Abstract
STAT3/mTOR pathway plays an important role in inflammation, cell growth, and proliferation. However, the role of STAT3/mTOR pathway in chronic kidney injury remains unclear. Folic acid was used to induce kidney injury C57BL/6 mouse model followed by analysis of serum creatinine, renal weight ratio changes, renal pathological changes and STAT3/mTOR pathway changes. Glomerular mesangial cells were divided into control group, model group, STAT3 inhibitor (S3I-201) group followed by analysis of cell proliferation by MTT assay, cell apoptosis by flow cytometry, formation of autophagosomes by electron microscopy, expression of STAT3/mTOR signaling proteins and autophagy proteins LC3II and p62 by Western blot, expression of E-cadherin and Vimentin by immunofluorescence. The serum creatinine and renal weight ratio was increased with obvious lesions and upregulated STAT3 and p-mTOR level. Compared with control group, the difference was statistically significant (P < 0.05). Folic acid-induced injury of mesangial cells showed inhibited cell proliferation, promoted apoptosis, increased LC3II expression, decreased p62 expression, increased autophagic vacuoles and expression of STAT3 and p-mTOR as well as decreased E-cadherin expression and increased Vimentin expression. The difference was statistically significant compared with control group (P < 0.05). All above changes were significantly reversed after treatment with STAT3 inhibitor S3I-201 (P < 0.05). Activated STAT3/mTOR pathway, enhanced autophagy, promoted apoptosis of mesangial cells and inhibited cell proliferation were found in mice with renal injury. Inhibition of STAT3/mTOR activation inhibits autophagy and cell apoptosis and promotes cell proliferation.
Keywords: Kidney injury, STAT3/mTOR, autophagy, apoptosis, proliferation
Introduction
Kidney injury is one of the common diseases and can cause different degrees of damage to the kidney and blood vessels, and is common in urinary tract injuries [1,2]. Kidney injury can be divided into acute kidney injury and chronic kidney injury according to the disease development progression. Chronic kidney injury is also more common in chronic kidney disease, which can lead to impaired renal function after acute kidney injury [3]. The kidney is an important organ of the body that can be used to remove metabolic byproducts, remove toxins, regulate body volume, electrolytes and systemic hemodynamics, and produce hormones such as erythropoietin and active vitamins. The internal environment of the body plays a central role in the balance [4,5]. In recent years, the incidence of kidney injury worldwide and the mortality caused by kidney damage have increased significantly, possessing serious health and economic problems for patients and their families and society. Therefore, how to effectively prevent kidney injury becomes a concern [6,7]. The mechanism of kidney injury is very complicated, involving inflammation, oxidative stress, ischemia and hypoxia which only determine the type of kidney injury, but also are closely related to the anatomical parts of the nephron damage (including glomeruli, fallopian tubes, mesangial, vascular system) [8,9]. Kidney injury leads to tubulointerstitial damage and fibrosis, resulting in progressive chronic kidney disease and end-stage renal failure [10]. Therefore, understanding the underlying mechanisms by which changes can lead to kidney injury has clinical significance to prevent and treat kidney disease.
The pathogenesis of kidney injury is complex and autophagy is of great significance in the process of kidney injury. Autophagy is an important regulatory mechanism for maintaining cell homeostasis. Degradation of cytosolic macromolecules and organelles by lysosomal enzymes is a highly conserved physiological mechanism [11,12]. There are many regulatory signals in autophagy. Signal transduction and transcriptional activator 3 (STAT3) is a highly conserved family of transcription factors that can be expressed in many tissues and cells such as the heart, brain, liver, skeletal muscle, fibroblasts, and immune cells, and participates in several processes such as cell proliferation, autophagy, and apoptosis [13,14]. The rapamycin target protein (mTOR) is an important autophagy-related factor whose activation can be involved in the process of autophagy [15,16]. However, the role of the STAT3/mTOR signaling pathway in kidney injury and whether it is associated with autophagy has not been reported.
Materials and methods
Experimental animals
20 healthy male C57BL/6 mice, 2 months old, SPF grade, body weight (20 ± 2) g, were purchased from the experimental animal center of the unit and fed IN SPF animal experiment center. Feeding conditions included maintaining the temperature at (21 ± 1)°C and maintaining relative humidity (50-70%) under constant temperature and constant humidity conditions, ensuring a 12/day cycle every 12 hours. Animal experiments were performed in strict accordance with the experimental design and performed by experienced technicians to minimize animal suffering. The study was approved by the Ethics Committee of our hospital and all tests met the animal welfare standards.
