Abstract
MicroRNAs are a class of short, non-coding RNAs that play a crucial role in normal physiology by attenuating translation or targeting messenger RNAs for degradation. Deregulation of miRNAs disturbs key molecular events in interconnected processes such as cell proliferation, tumor angiogenesis, self-renewal, apoptosis, metastasis and epithelial to mesenchymal transition. This process initiates, promotes and develops the pathophysiology of cancer. The modulation of miRNAs results in epigenetic changes in the genome, which eventually leads to cancer. Targeting deregulated miRNAs by natural products derived from plants is an ideal strategy to combat tumorigenesis. Owing to their fewer side effects, natural products have been used as chemotherapeutic agents against various cancers. These natural products modulate the dysregulated signaling pathways by downregulating the oncogenic miRNAs which play a crucial role in the development of tumorigenesis and maintain a fine balance of tumor suppressor miRNAs. This review article aims to highlight the key modifications of miRNAs which lead to tumorigenesis and the chemotherapeutic potential of natural products by targeting miRNAs and their possible mechanism of inhibition for developing an effective anti-cancer agent(s). They will have less damaging effects on normal cells for future chemotherapeutics.
Keywords: MicroRNA, natural products, cancer, signalling, oncomirs, metastasis
Introduction
MicroRNAs (miRNAs) are small intracellular, noncoding RNA molecules that regulate gene expression both at the posttranslational and posttranscriptional levels. Generally, they are 18-22 nucleotide long RNA molecules which attenuate translation by binding to the 3’- untranslated regions (UTRs) of target messenger RNA (mRNA) via imperfect matching, thereby causing mRNA degradation. The end result is inhibition of protein translation [1,2]. They can regulate the multi-gene expression individually or in combination with other miRNAs. To date, more than 2500 miRNAs have been identified in humans, and miRNA conserved targets regulate around one-third of all human genes [3]. Recent studies have documented that miRNAs are not only involved in the developmental processes like cell differentiation; they also play a crucial role in the tumor pathophysiology and been reported to have aberrant expression of miRNA profile in cancer patients compared to normal patients [4,5]. Moreover, miRNA is reported to be involved in the development of drug resistance against approved chemotherapeutic drugs [6]. Owing to these prominent features of miRNAs which link them with tumorigenesis, they constitute a promising target player in cancer diagnostics and therapeutics [6-8].
Natural products have been used from ancient times in a different clinical settings due to their novel structures, potential pharmacological activities and less damaging side effects on normal cells [9,10]. More than 47% of Food and Drug Administration (FDA) approved anti-cancer drugs originate from plants or modified plant products [11,12]. Natural products are non-nutritive plant secondary metabolites with various pharmacological activities that are anti-inflammatory, anti-diabetic and anti-cancer in character [11,13-17]. However, their mechanism is difficult to explain which limits their applicability in the current therapeutics [18]. Numerous recent studies have documented that natural products and their derivatives exhibit anti-tumor properties by regulating epigenetic modifications and affecting various signaling pathways [19,20]. Moreover, natural products have been documented to modulate miRNAs by various mechanisms, and thereby enhancing anti-cancer potential [21-23]. The present review focused on the regulation of miRNAs by natural products and their possible mechanisms of action for the diagnostics and therapeutics against this dreadful disease.
MiRNAs
MiRNAs are an evolutionarily conserved family of small, cytoplasmic, (18-22 nucleotides long), non-coding RNAs that regulate the expression of genes at the post-transcriptional level [24]. Mostly miRNAs are synthesized from intergenic regions and their localization varies from one species to another. The miRNAs play a key role in biological processes such as cellular, developmental and physiological ones [25,26]. MiRNAs can regulate many genes due to the presence of multiple miRNA binding sites within the functional genes, thereby influencing multiple signaling pathways involved in cellular processes [27,28]. Nearly 1/3rd of the protein population that is closely associated with many physiological processes are regulated by miRNAs [29]. Under normal physiological conditions miRNAs are vital components of feedback circuits and through their buffering effects provide robustness to crucial physiological processes [30]. This could serve to augment the fine-tuning of gene expression by reducing the translation of proteins to an extent that their effect will be under stringent regulation. Intriguingly, miRNAs could act as toggle switches to regulate various differentiation pathways. Apart from their wide functional importance, aberrant miRNAs expression could induce de-differentiation, cellular plasticity and oncogenic transformation in normal cells [31]. This characteristic feature makes them appealing targets in cancer therapeutics.
Biogenesis
The overview of the biogenesis of miRNAs is shown in Figure 1. The biogenesis starts with the synthesis of a large primary transcript called pri-miRNA from the respective transcribing gene with the help of RNA polymerase II. The transcript generated is protected with 5’ cap and 3’ polyadenylated tail. However, many pri-miRNAs transcripts were also synthesized by RNA polymerase III. The pri-miRNA transcripts are cleaved by a microprocessor which contains DiGeorge syndrome chromosomal region 8 (DGCR8) and Drosha into precursor miRNA (pre-miRNA). The translocation of pre-miRNA from the nucleus to the cytosol is guided by Ran/GTP/Exportin 5. Furthermore, Dicer processes pre-miRNA into approximately 20-25 nucleotide long miRNA duplex. Following the unwinding of this miRNA duplex, the mature strand of miRNA integrates with the protein complex called RNA-induced silencing complex (RISC). Consequently, it targets mRNA degradation [32].
Figure 1.
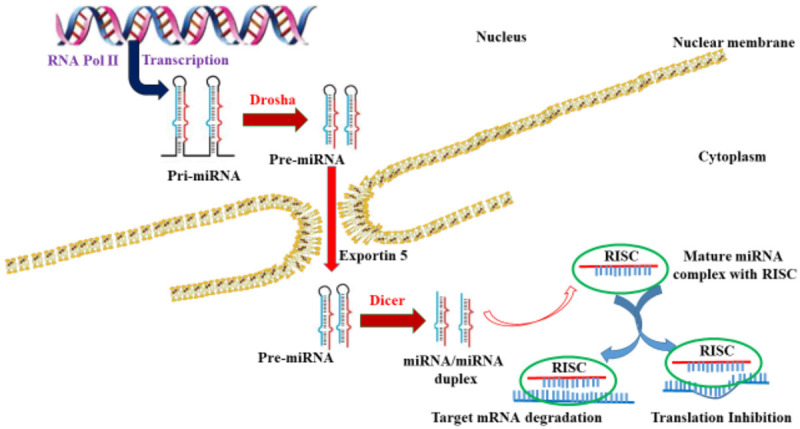
Schematic overview of the canonical pathway of miRNA biogenesis.
Types
Based on their functions, miRNAs are classified into two different categories, i.e. tumor suppressor miRNA and oncogenic miRNA which is also known as oncomir. The tumor suppressor miRNAs exert an inhibitory effect on tumor growth and are called gatekeepers. These miRNAs promote the suppressive role of oncogenic signaling pathways, thereby triggering a negative effect on tumorigenesis. Tumor suppressor miRNAs are either downregulated or completely lost in tumorigenesis [28]. However, oncomirs have an opposite effect, in that they are aberrantly overexpressed in carcinogenesis and promote oncogenic signaling pathways. Subsequently, cellular transformation and proliferation of cancer cells are induced [27].
Role of miRNAs in the development of tumorigenesis
As per the PubMed search, more than 10,000 articles have been published in diverse fields that are associated with miRNAs. A significant percentage of these articles focuses on miRNAs linked with tumorigenesis, both in terms of understanding the role and possible therapeutic approach associated with miRNAs. The fine regulation of complex cellular pathways by miRNAs constitute an Achilles heel, where aberration of a few miRNAs can strongly influence the expression pattern of proteins as well as genes and can drive cells towards oncogenic transformation [33]. They have been identified as regulating cancer-associated genes called proto-oncogenes by different means [34]. Recent reports suggest that nearly half of the miRNAs are associated with the regions of the genome which codes for oncogenes [24,34]. Microarray data from recent studies of human samples also point to the deregulated expression of miRNAs and results in defective miRNA processing, posttranscriptional regulation, mutation or epigenetic modifications [35,36]. The aberrant expression of miRNA results in decreased expression or inactivation of tumor suppressor miRNAs and activation or increased expression of oncomirs when compared to normal tissues [37]. Essentially, animal models attributing miRNA ablation or overexpression have revealed a causal link between miRNAs and tumorigenesis. Furthermore, miRNA deregulation influences the signaling pathways regulating cellular growth which induces epigenetic modification of tumor suppressors, enhances cell death resistance, inhibits apoptosis of tumorigenic cells and helps in drug resistance. Therefore all these events result in the development and progression of carcinogenesis [38,39]. Importantly, these findings point to miRNAs as interesting targets for clinical work, and could serve as promising biomarkers and putative targets in future therapeutics.
Role of natural product compounds in modulating miRNAs to prevent tumorigenesis
Ever since miRNAs were discovered in 1993, scientific research has established the foolproof role of miRNAs in the molecular mechanism of tumorigenesis events such as initiation, promotion, progression, and metastasis. Intriguingly, recent findings demonstrate that miRNAs not only play a fundamental role in the progression of tumorigenesis, they also exert a critical role in programmed cell death mechanisms and cellular differentiation by regulating tumor suppressor and oncogenes [40]. Owing to their ability to regulate the expression pattern of a myriad of genes, miRNAs simultaneously modulate many cellular pathways [32]. This evidence suggests that miRNAs are promising biological molecules that can serve as biomarkers for diagnostics, prognostics as well as cancer therapeutics. Natural product compounds and their derivatives have been widely used in various medical ailments, including diabetes, neurological disorders, gastrointestinal diseases, obesity, and cancer. Due to their potential biological activity, novel structure, and less deleterious effects on normal cells, natural product compounds exhibit an anti-tumor character by targeting multiple cellular signaling pathways [41]. The vast structural diversity of natural products adds to their challenging complex mechanism, which limits their application [9,42]. Moreover, the multifunctionality mechanism of natural products provides them with a great advantage to deal with tumorigeneses; a multistage process triggered by the deregulation of many cellular signaling pathways [17,43]. This is possibly one of the best plausible explanations of why single chemotherapy often fails in the therapeutics of cancer. Therefore, owing to exerting an effect on multiple target sites, the natural products are believed to be more efficacious in the therapeutics of cancer [44].
Besides exhibiting other potential biological activities, the recent data demonstrated that natural products exhibit anti-proliferative properties by modulating cancer epigenetics [45]. Interestingly, natural compounds exhibit anti-cancer effects by targeting multiple aberrated cellular signaling pathways [46]. Similarly, miRNAs modulate a plethora of biological processes such as tumor growth, progression, and cell death mechanisms [47]. Since both natural compounds and miRNAs wield an impact on multiple cellular targets. Consequently, there is a strong notion that natural products could modulate the miRNAs and pave the way for future therapeutics for many diseases including cancer. The data revealed that natural products display anti-cancer potential by modulating miRNA profiles via unknown mechanisms. Here, the present review highlights the natural products associated with miRNA regulation for preventing tumorigenesis and summarize their possible mechanism of action.
Flavonoid and isoflavonoid compounds
Resveratrol
Resveratrol is a natural polyphenolic, prenylflavonoid, or phytoalexin extracted from various plant sources such as mulberry, grapes, red wine, and peanut, etc., and displays numerous potential biological activities including anti-tumor activities [48]. Recently, resveratrol has been well documented to modulate miRNAs. Resveratrol reduces tumor growth of glioblastoma cells by downregulation of oncomirs (miR-19, miR-21, and miR-30a-5p) and restores the expression of tumor suppressor miRNAs [49]. A novel finding recently revealed that resveratrol exhibits an anti-tumor mechanism by augmenting the expression of tumor suppressors miR-34a, miR-424, and miR-503 in breast cancer cells modulating the p53 signaling pathway. This leads to suppression of tumorigenesis associated with heterogeneous nuclear ribonucleoprotein A1 (HNRNPA1). Intriguingly, resveratrol could directly modulate the HNRNPA1 associated pre-mRNA splicing pathway by inducing miR-424 and miR-503 to prevent breast cancer tumorigenesis [50]. Resveratrol sensitizes adriamycin resistant breast cancer cells, arrests cell cycle, induces cell killing by modulating tumor suppressor miR-122-5p-mediated regulation of cyclin-dependent kinases (CDKs) and anti-apoptotic proteins [51].
Resveratrol is able to regulate the cell cycle and apoptotic machinery in breast cancer cells by modulating key tumor suppressor miRNAs (miR-542-3p, miR-125b, miR-409-3p, miR-200c-3p, miR-122-5p). This induction of resveratrol mediated tumor suppressor miRNAs attenuates CDKs, X-linked inhibitor of apoptosis protein (XIAP), and B-cell lymphoma 2 (Bcl-2). It thereby curtails tumor growth and induces cell death in breast cancer cells [52]. Resveratrol augments the expression of major histocompatibility complex (MHC) class I chain A and B by abrogating miR-17-mediated c-Myc in breast cancer [53]. Resveratrol attenuates colorectal cancer (CRC) cell viability, invasion, EMT and induces cell death by modulating tumor suppressor miR-200c [54]. Resveratrol significantly exhibits anti-tumor effect in both in vitro and in vivo melanoma models by modulating tumorigenic miR-221-mediated nuclear factor-kappa-light-chain enhancer of activated B cells (NF-kB) signaling [55]. In acute lymphoblastic leukemia (ALL) cells, resveratrol inhibits cell proliferation, arrests cell cycle, induces cell killing by downregulating the expression of miR-196b and miR-1290 which targets 3’-UTR region of insulin growth factor binding protein 3 (IGFBP3) that plays a key role in ALL [56]. Triacetyl derivative of resveratrol specifically inhibits clonogenic property, induces apoptosis and EMT in pancreatic cell models by upregulating miR-200 family-mediated downregulation of sonic hedgehog (SHH) signaling proteins Zeb1, Snail, N-cadherin, Slug and upregulates E-cadherin expression [57]. However, it has been demonstrated that resveratrol suppresses reactive oxygen species (ROS) mediated invasion or migration in pancreatic cells by downregulating the miR-21 expression [58].
3,6-dihydroxyflavone (3,6-DHF)
3,6-DHF is a flavonoid natural product compound and exhibits anti-tumor activity both in in vitro and in vivo models. In the recent past 3,6-DHF breast cancer cell death by downregulating miR-21 expression and upregulates the miR-34a expression. Mechanistic studies revealed that 3-6-DHF simultaneously upregulates miR-34a by inhibits DNMT1 and regulates histone modification on miR-21 promoter at H3K9-11ac [59]. 3,6-DHF significantly abrogates TET1 mediated DNMT1, DNA hypermethylation and augments miR-34a expression in breast cancer [60].
Quercetin
Quercetin, is a flavonoid natural product compound found in apples, onions, red wine, and tea. Quercetin exhibits promising anti-tumor properties through various mechanisms. Recent data demonstrated that quercetin enhances anti-cancer outcomes by modulating more than 50 unique miRNAs. Quercetin upregulates the let-7c miRNA in pancreatic ductal carcinoma (PDA) cells (AsPC-1), thereby inducing NUMB like Endocytic Adaptor Protein (Numbl) expression, which abrogates the Notch signaling pathway and so prevents pancreatic tumorigenesis [61]. Quercetin upregulates tumor suppressor miR-200b-3p in PDA cells. The induction of miR-200b-3p switches the symmetric division of cancer stem cells (CSCs) of PDA to asymmetric cell division mode by abrogating Notch and augmenting Numbl [62]. Quercetin treatment significantly eliminates ROS, reduces miR-21 expression and boosts programmed cell death-4 (PCD4) in transformed bronchial alveolar (BEAS-2B) cells, which were earlier exposed to hexavalent chromium [Cr(VI)] [63].
