Abstract
Tissue engineering technology provides a promising approach for cartilage repair, and in this strategy, scaffolds play a pivotal role in directing cartilage regeneration. Fish collagen (FC) is currently considered an alternative source of mammalian collagen (MC) for tissue engineering due to its excellent biocompatibility, suitable biodegradability, inert immunogenicity, rich sources, low price and lack of risk for the transmission of zoonosis. Here, we fabricated three types of electrospun nanofibrous membranes composed of FC and polycaprolactone (PCL) with three different FC/PCL ratios (9/1, 7/3, 5/5) and investigated the feasibility of using the membranes with chondrocytes in cartilage regeneration. Our results demonstrated that increases in the FC content were associated with improvements in biodegradability, absorption, and cell adhesion capacity, but weaker mechanical properties. In addition, all three nanofibrous membranes showed satisfactory biocompatibility as evidenced by supporting chondrocyte proliferation and cartilage formation in vitro. Furthermore, all three membranes seeded with chondrocytes formed mature cartilage-like tissue after 8 weeks of in vivo culture, but satisfactory homogeneous cartilage regeneration was only achieved with the F9P1 group. The current results demonstrated that the electrospun FC/PCL membrane is a promising scaffold for cartilage regeneration and that the F9P1 group might represent a relatively suitable ratio. The research models established in the current study provide detailed information for the regeneration of cartilage and other tissue based on electrospun FC/PCL membranes.
Keywords: Fish collagen, electrospun, cartilage regeneration, tissue engineering, biomaterials
Introduction
The repair of cartilage defects poses a great challenge in clinical treatment due to the avascular, aneural, and alymphatic structure of cartilage tissue. Tissue engineering, a newly developed tissue-regenerating technique that involves the use of cells and biodegradable scaffolds, provides a promising approach for the repair and functional reconstruction of cartilage defects [1]. Mammal-derived collagen (MC), which is mainly sourced from bovine tendon and porcine skin, is a commonly used scaffold for cartilage tissue engineering [2-4]. However, the application of MC is restricted by zoonosis, religious issues, immunogenicity, and high cost [4]. Fish collagen (FC) has recently been considered an alternative source of MC due to its excellent biocompatibility, suitable biodegradability, inert immunogenicity, rich sources, low price, and lack of risk for the transmission of zoonosis [5].
Electrospinning is a simple and economical method for producing a connected and porous nanofibrous membrane that mimics the extracellular matrix (ECM) both structurally and mechanically [4]. Furthermore, an electrospun membrane is hopefully able to simultaneously meet all the requirements of an ideal scaffold: mechanical barrier, degradation rate, chondrogenesis and clinical maneuverability [6]. Many studies have indicated that FC-based electrospun scaffolds have wide applicability in the engineering of tissues or organs, such as periodontal tissue [3], skin [7] and bone regeneration [2]. However, whether FC-based electrospun scaffolds could be used to engineer cartilage remains uninvestigated.
In the current study, we fabricated electrospun FC/polycaprolactone (FC/PCL) nanofibrous membranes produced with three different ratios (9/1, 7/3, and 5/5) of these compounds. These nanofibrous membranes were then characterized by analyzing their morphological structure, mechanical strength, biodegradability, absorption and cell adhesion capacity, as well as biocompatibility. In addition, these FC/PCL nanofibrous membranes were employed for cartilage engineering in vitro and in vivo, and we further examined the influences of the FC/PCL ratio on cartilage regeneration.
Materials and methods
Animals
Tilapia was provided by the Shanghai Fisheries Research Institute (Shanghai, China). New Zealand white rabbits aged 2 months were purchased from Shanghai Jiagan Experimental Animal Raising Farm (Shanghai, China), and nude mice aged 6 weeks were purchased from Shanghai Slaccas Experimental Animal Ltd (Shanghai, China). All protocols used for the animal experiments were approved by the Animal Care and Experiment Committee of Weifang Medical University (Shandong, China).
Preparation of FC powder
After cleaning, the tilapia scales were treated with 30% HCl solution for 3 h and then washed with distilled water. The material was then preheated at 60°C for 3 h in distilled water and, enzymatically hydrolyzed with 0.1-0.3% complex protease at 60°C for 6 h. The FC powder used in the subsequent experiments was then obtained after filtration and drying.
Preparation of different electrospun FC/PCL membranes
PCL (MW = 80,000) was purchased from Aldrich. A series of FC/PCL membranes with FC:PCL weight ratios of 9:1, 7:3 and 5:5 (F9P1, F7P3, and F5P5, respectively) were prepared through electrospinning. Briefly, FC and PCL at different weight ratios were dissolved in hexafluoroisopropanol to prepare a 12% w/v solution and magnetically stirred for 24 h. The solution was then placed in a 1 mL syringe equipped with a 27-gauge needle and dispersed by a syringe pump at a feeding rate of 0.4 mL/h under 45-55% humidity at a temperature of 21-22°C. An electrospinning voltage of 9 kV DC was applied to the needle using a high-voltage power supply (TXR1020N30-30, Teslaman, Dalian, China). The distance between the tip of the syringe and the collector was 8 cm. The resultant electrospun membranes were collected on aluminum foil (200 mm × 200 mm) mounted on the surface of an adjustable lab jack. The prepared electrospun FC/PCL membranes were dried in a vacuum oven for 1 week at room temperature to remove all residual solvent before subsequent uses [4].
Characteristic analysis of the membranes
Mechanical analysis
The mechanical properties of the three membranes were determined using a tabletop uniaxial material testing machine (H5K-S, Hounsfield, United Kingdom) equipped with a 10-N load cell. Rectangle-shaped specimens (50 mm × 10 mm × 0.10-0.20 mm) were stretched at a constant cross-head speed of 10 mm/min. The tensile strength, strain at break, and Young’s modulus of all the groups were analyzed and calculated according to the stress-strain curve [8].
Scanning electron microscopy (SEM)
The three membrane types, F9P1, F7P3, and F5P5, were sputter-coated with gold for 50 s to increase their conductivity, and the samples were then examined by SEM (JEOL-6380LV, Japan).