Reagents and instruments
Sodium pentobarbital was purchased from Shanghai Zhaohui Pharmaceutical Co., Ltd. The mesangial cell line is provided by my laboratory and stored in liquid nitrogen. Folic acid and the STAT3 inhibitor S3I-201 were purchased from Sigma, USA. Fetal bovine serum (FBS) and cyan chain double antibody were purchased from Hyclone Corporation of the United States. Dimethyl sulfoxide, MTT powder was purchased from Gibco; trypsin-EDTA digest was purchased from Sigma, USA. Western blot related chemical reagents were purchased from Shanghai Biyuntian Biotechnology Co., Ltd., ECL reagents were purchased from Amersham Biosciences, rabbit anti-mouse RANKL monoclonal antibody, rabbit anti-mouse STAT3/mTOR monoclonal antibody, rabbit anti-mouse LC3II monoclonal antibody, rabbit anti-mouse p62 Monoclonal antibody, rabbit anti-mouse E-Cadherin monoclonal antibody and rabbit anti-mouse Vimentin monoclonal antibody, goat anti-rabbit horseradish peroxidase (HRP) labeled IgG secondary antibody were purchased from Cell signaling Corporation of the United States. Other commonly used reagents were purchased from Shanghai Shenggong Biological Co., Ltd. The Labsystem Version 1.3.1 microplate reader was purchased from Bio-rad Corporation of the United States. The clean workbench was purchased from Suzhou Purification Equipment Factory in Jiangsu Province. Surgical microscopy equipment was purchased from Suzhou Medical Instrument Factory. The Hera cell CO2 incubator was purchased from Thermo Company, Germany. BX43 fluorescence microscope and electron microscope were purchased from Olympus Corporation of Japan. The OLYMPUSAU 2700 automatic biochemical analyzer was purchased from Olympus Japan.
Preparation of kidney injury model
20 healthy male C57BL/6 mice were adaptively reared for 2 weeks and randomly divided into 2 groups. The model group was intraperitoneally injected with folic acid at a dose of 250 mg/kg (dissolved in 150 mM NaHCO3, folic acid concentration 24 mg/ml) for 3 consecutive days. Normal control group: NaHCO3 (150 mM, liquid amount 0.2 ml/10 g) was intraperitoneally injected. After modeling, 0.2 ml of mouse plasma was collected and the level of creatinine was measured by an automatic biochemical analyzer. Then mice were sacrificed and the kidney was weighed [17].
Sample collection
After treatment, mice in each group were weighed, and 3 ml of tail vein blood was taken and placed in a vacuum blood collection tube containing anticoagulant. The blood was placed in a 37°C incubator for 1 h and centrifuged at 3000 rpm for 5 min. The supernatant was placed in an EP tube and analyzed by fully automated biochemical analyzer, and the rest was stored in a -20°C refrigerator. Kidney tissues of mice from each group were collected, weighed, and stored in a -80°C refrigerator.
Masson staining
Tissues were fixed in deionized water at 37°C for 30 s and washed with deionized water. Two 1.5 ml EP tubes were prepared and 0.5 ml Sodium Nitrite Solution was added and mixed for 20 s. The mixture was allowed to stand at room temperature for 2 min, and the two were mixed. The prepared staining solution was poured into a dyeing tank, and the water was heated to 37°C. The climbing piece was placed in a dyeing tank and incubated at 37°C for 1 h followed by being counterstained in hematoxylin stain for 2 min and subsequent rinse with alkaline tap water, dry naturally, glycerin-sealed, and observation under the microscope.
Culture and grouping of mesangial cell lines
Human glomerular mesangial cell strain was stored in liquid nitrogen, thawed in 37°C water bath until the cells were completely thawed, centrifuged at 1000 rpm for 3 min, resuspended in 1 ml fresh culture medium, transferred to a 50 ml cell culture flask, followed by addition of 4 ml fresh culture solution and cultured at 37°C with 5% CO2 for 24-48 hours. The mesangial cells were cultured in DMEM containing 10% heat-inactivated fetal bovine serum and 10 U/mL penicillin and 10 μg/mL streptomycin in a 37°C, 5% CO2 incubator to maintain the number of cells at 1 × 106/culture flask. The subcultured DMEM cells were diluted to 1 × 106/ml, inoculated in a 35 mm culture dish, and cultured in a serum-free DMEM medium containing 100 ng/ml PMA and 0.3% BSA for 24 hours. The 3-8 generation logarithmic growth phase glomerular mesangial cells were randomly divided into 3 groups, control group (normal cultured cells); model group (2 μM folic acid was added to the cells to prepare the folate induced injury model); STAT3 inhibitor group (folate-induced injury model was treated with 5 μM STAT3 inhibitor S3I-201 [18]).
