Abstract
Introduction
The study aimed to evaluate the outcomes of artificial urinary sphincter ZSI 375 implantation for stress urinary incontinence, focusing on quality of life assessment (QoL).
Material and methods
The study had a prospective and non-randomized design. It was conducted in two urological centres in Poland. Between July 2013 and June 2019, artificial urinary sphincter ZSI 375 was implanted in 86 consecutive men with stress urinary incontinence. The follow up was completed in December 2019. The assessment of functional results was based on number of pads used and declared to have been used by patients. The quality of life was assessed on the basis of the ICIQ-SF questionnaire (International Consultation on Incontinence Questionnaire-Short Form), SF-36 questionnaire (Short Form 36 Health Survey Questionnaire) and the severity of pain by means of the NRS (numerical rating scale of pain intensity).
Results
The operations were performed in 86 patients aged 28 to 80 (median 69). With the median (SD; range) follow-up of 21 (20.2; 1–68) months, daily pad usage decreased significantly from ≥4 to 1.1 (±0.97 pads) per day. Seven (8.1%) patients achieved total continence, 60 (69.8%) social continence, 14 (16.3%) improvement and 5 (5.8%) failures (≥4 pads per day). 15 patients (17.5%) experienced complications after surgery. The study showed a significant improvement of QoL evaluated by ICIQ-UI SF and SF-36.
Conclusions
Therapy with the use of ZSI 375 device is successfully applied in surgical management of moderate to severe male stress urinary incontinence. The life quality of patients assessed using questionnaires is at a high level.
Keywords: artificial urinary sphincter, male incontinence, quality of life, ZSI 375
INTRODUCTION
Urinary incontinence (UI) affects up to 39% of men and increases with age [1, 2, 3]. The most common cause of stress urinary incontinence (SUI) in adult men is iatrogenically induced insufficiency of the external urethral sphincter, resulting predominantly from the radical treatment of prostate cancer [1, 2, 3]. The frequency of UI in men undergoing radical prostatectomy ranges widely, from 5 to 48% [2, 3], depending on the surgeon's experience, how the problem is assessed, and how the definition of UI is interpreted [1, 2, 3]. Initial management of male SUI consists of pelvic floor muscle training with biofeedback. Should the conservative approach fail, surgical intervention becomes inevitable. Currently, there are several products available for operative treatment of male SUI including artificial urinary sphincter (AUS) AMS 800™ (Boston Scientific, Marlborough, USA) which is considered the gold standard in the treatment of moderate to severe SUI in men [1, 2, 3, 15-21]. ZSI 375 (Zephyr Surgical Implants, Geneva, Switzerland) is a more recent invention, with the first implantation completed in March 2009 [4–9]. The device itself is a one-piece AUS, which is designed to be inserted, with a two-part device composition (cuff and pump connected via kink-resistant tubing) to ease the ZSI implantation process. With a lack of abdominal reservoir, the risk of damage to either the bladder or to the bowel is minimised and reduces operating time [4–9]. There is a dearth of literature assessing the outcomes and safety of ZSI implantation as well as studies assessing patient quality of life (QoL) after ZSI implantation. This study evaluates the outcomes of ZSI implantation for SUI, focusing on QoL assessment.
MATERIAL AND METHODS
The patient population for this study was 86 consecutive male patients with iatrogenic SUI undergoing ZSI artificial urinary sphincter (AUS) implantation in two urological centres who were included into this prospective cohort study. The pre-implantation evaluation comprised of the patient’s history, analysis of voiding diaries (time and voided volumes, number of pads used daily, UI episodes), clinical examination, urethrocystoscopy, urodynamic assessment, ICIQ-SF questionnaire (International Consultation on Incontinence Questionnaire-Short Form), and SF-36 questionnaire (Short Form 36 Health Survey Questionnaire). All patients had sterile urine at the time of surgery and no bladder obstruction in the form of urethral stricture or vesico-urethral anastomosis. The lack of manual ability to manipulate the artificial sphincter pump was very important. All men who suffered from SUI following radical treatment of prostate cancer had a stable level of prostate-specific antigen (PSA) for the previous year prior to the ZSI implantation. Before the ZSI implantation, 78 patients (90.6%) used ≥4 pads per day (severe incontinence). Postoperative assessment of patients included recording postoperative complications, the number of pads used per day, post-void residual volume, ICIQ-UI SF, SF-36 questionnaires, and a numerical rating scale for pain (NRS). Patient evaluation was carried out during outpatient visits at 1, 3, 6, and 12 months and every 6 months after activation of the ZSI. The continence spectrum is defined based on number of pads used per day, as follows:
total continence = 0 pads per day
social continence = 0 to 1 pad per day
incontinence = more than 1 pad per day
light incontinence = 2 pads per day
moderate incontinence = 3 pads per day
severe incontinence = 4 or more pads per day.
