Abstract
Transient Receptor Potential Melastatin 4 (TRPM4) is a nonselective channel conducting monovalent ions and indirectly regulates intracellular Ca2+. Aberrant expression has been reported in a number of cancers. However, the biological function of TRPM4 in endometrial carcinoma (EC) is still unknown. We find that decreased TRPM4 expression is significantly correlated with a poor prognosis, overall survival (OS, P<0.001) and recurrence-free survival (P=0.002) through The Cancer Genome Atlas (TCGA) datasets in mRNA level. Multivariate Cox regression analysis suggests that TRPM4 is an independent prognostic factor for OS in EC patients. In vitro assays show that TRPM4-deletion results in significant promotion of proliferation and migration in EC cells. We then conducted a gene set enrichment analysis (GSEA) and according to the results, the expression of TRPM4 is modulated by estrogen, which is inhibited by ER antagonist. Furthermore, the silencing of TRPM4 causes a decreased p53 and hyper-activation of EMT, PI3K/AKT/mTOR signaling pathway in EC, as demonstrated in vitro. Overall, these results indicate that TRPM4 is clinically useful in predicting EC prognosis and represent a potential candidate as a new therapeutic target.
Keywords: Endometrial carcinoma, TRPM4, TCGA, prognosis, GSEA, estrogen
Introduction
Endometrial carcinoma (EC) is one of the most common gynecological malignancy cancers worldwide and in China [1]. Importantly, the incidence and mortality rates for EC have been rising in both developed and developing countries [2]. For patients with metastasis or recurrence, regardless of the grade and stage, the prognosis is unfavorable. They are at a significantly higher risk of mortality and typically experience a poor quality of life, with a median overall survival time of less than 16 weeks [3]. It remains unclear how genetic regulatory networks modulate EC initiation. Although certain improvements have been made, including classifying patients by estrogen receptor status, it remains difficult to predict the prognosis and find an effective biomarker for patients with EC [4].
TRPM4 is a monovalent non-selective cation protein that is activated upon elevation of intracellular Ca2+ [5]. It performs a role in the regulation of intracellular Ca2+ overloading, the preservation of plasma membrane potential and the control of intracellular Ca2+ oscillations [6]. We previously found that the level of L-type Ca2+ channel subunit CACNA1D was overexpressed in EC tissues as compared to that in benign controls and calcium influx to EC cells through CACNA1D promoted their proliferation and migration [7]. Furthermore, recent studies found that TRPM4 may be involved in the pathophysiology of EC and is responsible for a prognostic prediction model of a panel of 7 genes, including TRPM4 [8]. Thus, we speculated that TRPM4 is also a key molecule in mediating the progression and metastasis of EC and may have an influence on the clinicopathologic characteristics.
PI3K/AKT/mTOR pathway is pivotal for the control of cell transcription, translation, migration, metabolism, proliferation, and survival [9]. Aberrant hyperactivation of PI3K/AKT/mTOR pathway is one of the most common tumor-related signaling pathways that can be activated in quite few tumor types, including endometrial carcinoma, breast cancer, bladder cancer and pancreatic cancer, mainly including mutations of PIK3CA and AKT1 [10-12]. A previous study suggested that TRPM4 can promote the cell proliferation in prostate cancer by regulating the AKT activity [13]. A previous study had revealed that the expression of TRPM4 could mediate certain behaviors of cancer cells such as migration and invasion [14]. But the biological process and the specific mechanisms in carcinoma still remain unknown. Here we focus on the association between TRPM4 expression and the clinicopathologic features and prognosis of patients in The Cancer Genome Atlas (TCGA). We also investigated the role of TRPM4 in EC cell migration and metastasis in vitro. Moreover, to explore the underlying mechanism of TRPM4-mediated cell migration, gene set enrichment analysis (GSEA) was conducted for TRPM4 with TCGA datasets. According to the results, we conclulded that TRPM4 is enriched in estrogen response and can activate the EMT, p53 and PI3K/AKT/mTOR pathways in EC cells. Finally, we validated the GSEA findings through in vitro assays.
Materials and methods
Data download and bioinformatics analysis
UCSC Xena browser (http://xena.ucsc.edu/) was used to obtain TRPM4 normalized level 3 IllumiaHiSeq RNASeqV2 microarray gene expression data in patients with EC and clinical data of TCGA datasets to evaluate TRPM4 mRNA expression in different subtypes of human EC. In total, 491 primary EC patients with detailed TRPM4 expression and whole clinical data were chosen from the database and 35 normal EC patients were excluded. Boxplots were used to visualize expression differences for discrete variables. TRPM4 high/low mRNA expression was retrieved and identified according to median value of gene expression.
Regents
TRPM4, N-cadherin, cytokeratin, vinmentin antibodies were purchased from Abcam (Cambridge, MA, USA). P53, E-cadherin, PI3K, phospho-PI3K, AKT, phospho-AKT, mTOR, phospho-mTOR antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Estrogen (soluble complexes) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Estrogen receptor antagonist ICI 182780 was purchased from Selleck (Shanghai, China). Opti-MEM medium and Lipofectamine RNAiMAX reagent were purchased from Thermo Fisher Scientific (Carlsbad, CA, USA).
Cell culture
The endometrial carcinoma cell line AN3CA was obtained from laboratory stocks and cultured in DMEM/F12 (Thermo Fisher Scientific) medium supplemented with 10% fetal bovine serum (Thermo Fisher Scientific). Cells were cultivated at 37°C, 5% CO2 and subcultured every 3 days, and used for the following experiments. In estrogen-stimulated experiments, to avoid the potential interference of hormones that were derived from the medium, the cells were cultured in phenol-red-free DMEM/F12 (HyClone) containing 10% dextran-charcoal fetal bovine serum (Biochrom AG, Berlin, Germany) for 24 hours before treatment.
