Abstract
Human amniotic epithelial cells (hAECs) show similar features to stem cells and have low immunogenicity. This study aims to investigate the therapeutic effect of hAEC transplantation on cyclophosphamide-induced primary ovarian insufficiency (POI) rats and evaluate the underlying mechanisms by mRNA sequencing of ovarian samples. Notably, hAECs mainly located in the interstitial area of the ovaries rather than follicles. hAEC transplantation led to a slight increase in body and ovary weight, normalized irregular estrous cycles, decreased serum follicle stimulating hormone (FSH) and increased anti-Mullerian hormone (AMH) level and restored follicle pools in POI rats. Ovarian expression of AMH, follicle stimulating hormone receptor (FSHR) and klotho in POI rats was also significantly upregulated following hAEC transplantation. Fetus number was higher in the hAEC transplantation group than the POI group. The mRNA sequencing results showed that hAEC transplantation led to the upregulation of several angiogenesis and inflammation molecules including interferon regulatory factor 7 (IRF7), Mx dynamin-like GTPase 1 (Mx1), vascular endothelial growth factor receptor (VEGFR)1 and VEGFR2. Moreover, hAEC therapy had an effect on ribosomes, protein digestion, protein absorption, neuroactive ligand-receptor interaction, cAMP signaling pathway and steroid biosynthesis pathways. The expression of several steroid biosynthesis proteins was significantly upregulated as measured by quantitative real-time polymerase chain reaction (RT-qPCR), immunohistochemical staining and Western blot analysis. In summary, hAECs can significantly restore ovarian function, and improve both ovarian reserve and fertility. This may be due to the paracrine effect of hAECs in regulating steroid biosynthesis, modulating follicle development from initiation to ovulation, promoting angiogenesis and reducing inflammation.
Keywords: Human amniotic epithelial cells, mRNA sequencing, ovarian function, primary ovarian failure/insufficiency, steroid biosynthesis
Introduction
Primary ovarian insufficiency/premature ovarian failure (POI/POF) is defined by persistently elevated gonadotropin levels and absent or irregular menstrual cycles before the age of 40 years, with an estimated 1% incidence [1-3]. POI is associated with both physical and mental manifestations including secondary infertility, menopausal symptoms, increased bone or cardiovascular disease, and psychologic suffering such as depression, anxiety and potential early decline in cognition [3,4]. Nowadays chemotherapy is commonly used in malignant tumors and unavoidably causes ovarian damage or POI in young female patients. Hormone replacement therapy (HRT) could partially ameliorate some discomforts but may also increase the risk of stroke, breast cancer, cardiovascular disease and recurrence in cancer survivors due to adverse side effects [3]. Therefore, it is crucial for researchers to develop efficacious treatment strategies to combat the irreversible process of POI.
Recently, various stem cell transplantation methods have been shown to effectively restore ovarian function and promote fertility in preclinical animal experiments [5-8]. Human amniotic epithelial cells (hAECs) digested from fetal membranes of placenta are the desired source of stem cells because of their pluripotent properties, low immunogenicity and abundance [9]. Studies have shown that hAECs can differentiate into follicle-stimulating hormone receptor (FSHR)-positive ovarian granulosa cells in vivo and secrete a broad range of chemokines and cytokines, thus making it possible to restore ovarian function via cell replacement or a paracrine effect [6,10]. However, the exact mechanism remains unclear.
In this experiment, we developed a cyclophosphamide (CTX) pretreated rat model to assess the effect of different methods of hAEC transplantation and explore the mechanisms underlying positive treatment effects via mRNA sequencing of ovarian samples. The data from this study provide a fresh perspective on the role of hAEC transplantation in treating POI.
Material and methods
Isolation and culture of hAECs
Human placentas were obtained from pregnant women who underwent uncomplicated caesarean sections after testing negative for hepatitis B, hepatitis C and human immunodeficiency virus (HIV)-I with written and informed consent. The indications for caesarean section were repeat operation, breech presentation, twins and fetal distress. The fetal surface of the amniotic membranes was mechanically cut from the placenta and placed in sterile dishes containing phosphate-buffered saline (PBS), then cut into 0.5-1.0 cm2 segments. The segments were washed until no obvious blood remained and then digested with 0.05% trypsin/ethylenediaminetetraacetic acid (EDTA) at 37°C for 15 min with gentle shaking to remove any residual blood. The amnion membrane segments were digested twice for 30 minutes each at 37°C with gentle shaking. The cell suspensions from both digestions were seeded in six-well plates in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS, Corning, USA), Penicillin-Streptomycin Solution (Beyotime biotechnology, Shanghai, China), and incubated at 37°C 5% CO2 in humidified air. When hAECs grew to 80-90% confluence, the cells were collected for subsequent experiments. The use of human amnions was approved by the institutional ethics committee.
Identification and characterization of hAECs
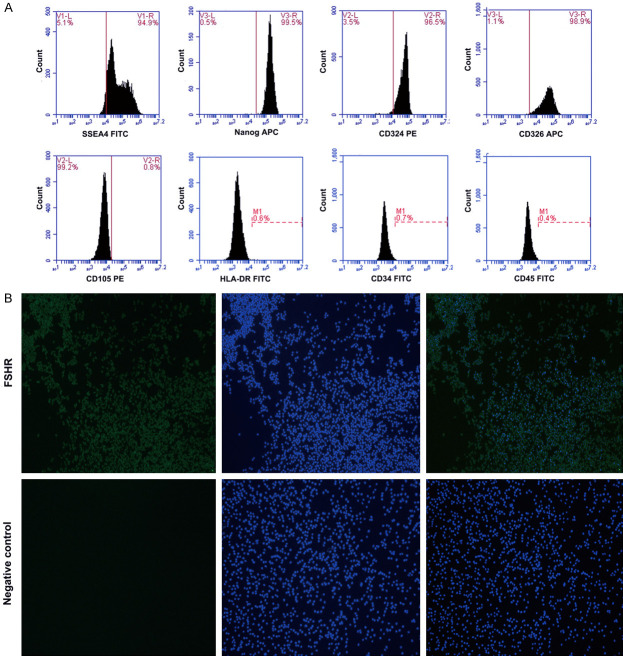
Fluorescence-activated cell sorting (FACS) was used to identify the phenotypes of hAECs. After staining with phycoerythrin (PE)-conjugated, fluorescein isothiocyanate (FITC) conjugated or allophycocyanin (APC)-conjugated anti-human SSEA-4, Nanog, CD324, CD326, CD105, CD34, CD45 or HLA-DR monoclonal antibodies (Bio Legend), the cells were sorted by flow cytometry. hAECs were seeded in 6-well plates and fixed with 4% paraformaldehyde for 20 minutes at room temperature, and then washed 3 times with PBS. Cells were permeabilized with 0.3% Triton X-100 for 20 minutes at room temperature and washed 3 times with PBS. The cells were immersed in goat serum for 30 minutes and incubated with anti-follicle stimulating hormone receptor (FSHR) (1:100 dilution; SAB; China) at 4°C overnight. After washing 3 times, the cells were incubated with PE/FITC/APC-labeled IgG, and fluorescence images were captured using a fluorescent microscope (Nikon Corporation, Tokyo, Japan). Negative control images from cells incubated with PBS and no primary antibody were also obtained.
Premature ovarian failure and insufficiency (POF/POI) model establishment
Female Sprague-Dawley (SD) sexually mature rats, 8-10 weeks-old (240-260 g by weight), were obtained from the Experimental Animal Center of Chongqing Medical University. In total, 80 SD rats were randomly assigned to 4 groups: POI group (n=20), intravenous (IV) hAEC group (n=20), in situ hAEC group (n=20) and control group (n=20). Overall, 60 SD rats from the first three groups were intraperitoneally injected with CTX (Shengdi medicine, Jiangsu, China) at a dosage of 50 mg/kg (re-suspended in normal saline) on the first day and 8 mg/kg/day for the following 14 days. Rats in the control group were administered an equivalent volume of saline. The animals were housed in an animal facility and all procedures were conducted according to the guidelines of the Institutional Animal Care and Use Committee of Chongqing Medical University.
Stem cell transplantation
Twenty-four hours after chemotherapy, the rats from the intravenous group received 0.6 mL PBS containing 4×106 hAECs via the tail vein and 50 μl PBS injected into each ovary through a microinjector while rats of the in situ group were administered 2×106 hAECs in 50 μl PBS per ovary and received 0.6 mL PBS in the tail vein. In the POI and control groups, the rats received a corresponding volume of PBS through the tail vein and in situ ovarian injection in the meantime.
Labeling and tracking of hAECs
In order to track and locate the hAECs in ovarian tissues, the transplanted cells were pre-labeled with PKH26 fluorescent dye (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions. The staining efficiency was measured by flow cytometry. The PKH26-labeled hAECs were administered via the tail vein and/or directly into ovaries as previously described. At both twenty-four hours and two weeks after cell injection, ovaries were fixed and cut into fresh slices. After fixation with pre-cooled acetone, the sections were rinsed and stained with 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI; Boster Biological Technology, Wuhan, China) for 10 minutes at room temperature. The sections were instantly examined under a fluorescent microscope (Nikon Corporation, Tokyo, Japan).