Quercetin upregulates the tumor suppressor miR-143, inhibits autophagy-related protein Gamma-aminobutyric acid receptor-associated protein-like 1 (GABARAPL1) which is commonly called Atg8. This inhibition impairs autophagy which eventually improves quercetin mediated efficacy in gastric cancer cells [64]. Quercetin in combination with Tamarix articulate drastically reduces the expression of insulin-like growth factor 2 mRNA binding protein 1 (IGF2BP1) and IGF2BP3; it also upregulates the tumor suppressor miRNA-1275 in Huh-7 cells [13]. However, in combination with cordycepin in lung cancer cells (A549), quercetin exhibits a suppressive effect on the expression of claudin-2 at the transcriptional level, by increases the expression of miR-16 and stimulates its binding to the 3’-UTR of claudin-2 [65]. Quercetin promotes osteosarcoma cell sensitivity to cisplatin and controls KRAS hyperactivation by modulating the expression of miR-217 [66]. Quercetin induces a significant induction of apoptosis in ovarian (SKOV-3) cancer cells by upregulating the miR-145 expression [67]. Quercetin reduces cell viability, abrogates tumor cell invasion, migration, and MMP-9, MMP-2 by upregulating miR-16 and HOXA10 in oral cancer cells [68]. Furthermore, quercetin exhibits an anti-tumor effect in oral squamous cell carcinoma (OSCC) by upregulating miR-22. Mechanistically, quercetin-mediated miR-22 upregulation suppresses Wnt1/β-catenin signaling, thus attenuating tumor growth both in in vitro and in vivo models [69].
Epigallocatechin-3-gallate (EGCG)
EGCG is a key polyphenol flavonoid natural product compound found in green tea. Besides having other pharmacological properties, EGCG exhibits potent anti-tumor activity [70]. EGCG is one of the most widely studied natural compounds in terms of regulating miRNAs expression. More than 205 miRNAs exhibited differential expression upon treatment with EGCG. However, next-generation sequencing analysis in NSCLC cells (A549) suggests that EGCG treatment identifies 4 putative novel, 115 known and 3 putative novel and 134 known miRNAs at 40 µM and 100 µM concentrations, respectively. Furthermore, these miRNAs modulate the mitogen-activated protein kinase (MAPK) signaling pathway and can act as biomarkers for the diagnostics, prognostics, and therapeutics for lung cancer [71]. EGCG exerts a suppressive effect on the growth of human cervical cancer cells (HeLa, SiHa, CaSki, and C33A) infected with subtypes of human papillomavirus (HPV) by upregulating the tumor suppressor miRNAs (miR-29a, miR-125b, miR-210, and miR-203) [72]. EGCG curtails the NF-ΚB pathway and sensitizes 5-fluorouracil (5-FU) resistant CRC cells to chemotherapy by: firstly, upregulating the expression of miR-155-5p; and secondly, subduing the gene expression of drug resistance acquired gene multi-drug resistance-1 (MDR1) [73]. However, in NSCLC cells, EGCG sensitizes cisplatin-mediated resistance and reduces CSC-like phenotypic features. It does this by upregulating and downregulating the expression of miR-485 and CD44, respectively [74].
EGCG upregulates miR-204 and targeting Slug and Sox4 in OSCC cells. Further analysis revealed that induction of miR-204 drastically reduces CD44+ and aldehyde dehydrogenase 1 (ALDH1+) cells, and inhibits EMT and self-renewal capacity of OSCC-CSCs upon EGCG treatment [75]. EGCG effectively targets tumor cells by enhancing the expression of hsa-mir-485-5p in NSCLC cells. The induction of hsa-mir-485-5p modulates retinoid X receptor alpha (RXRα), significantly represses CSC-like features and induces programmed cell death in lung cancer cells [76]. Additionally, EGCG treatment upregulates miRNA-15b expression in both human and murine T cells. Further, the study reveals that EGCG induced miRNA-15b downregulates store-operated calcium channel (SOCE) expression by binding with SRIM2. The interaction of SRIM2 and miRNA-15b drastically curtails the protein expression of Orai1 and STIM2 in murine T cells and induces apoptosis. This occurs by enhancing mitochondrial membrane depolarization and releasing cytochrome in murine and human T cells [77]. These studies suggest that EGCG exhibits promising anti-tumor potential by modulating miRNAs.
Genistein
Genistein is an isoflavone natural product compound abundantly found in soybeans. Genistein attenuates the expression of several oncomirs as well as restores the expression of tumor suppressor miRNAs. Genistein augments the expression of tumor suppressor let-7d and significantly reduces the expression of stellar cell fibrosis marker thrombospondin 1 (THBS1) by modulating its 3’-UTR, thus helping to prevent the formation of fibrosis which is one step away from pancreatic carcinogenesis [78]. Genistein enhances programmed cell death in laryngeal carcinoma cells by simultaneously upregulating p53 and miR-1469 expression and abrogates the expression of tumorigenesis associated the myeloid cell leukemia 1 (Mcl1) [79]. Genistein analog sensitizes resistant ovarian cancer cells by inhibiting PI3K/AKT signaling, reduces nuclear c-Myc expression by upregulating miR-7d [80]. In combination with propofol or alone in human gliosarcoma cells (U251), genistein exerts anti-proliferative effect and augments apoptosis mediated cell death by increasing the expression of miRNA-218. This upregulation of miRNA-218 increases the expression of apoptotic proteins Bax and Bad with concomitant reduction of inflammatory cytokines 1L-6, TNF-α and IL-1β proteins [81]. Genistein treatment drastically reduces clonogenic ability, cell viability, and growth of human retinoblastoma cells (Y79) and induces apoptosis. Mechanistically, genistein upregulates miR-145 and imposes an inhibitory effect on cellular growth via post-translational modulations of ATP binding cassette subfamily E member 1 (ABCE1) [82]. Genistein in combination with ox-LDL eliminates ROS production significantly and helps to reverse oxidative damage in HUVECs.
Moreover, the combination increases the expression of anti-oxidant enzymes such as catalase and superoxide dismutase via modulation of miR-34a/sirtuin-1 axis which eventually helps in translocation and epigenetic modulation, for instance deacetylation of forkhead box 03A (FOXO3A) [83]. Combined with miR-223 inhibitor, genistein sensitizes gemcitabine-resistant (GR) pancreatic cancer cells to chemotherapy, thereby causing the downregulation of miR-223, which eventually leads to the inhibition of cell migration and invasion of cells with EMT [84]. However, genistein downregulates miR-155 in breast cancer cells, which leads to the suppression of cell viability and induction of cell death with a concomitant increase in pro-apoptotic genes p21, FOXO3 and PTEN [85]. Interestingly, genistein has been reported to decrease multidrug resistance by increasing the upregulation of ABC drug transporters ABCG2 and ABCC1 at the translational level in breast cancer cells. Further, genistein increases the expression of ABC transporter by downregulating the expression of miR-181a, which negatively modulates the expression of drug transporters [86]. Genistein suppresses the NF-κB signaling pathway and upregulates miR-29b expression in multiple myeloma (MM) cells U266. This induction of miR-29b reduces cell viability and enhances apoptosis by cleaving the active product of caspase-3 [87]. Together these reports reveal that genistein is a promising anti-cancer natural product and generates a good outcome by modulating miRNAs and regulating various signaling pathways. Figure 2 illustrates the structure of flavonoid and isoflavonoid compounds modulating miRNAs, while Table 1 summarizes: firstly, the different flavonoid and isoflavonoid natural compounds modulating miRNA; and secondly, their mechanism of action in various cellular and animal models.
Figure 2.
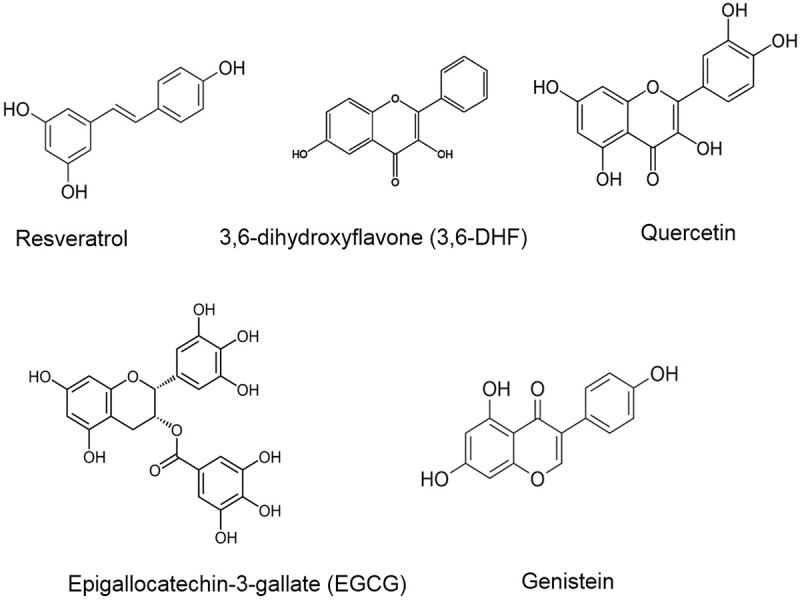
Flavonoid and Isoflavonoid compounds modulating various miRNAs.
Table 1.
Flavonoid and isoflavonoid natural compounds modulating miRNA and their mechanism of action in various cellular and animal models
| Natural Products | Modulation of miRNAs | Mechanism of action | Tumor type | References/Authors |
|---|---|---|---|---|
| Resveratrol | ↑miR-34a, miR-424, miR-503, miR-122-5p, miR-542-3p, miR-125b, miR-409-3p, miR-200c-3p, miR-200c, miR-200 family | Inhibits cell proliferation, arrests cycle in S-phase by targets PI3K/AKT/mTOR pathway, inhibits tumorigenesis by targeting HNRNPA1-related pre-mRNA splicing pathway, Sensitizes drug resistant cells by targeting CDKs, Bcl-2 and apoptosis, suppresses c-Myc expression, attenuates EMT, invasion, and motility of cancer cells. Inhibition of NF-kB signaling, Induction of apoptosis and inhibition of glycolysis | Intracranial glioma, breast cancer, CRC, melanoma, ALL, pancreatic cancer | Wang et al. 2015 [49], Otsuka et al. 2018 [50], Zhang et al. 2019 [51], Venkatadri et al. 2016 [52], Pan et al. 2017 [53], Karimi et al. [54], Wu et al. 2017 [55], Zhou et al. 2017 [56], Fu et al. 2019 [57], Yan et al. 2018 [58] |
| ↓miR-21, miR-30a-5p, miR-19, miR-17, miR-221, miR-196b, miR-1290 | ||||
| 3, 6-Dihydroxyflavone (3, 6-DHF) | ↑miR-34a | Suppresses PI3K/AKT/mTOR signaling and histone modification by inhibiting DNMT1 and hypermethylation | Breast cancer | Peng et al. 2015 [59], Peng et al. 2017 [60] |
| ↓miR-21 | Promotes apoptosis | |||
| Quercetin | ↑let-7c, miR-200b-3p, miR-143, miR-16, miR-217, miR-145, miR-22 | Growth, apoptosis, controls cell division, inhibits Notch signaling, ROS elimination and activates PDCD-4, sensitization and autophagy inhibition, claudin-2 inhibition, Sensitization of cells by inhibiting KRas, apoptosis induction, inhibits cell motility and invasion by regulating HOXA10 signaling, targets Wnt1/β-catenin signaling | Pancreatic adenocarcinoma, gastric cancer, lung cancer, osteosarcoma, ovarian cancer, oral cancer | Nwaeburu et al. 2016 [61], Nwaeburu et al. 2017 [62], Pratheeshkumar et al. 2017 [63], Du et al. 2015 [64], Sonoki et al. 2015 [65], Zhang et al. 2015 [66], Zhou et al. 2015 [67], Zhao et al. 2019 [68], Zhang et al. 2019 [69] |
| ↓miR-21 | ||||
| Epigallocatechin-3-gallate (EGCG) | ↑miR-210, miR-29, miR-125b, miR-155-5p, miR-485, miR-204, hsa-mir-485-5p, miR-15b, | Suprression of tumor cell growth, suppresses MDR1, GRP78 expression and upregulates apoptotic protein expression, inhibits CSC-like characteristics by targeting CD44, reduces stemness of OSCC-CSCs, Targets RXRα and inhibits CSC-like properties, targets store operated calcium entry | Cervical cancer, CRC, lung cancer, OSCC | Bardwaj et al. 2019 [71], Zhu et al. 2019 [72], La et al. 2019 [73], Jiang et al. 2018 [74], Yu et al. 2016 [75], Jiang et al. 2018 [76], Zhang et al. 2017 [77] |
| ↓miR-21 | ||||
| Genistein | ↑let-7d, miR-1469, miR-218, miR-145, miR-29b | Inhibits THBS1 expression by targeting its 3-UTR, targets p53-mediated apoptosis and inhibition of Mcl1, sensitizes tumor cells by targeting c-Myc, targets NF-kB signaling, targets post transcriptional regulation of ABCE1, sirtuin 1-mediated nuclear translocation and deacetylation of foxo3a, sensitizes gemcitabine-resistant tumor cells, activates apoptosis and inhibits metastatic potential of cancer cells, increase multidrug resistance and upregulation of ABC transporters, targets NF-kB pathway. | pancreatic carcinoma, laryngeal carcinoma, ovarian cancer, intracranial glioma, retinoblastoma, breast cancer, multiple myeloma | Asama et al. 2019 [78], Ma et al. 2018 [79], Ning et al. 2017 [80], Zheng et al. 2017 [81], Wei et al. 2017 [82], Zhang et al. 2017 [83], Ma et al. 2016 [84], de et al. 2016 [85], Rigalli et al. 2016 [86], Xie et al. 2016 [87] |
| ↓miR-34a, miR-223, miR-155, miR-181a |
Terpenoid and associated compounds
Oleanolic acid
Oleanolic acid is a pentacyclic triterpenoid natural product compound with potential anti-tumor activity. A recent study revealed that oleanolic acid exhibits antiproliferative potential by upregulating tumor suppressor miR-122 both in in vitro and in vivo models of lung carcinoma. However, oleanolic acid-miR-122 mediated anti-tumor effect was neutralized by overexpression of myocyte enhancer factor 2D (MEF2D) and cyclin G1 (CCNG1) in lung carcinoma cells. Oleanolic acid-mediated anti-tumor effect was indicated through the miR-122/MEF2D/CCNG1 pathway [88].
