Abstract
Peripheral nerve injury (PNI)-induced neuropathic pain is a prevalent and severe clinical problem. It has been shown that microglia-mediated neuroinflammation plays a crucial role in neuropathic pain. The present study investigated the abnormal expression of C-X-C motif chemokine receptor type 2 (CXCR2) in a rat L5 spinal nerve ligation (SNL) model and evaluated the role of SB225002, a specific antagonist of CXCR2, in repressing neuroinflammation and neuropathic pain. It was found that CXCR2 expression was significantly upregulated in the dorsal horn of L5-SNL rats compared with sham control. Moreover, CXCR2 expression was increased in spinal microglia of rats after L5-SNL. Based on these results, the present study further examined whether pharmacological inhibition of CXCR2 suppressed microglial activation and neuropathic pain. It was demonstrated that SB225002 treatment inhibited L5-SNL-induced microglia proliferation and activation. Furthermore, SB225002 also significantly suppressed the L5-SNL-induced pro-inflammatory response, as indicated by decreased production of tumor necrosis factor-α, interleukin (IL)-1β and IL-6 in spinal cord tissues. The results indicated that SB225002 also significantly inhibited microglial cell viability and lipopolysaccharide-induced production of pro-inflammatory cytokines in cultured microglia. Functionally, SB225002 treatment effectively repressed mechanical and cold hypersensitivity after peripheral nerve injury. Collectively, the present results suggested that pharmacological inhibition of CXCR2 by SB225002 suppressed L5-SNL-induced neuroinflammation and neuropathic pain, thus offering a potential therapeutic strategy for neuropathic pain treatment.
Keywords: Peripheral nerve injury, neuroinflammation, C-X-C motif chemokine receptor type 2, L5 spinal nerve ligation, SB225002, microglial
Introduction
Peripheral nerve injury (PNI) can lead to neuropathic pain in the clinic and is detrimental to the quality of life of the patient [1]. Several factors can cause PNI, such as ischemia, stretch injury and penetrating injury [2]. PNI causes damage to the myelin sheaths distal to the site of the injury and degeneration of damaged axons [3]. In the peripheral nervous system, neurotransmitters, cytokines, chemokines and neurotrophic factors are released, which further induce peripheral inflammation [4,5]. Ultimately, PNI results in chronic pain [6]. Currently, reducing neurosensitivity and relieving symptoms are the primary focus of treatments for chronic pain [1,7,8]. However, these types of therapy do not have a satisfactory therapeutic effect.
Microglia cells are the main form of immune defense when the nervous system is injured or exposed to toxic stimuli, and can initiate the neuroimmune response to propagate and release a diverse range of inflammatory factors, such as neurotropic factors and chemokines [9-11]. Microglia can also stimulate neurons and trigger neuronal hyperexcitability [12]. During the initiation of tissue healing, PNI and inflammation lead to microglia activation and proliferation [13-15]. However, after the completion of tissue repair, activated microglia and inflammatory factors remain residual in the peripheral nervous system, which results in chronic pain in the absence of stimulation [16,17].
Previous studies have revealed that chemokines, which are small secreted chemoattractant proteins, are related to peripheral nervous system diseases and participate in neuroinflammation progression and trigger chronic pain [18]. Furthermore, chemokines stimulate the migration and activation of microglia, and modulated the neuropathic pain process [19]. CXCR2 is a type of chemokine that is involved in inflammation and neuropathic pain [20,21]. Previous studies have also shown that CXCR2 relieves the inflammatory reaction and neuropathic pain. However, the underlying mechanism remains elusive.
The aim of the present study was to investigate the potential effect of SB225002 on CXCR2-related signaling pathways in neuropathic pain. It was found that the expression of CXCR2 was increased in spinal microglia of rats after L5 spinal nerve ligation (L5-SNL) and positively associated with the disease time course. Moreover, SB225002 inhibited L5-SNL-induced microglia proliferation and activation, and the pro-inflammatory response. The results also indicated that SB225002 effectively improved mechanical and cold hypersensitivity after PNI.
Materials and methods
L5-SNL model
Adult male Sprague Dawley rats (weight, 200-250 g) were purchased from Shanghai Model Organisms Center, Inc. All rat experiments were approved by the Ethics Committee of Affiliated Hospital of Nantong University. Rats were acclimated in plastic cages at 22°C and placed in a standard reversed 12:12 h light-dark cycle with free access to water and food 1 week before surgery. The surgery for SNL was performed as described previously [22]. Rats were anesthetized by intraperitoneal injection of 2% pentobarbital sodium (50 mg/kg). After a 2 cm incision was cut in along the back of the rats, the L6 transverse process was removed and L5 spinal nerves were exposed and ligated with 6-0 silk thread. Then, the incision was sutured layer by layer and a number of L5 SNL rats were treated with SB225002. Compared with the operation group, the sham operation group received the same operation except the unligated L5 nerve.