Estrous cycle examination
One week before CTX injection, one week after CTX injection and one week after hAEC transplantation, vaginal smears were performed at 9 am daily for at least 7 consecutive days. The smears were stained with hematoxylin and eosin (H&E) and observed under a light microscope. The type of estrous cycle was identified by the appearance and/or proportion of nucleated cells, keratinized epithelial cells and leukocytes. Cycles were characterized as regular (4-6 day cycle pattern; 1 day for proestrus, 1-2 days for estrous, 1 day for metestrus and 1-2 days for diestrus), irregular cycles presented as prolonged diestrus and normal or prolonged estrus (3-4 days for estrus, or 4-5 days for diestrus), and irregular cycles (>4 days for estrus or >5 days for diestrus or no cyclicity) [11].
Enzyme-linked immunosorbent assay
Two weeks after hAEC transplantation, rat blood samples were obtained by abdominal aorta puncture under anesthesia. Blood samples were placed on ice and centrifuged at 500× g for 15 minutes. The supernatant was obtained for sex hormone detection by FSH ELISA kits (Fusheng Industry Co., Ltd., Shanghai, China) and AMH kits (JYM, Wuhan, China) as described in the manual.
Ovarian histologic analysis and follicle count
Two weeks after cell transplantation, the rats were euthanized, and ovaries were collected. The ovaries were cut into slices and stained with H&E for histologic evaluation by light microscopy. Only the follicles presenting an oocyte with a clear nucleus were counted. Furthermore, the follicles were counted and recorded as preantral follicles (primordial, primary), antral follicles (secondary, mature) and atresia follicles, according to the described method [12]. Nine ovarian samples were included in each group and five sections of one entire ovary were counted for statistical analysis.
Immunohistochemical staining
Ovarian tissues were collected and cut into sections at a thickness of 5 μm. The immunohistochemistry staining procedure was performed in accordance with the manufacturer’s instructions using SPlink detection kits (ZSGB-BIO, Beijing, China). After dewaxing and antigen retrieval, the slides were blocked with goat serum for 30 minutes and incubated with rabbit primary polyclonal antibodies against mouse AMH (1:200 dilution; Abcam), FSHR (1:150 dilution; SAB; China) and klotho (1:100 dilution; ZEN BIO; China) overnight at 4°C. Subsequently, sections were hatched with biotinylated secondary IgG antibody and immunoreactivity was visualized following application of 0.05% diaminobenzidine (DAB, ZSGB-BIO, Beijing, China).
The German immunoreactive score criteria (IRS) was applied to assess the staining results. In brief, immunoreactivity intensity was scored as “0” (negative), “1” (weak), “2” (moderate) and “3” (strong); the area of positive cells was graded as “0” (<5%), “1” (5-25%), “2” (25-50%), “3” (50-75%) or “4” (>75%). The final score was calculated by adding together the values for staining intensity and area [13].
Fertility assay
Two weeks after hAEC transplantation, female rats of four groups were mated with male rats at a ratio of 3:1. Vaginal smears were performed every morning to verify the appearance of sperm. When sperm were found in a vaginal smear, mating would cease, and the time point was recorded as E0.5 (Embryonic Day 0.5). The pregnant rats were euthanized and sacrificed on E13.5 for fetus examination.
mRNA sequencing
Total mRNA was isolated from the ovaries of the POI group, intravenous hAECs group and in situ hAECs group using Direct-zol RNA miniPrep Kits (ZYMO research, California, USA). After concentration and purity testing, total mRNA in each sample was reverse transcribed by oligo T primers to synthesize the first strand of cDNA. Subsequently, the double stranded cDNAs were produced by the reaction of RNase H enzyme, DNA polymerase and T4 ligase. The double-stranded cDNAs were then fragmented by Tn5 enzyme and both ends were subjected to addition of sequencing adapters. The P5 and P7 primers were bound to sequencing adapters at both ends and enriched polymerase chain reaction (PCR) amplifications were subsequently performed. The complete library was sequenced with Illumina HiSeq X Ten strategy.
After filtration of all the reads using Trimmomatic [14], the clean data were aligned to the Rattus norvegicus genome to obtain gene expression data in the form of read counts. The data were normalized by converting the read counts to FPKM (fragments per kilobase of exon model per million mapped fragments) values. The differentially expressed genes (DEGs) were screened out among three groups using the |log2 fold change| ≥1 and the adjusted P<0.05 by comparing the FPKM values. Gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) analysis were performed to identify the potential functions and associated pathways of DEGs with corrected p values <0.05. Venn diagrams were constructed to identify common DEGs in the two treatment groups compared with the POI group.
RT-qPCR analysis
Total RNA was extracted from ovaries using Direct-zol RNA miniPrep Kits. The cDNAs were generated using PrimeScript™ RT reagent Kit with gDNA Eraser (Takara Bio, Beijing, China) according to the manufacturers’ instructions. Quantitative real-time PCR was performed using real-time fluorescence quantitative PCR Systems (Applied Biosystems). Each sample was analyzed 3 times. The primer sequences for target genes are listed in Table 1. The parameters for qPCR were set as follows: initial denaturation for 3 minutes at 95°C followed by 40 cycles of 15 seconds each at 95°C, 30 seconds at 60°C and 30 seconds at 72°C. The relative gene expression was calculated using the 2-ΔΔCT method. Ratios of gene expression were displayed as fold-change relative to the POI group after normalizing to the allogeneic glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene.
Table 1.
Primers for real-time PCR
| Gene | GenBank Accession number | Forward primer | Reverse primer |
|---|---|---|---|
| Tm7sf2 | NM_001013071.1 | TCGCCTCGGTTCCTTTGA | GAGGGCATCACCCACATACAG |
| Cyp51 | NM_012941.2 | CACGCTTAGCCTTGTCTACCTG | AAGTGAAAGTCTTGCCCACCA |
| Dhcr24 | NM_001080148.1 | TAGAGCCCAGCAAGCTGAATAG | GCCAAAGAGGTAGCGGAAGA |
| Sqle | NM_017136.2 | GGACAAGGAGACTGGGGACA | CCAACGAAGTGGGAGGAAAC |
| Hsd17b7 | NM_017235.3 | GGAGCAGAGGAAGTCAAGCG | CCTGGAACCCATCAGCAGTAA |
| Fdft1 | NM_019238.2 | GCAAGGAGAAGCACCGAGTAG | AGGCGAGAAAGGCCGATT |
| Msmo1 | NM_080886.1 | CTCGGCATCACGATTTCCA | GCTCCCCAGAAGCAATGTTAG |
| Nsdhl | NM_001009399.1 | AATGGGAAGAACCTGGTGGA | CGTTGGTGATGTGAAATGCC |
| Cited2 | NM_053698.2 | GCGAGCACATACACTACGGC | GGGTAGGGGTGATGGTTGAA |
| Irf7 | NM_001033691.1 | CCTCTGCTTTCTGGTGATGCT | TCAGGAAGGTGTTCTTGCTCC |
| Mfap3l | NM_001012049.1 | ACCTGTAGAGGCACCAGAACG | TAAGGAGCAGCCCCTGATTT |
| Mx1 | NM_001271058.1 | AGCAACTGAAGCAGGGAGAAA | GGTCACCCACAGCCACTCTT |
| GAPDH | NM_017008.4 | GCAAGTTCAACGGCACAG | CGCCAGTAGACTCCACGAC |
Western blotting analysis
Western blotting analysis was used to measure the protein expression of several key molecules identified from the mRNA sequencing results. In brief, two weeks after hAEC transplantation, ovarian proteins of interest in the POI and in situ hAEC groups were extracted with RIPA buffer (Beyotime) and the protein concentration was measured by Bradford’s method. Protein samples were electrically separated on 10% SDS-PAGE gels and transferred onto polyvinylidine fluoride (PVDF) membranes. After blocking with 8% skimmed milk for 2 hours, the membranes were kept overnight at 4°C with anti-squalene monooxygenase (SQLE) (1:500; ZEN BIO; China), anti-farnesyl-diphosphate farnesyltransferase 1 (FDFT1) (1:500; ZEN BIO; China) and anti-β-actin (1:1000; Boster; China) polyclonal antibodies. After washing three times, the membranes were immersed in secondary antibody solution at room temperature for two hours. Enhanced chemiluminescent Ultra reagents (NCM Biotech, Suzhou, China) were used to identify the protein bands. In addition, immunohistochemical staining was used to identify ovarian expression of SQLE and FDFT1 at a primary antibody dilution of 1:100 according to the protocol outlined earlier.
Data analysis
Data are expressed as mean ± standard deviation (SD). All analyses were performed using SPSS 18.0 software. Independent-sample t tests and ordinary one-way analysis of variance (ANOVA) were applied for two- and multiple-group comparisons, respectively. Post-hoc tests were performed using the Tukey HSD (homogeneity of variance) test or Dunnett T3 (heterogeneity of variance) test. P<0.05 was considered statistically significant.
Results
Characterization and identification of hAECs
The hAECs had high expression of stem cell markers (SSEA4, Nanog) and epithelial cell markers (CD324, CD326), and low expression of mesenchymal markers CD105, hematopoietic stem cell markers CD34 and CD45, and the immunologic marker HLA-DR (Figure 1A). These data show that the phenotype of the hAECs used in this study are consistent with most previous studies. Surprisingly, a portion of hAECs were positive for FSHR, which is a well-known specific marker of ovarian granulosa cells (Figure 1B).
Figure 1.
hAEC phenotypes. A. hAECs are positive for stem cell markers (SSEA4, Nanog) and epithelial cell markers (CD324, CD326), and negative for mesenchymal markers (CD105), hematopoietic stem cell markers (CD34, CD45) and immunologic (HLA-DR) markers by flow cytometry. B. hAECs are immunoreactive for FSHR as detected by immunofluorescence staining. FSHR, follicle stimulating hormone receptor; hAEC, human amniotic epithelial cell; hAECs, human amniotic epithelial cells.