MTT assay for detecting proliferation of glomerular mesangial cell
The logarithmic growth phase of mesangial cells was collected and cultured in a 96-well culture plate in 10% fetal bovine serum α-MEM culture medium at a concentration of 5 × 103, and the supernatant was discarded after 24 hours of culture. 20 ul of sterile MTT was added and 3 replicate wells were set at each time point. After culture for 4 h, the supernatant was completely removed followed by addition of DMSO 150 μl/well until the purple crystals were fully dissolved. Then, the absorbance (A) value was measured at a wavelength of 570 nm by a microplate reader and the proliferation rate of each group was calculated.
Flow cytometry detection of cell apoptosis
Cells were collected after addition of 1 ml of 0.25% trypsin to digest cells. After cells were rounded and some cells were suspended, PBS was added to terminate the digestion. The cells were collected in a flow tube, centrifuged at 1500 rpm for 5 min, the supernatant was discarded and cells were completely resuspended in 3 ml of 4°C pre-cooled PBS, centrifuged at 1500 rpm for 5 min, and the supernatant was discarded. The pellet was resuspended in 300 μL of Binding Buffer followed by addition of 5 μL of Annexin V-FITC and 5 μL of Propidium Iodide. The reaction was protected from light for 5-15 minutes at room temperature followed by analysis of cell apoptosis by flow cytometry.
Electron microscopy for autophagy
The cells were directly scraped off, centrifuged to discard the supernatant, washed twice with PBS, centrifuged and fixed in 2.5% glutaraldehyde at 4°C. Ultra-thin sectioning was then performed and autophagy was observed under an electron microscopy.
Immunofluorescence staining
Cells were fixed in 4% paraformaldehyde at room temperature for 20 minutes. The paraformaldehyde was aspirated and washed 3 times with PBS for 5 min each time. 0.5% Triton X-100 (prepared in PBS) was then added and incubated for 20 min at room temperature. The Triton X-100 was removed and washed 3 times with PBS for 5 min each. 5% BSA was blocked for 2 hours. The blocking solution was aspirated, and the primary antibody was added and incubated overnight in a 4°C wet box. The primary antibody was aspirated and washed 3 times with PBS for 5 min each time. Then, secondary antibody at the appropriate concentration was added and incubated at 37°C for 1 hour at room temperature in the dark. The secondary antibody was aspirated and washed 3 times with PBS for 5 min each time. DAPI was added to the slide, or Hoechst counterstained the nuclei and incubated for 5-10 min in the dark.
Western blot analysis of STAT3/mTOR, LC3II, and p62 protein expression
The kidney tissues of each group were extracted, ground under liquid nitrogen, and the mesangial cell protein was extracted: the lysate was added, and the protein was quantified and stored at -20°C for Western blot. Proteins were separated on 10% SDS-PAGE electrophoresis and transferred to PVDF membrane. After 1 h of blocking, the diluted primary antibody STAT3/mTOR, p-mTOR, LC3II, p62 protein (diluted concentrations: 1:1000, 1:1000, 1:1000, 1:2000, 1:1000) were added and incubated at 4°C overnight. After PBST washing, 1:2000 goat anti-rabbit secondary antibody was added and incubated for 30 min followed by washing with PBST, addition of chemiluminescence for 1 min, X-ray exposure and development. X-film and strip density measurements were separately scanned using protein image processing system software and Quantity one software. The experiment was repeated four times (n=4).
Statistical analysis
Data were analyzed by SPSS 16.0 statistical software. The count data was expressed as a percentage and a chi-square test was performed. Measurement data were expressed as mean ± standard deviation (SD) and comparison of multiple groups of samples was performed using one-way ANOVA. P < 0.05 was considered statistically significant.
Results
Changes in serum creatinine and kidney weight ratio in mice with kidney injury
In mice with kidney injury, serum creatinine and kidney weight ratio was increased with obvious lesions and increased STAT3 and p-mTOR expression. Compared with the control group, the difference was statistically significant (P < 0.05) (Figure 1).
Figure 1.