SiRNA plasmid transfections
The short interfering RNA (siRNA) against TRPM4 and negative control plasmid siRNA (NC) were chemically synthesized by Gene Pharma (Suzhou, China). Plasmid transfections were performed using Opti-MEM medium and Lipofectamine RNAiMAX reagent according to the manufacturer’s instructions. AN3CA cells were transfected with TRPM4-siRNA or the corresponding control vector, respectively, for RNA interference experiments. SiRNA sequences were as follows: TRPM4 siRNA sense: 5’-GCACGACGUUCAUAGUUGATT-3’ and antisense: 3’-GACGUGCUGCAAGUAUCAACU-5’.
Gene set enrichment analysis (GSEA)
The collected 491 EC samples from RNA-sequencing data were divided into 2 groups according to the median value of the expression of TRPM4 (high vs low expression). GSEA 3.0 was used to analyze data. C2.all.v6.2.symbols.gmt and hallmark.all.v6.2.symbols.gmt data clusters were downloaded from the GSEA website. GSEA analysis was carried out to elucidate the significant survival difference observed between high and low TRPM4 groups. Enri-chment analysis was performed on the sorted samples using default weighted enrichment statistics. Random assortment times were set to 1,000. Discovery Rate (FDR) was calculated. A gene set was considered significantly enriched when the FDR score was <0.25.
Western blotting analysis
AN3CA cells were harvested and lysed, and western blots were conducted as previously described [15]. Thirty micrograms of protein were run on SDS-PAGE (polyacrylamide gel electrophoresis) and transferred to NC (nitrocellulose) filter membranes. The membranes were incubated with TRPM4 (at 1:1,000 dilution), p53 (at 1:1,000 dilution), cytokeratin (at 1:1,000 dilution), E-cadherin (at 1:1,000 dilution), N-cadherin (at 1:1,000 dilution), vinmentin (at 1:1,000 dilution), cytokeratin (at 1:1,000 dilution), AKT (at 1:1,000 dilution), p-AKT (at 1:1,000 dilution), PI3K (at 1:1,000 dilution), p-PI3K (at 1:1,000 dilution), mTOR and p-mTOR (at 1:1,000 dilution) specific antibodies, followed by horseradish peroxidase-conjugated secondary antibody [(ZSGB-BIO, China)]. The ECL (Applygen, China) substrate was used to detect expression. The band intensities were analyzed using the Bio-Rad imaging system (Hercules, CA, USA).
Flow cytometry assay
At the indicated time points, AN3CA cells were trypsinized and washed with ice-cold PBS. For cell cycle distribution analysis, the cells were fixed with 70% ethanol and stained with PI/RNase/PBS (100 µg/mL PI and 10 µg/mL RNase A) buffer for 30 min at room temperature in the dark. The stained single cell suspension was analyzed on a BD LSRFortessa SORP flow cytometer (BD Biosciences, USA). Flow cytometry data were analyzed using the ModFit LT software (Verity Software House, Topsham, ME, USA).
In vitro cell proliferation assay
AN3CA cells of different groups were seeded in a 96-well plate [Corning, USA] at a density of 1,000 cells/200 µl/well in quadruplicate and maintained in DMEM/F12 containing 10% dextran-charcoal fetal bovine serum and evaluated following a period of incubation (overnight, day 3 and day 4). After removing the medium, 100 µl 10% Cell Counting Kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added to each well and incubated for an additional 2 h at 37°C according to the manufacturer’s instructions. Subsequently, cell viability was determined at 450 nm using a spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA).
In vitro cell migration assay
AN3CA Cells at a density of 5 × 105/ml/well were seeded into 6-well plates [Corning, USA] and cultured overnight at 37°C to form a confluent monolayer. Cells were artificially scraped with a 200 µl pipette tip and washed three times with PBS. Images were taken at 0 and 24 h and analyzed using Image J software (Rawak Software, Inc. Germany).
Transwell migration assay
Chamber dishes (Corning, USA) were prepared as previously described [16]. SiTRPM4 transfected and nonspecific-transfected AN3CA cells at a density of 105 cells per well were added to the upper chamber and then incubated for 36 hours. The cells were fixed with 4% paraformaldehyde for 30 minutes, stained with 0.1% crystal violet for 30 minutes, washed 3 times with PBS, and counted in 6 fields under the microscope. All the experiments were performed 3 times.
Statistics
Data was presented as mean ± standard deviation (SD) or median (interquartile) for continuous variables, and as frequency or percentage for categorical variables. The relationship between clinical pathologic features and the expression of TRPM4 were analyzed with the Wilcoxon signed-rank test and logistic regression. Clinicopathologic characteristics associated with survival status or recurrence-free survival/overall survival in TCGA patients using Cox regression and the Kaplan-Meier method. Multivariate Cox analysis was used to compare the influence of TRPM4 expression on survival along with other clinical characteristics (clinical stages, tumor grade, tumor status, lymph node metastasis, mymetrial invasion, and distant metastasis). The cut-off value of TRPM4 expression was determined by its median value. P<0.05 was considered statistically significant. All confidence intervals (CIs) were stated at the 95% confidence level. All statistical analyses were conducted using SPSS 21.0 (IBM, California, USA).
