Abstract
X-inactivation-specific transcript (XIST) is a long noncoding RNA (lncRNA) that functions as an indicator of various human tumors, including those of breast cancer. This study was conducted to characterize a novel regulatory network involving XIST in breast cancer cells. The mRNAs of XIST, miR-125b-5p, and NOD-like receptor family CARD domain containing 5 (NLRC5) in breast cancer cells and tissues were analyzed using quantitative real-time polymerase chain reaction. Cell proliferation, apoptosis, migration, and invasion were separately detected via cell counting kit-8, flow cytometry, and Transwell assays. The relationships between XIST, miR-125b-5p, and NLRC5 were predicted and then confirmed using the dual-luciferase reporter assay. NLRC5 protein expression was quantitated using western blot assays. XIST was found to be overexpressed in breast cancer tissues and cells, which was accompanied by miR-125b-5p downregulation and NLRC5 upregulation. XIST knockdown significantly repressed cell proliferation, anti-apoptosis, migration, and invasion activities in breast cancer cells, and the loss of miR-125b-5p had a similar effect. XIST was shown to sponge miR-125b-5p, which in turn targeted NLRC5. NLRC5, a breast cancer promotor, is negatively regulated by miR-125b-5p. Moreover, the downregulation of NLRC5 induced by the loss of XIST was significantly reversed by miR-125b-5p knockdown. In conclusion, the lncRNA XIST promotes the malignancy of breast cancer cells partly by competitively binding to miR-125b-5p, which then led to increased NLRC5 expression. Our study suggests that targeting XIST may be a possible treatment for breast cancer.
Keywords: LncRNA XIST, miR-125b-5p, NLRC5, breast cancer cell carcinoma
Introduction
Breast cancer is one of the most common cancers that results in malignant tumors in women [1]. Although the therapeutic strategies of surgery, radiotherapy, and chemotherapy have improved in the past years, the 5-year survival rate is low, and the mortality rate of breast cancer patients is still high [2]. Almost 500,000 people die of breast cancer every year, with the disease affecting younger patients [3]. Therefore, it is necessary to develop additional evidence-based and rational comprehensive treatments by identifying new biological indicators that are related to the prognoses and diagnoses of patients with breast cancer.
Long noncoding RNAs (lncRNAs) are key cis- or trans-regulators of gene expression with lengths exceeding 200 nucleotides. They play roles in the pathological processes of human carcinomas including breast cancer [4]. X-inactivation-specific transcript (XIST), a novel lncRNA located at the X-inactivation center, may alter heterochromatin stability, leading to changes in gene expression, thereby affecting cancer progression [5]. XIST-induced chromosome inactivation is related to a selective disadvantage in fibrosarcoma cells [6]. Previous studies have also reported that XIST overexpression is highly correlated with poorer prognoses in patients with various cancers, such as breast cancer [7], pancreatic cancer [8], and brain cancer [9]. However, the role that XIST plays in breast cancer has not been fully elucidated. A recent study revealed that numerous microRNAs (miRNAs) were involved in tumor progression as targets of lncRNAs [10]. Liu et al. reported that the capacity for epithelial-mesenchymal transition was inhibited by miR-486-5p upregulation induced by the loss of XIST in colorectal cancer cells [11]. Additionally, miR-125b-5p was reported to be downregulated in esophageal squamous cell carcinomas, and it negatively regulates HMGA2 expression [12]. However, the potential regulatory role of miR-125b-5p and its association with XIST in breast cancer have been rarely reported.
NOD-like receptor family CARD domain containing 5 (NLRC5) is involved in the body’s natural immune response and responses against pathogen infection [13]. NLRC5 may induce acute inflammatory or anti-inflammatory responses within a short period of time, as well as long-term effects, which trigger an imbalance in immune regulation [14] and may be related to the development of cancer [15]. NLRC5 may also play an important role in the development of liver cancer [16]. Studies have shown that the Wnt/β-catenin signaling pathway activates target genes downstream to trigger the development of liver cancer [17]. Given the importance of NLRC5 for cancer progression, it is important to characterize its potential role in breast cancer.
In the present study, we characterized XIST expression in breast cancer cells and tissues, as well as its functional roles in the cell proliferation, anti-apoptosis activity, migration, and invasion of breast cancer cells. In addition, the relationships between XIST, miR-125b-5p, and NLRC5 were elucidated to identify potential molecular targets for breast cancer treatments associated with the XIST/miR-125b-5p/NLRC5 axis.
Methods
Patients’ samples collection
The experiments were approved by the Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong University. Fifty-four paired breast cancer tissues and adjacent non-tumor tissues were collected from March, 2015 to May, 2019 in Shandong Provincial Hospital Affiliated to Shandong University. The written informed consent was obtained from every patient with breast cancer involved in our study and tissues were immediately stored at -80°C.
Cell culture and transfection
Three breast cancer cell lines (MCF-7, MDA-MB-231 and SKBR3 cells) and normal breast epithelial cell line (MCF-10A cells) were obtained from the Cell Bank, China Academy of Sciences (Shanghai, China) and incubated in DMEM (Invitrogen, Carlsbad, CA, USA) including 10% fetal bovine serum (FBS; Invitrogen) in a humidified incubator at 37°C with 5% CO2. Vectors or oligonucleotides (including small interference RNA (siRNA) against XIST (si-XIST), has-miR-125b-5p mimic/inhibitor, pcDNA-NLRC5 vector and each matched controls) were constructed by GenePharma (Shanghai, China) and transfected into MCF-7 and MDA-MB-231 cells with Lipofectamine® 2000 reagent (Invitrogen). The specific transfection steps referred to the instruction manual. At 48 h post transfection, cells were harvested for subsequent analyses.