Patients were considered ‘cured’ if they used no pads or used an occasional ‘security pad’, or considered ‘improved’ if they used both fewer than 2 pads per day and 50% fewer pads than at baseline. Otherwise, they were defined as ‘not improved’. Success was defined as finding the patient ‘cured’ and ‘improved’ following device activation. This study followed all applicable laws and regulations, good clinical practice, and ethical principles, as described in the Helsinki Declaration of 1975, and revised in Tokyo in 2008. The local Bioethical Committee approved the study protocol.
The device
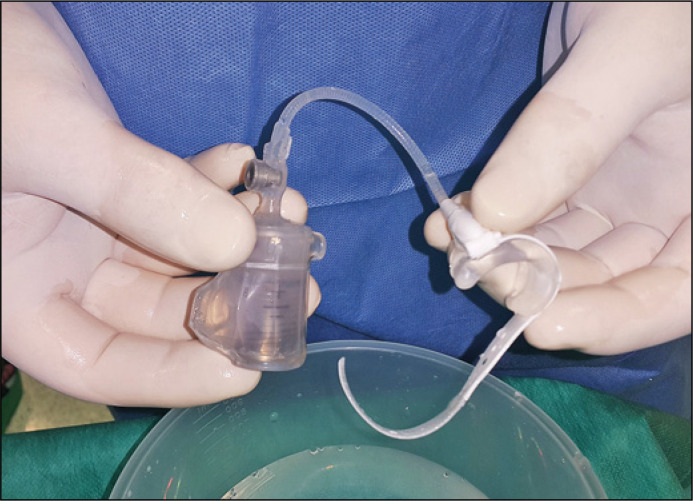
ZSI 375 is an AUS manufactured by Zephyr Surgical Implants (Geneva, Switzerland). It is a one-piece, two-part device made of medical silicone, equipped with an inflatable and adjustable cuff and a pressure-regulating tank (Figure 1). ZSI 375 has two circuits: a hydraulic circuit and a compensation pouch circuit separated by a piston. The patient presses the pump located in the scrotum to open the sphincter cuff surrounding the urethra. After micturition, the sphincter cuff automatically tightens around the urethra within 2–3 minutes.
Figure 1.
Artificial urinary sphincter ZSI 375.
Surgical technique
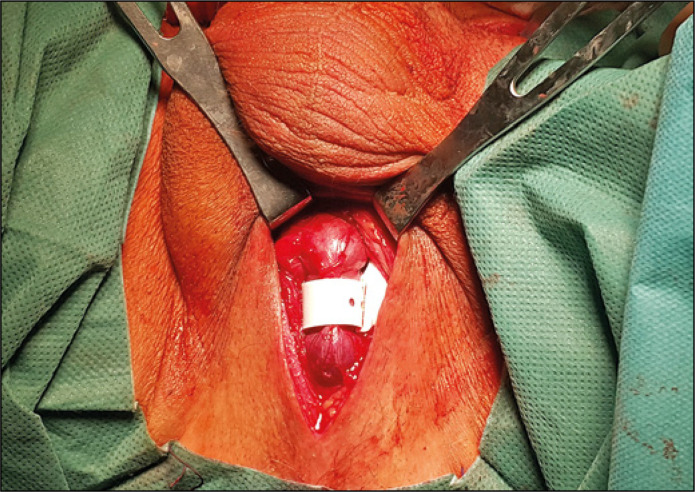
The surgical procedures were performed under general or regional anaesthesia, and a 16F Foley catheter was inserted for guidance. Patients were placed in the lithotomy position and a traditional surgical technique was used consisting of a perineal incision for cuff placement and inguinal incision for pump unit scrotal placement in all cases (Figure 2). A 12F Foley catheter was inserted at the final stage of the procedure and removed usually 24 hours afterwards. Eight weeks later the AUS was activated in an outpatient setting. A detailed description of the ZSI 375 artificial sphincter implantation procedure was presented in earlier publications [5, 7, 8].
