Abstract
Previous studies have demonstrated extracorporeal cardiac shock waves (ECSW) could induce angiogenesis and improves myocardial function in patients with coronary heart diseases as a safe, effective, and non-invasive angiogenic approach. The endothelial progenitor cells (EPCs) can migrate to the ischemic myocardium and differentiate into vascular endothelial cells, thus promoting the angiogenesis. Whether ECSW can improve the angiogenic ability of EPCs is unclear. This topic studied the effects of ECSW Therapy on EPCs functions and related signal transduction pathways. The bone marrow-derived EPCs of SD rats were isolated by the density centrifugation method. After treatment with ECSW (500 shots at 0.09 mJ/mm2), the cell viability, anti-apoptosis, migration, and tube formation of EPCs were significantly improved. In addition, the expressions of phosphorylated AKT and ERK were increased after ECSW treatment, the expressions of downstream signaling molecules eNOS and Bcl-2 were also increased, but the expressions of Bax and Caspase3 were decreased. However, these beneficial effects can be inhibited by PI3K/AKT inhibitor LY294002 and MEK/ERK inhibitor PD98059. Together, ECSW can promote the cell viability, migration, and angiogenic ability of EPCs and inhibit the apoptosis of EPCs through the PI3K/AKT and MEK/ERK signaling pathways. The mechanism may be related to promoting the expressions of downstream p-eNOS and anti-apoptotic protein Bcl-2 and inhibiting the expressions of pro-apoptotic protein Bax and Caspase3 through the PI3K/AKT and MEK/ERK signaling pathways.
Keywords: Extracorporeal cardiac shock waves, endothelial progenitor cells, cell function, signaling pathways
Introduction
Current drug therapies, percutaneous transluminal coronary angioplasty (PTCA), and coronary artery bypass grafting (CABG) are three major therapeutic options for coronary heart diseases. However, due to the existence of incomplete revascularization, complexity of surgical procedures, risk of surgery, and high cost of surgery, the prognosis for patients with severe coronary heart diseases without indications for PCI or CABG still remains poor. Even some patients having undergone conventional standard treatment may still occur myocardial ischemia [1-3]. Therefore, it is crucial to develop alternative therapeutic strategies for severe coronary heart diseases.
ECSW system (MODULITH SLC, STORZ MEDICAL, Switzerland) was developed and applied in coronary heart diseases based on multi-year experience of urinary lithotripsy treatment. ECSW has shown to induce angiogenesis and improve myocardial function in the cellular, animal and clinical studies, suggesting that ECSW is an effective and noninvasive therapeutic strategy for myocardial ischemia [4-11]. Despite this, further studies are needed to determine the precise molecular mechanism for ECSW-induced angiogenesis and recovery of myocardial function.
Bone marrow-derived stem cells have been used in recent trials for myocardial regeneration and neoangiogenesis [12]. However, bone marrow-derived stem cells are composed of a heterogeneous group of cells, and many controversies regarding the ideal subtype for cell therapy still remain [13]. Studies have indicated extracorporeal shock waves can enhance proliferation of bone marrow mesenchymal stem cells (MSCs), inducing conversion of MSCs into osteoblasts, which may be one of the effective mechanisms for treating avascular necrosis of the femoral head [14]. EPCs, also known as hemangioblasts, are the precursor cells of vascular endothelial cells. They are a group of cells with migratory characteristics that can self-renew, proliferate, and differentiate into vascular endothelial cells. The number and function of EPCs could be affected in patients with coronary heart diseases or cardiovascular risk factors, indicating that these risk factors not only directly alter the function of vascular endothelium but also impair EPCs-mediated endothelial repair [15-17]. Studies have suggested that improving the angiogenic ability of EPCs by drugs and other interventions is an effective new method for the prevention and treatment of ischemic heart diseases [18]. Therefore, whether non-invasive ECSW treatment can improve the function of EPCs, thus promoting the endothelial repair and angiogenesis in patients with coronary heart diseases, is worthy of further studies. The PI3K/AKT and MEK/ERK signaling pathways play important roles in promoting the mitotic transformation and regulating the expressions of various growth factors and downstream cytokines [19-21]. Studies targeting the effects of ECSW on EPCs function and related molecular mechanisms have not been reported. In the present study we have investigated the effects of ECSW on EPCs function in vitro and the related molecular mechanism and assessed whether these effects might be mediated by PI3K/AKT and MEK/ERK signaling pathways, aiming to provide theoretical basis for ECSW in promoting the angiogenesis.
Material and methods
Culture and identification of EPCs
Murine bone marrow-derived EPCs were isolated from 4-week-old SD rats by the density centrifugation method. The harvested cells were resuspended in complete medium (EGM-2) (lonza) and cultured at 37°C under an atmosphere of 5% CO2. Four hours later, non-adherent cells were removed, and the adherent cells were cultured continuously. Only adherent cells were used in further experiments. The medium was changed every three day. Third to fourth passage cells were then harvested for subsequent tests.
Cell identification: For characterization, after 7 days of culture, the cells were incubated with 10 mg/ml Dil-AcLDL for 4 hours, fixed with 4% paraformaldehyde and then incubated with 10 mg/ml FITC-UEA-1 for 1 h. Dual staining for Dil-AcLDL uptake and lectin from FITC-UEA-1 binding was used for EPC confirmation. Flow cytometry was further performed to identify cell markers of EPCs using the following mouse monoclonal antibodies: FITC-CD31, FITC-CD34, FITC-CD133, APC-VEGFR-2.
