Abstract
Airway remodeling represents the healing and alteration in the airway that occur as a consequence of chronic inflammation. Extracellular matrix synthesis regulated by transforming growth factor (TGF-β1) and vascular remodeling regulated by epidermal growth factor (EGF) are important factors for the airway remodeling. This study aimed to probe the effect of catalpol, a major component of Radix Rehmanniae Preparata (Shudihuang), on airway remodeling and expression of TGF-β1 and EGF in asthmatic mice. A mice model of asthma was induced by ovalbumin (OVA) treatment. BALB/c mice were randomly divided into blank control group, asthma model group, dexamethasone (DEX) group (positive control), high, medium and low dose of catalpol intervention group. Changes in lung histology were observed using hematoxylin and eosin staining. The levels of TGF-β1 and EGF in mouse sera and bronchoalveolar lavage fluid (BALF) were examined by ELISA. The EGF mRNA and protein levels in mice tissues were determined. The results indicated that catalpol improved general conditions and reduced the damage of lung tissues in asthmatic mice. Moreover, results of ELISA revealed that catalpol significantly reduced the OVA-induced levels of TGF-β1 and EGF in sera and in bronchoalveolar lavage fluid (BALF). Additionally, results indicated that catalpol decreased the OVA-induced EGF mRNA and protein expression in lung tissues in asthmatic mice. Catalpol at a high dose was more efficient in decreasing the level of TGF-β1 in mice sera and BALF comparing the DEX group. Current study has demonstrated that catalpol might effectively prevent airway remodeling in asthma via inhibiting TGF-β1 and EGF.
Keywords: Airway remodeling, catalpol, TGF-β1, EGF
Introduction
Asthma is a chronic disease of the airways in the lungs. Bronchial asthma is characterized by wheezing, coughing, bronchial contraction, chest tightness and shortness of breath [1-3]. During the decades, glucocorticoids, β2 receptor agonist, and theophylline have been considered as the primary agents for asthma treatment. However, their clinical applications are strongly restricted by low to little efficiency in treatment of severe symptoms and unexpected side effects associated with high dose or long-term use [4-10]. Therefore, it is extremely urgent to develop better and new medications to prevent asthma or relieve asthmatic symptoms after appropriate treatment.
Recently, traditional Chinese medicines have been developed to relieve asthmatic symptoms using animal model [11,12]. Rehmannia Glutinosa Libosch (Dihuang) is a perennial herb widely distributed along the suburban hilly area of China. The yellow root of Dihuang-Radix Rehmanniae Preparata (Shudihuang)-has been considered as a traditional Chinese medicine. The pharmacological effects of Shudihuang include hypoglycemia, hemostasis, anti-disseminated intravascular coagulation, and treatment of hepatitis and diphtheria, etc [11-23]. A study demonstrated that catalpol mitigated the symptoms of asthma in a mouse model via inhibition of eosinophil infiltration in the asthmatic mice [24]. Also, catalpol can improve the balance of the interleukin 4 (IL-4)/interferon gamma (IFN-γ) ratio in asthma [25]. In the studies using a combination of catapol with other methods or medicines, it has been found that catalpol injection combined with budesonide can result in a synergistic effect to further inhibit the airway inflammation of asthma [26] and catapol enhanced the treating effect of specific immune therapy (SIT) [27].
Airway remodeling or airway reconstruction has been considered as one of the primary pathological mythologies for asthma treatment [24-31]. Transforming growth factor (TGF-β1) and epidermal growth factor (EGF) are critical factors in the pathogenesis of airway remodeling. EGF can be induced by allergic reaction and the factor mediates vascular remodeling and angiogenesis, which are related to airway remodeling [32-35]. TGF-β1 has been known to involve in the synthesis of those matrix molecules which are associated with airway remodeling [36-41]. Therefore, the modulation of TGF-β1 and EGF expression might play a significant role in regulating airway remodeling of asthma.
In this study, the effects of catapol on general conditions and lung histopathology were investigated using an established mouse model of asthma. The possibility of underlying molecular mechanisms associated with its regulation of TGF-β1 and EGF expression were also studied and discussed. The findings support that catalpol may become a promising drug for the treatment of asthma.
Materials and methods
Experimental animals
Ninety male BALB/c mice (age: 5-6 weeks, weight: 50-60 g) were purchased from the Experimental Animal Center at First People’s Hospital of Jiujiang (Jiangxi Province, China). The mice were maintained and fed under specific conditions. Illumination and dark time period were both set to 12 h. There were no restrictions on drinking water and feeding. The animal experimental procedures were approved by the Ethics Committee of the First People’s Hospital of Jiujiang and the first affiliated hospital of Nanchang university. All experiments including animals were conducted strictly based on the guidelines of the Care and Use of Laboratory Animals by the National Institute of Health, China.