Primary microglia and BV2 cells culture
Primary microglia cells were isolated from L5-SNL rat model or the sham operation rat. The vertebral columns were separated and placed in Hank’s fluid. The tissue was crushed into 1 mm blocks, filtered (100-mm nylon screen) and centrifuged at 3,000×g for 5 min. Cells were resuspended in DMEM containing with 10% FBS, 1% penicillin/streptomycin and 2 mM L-glutamine. Cells were then seeded in 6-well plates (3×105) for 2 weeks. When the cells reached 95% confluence, lipopolysaccharide (LPS; Sigma-Aldrich; Merck KGaA; 100 ng/ml) was added to induce the pro-inflammatory response.
BV-2 microglial were obtained from the Cell Culture Center of Chinese Academy of Medical Science and maintained in DMEM supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin at 37°C in a humidified incubator with 5% CO2. BV-2 cells were treated with LPS (100 ng/ml) and SB225002 for a certain period of time.
Behavioral assessment
For the mechanical allodynia testing, a Plexiglas box (30×30×30 cm) with a clear floor was used. The floor had numerous holes (0.5 cm in diameter) and the distance between holes was 1.5 cm. In order to see the rat paws, the floor was placed on a mirror tilt at 45°. The experimental rats were placed in the testing chamber and allowed to adapt for 0.5 h, and then eight von Frey fibers of different forces were used to perform vertical mechanical stimulation on the paw for 5 sec in sequence. The 2 g force filament was used for the first test, and then a smaller or greater force was applied depending on whether or not the paw was retracted. Sudden withdrawal or retraction of the hind paw were defined as positive reactions and PWT was counted according to the ‘up-down method’. The behavioral tests were executed on the 1st, 3rd, 5th and 7th day after SNL.
The behavioral signs of cold allodynia were determined using a Thermal Place Preference system. In brief, 22°C water was used to estimate cold sensitivity of the paw. Rats were placed in the box and with wire mesh floor and allowed to adapt for 0.5 h before the experiment. The rear paw on the plantar surface was sprayed 50 μl water and paw withdrawals were tested for 1 min following water spray.
ELISA
Tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β ELISA kits were obtained from Invitrogen (Thermo Fisher Scientific, Inc.) and were used examine the protein levels of the inflammatory cytokines. The media of spinal primary microglia of L5-SNL rats or BV2 cells were first induced with LPS (100 ng/ml) for 24 h and then BV2 cells were treated with SB225002 for another 0.5 h. Rat specific antibodies were added to 24-well plates and the absorbance of the sample was measured at 450 nm with a microplate reader (Dynamica Scientific Ltd.).
Western blot analysis
Total protein of dorsal horn tissue of L5-SNL or sham-operation rats and microglia were exacted via RIPA buffer (Pierce; Thermo Fisher Scientific, Inc.) and the protein concentration was determined with a bicinchoninic acid kit (Pierce; Thermo Fisher Scientific, Inc.). Proteins were separated via centrifugation at 15,000×g at 4°C for 0.5 h, subjected to SDS-PAGE and transferred to PVDF membranes. The membranes were incubated overnight at 4°C with antibodies [CXCR2 (Abcam; cat. no. ab14935; 1:500), ionized calcium binding adaptor molecule 1 (Iba-1; Abcam; cat. no. ab178846; 1:2,000) and Actin (Abcam; cat. no. ab179467; 1:10,000)] and then incubated with Goat Anti-Rabbit IgG H&L (horseradish peroxidase-conjugated; cat. no. ab205718; 1:5,000; Abcam). Protein signal was assessed with an enhanced chemiluminescence detection system (Pierce; Thermo Fisher Scientific, Inc.).
Immunofluorescence
Transverse spinal cord sections of experimental rats were cut with a sharp instrument and used for immunofluorescence detection. Sections were washed with PBS and subsequently fixed with cold 4% PFA for 15 min at room temperature. The sections were incubated with primary antibodies: Rabbit-anti-CXCR2 (1:500; cat. no. ab14935; Abcam) or rabbit-anti-Iba1 (1:100; cat. no. ab178847; Abcam) overnight at 4°C. After washing three times with PBS, the samples were incubated with anti-rabbit Alexa Fluor® 488 (1:1,000; cat. no. ab150077; Abcam) for 1 h in the dark. The sections were counterstained with Vectashield mounting medium with DAPI (Sigma-Aldrich; Merck KGaA).