Experimental grouping and ECSW processing
The EPCs in logarithmic growth phase were harvested from the cultures and placed in a 5 ml cryotubes, and divided into 6 groups: the vehicle group (added with the same volume of DMSO); group ECSW (treated with ECSW); group LY294002 (pretreated with PI3K/AKT inhibitor [5 μM] LY294002 for 30 min, without ECSW treatment); group PD98059 (pretreated with MEK/ERK inhibitor [10 μM] PD98059 for 30 min, without ECSW treatment); group ECSW+LY294002 (pretreated with PI3K/AKT inhibitor [5 μM] LY294002 for 30 min, then treated with ECSW); group ECSW+PD98059 (pretreated with MEK/ERK inhibitor [10 μM] PD98059 for 30 min, then treated with ECSW). During the experiments, DMSO was used as the solvent of LY294002 and PD98059. We treated EPCs with a low-energy shock wave (0.09 mJ/mm2, approximately 10% of the energy for the lithotripsy treatment, total of 500 shots) generated by ECSW system (MODULITH SLC, STORZ MEDICAL, Switzerland). The cells were cultured for 24 hours at 37°C under an atmosphere of 5% CO2 before subsequent testing.
Cell viability assay
The cell viability was measured by MTT assay. 100 μL of cell suspension (about 5 × 104 cells/well) was seeded in a 96-well plate and incubated 24 hours after treated with different methods as described above. 10 μl of MTT solution (5 mg/ml) was added into each well and cultured for 4 hours. Then the cells were added with 150 μl of DMSO and incubated for another 20 min in one incubator. Finally, the absorbance (OD) at 490 nm was measured by ELISA.
Flow cytometry
The apoptosis was detected using the Annexin V-FITC/PI staining kit. After being collected, the cells were washed twice with PBS and resuspended in Annexin V-binding buffer. 100 μl of such cell suspension was added to new tubes, stained with 5 ul of Annexin V-FITC and PI for 15 min incubation at room temperature in darkness, then analyzed by flow cytometry (flow instrument model: Partec flow).
Cell migration assay
Transwell chamber was used to observe the cell migration. The cells in each group were collected, and resuspended in 2% FBS EGM-2 medium. 1 × 105 cells in 500 μL medium (2% FBS in EGM-2 medium+2 ng of VEGF) was added to the lower chamber, and 100 μL of the cell suspension was added to the upper chamber. The chamber was collected 24 hours later. The cells on the bottom of the Transwell membrane were fixed with 4% paraformaldehyde for 30 min and stained with 1% crystal violet for 15 min. Three randomly microscopic fields were selected to enumerate the cells under an inverted microscope.
Tube formation assay
Matrigel basement membrane matrix was used to evaluate the tube formation ability of the cells. The Matrigel was thawed overnight at 4°C and diluted with DMEM/F12 medium (ratio 1:1), then added into 96-well plates (50 μL/well). The Matrigel was then coagulated by placing in one 37°C incubator for 30 min. The cells in 100 μL of EGM-2 medium each group were inoculated into wells (104 cells per well), and incubated in an incubator for later observation. After overnight of incubation, three randomly microscopic fields were selected to observe the capillary-like structures and calculate the tube length.
Western blot analysis
After being lysed with the RIPA lysate containing protease inhibitors, the cell lysates were collected, and the supernatant was taken for protein concentration determination and Western blot analysis. The same amount of protein sample was subjected to SDS-PAGE electrophoresis, followed by membrane transferring and overnight blocking. The rabbit anti-human AKT antibody, Phospho-AKT antibody, ERK1/2 antibody, Phospho-ERK1/2 antibody, eNOS antibody, p-eNOS antibody, Bax antibody, Bcl-2 antibody, and Caspase3 antibody with appropriate dilution ratios were added onto the protein transfer membrane for overnight hybridization at 4°C; after washing, the horseradish peroxidase-labeled secondary antibody cross-linker was added and reacted at room temperature for 1-2 h. The above detection used β-actin as the internal reference. The protein transfer membrane was developed with ECL enhanced chemiluminescence reagent, and the gray scale data obtained by electronic computer gray scale scanning were then used for analysis.
Statistical analysis
Data analysis was performed using SPSS 17.0 statistical software. The experimental data were expressed as mean ± standard deviation (x ± s). All quantitative data were analyzed with one-way ANOVA, followed by Fisher’s least significant difference (LSD) method for pair-wise multiple comparisons. If the variance was not uniform, the rank sum test can be used. A = 0.05 was set as the test level, and P<0.05 was considered as statistical significance.
Results
Culture and identification of cells
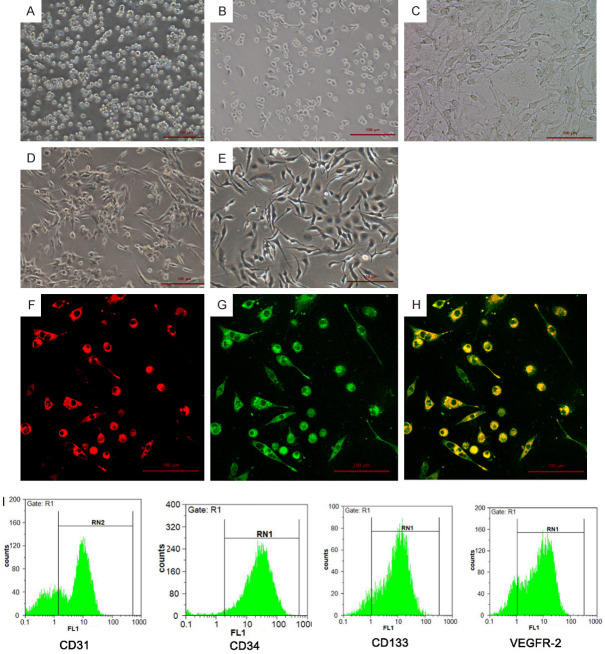
The isolated mononuclear cells were small while uniform in size, round and unevenly distributed at the bottom of culture flask (Figure 1A); on Day 3, adherent, oval or short spindle cells can be observed (Figure 1B). On Day 7, most of the cells became spindle and showed typical colony growth. The cells at the colony center were partially necrotic, and the surrounding cells arranged radially (Figure 1C). On Day 14, the cells around the colony center gradually merged and formed line-like arrangement (Figure 1D); on Day 20, the cells joined and formed capillary-like structure (Figure 1E).
Figure 1.
