Abstract
Curcumin is a safe, cost-effective natural agent with multiple targets that displays therapeutic potential in cancer. Recently, we reported a novel curcumin analog, Da0324, which exhibited significantly improved stability and anti-cancer activity. However, the molecular mechanism underlying the anti-cancer activity of Da0324 remains largely unknown. Long non-coding RNAs have been shown to play important roles in cancer development and progression and may be potential targets for cancer therapy. Here, we showed that Da0324 treatment down-regulated the expression of LINC01021 in gastric cancer cells. Da0324 treatment or knockdown of LINC01021 by antisense oligos significantly inhibited gastric cancer cell growth, and also up-regulated P53 expression and down-regulated Bcl-2 expression in vitro and in vivo. Furthermore, Da0324 treatment or knockdown of LINC01021 in gastric cancer cells suppressed cell migration, invasion and epithelial-mesenchymal transition (EMT), as well as induced apoptosis and autophagy. In addition, overexpression of LINC01021 promoted growth and EMT, inhibited P53 expression and increased Bcl-2 expression in gastric cancer cells. Finally, overexpression of LINC01021 reversed the anti-cancer effect of Da0324. Our findings indicate a novel anti-cancer mechanism for Da0324, and that LINC01021 might be a potential therapeutic target for the treatment of gastric cancer.
Keywords: Curcumin analog, Da0324, gastric cancer, LINC01021
Introduction
Gastric cancer (GC) is one of the most common malignant tumors in the world and has high mortality [1,2]. As methods of GC prevention and treatment have developed, the incidence of GC has been reduced, but it still remains one of the leading causes of cancer-related mortality in Asia [3]. One of the main treatments for GC is surgery, but most patients with early GC have a low rate of radical resection and a high recurrence rate [4]. Another major treatment option is chemotherapy, but its long-term application is likely to produce body resistance [5,6]. Therefore, there is an urgent need to develop new therapeutic targets and drugs for GC.
Long non-coding RNAs (lncRNAs) are defined as RNAs greater than 200 bases in length that have no obvious coding ability [7]. Recent analyses indicate that the human genome encodes approximately 32,000 lncRNAs, and that these play critical roles in various cellular and physiological functions [8]. LncRNAs affect gene regulation through a variety of mechanisms, but their main function is realized through interaction with RNA, DNA or protein [9]. Cytoplasmic lncRNAs bind to and regulate proteins or RNAs, some have been proposed to act as “miRNA sponges”, adsorbing and inactivating specific miRNAs, while others can work as scaffolds, combining with proteins to form complexes [9,10]. Nuclear-retained lncRNAs cooperate with proteins for the cis- or trans-regulation of chromatin status, which also directs proteins to specific genomic regions [11,12]. Accordingly, lncRNAs regulate a variety of cellular functions, including chromatin remodeling, transcription, mRNA precursor splicing, and RNA stability [10,13,14]. Accumulating evidence demonstrated that dysregulation of lncRNAs occurs in a variety of human cancers, and lncRNAs have been found to participate in GC development and progression [15]. Ultimately, lncRNAs have been revealed as a novel, potential therapeutic target for the treatment of cancer [16].
Since the application of chemotherapy to cancer treatment, natural compounds have become an important class of anti-tumor drugs [17]. Curcumin is an ancient spice and herb that is extracted from the roots of turmeric plants, part of the ginger’s family [18]. Curcumin has been received attention for its many biological activities, such as anti-inflammatory [19,20], antioxidant [21,22] and anti-tumor [21,23], which are realized either alone or in combination with drugs [24]. Unfortunately, the therapeutic potential of curcumin is limited by its low bioavailability, chemical instability and rapid metabolism [25]. A potentially effective strategy for solving these difficulties in the application of curcumin is to design a structural analog with superior pharmacokinetic and biological properties. In our previous study, we discovered a promising curcumin analog named Da0324, which displayed target selectivity for GC cells with improved stability and inhibited NF-ΚB activation in GC cells [26]. However, the molecular mechanisms underlying the anti-cancer activities of Da0324 are still not fully understood. In the present study, we report that Da0324 exerts anti-tumor activities against GC through downregulation of LINC01021. This finding provides an important direction for the development of clinical anticancer drugs and targeted therapies.
Materials and methods
Cell lines and culture
The human GC cell line KATO III and the human normal gastric mucosa epithelial cell line GES-1 were obtained from the Cell Bank of the Chinese Academy of Science (Shanghai, China). The cell lines BGC-823 and SGC-7901 were purchased from the China Center for Type Culture Collection (Wuhan, China). All cell lines were grown in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 100 U/mL penicillin, 10 mg/L streptomycin (Thermo Fisher Scientific), and 10% fetal bovine serum (FBS) (Sigma, Germany). All cells were cultured and maintained in a humidified incubator at 37°C under 5% CO2 atmosphere.
High-throughput sequencing of lncRNAs
A high-throughput sequencing assay was performed to screen differentially expressed lncRNAs among total RNAs extracted from SGC7901 cells treated with either DMSO or 4 μM Da0324 for 48 h in triplicate. In brief, total RNA was extracted using TRIzol® reagent (Thermo Fisher Scientific) and ribosomal RNA was depleted with the Epicentre Ribo-Zero Gold kit according to the manufacturer’s protocol (Illumina, San Diego, CA, USA). RNA fractions were fragmented into small pieces and then reverse-transcribed to create a complementary DNA (cDNA) library using the mRNA-Seq sample preparation kit (Illumina). Paired-end sequencing was performed using an Illumina HiSeq4000 sequencer (Illumina) by the service provider LianChuan Sciences (Hangzhou, China). Among differentially expressed lncRNAs, log2 (fold change) > 1 or log2 (fold change) < -1 with P-value < 0.05 was considered statistically significant.
Analysis of data from The Cancer Genome Atlas (TCGA)
LncRNA expression data from of 375 GC tissue samples and 32 normal stomach tissue samples were collected from the TCGA stomach adenocarcinoma dataset (STAD) and the level of LINC01021 expression determined.
Cell transfection
Antisense oligos (ASOs) targeting LINC01021 and siRNAs targeting P53, as well as corresponding negative controls (NC), were synthesized by RiboBio Biotech (Guangzhou, China). The oligo sequences were LINC01021-ASO-1: 5’-CCT GCG CAT ATT TAA CTA TC-3’; LINC01021-ASO-2: 5’-GTT CTT AAC CTG CGC ATA TT-3’; P53-siRNA: 5’-GAC TCC AGT GGT AAT CTA C-3’. The plasmid pcDNA-P53 was used to overexpress P53 in BGC823 and SGC7901 cells. Cell transfection was performed using Lipofectamine 3000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. Full-length LINC01021 was amplified and cloned into the lentiviral vector plasmid pLVX-Puro (Clontech, Mountain View, CA, USA) between the EcoRI and BamHI restriction enzyme recognition sites. Lentiviral particles were produced by co-transfection of HEK293T cells with pLVX-LINC01021, pMD2.G, pMDL-G/P-RRE and pRSV-REV [27].