Andrographolide
Andrographolide is a natural bioactive labdane diterpenoid natural compound known for pharmacological activity. Extracted from Andrographis paniculata, andrographolide exhibits promising anti-tumor potential for both in vitro and in vivo models of hepatocellular carcinoma. MiRNA chip analysis revealed that andrographolide treatment modulates the miRNA expression profile by upregulating 22 miRNAs and downregulating 10 miRNAs, thereby inhibits tumor growth both in vitro and in vivo. Furthermore, the study demonstrates that the upregulated miRNAs include miR-23a-5p, miR-222-3p, miR-30b-5p, and miR-106b-5p are mostly modulating signaling cascades such as focal adhesion MAPK pathway [89]. Andrographolide inhibits tumor angiogenesis which is a crucial process for facilitating the cancer cells in such a way to enter into the blood circulation and disseminate them to distant sites (metastasis). Andrographolide exerts an anti-angiogenesis effect by significantly reducing the neovascularization in the yolk sac membrane (YSM) and chorioallantoic membrane (CAM) model of the chick embryo. Mechanistically, andrographolide simultaneously downregulates the expression of miR-21-5p and targets tissue inhibitor of metalloproteases 3 (TIMP3) which eventually leads to inhibition of cell viability, cell migration, invasion and tube formation of endothelial cells [90]. Andrographolide significantly decreases the stemness potential and enhances the radiosensitivity of OCSCs by the upregulation of tumor suppressor miR-218. The upregulation of miR-218 mitigates the reduction in Bmi expression and leads to a significant reduction in the stemness of OCSCs. In this way tumor aggressiveness is curtailed [91].
Boswellic acid
A chief triterpene natural compound, boswellic acid is derived from a resin obtained from Boswellia serrata. Boswellic acid (3 acetyl-11-keto-β-boswellic acid-AKBA) and its derivatives have been reported to modulate the expression of miRNAs. One important study suggests that in colorectal cancer, AKBA upregulates some crucial tumor suppressor miRNAs (let-7, miR-34a, miR-27a, and miR-200 family), which in turn causes a drastic reduction in tumor growth in xenograft mouse models [92]. AKBA augments programmed cell death, triggers G1 cell cycle arrest and abrogates estrogen receptor-alpha (ER-α) by modulating miR-206 in transformed breast cancer cells [93]. This indicates AKBA mediated modulation of miRNAs is a very important mechanism that exhibits anti-tumor potential and needs further systematic and extensive evaluation.
Betulinic acid (BA)
BA is a pentacyclic triterpene lupane structural natural product compound obtained from various plant sources including fruits and vegetables. BA exhibits numerous pharmacological properties, and these can be hepatoprotective, anti-malarial, anti-inflammatory, immunomodulatory, anti-fibrotic, anti-HIV and anti-angiogenic [94]. Additionally, BA displays an anti-proliferative effect on a wide range of human cancer cell lines. Despite inducing cell killing in tumor cells by various anti-cancer mechanisms including induction of apoptosis, inhibition of cell migration and invasion, BA has been reported to exert an anti-cancer effect by modulating miRNAs [95]. BA exerts tumor inhibitory effect in HCC cells by simultaneously upregulates the expression of miR-21 and p66shc. The mechanistic study demonstrates that BA induces miR-21 mediated induction of p53. This induction of p53 activates pro-apoptotic proteins, eliminates ROS generation and increases depolarization of mitochondrial membrane potential. Furthermore, the study demonstrated the inhibition of N-nitrosodiethylamine/carbon tetrachloride (DEN/CCl4)-induced tumor growth in mice by BA inhibiting Sod2 signaling in HCC cells [96]. Another study revealed that a combination of BA with miR-101 and miR-24-2 in pancreatic cancer cells (Mia PaCa-2, PANC-1) exhibits an anti-tumor synergistic effect. This is revealed by significantly inhibiting cell viability and apoptosis in pancreatic cancer cells [97].
Cucurbitacin B
Cucurbitacin B belongs to the tetracyclic triterpene class of natural product compounds. Cucurbitacin B exhibits anti-cancer potential on various types of cancer cell lines [98]. Cucurbitacin B exerts an anti-proliferative effect in pancreatic cancer cells by downregulating the expression of actin filament-associated protein 1-antisense RNA 1 (AFAP1-ASI) and triggering cell cycle arrest at the G2M phase. Mechanistically cucurbitacin B upregulates the expression of tumor suppressor miR-146b-5p that binds to AFAP1-AS1 and prevents any tumorigenic effect to pancreatic cancer cells for both in vitro and in vivo models [99]. Another interesting study in gastric cancer cells discovered that long non-coding RNA (lncRNA) GACAT3 plays a key role in the tumorigenesis of gastric cancer. Interestingly, GACAT3 is believed to behave like the miRNA sponge for high mobility group A1 (HMGA1) which predominantly acts as an oncogene in most cancers. However, increased expression of GACAT3 decreases the cucurbitacin-mediated apoptosis in gastric cancer [100]. Cucurbitacin upregulates miR-143, miR-145 and miR-34a, thereby compromising cell viability, enhancing significant cell killing by programmed cell death type I (apoptosis) and triggering cell cycle arrest at G1S phase. It does this by upregulating and downregulating the protein expressions of p27, p21, and Rb, pRb, E6, CDK4, cyclin D1, respectively [101].
Paclitaxel
Paclitaxel is a diterpenoid compound, most commonly used as an anti-neoplastic drug. It is used to combat various types of malignancies. Mechanistically, paclitaxel induces programmed cell death in tumor cells by attenuating tubulin function, which eventually triggers cell cycle arrest and curtails neoplastic cells’ ability to divide [102]. Disappointingly, patients treated with paclitaxel usually develop more resistance to it, which becomes a problem for patients suffering from various malignancies and particularly lung carcinoma patients. Paclitaxel induced resistance in lung cancer cells (A549) upregulates oncomir miR-421 that helps in the reduction of KEAP1 expression and augments neoplastic features such as invasion, migration and clonogenic property of lung carcinoma cells. Further, the study demonstrates that miR-421 is regulated by β-catenin that acts as the upstream regulator of miR-421 [103]. Bioinformatics analysis suggests that paclitaxel-resistant NSCLC H460 cells modulated as many as 43 miRNAs. Of these, 28 miRNAs were downregulated and 15 miRNAs were upregulated when compared to normal H460 cells. Further analysis revealed that the three key miRNAs (miR-766-3p, miR-362-3p, miR-6507-3p) are dysregulated and might play a critical role in the development of paclitaxel resistance by targeting microtubule-associated protein tau (MAPT) in NSCLC (H460) cells [104]. The paclitaxel-mediated anti-tumor effect was curtailed by oncomir miR-4262 and targeting tumor suppressor protein PTEN, thereby hyperactivating downstream PI3K/Akt pathway in NSCLC cells [105].
However, the bioinformatic analysis demonstrated that the upregulation of tumor suppressor miR-5195-3p expression sensitizes paclitaxel-resistant triple-negative cancer cells (TNBC) to enhance the anti-proliferative effect, apoptosis and triggers cell cycle arrest [106]. Paclitaxel-induced drug resistance is overcome by tumor suppressor miR-107, through increasing the induction of apoptosis and reducing the expression of cyclin D1, Wnt1, and β-catenin in breast cancer cells [107]. Compelling evidence demonstrated that long intergenic noncoding RNA 00511 (LINC00511) regulates paclitaxel-resistant breast cancer cells by negatively regulating miR-29c. However, the simultaneous knockdown of LINC00511 and paclitaxel treatment upregulates miR-29c and augments the killing of breast cancer cells [108]. Additionally, miR-29c in nasopharyngeal carcinoma targets integrin beta-1 (ITBG1), overcomes paclitaxel resistance, sensitizes and induces apoptosis to nasopharyngeal cancer cells [109]. A combination of paclitaxel and miR-34a in CRC cells overcomes resistance, augments tumor cell killing by triggering cell cycle arrest [110]. Interestingly, miR-155-3p is dysregulated in breast cancer cells and is associated with a poor survival rate in breast cancer patients. Overexpression of miR-155-3p targets MYD88 and reverses paclitaxel drug resistance [111]. Collectively, this evidence demonstrated that paclitaxel is a promising anti-tumor agent and modulates miRNA expression which regulates various signaling pathways. Figure 3 represents the structure of terpenoids and associated compounds modulating miRNAs. Table 2 shows the different terpenoids and associated compounds modulating miRNA and their mechanism of action in various cellular and animal models.
Figure 3.
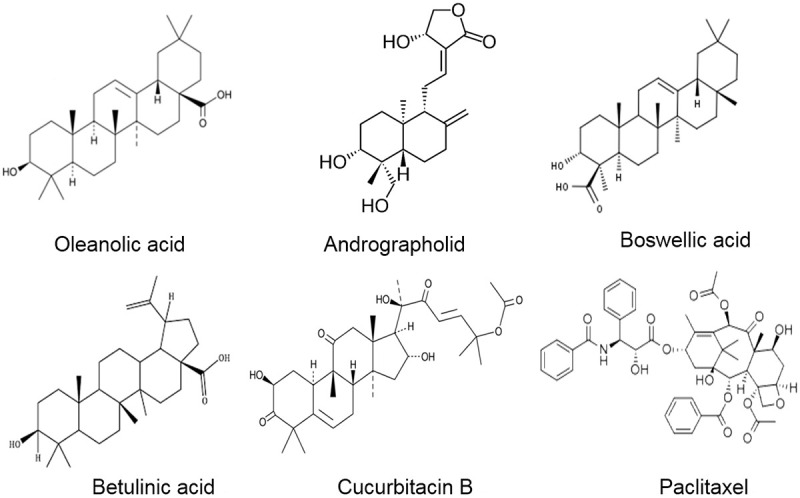
Structure of terpenoids and associated compounds modulating miRNAs.
Table 2.
Terpenoids and associated natural compounds modulating miRNA and their mechanism of action against various cellular and animal models
| Natural Products | Modulation of miRNAs | Mechanism of action | Tumor type | References/Authors |
|---|---|---|---|---|
| Oleanolic acid | ↑miR-122 | Suppresses tumor growth via CCNG1/MEF2D pathway | Lung cancer | Zhao et al. 2015 [88] |
| Andrographolide | ↑miR-222-3p, miR-23a-3p, miR-106b-5p and miR-30b-5p, miR-218 | Apoptosis to cancer cells, inhibits neovascularization, mitigates stemness and tumor aggressiveness, chemosensitization of cancer cells | HCC, oral carcinoma | Lu et al. 2016 [89], Dai et al. 2017 [90], Yang et al. 2017 [91] |
| ↓miR-21-5p | ||||
| Boswellic acid | ↑miR-34a | Enhances anti-tumor efficacy in xenografts, inhibits cell cycle and induces apoptosis | CRC, breast cancer | Toden et al. 2015 [92], Jiang et al. 2020 [93] |
| ↓miR-27a, miR-206 | ||||
| Betulinic acid (BA) | ↑miR-24-2, miR-21, miR-101 | P53 mediated apoptosis, eliminates ROS production, Activation of procaspase 3, PARP1 and PKM2 | HCC, pancreatic cancer | Yang et al. 2015 [96], Pandita et al. 2015 [97] |
| Cucurbitacin B | ↑miR-146b-5p, miR-143, miR-145, miR-34a | Decreases actin filament expression, triggers cell cycle arrest, decreases cell viability, upregulates pro-apoptotic proteins | Pancreatic cancer, cervical cancer | Zhou et al. 2019 [99], Sikander et al. 2016 [101] |
| Paclitaxel | ↑miR-5195-3p, miR-107, miR-29c, miR-155-3p, miR-34a | Sensitization of resistant cells to apoptosis, induces cell cycle arrest, attenuates β-catenin signaling pathway, targets integrin beta-1 (ITBG1), Increases neoplastic features like clonogenic, invasion and motility property of cells, PTEN downregulation | NSCLC, breast cancer, nasopharyngeal carcinoma, CRC | Duan et al. 2019 [103], Cai et al. 2019 [104], Sun et al. 2019 [105], Liu et al. 2019 [106], Ma et al. 2019 [107], Zhang et al. 2019 [108], Huang et al. 2019 [109], Soltani et al. 2019 [110], Zhang et al. 2019 [111] |
| ↓miR-421, miR-766-3p, miR-362-3p, miR-6507-3p, miR-4262 |
Alkaloids and associated compounds
Berberine (BBM) and Berbamine derivate BBMD3
BBM and BBMD3 are two important and commonly used isoquinoline alkaloids for pharmacological activities. Berberine is extensively used against various gastrointestinal associated ailments. Recent reports documented that BBM in combination with gefitinib inhibits HOTAIR-mediated EMT by simultaneous downregulation and upregulation of snail and miR-34a-5p in SCLCC cells [112]. BBM sensitizes p53 defective ALL cells to apoptosis by upregulating miR-24-3p and downregulating anti-apoptotic protein XIAP [113]. Additionally, berberine exhibits anti-cancer potential in multiple myeloma cells by suppressing the expression of miR-19a/92a [114,115]. Moreover, miRNA microarray analysis demonstrated that berberine downregulates the cluster of oncomir miR-106b/26 in multiple myeloma cells. These are involved in many tumor-associated signaling cascades and induction of tiny anti-miR106b/25 clusters, exerting a significant anti-tumor effect [116].
Berberine suppresses both in vitro and in vivo endometrial cancer cell proliferation, invasion, motility, and metastasis, by inducing tumor suppressor miR-101, which eventually causes abrogation of prostaglandin E2 (PGE2)/cyclooxygenase-2 (COX-2) signaling cascade [117]. Berberine in combination with miR-122 and stabilizing agent and nanocarrier polyethyleneimine (PEI)-cholesterol (PC) attenuates OSCC migration and invasion significantly when compared with berberine alone [118]. Berberine in combination with cisplatin downregulates miR-93 and thereby augments significant cell death, also triggering cell cycle arrest in the G0/G1 phase in ovarian cancer cells A2700 [119]. Berberine in combination with cisplatin in gastric cancer cells (SGC-7901, BGC-823) drastically induces caspase-dependent cell death and enhances cell sensitivity to cisplatin by upregulating tumor suppressor miR-203, that targets 3’-UTR region of Bcl-w to induce cell death in cisplatin-resistant gastric cancer cells [120]. A combination of berberine and evodiamine restores E-cadherin and par3 expression and downregulates the expression of oncomir miR-429 in CRC cells and animal tissues [121]. However, the combination modulates miRNA expression by influencing the expression of DNMTs at the post-transcriptional stage [122].
Camptothecin (CPT)
Camptothecin, an alkaloid natural product, extracted from Camptotheca acuminata bark, is reported to have potential anti-tumor activity against diverse cancers [123]. In myelogenous leukemia cells and human cervical carcinoma cells, CPT downregulates the expression of miR-125b, and this results in the upregulation of Bak1 and p53 and enhances apoptosis [124]. Camptothecin attenuates transcriptional factor hypoxia-inducible factor-1α (HIF-1α) in HeLa cells. This attenuation increases the expression of miR-17-5p, miR-155, and miR-18a to inhibit tumor growth [125]. However, in cervical cancer cells, hydroxycamptothecin (HCPT) mediated autophagy is attenuated by miR-30a and acts as oncomir by targets the 3’-UTR region of Beclin 1 which is a key tumor suppressor player in autophagy induction [126]. HCPT sensitizes resistant gastric cancer cells to promote programmed cell death by modulating at least 25 miRNAs (miR-7, miR-126, miR-196a, let-7g, miR-200, miR-31, and miR-338), which play a crucial role in the development of tumorigenesis and chemosensitivity [127]. In drug-resistant CRC cells, HCPT fails to kill the cells, but the overexpression of miR-506 in a combination with HCPT restores the anti-cancer potential of peroxisome proliferator-activated receptor (PPARα)-mediated by inducing cell killing in resistant CRC cells [128].