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from microglia using TRIzol® reagent (Thermo Fisher Scientific, Inc.) and was RT to cDNA using the PrimeScript™ RT reagent kit (Invitrogen; Thermo Fisher Scientific, Inc.). RT-qPCR was conducted using SYBR Premix Ex Taq (TaKaRa Bio, Inc.) and the corresponding primer sequences were: CXCR2 forward (F), CGCTGTCAATGCCTGAAG; and reverse (R), GGCGTCACACTCAAGCTCT; and GAPDH (internal standard) (F), TGGCAAAGTGGAGATTGTTGCC; and (R), AAGATGGTGATGGGCTTCCCG. Relative CXCR2 expression was normalized to GAPDH expression using the 2-ΔΔCq method.
Cell counting Kit-8 (CCK-8) assay
A CCK-8 assay was carried out to detect the relationship between BV2 cell activity and SB225002 dose or time. BV2 cells (1×103 cell per well) were seeded into 96-well plates and cultured for 1 day. Different doses of SB225002 (0, 0.1, 0.2, 0.5, 2 and 5 μM) were added to the medium for 48 h, or 0.5 μM for different treatment durations (12, 24, 48, 72 and 96 h). After changing the media, 10 μl CCK-8 solution (Dojindo Molecular Technologies, Inc.) was added to each well and plates were incubated for 2 h at 37°C. Absorbance was measured at 450 nm via a VersaMax microplate reader.
Statistical analyses
Data were analyzed using SPSS 11.5 software (SPSS, Inc.). Each experiment was carried out for ≥3 independent batches and data are presented as the mean ± SD. The Student’s t-test was performed to analyze comparisons between the two groups (Figures 1, 2 and 5) and one-way ANOVA followed by the Scheffé test (Figures 3, 4 and 6) was performed to analyze comparisons between multiple groups. P<0.05 was considered to indicate a statistically significant difference.
Figure 1.
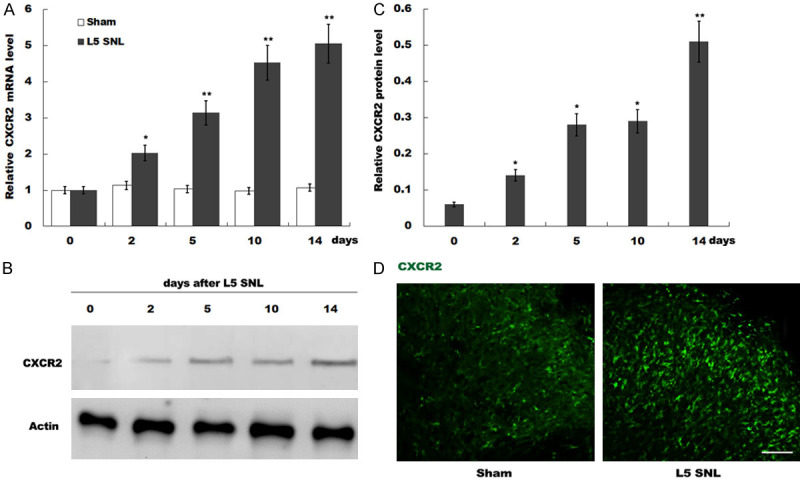
CXCR2 expression is significantly upregulated in the dorsal horn of L5-SNL rats. (A) qPCR analysis of CXCR2 mRNA expression in the dorsal horn after 0, 2, 5, 10 and 14 days in L5-SNL rats (n=3 rats in each group). The 2-ΔΔCt method was used to quantify relative CXCR2 level compared with sham-operated group. All samples were tested 3 times, and data were presented as mean ± SD. (B and C) Western blot (B) and quantitative analysis (C) of CXCR2 protein expression in the dorsal horn after 0, 2, 5, 10 and 14 days of L5-SNL (n=3 rats in each group). GAPDH was used as loading control in western blot analysis. (D) CXCR2 immunoreactivity (green) was detected in dorsal horn of rats after 5 days of L5-SNL compared with sham-operated group. *P<0.05, **P<0.01 vs. sham-operated group. CXCR2, C-X-C motif chemokine receptor type 2; L5-SNL, L5 spinal nerve ligation.
Figure 2.