Establishment of asthma model and catapol treatment
After on-site acclimatization for seven days, ninety experimental BALB/c mice were randomly divided into six groups with fifteen mice in each group. These groups are blank control group, asthma model group, dexamethasone (DEX) group, low dose of catapol (catapol-L) intervention group, medium dose of catapol (catapol-M) intervention group and high dose of catapol (catapol-H) intervention group. The mice in the blank control group were given intraperitoneal injection with normal saline and airway nebulization. The mice in the other groups were intraperitoneally injected and atomized with ovalbumin (OVA) liquid prepared on the same day to establish a mouse asthma model.
On the first day of the experiment, each mouse was intraperitoneally injected with 0.1 mL of 10% OVA solution (Sigma-Aldrich, St. Louis, MO, USA) in normal saline containing 5 mg of dry powder of aluminum hydroxide (Sinopharm). Two weeks after sensitization, 1% OVA solution which was prepared in physiological saline was administered by nebulization to induce asthma, the administration continued for three consecutive days, followed by stimulated once every other day for 20 min. The mice exhibited the following symptoms as respiratory acceleration, sneezing, perioral cyanosis, abdominal spasm and nodding breathing, suggesting that asthma was successfully stimulated in mice.
The OVA-sensitized mice were administered with drugs 30 min before aerosol inhalation challenge. The blank control group and the model group were treated with normal saline, and the other intervention groups were given the corresponding drugs for intervention. The mice in the DEX group were given DEX (0.85 mg/kg; Pharmaceutical Co., Ltd, China) daily for 21 days. The mouse in the catapol groups were administered with 3 mg/kg in normal saline (catapol-L), 7 mg/kg in normal saline (catapol-M) or 12 mg/kg in normal saline (catapol-H) of catapol (Melonepharma, Dalian, China) via intraperitoneal injection 0.5 h before each OVA challenge once a day for 21 days, whereas mice in the blank control and model group were treated with the same volume of physiological saline once a day for 21 days. During OVA sensitization and challenge, the general conditions of mice in each group were observed and monitored.
Analysis of serum samples
Mice blood was aseptically acquired from the abdominal aortas of the mice after the final challenge, and the serum were isolated by centrifuging the blood sample at 3000 rpm for 30 min. The serum was then collected and stored in a fridge at -10°C for future analysis. The serum levels of TGF-β1 and EGF were measured by commercial enzyme-linked immunosorbent assay (ELISA) kits (Elabscience, Wuhan, China) followed the manufacturer’s instructions.
Analysis of bronchoalveolar lavage fluid (BALF) samples
On experiment Day 35, mice were sacrificed by blood letting from femoral artery. Bronchoalveolar lavage was performed by washing the lungs three times using 5 mL normal saline. The fluid was centrifuged at 1000 rpm for 20 min at 5°C, the supernatant BALF was collected and stored at -10°C for future analysis. The levels of TGF-β1 and EGF in the BALF were measured using commercial ELISA kits (Elabscience, Wuhan, China).
Histological examination
The lung tissues were stripped from mice, and washed using normal saline, fixed in 4% paraformaldehyde solution, dehydrated with gradient alcohol, and embedded in paraffin. After deparaffinization and rehydration, the sections were subjected to Hematoxylin and Eosin (H&E) staining. The images were captured with an optical microscope (Olympus) under a 400× magnification.
Immunohistochemistry
The lung sections were dewaxed, rehydrated, and treated with trypsinization to retrieve the antigen. Hydrogen peroxide solution (3%) was added to quench endogenous peroxidase activity for 20 min at room temperature. Then the sections were blocked with goat serum for 15 min, then incubated with primary antibodies against eotaxin (1:50 in PBS, Santa Cruz, Dallas, TX, USA) at 4°C for overnight. The lung sections were stained with 3,3’-diaminobenzidine (DAB), counterstained with hematoxylin, dehydrated, and then embedded in paraffin. The images were captured with an optical microscope (Olympus) under a 400× magnification.
Statistical analysis
SPSS software 22.0 (Chicago, IL, USA) was used for statistical analyses. The experimental data were expressed by mean ± SD. Single-factor Analysis of Variance (ANOVA) was used to compare the difference between the control group and the experimental group, P<0.05 indicates that there are no significant differences between the data.
Results
Catalpol alleviated airway inflammation
The mice in the control group maintained glossy hair, with good appetite and mental conditions. However, the mice in the groups other than the control group exhibited various extents of lack of appetite, sneezing, nose scratching, irritability, shortness of breath, cyanosis of lips and abdominal spasm, suggesting that the asthma model was successfully established. Especially, the mice in the DEX group and catapol groups also exhibited irritability and dyspnea in the early stage of sensitization. However, with the extension of drug administration, the occurrence time of asthmatic symptoms was gradually increased, the respiratory rate was reduced, and the cyanosis symptoms were improved, especially for the DEX group and the catapol-H group.
