Abstract
De-differentiated liposarcoma (DDLPS) is a rare cancer with high rates of recurrence and metastasis. Currently, treatment with doxorubicin-ifosphamide, following surgical resection, is routinely performed. However, clinical treatment of these refractory cancers require further study. We investigated the treatment of mesenchymal stromal cells (MSC) transduced with dodecameric tumor necrosis factor receptor apoptosis-inducing ligand (dTRAIL) and herpes simplex virus thymidine kinase (HSV-TK) (MSC-TR/TK), as a method to approach DDLPS therapy. First, in order to assess the efficacy of this therapy, cell viability was evaluated by apoptosis analysis of a DDLPS cell line co-cultured with patient-derived cells (PDCs) and MSC-TR/TK in vitro. In vivo, we established a lung metastasis model using the DDLPS cell line and assessed the anti-tumorigenic efficiency of dTRAIL-TK by injecting MSC-TR/TK. Results confirmed that liposarcoma cells resistant to dTRAIL in PDCs, transformed by HSV-TK, induced apoptosis effectively after treatment with toxic ganciclovir (GCV). Meanwhile, we observed that treatment of GCV after injection of MSC-TR/TK effectively eliminated lung nodules in a lung metastasis model established from LPS246 cells resistant to dTRAIL. When mice were treated with GCV two days after double injection with MSC-TR/TK, the tumor suppression effect was even more pronounced.
Keywords: DDLPS, PDC, dTRAIL, HSV-TK, MSC-TR/TK, GCV
Introduction
Liposarcoma (LPS) is the most prevalent soft tissue sarcoma. Subtypes of LPS, well-differentiated liposarcoma (WDLPS) and dedifferentiated liposarcoma (DDLPS), have been genetically characterized by amplification of MDM2 and CDK4, localized in the 12q13-q15 region [1,2]. WDLPS has characteristics of an atypical tumor, whereas DDLPS is more aggressive, and can invade locally, as well as metastasize. A poor prognosis is associated with a DDLPS diagnosis. DDLPS has a local recurrence rate of over 80%, a 20-30% rate of distant metastasis, and a 40-60% 5-year overall survival rate [3]. Nonetheless, there is no definitive treatment for DDLPS, except surgical resection.
Herpes simplex virus thymidine kinase (HSV-TK)-mediated suicide gene therapy has been a widely-accepted strategy for cancer [4]. HSV-TK metabolizes the nontoxic nucleoside analog ganciclovir (GCV) to a toxic tri-phosphorylated form, causing apoptosis of target cells transduced by HSV-TK, as well as that of rapidly-dividing bystander cells [5]. TRAIL is a homotrimeric, type II transmembrane protein that induces apoptosis through both intrinsic and extrinsic death pathways [6]. TRAIL is known to induce selective apoptosis of transformed cells, without affecting normal cells [7]. Clinical applications of TRAIL as an anticancer agent have increased drastically. MSC-delivered TRAIL overcomes the limited half-life of TRAIL by systemically delivering recombinant TRAIL [8-10]. The effect of LPS on TRAIL appears to be heterogeneous [11]. Therefore, in the case of LPS resistance to TRAIL, a concomitant treatment is required to overcome the resistance. A previous study showed that TRAIL enhanced the efficacy of HSV-TK/GCV therapy by augmenting both target and bystander killing effects [11,12], further supporting the potential clinical use of combined treatment with HSV-TK/GCV gene therapy and TRAIL-based therapy.
In the present study, we analyzed the therapeutic effects of MSC-mediated combination gene therapy of TRAIL and HSV-TK in a human liposarcoma cell line and experimental model of lung metastasis. After establishing an in vivo model system, we assessed antitumor effects of engineered MSCs co-expressing dodecameric TRAIL (dTRAIL) and HSV-TK. Upon in vivo administration, engineered MSCs were found to be specifically localized in the lung, and dTRAIL and HSV-TK secreted in conjunction with GCV enhanced killing activity against the target liposarcoma. Also, we used two injections of MSC-TR/TK into liposarcoma-bearing mice to maximize the effect of dTRAIL and HSV-TK as anticancer drugs. Although clinical studies are required, the encouraging results from this study shows that MSC-TR/TK could be a therapeutic approach for treatment of liposarcoma patients with distant metastasis and TRAIL resistance.
