Abstract
Introduction
Patients upstaged to pT3 after partial nephrectomy (PN) may be at an increased risk of disease progression compared to those patients submitted to radical nephrectomy (RN). We sought to identify preoperative factors predicting pT3 upstaging in localized renal cell carcinoma.
Material and methods
Patients submitted to nephrectomy for clinically localized (cT1–cT2) renal cell carcinoma between 2011 and 2016 were identified from a prospective registry, those presenting with locally advanced or metastatic disease were excluded. Clinical factors, laboratory, and imaging using RENAL score, were analyzed. A multivariate analysis was performed looking for stage pT3a predictors.
Results
Two hundred and nine patients were included, 66% were men, with a mean age of 57 years. Mean tumor size was 49 ±31 mm. 19% were staged as pT3a. Of this group, 10% underwent a PN. Age, hypertension, presence of hematuria, creatinine levels, size and RENAL score were statistically associated with locally advanced stage. The variables of the RENAL score that were associated to pT3a stage were size, nearness to renal sinus/collector system and contact with main renal vessels. On the multivariate analysis, only age, size, and contact with renal vessels were found to predict upstaging. A model was developed which was able to predict stage pT3a with an area under the curve (AUC) of 0.864 in the ROC curve.
Conclusions
Upstaging to pT3a is fairly common in clinically localized tumors. A formula that includes tumor size, age and contact with the main vessels on imaging, can help predict it. This should be considered when deciding if the patient is a candidate for nephron sparing surgery.
Keywords: kidney neoplasms, nephrectomy, neoplasm staging, nomograms
INTRODUCTION
Partial nephrectomy (PN) has become the standard treatment for cT1 renal tumors [1]. Over the last years the technique has evolved and larger and more complex tumors are approached by PN [2], thereby a raise in pathologic upstaging to pT3a has been observed [3]. PN effectiveness for tumors with involvement of the perirenal fat, renal sinus or renal vessels (pT3a) has not been established [4]. Patients with clinically localized tumors (cT1) who show pathologic upstage to pT3a seem to have higher risk of recurrence and worse cancer-specific survival when submitted to PN [5–8]. In this context, preoperatively identifying patients who have locally advanced tumors (pT3a) is paramount, since this population would be bad candidates for PN. It seems interesting to assess if nephrometry using RENAL Score (RS) [9], which was originally created to estimate surgical difficulty, might serve as a predictor of locally advanced pathologic stage. The aim of this research was to find preoperative variables, and with these create a formula, which would allow us to predict upstaging to locally advanced disease (pT3a).
MATERIAL AND METHODS
We retrospectively collected data from patients who were submitted to nephrectomy (radical and partial) with confirmed renal cell carcinoma between 2011 and 2016. Patients with clear invasion of renal vessels or cava (cT3b-c), other organ invasion (cT4), nodal or metastatic compromise were excluded. Demographics, laboratory results, imaging reports (including RS), surgical protocol and histology report were evaluated. RS was calculated by urologists based on computed tomography.
We defined as ‘localized tumor’, those cases with a pT1–T2 pathology report according to the American Joint Committee on Cancer (AJCC) 2010 [10], and ‘locally advanced’ for those reported as pT3a (including any subtype). The study was approved by the local ethical committee. Preoperative features were compared between both groups to assess whether there was any significant association. In the bivariate analysis, we employed chi-square test to compare categorical variables. Normally distributed continuous variables (age and hematocrit) were analyzed with t-student and Mann-Whitney U test was utilized for non-normally ones (creatinine, tumor size, alkaline phosphate). A multivariate analysis with binary logistic regression was performed using the variables that were significantly associated to pT3 in the bivariate analysis. We used the Wald method with forward selection to incorporate variables to the model. A formula according to the model was constructed. Then we generated a ROC curve to assess the diagnostic precision of our predictive model and used the Youden index to find the most appropriate cut-off value. A nomogram was developed in order to easily apply the predictive model. A value of p ≤0.05 was considered statistically significant. Analysis was performed using SPSS, version 22.
RESULTS
We reviewed 288 patients submitted to nephrectomy (radical or partial) with the mentioned inclusion criteria. Seventy-nine cases were discarded due to unavailable imaging studies. The final analyzed group was composed of 209 patients. Demographic, clinical and imaging characteristics are shown in Table 1. Forty subjects (19%) were locally advanced. Partial nephrectomy was performed in 47.5% (56.7% in localized tumors compared to 10% in those locally advanced). Most patients were approached by laparoscopy (82.2%), with similar proportion between RN (84.1%) and PN (81.4%).