Catapol improved lung histological conditions of asthmatic mice
H&E staining method was conducted to evaluate morphological changes in mice lung tissues. Comparing the blank control group (see Figure 1A), an increase in airway smooth muscle mass (including hypertrophy and hyperplasia) was observed with a large number of inflammatory cell infiltration. Figure 1B shows disorder of airway epithelial structure and narrowing of the airway lumen in the asthma model group. These observations can be considered as key factors in asthmatic airway remodeling. In addition, comparing the model group, the DEX group (see Figure 1C) and catapol intervention groups (see Figure 1D-F), especially for the catapol-H and catapol-M groups, suggesting dramatically reduced infiltration of inflammatory cells, more regular arrangement of airway epithelial cells, and significantly improved lumen stenosis.
Figure 1.
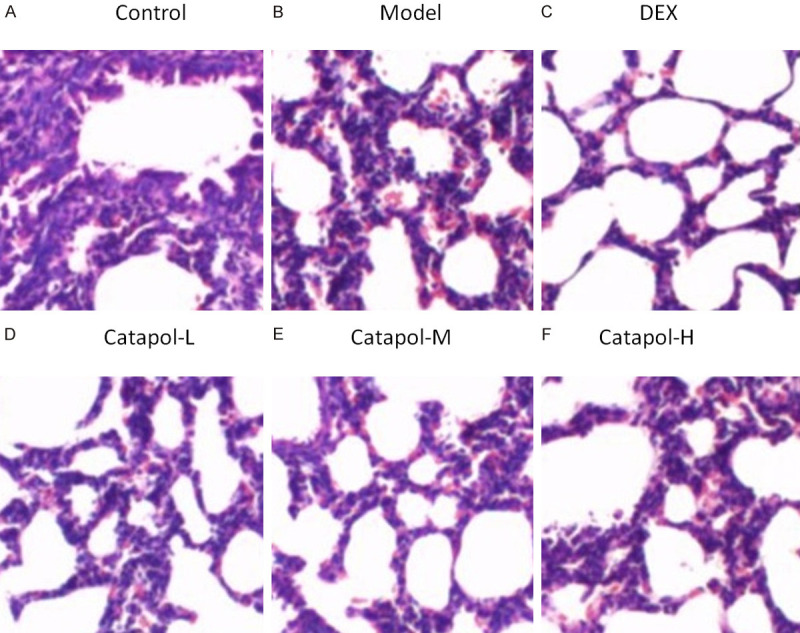
Images for morphological changes in lung tissues using H&E staining method (×400).
Determination of IgE and cell counts in BALF
The level of IgE in BALF was determined using the ELISA method. Figure 2A shows that the level of IgE in BLAF dramatically increased in the model group, and it was significantly higher than that in the control group. In the DEX group and catapol intervention groups, the IgE level was dramatically decreased, especially for the catapol-H and catapol-M groups. After sensitization and OVA-challenge, Figure 2B shows that the total number of cells in the model group and the catalpol group was significantly increased compared with the control group. The percentages of eosinophils and neutrophils in the catalpol group were increased significantly compared with the model group (see Figure 2C and 2D). As a comparison, the percentages of lymphocytes and macrophages in the catalpol group were significantly decreased compared with the model group (see Figure 2E and 2F). In all the cases, the catapol-H group exhibits a much pronounced effect comparing the catapol-L and catapol-M groups.
Figure 2.
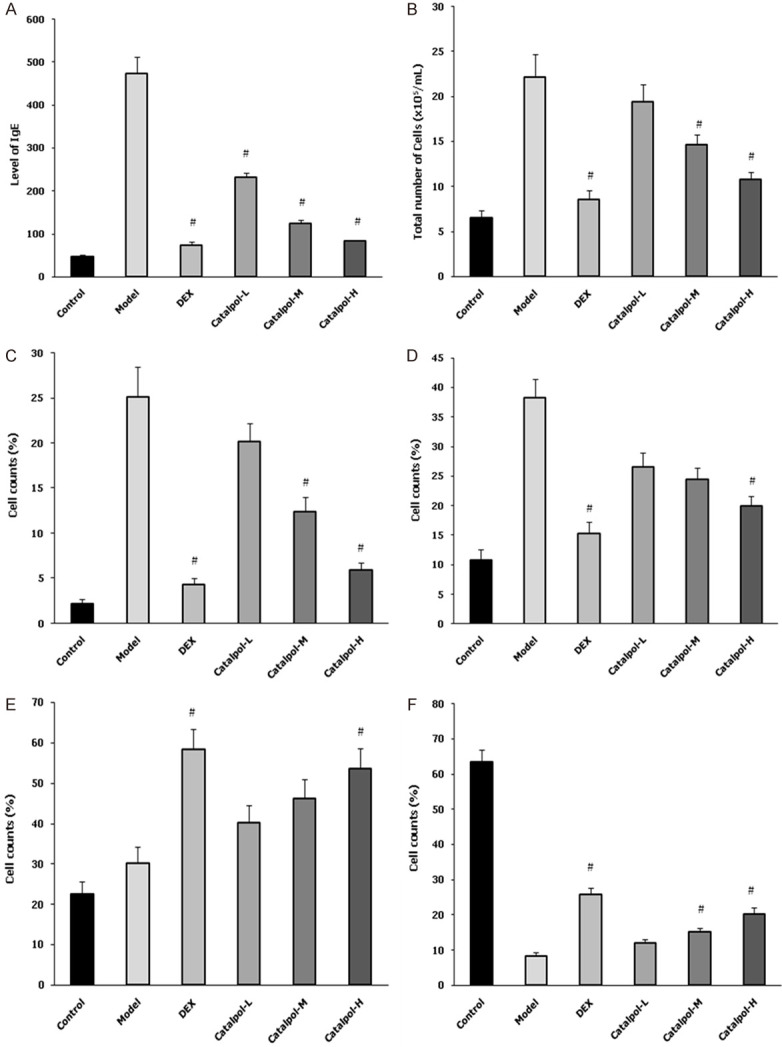
The level of IgE in BALF (A), total number of cells in BALF (B), and percentages of eosinophils (C), neutrophils (D), lymphocytes (E), and macrophages (F) in BALF for the experimental groups. “#” represents P<0.05 comparing the model group. Data are presented as the mean ± SD.