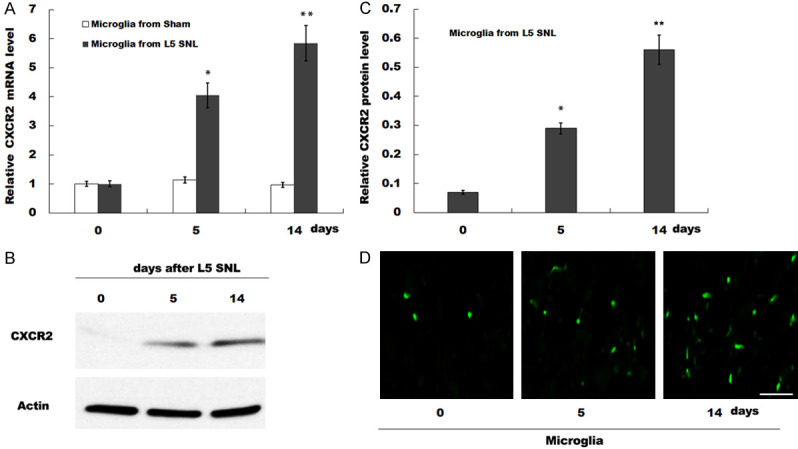
CXCR2 expression is increased in spinal microglia of rats after L5-SNL. (A) CXCR2 mRNA expression was detected by qPCR analysis in microglia isolated from 0, 5 and 14 days of L5 SNL rats (n=5) compared with sham-operated groups (n=3). The 2-ΔΔCt method was used to quantify relative CXCR2 level. All samples were tested 3 times, and data were presented as mean ± SD. (B and C) Western blot (B) and quantitative analysis (C) of CXCR2 protein expression in spinal microglia isolated from 0, 5 and 14 days of L5 SNL rats (n=3). GAPDH was used as loading control in western blot analysis. (D) CXCR2 immunoreactivity (green) was detected in dorsal horn isolated from 0, 5 and 14 days of L5 SNL rats (n=3). *P<0.05, **P<0.01 vs. sham-operated group. CXCR2, C-X-C motif chemokine receptor type 2; L5-SNL, L5 spinal nerve ligation.
Figure 5.
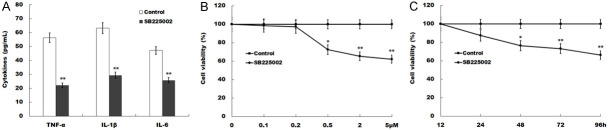
SB225002 inhibits microglial cell viability and LPS-induced production of pro-inflammatory cytokines of BV2 cells. (A) The levels of TNF-α, IL-1β and IL-6 were determined by ELISA assay using ELISA kits in BV2 cells induced with LPS for 24 h and treated with or without SB225002. (B) Cell viability of dose-related effect of SB225002 was measured by CCK-8 assay in BV2 cells induced with LPS for 24 h treated with 0, 0.1, 0.2, 0.5, 2 or 5 μM SB225002 for 48 h. (C) Cell viability of time-related effect of SB225002 was measured by CCK-8 assay in BV2 cells induced with LPS for 24 h treated with 0.5 μM SB225002 for 12, 24, 48, 72 or 96 h. *P<0.05 and **P<0.01 vs. control group. TNF, tumor necrosis factor; IL, interleukin. CCK-8, Cell Counting Kit-8.
Figure 3.
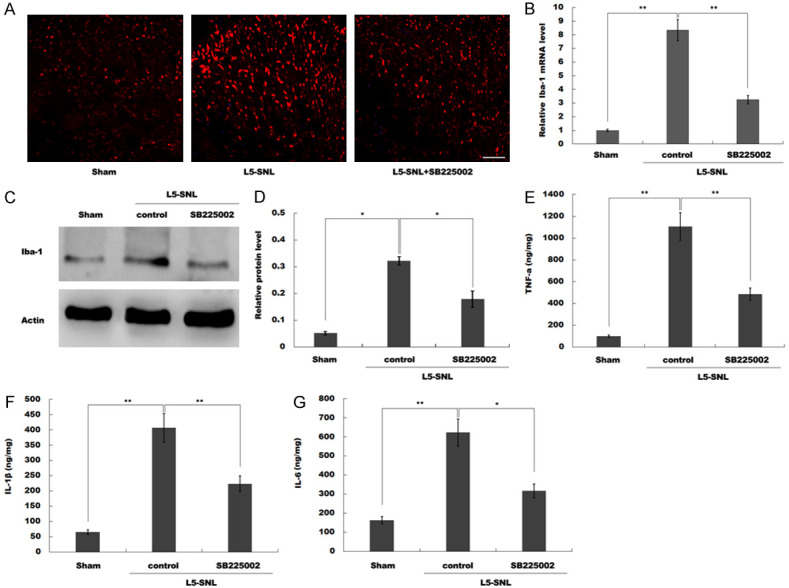
SB225002 treatment inhibits L5-SNL-induced microglia proliferation and activation and the pro-inflammatory response. (A) The number of microglia were analyzed by immunofluorescence in the spinal cord of L5-SNL rats with or without SB225002 treatment compared with sham-operated group. All samples were tested 3 times, and data were presented as mean ± SD. (B) mRNA expression of Iba-1 was assessed by qPCR analysis in spinal microglia isolated from L5-SNL rats with or without SB225002 treatment compared with sham-operated group. (C and D) Western blot (C) and quantitative analysis (D) of Iba-1 protein expression in spinal microglia isolated from L5-SNL rats with or without SB225002 treatment compared with sham-operated group (n=3 of each group). GAPDH was used as loading control in western blot analysis. (E-G) The levels of pro-inflammatory cytokines TNF-α (E), IL-1β (F) and IL-6 (G) were determined by ELISA assay using ELISA kits in spinal cord tissues of L5-SNL rats with or without SB225002 treatment compared with sham-operated group. *P<0.05, **P<0.01. L5-SNL, L5 spinal nerve ligation; TNF, tumor necrosis factor; IL, interleukin; Iba-1, ionized calcium binding adaptor molecule-1.
