Abstract
Noise pollution is a major public hazard. Previous studies have shown that environmental noise affects the reorganization of the auditory cortex and leads to behavioral abnormality; however, the effects of long-term environmental noise exposure on the inner ear and hearing remain to be elucidated. In this study, we simulated environmental noise with a long-term 70 dB sound pressure level “white” noise, observed its effect on the inner ears of C57BL/6J mice, and developed an in vitro model for mechanistic studies. We found that environmental noise increased the hearing threshold, decreased the auditory response amplitude, and aggravated the range and extent of age-related hearing loss (ARHL), especially in the intermediate frequency band in mice. Cochlear ribbon synapse is the primary site of inner ear injury caused by environmental noise. We also verified, through an in vitro simulation of the excitatory toxicity of glutamate and aging effects, that the activation of NLRP3 inflammasome plays a vital role in the cochlear ribbon synaptic damage. Our results show that long-term exposure to low-intensity environmental noise can lead to hearing loss via the disruption of ribbon synapses, which is caused by an inflammatory reaction. Additionally, environmental noise can further aggravate the progression of ARHL. This study expounded the pathogenesis of the inner ear damage caused by environmental noise exposure and provides a new direction for the prevention and treatment of hearing loss.
Keywords: Noise-induced hearing loss, age-related hearing loss, ribbon synapse, auditory nerve, environmental noise
Introduction
Noise-induced hearing loss (NIHL) is a common cause of deafness [1]. In the US, the Occupational Safety and Health Administration has recommended that sound levels should not exceed 90 dB on an 8-hour daily basis [2]. Most people are exposed to environmental noise below this standard. Recently, it has been proposed that environmental noise exposure can lead to behavioral disorders and substantially impair the function of the auditory cortex, resulting in a decline in sound recognition [3,4]. This raises the serious concern that environmental noise may not be as safe as once thought, especially for individuals who are exposed to long-term noise in their everyday life. However, the effects of environmental noise on the peripheral auditory system remain to be elucidated.
Identification of the pathogenic mechanisms provoked by environmental noise is critical for hearing protection. Previous studies have shown that NIHL is correlated with cochlear ribbon synapses [1]. Ribbon synapse is a vital structure between the inner hair cell (IHC) and spiral ganglion neuron (SGN), which is the primary synaptic structure in the sound conduction pathway and plays an important role in sound signal transmission [5]. Studies have shown that ribbon synapses are highly sensitive to noise exposure [6,7]. In certain cases, such as those of “hidden hearing loss”, a short-term high intensity noise exposure can lead to a temporary increase in hearing threshold and subsequent recovery [8,9]. In this process, noise exposure induces cochlear ribbon synapse damage, as well as degeneration of the SGN fibers without causing loss of hair cells (HCs), in which the synaptic connections can only partially recover [10,11]. Studies with repeated exposure to moderate intensity noise of 110 dB have shown that a threshold shift in the high-frequency similar to that in age-related hearing loss (ARHL) could be associated with the accumulated afferent terminal damage [12]. Moreover, in long-term observation after noise exposure, onset of ganglion cell death was delayed and the initial loss in synapses was consistent with the final loss of SGNs detected 2 years later [8]. Thus, NIHL is a cumulative process and has a synergistic relationship with ARHL [13]. ARHL presents as a progressive hearing loss starting from high frequencies. However, the alteration of threshold is not the only manifestation; individuals with advanced age also experience greater difficulty in speech comprehension in the clinical setting. In ARHL, morphologic alterations in the IHC synaptic terminals prior to the loss of SGNs and hearing sensitivity [14,15], and the nature and progression of this hearing loss are susceptible to noise effects [16]. Previous studies have shown that the damage to ribbon synapses caused by noise is plastic or accumulative; however, the effect of low-intensity, long-duration environmental noise on ribbon synapses remains unclear.
In this study, we aimed to investigate whether low-intensity environmental noise can disrupt hearing and the resulting pattern of hearing impairment. To accomplish this, adult mice were exposed to imitated environmental noise for different durations, and the effects on hearing and morphological features in the cochlea were assessed. Furthermore, we conducted in vitro culture experiments of the basilar membrane to explore the underlying mechanism of ribbon synapse disruption.
Materials and methods
Animals and noise exposure
Forty male C57BL/6J mice (8 weeks old) were obtained from the Experimental Animal Centre of Capital Medical University (Beijing, China). Hearing function was assessed prior to the experiments. No auricle or ear canal abnormality was identified. Mice were randomly divided into the control and exposure groups (40 cochleae per group). For the noise treatment, the mice of the exposure group were placed in a reverberation chamber and were receiving “white” noise of 70 dB sound pressure level (SPL) on an 8-hour daily basis for up to 3 months. The mice in the control group were placed in an isolated environment with background noise less than 30 dB for the same time period. All procedures were performed in accordance with the animal protocol approved by the Animal Care and Use Committee of Capital Medical University of China. For the comparison of the experimental results, mice were divided into the following four groups: unexposed 1-month (UNE1M) group; exposed 1-month (E1M) group; unexposed 3-month (UNE3M) group; and exposed 3-month (E3M) group.
Hearing examination
Auditory brainstem response (ABR) tests were conducted using TDT System (Tucker Davis Technologies, Alachua, FL, USA) in a soundproof shielded room to determine auditory thresholds, which were based on the reproducibility of Wave III. The SigGen/BioSig software (Tucker Davis Technologies) was used to generate acoustic stimuli and display the evoked potentials. The mice were anesthetized with intraperitoneal injection of ketamine (100 mg/Kg, Sigma, MO, USA) and xylazine (10 mg/Kg, Sigma) and were then kept warm with a heating pad during the ABR recordings. The electrodes were placed under the skin of both auricles and at the top of the head. Specific auditory stimuli with a rate of 20 beats per second, a scanning time of 20 ms, and an average of 1024 superposition times, bandwidth filtering at 100-3000 Hz, were delivered through plastic tubes in the ear canals. Auditory thresholds were obtained by varying the SPL in 5-dB steps to the lowest level that could be recognized. ABR thresholds were collected for the acoustic stimuli frequencies on 2, 4, 8, 12, 16, 24, and 32 kHz. The amplitude of Wave I was also analyzed to evaluate the cochlear function, as it has been proposed to reflect changes in synaptic potentials between the IHCs and auditory nerve fibers (ANFs). In this study, the amplitude of the ABR Wave I was measured as the value from the baseline to the peak (latency = 1.2-1.9 ms).
