Abstract
Hepatocellular carcinoma (HCC) has an extremely poor prognosis and is one of the most common malignancies worldwide. Immune checkpoint suppression has become the most effective treatment option for liver cancer. The strategies used for immune checkpoint inhibitor targeting cancer therapies have been affected by some significant successes, including blocking the advanced-stage malignant tumor by death protein 1 (PD-1)/programmed cell death ligand (PDL-1), and cytotoxic T-lymphocyte antigen-4 (CTLA4) pathways. T cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT) is an immune checkpoint that participates in tumor immune surveillance. Mainly expressed on T cells, natural killer (NK) cells, and other antigen-presenting cells (APCs), it diminishes cytokine production and exhibits strong suppressive properties. TIGIT achieves a more active antitumor immune response and highlights a pivotal role for cancer immunotherapy. Preclinical studies have found inhibitory effects using a targeted approach. Monotherapy targeting TIGIT or in combination with anti-PD-1/PD-L1 monoclonal antibodies for the treatment of patients with advanced solid malignancies have demonstrated improved antitumor immune responses. Due to the high tumor heterogeneity of liver cancer, immune checkpoint suppression therapy still needs further exploration. Therefore, we provide insights into the characteristics of TIGIT and the immune system in HCC.
Keywords: HCC, TIGIT, immune check point, immune cells
Background
Hepatocellular carcinoma (HCC) most commonly occurs with chronic virus inflammation such as hepatitis B virus (HBV) and hepatitis C virus, overconsumption of alcohol, aflatoxin B1 exposure, obesity-related nonalcoholic fatty liver disease, type 2 diabetes, and exposure to toxic chemical compounds in the environment. HCC is the fourth most common cause of cancer-related deaths worldwide [1,2]. Chronic HBV infection can lead to cirrhosis and advanced HCC [3]. Liver transplantation, resection, or radiofrequency ablation can be used during the early stages of HCC, but these treatments are associated with high rates of recurrence. Trans-arterial chemoembolization or radio-embolization can be applied during the intermediate stages, but the overall survival time is <20 months. HCC is usually diagnosed at an advanced stage when there are fewer available treatment options. Use of any of these options is associated with a dismal prognosis [4]. Advanced-stage HCC remains difficult to cure due to tumor heterogeneity and the lack of suitable therapeutic strategies [5]. The molecular mechanisms leading to the development of HCC are complex and not completely understood [6]. Therefore, HCC is an important area for immunotherapy research [7]. Clinical trials of anti-TIGIT agents have been performed (Table 1). Targeting immune checkpoint molecules represents a revolutionary approach for counteracting the immune invasion of tumor cells [8]. This review focuses on TIGIT, a promising novel immune checkpoint, presents the evidence that TIGIT expression contributes to HCC progression through tumor-associated immune suppression, and discusses the mechanisms via which HCC interacts with the immune microenvironment.
Table 1.
Clinical trials on anti-TIGIT agents
| NCT number | Intervention/treatment | Disease or condition | Phrases | Status |
|---|---|---|---|---|
| 04150965 | Drug: Elotuzumab | Multiple Myeloma | Phase I | Not yet recruiting |
| Drug: Pomalidomide | Relapsed Refractory | Phase II | ||
| Drug dexamethasone | Multiple Myeloma | |||
| Drug: Anti-LAG-3 | ||||
| Drug: Anti-LAG3 | ||||
| Drug: Anti-TIGIT | ||||
| 04047862 | Drug: BGB-A1217 | Metastatic Solid Tumors | Phase I/Ib | 39 Patients |
| Drug: Tislelizumab | ||||
| 03563716 | Drug: Atezolizumab | Non-small Cell Lung Cancer | Phase II | 135 participants |
| Drug: MTIG7192A | ||||
| Drug: Placebo | ||||
| 04256421 | Drug: Tiragolumab | Small Cell Lung Cancer | Phase III | 400 participants |
| Drug: Atezolizumab | ||||
| Drug: Carboplatin | ||||
| Drug: Etoposide | ||||
| Drug: Placebo |
The liver is an immune-tolerant organ that often encounters chronic infections and tumorigenesis [8]. As a naturally immune-tolerant organ, it has a specific immune-anatomy that facilitates the establishment of an immunosuppressive microenvironment [9]. However, HCC’s immune-biology, it effects on associated molecular mechanisms of the immune system, and tumor-associated immune checkpoint signaling make it highly suppressive to this microenvironment [7]. HCC is an inflammation-driven disease, and can be a consequence of virus infection-associated inflammation, liver fibrosis, and cirrhosis. HBV-DNA integration frequently occurs in patients with HBV-related HCC [1]. TIGIT blockade or deficiency can accelerate the progression of chronic liver inflammation and fibrosis and can increase with HBV Ag-specific CD8+T cell numbers. These characteristics indicate that TIGIT is a vital molecule in adaptive immunity-mediated tumor progression and liver tolerance to the effects of infection and tumor cell invasion [10]. This review focuses on the expression of TIGIT, a novel inhibitory immune checkpoint molecule that regulates cellular immune responses that maintain homeostasis. We also discuss the pathogenesis of HCC and associated immunopathological mechanisms.
Gene profile of TIGIT
The TIGIT gene is an important protein-coding gene. It encodes a member of the PVR (poliovirus receptor) family of immunoglobin proteins (https://www.genecards.org). Cell adhesion molecules (CAMs) and the T cell co-signaling pathway are two important associated pathways that regulate immune cell differentiation and tissue morphogenesis [11]. Gene ontology annotations related to this gene include signaling receptor binding. NECTIN2 is an important paralog of this gene. Gene features of TIGIT are presented in Table 2.
Table 2.
The gene profile of TIGIT
| Items | Status |
|---|---|
| Cytogenetic location | 3q13.31 |
| External IDs for TIGIT Gene | HGNC: Entrez Gene: 201633 |
| Ensembl: ENSG00000181847 | |
| OMIM: 612859 | |
| Genomic Locations for TIGIT Gene | UniProtKB: Q495A1 |
| chr3: 114,276,913-114,310,288 (GRCh38/hg38) | |
| Size: 33,376 bases | |
| chr3: 113,995,760-114,029,135 (GRCh37/hg19) | |
| Genes name | Size: 33,376 bases |
| Genomic coordinates | TIGIT, VSIG9, VSTM3, WUCAM |
| Protein names | 3: 114,291,101-114,329,746 |
| T-cell immunoreceptor with Ig and ITIM domains | |
| V-set and immunoglobulin domain-containing protein 9 | |
| Cloning and Expression | V-set and transmembrane domain-containing protein 3 |
| 244-amino acid TIGIT protein with an Ig domain, a type I transmembrane domain and an ITIM motif |
The structure and function of TIGIT
TIGIT binds with high affinity to the poliovirus receptor (PVR), which causes increased secretion of interleukin-10 (IL-10) [12], decreased secretion of IL-12B [13], and suppression of T cell activation by promoting generation of mature immunoregulatory dendritic cells. TIGIT has a crucial role in the antitumor and antiviral immune processes that maintain hemostasis. Xin et al. [14] used gene chip scanning to sequence and identify this cell surface protein complex, which is mainly detected on T cells and NK cells [15,16]. TIGIT is a novel member of the immunoglobulin (Ig) superfamily. It consists of three functional parts (i.e., an immunoglobulin variable fragment, a type I transmembrane protein domain, and the intracellular immune-receptor tyrosine-based inhibitory motif (ITIM)) [14]. Study findings indicate that the ITIM motif is a key domain, which is responsible for the inhibitory function of TIGIT expressed on NK cells and that results in the inhibition of NK cell killing [16].