In vivo tracking of hAECs
The hAECs were labeled with PKH26 before transplantation. Flow cytometry analysis indicated that the labeling rate was 93.96% (Figure 2A-C). Twenty-four hours after cell injection, a red fluorescent signal was mainly detected in the interstitial area of the ovaries rather than follicles both in the intravenous and in situ hAEC groups (Figure 2D). Moreover, labeled cells were still weakly fluorescent 2 weeks after cell transplantation in these groups (Figure 2E).
Figure 2.
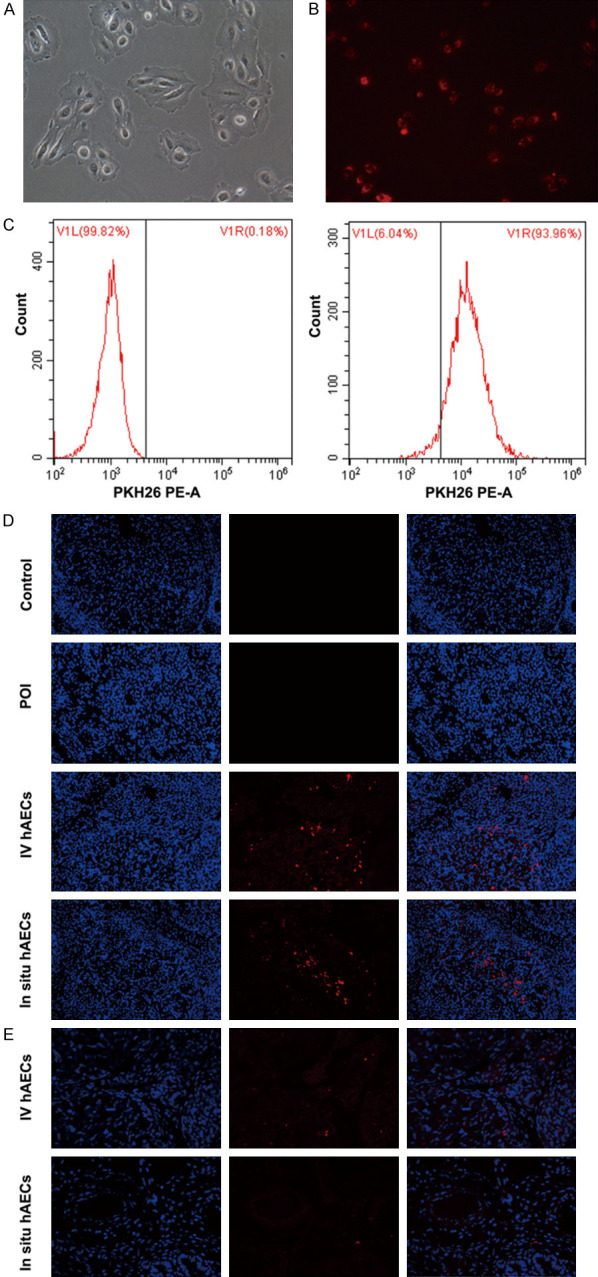
Morphology, PKH26 labelling and in vivo tracking of hAECs. (A) hAECs show epithelial morphology under bright-field microscope. (B) PKH26 pre-labeled hAECs under a fluorescent microscope (red fluorescence). (C) The labeling rate is measured by flow cytometry. (D, E) Transplanted hAECs are observed at twenty-four hours (D) and 2 weeks (E) in ovaries (200×). hAECs, human amniotic epithelial cells.
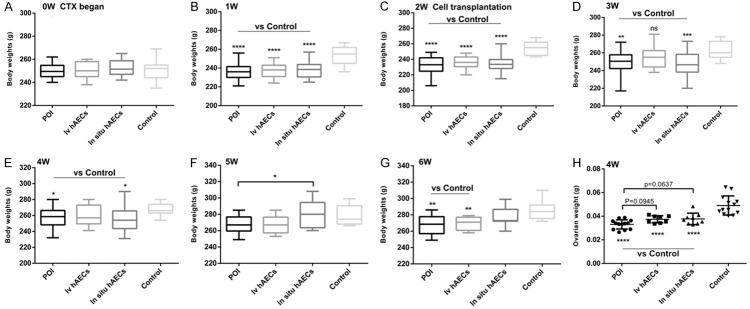
Body and ovary weight change in rats
There was no difference in body weight between the 4 groups before CTX injection (Figure 3A). One and 2 weeks after CTX administration, rats in the control group had higher body weights than the other 3 groups (Figure 3B and 3C, respectively; P<0.0001). In addition, 1-4 weeks after cell transplantation, rats in the intravenous or in situ hAEC group exhibited slightly increased body weights compared with those in the POI group (Figure 3D-G). Moreover, ovary weights in the POI group, intravenous group or in situ hAEC group were dramatically lower than those in the control group (P<0.0001), and there was a trend towards higher ovary weights in POI rats following hAEC transplantation (Figure 3H; P=0.0945 and P=0.0637, respectively). These data suggest that both intravenous and in situ injection of hAECs increases body and ovary weight in POI rats to some extent.
Figure 3.
Body and ovary weight in all rats. A-G. Body weight in each of 4 groups before and after cell transplantation. One- and 2-week exposure to CTX significantly decreased body weight of POI rats compared with normal rats. One to four weeks after cell transplantation, rats in the intravenous or in situ hAEC group exhibited slightly increased body weights compared with those in the POI group. H. Ovarian weight 2 weeks after cell transplantation. POI rats show significantly lower ovarian weight and there was a trend towards higher ovary weights following hAEC transplantation. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. CTX, cyclophosphamide; hAEC, human amniotic epithelial cell; POI, primary ovarian insufficiency.
Effects of hAEC transplantation on estrous cycles
Estrous cycles were measured before CTX injection, 1 week after CTX injection and 1 week after hAEC transplantation. Before chemotherapy, the estrous cycles of rats in each group were fairly regular, lasting for 4-6 days including proestrus for 1 day, estrus for 1-2 days, metestrus for 1 day and diestrus for 1-2 days. However, half of the rats showed abnormal cycles after 1 week of exposure to CTX. Furthermore, 1 week after hAEC transplantation, 71% of rats in the POI group had regular cycles whereas 92% and 100% of rats in the intravenous and in situ hAEC groups, respectively, had regular cycles. These findings imply that CTX causes irregular estrous cycles due to ovarian toxicity while hAEC transplantation can mitigate these effects (Figure 4A, 4B).
Figure 4.
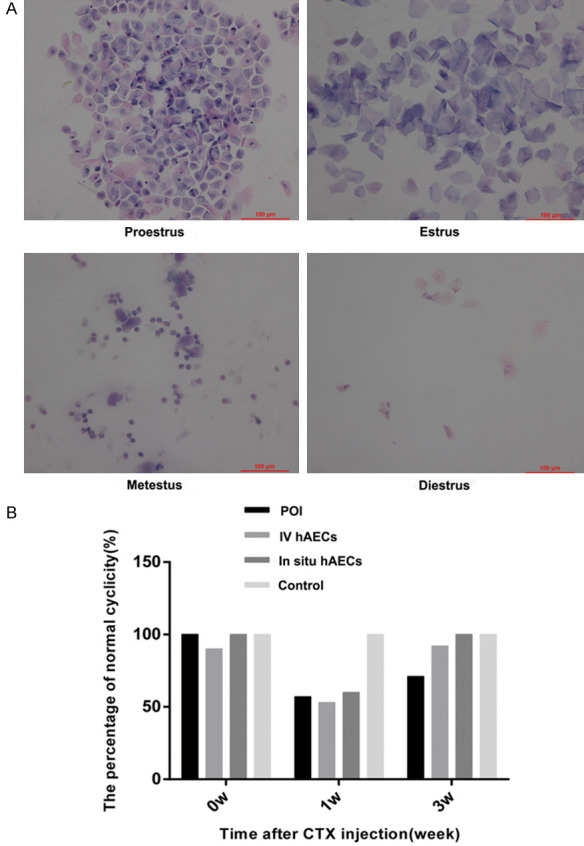
hAEC transplantation decreases the irregularity of estrous cycles. A. Representative photograph of proestrus, estrus, metestrus, and diestrus. B. The percentage of regular estrous cycles in rats before CTX injection, 1 week after CTX exposure and 1 week after cell transplantation in 4 groups. hAEC transplanted rats had more regular estrous cycles than POI rats. Scale bar: 100 μm. Magnification ×200. hAEC, human amniotic epithelial cell; POI, primary ovarian insufficiency.
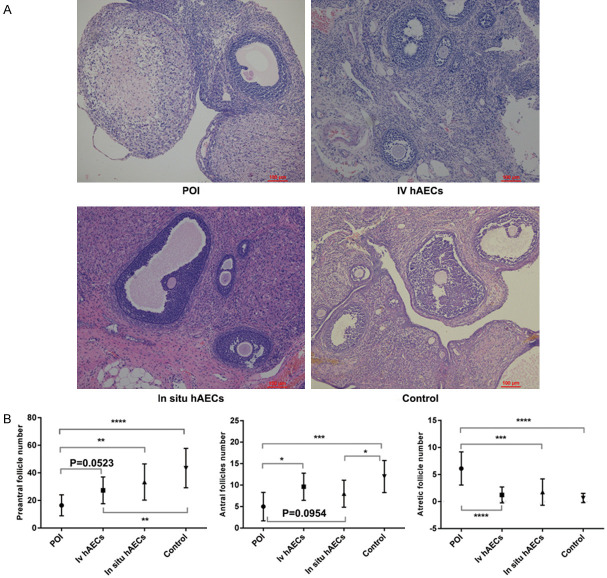
Histological examination and follicle counts
Ovaries were collected and stained for histological analysis and follicle counts. The ovaries of rats contained numerous fibrotic and granulosa cells that were arranged irregularly among follicles in the POI group. The morphological changes in the hAEC transplantation groups were better than the POI group (Figure 5A). Following hAEC transplantation, the POI group had more atretic follicles than the other 3 groups (all P<0.001). In addition, there were fewer preantral follicles (primordial follicles and primary follicles) in the POI group than the in situ hAEC group (P<0.01), control group (P<0.0001) and the intravenous hAEC group (P=0.0523). Similarly, there were more antral follicles (secondary follicles and mature follicles) in the intravenous group (P<0.05), the control group (P<0.001) and the in situ hAEC group (P=0.0954) than the POI group (Figure 5B).
