerosis Risk in Communities
- CI
confidence interval
- DSST
digit symbol substitution test
- DWRT
delayed word recall test
- WFT
word fluency test
Cognitive decline refers to the decrease in mental processes, such as attention, short-term and long-term memory, reasoning, movement coordination, and planning of tasks, which are important for the conduct of daily living activities (1, 2). Neurobiological and cognitive performance studies suggest that declines in cognitive function are gradual and begin in early adulthood (3–5). Faster rates of cognitive decline could lead to earlier onset of cognitive impairment and dementia, resulting in significant burden to those affected and their caregivers (6). By 2050, it is projected that the number of Americans aged 65 years or older will triple, and the United States will become more racially and ethnically diverse (7). Racial/ethnic disparities in dementia prevalence and incidence have been documented. Studies indicate that Black and other racial minority groups are disproportionately burdened with Alzheimer disease and other forms of cognitive impairment compared with Whites (8–11). To reduce the incidence of cognitive impairment and dementia, it is important to identify modifiable lifestyle risk factors that can prevent or delay the progression of cognitive decline.
The relationship between ethanol intake and cognitive decline (12–26) has been previously studied but remains poorly understood due to inconsistent findings. While heavy ethanol intake is associated with greater cognitive decline (26), low to moderate ethanol intake has been associated with less cognitive decline (13, 16, 17, 19–21, 24, 25) or no cognitive decline (12, 23, 26). Inconsistent findings might be attributable to the use of single measures of ethanol (12, 13, 16–18, 21, 23–25) and short follow-up (12, 13, 16, 19–21, 23). Few studies have investigated the association of ethanol on cognitive decline in Black populations despite their disproportionate burden of Alzheimer disease and other forms of cognitive impairment (20, 25). Furthermore, few studies have investigated the association of midlife ethanol intake with late-life cognition (18, 26). Prospective studies, including the Atherosclerosis Risk in Communities (ARIC) study, have demonstrated that midlife vascular risk factors are most strongly associated with late-life cognitive decline (27–30). Attrition is a methodological concern in longitudinal studies of cognitive decline (31). Most studies of ethanol intake and cognitive decline (attrition range: 11%–57%) did not adjust for attrition (12–26), which could have biased their association estimates.
Studies that used a single measure of ethanol intake to define the drinking behavior of participants assume that drinking behavior is static thereafter. However, individuals’ drinking habits change over time (32, 33), which can affect their risk of developing disease (34, 35). Therefore, not accounting for long-term drinking pattern or changes in ethanol intake can introduce bias in the study (36–38).
Using repeat assessments of ethanol intake over 9 years and repeat measurements of cognitive function over 15 years, our aims were: 1) to characterize temporal trajectories of ethanol intake during midlife in Black and White adults, 2) to examine whether temporal trajectories of ethanol intake in midlife are associated with 15-year rate of decline in cognition from mid- to late life among Black and White adults, and 3) to examine whether short-term ethanol intake in midlife shows comparable associations with 15-year cognitive decline.
METHODS
Study population
The ARIC study is a community-based, prospective cohort study of, 15,792 middle-aged adults selected through probability sampling from 4 US communities: Washington County, Maryland; Forsyth County, North Carolina; suburbs of Minneapolis, Minnesota; and Jackson, Mississippi. Participants were seen at 4 study visits approximately 3 years apart from 1987–1989 through 1996–1998, and a fifth examination visit was conducted in 2011–2013 (Figure 1).
Figure 1.
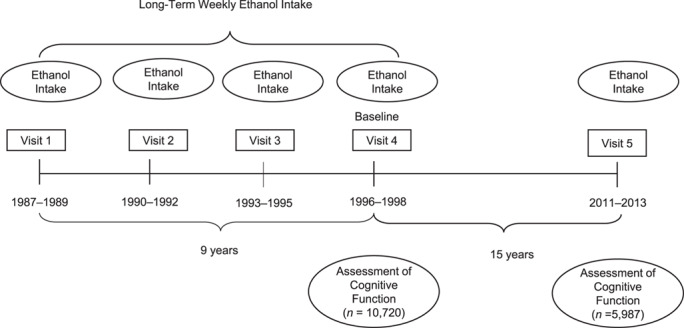
Timeline for assessment of alcohol consumption and cognitive functioning, Atherosclerosis Risk in Communities, United States, 1987–2013.
The baseline for the present analysis was visit 4, which allows for the investigation of the association of trajectories of ethanol intake across 9 years in midlife and subsequent 15-year cognitive decline from mid- to late life (Figure 1). Of the 11,625 Black and White participants who attended visit 4, we excluded Blacks from Minnesota and Washington County due to small sample size (n = 38) (39), those who were missing 1 or more cognitive function tests at study baseline (n = 625), and those with missing covariates (n = 86), giving a final sample size of 10,876 participants at baseline.
Assessment of ethanol intake
Ethanol intake was assessed at all visits by means of an interviewer-administered questionnaire (40). During the exam, participants were asked the following questions: “Do you presently drink alcoholic beverages?” and “Have you ever consumed alcoholic beverages?” Individuals replying no to both questions were classified as never drinkers. Those who replied “no” to the first question and “yes” to the second question were classified as former drinkers.
Current drinkers were asked how often they usually drank wine, beer, or hard liquor. The amount of ethanol consumed (in grams per week) was calculated assuming the following ethanol content: 4 oz of wine = 10.8 g; 12 oz of beer = 13.2 g; and 1.5 oz of distilled spirits = 15.1 g. For a drinker who reported less than 1 drink per week, the ethanol intake was recorded as 0 grams per week.
Categories of ethanol intake at each visit were created using the US Department of Agriculture and US Department of Health and Human Services Dietary Guidelines for Americans, 2015–2020 guideline for low to moderate drinking (≤210 g/week for men and ≤105 g/week for women) and heavy drinking (>210 g/week for men and >105 g/week for women) (41). Utilizing ethanol intake categories across visits 1–4, trajectories of ethanol intake were then classified as 1) stable, never drinkers; 2) stable, low to moderate drinkers; 3) stable, heavy drinkers; 4) stable, former drinkers; 5) mostly low to moderate drinkers; 6) mostly heavy drinkers; and 7) mostly former drinkers. In creating the “mostly” ethanol intake trajectories, ethanol intake status at study baseline (visit 4) was taken in account. Participants who did not have a current drinking status (i.e., never or former) across visits 1–3, but who reported current drinking at visit 4 (i.e., low to moderate or heavy) were assigned to the “mostly” ethanol intake category at visit 4 (i.e., low to moderate or heavy). Participants who had a current drinking status (i.e., low to moderate or heavy) across visits 1–3 but who reported former drinking at visit 4 were assigned to the “mostly” former long-term pattern of ethanol intake. Participants who reported 2 visits of current drinking (i.e., low to moderate or heavy) and 2 periods of former drinking were assigned to the “mostly” ethanol intake category at visit 4 (i.e., former low to moderate or heavy).
Definition and counts for this long-term categorization are presented in Table 1. Average ethanol intake across 9 years in midlife was calculated for each participant by averaging weekly ethanol intake reported in ARIC visits 1–4.
Table 1.