Culture and identification of rat bone-derived EPCs. A-E. On Day 1, Day 3, Day 7, Day 14, and Day 20 of culture, the morphology of EPCs cells is observed under an inverted microscope (magnification ×100). F-H. Immunofluorescence staining of EPCs double positive for Dil-AcLDL and FITC-UEA-1. Scale bar =100 μm. I. Cell markers (CD31, CD34, CD133 and VEGFR-2) of EPCs were identified by flow cytometry.
After 7 days of culture (typical culture period before further experiments), adherent EPCs were characterized by immunofluorescence. They were double positive staining for Dil-AcLDL and FITC-UEA-1 (Figure 1F-H). Flow cytometry assay showed that the majority of EPCs were positive for CD31, CD34, CD133 and VEGFR-2 (Figure 1I), indicating that EPCs were successfully isolated from rat bone marrow.
ECSW promotes the cell viability of EPCs and inhibits the apoptosis of EPCs
The cell viability of EPCs in each group was detected by MTT assay. The results (Figure 2A) showed that ECSW promoted the cell viability of EPCs while pretreatment with PI3K/AKT inhibitor LY294002 or MEK/ERK inhibitor PD98059 inhibited this effect of ECSW.
Figure 2.
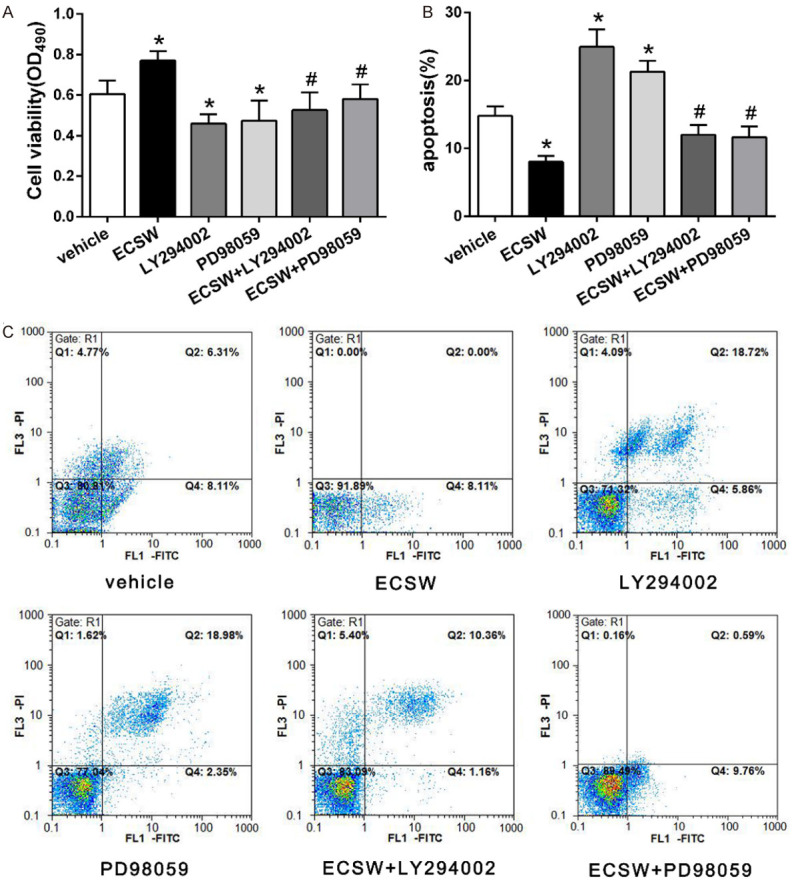
ECSW promotes the cell viability of EPCs and inhibits the apoptosis through the PI3K/AKT and MEK/ERK signaling pathways. A. Quantitative analysis of the cell viability of EPCs in each group. ECSW promoted the cell viability of EPCs while pretreatment with PI3K/AKT inhibitor LY294002 or MEK/ERK inhibitor PD98059 inhibited this effect. Data are presented as mean ± SD, N=5. *P<0.05 vs group vehicle, #P<0.05 vs group ECSW. B. The percentage of apoptotic cells with Annexin V-FITC positive expression was used to assess EPC apoptosis. ECSW protected EPCs from apoptosis while pretreatment with PI3K/AKT inhibitor LY294002 or MEK/ERK inhibitor PD98059 inhibited this effect of ECSW. Data are presented as mean ± SD, N=3. *P<0.05 vs group vehicle, #P<0.05 vs group ECSW. C. Cell apoptosis was detected by Annexin V-FITC and PI double staining and analyzed with flow cytometry.
Cell apoptosis assay with Annexin V-FITC and PI double staining (Figure 2B, 2C) revealed that ECSW protected EPCs from apoptosis while pretreatment with PI3K/AKT inhibitor LY294002 or MEK/ERK inhibitor PD98059 inhibited this effect of ECSW.
ECSW promotes the migration and tube formation of EPCs
The Transwell chamber assay was used to detect the migration ability of EPCs in different groups. As shown in Figure 3, ECSW improved the migration of EPCs while pretreatment with PI3K/AKT inhibitor LY294002 or MEK/ERK inhibitor PD98059 inhibited this effect of ECSW.
Figure 3.
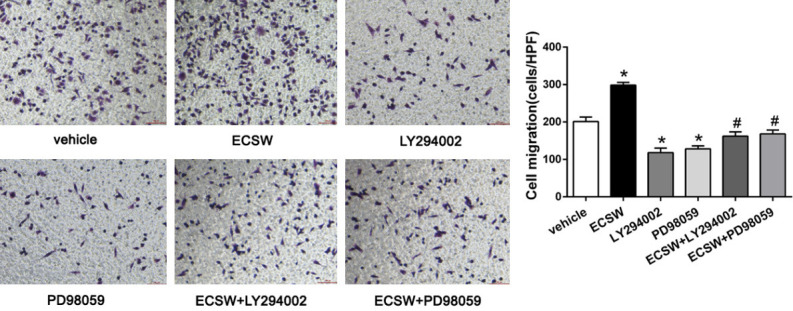
ECSW promotes the migration of EPCs through the PI3K/AKT and MEK/ERK signaling pathways. The transwell chamber assay was used to measure the migration capacity of EPCs in different groups, and the number of migrated cells was counted using an inverted microscope. ECSW improved the migration of EPCs while pretreatment with PI3K/AKT inhibitor LY294002 or MEK/ERK inhibitor PD98059 inhibited this effect of ECSW. Scale bar =100 μm. Data are presented as mean ± SD, N=3. *P<0.05 vs group vehicle, #P<0.05 vs group ECSW.