CD155 (PVR/necl5/Tage4), a TIGIT ligand and member of the nectin-like family [17], is expressed at high levels in several human malignancies. The presence of CD155 is associated with a poor prognosis [18,19]. It acts as a significantly important immune ligand via interaction with the DNAM-1 (co-activating)/TIGIT (co-inhibitory)/ligand axis during regulation of T cell and NK cell functions [20].
The PVR family, which includes PVR (CD155, CD96), PVRL 2 (CD122), PVRIG (CD122R), and the DNAX accessory molecule-1 (DNAM-1) [21]. The glycoproteins NECL5 and CD155 are PVRs and are key Ig superfamily members. They contain three extracellular Ig-like domains and can be grouped into a nectin subfamily that has a central role in cell-to-cell communication [22,23]. CD155 is highly expressed on dendritic cells (DCs), human vascular endothelial cells, and some human tumor cells [24]. CD155 participates in sending co-inhibitory or co-stimulatory signals via binding of different receptors [25]. It is expressed on DCs that are professional antigen-presenting cells involved with antigen presentation, immune cell migration, and production of numerous cytokines that contribute to T cell and NK cell activation [26]. The TIGIT/CD155 pathway decreases DC cytotoxicity via promotion of IL-10 secretion and induction of T cell dysfunction [14]. Similar to TIGIT, the co-inhibitory CD96 binds to ligand CD155 to down regulate immune responses against tumors [3,27,28]. The co-stimulatory molecule, CD226, competes with TIGIT and CD96 also binds to the common ligand CD155 to deliver activating signals. Binding of CD155/CD226 activates the killing activity of NK cells [29] and CD8+T cell-associated tumor killing capability [30]. PVRs have high affinity for TIGIT, which effectively interrupts the communication of the PVR with its other receptors (e.g., CD226 and CD96) [31]. In contrast, compared with the TIGIT/CD155 interaction, CD226 has less affinity with CD155 [32,33]. CD155 also acts as an immune receptor, which is recognized as a promising target of tumor-associated immunotherapy. TIGIT is an inhibitory receptor, and the expression of TIGIT on T cells, NK cells, and APCs has been detected at high levels [34]. Taken together, these results suggest that TIGIT has a vital function during chronic inflammation and in promoting tumorigenesis through direct or indirect pathways [10]. Low-level expression of TIGIT on naïve T cells has a particularly important role in T cell exhaustion in the tumor microenvironment. Various immune cells participate in the complex liver immune microenvironment pathways. These immune cells are part of a vital HCC microenvironment. Co-signaling molecules combine with their ligands during tumor immunity.
The function of TIGIT expressed on T cells with HCC
The cytotoxic T-lymphocyte response is a critical component of the immune response to tumors [35]. T cell responses rely on T cell receptor (TCR) binding with co-signal molecules that can direct and fine-tune essential communication with the host cell [36,37]. This process results in a cascade of downstream responses [38]. T cell function is activated by two different pathways. The first pathway includes TCR contact with antigenic peptide/major histocompatibility complex, which is mainly expressed on the APC surface. The second classical pathway involves antigen-independent regulator molecules. The co-signals are divided into co-stimulators and co-inhibitors that participate in T cell priming, cell growth, initial differentiation, and maturation of function during the immune response [38,39]. In the normal state, TCR binding of TIGIT or other co-inhibitors minimizes damage and prevents harmful auto-immunity [40,41]. TIGIT participates in direct inhibition of T cell activation and proliferation. TIGIT can be detected on T cells (e.g., activated CD4+T cells, CD8+T cells, and Foxp3+T regulatory (Treg) cells). The subsets of type 1 regulatory T cells and follicular helper T (Tfh) cells can also express TIGIT, which delivers inhibitory signals and down regulates the production of cytotoxicity and cytokine expression [42]. The CD8+T cell expresses TIGIT at high levels, which are correlated with the degree of tumor malignancy [43]. This inhibitory function of TIGIT can be targeted during treatments based on immunotherapeutic intervention. TIGIT highly expressed on T cells and the correlation between tumor cells and T cells are illustrated in Figures 1 and 2.
Figure 1.
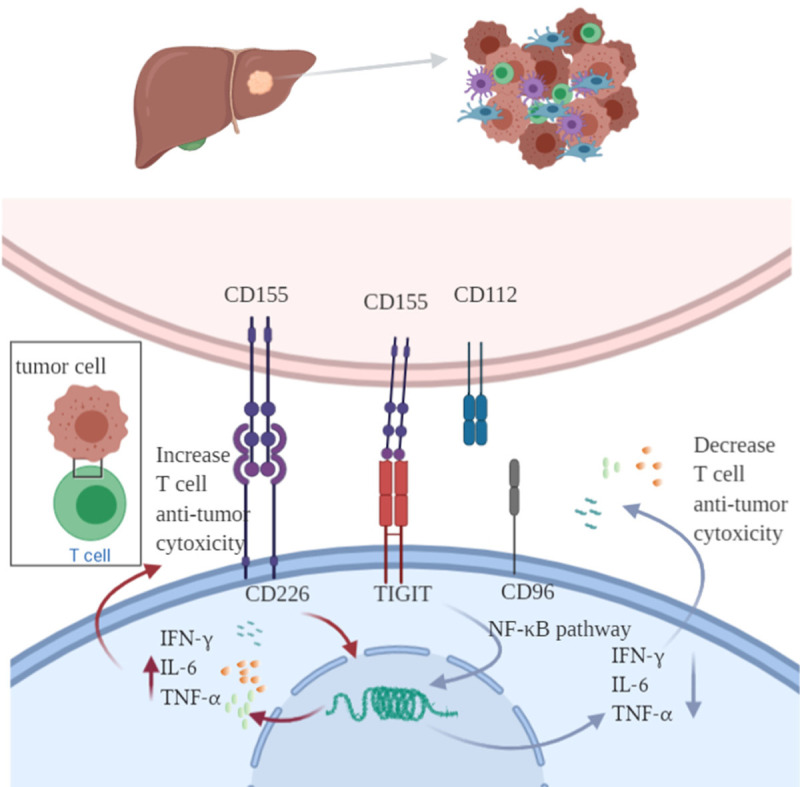
Various immune cells such as T lymphocytes, NK cells, macrophages, dendritic cells, Kupffer cells participant in the liver immune microenvironment pathways. TIGIT participant in tumor immune surveillance, exhibit an inhibitory signal through the ligation of TIGIT with CD155 or CD112, TIGIT has higher affinity of CD155 trigger inhibitory signaling via its cytoplasmic ITIM or ITT-like motif. TIGIT competes with the co-stimulatory CD226 for combination with CD155 and CD112. CD96 competes with CD226 for CD155 binding, however Interaction of CD96 with TIGIT inhibit immune cells activities. TIGIT can expressed on both immune cells and hepatocellular carcinoma cells in tumor associated environment.
Figure 2.