Cell counting kit-8 assay
Cell proliferation was detected using cell counting kit-8 assay (CCK-8; Dojindo, Kumamoto, Japan). Briefly, transfected cells were seeded into a 96-well plate at a density of 5×103/well in triplicate. At 24 h, 48 h and 72 h after incubation, cell proliferation was assessed according to the manufacturer’s instructions. A microplate reader (Bio-Rad) was used to measure the optical density (OD value) at an absorbance of 450 nm.
Flow cytometry assay
Annexin V-FITC apoptosis detection kit (Thermo Fisher Scientific) was used to detect cell apoptosis. The cells were collected and the concentration was adjusted to 1×106 cells/mL. 200 µL cell suspension was used for assay and was re-suspended in 300 µL binding buffer and gently mixed with 5 µL Annexin V-FITC/PI for 15 min in the dark, the apoptosis of stained cells was observed using a flow cytometer.
Transwell assay
Transwell assay was used to determine the abilities of migration and invasion. 1×105 cells/100 µL/well were seeded into the upper chambers for migration test or into the upper chamber already coated with 10 µL Matrigel for invasion test. After 48-h conventional incubation, transwell chambers were taken out and the inner cells were lightly wiped off and then put into a new 24 well-plate with 4% paraformaldehyde (600 µL). Until fixed for 5 min, cells were stained with 0.1% crystal violet for 10 min. Additionally, cells were calculated in five random fields under an inverted microscope.
Dual-luciferase reporter assay
The sequence of XIST or NLRC5 was synthesized and cloned into the pGL3 Dual-luciferase reporter vector (Promega, Madison, MI, USA). The generated new reporter vectors were named as pGL3-XIST-WT (wild-type of XIST), pGL3-XIST-MUT (mutant-type of XIST), pGL3-NLRC5-WT (wild-type of NLRC5), and pGL3-NLRC5-MUT (mutant-type of NLRC5), respectively. The pGL3-XIST-WT and pGL3-NLRC5-WT contained the predicted binding sites of miR-125b-5p. Above reporter vectors were co-transfected into MCF-7 and MDA-MB-231 cells with miR-NC or miR-125b-5p mimic using Lipofectamine® 2000. After 48 h, the relative luciferase activity was detected using a dual-luciferase reporter assay kit (Promega), normalized to that of Renilla.
RNA isolation and quantitative reverse transcription polymerase (qRT-PCR)
Total RNA was extracted with TRIZOL reagent (Invitrogen) in accordance with the manufacturer’s instructions, and then qRT-PCR was performed with the SYBR Green PCR kit (Takara, Otsu, Japan) following the manufacturer’s protocol. 2-ΔΔCT method was used to calculate the levels of XIST, miR-125b-5p and NLRC5, normalized to U6 small nuclear RNA (U6-snRNA) and housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), separately. The involved primer sequences were as follows: XIST, forward 5’-CTT GGA TGG GTT GCC AGC TA-3’, reverse 5’-TCA TGC CCC ATC TCC ACC TA-3’; miR-125b-5p, forward 5’-CTC AGT CCC TGA GAC CCT AAC-3’, reverse 5’-CAA GAG CCT AAC CCG TGG AT-3’; NLRC5, forward 5’-GCT CGG CAA CAA GAA CCT GT-3’, reverse 5’-GGT CCA AGG TCT CGT TCC T-3’; GAPDH, forward 5’-ACA ACT TTG GTA TCG TGG AAG G-3’, reverse 5’-GCC ATC ACG CCA CAG TTT C-3’; U6, forward 5’-CTC GCT TCG GCA GCA CA-3’, reverse 5’-AAC GCT TCA CGA ATT TGC GT-3’.
Western blot analysis
Proteins of tissues and cells were lysed with the radioimmunoprecipitation assay (RIPA) (Beyotime Biotech, Shanghai, China) and the concentration of the total proteins was quantified by the bicinchoninic acid (BCA) method using a BCA-kit (Pierce Biotechnology, Inc., Rockford, IL, USA). The proteins were then electro-transferred onto the polyvinylidene fluoride (PVDF) membranes (Amersham Biosciences, Little Chalfont, UK). The membranes were blocked with 5% skimmed milk for 1 h at room temperature. And then the membranes were incubated with primary antibodies against NLRC5 or GAPDH at 4°C overnight. After they were washed three times with PBST, the membranes were incubated with horseradish peroxidase-labeled secondary antibody for 1 h at 37°C. The protein bands were visualized with electrochemiluminescence (ECL; Pierce) and quantified using LabworksTM Analaysis Software 4.0 (Labworks, Upland, CA, USA).
Statistical analysis
All data were presented as mean ± standard deviation (SD) and analyzed using a professional SPSS software 20.0 (SPSS Inc., Chicago, Ull, USA). Differences between two groups were analyzed using student’s t-test and among three or more groups were analyzed by oneway ANOVA. P < 0.05 was considered statistically significant.