Figure 2.
ZSI 375 sphincter cuff located around the urethra.
Questionnaires and quality of life assessment
ICIQ-UI SF is a questionnaire used to assess the frequency, severity, and impact on quality of life of men and women with UI in both clinical trials and daily practice [10]. ICIQ-UI SF is validated for the assessment of UI after surgery in both men and women with SUI (validation level: A). The ICIQ-UI SF questionnaire allows evaluation changes after conservative or interventional treatment. The questionnaire consists of 6 questions, 4 of which relate to UI, and the score is the sum of the point value from questions 3, 4, and 5, with a values range from 0 to 21 points. A higher value means worse incontinence-related QoL [10].
The SF-36 questionnaire is used for subjective health assessments [11, 12]. It consists of 11 questions containing a total of 36 statements to assess 8 indicators of QoL: physical functioning, limitations due to physical health, limitations due to emotional problems, vitality, emotional well-being, social functioning, pain, and general health. Each category is placed on a scale of 0 to 100, with higher values meaning less disturbance in health [11, 12].
Statistical analysis
The following methods were used in the statistical analysis: Spearman rank correlations, Friedman ANOVA and Kendall compliance coefficient, Mann-Whitney test, Kruskal-Wallis test, multivariate tables – chi-squared test with appropriate corrections. For statistical evaluations the following was used: TIBCO Software Inc. (2017), Statistica (data analysis software system), version 13. The level of statistical significance used for all analyses is alpha = 0.05 (it was assumed that the results are significant for the test probability value, P-value <0.05).
RESULTS
A total of 86 patients with a median age of 69 years (Q1 = 65.0; Q3 = 72.0; range: 28–80), had a ZSI 375 AUS device inserted due to iatrogenic SUI at two Polish urological centres between July 2013 and June 2019. Of these, 50 (58.1%) reported incontinence after radical prostatectomy (RP), 23 (26.7%) after RP and adjuvant radiotherapy, 7 (8.1%) after transurethral resection of the prostate (TURP), 2 (2.3%) after radical radiotherapy, and 4 (4.7%) were incontinent after other procedures (rectal surgery with urinary tract injury, high-intensity focused ultrasound) (Table 1). No patient included in the study had detrusor overactivity or urethral or vesico-urethral anastomotic narrowing. The median (Q1; Q3; range) period of incontinence was 36 months (Q1 = 24.0; Q3 = 72.0; 11–204), and 79 patients (91.6%) were incontinent >1 year before implantation. The follow-up finished in December 2019 and the median follow-up time was 21 months (Q1 = 8,0; Q3 = 40,0; range: 1–68). Among the total patient population, 78 patients (90.6 %) were diagnosed with severe incontinence and used ≥4 pads a day at baseline. The others (8 patients; 9.4%) suffered from moderate incontinence (Table 1). The mean operative time was 89 minutes (SD: 24.1 min; range 55–150 min).
Table 1.