Histological preparation
After the ABR test, mice were sacrificed by cervical dislocation under deep anesthesia. The temporal bone was removed, and the cochlea was separated quickly. We opened the round and oval windows and removed the bone fragment over the apical turn to allow a rapid flushing of 4% paraformaldehyde inward through the cochlea roof. The cochleae were then fixed in the same fixative overnight at 4°C and were finally decalcified for 12-18 hours in 10% ethylenediaminetetraacetic acid (EDTA) solutions. After decalcification, we dissected the cochlea and pulled off the tectorial and Reissner’s membranes.
Cochlear frequency mapping
A cochlear frequency map was computed according to previous studies, and the method description partly reproduces their wording [17,18]. The basilar membrane was cut into three segments with the length of each segment (l1, l2, l3) being measured along the Corti organ using a low magnification microscope; the sum of each segment was the total length of the basilar membrane (L = l1 + l2 + l3). The total length (L) was divided into 10 parts starting from the cochlear apex: nL/10 (n = 1, 2, 3…10), while 11 adjacent points were selected and the distance from the apex were marked as 0, 10%, 20%....90%, and 100%, respectively. Because the range of the audible spectrum in mice (1-64 kHz) sequentially covered the full cochlear length, the 1-64 kHz range corresponded to the equidistant parts along the entire length of the cochlea (0-100%). Thus, the cochlear frequency mapping was established by the correspondence between the whole length of the cochlear basilar membrane and the span of the auditory spectrum. When calculating the number of synapses, we used the synaptic counts near to 10%, 30%, 50%, 70%, and 90% points to represent the number of synapses in each 2 L/10 range, respectively.
In vitro culture of the cochlea basilar membrane
P3 C57BL/6J mice were euthanized by carbon dioxide and disinfected with 75% ethanol. Then, their heads were removed and the brain tissue was separated. The cochlear structure was separated under a stereoscopic anatomical microscope, and the basilar membrane of the cochlea was rapidly dissected. Next, the basilar membrane was transferred into Dulbecco’s modified Eagles medium (DMEM)/F12 medium (Sigma-Aldrich, St. Louis, USA) supplemented with 10% bovine serum albumin (Sigma-Aldrich), and were incubated for 24 hours at 37°C in 95% oxygen and 5% CO2. After 1 day of in vitro cultivation, the cochlear explants were used in the subsequent experiments.
The cochlear explants of the control group remained in the DMEM/F12 medium for 3, 5 days (the medium was replaced daily). Noise-induced synaptic disorder is a type of glutamate excitotoxicity [19]. To examine excitotoxic damage, the explants were left in the DMEM/F12 medium for 48 hours and were then treated with 0.5 mM NK medium [20,21] for 12 hours, which consists of 0.5 mM N-methyl-D-aspartate (0114, Tocris Bioscience, UK) and 0.5 mM kainate (K2389, Sigma-Aldrich, USA). To simulate aging, explants were left in the DMEM/F12 medium for additional 12 hours and then transferred in medium containing 40 mg/mL D-galactose (D-g; Sigma-Aldrich, USA) for 48 hours. The D-g-induced senescence model has been widely used to study the mechanisms and interventions of aging in the auditory system [22,23]. To simulate the combined influence of noise and aging, explants were cultured first in the 40 mg/ml D-g medium for 48 hours, and next in the 0.5 mM NK medium for 12 hours. The cochleae of all four groups were removed from the medium and immobilized simultaneously.
Immunohistochemistry
The samples were washed three times in 0.01 M phosphate-buffered saline (PBS) and incubated in a blocking solution comprised of 10% goat serum with 0.25% Triton X-100 for 30 min at 25°C. Then, the samples were incubated overnight at 4°C with the following antibodies: (1) anti-c-terminal binding protein 2 (CtBP2; 612044, BD Biosciences, USA; 1:500), (2) anti-myosin-VIIa (25-6790, Proteus Biosciences, USA; 1:300), and (3) anti-β3-Tubulin (ab78078, Abcam, USA; 1:300). Next, the specimens were washed in 0.01 M PBS three times, and incubated at 25°C for 2 hours with the secondary antibodies, including goat anti-mouse IgG1 Alexa Fluor 568, goat anti-mouse IgG2a Alexa Fluor 488, and goat anti-rabbit IgG(H+L) Alexa Fluor 647 (A21124, A21131, A21244, Invitrogen/Molecular Probes, USA; 1:300). Then, the samples were washed in PBS three times, and approximately 40 μL of 4, 6-diamidino-2-phenylindole (DAPI; ZLI9557, ZSGB-BIO, China) was applied in dark to stain the nuclei. After immunostaining, microdissected segments of the basilar membrane were mounted on slides and a coverslip was applied under a dissecting microscope.
Confocal microscopy imaging
A laser scanning confocal microscope (Leica, Wetzlar, Germany) with a ×63 oil immersion objective lens was used. The excitation wavelengths used were 488, 568, and 647 nm; local images were digitally magnified by twofold, and Z stacks of the IHC base were captured. Sequence scanning was performed from the apex of the basilar membrane at 0.3 μm intervals. All digital images were acquired under the same conditions.
Scanning electron microscopy
Mice were sacrificed as previously described and the cochleae were separated. The whole cochlea was fixed in 2.5% glutaraldehyde and was then placed in 1% osmium tetroxide at room temperature for 2 hours. After thoroughly rinsing with 0.1 M PBS, the cochlea was dissected to expose the basilar membrane. After dehydration in graded alcohol series, the sample was critical-point dried with CO2 and coated with platinum; they were then observed under a scanning electron microscope (JEOL JSM-35C, Hitachi, Japan). The images were digitally photographed and recorded.
Hematoxylin and eosin staining
Cochlear tissues were immobilized with 4% paraformaldehyde in PBS overnight at 4°C. The specimens were decalcified for 12 hours in 10% EDTA and then were dehydrated in graded alcohol series, transparentized with xylene, and embedded in paraffin. The paraffin blocks were cut into 10 μm thick slices and stained with hematoxylin and eosin.