We examined the tube formation of EPCs by using Matrigel assay, and the tube length was used to express the ability of angiogenesis. As shown in Figure 4, ECSW improved the tube formation of EPCs while pretreatment with PI3K/AKT inhibitor LY294002 or MEK/ERK inhibitor PD98059 inhibited this effect of ECSW.
Figure 4.
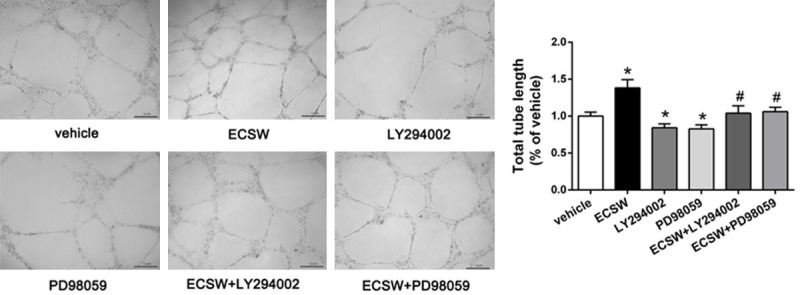
ECSW promotes the tube formation of EPCs through the PI3K/AKT and MEK/ERK signaling pathways. The Matrigel assay in vitro was used to investigate the effect of ECSW on angiogenesis in EPCs, three randomly microscopic fields were selected to observe the capillary-like structures and calculate the tube length. ECSW improved the tube forming ability of EPCs while pretreatment with PI3K/AKT inhibitor LY294002 or MEK/ERK inhibitor PD98059 inhibited this effect of ECSW. Scale bar =50 μm. Data are presented as mean ± SD, N=3. *P<0.05 vs group vehicle, #P<0.05 vs group ECSW.
ECSW activates the EPCs PI3K/AKT and MEK/ERK signaling pathways, promotes the expressions of p-eNOS and Bcl-2, and inhibits the expressions of Bax and Caspase3
Western blot assay was used to assessed the effects of ECSW on EPCs’ function might be mediated by PI3K/AKT and MEK/ERK signaling pathways. The results (Figure 5A-C) showed that the phosphorylation levels of AKT and ERK in group ECSW were significantly higher than those in group vehicle, but the phosphorylation levels of AKT and ERK were decreased after LY294002 and PD98059 were added respectively.
Figure 5.
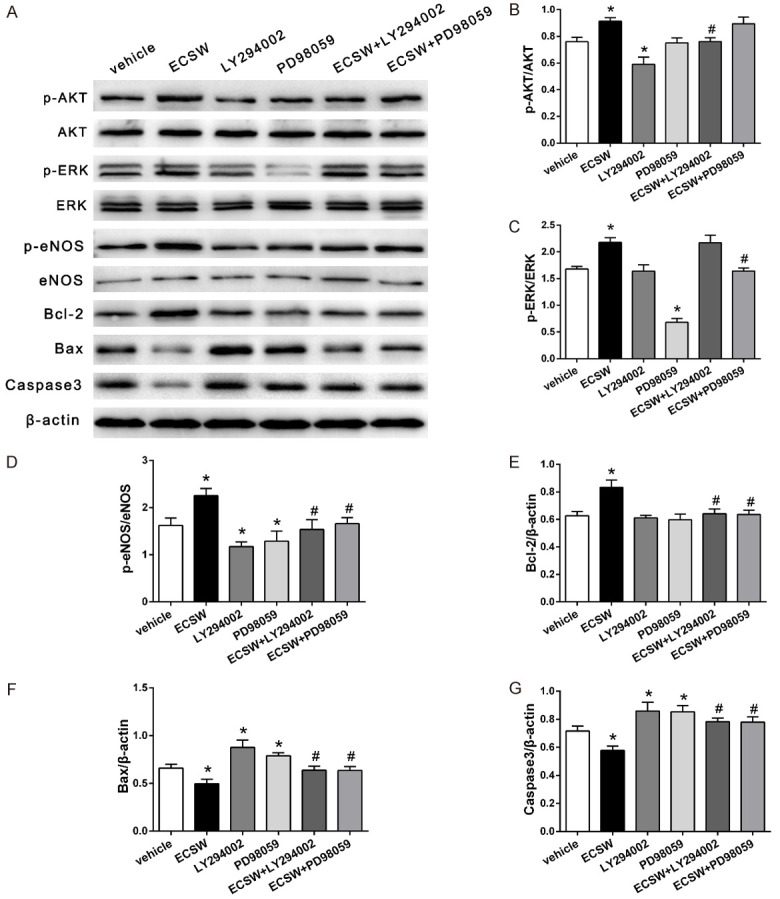
ECSW promotes the expressions of p-eNOS and Bcl-2 and inhibits the expressions of apoptotic factor Bax and Caspase3 through the PI3K/AKT and MEK/ERK signaling pathways. A. Western blotting analysis of AKT, p-AKT, ERK, p-ERK, P-eNOS, eNOS, Bcl-2, Bax, and caspase3 protein in EPCs. B-G. Quantification analysis of the expression levels of p-AKT, p-ERK, P-eNOS, Bcl-2, Bax, and caspase3 based on the Western blotting analysis results. the expressions of p-AKT and p-ERK were increased after ECSW treatment, the expressions of downstream signaling molecules eNOS and Bcl-2 were also increased, but the expressions of Bax and Caspase3 were decreased. However, these beneficial effects can be inhibited by PI3K/AKT inhibitor LY294002 and MEK/ERK inhibitor PD98059. Data are presented as mean ± SD. *P<0.05 vs group vehicle, #P<0.05 vs group ECSW.