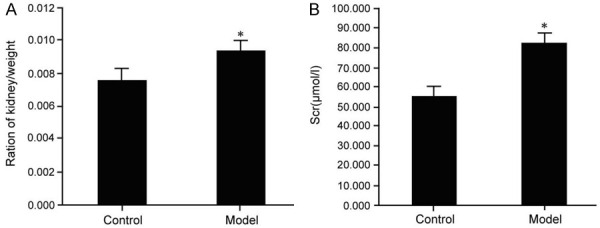
Changes in kidney weight ratio and serum creatinine in mice with kidney injury. Kidney injury mice model was established through injection of folic acid followed by analysis of mice kidney weight ratio (A) and serum creatinine level (B); compared with the control group, *P < 0.05.
Pathological changes in mice with kidney injury
Pathological changes in injured mice were analyzed by Masson staining. There were no lesions in the kidney glomeruli, renal tubules and renal interstitial in the control group. In the model group, most of the glomeruli of the kidneys showed mesangial cell proliferation and increased matrix. Some of the glomerular capillaries were compressed and lobulated, and the morphology was irregular. Fibrin exudate was found in the Bowman’s capsule. A small amount of inflammatory cells was infiltrated into the tubule interstitial, the renal tubules in the concentrated area of inflammation disappeared, the surrounding renal tubular epithelium was swollen and vacuolated, and the lumen was occluded (Figure 2).
Figure 2.

Pathological changes in mice with masson-stained kidney injury (× 100). Kidney injury mice model was established through injection of folic acid followed by analysis of the kidney pathological changes in control mice and model mice.
Analysis of STAT3/mTOR pathway changes in mice with kidney injury
Western blot analysis of STAT3/mTOR pathway changes in mice with kidney injury showed that STAT3/mTOR pathway was activated as demonstrated by increased STAT3 expression and p-mTOR phosphorylation in mice with kidney injury. The difference was statistically significant compared with control group (P < 0.05) (Figure 3).
Figure 3.
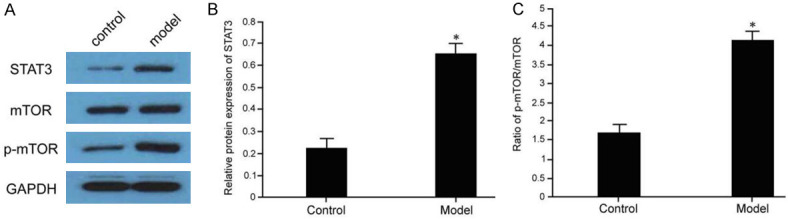
Analysis of STAT3/mTOR pathway changes in mice with kidney injury. The kidney tissues were collected from control or model mice followed by western blot analysis of STAT3/mTOR signaling protein expression (A) and subsequent statistical analysis of STAT3 expression (B) and mTOR phosphorylation level (C); compared with the control group, *P < 0.05.
Effect of STAT3/mTOR pathway on proliferation of injured mesangial cells
MTT assay was used to analyze the effect of STAT3/mTOR pathway on the proliferation of mesangial cells induced by folic acid. After folic acid injury, the proliferation of mesangial cells in the model group was inhibited. Compared with the control group, the difference was statistically significant (P < 0.05); Addition of STAT3 inhibitor can promote the proliferation of injured mesangial cells, compared with the model group, the difference was statistically significant (P < 0.05) (Figure 4).
Figure 4.
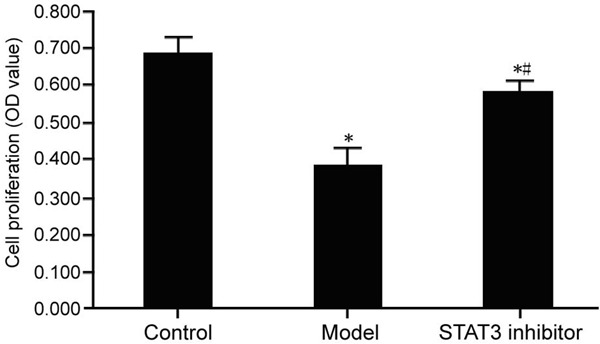
Effect of STAT3/mTOR pathway on proliferation of injured mesangial cells. The glomerular mesangial cells were randomly divided into control group (normal cultured cells); model group (2 μM folic acid was added to the cells to prepare the folate induced injury model); and STAT3 inhibitor group (folate-induced injury model was treated with 5 μM STAT3 inhibitor S3I-201) followed by measuring cell proliferation by MTT assay. Compared with the control group, *P < 0.05; compared with the model group, #P < 0.05.