Western blotting
Basilar membrane tissues were crushed and homogenized in RIPA Lysis Buffer (Beyotime, China) at 4°C for 30 min. Insoluble materials were removed by centrifugation. Bicinchoninic acid protein assay kit (Beyotime, China) was used to determine the protein concentration of each specimen. Homogenates were separated on a 12% acrylamide gel and transferred to a polyvinylidene fluoride membrane. Blotto [3% bull serum albumin in tris buffered saline Tween (TBST), 0.1% Tween 20, and 150 mM NaCl] was used to block the blots for 2 hours; then, the blots were incubated overnight at 4°C with the following antibodies: NLRP3, caspase-1, IL-1β (ab214185, ab207802, ab9722, Abcam, USA; 1:500), and β-actin (CST, USA; 1:1000). The blots were washed three times in TBST and incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies (ZSGB-BIO, China; 1:5000) for 2 hours. After washing three times in TBST, the protein bands were visualized with a chemiluminescence reagent (Applygen Technologies Inc., China); β-actin was used as the internal control.
Enzyme linked immunosorbent assay
TNF-α, IL-18, and IL-1β proteins were measured using the TNF-α, IL-18, and IL-1β ELISA kit (Shanghai Lianshuo Biological Technological Co., Ltd., China) as per the manufacturer’s instructions. All experiments were repeated at least three times.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software Inc., LaJolla, CA, USA). All data are presented as the mean ± standard deviation (SD). After meeting the assumption of normality and homogeneity of the variances, a parameter test was performed. The Kolmogorov-Smirnov test was used for the evaluation of normal distribution and the Bartlett test for the homogeneity of the variances. Statistical analyses were conducted using independent sample t-tests for two-group comparison and the one-way analysis of variance (followed by Student-Newman-Keuls test) for multiple group comparison. P-values of <0.05 were considered as statistically significant.
Results
Long-term environmental noise exposure caused hearing loss via ribbon synapse disruption
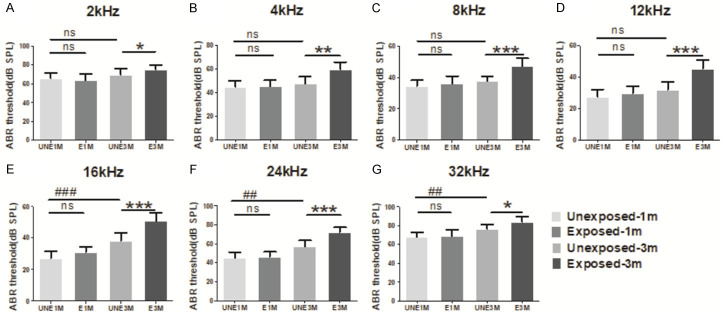
The ABR thresholds were measured in frequencies of 2-32 kHz (Figure 1). There were no significant differences in the ABR threshold between the UNE1M and E1M groups. However, significant differences in the ABR threshold were observed at all frequencies between the UNE3M and E3M groups (P<0.05); the largest increase was found at the 8-24 kHz frequency range in the E3M group (P<0.001); this corresponds to the middle frequency of cochleae in mice and suggests that the middle frequency region in mice is most susceptible to environmental noise exposure.
Figure 1.
ABR thresholds in different frequencies. No significant difference was observed between the UNE1M and E1M groups; the threshold of the UNE3M group is higher than that of the UNE1M group at 16 kHz or higher; compared to the UNE3M group, the threshold of the E3M group at all frequencies increased to varying degrees, which was primarily concentrated at the intermediate frequency (8-24 kHz) of the audio spectrum (n = 7 per group). Data are presented as mean ± SD. ##P<0.01, ###P<0.001 vs. UNE1M; *P<0.05, **P<0.01, ***P<0.001 vs. E3M. UNE1M, unexposed 1-month; E1M, exposed 1-month; UNE3M, unexposed 3-month; E3M, exposed 3-month.
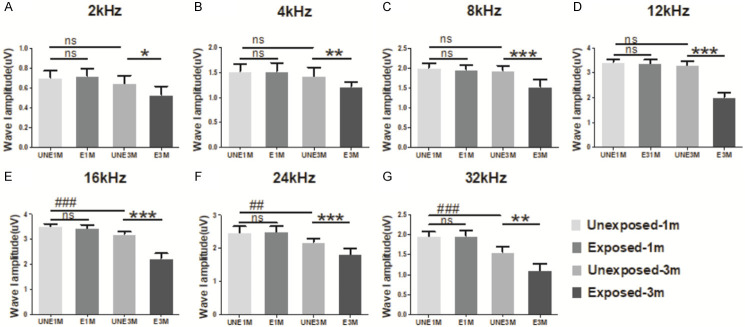
Consistent with our finding of an increase in the ABR threshold, there was a reduction in the full range of ABR Wave I amplitude in the E3M group (Figure 2). The most significant reduction was observed at frequencies of 8-24 kHz in the E3M group compared to the UNE3M group (P<0.001). This frequency range corresponds to the highest elevated range in ABR thresholds.
Figure 2.
Variation of ABR wave I amplitude of different frequencies at 90 dB SPL. Almost all frequencies showed no significant change after 1 month of exposure. Compared to the UNE1M group, the amplitude of wave I for the UNE3M group decreased from 16 kHz to 32 kHz. Compared to the UNE3M group, the amplitudes of all frequencies in the E3M group were reduced and the frequencies of 8-24 kHz were more significant (n = 6 per group). Data are presented as mean ± SD. ##P<0.01, ###P<0.001 vs. UNE1M; *P<0.05, **P<0.01, ***P<0.001 vs. E3M.
We quantitatively evaluated the changes in ribbon synapses through the whole length of the cochlea (Figure 3). There was no significant difference in the loss of ribbon synapses between the E1M and UNE1M groups. However, there was a significant difference in the loss of ribbon synapses between the E3M and UNE3M groups, and the maximal loss of ribbon synapses occurred at the 30-70% cochlear region from the apex (P<0.001); this approximately corresponds to the middle spectrum distribution of hearing loss. These data suggest that the quantitative loss and functional impairment of the ribbon synapses were responsible for the hearing loss along the length of the cochlear.
Figure 3.