Materials and methods
Cells and reagents
Human recombinant TRAIL protein (rhTRAIL) was purchased from R&D Systems (R&D Systems, Minneapolis, MN, USA). The isolation of mesenchymal stromal cells (MSC) from human tissues has been described previously [13]. The established DDLPS cell lines, LPS246 were kindly provided by Dr. Dina Lev (The University of Texas, MD Anderson Cancer Center, Houston). DDLPS patient-derived cells (PDCs; 11GS-079, 11GS-099, 11GS-106, 14GS-076) were isolated from the tumor tissues of patients from Samsung Medical Center (SMC; Korea republic) (Table 1). The tumor material was excised aseptically, then processed for primary tissue cultivation, as described previously. DDLPS PDCs were characterized via fluorescence in situ hybridization (FISH), karyotyping, and array comparative genomic hybridization (CGH) [14].
Table 1.
Case materials
| Case | No. | Sex | Age | Pathological Diagnosis | Disease status |
|---|---|---|---|---|---|
| 1 | 11GS-079 | M | 67 | DDLPS | Retroperitonium |
| 2 | 11GS-099 | M | 62 | DDLPS | Retroperitonium |
| 3 | 11GS-106 | F | 74 | DDLPS | Retroperitonium |
| 4 | 14GS-076 | F | 51 | DDLPS | Retroperitonium |
Adenoviruses and transduction
All the engineered MSC used were produced as in previous studies [12]. All rAd concentrations used for the transduction of cells are provided as multiplicity of infection (MOI) in plaque-forming unit per cells (PFU/cell). For MSC transduction, a mixture of 20 MOI of rAd and 50 µmol/L of Fe3+ was preincubated in serum-free medium at room temperature for 30 minutes, then infected into MSCs for 30 minutes. Transduction efficiency of adenovirus in MSCs was about 80 percent. Levels of secreted TRAIL from MSC/dTRAIL-TK (MSC-TR/TK) culture supernatants were analyzed by enzyme-linked immunosorbent assay (ELISA) using purified anti-human TRAIL antibody (Peprotech, Rocky Hill, New Jersey USA) and biotinylated anti-human TRAIL antibody (Peprotech, Rocky Hill, New Jersey USA), according to the manufacturer’s instructions. To measure the concentration of TRAIL secreted from MSC-TR/TK upon GCV (Sigma, St. Louis, MO, USA) treatment, culture supernatants were harvested every day and changed, with addition of fresh GCV. Collected supernatant were subjected to ELISA as described earlier. To analyze the gene expression of HSV-TK, reverse transcription PCR assays were conducted using primers for codon optimized HSV-TK cDNA, (forward, 5’-GCCTTCGACCAGGCCGCTAG-3’ and reverse, 5’-CCATGCCGTGGGGTCCATCG-3’).
In vitro viability assay
To measure the effects of dTRAIL-TK on MSC viability, MSC were transduced with different doses (0, 10, 50, and 100 MOI) of rAd/mock and rAd/dTRAIL-TK and allowed to grow for three days. Cell viability was measured with a cell counting kit (CCK8, Dojindo Molecular Technology, Kumamoto, Japan). The cytotoxicity of GCV results on engineered MSCs were the same as in previous [11].
In vitro co-culture assay
LPS246 and patient-derived cells (PDCs) were labeled with 0.2 µmol/L DiIC18(5)-DS (Invitrogen, Waltham, MA, USA) for 1 h at 37°C, then directly co-cultured with MSC-TR/TK, and MSC-Mock at a ratio of 1:1 for 48 hours (2 × 105 cells of each cell). After treatment with 100 µmol/L GCV for 48 hours, all floating and adherent cells were harvested. Cells were stained with 5 mL of allophycocyanin-conjugated annexin V (eBioscience, San Diego, CA, USA) and 7-aminoactinomycin D (7-AAD; BD Biosciences, Franklin Lakes, NJ, USA) in 100 mL binding buffer, and analyzed by Calibur flow cytometer (FACS; BD Biosciences, Franklin Lakes, NJ, USA) to determine the proportion of apoptotic tumor cells.