Trajectories of Ethanol Intake Across Midlife, According to Race and Overall, Atherosclerosis Risk in Communities, United States, 1987–1998
| Race | |||||||
|---|---|---|---|---|---|---|---|
| Black (n = 2,169; 19.9%) | White (n = 8,707; 80.1%) | Overall (n = 10,876) | |||||
| Long-TermEthanol Intake | Weekly Ethanol Intake | No. a | % a | No. a | % a | No. a | % a |
| Stable, never | 0 g/week at each visit | 472 | 21.8 | 1,033 | 11.8 | 1,505 | 13.8 |
| Stable, low to moderate | At each visit: ≤210 g/week for men; ≤105 g/week for women | 178 | 8.2 | 3,095 | 35.5 | 3,273 | 30.1 |
| Stable, heavy | At each visit: >210 g/week for men and >105 g/week for women at each visit | 15 | 0.7 | 176 | 2.0 | 191 | 1.8 |
| Stable, former | At each visit: classified as former drinker, visits 1–4 | 191 | 8.8 | 664 | 7.6 | 855 | 7.9 |
| Mostly low to moderate | Majority of visits: classified as low to moderate ethanol drinking | 314 | 14.5 | 1,091 | 12.5 | 1,405 | 12.9 |
| Mostly heavy | Majority of visits: classified as heavy ethanol drinking | 53 | 2.4 | 511 | 5.9 | 564 | 5.2 |
| Mostly former | Majority of visits: classified as former ethanol drinking | 561 | 25.9 | 1,687 | 19.4 | 2,248 | 20.7 |
| Unclassifiedb | 385 | 17.7 | 450 | 5.2 | 835 | 7.7 | |
Abbreviation: ARIC, Atherosclerosis Risk in Communities.
a Counts and percentages were calculated based on data prior to using multiple imputation using chained equations to impute missing weekly ethanol intake data for ARIC visits 1–4
b Unclassified: study participants’ long-term ethanol intake could not be classified according to table categories or any well-established drinking category found in published literature.
Assessment of cognitive function
Cognitive function was assessed at visit 2 (1990–1992, at ages 48–67 years), visit 4 (1996–1998, at ages 54–73 years), and visit 5 (as part of the ARIC Neurocognitive Study, 2011–2013, at ages 70–89 years) using 3 standardized cognitive tests. All 3 tests were administered by trained examiners using standardized protocols in a quiet room. Recordings were reviewed for quality control.
Verbal learning and recent memory were assessed by the delayed word recall test (DWRT). Participants were asked to learn 10 nouns, and after a 5-minute delay were given 60 seconds to recall the words. The DWRT score is the number of words recalled (0–10) (42).
Executive function and processing speed were assessed by the digit symbol substitution test (DSST). Participants were given 90 seconds to fill in blank squares with symbols corresponding to digits from 1–9 using a key that matches digits to symbols (43).
Executive function and expressive language were assessed by the word fluency test (WFT), during which participants generate as many words starting with the letters F, A, and S as possible within 60 seconds, with 1 trial per letter (44). The WFT score is the total number of acceptable words generated for the 3 letters (45).
To facilitate comparison across cognitive tests, z scores standardized to visit 2 were calculated for each test by subtracting the participant’s overall mean test score at visit 2 from their test score at each visit and then dividing by the standard deviation of the visit 2 scores.
Using data from these tests in a factor analysis, factor scores for general cognitive performance were derived (46). Briefly, the factor analysis is a structured approach for identifying common covariation between specific indicators, in this case the cognitive tests, to reduce measurement error when combining data across multiple cognitive tests. The interpretations of factor scores are similar to that for z scores because they were scaled to have a mean of 0 and variance of 1 at ARIC visit 2, when the participant’s cognitive function was first tested.
Covariates
Potential confounders were identified from literature (2, 47, 48) and directed-acyclic-graph analysis (49).
Age, sex, and educational attainment (less than high school, high-school graduation, beyond high school), and smoking status (current, former, never) were assessed at visit 4 via self-report from the home interviews. Time spent in moderate to vigorous physical activity in metabolic-equivalent minutes/week was measured at visits 1 and 3 using the modified Baecke questionnaire (50). Apolipoprotein E  4 (APOE
4 (APOE 4) (0, 1, 2) was genotyped by TaqMan assay (Applied Biosystems, Foster City, California) (51, 52). Body mass index was calculated at visit 4 as weight (kg) divided by height (m) squared. Diabetes (yes, no) was defined at visit 4 as self-reported history of a physician’s diagnosis of diabetes, fasting blood glucose level of ≥126 mg/dL, nonfasting blood glucose level of ≥200 mg/dL, or diabetes medication use in the past 2 weeks. Stroke was defined by a self-reported history at visit 1 or an adjudicated event between visits 1 and 4 (53). Dietary factors were assessed using an interviewer-administered 66-item food frequency questionnaire measuring usual intake of foods over the past year at visit 1 (1987–1989) and visit 3 (1993–1995). We calculated the Healthy Food Score, adapted from Steffen et al., described elsewhere (54, 55).
4) (0, 1, 2) was genotyped by TaqMan assay (Applied Biosystems, Foster City, California) (51, 52). Body mass index was calculated at visit 4 as weight (kg) divided by height (m) squared. Diabetes (yes, no) was defined at visit 4 as self-reported history of a physician’s diagnosis of diabetes, fasting blood glucose level of ≥126 mg/dL, nonfasting blood glucose level of ≥200 mg/dL, or diabetes medication use in the past 2 weeks. Stroke was defined by a self-reported history at visit 1 or an adjudicated event between visits 1 and 4 (53). Dietary factors were assessed using an interviewer-administered 66-item food frequency questionnaire measuring usual intake of foods over the past year at visit 1 (1987–1989) and visit 3 (1993–1995). We calculated the Healthy Food Score, adapted from Steffen et al., described elsewhere (54, 55).
Statistical analysis
Multiple imputation.
Missing data due to attrition were imputed by multiple imputation using chained equations (MICE) (56). Missing ethanol intake and cognitive data across ARIC visits were imputed based on the observed values of key covariates for a given individual, as well as the relationships observed in the data for other participants. To account for the uncertainty of the imputation and ensure correct standard error estimation (56), 25 data sets were imputed (57). Validation of the MICE approach for cognitive outcome in ARIC has been previously reported, and it has been determined that MICE produced unbiased imputed values using 25 imputed data sets (57).
Statistical modeling.
To evaluate change in general cognitive performance, DSST, DWRT, and WFT tests between visits 4 and 5, multivariable linear regression models were used, with the outcome being visit-5 z score minus visit-4 z score. All models were race-stratified and adjusted for age, age squared, sex, race-center, educational attainment, smoking status, body mass index, diabetes, history of stroke, diet score, physical activity, and the APOE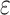 4 allele.
4 allele.
Statistical analyses were performed with Stata, version 15 (StataCorp LLC, College Station, Texas) (58), and SAS, version 9.4 (SAS Institute, Cary, North Carolina), across 25 imputed data sets. The results from each imputed data set were summarized using Rubin’s rule (59) into an overall estimate accounting for both within and between imputation variances.
RESULTS
The trajectories of ethanol during midlife observed in our study sample were stable, never drinking (13.8%); stable, low to moderate drinking (30.1%); stable, heavy drinking (1.8%); stable, former drinking (7.9%); mostly low to moderate drinking (12.9%); mostly heavy drinking (5.2%); and mostly former drinking (20.7%) (Table 1). Overall, 7.7% of our study population’s long-term ethanol intake could not be classified as “stable” or “mostly” drinking, or any other drinking category used in ethanol research literature.
The mean age of participants at baseline was 63 years, 56% were female, and 20% were Black (Web Tables 1 and 2, available at https://academic.oup.com/aje). Stable, never drinkers were more likely to be never smokers and Black and were less likely to engage in physical activity. Stable, heavy drinkers were more likely to be current smokers and had a higher prevalence of hypertension and stroke, among Blacks only. Participants who attended visit 5 were, at baseline, younger and healthier, had higher cognitive performance, and were less likely to be heavy drinkers compared with those who died before visit 5 or were alive but did not attend visit 5 (Web Table 3).
Nine-year ethanol drinking trajectories and 15-year cognitive decline
Results from the multivariable linear regression models suggest no overall association between 9-year trajectories of ethanol intake in midlife and 15-year change in general cognitive performance, DWRT, WFT, and DSST z scores (Web Tables 4 and 5, and Figure 2).
Figure 2.