Figure 4.
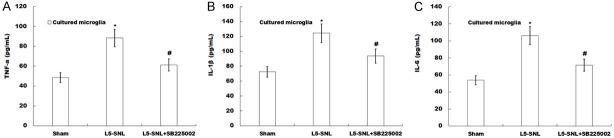
SB225002 inhibits LPS-induced production of pro-inflammatory cytokines. (A-C) The levels of TNF-α (A), IL-1β (B) and IL-6 (C) were determined by ELISA assay using ELISA kits in media of spinal primary microglia isolated from L5-SNL rats with or without SB225002 treatment compared with sham-operated group. *P<0.05 vs. sham-operated group, #P<0.05 vs. L5-SNL.
Figure 6.
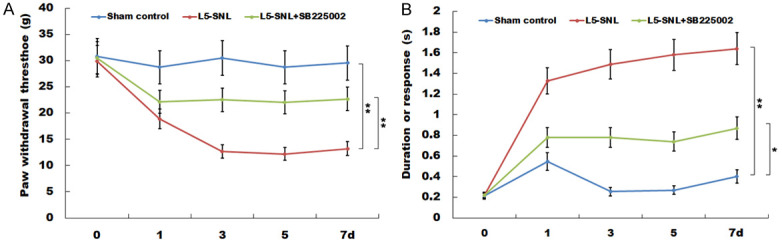
SB225002 treatment effectively represses mechanical and cold hypersensitivity after PNI. (A) Response threshold of mechanical stimulation and (B) the time of contraction response to cold stimulation were measured by von Frey fibers in L5-SNL rats with or without SB225002 treatment compared with sham-operated group (n=3 of each group). *P<0.05 and **P<0.01. L5-SNL, L5 spinal nerve ligation.
Results
CXCR2 expression is significantly upregulated in the dorsal horn of L5-SNL rats
The present study examined CXCR2 expression in the rat model of L5-SNL. Compared with the sham control, the mRNA expression of CXCR2 in the dorsal horn of L5-SNL rats was significantly increased (Figure 1A). The western blotting results indicated that the protein expression of CXCR2 in the dorsal horn was significantly upregulated after L5-SNL (Figure 1B and 1C). In addition, the expression of CXCR2 was assessed by immunofluorescence staining in the dorsal horn of the rats after 5 days of L5-SNL. It was found that the expression of CXCR2 in the dorsal horn was significantly increased (Figure 1D). Collectively, the results suggested that the expression of CXCR2 was enhanced in the dorsal horn of L5-SNL rats in a time-dependent manner.
CXCR2 expression is increased in spinal microglia of rats after L5-SNL
Microglia-mediated neuroinflammation plays a crucial role in neuropathic pain [23]. Therefore, the expression of CXCR2 was assessed in isolated microglia from spinal cord of rats. It was demonstrated that the expression of CXCR2 in spinal microglia was significantly increased in L5-SNL group compared with the sham group (Figure 2A). Moreover, the protein expression of CXCR2 in spinal microglia was examined by western blotting, and it was found that the expression CXCR2 was upregulated in rats with L5-SNL (Figure 2B and 2C). The expression of CXCR2 in spinal microglia was further examined by immunofluorescence staining, and the results indicated that the expression of CXCR2 in spinal microglia was increased after L5-SNL (Figure 2D). Therefore, the results suggested that CXCR2 expression was upregulated in spinal microglia of rats after L5-SNL.
SB225002 treatment inhibits L5-SNL-induced microglia proliferation and activation and the pro-inflammatory response
Previous studies have shown that the proliferation and activation of microglia play critical roles in neuropathic pain, which contributes to disease development and maintenance [24]. The immunofluorescence staining results identified that the number of microglia in the spinal cord of rats was significantly increased after L5-SNL. However, after administration of SB225002, the number of spinal microglia was significantly decreased (Figure 3A).
Iba-1 is an important biomarker of microglia; the proliferation and activation of microglia is indicated by increased expression of Iba-1 [25]. mRNA expression of Iba-1 was assessed by RT-qPCR and it was found that SB225002 significantly repressed the elevation of Iba-1 in rat spinal microglia after L5-SNL (Figure 3B). Moreover, western blotting results indicated that the protein expression of Iba-1 in spinal microglia was significantly downregulated after administration of SB225002 (Figure 3C and 3D), which suggested that the SB225002 inhibited the proliferation and activation of spinal microglia induced by L5-SNL.