Causes and degree of urinary incontinence
| Number of patients | Percentage | Confidence interval |
Percentage with 95% confidence interval | ||
|---|---|---|---|---|---|
| -95% CI | +95% CI | ||||
| Patients with the AUS ZSI 375 | 86 | ||||
| Causes of urinary incontinence | |||||
| RP | 50 | 58.1% | 47.0% | 68.7% | 58.1 (47.0–68.7)% |
| RP+RTH | 23 | 26.7% | 17.8% | 37.4% | 26.7 (17.8–37.4)% |
| RTH | 2 | 2.3% | 0.3% | 8.2% | 2.3 (0.3–8.2)% |
| TURP | 7 | 8.1% | 3.3% | 16.1% | 8.1 (3.3–16.1)% |
| Rect. surg. | 1 | 1.2% | 0.0% | 6.3% | 1.2 (0.0–6.3)% |
| HIFU | 1 | 1.2% | 0.0% | 6.3% | 1.2 (0.0–6.3)% |
| Injury | 2 | 2.3% | 0.3% | 8.2% | 2.3 (0.3–8.2)% |
| Number of pads used before implantation | |||||
| ≥4 | 78 | 90.6% | 82.3% | 95.9% | 90.6 (82.3–95.9)% |
| <4 | 8 | 9.4% | 4.2% | 17.7% | 9.4 (4.2–17.7)% |
AUS – artificial urinary sphincter; RP – radical prostatectomy; RTH – radiation therapy; TURP – transurethral resection of the prostate; HIFU – high–intensity focused ultrasound
At the median (Q1; Q3; range) follow-up of 21 months (Q1 = 8.0; Q3 = 40.0; 1–68), daily pad usage decreased significantly from ≥4 to 1.1 (±0.97 pads) per day at the last visit. Seven (8.1%) patients achieved total continence, 60 (69.8%) social continence, 14 (16.3%) improvement, and 5 (5.8%) failures (≥4 pads per day) (Table 2). According to postoperative reductions in the number of pads used per day, 67 patients (77.9%) were considered cured (social continence including total continence), and 14 patients (16.3%) had improved by the last visit. The ZSI system was considered successful (i.e. cured or improved) in 81 (94.2%) patients by reducing daily pad usage from ≥4 to 1.08 (±0.87) (Table 2). Analysis of the demographic and clinical variables in both successful and failure groups did not show statistically significant differences with regards to the mean age of the patients, preoperative pad usage, and duration of post-implantation follow-up period. No male experienced bladder overactivity, chronic urinary retention, scrotal discomfort caused by the pump size, or any other adverse effect following the sphincter activation. Fifteen patients (17.5%) experienced complications after surgery. There were no cases of infection reported, but in 11 (12.8%) cases urethral erosion was identified and it occurred on average at 13.5 months. Mechanical failure resulting in re-implantation of the ZSI occurred in 4 patients (4.7%) (Table 2).
Table 2.
Treatment results and complications
| Number of patients | Percentage | Confidence interval |
Percentage with 95% confidence interval | ||
|---|---|---|---|---|---|
| -95% CI | +95% CI | ||||
| Divisions of patients into a category of success and failure | |||||
| Success | 81 | 94.2% | 87.0% | 98.1% | 94.2 (87.0–98.1)% |
| Total continence | 7 | 8.1% | 3.3% | 16.1% | 8.1 (3.3–16.1)% |
| Social continence | 60 | 69.8% | 58.9% | 79.2% | 69.8 (58.9–79.2)% |
| Improvement | 14 | 16.3% | 9.2% | 25.8% | 16.3 (9.2–25.8)% |
| Failure | 5 | 5.8% | 1.9% | 13.0% | 5.8 (1.9–13.0)% |
| Complications | |||||