Results
XIST expression was upregulated in breast cancer tissues and cell lines
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to quantify the expression of XIST in breast cancer tissues and matched adjacent normal tissues (n = 54). XIST levels were significantly upregulated in breast cancer tissues compared with levels in the adjacent normal tissues (Figure 1A, P < 0.01). Moreover, qRT-PCR analysis also confirmed that levels of XIST were higher in breast cancer cell lines (MCF-7 and MDA-MB-231 cells) than in breast epithelial cells (MCF-10A cells) (Figure 1B). The data suggest that XIST, as an oncogene in breast cancer, can be a potential predictor of breast cancer development.
Figure 1.
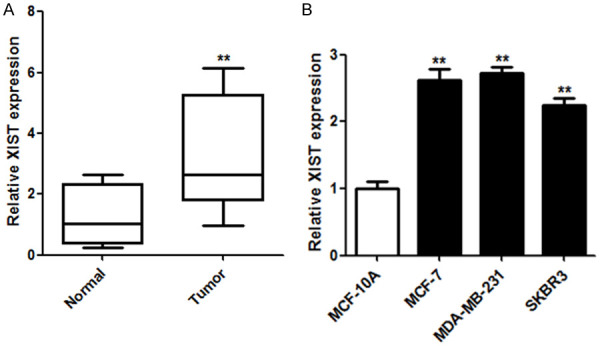
XIST expression was upregulated in breast cancer tissues and cells. A. XIST expression levels in breast cancer tissues and matched normal tissues were determined using qRT-PCR (n = 54). B. XIST expression levels in breast cancer cells (MCF-7 and MDA-MB-231 cells) and breast epithelial cells (MCF-10A cells) were also determined using qRT-PCR. **P < 0.01.
Interfering with XIST expression arrested proliferation, migration, and invasion, and induced cell apoptosis in breast cancer cells
Next, experiments were conducted to identify the function of XIST in breast cancer cells. XIST expression was downregulated in breast cancer cells transfected with the small interfering-XIST vector (Figure 2A). In addition, cell proliferation was inhibited in MCF-7 and MDA-MB-231 cells when XIST was suppressed (Figure 2B). Flow cytometry revealed that cell apoptosis was induced by the downregulation of XIST (Figure 2C). Moreover, cell migration and invasion decreased after XIST knockdown in MCF-7 and MDA-MB-231 cells (Figure 2D and 2E). Taken together, the results showed that XIST may be a potential target for breast cancer treatment.
Figure 2.
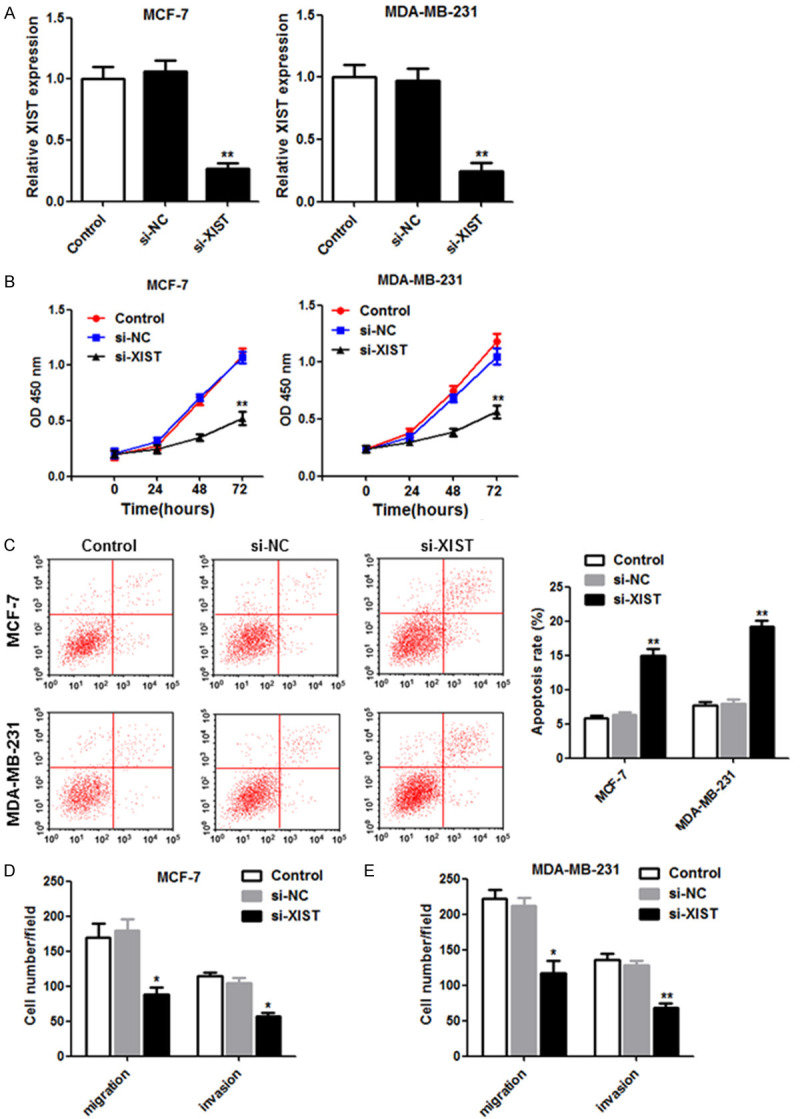
Knockdown of XIST decreased cell proliferation, migration and invasion, and induced apoptosis in breast cancer cells. (A) Si-NC or si-XIST was transfected into MCF-7 and MDA-MB-231 cells, and the interference effects were detected using qRT-PCR at 48 h post-transfection. (B) Cell proliferation was measured using the CCK-8 assay, and (C) flow cytometry was used to detect cell apoptosis. The Transwell assay was used to measure the migration (D) and invasion (E) activities exhibited by the transfected cells. *P < 0.05; **P < 0.01.