Results
Patient statistics
Table 1 shows 491 patients with both clinical and gene expression data from the TCGA endometrial cancer. Average age of this patient cohort at diagnosis was 63.82 ± 11.09 years (range from 31 to 90). Stage I was found in 315 patients (64.15%), stage II in 46 (9.37%), stage III in 105 (21.38%) and stage IV in 25 (5.09%). Grade 1, grade 2 and grade 3 comprised 19.96%, 23.22%, 56.82%, respectively. For the patients’ tumor status, 400 patients (81.47%) were tumor free and 91 patients (18.53%) were living with tumor. Patients whose myometrial invasion <50% account for 59.47% and ≥50% myometrial invasion account for 40.53%. Surgical approach of the majority of the patients (60.08%) was open surgery, and the rest went for minimally invasive surgery (39.92%). 73 patients (14.87%) had lymph node (pelvic and para-aortic) metastasis and 20 patients (4.07%) had distant metastasis.
Table 1.
Characteristics of TCGA endometrial carcinoma patient
| Clinical characteristics | Total (n=491) | Percentage (%) | |
|---|---|---|---|
| Age (Years) | 63.82 ± 11.09 | ||
| Clinical stage | Stage I | 315 | 64.15 |
| Stage II | 46 | 9.37 | |
| Stage III | 105 | 21.38 | |
| Stage IV | 25 | 5.09 | |
| Tumor grade | G1 | 98 | 19.96 |
| G2 | 114 | 23.22 | |
| G3 | 279 | 56.82 | |
| Tumor status | Tumor free | 400 | 81.47 |
| With tumor | 91 | 18.53 | |
| Myometrial invasion | <50% | 292 | 59.47 |
| ≥50% | 199 | 40.53 | |
| Surgical approach | Mini invasive | 196 | 39.92 |
| Open | 295 | 60.08 | |
| Lymph node metastasis (pelvic or para-aortic) | Negative | 418 | 85.13 |
| Positive | 73 | 14.87 | |
| Distant metastasis | Negative | 471 | 95.93 |
| Positive | 20 | 4.07 |
TCGA, The Cancer Genome Atlas; G1/2/3, grade 1/2/3.
Poor clinical outcomes and survival was associated with down-regulated TRPM4
A total of 491 EC samples with TRPM4 expression and clinical data were analyzed from TCGA. Normal endometrial samples were excluded. As shown in Figures 1 and S1, decreased expression of TRPM4 varied significantly depending on the clinical stage (P=0.002), tumor grade (P<0.001), tumor status (P<0.001), lymph node metastasis (P<0.001), myometrial invasion (P<0.001), and distant metastasis (P<0.001). Univariate analysis of TRPM4 expression as a categorical dependent variable (based on median expression value 10.8) was correlated with poor prognostic clinicopathologic characteristics (Table 2). Decreased TRPM4 expression in EC had significantly been associated with advanced stage (OR=0.208 for Stage I vs Stage IV), high grade (OR=0.133 for G1/2 vs G3), living with tumor (OR=0.215 for Tumor free vs With tumor), deep myometrial invasion (OR=0.358 for <50% vs ≥50%), lymph node metastasis (OR=0.296 for Negative vs Positive), and distant metastasis (OR=0.318 for Negative vs Positive). These results indicated that reduced TRPM4 tended to progress with a poor prognosis in EC patients.
Figure 1.
Down regulation of TRPM4 is significantly related with poor prognosis in TCGA. (A) TRPM4 expression in clinical stage I/II/III/IV. (B) TRPM4 expression in tumor grade 1/2/3. (C) TRPM4 expression in patients with tumor or tumor free status. (D) TRPM4 expression in people with or without lymph node metastasis. (E) Kaplan-Meier curve showing that low TRPM4 expression group is significantly associated with recurrence-free survival and (F) overall survival (491 patients with exact follow-up data were all included). *, P<0.05; **, P<0.01; ***, P<0.001. TCGA, The Cancer Genome Atlas; G1/2/3, grade 1/2/3.
Table 2.
TRPM4 expression associated with clinical pathological characteristics in TCGA
| Clinical characteristics | Odds ratio in TRPM4 expression | P-value |
|---|---|---|
| Clinical stage (Stage I vs Stage IV) | 0.208 (0.081-0.535) | <0.001* |
| Tumor grade (G1+G2 vs G3) | 0.133 (0.088-0.200) | <0.001* |
| Tumor status (Tumor Free vs With Tumor) | 0.251 (0.149-0.421) | <0.001* |
| Myometrial invasion (<50% vs ≥50%) | 0.358 (0.246-0.520) | <0.001* |
| Surgical approach (Mini Invasive vs Open) | 1.193 (0.831-1.713) | 0.338 |
| Lymph node metastasis (Negative vs Positive) | 0.296 (0.170-0.517) | <0.001* |
| Distant metastasis (Negative vs Positive) | 0.318 (0.114-0.889) | 0.022 |
The statistically significant values are with *, categorical dependent variable, greater or less than the TRPM4 median expression level. TCGA, The Cancer Genome Atlas; G1/2/3, grade 1/2/3.
Low expression of TRPM4 was an independent risk factor in multivariate analysis for survival
A total of 419 EC patients with both TRPM4 expression and survival data were available for analysis. As shown in Figure 1E and 1F, Kaplan-Meier survival analysis indicated that low TRPM4 expression was associated with worse recurrence free survival (RFS, P=0.002) and overall survival (OS, P<0.001), with a median survival of 112 months. When taking survival status as a categorical dependent variable factor (Table S1), univariate analysis showed that low TRPM4 expression correlated significantly with poor OS (HR=0.340; 95% CI: 0.202-0.573, P<0.001). Other clinicopathologic variables associated with poor survival included advanced clinical stage, higher tumor grade, with tumor status, deep myometrial invasion, positive lymph node, and distant metastasis.
When put all these significant indexes into a multivariate analysis (Table 3), TRPM4 expression remained an independent prognostic variable for overall survival, with an HR of 0.413, along with clinical stage and tumor status.
Table 3.