Animal model and in vivo MSC migration assay
NOD.Cg-PrkdcscidIl2rgtmlWjl/Sz (NOD-SCID IL2rγnull; NSG) mice were obtained from the Genexine (Seoul, Korea Republic). To establish experimental lung metastases, 1 × 106 of LPS246 cells were intravenously injected into the lateral tail vein. To investigate the antitumor effects of engineered MSCs in the metastatic LPS model, mice were injected intravenously with 1 × 106 of MSC-TR/TK or MSC-Mock cells into the tail vein. Antitumor effects of sequential therapy were evaluated by additional injections of 1 × 106 of MSC-TR/TK cells were made at two week intervals. GCV at 50 mg/kg was injected intraperitoneally for five consecutive days, starting on days 2 and 16; or days 2 and 7. After treatment with engineered MSC. On day 63 after the tumor injection, lungs of tumor-bearing mice were isolated, stained with picric acid, fixed in acetic acid solution, and the number of metastatic tumor nodules on the lung surface was counted. The mass of tumor tissue was also weighed at this time. The NSG mice were weighed every three days. Mice were humanely euthanized 8-9 weeks post-tumor implantation, and tumor-bearing lungs, livers and kidneys were weighed. The tumor-bearing organs were embedded in paraffin and examined histologically.
Statistics
One-way analysis of variance (ANOVA) was used to compare tumor mass, followed by Tukey post-hoc multiple comparison tests. All data are presented as the mean ± standard deviation (SD). Statistical analysis was performed using GraphicPad Prism5, and a probability value of < 0.05 was considered statistically significant.
Result
Engineered MSC expressing HSV-TK and secreting TRAIL proteins
We analyzed apoptosis to evaluate the cytotoxic effect toward MSCs themselves. Cytotoxicity resulting from expression of TRAIL was also measured. To obtain these data, we developed transduction conditions in vitro and in vivo using MSC-TR/TK. First, ELISA was performed to detect TRAIL secreted into the media (Figure 1A). Based on previous data [11], MSCs (3 × 104 in 300 μL serum free media) with respective MOI values were incubated with 50 μmol/L of Fe3+ for 48 hours. TRAIL secreted from the engineered MSCs was recovered from the media and measured. The amount of TRAIL secreted was dependent on the MOI (from 200 ng/mL in 10 MOI to 780 ng/mL in 100 MOI). Next, correlation between TRAIL concentration and apoptosis was determined (Figure 1B). MSC was found to dose-dependently lower viability by expression of TRAIL. Using FACS, significant results were observed with early stage apoptosis (7AAD) and late stage apoptosis (annexin V) (Figure 1C). These data showed low levels of viability in the TR/TK_50 MOI and 100 MOI groups, which secreted high levels of of TRAIL. For these reasons, we chose the engineered MSC with 12% apoptosis and a 70% survival rate, resulting from a 20 MOI viral dose, for in vitro assays.
Figure 1.