| Occurrence of complications | 15 | 17.5% | 10.1% | 27.1% | 17.5 (10.1–27.1)% |
| No complications | 71 | 82.5% | 72.9% | 89.9% | 82.5 (72.9–89.9)% |
| Division of complications | |||||
| Urethral erosion | 11 | 12.8% | 6.6% | 21.7% | 12.8 (6.6–21.7)% |
| Mechanical failure | 4 | 4.7% | 1.3% | 11.5% | 4.7 (1.3–11.5)% |
Quality of life assessments
ICIQ-UI SF questionnaire
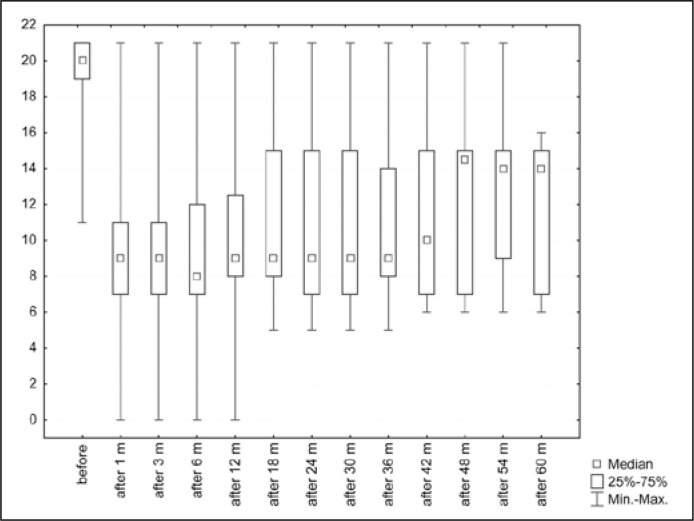
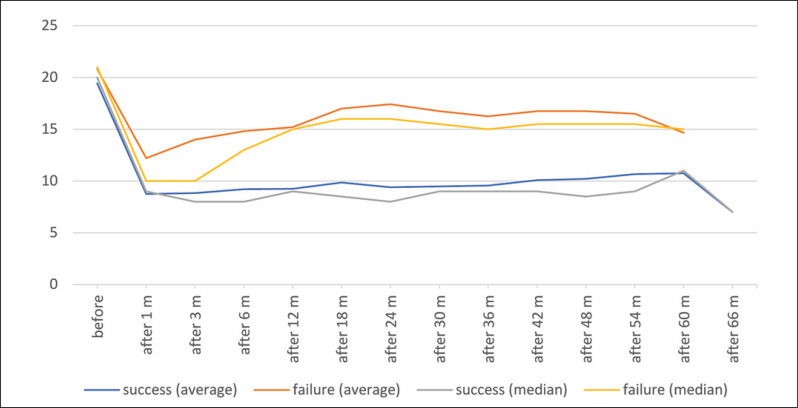
A higher point value (0–21) in the ICIQ-UI SF questionnaire means greater problems with UI and thus a lower QoL [10]. The analysis looked at the groups of patients divided into success or failure, and the group as a whole. In the group of all patients, the median point value before implantation of ZSI was 20.0 (average 19.5) (Table 3). A statistically significant difference (P <0.0001, Friedman ANOVA and Kendall compliance coefficient) was shown in the results of the questionnaire between the period before the implantation of ZSI and observation periods after the activation of the artificial sphincter, as shown in the Figure 3. When considering only the success group, a statistically significant difference (P <0.0001) was also shown from before and after implantation of the ZSI (Figure 4). However, this significance was not demonstrated in the failure group. The difficulty of this analysis was the very small (especially in the long term) number of observations in the failure group. A significant difference was shown between the success group and the failure group for almost all variables, except age, follow-up time at ‘1m’ and ‘54m’, and ‘after 60m’ (Figure 4). Figure 4 shows the average and median QoL (based on the ICIQ-UI SF questionnaire) at particular moments with a distinction between ‘success’ and ‘failure’ patients.
Table 3.
Results of the ICIQ-UI SF questionnaire (International Consultation on Incontinence Questionnaire-Short Form)
| Observation time | Results of the ICIQ-UI SF questionnaire (0–21) |
||||
|---|---|---|---|---|---|
| Average | Median | Minimum | Maximum | Standard deviation | |
| Before implantation | 19.5 | 20.0 | 11.0 | 21.0 | 1.7 |
| 1 month after activation | 9 | 9.0 | 0.0 | 21.0 | 3.8 |
| 3 months after activation | 9.2 | 9.0 | 0.0 | 21.0 | 3.5 |
| 6 months after activation | 9.6 | 8.0 | 0.0 | 21.0 | 3.8 |
| 12 months after activation | 9.8 | 9.0 | 0.0 | 21.0 | 3.6 |
| 18 months after activation | 10.7 | 9.0 | 5.0 | 21.0 | 3.9 |
| 24 months after activation | 10.6 | 9.0 | 5.0 | 21.0 | 4.2 |
| 30 months after activation | 10.6 | 9.0 | 5.0 | 21.0 | 4.2 |
| 36 months after activation | 10.8 | 9.0 | 5.0 | 21.0 | 4.1 |
| 42 months after activation | 11.5 | 10.0 | 6.0 | 21.0 | 4.3 |
| 48 months after activation | 12.1 | 14.5 | 6.0 | 21.0 | 4.7 |
| 54 months after activation | 12.5 | 14.0 | 6.0 | 21.0 | 4.6 |
| 60 months after activation | 11.8 | 14.0 | 6.0 | 16.0 | 4.1 |
| 66 months after activation | 7.0 | 7.0 | 7.0 | 7.0 | – |
ICIQ-UI SF – International Consultation on Incontinence Questionnaire-Short Form
Figure 3.