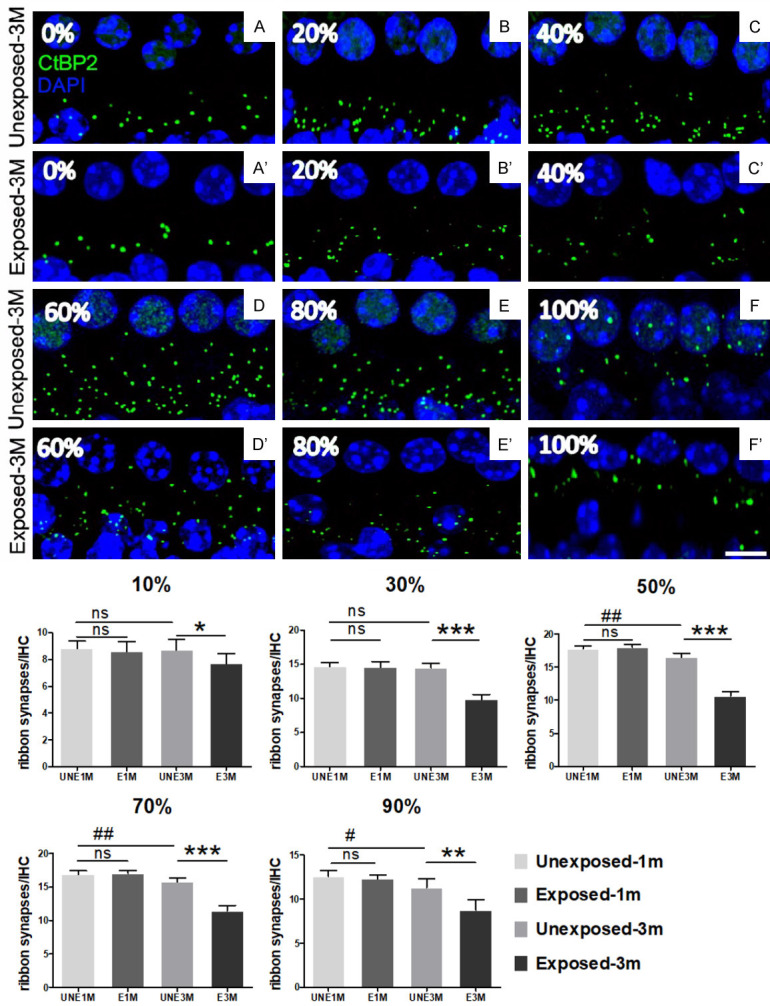
Quantitative changes in the IHC ribbon synapses induced by noise exposure. The ribbon synapses were labeled with anti-CtBP2 (green). The cell nuclei were labeled with DAPI (blue). A significant reduction in ribbon synapses immunostaining spots was shown in the 3-month exposed group, especially near the middle range (scale bar = 5 μm). The average number of ribbon synapses in each IHC was calculated. No significant difference was observed between the UNE1M and E1M groups. Compared to the UNE1M group, the number of ribbon synapses decreased from the 50% location to the bottom in the UNE3M group. Compared to the UNE3M group, the loss of synapses in the E3M group affected almost the entire length of the cochlea, with the most severe loss presented in the middle region (30-70% from the apex) (n = 5 per group). Data are presented as mean ± SD. #P<0.05, ##P<0.01 vs. UNE1M; *P<0.05, **P<0.01, ***P<0.001 vs. E3M.
Combined long-term environmental noise exposure and aging aggravated hearing loss
The ABR thresholds were higher in the UNE3M group than in the UNE1M group for frequencies over 16 kHz (P<0.05); however, the thresholds further increased in the E3M group, as previously mentioned. In the E3M group, the threshold value increased across the full frequency range and was most concentrated at the 8-24 kHz region (Figure 1). Thus, the changes in hearing threshold were more extensive and drastic after long-term exposure to environmental noise.
For Wave I, which represents cochlear function, the amplitude at high frequencies over 16 kHz were lower in the UNE3M than in the UNE1M group (P<0.05). However, in the E3M group, the amplitude decreased compared with the UNE3M group in the full frequency range, with a maximum decrease between 8 and 24 kHz (Figure 2), which exactly overlapped with the frequencies of ABR thresholds.
The decrease in the synapse number in the two unexposed groups were consistent with the declines in the Wave I amplitude. Compared to the UNE1M group, the UNE3M group exhibited a significant downward trend at the cochlea over the 50% region from the apex (P<0.05). The E3M group showed a greater decrease in the number of synapses than the UNE3M group; this decrease was reflected along the whole length of the cochlea, with the most concentrated area at 30%-70% (Figure 3).
Long-term environmental noise exposure caused hearing loss without changes in cochlear morphology
The disruptions of HCs and SGNs are usually considered to underlie hearing loss. We, therefore, investigated whether the cochlear morphology was affected by noise exposure. We compared the full length of the cochlea in E1M, E3M, and normal 8-week old mice; no obvious damage in the cilia bundles of the outer hair cells (OHCs) and IHCs was identified (Figure 4A-F), and the arrangement of HCs showed no significant change (Figure 4G-I). We also observed SGNs by cochlea slices and found no obvious change (Figure 4J-L). This suggests that environmental noise exposure does not cause a loss of OHCs, IHCs, and SGNs over the 3-month course.
Figure 4.
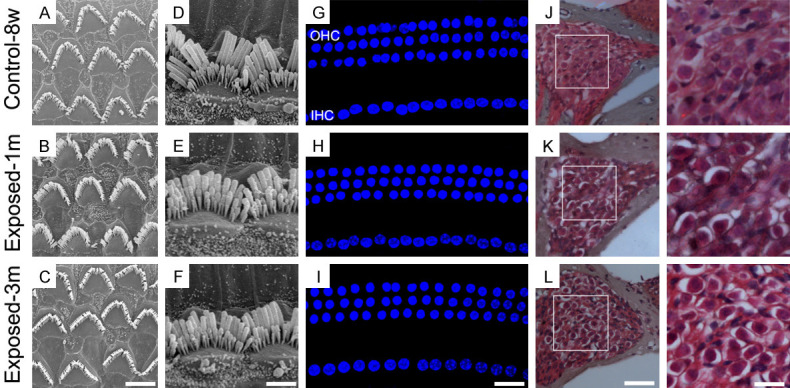
The morphology of the cochlea after 1- and 3-month noise exposure, respectively. Compared to normal 8-week-old mice, there were no significant changes in the HCs and SGNs of the two exposed groups. A-C: Electron microscopy image showing the cilia bundles of OHCs (scale bar = 5 μm); D-F: Electron microscopy image showing the cilia bundles of the IHCs (scale bar = 4 μm); G-I: The arrangement of the HCs labeled with DAPI (scale bar = 10 μm); J-L: The density of SGNs as shown by HE staining (scale bar = 50 μm and 20 μm).