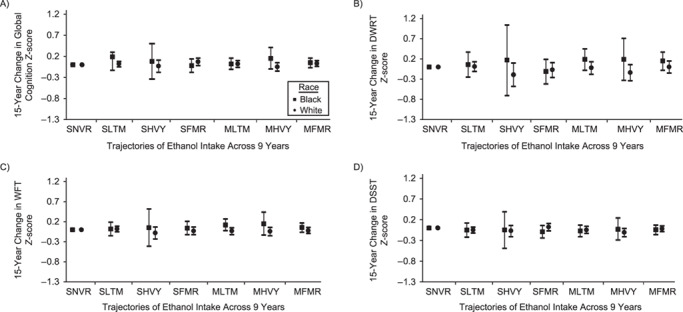
Change in cognitive performance in midlife, Atherosclerosis Risk in Communities, United States, 1987–2013. Adjusted mean difference in 15-year change in cognitive performance by 9-year trajectories of ethanol intake in midlife is shown relative to those who reported stable, never drinking. A) General cognitive performance; B) delayed word recall test (DWRT); C) word fluency test (WFT); and D) digit symbol substitution test (DSST). Multivariable linear regression models adjusted for age, age squared, sex, race-center, educational attainment, diet quality score, physical activity, smoking status, body mass-index, diabetes, history of stroke and the apolipoprotein E 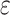 4 (APOE
4 (APOE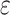 4) allele. All estimates are averages from 25 rounds of multiple imputation combined using Rubin’s rule and the variance of a function of the within and between completed data set variances. Sample sizes: Blacks (global cognition, n = 2,342; DWRT, n = 2,340; WFT, n = 2,334; and DSST, n = 2,325) and Whites (global cognition, n = 8,709; DWRT, n = 8,706; WFT, n = 8,702; and DSST, n = 8,691). MFMR, mostly former drinker; MHVY, mostly heavy drinker; MLTM, mostly low to moderate drinker; SFMR, stable, former drinker; SHVY, stable, heavy drinker; SLTM, stable, low to moderate drinker; SNVR, stable, never drinker.
4) allele. All estimates are averages from 25 rounds of multiple imputation combined using Rubin’s rule and the variance of a function of the within and between completed data set variances. Sample sizes: Blacks (global cognition, n = 2,342; DWRT, n = 2,340; WFT, n = 2,334; and DSST, n = 2,325) and Whites (global cognition, n = 8,709; DWRT, n = 8,706; WFT, n = 8,702; and DSST, n = 8,691). MFMR, mostly former drinker; MHVY, mostly heavy drinker; MLTM, mostly low to moderate drinker; SFMR, stable, former drinker; SHVY, stable, heavy drinker; SLTM, stable, low to moderate drinker; SNVR, stable, never drinker.
Among Blacks, stable, low to moderate drinkers (adjusted 15-year decline: −0.61, 95% confidence interval (CI): −1.03, −0.20), stable, heavy drinkers (adjusted 15-year decline: −0.57, 95% CI: −1.14, 0.00), mostly low to moderate drinkers (adjusted 15-year decline: −0.62, 95% CI: −1.03, −0.21), mostly heavy drinkers (adjusted 15-year decline: −0.49, 95% CI: −0.95, −0.03), and mostly former drinkers (adjusted 15-year decline: −0.60, 95% CI: −1.00, −0.19) had nominally lower 15-year decline in general cognitive performance z scores than stable, never drinkers (adjusted 15-year decline: −0.64, 95% CI: −1.05, −0.24), equivalent to 5%, 12%, 4%, 24%, and 7% lesser declines, respectively. However, slightly greater 15-year decline in general cognitive performance was observed for stable, former drinkers (adjusted 15-year decline: −0.67, 95% CI: −1.08, −0.25) than stable, never drinkers adjusted 15-year decline: (−0.64, 95% CI: −1.05, −0.24), equivalent to 3% greater decline (Web Table 4).
Among Whites, for 15-year decline in general cognitive performance, stable, low to moderate drinkers (adjusted 15-year decline: −0.84, 95% CI: −1.08, −0.60), stable, former drinkers (adjusted 15-year decline: −0.78, 95% CI: −1.03, −0.54), mostly low to moderate drinkers (adjusted 15-year decline: −0.84, 95% CI: −1.08, −0.59), and mostly former drinkers (adjusted 15-year decline: −0.83, 95% CI: −1.07, −0.58) had nominally lower 15-year decline in general cognitive performance than stable, never drinkers (adjusted 15-year decline: −0.86, 95% CI: −1.10, −0.62), equivalent to 2%, 8%, 2%, and 4% lesser declines, respectively. However, among Whites, slightly greater 15-year declines in general cognitive performance were observed for stable heavy drinkers (adjusted 15-year decline: −0.89, 95% CI: −1.18, −0.60) and mostly heavy drinkers (adjusted 15-year decline: −0.90, 95% CI: −1.15, −0.66) than for stable, never drinkers (adjusted 15-year decline: −0.86, 95% CI: −1.10, −0.62), equivalent to 4% and 5% greater declines, respectively. In addition, Whites who were mostly heavy drinkers (adjusted 15-year decline: −0.89, 95% CI: −1.18, −0.61) had a greater 15-year decline in DSST z scores than Whites who were stable, never drinkers (adjusted 15-year decline: −0.78, 95% CI: −1.06, −0.51), equivalent to 14% greater decline (Web Table 5).
Average ethanol intake across 9 years and 15-year cognitive decline
We observed no overall association between average ethanol intake across 9 years in midlife and 15-year change in general cognitive performance, DWRT, DSST, and WFT z scores from mid- to late life among Blacks (Table 2) and Whites (Table 3). There was no evidence of trends of increased 15-year change in general cognitive performance, DWRT, WFT, and DSST scores across quartiles of average ethanol intake across 9 years, among Blacks and Whites (Tables 2 and 3) (Web Figure 1).
Table 2.
Adjusted Mean Difference in 15-Year Change in Cognitive Performance Among Black Participants According to Quartiles of Cumulative Average Ethanol Intake, Atherosclerosis Risk in Communities, United States, 1987–2013
| Test and Quartile a | No. | Baseline Cognitive Score | 15-Year Decline b | Adjusted Mean Difference b , c , d | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Estimate | 95% CI | Estimate | 95% CI | % e | P for trend f | ||
| Global factor score z score | 2,342 | −0.73 (0.75) | 0.79 | |||||
| Quartile 1 | 300 | −0.55 (0.75) | −0.66 | −1.23, −0.09 | 0 | Referent | 0 | |
| Quartile 2 | 271 | −0.56 (0.79) | −0.69 | −1.26, −0.11 | −0.02 | −0.17, 0.13 | 3 | |
| Quartile 3 | 263 | −0.66 (0.82) | −0.67 | −1.24, −0.10 | 0.00 | −0.15, 0.14 | 1 | |
| Quartile 4 | 279 | −0.77 (0.79) | −0.67 | −1.24, −0.10 | −0.01 | −0.17, 0.15 | 1 | |
| P for differenceg | 0.99 | |||||||
| Delayed word recall z score | 2,340 | −0.37 (1.11) | 0.83 | |||||
| Quartile 1 | 301 | −0.30 (1.08) | −0.78 | −1.85, 0.28 | 0 | Referent | 0 | |
| Quartile 2 | 270 | −0.27 (1.11) | −0.75 | −1.81, 0.32 | 0.04 | −0.25, 0.32 | −4 | |
| Quartile 3 | 261 | −0.38 (1.08) | −0.86 | −1.90, 0.19 | −0.07 | −0.38, 0.23 | 9 | |
| Quartile 4 | 279 | −0.49 (1.21) | −0.84 | −1.88, 0.20 | −0.06 | −0.39, 0.27 | 7 | |
| P for differenceg | 0.88 | |||||||
| Word fluency z score | 2,334 | −0.39 (1.06) | 0.71 | |||||
| Quartile 1 | 300 | −0.15 (1.05) | −0.64 | −1.23, −0.05 | 0 | Referent | 0 | |
| Quartile 2 | 269 | −0.24 (1.06) | −0.62 | −1.2, −0.05 | 0.02 | −0.13, 0.17 | −2 | |
| Quartile 3 | 259 | −0.32 (1.14) | −0.62 | −1.21, −0.03 | 0.02 | −0.14, 0.18 | −3 | |
| Quartile 4 | 279 | −0.41 (1.08) | −0.66 | −1.23, −0.09 | −0.02 | −0.19, 0.15 | 3 | |
| P for differenceg | 0.96 | |||||||
| Digit symbol substitution z score | 2,325 | −0.97 (0.94) | 0.87 | |||||
| Quartile 1 | 299 | −0.74 (0.91) | −0.60 | −1.2, 0.00 | 0 | Referent | 0 | |
| Quartile 2 | 267 | −0.72 (0.94) | −0.62 | −1.22, −0.03 | −0.02 | −0.17, 0.13 | 4 | |
| Quartile 3 | 266 | −0.90 (1.03) | −0.58 | −1.17, 0.00 | 0.02 | −0.14, 0.18 | −3 | |
| Quartile 4 | 273 | −1.00 (0.97) | −0.61 | −1.19, −0.03 | −0.01 | −0.17, 0.16 | 1 | |
| P for differenceg | 0.96 | |||||||
Abbreviations: CI, confidence interval; SD, standard deviation.