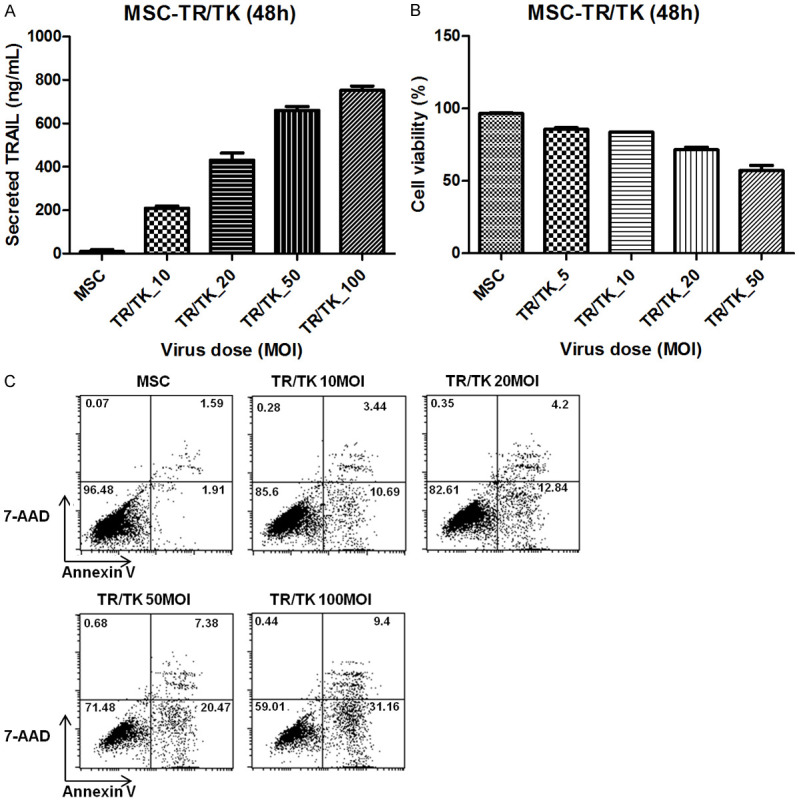
Comparison of secreted TRAIL and cell viability. MSC was transduced with increasing rAd/dTRAIL concentrations in the presence of Fe3+ (50 µmol/L) for 30 minutes. Forty eight hours after transduction, the amount of TRAIL secreted by each virus dose was measured by ELISA (A). Varying doses of virus (10, 20, 50, 100 MOI) were used to treat MSCs, and cell viability of engineered MSC was measured by the Cell Counting Assay Kit 8 48 hours later (B). The percentage of apoptosis in engineered MSC cells caused by TRAIL was quantified by 7AAD (early apoptosis) and annexin V (late apoptosis) (C).
Engineered MSC inducing apoptosis in LPS cell line and PDCs
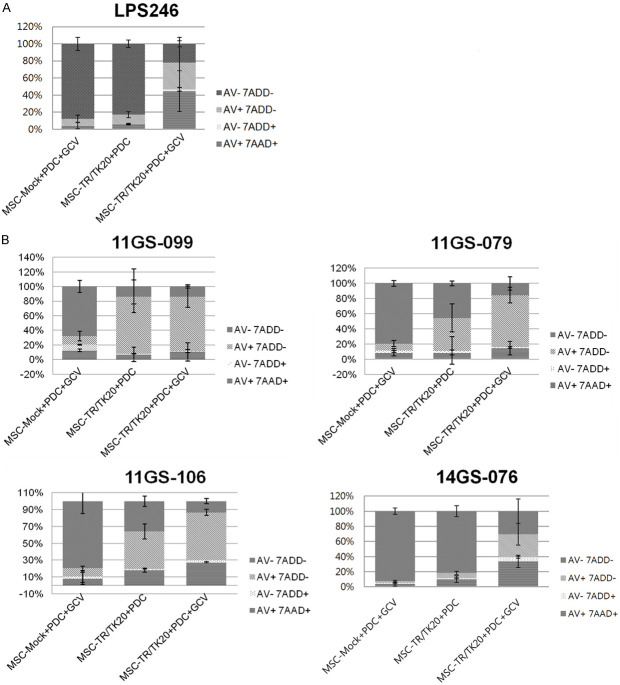
We investigated the effect on TRAIL in the liposarcoma cell line and PDCs (patient derived cells) co-cultured with engineered MSC (Mock and dTRAIL-TK expressing). In order to confirm whether engineered MSCs induced apoptosis of liposarcoma cells in vitro, the cells were co-cultured for 48 h with 100 μmol/L of GCV, based on previous data [11]. The LPS246 cell line showed resistance to TRAIL (~20%). However, when the cells were treated with GCV to induce TK, the induced toxic form of triphosphorylated GCV, apoptosis was significantly increased (~80%) in this cell line (Figures 2A, S1A). On the other hand, PDCs (11GS079, 11GS099, and 11GS106) showed high levels of apoptosis (53%~85%) after treatment with engineered MSCs, except for 14GS076 (Figures 2B, S1B, S1C). This result suggests that PDCs were sensitive to TRAIL, while 14GS076, which was resistant to TRAIL, induced apoptosis (~64%) by GCV treatment in LPS246 cells. From these results, we found that the resistance to TRAIL could be overcome by inducing apoptosis through transduction of GCV activation by TK where have resistance to TRAIL.