Relationship between clinicopathologic features and overall survival in patients by Cox regression. b. Multivariate analysis after variable selection
| Clinicopathologic variables | HR (95% CI) | P-Value |
|---|---|---|
| a | ||
| Age | 1.056 (1.015-1.100) | 0.008* |
| Expression of TRPM4 (Low vs High) | 0.263 (0.098-0.708) | 0.008* |
| Clinical stage (Stage I vs Stage IV) | 3.889 (1.024-14.777) | 0.046* |
| Tumor grade (G1/G2 vs G3) | 0.540 (0.194-1.502) | 0.238 |
| Tumor status (Tumor Free vs With Tumor) | 16.140 (6.043-43.108) | <0.001* |
| Lymph node metastasis(Negative vs Positive) | 0.867 (0.106-7.074) | 0.894 |
| Myometrial invasion (<50% vs ≥50%) | 1.166 (0.310-4.387) | 0.820 |
| Distant metastasis (Negative vs Positive) | 0.661 (0.084-5.224) | 0.695 |
| b | ||
| Expression of TRPM4 (Low vs High) | 0.332 (0.137-0.804) | 0.015* |
| Clinical stage (Stage I vs Stage IV) | 3.165 (1.012-9.904) | 0.048* |
| Tumor status (Tumor Free vs With Tumor) | 14.153 (5.480-36.550) | <0.001* |
With significant difference.
Blocking TRPM4 promoted cells proliferation and migration in EC cell line
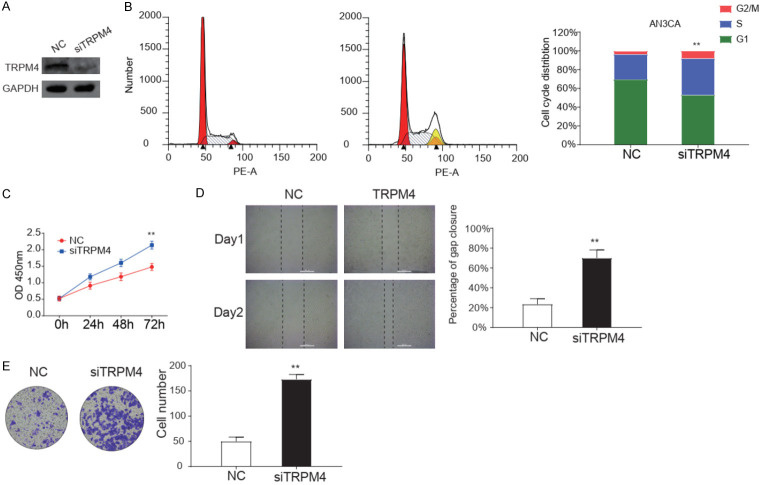
Endogenous TRPM4 and protein levels were examined in five EC cell lines, among which AN3CA, ishikawa, HEC-1A, and HEC-1B cell lines showed significantly higher levels of TRPM4 protein than RL9-52 cells (Figure 3A). AN3CA cell line was chosen for further study. We then assessed the biological functions of TRPM4 by performing the methods of deletion in AN3CA cells. The expression of TRPM4 was significantly depleted in AN3CA at protein and RNA level (Figures 2A, S2). TRPM4 significant decreased the portion of G2/M phase, but increased the portion of M phase in AN3CA cells, as demonstrated by cell cycle analysis (Figure 2B). Meanwhile, the CCK-8 assay showed that TRPM4 deletion increased cell viability (Figure 2C). These results indicated that TRPM4 hampered EC cells proliferation primarily by inducing cell cycle arrest. Migration ability affected the metastasis of cancer cells. Wound healing assay revealed that TRPM4 knockdown increased the migration rate in AN3CA cells monolayer. Figure 2D showed the representative images of the migration assays. Transwell migration assay indicated that siRNA-mediated knockdown of TRPM4 cells enhanced cell migration ability compared with the negative control group (Figure 2E). After all, TRPM4 significant inhibited the proliferation and migration of EC AN3CA cells.
Figure 3.
High expression of TRPM4 is enriched in estrogen response. A. Representative images of western blotting analysis of endogenous expression of TRPM4 in a panel of endometrial carcinoma cell lines. B, C. GSEA analysis was conducted to evaluate significant enrichment of genes on the early estrogen response and late estrogen response. D. The expression of TRPM4 decreases with the elevation of estrogen concentration verified by western blot. E. The decreased effect of estrogen on TRPM4 expression is blocked by estrogen antagonist ICI182780 (1 nmol/L).
Figure 2.
Knockdown of TRPM4 significantly increases the malignancy of AN3CA cells. A. Representative images of western blotting analysis of knocking down TRPM4 in protein level in AN3CA cells. B. Cycle distribution analysis of AN3CA cells with negative control or deletion of TRPM4 expression were performed by flow cytometry. C. CCK-8 assay was used to evaluate viability of AN3CA cells after being knocking down TRPM4. D. Wound healing assays for AN3CA cells after transfection of negative control (NC) or siTRPM4 plasmid for 24 h. E. Transwell migration assay for AN3CA cells after transfection for 24 h. Data is based on at least three independent experiments, and shown as the mean ± S.D. (*, P<0.05; **, P<0.01).