a Quartiles of ethanol intake, in grams/week. Global factor score z-score quartile 1, 0; quartile 2, 2.7–26.4; quartile 3, 26.4–86.2; and quartile 4, 86.3–1,559.4. Delayed word recall z score quartile 1, 0; quartile 2, 2.7–26.4; quartile 3, 26.4–85.8; and quartile 4, 86.1–1,526.1. Word fluency z score quartile 1, 0; quartile 2, 2.7–26.4; quartile 3, 26.4–85.8; and quartile 4, 86.0–1,565.1. Digit symbol substitution z score quartile 1, 0; quartile 2, 2.7–26.4; quartile 3, 26.4–87.3; and quartile 4: 87.9–1,566.9.
b Estimates were averages from 25 rounds of multiple imputation combined using Rubin’s rule and the variance of a function of the within and between completed data set variances.
c Multivariable linear regression models adjusted for age, age squared, sex, race-center, educational attainment, diet quality score, physical activity, smoking status, body mass index, diabetes, history of stroke, and the apolipoprotein E 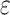 4 allele.
4 allele.
d Difference modeled as the follow-up neurocognitive exam (visit 5; 2011–2013) z score minus study baseline (visit 4; 1996–1998) z score. Negative values correspond to greater decline compared with the referent (lowest quartile).
e Positive values represent % greater decline relative to the reference group.
f P value for trend from multivariable linear regression model with cumulative average ethanol intake modeled as quartiles.
g P value for t test of equality of mean difference in 15-year change in cognitive performance across quartiles of cumulative average ethanol intake.
Table 3.
Adjusted Mean Difference in 15-Year Change in Cognitive Performance Among White Participants According to Quartiles of Cumulative Average Ethanol Intake, Atherosclerosis Risk in Communities, United States, 1987–2013
| Test and Quartile a | No. | Baseline Cognitive Score | 15-Year Decline b | Adjusted MeanDifference b , c , d | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Estimate | 95% CI | Estimate | 95% CI | % e | P for trend f | ||
| Global factor score z score | 8,709 | 0.12 (0.72) | 0.83 | |||||
| Quartile 1 | 1,706 | 0.21 (0.70) | −0.84 | −1.10, −0.57 | 0 | Referent | 0 | |
| Quartile 2 | 1,520 | 0.25 (0.69) | −0.84 | −1.10, −0.58 | 0.00 | −0.06, 0.05 | 1 | |
| Quartile 3 | 1,609 | 0.21 (0.70) | −0.83 | −1.09, −0.57 | 0.00 | −0.06, 0.06 | −0.30 | |
| Quartile 4 | 1,612 | 0.09 (0.71) | −0.87 | −1.13, −0.60 | −0.03 | −0.09, 0.03 | 4 | |
| P for differenceg | 0.64 | 0 | ||||||
| Delayed word recall z score | 8,706 | 0.06 (1.00) | 0.39 | |||||
| Quartile 1 | 1,704 | 0.15 (0.96) | −1.46 | −2.05, −0.86 | 0 | Referent | 0 | |
| Quartile 2 | 1,518 | 0.12 (0.99) | −1.44 | −2.02, −0.86 | 0.02 | −0.10, 0.14 | −1 | |
| Quartile 3 | 1,611 | 0.08 (0.98) | −1.40 | −1.99, −0.81 | 0.06 | −0.06, 0.18 | −4 | |
| Quartile 4 | 1,612 | 0.00 (1.03) | −1.50 | −2.09, −0.92 | −0.05 | −0.17, 0.08 | 3 | |
| P for differenceg | 0.34 | |||||||
| Word fluency z score | 8,702 | 0.14 (0.95) | 0.53 | |||||
| Quartile 1 | 1,704 | 0.14 (0.91) | −0.53 | −0.85, −0.21 | 0 | Referent | 0 | |
| Quartile 2 | 1,517 | 0.25 (0.93) | −0.52 | −0.84, −0.20 | 0.01 | −0.06, 0.08 | −2 | |
| Quartile 3 | 1,611 | 0.24 (0.97) | −0.52 | −0.83, −0.20 | 0.02 | −0.05, 0.08 | −3 | |
| Quartile 4 | 1,610 | 0.23 (1.00) | −0.56 | −0.88, −0.23 | −0.02 | −0.09, 0.04 | 5 | |
| P for differenceg | 0.64 | |||||||
| Digit symbol substitution z score | 8,691 | 0.17 (0.79) | 0.89 | |||||
| Quartile 1 | 1,702 | 0.27 (0.78) | −0.80 | −1.07, −0.52 | 0 | Referent | 0 | |
| Quartile 2 | 1,524 | 0.30 (0.77) | −0.80 | −1.08, −0.53 | −0.01 | −0.06, 0.05 | 1 | |
| Quartile 3 | 1,603 | 0.27 (0.77) | −0.79 | −1.06, −0.51 | 0.01 | −0.04, 0.06 | −1 | |
| Quartile 4 | 1,607 | 0.10 (0.77) | −0.82 | −1.10, −0.54 | −0.02 | −0.09, 0.04 | 3 | |
| P for differenceg | 0.70 | |||||||
Abbreviations: CI, confidence interval; SD, standard deviation.
a Quartiles of ethanol intake, in grams/week. Global factor score z-score quartile 1, 0; quartile 2, 2.7–24.1; quartile 3, 24.3–80.2; and quartile 4, 80.3–1,071.6. Delayed word recall z score quartile 1, 0; quartile 2, 2.7–24.0; quartile 3, 24.1–80.2; and quartile 4, 80.2–1,072.8. Word fluency z score quartile 1, 0; quartile 2, 2.7–24.0; quartile 3, 24.1–80.2; and quartile 4, 80.3–1,071.6. Digit symbol substitution z score quartile 1, 0; quartile 2, 2.7–24.3; quartile 3, 24.3–80.2; and quartile 4: 80.2–1,072.8
b Estimates were averages from 25 rounds of multiple imputation combined using Rubin’s rule and the variance of a function of the within and between completed data set variances.
c Multivariable linear regression models adjusted for age, age squared, sex, race-center, educational attainment, diet quality score, physical activity, smoking status, body mass index, diabetes, history of stroke, and the apolipoprotein E 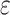 4 allele.
4 allele.
d Difference modeled as the follow-up neurocognitive exam (visit 5; 2011–2013) z score minus study baseline (visit 4; 1996–1998) z score. Negative values correspond to greater decline compared with the referent (lowest quartile).
e Positive values represent % greater decline relative to the reference group.
f P value for trend from multivariable linear regression model with cumulative average ethanol intake modeled as quartiles.
g P value for t test of equality of mean difference in 15-year change in cognitive performance across quartiles of cumulative average ethanol intake.
Ethanol intake measured at study baseline and 15-year cognitive decline
We also determined whether ethanol intake levels measured at study baseline (visit 4) showed similar trajectories of associations with 15-year change in general cognitive performance, DWRT, WFT, and DSST z scores. Findings based on ethanol intake levels at study baseline were similar to those observed using 9-year ethanol drinking trajectories for Blacks and Whites (Tables 4 and 5) (Web Figure 2).
Table 4.