Effect of STAT3/mTOR pathway on apoptosis of injured mesangial cells
After folic acid injury, the apoptosis of glomerular mesangial cells in the model group was increased, and the apoptosis rate was increased. Compared with the control group, the difference was statistically significant (P < 0.05). After addition of STAT3 inhibitor, it could inhibit the apoptosis of glomerular mesangial cells and decrease the apoptotic rate. Compared with the model group, the difference was statistically significant (P < 0.05) (Figure 5).
Figure 5.

Effect of STAT3/mTOR pathway on apoptosis of injured mesangial cells. The glomerular mesangial cells were randomly divided into control group, model group (2 μM folic acid was added to the cells to prepare the folate induced injury model); and STAT3 inhibitor group (folate-induced injury model was treated with 5 μM STAT3 inhibitor S3I-201) and then cell apoptosis was measured by flow cytometry using Annexin V-FITC and Propidium Iodide (A). Quantification of cell apoptosis was shown in panel (B). Compared with the control group, *P < 0.05; compared with the model group, #P < 0.05.
Electron microscopic analysis of the effect of STAT3/mTOR pathway on autophagy of injured mesangial cells
We found that in the control group, the cell morphology was normal and there were fewer autophagosomes, while a large number of autophagic lysosomal vesicles were found in the model group. In the STAT3 inhibitor group, the damaged mesangial membrane in the model group was significantly reduced with decreased autophagosome formation in cells (Figure 6).
Figure 6.
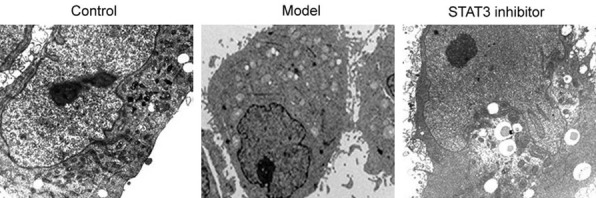
Electron microscopic analysis of autophagy in injured mesangial cells. The glomerular mesangial cells were randomly divided into control group, model group (2 μM folic acid was added to the cells to prepare the folate induced injury model); and STAT3 inhibitor group (folate-induced injury model was treated with 5 μM STAT3 inhibitor S3I-201) followed by analysis of autophagy of mesangial cells by electron microscopic.
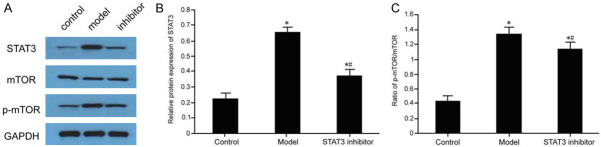
Alteration analysis of STAT3/mTOR pathway in injured mesangial cells
Western blot analysis of STAT3/mTOR pathway changes in injured mesangial cells showed that STAT3/mTOR pathway was activated, STAT3 expression was increased, and p-mTOR phosphorylation was increased in the injured mesangial cell model group. The difference was statistically significant compared with control group (P < 0.05). After addition of STAT3 inhibitor, STAT3/mTOR pathway activation was inhibited, STAT3 expression and p-mTOR phosphorylation was reduced. The difference was statistically significant compared with model group (P < 0.05) (Figure 7).
Figure 7.
Alteration analysis of STAT3/mTOR pathway in injured mesangial cells. Total protein was isolated of cells from control, model and STAT3 inhibitor group followed by western blot analysis of STAT3/mTOR signaling protein expression (A) and subsequent statistical analysis of STAT3 level (B) and mTOR phosphorylation level (C); compared with the control group, *P < 0.05; compared with the model group, #P < 0.05.
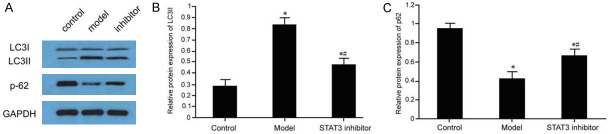
Effect of STAT3/mTOR pathway on autophagy protein in injured mesangial cells
The expression of LC3II and the expression of p62 protein in glomerular mesangial cells were decreased in folic acid-induced injury. Compared with the control group, the difference was statistically significant (P < 0.05). After addition of STAT3 inhibitor, it could inhibit the expression of LC3II in injured mesangial cells and promote the increase of p62 protein expression, compared with the model group, the difference was statistically significant (P < 0.05) (Figure 8).