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using TRIzol® reagent (Thermo Fisher Scientific). For detection of the subcellular distribution of LINC01021, cytoplasmic and nuclear RNA were isolated by PARIS™ Kit (Thermo Fisher Scientific). Total RNA was reverse transcribed to cDNA using the Revert Aid First-Strand cDNA Synthesis Kit (Thermo Fisher Scientific). For LINC01021, reverse transcription was performed with the lnRcute lncRNA First-Strand cDNA Synthesis Kit (Tiangen, China). Quantitative PCR was performed on a CFX96 Real-time PCR system (Bio-Rad, Berkeley, CA, USA) using SYBR Premix Ex Taq (Takara, Japan). The relative LINC01021 expression was calculated by a 2-ΔΔCt method. U6 was used as an internal reference. The specific primer sequences are: LINC01021-forward: 5’-GGA ACC CCT CTT GCT TTG CA-3’, LINC01021-reverse: 5’-ACG GGC ACA TTG AAG GGT CA-3’; U6-forward: 5’-CGC TTC ACG AAT TTG CGT GTC AT-3’, U6-reverse: 5’-CGC TTC ACG AAT TTG CGT GTC AT-3’.
Western blot analysis
Proteins were extracted from GC cells or xenograft tumor tissues using radioimmunoprecipitation assay (RIPA) lysis buffer supplemented with phenylmethylsulfonyl fluoride, phosphatase inhibitor and protease inhibitor. Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes. After that, the membranes were blocked with 5% skim milk at room temperature for 2 h and further incubated at 4°C overnight with specific primary antibodies against E-cadherin, N-cadherin, Bcl-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), vimentin (BD Biosciences, San Jose, CA, USA), P53, LC3B, P62, p-mTOR, mTOR, GAPDH, and β-actin (Cell Signaling Technology, Bioke, The Netherlands). GAPDH or β-actin was used as an internal control. Then, the membranes were successively incubated with secondary antibodies (Santa Cruz Biotechnology) and ECL reagent. Finally, specific bands were detected and analyzed by a scanning imaging system (Bio-Rad).
Cell viability and proliferation assays
For the CCK-8 assays, GC cells (5000 cells per well) were seeded into 96-well plates and incubated in RPMI-1640 medium containing different dosages of Da0324 for 24 h, 48 h, or 72 h. CCK-8 solution (10 μl/well, Dojindo, Japan) was added to each well at 2 h before the indicated time point, and the absorbance was measured at 450 nm according to the manufacturer’s protocols. For clone formation assays, transfected or Da0324-treated cells were seeded in six-well plates (800 cells/well) and cultured for two weeks. At the end of the experiment, cells were fixed with 4% paraformaldehyde and stained with crystal violet solution. Colony numbers were used to assess cell proliferation.
Apoptosis assay
In brief, after treatment with Da0324 (4 μM) or transfection for 48 h, cells were harvested and then stained with Annexin V-FITC and propidium iodide (PI) using an Annexin V-FITC/PI Apoptosis Detection Kit (BD Biosciences). Cell apoptosis was assessed by flow cytometry According to the manufacturer’s instructions (FACSCalibur, Becton Dickinson, Franklin Lakes, NJ, USA).
Cell migration and invasion assays
The migratory and invasive abilities of GC cells were evaluated using a transwell chamber with or without matrigel coating (BD Bioscience). GC cells (5×105 cells/well) were seeded in the upper chamber in RPMI-1640 medium without FBS and treated with Da0324, while 800 µL culture medium containing Da0324 and 10% FBS was added to the lower chamber. After incubation for 24 h at 37°C, the transwell chambers were successively fixed with 4% paraformaldehyde for 30 min and stained with crystal violet for another 30 min. Five random fields were counted per chamber using a microscope.
Animal experiments
Female BALB/c-nu mice (4-5 weeks old) were prepared for making a xenograft model. After resuspension in PBS, KATO III cells (5×106 cells/mouse) were injected subcutaneously into the right dorsal flank of the nude mice. When the tumor volume reached approximately 50 mm3, the nude mice were randomized into four groups that received the following treatments: (1) PBS as vehicle control (n = 6); (2) Da0324 (dissolved in 6% castor oil and 94% PBS, 20 mg/kg, n = 6); (3) ASO-control (dissolved in PBS, 250 nmol/kg, n = 5); (4) ASO-LINC01021 (dissolved in PBS, 250 nmol/kg, n = 5). Vehicle or Da0324 was given via intraperitoneal injection every day for 18 days. ASO-control and ASO-LINC01021 were administered through intratumoral injection at three-day intervals, and the ASO-control treatment was used as a control group. Tumors were excised after 24 days of treatment. Tumor volumes and animal body weights were measured and recorded every other day. Tumor volumes were calculated according to the following formula: volume = 0.5×L×W2 (L, longest diameter; W, diameter perpendicular to the longest diameter). At the end of the treatment, the mice were euthanized, and the xenograft tumors were dissected and weighed. All operations for animal experiments were approved by the Animal Experimental Ethics Committee of Wenzhou Medical University.
Statistical analysis
All statistical analyses were carried out using GraphPad Prism7.0 (GraphPad Prism, Inc., La Jolla, CA, USA). Student’s t-test was used to analyze differences between two groups. One-way analysis of variance was used to calculate statistical significance of differences between three groups. The results were expressed as means ± SD. A P-value < 0.05 was considered statistically significant.
Results
Da0324 inhibits proliferation and induces apoptosis and autophagy in GC cells
To verify whether Da0324 exhibited a cytostatic effect of GC cells, CCK-8 assays were performed to assess cell viability in response to Da0324 treatment in BGC823, SGC7901 and KATO III GC cells. The results showed that Da0324 significantly reduced the viability of all three cell lines in a time- and dose-dependent manner (Figure 1A), At 48 h to Da0324 exposure, the half maximal inhibitory concentration (IC50) values were 3.48 ± 0.59, 3.72 ± 0.20 and 4.69 ± 0.02 µM for BGC823, SGC7901, and KATO III cells, respectively (Figure 1B). Consistently, proliferation of GC cells was inhibited with Da0324 treatment, as evidenced by reduced colony formation (Figure 1C). In addition, the apoptosis-inducing effects of Da0324 were examined using Annexin-V/PI double staining. As shown in Figure 1D, Da0324 induced significant apoptotic effects in BGC823, SGC7901 and KATO III cells. The pro-apoptotic effect of Da0324 was supported by increased expression levels of P53 and decreased expression of the apoptosis-related protein Bcl-2 in Da0324-treated cells (Figure 1E). To test whether Da0324 treatment induced autophagy in GC cells, we analyzed the specific autophagy markers microtubule-associated protein light chain3B (LC3B) and P62 along with the autophagy-associated proteins mTOR and phosphorylated mTOR (p-mTOR) by western blotting. The LC3B protein has two variants: LC3B-I and LC3B-II, and turnover of LC3B-I to LC3B-II is considered a hallmark of autophagy [28]. As indicated in Figure 1E, protein levels of p-mTOR and P62 were reduced and the ratio of LC3B-II/LC3B-I was elevated in BGC823 and SGC7901 cells treated with Da0324, suggesting that Da0324 could induce autophagy in GC cells.
Figure 1.