Activated spinal microglia release a variety of pro-inflammatory mediators, such as TNF-α, IL-1β and IL-6, which all play important roles in neuropathic pain. Therefore, the present study determined the levels of TNF-α, IL-1β and IL-6 in spinal cord tissues using ELISA. The results suggested that the levels of TNF-α, IL-1β and IL-6 in spinal cord tissues were significantly increased by L5-SNL, whereas SB225002 treatment inhibited L5-SNL-induced upregulation of TNF-α, IL-1β and IL-6 (Figure 3E-G).
SB225002 inhibits LPS-induced production of pro-inflammatory cytokines
To determine whether SB225002 inhibited LPS-induced productions of pro-inflammatory cytokines, spinal primary microglia were isolated and the levels of TNF-α, IL-1β and IL-6 in the media were measured. It was found that levels of TNF-α, IL-1β and IL-6 in media of spinal primary microglia of L5-SNL rats were significantly higher compared with those in media of spinal primary microglia of sham-operated rats. Furthermore, these pro-inflammatory cytokines from spinal primary microglia of L5-SNL rats were reduced after administration of SB225002 (Figure 4A-C). Thus, it was speculated that SB225002 inhibited the LPS-induced microglial pro-inflammatory response.
SB225002 inhibits microglial cell viability and LPS-induced production of pro-inflammatory cytokines of BV2 cells
To further assess whether SB225002 inhibited microglial cell viability and LPS-induced production of pro-inflammatory cytokines, the levels TNF-α, IL-1β and IL-6 in media of BV2 cells were examined. The results indicated that treatment of SB225002 inhibited LPS-induced production of these pro-inflammatory cytokines (Figure 5A). Moreover, cell viability was measured by CCK-8 and it was found that SB225002 repressed microglia cell viability in dose-dependent and time-dependent manner (Figure 5B and 5C).
SB225002 treatment effectively represses mechanical and cold hypersensitivity after PNI
The results indicate that, compared with the sham-operated rats, the response threshold of mechanical stimulation was significantly reduced in L5-SNL rats (Figure 6A). However, after administration of SB225002, the response threshold of mechanical stimulation was significantly increased. Furthermore, it was demonstrated that the time of contraction response to cold stimulation was significantly increased after L5-SNL (Figure 6B). The results also suggested that the increase in response time to cold stimulation in L5-SNL rats was repressed with administration of SB225002.
Discussion
The present study detected CXCR2 expression in rats of L5-SNL, and found that CXCR2 expression was increased in spinal microglia of rats after L5-SNL. Therefore, the results suggested that SB225002 inhibited the proliferation and activation of spinal microglia induced by L5-SNL and the LPS-induced microglial pro-inflammatory response. In vitro it was found that SB225002 inhibited BV2 cell viability and LPS-induced production of pro-inflammatory cytokines. Moreover, in vivo, the results indicated that SB225002 increased the responsive sensibility of cold stimulation and threshold of mechanical stimulation in L5-SNL rats.
Neuropathic pain is a common disease, but the mechanisms of its development are complex and not fully understood; therefore, the effectiveness of conventional drug treatment is limited [26]. Previous studies have focused on the mechanisms of neuropathic pain development, and it has been reported that microRNA-34c inhibits neuroinflammation and alleviates neuropathic pain by directly targeting the NOD-like receptor protein 3 (NLRP3) 3’-untranslated region, which inhibits the expression of NLRP3 protein [27]. In addition, the expression levels of autophagy-related proteins are associated with neuropathic pain, such as Beclin 1 and LC3-phosphatidylethanolamine conjugate. The present study focused on the regulatory mechanism of chemokines in neuropathic pain. Previous studies have reported that chemokines, such as CXCL1, CCL2, CXCL5 and CXCL11, play a crucial role in genesis and development of neuropathic pain [28]. Moreover, the present results suggested that CXCR2 expression was increased in spinal microglia of rats after L5-SNL and mechanical and cold hypersensitivity after PNI was effectively repressed, while the expression of CXCR2 was suppressed by SB225002.
Previous studies have shown that PNI contributes to peripheral nerve inflammation and triggers chronic neuropathic pain and immune cell release in the peripheral nervous system [29]. Furthermore, microglial, which are strongly activated following PNI in the ipsilateral dorsal horn of the spinal cord, differentiate into different phenotypes and can trigger tissue repair or damage. In experimental animal models of traumatic injury, microglial secreted anti-inflammatory cytokines, including IL-4, transforming growth factor-β and IL-10, and act as a neuroprotective factor [30-32]. However, microglial can also mediate inflammatory reactions in injured nerves by increasing neuronal hyperexcitability, which leads to neuropathic pain. The present results demonstrated that SB225002 treatment inhibited L5-SNL-induced microglia proliferation and activation, and significantly suppressed L5-SNL-induced pro-inflammatory response.