XIST sponged miR-125b-5p, which was poorly expressed in breast cancer
XIST was predicted to potentially target miR-125b-5p (Figure 3A). A dual-luciferase reporter assay was conducted by separately co-transfecting wild-type or mutant XIST (XIST-WT and XIST-Mut, respectively) with miR-NC or mature miR-125b-5p into MCF-7 and MDA-MB-231 cells. Luciferase activity decreased significantly in MCF-7 and MDA-MB-231 cells co-transfected with XIST-WT and miR-125b-5p, but not in the other groups (Figure 3B). Moreover, we further characterized the relationship between miR-125b-5p and XIST in breast cancer cells by transfecting small interfering (si)-XIST. The expression of miR-125b-5p increased significantly with the loss of XIST in MCF-7 and MDA-MB-231 cells (Figure 3C). Decreased miR-125b-5p expression in breast cancer cells and tissues was also observed (Figure 3D and 3E). Overall, the results suggest that miR-125b-5p may be a potential tumor suppressor that is negatively regulated by XIST.
Figure 3.
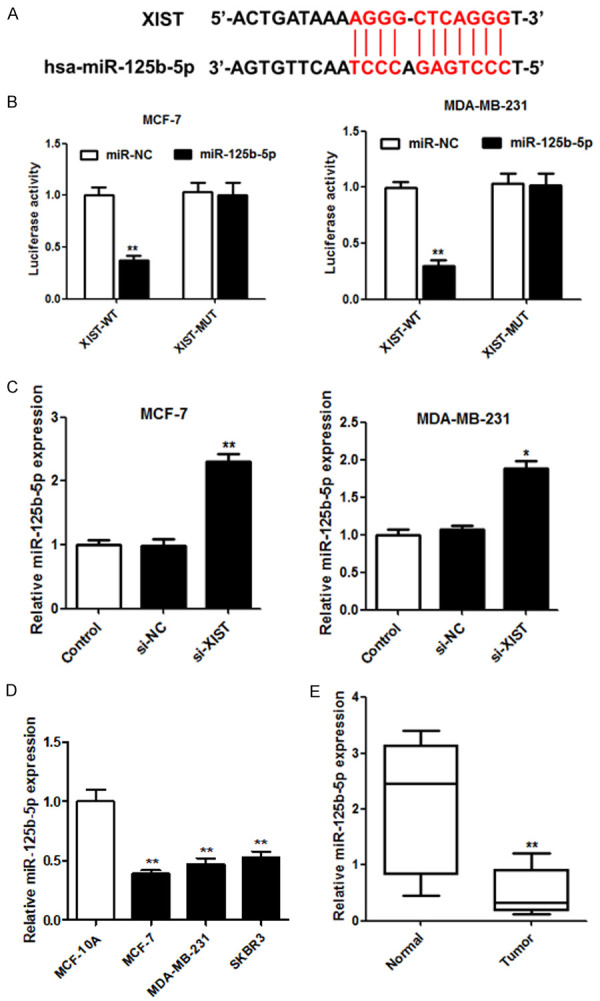
XIST sponged miR-125b-5p in breast cancer cells. (A) The binding site alignment of miR-125b-5p was predicted for XIST. (B) Luciferase activity was measured using a dual-luciferase reporter assay in cells co-transfected with pGL3-XIST-Wt or pGL3-XIST-Mut vectors and miR-NC or miR-125b-5p. (C) The relationship between miR-125b-5p and XIST in breast cancer cells was detected using qRT-PCR. The relative expression of miR-125b-5p in breast cancer cells (D) and tissues (E) (n = 54) was examined using qRT-PCR. *P < 0.05; **P < 0.01.
Downregulation of miR-125b-5p abolished si-XIST-mediated repression of breast cancer cells
The regulatory role of miR-125b-5p in XIST expression was further confirmed in MCF-7 and MDA-MB-231 cells by transfecting si-XIST with or without the anti-miR-125b-5p mimic. From the qRT-PCR analysis, miR-125b-5p expression was significantly elevated in MCF-7 and MDA-MB-231 cells with the loss of XIST, but the result was abrogated by anti-miR-125b-5p (Figure 4A). In addition, cell viability was inhibited in MCF-7 and MDA-MB-231 cells by si-XIST, whereas knockdown of miR-125b-5p coupled with XIST downregulation abolished the si-XIST-mediated arrest of cell proliferation (Figure 4B). Flow cytometry revealed that anti-miR-125b-5p reversed the apoptosis promoted by XIST downregulation in MCF-7 and MDA-MB-231 cells (Figure 4C).
Figure 4.