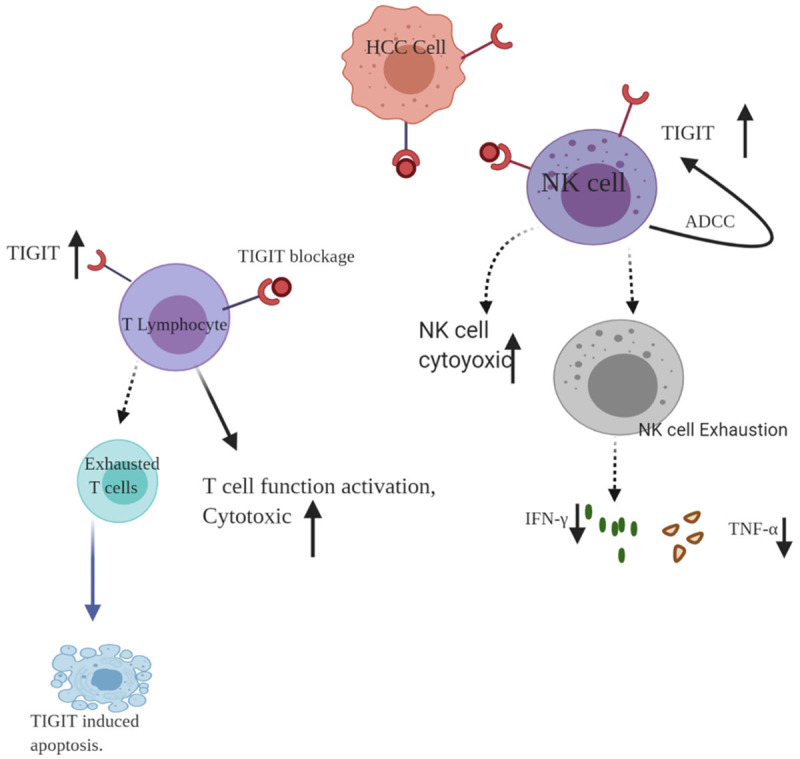
The regulation between TIGIT and immune cells. TIGIT could expressed on both cancer cell and immune cells such as T cell and NK cell. TIGIT inhibitors could suppress the inhibitory function and enhance the T cell and NK cell cytotoxic and anti-tumor function through upregulating the production of IFN-γ and TNF-α. The over expression of TIGIT of T cell could also cause T cell exhaustion, and down regulate the T cell cytotoxic. The NK cell activity could also be upregulation via antibody-dependent cellular cytotoxicity pathway.
Accumulating evidence reveals that Foxp3+Treg cells interact with other cells and release immune suppressive cytokines (e.g., IL-10 and transforming growth factor β (TGF-β)), which are crucial for immune homeostasis [44]. Treg cells are subsets of CD4+T cells and have crucial roles in the regulation of immune stability and immune suppressive functions [45]. Song et al. found that CD226 TIGIT functional subpopulations are significantly higher in patients infected with HBV [46]. TIGIT is a Treg cell inhibitory molecule that can inhibit T helper 1 and T helper 17 cell functions [47]. Fibrinogen-like protein 2 (Fgl2) promotes Treg cell-mediated inhibitory signals for T effector cell proliferation and cytokine production. TIGIT signaling induces Fgl2 transcription in TIGIT+Treg cells. TIGIT ligation triggers secretion of Fgl2 by Treg cells, which enables them to act as potent suppressors [47]. To promote T cell proliferation, TIGIT also participates in regulating the T cell anti-apoptosis pathway via upregulation of the related key protein, Bcl-xL. Interrupting both TIGIT and PD-1 can upregulate tumor antigen-specific CD8+T cell responses and the effects of CD8+ effector memory cells. Compared with other memory cell subsets, they express higher levels of granzyme B and perforin, which has a protective function with a positive correlation with a good prognosis [48,49]. Su C et al. found that TIGIT is highly expressed in both CD4+T cells and Treg cells of patients with HCC [43]. They also found that TIGIT expression level accelerates and declines along with the degree of cancerous differentiation, from high rates of expression and differentiation to low rates of expression and differentiation. The expression level of TIGIT is also positively related to α-fetoprotein (AFP) expression [43]. During the pathogenesis of HBV-associated HCC, the growth of PD-1+TIGIT+T cell populations accelerates and is co-related to the degree of malignancy in patients with advanced-stage HCC. TIGIT also has a relevant role in HBV-related HCC [50]. Yang Z et al. [50] found that TIGIT CD8 T+ cell population expression is associated with accelerated disease progression and a poor outcome in patients with HBV-HCC. Tian Z et al. [10] validated that TIGIT functions as a safeguard maintaining homeostasis of the hepatic immune system, and leading to CD8+T cell exhaustion. This process provides protection from immune-mediated injury and carcinogenic initiation [10,43]. Previous studies found that HCC can escape from the host’s immune attack and protects itself through the secretion of inhibitory cytokines, tumor specific antigens, and changes to the tumor microenvironment [10]. Examination of cancerous tissues from patients with HCC revealed that the degree of tumor differentiation is negatively correlated with expression levels of TIGIT and CD155 [51]. The TIGIT+CD4+T cells and TIGIT+Treg cells are crucial populations among those that decrease. Therefore, in the future, the level of TIGIT expressed on CD4+T cells or Treg cells may serve as a potential predictive biomarker for an early-stage diagnosis, treatment, and prognostic indicator in patients with HCC [51]. Blocking STAT3 might prevent HCC-mediated exhaustion of T cells and NK cells, illustrated with low expression of TIGIT on T cells and NK cells in the immunized HCC mice model [52]. TIGIT is predominantly expressed on immune cells with multiple roles. In one cell type, it can participate during various stages and on different occasions [53]. Altogether, TIGIT not only has significantly important roles in clinical prognosis, it also provides novel insights into strategies in immunotherapy.
The function of TIGIT expressed on NK cells with HCC
NK cells are abundant in the liver; they participate in immune surveillance and attack tumor cells via production of cytotoxicity. However, the numbers of NK cells in tumor tissues is less than in non-tumor tissues. Tumor-infiltrating NK cells also display a low activation status with an impaired tumor cell killing capacity and cytotoxic factor production [51]. TIGIT [14], CD96 [54,55], CD226 [55,56], and I-restricted T cell-associated molecule [57] are key regulators of NK and T cell functions, especially in the tumor microenvironment. CD96 binding CD155 can suppress the immune response. In patients with HCC, CD96+NK cells are functionally exhausted and IFN-γ and TNF-α expression are at low levels, as are T-bet, IL-15, perforin, and granzyme B. Inhibitory factors such as IL-10 and TGF-β1 are highly expressed. Recent study results indicate that the TIGIT motif binds with co-inhibition receptor PVR with high affinity; the TIGIT-mediated inhibition signal leads to suppression of the production of NK cell cytotoxicity [58]. CD96 is expressed not only on primary human peripheral NK cells, but also on various NK cell lines [51]. Overexpression of CD96+NK cells can mainly be detected in hematological malignancies like acute myeloid leukemia [54] and lung squamous cell carcinoma [59]. During chronic HBV infection, liver cirrhosis, and HCC, increased levels of CD96+NK cells can also be detected. A dynamic balance exists between CD96, TIGIT, and CD226 on intra-tumor NK cells of patients with HCC. In these patients, the expression of CD96 increases, while the expression of TIGIT decreases. Under the activation of TGF-β1, the NK phenotype can change from TIGIT+NK cells to CD96+NK cells. Co-inhibition of CD96 with other checkpoint receptors may provide a more efficient therapeutic approach during the use of immunotherapy for liver cancer. Some researchers have found that there is also a novel population of NK cells (i.e., CD49a+liver-resident NK cells) that is positively correlated with the expression level of checkpoint molecules (e.g., PD-1, CD96, and TIGIT) on tumor cell surfaces in patients with HCC [60-62]. Tissue-resident CD49a+NK cells accumulate in the tumor microenvironment and exhibit immune-exhausted regulatory characteristics that negatively regulate the immune response and progress, predict tumor progression, and a poor clinical outcome [51]. Blockade of TIGIT of NK cells can enhance the oncolytic adenovirus during ovarian cancer therapy [51]. OhsI et al. found that TIGIT blockers combined with IL12 can help reestablish NK cell antitumor capacity and cytotoxicity [63]. TIGIT is also upregulated upon NK cell activation through antibody-dependent cell-mediated cytotoxicity in human breast cancer [64]. He Y et al. found that if TIGIT NK cells are suppressed and immature, the combination of TIGIT and CD155 promotes NK cell maturation [65]. In the tumor-associated environment, NK cell cytotoxic capability can be suppressed by TIGIT upregulation and enhanced by TIGIT inhibitors (Figure 2).
