Abstract
Objective: Polycystic ovary syndrome (PCOS) is associated with alteration of Apelin signaling in ovarian granulosa cells (GCs). However, the molecular mechanisms regulating Apelin expression remain poorly understood. This study aims to investigate the role of miR-424 in modulating Apelin expression and GC functions. Methods: miRNA expression in GCs was altered by transfection with specific miR-424 mimics and inhibitors. Apelin level was determined by ELISA. miR-424 and mRNA expression were analyzed by quantitative RT-PCR. Protein abundance was measured by western blotting. Genomic sequence targeted by miR-424 was validated by dual-luciferase reporter assay. Apelin gene was overexpressed by transfection of LV-003 vector carrying its cDNA. GC proliferation was analyzed by MTS method, and its cell cycle progression and apoptosis were measured by flow cytometry. Results: Apelin concentration was increased in serum and follicular fluid from PCOS patients, accompanied by upregulated APJ (Apelin receptor) expression and suppressed miR-424 expression in GCs. miR-424 mimics suppressed Apelin and APJ expression in KGN cells by targeting 3’ UTR of Apelin and APJ, whereas miR-424 inhibitors had the opposite effects. miR-424 inhibited KGN cell proliferation and cell cycle progression by down-regulating Cyclin-D/E expression. Moreover, miR-424 promoted KGN cell apoptosis by increasing truncated Caspase-3 level. The regulation of KGN cell proliferation and apoptosis by miR-424 was mediated by directly suppressing Apelin gene expression, instead of inhibiting Apelin peptide activity. Conclusion: miR-424 suppresses proliferation and promotes apoptosis of human ovarian granulosa cells by directly targeting and inhibiting Apelin and APJ expression.
Keywords: miR-424, Apelin, APJ, granulosa cell, proliferation, apoptosis
Introduction
Polycystic ovary syndrome (PCOS) is a condition in women involving abnormally elevated androgens (male hormones), which can manifest as irregular menstrual periods, pelvic pain, excess body hair, acne, heavy periods, diabetes mellitus, high blood pressure, difficulty in getting pregnant and endometrial cancer [1,2]. Previous epidemiological studies have shown that development of polycystic ovary syndrome is associated with a range of genetic and environmental factors such as insulin resistance, obesity, lack of physical exercise and genetic predisposition of the fetal ovary to hyper-secreting androgens [3,4]. PCOS is currently the most common endocrine disease affecting women aged between 18 and 44 years old, among whom the incidence can reach as high as 20% [5,6]. No cure for PCOS is currently available, although birth control pills, anti-androgens, metformin and clomiphene have all been tried [6,7]. This lack of effective treatment is partially due to our limited understanding of the underlying pathogenic mechanisms.
Apelin, also known as APLN, is a group of peptides encoded by the human APLN gene, which play their pleiotropic biological and pathogenic roles by binding to G protein-coupled APJ receptors [8,9]. It has been well documented that the APJ receptor is widely expressed in human organs and tissues, including vascular vessels, cardiac cells, neurons and adipocytes [8-11]. Consistent with their wide distribution, Apelin and the APJ receptor have been reported to be associated with various human disorders including endothelial dysfunction, digestive pathology, oxidative stress-related inflammation disease, obesity, Alzheimer disease, and cancers [9-12]. Previous reports have shown that dysfunction of the Apelin and APJ receptor signaling pathway is closely linked to PCOS [13-15]. APLN concentration in follicular fluid and granulosa cells (GCs), and APJ receptor expression in GCs were found to be significantly higher in patients with PCOS, compared with normal controls [14,15]. Overproduction of APLN in GCs was shown to promote insulin-like growth factor 1 (IGF1)-induced steroidogenesis, through the elevation of 3-beta-hydrosteroid dehydrogenase (HSD3B) expression and activation of the MAPK and Akt signaling pathways [15]. Apelin release in PCOS has been shown to be influenced by nutritional status, such as alterations of leptin and resistin secretion, resulting in disturbance of the pituitary-ovarian axis [16]. However, the role of Apelin and the APJ receptor pathway in PCOS pathogenesis, as well as the regulation of their expression and function, are still poorly understood.
microRNAs, also known as miRNAs, are non-coding RNA molecules usually containing 22 nucleotides, which are widely expressed in various species [17]. It has been shown that miRNAs can post-transcriptionally suppress gene expression by base pairing with the mRNA of target genes, thus producing a range of biological and pathogenic effects [18-20]. For example, miR-424 and miR-503 in pulmonary artery endothelial cells, which are regulated by APLN, ameliorated pulmonary hypertension by directly targeting the fibroblast growth factor 2 (FGF2) and FGFR1 (fibroblast growth factor receptor 1) [21]. Recent reports also demonstrated that APLN expression can be repressed by miR-497, which contributes to the lipid accumulation in macrophages induced by the accelerated oxidization of low-density lipoproteins, suggesting a potential therapeutic target for atherosclerosis treatment [22]. APLN expression can also be targeted and suppressed by miR-503 during endogenous blood vessel repair and ischemic damage, resulting in the inhibition of hypoxia-induced endothelial progenitor cell proliferation and migration [23]. Although miRNAs have been shown to be critical regulators of APLN expression and function, miRNA-mediated APLN regulation during PCOS development has not yet been reported.
Alterations in miRNA profiles have been reported during PCOS development and treatment [24]. More than 200 miRNAs were shown to be differentially expressed in human follicular fluid from patients with PCOS. These miRNAs are involved in a number of biological processes, such as insulin regulation and inflammation [25]. However, their role in the pathogenesis of PCOS remains largely unexplored. In the present study, we aimed to investigate the role of miR-424 in regulating Apelin and APJ receptor expression in the context of PCOS, and their involvement in granulosa cell proliferation and apoptosis, in order to provide new insights into the molecular mechanisms underlying PCOS pathogenesis, which will lead to new therapeutic targets for PCOS treatment.
Materials and methods
Patient cohort and granulosa cell purification
The follicular fluid and serum samples used in this study were collected from patients with PCOS who were registered with the Reproductive Medical Center of Boai Hospital of Zhongshan (Zhongshan, Guangdong Province, China) between June 1st, 2015 and Dec 31st, 2018. Healthy volunteers were included in the control group, and subjected to follicular fluid and serum collection using the same procedures. Written informed consent was obtained from each participant in advance, and the research protocol was approved by the Ethics Committee of the Boai Hospital of Zhongshan. The purification of GCs from follicular fluid samples from both PCOS patients and the control group was carried out following previously described procedures [26].