Effect of catapol on EGF expression
EGF is a very important mediator of airway remodeling in asthma [11]. Figure 3 shows that serum level of TGF-β1 in the asthma model group was significantly increased comparing the blank control group. Moreover, catapol intervention significantly and dose-dependently decreased serum EGF level in asthma mice model (see Figure 3A). EGF level in BALF and in lung tissues exhibited a similar expression tendency (see Figure 3B and 3C). In addition, comparing the DEX group, EGF levels in sera and in BALF were both significantly decreased in the catapol-H group (see Figure 3A and 3B). The EGF expression was analyzed through measuring average OD values from 3 randomly selected fields at ×400 magnification, the results were shown in Figure 4.
Figure 3.
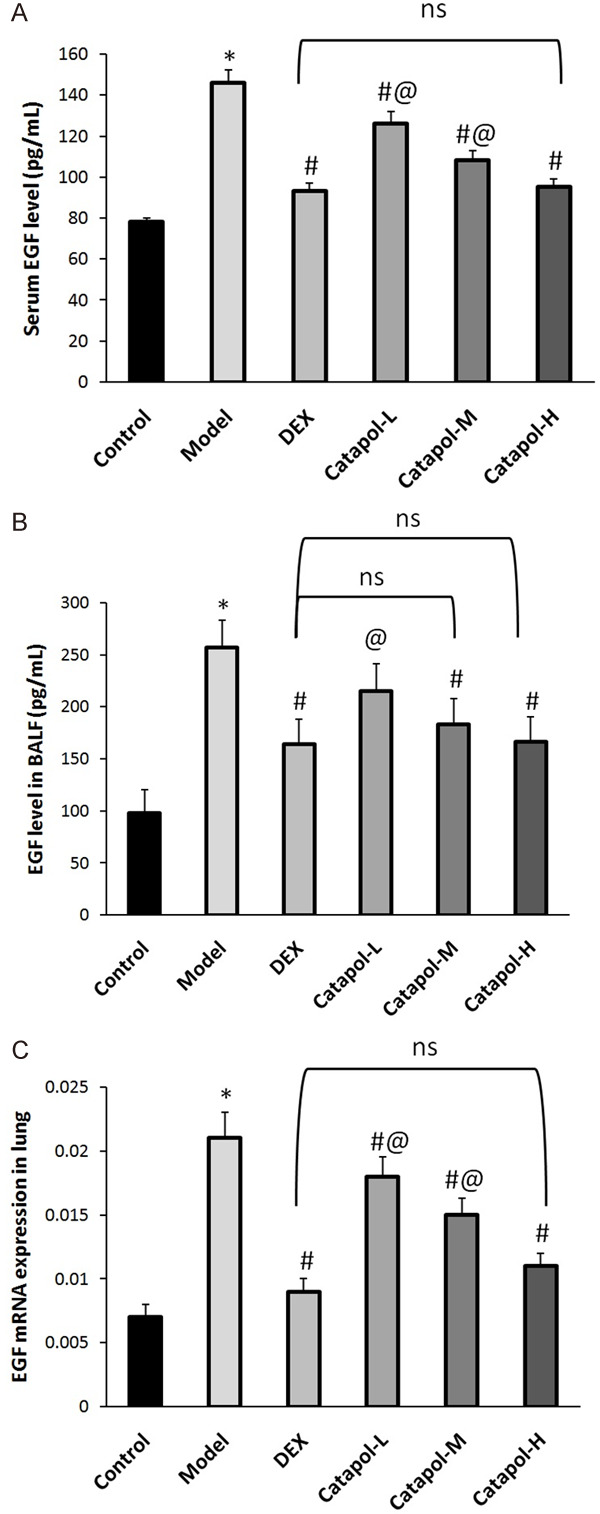
EGF expression in sera (A), BALF (B), and lung tissues (C). “*” represents P<0.05 comparing the control group, “#” represents P<0.05 comparing the model group, “@” represents P<0.05 comparing the DEX group, “ns” represents no significant difference. Data are presented as the mean ± SD.