Adjusted Mean Difference in 15-year Change in Cognitive Performance Among Black Participants According to Visit 4 Ethanol Intake Status, Atherosclerosis Risk in Communities, United States, 1987–2013
| Test and Drinking Status at Visit 4 | No. | Baseline Cognitive Score | 15-Year Decline a | Adjusted Mean Difference a,b , c | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Estimate | 95% CI | Estimate | 95% CI | % d | P for Difference e | ||
| Global factor score z score | 2,342 | −0.73 (0.75) | 0.697 | |||||
| Never drinking | 817 | −0.85 (0.71) | −0.53 | −0.91, −0.15 | 0 | Referent | 0 | |
| Low to moderate drinking | 531 | −0.55 (0.77) | −0.52 | −0.90, −0.14 | 0.01 | −0.10, 0.13 | −2 | |
| Heavy drinking | 78 | −0.62 (0.91) | −0.40 | −0.82, 0.03 | 0.13 | −0.09, 0.35 | −25 | |
| Former drinking | 915 | −0.75 (0.74) | −0.52 | −0.90, −0.14 | 0.01 | −0.09, 0.11 | −1 | |
| Delayed word recall z score | 2,340 | −0.37 (1.11) | 0.814 | |||||
| Never drinking | 816 | −0.39 (1.12) | −0.95 | −1.72, −0.18 | 0 | Referent | 0 | |
| Low to moderate drinking | 530 | −0.31 (1.16) | −0.86 | −1.63, −0.09 | 0.09 | −0.12, 0.31 | −10 | |
| Heavy drinking | 78 | −0.38 (1.13) | −0.79 | −1.62, 0.04 | 0.16 | −0.30, 0.62 | −17 | |
| Former drinking | 916 | −0.38 (1.06) | −0.89 | −1.65, −0.12 | 0.06 | −0.13, 0.26 | −6 | |
| Word fluency z score | 2,334 | −0.39 (1.06) | 0.609 | |||||
| Never drinking | 814 | −0.53 (1.02) | −0.40 | −0.83, 0.03 | 0 | Referent | 0 | |
| Low to moderate drinking | 529 | −0.16 (1.1) | −0.34 | −0.77, 0.10 | 0.06 | −0.06, 0.18 | −16 | |
| Heavy drinking | 77 | −0.28 (1.13) | −0.27 | −0.75, 0.22 | 0.13 | −0.12, 0.38 | −33 | |
| Former drinking | 914 | −0.41 (1.05) | −0.38 | −0.80, 0.05 | 0.02 | −0.08, 0.13 | −6 | |
| Digit symbol substitution z score | 2,325 | −0.97 (0.94) | 0.614 | |||||
| Never drinking | 812 | −1.13 (0.91) | −0.26 | −0.69, 0.17 | 0 | Referent | 0 | |
| Low to moderate drinking | 528 | −0.74 (0.94) | −0.32 | −0.74, 0.11 | −0.06 | −0.17, 0.06 | 21 | |
| Heavy drinking | 77 | −0.75 (1.06) | −0.27 | −0.74, 0.20 | −0.01 | −0.23, 0.22 | 3 | |
| Former drinking | 908 | −0.98(0.92) | −0.32 | −0.74, 0.10 | −0.06 | −0.15, 0.03 | 22 | |
Abbreviations: CI, confidence interval; SD, standard deviation.
a Multivariable linear regression models adjusted for age, age squared, sex, race-center, educational attainment, diet quality score, physical activity, smoking status, body mass-index, diabetes, history of stroke and the apolipoprotein E 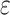 4 allele.
4 allele.
b Estimates were averages from 25 rounds of multiple imputation combined using Rubin’s rule and the variance of a function of the within and between completed data set variances.
c Difference modeled as the follow-up neurocognitive exam (visit 5; 2011–2013) z score minus study baseline (visit 4; 1996–1998) z score. Negative values correspond to greater decline compared with the referent (never drinker).
d Percent, positive values represent % greater decline relative to the referent group.
e P value for t test of equality of mean difference in 15-year change in cognitive performance across visit 4 ethanol intake.
Table 5.
Adjusted Mean Difference in 15-year Change in Cognitive Performance by Visit-4 Ethanol Intake Status for Atherosclerosis Risk in Communities (ARIC) Study White Participants, United States, 1987–2013
| Test and Drinking Status at Visit 4 | No. | Baseline Cognitive Score | 15-Year Decline a | Adjusted Mean Difference a,b , c | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Estimate | 95% CI | Estimate | 95% CI | % d | P for Difference e | ||
| Global factor score z score | 8,709 | 0.12 (0.72) | 0.072 | |||||
| Never drinking | 1,450 | −0.02 (0.70) | −0.84 | −1.07, −0.61 | 0 | Referent | 0 | |
| Low to moderate drinking | 4,247 | 0.25 (0.69) | −0.83 | −1.06, −0.59 | 0.01 | −0.04, 0.07 | −1 | |
| Heavy drinking | 629 | 0.23 (0.69) | −0.90 | −1.14, −0.65 | −0.06 | −0.15, 0.03 | 7 | |
| Former drinking | 2,383 | −0.05 (0.72) | −0.80 | −1.04, −0.57 | 0.04 | −0.02, 0.10 | −4 | |
| Delayed word recall z score | 8,706 | 0.06 (1.00) | 0.177 | |||||
| Never drinking | 1,450 | 0.01 (1.01) | −1.37 | −1.91, −0.83 | 0 | Referent | 0 | |
| Low to moderate drinking | 4,246 | 0.12 (0.98) | −1.36 | −1.89, −0.83 | 0.02 | −0.09, 0.13 | −1 | |
| Heavy drinking | 627 | 0.15 (1.01) | −1.53 | −2.08, −0.98 | −0.16 | −0.34, 0.03 | 11 | |
| Former drinking | 2,383 | −0.02 (1.03) | −1.38 | −1.91, −0.84 | 0.00 | −0.13, 0.12 | 0.4 | |
| Word fluency z score | 8,702 | 0.14 (0.95) | 0.323 | |||||
| Never drinking | 1,450 | −0.11 (0.90) | −0.43 | −0.74, −0.12 | 0 | Referent | 0 | |
| Low to moderate drinking | 4,245 | 0.27 (0.95) | −0.43 | −0.73, −0.13 | 0.00 | −0.07, 0.06 | 1 | |
| Heavy drinking | 628 | 0.36 (0.96) | −0.50 | −0.80, −0.20 | −0.07 | −0.17, 0.02 | 17 | |
| Former drinking | 2,379 | −0.01 (0.95) | −0.45 | −0.75, −0.15 | −0.03 | −0.09, 0.04 | 6 | |
| Digit symbol substitution z score | 8,691 | 0.17 (0.79) | 0.026 | |||||
| Never drinking | 1,447 | 0.03 (0.78) | −0.77 | −1.04, −0.50 | 0 | Referent | 0 | |
| Low to moderate drinking | 4,242 | 0.30 (0.77) | −0.81 | −1.07, −0.55 | −0.04 | −0.10, 0.02 | 5 | |
| Heavy drinking | 627 | 0.24 (0.75) | −0.87 | −1.15, −0.60 | −0.10 | −0.19, −0.02 | 13 | |
| Former drinking | 2,375 | −0.02 (0.80) | −0.77 | −1.04, −0.51 | 0.00 | −0.06, 0.06 | 0 | |
Abbreviations: CI, confidence interval; SD, standard deviation.
a Multivariable linear regression models adjusted for age, age squared, sex, race-center, educational attainment, diet quality score, physical activity, smoking status, body mass-index, diabetes, history of stroke and the apolipoprotein E 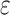 4 allele.
4 allele.
b Estimates were averages from 25 rounds of multiple imputation combined using Rubin’s rule and the variance of a function of the within and between completed data set variances.
c Difference modeled as the follow-up neurocognitive exam (visit 5; 2011–2013) z score minus study baseline (visit 4; 1996–1998) z score. Negative values correspond to greater decline compared with the referent (never drinker).
d Percent, positive values represent % greater decline relative to the referent group.
e P value for t test of equality of mean difference in 15-year change in cognitive performance across visit 4 ethanol intake.
Among Blacks, we observed no overall association between ethanol intake measured at study baseline and 15-year change in general cognitive performance (P = 0.697), DWRT (P = 0.814), WFT (P = 0.609), and DSST (P = 0.614) z scores (Table 4).