Enzyme-Linked ImmunoSorbent Assay (ELISA)
The Apelin concentration in serum, follicular fluid and cell lysates was determined by ELISA (Enzyme-Linked ImmunoSorbent Assay) using Human Apelin ELISA Kits (#SBJ-H0192; Mr Ng Nangjing Biological, Nanjing, China) following the manufacturer’s instructions. Briefly, serum, follicular fluid and cell lysates were incubated with enzyme-labeled reagent at 37°C for 30 min, washed five times, incubated with chromogenic solutions A and B at 37°C for 30 min, and finally measured using a microplate reader.
Cell culture and transfection
Ovarian granulosa cell line KGN obtained from the Riken Biosource Center (Riken, Japan) was cultured in DMEM medium supplemented with 5% FBS at 37°C in a humidified cell culture chamber under 5% CO2. For modulation of miRNA expression in KGN cells, an miR-424 mimic (5’-CAGCAGCAAUUCAUGUUUUGAA CAAAACAUGAAUUGCUGCUGUU-3’), miR-424 inhibitor (5’-UUCAAAACAUGAAUUGCUGCUG-3’), and the negative controls (NC), were synthesized by the GenePharma Company (Suzhou, China). Mimics and inhibitors of microRNAs were transfected into KGN cells using the Lipofectamine 2000 reagent (Invitrogen, USA) following the manufacturer’s instructions. Apelin-expressing plasmids were synthesized in the General Biosystems Company (Anhui, China), which were then transfected into the KGN cells using Lipofectamine 2000 reagent, as instructed by the manufacturer. The expression of miRNAs was validated by quantitative RT-PCR, and alterations of Apelin expression were verified by ELISA assay, 48 hours after cell transfection.
Quantitative RT-PCR assay (qRT-PCR)
mRNA and miRNA expression was analyzed using qRT-PCR with primer pairs listed in Table 1. Briefly, total RNA was extracted from GCs using Trizol reagent (#15596026; Thermo Fishier Scientific) following the manufacturer’s instructions. Subsequently, 2.5 µg of total RNA was used for cDNA synthesis using the miScript II RT Kit (Cat No: 218160; QIAGEN) following the manufacturer’s instructions. Relative expression levels of mRNA and miRNA were measured using PCR with QuantiNova SYBR® Green PCR Kits (Cat No./ID: 208052) following instructions by the manufacturer. β-actin and U6 were included as the internal standard. The relative expressional levels of miRNA and mRNA were calculated by the standard 2-ΔΔCt method. Results from at least three biological replicates were collected for statistical analysis of differential expression.
Table 1.
Sequences of primers used for quantitative RT-PCR assays
| Genes | Forward primer (5’-3’) | Reverse primer (5’-3’) |
|---|---|---|
| APJ | CCGCAAGGAACGCATCG | GTGGTAGGGCATCCAGCACA |
| hsa-miR-424-5p | GCAGCAGCAATTCATGTTT | GTGCAGGGTCCGAGGT |
| hsa-mir-503 | GTAGCAGCGGGAACAGTT | GTGCAGGGTCCGAGGT |
| Apelin | GCTGCTCTGGCTCTCCT | TTCCTCCGACCTCCCT |
| ERK-MAPK1 | ACACGTTGGTACAGGGCTC | GGTCTTCTTGTGATGGGGA |
| PCNA | AACCTGCAGAGCATGGACTC | TCATTGCCGGCGCATTTTAG |
| Cyclin D1 | GCTGCGAAGTGGAAACCATC | CCTCCTTCTGCACACATTTGAA |
| Cyclin E | GCCAGCCTTGGGACAATAATG | CTTGCACGTTGAGTTTGGGT |
| GAPDH | GAGTCAACGGATTTGGTCGT | GACAAGCTTCCCGTTCTCAG |
Immunoblotting
Relative protein abundance in KGN cells was determined by Western blotting using specific primary antibodies. Briefly, total protein was extracted from cultured KGN cells using Tissue or Cell Total Protein Extraction Kits (C510003; Sangon, Shanghai) following the protocol provided by the manufacturer. Protein concentration was measured by the BCA method, and 30 µg of protein from each group was heated at 100°C for 5 min, before being separated by 10% SDS-PAGE. Proteins on SDS-PAGE gels were then transferred onto PVDF membranes (Millipore), which were then blocked with 5% lipid-free milk in PBS solution, incubated with diluted primary antibodies for 2 h at room temperature, washed 5 min with PBS solution three times, and then incubated with the appropriate secondary antibodies at room temperature for 2 h. The immunocomplexes were incubated with enhanced chemiluminescence (ECL) solution (Thermo Fishier Scientific) as suggested by the manufacturer. GAPDH was used as the internal standard for the relative comparison of protein levels, and at least three biological repeats were carried out for statistical analysis. The primary antibodies used in this study include anti-Cyclin D1 (Abcam), anti-Cyclin E1 (Bioss), anti-p-AMPKα (Thr172) (CST), anti-Caspase3 (CST), anti-AMPK alpha (CST), anti-PCNA (Santa Cruz), anti-p-ERK1/2 (CST), anti-ERK1/2 (CST), anti-Apelin R (APJ) (Millipore) and anti-GAPDH (Life).
Dual-luciferase reporter assay
The association of miR-424 with 3’-UTR of Apelin or the APJ was validated by dual-luciferase reporter assay using the pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega) following the manufacturer’s instructions. The recombinant pmirGLO-miR-424-mimics or pmirGLO-miR-424-NC plasmids, as well as recombinant plasmids expressing wild type (WT) or mutant (MUT) Apelin or APJ 3’-UTR sequences, were co-transfected into KGN cells using Lipofectamine 2000 reagent (Invitrogen) following the manufacturer’s instructions. Luciferase activity was detected using a GloMax® 20/20 Luminometer with the Nano-Glo® Dual-Luciferase® Reporter (NanoDLR) Assay (Promega). The luciferase reporter assay was repeated at least three times for the analysis of association between miRNA and genomic sequence.