Figure 5.
Histopathological examination and follicle counts in ovaries. A. The pathological changes in ovaries were identified by H&E staining 2 weeks after cell transplantation. The ovaries of rats contained numerous fibrotic and granulosa cells that were arranged irregularly among follicles in the POI group and get better in the hAEC groups. B. The number of follicles (preantral/antral/atretic follicles) were counted and compared between groups. hAEC treatment groups show more preantral/antra follicles and less atretic follicles than POI group. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Scale bar: 100 μm. Magnification ×200. hAEC, human amniotic epithelial cell; H&E, hematoxylin and eosin staining.
Serum levels of FSH and AMH
Following CTX treatment, serum FSH level was increased and the AMH level was decreased in the POI group compared with the control group, and the differences were significant (P<0.05 and P<0.01 respectively). After hAEC treatment, the FSH level was significantly lower while the AMH level was significantly higher in the intravenous and in situ hAEC groups compared with the POI group (P<0.01) (Figure 6A, 6B).
Figure 6.
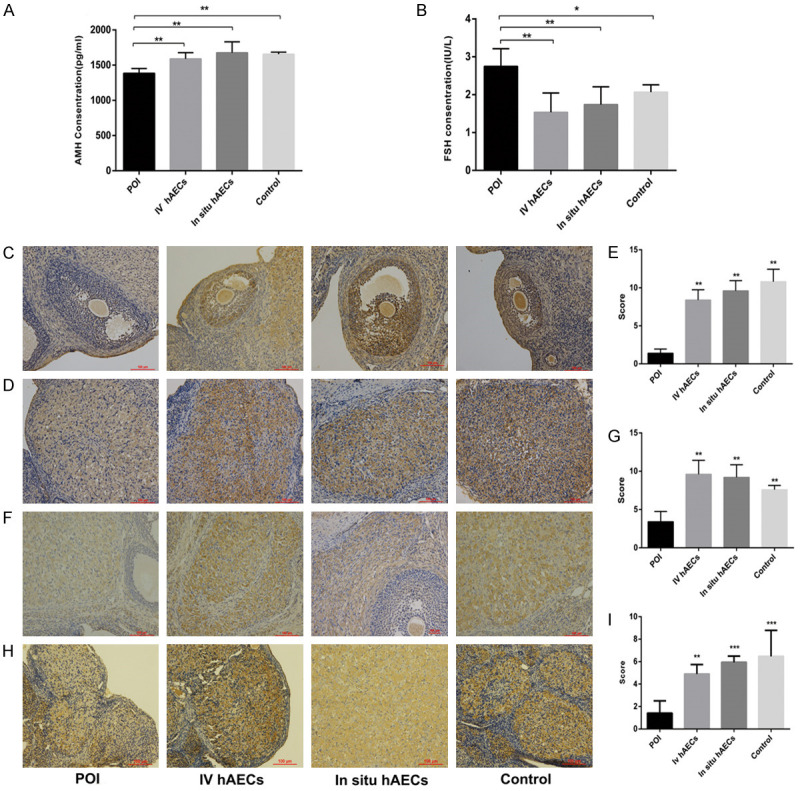
Effect of hAEC transplantation on serum levels of FSH and AMH (A, B) and ovarian expression of AMH, FSHR and KL (C-I) in POI rats. (A, B) Serum AMH (A) concentration increase while FSH (B) concentration decrease in hAEC transplantation groups compared with POI group. (C-E) AMH, (F, G) FSHR and (H, I) KL expression in ovaries. Upregulated expression of ovarian AMH, FSHR and KL was observed following hAEC transplantation in POI rats. Cell nuclei were stained blue. *P<0.05, **P<0.01, ***P<0.001. Scale bar: 100 μm. Magnification ×200. AMH, anti-Müllerian hormone; FSHR, follicle stimulating hormone receptor; hAEC, human amniotic epithelial cell; KL, klotho; POI, primary ovarian insufficiency.
Expression of AMH, FSHR and KL in ovaries
To further verify the changes in AMH and FSH levels, the ovarian expression of AMH and FSHR were detected by immunohistochemistry. AMH expression was mainly located in the preantral, small antral follicles and corpus luteum. FSHR expression was predominantly observed in the corpus luteum. The data showed that ovarian expression of AMH and FSHR was reduced in the POI group compared with the control group. However, there was significantly higher expression of AMH and FSHR either in the intravenous or in situ hAEC group than the POI group (P<0.01) (Figure 6C-G). We also determined klotho (KL) expression for the first time. We found that it was mainly expressed in corpus luteum cells and a significant decrease was observed following CTX administration, whereas KL level was significantly increased in the intravenous or in situ hAEC groups compared with the POI group (P<0.01) (Figure 6H, 6I).
Fertility outcomes
The fertility of rats in each of the 4 groups was assessed according to the mating test. Our study revealed that number of fetuses in the POI group (11.67±1.366), the intravenous group (13.83±0.4082) and the in-situ treatment group (12.57±0.9759) was significantly lower than the control group (16.14±1.864). However, the number in the intravenous group was significantly higher than in the POI group (P<0.05), while a slight increase was observed in the direct ovarian injection group compared with the POI group (P=0.22) (Figure 7A, 7B).
Figure 7.
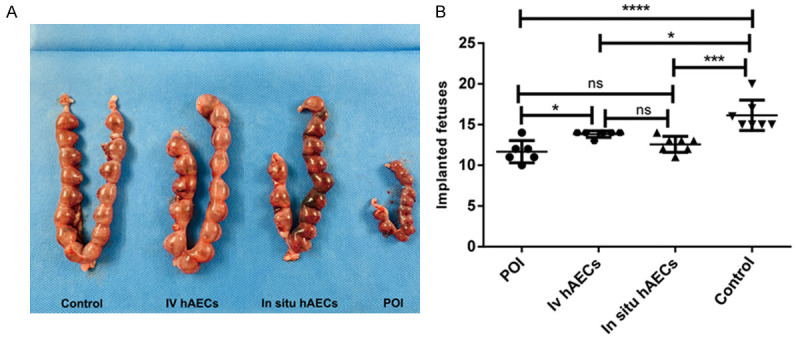
hAECs facilitate improved fertility in POI rats. A. Representative image of implanted fetuses in the 4 groups. B. Comparison of fetus number in the 4 groups. The control group has significantly more fetus than the other 3 groups. hAEC transplantation increases the fetus number and reaches a significant level in the intravenous group. *P<0.05, ***P<0.001, ****P<0.0001. ns means not significant. POI, primary ovarian insufficiency.