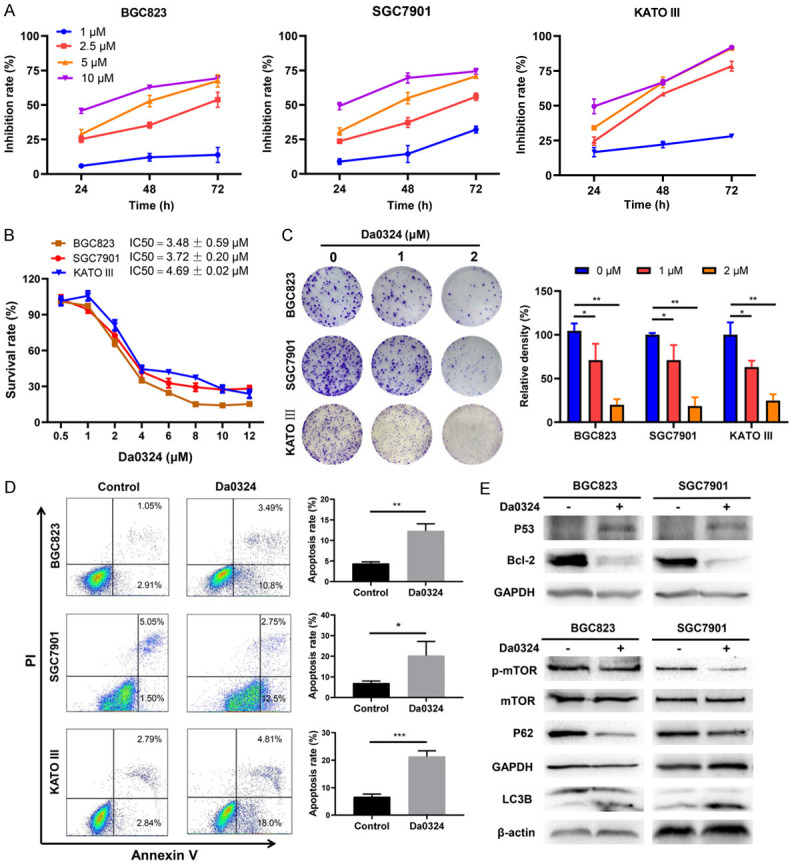
Da0324 inhibits proliferation and induces apoptosis and autophagy in gastric cancer cells. A. Gastric cancer cell lines (BGC823, SGC7901 and KATO III) were treated with the indicated concentration of Da0324 for 24 h, 48 h, or 72 h. Cell viability was measured using a Cell Counting Kit-8 (CCK-8) and Growth inhibition rates of Da0324 on GC cells were in comparison with untreated cells. B. BGC823, SGC7901 and KATO III cells were treated with 0, 0.5, 1, 2, 4, 6, 8, 10, or 12 mM Da0324 for 48 h, and cell viability was measured by CCK-8 assays. The half maximal inhibitory concentration (IC50) values were calculated with GraphPad statistical software. C. Clonogenic assay showed the effects of Da03224 treatment on clonogenic formation in gastric cancer cells. BGC823, SGC7901 and KATO III cells were treated with Da0324 (1 or 2 µM) for 48 h, then cultured for two weeks in complete medium and the colony density calculated. Representative images of clonogenic assay (left panel) and quantitative analysis (right panel). All data are representative of three independent experiments and are presented as the means ± SD. *, P < 0.05; **, P < 0.01. D. BGC823, SGC7901 and KATO III cells were treated with 4 μM Da0324 for 48 h. Cells were fixed and stained with Annexin V/PI and apoptosis was analyzed by flow cytometry. Representative dot plots of Annexin V/PI staining are shown in the left panel, and quantitative data are presented in the right panel. All data are representative of three independent experiments and are presented as the means ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001. E. BGC823 and SGC7901 cells were treated with 4 μM Da0324 or control for 48 h and then harvested for analysis by western blot using P53, Bcl-2, p-mTOR, mTOR, P62, and LC3B antibodies. GAPDH or β-actin was used as an internal control.
Da0324 inhibits migration, invasion, and epithelial-mesenchymal transition (EMT) in GC cells
The effects of Da0324 on migration and invasion of GC cells were assessed with transwell assays, in which Da0324 significantly inhibited the migration and invasion of BGC823, SGC7901 and KATO III cells (Figure 2A). EMT is important for tumor migration and invasion, thus the effect of Da0324 on the expression of EMT markers in GC cells was also analyzed by western blotting. As shown in Figure 2B, Da0324 treatment of GC cells increased expression of E-cadherin and decreased expression of N-cadherin and vimentin.
Figure 2.
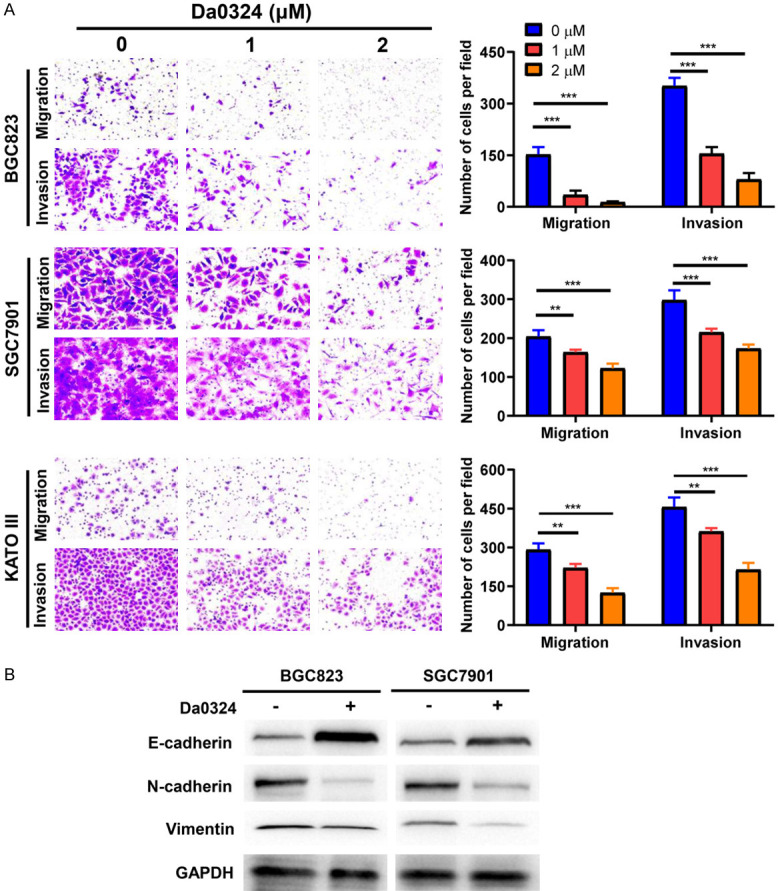
Da0324 inhibits migration, invasion, and EMT in GC cells. A. BGC823, SGC7901 and KATO III cells were treated with Da0324 (1 or 2 µM) for 24 h and transwell migration and matrigel invasion assays were performed. Representative image (left panel) and quantitative data (right panel) of cell migration and invasion are shown. Bar graphs are representative of three independent experiments and the data are presented as the means ± SD. **, P < 0.01; ***, P < 0.001. B. Western blot analysis for the expression of E-cadherin, vimentin, and N-cadherin in BGC823 and SGC7901 cells treated with or without 2 µM Da0324. GAPDH was used as an internal control.
Da0324 treatment suppresses long non-coding RNA LINC01021 expression in GC cells
High-throughput sequencing was used to identify differentially expressed lncRNAs in SGC7901 cells treated with DMSO (control) or 4 μM Da0324 for 48 h. A total of 280 differentially expressed lncRNAs were identified (152 upregulated and 128 downregulated, P < 0.05, fold change > 2 or < -2) (Figure 3A). Figure 3B shows the top 40 most significantly differentially expressed lncRNAs. To verify the consistency of the lncRNA sequence data, ten lncRNAs were selected for qRT-PCR analysis, of which eight were validated (Figure 3C). LINC01021 was amongst the most highly down-regulated lncRNAs, and was selected for further study based on the following considerations. First, expression of LINC01021 was significantly downregulated in BGC823, SGC7901 and KATO III cells treated with Da0324 for 48 h (Figure 3D). Second, according to data from TCGA, LINC01021 was remarkably overexpressed in GC tissues and cells when compared with normal tissues and normal gastric epithelial cells (Figure 3E and 3F). Finally, qRT-PCR of cytoplasmic and nuclear fractions from GC cells indicated that most LINC01021 was present in the nucleus (Figure 3G).