TIGIT expressed on APCs in HCC
The tumor-associated macrophages (TAMs) that highly infiltrate most solid tumors lead to tumor progression. TAMs express inhibitory signals that shape antitumor immunity and promote tumor proliferation by expressing cytokines and chemokines; they are closely related to the occurrence and development of lymphoma [9,66]. Macrophages are the dominant leukocyte population found in the tumor microenvironment, including HCC TAMs. Preclinical studies revealed that TIGIT has a key role in the regulation of immune cell recruitment, monocyte phenotype polarization, and autophagy of TAMs during tumor progression.
TAM numbers are negatively correlated with survival and prognosis in patients with HCC. However, no clear evidence indicates that TIGIT directly regulates macrophages in patients with HCC. Macrophage co-culture with porcine aortic endothelial cells revealed that high TIGIT expression does not occur on M1 macrophages [67]. But, TIGIT is highly expressed on M2 macrophages. Study findings suggest it participates in the down-regulation of the release of TNF-α, IL-β, and IL-12, and suppresses macrophage-mediated cytotoxicity. Study results also suggest that the phosphorylation of SHP-1 (a tail of TIGIT) and TIGIT-CD155 binding participates in this inhibitory signal pathway on macrophages [67]. LPS-stimulated macrophages (i.e., M2-like cells) release anti-inflammatory factors (e.g., IL-10) and significantly reduce various pro-inflammatory cytokines (e.g., TNF-α) [12]. Human regulatory macrophages (Mreg) are a novel subpopulation that reflect a specific phenotype of macrophage differentiation, which is characterized by stable suppressive activity. Mregs strongly induce TIGIT+T cells through a partly immunosuppressive agent-dependent mechanism in solid organ transplantation [68]. The TIGIT is highly expressed on APCs, especially on DCs. Some endothelial cells (e.g., Kupffer cells) also have high levels of TIGIT expression [16]. The release of inhibitory factors from DCs occurs via the ligand of CD155 and TIGIT on DCs; it results in an inhibitory immune response in T cells. The combination of TIGIT and CD155 does not weaken the DC maturation process, but it does impair the effectiveness of activating and modulating the antigen-specific T cells and induces tolerance in T cells [69]. DCs not only contribute to the anti-inflammation process. They also function to restore the antiviral immunity of HBV-specific T cells [70]. The details of the relationships between the inhibitory molecule TIGIT expressed on DCs and HBV-related HCC progression remain to be determined.
PD-1: the classical co-inhibitory partner
PD-1 is a well-studied cell surface protein. It is also an immune checkpoint expressed by T cells, B cells, and macrophages. PD-1 is predominantly co-expressed with TIGIT in CD8+T cells [71,72]. It acts as a key regulator in balancing physiological immunity and pathological immunity, and maintains homeostasis and tolerance [73]. Accumulating evidence indicates that immune checkpoint targeting of PD-1 results in substantial clinical benefits in patients with solid tumors. In patients with HBV-related HCC, overexpression of CD8+T cells results in immune exhaustion, a dysfunctional phenotype, and causes immune damage to liver tissue [74]. PD-1 is highly expressed in tumor-surrounding tissues, especially in the liver portal region. It is also positively correlated with tumor size and the degree of differentiation [74]. PD-1 overexpression on tumor-infiltrating T lymphocytes inhibits proliferation and metastasis of HCC cells [75,76]. Expression of PD-1 from high to low correlates with distinct gene expression profiles on CD8+T cells. The accelerated levels of PD-1 expression on CD8+T cells accompanied by higher levels of the exhaustion of gene expression negatively regulates T cell function [77]. PD-1-high CD8+T cells also highly express other inhibitory immune checkpoints (e.g., TIGIT) and decrease production of IFN-γ and TNF-α in response to anti-CD3 [78]. Thymocyte selection-associated high mobility group box protein (TOX) is as a transcription factor that participates in the regulation of T cell differentiation [79]. TOX binds with PD-1 in the cytoplasm and promotes endocytic recycling of PD-1. This process results in higher expression of PD-1 in the cell membrane and an impaired CD8+T cell tumor killing capability [80]. Combining the inhibitory receptors of TIGIT and PD-1 might be used to enhance the immunosuppressive characteristics of cancer immune therapy.
The role of TIGIT in other diseases
In addition to targeting checkpoint inhibition for solid tumor immunotherapy, the role of TIGIT in myeloma treatment merits a fresh look. In adult acute lymphoblastic leukemia, the levels of TIGIT on n CD4+CD25+T cells and CD8+T cells are significantly upregulated, compared with a healthy control group. Similar to TIGIT, PD-1 and Tim-3 are also highly expressed. Together with PD-1, Tim3, Lag3, and TIGIT have co-inhibitory effects during the regulation of T cell function [81-83]. Guillerey et al. [84] were the first to find that the percentage of TIGIT expressed in CD8+T cells is related to myeloma burden in a murine V k*MYC myeloma model. They also found that the malignant progression of multiple myeloma is correlated with overexpression of TIGIT on CD8+T cells in both mice and humans. TIGIT shows high levels of expression in CD8+T cells, compared with other immune checkpoint molecules that negatively regulate T cell killing function. More importantly, monoclonal antibodies that block TIGIT can reduce the malignant progression of multiple myeloma. Minnie et al.’s study also found that the progression of myeloma is closely related to inhibitory receptor expression on CD8+T cells, while the co-stimulatory receptor CD226 (DNAM-1) is detected at low levels on CD8+T cells that have an exhausted phenotype and high levels of IL-10; the latter have a role in the T cell exhaustion [85]. Notably, the IL-10 that causes relapsed myeloma originates from myeloid cell subsets (DCs) that lack major histocompatibility complex class II expression (but expressed PD-L1) and CD641 macrophages [85].