Results of the ICIQ-UI SF questionnaire before implantation of the ZSI 375 and after activation of the ZSI 375.
Figure 4.
Results of the ICIQ-UI SF questionnaire before implantation of the ZSI 375 and after activation of the ZSI 375. Division into a group of success and failure.
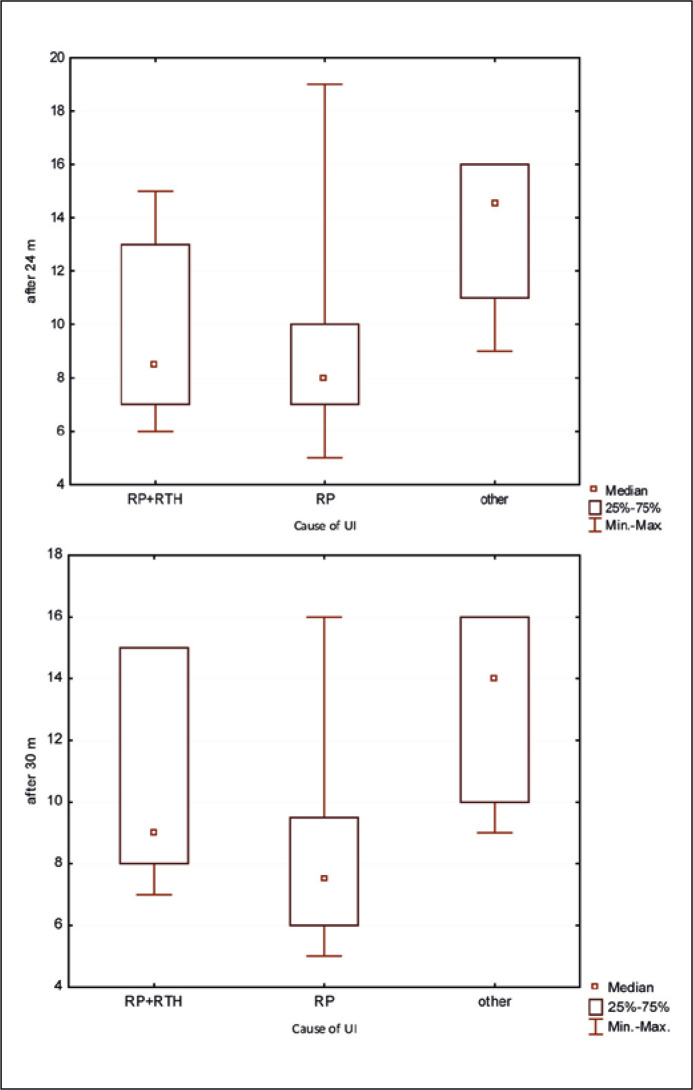
The authors also investigated whether the cause of iatrogenic UI influences the QoL of patients according to the ICIQ-UI SF questionnaire. Patients were divided into three groups: RP (50 – SUI after radical prostatectomy), RP + RTH (23 – SUI after radical prostatectomy and radiation therapy), other (13 – SUI for any other iatrogenic cause). A statistically significant difference (Kruskal-Wallis test) was found only after the 24 (P = 0.04) and 30 month (P = 0.03) observations between the RP and other groups. Better QoL (lower score) was demonstrated in the RP group (Figure 5).