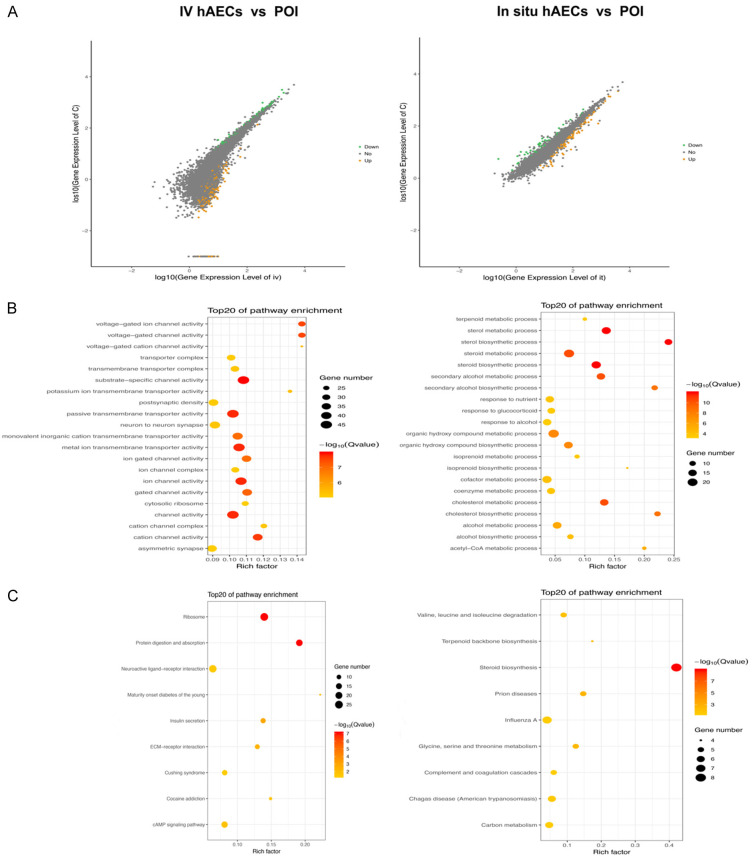
Differentially expressed mRNA screening
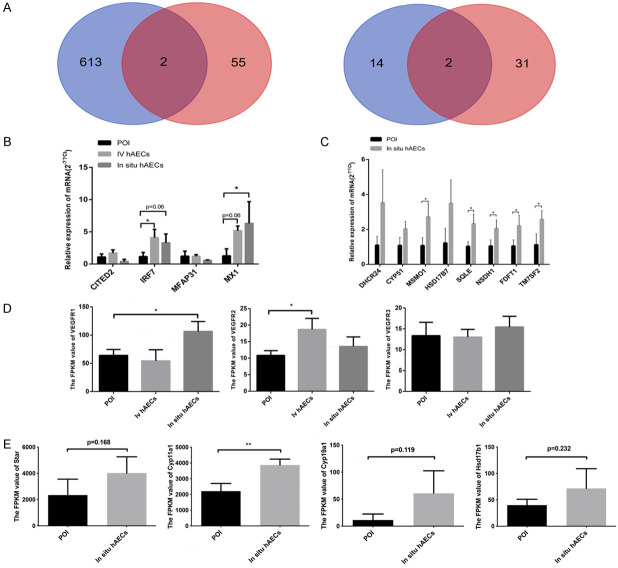
To identify genes involved in hAEC treatment, 3 samples from each group (intravenous hAEC group, in situ hAEC group and POI group) were analyzed using mRNA sequencing. DEGs between groups were screened out according to the adjusted p value and fold change. In total, 783 DEGs were found between the intravenous hAEC and POI group, including 619 upregulated and 164 downregulated genes. Similarly, 116 upregulated DEGs and 52 downregulated DEGs were identified between the in situ hAEC and POI group (Figure 8A). GO analysis showed that the DEGs (intravenous hAEC group vs POI group) were mainly related to channel activity and transmembrane transporter activity while the DEGs (in situ hAEC group vs POI group) were associated with sterol biosynthetic and sterol metabolism (Figure 8B). KEGG analysis showed that the DEGs (intravenous hAEC group vs POI group) were associated with ribosomes, protein digestion and absorption, neuroactive ligand-receptor interaction, insulin secretion, ECM-receptor interaction, Cushing syndrome and cAMP signaling pathway; whereas the DEGs for the in situ hAEC vs the POI group were mainly associated with steroid biosynthesis, prion diseases, glycine/serine/threonine metabolism, terpenoid backbone biosynthesis, valine/leucine/isoleucine degradation, Chagas disease, influenza A, complement and coagulation cascades, carbon metabolism (Figure 8C). Moreover, 4 DEGs were differentially expressed in both the IV hAEC and in situ hAEC groups compared with the POI group, including 2 upregulated and 2 downregulated genes (regulatory factor 7 [IRF7]/Mx dynamin-like GTPase 1 [Mx1], microfibril-associated glycoprotein 3-like [MFAP3L]/cAMP-responsive element-binding protein (CREBBP)/p300-interactingtrans-activator 2 with glutamic acid (E) and aspartic acid (D)-rich tai [CITED2], respectively) (Figure 9A). Vascular endothelial growth factor receptor 1 (VEGFR1) (in situ hAEC group) and VEGFR2 (IV hAEC group) expression were significantly increased compared with the POI group, respectively (both P<0.05), while no difference in VEGFR3 expression was observed among the 3 groups (Figure 9D).
Figure 8.
mRNA sequencing results. (A) scatter plots show DEGs between the IV hAEC and POI groups and the in situ hAEC vs POI groups. Yellow plots indicate upregulated DEGs, green plots represent downregulated DEGs and gray plots suggest no significantly changed genes. DEGs were calculated by |log2 fold change| ≥1 and adjusted p<0.05 by comparing the FPKM values. (B, C) GO (B) and KEGG (C) analysis of DEGs were performed. Corrected p<0.05. DEGs, differentially expressed genes; GO, gene ontology; IV hAEC, intravenous human amniotic epithelial cell; KEGG, Kyoto encyclopedia of genes and genomes; POI, primary ovarian insufficiency.
Figure 9.
Verification of mRNA sequencing results by RT-qPCR analysis. (A) Commonly DEGs between the IV hAEC vs POI group and the in situ hAEC vs POI group were visualized by Venn diagrams. (B) Verification of 4 commonly DEGs among 3 groups. (C) Eight enzymes were differentially expressed between the in situ hAEC group and the POI group and associated with steroid biosynthesis. (D, E) FPKM values for VEGFR1, VEGFR2, VEGFR3 (D) in 3 groups and Star, Cyp11a1, Cyp19a1 and Hsd17b1 (E) in 2 groups by mRNA sequencing. *P<0.05, **P<0.01 indicates the significant difference between groups. DEGs, differentially expressed genes; FPKM, fragments per kilobase of exon model per million mapped fragments; POI, primary ovarian insufficiency; VEGFR, vascular endothelial growth factor receptor.
Verification of mRNA sequencing results
Several key molecules were analyzed in the following experiments. Four commonly expressed genes in the intravenous hAEC vs the POI group and the in situ hAEC vs POI group were verified by real-time PCR analysis. IRF7 and Mx1 were upregulated in the intravenous hAEC and in situ hAEC groups compared with the POI group, which is consistent with the mRNA screening results. However, the other 2 simultaneously DEGs were not significantly changed at the mRNA level as they were in the sequencing analysis (Figure 9B). Moreover, 8 enzymes associated with steroid biosynthesis were confirmed by real-time PCR, immunohistochemical analysis and western blotting. Five of these (Msmo1, SQLE, NSDH1, FDFT1, TM7SF2) were significantly upregulated in the in situ hAEC group (P<0.05) while 3 (DHCR24, CYP51, HSD17B7) were increased compared with the POI group as confirmed by real-time PCR analysis (Figure 9C). SQLE and FDFT1 were mainly expressed in granulosa cells and dramatically increased in the in situ hAEC group compared with the POI group by immunohistochemical and western blotting analysis (P<0.01) (Figure 10A-F). The FPKM values for Star, Cyp11a1, Cyp19a1 and Hsd17b1, which are key enzymes in estrogen and progesterone production, were also increased in the in situ hAEC group compared with the POI group (Figure 9E).
Figure 10.
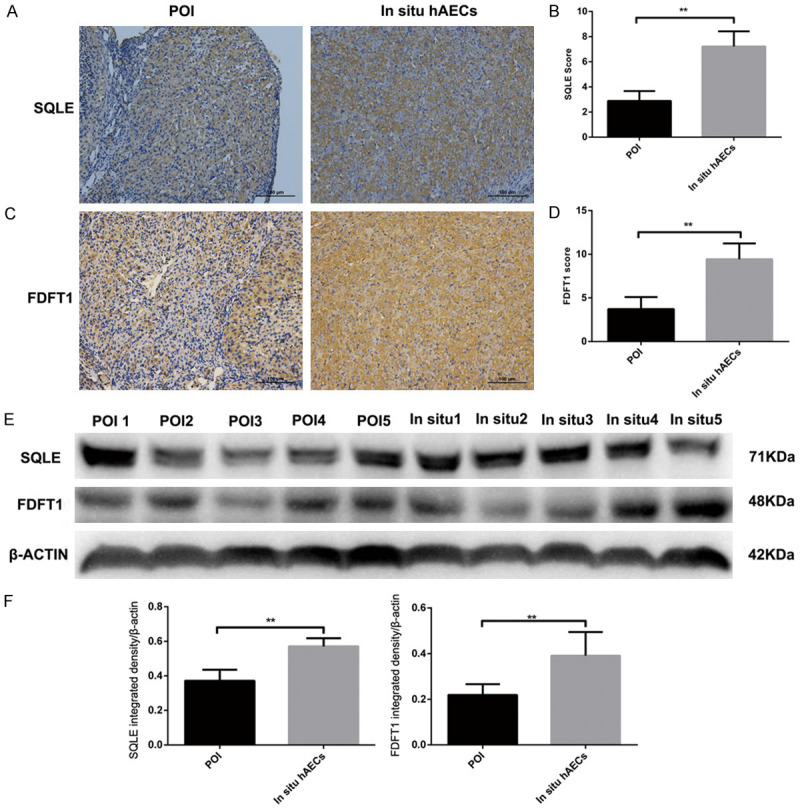
SQLE and FDFT1 expression by immunohistochemical staining and Western blotting analysis. (A-D) SQLE (A, B) and FDFT1 (C, D) expression are upregulated in the in situ hAEC group compared with the POI group by immunohistochemical analysis. (E, F) SQLE and FDFT1 were determined by Western blotting. The same trend as in the immunohistochemical analysis result was observed. **P<0.01. FDFT1, farnesyl-diphosphate farnesyltransferase 1; SQLE, squalene monooxygenase.