Figure 4.
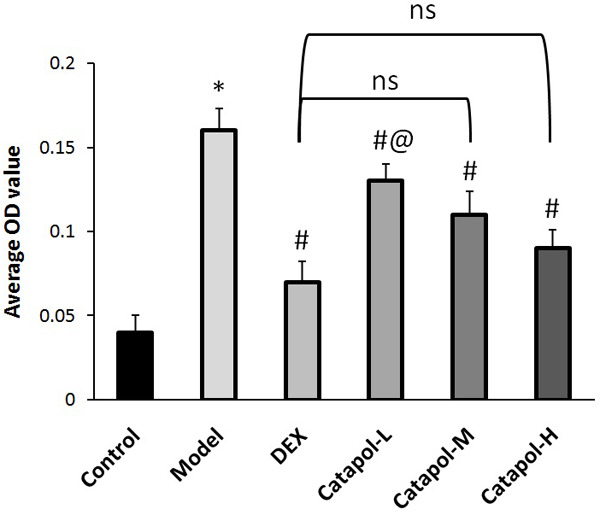
The average OD values for EGF expression. “*” represents P<0.05 comparing the control group, “#” represents P<0.05 comparing the model group, “@” represents P<0.05 comparing the DEX group, “ns” represents no significant difference. Data are presented as the mean ± SD.
Effect of catapol on TGF-β1 expression
TGF-β1 is a key angiogenic molecule and can notably contributes to airway remodeling in asthma [24-27]. Hence, it is necessary to investigate whether the protective effect of catapol on asthmatic mice was associated with its regulation of TGF-β1. The TGF-β1 levels in sera and in BALF were measured using ELISA, and protein expression in lungs was studied using immunohistochemistry. Figure 5 shows that serum level of TGF-β1 in the asthma model group was significantly higher than that in the blank control group. Comparing the model group, the serum EGF levels were dramatically decreased in the DEX and catapol treatment groups. Moreover, catapol intervention decreased serum TGF-β1 level in a dose-dependent manner. Similar expression pattern of TGF-β1 was acquired in BALF, as well as in mRNA expression in lung tissues. In addition, there was no significant difference in TGF-β1 expression between the DEX and the catapol-H group.
Figure 5.
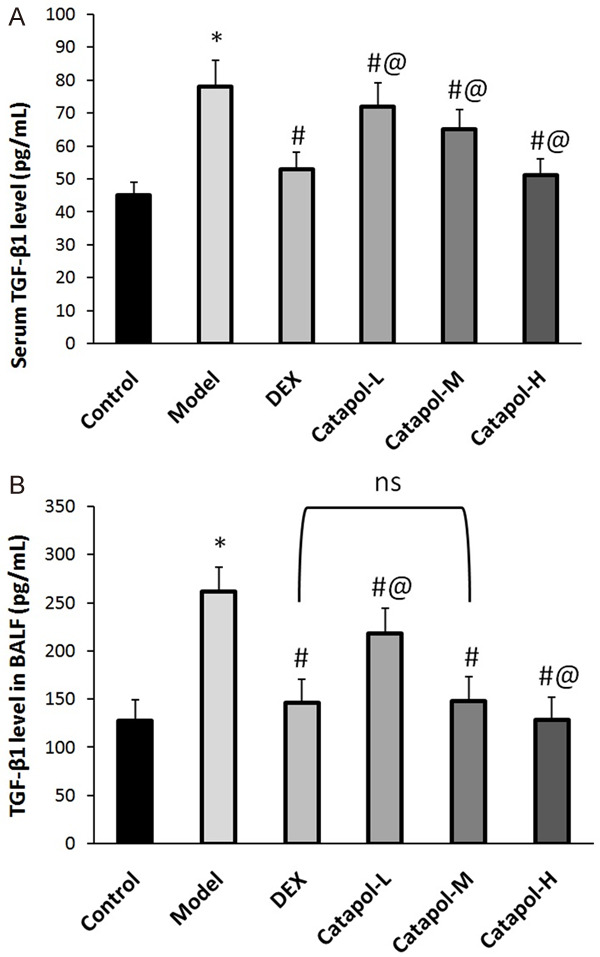
TGF-β1 expression in sera (A) and BALF (B). “*” represents P<0.05 comparing the control group, “#” represents P<0.05 comparing the model group, “@” represents P<0.05 comparing the DEX group, “ns” represents no significant difference. Data are presented as the mean ± SD.
Discussion
Catapol is a main component in the traditional Chinese medicine Radix Rehmanniae Preparata. In previous publications [24,25], it has been shown that large doses of catalpol injection can mitigate the asthma-induced symptoms with an OVA-induced rat model, catalpol has been found to inhibit eosinophil infiltration in the asthmatic rats, which might be related to an anti-asthmatic effect. Furthermore, the level of IFN-γ in the serum of rats after catalpol treatment was increased, but the level of IL-4 was decreased, indicating that catalpol improved the balance of the IL-4/IFN-γ ratio. The current study demonstrated obvious evidences that catapol improved general conditions of asthmatic mice. Specifically, the occurrence time of asthmatic symptoms was extended; the respiratory rate was reduced; and the cyanosis symptoms were improved. Moreover, catapol decreased airway smooth muscle mass and inflammatory cell infiltration, and improved disorder of airway epithelial structure and narrowing of the airway lumen of asthmatic mice, suggesting that catapol might have an inhibitory function in asthmatic airway remodeling. Additionally, the anti-remodeling effect of catapol was comparable to that of DEX as a positive control drug.