Figure 3.
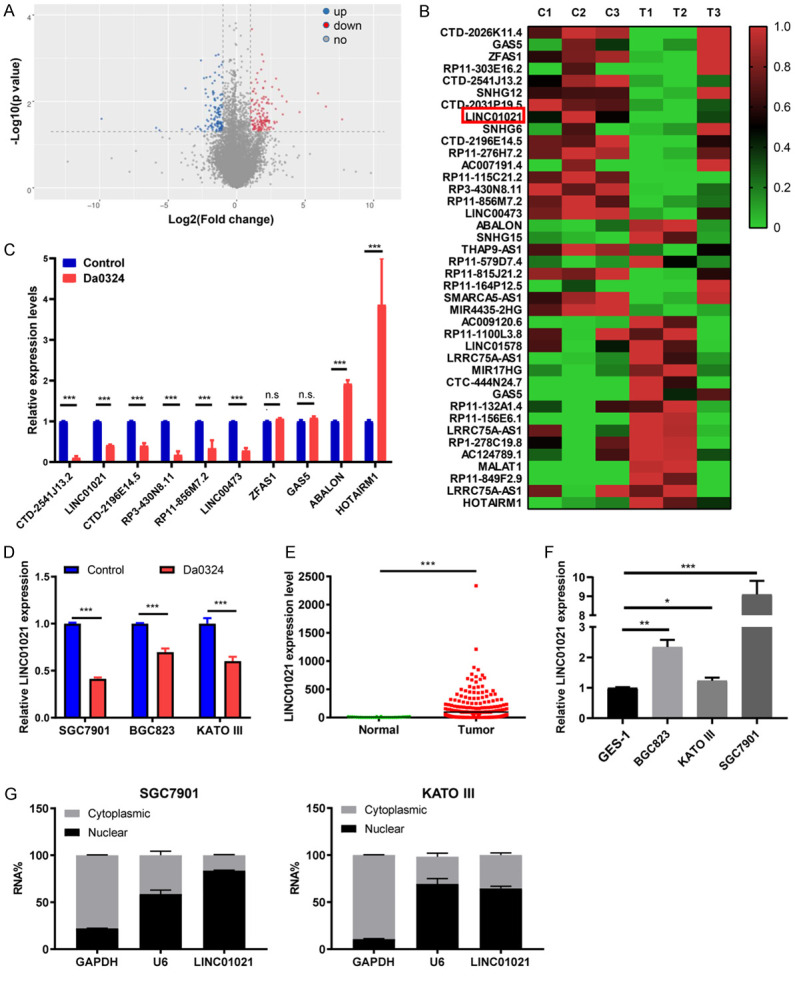
Long non-coding RNA LINC01021 was downregulated by Da0324 treatment in gastric cancer cells. A. Volcano plots of lncRNAs differentially expressed between the control and Da0324 treatment groups. SGC7901 cells were treated with DMSO (control) or 4 μM Da0324 for 48 h and high-throughput sequencing assay was performed. The X-axis represents log2 of fold changes. The Y-axis represents -log10 of P values. Red spots denote upregulated lncRNAs, blue spots denote downregulated lncRNAs. B. Heatmap of the top 40 lncRNAs most significantly differentially expressed in SGC7901 cells with Da0324 treatment. C. Ten differentially expressed lncRNAs regulated by Da0324 were validated by qRT-PCR. SGC7901 cells were treated with DMSO (control) or 4 μM Da0324 for 48 h. RNAs were isolated and converted to cDNA. Quantitative real-time PCR was performed to determine the expression level of lncRNAs. GAPDH was used as a housekeeping gene. All bars represent relative expression levels and data represent mean ± SD. ***, P < 0.001; n.s. means no significant difference. D. LINC01021 expression by qRT-PCR in BGC823, SGC7901 and KATO III cells treated with Da0324 or DMSO (control) for 48 h. All bars represent relative expression levels and data represent mean ± SD. ***, P < 0.001. E. TCGA data for the expression of LINC01021 in gastric cancer tissues (n = 375) and normal tissues (n = 32). F. LINC01021 expression by qRT-PCR in the normal gastric epithelial cell line GES-1 and in gastric cancer cell lines (BGC823, SGC7901, and KATO III). All bars represent relative expression levels and data represent mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001. G. The subcellular location of LINC01021 in SGC7901 and KATO III cells was determined by qRT-PCR. GAPDH was used as a positive control for cytoplasmic RNA localization, U6 for nuclear RNAs. The data are shown as means ± SD. lncRNA, long non-coding RNA; qRT-PCR, quantitative reverse transcription-polymerase chain reaction.
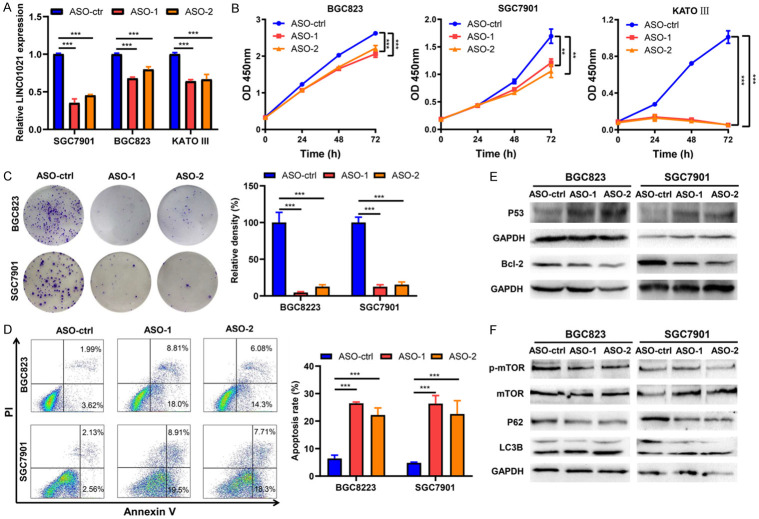
ASO-mediated silencing of LINC01021 inhibits cell proliferation and induces apoptosis and autophagy in GC cells
To explore the biologic roles of LINC01021 in GC, we knocked down LINC01021 in BGC823, SGC7901, and KATO III cells by transfection with ASOs specific for LINC01021. The knockdown efficiency of LINC01021-specific ASO-1 and LINC01021-specific ASO-2 was verified by qRT-PCR (Figure 4A). Growth curves generated from CCK-8 assays revealed that the ablation of LINC01021 by LINC01021-specific ASO-1 and ASO-2 inhibited the growth of all three GC cell lines (Figure 4B). Similarly, knockdown of LINC01021 decreased the clonogenic survival of BGC823 and SGC7901 cells in colony formation assays (Figure 4C). As apoptosis is a contributing factor for cancer cell growth inhibition, we evaluated apoptosis by flow cytometry analysis and found that BGC823 and SGC7901 cells transfected with LINC01021-specific ASO-1 and ASO-2 had higher apoptotic rates than did the same cells transfected with ASO-control (Figure 4D), demonstrating that knockdown of LINC01021 induced apoptosis in GC cells. Moreover, BGC823 and SGC7901 cells transfected with LINC01021-specific ASO-1 and ASO-2 expressed significantly higher levels of P53 and lower levels of Bcl-2 protein (Figure 4E). Knockdown of LINC01021 also increased the conversion of LC3B-I into LC3B-II, and decreased the levels of P62 and phosphorylation of mTOR (Figure 4F). These data confirm that reduced expression of LINC01021 inhibits cell growth and promotes apoptosis and autophagy in GC cells in vitro.