Cell cycle, proliferation, and apoptosis
KGN cell cycle progression was evaluated using Tali™ Cell Cycle Kit (Thermo Fishier Scientific) and quantitatively analyzed by flow cytometry. KGN cells were incubated with the Cell Cycle Solution in darkness for 20-25 min, and the flow cytometry was then performed to quantify the percentage of KGN cells at different stages of the cell cycle. The proliferation rate of KGN cells in this study was measured using MTS Assay Kit (#ab197010; Abcam) following the manufacturer’s instructions. KGN cells cultured in 96-well plates were incubated with 20 µL/well MTS Reagent for 2-3 h under normal culture conditions at 37°C, and cell proliferation was evaluated by measuring the absorbance at 490 nm wavelength using a plate reader. Apoptosis in KGN cells was analyzed using Annexin V-FITC/propidium iodide (PI) apoptosis detection kit (Sigma-Aldrich) with a flow cytometer, following the manufacturer’s instructions. KGN cells were incubated with Annexin V-FITC and PI solution for 12-15 min, and then subjected to flow cytometry analysis. At least three biological replicates were carried out for statistical analysis of cell cycle progression, proliferation, and apoptosis.
Statistical analysis
Data generated in this study were analyzed using the GraphPad Prism 7.0 (GraphPad Software Inc., La Jolla, CA, USA) and SPSS 18.0 software (IBM, USA), with data presented as mean ± standard deviation (SD) from at least three biological replicates. Student’s t-tests or one-way analysis of variation were performed for the analysis of significant difference, whenever appropriate. Significant difference between groups was defined by a P value of < 0.05 or < 0.01.
Results
Differential expression of Apelin and miR-424 in patients with PCOS
To investigate the role of miR-424 in Apelin expression and PCOS development, we first measured the expression of Apelin in serum and follicular fluid from patients with PCOS. We found that Apelin level in the serum of PCOS patients was greatly elevated compared with the control group (Figure 1A). The Apelin concentration in follicular fluid from patients with PCOS was also significantly higher than that in the control group (Figure 1B). However, we found the miR-424 expression in ovarian GCs purified from the follicular fluids of PCOS patients was significantly lower than that of the control group (Figure 1C). In the GCs from follicular fluid of PCOS patients, the expression of the Apelin receptor APJ was greatly increased, in comparison with the control group (Figure 1D). The significantly increased expression of Apelin and APJ, and reduced level of miR-424 indicate the critical roles of these molecules in GCs during the development of PCOS. The opposite changes in miR-424 and Apelin level in PCOS patients suggested miR-424 targets at Apelin or APJ gene sequences.
Figure 1.
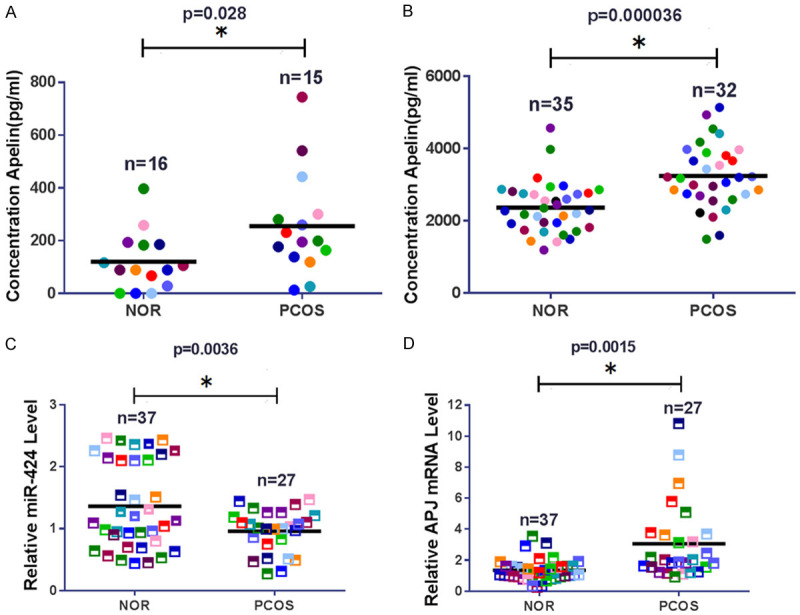
Differential expression of Apelin, APJ, and miR-424 in PCOS patients. A. Elevated expression of Apelin in serum from PCOS patients. Apelin concentration in human serum was determined by ELISA. B. Elevated expression of Apelin in follicular fluid collected from patients with PCOS. Apelin concentration in follicular fluids was measured by ELISA. C. Decreased miR-424 levels in granulosa cells (GCs) from follicular fluid of PCOS patients. D. Increased expression of APJ in GCs purified from follicular fluid in PCOS patients. PCOS: polycystic ovary syndrome; APJ: Apelin receptor; NOR: normal control; * indicates P < 0.05.
miR-424 suppresses Apelin and APJ expression in human GCs
To investigate the regulatory effects of miRNAs on Apelin and its receptor, the expression of miR-424 was modulated in human granulosa cell line KGN by transfection with specific mimics and inhibitors. Figure 2A showed that the expression of miR-424 was up-regulated when the KGN cells were transfected with mimics. As expected, we found Apelin in KGN cells was significantly down-regulated by transfection with miR-424 mimics, compared with those transfected with NC (Figure 2B). In contrast, Apelin expression in KGN cells was greatly up-regulated by transfection with miR-424 inhibitors, compared with cells transfected with NC (Figure 2C). The expression of APJ in KGN cells was also significantly suppressed by miR-424 mimics, but markedly up-regulated by miR-424 inhibitors (Figure 2D). The changes in APJ protein abundance in KGN cells induced by mimics or inhibitors of miR-424 were further confirmed by Western blotting (Figure 2E and 2F). These results suggest miR-424 suppresses the expression of both Apelin and its receptor in GCs.
Figure 2.