Excitotoxic and senescent effects on the in vitro model
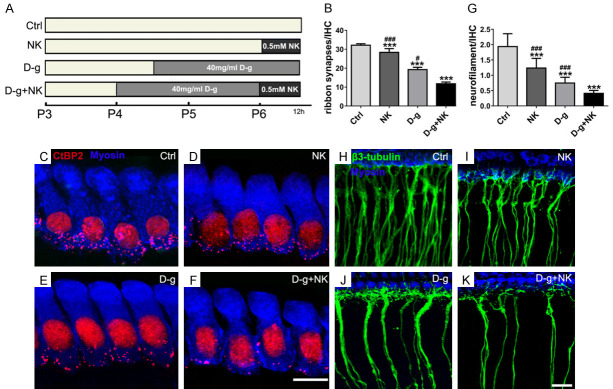
To study further the combined effects of noise and aging on the synapses of the IHCs and peripheral auditory nerves, we prepared an in vitro culture of the basilar membrane (Figure 5A). Because synapses and nerve filaments were distributed differently in different regions of the basilar membrane, the synapses were mostly concentrated in the middle segment as shown earlier. Therefore, the middle segment of the basilar membrane was used for counting to reduce the effects of distribution variability.
Figure 5.
Variations of ribbon synapses and ANFs of the basilar membrane cultured in vitro. Immunofluorescence staining showed that HCs were labeled with Myo-VIIa (blue), cell nuclei and ribbon synapse were labeled with Anti-CtBP2 (red), and ANFs were labeled with β3-Tubulin (green). A: The process of in vitro culture for each group. B: Upon comparing the number of ribbon synapses in each group, in the D-g+NK group was significantly lower than the control group, and there were significant differences in the NK and D-g groups compared to the control or the double-intervention group (n = 4 per group). C-F: The decrease in ribbon synapse differed in each intervention group. Scale bar = 5 μm. G: The numbers of ANFs were compared and were consistent with the changes in ribbon synapses (n = 5 per group). H-K: The counts of ANFs varied in each intervention group. Scale bar = 10 μm. Data are presented as mean ± SD. ***P<0.001 vs. Ctrl. #P<0.05, ###P<0.001 vs. D-g+NK. Ctrl, control; NK, N-methyl-D-aspartate + kainate; D-g, D-galactose.
In the in vitro control group, the mean number of ribbon synapses per IHC was 32.16±0.74 (Figure 5B, 5C). We subjected cochlear explants to NK treatment, as described in the method section, to simulate the excitotoxic effect similar to that caused by noise. As a result, the mean number of ribbon synapses reduced to 28.35±2.01 (Figure 5B, 5D), which was significantly different from that of the control group (P<0.001). After D-g treatment to simulate senescence, the mean number of ribbon synapses reduced to 19.25±1.24 (Figure 5B, 5E), which significantly differed from that of the control group (P<0.001). Finally, when both D-g and NK were administered, the number of ribbon synapses reduced to 11.78±0.90 (Figure 5B, 5F), which was significantly different from that of the control and the other two groups (P<0.001). Along with synaptic changes, nerve filaments were also counted. The reduction tendency within each group was the same as that of the ribbon synapses; however, the changes in the number of nerve filaments was greater than that of the ribbon synapses (Figure 5G-K).
Combined effect of excitotoxicity and senescence on basilar membrane impairment was caused by inflammation
Inflammation is one of the major mechanisms underlying neurological damage [24]; NLRP3 inflammasome activation causes caspase-1 activation and cleavage of specific pro-cytokines, such as IL-1β, into their mature forms. We found significantly increased NLRP3 expression in the NK and D-g groups compared to the control group (P<0.01 and P<0.001, respectively). Moreover, the D-g plus NK group presented the most intense increase (P<0.001). The NLRP3 activation also led to the activation of caspase-1 and yielded a significant increase in IL-1β expression (Figure 6A, 6B). Cytokine activation can be induced either by NK or D-g, and small increases in cytokine levels can cause inflammatory damage. Additionally, cytokine levels in the basilar membrane were measured (Figure 6C), and TNF-α, and IL-18 levels were found increased in the NK and D-g groups. However, the D-g plus NK group experienced the most significant inflammatory injury.
Figure 6.
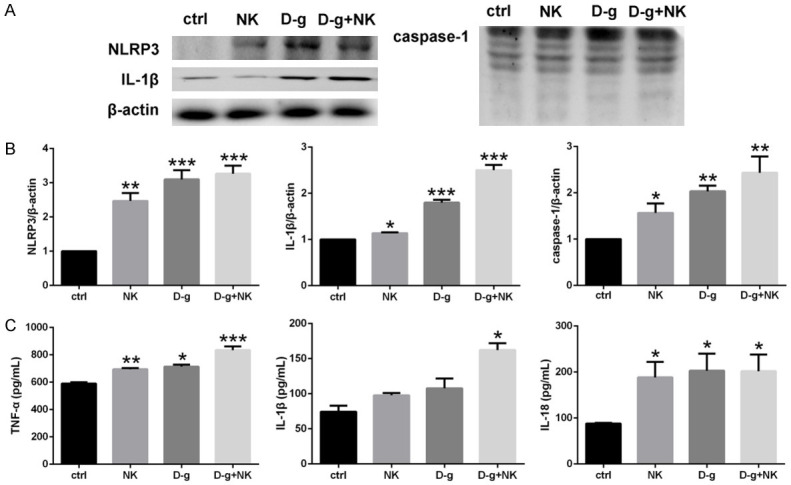
NLRP3 inflammasome pathway and cytokines. A, B: Western blot analysis and densitometric quantification of NLRP3, IL-1β, and caspase-1 proteins. The NLRP3 and cytokines of each intervention group significantly increased compared to the control group, while the level of the D-g+NK group increased the most (n = 3 per group). C: ELISA results of TNF-α, IL-1β, and IL-18 showed that the most significant increase was observed in the D-g+NK group (n = 3 per group). Data are presented as mean ± SD. *P<0.05, **P<0.01 *** P<0.001 vs. ctrl.