Figure 8.
Effect of STAT3/mTOR pathway on autophagy protein in injured mesangial cells. Total protein was isolated of cells from control, model and STAT3 inhibitor group followed by western blot analysis of autophagy protein level in injured mesangial cells (A) and subsequent statistical analysis of LC3II expression (B) and p62 expression (C); compared with control group, *P < 0.05; Comparison, #P < 0.05.
Effect of STAT3/mTOR pathway on depolarization of injured glomerular mesangial cells
The effect of STAT3/mTOR pathway on the expression of depolarization-related proteins in injured mesangial cells was analyzed by immunofluorescence. The results showed that the expression of E-cadherin was decreased and the expression of Vimentin was increased after folic acid-induced injury. The addition of S3I-201 reversed the kidney injury, increased the expression of E-cadherin and decreased the expression of Vimentin (Figure 9).
Figure 9.

Immunofluorescence assay for the effect of STAT3/mTOR pathway on loss of mesangial cells EMT. Cells from control, model and STAT3 inhibitor group were fixed in 4% paraformaldehyde and permeabilized by 0.5% Triton X-100 followed by immunofluorescence analysis of depolarization of injured glomerular mesangial cells.
Discussion
STAT3 and tyrosine phosphorylation signal pathways are coupled to form a bifunctional protein and participate in the regulation of the expression of several functional proteins which are associated with cell proliferation and apoptosis. Activation of STAT3 can lead to abnormal cell proliferation and apoptosis and evasion [19]. Mammalian mTOR is also present in the cytoplasm and belongs to the silk threonine protein kinase. Different stimulation signals can activate or block mTOR activation [20]. The STAT3/mTOR pathway can cause inflammation and damage in the body [21]. This study confirmed that the mouse model of chronic kidney injury prepared by folic acid can cause an increase in the kidney weight ratio of injured mice and elevated serum creatinine in most glomeruli of the kidney. It is confirmed that the kidney injury model was successfully prepared, and further studies have shown that during kidney injury process, the STAT3/mTOR pathway was activated, suggesting that the STAT3/mTOR pathway might be involved in the kidney injury process.
Mesangial cells are an important part of the kidney. The kidney is a high-energy and high-metabolizing organ. Mesangial cell is a highly differentiated cell and susceptible to various diseases such as hypoxia and metabolic disorders. Many studies have found that chronic kidney injury-induced mall mesangial cell damage is an important cause of chronic fibrosis [22]. At the time of injury, after glomerular mesangial cells are damaged, proliferation is reduced, apoptosis is increased, depolarization occurs, and typical epithelial proteins such as E-cadherin are lost, while vimentin (Vimentin) expression is increased which can promote the occurrence of renal interstitial fibrosis [23]. This study confirmed that folic acid-induced kidney injury leads to increased apoptosis of mesangial cells, inhibited cell proliferation, decreased expression of E-cadherin, and increased expression of Vimentin, suggesting that glomerular mesangial cells are depolarized during kidney injury, resulting in the appearance of interstitial cell phenotype, causing kidney damage. After adding STAT3 inhibitor, the degeneration of mesangial cells caused by kidney injury can be reversed. Further studies have shown that a large number of autophagic lysosomal vesicles are present in the mesangial cells caused by kidney injury, and the expression of LC3II is increased and the expression of p62 protein is decreased. In the STAT3 inhibitor group, the damaged kidneys in the model group can be significantly reduced. The formation of autophagosomes in the mesangial cells and LC3II level was decreased, and the expression of p62 protein was increased, suggesting that STAT3/mTOR pathway is involved in the autophagy process of kidney injury. The reduction in the clearance of misfolded proteins and damaged organelles due to defects in autophagy eventually leads to a decrease in the number of mesangial cells and aggravation of depolarization, which in turn causes kidney damage [24,25]. The STAT3/mTOR pathway affects autophagy, which in turn affects depolarization and proliferation of mesangial cells, thereby delaying kidney damage.
Conclusion
STAT3/mTOR pathway is activated in mice with kidney injury and addition of STAT3 inhibitor S3I-201 inhibits STAT3/mTOR pathway activation, inhibits autophagy and apoptosis and promotes cell proliferation.
Disclosure of conflict of interest
None.