An asthmatic mice model was established in current study by repeated sensitization and OVA-challenge. A great number of inflammatory cells, including eosinophils and lymphocytes, infiltrated around bronchioles and capillaries have been observed. The level of IgE in the model group was higher than that of the control group. These results suggest that the animal model was successfully established and replicated. The airway inflammation was significantly alleviated after catalpol treatment, and the level of IgE in the BALF for the catalpol groups was significantly lower than that in the model group.
Both TGF-β1 and EGF are mediators of airway remodeling in asthma [24,25]. TGF-β1 contributes mainly to abnormal airway smooth muscle function in asthma by inducing airway smooth muscle cell proliferation and hypertrophy and by promoting the release of angiogenic, fibrogenic and inflammatory mediators [24,28-32]. EGF is a key angiogenic molecule and can stimulate the proliferation and migration of vascular endothelial to form new vascular, and greatly contributes to airway inflammation and airway remodeling in asthma [33-36]. Moreover, levels of TGF-β1 and EGF have been found to increase in chronic lung diseases such as asthma [24-36]. The results in this study are in agreement with previous findings, an increase in levels of TGF-β1 and EGF in sera and in BALF, and an increase in mRNA and protein levels of EGF in lung tissues of OVA-induced asthmatic mice were observed. The important function of TGF-β1 and EGF in mediating airway remodeling suggests that the modulation of TGF-β1 and EGF expression might play a significant role in regulating airway remodeling and consequently affect the progress of asthma. The interesting results in this study demonstrated that catapol greatly reduced the OVA-induced levels of TGF-β1 and EGF in asthmatic mice. These results indicate that inhibition of TGF-β1 and EGF may be involved in the anti-airway remodeling effect of catapol on asthma.
In addition, the findings have demonstrated, for the first time, the interaction between TGF-β1 and EGF caused by catapol. It has been reported that inhibition of EGF hinders the production of TGF-β1 in an animal model with allergic airway disease [24,25]. Also, TGF-β1 facilitates the release of EGF via airway smooth muscle cells with a time-dependent manner [25]. These conclusions support a direct interaction between TGF-β1 and EGF on regulating airway remodeling in asthma.
Previously, some traditional Chinese medicines have been found to mitigate asthmatic symptoms and relieve airway responsiveness, inflammation and remodeling in asthmatic animal models [11,12,24-28]. Radix Rehmanniae Preparata is composed of many compounds as traditional Chinese herbs, catapol as a primary component has been shown to suppress inflammation, to relieve cough, and to expel phlegm. Some publications together with the current study have successfully demonstrated the anti-asthmatic effects of catapol [24-27]. Therefore, catalpol might become a potential drug in clinical treatment of asthma.
In summary, the increased expression of TGF-β1 and EGF in asthmatic mice indicates that TGF-β1 and EGF may be involved in airway remodeling. Early administration of catapol in the treatment of asthma might reduce expression of TGF-β1 and EGF and correspondingly mitigate the symptoms of asthma in an OVA-induced mouse model. The findings in this study support a potential application of catapol as a therapeutic drug for the patients suffering from asthma.
Acknowledgements
This work was supported by Key Project (20171ACG70003) from Department of Science and Technology of Jiangxi Province.
Disclosure of conflict of interest
None.