Figure 2.
Comparison of the percentage of apoptosis in the DDLPS cell line and PDCs co-cultured with engineered MSC. LPS246 in the DDLPS cell line (A) and 14GS-076, 11GS-079, 11GS-099 and 11GS-106, in the PDCs (B) were co-cultured with engineered MSCs at a ratio of 1:1 for 48 hours, then treated with 100 μmol/L GCV. At 48 hours after GCV addition, all floating and attached cells were harvested. The percentages of apoptotic cells were quantified by 7 AAD and annexin V staining. Data represent mean ± SD of three independent experiments. MSC-MOCK+PDC+GCV: group co-cultured with MSC-Mock (GCV); MSC-TR/TK20+PDC: group co-cultured with 20 MOI of MSC-TR/TK (without GCV); MSC-TR/TK20+PDC+GCV: group co-cultured with 20 MOI of MSC-TR/TK (GCV).
Change in amount of secreted TRAIL on engineered MSCs with time
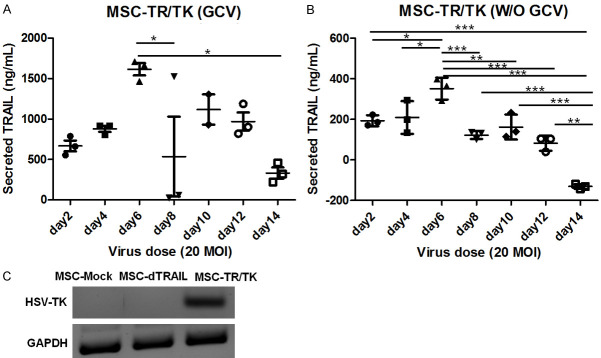
To analyze transduction of TRAIL in the in vivo model, we measured the amount of TRAIL secreted each day (0 d-14 d). As shown in Figure 3A and 3B, secretion of TRAIL was highly increased on day 6 after transduction of MSC with dTRAIL-TK/GCV or dTRAIL-TK. Moreover, the highest levels of TRAIL were secreted on 6 day in both groups of MSCs infected with 20 MOI. Levels of secreted TRAIL significantly decreased after 6 days. At 6 days the expression levels were also decreased, and remained at these levels until day 12. Next, we investigated the RNA expressions of HSV-TK by RT-PCR (reverse transcriptase-polymerase chain reaction). Only the MSC-TR/TK group showed RNA expression of HSV-TK (Figure 3C). From the above results, cells were treated with GCV on 5 consecutive days, starting 6 days after the engineered MSC treatment. Cells were also treated with GCV daily, starting 2 days after engineered MSC treatment. To maximize the therapeutic effect, two injections of engineered MSCs were made, through separate experimental designs (as described in Methods). Thus, engineered MSCs were administered one week and two weeks after LPS246 administration, and the therapeutic effect was observed after 5 consecutive administrations of GCV (Figure 4A).
Figure 3.
Changes in TRAIL secretion with day and expression of HSV-TK in engineered MSCs. To evaluate the amount of secreted TRAIL, 20 MOI of rAd/dTRAIL-TK was transduced on MSCs with 50 µmol/L of Fe3+ for 30 minutes. Changes in amounts of secreted TRAIL were measured at days indicated, with GCV (A) or without GCV (B). After rAd/dTRAIL-TK was transduced on MSCs for 48 hours, the expression level of mRNA of HSV-TK was analyzed by reverse transcriptase PCR (C).