Among Whites, we observed no overall association between ethanol intake and 15-year change in general cognitive performance (P = 0.072), DWRT (P = 0.177), and WFT (P = 0.323) z scores (Table 5). However, an association was observed between ethanol intake measured at study baseline and 15-year change in DSST z scores (P = 0.026). The difference in 15-year change in DSST z scores between heavy drinkers and never drinkers at study baseline was −0.10 (95% CI: −0.19, −0.02), equivalent to 13% greater decline. Declines in cognitive performance were slighter higher for long-term trajectories of ethanol intake than ethanol intake at study baseline.
DISCUSSION
This study, conducted in a community cohort, found no evidence that stable, low to moderate drinking and mostly low to moderate drinking in midlife were associated with lesser 15-year cognitive decline from mid- to late life compared with stable, never drinking. There was no evidence that stable, heavy drinking; mostly heavy drinking; stable, former drinking; and mostly former drinking were associated with greater 15-year cognitive decline from mid- to late life compared with stable, never drinking. No association was found for ethanol intake averaged across 9 years during midlife and 15-year cognitive decline from mid- to late life. Further, we did not observe an association with ethanol intake at baseline and 15-year cognitive decline, except for DSST in Whites. The difference in 15-year change in DSST z scores among heavy drinkers compared with never drinkers at study baseline was −0.10 (95% CI: −0.19, −0.02), equivalent to 13% greater decline. In Blacks and Whites, we observed similar declines in cognitive performance for stable drinking categories and drinking categories measured at study baseline. Overall, a slightly lesser rate of decline was observed among Blacks.
Cross-sectional studies suggest a protective association of moderate ethanol intake on cognitive function (60–62). However, cross-sectional studies are vulnerable to reverse causation, selection bias, and residual confounding. A cross-sectional analysis of the association of ethanol intake with magnetic resonance imaging–defined cerebral abnormalities conducted in the ARIC study reported no significant neuroprotective association of low to moderate drinking on White matter grade in middle-aged adults (63). White matter hyperintensities are a well-established marker of cerebral small vessel disease (64–66) and subsequent cognitive impairment (67).
Previous studies of ethanol intake and cognitive decline (12–26) were conducted primarily in older populations (≥65 years at study baseline), had short follow-up (<5 years) (12, 13, 16, 19–21, 23), and used single measures of ethanol intake (12, 13, 16–18, 21, 23–25). Most studies assessed cognitive function using the Mini-Mental State Examination (13, 17, 21, 23, 25), which has known “ceiling effects” (68). Most studies did not adjust for the APOE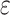 4 allele (16, 17, 19–21, 24, 25), a strong risk factor for cognitive impairment (69). Previous studies estimate that the association of ethanol intake with cognitive decline might reflect survival bias, given their older study populations, and attrition bias, which was not accounted for in their analyses. Some studies that reported a protective association of low to moderate ethanol intake on cognitive decline used a reference group of nondrinkers including former drinkers who might have abstained from ethanol due to poor health (“sick quitters”) (13, 24); this increases risk in the nondrinkers group and therefore biases estimates of moderate versus nondrinking toward a protective association (70–74).
4 allele (16, 17, 19–21, 24, 25), a strong risk factor for cognitive impairment (69). Previous studies estimate that the association of ethanol intake with cognitive decline might reflect survival bias, given their older study populations, and attrition bias, which was not accounted for in their analyses. Some studies that reported a protective association of low to moderate ethanol intake on cognitive decline used a reference group of nondrinkers including former drinkers who might have abstained from ethanol due to poor health (“sick quitters”) (13, 24); this increases risk in the nondrinkers group and therefore biases estimates of moderate versus nondrinking toward a protective association (70–74).
Our study differs from previous studies of ethanol intake and cognitive decline by investigating long-term trajectories of ethanol intake across 9 years in midlife with cognitive decline from mid- to late life over a 15-year course of prospective follow-up in a biracial sample of middle-aged Black and White adults. Our study assessed cognitive function using cognitive tests that, in contrast to the Mini-Mental State Examination, have no “ceiling effects.” Unlike some previous studies of ethanol intake and cognitive decline (13, 24), we differentiated never drinkers from former drinkers, and we adjusted for the APOE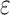 4 allele. We also adjusted for educational attainment, a proxy for socioeconomic status (75), as has been done in previous studies (12–26). Among participants with a high-school or greater education, we observed higher levels of ethanol intake and better cognitive performance than among those with a less than high-school education. Finally, we adjusted for attrition, which might have produced less-biased estimates of the association of ethanol intake with cognitive decline.
4 allele. We also adjusted for educational attainment, a proxy for socioeconomic status (75), as has been done in previous studies (12–26). Among participants with a high-school or greater education, we observed higher levels of ethanol intake and better cognitive performance than among those with a less than high-school education. Finally, we adjusted for attrition, which might have produced less-biased estimates of the association of ethanol intake with cognitive decline.
Low to moderate drinking is hypothetically associated with cognitive decline through cerebrovascular and cardiovascular pathways, involving effects that play out over an extended period of time (76, 77). Heavy ethanol intake on the other hand has detrimental short- and long-term effects on the brain (78, 79).
Several limitations of this study should be mentioned. Ethanol intake was self-reported and therefore could be underreported (80). Our study findings of a lack of association between ethanol intake and cognitive decline in general cognitive performance might be the result of error in the measurement of ethanol intake that could have attenuated the association estimates; the use of a standardized instrument administered by trained personnel and the availability of repeat measurements mitigates this concern. Given that participants who died before visit 5 had higher levels of ethanol intake at baseline, it is possible that survivorship bias could have distorted our results (Web Table 3). Cohort attrition over the prolonged follow-up could have biased an association toward the null, if nondifferentially related to ethanol intake. No clear pattern of association of ethanol intake with attrition was observed, and sensitivity analysis indicated that missing-data patterns were effectively corrected by multiple imputation using chained equations (Web Tables 6–11). Last, the low prevalence of heavy drinking in our study population limited our ability to estimate the impact of heaving drinking on cognitive performance over time.
Strengths of this study include a prospective design and 15 years of follow-up, a biracial population sample, repeated measurements of ethanol intake and well-characterized cognitive function, and rich covariate data.
While low to moderate ethanol intake might reduce the risk of some cardiovascular outcomes (81), harmful effects exists even at low doses for various cancers (82). As a result, the American Heart Association (AHA) does not recommend initiating of ethanol intake for cardiovascular disease and cancer prevention (83). Our findings are consistent with previous reports indicating that moderate ethanol intake might not be protective against cognitive decline, and therefore support the AHA recommendation that low to moderate ethanol intake should not be recommended for the prevention of cognitive decline.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina (Shelly-Ann M. Love, Kari E. North, Anna Kucharska-Newton, Mariaelisa Graff, Laura Loehr, Sarah B. Jones, Gerardo Heiss); Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina (Donglin Zeng); Aetion, Boston, Massachusetts (Natalia Petruski-Ivleva); Department of Epidemiology, College of Public Health, University of Kentucky, Lexington, Kentucky (Anna Kucharska-Newton); Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, New York (Priya Palta); Department of Medicine, Columbia University, New York, New York (Priya Palta); and Department of Medicine, University of North Carolina, Chapel Hill, North Carolina (Laura Loehr).
This work was funded by the National Institute of Alcohol Abuse Alcoholism (F31 Predoctoral Individual National Research Service Grant Award 1F31AA024971-01) and the National Heart, Lung, and Blood Institute (training grant T32HL129982). The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by the National Heart, Lung, and Blood Institute (contracts HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Neurocognitive data is supported by the National Heart, Lung, and Blood Institute, National Institute of Neurological Disorders and Stroke, National Institute on Aging, and National Institute on Deafness and Other Communication Disorders (grants U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, NINDS, NIA and NIDCD), with previous brain magnetic resonance imaging examinations funded by the National Heart, Lung, and Blood Institute (grant R01-HL70825).
Conflict of interest: none declared.