In vitro degradation
Several wafer-shaped F9P1, F7P3, and F5P5 samples were suspended in PBS containing 0.6 mg/mL collagenase (pH 7.4) in a shaking water bath at 37°C for 4 days. The samples were regularly washed with deionized water and dried every day. The degree of degradation was determined by the dry-weight change and the statistical significance of the differences among the groups was determined by one-way analysis of variance.
Hydrophilicity evaluation
The hydrophilicity was evaluated by analyzing the contact angles of water droplets on the F9P1, F7P3, and F5P5 membranes. Deionized water (0.5 mL) was automatically dropped onto the surface of the flat membranes. The contact angle at the fourth second was measured using a video-enabled Goniometer (VCA-optima, AST, Inc.) and calculated automatically [8,9].
In vitro protein adsorption test
The total amount of protein adsorbed in the membranes was determined using a modified bicinchoninic acid protein test as described previously [9,10]. The amount of adsorbed protein on the scaffolds was measured using a BCA protein assay kit (Beyotime, China), and the statistical significance of the differences among the groups was determined by one-way analysis of variance.
Isolation and culture of chondrocytes
The auricular cartilage samples harvested from New Zealand white rabbits were minced into approximately 1.0-mm3 pieces and digested for 8 h with 0.15% type II collagenase (Gibco) in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) at 37°C. The isolated chondrocytes were cultured in DMEM supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin-streptomycin (Gibco) at 37°C under an atmosphere containing 5% CO2. Chondrocytes at passage two (P2) were used for the in vitro and in vivo cartilage evaluations [11].
Cell proliferation assay
To evaluate the biocompatibility of the three membranes, chondrocytes were seeded onto the membranes at a density of 5 × 104 cells/cm2 in DMEM with 10% FBS and then cultured at 37°C with 5% CO2 for 7 days. After in vitro cultured for 1, 4, and 7 days, the viability of chondrocytes in the membranes was determined through a Live & Dead Cell Viability Assay (Invitrogen, USA) and subsequent examination using a confocal microscope (Nikon, A1RMP, Japan). The viable cells were analyzed using Cell Counting Kit-8 (CCK-8; Dojindo, Japan) according to the manufacturer’s instructions. The optical density (OD) was measured at 450 nm, and the mean value derived from five wells was calculated [12]. The statistical significance of the differences among the groups was determined by one-way analysis of variance.
Adherence rate
After 24 h of incubation, the chondrocyte-membrane constructs generated as described above were gently rinsed with PBS to remove dead cells. The rinsing solution and cells remaining in the culture dish were collected and counted, and the result was designated N. The cell seeding efficiencies of the scaffolds were calculated based on the following formula: (total cell number-N)/total cell number × 100% [13]. The statistical significance of the differences among the groups was determined by one-way analysis of variance.
Preparation of chondrocyte-membrane constructs
Chondrocyte-FC/PCL membrane constructs were prepared using a sandwich model as previously reported [12]. Briefly, one piece of FC/PCL membrane was placed in a culture dish, and seeded with 5 mL of a chondrocyte suspension with 100 × 106 cells/mL. Another FC/PCL membrane was then stacked on top of the layer and seeded with the same number of cells. A chondrocyte-FC/PCL membrane sandwich was eventually achieved after stacking three layers of the FC/PCL membranes. The constructs were maintained in the culture dish for 1 h, and DMEM with 10% FBS was then gently added to cover the constructs. The constructs were incubated at 37°C in 5% CO2. The medium was exchanged every 3 days. After 2 weeks of in vitro culture, the constructs were either maintained in culture for another 2 weeks or subcutaneously implanted into nude mice for 8 weeks.
In vivo implantation
After the induction of anesthesia thorough the administration of 0.3 mL of 1% pentobarbital sodium, nude mice underwent aseptic preparation on their back, the skin was incised, and the subcutaneous tissue was separated to form a pocket. The constructs were then placed directly into the pocket, the incision was closed, and the animals were allowed to recover from anesthesia.
Gross views
The three types of electrospun membranes were trimmed to a wafer shape (8 mm in diameter) for photograph. The in vivo samples were carefully stripped to remove the surrounding fibrous tissue and information on their appearance, such as color, texture, and elasticity, was recorded.
Histological and immunohistochemical analyses
The regenerated cartilage was harvested and subjected to histological and immunohistochemical analyses as described previously [14]. Hematoxylin and eosin (HE) and safranin-O staining was performed to evaluate the histological structure and glycosaminoglycan (GAG) deposition, respectively. The expression of collagen II was detected using a rabbit polyclonal antibody against collagen II (ab34712, 1:100, Abcam, Cambridge, UK), followed by a horseradish peroxidase-conjugated anti-rabbit antibody (1:100, Dako, Denmark). Both antibodies were diluted in PBS and then colorized with diaminobenzidine tetrahydrochloride (DAB, Dako).
Biomechanical analyses
To evaluate the mechanical properties of engineered cartilage, Young’s modulus was detected and analyzed using a constant compressive strain rate obtained from a biomechanical analyzer (Instron-5542, Canton, MA, USA) [15]. The statistical significance of the differences among the groups was determined by one-way analysis of variance.
Biochemical analyses
The specimens were digested in papain solution (Sigma-Aldrich) at 65°C. The sulfated glycosaminoglycan (GAG) content of engineered cartilage in subcutaneous sites and, in native rabbit auricular cartilage was quantified using the Alcian Blue method [15,16]. Genomic DNA was recovered in elution buffer after ethanol extraction and column adsorption. The DNA content was detected using a nucleic acid protein quantitation detector (Nanodrop 2000). Each sample was analyzed three times. The content of total collagen in the different groups was quantified using a hydroxyproline assay. The samples were prepared by alkaline hydrolysis, and free hydroxyproline hydrolysates were assayed according to previously described methods [17]. The statistical significance of the differences among the groups was determined by one-way analysis of variance.
Statistical analysis
All quantitative data are presented as the means ± standard deviations, and the statistical significance of the differences among the groups was determined by one-way analysis of variance. The data were analyzed using GraphPad Prism 5.0, and a p value < 0.05 was considered to indicate statistical significance.