Figure 4.
ASO-mediated silencing of LINC01021 inhibits cell proliferation and induces apoptosis and autophagy in gastric cancer cells. A. BGC823, SGC7901 and KATO III cells were transfected with 50 nM ASO-control, LINC01021-specific ASO-1, or LINC01021-specific ASO-2 for 48 h. LINC01021 expression were determined by qRT-PCR. B. BGC823, SGC7901 and KATO III cells were transfected with 50 nM ASO-control, LINC01021-specific ASO-1, or LINC01021-specific ASO-2. At 0, 24, 48 or 72 h post-transfection, cell viability was determined by the CCK-8 assays. The data were expressed as mean ± SD. All data are representative of three independent experiments. **, P < 0.01; ***, P < 0.001. C. The proliferation of gastric cancer cells (BGC823 and SGC7901) transfected with LINC01021-ASOs or ASO-control was determined by colony formation assays. The data were expressed as mean ± SD. All data are representative of three independent experiments. **, P < 0.01; ***, P < 0.001. D. Flow cytometry analysis of cell apoptosis in gastric cancer cells (BGC823 and SGC7901). Cells were transfected with 50 nM LINC01021-ASOs or ASO-control for 48 h and stained with Annexin V/PI. Representative dot plots of Annexin V/PI staining are shown in the left panel, and quantitative data are presented in the right panel. All data are representative of three independent experiments and are presented as the means ± SD. ***, P < 0.001. E, F. Western blot analysis of P53, Bcl-2, mTOR, p-mTOR, P62, and LC3B expression in BGC823 and SGC7901 cells transfected with LINC01021-ASOs or ASO-control. qRT-PCR, quantitative reverse transcription-polymerase chain reaction; ASO, antisense oligo; LC3B, light chain3B; p-mTOR, phosphorylated mTOR.
ASO-mediated silencing of LINC01021 inhibits GC cell migration, invasion, and EMT
Next, we performed transwell assays to determine the migration and invasion capacity of BGC823 and SGC7901 cells following LINC01021 knockdown. We observed that the migratory and invasive properties of both cell lines were significantly impaired after knockdown with LINC01021-specific ASO-1 and ASO-2 (Figure 5A). Additionally, as determined by western blotting of EMT-related markers, LINC01021 knockdown increased E-cadherin protein expression level and inhibited N-cadherin and vimentin protein expression (Figure 5B).
Figure 5.
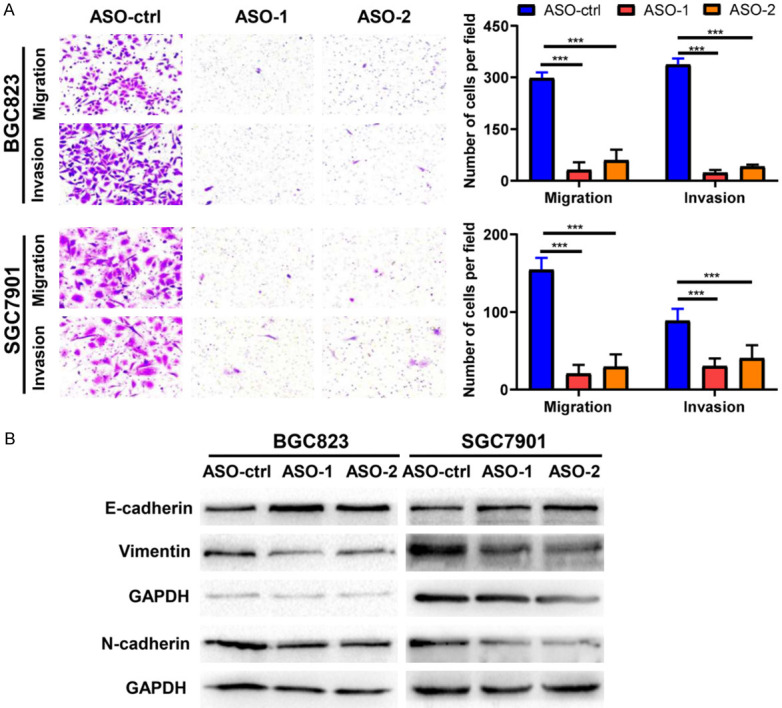
ASO-mediated silencing of LINC01021 inhibits gastric cancer cell migration, invasion, and EMT. A. BGC823 and SGC7901 cells were transfected with 50 nM ASO-control, LINC01021-specific ASO-1 or LINC01021-specific ASO-2 for 48 h and transwell migration and matrigel invasion assays were performed. Representative image (left panel) and quantitative data (right panel) of cell migration and invasion are shown. All data are presented as the means ± SD. ***, P < 0.001. B. Western blot analysis of the expression of E-cadherin, N-cadherin and vimentin proteins in BGC823 and SGC7901 cells transfected with ASO-control, LINC01021-specific ASO-1 or LINC01021-specific ASO-2. ASO-ctrl, ASO-control; EMT, epithelial-mesenchymal transition.
Da0324 or LINC01021-specific ASO treatment suppresses tumor growth of human GC xenografts in vivo
To investigate whether Da0324 inhibited tumor growth in vivo, KATO III cells were used to create a subcutaneous xenograft model in nude mice. As shown in Figure 6A and 6B, relative to the control group, treatment with Da0324 substantially reduced the volume, size, and weight of tumors in KATO III xenografted mice. Meanwhile, there was no significant difference in body weight between the control group and the Da0324 treatment group, indicating no apparent toxicity of Da0324 in vivo (Figure 6C). Results from qRT-PCR showed that Da0324 treatment downregulated expression of LINC01021 (Figure 6D). In addition, Da0324 treatment significantly upregulated the protein expression of P53 and suppressed the expression of Bcl-2 (Figure 6E). To determine whether LINC01021 affected tumorigenesis, KATO III cells were inoculated subcutaneously into nude mice and ASOs administered by intratumoral injection. Both xenograft tumor weight and volume were remarkably reduced in the ASO-LINC01021 treatment group when compared to the control group (Figure 6F and 6G). As shown in Figure 6H, ASO-LINC01021 treatment does not affect the weight of nude mice. Furthermore, qRT-PCR confirmed that ASO-LINC01021 treatment suppressed the expression of LINC01021 in tumor tissues (Figure 6I). Simultaneously, P53 protein level was increased and Bcl-2 protein level was decreased in the ASO-LINC01021 group relative to the ASO-control group (Figure 6J).
Figure 6.