Table 1.
Characteristics of the studied population based on pathological stage
| Characteristics | Pathologic stage |
P value | ||
|---|---|---|---|---|
| All | Localized (pT1–T2) | Locally advanced (pT3a) | ||
| N (%) | 209 (100) | 169 (81) | 40 (19) | |
| Age x (SD) | 57.21 (11.8) | 55.6 (11.7) | 64 (9.7) | <.001 |
| Gender (%) Male Female |
139 (66.5) 70 (33.5) |
112 (66.3) 57 (33.7) |
27 (67.5) 13 (32.5) |
0.88 |
| Arterial hypertension (%) | 93 (45.8) | 66 (40.2) | 27 (69.2) | <.001 |
| Diabetes (%) | 38 (18.7) | 28 (17.1) | 10 (25.6) | 0.22 |
| Smoking habit (%) | 36 (18) | 31 (19.1) | 5 (13.2) | 0.39 |
| Hematuria (%) | 28 (14.3) | 17 (10.8) | 11 (28.2) | .006 |
| Pain | 55 (28.4) | 46 (29.3) | 9 (24.3) | 0.55 |
| Palpable mass | 4 (2.1) | 2 (1.3) | 3 (5.4) | 0.11 |
| Surgery (%) Radical nephrectomy Partial nephrectomy |
107 (52.5) 97 (47.5) |
71 (43.3) 93 (56.7) |
36 (90) 4 (10) |
<.001 |
| Hematocrit (x & SD) | 41.8 (5.3) | 42 (5.1) | 40.7 (6.5) | 0.31 |
| Creatinine (x & SD) | 1.23 (1.8) | 1.25 (1.9) | 1.16 (0.4) | .007 |
| Alkaline phosphate (x & SD) | 90.4 (29.9) | 89.8 (30) | 92.7 (29.9) | 0.57 |
| Size mm (x y DS) | 49.6 (31.5) | 44.3 (29.5) | 71.7 (30.3) | <.001 |
| RENAL score (M & range) | 8 (4–12) | 8 (4–12) | 10 (4–12) | <.001 |
| Radius (%) ≤4 cm 4-7 cm ≥7 cm |
105 (50.2) 59 (28.2) 45 (21.5) |
101 (59.8) 45 (26.6) 23 (13.6) |
4 (10) 14 (35) 22 (55) |
<.001 |
| Exophytic (%) ≥50% <50% |
95 (45.5) 76 (36.4) |
78 (46.2) 64 (37.9) |
17 (42.5) 12 (30) |
0.22 |
| Endophytic 100% | 38 (18.2) | 27 (16) | 11 (27.5) | |
| Nearness to the collecting system or sinus (%) ≥7 mm 4–7mm ≤4 mm |
56 (26.8) 35 (16.7) 118 (56.5) |
52 (30.8) 30 (17.8) 87 (51.5) |
4 (10) 5 (12.5) 31 (77.5) |
0.008 |
| Location relative to polar lines (%) Does not cross polar line <50% crosses polar line >50% crosses polar line |
64 (30.6) 46 (22) 99 (47.4) |
56 (33.1) 36 (21.3) 77 (45.6) |
8 (20) 10 (25) 22 (55) |
0.27 |
| Suffix ‘h’ Does not touch main vessels Touches main vessels |
195 (93.3) 14 (6.7) |
163 (96.4) 6 (3.5) |
32 (80) 8 (20) |
<.001 |
*P Values in bold letters represent values under 0.05; M – median; x – average; SD – standard deviation
In the bivariate analysis, age, arterial hypertension, hematuria, lower serum creatinine, and tumor size were associated with locally advanced pathologic stage. pT3a group had higher RS [median of 10 vs. 8 points (p <0.001)]. Among RS variables, tumor size, nearness to renal sinus/collector system, and contact with the renal vessel, were statistically associated to locally advanced tumors (Table 1).
On multivariate analysis, age, radius and contact with the main vessels (suffix ‘h’) were independent predictors of locally advanced tumors (Table 2). Even though the RENAL score was a predictor of upstage as a whole, when the variables within the RS were analyzed separately, only the tumor size and the contact with the main vessels remained independently and significantly associated to pT3a upstage.
Table 2.