Results
Characteristic analysis of electrospun membranes
The influence of the FC/PCL ratio on the basic properties of the electrospun membranes was first investigated. According to the current results, all the groups presented similar gross views and microstructures (Figure 1A) despite the differences in their FC/PCL ratio. Nevertheless, the changes in the FC/PCL ratio had an obvious influence on the mechanical properties of the membranes, as demonstrated by the findings that the stress-strain curves in the different groups presented quite different profiles (Figure 1B-E). The mechanical strength of the scaffolds, such as the tensile strength (Figure 1C) and Young’s modulus (Figure 1D), increased significantly with decreases in the FC content, whereas the opposite trend was found for the strain at break (Figure 1E). All these results indicated that the mechanical properties of the membrane were substantially improved by the incorporation of PCL.
Figure 1.
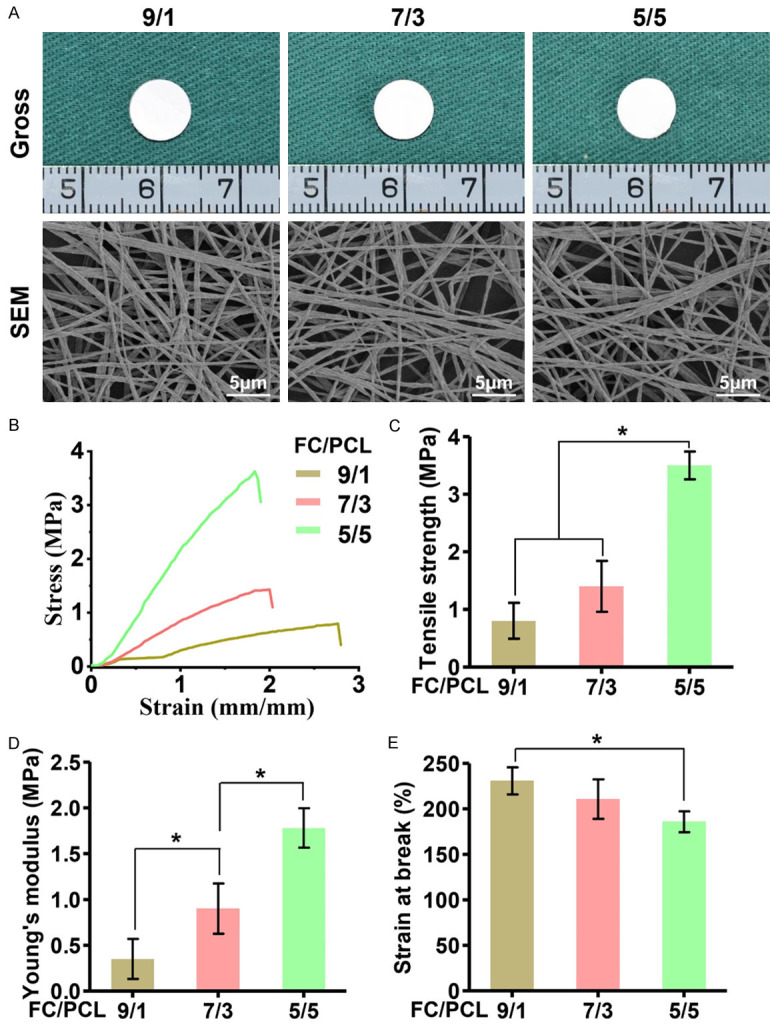
Morphological and mechanical analyses of three different FC/PCL membranes. Gross view and SEM images of the membranes indicated that all the groups presented similar macro- and microstructures (A). The stress-strain curves of the different groups at the wet state showed quite different profiles in (B). Increases in the PCL content of the membranes led to concomitant increases in the tensile strength (C) and Young’s modulus (D) but decreases in the strain at break (E). *P < 0.05.
Biodegradability is an important factor for designing scaffolds that will be used in cartilage tissue engineering. Our results revealed that the degradation rates of the F9P1, F7P3 and F5P5 groups were 92.8 ± 4.1%, 77.4 ± 5.2% and 53.2 ± 4.7%, respectively (Figure 2A), which indicated that the biodegradability decreased with increases in the PCL content.
Figure 2.
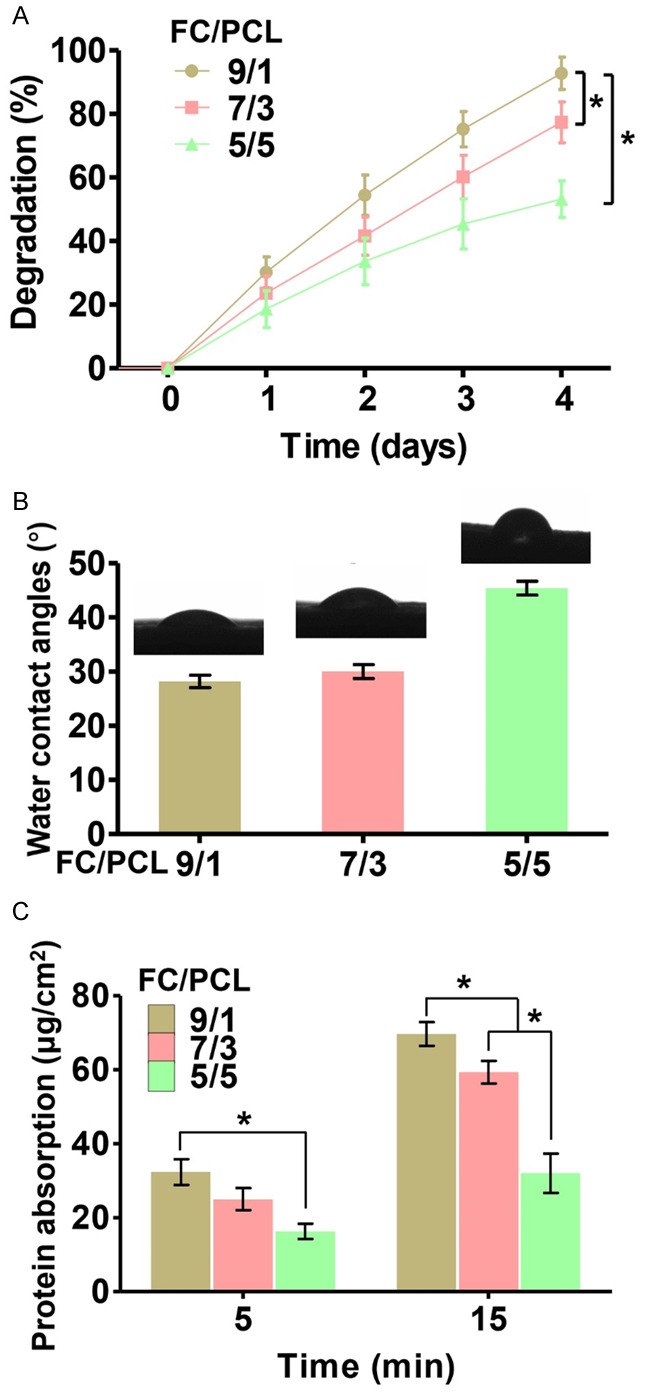
Increasing in the FC content resulted in increases in the degradation rate (A), water contact angles (B) and protein adsorption capacity (C) of the membranes. *P < 0.05.