REFERENCES
- 1. Committee on the Public Health Dimensions of Cognitive, Aging Board on Health Sciences Policy, Institute of Medicine, Blazer DG, Yaffe K, et al. . Cognitive Aging: Progress in Understanding and Opportunities for Action. The National Academies Collection: Reports Funded by National Institutes of Health.Washington, DC: National Academies Press; 2015. [PubMed] [Google Scholar]
- 2. Beydoun MA, Beydoun HA, Gamaldo AA, et al. . Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. 2014;14:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salthouse TA. When does age-related cognitive decline begin? Neurobiol Aging. 2009;30(4):507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Christensen H, O'Brien J. Age-related cognitive decline and its relationship to dementia In: O'Brien J, Ames D, Burns A, eds. Dementia. 2nd ed. London, UK: Arnold; 2000:15–28. [Google Scholar]
- 5. Schaie KW. The optimization of cognitive functioning in old age: Predictions based on cohort-sequential and longitudinal data In: Baltes MM, Baltes PB, eds. Successful Aging: Perspectives from the Behavioral Sciences. Cambridge, UK: Cambridge University Press; 1990:94–117. [Google Scholar]
- 6. Alzheimer’s Association 2014 Alzheimer's disease facts and figures. Alzheimers Dement. 2014;10(2):e47–e92. [DOI] [PubMed] [Google Scholar]
- 7. Ortman JM, Velkoff VA, Hogan H An aging nation: the older population in the United States. https://www.census.gov/prod/2014pubs/p25-1140.pdf. Accessed January 8, 2020.
- 8. Schwartz BS, Glass TA, Bolla KI, et al. . Disparities in cognitive functioning by race/ethnicity in the Baltimore Memory Study. Environ Health Perspect. 2004;112(3):314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang MX, Cross P, Andrews H, et al. . Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49–56. [DOI] [PubMed] [Google Scholar]
- 10. Bowen ME. Childhood socioeconomic status and racial differences in disability: evidence from the Health and Retirement Study (1998–2006). Soc Sci Med. 2009;69(3):433–441. [DOI] [PubMed] [Google Scholar]
- 11. Kelley-Moore JA, Ferraro KF. The black/white disability gap: persistent inequality in later life? J Gerontol B Psychol Sci Soc Sci. 2004;59(1):S34–S43. [DOI] [PubMed] [Google Scholar]
- 12. Herbert LE, Scherr PA, Beckett LA, et al. . Relation of smoking and low-to-moderate alcohol consumption to change in cognitive function: a longitudinal study in a defined community of older persons. Am J Epidemiol. 1993;137(8):881–891. [DOI] [PubMed] [Google Scholar]
- 13. Dufouil C, Tzourio C, Brayne C, et al. . Influence of apolipoprotein E genotype on the risk of cognitive deterioration in moderate drinkers and smokers. Epidemiology. 2000;11(3):280–284. [DOI] [PubMed] [Google Scholar]
- 14. Leroi I, Sheppard JM, Lyketsos CG. Cognitive function after 11.5 years of alcohol use: relation to alcohol use. Am J Epidemiol. 2002;156(8):747–752. [DOI] [PubMed] [Google Scholar]
- 15. Bond GE, Burr R, McCurry SM, Rice MM, et al. . Alcohol, gender, and cognitive performance: a longitudinal study comparing older Japanese and non-Hispanic white Americans. J Aging Health. 2004;16(5):615–640. [DOI] [PubMed] [Google Scholar]
- 16. Espeland MA, Gu L, Masaki KH, et al. . Association between reported alcohol intake and cognition: results from the Women’s Health Initiative Memory Study. Am J Epidemiol. 2005;161(3):228–238. [DOI] [PubMed] [Google Scholar]
- 17. Ganguli M, Vander Bilt J, Saxton JA, et al. . Alcohol consumption and cognitive function in late life: a longitudinal community study. Neurology. 2005;65(8):1210–1217. [DOI] [PubMed] [Google Scholar]
- 18. Richards M, Hardy R, Wadsworth ME. Alcohol consumption and midlife cognitive change in the British 1946 birth cohort study. Alcohol Alcohol. 2005;40(2):112–117. [DOI] [PubMed] [Google Scholar]
- 19. Stampfer MJ, Kang JH, Chen J, et al. . Effects of moderate alcohol consumption on cognitive function in women. N Engl J Med. 2005;352(3):245–253. [DOI] [PubMed] [Google Scholar]
- 20. Wright CB, Elkind MS, Luo X, et al. . Reported alcohol consumption and cognitive decline: the Northern Manhattan Study. Neuroepidemiology. 2006;27(4):201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stott DJ, Falconer A, Kerr GD, et al. . Does low to moderate alcohol intake protect against cognitive decline in older people? J Am Geriatr Soc. 2008;56(12):2217–2224. [DOI] [PubMed] [Google Scholar]
- 22. Yaffe K, Fiocco AJ, Lindquist K, et al. . Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology. 2009;72(23):2029–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lobo E, Dufouil C, Marcos G, et al. . Is there an association between low-to-moderate alcohol consumption and risk of cognitive decline? Am J Epidemiol. 2010;172(6):708–716. [DOI] [PubMed] [Google Scholar]
- 24. Zanjani F, Downer BG, Kruger TM, et al. . Alcohol effects on cognitive change in middle-aged and older adults. Aging Ment Health. 2013;17(1):12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beydoun MA, Gamaldo AA, Beydoun HA, et al. . Caffeine and alcohol intakes and overall nutrient adequacy are associated with longitudinal cognitive performance among U.S. adults. J Nutr. 2014;144(6):890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sabia S, Elbaz A, Britton A, et al. . Alcohol consumption and cognitive decline in early old age. Neurology. 2014;82(4):332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Swan GE, De Carli C, Miller BL, et al. . Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51(4):986–993. [DOI] [PubMed] [Google Scholar]
- 28. Launer LJ, Masaki K, Petrovitch H, et al. . The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA. 1995;274(23):1846–1851. [PubMed] [Google Scholar]
- 29. Gottesman RF, Schneider AL, Albert M, et al. . Midlife hypertension and 20-year cognitive change: the Atherosclerosis Risk in Communities Neurocognitive Study. JAMA Neurol. 2014;71(10):1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rawlings AM, Sharrett AR, Schneider AL, et al. . Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med. 2014;161(11):785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weuve J, Tchetgen Tchetgen EJ, Glymour MM, et al. . Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23(1):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Britton A, Ben-Shlomo Y, Benzeval M, et al. . Life course trajectories of alcohol consumption in the United Kingdom using longitudinal data from nine cohort studies. BMC Med. 2015;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Britton A, Bell S. Reasons why people change their alcohol consumption in later life: findings from the Whitehall II Cohort Study. PLoS One. 2015;10(3):e0119421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Britton A, Marmot MG, Shipley MJ. How does variability in alcohol consumption over time affect the relationship with mortality and coronary heart disease? Addiction. 2010;105(4):639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Howe CJ, Sander PM, Plankey MW, et al. . Effects of time-varying exposures adjusting for time-varying confounders: the case of alcohol consumption and risk of incident human immunodeficiency virus infection. Int J Public Health. 2010;55(3):227–228. [DOI] [PubMed] [Google Scholar]
- 36. Fibrinogen Studies Collaboration, Wood AM, White I, et al. . Regression dilution methods for meta-analysis: assessing long-term variability in plasma fibrinogen among 27,247 adults in 15 prospective studies. Int J Epidemiol. 2006;35(6):1570–1578. [DOI] [PubMed] [Google Scholar]
- 37. Bell S, Britton A. The role of alcohol consumption in regulating circulating levels of adiponectin: a prospective cohort study. J Clin Endocrinol Metab. 2015;100(7):2763–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Greenfield TK, Kerr WC. Commentary on Liang & Chikritzhs (2011): quantifying the impacts of health problems on drinking and subsequent morbidity and mortality— life-course measures are essential. Addiction. 2011;106(1):82–83. [DOI] [PubMed] [Google Scholar]
- 39. The ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 40. Willett WC, Sampson L, Browne ML, et al. . The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127(1):188–199. [DOI] [PubMed] [Google Scholar]
- 41. US Department of Agriculture 2015–2020 Dietary Guidelines for Americans. 8th ed. Washington, DC: US Department of Health and Human Services; 2015. [Google Scholar]
- 42. Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. 1989;46(2):141–145. [DOI] [PubMed] [Google Scholar]
- 43. Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th ed. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 44. Lezar M. Neuropsychological Assessment. 2nd ed. New York, NY: Oxford University Press; 1983. [Google Scholar]
- 45. Benton AL, Hamsher K dZ. Multilingual Aphasia Examination. Manual of Instructions. 2nd ed. Iowa City, IA: AJA Associates; 1989. [Google Scholar]
- 46. Gross AL, Power MC, Albert MS, et al. . Application of latent variable methods to the study of cognitive decline when tests change over time. Epidemiology. 2015;26(6):878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Plassman BL, Williams JW Jr, Burke JR, et al. . Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med. 2010;153(3):182–193. [DOI] [PubMed] [Google Scholar]
- 48. Baumgart M, Snyder HM, Carrillo MC, et al. . Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11(6):718–726. [DOI] [PubMed] [Google Scholar]
- 49. Textor J, Hardt J, Knüppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22(5):745. [DOI] [PubMed] [Google Scholar]
- 50. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. [DOI] [PubMed] [Google Scholar]
- 51.Gene expression analysis using TaqMan assays. https://www.thermofisher.com/us/en/home/life-science/pcr/real-time-pcr/real-time-pcr-assays/taqman-gene-expression.html. Accessed January 8, 2020.