Recent findings indicate that there is a novel mode of cross-talk between the immune system and the neuroendocrine system. As physiological stress increases, the immunological status may show protective immunity. Glucocorticoid-induced immunosuppression of TIGIT may be operational in various subsets of immune effector cells that participate in the resolution of inflammation when faced with threats, such as infections with viruses, and cancer cell invasions [86]. TIGIT is also co-expressed with CD11b or CD11c in tumor-infiltrating stromal cells. In colorectal tumors, the tumor-intrinsic TIGIT is highly expressed on CD4+T cells, CD8+T cells, and NK cells, and inhibits the function of these cells via interaction with CD155 (PVR) [87]. The tumor-intrinsic TIGIT not only has a suppressing function on immune cells; it also enhances tumor growth and significantly impairs tumor genesis via weakening the immune cell cytotoxicity [86], granule polarization, and cytokine release. In patients with head and neck squamous cell carcinoma [88], the expression of TIGIT on tumor-infiltrating T cells is higher compared with healthy controls. Similar results have been found for CD155. Blocking TIGIT/CD155 signaling can alleviate CTL exhaustion, decrease suppression by Tregs and MDSCs, inhibit tumor progression [75], and manifest antitumor immunity during progression of malignant tumors. Zhang et al. [89]. found that in patients with E. multilocularis-associated alveolar echinococcosis (AE), the expression of TIGIT is highly expressed in both blood and liver-infiltrating T cells. TIGIT causes T cell functional exhaustion through its CD155 ligand. In patients with AE, the expression of TIGIT shows apparently higher levels, compared with healthy people. This expression is correlated with lesion activity. The TIGIT-CD155 interaction has a key role in AE-related T cell exhaustion [89].
Conclusions and future perspectives
Most cases of HCC are diagnosed at an advanced stage, but use of immune checkpoint inhibitors may benefit patients and improve survival times [90,91]. HCC is associated with highly heterogeneous lesions and multiple pathologies, including multiple etiologically-induced liver-related diseases [92]. The notably robust upregulation of TIGIT in the liver corresponds with stresses and regulates its function. Cell-to-cell communication includes abundant intricate signal pathways to maintain hemostasis. Blocking one signal pathway results in compensation by other pathways. Evidence indicates that targeting the exhausted T cell’s inhibitory signal may lead to overexpression of other signals. Although clinically-important achievements have been gained from the use of immune therapy, the proper combinations of these strategies remain to be determined. The immune checkpoint targeting therapies and their irreplaceable precision targeting and profound immune responsiveness have the potential to change the conventional treatment approaches.
Acknowledgements
This study was supported by funds from the National Science and Technology Major Project of China (2018ZX10302206) and National Science and Technology Major Project of China (2017ZX10202203).
Disclosure of conflict of interest
None.
References
- 1.Caruso S, O’Brien DR, Cleary SP, Roberts LR, Zucman-Rossi J. Genetics of HCC: novel approaches to explore molecular diversity. Hepatology. 2020 doi: 10.1002/hep.31394. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Hiwatashi K, Ueno S, Sakoda M, Iino S, Minami K, Yamasaki Y, Okubo K, Noda M, Kurahara H, Mataki Y, Maemura K, Shinchi H, Natsugoe S. Problems of long survival following surgery in patients with nonBNonC-HCC: comparison with HBV and HCV related-HCC. J Cancer. 2015;6:438–447. doi: 10.7150/jca.10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tout I, Loureiro D, Mansouri A, Soumelis V, Boyer N, Asselah T. Hepatitis B surface antigen seroclearance: immune mechanisms, clinical impact, importance for drug development. J Hepatol. 2020;73:409–422. doi: 10.1016/j.jhep.2020.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Pei W, Chen J, Wang C, Qiu S, Zeng J, Gao M, Zhou B, Li D, Sacks MS, Han L, Shan H, Hu W, Feng Y, Zhou G. Regional biomechanical imaging of liver cancer cells. J Cancer. 2019;10:4481–4487. doi: 10.7150/jca.32985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown ZJ, Heinrich B, Greten TF. Mouse models of hepatocellular carcinoma: an overview and highlights for immunotherapy research. Nat Rev Gastroenterol Hepatol. 2018;15:536–554. doi: 10.1038/s41575-018-0033-6. [DOI] [PubMed] [Google Scholar]
- 7.Wood NJ. Immunotherapy: therapeutic potential of genetically modified HBV-specific T cells for chronic HBV infection and HBV-related HCC. Nat Rev Gastroenterol Hepatol. 2011;8:61. doi: 10.1038/nrgastro.2010.221. [DOI] [PubMed] [Google Scholar]
- 8.Kubes P, Jenne C. Immune responses in the liver. Annu Rev Immunol. 2018;36:247–277. doi: 10.1146/annurev-immunol-051116-052415. [DOI] [PubMed] [Google Scholar]
- 9.Lu C, Rong D, Zhang B, Zheng W, Wang X, Chen Z, Tang W. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: challenges and opportunities. Mol Cancer. 2019;18:130. doi: 10.1186/s12943-019-1047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zong L, Peng H, Sun C, Li F, Zheng M, Chen Y, Wei H, Sun R, Tian Z. Breakdown of adaptive immunotolerance induces hepatocellular carcinoma in HBsAg-tg mice. Nat Commun. 2019;10:221. doi: 10.1038/s41467-018-08096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gur C, Maalouf N, Gerhard M, Singer BB, Emgård J, Temper V, Neuman T, Mandelboim O, Bachrach G. Helicobacter pyloriThe HopQ outermembrane protein inhibits immune cell activities. Oncoimmunology. 2019;8:e1553487. doi: 10.1080/2162402X.2018.1553487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Lu PH, Liu L, Fang ZM, Duan W, Liu ZL, Wang CY, Zhou P, Yu XF, He WT. TIGIT negatively regulates inflammation by altering macrophage phenotype. Immunobiology. 2016;221:48–55. doi: 10.1016/j.imbio.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Sun H, Sun C, Xiao W. Expression regulation of co-inhibitory molecules on human natural killer cells in response to cytokine stimulations. Cytokine. 2014;65:33–41. doi: 10.1016/j.cyto.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, Eaton D, Grogan JL. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 15.Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, Sharpe AH, Kuchroo VK. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186:1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, Stern-Ginossar N, Tsukerman P, Jonjic S, Mandelboim O. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2009;106:17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bi J, Zhang Q, Liang D, Xiong L, Wei H, Sun R, Tian Z. T-cell Ig and ITIM domain regulates natural killer cell activation in murine acute viral hepatitis. Hepatology. 2014;59:1715–1725. doi: 10.1002/hep.26968. [DOI] [PubMed] [Google Scholar]