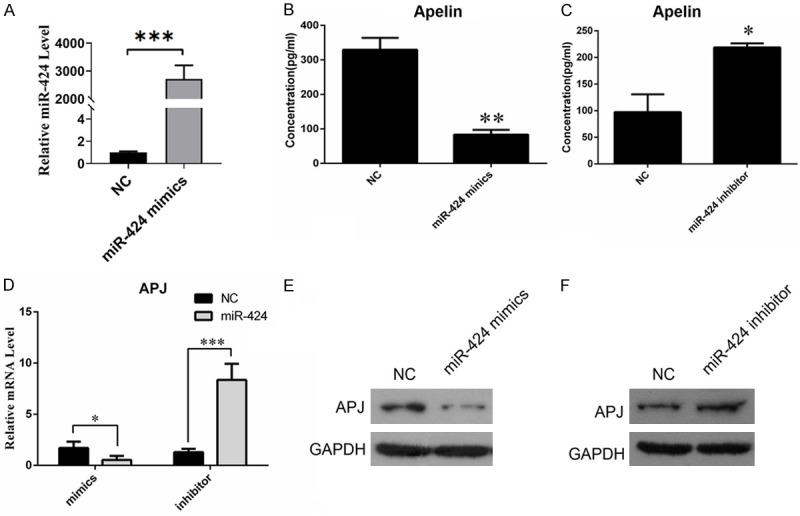
Suppression of Apelin and APJ expression in KGN cells by miR-424. (A) qRT-PCR was used to measure the expression of miR-424. (B) Decreased expression of Apelin in KGN cells transfected with miR-424 mimics. Apelin concentration in KGN cells was determined by ELISA. (C) Elevated expression of Apelin in KGN cells transfected with miR-424 inhibitors. Apelin concentration in KGN cells was measured by ELISA. (D) Alterations of APJ expression in KGN cells transfected with miR-424 mimics and inhibitors. qRT-PCR was used to analyze APJ gene expression. (E, F) APJ protein abundance in KGN cells transfected with miR-424 mimics (E) and miR-424 inhibitors (F). APJ: Apelin receptor; NC: negative control; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; * and ** indicates P < 0.05 and < 0.01, respectively.
miR-424 directly targets 3’-UTR of Apelin and APJ genes
Based on the inhibition of Apelin and APJ expression by miR-424, we hypothesized that Apelin and APJ genes are direct targets of miR-424. To test our hypothesis, we first used the TargetScanHuman7.1 software (http://www.targetscan.org/vert_71/) to predict the binding sites of miR-424 at the Apelin and APJ loci. Next we performed a dual-luciferase reporter assay to analyze their association. Based on our bioinformatics analyses, the wild-type (WT) 3’-UTR sequence of the Apelin gene and a mutant version were cloned into the recombinant plasmids for dual-luciferase reporter assays (Figure 3A). We found that transfection with miR-424 mimics significantly decreased luciferase activity in KGN cells expressing the WT 3’ UTR of Apelin, but not in KGN cells expressing the mutant 3’ UTR (Figure 3B). The dual-luciferase reporter assay result confirmed a direct regulation between miR-424 and 3’-UTR of the Apelin gene (Figure 3C). Using a combination of bioinformatics and dual-luciferase reporter assays, we also demonstrated that miR-424 directly targets 3-UTR of the APJ gene (Figure 3D-F). Therefore, we conclude that miR-424 functions as an inhibitory regulator of both Apelin and APJ expression in KGN cells.
Figure 3.
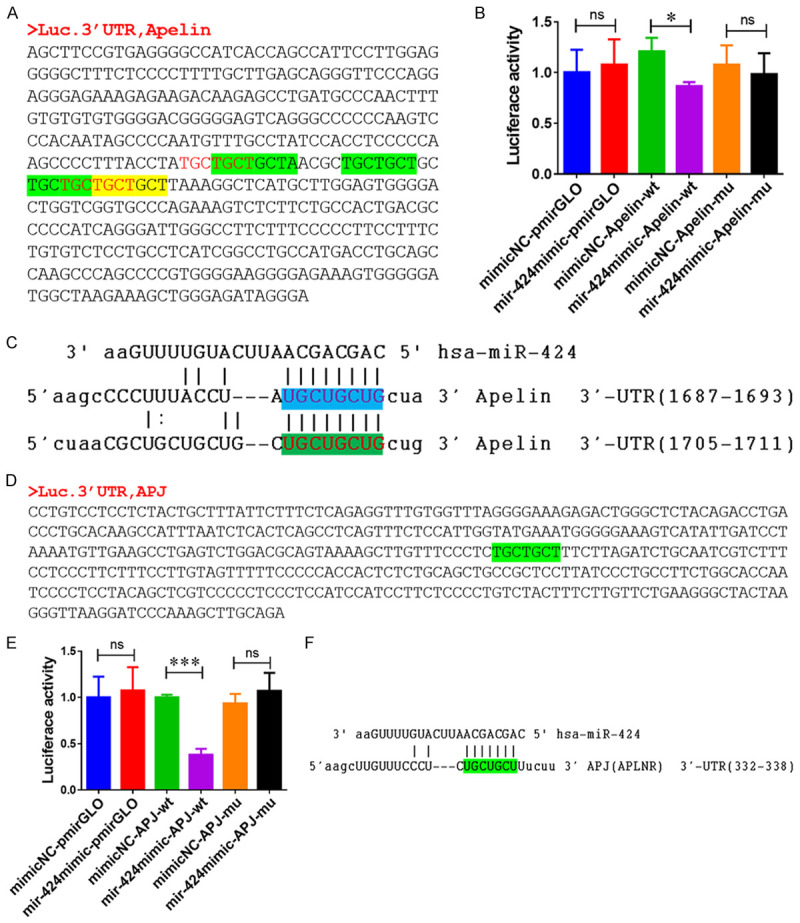
miR-424 directly targets 3’-UTR of Apelin and APJ genes. A. The 3’-UTR of the Apelin gene used for construction of recombinant plasmids for dual-luciferase reporter assays. B. Direct targeting of the 3’ UTR of Apelin gene by miR-424 in human KGN cells. C. The binding sites of miR-424 on the Apelin gene was predicted by TargetScanHuman 7.1. D. The 3’-UTR of the APJ gene used for construction of recombinant plasmids for dual-luciferase reporter assays. E. Direct targeting of 3’-UTR of APJ by miR-424 in human KGN cells, shown by dual-luciferase reporter assays. F. Binding of miR-424 to 3’ UTR of APJ gene predicted by TargetScanHuman 7.1 software. APJ: Apelin receptor; NC: negative control; WT: wild type; MUT: mutant; UTR: untranslated region; * and ** indicates P < 0.05 and < 0.01, respectively.