CPT exerts anti-tumor effect by augmenting apoptosis and autophagy in cervical cancer cells. Mechanistically, CPT upregulates the expression of tumor suppressor miR-16 and miR-15a, which augments autophagy by targeting a key component of the mammalian target of rapamycin complex 2 (mTORC2) called Rictor. Suppression of Rictor abrogates mTORC1 and p70S6K associated phosphorylation and consequently reduces cell viability and triggers cell cycle arrest in the G1/S phase, thus augmenting CPT efficacy [129]. CPT suppresses HIF-1α protein expression by upregulating miR-155 and miR-17-5p, which targets the HIF-1α mRNA 3’-UTR region and impairing protein expression [125]. In a similar study, CPT restores mitochondrial-mediated apoptosis in cancer cells by mitigating the expression of oncomir miR-125b, which targets the 3’-UTR region of key proteins of mitochondrial-dependent apoptosis signaling pathway. Examples are p53, Mcl1, and Bak1 and prevents any activation of intrinsic apoptosis [124].
Vincristine
Vincristine is a vinca alkaloid derived from Catharanthus roseus and it has been utilized as a chemotherapeutic agent for the last 40 years against a broad spectrum of cancers, particularly leukemia-associated malignancies. Mechanistically, vincristine affects microtubules and interferes in spindle formation during the cell division of neoplastic cells. Despite interference from microtubules and spindle formation during the division of neoplastic cells, a recent study demonstrated that vincristine modulates various miRNAs [130]. Vincristine sensitizes drug-resistant diffuse large B-cell lymphoma (DLBCL) to programmed cell death by upregulating miR-155 expression and improving the outcome of those B-cell lymphoma patients categorized under DLBCL [130]. A combination of vinca alkaloids vincristine, vinblastine, and vinorelbine exhibits p53 upregulation in breast cancer cells. However, the mechanistic study demonstrated that a combination of all three vinca alkaloids triggers apoptosis in breast cancer cells by upregulating tumor suppressor miR-222-3p [131]. Deep sequencing identification of vincristine associated drug resistance in CRC cells (HCT-8) demonstrated that 24 miRNAs were significantly modulated, of which 7 and 17 miRNAs were downregulated and upregulated, respectively [132]. However, co-expression of miR-99a and miR-125b helps in developing resistance of megakaryoblastic leukemia cells (CMK) to vincristine treatment [133]. Collectively, these reports suggest that vincristine is a promising anti-cancer drug and modulates expression of key miRNAs which regulates tumorigenesis associated signaling pathways. Figure 4 depicts the structure of alkaloids and associated compounds modulating miRNAs. Table 3 summarizes the different alkaloids and associated compounds modulating miRNA and their mechanism of action in various cellular and animal models.
Figure 4.
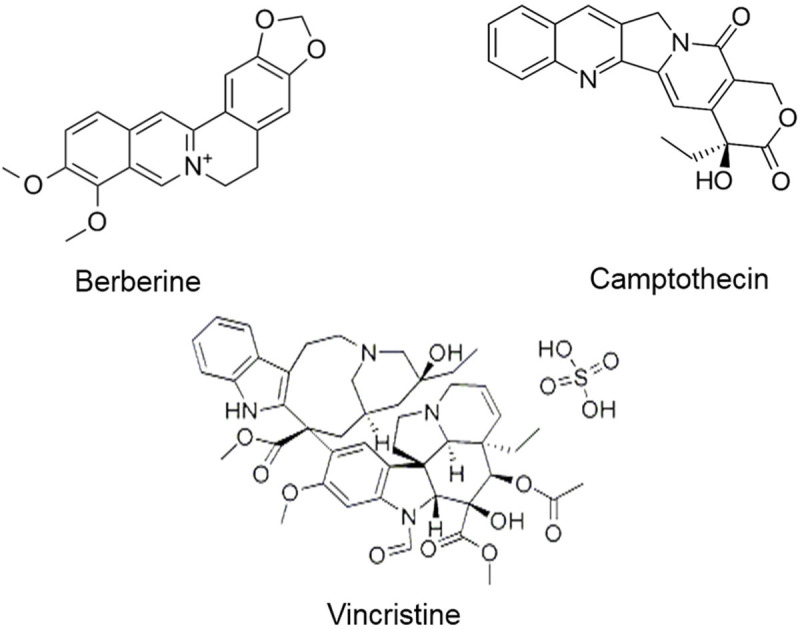
Structure of alkaloids and associated compounds modulating miRNAs.
Table 3.
Alkaloids and associated natural compounds modulating miRNA and their mechanism of action against various cellular and animal models
| Natural Products | Modulation of miRNAs | Mechanism of action | Tumor type | References/Authors |
|---|---|---|---|---|
| Berberine (BBM) and Berbamine derivate (BBMD3) | ↑miR-34a-5p, miR-24-3p, miR-152, miR-429, miR-29a, miR-34a, miR-154, miR-26a, miR-124, miR-101, miR-122, miR-203 | Inhibits snail-mediated EMT, induction of apoptosis by targeting XIAP, p53-mediated apoptosis induction, suppresses cell cycle proteins, targets cyclooxygenase-2 (COX-2), arrests cell cycle and sensitizes tumor cells to cisplatin therapy, modulates DNMTs | NSCLC, AML, CRC, melanoma, multiple myeloma, endometrial carcinoma, oral squamous cell carcinoma, gastric carcinoma | Zheng et al. 2020 [112], Liu et al. 2020 [113], Dong et al. 2019 [114], Yin et al. [115], Gu et al. 2017 [116], Wang et al. 2018 [117], Li et al. 2018 [118], Chen et al. 2015 [119], You et al. 2016 [120], Liu et al. 2016 [121], Huang et al. 2017 [122] |
| ↓miR-19a/92a, miR-106b/26, miR-93, miR106b/25 | ||||
| Camptothecin (CPT) | ↑miR-155, miR-17-5p, miR-506 | Induces apoptosis, autophagy and triggers cell cycle arrest at G1/S phase, reduces HIF-1α protein expression and activity, sensitization of cells to apoptosis | Myeloid leukemia, Cervical carcinoma, gastric carcinoma, CRC | Zeng et al. 2012 [124], Bertozzi et al. 2014 [125], Cheng et al. 2015 [126], Wu et al. 2011 [127], Tong et al. 2011 [128], Huang et al. 2015 [129] |
| ↓miR-125b, miR-30a, miR-125b | Inhibits apoptosis and autophagy, prevents intrinsic apoptosis | |||
| Vincristine | ↑miR-155, miR-222-3p | Induces apoptosis, p53 upregulation, Drug-resistance, downregulation of apoptotic pathways | Diffuse large B-cell lymphoma, breast cancer, CRC, megakaryoblastic leukemia, ALL | Due et al. 2019 [130], Mavrogiannis et al. 2018 [131], Dong et al. 2015 [132], Kandi et al. 2015 [133] |
| ↓miR-99a, miR-125b, miR-100 |
Other natural compounds
Curcumin
Curcumin is a very important natural product compound found in turmeric. It is abundant in polyphenolics and has numerous health benefits, for instance, anti-tumor, anti-inflammatory, and anti-oxidant properties. Preclinical data suggest that curcumin exhibits anti-cancer potential on diverse cancers [48,134]. Recent studies revealed that curcumin exhibits anti-tumor activity on a wide range of cancers by modulating epigenetics [135]. Curcumin sensitizes tumorigenic NF-κB signaling in glioblastoma cells to the drug of choice TMZ, thereby inducing miR-146a which results in inhibited tumor cell growth and survival [136]. Curcumin modulates exosome-mediated miR-21 expression in chronic myelogenous leukemia (CML) cells and causes a drastic reduction in tumor cells [137]. Microarray analysis demonstrated that curcumin when combined with radiation treatment in breast cancer cells (T24 and HT-1376) modulates as many as 17 miRNA expressions. Among them, the combination significantly downregulates the key oncomir miR-1246 expression and markedly reduces the cell viability and clonogenic property, thereby triggering cell cycle arrest at the G0/G1 phase. Further analysis revealed that miR-1246 modulates the 3’-UTR region of p53 and promotes tumorigenesis [138].
However, in prostate cancer cells curcumin upregulates tumor suppressor miR-34a, which eventually inhibits β-catenin and cell cycle-associated regulatory proteins. Consequently, cell cycle arrest and apoptosis is triggered [139]. Curcumin has the potential for triggering cell apoptosis in gastric carcinoma cells (MGC-803) by negatively regulating the miR-21 and decreasing phosphorylation of Akt and upregulating tumor suppressor protein PTEN. Curcumin negatively regulates cell viability, abrogates tumor cell invasion and migration by upregulating tumor suppressor miR-99a in gastric cancer cells (SO-Rb50, Y79) [140]. Curcumin affects tumor cell invasion and inhibiting EMT in breast cancer cells by modulating miR-34a expression which leads to the downregulation of Slug, Axl and CD24 genes which are key mediators of EMT [141]. Furthermore curcumin suppresses growth and motility capability of thymic carcinoma cells and abrogates Notch and mTOR signaling pathways by downregulating the expression of miR-27a [142]. However, the anti-tumor effect was greatly enhanced in combination with MAPK inhibitor PD98059 [143]. It is found that curcumin upregulates tumor suppressor miR-344a-3p in schwannoma cells (RT4), thereby augmenting apoptosis by activating caspase-3, -9 and downregulating anti-apoptotic proteins Bcl-2 [144].
Docosahexaenoic acid (DHA)
An unsaturated fatty acid, DHA is abundant in seaweed oil, egg yolk, etc., and is very common and a key constituent of infant formula for the development of intelligence and vision in infants. Although DHA is used in the therapeutics of breast cancer, the mechanism of action is still not well understood. A recent report suggests that DHA attenuates tumor angiogenesis by suppressing neovascularization. MiRNAs are reported to be secreted in exosomes and translocated to endothelial cells, which facilitates the proangiogenic effect. A recent study revealed that the treatment of MCF-7 cells with DHA increases both miRNAs (let7a, miR-21, miR-23b, miR-27b, and miR-320b) secretion and miRNA enclosed exosome secretion. Through this mechanism DHA might facilitate more exosomal miRNAs to endothelial cells in breast cancer, in this way modulating miRNAs and attenuating tumor angiogenesis [145]. Combination with other polyunsaturated fatty acids, DHA reduces cellular growth of breast cancer cells (MCF-7) by downregulating the expression of oncomir miR-21 [146]. A combination of DHA and eicosapentaenoic acid sensitizes multiple myeloma cells to dexamethasone chemotherapy, thereby upregulating miR-34a. Mechanistically, this combination modulates miR-34a mediated-p53 activation which in turn regulates the suppression of anti-apoptotic protein Bcl-2 [147]. A combination of DHA avoids the detrimental effect of docetaxel in gastric cancer cells on MHC class I chain-related protein A (MICA) by downregulating miR-20a. The restoration of MICA enhances the anti-tumor immune response to the combination of DHA with docetaxel and does not allow docetaxel to negatively regulate MICA [148].
6)-Gingerol (6G)
6G is a polyphenolic alkanone natural product compound with potential pharmacological properties. The aforementioned reports suggest that the anti-tumor activity of 6G is associated with miRNA expression in tumor cells [149]. In myeloid leukemia cells, G6 subdues mitochondrial complex (MRC1), followed by induction of ROS and concomitantly induces DNA damage and upregulation of miR-27b. The G6-mediated upregulated miR-27b attenuates the PPARγ-NF-kB pathway and induces apoptosis in tumor cells [150]. 6G promotes neuroprotection by upregulating miR-103 expression in cerebral ischemic cells (PC-12). Another study demonstrated that 6G-mediated upregulation of miR-103 mitigates Bcl-2 interacting protein 3 (BNIP3) regulation, thus regulating c-Jun-N-terminal kinase (JNK) and p38 MAPK pathways negatively [151].
Diaporine A
Diaporine A is another pharmacologically active natural product compound, extracted from endophytic fungus 3lp-10. In a recent study of NSCLC cells, diaporine A exhibits anti-tumor activity by upregulating miR-99a. This upregulated expression of miR-99a subdues the tumorigenic mTOR signaling pathway, thus controlling tumor growth in NSCLC [152].
Prodigiosene
Prodigiosene (methyl-3-pentyl-6methoxyprodigiosene) is a type of natural product compound obtained from the Serratia marcescens. Prodigiosene exhibits anti-tumor activity by upregulating miR-16-1 in CRC stem-like cells. The upregulation of miR-16-1 drastically silences its target gene survivin and concomitantly sensitizes and promotes apoptosis in CRC cells. It is believed that prodigiosene could be the drug of choice in countries where: firstly, highly expressed survivin in CRC stem cells becomes the main obstacle in chemotherapeutic; and secondly, causes high morbidity and mortality of CRC patients [153].
Aplysin
A marine bromide natural compound, Aplysin not only suppresses tumorigenesis but also sensitizes and eliminates drug-resistant tumor cells. Aplysin sensitizes and eliminates TMZ resistant glioma cells by upregulating the tumor suppressor miR-181 which induces downregulation of mitogen-activated protein kinase kinase 1 (MEK1) [154].
Methyl jasmonate
Recently, a combination of drugs in clinical therapeutics is one of the key advances made in the field of medicine. The synergic effect of the drugs is preferred over single drug therapeutics in cancer and these combinations considerably enhance drugs’ anti-tumor potential. A recent analysis suggests that the combination of two natural products, methyl jasmonate and gambogic acid (GA) sensitizes and triggers resistant bladder cancer cells to programmed cell death. Mechanistically, this combination increases the tumor suppressor miR-101 expression by deregulating EZH2, which results in a significant induction of tumor efficacy compared to the single drug [155]. A similar study in CRC cells (SW620) revealed that methyl jasmonate exerts an inhibitory effect on cell proliferation and enhances cell apoptosis by upregulating miR-101 and downregulates EZH2 protein expression [156].
Ellagitannin (BJA3121) and BJA32515
The polyphenolic natural product compounds, Ellagitannin (1,3-Di-O-galloyl-4,6-(s)-HHDP-bD-glucopyranose, BJA3121) and BJA32515 (1,3,4-triO-galloyl-6-O-caffeoyl-beta-D-glucopyranose) are obtained from Balanophora Japonica Makino. These compounds display strong anti-proliferative potential. A recent study demonstrates that ellagitannin BJA3121 and BJA32515 exhibit tumor growth inhibition in HepG2 tumor xenograft by modulating the expression profile of miRNAs. Ellagitannin derivative methylated urolithin A exhibits anti-proliferative potential, upregulates PTEN and programmed cell death protein 4 (Pdcd4) protein expression. Mechanistically, ellagitannin derivative treatment downregulates miR-21 expression and subsequently causes simultaneous upregulation of tumor suppressor PTEN, FOXO3a and downregulation of two things: tumorigenic Akt phosphorylation and Wnt/β-catenin in prostate cancer cells (DU 145) [157].