Figure 4.
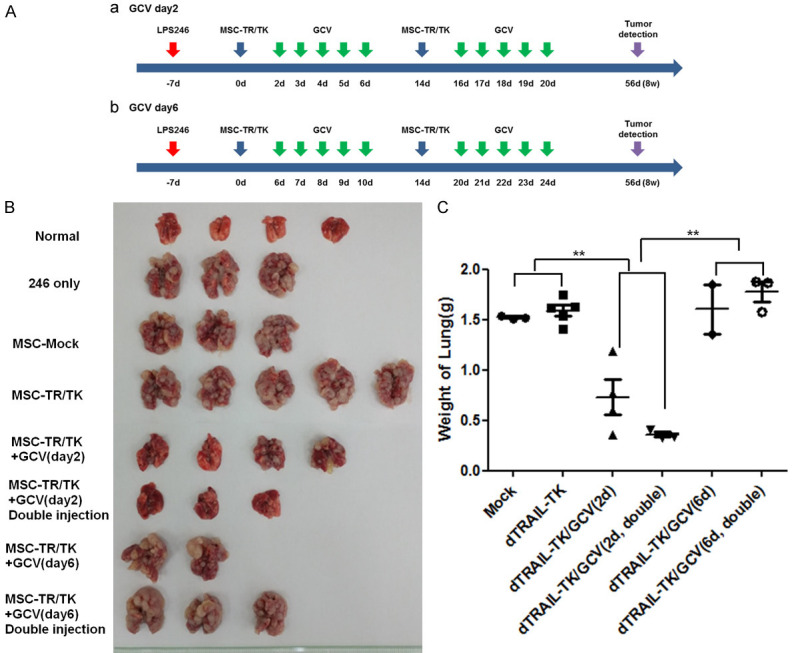
Suppressive effect of MSC-TK on lung metastasis. Schematic illustration of lung metastasis model. MSC-Mock or MSC-TR/TK was intravenously injected into mice, either once or repeatedly over 2 week intervals, starting one week after intravenous injection of LPS246 cells. After engineered MSC treatment, 50 mg/kg of GCV was intraperitoneally injected for 5 consecutive days starting on day 2 (Aa) or on day 16 (Ab). Gross images of tumor burden in the lung of each group after 8 weeks of treatment (n = 2-5 mice/group) (B). Tumor-bearing lung weights (C). **P < 0.01, values are mean ± SD.
Anti-metastatic effect by MSC-TR/TK
LPS246 cells were injected using the above method, followed by intravenous injection of MSCs one week later. MSCs were also engrafted on the basis of Figure 3 and previous experiments [11,12], and 50 mg/kg GCV was injected intraperitoneally 2 days and 6 days after engrafting the engineered MSCs (Figure 4A). Only the MSC-TR/TK with GCV group showed a decreased number of tumor lung nodules on the second day after MSC engrafting. In addition, MSC-TR/TK was marginally affected in the group administered at 1 or 2 week intervals (double injection) sequentially (Figures 4B, 4C, S2). Finally, when the number of metastatic tumor nodules was counted on day of 63 after tumor injection, we found that the group of MSC-TR/TK mice with double injections of GCV (starting day 2 at 2 weeks intervals) significantly eliminated tumor nodules in the lung compared to MSC-Mock, the MSC-TR/TK only and MSC-TR/TK with GCV groups (treated on day 6).