MiR-424 represses granulosa cell cycle and proliferation by suppressing Cyclin D/E downstream of APJ
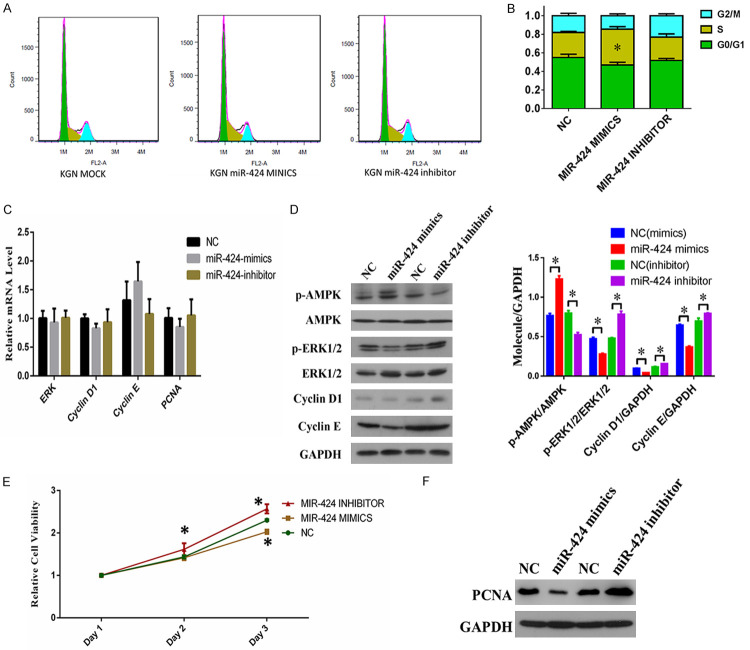
To further investigate the cellular functions of miR-424 during PCOS development, we analyzed the effect of miR-424 expression on KGN cell cycle progression and proliferation. We showed, using flow cytometry, that transfection with miR-424 mimics caused a significant increase in the number of KGN cells at the S phase during cell cycle progression, compared with those transfected with the negative control (Figure 4A and 4B). Next we measured the expression of major regulators of cell proliferation and cell cycle progression, including ERKs (extracellular signal-regulated kinases), CCND1 (cyclin D1), CCNE (cyclin E), PCNA (Proliferating cell nuclear antigen), and phosphorylated forms of AMPK (AMP-activated protein kinase) and ERK1/2. Although we observed no significant changes at the mRNA level (Figure 4C), we found that the abundance of phosphorylated ERK1/2, CCND1, and CCNE proteins in KGN cells was suppressed by miR-424 mimics, and increased by miR-424 inhibitors (Figure 4D). We also found that the proliferation capability of KGN cells was significantly repressed by transfection with miR-424 mimics, but was increased by transfection with miR-424 inhibitors (Figure 4E). In addition, we observed an increase in PCNA expression in KGN cells induced by the miR-424 inhibitor (Figure 4F). These results indicate that miR-424 inhibits granulosa cell proliferation and cell cycle progression by suppressing the expression of CCND1, CCNE and phosphorylated ERK1/2, which function downstream of the APJ receptor.
Figure 4.
Suppression of KGN cell cycle progression and proliferation by miR-424. A. KGN cell cycle progression after transfection with miR-424 mimics and inhibitors. More KGN cells at S stage were observed using flow cytometry after miR-424 mimics transfection. B. Quantitation of KGN cells at different stages of cell cycle following transfection of miR-424 mimics and inhibitors. C. Relative mRNA levels of ERK1/2, CCND1, CCNE, and PCNA genes in KGN cells transfected with miR-424 mimics and inhibitors. Gene expression was analyzed by quantitative RT-PCR. D. Protein abundances of p-AMPK, p-ERK1/2, CCND1, and CCNE in KGN cells transfected with miR-424 mimics and inhibitors. E. Proliferation of KGN cells transfected with miR-424 mimics and inhibitors by MTS. F. PCNA protein abundance in KGN cells transfected with miR-424 mimics and inhibitors, determined by Western blotting. NC: negative control; ERKs: extracellular signal-regulated kinases; CCND1: cyclin D1; CCNE: cyclin E; PCNA: Proliferating cell nuclear antigen; AMPK: AMP-activated protein kinase; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; *P < 0.05.
MiR-424 promotes granulosa cell apoptosis mediated by Caspase-3 truncation
To gain more insight into the role of miR-424 on GCs, we investigated how expression changes in miR-424 affect KGN cell apoptosis. We observed that transfection with miR-424 significantly increased the proportion of apoptotic KGN cells in comparison with negative control, whereas miR-424 inhibitor suppressed the apoptosis of KGN cells (Figure 5A). We found that the percentage of KGN cells in the late stage of apoptosis was significantly increased after transfection with miR-424 mimics (Figure 5B). Consistent with the change in the percentage of apoptotic cells, we confirmed by Western blotting that the amount of truncated Caspase-3 protein was markedly increased in KGN cells after transfection with miR-424 mimics, and greatly suppressed by transfection with miR-424 inhibitors (Figure 5C). These results show that miR-424 effectively promotes granulosa cell apoptosis mediated by the increase in truncated Caspase-3 protein.
Figure 5.
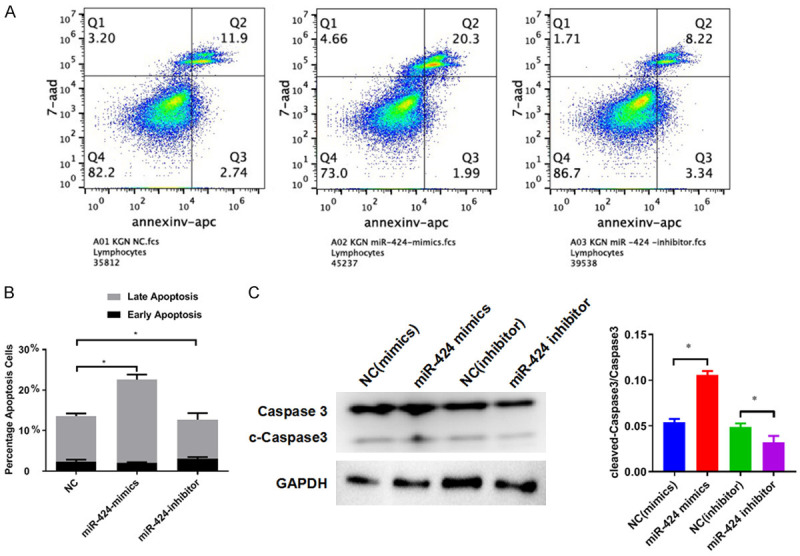
miR-424 promotes KGN cell apoptosis by inducing Caspase-3 truncation. A. Alteration of KGN cell apoptosis induced by transfection with miR-424 mimics and inhibitors. Cell apoptosis was analyzed by flow cytometry. B. Quantitation of apoptotic KGN cells transfected with miR-424 mimics and inhibitors. C. The abundance of Caspase-3 protein and truncated Caspase-3 protein in KGN cells transfected with miR-424 mimics and inhibitors. Western blotting was performed to analyze protein abundance. NC: negative control; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; *P < 0.05.