References
- 1.Pilarczyk K, Carstens H, Papathanasiou M, Ludike P, Koch A, Jakob H, Kamler M, Pizanis N. Prediction of acute kidney injury after left ventricular assist device implantation: evaluation of clinical risk scores. Artif Organs. 2020;44:162–173. doi: 10.1111/aor.13548. [DOI] [PubMed] [Google Scholar]
- 2.Sedaghat Z, Kadkhodaee M, Seifi B, Salehi E. Inducible and endothelial nitric oxide synthase distribution and expression with hind limb per-conditioning of the rat kidney. Arch Med Sci. 2019;15:1081–1091. doi: 10.5114/aoms.2019.85651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castellano G, Stasi A, Franzin R, Sallustio F, Divella C, Spinelli A, Netti GS, Fiaccadori E, Cantaluppi V, Crovace A, Staffieri F, Lacitignola L, Grandaliano G, Simone S, Pertosa GB, Gesualdo L. LPS-binding protein modulates acute renal fibrosis by inducing pericyte-to-myofibroblast trans-differentiation through TLR-4 signaling. Int J Mol Sci. 2019;20:3682. doi: 10.3390/ijms20153682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao L, Zhi D, Han J, Kumar Sah S, Xie Y. Combinational effect of curcumin and metformin against gentamicin-induced nephrotoxicity: involvement of antioxidative, anti-inflammatory and antiapoptotic pathway. J Food Biochem. 2019;43:e12836. doi: 10.1111/jfbc.12836. [DOI] [PubMed] [Google Scholar]
- 5.Shen Z, Lin J, Teng J, Zhuang Y, Zhang H, Wang C, Zhang Y, Ding X, Zhang X. Association of urinary ionomic profiles and acute kidney injury and mortality in patients after cardiac surgery. J Thorac Cardiovasc Surg. 2020;159:918–926. e5. doi: 10.1016/j.jtcvs.2019.02.095. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Mao L, Xu Y, Wu X. Endothelial-specific deletion of Brahma-related gene 1 (BRG1) assuages unilateral ureteral obstruction induced renal injury in mice. Biochem Biophys Res Commun. 2019;517:244–252. doi: 10.1016/j.bbrc.2019.07.077. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Meseguer J, Torres-Gonzalez L, Gutierrez-Gonzalez JA, Alarcon-Galvan G, Zapata-Chavira H, Waksman-de Torres N, Moreno-Pena DP, Munoz-Espinosa LE, Cordero-Perez P. Anti-inflammatory and nephroprotective activity of Juglans mollis against renal ischemia-reperfusion damage in a Wistar rat model. BMC Complement Altern Med. 2019;19:186. doi: 10.1186/s12906-019-2604-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rewa OG, Tolwani A, Mottes T, Juncos LA, Ronco C, Kashani K, Rosner M, Haase M, Kellum J, Bagshaw SM ADQI Consensus Meeting Members on behalf of ADQI XXII. Quality of care and safety measures of acute renal replacement therapy: workgroup statements from the 22nd acute disease quality initiative (ADQI) consensus conference. J Crit Care. 2019;54:52–57. doi: 10.1016/j.jcrc.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Lu M, Wang P, Qiao Y, Jiang C, Ge Y, Flickinger B, Malhotra DK, Dworkin LD, Liu Z, Gong R. GSK3beta-mediated Keap1-independent regulation of Nrf2 antioxidant response: a molecular rheostat of acute kidney injury to chronic kidney disease transition. Redox Biol. 2019;26:101275. doi: 10.1016/j.redox.2019.101275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chua JT, Argueta DA, DiPatrizio NV, Kovesdy CP, Vaziri ND, Kalantar-Zadeh K, Moradi H. Endocannabinoid system and the kidneys: from renal physiology to injury and disease. Cannabis Cannabinoid Res. 2019;4:10–20. doi: 10.1089/can.2018.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong CW, Zhilenkov AV, Kraevaya OA, Mischenko DV, Troshin PA, Hsu SH. Toward understanding the antitumor effects of water-soluble fullerene derivatives on lung cancer cells: apoptosis or autophagy pathways? J Med Chem. 2019;62:7111–7125. doi: 10.1021/acs.jmedchem.9b00652. [DOI] [PubMed] [Google Scholar]