The hydrophilicity of the membranes was evaluated by testing the water contact angle. The water contact angles at the fourth second obtained for the F9P1, F7P3, and F5P5 membranes were 28.2 ± 2.3%, 30.0 ± 2.6% and 45.4 ± 2.5%, respectively (Figure 2B). This result indicated that the hydrophilicity was significantly increased with increases in the FC content. Moreover, the protein absorption index values also tended to significantly increase with increases in the FC content (Figure 2C), which indicated that FC enhanced the adsorption capacity and might positively affect cell attachment. Collectively, these results indicated that the high PCL content was favorable for the mechanical strength of FC/PCL membranes but unfavorable for their hydrophilicity.
Evaluation of the biocompatibility of the membranes
Biocompatibility, which includes the cell adherence rate, cell viability, and cytotoxicity, is an important aspect for evaluating whether a FC/PCL membrane is suitable for tissue regeneration. The current results revealed that the cell adherence rate tended to significantly increase with increase in the FC content (Figure 3B). Cell viability assays further confirmed that membranes with higher FC content displayed higher numbers of chondrocytes (Figure 3A). Noticeably, the cell viability assays also showed that chondrocytes grew well on all three membranes and exhibited significant proliferation over time, and very few dead cells were observed at any of the investigated time points, which was further confirmed by cytotoxicity analysis (Figure 3C). Collectively, these results indicated that all FC/PCL membranes presented satisfactory biocompatibility and that the F9P1 group exhibited the most superior chondrocyte adherence capacity.
Figure 3.
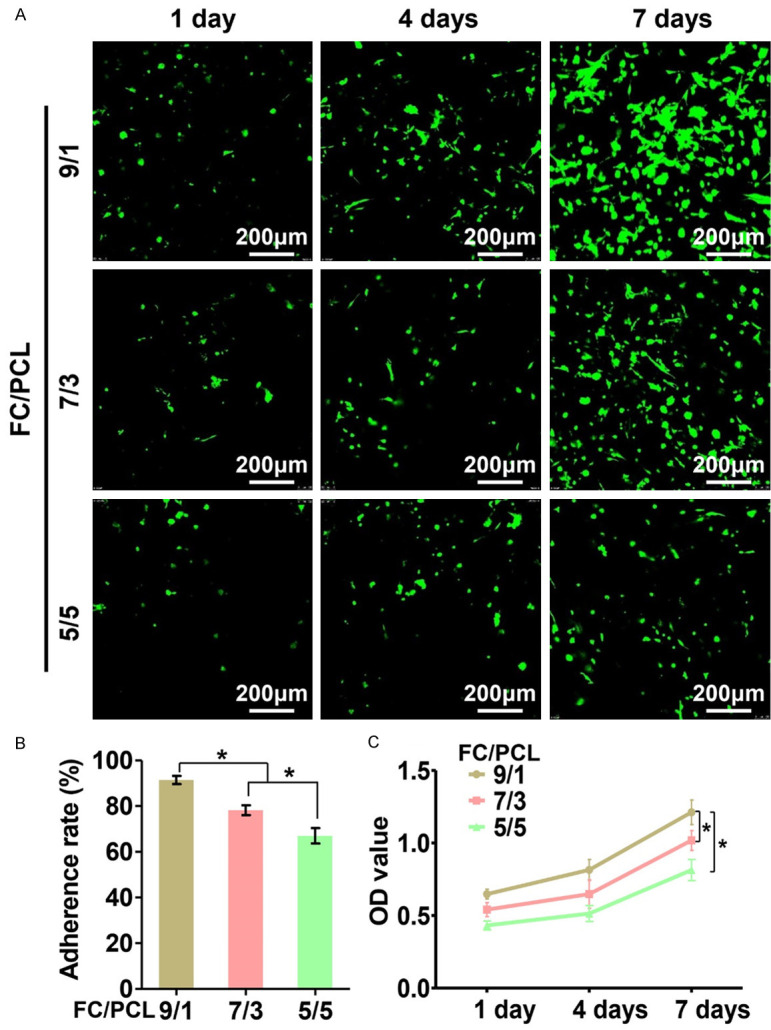
Biocompatibility evaluations of three different FC/PCL membranes. Different FC contents resulted in comparable levels of good biocompatibility, as evidenced by Live & Dead staining (A) and CCK-8 assay (C) results obtained for the three membranes at 1 to 7 days after chondrocyte inoculation, but led to increases in the adherence rate of the membranes (B). *P < 0.05.