Discussion
This study presents several critical points on environmental noise and hearing loss, which are the following: I) Long-term environmental noise exposure can cause significant hearing loss. II) Continuous low-intensity noise exposure caused maximal elevations of the ABR threshold at the 8-24 kHz range, which corresponded to a maximal reduction in ribbon synapses at the 30-70% cochlear region from the apex; moreover, the amplitudes of Wave I changed in parallel with the number of ribbon synapses and ABR thresholds, which indicates that synaptic changes (in the number and function) were closely related to hearing loss. III) Aging caused hearing threshold deterioration and a decrease in the number of synapses however, after noise exposure, the damage pattern changed from ≥16 kHz to almost the entire frequency range; there was a corresponding decrease in the number of synapses at ≥50% from the apex to nearly the full length, which indicates that the combination of noise and aging enhanced the pathogenicity. IV) Low-intensity environmental noise exposure leading to hearing loss was not necessarily accompanied by morphological changes in cochlear components, including the HCs, HC stereocilia, and SGNs. V) The combined effect of excitotoxicity and senescence stimulation of the basilar membrane manifested as a decrease in the number of ribbon synapses and spiral nerve fibers, additional to the inflammatory reaction, which is the mechanism underlying this combined effect on the basilar membrane.
Currently, it is still unclear whether exposure to environmental noise can damage hearing ability. However, emerging evidence has shown that environmental noise exposure may not be totally safe. For example, adult rats living in low-level ambient noise were found to have impaired fine pitch discrimination [3], which suggests that a moderate noise level could be harmful to hearing. Another study has reported that chronic exposure to moderate-level noise during adulthood impaired behavioral and neuronal discrimination of sounds in the temporal domain, with no peripheral deficits however [25]. In the present study, we demonstrated that exposure to continuous and unstructured (“white”) noise impairs peripheral hearing.
One of the critical findings of this study is that long-term imitated environmental sound exposure (“white” noise of 70 dB SPL) affected the cochlear space unequally. In the unexposed groups, the maximal number of ribbon synapses was found in the middle region of the cochlea [17]. After long-term noise exposure, there was a maximal reduction in ribbon synapses approximately in the same region. Meanwhile, the amplitudes of the ABR Wave I, which is indicative of the connections between the IHCs and the auditory nerve, were consistent with the presence of synaptic changes. These results indicate that synapses in the middle region of the cochlear are most susceptible to sound insult. Similar cases in humans have been reported. For example, the presence of notch at frequencies of 3-6 kHZ in elderly individuals was associated with a history of long-term noise exposure [26]. Additional fMRI data have supported this conclusion, and have demonstrated that various forms of long-term, but not passive, acoustic exposure, such as long-term musical training, can alter responsive patterns to acoustic stimuli [27].
Interestingly, we did not find morphological changes associated with hearing loss in the noise-exposed groups; this could be because the impairment occurs in early stages and does not result in loss of HCs. In accordance with this, a previous study has also shown that noise exposure (110 dB) did not cause any loss of HCs [28]. However, even this early lesion can cause hearing impairment. Individuals with a noisy occupation were reported to have impaired speech-sound discrimination compared to those who work under quiet conditions [29]. For example, people working in the armchair industry (with a background noise level of 70-80 dB) presented different mismatch negativity responses to deviant and speech sounds than those working in quiet conditions, which indicates that they had impaired speech-sound discrimination [30]. Therefore, lesion without cell loss is more harmful to hearing than previously thought.
In this study, the age of mice increased with the duration of noise exposure. Aging is a contributing factor to hearing loss [31], and synaptic decline is one of the important mechanisms implicated in ARHL [15,32]. Aging also affects the communication between the IHCs and the terminal of the auditory nerve [14]. Our results showed that the hearing threshold increased at higher frequencies due to aging, and the corresponding ribbon synapses were also impaired near the bottom of the cochlear. However, the combined effect of noise and aging resulted in a different pattern of hearing loss, with more extensive and severe damage. Moreover, there was a corresponding greater loss in the number and function of ribbon synapses. This result indicates that pathogenicity of hearing loss, in other words, environmental noise aggravated ARHL, and ribbon synapses were the common targets of noise and aging-induced hearing loss. Noise over exposures at an earlier age have long-term consequences on hearing in life span [8]. It follows that if there has been continuous exposure to noise from youth, even at low levels, ARHL could be easily aggravated.
It is well known that noise induces excitatory ototoxicity via glutamate [33,34], which causes denaturation of the ANFs; ANFs then lose contact with the IHCs causing loss of ribbon synapses. Ribbon synapses are directly correlated to IHC structure and function [5,35]. The in vitro experiment simulating an injured basilar membrane revealed that nerve fibers and synapses had synchronous damage under excitotoxic and senescent factors, and the degree of nerve fiber damage was more serious than that of the ribbon synapses. Among these, the superposition of excitotoxicity and senescence leads to the most serious damage of nerve fibers and synapses. Other studies using in vitro NK models, have indicated that the loss of presynaptic structure was not significant [20,21], whereas our results showed a significant decline in presynaptic structure. Two possible reasons for this are that the NK treatment lasted longer, and that the combined effects of excitotoxicity and senescence further increased the damage. This is also consistent with the results of using long-term noise and its aggravation of ARHL in the in vivo studies. The interaction between noise exposure and aging causes neural damage [16]. In a long-term observation, noise exposure-induced damage to the IHC synapses further deteriorated with age rather than recovered; additionally, the frequency distribution of damage tended to expand from high to low frequencies, after which there was a slow loss of SGNs [13]. Our in vivo experiments revealed no significant decrease in the SGNs at 3 months after noise exposure, possibly due to the short observation time.