GSEA indicated an estrogen-related TRPM4 expression
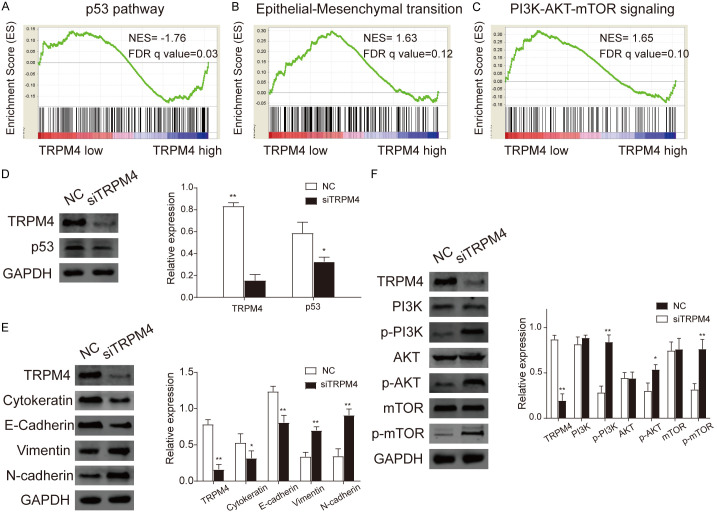
To identify the TRPM4 possible pathway and function in EC, we conducted Gene Set Enrichment Analysis between low and high TRPM4 expression data sets (Table S2). GSEA results revealed that early and late estrogen responses were enriched in high expression of TRPM4 (Figure 3B, 3C). We then confirmed their connection by western blot. Figure 3D showed that the level of TRPM4 was significantly reduced with the increasing concentration of estrogen. Furthermore, we examined whether estrogen receptor antagonist ICI182780 could block the effect of estrogen on TRPM4. As shown in Figure 3E, TRPM4 expression was no longer reduced by estrogen.
TRPM4 silencing increased the epithelial-mesenchymal transition in endometrial cancer cells
GSEA findings indicated that TRPM4 is correlated to epithelial-mesenchymal transition (EMT) (Figure 4B). Therefore, protein expression of EMT markers was investigated by the methods of western blot. E-cadherin, as the most essential epithelial marker, was lower in siTRPM4 cells compared with control group. Accompanied with TRPM4 silencing, N-cadherin and vimentin, the mesenchymal markers, were up-regulated in TRPM4 deleted cells. Moreover, the expression of cytokeratin was decreased significant in the TRPM4-depletion group when compared with the control group (Figure 4E). The correlation analysis between the expression of TRPM4 and E-cadherin (R=0.198, P=0.012) or N-cadherin (R=-0.216, P<0.001) in TCGA revealed the same results (Figure S3). These data demonstrated that TRPM4 silencing increased EMT progression in EC cells.
Figure 4.
A. High expression of TRPM4 is enriched in p53 pathway by GSEA analysis (NES=-1.67, FDR q=0.03). B. GSEA result also shows that Epithelial-Mesenchymal transition is enriched in TRPM4 low expression (NES=1.63, FDR q=0.12). C. PI3K/AKT/mTOR signaling pathway is enriched in TRPM4 low expression (NES=1.65, FDR q=0.10). D. Validation by western blot analysis of AN3CA cells transfected with negative control (NC) or siTRPM4 plasmid in p53 pathway. E. The expression of mesenchymal markers including N-cadherin significantly increased and epithelial markers cytokeratin and E-cadherin decreased in TRPM4 NC and siTRPM4 groups in protein level by western blotting. F. In vitro validation of western blot analysis of AN3CA cells with or without deletion of TRPM4 in PI3K/AKT/mTOR signaling pathway. Results showed that the expression of p-PI3K, p-AKT, p-mTOR were elevated in siTRPM4 group. (*, P<0.05; **, P<0.01).
TRPM4 participated in p53 and PI3K/AKT/mTOR signaling pathway in EC cells
To investigate the signaling pathway which TRPM4 participated in, we examined classic signaling pathway associated with tumor genesis with GSEA (Figure 4A, 4C). Results showed that low TRPM4 expression was enriched in p53 and PI3K/AKT/mTOR signaling pathway. Therefore, we further confirmed the effect of TRPM4 on p53 and PI3K/AKT/mTOR signal pathway in vivo. The outcomes suggested that down-regulation of TRPM4 decreased p53 level and promoted the phosphorylation of PI3K, AKT and mTOR in AN3CA cells (Figure 4D, 4F). The findings indicated that in EC, the expression of TRPM4 was significantly associated with p53 and PI3K/AKT/mTOR.
Discussion
In the present study, we found TRPM4 channel was a calcium-activated nonselective cation channel as it allowed transportation of only monovalent cations (e.g. Na+, K+), hence, modulating the transmembrane electrical potential [17,18]. TRPM4 could also be activated by SOCE, when its signals exceeded a certain threshold [19]. Its activity is responsible of a local increase of intracellular calcium for the membrane depolarization, affecting the driving force for external calcium entry and modulating several intracellular calcium-dependent signaling pathways [20]. Under physiological conditions, TRPM4 is involved in different cellular functions. For instance, TRPM4 participated in insulin secretion, T-cell proliferation, mast cell activation, and dendritic cell migration [21-24]. It is involved in the migration of prostate cancer cells and proliferation of breast cancer cells [5]. Also, TRPM4 has been described as being localized in the focal adhesion (FA) complexes, playing a pivotal role in FA turnover and lamellipodial actin cytoskeleton dynamics [25]. Known functions of TRPM4 also include the prognostic risk of biochemical recurrence after the radical prostatectomy and overexpression of TRPM4 in prostate cancer is also associated with increased risk of recurrence [26]. In the present study, TRPM4 expression was found markedly declined in different phenotypes of EC in TCGA datasets while lower TRPM4 expression was correlated with reduced overall and recurrence-free survival. Moreover, TRPM4-depletion promoted EC cell proliferation and metastasis in vitro and TRPM4 expression declined with the increasing concentration of estrogen. Finally, TRPM4 might influence EC cells functions through EMT, p53 and PI3K/AKT/mTOR pathways. Our results highlighted TRPM4 as the proliferation and metastasis indicator in EC.