In vitro cartilage formation
Despite their above-described promising potential, whether FC/PCL membranes are suitable for in vitro cartilage formation remains uncertain. The feasibility of cartilage regeneration was then explored in vitro using a sandwich model of FC/PCL membranes seeded with chondrocytes. After 4 weeks of culture, histological examinations showed the formation of preliminary cartilage-like tissue, which displayed typical lacunae structures (Figure 4A1-C1) and cartilage-specific ECM deposition, as evidenced by positive staining for GAG (Figure 4A2-C2) and type collagen II (Figure 4A3-C3). Abundant undegraded membranes were observed in all three groups, and the remaining scaffolds presented a continuous, approximately parallel zonal distribution (Figure 4). Notably, the thickness of the neocartilage layer presented a significantly increasing trend with increases in the FC content, which is consistent with the results obtained for the cell adherence rate. These results indicated that all the FC/PCL membranes were suitable for in vitro cartilage formation whereas the F9P1 group exhibited the most promising potential for robust cartilage regeneration.
Figure 4.
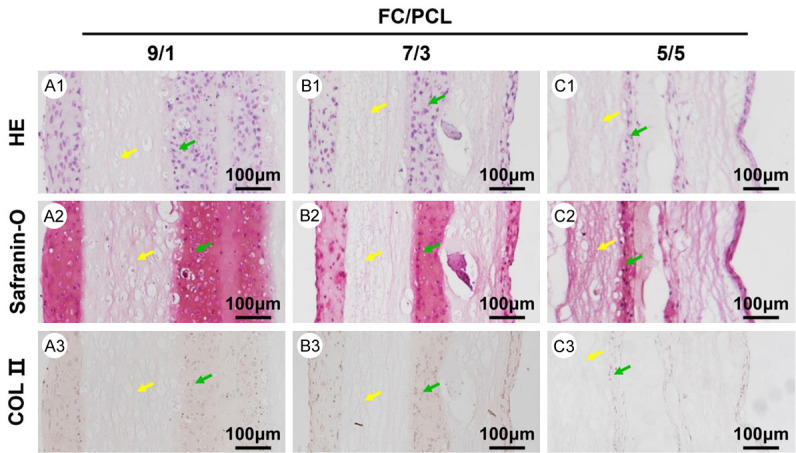
Histological examination of in vitro cartilage formation at 4 weeks. The histological assessment, including the HE, safranin-O and collagen II staining of the F9P1 (A1-A3), F7P3 (B1-B3), and F5P5 (C1-C3) samples, indicated that increases in the FC content led to increases in the thickness of the neocartilage layer. Yellow arrows indicate electrospun membranes. Green arrows indicate neocartilage.
In vivo cartilage regeneration
Cartilage regeneration in vivo is the most important criterion for determining whether an electrospun membrane can be used for cartilage engineering. The different membranes were constructed into a 3D patch structure using a classical sandwich model [17]. All the samples basically maintained their cylindrical shapes and formed cartilage-like tissue within 8 weeks after in vivo implantation (Figure 5A1-C1). Histological analyses revealed that all the samples displayed cartilage-specific ECM deposition and typical lacunae structures, as observed by positive GAG and type II collagen staining (Figure 5A2-C2, 5A3-C3 and 5A4-C4). Notably, the F9P1 samples showed a relatively homogeneous cartilage structure with almost no visible residual membranes (Figure 5A2-A4). In stark contrast, the F7P3 (Figure 5A2-A4) and F5P5 (Figure 5A2-A4) samples presented an obviously heterogeneous structure with abundant residual undegraded membranes and positive safranin-O and type II collagen staining only in some neocartilage regions.
Figure 5.
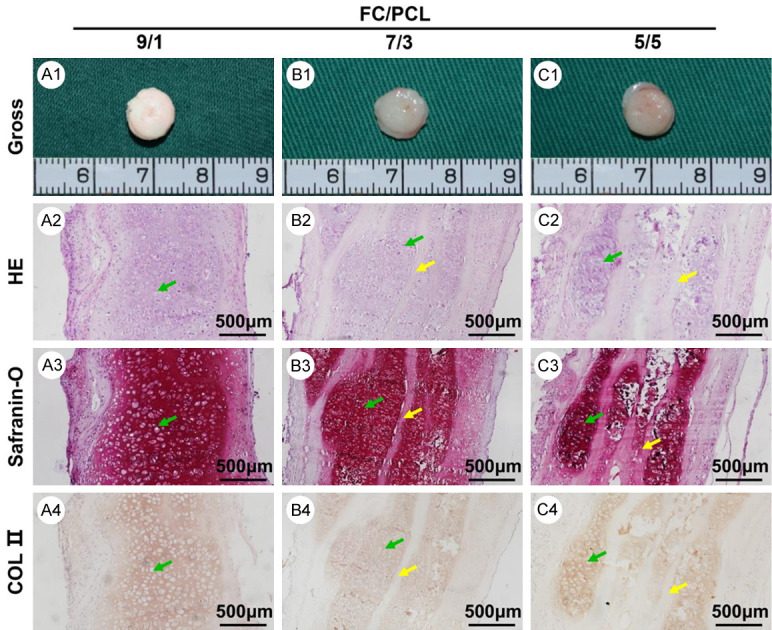
In vivo neocartilage evaluation at 8 weeks. Gross view (A1-C1) of all three groups presented similar ivory-white appearances. The histological assessment, including HE (A2-C2), safranin-O (A3-C3), and collagen II (A4-C4) staining of all three samples, revealed that increases in the FC content led to a more homogeneous cartilage-specific extracellular matrix distribution and fewer residual electrospun membranes. Yellow arrows indicate undegraded electrospun membranes. Green arrows indicate neocartilage.
Quantitative analyses showed that the Young’s moduli and the DNA, GAG, and total collagen contents, presented a significant trend with increases in the FC content (Figure 6). It is also worth noting that the values of these quantitative indexes obtained for the F9P1 group are close to those found for the native auricular cartilage group. These results indicated that the electrospun FC/PCL membranes represent an ideal scaffold for cartilage regeneration in vivo and that a high PCL content was favorable for cartilage formation and ECM production.
Figure 6.