1’S-1’-acetoxychavicol acetate (ACA)
ACA is a very important natural product and has been reported to sensitize resistant tumor cells to programmed cell death. ACA induces anti-cancer activity by sensitizing cisplatin-resistant human cervical carcinoma cell line Ca Ski. ACA in combination with cisplatin modulates miRNAs that target genes linked to cell cycle progression and apoptosis. The change in the expression level of miRNAs upon treatment with cisplatin and ACA is significant for inducing apoptosis in these resistant cells [158]. However, ACA downregulates oncomir miR-210 expression and promotes the anti-tumor effect by negatively regulating cell proliferation and exhibits significant cell killing in cervical cancer cells. Mechanistically, ACA inhibits the interaction of miR-210 with 3’-UTR region of SMAD4 and helps in the restoration of SMAD4 expression, which eventually enhances anti-proliferative effect and induction of cell apoptosis [159]. Another study done on cervical cancer cells demonstrated that the downregulation of miR-629 by its respective inhibitor increases the ACA sensitization of resistant cervical cancer cells to anti-tumor effect and apoptosis. The mechanistic study revealed that upon miR-629 inhibition, ACA treated cervical cancer cells to restore the Ras suppressor 1 (RSU1) protein expression, thereby increasing anti-proliferative and apoptosis-inducing ability in cervical cancer cells [160].
10’ (Z), 13’ (E), 15’ (E) heptadecatrienylhydroquinone [HQ17 (3)]
HQ17 (3) is a natural product compound obtained from the lacquer tree sap of the species Rhus succedanea. The compound exhibits anti-leukemia activity by regulating c-Myc expression. Mechanistically, HQ17 (3) attenuates c-Myc expression, resulting in the downregulation of miR-17-92 cluster expression and so inhibiting leukemia cells [161].
Indole-3-Carbinol (I3C) and Diindolylmethane (DIM)
DIM is a very active, natural product compound present in cruciferous vegetables like kale, cabbage, radish, broccoli, turnip, cauliflower, and Brussels sprouts [162,163]. DIM modulates the expression of various miRNAs intricated in tumorigenesis [164]. DIM in combination with tetrachloridebenzo-p-dioxin (TCDD) upregulates the expression of aryl hydrocarbon receptor (Ahr) by modulating miR-150-5p expression. The upregulation of miR-150-5p regulates MAPK pathways and can inhibit prostate cancer cells’ viability and invasion [165]. Further, this combination upregulates the Ahr-miRNA cluster (miR-212/132). The synergistic effect of miR-212/132 targets pro-metastatic SRY-related HMG-box4 (SOX4) at the 3’-UTR region and exerts an anti-invasive and anti-migration effect on tumor cells [166]. DIM modulates miR-30e in gastric cancer cells. Mechanistic studies demonstrate that DIM disrupts the interaction of miR-30e with the 3-UTR region of autophagy-related 5 (ATG-5). It, firstly, activates autophagy associated conversion of microtubule-associated protein 1A/1B-light chain 3 (LC3I) to LC3II and ATG5, and secondly, exerts an inhibitory effect on the proliferation of gastric cancer cells [167]. Collectively, these promising results indicated that DIM and I3C might be harnessed as potential anti-cancer chemotherapeutic agents by modulating crucial miRNAs regulating tumorigenesis. Figure 5 presents the structure of natural compounds modulating miRNAs. Table 4 shows the different natural compounds modulating miRNA and their mechanism of action in various cellular and animal models.
Figure 5.
Chemical structures of other natural product compounds which modulate miRNA expression.
Table 4.
Modulation of miRNA expression by other natural product compounds and their possible mechanism of action
| Natural Products | Modulation of miRNAs | Mechanism of action | Tumor type | References/Authors |
|---|---|---|---|---|
| Curcumin | ↑miRNA-146a, MiR-196b, miR-1264, miR-34a, miR-99a, miR-344a-3p | Sensitization of cells to TMZ-mediated apoptosis, Bcr-Abl downregulation, radiosensitization-mediated p53 activation in cells, targets JAK/STAT pathway, inhibits cell invasion and motility, apoptosis induction | Glioblastoma, CML, bladder carcinoma, prostate cancer, retinoblastoma, thymic carcinoma, gastric cancer, schwannoma | Wu et al. 2015 [136], Taverna et al. 2015 [137], Xu et al. 2019 [138], Zhu et al. 2019 [139], Li et al. 2018 [140], Gallardo et al. 2020 [141], Han et al. 2020 [142], Qiang et al. 2019 [143], Sohn et al. 2018 [144] |
| ↓miR-27a, miR-21 | ||||
| Docosahexaenoic acid (DHA) | ↑miR-34a | Attenuates tumor angiogenesis, sensitization of cells, restores MICA expression and enhances anti-tumor effect | Breast carcinoma, multiple myeloma, gastric carcinoma | LeMay et al. 2018 [146], Dai et al. 2017 [147], Shekari et al. 2019 [148] |
| ↓miR-21, miR-20a | ||||
| 6-gingerol (6G) | ↑miR-27b, miR-103 | Blocks PPARγ-NF-kB pathway, mitigates BNIP3 regulation associated JNK and p38MAPK signaling pathway | Myeloid leukemia, neuroblastoma | Rastogi et al. 2014 [150], Kang et al. 2019 [151] |
| Diaporine A | ↑miR-99a | Suppresses mTOR pathway | SCLC | Lin et al. 2017 [152] |
| Prodigiosene | ↑miR-16-1 | Targets surviving | CRC | Sam et al. 2016 [153] |
| Aplysin | ↑miR-181, miR-181b | Sensitize cells towards apoptosis | Glioblastoma | Gong et al. 2014 [154] |
| Methyl jasmonate | ↑miR-101 | Triggers apoptosis, downregulates EZH2 | Bladder cancer, CRC | Wang et al. 2014 [155], Peng et al. 2017 [156] |
| Ellagitannin (BJA321) and BJA32515 | ↓miR-21 | Activates Pdcd4 by upregulating PTEN | Prostate cancer | Zhou et al. 2016 [157] |
| 1’S-1’-acetoxychavicol acetate (ACA) | ↓miR-210, miR-629 | Sensitization of resistant cells | Cervical carcinoma | Phuah et al. 2017 [159], Phuah et al. 2017 [160] |
| 10’(Z), 13’(E), 15’(E)-heptadecatrienylhydroquinone [HQ17 (3)] | ↑miR-17-92 | Downregulation of c-Myc-mediated apoptosis | Leukemia | Liao et al. 2014 [161] |
| Indole-3-Carbinol (I3C) and Diindolylmethane (DIM) | ↑miR-150-5p, miR-212/132 | Targets MAP3K12 pathway, suppresses metastasis by targeting SOX4, activates autophagy | Prostate cancer, breast cancer, gastric cancer | Yu et al. 2018 [165], Hanieh et al. 2015 [166], Ye et al. 2016 [167], Royam et al. 2019 [168], Sayeed et al. 2017 [169] |
| ↓miR-30e |
Conclusion and future perspectives
Besides the importance of miRNAs, epigenetic modifications and their importance in tumorigenesis, the present review highlights the role of natural product compounds modulating tumorigenic miRNAs in favor of chemoprevention. The miRNAs have played a remarkable role in tumorigenesis by binding to the 3’UTR of target mRNA, thereby degrading mRNA or inhibiting translation. Besides miRNAs other noncoding miRNAs (lncRNAs, circular RNAs, etc.) have a crucial role to play in tumorigenesis by modulating the epigenetics of oncomirs and tumor suppressor miRNAs in favor of tumorigenesis. However, miRNAs and other related RNAs not only have a role in tumorigenesis; they also play a key role in diagnostics, prognostics and cancer therapeutics. For this reason miRNAs have much potential in cancer therapeutics.
Despite other pharmacological properties, natural products exhibit anti-tumor activity by modulating the expression of both oncomirs and tumor suppressor miRNAs, thereby impedes cancer signaling pathways and attenuates tumorigenesis. Additionally, the natural products increase the efficacy of conventional cancer therapies by modulating miRNAs (Figure 6). Thus, modulating miRNAs by natural products could open up innovative opportunities for future therapeutics concerning cancer. Owing to their structural complexity, natural products have a unique ability to deal with tumorigenesis by modulating the miRNA expression through numerous mechanisms such as miRNA processing and epigenetic transcriptional modifications. The natural products exhibit anti-tumor growth activity, apoptosis induction, reductions in cell stemness and increasing drug sensitivity by modulating the expression profile of miRNAs.
Figure 6.
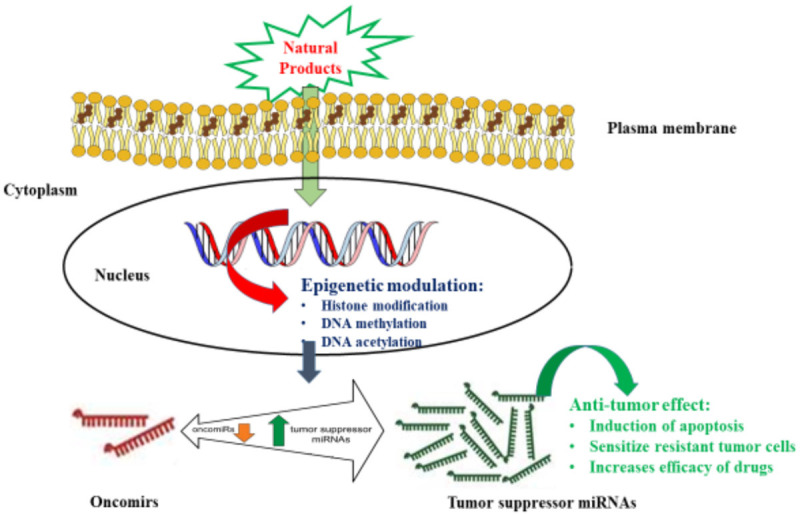
Schematic diagram showing the mechanism of natural product compounds modulating miRNAs in cancer cells.
Besides these pharmacological activities, modulation of miRNAs by natural products could open up new avenues in the field of combination drug design with FDA approved conventional therapeutics for the prevention, sensitization of resistant cells and recurrent patients. The objective is to enhance complete remission of cells and successful treatment outcomes for cancer patients. Despite the expectations of the promising and worthy characteristics of natural products’ anti-tumor activities, there are many challenges to resolve such as poor bioavailability of natural products. To this end, diverse methodologies will be indispensable to overcome these limitations, including synthetic formulations, chemical modifications, and delivery of liposomes and nanoparticles. Thus, it is expected that these novel strategies will avert tumor recurrence and resistance in future therapies of drug development.
Acknowledgements
The researcher would like to thank the Deanship of Scientific Research, Qassim University for funding publication of this project.
Disclosure of conflict of interest
None.
References
- 1.Lui PY, Jin DY, Stevenson NJ. MicroRNA: master controllers of intracellular signaling pathways. Cell Mol Life Sci. 2015;72:3531–3542. doi: 10.1007/s00018-015-1940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maher SG, Bibby BA, Moody HL, Reid G. Epigenetic Cancer Therapy. Elsevier; 2015. MicroRNAs and cancer; pp. 67–90. [Google Scholar]
- 3.Kamphuis WW, Derada Troletti C, Reijerkerk A, Romero IA, de Vries HE. The blood-brain barrier in multiple sclerosis: microRNAs as key regulators. CNS Neurol Disord Drug Targets. 2015;14:157–167. doi: 10.2174/1871527314666150116125246. [DOI] [PubMed] [Google Scholar]
- 4.Ivkovic TC, Voss G, Cornella H, Ceder Y. microRNAs as cancer therapeutics: a step closer to clinical application. Cancer Lett. 2017;407:113–122. doi: 10.1016/j.canlet.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther. 2016;1:1–9. doi: 10.1038/sigtrans.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu X, Liu R, Wang M, Kumar AK, Pan F, He L, Hu Z, Guo Z. MicroRNA-140 impedes DNA repair by targeting FEN1 and enhances chemotherapeutic response in breast cancer. Oncogene. 2020;39:234–247. doi: 10.1038/s41388-019-0986-0. [DOI] [PubMed] [Google Scholar]
- 7.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 8.Jiang C, Chen X, Alattar M, Wei J, Liu H. MicroRNAs in tumorigenesis, metastasis, diagnosis and prognosis of gastric cancer. Cancer Gene Ther. 2015;22:291–301. doi: 10.1038/cgt.2015.19. [DOI] [PubMed] [Google Scholar]
- 9.Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov. 2015;14:111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 10.Shen B. A new golden age of natural products drug discovery. Cell. 2015;163:1297–1300. doi: 10.1016/j.cell.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seca AM, Pinto DC. Plant secondary metabolites as anticancer agents: successes in clinical trials and therapeutic application. Int J Mol Sci. 2018;19:263. doi: 10.3390/ijms19010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Twilley D, Lall N. Natural Products and Drug Discovery. Elsevier; 2018. The role of natural products from plants in the development of anticancer agents; pp. 139–178. [Google Scholar]
- 13.Alnuqaydan AM, Rah B. Tamarix articulata (T. articulata)-an important halophytic medicinal plant with potential pharmacological properties. Curr Pharm Biotechnol. 2019;20:285–292. doi: 10.2174/1389201020666190318120103. [DOI] [PubMed] [Google Scholar]
- 14.Rah B, Amin H, Yousuf K, Khan S, Jamwal G, Mukherjee D, Goswami A. A novel MMP-2 inhibitor 3-azidowithaferin A (3-azidoWA) abrogates cancer cell invasion and angiogenesis by modulating extracellular Par-4. PLoS One. 2012;7:e44039. doi: 10.1371/journal.pone.0044039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rah B, Lone SH, Rasool RU, Farooq S, Nayak D, Chikan NA, Chakraborty S, Behl A, Mondhe DM, Goswami A. Design and synthesis of antitumor heck-coupled Sclareol analogues: modulation of BH3 family members by SS-12 in autophagy and apoptotic cell death. J Med Chem. 2015;58:3432–3444. doi: 10.1021/jm501942m. [DOI] [PubMed] [Google Scholar]
- 16.Rah B, Rasool Ru, Nayak D, Yousuf SK, Mukherjee D, Kumar LD, Goswami A. PAWR-mediated suppression of BCL2 promotes switching of 3-azido withaferin A (3-AWA)-induced autophagy to apoptosis in prostate cancer cells. Autophagy. 2015;11:314–331. doi: 10.1080/15548627.2015.1017182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alnuqaydan AM, Rah B, Almutary AG, Chauhan SS. Synergistic antitumor effect of 5-fluorouracil and withaferin-A induces endoplasmic reticulum stress-mediated autophagy and apoptosis in colorectal cancer cells. Am J Cancer Res. 2020;10:799–815. [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma SV, Haber DA, Settleman J. Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat Rev Cancer. 2010;10:241–53. doi: 10.1038/nrc2820. [DOI] [PubMed] [Google Scholar]