Logistic regression of predictors for tumor upstaging
| Variable | OR | CI 95% | P value |
|---|---|---|---|
| Age | 1.071 | 1.031–1.113 | <.001 |
| Suffix ‘h’ (touches main vessels) | 6.44 | 1.505–27.56 | .01 |
| Radius ≤4 cm 4–7 cm ≥7 cm |
5.89 21.72 |
1.75–19.82 6.472–72.902 |
.004 <.001 |
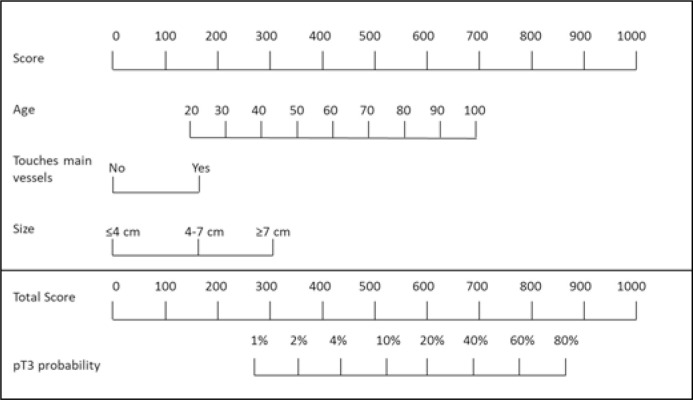
In our model, each year of increased age had a 7.1% increased risk of harboring locally advanced disease. Likewise, tumor size ≥7cm, showed a risk 21.7 times higher of being pT3a compared to tumors under ≤4 cm. Derived from this model a score and nomogram were confectioned (Figures 1 and 2).
Figure 1.
Formula to calculate pT3 predictive score.
Figure 2.
pT3 stage predictive nomogram.
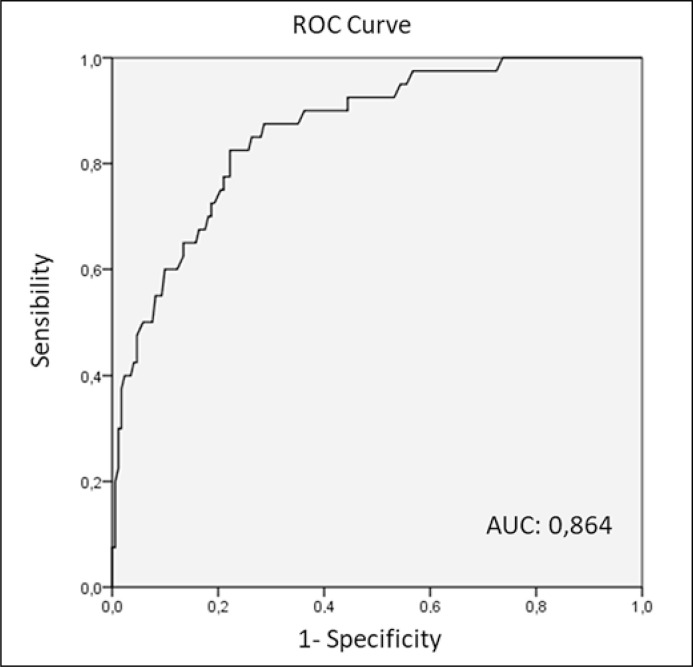
By applying this formula to our population, we obtained scores ranging from 214 to 943. The area under the ROC curve for predicting locally advanced stage using that formula was 0.864 (Figure 3). Using a cut-off point of 598, we achieved a sensitivity of 83% and a specificity of 78.1% for detecting pT3.
Figure 3.
Predictive model ROC curve.
The nomogram derived from this formula allows us to quickly asses the risk of pT3a (Figure 2).
DISCUSSION
A comparative analysis was conducted between the patients upstaged to pT3a and non-upstaged patients submitted to any kind of nephrectomy (radical/partial; open/laparoscopic) in an academic center. Traditionally, PN has been considered oncologically equivalent to RN in cT1 RCC [11] but recent literature suggest that this is not the case for upstaged patients [5–8]. We emphasize three different predictive variables that, put together in a model, may be useful to suspect the presence of a concealed pT3a with fair accuracy (AUC 0.864).