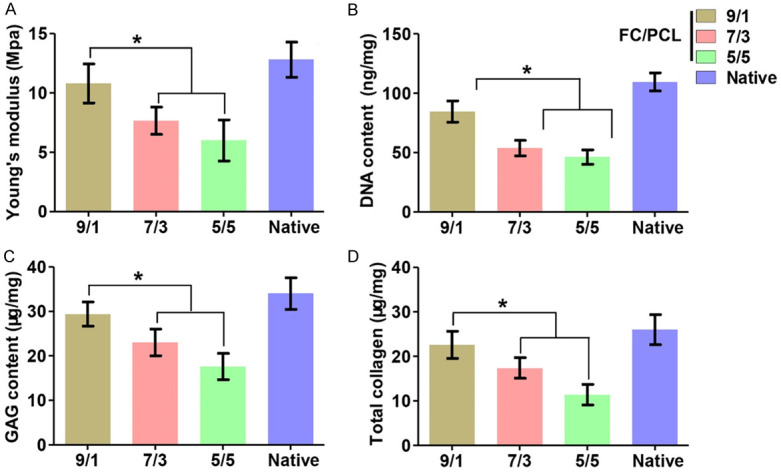
Quantitative in vivo analyses of neocartilage. Increases in the FC content led to increases in quantitative indexes, including Young’s modulus (A), DNA content (B), GAG content (C) and total collagen content (D). The values of all of these indexes obtained for the FC/PCL 9/1 group were even comparable to those found for the native rabbit auricular cartilage group. *P < 0.05.
Discussion
Fish discards that are returned to the sea involve an underutilization of marine resources and represent a serious obstacle to the sustainability of fisheries [18]. Fish discards can potentially be used to isolate collagen, which is further used in various biomedical fields, such as cartilage regeneration. Therefore, in this study, FC was reinforced with PCL to fabricate multifunctional and inexpensive electrospun membranes with sound mechanical strength, proper biodegradability, excellent hydrophilicity, suitable absorption and cell adhesion capacities, satisfactory biocompatibility, and the ability to promote cartilage regeneration in vitro and in vivo.
Collagen, which is the most abundant protein in the animal body and the main constituent of the ECM of most tissue types, exhibits excellent biological properties such as noncytotoxicity, biodegradability, and bioabsorbability [19,20]. In recent decades, MC has been widely used as a biomaterial in biomedical fields and as an active ingredient for modifying other biomaterials. However, the use of MC is challenging due to the potential risk of transmissible diseases from mammalian tissues to human recipients as well as religious issues [21,22]. Furthermore, previous studies have provided evidence that terrestrial animals and humans share a common important immunodominant epitope, which is likely to cause immunological cross-reaction [23]. FC has recently been considered an alternative source of MC due to its excellent biocompatibility, suitable biodegradability, inert immunogenicity, rich sources, low price and lack of risk for the transmission of zoonoses [22,24]. The rapid development of fish product processes has resulted in the production of numerous wastes, which not only pollute the environment but also waste resources. Consequently, the processing of FC into biomaterials is of great significance for making full use of its residual value. FC exerts a positive effect on osteoblastic cells and accelerates matrix mineralization [25]. FC incorporated with chito-oligosaccharide exhibits good antibactericidal activity and satisfactory biocompatibility in vitro and supports the proliferation of fibroblasts [26]. These results indicate that FC represents a promising scaffold for use in tissue engineering.
Numerous studies have attempted to use FC as a biomaterial, such as for application in bone tissue engineering [5,27-29], corneal tissue engineering [30], skin tissue engineering [31-33], blood and lymphatic vessels [34], and periodontal tissue regeneration [35]. However, limited progress has been achieved in cartilage regeneration based on FC, and the main obstacles in this process are weak mechanical properties and a fast degradation rate [2]. In the current study, FC was enzymatically hydrolyzed into smaller molecule proteins, which also significantly impaired its mechanical properties, accelerated its degradation rate, and further impeded its application for cartilage regeneration [36]. PCL has been approved by the US Food and Drug Administration for internal use in the human body and exhibits sound mechanical properties and a low degradation rate [1]. Consequently, through reinforcement with PCL, FC/PCL composite can simultaneously overcome all the above-mentioned obstacles. Our results indicated that increases in the PCL content improved the mechanical properties but postponed the biodegradability. By adjusting the FC/PCL component ratios, a balance between the membrane degradation rate and the cartilage ECM formation rate is hopefully achieved.
Therefore, whether FC/PCL membranes are suitable for cartilage regeneration is a major concern. Biocompatibility evaluations-including the cell adhesion rate, cell viability, proliferation rate, and production of ECM-are generally used to investigate whether a scaffold is suitable for cartilage regeneration. As expected, FC/PCL showed good cell affinity, low toxicity and a strong ability to promote chondrocyte proliferation. Furthermore, our results demonstrated that the FC/PCL membranes combined with chondrocytes formed mature cartilage-like tissue with typical cartilage lacunae and cartilage-specific ECM both in vitro and in vivo, which supported our hypothesis that these membranes would be suitable for cartilage regeneration. Noticeably, after 8 weeks of in vivo culture, satisfactory homogeneous cartilage regeneration was only achieved within the F9P1 group, whereas an inconsistent distribution of neocartilage was observed in the membranes with a high PCL content. Favorable cartilage regeneration might be attributed to the following aspects: i) FC and PCL, as the main components of the membranes, exhibit good biocompatibility; ii) the FC scaffold mimics the main tissue components of cartilage and supports the chondrogenic differentiation of chondrocytes [37]; iii) the electrospun nanofibrous structure provides a biomimetic topology and appropriate porosity, and these physical characteristics enhance cell attachment, proliferation, and cartilage ECM secretion; and iiii) the optimal degradation rate of the FC/PCL 9/1 group matches the neo-ECM formation rate. Our previous study also indicated that a high PCL content is unfavorable for cartilage formation [1] because a high PCL content decreased the hydrophilic property of the membranes, leading to lower cell adhesion. Additionally, the relatively slow degradation rate of PCL hampers the formation of neocartilage.
Conclusion
In summary, the current study demonstrated that an electrospun FC/PCL membrane was suitable for cartilage regeneration and that the ratio of the F9P1 group might be relatively suitable for homogeneous cartilage regeneration. The current study provides detailed and useful information for the regeneration of cartilage and other tissues based on electrospun FC/PCL membranes.