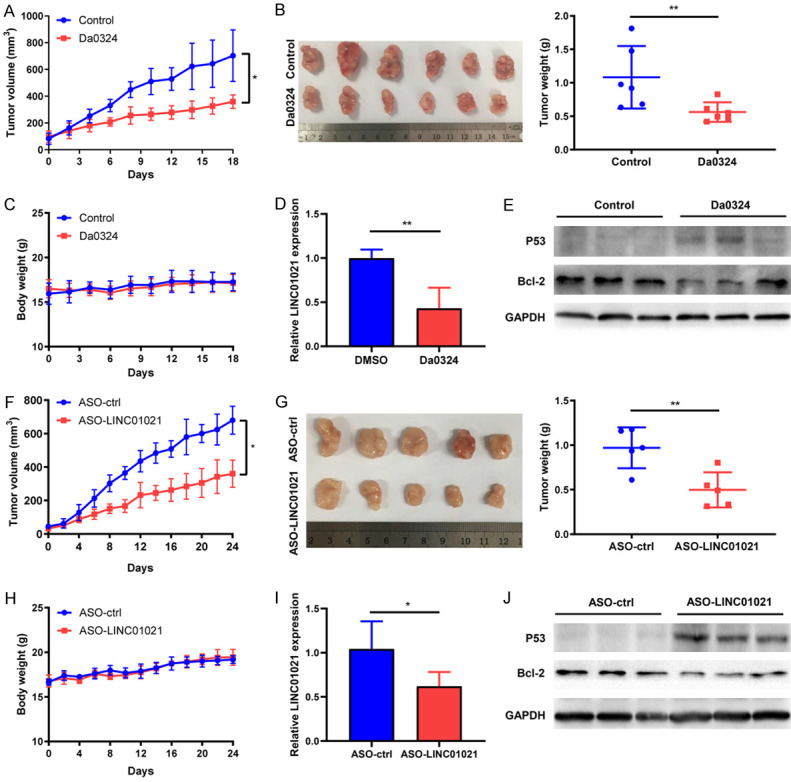
Da0324 or LINC01021-specific ASO treatment suppresses tumor growth of GC cells in vivo. A. Effects of Da0324 treatment on tumor volume in subcutaneous xenograft mouse models using KATO III cells. Tumor volume as measured by caliper of Da0324 (20 mg/kg, intraperitoneal injection every day) or vehicle administered for 18 days in a subcutaneous xenograft model. The data were expressed as mean ± SD. *, P < 0.05. B. Tumors were excised after 18 days of treatment. Images and weight of the dissected xenograft tumors are shown. C. Body weight of nude mice. D. Determination of tumor LINC01021 expression by qRT-PCR for both the control and treatment groups. The data were expressed as mean ± SD. **, P < 0.01. E. Western blot analysis of the protein expression of P53 and Bcl-2 in control or Da0324-treated mouse xenograft tumors. GAPGH was used as an internal control. F. Tumor volume of subcutaneous xenograft mouse models using KATO III cells treated with LINC01021-specific ASO or ASO-control. Tumor volume as measured by caliper of LINC01021-specific ASO or ASO-control (250 nmol/kg, intratumoral injection at three-day intervals) administered for 24 days in a subcutaneous xenograft model. The data were expressed as mean ± SD. *, P < 0.05. G. Tumors were excised after 24 days of treatment. Images and weight of the dissected xenograft tumors are shown. The data were expressed as mean ± SD. **, P < 0.01. H. Body weight of nude mice from ASO-control and ASO-LINC01021 treatment groups. I. Determination of LINC01021 expression by qRT-PCR in ASO-control and ASO-LINC01021 treatment xenografts. *, P < 0.05. J. Western blot analysis of the protein expression of P53 and Bcl-2 in tumor tissues from ASO-control and ASO-LINC01021 treatment groups. GAPGH was used as an internal control. ASO-ctrl, ASO-control; qRT-PCR, quantitative reverse transcription-polymerase chain reaction.
Overexpression of LINC01021 reverses Da0324-mediated cytotoxic effects in GC cells
To clarify whether LINC01021 is involved in the inhibitory effect of Da0324 on GC cells, we established BGC823 and SGC7901 cell lines with stably overexpressed LINC01021 via lentiviral infection. This overexpression was verified by qRT-PCR (Figure 7A) and significantly promoted the growth of BGC823 and SGC7901 cells (Figure 7B). Further in vitro cell viability and colony formation assays showed that overexpression of LINC01021 reversed the Da0324-mediated growth inhibition of BGC823 and SGC7901 cells (Figure 7C-E). As shown in Figure 7F, overexpression of LINC01021 also inhibited P53 protein expression and increased Bcl-2 protein expression in both cell lines. Moreover, overexpression of LINC01021 induced EMT in both cell lines, evidenced by the epithelial marker E-cadherin expression being downregulated and the mesenchymal marker N-cadherin upregulated (Figure 7F). These findings support the role of LINC01021 in Da0324-mediated cytotoxicity in GC cells.
Figure 7.
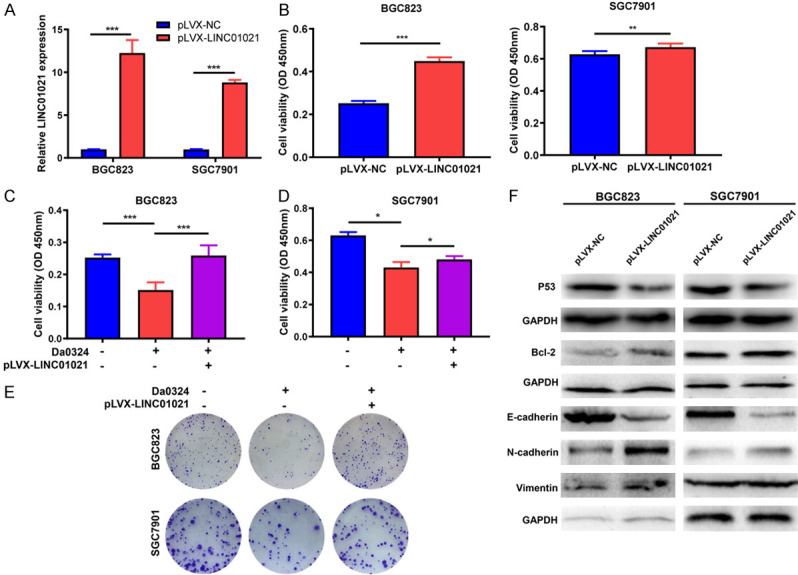
Overexpression of LINC01021 reverses Da0324-mediated cytotoxic effects in gastric cancer cells. A. BGC823 and SGC7901 cells were transfected with negative control vector (pLVX-NC) or overexpressing LINC01021 vector (pLVX-LINC01021) for 48 h. LINC01021 expression was determined by qRT-PCR analysis. The data represent the mean ± SD from three independent experiments. ***, P < 0.001. B. BGC823 and SGC7901 cells were transfected with pLVX-NC or pLVX-LINC01021 for 48 h. Cell viability was evaluated by CCK-8 assays. The data represent the mean ± SD from three independent experiments. **, P < 0.01; ***, P < 0.001. C, D. Overexpression of LINC01021 reversed Da0324-mediated growth inhibition of gastric cancer cells. BGC823 and SGC7901 cells were transfected with pLVX-NC or pLVX-LINC01021 for 24 h and then treated with Da0324 (4 µM) for 24 h. Cell viability was determined by CCK-8 assays. The data represent the mean ± SD from three independent experiments. *, P < 0.05; ***, P < 0.001. E. BGC823 and SGC7901 cells transfected with pLVX-NC or pLVX-LINC01021 were exposed to Da0324 for 48 h and colony formation was assessed. F. BGC823 and SGC7901 cells were transfected with pLVX-NC or pLVX-LINC01021 for 48 h and the expression levels of P53, Bcl-2, E-cadherin, N-cadherin and vimentin were determined by western blot analysis. GAPGH was used as an internal control. qRT-PCR, quantitative reverse transcription-polymerase chain reaction.
Discussion
The use of natural compounds in cancer treatment is a hot research area, and studies have reported successful use of some plant extracts and pure compounds to treat various cancers [29,30]. Considering that curcumin is a safe and cost-effective natural agent with multiple targets in cancer, it is a suitable drug for exploring in the context of cancer treatment and can bring enormous clinical benefits [31]. However, the poor systemic bioavailability of curcumin hinders its therapeutic potential. In our previous study, we reported a novel curcumin analog, Da0324, which exhibited significantly improved stability and anti-cancer activity against GC cells in vitro and reduced toxicity to normal gastric mucous epithelial cells [26]. In this report, we further showed that Da0324 inhibited cell proliferation and colony formation of BGC823, SGC7901, and KATO III cell lines in a dose-dependent manner. Also, we discovered that Da0324 induced apoptosis and autophagy, as well as inhibited migration, invasion, and EMT in GC cells. Moreover, Da0324 significantly suppressed tumor growth of human GC xenografts in vivo.