- 12.Tseng TY, Chiou HL, Lin CW, Chen YS, Hsu LS, Lee CH, Hsieh YH. Repression of metastasis-associated protein 2 for inhibiting metastasis of human oral cancer cells by promoting the p-cofilin-1/LC3-II expression. J Oral Pathol Med. 2019;48:959–966. doi: 10.1111/jop.12941. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Chen C, Xu XD, Li H, Chen MH, Liu J, Tang LJ. Levels of miR-125a-5p are altered in Mycobacterium avium-infected macrophages and associate with the triggering of an autophagic response. Microbes Infect. 2020;22:31–39. doi: 10.1016/j.micinf.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Larrue C, Heydt Q, Saland E, Boutzen H, Kaoma T, Sarry JE, Joffre C, Recher C. Oncogenic KIT mutations induce STAT3-dependent autophagy to support cell proliferation in acute myeloid leukemia. Oncogenesis. 2019;8:39. doi: 10.1038/s41389-019-0148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miao B, Qi WJ, Zhang SW, Wang H, Wang C, Hu L, Huang GW, Li SR. miR-148a suppresses autophagy by down-regulation of IL-6/STAT3 signaling in cerulein-induced acute pancreatitis. Pancreatology. 2019;19:557–565. doi: 10.1016/j.pan.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Yuan W, Chang H, Liu X, Wang S, Liu H, Xuan H. Brazilian green propolis inhibits Ox-LDL-stimulated oxidative stress in human umbilical vein endothelial cells partly through PI3K/Akt/mTOR-Mediated Nrf2/HO-1 pathway. Evid Based Complement Alternat Med. 2019;2019:5789574. doi: 10.1155/2019/5789574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egli-Spichtig D, Zhang MYH, Perwad F. Fibroblast growth factor 23 expression is increased in multiple organs in mice with folic acid-induced acute kidney injury. Front Physiol. 2018;9:1494. doi: 10.3389/fphys.2018.01494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durlacher-Betzer K, Hassan A, Levi R, Axelrod J, Silver J, Naveh-Many T. Interleukin-6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney Int. 2018;94:315–325. doi: 10.1016/j.kint.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 19.Chung BH, Kim BM, Doh KC, Cho ML, Kim KW, Yang CW. Protective effect of 1alpha,25-dihydroxyvitamin D3 on effector CD4+ T cell induced injury in human renal proximal tubular epithelial cells. PLoS One. 2017;12:e0172536. doi: 10.1371/journal.pone.0172536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreucci M, Faga T, Russo D, Bertucci B, Tamburrini O, Pisani A, Sabbatini M, Fuiano G, Michael A. Differential activation of signaling pathways by low-osmolar and iso-osmolar radiocontrast agents in human renal tubular cells. J Cell Biochem. 2014;115:281–289. doi: 10.1002/jcb.24662. [DOI] [PubMed] [Google Scholar]
- 21.Wang SY, Wang WJ, Liu JQ, Song YH, Li P, Sun XF, Cai GY, Chen XM. Methionine restriction delays senescence and suppresses the senescence-associated secretory phenotype in the kidney through endogenous hydrogen sulfide. Cell Cycle. 2019;18:1573–1587. doi: 10.1080/15384101.2019.1618124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xing JJ, Hou JG, Ma ZN, Wang Z, Ren S, Wang YP, Liu WC, Chen C, Li W. Ginsenoside Rb3 provides protective effects against cisplatin-induced nephrotoxicity via regulation of AMPK-/mTOR-mediated autophagy and inhibition of apoptosis in vitro and in vivo. Cell Prolif. 2019;52:e12627. doi: 10.1111/cpr.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao W, Wang Z, Fu Z, Ma H, Jiang M, Xu A, Zhang W. p62/SQSTM1 protects against cisplatin-induced oxidative stress in kidneys by mediating the cross talk between autophagy and the Keap1-Nrf2 signalling pathway. Free Radic Res. 2019;53:800–814. doi: 10.1080/10715762.2019.1635251. [DOI] [PubMed] [Google Scholar]
- 24.Kim JH, Kim KM, Jeong JU, Shin JH, Shin JM, Bang KT. Nrf2-Heme oxygenase-1 modulates autophagy and inhibits apoptosis triggered by elevated glucose levels in renal tubule cells. Kidney Res Clin Pract. 2019;38:318–325. doi: 10.23876/j.krcp.18.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan P, Sun ZM, Luo LF, Zhou J, Yang S, Zhao YS, Yu FY, An JR, Wang N, Ji ES. Hydrogen protects against chronic intermittent hypoxia induced renal dysfunction by promoting autophagy and alleviating apoptosis. Life Sci. 2019;225:46–54. doi: 10.1016/j.lfs.2019.04.005. [DOI] [PubMed] [Google Scholar]