TRPM4 transcripts are up-regulated in many types of cancers such as cervical cancer, prostate cancer, B cell lymphoma, colorectal cancer and bladder cancer [27-29]. Moreover, it has also been shown to be up-regulated in prostate cancer associated with migration of prostate cancer [30]. In this study, we conducted a high throughput RNA-sequencing data from TCGA and proved that decreased TRPM4 expression was associated with poor clinicopathologic factors (clinical stage, tumor grade, tumor status, lymph node, myometrial invasion, and distant metastasis) and survival. Multivariate analysis indicated that TRPM4 expression was an independent risk factor. This suggested that TRPM4 may serve as a potential prognostic marker for prognosis and therapeutic target in EC.
The dysregulation of gene expression caused malignant phenotype, including proliferation and migration. In prostate cancer, silence of TRPM4 resulted in significant reduction of proliferation, migration, invasion, through microRNA-150 [31]. Another report demonstrated that TRPM4 was up-regulated in prostate cancer compared with normal prostate epithelium, and it was also a candidate gene which was considered to be involved in the early stage of prostatic carcinogenesis [32]. Adversely, TRPM4-deletion improved the growth, migration ability and drove G1 to S phase cell cycle transition in EC cells. Taken together, these data represented that TRPM4 inhibited proliferation, cell cycle as well as movement of malignant endometrial cells.
High level of estrogen and lacking of progesterone had been proposed as the main hormonal aberrations to affect endometrial proliferation and cell survival that could result in an increased risk of epithelial cell transformation [33]. Among these reasons, long-term exposure to estrogen is the most direct one [34]. Estrogens regulated proliferation, regeneration and function of normal endometrial cells, therefore are implicated in endometrial carcinogenesis directly or via influencing other hormones and metabolic pathways. To further investigate the pathways and functions of TRPM4, we then used GSEA to explore elevated TRPM4 expression groups. GSEA findings demonstrated that early and late estrogen responses were positively associated with high TRPM4 expression. Western blot was then conducted to validate the GSEA findings. The results suggested that the content of TRPM4 decreased with the elevation of estrogen. These effects were inhibited by the ER antagonist ICI182780, indicating that membrane ERs may be involved in these processes. For the first time, we showed that estrogen was a negative regulator of TRPM4 in EC cells, not only by bioinformatics methods, but also in in vitro models.
TRPM4 also acted to maintain endothelial features and its loss promoted fibrotic conversion via TGF-β production [6]. Previous studies showed that TRPM4 increased EMT by inducing the expression of Snail1 gene in prostate cancer [26]. Recently Hong et al. reported that microRNA-150 suppressed EMT, invasion and metastasis in prostate cancer through the TRPM4-mediated β-catenin signaling pathway [27]. The TRPM4 induced up-regulation of β-catenin also enhanced cell proliferation in T-REx 293 cells [35]. We found that low-TRPM4 expression was enriched in EMT by GSEA. Furthermore, we proved that the expression of E-cadherin and cytokeratin were diminished after the TRPM4 depleting in AN3CA cells, meanwhile N-cadherin and vimentin were increased. These findings revealed that TRPM4 negatively regulated EMT in EC.
To detect the functional effects of TRPM4 knockdown, we assessed the gene expression profiles of low and high level of TRPM4 in TCGA by GSEA. Our data indicated that TRPM4 is implicated in the regulation of several essential pathways, including the p53 and PI3K/AKT/mTOR signaling pathways. P53 was a tumor suppressor protein that caused cell cycle arrest in case of DNA damage to allow DNA repair or induction of apoptosis in case of substantial damage. It had been early reported to play an essential role in the progression of many cancers [36]. In patients with EC, p53 pathway stimulates EC cell proliferation and was associated with clinicopathologic features [37]. PI3K/AKT/mTOR signaling pathway activation was heavily implicated in EC pathogenesis [38]. The activation of PI3K and mTOR were two of the most common events in the development of human cancer, including EC [39]. Alteration of the PI3K/AKT/mTOR signaling pathway was involved in EC pathogenesis [40]. Previous studies had demonstrated that the FAM83B and CLDN6 promoted cell proliferation and migration via PI3K/AKT/mTOR signaling pathway [41,42]. Furthermore, our western blot analysis confirmed that when TRPM4 was depleted in EC cells, the activity of p53 was significantly inhibited and phospho-PI3K/AKT/mTOR signaling pathway was strongly activated, as represented by the decreased protein level of p53 and increased protein level of p-PI3K, p-AKT and p-mTOR.