The expressions of downstream signaling molecules p-eNOS, eNOS, Bcl-2, Bax and Caspase3 in different groups were further examined by Western blot. These factors play important roles in regulating the function of EPCs. The results (Figure 5A, 5D-G) showed that the phosphorylation of eNOS and the expression of Bcl-2 were increased after ECSW treatment, but the protein expressions of Bax and Caspase3 were decreased. After LY294002 and PD98059 were added, respectively, these beneficial effects were inhibited.
Discussion
When the myocardium occurs ischemia and necrosis, the body can compensatively form new capillaries, but the reconstruction of this microcirculation is not enough to meet the needs of the body to improve the myocardial blood supply. Finding a new non-invasive method to induce ischemic myocardial angiogenesis and improve myocardial perfusion could be important to improve myocardial function in patients with coronary heart diseases, and is also a hotspot in the research of cardiovascular disease treatment in recent years. In order to accomplish the revascularization of ischemic myocardium, it is imperative that an effective therapy for ischemic cardiomyopathy be developed. In recent years, animal experiments and clinical studies have confirmed that the low-energy extracorporeal cardiac shock wave which level is approximately 10% of that used for urinary lithotripsy treatment can effectively induces angiogenesis and improves myocardial perfusion and myocardial function without any major adverse effects, its optimal energy is 0.09 mJ/mm2 [4-11]. ECSW exerts a ‘cavitation effect’ (a mm-sized violent collapse of bubbles inside and outside the cells) and was shown to induce localized stress on cell membranes that resembles shear stress, due to the localized nature of the physical forces generated by cavitation [10,11]. It has been found that ECSW can upregulate the expressions of vascular endothelial growth factor (VEGF) mRNA and its receptor fms-like tyrosine-1 (flt-1), nitric oxide synthase (eNOS) in vitro [10,22,23]. Because the VEGF-flt-1 system is essential in initiating vasculogenesis and angiogenesis, the effect of ECSW may explain in part the underlying mechanisms for ECSW-induced angiogenesis. However, detailed intracellular mechanisms of ECSW action remain to be elucidated.
The pathological basis of coronary heart diseases is mainly atherosclerotic formation, and the damage of vascular endothelial cell is the starting point of atherosclerosis [24,25]. Repair of endothelium is closely related to the development and prognosis of cardiovascular diseases. Mature endothelial cells have lower proliferative capacity and cannot provide sufficient numbers of cells to repair the vascular endothelial cell have been damaged. The precursor cells of endothelial cells, EPCs, can mobilize, proliferate, migrate, and differentiate into endothelial cells from the bone marrow, playing an important role in maintaining the integrity of vascular endothelium, repairing endothelial damage, and promoting vasculogenesis and angiogenesis. The number and function of endothelial progenitor cells can indicate the ability of repairing vascular endothelial damage [26]. EPCs can differentiate into mature endothelial cells, which not only participate in the repair of vascular endothelial damage but also the formation of microvessel.
Previous our research has found that ECSW can promote the proliferation of EPCs and improve the cardiac function [27]. However, the effects of ECSW on the apoptosis, migration, and angiogenesis of EPCs and related molecular mechanisms have not been reported so far. The PI3K/AKT and MEK/ERK signaling pathways are two important pathways in the mitogen-activated protein kinases (MAPKs) signaling pathways, which exist in most cells, including EPCs, and can regulate the cell viability, proliferation, apoptosis, migration, differentiation, and other functions by activating downstream signals [19-21,28,29]. In this study, the effects of ECSW on the cell viability, apoptosis, migration, and angiogenesis of EPCs were first observed. Through culturing the mononuclear cells isolated from the bone marrow, we could obtain the endothelial progenitor cells. In this study, we preliminarily indicate that ECSW can promote the cell viability, migration, and tube formation and inhibit the apoptosis of EPCs, suggesting that ECSW promote the function of EPCs. To further investigate whether these effects of ECSW might be mediated by PI3K/AKT and MEK/ERK signaling pathways, Western blot assay results revealed that ECSW can promote the phosphorylation of AKT and ERK in EPCs, and LY294002 and PD98059 can significantly inhibit the phosphorylation of AKT and ERK in EPCs. Similarly, the cell viability, anti-apoptosis, migration, and angiogenic ability of EPCs were also inhibited, indicating that ECSW promote the function of EPCs by activating the PI3K/AKT and MEK/ERK signaling pathways.
Under simulated pathophysiological conditions (such as 1 mM H2O2 and 10 mM L-Arg), ECSW stimulation can synthesize NO in a relatively gentle manner without the catalysis of NO synthase [30]. It has been shown that the use of low-energy shock waves in human umbilical vein endothelial cells (HUVECs) can rapidly enhance eNOS activity, accelerate the production of NO by affecting tyrosine-phosphorylation of eNOS and suppressing NF-λB activation [31]. The study found that ECSW can promote the expression of eNOS in EPCs. The expression of eNOS was significantly decreased in EPCs after pretreatment of LY294002 and PD98059, indicating that ECSW can up-regulate the expression of eNOS by activating the PI3K/AKT and MEK/ERK signaling pathways. The downstream molecular of PI3K/AKT and MEK/ERK signaling pathways, eNOS, is involved in various biological functions of EPCs and plays an important role in repairing endothelial function [32-35]. eNOS can catalyze L-arginine to produce NO, playing a critical role in a number of fundamental events such as inflammation, angiogenesis, platelet aggregation and vasodilatation, and is of great importance for the maintenance of normal physiological functions of EPCs [36,37]. Changes in NO content and biological activity can reflect the state of EPCs [38,39]. Therefore, it is conceivable that one of the possible molecular mechanisms of action behind these beneficial effects of ESW seems to be related to the enhancement of eNOS activity, keeping NO contents at a physiological level.