Discussion
About 1% of adult women are affected by POF which can impact both mental and physical health [1-3]. Various factors lead to POF or POI including chemotherapy for cancer. Of the major kinds of chemotherapeutic drugs, the alkylators are considered extremely gonadotoxic and lead to damage in resting primordial follicle pools because they are not cell cycle-specific, thus resulting in decreased ovarian reserves or even POF [15]. Cyclophosphamide, a specific type of alkylator, exerts its anticancer effects via double-stranded DNA damage and subsequent cell death if the defects are not repaired [16]. In addition, chemotherapy drugs excessively stimulate the primordial follicles, leading to the depletion of the primordial follicle pool, and chemotherapy drugs also damage ovarian blood vessels, resulting in loss of follicles [17,18]. Until now, no effective methods for the prevention or treatment of POF have been identified. HRT is widely used to alleviate perimenopausal or postmenopausal syndrome (characterized by symptoms such as hot flashes, vaginal dryness, night sweats and insomnia), decrease bone or cardiovascular diseases, and to combat mood disorders such as anxiety, depression and cognitive decline [3]. However, HRT is not effective for continuous elevation of FSH or against damaged fertility because elevated basal FSH levels are associated with extremely low pregnancy rates [19]. Moreover, the long-term application of hormones increases the risk of gynecological tumors. Various stem cell treatments have been evaluated for the treatment of POF in animal models that were shown to restore ovarian function and fertility to a certain extent. hAECs are stem cells that exhibit great advantages for the treatment of POF because of their abundance and low immunogenicity [9]. In the current study, we developed a CTX pretreated rat model and investigated the effect of hAEC transplantation on POI rats. The results demonstrated that hAECs mainly located in the interstitial area of the ovary. hAEC transplantation slightly increased body and ovary weight and normalized irregular estrous cycles. In addition, hAEC transplantation decreased serum FSH levels and increased AMH levels and led to the restoration of follicle pools in POI rats. Ovarian AMH and FSHR expression in POI rats was also significantly upregulated following hAEC transplantation, which was consistent with serum levels. The expression of KL, which is a protein relevant to ageing in women, was increased after hAEC treatment. Moreover, hAEC treatment ultimately increased the average fetus number which suggests improved fertility.
AMH belongs to the transforming growth factor beta (TGFβ) superfamily, and currently is the only negative regulator for primordial follicle recruitment and subsequently transition to primary follicles [20]. AMH is mainly secreted by granulosa cells in the preantral and small antral follicles [20,21]. It has been reported that serum AMH level is a predictor of the growing follicle population and closely relates to the number of primordial follicles [22]. Previous reports indicated that CTX excessively activated the primordial follicles, leading to depletion of ovarian reserves and subsequent POI/POF [23,24]. The AMH expression in the serum and ovaries was obviously increased following hAEC transplantation, causing a decrease in the transition of primordial follicles to primary follicles thus effectively preserving the primordial follicle pools and ovarian reserves. In other words, hAECs may protect ovarian reserves from CTX insult by the upregulation of AMH.
The FSHR is an important member of the G protein-coupled receptor (GPCR) superfamily and connects to a complex signaling network which involves several G protein subtypes; therefore, it is likely that it interacts with other receptors, including the IGF-1 receptor (IGF-1R), the epidermal growth factor receptor (EGFR) and associated proteins [25-28]. The FSHR is highly expressed in ovarian granulosa cells and is mainly stimulated by FSH to regulate the growth and maturation of follicles, as well as the production of estrogen [29]. Studies showed that reduced expression of FSHR was correlated with poor response to FSH, less mature oocytes and lower peak E2 levels [30]. In contrast, FSHR expression induced by BMP15 through Smad or non-Smad pathways increased follicular growth and oocyte ovulation [30,31]. In our study, hAECs enhanced FSHR expression and increased preovulatory and ovulatory follicles. Various factors could decrease ovarian FSHR expression, including chemotherapy drugs, immune factors and heat stress [21,32]. One possible reason is the apoptosis of granulosa cells where FSHR is predominantly expressed. This finding suggests that hAEC therapy may be an effective method to modulate the function of granulosa cells, which is extremely crucial for follicle growth and maturation.
The Klotho (KL) gene was first described in 1997 as an ageing-suppressor gene [33]. Afterwards it was found that overexpression or knockout of KL correlated with prolonged or shortened lifespan, respectively [34,35]. Research indicates that CTX inhibited KL expression in granulosa cells of the mouse ovary, and KL-/- mice exhibited pathological manifestations similar to cyclophosphamide-induced POF mice, which were both due to the reduced ability of ovarian granulosa cells to induce autophagy and ROS scavenging [36]. This is consistent with our results which showed that CTX treatment decreased KL expression in ovarian granulosa cells. As such, KL may play a vital role in POI. Our study firstly showed that hAEC transplantation significantly increased KL level in granulosa cells, which may contribute to the recovery of ovarian function by regulating ovarian granulosa cells activities.
To further explore the mechanism of hAEC transplantation in repairing ovarian function in POI rats, we performed mRNA sequencing of ovarian samples from 3 groups to identify potential key genes and pathways. The results showed that IRF7 and Mx1 were consistently upregulated both in the intravenous hAEC group and the in situ hAEC group compared with the POI group.
IRF7 is an important member of the IRF family, which is involved in IFNα/β induction via toll-like receptors (TLRs) [37]. Previous research revealed that IRF7 is important for angiogenesis since IRF7 overexpression induced the expression of inflammatory cytokines including IL6, CCL2 and CXCL1, hence increased tumor formation through increased angiogenesis and the acquisition of glioma stem cell properties [38]. Experiments in epithelial PK15 cell lines of the porcine kidney showed that overexpression of IRF3/IRF7 decreased IL-6 expression and increased IFNα production by lipopolysaccharide (LPS) stimulation either independent or dependent of the TLR4/TBK1 pathway, respectively, thus playing an anti-inflammatory role [39]. Interestingly, depletion of IRF3 and IRF7 in C57BL/6 mice showed attenuated angiogenesis and arteriogenesis via decreased secretion of pro-inflammatory factors (TNFα, IL6, CCL2) and growth factor receptor (VEGFR2) after unilateral hind limb ischemia injury [40]. In addition, IRF7 was upregulated in different heart failure models including myocarditis, myocardial infarction and diabetes mellitus [41]. After human umbilical endothelial cells (HUVECs) were exposed to ionizing radiation, microarray analysis showed that upregulated genes were particularly linked with the type-1 interferon response and an upregulated gene network containing IRF7 as well as its transcriptional targets Mx1 [42]. Therefore, IRF7 and its transcript Mx1 are likely involved in inflammatory responses and in angiogenesis. Previous studies have verified that CTX could significantly impair angiogenesis and cause inflammation [43]. Our data showed upregulated expression of IRF7, Mx1, VEGFR1 and VEGFR2 in the treatment groups which may imply that hAEC therapy could increase angiogenesis and attenuate inflammation, thus leading to recovery of ovarian function.
Interestingly, pathway analysis revealed that the DEGs in the intravenous hAEC vs the POI group were significantly associated with ribosomes, protein digestion and absorption, neuroactive ligand-receptor interaction and cAMP signaling pathway; whereas the upregulated DEGs in the in situ hAEC vs POI group were mainly associated with cholesterol and steroid biosynthesis. The neuroactive ligand-receptor interaction pathway is related with signaling interaction and signal transduction, reproduction and gonadal development, and ovulation. Of these enriched genes, corticotropin releasing hormone receptor 1 (CRHR1), 5-hydroxytryptamine receptor 4 (HTR4), pituitary adenylate cyclase-activating polypeptide type 1 receptor (PAC1-R) and neuropeptide FF receptor 1 (Npffr1) are all highly expressed in ovaries, which are involved in proliferation and apoptosis of ovarian cells and play important roles in follicle development and ovulation of the ovaries [44-50]. The cAMP signaling pathway is involved in steroidogenesis of the antral follicular stage [51]. It is well known that hormone biosynthesis is modulated by a series of ovary steroidogenic enzymes which are critical for reproductive function including follicle development and ovulation [52,53]. Previous evidence suggest that oocytes were not efficient in cholesterol synthesis and required cumulus cells to provide cholesterol biosynthetic products, while BMP15 and GDF9 originating from oocytes promoted cholesterol synthesis in cumulus cells [54]. It is believed that the antral follicle and the corpus luteum are both steroidogenic glands and within them the theca and granulosa cells cooperate to promote ovarian steroidogenesis [55]. To be specific, first the luteinizing hormone (LH) receptors of theca cells receive LH signals by increasing related enzymes such as Star and Cyp11A1 for the conversion of cholesterol to progesterone. Subsequently, FSH receptors of granulosa cells bind to FSH and elevate the expression of enzymes such as CYP19 and HSD17B to convert the theca-derived androgens into estrogens [55,56]. Our data show that cholesterol and steroid biosynthesis enzymes were upregulated at both the gene and protein level following in situ hAEC transplantation thus probably resulting in increased ovarian steroidogenesis and restored ovarian endocrine function. Observations demonstrate that progesterone and estradiol both inhibit the excessive transition of primordial follicles to primary follicles by hampering coordinated oocyte apoptosis, which is required for primordial follicle assembly, hence preventing conditions such as POF or premature onset of menopause [20]. This may also indicate that hAECs could not only restore ovarian endocrine function but also preserve ovarian reserves through the upregulation of steroidogenesis enzymes.