In conclusion, the present results indicated that SB225002 may be used for nerve pain therapy. Moreover, it was found that the expression of CXCR2 was enhanced in the dorsal horn and spinal microglia of rats after L5-SNL. In addition, SB225002 treatment inhibited microglia activation and proliferation, and also the pro-inflammatory responses in vitro and in vivo. Furthermore, after PNI, SB225002 administration significantly improved mechanical and cold hypersensitivity in a inflammatory neuropathic pain model.
Acknowledgements
The study was funded by the National Natural Science Foundation of China’s Youth Foundation (grant no. 81701105), the Jiangsu Postdoctoral Research Support Program (grant no. 2018K257C) and the Nantong Municipal Science and Technology Plan to Fund Projects (grant no. MS12017013).
Disclosure of conflict of interest
None.
References
- 1.Han GH, Peng J, Liu P, Ding X, Wei S, Lu S, Wang Y. Therapeutic strategies for peripheral nerve injury: decellularized nerve conduits and Schwann cell transplantation. Neural Regen Res. 2019;14:1343–1351. doi: 10.4103/1673-5374.253511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isaacs J, Cochran AR. Nerve transfers for peripheral nerve injury in the upper limb: a case-based review. Bone Joint J. 2019;101-B:124–131. doi: 10.1302/0301-620X.101B2.BJJ-2018-0839.R1. [DOI] [PubMed] [Google Scholar]
- 3.Imperadore P, Shah SB, Makarenkova HP, Fiorito G. Nerve degeneration and regeneration in the cephalopod mollusc octopus vulgaris: the case of the pallial nerve. Sci Rep. 2017;7:46564. doi: 10.1038/srep46564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie X, Luo X, Liu N, Li X, Lou F, Zheng Y, Ren Y. Monocytes, microglia, and CD200-CD200R1 signaling are essential in the transmission of inflammation from the periphery to the central nervous system. J Neurochem. 2017;141:222–235. doi: 10.1111/jnc.13972. [DOI] [PubMed] [Google Scholar]
- 5.Ma Q, Xing C, Long W, Wang HY, Liu Q, Wang RF. Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J Neuroinflammation. 2019;16:53. doi: 10.1186/s12974-019-1434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matamala-Gomez M, Diaz Gonzalez AM, Slater M, Sanchez-Vives MV. Decreasing pain ratings in chronic arm pain through changing a virtual body: different strategies for different pain types. J Pain. 2019;20:685–697. doi: 10.1016/j.jpain.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Miao J, Xiao W, Wang L, Han F, Wu H, Deng X, Guo X, Zhao C. The value of the Prognostic Nutritional Index (PNI) in predicting outcomes and guiding the treatment strategy of nasopharyngeal carcinoma (NPC) patients receiving intensity-modulated radiotherapy (IMRT) with or without chemotherapy. J Cancer Res Clin Oncol. 2017;143:1263–1273. doi: 10.1007/s00432-017-2360-3. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Xia L, Wang Y, Hong S, Chen H, Liang S, Peng P, Chen Y. Low prognostic nutritional index (PNI) predicts unfavorable distant metastasis-free survival in nasopharyngeal carcinoma: a propensity score-matched analysis. PLoS One. 2016;11:e0158853. doi: 10.1371/journal.pone.0158853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puga DA, Tovar CA, Guan Z, Gensel JC, Lyman MS, McTigue DM, Popovich PG. Stress exacerbates neuron loss and microglia proliferation in a rat model of excitotoxic lower motor neuron injury. Brain Behav Immun. 2015;49:246–254. doi: 10.1016/j.bbi.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calvo M, Bennett DL. The mechanisms of microgliosis and pain following peripheral nerve injury. Exp Neurol. 2012;234:271–282. doi: 10.1016/j.expneurol.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Sierra A, Beccari S, Diaz-Aparicio I, Encinas JM, Comeau S, Tremblay ME. Surveillance, phagocytosis, and inflammation: how never-resting microglia influence adult hippocampal neurogenesis. Neural Plast. 2014;2014:610343. doi: 10.1155/2014/610343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Shi XQ, Fan A, West B, Zhang J. Targeting macrophage and microglia activation with colony stimulating factor 1 receptor inhibitor is an effective strategy to treat injury-triggered neuropathic pain. Mol Pain. 2018;14:1744806918764979. doi: 10.1177/1744806918764979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohno K, Kitano J, Kohro Y, Tozaki-Saitoh H, Inoue K, Tsuda M. Temporal kinetics of microgliosis in the spinal dorsal horn after peripheral nerve injury in rodents. Biol Pharm Bull. 2018;41:1096–1102. doi: 10.1248/bpb.b18-00278. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Muriana A, Mancuso R, Francos-Quijorna I, Olmos-Alonso A, Osta R, Perry VH, Navarro X, Gomez-Nicola D, Lopez-Vales R. CSF1R blockade slows the progression of amyotrophic lateral sclerosis by reducing microgliosis and invasion of macrophages into peripheral nerves. Sci Rep. 2016;6:25663. doi: 10.1038/srep25663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rotterman TM, Akhter ET, Lane AR, MacPherson KP, Garcia VV, Tansey MG, Alvarez FJ. Spinal motor circuit synaptic plasticity after peripheral nerve injury depends on microglia activation and a CCR2 mechanism. J Neurosci. 2019;39:3412–3433. doi: 10.1523/JNEUROSCI.2945-17.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(Suppl 1):S10–28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Backryd E, Lind AL, Thulin M, Larsson A, Gerdle B, Gordh T. High levels of cerebrospinal fluid chemokines point to the presence of neuroinflammation in peripheral neuropathic pain: a cross-sectional study of 2 cohorts of patients compared with healthy controls. Pain. 2017;158:2487–2495. doi: 10.1097/j.pain.0000000000001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Deng G, Wang H, Yang M, Yang R, Li X, Zhang X, Yuan H. Interleukin-1beta pre-treated bone marrow stromal cells alleviate neuropathic pain through CCL7-mediated inhibition of microglial activation in the spinal cord. Sci Rep. 2017;7:42260. doi: 10.1038/srep42260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown AJ, Joseph PR, Sawant KV, Rajarathnam K. Chemokine CXCL7 heterodimers: structural insights, CXCR2 receptor function, and glycosaminoglycan interactions. Int J Mol Sci. 2017;18:748. doi: 10.3390/ijms18040748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao DL, Qian B, Zhang ZJ, Gao YJ, Wu XB. Chemokine receptor CXCR2 in dorsal root ganglion contributes to the maintenance of inflammatory pain. Brain Res Bull. 2016;127:219–225. doi: 10.1016/j.brainresbull.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 23.Malko P, Syed Mortadza SA, McWilliam J, Jiang LH. TRPM2 channel in microglia as a new player in neuroinflammation associated with a spectrum of central nervous system pathologies. Front Pharmacol. 2019;10:239. doi: 10.3389/fphar.2019.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomes C, Ferreira R, George J, Sanches R, Rodrigues DI, Goncalves N, Cunha RA. Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. J Neuroinflammation. 2013;10:16. doi: 10.1186/1742-2094-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stankov A, Belakaposka-Srpanova V, Bitoljanu N, Cakar L, Cakar Z, Rosoklija G. Visualisation of microglia with the use of immunohistochemical double staining method for CD-68 and Iba-1 of cerebral tissue samples in cases of brain contusions. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2015;36:141–145. doi: 10.1515/prilozi-2015-0062. [DOI] [PubMed] [Google Scholar]
- 26.Duffy SS, Lees JG, Perera CJ, Moalem-Taylor G. Managing neuropathic pain in multiple sclerosis: pharmacological interventions. Med Chem. 2018;14:106–119. doi: 10.2174/1573406413666170906122508. [DOI] [PubMed] [Google Scholar]
- 27.Xu L, Wang Q, Jiang W, Yu S, Zhang S. MiR-34c ameliorates neuropathic pain by targeting NLRP3 in a mouse model of chronic constriction injury. Neuroscience. 2019;399:125–134. doi: 10.1016/j.neuroscience.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 28.Rojewska E, Ciapala K, Mika J. Kynurenic acid and zaprinast diminished CXCL17-evoked pain-related behaviour and enhanced morphine analgesia in a mouse neuropathic pain model. Pharmacol Rep. 2019;71:139–148. doi: 10.1016/j.pharep.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Moreau N, Mauborgne A, Bourgoin S, Couraud PO, Romero IA, Weksler BB, Villanueva L, Pohl M, Boucher Y. Early alterations of Hedgehog signaling pathway in vascular endothelial cells after peripheral nerve injury elicit blood-nerve barrier disruption, nerve inflammation, and neuropathic pain development. Pain. 2016;157:827–839. doi: 10.1097/j.pain.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 30.Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siqueira Mietto B, Kroner A, Girolami EI, Santos-Nogueira E, Zhang J, David S. Role of IL-10 in resolution of inflammation and functional recovery after peripheral nerve injury. J Neurosci. 2015;35:16431–16442. doi: 10.1523/JNEUROSCI.2119-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]