The Bcl-2 family proteins are a class of proteins that play a crucial role in the process of apoptosis, including anti-apoptotic proteins and pro-apoptotic proteins. The Bax protein is present in the cytoplasm of normal cells. When stimulated by a series of apoptotic signals, it initiates the Caspase cascade and activates the downstream factor Caspase3, leading to apoptosis [40-42]. In contrast, Bcl-2 has the ability to suppress this Bax-induced release of Caspase3, and expression ratio of Bcl-2 to Bax was reported to determine cell apoptosis following apoptotic signals. Studies have shown that the PI3K/AKT and MEK/ERK signaling pathways are involved in the regulation of downstream apoptosis factors such as Bcl-2, Bax, and Caspase3 [28,43-46]. Therefore, this study also investigated whether ECSW could inhibit the apoptosis of EPCs by regulating the PI3K/AKT and MEK/ERK signaling pathways. The results of this study showed that ECSW can promote the expression of anti-apoptotic proteins Bcl-2 and inhibit the expressions of pro-apoptotic proteins Bax and Caspase3, while pretreatment with LY294002 or PD98059 inhibited the beneficial effects of ECSW, indicating that ECSW can inhibit the apoptosis of EPCs through the PI3K/AKT and MEK/ERK signaling pathways.
In summary, our results demonstrated that ECSW plays an important role in promoting EPCs function. The mechanism may be related to promoting the expressions of p-eNOS and Bcl-2 and inhibiting the expressions of Bax and Caspase3 through the PI3K/AKT and MEK/ERK signaling pathways. ECSW can promote the angiogenesis of ischemic tissues for the treatment of coronary heart diseases as it can promote the angiogenic function of EPCs by regulating PI3K/AKT and MEK/ERK signaling pathways. This can provide evidence of the applications of ECSW in treatment of coronary heart disease.
Acknowledgements
This study was supported by National Natural Science Foundation of China (No. 81560072, No. 81760067), Yunnan Provincial Science and Technology Department (No. 2013FB128, 2014FB037) and Yunnan Provincial Medical Science Leader Training Project (D-201622).
Disclosure of conflict of interest
None.
References
- 1.Waldo SW, Brenner DA, Li S, Alexander K, Ganz P. Reperfusion times and in-hospital outcomes among patients with an isolated posterior myocardial infarction: insights from the National Cardiovascular Data Registry (NCDR) Am Heart J. 2014;167:350–354. doi: 10.1016/j.ahj.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Deb S, Wijeysundera HC, Ko DT, Tsubota H, Hill S, Fremes SE. Coronary artery bypass graft surgery vs percutaneous interventions in coronary revascularization: a systematic review. JAMA. 2013;310:2086–2095. doi: 10.1001/jama.2013.281718. [DOI] [PubMed] [Google Scholar]
- 3.Cheng K, de Silva R. New advances in the management of refractory angina pectoris. Eur Cardiol. 2018;13:70–79. doi: 10.15420/ecr.2018:1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu M, Sun CK, Lin YC, Wang CJ. Extracorporeal shock wave therapy reverses ischemia-related left ventricular dysfunction and remodeling: molecular-cellular and functional assessment. PLoS One. 2011;6:e24342. doi: 10.1371/journal.pone.0024342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaller M, Faber L, Bogunovic N, Horstkotte D, Burchert W, Lindner O. Cardiac shock wave therapy and myocardial perfusion in severe coronary artery disease. Clin Res Cardiol. 2015;104:843–849. doi: 10.1007/s00392-015-0853-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holfeld J, Zimpfer D, Albrecht-Schgoer K, Stojadinovic A, Paulus P, Dumfarth J, Thomas A, Lobenwein D, Tepekoylu C, Rosenhek R, Schaden W, Kirchmair R, Aharinejad S, Grimm M. Epicardial shock-wave therapy improves ventricular function in a porcine model of ischaemic heart disease. J Tissue Eng Regen Med. 2016;10:1057–1064. doi: 10.1002/term.1890. [DOI] [PubMed] [Google Scholar]
- 7.Gollmann-Tepekoylu C, Lobenwein D, Theurl M, Primessnig U, Lener D, Kirchmair E, Mathes W, Graber M, Polzl L, An A, Koziel K, Pechriggl E, Voelkl J, Paulus P, Schaden W, Grimm M, Kirchmair R, Holfeld J. Shock wave therapy improves cardiac function in a model of chronic ischemic heart failure: evidence for a mechanism involving VEGF signaling and the extracellular matrix. J Am Heart Assoc. 2018;7:e010025. doi: 10.1161/JAHA.118.010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang W, He Y, Gan L, Zhang F, Hua B, Yang P, Liu J, Yang L, Guo T. Cardiac shock wave therapy promotes arteriogenesis of coronary micrangium, and ILK is involved in the biomechanical effects by proteomic analysis. Sci Rep. 2018;8:1814. doi: 10.1038/s41598-018-19393-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kikuchi Y, Ito K, Ito Y, Shiroto T, Tsuburaya R, Aizawa K, Hao K, Fukumoto Y, Takahashi J, Takeda M, Nakayama M, Yasuda S, Kuriyama S, Tsuji I, Shimokawa H. Double-blind and placebo-controlled study of the effectiveness and safety of extracorporeal cardiac shock wave therapy for severe angina pectoris. Circ J. 2010;74:589–591. doi: 10.1253/circj.cj-09-1028. [DOI] [PubMed] [Google Scholar]