Conclusions
These results indicated that low TRPM4 level was correlated with poor clinical outcomes and survival, and TRPM4 was also an independent risk factor in EC patients. TRPM4 played as a tumor suppressor gene in EC cells and the silencing of TRPM4 promoted proliferation and migration. Estrogen might act as an antagonist to decline the expression of TRPM4. The mechanism of TRPM4 might work through EMT or p53, and PI3K/AKT/mTOR signaling pathway. Finally, it also represented a potential therapeutic target for the EC treatment.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Grant No 81874108, 81802607).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Makker A, Goel MM. Tumor progression, metastasis, and modulators of epithelial-mesenchymal transition in endometrioid endometrial carcinoma: an update. Endocr Relat Cancer. 2016;23:R85–R111. doi: 10.1530/ERC-15-0218. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhry P, Asselin E. Resistance to chemotherapy and hormone therapy in endometrial cancer. Endocr Relat Cancer. 2009;16:363–380. doi: 10.1677/ERC-08-0266. [DOI] [PubMed] [Google Scholar]
- 4.Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094–1108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- 5.Holzmann C, Kappel S, Kilch T, Jochum MM, Urban SK, Jung V, Stockle M, Rother K, Greiner M, Peinelt C. Transient receptor potential melastatin 4 channel contributes to migration of androgen-insensitive prostate cancer cells. Oncotarget. 2015;6:41783–41793. doi: 10.18632/oncotarget.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Echeverria C, Montorfano I, Cabello-Verrugio C, Armisen R, Varela D, Simon F. Suppression of transient receptor potential melastatin 4 expression promotes conversion of endothelial cells into fibroblasts via transforming growth factor/activin receptor-like kinase 5 pathway. J Hypertens. 2015;33:981–992. doi: 10.1097/HJH.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 7.Hao J, Bao X, Jin B, Wang X, Mao Z, Li X, Wei L, Shen D, Wang JL. Ca2+ channel subunit alpha 1D promotes proliferation and migration of endometrial cancer cells mediated by 17beta-estradiol via the G protein-coupled estrogen receptor. FASEB J. 2015;29:2883–2893. doi: 10.1096/fj.14-265603. [DOI] [PubMed] [Google Scholar]
- 8.Liu L, Lin J, He H. Identification of potential crucial genes associated with the pathogenesis and prognosis of endometrial cancer. Front Genet. 2019;10:373. doi: 10.3389/fgene.2019.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saitoh M, Ohmichi M, Takahashi K, Kawagoe J, Ohta T, Doshida M, Takahashi T, Igarashi H, Mori-Abe A, Du B, Tsutsumi S, Kurachi H. Medroxyprogesterone acetate induces cell proliferation through up-regulation of cyclin D1 expression via phosphatidylinositol 3-kinase/Akt/nuclear factor-kappaB cascade in human breast cancer cells. Endocrinology. 2005;146:4917–4925. doi: 10.1210/en.2004-1535. [DOI] [PubMed] [Google Scholar]
- 10.Sathe A, Nawroth R. Targeting the PI3K/AKT/mTOR pathway in bladder cancer. Methods Mol Biol. 2018;1655:335–350. doi: 10.1007/978-1-4939-7234-0_23. [DOI] [PubMed] [Google Scholar]
- 11.Fujiwara M, Izuishi K, Sano T, Hossain MA, Kimura S, Masaki T, Suzuki Y. Modulating effect of the PI3-kinase inhibitor LY294002 on cisplatin in human pancreatic cancer cells. J Exp Clin Cancer Res. 2008;27:76. doi: 10.1186/1756-9966-27-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hubbard PA, Moody CL, Murali R. Allosteric modulation of ras and the PI3K/AKT/mTOR pathway: emerging therapeutic opportunities. Front Physiol. 2014;5:478. doi: 10.3389/fphys.2014.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sagredo AI, Sagredo EA, Cappelli C, Baez P, Andaur RE, Blanco C, Tapia JC, Echeverria C, Cerda O, Stutzin A, Simon F, Marcelain K, Armisen R. TRPM4 regulates Akt/GSK3-beta activity and enhances beta-catenin signaling and cell proliferation in prostate cancer cells. Mol Oncol. 2018;12:151–165. doi: 10.1002/1878-0261.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Y, Liao P. TRPM4 channel and cancer. Cancer Lett. 2019;454:66–69. doi: 10.1016/j.canlet.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Li X, Zhao L, Zhang L, Zhang G, Wang J, Wei L. Nongenomic effect of estrogen on the MAPK signaling pathway and calcium influx in endometrial carcinoma cells. J Cell Biochem. 2009;106:553–562. doi: 10.1002/jcb.22017. [DOI] [PubMed] [Google Scholar]
- 16.Bao XX, Xie BS, Li Q, Li XP, Wei LH, Wang JL. Nifedipine induced autophagy through Beclin1 and mTOR pathway in endometrial carcinoma cells. Chin Med J (Engl) 2012;125:3120–3126. [PubMed] [Google Scholar]
- 17.Nilius B, Prenen J, Droogmans G, Voets T, Vennekens R, Freichel M, Wissenbach U, Flockerzi V. Voltage dependence of the Ca2+-activated cation channel TRPM4. J Biol Chem. 2003;278:30813–30820. doi: 10.1074/jbc.M305127200. [DOI] [PubMed] [Google Scholar]
- 18.Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. doi: 10.1016/s0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- 19.Kilch T, Kappel S, Peinelt C. Regulation of Ca(2+) signaling in prostate cancer cells. Channels (Austin) 2016;10:170–171. doi: 10.1080/19336950.2015.1137176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guinamard R, Demion M, Launay P. Physiological roles of the TRPM4 channel extracted from background currents. Physiology (Bethesda) 2010;25:155–164. doi: 10.1152/physiol.00004.2010. [DOI] [PubMed] [Google Scholar]