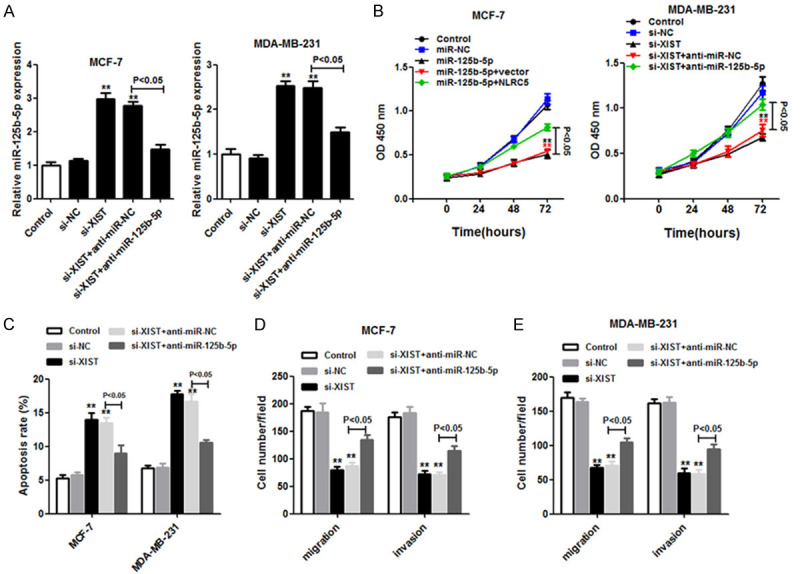
Anti-miR-125b-5p reversed the effect of XIST loss in MCF-7 and MDA-MB-231 cells. MCF-7 and MDA-MB-231 cells transfected with si-NC, si-XIST, si-XIST+anti-miR-NC, or si-XIST+anti-miR-125b-5p were classified into four groups. A. The miR-125b-5p expression was quantitated by using the quantitative real-time polymerase chain reaction. B. Cell proliferation was examined using the CCK-8 assay. C. Flow cytometry was used to detect the apoptosis rate of cells. D and E. Analysis of cell migration and invasion was conducted using the Transwell assay. **P < 0.01.
In a similar manner, the migration and invasion of breast cancer cells with XIST loss were restored by the knockdown of miR-125b-5p (Figure 4D and 4E). Taken together, the results indicate that miR-125b-5p inhibition significantly promotes cell proliferation, anti-apoptosis, migration, and invasion activities by targeting XIST in breast cancer cells.
The NLRC5 oncogene target was suppressed by miR-125b-5p
As shown in Figure 5A, hsa-miR-125b-5p is predicted to be bound to the 3’-UTR of NLRC5. The wild-type and mutant NLRC5 reporter vectors were named pGL3-NLRC5-3’-UTR-WT and pGL3-NLRC5-3’-UTR-Mut, respectively, and these vectors were transfected into MCF-7 and MDA-MB-231 cells, along with miR-NC or the miR-125b-5p mimic to characterize the binding of miR-125b-5p to NLRC5. Luciferase activity decreased significantly in cells treated with NLRC5-WT and miR-125b-5p compared with cells treated with miR-NC or NLRC5-Mut (Figure 5B). Accordingly, western blot analysis revealed that miR-125b-5p overexpression effectively repressed NLRC5 expression in MCF-7 and MDA-MB-231 cells (Figure 5C). Also, the expression of NLRC5 was higher in breast cancer cells (MCF-7 and MDA-MB-231 cells) than in breast epithelial cells (MCF-10A cells) (Figure 5D). Moreover, NLRC5 protein expression was upregulated in breast cancer tissues compared to expression levels in paired normal tissues. Overall, our results indicate that NLRC5 expression may be directly repressed by miR-125b-5p.
Figure 5.
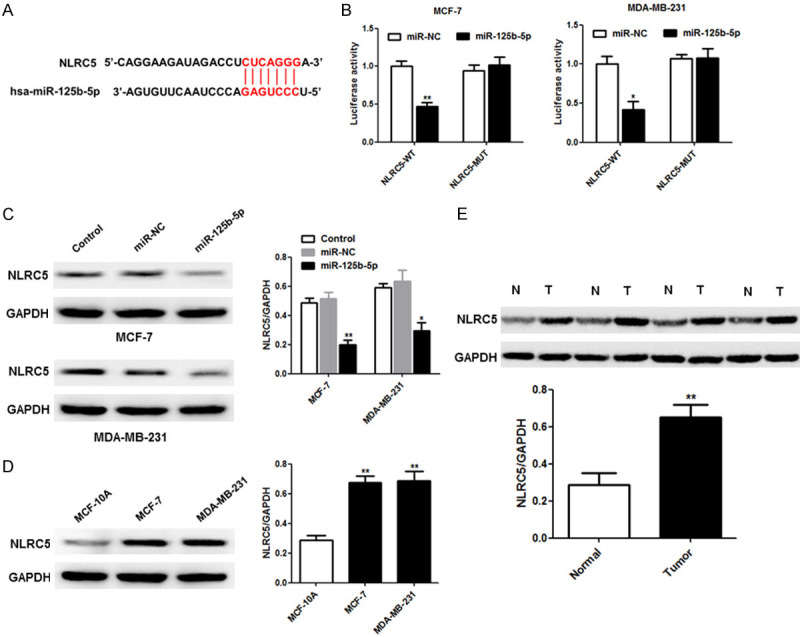
NLRC5 is a novel target of miR-125b-5p in breast cancer cells. (A) The predicted potential binding sites of miR-125b-5p in the 3’-UTR of NLRC5. (B) Luciferase activity was detected in MCF-7 and MDA-MB-231 cells transfected with pGL3-NLRC5-3’-UTR-WT and pGL3-NLRC5-3’-UTR-Mut reporter vectors, along with miR-125b-5p or miR-NC. (C) The western blot assay was used to characterize NLRC5 expression in MCF-7 and MDA-MB-231 cells with or without miR-125b-5p. The protein levels of NLRC5 were determined in breast cancer cells and MCF-10A cells (D) as well as in breast cancer tissues and paired normal tissues (E). *P < 0.05; **P < 0.01.