- 10.Nishida T, Shimokawa H, Oi K, Tatewaki H, Uwatoku T, Abe K, Matsumoto Y, Kajihara N, Eto M, Matsuda T, Yasui H, Takeshita A, Sunaqawa K. Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation. 2004;110:3055–3061. doi: 10.1161/01.CIR.0000148849.51177.97. [DOI] [PubMed] [Google Scholar]
- 11.Ito K, Fukumoto Y, Shimokawa H. Extracorporeal shock wave therapy for ischemic cardiovascular disorders. Am J Cardiovasc Drugs. 2011;11:295–302. doi: 10.2165/11592760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Gao F, Hou H, Liang H, Weinreb RN, Wang H, Wang Y. Bone marrow-derived cells in ocular neovascularization: contribution and mechanisms. Angiogenesis. 2016;19:107–118. doi: 10.1007/s10456-016-9497-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strauer BE, Brehm M, Zeus T, Köstering M, Hernandez A, Sorg RV, Kögler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 14.Zhai L, Sun N, Zhang B, Liu ST, Zhao Z, Jin HC, Ma XL, Xing GY. Effects of focused extracorporeal shock waves on bone marrow mesenchymal stem cells in patients with avascular necrosis of the femoral head. Ultrasound Med Biol. 2016;42:753–762. doi: 10.1016/j.ultrasmedbio.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt-Lucke C, Rössig L, Fichtlscherer S, Vasa M, Britten M, Kämper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predict future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 16.Nollet E, Hoymans VY, Rodrigus IR, De Bock D, Dom M, Vanassche B, Van Hoof VO, Cools N, Van Ackeren K, Wouters K, Vermeulen K, Vrints CJ, Van Craenenbroeck EM. Bone marrow-derived progenitor cells are functionally impaired in ischemic heart disease. J Cardiovasc Transl Res. 2016;9:266–278. doi: 10.1007/s12265-016-9707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui Y, Sun Q, Liu Z. Ambient particulate matter exposure and cardiovascular diseases: a focus on progenitor and stem cells. J Cell Mol Med. 2016;20:782–793. doi: 10.1111/jcmm.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parzonko A, Oświt A, Bazylko A, Naruszewicz M. Anthocyans-rich Aronia melanocarpa extract possesses ability to protect endothelial progenitor cells against angiotensin II induced dysfunction. Phytomedicine. 2015;22:1238–1246. doi: 10.1016/j.phymed.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Chen WC, Chung CH, Lu YC, Wu MH, Chou PH, Yen JY, Lai YW, Wang GS, Liu SC, Cheng JK, Wu YJ, Yeh HI, Wang LY, Wang SW. BMP-2 induces angiogenesis by provoking integrin α6 expression in human endothelial progenitor cells. Biochem Pharmacol. 2018;150:256–266. doi: 10.1016/j.bcp.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Zou HX, Jia J, Zhang WF, Sun ZJ, Zhao YF. Propranolol inhibits endothelial progenitor cell homing: a possible treatment mechanism of infantile hemangioma. Cardiovascular Pathology. 2012;22:203–210. doi: 10.1016/j.carpath.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Dai T, Hu Y, Zheng H. Hypoxia increases expression of CXC chemokine receptor 4 via activation of PI3K/Akt leading to enhanced migration of endothelial progenitor cells. Eur Rev Med Pharmacol Sci. 2017;21:1820–1827. [PubMed] [Google Scholar]
- 22.Zhao Y, Wang J, Wang M, Sun P, Chen J, Jin X, Zhang H. Activation of bone marrow-derived mesenchymal stromal cells-a new mechanism of defocused low-energy shock wave in regenerative medicine. Cytotherapy. 2013;15:1449–1457. doi: 10.1016/j.jcyt.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Suhr F, Delhasse Y, Bungartz G, Schmidt A, Pfannkuche K, Bloch W. Cell biological effects of mechanical stimulations generated by focused extracorporeal shock wave applications on cultured human bone marrow stromal cells. Stem Cell Res. 2013;11:951–964. doi: 10.1016/j.scr.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Li F, Chen Q, Song X, Zhou L, Zhang J. MiR-30b is involved in the homocysteine-induced apoptosis in human coronary artery endothelial cells by regulating the expression of caspase 3. Int J Mol Sci. 2015;16:17682–17695. doi: 10.3390/ijms160817682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urban I, Turinsky M, Gehrmann S, Morgenstern J, Brune M, Milewski MR, Wagner AH, Rumig C, Fleming T, Leuschner F, Gleissner CA, Hecker M. 15-deoxy-delta12,14-prostaglandin J2 reinforces the anti-inflammatory capacity of endothelial cells with a genetically determined no deficit. Circ Res. 2019;125:282–294. doi: 10.1161/CIRCRESAHA.118.313820. [DOI] [PubMed] [Google Scholar]
- 26.Povsic TJ, Goldschmidt-Clermont PJ. Endothelial progenitor cells: markers of vascular reparative capacity. Ther Adv Cardiovasc Dis. 2008;2:199–213. doi: 10.1177/1753944708093412. [DOI] [PubMed] [Google Scholar]
- 27.Cai HY, Li L, Guo T, Wang YU, Ma TK, Xiao JM, Zhao L, Fang Y, Yang P, Zhao HU. Cardiac shockwave therapy improves myocardial function in patients with refractory coronary artery disease by promoting VEGF and IL-8 secretion to mediate the proliferation of endothelial progenitor cells. Exp Ther Med. 2015;10:2410–2416. doi: 10.3892/etm.2015.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y, Zhang X, Cui H, Zhang C, Zhu C, Li L. Apelin-13 protects the brain against ischemia/reperfusion injurythrough activating PI3K/Akt and ERK1/2 signaling pathways. Neurosci Lett. 2014;568:44–49. doi: 10.1016/j.neulet.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 29.Lee SJ, Namkoong S, Kim YM, Kim CK, Lee H, Ha KS, Chung HT, Kwon YG, Kim YM. Fractalkine stimulates angiogenesis by activating the Raf-1/MEK/ERK-and PI3K/Akt/eNOS-dependent signal pathways. Am J Physiol Heart Circ Physiol. 2006;291:H2836–H2846. doi: 10.1152/ajpheart.00113.2006. [DOI] [PubMed] [Google Scholar]