- 19.Chang LC, Yu YL. Dietary components as epigenetic-regulating agents against cancer. Biomedicine. 2016;6:1–8. doi: 10.7603/s40681-016-0002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lernoux M, Schnekenburger M, Dicato M, Diederich M. Anti-cancer effects of naturally derived compounds targeting histone deacetylase 6-related pathways. Pharmacol Res. 2018;129:337–356. doi: 10.1016/j.phrs.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Ozbey U, Attar R, Romero MA, Alhewairini SS, Afshar B, Sabitaliyevich UY, Hanna-Wakim L, Ozcelik B, Farooqi AA. Apigenin as an effective anticancer natural product: spotlight on TRAIL, WNT/β-catenin, JAK-STAT pathways, and microRNAs. J Cell Biochem. 2019;120:1060–1067. doi: 10.1002/jcb.27575. [DOI] [PubMed] [Google Scholar]
- 22.Samec M, Liskova A, Kubatka P, Uramova S, Zubor P, Samuel SM, Zulli A, Pec M, Bielik T, Biringer K. The role of dietary phytochemicals in the carcinogenesis via the modulation of miRNA expression. J Cancer Res Clin Oncol. 2019;145:1665–1679. doi: 10.1007/s00432-019-02940-0. [DOI] [PubMed] [Google Scholar]
- 23.Yi J, Li S, Wang C, Cao N, Qu H, Cheng C, Wang Z, Wang L, Zhou L. Potential applications of polyphenols on main ncRNAs regulations as novel therapeutic strategy for cancer. Biomed Pharmacother. 2019;113:108703. doi: 10.1016/j.biopha.2019.108703. [DOI] [PubMed] [Google Scholar]
- 24.Reddy KB. MicroRNA (miRNA) in cancer. Cancer Cell Int. 2015;15:38. doi: 10.1186/s12935-015-0185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliveto S, Mancino M, Manfrini N, Biffo S. Role of microRNAs in translation regulation and cancer. World J Biol Chem. 2017;8:45–56. doi: 10.4331/wjbc.v8.i1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, Zhang B. MicroRNAs in control of plant development. J Cell Physiol. 2016;231:303–313. doi: 10.1002/jcp.25125. [DOI] [PubMed] [Google Scholar]
- 27.Bracken CP, Scott HS, Goodall GJ. A network-biology perspective of microRNA function and dysfunction in cancer. Nat Rev Genet. 2016;17:719. doi: 10.1038/nrg.2016.134. [DOI] [PubMed] [Google Scholar]
- 28.Gebert LF, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Genet. 2019;20:21–37. doi: 10.1038/s41580-018-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung HJ, Suh Y. Regulation of IGF-1 signaling by microRNAs. Front Genet. 2015;5:472. doi: 10.3389/fgene.2014.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta A, Baltimore D. MicroRNAs as regulatory elements in immune system logic. Nat Rev Immunol. 2016;16:279–94. doi: 10.1038/nri.2016.40. [DOI] [PubMed] [Google Scholar]
- 31.Khan IN, Al-Karim S, Bora RS, Chaudhary AG, Saini KS. Cancer stem cells: a challenging paradigm for designing targeted drug therapies. Drug Discov Today. 2015;20:1205–1216. doi: 10.1016/j.drudis.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat Rev Mol Cell Biol. 2019;20:5–20. doi: 10.1038/s41580-019-0106-6. [DOI] [PubMed] [Google Scholar]
- 33.Shah MY, Ferrajoli A, Sood AK, Lopez-Berestein G, Calin GA. microRNA therapeutics in cancer-an emerging concept. EBioMedicine. 2016;12:34–42. doi: 10.1016/j.ebiom.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou K, Liu M, Cao Y. New insight into microRNA functions in cancer: oncogene-microRNA-tumor suppressor gene network. Front Mol Biosci. 2017;4:46. doi: 10.3389/fmolb.2017.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gulyaeva LF, Kushlinskiy NE. Regulatory mechanisms of microRNA expression. J Transl Med. 2016;14:143. doi: 10.1186/s12967-016-0893-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Syed SN, Brune B. MicroRNAs as emerging regulators of signaling in the tumor microenvironment. Cancers (Basel) 2020;12:911. doi: 10.3390/cancers12040911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knackmuss U, Lindner S, Aneichyk T, Kotkamp B, Knust Z, Villunger A, Herzog S. MAP3K11 is a tumor suppressor targeted by the oncomiR miR-125b in early B cells. Cell Death Differ. 2016;23:242–252. doi: 10.1038/cdd.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slabáková E, Culig Z, Remšík J, Souček K. Alternative mechanisms of miR-34a regulation in cancer. Cell Death Dis. 2017;8:e3100–e3100. doi: 10.1038/cddis.2017.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao Y, Lin L, Li T, Yang J, Wei Y. The role of miRNA-223 in cancer: function, diagnosis and therapy. Gene. 2017;616:1–7. doi: 10.1016/j.gene.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 40.Iqbal MA, Arora S, Prakasam G, Calin GA, Syed MA. MicroRNA in lung cancer: role, mechanisms, pathways and therapeutic relevance. Mol Aspects Med. 2019;70:3–20. doi: 10.1016/j.mam.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Efferth T, Saeed ME, Kadioglu O, Seo EJ, Shirooie S, Mbaveng AT, Nabavi SM, Kuete V. Collateral sensitivity of natural products in drug-resistant cancer cells. Biotechnol Adv. 2020;38:107342. doi: 10.1016/j.biotechadv.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Smanski MJ, Zhou H, Claesen J, Shen B, Fischbach MA, Voigt CA. Synthetic biology to access and expand nature’s chemical diversity. Nat Rev Microbiol. 2016;14:135–49. doi: 10.1038/nrmicro.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rah B, Nayak D, Rasool R, Chakraborty S, Katoch A, Amin H, Goswami A. Reprogramming of molecular switching events in upr driven er stress: scope for development of anticancer therapeutics. Curr Mol Med. 2016;16:690–701. doi: 10.2174/1566524016666160829152658. [DOI] [PubMed] [Google Scholar]
- 44.Singh I, Amin H, Rah B, Goswami A. Targeting EGFR and IGF 1R: a promising combination therapy for metastatic cancer. Front Biosci (Schol Ed) 2013;5:231–46. doi: 10.2741/s369. [DOI] [PubMed] [Google Scholar]
- 45.Li S, Kuo HD, Yin R, Wu R, Liu X, Wang L, Hudlikar R, Peter RM, Kong AN. Epigenetics/epigenomics of triterpenoids in cancer prevention and in health. Biochem Pharmacol. 2020;175:113890. doi: 10.1016/j.bcp.2020.113890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan H, Reale M, Ullah H, Sureda A, Tejada S, Wang Y, Zhang ZJ, Xiao J. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: updates and future directions. Biotechnol Adv. 2020;38:107385. doi: 10.1016/j.biotechadv.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 47.Yuan M, Zhang X, Zhang J, Wang K, Zhang Y, Shang W, Zhang Y, Cui J, Shi X, Na H. DC-SIGN-LEF1/TCF1-miR-185 feedback loop promotes colorectal cancer invasion and metastasis. Cell Death Differ. 2020;27:379–395. doi: 10.1038/s41418-019-0361-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luca SV, Macovei I, Bujor A, Miron A, Skalicka-Woźniak K, Aprotosoaie AC, Trifan A. Bioactivity of dietary polyphenols: the role of metabolites. Crit Rev Food Sci Nutr. 2020;60:626–659. doi: 10.1080/10408398.2018.1546669. [DOI] [PubMed] [Google Scholar]
- 49.Wang G, Dai F, Yu K, Jia Z, Zhang A, Huang Q, Kang C, Jiang H, Pu P. Resveratrol inhibits glioma cell growth via targeting oncogenic microRNAs and multiple signaling pathways. Int J Oncol. 2015;46:1739–1747. doi: 10.3892/ijo.2015.2863. [DOI] [PubMed] [Google Scholar]
- 50.Otsuka K, Yamamoto Y, Ochiya T. Regulatory role of resveratrol, a microRNA-controlling compound, in HNRNPA1 expression, which is associated with poor prognosis in breast cancer. Oncotarget. 2018;9:24718–24730. doi: 10.18632/oncotarget.25339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang W, Jiang H, Chen Y, Ren F. Resveratrol chemosensitizes adriamycin-resistant breast cancer cells by modulating miR-122-5p. J Cell Biochem. 2019;120:16283–16292. doi: 10.1002/jcb.28910. [DOI] [PubMed] [Google Scholar]
- 52.Venkatadri R, Muni T, Iyer AK, Yakisich JS, Azad N. Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis. 2016;7:e2104. doi: 10.1038/cddis.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pan J, Shen J, Si W, Du C, Chen D, Xu L, Yao M, Fu P, Fan W. Resveratrol promotes MICA/B expression and natural killer cell lysis of breast cancer cells by suppressing c-Myc/miR-17 pathway. Oncotarget. 2017;8:65743–65758. doi: 10.18632/oncotarget.19445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karimi Dermani F, Saidijam M, Amini R, Mahdavinezhad A, Heydari K, Najafi R. Resveratrol inhibits proliferation, invasion, and epithelial-mesenchymal transition by increasing miR-200c expression in HCT-116 colorectal cancer cells. J Cell Biochem. 2017;118:1547–1555. doi: 10.1002/jcb.25816. [DOI] [PubMed] [Google Scholar]
- 55.Wu F, Cui L. Resveratrol suppresses melanoma by inhibiting NF-κB/miR-221 and inducing TFG expression. Arch Dermatol Res. 2017;309:823–831. doi: 10.1007/s00403-017-1784-6. [DOI] [PubMed] [Google Scholar]
- 56.Zhou W, Wang S, Ying Y, Zhou R, Mao P. miR-196b/miR-1290 participate in the antitumor effect of resveratrol via regulation of IGFBP3 expression in acute lymphoblastic leukemia. Oncol Rep. 2017;37:1075–1083. doi: 10.3892/or.2016.5321. [DOI] [PubMed] [Google Scholar]
- 57.Fu J, Shrivastava A, Shrivastava SK, Srivastava RK, Shankar S. Triacetyl resveratrol upregulates miRNA-200 and suppresses the Shh pathway in pancreatic cancer: a potential therapeutic agent. Int J Oncol. 2019;54:1306–1316. doi: 10.3892/ijo.2019.4700. [DOI] [PubMed] [Google Scholar]
- 58.Yan B, Cheng L, Jiang Z, Chen K, Zhou C, Sun L, Cao J, Qian W, Li J, Shan T, Lei J, Ma Q, Ma J. Resveratrol inhibits ROS-promoted activation and glycolysis of pancreatic stellate cells via suppression of miR-21. Oxid Med Cell Longev. 2018;2018:1346958. doi: 10.1155/2018/1346958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng X, Chang H, Gu Y, Chen J, Yi L, Xie Q, Zhu J, Zhang Q, Mi M. 3, 6-Dihydroxyflavone suppresses breast carcinogenesis by epigenetically regulating miR-34a and miR-21. Cancer Prev Res (Phila) 2015;8:509–517. doi: 10.1158/1940-6207.CAPR-14-0357. [DOI] [PubMed] [Google Scholar]
- 60.Peng X, Chang H, Chen J, Zhang Q, Yu X, Mi M. 3, 6-Dihydroxyflavone regulates microRNA-34a through DNA methylation. BMC Cancer. 2017;17:619. doi: 10.1186/s12885-017-3638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nwaeburu CC, Bauer N, Zhao Z, Abukiwan A, Gladkich J, Benner A, Herr I. Up-regulation of microRNA Let-7c by quercetin inhibits pancreatic cancer progression by activation of Numbl. Oncotarget. 2016;7:58367–58380. doi: 10.18632/oncotarget.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nwaeburu CC, Abukiwan A, Zhao Z, Herr I. Quercetin-induced miR-200b-3p regulates the mode of self-renewing divisions in pancreatic cancer. Mol Cancer. 2017;16:23. doi: 10.1186/s12943-017-0589-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Pratheeshkumar P, Son YO, Divya SP, Wang L, Turcios L, Roy RV, Hitron JA, Kim D, Dai J, Asha P. Quercetin inhibits Cr (VI)-induced malignant cell transformation by targeting miR-21-PDCD4 signaling pathway. Oncotarget. 2017;8:52118–52131. doi: 10.18632/oncotarget.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Du F, Feng Y, Fang J, Yang M. MicroRNA-143 enhances chemosensitivity of Quercetin through autophagy inhibition via target GABARAPL1 in gastric cancer cells. Biomed Pharmacother. 2015;74:169–177. doi: 10.1016/j.biopha.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 65.Sonoki H, Sato T, Endo S, Matsunaga T, Yamaguchi M, Yamazaki Y, Sugatani J, Ikari A. Quercetin decreases claudin-2 expression mediated by up-regulation of microRNA miR-16 in lung adenocarcinoma A549 cells. Nutrients. 2015;7:4578–4592. doi: 10.3390/nu7064578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang X, Guo Q, Chen J, Chen Z. Quercetin enhances cisplatin sensitivity of human osteosarcoma cells by modulating microRNA-217-KRAS axis. Mol Cells. 2015;38:638–42. doi: 10.14348/molcells.2015.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou J, Gong J, Ding C, Chen G. Quercetin induces the apoptosis of human ovarian carcinoma cells by upregulating the expression of microRNA-145. Mol Med Rep. 2015;12:3127–3131. doi: 10.3892/mmr.2015.3679. [DOI] [PubMed] [Google Scholar]
- 68.Zhao J, Fang Z, Zha Z, Sun Q, Wang H, Sun M, Qiao B. Quercetin inhibits cell viability, migration and invasion by regulating miR-16/HOXA10 axis in oral cancer. Eur J Pharmacol. 2019;847:11–18. doi: 10.1016/j.ejphar.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 69.Zhang C, Hao Y, Sun Y, Liu P. Quercetin suppresses the tumorigenesis of oral squamous cell carcinoma by regulating microRNA-22/WNT1/β-catenin axis. J Pharmacol Sci. 2019;140:128–136. doi: 10.1016/j.jphs.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 70.Toden S, Tran HM, Tovar-Camargo OA, Okugawa Y, Goel A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget. 2016;7:16158–71. doi: 10.18632/oncotarget.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhardwaj V, Mandal AKA. Next-generation sequencing reveals the role of epigallocatechin-3-gallate in regulating putative novel and known microRNAs which target the MAPK pathway in non-small-cell lung cancer A549 cells. Molecules. 2019;24:368. doi: 10.3390/molecules24020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu Y, Huang Y, Liu M, Yan Q, Zhao W, Yang P, Gao Q, Wei J, Zhao W, Ma L. Epigallocatechin gallate inhibits cell growth and regulates miRNA expression in cervical carcinoma cell lines infected with different high-risk human papillomavirus subtypes. Exp Ther Med. 2019;17:1742–1748. doi: 10.3892/etm.2018.7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.La X, Zhang L, Li Z, Li H, Yang Y. (-)-epigallocatechin gallate (EGCG) enhances the sensitivity of colorectal cancer cells to 5-FU by inhibiting GRP78/NF-κB/miR-155-5p/MDR1 pathway. J Agric Food Chem. 2019;67:2510–2518. doi: 10.1021/acs.jafc.8b06665. [DOI] [PubMed] [Google Scholar]
- 74.Jiang P, Xu C, Chen L, Chen A, Wu X, Zhou M, Haq IU, Mariyam Z, Feng Q. EGCG inhibits CSC-like properties through targeting miR-485/CD44 axis in A549-cisplatin resistant cells. Mol Carcinog. 2018;57:1835–1844. doi: 10.1002/mc.22901. [DOI] [PubMed] [Google Scholar]