Most of the cochlear damages are induced by different molecular mechanisms, such as increased mitochondrial ROS, inflammation, autophagy, and cell apoptosis [23,36-38]. Noise can increase the expression of pro-inflammatory cytokines in the peripheral and central auditory systems, which have been implicated in the initiation and progression of hearing loss [39,40]. Inflammation also plays an important role in the pathological process of ARHL [23]. NLRP3 has been reported to be involved in the inner ear injury in mice with ARHL [41]; moreover, inflammatory cytokines in the serum have also been associated with the incidence of ARHL [42]. Our in vitro experiments revealed that NK and D-g activated the NLRP3 inflammasome pathway of the basilar membrane and promoted the production of inflammatory cytokines; these cytokines may possibly interact and damage the cochlea. NK and D-g simulated both noise and aging factors; these results indicate that noise and aging combined enhance cochlear inflammation. The inflammatory response is an important mechanism underlying nerve injury, and inflammation-induced damage of the ANFs can cause further disorder in ribbon synapses. Consequently, the resulting deterioration in cochlear function can lead to permanent hearing loss.
In summary, our results suggest that the accumulative effect of long-term exposure to environmental noise and aging leads to permanent hearing loss via an inflammation-induced disruption in the connections between the IHC ribbon synapses and ANFs. Therefore, strategies for the effective prevention and control of environmental noise should be implemented. Furthermore, future work should focus on the development of effective drugs to reduce the inflammatory response of nerves and promote restoration of synaptic connections between the terminal of the auditory nerve and IHCs to treat deafness caused by chronic noise exposure.
Acknowledgements
We would like to thank the Editage company for its linguistic assistance during the preparation of this manuscript. This work was supported by the National Natural Science Foundation of China [grant number 81770997; 81830030]; and the joint funding project of Beijing Natural Science Foundation and Beijing Education Committee [grant number KZ201810025040].
Disclosure of conflict of interest
None.
References
- 1.Kujawa SG, Liberman MC. Translating animal models to human therapeutics in noise-induced and age-related hearing loss. Hear Res. 2019;377:44–52. doi: 10.1016/j.heares.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liberman MC. Hidden hearing loss. Sci Am. 2015;313:48–53. doi: 10.1038/scientificamerican0815-48. [DOI] [PubMed] [Google Scholar]
- 3.Zheng W. Auditory map reorganization and pitch discrimination in adult rats chronically exposed to low-level ambient noise. Front Syst Neurosci. 2012;6:65. doi: 10.3389/fnsys.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou X, Merzenich MM. Environmental noise exposure degrades normal listening processes. Nat Commun. 2012;3:843. doi: 10.1038/ncomms1849. [DOI] [PubMed] [Google Scholar]
- 5.Matthews G, Fuchs P. The diverse roles of ribbon synapses in sensory neurotransmission. Nat Rev Neurosci. 2010;11:812–822. doi: 10.1038/nrn2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan AF, Kujawa SG, Hammill T, Le Prell C, Kil J. Temporary and permanent noise-induced threshold shifts: a review of basic and clinical observations. Otol Neurotol. 2016;37:e271–275. doi: 10.1097/MAO.0000000000001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Yin S, Chen H, Shi L. Noise-induced cochlear synaptopathy and ribbon synapse regeneration: repair process and therapeutic target. Adv Exp Med Biol. 2019;1130:37–57. doi: 10.1007/978-981-13-6123-4_3. [DOI] [PubMed] [Google Scholar]
- 8.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin HW, Furman AC, Kujawa SG, Liberman MC. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol. 2011;12:605–616. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liberman MC, Kujawa SG. Cochlear synaptopathy in acquired sensorineural hearing loss: manifestations and mechanisms. Hear Res. 2017;349:138–147. doi: 10.1016/j.heares.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi L, Liu L, He T, Guo X, Yu Z, Yin S, Wang J. Ribbon synapse plasticity in the cochleae of Guinea pigs after noise-induced silent damage. PLoS One. 2013;8:e81566. doi: 10.1371/journal.pone.0081566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Ren C. Effects of repeated “benign” noise exposures in young CBA mice: shedding light on age-related hearing loss. J Assoc Res Otolaryngol. 2012;13:505–515. doi: 10.1007/s10162-012-0329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez KA, Jeffers PW, Lall K, Liberman MC, Kujawa SG. Aging after noise exposure: acceleration of cochlear synaptopathy in “recovered” ears. J Neurosci. 2015;35:7509–7520. doi: 10.1523/JNEUROSCI.5138-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stamataki S, Francis HW, Lehar M, May BJ, Ryugo DK. Synaptic alterations at inner hair cells precede spiral ganglion cell loss in aging C57BL/6J mice. Hear Res. 2006;221:104–118. doi: 10.1016/j.heares.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci. 2013;33:13686–13694. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26:2115–2123. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L, Chen D, Qu T, Ding T, Yan A, Gong P, Liu Y, Zhang J, Gong S, Yang S, Peng H, Liu K. Maximal number of pre-synaptic ribbons are formed in cochlear region corresponding to middle frequency in mice. Acta Otolaryngol. 2018;138:25–30. doi: 10.1080/00016489.2017.1367417. [DOI] [PubMed] [Google Scholar]
- 18.Muller M, von Hunerbein K, Hoidis S, Smolders JW. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res. 2005;202:63–73. doi: 10.1016/j.heares.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss. Hear Res. 2015;330:191–199. doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q, Green SH. Functional role of neurotrophin-3 in synapse regeneration by spiral ganglion neurons on inner hair cells after excitotoxic trauma in vitro. J Neurosci. 2011;31:7938–7949. doi: 10.1523/JNEUROSCI.1434-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamahara K, Asaka N, Kita T, Kishimoto I, Matsunaga M, Yamamoto N, Omori K, Nakagawa T. Insulin-like growth factor 1 promotes cochlear synapse regeneration after excitotoxic trauma in vitro. Hear Res. 2019;374:5–12. doi: 10.1016/j.heares.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Du Z, Yang Q, Liu L, Li S, Zhao J, Hu J, Liu C, Qian D, Gao C. NADPH oxidase 2-dependent oxidative stress, mitochondrial damage and apoptosis in the ventral cochlear nucleus of D-galactose-induced aging rats. Neuroscience. 2015;286:281–292. doi: 10.1016/j.neuroscience.2014.11.061. [DOI] [PubMed] [Google Scholar]