- 18.Kučan Brlić P, Lenac Roviš T, Cinamon G, Tsukerman P, Mandelboim O, Jonjić S. Targeting PVR (CD155) and its receptors in anti-tumor therapy. Cell Mol Immunol. 2019;16:40–52. doi: 10.1038/s41423-018-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stamm H, Oliveira-Ferrer L, Grossjohann EM, Muschhammer J, Thaden V, Brauneck F, Kischel R, Müller V, Bokemeyer C, Fiedler W, Wellbrock J. Targeting the TIGIT-PVR immune checkpoint axis as novel therapeutic option in breast cancer. Oncoimmunology. 2019;8:e1674605. doi: 10.1080/2162402X.2019.1674605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Donnell JS, Madore J, Li XY, Smyth MJ. Tumor intrinsic and extrinsic immune functions of CD155. Semin Cancer Biol. 2019 doi: 10.1016/j.semcancer.2019.11.013. S1044-579X(19)30403-1. [DOI] [PubMed] [Google Scholar]
- 21.Tahara-Hanaoka S, Shibuya K, Onoda Y, Zhang H, Yamazaki S, Miyamoto A, Honda S, Lanier LL, Shibuya A. Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112) Int Immunol. 2004;16:533–538. doi: 10.1093/intimm/dxh059. [DOI] [PubMed] [Google Scholar]
- 22.Takai Y, Irie K, Shimizu K, Sakisaka T, Ikeda W. Nectins and nectin-like molecules: roles in cell adhesion, migration, and polarization. Cancer Sci. 2003;94:655–667. doi: 10.1111/j.1349-7006.2003.tb01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki K, Hu D, Bustos T, Zlotogora J, Richieri-Costa A, Helms JA, Spritz RA. Mutations of PVRL1, encoding a cell-cell adhesion molecule/herpesvirus receptor, in cleft lip/palate-ectodermal dysplasia. Nat Genet. 2000;25:427–430. doi: 10.1038/78119. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs A, Colonna M. The role of NK cell recognition of nectin and nectin-like proteins in tumor immunosurveillance. Semin Cancer Biol. 2006;16:359–366. doi: 10.1016/j.semcancer.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Sakisaka T, Takai Y. Biology and pathology of nectins and nectin-like molecules. Curr Opin Cell Biol. 2004;16:513–521. doi: 10.1016/j.ceb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Pende D, Castriconi R, Romagnani P, Spaggiari GM, Marcenaro S, Dondero A, Lazzeri E, Lasagni L, Martini S, Rivera P, Capobianco A, Moretta L, Moretta A, Bottino C. Expression of the DNAM-1 ligands, Nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: relevance for natural killer-dendritic cell interaction. Blood. 2006;107:2030–2036. doi: 10.1182/blood-2005-07-2696. [DOI] [PubMed] [Google Scholar]
- 27.Meyer D, Seth S, Albrecht J, Maier MK, du Pasquier L, Ravens I, Dreyer L, Burger R, Gramatzki M, Schwinzer R, Kremmer E, Foerster R, Bernhardt G. CD96 interaction with CD155 via its first Ig-like domain is modulated by alternative splicing or mutations in distal Ig-like domains. J Biol Chem. 2009;284:2235–2244. doi: 10.1074/jbc.M807698200. [DOI] [PubMed] [Google Scholar]
- 28.Seth S, Maier MK, Qiu Q, Ravens I, Kremmer E, Förster R, Bernhardt G. The murine pan T cell marker CD96 is an adhesion receptor for CD155 and nectin-1. Biochem Biophys Res Commun. 2007;364:959–965. doi: 10.1016/j.bbrc.2007.10.102. [DOI] [PubMed] [Google Scholar]
- 29.Gong J, Fang L, Liu R, Wang Y, Xing J, Chen Y, Zhuang R, Zhang Y, Zhang C, Yang A, Zhang X, Jin B, Chen L. UPR decreases CD226 ligand CD155 expression and sensitivity to NK cell-mediated cytotoxicity in hepatoma cells. Eur J Immunol. 2014;44:3758–3767. doi: 10.1002/eji.201444574. [DOI] [PubMed] [Google Scholar]
- 30.Tahara-Hanaoka S, Shibuya K, Kai H, Miyamoto A, Morikawa Y, Ohkochi N, Honda S, Shibuya A. Tumor rejection by the poliovirus receptor family ligands of the DNAM-1 (CD226) receptor. Blood. 2006;107:1491–1496. doi: 10.1182/blood-2005-04-1684. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Luo J, Chen Y, Cui J, Lei Y, Cui Y, Jiang N, Jiang W, Chen L, Chen Y, Kuang Y, Tang K, Ke Z. Combined evaluation of the expression status of CD155 and TIGIT plays an important role in the prognosis of LUAD (lung adenocarcinoma) Int Immunopharmacol. 2020;80:106198. doi: 10.1016/j.intimp.2020.106198. [DOI] [PubMed] [Google Scholar]
- 32.Seth S, Ravens I, Kremmer E, Maier MK, Hadis U, Hardtke S, Förster R, Bernhardt G. Abundance of follicular helper T cells in Peyer’s patches is modulated by CD155. Eur J Immunol. 2009;39:3160–3170. doi: 10.1002/eji.200939470. [DOI] [PubMed] [Google Scholar]
- 33.Liu T, Zhang D, Zhang Y, Xu X, Zhou B, Fang L, Zhang Y, Su Y, Jin B, Zhuang R, Guo S. Blocking CD226 promotes allogeneic transplant immune tolerance and improves skin graft survival by increasing the frequency of regulatory T cells in a murine model. Cell Physiol Biochem. 2018;45:2338–2350. doi: 10.1159/000488182. [DOI] [PubMed] [Google Scholar]
- 34.Harjunpää H, Guillerey C. TIGIT as an emerging immune checkpoint. Clin Exp Immunol. 2020;200:108–119. doi: 10.1111/cei.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z, Yu J, Carson WE 3rd, Bai XF. The role of IL-27 in the induction of anti-tumor cytotoxic T lymphocyte response. Am J Transl Res. 2013;5:470–480. [PMC free article] [PubMed] [Google Scholar]
- 36.Köksal H, Dillard P, Josefsson SE, Maggadottir SM, Pollmann S, Fåne A, Blaker YN, Beiske K, Huse K, Kolstad A, Holte H, Kvalheim G, Smeland EB, Myklebust JH, Inderberg EM, Wälchli S. T cells expressing checkpoint receptor TIGIT are enriched in follicular lymphoma tumors and characterized by reversible suppression of T-cell receptor signaling. Blood Adv. 2018;24:870–881. doi: 10.1158/1078-0432.CCR-17-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samanta D, Guo H, Rubinstein R, Ramagopal UA, Almo SC. Structural, mutational and biophysical studies reveal a canonical mode of molecular recognition between immune receptor TIGIT and nectin-2. Mol Immunol. 2017;81:151–159. doi: 10.1016/j.molimm.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 39.Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18:155. doi: 10.1186/s12943-019-1091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX, Weissman IL. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer. 2019;19:568–586. doi: 10.1038/s41568-019-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li M, Xia P, Du Y, Liu S, Huang G, Chen J, Zhang H, Hou N, Cheng X, Zhou L, Li P, Yang X, Fan Z. T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-γ production of natural killer cells via β-arrestin 2-mediated negative signaling. J Biol Chem. 2014;289:17647–17657. doi: 10.1074/jbc.M114.572420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duan X, Liu J, Cui J, Ma B, Zhou Q, Yang X, Lu Z, Du Y, Su C. Expression of TIGIT/CD155 and correlations with clinical pathological features in human hepatocellular carcinoma. Mol Med Rep. 2019;20:3773–3781. doi: 10.3892/mmr.2019.10641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo D, Liu X, Zeng C, Cheng L, Song G, Hou X, Zhu L, Zou K. Estrogen receptor β activation ameliorates DSS-induced chronic colitis by inhibiting inflammation and promoting Treg differentiation. Int Immunopharmacol. 2019;77:105971. doi: 10.1016/j.intimp.2019.105971. [DOI] [PubMed] [Google Scholar]