- 52. Volcik KA, Barkley RA, Hutchinson RG, et al. . Apolipoprotein E polymorphisms predict low density lipoprotein cholesterol levels and carotid artery wall thickness but not incident coronary heart disease in 12,491 ARIC study participants. Am J Epidemiol. 2006;164(4):342–348. [DOI] [PubMed] [Google Scholar]
- 53. Toole JF, Lefkowitz DS, Chambless LE, et al. . Self-reported transient ischemic attack and stroke symptoms: methods and baseline prevalence. The ARIC Study, 1987–1989. Am J Epidemiol. 1996;144(9):849–856. [DOI] [PubMed] [Google Scholar]
- 54. Weng LC, Steffen LM, Szklo M, et al. . A diet pattern with more dairy and nuts, but less meat is related to lower risk of developing hypertension in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Nutrients. 2013;5(5):1719–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Steffen LM, Kroenke CH, Yu X, et al. . Associations of plant food, dairy product, and meat intakes with 15-y incidence of elevated blood pressure in young black and white adults: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Clin Nutr. 2005;82(6):1169–1177. [DOI] [PubMed] [Google Scholar]
- 56. Little RJA, Rubin DB. Statistical Analysis With Missing Data. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 57. Rawlings AM, Sang Y, Sharrett AR, et al. . Multiple imputation of cognitive performance as a repeatedly measured outcome. Eur J Epidemiol. 2017;32(1):55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. StataCorp Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 59. Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 60. Dufouil C, Ducimetière P, Alpérovitch A. Sex differences in the association between alcohol consumption and cognitive performance. EVA Study Group. Epidemiology of Vascular Aging. Am J Epidemiol. 1997;146(5):405–412. [DOI] [PubMed] [Google Scholar]
- 61. Elias PK, Elias MF, D’Agostino RB, et al. . Alcohol consumption and cognitive performance in the Framingham Heart Study. Am J Epidemiol. 1999;150(6):580–589. [DOI] [PubMed] [Google Scholar]
- 62. Lang I, Wallace RB, Huppert FA, et al. . Moderate alcohol consumption in older adults is associated with better cognition and well-being than abstinence. Age Ageing. 2007;36(3):256–261. [DOI] [PubMed] [Google Scholar]
- 63. Ding J, Eigenbrodt ML, Mosley TH Jr, et al. . Alcohol intake and cerebral abnormalities on magnetic resonance imaging in a community-based population of middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2004;35(1):16–21. [DOI] [PubMed] [Google Scholar]
- 64. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701. [DOI] [PubMed] [Google Scholar]
- 65. Wardlaw JM, Smith EE, Biessels GJ, et al. . Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yilmaz P, Ikram MK, Niessen WJ, et al. . Practical small vessel disease score relates to stroke, dementia, and death. Stroke. 2018;49(12):2857–2865. [DOI] [PubMed] [Google Scholar]
- 67. Power MC, Tingle JV, Reid RI, et al. . Midlife and late-life vascular risk factors and white matter microstructural integrity: the Atherosclerosis Risk in Communities Neurocognitive Study. J Am Heart Assoc. 2017;6(5):e005608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schneider AL, Sharrett AR, Patel MD, et al. . Education and cognitive change over 15 years: the Atherosclerosis Risk in Communities study. J Am Geriatr Soc. 2012;60(10):1847–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bunce D, Fratiglioni L, Small BJ, et al. . APOE and cognitive decline in preclinical Alzheimer disease and non-demented aging. Neurology. 2004;63(5):816–821. [DOI] [PubMed] [Google Scholar]
- 70. Fillmore KM, Golding JM, Graves KL, et al. . Alcohol consumption and mortality. III. Studies of female populations. Addiction. 1998;93(2):219–229. [DOI] [PubMed] [Google Scholar]
- 71. Leino EV, Romelsjö A, Shoemaker C, et al. . Alcohol consumption and mortality. II. Studies of male populations. Addiction. 1998;93(2):205–218. [DOI] [PubMed] [Google Scholar]
- 72. Fillmore KM, Golding JM, Graves KL, et al. . Alcohol consumption and mortality. I. Characteristics of drinking groups. Addiction. 1998;93(2):183–203. [DOI] [PubMed] [Google Scholar]
- 73. O’Mahony JF, Doherty B. Intellectual impairment among recently abstinent alcohol abusers. Br J Clin Psychol. 1996;35(1):77–83. [DOI] [PubMed] [Google Scholar]
- 74. Goldman E, Najman JM. Lifetime abstainers, current abstainers and imbibers: a methodological note. Br J Addict. 1984;79(3):309–314. [DOI] [PubMed] [Google Scholar]
- 75. Oakes JM, Rossi PH. The measurement of SES in health research: current practice and steps toward a new approach. Soc Sci Med. 2003;56(4):769–784. [DOI] [PubMed] [Google Scholar]
- 76. Pinder RM, Sandler M. Alcohol, wine and mental health: focus on dementia and stroke. J Psychopharmacol. 2004;18(4):449–456. [DOI] [PubMed] [Google Scholar]
- 77. Collins MA, Neafsey EJ, Mukamal KJ, et al. . Alcohol in moderation, cardioprotection, and neuroprotection: epidemiological considerations and mechanistic studies. Alcohol Clin Exp Res. 2009;33(2):206–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhu W, Volkow ND, Ma Y, et al. . Relationship between ethanol-induced changes in brain regional metabolism and its motor, behavioural and cognitive effects. Alcohol Alcohol. 2004;39(1):53–58. [DOI] [PubMed] [Google Scholar]
- 79. Panza F, Frisardi V, Seripa D, et al. . Alcohol consumption in mild cognitive impairment and dementia: harmful or neuroprotective? Int J Geriatr Psychiatry. 2012;27(12):1218–1238. [DOI] [PubMed] [Google Scholar]
- 80. Devos-Comby L, Lange JE. "My drink is larger than yours"? A literature review of self-defined drink sizes and standard drinks. Curr Drug Abuse Rev. 2008;1(2):162–176. [DOI] [PubMed] [Google Scholar]
- 81. Koppes LL, Dekker JM, Hendriks HF, et al. . Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care. 2005;28(3):719–725. [DOI] [PubMed] [Google Scholar]
- 82. Allen NE, Beral V, Casabonne D, et al. . Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101(5):296–305. [DOI] [PubMed] [Google Scholar]
- 83. American Heart Association Is drinking alcohol part of a healthy lifestyle?. http://www.heart.org/HEARTORG/GettingHealthy/NutritionCenter/HealthyEating/Alcohol-and-Heart-Health_UCM_305173_Article.jsp. Accessed January 8, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