Overexpression of NLRC5 reversed miR-125b-5p-mediated effects on breast cancer cells
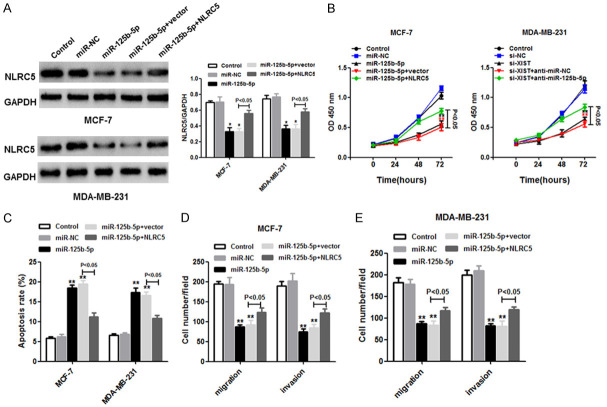
To determine whether miR-125b-5p is a tumor inhibitor that targets NLRC5, we divided MCF-7 and MDA-MB-231 cells into five groups each: control, miR-NC, miR-125b-5p, miR-125b-5p+vector, and miR-125b-5p+NLRC5. NLRC5 expression decreased significantly in the miR-125b-5p group compared to the miR-NC group, whereas NLRC5 expression increased in the miR-125b-5p+NLRC5 group compared to the miR-125b-5p+vector group, in both MCF-7 and MDA-MB-231 cells, based on western blot (Figure 6A). Moreover, miR-125b-5p repressed cell proliferation (Figure 6B) and induced cell apoptosis (Figure 6C), according to the Cell Counting Kit-8 assay and flow cytometry, respectively. Interestingly, the effects might have been partially inhibited by NLRC5 overexpression in MCF-7 and MDA-MB-231 cells. In addition, cell migration and invasion were suppressed in the miR-125b-5p group, but the opposite result was observed in the miR-125b-5p+NLRC5 group, as determined using the Transwell assay (Figure 6D and 6E). In summary, the results indicate that NLRC5 may promote the progression of breast cancer cells and that NLRC5 binds directly to miR-125b-5p.
Figure 6.
NLRC5 was negatively correlated with miR-125b-5p in breast cancer cells. MCF-7 and MDA-MB-231 cells were transfected with miR-NC, miR-125b-5p, miR-125b-5p+vector, or miR-125b-5p+NLRC5 vector (four groups per cell type). (A) The western blot assay was used to detect NLRC5 expression in transfected cells. (B) The CCK-8 assay was used to measure cell proliferation. (C) Flow cytometry was performed to detect cell apoptosis in transfected cells. Migration (D) and invasion (E) activities in MCF-7 and MDA-MB-231 cells were assessed using the Transwell assay. *P < 0.05; **P < 0.01.
XIST downregulation suppressed NLRC5 expression as miR-126b-5p was not sponged
We further characterized the potential relationships between XIST, miR-125b-5p, and NLRC5 in breast cancer cells. NLRC5 mRNA and protein were found in MCF-7 and MDA-MB-231 cells with si-XIST, and the suppression of miR-125b-5p produced similar results (Figure 7). The above results indicate that XIST downregulation inhibits NLRC5 expression as miR-125b-5p does not get sponged in breast cancer cells.
Figure 7.
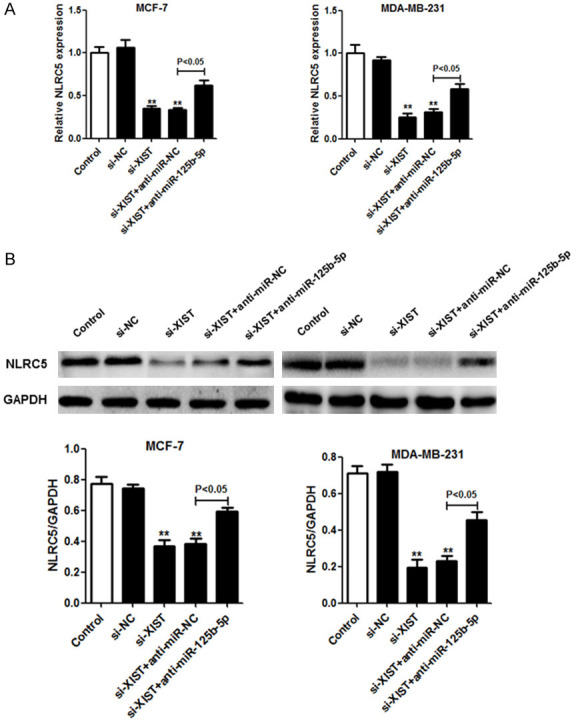
The loss of XIST induced NLRC5 repression as XIST usually binds to miR-125b-5p in breast cancer cells. A. NLRC5 expression was quantified using qRT-PCR at 48 h after si-NC, si-XIST, si-XIST+anti-miR-NC, or si-XIST+anti-miR-125b-5p were transfected into MCF-7 and MDA-MB-231 cells. B. Protein expression levels of NLRC5 in the transfected cells were determined using the western blot assay. *P < 0.05; **P < 0.01.