- 45.Di Blasi D, Boldanova T, Mori L, Terracciano L, Heim MH, De Libero G. Unique T-cell populations define immune-inflamed hepatocellular carcinoma. Cell Mol Gastroenterol Hepatol. 2020;9:195–218. doi: 10.1016/j.jcmgh.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu C, Xu L, Xia C, Long Y, Liu C, Lu S, Song Y. Increased proportion of functional subpopulations in circulating regulatory T cells in patients with chronic hepatitis B. Hepatol Res. 2020;50:439–452. doi: 10.1111/hepr.13472. [DOI] [PubMed] [Google Scholar]
- 47.Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, Xia J, Tan TG, Sefik E, Yajnik V, Sharpe AH, Quintana FJ, Mathis D, Benoist C, Hafler DA, Kuchroo VK. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40:569–581. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dougall WC, Kurtulus S, Smyth MJ, Anderson AC. TIGIT and CD96: new checkpoint receptor targets for cancer immunotherapy. Immunol Rev. 2017;276:112–120. doi: 10.1111/imr.12518. [DOI] [PubMed] [Google Scholar]
- 49.Williams MA. Instant recall: a key role for effector-phenotype CD8+ memory T cells in immune protection. Immunity. 2013;38:1090–1091. doi: 10.1016/j.immuni.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Li M, Wang X, Dang Z, Jiang Y, Wang X, Kong Y, Yang Z. PD-1 TIGIT CD8 T cells are associated with pathogenesis and progression of patients with hepatitis B virus-related hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68:2041–2054. doi: 10.1007/s00262-019-02426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun H, Huang Q, Huang M, Wen H, Lin R, Zheng M, Qu K, Li K, Wei H, Xiao W, Sun R, Tian Z, Sun C. Human CD96 correlates to natural killer cell exhaustion and predicts the prognosis of human hepatocellular carcinoma. Hepatology. 2019;70:168–183. doi: 10.1002/hep.30347. [DOI] [PubMed] [Google Scholar]
- 52.Han Q, Wang Y, Pang M, Zhang J. STAT3-blocked whole-cell hepatoma vaccine induces cellular and humoral immune response against HCC. J Exp Clin Cancer Res. 2017;36:156. doi: 10.1186/s13046-017-0623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cox MA, Nechanitzky R, Mak TW. Check point inhibitors as therapies for infectious diseases. Curr Opin Immunol. 2017;48:61–67. doi: 10.1016/j.coi.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 54.Chávez-González A, Dorantes-Acosta E, Moreno-Lorenzana D, Alvarado-Moreno A, Arriaga-Pizano L, Mayani H. Expression of CD90, CD96, CD117, and CD123 on different hematopoietic cell populations from pediatric patients with acute myeloid leukemia. Arch Med Res. 2014;45:343–350. doi: 10.1016/j.arcmed.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Chan CJ, Martinet L, Gilfillan S, Souza-Fonseca-Guimaraes F, Chow MT, Town L, Ritchie DS, Colonna M, Andrews DM, Smyth MJ. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat Immunol. 2014;15:431–438. doi: 10.1038/ni.2850. [DOI] [PubMed] [Google Scholar]
- 56.Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, McClanahan T, Kitamura T, Nicholl J, Sutherland GR, Lanier LL, Phillips JH. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4:573–581. doi: 10.1016/s1074-7613(00)70060-4. [DOI] [PubMed] [Google Scholar]
- 57.Kennedy J, Vicari AP, Saylor V, Zurawski SM, Copeland NG, Gilbert DJ, Jenkins NA, Zlotnik A. A molecular analysis of NKT cells: identification of a class-I restricted T cell-associated molecule (CRTAM) J Leukoc Biol. 2000;67:725–734. doi: 10.1002/jlb.67.5.725. [DOI] [PubMed] [Google Scholar]
- 58.Stanietsky N, Rovis TL, Glasner A, Seidel E, Tsukerman P, Yamin R, Enk J, Jonjic S, Mandelboim O. Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. Version 2. Eur J Immunol. 2013;43:2138–2150. doi: 10.1002/eji.201243072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, Eaton DL, Grogan JL. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 60.Judge SJ, Dunai C, Aguilar EG, Vick SC, Sturgill IR, Khuat LT, Stoffel KM, Van Dyke J, Longo DL, Darrow MA, Anderson SK, Blazar BR, Monjazeb AM, Serody JS, Canter RJ, Murphy WJ. Minimal PD-1 expression in mouse and human NK cells under diverse conditions. J Clin Invest. 2020;130:3051–3068. doi: 10.1172/JCI133353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shahbaz S, Dunsmore G, Koleva P, Xu L, Houston S, Elahi S. Galectin-9 and VISTA expression define terminally exhausted T cells in HIV-1 infection. J Immunol. 2020;204:2474–2491. doi: 10.4049/jimmunol.1901481. [DOI] [PubMed] [Google Scholar]
- 62.Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martínez-Colón GJ, McKechnie JL, Ivison GT, Ranganath T, Vergara R, Hollis T, Simpson LJ, Grant P, Subramanian A, Rogers AJ, Blish CA. TIGIT is upregulated by HIV-1 infection and marks a highly functional adaptive and mature subset of natural killer cells. Nat Med. 2020;34:801–813. doi: 10.1097/QAD.0000000000002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohs I, Ducimetière L, Marinho J, Kulig P, Becher B, Tugues S. Restoration of natural killer cell antimetastatic activity by IL12 and checkpoint blockade. Cancer Res. 2017;77:7059–7071. doi: 10.1158/0008-5472.CAN-17-1032. [DOI] [PubMed] [Google Scholar]
- 64.Xu F, Sunderland A, Zhou Y, Schulick RD, Edil BH, Zhu Y. Blockade of CD112R and TIGIT signaling sensitizes human natural killer cell functions. Cancer Immunol Immunother. 2017;66:1367–1375. doi: 10.1007/s00262-017-2031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He Y, Peng H, Sun R, Wei H, Ljunggren HG, Yokoyama WM, Tian Z. Contribution of inhibitory receptor TIGIT to NK cell education. J Autoimmun. 2017;81:1–12. doi: 10.1016/j.jaut.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 66.Dunn SE, Bhat R, Straus DS, Sobel RA, Axtell R, Johnson A, Nguyen K, Mukundan L, Moshkova M, Dugas JC, Chawla A, Steinman L. Peroxisome proliferator-activated receptor delta limits the expansion of pathogenic Th cells during central nervous system autoimmunity. J Exp Med. 2010;207:1599–1608. doi: 10.1084/jem.20091663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noguchi Y, Maeda A, Lo PC, Takakura C, Haneda T, Kodama T, Yoneyama T, Toyama C, Tazuke Y, Okuyama H, Miyagawa S. Human TIGIT on porcine aortic endothelial cells suppresses xenogeneic macrophage-mediated cytotoxicity. Immunobiology. 2019;224:605–613. doi: 10.1016/j.imbio.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 68.Riquelme P, Haarer J, Kammler A, Walter L, Tomiuk S, Ahrens N, Wege AK, Goecze I, Zecher D, Banas B, Spang R, Fändrich F, Lutz MB, Sawitzki B, Schlitt HJ, Ochando J, Geissler EK, Hutchinson JA. TIGIT iTregs elicited by human regulatory macrophages control T cell immunity. Nat Commun. 2018;9:2858. doi: 10.1038/s41467-018-05167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu X, Liu J, Cui P, Liu T, Piao C, Xu X, Zhang Q, Xiao M, Liu X, Wang Y, Yang L. Co-inhibition of TIGIT, PD1, and Tim3 reverses dysfunction of Wilms tumor protein-1 (WT1)-specific CD8+T lymphocytes after dendritic cell vaccination in gastric cancer. Am J Cancer Res. 2018;8:1564–1575. [PMC free article] [PubMed] [Google Scholar]