miR-424 represses proliferation and promotes apoptosis of GCs by repressing Apelin expression
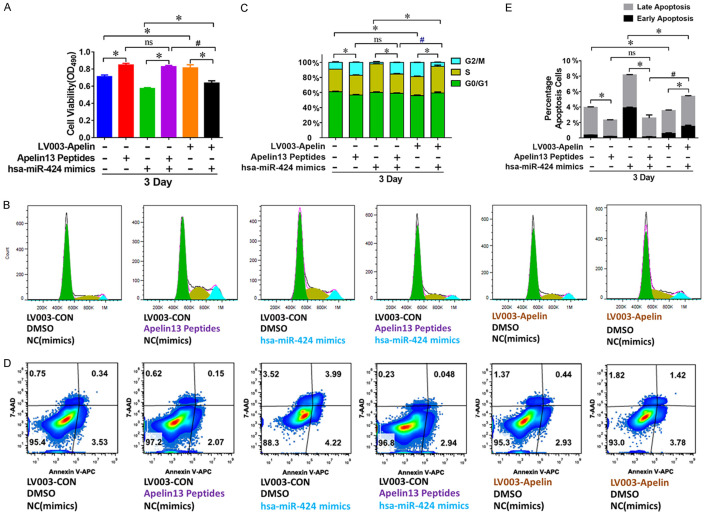
To investigate the role of Apelin expression in the regulation of granulosa cell proliferation and apoptosis by miR-424, we further analyzed the KGN cells after combined treatment with miR-424 mimics and synthesized Apelin 13 peptides or Apelin-overexpressing plasmid (LV003-Apelin). We found that both Apelin 13 peptides and LV003-Apelin plasmid induced significant increase in KGN cell proliferation rate (Figure 6A). The proliferation rate of KGN cells treated with the combination of Apelin 13 peptides and miR-424 mimics showed significant increases compared with those treated with miR-424 mimics alone (Figure 6A). The proliferation rate of KGN cells treated with the combination of LV003-Apelin plasmid and miR-424 mimics also exhibited a significant increase compared with those treated with miR-424 mimics alone (Figure 6A). Consistent alterations in KGN cell cycle progression and apoptosis were also observed in cells under the same set of treatments (Figure 6B-E). These results demonstrate that the effects of miR-424 on KGN cell proliferation and apoptosis were mediated by Apelin.
Figure 6.
miR-424 modulates KGN cell proliferation and apoptosis by suppressing Apelin expression. A. Changes in KGN cell proliferation rate after being treated with multiple combinations of miR-424 inhibitors, LV003-Apelin overexpression construct, and Apelin 13 peptides. Cell proliferation was analyzed by MTS. B, C. Alterations in KGN cell cycle progression after being treated with multiple combinations of miR-424 inhibitors, LV003-Apelin construct, and Apelin 13 peptides. Cell cycle progression was assessed by flow cytometry. D, E. Regulation of KGN cell apoptosis by multiple combinations of miR-424 inhibitors, LV003-Apelin construct, and Apelin 13 peptides. Cell apoptosis was evaluated by flow cytometry. NC: negative control; ns: non-significant difference; * and # indicate P < 0.05.
Compared with KGN cells treated with V003-Apelin plasmid alone, the transfection of V003-Apelin plasmid combined with miR-424 mimics induced a significant decrease in KGN cell proliferation (Figure 6A). However, in comparison with KGN cells treated with Apelin 13 peptide alone, the combination of Apelin 13 peptide and miR-424 mimics caused no decrease in KGN cell proliferation rate (Figure 6A). The proliferation rate of KGN cells treated with a combination of V003-Apelin plasmid and miR-424 mimics was significantly lower than that of cells treated with a combination of Apelin 13 peptide and miR-424 mimics (Figure 6A). The cell cycle progression and apoptosis of KGN cells showed corresponding changes after the same set of treatments (Figure 6B-E). Together, these results support that miR-424 suppresses proliferation and promotes apoptosis in KGN cells by inhibiting the expression of the Apelin gene, instead of by repressing the activity of Apelin peptides.
Discussion
Human ovarian GCs are located on the surface of ovarian follicles, and play a critical role in follicular formation and development. They secrete estrogen to maintain the physiological function of ovarian follicles [27]. Previous reports have demonstrated that dysregulated proliferation and apoptosis of ovarian GCs are closely associated with the pathogenesis of PCOS [27]. Therefore, human ovarian granulosa cell lines such as KGN have been studied to understand PCOS pathogenesis and treatment [28,29]. During the development of PCOS, ovarian GCs usually show increased cell proliferation and suppressed apoptosis [28]. However, the molecular mechanisms modulating the proliferation and apoptosis of human ovarian GCs in PCOS remain poorly understood.
In this present study, we found that miR-424 expression was greatly down-regulated in ovarian GCs from PCOS patients, while Apelin and APJ expression showed the opposite change. Further investigation showed that miR-424 directly targets 3’ UTR of both Apelin and APJ genes in the KGN cells. These results suggested that the regulation of Apelin and APJ gene expression by miR-424 plays a critical role during PCOS development. Previous reports have shown that Apelin and its receptor were regulated by a number of miRNAs such as miR-497 and miR-503 [22,23]; however, no microRNAs were shown to regulate Apelin signaling in the context of PCOS. Here, we showed that Apelin and APJ were regulated by miR-424 in human ovarian GCs, consistent with previous notions that Apelin pathways were modulated by various miRNAs during different pathogenic processes. Apelin signaling was also shown to regulate the function of miRNAs, including miR-424 and miR-503 [21]. Whether the same is true in ovarian GCs during PCOS pathogenesis requires further investigation.