Numerous studies have demonstrated that lncRNAs play important roles in cancer development [32]. In GC, dysregulation of lncRNAs is a common event and has been shown to affect tumor progression [33,34]. LncRNAs may be potential targets for cancer therapy development [35]. During recent years, curcumin has been explored for potential in regulating lncRNAs [36]. For example, curcumin inhibits the Wnt and mTOR pathways in A549 cells through downregulation of the lncRNA UCA1 [37]. Curcumin has also been reported to sensitize pancreatic cancer cells to gemcitabine by inhibiting expression of the lncRNA PVT1 [38]. Furthermore, Esmatabadi et al. observed that dendrosomal curcumin (DNC) is an activator for GAS5, which decreases the chemotherapeutic effect of DNC in breast cancer cells [39], and Zamani et al. found that DNC increases expression of the lncRNA gene MEG3 in hepatocellular cancer, thereby impeding cancerous tumult [40].
To investigate the role of lncRNAs in the anticancer activity of Da03242, we performed high-throughput sequencing to identify lncRNAs differentially expressed in Da03242-treated GC cells. Interestingly, we found that Da0324 downregulated LINC01021, also named PURPL (P53 upregulated regulator of P53 levels), which is a direct P53 target [41]. LINC01021 has also been reported to inhibit the basal level of P53 by binding to MYBBP1A, a protein that binds and activates P53 [41]. There have been reports that knockdown of LINC01021 increases the sensitivity of the colorectal cancer cell line HCT116 to chemotherapeutic drugs [42]. Although LINC01021 plays an important role in colorectal cancer and liver cancer, little is known about the functionality of this lncRNA in GC [43]. In our study, we found that ASO-mediated silencing of LINC01021 inhibited cell proliferation and induces apoptosis and autophagy in GC cells. We also found that LINC01021-specific ASO inhibits GC cell migration, invasion, and EMT, as well as the tumor growth of human GC xenografts in vivo. These results suggest that LINC01021 may be a promising target for future GC treatment.
P53 is well known as a tumor suppressor gene involved in diverse metabolic processes [44]. More than 50% of tumors in humans have lost the protection of P53, resulting in resistance to apoptosis and infinite proliferation [45]. Furthermore, the proto-oncogene Bcl-2 is a key factor in inhibiting apoptosis and a p53 target [46]. Previous studies showed that curcumin activates P53 and downregulates Bcl-2 expression in some cancer cells [47-49]. In this study, we observed that Da0324 treatment or silencing of LINC01021 also led to increased P53 expression and decreased Bcl-2 expression in GC cells both in vitro and in vivo. Conversely, overexpression of LINC01021 inhibited P53 expression and increased Bcl-2 expression in GC cells, as well as inducing EMT. Moreover, overexpression of LINC01021 significantly promoted GC cell growth and reversed the Da0324-mediated cytotoxic effects, suggesting that Da0324 exerts anticancer effects by regulating LINC01021, which can modulate the expression of P53 and Bcl-2.
In conclusion, our results demonstrate that Da0324 exerts anticancer activities against GC via downregulation of LINC01021, which leads to inhibition of the growth, migration, invasion, and EMT of GC cells and to increased cell apoptosis and autophagy through activation of P53. LINC01021 is expected to be a new candidate for the treatment of GC. These findings reveal a novel anti-cancer mechanism of Da0324 a potential therapeutic target in the form of LINC01021 for the treatment of GC.
Acknowledgements
This work was supported by the Zhejiang Provincial Natural Science Foundation of China [No. LY19H160025 and LY19H160024], Medicine and Health Science and Technology Program of Zhejiang Province (2020KY635), National Natural Science Foundation of China [No. 81672385], and Wenzhou Science & technological Project [No. Y20170180].
Disclosure of conflict of interest
None.
Abbreviations
- ASO
Antisense oligo
- cDNA
complementary DNA
- EMT
Epithelial-mesenchymal transition
- FBS
fetal bovine serum
- GC
gastric cancer
- LC3B
light chain3B
- lncRNA
Long non-coding RNA
- p-mTOR
phosphorylated mTOR
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Hayakawa Y, Sethi N, Sepulveda AR, Bass AJ, Wang TC. Oesophageal adenocarcinoma and gastric cancer: should we mind the gap? Nat Rev Cancer. 2016;16:305–318. doi: 10.1038/nrc.2016.24. [DOI] [PubMed] [Google Scholar]
- 3.El-Sedfy A, Brar SS, Coburn NG. Current role of minimally invasive approaches in the treatment of early gastric cancer. World J Gastroenterol. 2014;20:3880–3888. doi: 10.3748/wjg.v20.i14.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H, Chen X, Sun J, Gao P, Song Y, Zhang N, Lu X, Xu H, Wang Z. The efficacy and toxicity of paclitaxel plus S-1 compared with paclitaxel plus 5-FU for advanced gastric cancer: a PRISMA systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2014;93:e164. doi: 10.1097/MD.0000000000000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 6.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J. Clin. Oncol. 2006;24:2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 7.Mattick JS, Rinn JL. Discovery and annotation of long noncoding RNAs. Nat Struct Mol Biol. 2015;22:5–7. doi: 10.1038/nsmb.2942. [DOI] [PubMed] [Google Scholar]
- 8.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atianand MK, Caffrey DR, Fitzgerald KA. Immunobiology of long noncoding RNAs. Annu Rev Immunol. 2017;35:177–198. doi: 10.1146/annurev-immunol-041015-055459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9:703–719. doi: 10.4161/rna.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toiber D, Leprivier G, Rotblat B. Long noncoding RNA: noncoding and not coded. Cell Death Discov. 2017;3:16104. doi: 10.1038/cddiscovery.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geisler S, Coller J. RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol. 2013;14:699–712. doi: 10.1038/nrm3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172:393–407. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fanelli GN, Gasparini P, Coati I, Cui R, Pakula H, Chowdhury B, Valeri N, Loupakis F, Kupcinskas J, Cappellesso R, Fassan M. Long-noncoding RNAs in gastroesophageal cancers. Noncoding RNA Res. 2018;3:195–212. doi: 10.1016/j.ncrna.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77:3965–3981. doi: 10.1158/0008-5472.CAN-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nobili S, Lippi D, Witort E, Donnini M, Bausi L, Mini E, Capaccioli S. Natural compounds for cancer treatment and prevention. Pharmacol Res. 2009;59:365–378. doi: 10.1016/j.phrs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Priyadarsini KI. The chemistry of curcumin: from extraction to therapeutic agent. Molecules. 2014;19:20091–20112. doi: 10.3390/molecules191220091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karimian MS, Pirro M, Majeed M, Sahebkar A. Curcumin as a natural regulator of monocyte chemoattractant protein-1. Cytokine Growth Factor Rev. 2017;33:55–63. doi: 10.1016/j.cytogfr.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Cavaleri F. Presenting a new standard drug model for turmeric and its prized extract, curcumin. Int J Inflam. 2018;2018:5023429. doi: 10.1155/2018/5023429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panahi Y, Saadat A, Beiraghdar F, Sahebkar A. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: a randomized double-blind placebo-controlled trial. Phytother Res. 2014;28:1461–1467. doi: 10.1002/ptr.5149. [DOI] [PubMed] [Google Scholar]