Discussion
The following describes results obtained from this study. First, patient-derived cells of liposarcoma (PDCs) were co-cultured with engineered MSCs and treated with GCV, generating a TRAIL-resistant cell group and a TRAIL-sensitive group. We found that resistance to TRAIL could be overcome by activating GCV through HSV-TK. This suggests that HSV-TK plays an important role in eradication of TRAIL-resistant cells. Second, according to previous data, the therapeutic effect of TRAIL/TK on slow-growing cells was observed to be sensitive to TRAIL [15], and breast, colon, and skin neoplasms, in particular, were reported to be comprised of heterogeneous populations [16]. According to this report, most of the groups located in the side population of these cells were resistant to HSV-TK. These cells also grew more slowly and underwent tumor initiation and tumor formation [17]. In other words, these cancer stem cells were observed to be repopulating as recurrent tumors after treatment with HSV-TK [18]. These groups were found to be sensitive to TRAIL [19,20]. Though, further studies are needed to determine the extent of liposarcoma cancer stem cells in TRAIL-sensitive PDCs, we think that the clinical effects of specific targeting of these cells would be very interesting. Fourth, other studies have reported that when magnoxide 675-labeled MSCs were intravenously injected into mice, the cells remained in the lungs for up to 7 days. After 7 days, the signal gradually changed [12]. Based on these observations, we assume that engrafted engineered MSCs will decrease significantly in the mouse after 7 days. Investigations into tropism and residual timing of engineered MSC administration to humans are required before clinical application. We believe that the administration of GCV starting day 2 after engraftment of engineered MSC was sufficient to verify the therapeutic effect in the preclinical stage. Finally, an anticancer effect of MSC-TK was observed in experiments using DDLPS cell lines. Therefore, the anti-tumorigenic effect on PDCs showing resistance to TRAIL was demonstrated by inducing toxic triphosphate GCV and the bystander effect through TK. We have shown that treatment with MSC-TR/TK and GCV have a therapeutic effect on TRAIL-resistant PDCs. Although we could only derive the results from in vivo DDLPS cell line data, we believe that this result sufficiently supports the in vitro data.
In conclusion, studies on the anticancer effect through TRAIL have been intensively conducted to apply the proven therapeutic effects to clinical practice [21]. We have carried out studies to apply this TRAIL therapy to cells from liposarcoma patients. Specifically, through this study, we used PDCs for clinical applications. Also, through this experiment using PDCs, we demonstrated that the effects of TRAIL-resistant cells could be overcome by toxic GCV generated by TK. This result is expected to facilitate the therapeutic approach of TRAIL in patients with liposarcoma.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2015R1D1A1A01059879).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Tseng WW, Somaiah N, Lazar AJ, Lev DC, Pollock RE. Novel systemic therapies in advanced liposarcoma: a review of recent clinical trial results. Cancers (Basel) 2013;5:529–549. doi: 10.3390/cancers5020529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman A, Lazar AJ, Pollock RE, Lev D. New frontiers in the treatment of liposarcoma, a therapeutically resistant malignant cohort. Drug Resist Updat. 2011;14:52–66. doi: 10.1016/j.drup.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Toulmonde M, Le Cesne A, Piperno-Neumann S, Penel N, Chevreau C, Duffaud F, Bellera C, Italiano A. Aplidin in patients with advanced dedifferentiated liposarcomas: a French sarcoma group single-arm phase II study. Ann Oncol. 2015;26:1465–1470. doi: 10.1093/annonc/mdv195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fillat C, Carrio M, Cascante A, Sangro B. Suicide gene therapy mediated by the herpes simplex virus thymidine kinase gene/ganciclovir system: fifteen years of application. Curr Gene Ther. 2003;3:13–26. doi: 10.2174/1566523033347426. [DOI] [PubMed] [Google Scholar]