References
- 1.Braman SS. The global burden of asthma. Chest. 2006;130:4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 2.Asher I, Pearce N. Global burden of asthma among children. Int J Tuberc Lung Dis. 2014;18:1269–1278. doi: 10.5588/ijtld.14.0170. [DOI] [PubMed] [Google Scholar]
- 3.Noble PB, Ansell TK, James AL, McFawn PK, Mitchell HW. Airway smooth muscle dynamics and hyperresponsiveness: in and outside the clinic. J Allergy (Cairo) 2012;2012:157047. doi: 10.1155/2012/157047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desai D, Brightling C. Cytokine and anti-cytokine therapy in asthma: ready for the clinic? Clin Exp Immunol. 2009;158:10–19. doi: 10.1111/j.1365-2249.2009.03998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wardlaw AJ, Brightling C, Green R, Woltmann G, Pavord I. Eosinophils in asthma and other allergic disease. Br Med Bull. 2000;56:985–1003. doi: 10.1258/0007142001903490. [DOI] [PubMed] [Google Scholar]
- 6.Wardlaw AJ, Brightling CE, Green R, Woltmann G, Bradding P, Pavord ID. New insights into the relationship between airway inflammation and asthma. Clin Sci (London) 2012;103:201–211. doi: 10.1042/cs1030201. [DOI] [PubMed] [Google Scholar]
- 7.Brightling CE, Bradding P, Pavord ID, Wardlaw AJ. New insights into the role of the mast cell in asthma. Clin Exp Allergy. 2003;33:550–556. doi: 10.1046/j.1365-2222.2003.01636.x. [DOI] [PubMed] [Google Scholar]
- 8.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 9.Venarske D, deShazo RD. Molecular mechanisms of allergic disease. South Med J. 2003;96:1049–1054. doi: 10.1097/01.SMJ.0000097887.04639.39. [DOI] [PubMed] [Google Scholar]
- 10.Zhang RX, Li MX, Jia ZP. Rehmannia glutinosa: review of botany, chemistry and pharmacology. J Ethnopharmacol. 2008;117:199–214. doi: 10.1016/j.jep.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Lee HK, Oh SR, Ahn KS, Lee SK, Lee JK, Kwon OK, Kim DY, Joung H, Quan GH, Kim MJ, Park BY. Pharmaceutical composition comprising an extract of pseudolysimachion longifolium and the catalpol derivatives isolated therefrom having antiinflammatory, antiallergic and antiasthmatic activity. 2013:US8455541B2. [Google Scholar]
- 12.Park EJ, Lee HS, Oh SR, Lee HK, Lee HS. Pharmacokinetics of verproside after intravenous and oral administration in rats. Arch Pharm Res. 2009;32:559–564. doi: 10.1007/s12272-009-1412-x. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Xu Z, Jiang Z, Sun L, Ji J, Miao J, Zhang X, Huang S, Wang T, Zhang L. Hypoglycemic effect of catalpol on high-fat diet/streptozotocin-induced diabetic mice by increasing skeletal muscle mitochondrial biogenesis. Acta Biochim Biophys Sin Shanghai. 2014;46:738–748. doi: 10.1093/abbs/gmu065. [DOI] [PubMed] [Google Scholar]
- 14.Tian YY, An LJ, Jiang L, Duan YL, Chen J, Jiang B. Catalpol protects dopaminergic neurons from LPS-induced neurotoxicity in mesencephalic neuron-glia cultures. Life Sci. 2006;80:193–199. doi: 10.1016/j.lfs.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Jiang B, Du J, Liu JH, Bao YM, An LJ. Catalpol attenuates the neurotoxicity induced by beta-amyloid(1-42) in cortical neuron-glia cultures. Brain Res. 2008;1188:139–147. doi: 10.1016/j.brainres.2007.07.105. [DOI] [PubMed] [Google Scholar]
- 16.Jin D, Cao M, Mu X, Yang G, Xue W, Huang Y, Chen H. Catalpol inhibited the proliferation of T24 human bladder cancer cells by inducing apoptosis through the blockade of Akt-mediated anti-apoptotic signaling. Cell Biochem Biophys. 2015;71:1349–1356. doi: 10.1007/s12013-014-0355-0. [DOI] [PubMed] [Google Scholar]
- 17.Gao N, Tian JX, Shang YH, Zhao DY, Wu T. Catalpol suppresses proliferation and facilitates apoptosis of OVCAR-3 ovarian cancer cells through upregulating microRNA-200 and downregulating MMP-2 expression. Int J Mol Sci. 2014;15:19394–19405. doi: 10.3390/ijms151119394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bi J, Jiang B, Zorn A, Zhao RG, Liu P, An LJ. Catalpol inhibits LPS plus IFN-γ-induced inflammatory response in astrocytes primary cultures. Toxicol In Vitro. 2013;27:543–550. doi: 10.1016/j.tiv.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J, Xu G, Ma S, Li F, Yuan M, Xu H, Huang K. Catalpol ameliorates high-fat diet-induced insulin resistance and adipose tissue inflammation by suppressing the JNK and NF-kappaB pathways. Biochem Biophys Res Comm. 2015;467:853–858. doi: 10.1016/j.bbrc.2015.10.054. [DOI] [PubMed] [Google Scholar]