The development of PCOS has been shown to be closely associated with human ovarian granulosa cell survival and proliferation [27,30]. Proliferation in the granulosa cell population in PCOS patients was found to be significantly accelerated, accompanied by largely increased expression of cell survival factors [27]. The suppression of ovarian granulosa cell proliferation has been conjectured to be an effective approach for inhibiting PCOS progression. microRNA-145 was shown to negatively regulate the expression of insulin receptor substrate 1 (IRS1) and the proliferation of ovarian GCs from PCOS patients [31]. These results indicate the potential of miR-145 as a novel and promising molecular target for PCOS treatment. In the present study we showed that the proliferation of human ovarian GCs was significantly repressed by miR-145 mimics [32]. Cyclins D and E are critical regulators of cell cycle progression associated with cell proliferation in various biological and pathogenic processes [33-35]. In this study, we showed that miR-424 effectively suppressed the expression of Cyclin D and E, providing additional molecular evidence supporting the role of miR-424 in modulating human ovarian granulosa cell proliferation. We demonstrated that miR-424 functions as a previously unknown inhibitor of human ovarian granulosa cell proliferation during PCOS development.
In addition to the accelerated proliferation, the apoptosis of human ovarian GCs was reported to be inhibited during PCOS development, which is an additional cellular mechanism underlying PCOS pathogenesis [27,36]. Apoptosis of ovarian GCs could also be regulated by miRNA molecules. For example, miR-141-3p, which was significantly down-regulated in the ovaries of a PCOS rat model, was shown to inhibit the apoptosis of rat ovarian GCs, thus being implicated in the etiology of PCOS [37]. In the present study, we found that the apoptosis of human ovarian GCs was promoted by miR-424 mimics, and inhibited by miR-424 inhibitors. Cell apoptosis in various tissues was promoted by the Caspase-related signaling pathways, especially the truncation and activation of Caspase-3 [38]. Here we found that the acceleration of apoptosis in human ovarian granulosa cell by miR-424 was accompanied by Caspase-3 activation. Combined with the regulation of ovarian granulosa cell proliferation described above, our results showed that miR-424 serves as an important hub exerting pleiotropic effects on human ovarian GCs, including cell proliferation, cell cycle progression, and apoptosis. These findings suggested that miR-424 is a promising target for new therapeutic regimens for PCOS patients.
We also showed that the regulation of human ovarian granulosa cell proliferation and apoptosis by miR-424 was mediated by modulating Apelin expression, instead of inhibiting activity of synthesized Apelin peptides. These results provided additional information on the molecular events during miR-424-regulated PCOS pathogenesis, which could lead to new drug development and treatment. In this study we have demonstrated that miR-424, which is suppressed in PCOS development, inhibit proliferation and promote apoptosis of human ovarian GCs via targeting Apelin expression and its APJ receptor. These observations provide new insights into microRNA-mediated PCOS pathogenesis and a basis for the development of new treatments for PCOS patients by targeting miRNAs and Apelin signaling.
Acknowledgements
This work was supported by the Zhongshan Science and Technology Project [grant numbers 2015B1037]; Zhongshan Social Public Welfare Science and Technology Research Project [grant numbers 2016B1007]; and Guangdong Medical Science and Technology Research Fund Project [grant numbers A2017603].
Disclosure of conflict of interest
None.
References
- 1.Ginsburg J, Havard CW. Polycystic ovary syndrome. Lancet. 2011;51:415–422. [Google Scholar]
- 2.Li R, Zhang Q, Yang D, Li S, Lu S, Wu X, Wei Z, Song X, Wang X, Fu S. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Human Reproduction. 2013;28:2562–2569. doi: 10.1093/humrep/det262. [DOI] [PubMed] [Google Scholar]
- 3.Carroll J, Saxena R, Welt CK. Environmental and genetic factors influence age at menarche in women with polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2012;25:459–466. doi: 10.1515/jpem-2012-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franks S, Mccarthy MI, Hardy K. Development of polycystic ovary syndrome: involvement of genetic and environmental factors. Int J Androl. 2006;29:278–285. doi: 10.1111/j.1365-2605.2005.00623.x. discussion 286-290. [DOI] [PubMed] [Google Scholar]
- 5.Burks HR, Wild RA. Polycystic Ovary Syndrome. Springer; 2014. Diagnostic criteria and epidemiology of PCOS; pp. 3–10. [Google Scholar]
- 6.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6:1–13. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Legro RS. Obesity and PCOS: implications for diagnosis and treatment. Semin Reprod Med. 2012;30:496–506. doi: 10.1055/s-0032-1328878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao J, Li H, Chen L. Targeting drugs to APJ receptor: the prospect of treatment of hypertension and other cardiovascular diseases. Current Drug Targets. 2015;16:148–55. doi: 10.2174/1389450115666141128120053. [DOI] [PubMed] [Google Scholar]
- 9.Kurowska P, Barbe A, Różycka M, Chmielińska J, Dupont J, Rak A. Apelin in reproductive physiology and pathology of different species: a critical review. Int J Endocrinol. 2018;2018:9170480. doi: 10.1155/2018/9170480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masoumi J, Abbasloui M, Parvan R, Mohammadnejad D, Pavon-Djavid G, Barzegari A, Abdolalizadeh J. Apelin, a promising target for Alzheimer disease prevention and treatment. Neuropeptides. 2018;70:76–86. doi: 10.1016/j.npep.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Wysocka MB, Pietraszekgremplewicz K, Nowak D. The role of apelin in cardiovascular diseases, obesity and cancer. Front Physiol. 2018;9:557. doi: 10.3389/fphys.2018.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Q, Cao J, Chen L. Apelin/APJ system: a novel therapeutic target for oxidative stress-related inflammatory diseases (Review) Int J Mol Med. 2016;37:1159. doi: 10.3892/ijmm.2016.2544. [DOI] [PubMed] [Google Scholar]