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFC1105800), the National Natural Science Foundation of China (81771568, 81301647), the Opening Foundation of Shandong Provincial Key Laboratory of Plastic and Microscopic Repair Technology (KF2017006), the Project of Scientific Research and Development of Universities in Shandong Province (J18KB112), and the Doctoral Research Startup Fund of the First Affiliated Hospital of Xinxiang Medical University (10240).
Disclosure of conflict of interest
None.
References
- 1.Zheng R, Duan H, Xue J, Liu Y, Feng B, Zhao S, Zhu Y, Liu Y, He A, Zhang W, Liu W, Cao Y, Zhou G. The influence of Gelatin/PCL ratio and 3-D construct shape of electrospun membranes on cartilage regeneration. Biomaterials. 2014;35:152–164. doi: 10.1016/j.biomaterials.2013.09.082. [DOI] [PubMed] [Google Scholar]
- 2.Jin S, Sun F, Zou Q, Huang J, Zuo Y, Li Y, Wang S, Cheng L, Man Y, Yang F, Li J. Fish Collagen and hydroxyapatite reinforced poly (lactide- co-glycolide) fibrous membrane for guided bone regeneration. Biomacromolecules. 2019;20:2058–2067. doi: 10.1021/acs.biomac.9b00267. [DOI] [PubMed] [Google Scholar]
- 3.Zhou T, Liu X, Sui B, Liu C, Mo X, Sun J. Development of fish collagen/bioactive glass/chitosan composite nanofibers as a GTR/GBR membrane for inducing periodontal tissue regeneration. Biomed Mater. 2017;12:055004. doi: 10.1088/1748-605X/aa7b55. [DOI] [PubMed] [Google Scholar]
- 4.Choi DJ, Choi SM, Kang HY, Min HJ, Lee R, Ikram M, Subhan F, Jin SW, Jeong YH, Kwak JY, Yoon S. Bioactive fish collagen/polycaprolactone composite nanofibrous scaffolds fabricated by electrospinning for 3D cell culture. J Biotechnol. 2015;205:47–58. doi: 10.1016/j.jbiotec.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Im J, Choi CH, Mun F, Lee J, Kim H, Jung WK, Jang CH, Kim G. A polycaprolactone/fish collagen/alginate biocomposite supplemented with phlorotannin for hard tissue regeneration. RSC Adv. 2017;7:2009–2018. [Google Scholar]
- 6.Xue J, Feng B, Zheng R, Lu Y, Zhou G, Liu W, Cao Y, Zhang Y, Zhang WJ. Engineering ear-shaped cartilage using electrospun fibrous membranes of gelatin/polycaprolactone. Biomaterials. 2013;34:2624–2631. doi: 10.1016/j.biomaterials.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Zhou T, Sui B, Mo X, Sun J. Multifunctional and biomimetic fish collagen/bioactive glass nanofibers: fabrication, antibacterial activity and inducing skin regeneration in vitro and in vivo. Int J Nanomedicine. 2017;12:3495–3507. doi: 10.2147/IJN.S132459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng B, Tu H, Yuan H, Peng H, Zhang Y. Acetic-acid-mediated miscibility toward electrospinning homogeneous composite nanofibers of GT/PCL. Biomacromolecules. 2012;13:3917–3925. doi: 10.1021/bm3009389. [DOI] [PubMed] [Google Scholar]
- 9.Lin LQ, Xu YW, Li YQ, Gong XD, Wei M, Zhang W, Zhang X, Xu Y. Nanofibrous Wharton’s jelly scaffold in combination with adipose-derived stem cells for cartilage engineering. Mater Design. 2020;186:108216. [Google Scholar]
- 10.Jee KS, Park HD, Park KD, Kim YH, Shin JW. Heparin conjugated polylactide as a blood compatible material. Biomacromolecules. 2004;5:1877–1881. doi: 10.1021/bm049795i. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Li YQ, Liu YQ, Li H, Jia ZH, Tang Y, Jiang GN, Zhang X, Duan L. Surface modification of decellularized trachea matrix with collagen and laser micropore technique to promote cartilage regeneration. Am J Trans Res. 2019;11:5390–5403. [PMC free article] [PubMed] [Google Scholar]
- 12.Xue JX, Feng B, Zheng R, Lu Y, Zhou GD, Liu W, Cao YL, Zhang YZ, Zhang WJ. Engineering ear-shaped cartilage using electrospun fibrous membranes of gelatin/polycaprolactone. Biomaterials. 2013;34:2624–2631. doi: 10.1016/j.biomaterials.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Zhang YJ, Xu Y, Liu YQ, Li D, Yin ZQ, Huo YY, Jiang GN, Yang Y, Wang ZX, Li YQ, Lu FJ, Liu Y, Duan L, Zhou GD. Porous decellularized trachea scaffold prepared by a laser micropore technique. J Mech Behav Biomed. 2019;90:96–103. doi: 10.1016/j.jmbbm.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Duan H, Li YQ, She YL, Zhu JJ, Zhou GD, Jiang GN, Yang Y. Nanofibrillar decellularized wharton’s jelly matrix for segmental tracheal repair. Adv Funct Mater. 2020;30:1910067. [Google Scholar]
- 15.Yan D, Zhou G, Zhou X, Liu W, Zhang WJ, Luo X, Zhang L, Jiang T, Cui L, Cao Y. The impact of low levels of collagen IX and pyridinoline on the mechanical properties of in vitro engineered cartilage. Biomaterials. 2009;30:814–821. doi: 10.1016/j.biomaterials.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 16.Zhou G, Liu W, Cui L, Wang X, Liu T, Cao Y. Repair of porcine articular osteochondral defects in non-weightbearing areas with autologous bone marrow stromal cells. Tissue Eng. 2006;12:3209–3221. doi: 10.1089/ten.2006.12.3209. [DOI] [PubMed] [Google Scholar]
- 17.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–229. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Galvez R, Garcia-Moreno PJ, Morales-Medina R, Guadix A, Guadix EM. Bile acid binding capacity of fish protein hydrolysates from discard species of the west mediterranean sea. Food Funct. 2015;6:1261–1267. doi: 10.1039/c4fo01171f. [DOI] [PubMed] [Google Scholar]