- 75.Yu CC, Chen PN, Peng CY, Yu CH, Chou MY. Suppression of miR-204 enables oral squamous cell carcinomas to promote cancer stemness, EMT traits, and lymph node metastasis. Oncotarget. 2016;7:20180–92. doi: 10.18632/oncotarget.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang P, Xu C, Chen L, Chen A, Wu X, Zhou M, Haq IU, Mariyam Z, Feng Q. Epigallocatechin-3-gallate inhibited cancer stem cell-like properties by targeting hsa-mir-485-5p/RXRα in lung cancer. J Cell Biochem. 2018;119:8623–8635. doi: 10.1002/jcb.27117. [DOI] [PubMed] [Google Scholar]
- 77.Zhang S, al-Maghout T, Bissinger R, Zeng N, Pelzl L, Salker MS, Cheng A, Singh Y, Lang F. Epigallocatechin-3-gallate (EGCG) up-regulates miR-15b expression thus attenuating store operated calcium entry (SOCE) into murine CD4+ T cells and human leukaemic T cell lymphoblasts. Oncotarget. 2017;8:89500–89514. doi: 10.18632/oncotarget.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Asama H, Suzuki R, Hikichi T, Takagi T, Masamune A, Ohira H. MicroRNA let-7d targets thrombospondin-1 and inhibits the activation of human pancreatic stellate cells. Pancreatology. 2019;19:196–203. doi: 10.1016/j.pan.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 79.Ma CH, Zhang YX, Tang LH, Yang XJ, Cui WM, Han CC, Ji WY. MicroRNA-1469, a p53-responsive microRNA promotes Genistein induced apoptosis by targeting Mcl1 in human laryngeal cancer cells. Biomed Pharmacother. 2018;106:665–671. doi: 10.1016/j.biopha.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 80.Ning YX, Luo X, Xu M, Feng X, Wang J. Let-7d increases ovarian cancer cell sensitivity to a genistein analog by targeting c-Myc. Oncotarget. 2017;8:74836–74845. doi: 10.18632/oncotarget.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng Y, Liu H, Liang Y. Genistein exerts potent antitumour effects alongside anaesthetic, propofol, by suppressing cell proliferation and nuclear factor-κB-mediated signalling and through upregulating micro RNA-218 expression in an intracranial rat brain tumour model. J Pharm Pharmacol. 2017;69:1565–1577. doi: 10.1111/jphp.12781. [DOI] [PubMed] [Google Scholar]
- 82.Wei D, Yang L, Lv B, Chen L. Genistein suppresses retinoblastoma cell viability and growth and induces apoptosis by upregulating miR-145 and inhibiting its target ABCE1. Mol Vis. 2017;23:385–394. [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang H, Zhao Z, Pang X, Yang J, Yu H, Zhang Y, Zhou H, Zhao J. MiR-34a/sirtuin-1/foxo3a is involved in genistein protecting against ox-LDL-induced oxidative damage in HUVECs. Toxicol Lett. 2017;277:115–122. doi: 10.1016/j.toxlet.2017.07.216. [DOI] [PubMed] [Google Scholar]
- 84.Ma J, Zeng F, Ma C, Pang H, Fang B, Lian C, Yin B, Zhang X, Wang Z, Xia J. Synergistic reversal effect of epithelial-to-mesenchymal transition by miR-223 inhibitor and genistein in gemcitabine-resistant pancreatic cancer cells. Am J Cancer Res. 2016;6:1384–95. [PMC free article] [PubMed] [Google Scholar]
- 85.de la Parra C, Castillo-Pichardo L, Cruz-Collazo A, Cubano L, Redis R, Calin GA, Dharmawardhane S. Soy isoflavone genistein-mediated downregulation of miR-155 contributes to the anticancer effects of genistein. Nutr Cancer. 2016;68:154–164. doi: 10.1080/01635581.2016.1115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rigalli JP, Tocchetti GN, Arana MR, Villanueva SSM, Catania VA, Theile D, Ruiz ML, Weiss J. The phytoestrogen genistein enhances multidrug resistance in breast cancer cell lines by translational regulation of ABC transporters. Cancer Lett. 2016;376:165–172. doi: 10.1016/j.canlet.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 87.Xie J, Wang J, Zhu B. Genistein inhibits the proliferation of human multiple myeloma cells through suppression of nuclear factor-κB and upregulation of microRNA-29b. Mol Med Rep. 2016;13:1627–1632. doi: 10.3892/mmr.2015.4740. [DOI] [PubMed] [Google Scholar]
- 88.Zhao X, Liu M, Li D. Oleanolic acid suppresses the proliferation of lung carcinoma cells by miR-122/Cyclin G1/MEF2D axis. Mol Cell Biochem. 2015;400:1–7. doi: 10.1007/s11010-014-2228-7. [DOI] [PubMed] [Google Scholar]
- 89.Lu B, Sheng Y, Zhang J, Zheng Z, Ji L. The altered microRNA profile in andrographolide-induced inhibition of hepatoma tumor growth. Gene. 2016;588:124–133. doi: 10.1016/j.gene.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 90.Dai J, Lin Y, Duan Y, Li Z, Zhou D, Chen W, Wang L, Zhang QQ. Andrographolide inhibits angiogenesis by inhibiting the Mir-21-5p/TIMP3 signaling pathway. Int J Biol Sci. 2017;13:660–668. doi: 10.7150/ijbs.19194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang PY, Hsieh PL, Wang TH, Yu CC, Lu MY, Liao YW, Lee TH, Peng CY. Andrographolide impedes cancer stemness and enhances radio-sensitivity in oral carcinomas via miR-218 activation. Oncotarget. 2017;8:4196–4207. doi: 10.18632/oncotarget.13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Toden S, Okugawa Y, Buhrmann C, Nattamai D, Anguiano E, Baldwin N, Shakibaei M, Boland CR, Goel A. Novel evidence for curcumin and boswellic acid-induced chemoprevention through regulation of miR-34a and miR-27a in colorectal cancer. Cancer Prev Res (Phila) 2015;8:431–443. doi: 10.1158/1940-6207.CAPR-14-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiang X, Liu Y, Zhang G, Lin S, Yuan N, Wu J, Yan X, Ma Y, Ma M. Acetyl-11-keto-β-boswellic acid inhibits precancerous breast lesion MCF-10AT cells via regulation of LINC00707/miR-206 that reduces estrogen receptor-α. Cancer Manag Res. 2020;12:2301–2314. doi: 10.2147/CMAR.S238051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luo R, Fang D, Chu P, Wu H, Zhang Z, Tang Z. Multiple molecular targets in breast cancer therapy by betulinic acid. Biomed Pharmacother. 2016;84:1321–1330. doi: 10.1016/j.biopha.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 95.Kumar P, Bhadauria AS, Singh AK, Saha S. Betulinic acid as apoptosis activator: molecular mechanisms, mathematical modeling and chemical modifications. Life Sci. 2018;209:24–33. doi: 10.1016/j.lfs.2018.07.056. [DOI] [PubMed] [Google Scholar]
- 96.Yang J, Qiu B, Li X, Zhang H, Liu W. p53-p66shc/miR-21-Sod2 signaling is critical for the inhibitory effect of betulinic acid on hepatocellular carcinoma. Toxicol Lett. 2015;238:1–10. doi: 10.1016/j.toxlet.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 97.Pandita A, Manvati S, Singh SK, Vaishnavi S, Bamezai RN. Combined effect of microRNA, nutraceuticals and drug on pancreatic cancer cell lines. Chem Biol Interact. 2015;233:56–64. doi: 10.1016/j.cbi.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 98.Dandawate P, Subramaniam D, Panovich P, Standing D, Krishnamachary B, Kaushik G, Thomas SM, Dhar A, Weir SJ, Jensen RA. Cucurbitacin B and I inhibits colon cancer growth by targeting the Notch signaling pathway. Sci Rep. 2020;10:1–15. doi: 10.1038/s41598-020-57940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou J, Liu M, Chen Y, Xu S, Guo Y, Zhao L. Cucurbitacin B suppresses proliferation of pancreatic cancer cells by ceRNA: effect of miR-146b-5p and lncRNA-AFAP1-AS1. J Cell Physiol. 2019;234:4655–4667. doi: 10.1002/jcp.27264. [DOI] [PubMed] [Google Scholar]
- 100.Lin Y, Li J, Ye S, Chen J, Zhang Y, Wang L, Xi Y, Bu S, Qiu X. LncRNA GACAT3 acts as a competing endogenous RNA of HMGA1 and alleviates cucurbitacin B-induced apoptosis of gastric cancer cells. Gene. 2018;678:164–171. doi: 10.1016/j.gene.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 101.Sikander M, Hafeez BB, Malik S, Alsayari A, Halaweish FT, Yallapu MM, Chauhan SC, Jaggi M. Cucurbitacin D exhibits potent anti-cancer activity in cervical cancer. Sci Rep. 2016;6:36594. doi: 10.1038/srep36594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abu Samaan TM, Samec M, Liskova A, Kubatka P, Büsselberg D. Paclitaxel’s mechanistic and clinical effects on breast cancer. Biomolecules. 2019;9:789. doi: 10.3390/biom9120789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duan FG, Wang MF, Cao YB, Dan Li, Li RZ, Fan XX, Khan I, Lai HL, Zhang YZ, Hsiao WW, Yao XJ, Wu QB, Liu L, Tang YJ, Leung EL. MicroRNA-421 confers paclitaxel resistance by binding to the KEAP1 3’UTR and predicts poor survival in non-small cell lung cancer. Cell Death Dis. 2019;10:1–14. doi: 10.1038/s41419-019-2031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cai Y, Jia R, Xiong H, Ren Q, Zuo W, Lin T, Lin R, Lei Y, Wang P, Dong H. Integrative gene expression profiling reveals that dysregulated triple microRNAs confer paclitaxel resistance in non-small cell lung cancer via co-targeting MAPT. Cancer Manag Res. 2019;11:7391–7404. doi: 10.2147/CMAR.S215427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun H, Zhou X, Bao Y, Xiong G, Cui Y, Zhou H. Involvement of miR-4262 in paclitaxel resistance through the regulation of PTEN in non-small cell lung cancer. Open Biol. 2019;9:180227. doi: 10.1098/rsob.180227. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106.Liu M, Gong C, Xu R, Chen Y, Wang X. MicroRNA-5195-3p enhances the chemosensitivity of triple-negative breast cancer to paclitaxel by downregulating EIF4A2. Cell Mol Biol Lett. 2019;24:47. doi: 10.1186/s11658-019-0168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ma C, Shi X, Guo W, Niu J, Wang G. miR-107 enhances the sensitivity of breast cancer cells to paclitaxel. Open Med. 2019;14:456–466. doi: 10.1515/med-2019-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang H, Zhao B, Wang X, Zhang F, Yu W. LINC00511 knockdown enhances paclitaxel cytotoxicity in breast cancer via regulating miR-29c/CDK6 axis. Life Sci. 2019;228:135–144. doi: 10.1016/j.lfs.2019.04.063. [DOI] [PubMed] [Google Scholar]
- 109.Huang L, Hu C, Chao H, Wang R, Lu H, Li H, Chen H. miR-29c regulates resistance to paclitaxel in nasopharyngeal cancer by targeting ITGB1. Exp Cell Res. 2019;378:1–10. doi: 10.1016/j.yexcr.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 110.Soltani-Sedeh H, Irani S, Mirfakhraie R, Soleimani M. Potential using of microRNA-34A in combination with paclitaxel in colorectal cancer cells. J Cancer Res Ther. 2019;15:32–37. doi: 10.4103/jcrt.JCRT_267_17. [DOI] [PubMed] [Google Scholar]
- 111.Zhang L, Chen T, Yan L, Xu H, Wang Y, Li Y, Wang H, Chen S, Wang W, Chen C. MiR-155-3p acts as a tumor suppressor and reverses paclitaxel resistance via negative regulation of MYD88 in human breast cancer. Gene. 2019;700:85–95. doi: 10.1016/j.gene.2019.02.066. [DOI] [PubMed] [Google Scholar]
- 112.Zheng F, Li J, Ma C, Tang X, Tang Q, Wu J, Chai X, Xie J, Yang XB, Hann SS. Novel regulation of miR-34a-5p and HOTAIR by the combination of berberine and gefitinib leading to inhibition of EMT in human lung cancer. J Cell Mol Med. 2020;24:5578–5592. doi: 10.1111/jcmm.15214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu J, Chen Z, Cui Y, Wei H, Zhu Z, Mao F, Wang Y, Liu Y. Berberine promotes XIAP-mediated cells apoptosis by upregulation of miR-24-3p in acute lymphoblastic leukemia. Aging (Albany NY) 2020;12:3298–3311. doi: 10.18632/aging.102813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dong Y, Chen WH, Gao LJ, Liu MY, Li J, Wang J. Bioactive ingredients in Chinese herbal medicines that target non-coding RNAs: promising new choices for disease treatment. Front Pharmacol. 2019;10:515. doi: 10.3389/fphar.2019.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yin Z, Yang J, Ning R, Liu Y, Feng M, Gu C, Fei J, Li Y. Signal pathways, diseases, and functions associated with the miR-19a/92a cluster and the use of berberine to modulate the expression of this cluster in multiple myeloma cells. J Biochem Mol Toxicol. 2018;32:e22057. doi: 10.1002/jbt.22057. [DOI] [PubMed] [Google Scholar]
- 116.Gu C, Li T, Yin Z, Chen S, Fei J, Shen J, Zhang Y. Integrative analysis of signaling pathways and diseases associated with the miR-106b/25 cluster and their function study in berb erine-induced multiple myeloma cells. Funct Integr Genomics. 2017;17:253–262. doi: 10.1007/s10142-016-0519-7. [DOI] [PubMed] [Google Scholar]
- 117.Wang Y, Zhang S. Berberine suppresses growth and metastasis of endometrial cancer cells via miR-101/COX-2. Biomed Pharmacother. 2018;103:1287–1293. doi: 10.1016/j.biopha.2018.04.161. [DOI] [PubMed] [Google Scholar]
- 118.Li L, Li X, Huang X, Jiang W, Liu L, Hou C, Yang Y, Zhang L, Zhang X, Ye L. Synergistic anticancer effects of nanocarrier loaded with berberine and miR-122. Biosci Rep. 2018;38:BSR20180311. doi: 10.1042/BSR20180311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen Q, Qin R, Fang Y, Li H. Berberine sensitizes human ovarian cancer cells to cisplatin through miR-93/PTEN/Akt signaling pathway. Cell Physiol Biochem. 2015;36:956–965. doi: 10.1159/000430270. [DOI] [PubMed] [Google Scholar]
- 120.You HY, Xie XM, Zhang WJ, Zhu HL, Jiang FZ. Berberine modulates cisplatin sensitivity of human gastric cancer cells by upregulation of miR-203. In Vitro Cell Dev Biol Anim. 2016;52:857–63. doi: 10.1007/s11626-016-0044-y. [DOI] [PubMed] [Google Scholar]
- 121.Liu H, Huang C, Wu L, Wen B. Effect of evodiamine and berberine on miR-429 as an oncogene in human colorectal cancer. Onco Targets Ther. 2016;9:4121–7. doi: 10.2147/OTT.S104729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang C, Liu H, Gong XL, Wu LY, Wen B. Effect of evodiamine and berberine on the interaction between DNMTs and target microRNAs during malignant transformation of the colon by TGF-β1. Oncol Rep. 2017;37:1637–1645. doi: 10.3892/or.2017.5379. [DOI] [PubMed] [Google Scholar]