Discussion
In this study, we identified the lncRNA XIST as a novel predictor of breast cancer. We found that XIST was significantly upregulated in breast cancer tissues and cell lines. In addition, silencing the XIST gene slowed down cell proliferation, as well as weakened the anti-apoptosis, migration, and invasion abilities of breast cancer cells, which further confirms the promotional role XIST plays in breast cancer.
Recently, both miRNA and lncRNA have been shown to be key gene expression regulatory molecules that play important roles in tumorigenesis [18] and are closely correlated. The lncRNA can act as a miRNA sponge, thus inhibiting miRNA activity and affecting the development of tumors [19]. It has been suggested that miRNAs are closely associated with XIST [20]. Using online software, we showed that miR-125b-5p, a target of XIST, was minimally expressed in breast cancer cells and tissues. When si-XIST was transfected into breast cancer cells, it induced the upregulation of miR-125b-5p, thereby arresting breast cancer growth and metastasis. Conversely, downregulating miR-125b-5p expression abolished the effect induced by the loss of XIST. These data indicate that XIST promotes breast cancer progression by directly affecting miR-125b-5p expression.
NLRC5 has been reported to play a pivotal role in the etiology of many cancers [21]. NLRC5 expression is elevated in hepatocellular carcinoma (HCC), and NLRC5 knockdown was reported to significantly suppress HCC cell proliferation, migration, and invasion as well as arrest cells at the G0/G1 phase. Moreover, NLRC5 overexpression promoted HCC cell proliferation, migration, and invasion [22]. It has also been reported that NLRC5 is widely expressed in gastric cancer tissues and is involved in invasion and metastasis in gastric cancer [23]. We found that hsa-miR-125b-5p is bound to the 3’-UTR of NLRC5, and NLRC5 was weakly expressed in breast cancer cells with upregulated miR-125b-5p expression. Notably, NLRC5 was strongly expressed in breast cancer cells and tissues. Furthermore, the results of our rescue experiments revealed that the upregulation of NLRC5 reversed the inhibitory effect of miR-125b-5p overexpression on proliferation, anti-apoptosis activity, and metastasis in breast cancer cells. Mechanistically, NLRC5 expression was upregulated in cells treated with si-XIST, and the loss of miR-125b-5p had a similar effect. Thus, the results showed that silencing XIST downregulated NLRC5 expression as XIST normally sponges miR-125b-5p in breast cancer cells. Due to practical limitations, we will conduct related in vivo experiments in the future.
Both XIST (a lncRNA) and NLRC5 are overexpressed, and miR-125b-5p is downregulated, in breast cancer tissues and cells. XIST suppression decreased cell growth, cell metastasis, and anti-apoptosis activity. Both XIST and NLRC5 contain binding sites for miR-125b-5p, as initially predicted in our study. Decreasing XIST levels increased miR-125b-5p expression, and the upregulation of miR-125b-5p produced similar inhibitory effects on breast cancer cells as that mediated by the loss of XIST. Additionally, miR-125b-5p negatively regulates NLRC5 and reverses the NLRC5 expression induced by XIST in breast cancer cells. Based on our results, there is strong evidence that the XIST/miR-125b-5p/NLRC5 network can be a therapeutic target for breast cancer.
Acknowledgements
This study was supported by Shandong Province Key and Development Program (2019GSF108086).
Disclosure of conflict of interest
None.
References
- 1.Mourtada-Maarabouni M, Pickard MR, Hedge VL, Farzaneh F, Williams GT. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene. 2008;28:195–208. doi: 10.1038/onc.2008.373. [DOI] [PubMed] [Google Scholar]
- 2.Rezaianzadeh A, Jalali M, Maghsoudi A, Mokhtari AM, Azgomi SH, Dehghani SL. The overall 5-year survival rate of breast cancer among iranian women: a systematic review and meta-analysis of published studies. Breast Dis. 2017;37:63–68. doi: 10.3233/BD-160244. [DOI] [PubMed] [Google Scholar]
- 3.Razum O, Zeeb H, Beck K, Becher H, Stegmaier C. Combining a name algorithm with a capture-recapture method to retrieve cases of Turkish descent in a German population-based cancer registry. Eur J Cancer. 2001;36:2380–2384. doi: 10.1016/s0959-8049(00)00333-6. [DOI] [PubMed] [Google Scholar]
- 4.Jiao ZY, Tian Q, Li N, Wang HB, Li KZ. Plasma long non-coding RNAs (lncRNAs) serve as potential biomarkers for predicting breast cancer. Eur Rev Med Pharmacol Sci. 2018;22:1994–1999. doi: 10.26355/eurrev_201804_14727. [DOI] [PubMed] [Google Scholar]
- 5.De La Fuente R, Hahnel A, Basrur PK, King WA. X inactive-specific transcript (Xist) expression and X chromosome inactivation in the preattachment bovine embryo. Biol Reprod. 1999;60:769–775. doi: 10.1095/biolreprod60.3.769. [DOI] [PubMed] [Google Scholar]