Stem cells mainly exert their recovery effects by trans-differentiation ability, cell fusion events and paracrine effects. hAECs were shown to occasionally differentiate into granulosa cells in a POF mouse model [6]. In this study, the authors showed that human specific FSHR expression patterns were located in granulosa cells of the Graafian follicles in mouse ovaries. Interestingly, FSHR was detected in hAECs by immunofluorescence staining in our study, which is in accordance with Stilley’s findings that FSHR staining was positive in extragonadal tissues, including hAECs [57]. Therefore, further investigation may be necessary to prove the trans-differentiation ability of hAECs to granulosa cells and their role in recovery of ovarian function. Cell fusion and reprogramming between stem cells and host cells is a rare but possible event by which a mature phenotype can be generated [58-60]. Studies have shown that transplanted bone marrow cells can fuse with epithelial cells, Purkinje neurons, cardiomyocytes and hepatocytes [61-64]. However, Marongiu reported that rat-derived amniotic epithelial cells did not fuse with liver cells in vivo [65]. Because of the extremely low rates of cell fusion, this is not likely to be the main mechanism by which hAECs regulate ovarian function. hAECs secrete a broad range of cytokines which regulate multiple immune-related processes such as proliferation, immune response, cell apoptosis and angiogenesis [66], and restore ovarian function via intravenous injection, in situ injection or intraperitoneal injection of hAECs or hAECs-conditioned medium. This suggests that hAECs regulate ovarian function partly through paracrine effects. In addition, various factors have been proven to regulate steroidogenesis in granulosa cells including the TGF-β superfamily [67-69], brain-derived neurotrophic factor (BDNF) [70,71], growth differentiation factor 9 [72] and growth differentiation factor 8 [73]. Therefore, it is most likely that hAECs could secrete multiple cytokines which may be involved in steroidogenesis in granulosa cells, modulating follicle fate from initiation to ovulation, regulating angiogenesis and inflammation of the ovary thus leading to recovery of ovarian function. However, further in vitro investigation will be needed to elucidate the exact mechanisms.
In summary, our findings highlight the effectiveness of hAECs in improving ovarian reserves, function and fertility. The results also indicate that hAECs upregulate ovarian AMH, FSHR and KL expression, which may prevent overactivation of primordial follicles and promote the development and maturation of growing follicles. Finally, high-throughput sequencing showed that hAEC transplantation had an effect on ribosomes, protein digestion, protein absorption, neuroactive ligand-receptor interaction, cAMP signaling pathway and steroid biosynthesis pathways, which are involved in steroidogenesis, follicle development and ovulation. hAEC treatment also increased the expression of the vascular and anti-inflammatory factors IRF7, Mx1, VEGFR1 and VEGFR2, which participate in angiogenesis and inflammation.
Conclusion
hAEC transplantation may help to recover ovarian function, protect ovarian reserves and promote fertility, thus making them a promising source of stem cell therapy. This study provides a fresh perspective on the role of hAEC transplantation in treating POI.
Acknowledgements
We thank Prof. Tinghe Yu for the technical help and advice. This research did not receive any specific grant from funding agencies.
Disclosure of conflict of interest
None.
References
- 1.Sadeghi MR. New hopes for the treatment of primary ovarian insufficiency/premature ovarian failure. J Reprod Infertil. 2013;14:1–2. [PMC free article] [PubMed] [Google Scholar]
- 2.De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. 2010;376:911–921. doi: 10.1016/S0140-6736(10)60355-8. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan SD, Sarrel PM, Nelson LM. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil Steril. 2016;106:1588–1599. doi: 10.1016/j.fertnstert.2016.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee HJ, Selesniemi K, Niikura Y, Niikura T, Klein R, Dombkowski DM, Tilly JL. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J. Clin. Oncol. 2007;25:3198–3204. doi: 10.1200/JCO.2006.10.3028. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, Wang L, Yao X, Lai D, Guo L. Human amniotic epithelial cells can differentiate into granulosa cells and restore folliculogenesis in a mouse model of chemotherapy-induced premature ovarian failure. Stem Cell Res Ther. 2013;4:124. doi: 10.1186/scrt335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun M, Wang S, Li Y, Yu L, Gu F, Wang C, Yao Y. Adipose-derived stem cells improved mouse ovary function after chemotherapy-induced ovary failure. Stem Cell Res Ther. 2013;4:80. doi: 10.1186/scrt231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q, Xu M, Yao X, Li T, Wang Q, Lai D. Human amniotic epithelial cells inhibit granulosa cell apoptosis induced by chemotherapy and restore the fertility. Stem Cell Res Ther. 2015;6:152. doi: 10.1186/s13287-015-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Germanio C, Bernier M, de Cabo R, Barboni B. Amniotic epithelial cells: a new tool to combat aging and age-related diseases? Front Cell Dev Biol. 2016;4:135. doi: 10.3389/fcell.2016.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao X, Guo Y, Wang Q, Xu M, Zhang Q, Li T, Lai D. The paracrine effect of transplanted human amniotic epithelial cells on ovarian function improvement in a mouse model of chemotherapy-induced primary ovarian insufficiency. Stem Cells Int. 2016;2016:4148923. doi: 10.1155/2016/4148923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17:555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- 13.Remmele W, Stegner HE. [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue] . Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 14.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonmezer M, Oktay K. Fertility preservation in young women undergoing breast cancer therapy. Oncologist. 2006;11:422–434. doi: 10.1634/theoncologist.11-5-422. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen QN, Zerafa N, Liew SH, Morgan FH, Strasser A, Scott CL, Findlay JK, Hickey M, Hutt KJ. Loss of PUMA protects the ovarian reserve during DNA-damaging chemotherapy and preserves fertility. Cell Death Dis. 2018;9:618. doi: 10.1038/s41419-018-0633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedoschi G, Navarro PA, Oktay K. Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Future Oncol. 2016;12:2333–2344. doi: 10.2217/fon-2016-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spears N, Lopes F, Stefansdottir A, Rossi V, De Felici M, Anderson RA, Klinger FG. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update. 2019;25:673–693. doi: 10.1093/humupd/dmz027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Licciardi FL, Liu HC, Rosenwaks Z. Day 3 estradiol serum concentrations as prognosticators of ovarian stimulation response and pregnancy outcome in patients undergoing in vitro fertilization. Fertil Steril. 1995;64:991–994. doi: 10.1016/s0015-0282(16)57916-3. [DOI] [PubMed] [Google Scholar]
- 20.Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005;11:461–471. doi: 10.1093/humupd/dmi020. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Luo Q, Lu X, Yin N, Zhou D, Zhang L, Zhao W, Wang D, Du P, Hou Y, Zhang Y, Yuan W. Effects of hPMSCs on granulosa cell apoptosis and AMH expression and their role in the restoration of ovary function in premature ovarian failure mice. Stem Cell Res Ther. 2018;9:20. doi: 10.1186/s13287-017-0745-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Marcello MF, Nuciforo G, Romeo R, Di Dino G, Russo I, Russo A, Palumbo G, Schiliro G. Structural and ultrastructural study of the ovary in childhood leukemia after successful treatment. Cancer. 1990;66:2099–2104. doi: 10.1002/1097-0142(19901115)66:10<2099::aid-cncr2820661010>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, Wolf I, Kanety H, Sredni B, Meirow D. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;5:185ra162. doi: 10.1126/scitranslmed.3005402. [DOI] [PubMed] [Google Scholar]
- 24.Roness H, Gavish Z, Cohen Y, Meirow D. Ovarian follicle burnout: a universal phenomenon? Cell Cycle. 2013;12:3245–3246. doi: 10.4161/cc.26358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wayne CM, Fan HY, Cheng X, Richards JS. Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol Endocrinol. 2007;21:1940–1957. doi: 10.1210/me.2007-0020. [DOI] [PubMed] [Google Scholar]
- 26.Baumgarten SC, Convissar SM, Fierro MA, Winston NJ, Scoccia B, Stocco C. IGF1R signaling is necessary for FSH-induced activation of AKT and differentiation of human Cumulus granulosa cells. J Clin Endocrinol Metab. 2014;99:2995–3004. doi: 10.1210/jc.2014-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou J, Chin E, Bondy C. Cellular pattern of insulin-like growth factor-I (IGF-I) and IGF-I receptor gene expression in the developing and mature ovarian follicle. Endocrinology. 1991;129:3281–3288. doi: 10.1210/endo-129-6-3281. [DOI] [PubMed] [Google Scholar]
- 28.Zhou P, Baumgarten SC, Wu Y, Bennett J, Winston N, Hirshfeld-Cytron J, Stocco C. IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol Endocrinol. 2013;27:511–523. doi: 10.1210/me.2012-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulloa-Aguirre A, Reiter E, Crepieux P. FSH receptor signaling: complexity of interactions and signal diversity. Endocrinology. 2018;159:3020–3035. doi: 10.1210/en.2018-00452. [DOI] [PubMed] [Google Scholar]