- 123.Mei C, Lei L, Tan LM, Xu XJ, He BM, Luo C, Yin JY, Li X, Zhang W, Zhou HH. The role of single strand break repair pathways in cellular responses to camptothecin induced DNA damage. Biomed Pharmacother. 2020;125:109875. doi: 10.1016/j.biopha.2020.109875. [DOI] [PubMed] [Google Scholar]
- 124.Zeng CW, Zhang XJ, Lin KY, Ye H, Feng SY, Zhang H, Chen YQ. Camptothecin induces apoptosis in cancer cells via microRNA-125b-mediated mitochondrial pathways. Mol Pharmacol. 2012;81:578–586. doi: 10.1124/mol.111.076794. [DOI] [PubMed] [Google Scholar]
- 125.Bertozzi D, Marinello J, Manzo SG, Fornari F, Gramantieri L, Capranico G. The natural inhibitor of DNA topoisomerase I, camptothecin, modulates HIF-1α activity by changing miR expression patterns in human cancer cells. Mol Cancer Ther. 2014;13:239–248. doi: 10.1158/1535-7163.MCT-13-0729. [DOI] [PubMed] [Google Scholar]
- 126.Cheng Y, Chen G, Hu M, Huang J, Li B, Zhou L, Hong L. Has-miR-30a regulates autophagic activity in cervical cancer upon hydroxycamptothecin exposure. Biomed Pharmacother. 2015;75:67–74. doi: 10.1016/j.biopha.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 127.Wu XM, Shao XQ, Meng XX, Zhang XN, Zhu L, Liu SX, Lin J, Xiao HS. Genome-wide analysis of microRNA and mRNA expression signatures in hydroxycamptothecin-resistant gastric cancer cells. Acta Pharmacol Sini. 2011;32:259–69. doi: 10.1038/aps.2010.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tong JL, Zhang CP, Nie F, Xu XT, Zhu MM, Xiao SD, Ran ZH. MicroRNA 506 regulates expression of PPAR alpha in hydroxycamptothecin-resistant human colon cancer cells. FEBS Lett. 2011;585:3560–3568. doi: 10.1016/j.febslet.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 129.Huang N, Wu J, Qiu W, Lyu Q, He J, Xie W, Xu N, Zhang Y. MiR-15a and miR-16 induce autophagy and enhance chemosensitivity of Camptothecin. Cancer Biol Ther. 2015;16:941–948. doi: 10.1080/15384047.2015.1040963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Due H, Schönherz AA, Ryø L, Primo MN, Jespersen DS, Thomsen EA, Roug AS, Xiao M, Tan X, Pang Y. MicroRNA-155 controls vincristine sensitivity and predicts superior clinical outcome in diffuse large B-cell lymphoma. Blood Adv. 2019;3:1185–1196. doi: 10.1182/bloodadvances.2018029660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mavrogiannis AV, Kokkinopoulou I, Kontos CK, Sideris DC. Effect of vinca alkaloids on the expression levels of microRNAs targeting apoptosis-related genes in breast cancer cell lines. Curr Pharm Biotechnol. 2018;19:1076–1086. doi: 10.2174/1389201019666181112103204. [DOI] [PubMed] [Google Scholar]
- 132.Dong WH, Li Q, Zhang XY, Guo Q, Li H, Wang TY. Deep sequencing identifies deregulation of microRNAs involved with vincristine drug-resistance of colon cancer cells. Int J Clin Exp Pathol. 2015;8:11524–30. [PMC free article] [PubMed] [Google Scholar]
- 133.Kandi R, Gutti U, Saladi R, Gutti RK. MiR-125b and miR-99a encoded on chromosome 21 co-regulate vincristine resistance in childhood acute megakaryoblastic leukemia. Hematol Oncol Stem Cell Ther. 2015;8:95–7. doi: 10.1016/j.hemonc.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 134.Wan Mohd Tajuddin WNB, Lajis NH, Abas F, Othman I, Naidu R. Mechanistic understanding of curcumin’s therapeutic effects in lung cancer. Nutrients. 2019;11:2989. doi: 10.3390/nu11122989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mirzaei H, Masoudifar A, Sahebkar A, Zare N, Sadri Nahand J, Rashidi B, Mehrabian E, Mohammadi M, Mirzaei HR, Jaafari MR. MicroRNA: a novel target of curcumin in cancer therapy. J Cell Physiol. 2018;233:3004–3015. doi: 10.1002/jcp.26055. [DOI] [PubMed] [Google Scholar]
- 136.Wu H, Liu Q, Cai T, Chen YD, Wang ZF. Induction of microRNA-146a is involved in curcumin-mediated enhancement of temozolomide cytotoxicity against human glioblastoma. Mol Med Rep. 2015;12:5461–5466. doi: 10.3892/mmr.2015.4087. [DOI] [PubMed] [Google Scholar]
- 137.Taverna S, Giallombardo M, Pucci M, Flugy A, Manno M, Raccosta S, Rolfo C, De Leo G, Alessandro R. Curcumin inhibits in vitro and in vivo chronic myelogenous leukemia cells growth: a possible role for exosomal disposal of miR-21. Oncotarget. 2015;6:21918–33. doi: 10.18632/oncotarget.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xu R, Li H, Wu S, Qu J, Yuan H, Zhou Y, Lu Q. MicroRNA-1246 regulates the radio-sensitizing effect of curcumin in bladder cancer cells via activating P53. Int Urol Nephrol. 2019;51:1771–1779. doi: 10.1007/s11255-019-02210-5. [DOI] [PubMed] [Google Scholar]
- 139.Zhu M, Zheng Z, Huang J, Ma X, Huang C, Wu R, Li X, Liang Z, Deng F, Wu J. Modulation of miR-34a in curcumin-induced antiproliferation of prostate cancer cells. J Cell Biochem. 2019;120:15616–15624. doi: 10.1002/jcb.28828. [DOI] [PubMed] [Google Scholar]
- 140.Li Y, Sun W, Han N, Zou Y, Yin D. Curcumin inhibits proliferation, migration, invasion and promotes apoptosis of retinoblastoma cell lines through modulation of miR-99a and JAK/STAT pathway. BMC Cancer. 2018;18:1230. doi: 10.1186/s12885-018-5130-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 141.Gallardo M, Kemmerling U, Aguayo F, Bleak TC, Muñoz JP, Calaf GM. Curcumin rescues breast cells from epithelial-mesenchymal transition and invasion induced by anti-miR-34a. Int J Oncol. 2020;56:480–493. doi: 10.3892/ijo.2019.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Han Z, Zhang J, Zhang K, Zhao Y. Curcumin inhibits cell viability, migration, and invasion of thymic carcinoma cells via downregulation of microRNA-27a. Phytother Res. 2020 doi: 10.1002/ptr.6629. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 143.Qiang Z, Meng L, Yi C, Yu L, Chen W, Sha W. Curcumin regulates the miR-21/PTEN/Akt pathway and acts in synergy with PD98059 to induce apoptosis of human gastric cancer MGC-803 cells. J Int Med Res. 2019;47:1288–1297. doi: 10.1177/0300060518822213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sohn EJ, Bak KM, Nam YK, Park HT. Upregulation of microRNA 344a-3p is involved in curcumin induced apoptosis in RT4 schwannoma cells. Cancer Cell Int. 2018;18:199. doi: 10.1186/s12935-018-0693-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hannafon BN, Carpenter KJ, Berry WL, Janknecht R, Dooley WC, Ding WQ. Exosome-mediated microRNA signaling from breast cancer cells is altered by the anti-angiogenesis agent docosahexaenoic acid (DHA) Mol Cancer. 2015;14:133. doi: 10.1186/s12943-015-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.LeMay-Nedjelski L, Mason-Ennis J, Taibi A, Comelli E, Thompson L. Omega-3 polyunsaturated fatty acids time-dependently reduce cell viability and oncogenic microRNA-21 expression in estrogen receptor-positive breast cancer cells (MCF-7) Int J Mol Sci. 2018;19:244. doi: 10.3390/ijms19010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dai X, Li M, Geng F. Omega-3 polyunsaturated fatty acids eicosapentaenoic acid and docosahexaenoic acid enhance dexamethasone sensitivity in multiple myeloma cells by the p53/miR-34a/Bcl-2 axis. Biochemistry (Moscow) 2017;82:826–833. doi: 10.1134/S0006297917070082. [DOI] [PubMed] [Google Scholar]
- 148.Shekari N, Javadian M, Ghaffari S, Baradaran B, Darabi M, Kazemi T. DHA abolishes the detrimental effect of docetaxel on downregulation of the mica via decreasing the expression level of microRNA-20a in gastric cancer. J Gastrointest Cancer. 2020;51:545–551. doi: 10.1007/s12029-019-00280-3. [DOI] [PubMed] [Google Scholar]
- 149.de Lima RMT, Dos Reis AC, de Menezes APM, Santos JVO, Filho JWGO, Ferreira JRO, de Alencar MVOB, da Mata AMOF, Khan IN, Islam A, Uddin SJ, Ali ES, Islam MT, Tripathi S, Mishra SK, Mubarak MS, Melo-Cavalcante AAC. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6] -gingerol in cancer: a comprehensive review. Phytother Res. 2018;32:1885–1907. doi: 10.1002/ptr.6134. [DOI] [PubMed] [Google Scholar]
- 150.Rastogi N, Gara RK, Trivedi R, Singh A, Dixit P, Maurya R, Duggal S, Bhatt M, Singh S, Mishra DP. (6)-Gingerolinduced myeloid leukemia cell death is initiated by reactive oxygen species and activation of miR-27b expression. Free Radic Biol Med. 2014;68:288–301. doi: 10.1016/j.freeradbiomed.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 151.Kang C, Kang M, Han Y, Zhang T, Quan W, Gao J. 6-Gingerols (6G) reduces hypoxia-induced PC-12 cells apoptosis and autophagy through regulation of miR-103/BNIP3. Artif Cells Nanomed Biotechnol. 2019;47:1653–1661. doi: 10.1080/21691401.2019.1606010. [DOI] [PubMed] [Google Scholar]
- 152.Lin Q, Ma L, Liu Z, Yang Z, Wang J, Liu J, Jiang G. Targeting microRNAs: a new action mechanism of natural compounds. Oncotarget. 2017;8:15961–15970. doi: 10.18632/oncotarget.14392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sam S, Sam MR, Esmaeillou M, Safaralizadeh R. Effective targeting survivin, caspase-3 and microRNA-16-1 expression by methyl-3-pentyl-6-methoxyprodigiosene triggers apoptosis in colorectal cancer stem-like cells. Oncol Res. 2016;22:715–723. doi: 10.1007/s12253-016-0055-8. [DOI] [PubMed] [Google Scholar]
- 154.Gong A, Ge N, Yao W, Lu L, Liang H. Aplysin enhances temozolomide sensitivity in glioma cells by increasing miR-181 level. Cancer Chemother Pharmacol. 2014;74:531–538. doi: 10.1007/s00280-014-2534-5. [DOI] [PubMed] [Google Scholar]
- 155.Wang Y, Xiang W, Wang M, Huang T, Xiao X, Wang L, Tao D, Dong L, Zeng F, Jiang G. Methyl jasmonate sensitizes human bladder cancer cells to gambogic acid-induced apoptosis through down-regulation of EZH 2 expression by miR-101. Br J Pharmacol. 2014;171:618–635. doi: 10.1111/bph.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Peng Z, Zhang Y. Methyl jasmonate induces the apoptosis of human colorectal cancer cells via downregulation of EZH2 expression by microRNA-101. Mol Med Rep. 2017;15:957–962. doi: 10.3892/mmr.2016.6061. [DOI] [PubMed] [Google Scholar]
- 157.Zhou B, Wang J, Zheng G, Qiu Z. Methylated urolithin A, the modified ellagitannin-derived metabolite, suppresses cell viability of DU145 human prostate cancer cells via targeting miR-21. Food Chem Toxicol. 2016;97:375–384. doi: 10.1016/j.fct.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 158.Phuah NH, In LL, Azmi MN, Ibrahim H, Awang K, Nagoor NH. Alterations of microRNA expression patterns in human cervical carcinoma cells (Ca Ski) toward 1’ S-1’-acetoxychavicol acetate and cisplatin. Reprod Sci. 2013;20:567–578. doi: 10.1177/1933719112459220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Phuah NH, Azmi MN, Awang K, Nagoor NH. Down-regulation of microRNA-210 confers sensitivity towards 1’s-1’-acetoxychavicol acetate (ACA) in cervical cancer cells by targeting SMAD4. Mol Cells. 2017;40:291–298. doi: 10.14348/molcells.2017.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Phuah NH, Azmi MN, Awang K, Nagoor NH. Suppression of microRNA-629 enhances sensitivity of cervical cancer cells to 1’ S-1’-acetoxychavicol acetate via regulating RSU1. Onco Targets Ther. 2017;10:1695–1705. doi: 10.2147/OTT.S117492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liao YC, Lin TH, Chen CY, Lin SB, Au LC. The antileukemia activity of natural product HQ17 (3) is possibly associated with downregulation of miR-17-92 cluster. Biomed Res Int. 2014;2014:306718. doi: 10.1155/2014/306718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mousa DS, El-Far AH, Saddiq AA, Sudha T, Mousa SA. Nanoformulated bioactive compounds derived from different natural products combat pancreatic cancer cell proliferation. Int J Nanomedicine. 2020;15:2259–2268. doi: 10.2147/IJN.S238256. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 163.Munakarmi S, Chand L, Shin HB, Jang KY, Jeong YJ. Indole-3-carbinol derivative dim mitigates carbon tetrachloride-induced acute liver injury in mice by inhibiting inflammatory response, apoptosis and regulating oxidative stress. Int J Mol Sci. 2020;21:2048. doi: 10.3390/ijms21062048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sayeed MA, Bracci M, Lucarini G, Lazzarini R, Di Primio R, Santarelli L. Regulation of microRNA using promising dietary phytochemicals: possible preventive and treatment option of malignant mesothelioma. Biomed Pharmacother. 2017;94:1197–1224. doi: 10.1016/j.biopha.2017.07.075. [DOI] [PubMed] [Google Scholar]
- 165.Yu J, Feng Y, Wang Y, An R. Aryl hydrocarbon receptor enhances the expression of miR-150-5p to suppress in prostate cancer progression by regulating MAP3K12. Arch Biochem Biophys. 2018;654:47–54. doi: 10.1016/j.abb.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 166.Hanieh H. Aryl hydrocarbon receptor-microRNA-212/132 axis in human breast cancer suppresses metastasis by targeting SOX4. Mol Cancer. 2015;14:172. doi: 10.1186/s12943-015-0443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ye Y, Fang Y, Xu W, Wang Q, Zhou J, Lu R. 3,3’-Diindolylmethane induces anti-human gastric cancer cells by the miR-30e-ATG5 modulating autophagy. Biochem Pharmacol. 2016;115:77–84. doi: 10.1016/j.bcp.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 168.Madurantakam Royam M, Ramesh R, Shanker R, Sabarimurugan S, Kumarasamy C, Ramesh N, Gothandam KM, Baxi S, Gupta A, Krishnan S, Jayaraj R. Mirna predictors of pancreatic cancer chemotherapeutic response: a systematic review and meta-analysis. Cancers (Basel) 2019;11:900. doi: 10.3390/cancers11070900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sayeed MA, Bracci M, Lazzarini R, Tomasetti M, Amati M, Lucarini G, Di Primio R, Santarelli L. Use of potential dietary phytochemicals to target miRNA: promising option for breast cancer prevention and treatment? J Funct Foods. 2017;28:177–193. [Google Scholar]