- 30.Gotte G, Amelio E, Russo S, Marlinghaus E, Musci G, Suzuki H. Short-time non-enzymatic nitric oxide synthesis from L-arginine and hydrogen peroxide induced by shock waves treatment. FEBS Lett. 2002;520:153–155. doi: 10.1016/s0014-5793(02)02807-7. [DOI] [PubMed] [Google Scholar]
- 31.Mariotto S, Cavalieri E, Amelio E, Ciampa AR, de Prati AC, Marlinghaus E, Russo S, Suzuki H. Extracorporeal shock waves: from lithotripsy to anti-inXammatory action by no production. Nitric Oxide. 2004;12:89–96. doi: 10.1016/j.niox.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 32.de Resende MM, Huw LY, Qian HS, Kauser K. Role of endothelial nitric oxide in bone marrow-derived progenitor cell mobilization. Handb Exp Pharmacol. 2007:37–44. doi: 10.1007/978-3-540-68976-8_2. [DOI] [PubMed] [Google Scholar]
- 33.Shi Y, Lv X, Liu Y, Li B, Liu M, Yan M, Liu Y, Li Q, Zhang X, He S, Zhu M, He J, Zhu Y, Zhu Y, Ai D. Elevating ATP-binding cassette transporter G1 improves re-endothelialization function of endothelial progenitor cells via Lyn/Akt/eNOS in diabetic mice. FASEB J. 2018;32:6525–6536. doi: 10.1096/fj.201800248RR. [DOI] [PubMed] [Google Scholar]
- 34.Li W, Du D, Wang H, Liu Y, Lai X, Jiang F, Chen D, Zhang Y, Zong J, Li Y. Silent information regulator 1 (SIRT1) promotes the migration and proliferation of endothelial progenitor cells through the PI3K/Akt/eNOS signaling pathway. Int J Clin Exp Pathol. 2015;8:2274–2287. [PMC free article] [PubMed] [Google Scholar]
- 35.Shen C, Li Q, Zhang YC, Ma G, Feng Y, Zhu Q, Dai Q, Chen Z, Yao Y, Chen L, Jiang Y, Liu N. Advanced glycation endproducts increase EPC apoptosis and decrease nitric oxide release via MAPK pathways. Biomed Pharmacother. 2010;64:35–43. doi: 10.1016/j.biopha.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Chen Q, Wang L, Li G. Ghrelin induces cell migration through GHSR1a-mediated PI3K/Akt /eNOS/NO signaling pathway in endothelial progenitor cells. Metabolism. 2013;62:743–752. doi: 10.1016/j.metabol.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Siragusa M, Fleming I. The eNOS signalosome and its link to endothelial dysfunction. Pflugers Arch. 2016;468:1125–1137. doi: 10.1007/s00424-016-1839-0. [DOI] [PubMed] [Google Scholar]
- 38.Lu A, Wang L, Qian L. The role of eNOS in the migration and proliferation of bone-marrow derived endothelial progenitor cells and in vitro angiogenesis. Cell Biol Int. 2015;39:484–490. doi: 10.1002/cbin.10405. [DOI] [PubMed] [Google Scholar]
- 39.Gao L, Li P, Zhang J, Hagiwara M, Shen B, Bledsoe G, Chang E, Chao L, Chao J. Novel role of kallistatin in vascular repair by promoting mobility, viability, and function of endothelial progenitor cells. J Am Heart Assoc. 2014;3:e001194. doi: 10.1161/JAHA.114.001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Tang L, Wang Y, Wang L, Liu X, Liu X, Chen Z, Liu L. Exendin-4 protects HUVECs from t-BHP-induced apoptosis via PI3K/Akt-Bcl-2-caspase-3 signaling. Endocr Res. 2016;41:229–235. doi: 10.3109/07435800.2015.1110162. [DOI] [PubMed] [Google Scholar]
- 41.Tsuruta F, Masuyama N, Gotoh Y. The phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondria. J Biol Chem. 2002;277:14040–14047. doi: 10.1074/jbc.M108975200. [DOI] [PubMed] [Google Scholar]
- 42.Nishikawa S, Tatsumi T, Shiraishi J, Matsunaga S, Takeda M, Mano A, Kobara M, Keira N, Okigaki M, Takahashi T, Matsubara H. Nicorandil regulates Bcl-2 family proteins and protects cardiac myocytes against hypoxia-induced apoptosis. J Mol Cell Cardiol. 2006;40:510–519. doi: 10.1016/j.yjmcc.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 43.Yu W, Shen T, Liu B, Wang S, Li J, Dai D, Cai J, He Q. Cardiac shock wave therapy attenuates H9c2 myoblast apoptosis by activating the AKT signal pathway. Cell Physiol Biochem. 2014;33:1293–1303. doi: 10.1159/000358697. [DOI] [PubMed] [Google Scholar]
- 44.Shebaby WN, Bodman-Smith KB, Mansour A, Mroueh M, Taleb RI, El-Sibai M, Daher CF. Daucus carota pentane-based fractions suppress proliferation and induce apoptosis in human colon adenocarcinoma HT-29 cells by inhibiting the MAPK and PI3K pathways. J Med Food. 2015;18:745–752. doi: 10.1089/jmf.2014.3225. [DOI] [PubMed] [Google Scholar]
- 45.Guo JR, Li W, Wu Y, Wu LQ, Li X, Guo YF, Zheng XH, Lian XL, Huang HF, Chen YZ. Hepatocyte growth factor promotes proliferation, invasion, and metastasis of myeloid leukemia cells through PI3K-AKT and MAPK/ERK signaling pathway. Am J Transl Res. 2016;8:3630–3644. [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang S, He Y, Tong Q, Chen Q, Wu X, Huang W. Deltonin induces apoptosis in MDAMB231 human breast cancer cells via reactive oxygen speciesmediated mitochondrial dysfunction and ERK/AKT signaling pathways. Mol Med Rep. 2013;7:1038–1044. doi: 10.3892/mmr.2013.1273. [DOI] [PubMed] [Google Scholar]