- 70.Martinet J, Leroy V, Dufeu-Duchesne T, Larrat S, Richard MJ, Zoulim F, Plumas J, Aspord C. Plasmacytoid dendritic cells induce efficient stimulation of antiviral immunity in the context of chronic hepatitis B virus infection. Hepatology. 2012;56:1706–1718. doi: 10.1002/hep.25879. [DOI] [PubMed] [Google Scholar]
- 71.Tang X, Li Q, Zhu Y, Zheng D, Dai J, Ni W, Wei J, Xue Y, Chen K, Hou W, Zhang C, Feng X, Liang Y. The advantages of PD1 activating chimeric receptor (PD1-ACR) engineered lymphocytes for PDL1(+) cancer therapy. Am J Transl Res. 2015;7:460–473. [PMC free article] [PubMed] [Google Scholar]
- 72.Kung-Chun Chiu D, Wai-Hin Yuen V, Wing-Sum Cheu J, Wei LL, Ting V, Fehlings M, Sumatoh H, Nardin A, Newell EW, Oi-Lin Ng I, Chung-Cheung Yau T, Wong CM, Chak-Lui Wong C. Hepatocellular carcinoma cells upregulate PVRL1, stabilizing PVR and inhibiting the cytotoxic T-cell response via TIGIT to mediate tumor resistance to PD1 inhibitors in mice. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.074. S0016-5085(20)30461-3. [DOI] [PubMed] [Google Scholar]
- 73.Benson DM Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang J, Yu J, Smith MK, Greenfield CN, Porcu P, Devine SM, Rotem-Yehudar R, Lozanski G, Byrd JC, Caligiuri MA. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116:2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodríguez-Iñigo E, Bartolomé J, Ortiz-Movilla N, Platero C, López-Alcorocho JM, Pardo M, Castillo I, Carreño V. Hepatitis C virus (HCV) and hepatitis B virus (HBV) can coinfect the same hepatocyte in the liver of patients with chronic HCV and occult HBV infection. J Virol. 2005;79:15578–15581. doi: 10.1128/JVI.79.24.15578-15581.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Z, Li B, Peng D, Xing H, Wang G, Li P, Wang J, Ye G, Chen J. Expression and clinical significance of PD1 in hepatocellular carcinoma tissues detected by a novel mouse anti-human PD1 monoclonal antibody. Int J Oncol. 2018;52:2079–2092. doi: 10.3892/ijo.2018.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim HD, Song GW, Park S, Jung MK, Kim MH, Kang HJ, Yoo C, Yi K, Kim KH, Eo S, Moon DB, Hong SM, Ju YS, Shin EC, Hwang S, Park SH. Association between expression level of PD1 by tumor-infiltrating CD8 T cells and features of hepatocellular carcinoma. Gastroenterology. 2018;155:1936–1950. e1917. doi: 10.1053/j.gastro.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 78.Song Y, Wang B, Song R, Hao Y, Wang D, Li Y, Jiang Y, Xu L, Ma Y, Zheng H, Kong Y, Zeng H. T-cell immunoglobulin and ITIM domain contributes to CD8 T-cell immunosenescence. Aging Cell. 2018;17:e12716. doi: 10.1111/acel.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Page N, Klimek B, De Roo M, Steinbach K, Soldati H, Lemeille S, Wagner I, Kreutzfeldt M, Di Liberto G, Vincenti I, Lingner T, Salinas G, Brück W, Simons M, Murr R, Kaye J, Zehn D, Pinschewer DD, Merkler D. Expression of the DNA-binding factor TOX promotes the encephalitogenic potential of microbe-induced autoreactive CD8 T cells. Immunity. 2019;50:763. doi: 10.1016/j.immuni.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 80.Wang X, He Q, Shen H, Xia A, Tian W, Yu W, Sun B. TOX promotes the exhaustion of antitumor CD8 T cells by preventing PD1 degradation in hepatocellular carcinoma. J Hepatol. 2019;71:731–741. doi: 10.1016/j.jhep.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 81.Joller N, Kuchroo VK. Tim-3, Lag-3, and TIGIT. Curr Top Microbiol Immunol. 2017;410:127–156. doi: 10.1007/82_2017_62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fromentin R, Bakeman W, Lawani MB, Khoury G, Hartogensis W, DaFonseca S, Killian M, Epling L, Hoh R, Sinclair E, Hecht FM, Bacchetti P, Deeks SG, Lewin SR, Sékaly RP, Chomont N. CD4+T cells expressing PD-1, TIGIT and LAG-3 contribute to HIV persistence during ART. PLoS Pathog. 2016;12:e1005761. doi: 10.1371/journal.ppat.1005761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guillerey C, Harjunpää H, Carrié N, Kassem S, Teo T, Miles K, Krumeich S, Weulersse M, Cuisinier M, Stannard K, Yu Y, Minnie SA, Hill GR, Dougall WC, Avet-Loiseau H, Teng MWL, Nakamura K, Martinet L, Smyth MJ. TIGIT immune checkpoint blockade restores CD8 T-cell immunity against multiple myeloma. Blood. 2018;132:1689–1694. doi: 10.1182/blood-2018-01-825265. [DOI] [PubMed] [Google Scholar]
- 85.Manieri NA, Chiang EY, Grogan JL. TIGIT: a key inhibitor of the cancer immunity cycle. Trends Immunol. 2017;38:20–28. doi: 10.1016/j.it.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 86.Zhou XM, Li WQ, Wu YH, Han L, Cao XG, Yang XM, Wang HF, Zhao WS, Zhai WJ, Qi YM, Gao YF. In vivoIntrinsic expression of immune checkpoint molecule TIGIT could help tumor growth by suppressing the function of NK and CD8 T cells. Front Immunol. 2018;9:2821. doi: 10.3389/fimmu.2018.02821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Érsek B, Silló P, Cakir U, Molnár V, Bencsik A, Mayer B, Mezey E, Kárpáti S, Pós Z, Németh K. Melanoma-associated fibroblasts impair CD8+T cell function and modify expression of immune checkpoint regulators via increased arginase activity. Cell Mol Life Sci. 2020 doi: 10.1007/s00018-020-03517-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu L, Mao L, Liu JF, Chen L, Yu GT, Yang LL, Wu H, Bu LL, Kulkarni AB, Zhang WF, Sun ZJ. Blockade of TIGIT/CD155 signaling reverses T-cell exhaustion and enhances antitumor capability in head and neck squamous cell carcinoma. Cancer Immunol Res. 2019;7:1700–1713. doi: 10.1158/2326-6066.CIR-18-0725. [DOI] [PubMed] [Google Scholar]
- 89.Zhang C, Lin R, Li Z, Yang S, Bi X, Wang H, Aini A, Zhang N, Abulizi A, Sun C, Li L, Zhao Z, Qin R, Li X, Li L, Aji T, Shao Y, Vuitton DA, Tian Z, Wen H. Immune exhaustion of T cells in alveolar echinococcosis patients and its reversal by blocking checkpoint receptor TIGIT in a murine model. Hepatology. 2020;71:1297–1315. doi: 10.1002/hep.30896. [DOI] [PubMed] [Google Scholar]
- 90.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 91.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang JH, Jiao LY, Li TJ, Zhu YY, Zhou JW, Tian J. In vitroGSK-3β suppresses HCC cell dissociation by upregulating epithelial junction proteins and inhibiting Wnt/β-catenin signaling pathway. J Cancer. 2017;8:1598–1608. doi: 10.7150/jca.18744. [DOI] [PMC free article] [PubMed] [Google Scholar]