- 30.Cai J, Lou HY, Dong MY, Lu XE, Zhu YM, Gao HJ, Huang HF. Poor ovarian response to gonadotropin stimulation is associated with low expression of follicle-stimulating hormone receptor in granulosa cells. Fertil Steril. 2007;87:1350–1356. doi: 10.1016/j.fertnstert.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu K, Nakamura T, Bayasula , Nakanishi N, Kasahara Y, Nagai T, Murase T, Osuka S, Goto M, Iwase A, Kikkawa F. Molecular mechanism of FSHR expression induced by BMP15 in human granulosa cells. J Assist Reprod Genet. 2019;36:1185–1194. doi: 10.1007/s10815-019-01469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Guo S, Cai L, Ma W, Shi Z. Lipopolysaccharide and heat stress impair the estradiol biosynthesis in granulosa cells via increase of HSP70 and inhibition of smad3 phosphorylation and nuclear translocation. Cell Signal. 2017;30:130–141. doi: 10.1016/j.cellsig.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 34.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y, Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr Rev. 2015;36:174–193. doi: 10.1210/er.2013-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu T, Liu Y, Huang Y, Chen J, Yu Z, Chen C, Lai L. miR-15b induces premature ovarian failure in mice via inhibition of alpha-Klotho expression in ovarian granulosa cells. Free Radic Biol Med. 2019;141:383–392. doi: 10.1016/j.freeradbiomed.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 38.Jin X, Kim SH, Jeon HM, Beck S, Sohn YW, Yin J, Kim JK, Lim YC, Lee JH, Kim SH, Kang SH, Pian X, Song MS, Park JB, Chae YS, Chung YG, Lee SH, Choi YJ, Nam DH, Choi YK, Kim H. Interferon regulatory factor 7 regulates glioma stem cells via interleukin-6 and Notch signalling. Brain. 2012;135:1055–1069. doi: 10.1093/brain/aws028. [DOI] [PubMed] [Google Scholar]
- 39.Chen PG, Guan YJ, Zha GM, Jiao XQ, Zhu HS, Zhang CY, Wang YY, Li HP. Swine IRF3/IRF7 attenuates inflammatory responses through TLR4 signaling pathway. Oncotarget. 2017;8:61958–61968. doi: 10.18632/oncotarget.18740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simons KH, de Vries MR, de Jong RCM, Peters HAB, Jukema JW, Quax PHA. IRF3 and IRF7 mediate neovascularization via inflammatory cytokines. J Cell Mol Med. 2019;23:3888–3896. doi: 10.1111/jcmm.14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becher PM, Hinrichs S, Fluschnik N, Hennigs JK, Klingel K, Blankenberg S, Westermann D, Lindner D. Role of Toll-like receptors and interferon regulatory factors in different experimental heart failure models of diverse etiology: IRF7 as novel cardiovascular stress-inducible factor. PLoS One. 2018;13:e0193844. doi: 10.1371/journal.pone.0193844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furusawa Y, Zhao QL, Hattori Y, Tabuchi Y, Iwasaki T, Nomura T, Kondo T. Comprehensive and computational analysis of genes in human umbilical vein endothelial cells responsive to X-irradiation. Genom Data. 2016;8:126–130. doi: 10.1016/j.gdata.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ling L, Feng X, Wei T, Wang Y, Wang Y, Zhang W, He L, Wang Z, Zeng Q, Xiong Z. Effects of low-intensity pulsed ultrasound (LIPUS)-pretreated human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation on primary ovarian insufficiency in rats. Stem Cell Res Ther. 2017;8:283. doi: 10.1186/s13287-017-0739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen H, Feng W, Chen K, Qiu X, Xu H, Mao G, Zhao T, Ding Y, Wu X. Transcriptomic analysis reveals potential mechanisms of toxicity in a combined exposure to dibutyl phthalate and diisobutyl phthalate in zebrafish (Danio rerio) ovary. Aquat Toxicol. 2019;216:105290. doi: 10.1016/j.aquatox.2019.105290. [DOI] [PubMed] [Google Scholar]
- 45.Xu S, Wang D, Zhou D, Lin Y, Che L, Fang Z, Wu D. Reproductive hormone and transcriptomic responses of pituitary tissue in anestrus gilts induced by nutrient restriction. PLoS One. 2015;10:e0143219. doi: 10.1371/journal.pone.0143219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen B, Liang G, Zhu X, Tan Y, Xu J, Wu H, Mao H, Zhang Y, Chen J, Rao Y, Zhou M, Liu S. Gene expression profiling in ovaries and association analyses reveal HEP21 as a candidate gene for sexual maturity in chickens. Animals (Basel) 2020;10:181. doi: 10.3390/ani10020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tao Z, Song W, Zhu C, Xu W, Liu H, Zhang S, Huifang L. Comparative transcriptomic analysis of high and low egg-producing duck ovaries. Poult Sci. 2017;96:4378–4388. doi: 10.3382/ps/pex229. [DOI] [PubMed] [Google Scholar]
- 48.Zhou R, Tsang AH, Lau SW, Ge W. Pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptors in the zebrafish ovary: evidence for potentially dual roles of PACAP in controlling final oocyte maturation. Biol Reprod. 2011;85:615–625. doi: 10.1095/biolreprod.111.091884. [DOI] [PubMed] [Google Scholar]
- 49.Barberi M, Di Paolo V, Latini S, Guglielmo MC, Cecconi S, Canipari R. Expression and functional activity of PACAP and its receptors on cumulus cells: effects on oocyte maturation. Mol Cell Endocrinol. 2013;375:79–88. doi: 10.1016/j.mce.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 50.Wilsterman K, Bentley GE, Comizzoli P. RFRP3 influences basal lamina degradation, cellular death, and progesterone secretion in cultured preantral ovarian follicles from the domestic cat. PeerJ. 2019;7:e7540. doi: 10.7717/peerj.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riccetti L, Sperduti S, Lazzaretti C, Casarini L, Simoni M. The cAMP/PKA pathway: steroidogenesis of the antral follicular stage. Minerva Ginecol. 2018;70:516–524. doi: 10.23736/S0026-4784.18.04282-X. [DOI] [PubMed] [Google Scholar]
- 52.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 53.Tu X, Liu M, Tang J, Zhang Y, Shi Y, Yu L, Sun Z. The ovarian estrogen synthesis function was impaired in Y123F mouse and partly restored by exogenous FSH supplement. Reprod Biol Endocrinol. 2018;16:44. doi: 10.1186/s12958-018-0365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su YQ, Sugiura K, Wigglesworth K, O’Brien MJ, Affourtit JP, Pangas SA, Matzuk MM, Eppig JJ. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development. 2008;135:111–121. doi: 10.1242/dev.009068. [DOI] [PubMed] [Google Scholar]
- 55.Craig ZR, Wang W, Flaws JA. Endocrine-disrupting chemicals in ovarian function: effects on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction. 2011;142:633–646. doi: 10.1530/REP-11-0136. [DOI] [PubMed] [Google Scholar]
- 56.Liu Z, Rudd MD, Hernandez-Gonzalez I, Gonzalez-Robayna I, Fan HY, Zeleznik AJ, Richards JS. FSH and FOXO1 regulate genes in the sterol/steroid and lipid biosynthetic pathways in granulosa cells. Mol Endocrinol. 2009;23:649–661. doi: 10.1210/me.2008-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stilley JA, Christensen DE, Dahlem KB, Guan R, Santillan DA, England SK, Al-Hendy A, Kirby PA, Segaloff DL. FSH receptor (FSHR) expression in human extragonadal reproductive tissues and the developing placenta, and the impact of its deletion on pregnancy in mice. Biol Reprod. 2014;91:74. doi: 10.1095/biolreprod.114.118562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 59.Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- 60.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 61.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 62.Johansson CB, Youssef S, Koleckar K, Holbrook C, Doyonnas R, Corbel SY, Steinman L, Rossi FM, Blau HM. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat Cell Biol. 2008;10:575–583. doi: 10.1038/ncb1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 64.Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, Peister A, Wang MY, Prockop DJ. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci U S A. 2003;100:2397–2402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marongiu M, Serra MP, Contini A, Sini M, Strom SC, Laconi E, Marongiu F. Rat-derived amniotic epithelial cells differentiate into mature hepatocytes in vivo with no evidence of cell fusion. Stem Cells Dev. 2015;24:1429–1435. doi: 10.1089/scd.2014.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Q, Bu S, Sun J, Xu M, Yao X, He K, Lai D. Paracrine effects of human amniotic epithelial cells protect against chemotherapy-induced ovarian damage. Stem Cell Res Ther. 2017;8:270. doi: 10.1186/s13287-017-0721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang HM, Cheng JC, Huang HF, Shi FT, Leung PC. Activin A, B and AB decrease progesterone production by down-regulating StAR in human granulosa cells. Mol Cell Endocrinol. 2015;412:290–301. doi: 10.1016/j.mce.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 68.Fang L, Chang HM, Cheng JC, Leung PC, Sun YP. TGF-beta1 downregulates StAR expression and decreases progesterone production through Smad3 and ERK1/2 signaling pathways in human granulosa cells. J Clin Endocrinol Metab. 2014;99:E2234–2243. doi: 10.1210/jc.2014-1930. [DOI] [PubMed] [Google Scholar]
- 69.Nagao S, Iwata N, Soejima Y, Takiguchi T, Aokage T, Kozato Y, Nakano Y, Nada T, Hasegawa T, Otsuka F. Interaction of ovarian steroidogenesis and clock gene expression modulated by bone morphogenetic protein-7 in human granulosa cells. Endocr J. 2019;66:157–164. doi: 10.1507/endocrj.EJ18-0423. [DOI] [PubMed] [Google Scholar]
- 70.Xie M, Li M, Zhou J, Ding X, Shao Y, Jing J, Liu Y, Yao B. Brain-derived neurotrophic factor promotes human granulosa-like tumor cell steroidogenesis and proliferation by activating the FSH receptor-mediated signaling pathway. Sci Rep. 2017;7:180. doi: 10.1038/s41598-017-00203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen S, Wang F, Liu Z, Zhao Y, Jiang Y, Chen L, Li C, Zhou X. Brain-derived neurotrophic factor promotes proliferation and progesterone synthesis in bovine granulosa cells. J Cell Physiol. 2019;234:8776–8787. doi: 10.1002/jcp.27536. [DOI] [PubMed] [Google Scholar]
- 72.Li J, Luo W, Huang T, Gong Y. Growth differentiation factor 9 promotes follicle-stimulating hormone-induced progesterone production in chicken follicular granulosa cells. Gen Comp Endocrinol. 2019;276:69–76. doi: 10.1016/j.ygcen.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 73.Chang HM, Fang L, Cheng JC, Taylor EL, Sun YP, Leung PC. Effects of growth differentiation factor 8 on steroidogenesis in human granulosa-lutein cells. Fertil Steril. 2016;105:520–528. doi: 10.1016/j.fertnstert.2015.10.034. [DOI] [PubMed] [Google Scholar]