- 6.Hall LL, Byron M, Sakai K, Carrel L, Willard HF, Lawrence JB. An ectopic human XIST gene can induce chromosome inactivation in postdifferentiation human HT-1080 cells. Proc Natl Acad Sci U S A. 2002;99:8677–8682. doi: 10.1073/pnas.132468999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng R, Lin S, Guan L, Yuan H, Zhang R. Long non-coding RNA XIST inhibited breast cancer cell growth, migration, and invasion via miR-155/CDX1 axis. Biochem Biophys Res Commun. 2018;498:1002–1008. doi: 10.1016/j.bbrc.2018.03.104. [DOI] [PubMed] [Google Scholar]
- 8.Wei W, Liu Y, Lu Y, Yang B, Tang L. LncRNA XIST promotes pancreatic cancer proliferation through miR-133a/EGFR. J Cell Biochem. 2017;118:3349–3358. doi: 10.1002/jcb.25988. [DOI] [PubMed] [Google Scholar]
- 9.Xing F, Liu Y, Wu SY, Wu K, Sharma S, Mo YY, Feng J, Sanders S, Jin G, Singh R, Vidi PA, Tyagi A, Chan MD, Ruiz J, Debinski W, Pasche BC, Lo HW, Metheny-Barlow LJ, D’Agostino RB Jr, Watabe K. Loss of XIST in breast cancer activates MSN-c-Met and reprograms microglia via exosomal miRNA to promote brain metastasis. Cancer Res. 2018;78:4316–4330. doi: 10.1158/0008-5472.CAN-18-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie L, Yao Z, Zhang Y, Li D, Hu F, Liao Y, Zhou L, Zhou Y, Huang Z, He Z, Han L, Yang Y, Yang Z. Deep RNA sequencing reveals the dynamic regulation of miRNA, lncRNAs, and mRNAs in osteosarcoma tumorigenesis and pulmonary metastasis. Cell Death Dis. 2018;9:772. doi: 10.1038/s41419-018-0813-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu A, Liu L, Lu H. LncRNA XIST facilitates proliferation and epithelial-mesenchymal transition of colorectal cancer cells through targeting miR-486-5p and promoting neuropilin-2. J Cell Physiol. 2019;234:13747–13761. doi: 10.1002/jcp.28054. [DOI] [PubMed] [Google Scholar]
- 12.Mei LL, Wang WJ, Qiu YT, Xie XF, Shi ZZ. miR-125b-5p functions as a tumor suppressor gene partially by regulating HMGA2 in esophageal squamous cell carcinoma. PLoS One. 2017;12:e0185636. doi: 10.1371/journal.pone.0185636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mótyán JA, Bagossi P, Benk S, Zsér JT. A molecular model of the full-length human NOD-like receptor family CARD domain containing 5 (NLRC5) protein. BMC Bioinformatics. 2013;14:275. doi: 10.1186/1471-2105-14-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez GM, Bobbala D, Serrano D, Mayhue M, Ilangumaran S. NLRC5 elicits antitumor immunity by enhancing processing and presentation of tumor antigens to CD8(+) T lymphocytes. OncoImmunology. 2016;5:e1151593. doi: 10.1080/2162402X.2016.1151593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chelbi ST, Guarda G. NLRC5, a promising new entry in tumor immunology. J Immunother Cancer. 2016;4:39. doi: 10.1186/s40425-016-0143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He YH, Li MF, Zhang XY, Meng XM, Huang C, Li J. NLRC5 promotes cell proliferation via regulating the AKT/VEGF-A signaling pathway in hepatocellular carcinoma. Toxicology. 2016;359-360:47–57. doi: 10.1016/j.tox.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Nejak-Bowen K, Monga SP. Wnt/β-catenin signaling in hepatic organogenesis. Organogenesis. 2008;4:92–99. doi: 10.4161/org.4.2.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhai W, Sun Y, Guo C, Hu G, Wang M, Zheng J, Lin W, Huang Q, Li G, Zheng J. LncRNA-SARCC suppresses renal cell carcinoma (RCC) progression via altering the androgen receptor (AR)/miRNA-143-3p signals. Cell Death Differ. 2017;24:1502–1517. doi: 10.1038/cdd.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheng-Can C, Bing-Xiu X, Jun-Ming G. Roles of interaction between miRNA and lncRNA in tumorigenesis. Chinese Journal of Biochemistry and Molecular Biology. 2014;11:1–10. [Google Scholar]
- 20.Sun K, Jia Z, Duan R, Yan Z, Jin Z, Yan L, Li Q, Yang J. Long non-coding RNA XIST regulates miR-106b-5p/P21 axis to suppress tumor progression in renal cell carcinoma. Biochem Biophys Res Commun. 2019;510:416–420. doi: 10.1016/j.bbrc.2019.01.116. [DOI] [PubMed] [Google Scholar]
- 21.Yoshihama S, Vijayan S, Sidiq T, Kobayashi KS. NLRC5/CITA: a key player in cancer immune surveillance. Trends Cancer. 2017;3:28–38. doi: 10.1016/j.trecan.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng YY, He YH, Chen C, Xu T, Li L, Ni MM, Meng XM, Huang C, Li J. NLRC5 regulates cell proliferation, migration and invasion in hepatocellular carcinoma by targeting the Wnt/β-catenin signaling pathway. Cancer Lett. 2016;376:10–21. doi: 10.1016/j.canlet.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Zhang M, Zheng X. High expression of NLRC5 is associated with prognosis of gastric cancer. Open Med (Wars) 2018;13:443–449. doi: 10.1515/med-2018-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]