- 5.Rath P, Shi H, Maruniak JA, Litofsky NS, Maria BL, Kirk MD. Stem cells as vectors to deliver HSV/tk gene therapy for malignant gliomas. Curr Stem Cell Res Ther. 2009;4:44–49. doi: 10.2174/157488809787169138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amm HM, Zhou T, Steg AD, Kuo H, Li Y, Buchsbaum DJ. Mechanisms of drug sensitization to TRA-8, an agonistic death receptor 5 antibody, involve modulation of the intrinsic apoptotic pathway in human breast cancer cells. Mol Cancer Res. 2011;9:403–417. doi: 10.1158/1541-7786.MCR-10-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y, Liang B, Jiang Q, Chen C, Yang K, Li C, Zheng A. Tumoricidal activity of combining the agonistic DR5 antibody D-6 with cisplatin in C30 cisplatin-resistant ovarian cancer in vitro and in vivo. Mol Med Rep. 2014;10:183–190. doi: 10.3892/mmr.2014.2193. [DOI] [PubMed] [Google Scholar]
- 8.Lim SM, Kim TH, Jiang HH, Park CW, Lee S, Chen X, Lee KC. Improved biological half-life and anti-tumor activity of TNF-related apoptosis-inducing ligand (TRAIL) using PEG-exposed nanoparticles. Biomaterials. 2011;32:3538–3546. doi: 10.1016/j.biomaterials.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Hampl M, Albert P, Fine HA. Antitumor activity and prolonged expression from a TRAIL-expressing adenoviral vector. Neoplasia. 2002;4:312–323. doi: 10.1038/sj.neo.7900245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loebinger MR, Eddaoudi A, Davies D, Janes SM. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69:4134–4142. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H, Jo EB, Kim SJ, Yang HM, Kim YM, Sung YC, Park JB, Hong D, Park H, Choi YL, Kim SJ. Therapeutic strategies for locally recurrent and metastatic de-differentiated liposarcoma with herpes simplex virus-thymidine kinase-expressing mesenchymal stromal cells. Cytotherapy. 2017;19:1035–1047. doi: 10.1016/j.jcyt.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Kim SW, Kim SJ, Park SH, Yang HG, Kang MC, Choi YW, Kim SM, Jeun SS, Sung YC. Complete regression of metastatic renal cell carcinoma by multiple injections of engineered mesenchymal stem cells expressing dodecameric TRAIL and HSV-TK. Clin Cancer Res. 2013;19:415–427. doi: 10.1158/1078-0432.CCR-12-1568. [DOI] [PubMed] [Google Scholar]
- 13.Yang HM, Sung JH, Choi YS, Lee HJ, Roh CR, Kim J, Shin M, Song S, Kwon CH, Joh JW, Kim SJ. Enhancement of the immunosuppressive effect of human adipose tissue-derived mesenchymal stromal cells through HLA-G1 expression. Cytotherapy. 2012;14:70–79. doi: 10.3109/14653249.2011.613926. [DOI] [PubMed] [Google Scholar]
- 14.Jo EB, Hong D, Lee YS, Lee H, Park JB, Kim SJ. Establishment of a novel PDX mouse model and evaluation of the tumor suppression efficacy of bortezomib against liposarcoma. Transl Oncol. 2018;12:269–281. doi: 10.1016/j.tranon.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu W, Liu W. Side populations of glioblastoma cells are less sensitive to HSV-TK/GCV suicide gene therapy system than the non-side population. In Vitro Cell Dev Biol Anim. 2010;46:497–501. doi: 10.1007/s11626-010-9274-6. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Wang K, Jensen TD, Li S, Bolund L, Wiuf C. Tumor heterogeneity in neoplasms of breast, colon, and skin. BMC Res Notes. 2010;3:321. doi: 10.1186/1756-0500-3-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 18.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sussman RT, Ricci MS, Hart LS, Sun SY, El-Deiry WS. Chemotherapy-resistant side-population of colon cancer cells has a higher sensitivity to TRAIL than the non-SP, a higher expression of c-Myc and TRAIL-receptor DR4. Cancer Biol Ther. 2007;6:1490–1495. doi: 10.4161/cbt.6.9.4905. [DOI] [PubMed] [Google Scholar]
- 20.Prabhu VV, Allen JE, Dicker DT, El-Deiry WS. Small-molecule ONC201/TIC10 targets chemotherapy-resistant colorectal cancer stem-like cells in an akt/foxo3a/trail-dependent manner. Cancer Res. 2015;75:1423–1432. doi: 10.1158/0008-5472.CAN-13-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Karstedt S, Montinaro A, Walczak H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat Rev Cancer. 2017;17:352–366. doi: 10.1038/nrc.2017.28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.