- 21.Cheng H, Beck A, Launay P, Gross SA, Stokes AJ, Kinet JP, Fleig A, Penner R. TRPM4 controls insulin secretion in pancreatic beta-cells. Cell Calcium. 2007;41:51–61. doi: 10.1016/j.ceca.2006.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Launay P, Cheng H, Srivatsan S, Penner R, Fleig A, Kinet JP. TRPM4 regulates calcium oscillations after T cell activation. Science. 2004;306:1374–1377. doi: 10.1126/science.1098845. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu T, Owsianik G, Freichel M, Flockerzi V, Nilius B, Vennekens R. TRPM4 regulates migration of mast cells in mice. Cell Calcium. 2009;45:226–232. doi: 10.1016/j.ceca.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Barbet G, Demion M, Moura IC, Serafini N, Leger T, Vrtovsnik F, Monteiro RC, Guinamard R, Kinet JP, Launay P. The calcium-activated nonselective cation channel TRPM4 is essential for the migration but not the maturation of dendritic cells. Nat Immunol. 2008;9:1148–1156. doi: 10.1038/ni.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caceres M, Ortiz L, Recabarren T, Romero A, Colombo A, Leiva-Salcedo E, Varela D, Rivas J, Silva I, Morales D, Campusano C, Almarza O, Simon F, Toledo H, Park KS, Trimmer JS, Cerda O. TRPM4 is a novel component of the adhesome required for focal adhesion disassembly, migration and contractility. PLoS One. 2015;10:e0130540. doi: 10.1371/journal.pone.0130540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berg KD, Soldini D, Jung M, Dietrich D, Stephan C, Jung K, Dietel M, Vainer B, Kristiansen G. TRPM4 protein expression in prostate cancer: a novel tissue biomarker associated with risk of biochemical recurrence following radical prostatectomy. Virchows Arch. 2016;468:345–355. doi: 10.1007/s00428-015-1880-y. [DOI] [PubMed] [Google Scholar]
- 27.Schinke EN, Bii V, Nalla A, Rae DT, Tedrick L, Meadows GG, Trobridge GD. A novel approach to identify driver genes involved in androgen-independent prostate cancer. Mol Cancer. 2014;13:120. doi: 10.1186/1476-4598-13-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loo SK, Ch’ng ES, Md Salleh MS, Banham AH, Pedersen LM, Moller MB, Green TM, Wong KK. TRPM4 expression is associated with activated B cell subtype and poor survival in diffuse large B cell lymphoma. Histopathology. 2017;71:98–111. doi: 10.1111/his.13204. [DOI] [PubMed] [Google Scholar]
- 29.Sozucan Y, Kalender ME, Sari I, Suner A, Oztuzcu S, Arman K, Yumrutas O, Bozgeyik I, Cengiz B, Igci YZ, Balakan O, Camci C. TRP genes family expression in colorectal cancer. Exp Oncol. 2015;37:208–212. [PubMed] [Google Scholar]
- 30.Sagredo AI, Sagredo EA, Pola V, Echeverria C, Andaur R, Michea L, Stutzin A, Simon F, Marcelain K, Armisen R. TRPM4 channel is involved in regulating epithelial to mesenchymal transition, migration, and invasion of prostate cancer cell lines. J Cell Physiol. 2019;234:2037–2050. doi: 10.1002/jcp.27371. [DOI] [PubMed] [Google Scholar]
- 31.Hong X, Yu JJ. MicroRNA-150 suppresses epithelial-mesenchymal transition, invasion, and metastasis in prostate cancer through the TRPM4-mediated beta-catenin signaling pathway. Am J Physiol Cell Physiol. 2019;316:C463–C480. doi: 10.1152/ajpcell.00142.2018. [DOI] [PubMed] [Google Scholar]
- 32.Ashida S, Nakagawa H, Katagiri T, Furihata M, Iiizumi M, Anazawa Y, Tsunoda T, Takata R, Kasahara K, Miki T, Fujioka T, Shuin T, Nakamura Y. Molecular features of the transition from prostatic intraepithelial neoplasia (PIN) to prostate cancer: genome-wide gene-expression profiles of prostate cancers and PINs. Cancer Res. 2004;64:5963–5972. doi: 10.1158/0008-5472.CAN-04-0020. [DOI] [PubMed] [Google Scholar]
- 33.Kamal A, Tempest N, Parkes C, Alnafakh R, Makrydima S, Adishesh M, Hapangama DK. Hormones and endometrial carcinogenesis. Horm Mol Biol Clin Investig. 2016;25:129–148. doi: 10.1515/hmbci-2016-0005. [DOI] [PubMed] [Google Scholar]
- 34.Edey KA, Rundle S, Hickey M. Hormone replacement therapy for women previously treated for endometrial cancer. Cochrane Database Syst Rev. 2018;5:CD008830. doi: 10.1002/14651858.CD008830.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armisen R, Marcelain K, Simon F, Tapia JC, Toro J, Quest AF, Stutzin A. TRPM4 enhances cell proliferation through up-regulation of the beta-catenin signaling pathway. J Cell Physiol. 2011;226:103–109. doi: 10.1002/jcp.22310. [DOI] [PubMed] [Google Scholar]
- 36.Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer. 2009;9:701–713. doi: 10.1038/nrc2693. [DOI] [PubMed] [Google Scholar]
- 37.Jongen VH, Briet JM, de Jong RA, Joppe E, ten Hoor KA, Boezen HM, Evans DB, Hollema H, van der Zee AG, Nijman HW. Aromatase, cyclooxygenase 2, HER-2/neu, and p53 as prognostic factors in endometrioid endometrial cancer. Int J Gynecol Cancer. 2009;19:670–676. doi: 10.1111/IGC.0b013e3181a47c25. [DOI] [PubMed] [Google Scholar]
- 38.Lin Q, Wang Y, Chen D, Sheng X, Liu J, Xiong H. Cisplatin regulates cell autophagy in endometrial cancer cells via the PI3K/AKT/mTOR signalling pathway. Oncol Lett. 2017;13:3567–3571. doi: 10.3892/ol.2017.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15:5308–5316. doi: 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slomovitz BM, Coleman RL. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin Cancer Res. 2012;18:5856–5864. doi: 10.1158/1078-0432.CCR-12-0662. [DOI] [PubMed] [Google Scholar]
- 41.Lin Q, Chen H, Zhang M, Xiong H, Jiang Q. Knocking down FAM83B inhibits endometrial cancer cell proliferation and metastasis by silencing the PI3K/AKT/mTOR pathway. Biomed Pharmacother. 2019;115:108939. doi: 10.1016/j.biopha.2019.108939. [DOI] [PubMed] [Google Scholar]
- 42.Cao X, He GZ. Knockdown of CLDN6 inhibits cell proliferation and migration via PI3K/AKT/mTOR signaling pathway in endometrial carcinoma cell line HEC-1-B. Onco Targets Ther. 2018;11:6351–6360. doi: 10.2147/OTT.S174618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.