Our series shows a higher proportion of upstaged patients (19%) compared to other publications mainly because we included almost every nephrectomy for cancer, excluding only those metastatic or with clear renal vessels/other organ involvement (cT3b-c/cT4). As an academic center, we admit patients derived from less complex hospitals which contribute to this higher proportion for pT3a patients. The experiences that include only cT1 patients with partial nephrectomies show around 5% upstaging [3, 6, 7, 8].
The first evidence suggesting PN has a detrimental outcome in upstaged patients was a study with 1250 cT1 patients undergoing PN or RN (73% and 27%) which found pT3 upstaging in 11%. After 37 months of follow-up, 7% had a local recurrence. In patients with upstage to pT3, PN was associated with a mayor risk of recurrence compared to RN (HR: 5.39 p: 0.001) [5]. Choosing a partial nephrectomy for a pT3 tumor might not be innocuous. 10% for our upstaged patients (n = 40) underwent PN which might have put them on a higher risk of recurrence.
Age being associated to pT3 tumors is not a novel finding. Using de SEER registry, Kates et al. [12], studied 15,000 renal tumors ≤3 cm, searching for predictive factors of locally advanced or metastatic disease. 6.3% were locally advanced. This subgroup was 3.6 years older than the reference group. Each year granted a 3% extra risk for non-local disease. Beksac et al., using a national database, also found older age to be associated to pT3 upstage (OR: 1.04) (6). In our study each increasing year determined a 7.1% extra risk for locally advanced tumor (OR 1.07). This observation is concordant with other reports [13, 14].
Nowadays there is a trend to offer active surveillance to elderly patients (a median of 8 years older than the surgical population [15]). This fact could be exposing them to a higher oncologic risk. Age being related to a more advanced stage, could be partially explained by the fact that these are asymptomatic tumors and they have a slow rate of growth, close to 0.3 cm/year [16]. One could think that elderly patients may have tumors growing for longer periods of time, putting them at higher risk of pT3.
CT scan has limitations when defining T-stage making the prediction of pT3 a useful tool. Bradley [17] observed that in 29% out of 92 cases had an incorrect staging compared to the biopsy report. They looked for pT3 imaging predictors. Perinephric stranding and tumor necrosis were not reliable signs for pT stage >T3a, however, thickening of Gerota's fascia and the presence of collateral vessels in the perinephric fat had a positive predictive value of 82% and 88%, respectively. In counterpart, a study by two radiologists who analyzed 117 cases with 47% of pT3a, obtained a sensibility for compromise of sinus fat, perinephric fat, and renal vein around 71–88%, 68–83%, 59–69%, respectively. The inter-observer concordance was moderate (kappa: 0.41). They also looked for pT3 predictors. They found that irregularity of the tumor edge and direct tumor contact with perirenal fascia or sinus fat increased the odds of local invasion with an OR: 2.5–3.9 [18]. The important inter-observer variability made these factors not amenable for clinical use.
Many studied have associated the RENAL score with the risk of upstaging [8, 19]. When each variable of this score was analyzed separately, only tumor size, the nearness to the sinus fat/collecting system and the contact with the renal vessels remained statistically related to upstaging. However, in the multivariable analysis, the nearness was not an independent predictor. A possible explanation for this might be that the important factor is the hilar location of the tumor and not the fact that the tumor approaches the superior or inferior calyx. This observation is concordant with Gorin et al. who found that the only independently associated variables within the RENAL score were size and hilar location [3]. As the RENAL score is widely used in our clinical practice, calculating our formula might result to be an easy task.
The limitations of our study are inherent to the retrospective modality, depending on accuracy of clinical records. We could not assess the follow-up and progression rate which could be approached in future studies. Currently we are elaborating an accurate registry in our institution which will allow us to refine our model with a bigger sample. In the future, we will apply this model to an external cohort in order to calibrate and make an external validation.