- 22.Panahi Y, Alishiri GH, Parvin S, Sahebkar A. Mitigation of systemic oxidative stress by curcuminoids in osteoarthritis: results of a randomized controlled trial. J Diet Suppl. 2016;13:209–220. doi: 10.3109/19390211.2015.1008611. [DOI] [PubMed] [Google Scholar]
- 23.Momtazi AA, Shahabipour F, Khatibi S, Johnston TP, Pirro M, Sahebkar A. Curcumin as a microRNA regulator in cancer: a review. Rev Physiol Biochem Pharmacol. 2016;171:1–38. doi: 10.1007/112_2016_3. [DOI] [PubMed] [Google Scholar]
- 24.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shetty D, Kim YJ, Shim H, Snyder JP. Eliminating the heart from the curcumin molecule: monocarbonyl curcumin mimics (MACs) Molecules. 2014;20:249–292. doi: 10.3390/molecules20010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin R, Xia Y, Chen Q, Li W, Chen D, Ye H, Zhao C, Du X, Shi D, Wu J, Liang G. Da0324, an inhibitor of nuclear factor-kappaB activation, demonstrates selective antitumor activity on human gastric cancer cells. Drug Des Devel Ther. 2016;10:979–995. doi: 10.2147/DDDT.S90081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuo Z, Zhang P, Lin F, Shang W, Bi R, Lu F, Wu J, Jiang L. Interplay between Trx-1 and S100P promotes colorectal cancer cell epithelial-mesenchymal transition by up-regulating S100A4 through AKT activation. J Cell Mol Med. 2018;22:2430–2441. doi: 10.1111/jcmm.13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanida I, Ueno T, Kominami E. LC3 and autophagy. Methods Mol Biol. 2008;445:77–88. doi: 10.1007/978-1-59745-157-4_4. [DOI] [PubMed] [Google Scholar]
- 29.Talib WH. Regressions of breast carcinoma syngraft following treatment with piperine in combination with thymoquinone. Sci Pharm. 2017;85:27. doi: 10.3390/scipharm85030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talib WH. Consumption of garlic and lemon aqueous extracts combination reduces tumor burden by angiogenesis inhibition, apoptosis induction, and immune system modulation. Nutrition. 2017;43-44:89–97. doi: 10.1016/j.nut.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Bordoloi D, Kunnumakkara AB. The potential of curcumin: a multitargeting agent in cancer cell chemosensitization. Role of Nutraceuticals in Chemoresistance to Cancer. 2018:31–60. [Google Scholar]
- 32.Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 33.Song YX, Sun JX, Zhao JH, Yang YC, Shi JX, Wu ZH, Chen XW, Gao P, Miao ZF, Wang ZN. Non-coding RNAs participate in the regulatory network of CLDN4 via ceRNA mediated miRNA evasion. Nat Commun. 2017;8:289. doi: 10.1038/s41467-017-00304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun TT, He J, Liang Q, Ren LL, Yan TT, Yu TC, Tang JY, Bao YJ, Hu Y, Lin Y, Sun D, Chen YX, Hong J, Chen H, Zou W, Fang JY. LncRNA GClnc1 promotes gastric carcinogenesis and may act as a modular scaffold of WDR5 and KAT2A complexes to specify the histone modification pattern. Cancer Discov. 2016;6:784–801. doi: 10.1158/2159-8290.CD-15-0921. [DOI] [PubMed] [Google Scholar]
- 35.Jiang MC, Ni JJ, Cui WY, Wang BY, Zhuo W. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am J Cancer Res. 2019;9:1354–1366. [PMC free article] [PubMed] [Google Scholar]
- 36.Mishra S, Verma SS, Rai V, Awasthee N, Chava S, Hui KM, Kumar AP, Challagundla KB, Sethi G, Gupta SC. Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases. Cell Mol Life Sci. 2019;76:1947–1966. doi: 10.1007/s00018-019-03053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang WH, Chen J, Zhang BR, Lu SJ, Wang F, Peng L, Dai JH, Sun YZ. Curcumin inhibits proliferation and enhances apoptosis in A549 cells by downregulating lncRNA UCA1. Pharmazie. 2018;73:402–407. doi: 10.1691/ph.2018.8402. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida K, Toden S, Ravindranathan P, Han H, Goel A. Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis. 2017;38:1036–1046. doi: 10.1093/carcin/bgx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esmatabadi MJD, Motamedrad M, Sadeghizadeh M. Down-regulation of lncRNA, GAS5 decreases chemotherapeutic effect of dendrosomal curcumin (DNC) in breast cancer cells. Phytomedicine. 2018;42:56–65. doi: 10.1016/j.phymed.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 40.Zamani M, Sadeghizadeh M, Behmanesh M, Najafi F. Dendrosomal curcumin increases expression of the long non-coding RNA gene MEG3 via up-regulation of epi-miRs in hepatocellular cancer. Phytomedicine. 2015;22:961–967. doi: 10.1016/j.phymed.2015.05.071. [DOI] [PubMed] [Google Scholar]
- 41.Li XL, Subramanian M, Jones MF, Chaudhary R, Singh DK, Zong X, Gryder B, Sindri S, Mo M, Schetter A, Wen X, Parvathaneni S, Kazandjian D, Jenkins LM, Tang W, Elloumi F, Martindale JL, Huarte M, Zhu Y, Robles AI, Frier SM, Rigo F, Cam M, Ambs S, Sharma S, Harris CC, Dasso M, Prasanth KV, Lal A. Long noncoding RNA PURPL suppresses basal p53 levels and promotes tumorigenicity in colorectal cancer. Cell Rep. 2017;20:2408–2423. doi: 10.1016/j.celrep.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaller M, Gotz U, Hermeking H. Loss of p53-inducible long non-coding RNA LINC01021 increases chemosensitivity. Oncotarget. 2017;8:102783–102800. doi: 10.18632/oncotarget.22245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu X, Wang Y, Wu G, Zhang W, Xu S, Wang W. Long noncoding RNA PURPL promotes cell proliferation in liver cancer by regulating p53. Mol Med Rep. 2019;19:4998–5006. doi: 10.3892/mmr.2019.10159. [DOI] [PubMed] [Google Scholar]
- 44.Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer. 2014;14:359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim S, An SS. Role of p53 isoforms and aggregations in cancer. Medicine (Baltimore) 2016;95:e3993. doi: 10.1097/MD.0000000000003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischer M. Census and evaluation of p53 target genes. Oncogene. 2017;36:3943–3956. doi: 10.1038/onc.2016.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He YC, He L, Khoshaba R, Lu FG, Cai C, Zhou FL, Liao DF, Cao D. Curcumin nicotinate selectively induces cancer cell apoptosis and cycle arrest through a P53-mediated mechanism. Molecules. 2019;24:4179. doi: 10.3390/molecules24224179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talib WH, Al-Hadid SA, Ali MBW, Al-Yasari IH, Ali MRA. Role of curcumin in regulating p53 in breast cancer: an overview of the mechanism of action. Breast Cancer (Dove Med Press) 2018;10:207–217. doi: 10.2147/BCTT.S167812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J, Cao Y, Sun J, Zhang Y. Curcumin reduces the expression of Bcl-2 by upregulating miR-15a and miR-16 in MCF-7 cells. Med Oncol. 2010;27:1114–1118. doi: 10.1007/s12032-009-9344-3. [DOI] [PubMed] [Google Scholar]