- 13.Chang CY, Tsai YC, Lee CH, Chan TF, Wang SH, Su JH. Lower serum apelin levels in women with polycystic ovary syndrome. Fertil Steril. 2011;95:2520–2523. doi: 10.1016/j.fertnstert.2011.04.044. [DOI] [PubMed] [Google Scholar]
- 14.Goren K, Sagsoz N, Noyari V, Yucel A, Caglayan O, Bostanci MS. Plasma apelin levels in patients with polycystic ovary syndrome/Polikistik over sendromlu hastalarda plazma apelin duzeyleri. J Turk Ger Gynecol Assoc. 2012;13:27–31. doi: 10.5152/jtgga.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roche J, Ramé C, Reverchon M, Mellouk N, Cornuau M, Guerif F, Froment P, Dupont J. Apelin (APLN) and Apelin Receptor (APLNR) in human ovary: expression, signaling, and regulation of steroidogenesis in primary human luteinized granulosa cells. Biol Reprod. 2016;95:104. doi: 10.1095/biolreprod.116.141754. [DOI] [PubMed] [Google Scholar]
- 16.Olszanecka-Glinianowicz M, Madej P, Nylec M, Owczarek A, Szanecki W, Skałba P, Chudek J. Circulating apelin level in relation to nutritional status in polycystic ovary syndrome and its association with metabolic and hormonal disturbances. Clin Endocrinol (Oxf) 2013;79:238–242. doi: 10.1111/cen.12120. [DOI] [PubMed] [Google Scholar]
- 17.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 18.Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 20.Kaur K, Vig S, Srivastava R, Mishra A, Singh VP, Srivastava AK, Datta M. Elevated hepatic miR-22-3p expression impairs gluconeogenesis by silencing the wnt-responsive transcription factor Tcf7. Diabetes. 2015;64:3659–3669. doi: 10.2337/db14-1924. [DOI] [PubMed] [Google Scholar]
- 21.Jongmin K, Yujung K, Yoko K, Lighthouse JK, Xiaoyue H, Aldred MA, Mclean DL, Hyekyung P, Comhair SA, Greif DM. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med. 2013;19:74–82. doi: 10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui J, Ren Z, Zou W, Jiang Y. miR-497 accelerates oxidized low-density lipoprotein-induced lipid accumulation in macrophages by repressing the expression of apelin. Cell Biol Int. 2017;41:1012–1019. doi: 10.1002/cbin.10808. [DOI] [PubMed] [Google Scholar]
- 23.Wen Y, Chen R, Zhu C, Qiao H, Liu Y, Ji H, Miao J, Chen L, Liu X, Yang Y. MiR-503 suppresses hypoxia-induced proliferation, migration and angiogenesis of endothelial progenitor cells by targeting Apelin. Peptides. 2018;105:58–65. doi: 10.1016/j.peptides.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Sørensen AE, Wissing ML, Salö S, Englund AL, Dalgaard LT. MicroRNAs related to polycystic ovary syndrome (PCOS) Genes. 2014;5:684. doi: 10.3390/genes5030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roth LW, Mccallie B, Alvero R, Schoolcraft WB, Minjarez D, Katz-Jaffe MG. Altered microRNA and gene expression in the follicular fluid of women with polycystic ovary syndrome. J Assist Reprod Genet. 2014;31:355–362. doi: 10.1007/s10815-013-0161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinn MC, Mcgregor SB, Stanton JL, Hessian PA, Gillett WR, Green DP. Purification of granulosa cells from human ovarian follicular fluid using granulosa cell aggregates. Reprod Fertil Dev. 2006;18:501. doi: 10.1071/rd05051. [DOI] [PubMed] [Google Scholar]
- 27.Das M, Djahanbakhch O, Hacihanefioglu B, Saridogan E, Ikram M, Ghali L, Raveendran M, Storey A. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;93:881–887. doi: 10.1210/jc.2007-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang L, Huang J, Li L, Chen Y, Chen X, Zhao X, Yang D. MicroRNA-93 promotes ovarian granulosa cells proliferation through targeting CDKN1A in polycystic ovarian syndrome. J Clin Endocrinol Metab. 2015;100:E729. doi: 10.1210/jc.2014-3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang M, Sun J, Xu B, Chrusciel M, Gao J, Bazert M, Stelmaszewska J, Xu Y, Zhang H, Pawelczyk L. Functional characterization of microRNA-27a-3p expression in human polycystic ovary syndrome. Endocrinology. 2018;159:297–309. doi: 10.1210/en.2017-00219. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Guo JH, Zhang XH, Chan HC. Defective CFTR-regulated granulosa cell proliferation in polycystic ovarian syndrome. Reproduction. 2015;149:393–401. doi: 10.1530/REP-14-0368. [DOI] [PubMed] [Google Scholar]
- 31.Cai G, Ma X, Chen B, Huang Y, Liu S, Yang H, Zou W. MicroRNA-145 negatively regulates cell proliferation through targeting IRS1 in isolated ovarian granulosa cells from patients with polycystic ovary syndrome. Reprod Sci. 2016;24:902. doi: 10.1177/1933719116673197. [DOI] [PubMed] [Google Scholar]
- 32.Jia X, Glazener C, Mowatt G, MacLennan G, Bain C, Fraser C, Burr J. Efficacy and safety of using mesh or grafts in surgery for anterior and/or posterior vaginal wall prolapse: systematic review and meta-analysis. BJOG. 2008;115:1350–1361. doi: 10.1111/j.1471-0528.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 33.Alevizopoulos K, Vlach J, Hennecke S, Amati B. Cyclin E and c-Myc promote cell proliferation in the presence of p16INK4a and of hypophosphorylated retinoblastoma family proteins. EMBO J. 2014;16:5322–5333. doi: 10.1093/emboj/16.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai J, Wei RJ, Li R, Feng JB, Yu YL, Liu PS. A study of CCND1 with epithelial ovarian cancer cell proliferation and apoptosis. Eur Rev Med Pharmacol Sci. 2016;20:4230. [PubMed] [Google Scholar]
- 35.Kozar K, Ciemerych MA, Rebel VI, Shigematsu H, Zagozdzon A, Sicinska E, Geng Y, Yu Q, Bhattacharya S, Bronson RT. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118:477–491. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 36.Cui X, Jing X, Wu X, Bi X, Liu J, Long Z, Zhang X, Zhang D, Jia H, Su D. Abnormal expression levels of BMP15/Smad1 are associated with granulosa cell apoptosis in patients with polycystic ovary syndrome. Mol Med Rep. 2017;16:8231–8236. doi: 10.3892/mmr.2017.7658. [DOI] [PubMed] [Google Scholar]
- 37.Li D, Xu D, Xu Y, Chen L, Li C, Dai X, Zhang L, Zheng L. MicroRNA-141-3p targets DAPK1 and inhibits apoptosis in rat ovarian granulosa cells. Cell Biochem Funct. 2017;35:197–201. doi: 10.1002/cbf.3248. [DOI] [PubMed] [Google Scholar]
- 38.Glamočlija V, Vilović K, Saraga-Babić M, Baranović A, Sapunar D. Apoptosis and active caspase-3 expression in human granulosa cells. Fertil Steril. 2005;83:426–431. doi: 10.1016/j.fertnstert.2004.06.075. [DOI] [PubMed] [Google Scholar]