CONCLUSIONS
Upstaging to pT3a is fairly common in clinically localized tumors (cT1–2). A formula that includes tumor size, age and contact with the main vessels on imaging can help predict it. This should be considered when deciding whether the patient is a good candidate for nephron-sparing surgery.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Campbell Steven, Uzzo Robert G, Allaf Mohamad E, et al. Renal Mass and Localized Renal Cancer: AUA Guideline. J Urol. 2017;198:520–529. doi: 10.1016/j.juro.2017.04.100. [DOI] [PubMed] [Google Scholar]
- 2.Mir MC, Derweesh I, Porpiglia F, Zargar H, Mottrie A, Autorino R. Partial Nephrectomy Versus Radical Nephrectomy for Clinical T1b and T2 Renal Tumors: A Systematic Review and Meta-analysis of Comparative Studies. Eur Urol. 2017;71:606–617. doi: 10.1016/j.eururo.2016.08.060. [DOI] [PubMed] [Google Scholar]
- 3.Gorin MA, Ball MW, Pierorazio PM, et al. Outcomes and predictors of clinical T1 to pathological T3a tumor up-staging after robotic partial nephrectomy: a multi-institutional analysis. J Urol. 2013;190:1907–1911. doi: 10.1016/j.juro.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Patard J-J, Shvarts O, Lam JS, et al. Safety and efficacy of partial nephrectomy for all T1 tumors based on an international multicenter experience. J Urol. 2004;171(6 Pt 1):2181–2185. doi: 10.1097/01.ju.0000124846.37299.5e. [DOI] [PubMed] [Google Scholar]
- 5.Shah PH, Moreira DM, Patel VR, et al. Partial Nephrectomy is Associated with Higher Risk of Relapse Compared with Radical Nephrectomy for Clinical Stage T1 Renal Cell Carcinoma Pathologically Up Staged to T3a. J Urol. 2017;198:289–296. doi: 10.1016/j.juro.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Beksac AT, Paulucci DJ, Gul Z, et al. Risk factors and prognostic implications for pathologic upstaging to T3a after partial nephrectomy. Minerva Urol E Nefrol Ital J UrolNephrol. 2019;71:395–405. doi: 10.23736/S0393-2249.18.03210-1. [DOI] [PubMed] [Google Scholar]
- 7.Russell CM, Lebastchi AH, Chipollini J, et al. Multi-institutional Survival Analysis of Incidental Pathologic T3a Upstaging in Clinical T1 Renal Cell Carcinoma Following Partial Nephrectomy. Urology. 2018;117:95–100. doi: 10.1016/j.urology.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Veccia A, Antonelli A, Minervini A, et al. Upstaging to pT3a disease in patients undergoing robotic partial nephrectomy for cT1 kidney cancer: Outcomes and predictors from a multi-institutional dataset. Urol Oncol. 2020;38:286–292. doi: 10.1016/j.urolonc.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 9.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844–853. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 10.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 11.Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors - is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181:55–61. doi: 10.1016/j.juro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kates M, Korets R, Sadeghi N, Pierorazio PM, McKiernan JM. Predictors of locally advanced and metastatic disease in patients with small renal masses. BJU Int. 2012;109:1463–1467. doi: 10.1111/j.1464-410X.2011.10553.x. [DOI] [PubMed] [Google Scholar]
- 13.Jeong S-H, Kim JK, Park J, et al. Pathological T3a Upstaging of Clinical T1 Renal Cell Carcinoma: Outcomes According to Surgical Technique and Predictors of Upstaging. PloS One. 2016;11:e0166183. doi: 10.1371/journal.pone.0166183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nayak JG, Patel P, Saarela O, et al. Pathological Upstaging of Clinical T1 to Pathological T3a Renal Cell Carcinoma: A Multi-institutional Analysis of Short-term Outcomes. Urology. 2016;94:154–160. doi: 10.1016/j.urology.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 15.Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma - a meta-analysis and review. J Urol. 2008;179:1227–1233. doi: 10.1016/j.juro.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 16.Mir MC, Capitanio U, Bertolo R, et al. Role of Active Surveillance for Localized Small Renal Masses. Eur Urol Oncol. 2018;1:177–187. doi: 10.1016/j.euo.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Bradley AJ, MacDonald L, Whiteside S, Johnson RJ, Ramani VAC. Accuracy of preoperative CT T staging of renal cell carcinoma: which features predict advanced stage? Clin Radiol. 2015;70:822–829. doi: 10.1016/j.crad.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Sokhi HK, Mok WY, Patel U. Stage T3a renal cell carcinoma: staging accuracy of CT for sinus fat, perinephric fat or renal vein invasion. Br J Radiol. 2015;88:20140504. doi: 10.1259/bjr.20140504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tay MHW, Thamboo TP, Wu FMW, et al. High R.E.N.A.L. Nephrometry scores are associated with pathologic upstaging of clinical T1 renal-cell carcinomas in radical nephrectomy specimens: implications for nephron-sparing surgery. J Endourol. 2014;28:1138–1142. doi: 10.1089/end.2014.0123. [DOI] [PubMed] [Google Scholar]