- 23.He ZH, Zou SY, Li M, Liao FL, Wu X, Sun HY, Zhao XY, Hu YJ, Li D, Xu XX, Chen S, Sun Y, Chai RJ, Kong WJ. The nuclear transcription factor FoxG1 affects the sensitivity of mimetic aging hair cells to inflammation by regulating autophagy pathways. Redox Biol. 2020;28:101364. doi: 10.1016/j.redox.2019.101364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakraborty S, Kaushik DK, Gupta M, Basu A. Inflammasome signaling at the heart of central nervous system pathology. J Neurosci Res. 2010;88:1615–1631. doi: 10.1002/jnr.22343. [DOI] [PubMed] [Google Scholar]
- 25.Lau C, Pienkowski M, Zhang JW, McPherson B, Wu EX. Chronic exposure to broadband noise at moderate sound pressure levels spatially shifts tone-evoked responses in the rat auditory midbrain. Neuroimage. 2015;122:44–51. doi: 10.1016/j.neuroimage.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 26.Gates GA, Schmid P, Kujawa SG, Nam B, D’Agostino R. Longitudinal threshold changes in older men with audiometric notches. Hear Res. 2000;141:220–228. doi: 10.1016/s0378-5955(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 27.Herdener M, Esposito F, di Salle F, Boller C, Hilti CC, Habermeyer B, Scheffler K, Wetzel S, Seifritz E, Cattapan-Ludewig K. Musical training induces functional plasticity in human hippocampus. J Neurosci. 2010;30:1377–1384. doi: 10.1523/JNEUROSCI.4513-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi L, Liu K, Wang H, Zhang Y, Hong Z, Wang M, Wang X, Jiang X, Yang S. Noise induced reversible changes of cochlear ribbon synapses contribute to temporary hearing loss in mice. Acta Otolaryngol. 2015;135:1093–1102. doi: 10.3109/00016489.2015.1061699. [DOI] [PubMed] [Google Scholar]
- 29.Kujala T, Shtyrov Y, Winkler I, Saher M, Tervaniemi M, Sallinen M, Teder-Salejarvi W, Alho K, Reinikainen K, Naatanen R. Long-term exposure to noise impairs cortical sound processing and attention control. Psychophysiology. 2004;41:875–881. doi: 10.1111/j.1469-8986.2004.00244.x. [DOI] [PubMed] [Google Scholar]
- 30.Brattico E, Kujala T, Tervaniemi M, Alku P, Ambrosi L, Monitillo V. Long-term exposure to occupational noise alters the cortical organization of sound processing. Clin Neurophysiol. 2005;116:190–203. doi: 10.1016/j.clinph.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 31.Fu X, Sun X, Zhang L, Jin Y, Chai R, Yang L, Zhang A, Liu X, Bai X, Li J, Wang H, Gao J. Tuberous sclerosis complex-mediated mTORC1 overactivation promotes age-related hearing loss. J Clin Invest. 2018;128:4938–4955. doi: 10.1172/JCI98058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Qin Y, Zhang Y, Cui W, Lei Y, Ma X, Cheng Y, Shi L, Lv M. Decreased levels of superoxide dismutase in inner pillar cells contribute to ribbon synapse impairment in presbycusis. Am J Transl Res. 2019;11:2403–2412. [PMC free article] [PubMed] [Google Scholar]
- 33.Puel JL, Ruel J, Gervais d’Aldin C, Pujol R. Excitotoxicity and repair of cochlear synapses after noise-trauma induced hearing loss. Neuroreport. 1998;9:2109–2114. doi: 10.1097/00001756-199806220-00037. [DOI] [PubMed] [Google Scholar]
- 34.Pujol R, Puel JL. Excitotoxicity, synaptic repair, and functional recovery in the mammalian cochlea: a review of recent findings. Ann N Y Acad Sci. 1999;884:249–254. doi: 10.1111/j.1749-6632.1999.tb08646.x. [DOI] [PubMed] [Google Scholar]
- 35.Khimich D, Nouvian R, Pujol R, Tom Dieck S, Egner A, Gundelfinger ED, Moser T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434:889–894. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Li W, He Z, Wang Y, Shao B, Cheng C, Zhang S, Tang M, Qian X, Kong W, Wang H, Chai R, Gao X. Pre-treatment with fasudil prevents neomycin-induced hair cell damage by reducing the accumulation of reactive oxygen species. Front Mol Neurosci. 2019;12:264. doi: 10.3389/fnmol.2019.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He Z, Guo L, Shu Y, Fang Q, Zhou H, Liu Y, Liu D, Lu L, Zhang X, Ding X, Liu D, Tang M, Kong W, Sha S, Li H, Gao X, Chai R. Autophagy protects auditory hair cells against neomycin-induced damage. Autophagy. 2017;13:1884–1904. doi: 10.1080/15548627.2017.1359449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao S, Cheng C, Wang M, Jiang P, Zhang L, Wang Y, Wu H, Zeng X, Wang H, Gao X, Ma Y, Chai R. Blebbistatin inhibits neomycin-induced apoptosis in hair cell-like HEI-OC-1 cells and in cochlear hair cells. Front Cell Neurosci. 2020;13:590. doi: 10.3389/fncel.2019.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujioka M, Kanzaki S, Okano HJ, Masuda M, Ogawa K, Okano H. Proinflammatory cytokines expression in noise-induced damaged cochlea. J Neurosci Res. 2006;83:575–583. doi: 10.1002/jnr.20764. [DOI] [PubMed] [Google Scholar]
- 40.Fuentes-Santamaria V, Alvarado JC, Melgar-Rojas P, Gabaldon-Ull MC, Miller JM, Juiz JM. The role of glia in the peripheral and central auditory system following noise overexposure: contribution of TNF-α and IL-1β to the pathogenesis of hearing loss. Front Neuroanat. 2017;11:9. doi: 10.3389/fnana.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi X, Qiu S, Zhuang W, Yuan N, Wang C, Zhang S, Sun T, Guo W, Gao F, Yang S, Qiao Y. NLRP3-inflammasomes are triggered by age-related hearing loss in the inner ear of mice. Am J Transl Res. 2017;9:5611–5618. [PMC free article] [PubMed] [Google Scholar]
- 42.Nash SD, Cruickshanks KJ, Zhan W, Tsai MY, Klein R, Chappell R, Nieto FJ, Klein BE, Schubert CR, Dalton DS, Tweed TS. Long-term assessment of systemic inflammation and the cumulative incidence of age-related hearing impairment in the epidemiology of hearing loss study. J Gerontol A Biol Sci Med Sci. 2014;69:207–214. doi: 10.1093/gerona/glt075. [DOI] [PMC free article] [PubMed] [Google Scholar]