- 20.Choi HJ, Jang HJ, Chung TW, Jeong SI, Cha J, Choi JY, Han CW, Jang YS, Joo M, Jeong HS, Ha KT. Catalpol suppresses advanced glycation end-products-induced inflammatory responses through inhibition of reactive oxygen species in human monocytic THP-1 cells. Fitoterapia. 2013;86:19–28. doi: 10.1016/j.fitote.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Park KS. Catalpol reduces the production of inflammatory mediators via PPARgamma activation in human intestinal Caco-2 cells. J Nat Med. 2016;70:620–626. doi: 10.1007/s11418-016-0988-y. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Jin C, Li Y, Guan S, Han F, Zhang S. Catalpol improves cholinergic function and reduces inflammatory cytokines in the senescent mice induced by D-galactose. Food Chem Toxicol. 2013;58:50–55. doi: 10.1016/j.fct.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Zhu J, Chen X, Wang H, Yan Q. Catalpol protects mice against renal ischemia/reperfusion injury via suppressing PI3K/Akt-eNOS signaling and inflammation. Int J Clin Exp Med. 2015;8:2038–2044. [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Zhang Y, Xu M, Luan J, Piao S, Chi S, Wang H. Catalpol alleviates ovalbumin-induced asthma in mice: reduced eosinophil infiltration in the lung. Int Immunopharmacol. 2017;43:140–146. doi: 10.1016/j.intimp.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Wang H, Yang X. Effects of catalpol on bronchial asthma and its relationship with cytokines. J Cell Biochem. 2019;120:8992–8998. doi: 10.1002/jcb.28170. [DOI] [PubMed] [Google Scholar]
- 26.Zhu H, Wang H. Inhibitory effects of catalpol coordinated with budesonide and their relationship with cytokines and Interleukin-13 expression. Am J Transl Res. 2019;11:6413–6421. [PMC free article] [PubMed] [Google Scholar]
- 27.Zou S, Hong J, Liu D, Lai G, Ye J, Song Y. Enhanced effect of catalpol on specific immune therapy in treatment of asthmatic mice. Am J Transl Res. 2019;11:2463–2469. [PMC free article] [PubMed] [Google Scholar]
- 28.Wei Y, Luo QL, Sun J, Chen MX, Liu F, Dong JC. Bu-Shen-Yi-Qi formulae suppress chronic airway inflammation and regulate Th17/Treg imbalance in the murine ovalbumin asthma model. J Ethnopharmacol. 2015;164:368–377. doi: 10.1016/j.jep.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Zhang XL, Jiang B, Li ZB, Hao S, An LJ. Catalpol ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by D-galactose. Pharmacol Biochem Behav 2007: 88:64–72. doi: 10.1016/j.pbb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Bi J, Jiang B, Liu JH, Lei C, Zhang XL, An LJ. Protective effects of catalpol against H2O2-induced oxidative stress in astrocytes primary cultures. Neurosci Lett. 2008;442:224–227. doi: 10.1016/j.neulet.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Bao Y, Jiang B, Wang Z, Liu Y, Zhang C, An L. Catalpol protects primary cultured astrocytes from in vitro ischemia-induced damage. Int J Dev Neurosci. 2008;26:309–317. doi: 10.1016/j.ijdevneu.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Fu K, Piao T, Wang M, Zhang J, Jiang J, Wang X, Liu H. Protective effect of catalpol on lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol. 2014;23:400–406. doi: 10.1016/j.intimp.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Durrant DM, Metzger DW. Emerging roles of T helper subsets in the pathogenesis of asthma. Immunol Investig. 2010;39:526–549. doi: 10.3109/08820131003615498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray A, Cohn L. Th2 cells and GATA-3 in asthma: new insights into the regulation of airway inflammation. J Clin Invest. 1999;104:985–993. doi: 10.1172/JCI8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnes PJ. Th2 cytokines and asthma: an introduction. Respir Res. 2001;2:64–65. doi: 10.1186/rr39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi H, Qin S, Huang G, Chen Y, Xiao C, Xu H, Liang G, Xie Z, Qin X, Wu J, Li G, Zhang C. Infiltration of eosinophils into the asthmatic airways caused by interleukin 5. Am J Respir Cell Mol Biol. 1997;16:220–224. doi: 10.1165/ajrcmb.16.3.9070605. [DOI] [PubMed] [Google Scholar]
- 37.Delphin S, Stavnezer J. Regulation of antibody class switching to IgE: characterization of an IL-4-responsive region in the immunoglobulin heavy-chain germline epsilon promoter. Ann N Y Acad Sci. 1995;764:123–135. doi: 10.1111/j.1749-6632.1995.tb55815.x. [DOI] [PubMed] [Google Scholar]
- 38.Garcia G, Taille C, Laveneziana P, Bourdin A, Chanez P, Humbert M. Antiinterleukin-5 therapy in severe asthma. Eur Respir Rev. 2013;22:251–257. doi: 10.1183/09059180.00004013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wegmann M. Targeting eosinophil biology in asthma therapy. Am J Respir Cell Mol Biol. 2011;45:667–674. doi: 10.1165/rcmb.2011-0013TR. [DOI] [PubMed] [Google Scholar]
- 40.Huang WC, Liou CJ. Dietary acacetin reduces airway hyperresponsiveness and eosinophil infiltration by modulating eotaxin-1 and th2 cytokines in a mouse model of asthma. Evid Based Complement Alternat Med. 2012;2012:910520. doi: 10.1155/2012/910520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ok IS, Kim SH, Kim BK, Lee JC, Lee YC. Pinellia ternata, citrus reticulata, and their combinational prescription inhibit eosinophil infiltration and airway hyperresponsiveness by suppressing CCR3+ and Th2 cytokines production in the ovalbumin-induced asthma model. Mediat Inflamm. 2009;2009:413270. doi: 10.1155/2009/413270. [DOI] [PMC free article] [PubMed] [Google Scholar]