- 19.Bhuiyan D, Jablonsky MJ, Kolesov I, Middleton J, Wick TM, Tannenbaum R. Novel synthesis and characterization of a collagen-based biopolymer initiated by hydroxyapatite nanoparticles. Acta Biomater. 2015;15:181–190. doi: 10.1016/j.actbio.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 20.Pina S, Oliveira JM, Reis RL. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: a review. Adv Mater. 2015;27:1143–1169. doi: 10.1002/adma.201403354. [DOI] [PubMed] [Google Scholar]
- 21.Hassanbhai AM, Lau CS, Wen F, Jayaraman P, Goh BT, Yu N, Teoh SH. In vivo immune responses of cross-linked electrospun tilapia collagen membrane. Tissue Eng Part A. 2017;23:1110–1119. doi: 10.1089/ten.tea.2016.0504. [DOI] [PubMed] [Google Scholar]
- 22.Yamada S, Yamamoto K, Ikeda T, Yanagiguchi K, Hayashi Y. Potency of fish collagen as a scaffold for regenerative medicine. Biomed Res Int. 2014;2014:302932. doi: 10.1155/2014/302932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pal GK, Suresh PV. Sustainable valorisation of seafood by-products: recovery of collagen and development of collagen-based novel functional food ingredients. Innov Food Sci Emerg. 2016;37:201–215. [Google Scholar]
- 24.Pati F, Datta P, Adhikari B, Dhara S, Ghosh K, Das Mohapatra PK. Collagen scaffolds derived from fresh water fish origin and their biocompatibility. J Biomed Mater Res A. 2012;100:1068–1079. doi: 10.1002/jbm.a.33280. [DOI] [PubMed] [Google Scholar]
- 25.Yamada S, Nagaoka H, Terajima M, Tsuda N, Hayashi Y, Yamauchi M. Effects of fish collagen peptides on collagen post-translational modifications and mineralization in an osteoblastic cell culture system. Dent Mater J. 2013;32:88–95. doi: 10.4012/dmj.2012-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Zhang CL, Zhang Q, Li P. Composite electrospun nanomembranes of fish scale collagen peptides/chito-oligosaccharides: antibacterial properties and potential for wound dressing. Int J Nanomedicine. 2011;6:667–676. doi: 10.2147/IJN.S17547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoyer B, Bernhardt A, Heinemann S, Stachel I, Meyer M, Gelinsky M. Biomimetically mineralized salmon collagen scaffolds for application in bone tissue engineering. Biomacromolecules. 2012;13:1059–1066. doi: 10.1021/bm201776r. [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Sun J. Potential application of hydrolyzed fish collagen for inducing the multidirectional differentiation of rat bone marrow mesenchymal stem cells. Biomacromolecules. 2014;15:436–443. doi: 10.1021/bm401780v. [DOI] [PubMed] [Google Scholar]
- 29.Elango J, Zhang J, Bao B, Palaniyandi K, Wang S, Wenhui W, Robinson JS. Rheological, biocompatibility and osteogenesis assessment of fish collagen scaffold for bone tissue engineering. Int J Biol Macromol. 2016;91:51–59. doi: 10.1016/j.ijbiomac.2016.05.067. [DOI] [PubMed] [Google Scholar]
- 30.van Essen TH, Lin CC, Hussain AK, Maas S, Lai HJ, Linnartz H, van den Berg TJ, Salvatori DC, Luyten GP, Jager MJ. A fish scale-derived collagen matrix as artificial cornea in rats: properties and potential. Invest Ophthalmol Vis Sci. 2013;54:3224–3233. doi: 10.1167/iovs.13-11799. [DOI] [PubMed] [Google Scholar]
- 31.Cao H, Chen MM, Liu Y, Liu YY, Huang YQ, Wang JH, Chen JD, Zhang QQ. Fish collagen-based scaffold containing PLGA microspheres for controlled growth factor delivery in skin tissue engineering. Colloids Surf B Biointerfaces. 2015;136:1098–1106. doi: 10.1016/j.colsurfb.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Zhou T, Wang N, Xue Y, Ding T, Liu X, Mo X, Sun J. Electrospun tilapia collagen nanofibers accelerating wound healing via inducing keratinocytes proliferation and differentiation. Colloids Surf B Biointerfaces. 2016;143:415–422. doi: 10.1016/j.colsurfb.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 33.Chen JJ, Gao KL, Liu S, Wang SJ, Elango J, Bao B, Dong J, Liu N, Wu WH. Fish collagen surgical compress repairing characteristics on wound healing process in vivo. Mar Drugs. 2019;17:33. doi: 10.3390/md17010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang JK, Yeo KP, Chun YY, Tan TTY, Tan NS, Angeli V, Choong C. Fish scale-derived collagen patch promotes growth of blood and lymphatic vessels in vivo. Acta Biomater. 2017;63:246–260. doi: 10.1016/j.actbio.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Zhou T, Liu X, Sui BY, Liu C, Mo XM, Sun J. Development of fish collagen/bioactive glass/chitosan composite nanofibers as a GTR/GBR membrane for inducing periodontal tissue regeneration. Biomed Mater. 2017;12:055004. doi: 10.1088/1748-605X/aa7b55. [DOI] [PubMed] [Google Scholar]
- 36.Xu Y, Li D, Yin Z, He A, Lin M, Jiang G, Song X, Hu X, Liu Y, Wang J, Wang X, Duan L, Zhou G. Tissue-engineered trachea regeneration using decellularized trachea matrix treated with laser micropore technique. Acta Biomater. 2017;58:113–121. doi: 10.1016/j.actbio.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Pustlauk W, Paul B, Gelinsky M, Bernhardt A. Jellyfish collagen and alginate: combined marine materials for superior chondrogenesis of hMSC. Mater Sci Eng C Mater Biol Appl. 2016;64:190–198. doi: 10.1016/j.msec.2016.03.081. [DOI] [PubMed] [Google Scholar]