Figure 5.
Results of the ICIQ-UI SF questionnaire in 24. and 30. months after activation of the ZSI 375. Division according to the cause of urinary incontinence.
SF-36
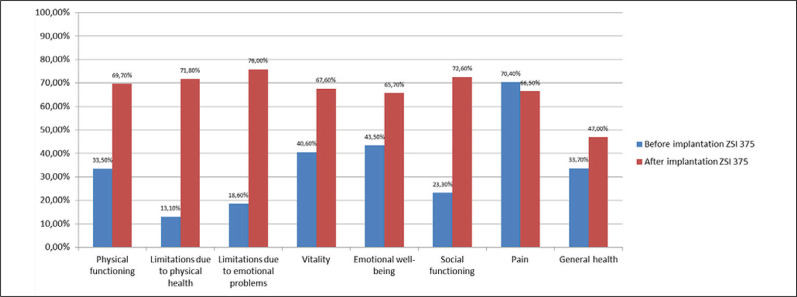
Based on the SF-36 questionnaire, an analysis of the differences between the results before and after implantation was made (3 months after activation). Significant improvement in patients' QoL was demonstrated (Table 4). Statistically significant differences (p <0.0001; Wilcoxon test, t test) before and after implantation in seven categories (physical functioning, limitations due to physical health, limitations due to emotional problems, vitality, emotional well-being, social functioning, general health), but not in the category for pain (p = 0.27) (Figure 6). There was no statistically significant difference before and after implantation in the ‘pain’ category when the groups were divided into ‘successes’ and ‘failures’. In terms of ‘physical functioning’, ‘social functioning’, and ‘general health’ statistically significant differences were shown in both the ‘success’ and ‘failure’ groups. In the remaining categories, statistical significance occurred only in the ‘success’ group (Figure 6).
Table 4.
Results of the SF-36 questionnaire (Short Form 36 Health Survey Questionnaire)
| Results of the SF-36 questionnaire |
|||||
|---|---|---|---|---|---|
| Average | Median | Minimum | Maximum | Standard deviation | |
| Physical functioning | |||||
| Before implantation | 33.5 | 30.0 | 0.0 | 80.0 | 21.6 |
| After implantation | 69.7 | 75.0 | 15.0 | 100.0 | 18.0 |
| Role limitations due to physical health | |||||
| Before implantation | 13.1 | 0.0 | 0.0 | 100.0 | 19.1 |
| After implantation | 71.8 | 75.0 | 0.0 | 100.0 | 29.3 |
| Role limitations due to emotional problems | |||||
| Before implantation | 18.6 | 0.0 | 0.0 | 100.0 | 23.8 |
| After implantation | 76.0 | 100.0 | 0.0 | 100.0 | 29.7 |
| Energy/fatigue | |||||
| Before implantation | 40.6 | 40 | 20.0 | 75.0 | 11.8 |
| After implantation | 67.6 | 65.0 | 30.0 | 100.0 | 15.7 |
| Emotional well-being | |||||
| Before implantation | 43.5 | 48.0 | 24.0 | 84.0 | 12.7 |
| After implantation | 65.7 | 68.0 | 32.0 | 88.0 | 13.0 |
| Social functioning | |||||
| Before implantation | 23.3 | 12.5 | 0.0 | 87.5 | 22.5 |
| After implantation | 72.6 | 75.0 | 22.5 | 100.0 | 19.2 |
| Pain | |||||
| Before implantation | 70.4 | 77.5 | 12.5 | 100.0 | 28.3 |
| After implantation | 66.5 | 67.5 | 10.0 | 100.0 | 29.2 |
| General health | |||||
| Before implantation | 33.7 | 32.5 | 20.0 | 55.0 | 10.0 |
| After implantation | 47.0 | 45.0 | 25.0 | 65.0 | 9.9 |
Figure 6.
Results of the SF-36 questionnaire.
Numerical rating scale of pain intensity
The degree of pain intensity was assessed with the use of the numerical rating scale (NRS, 0–10) as well as one of the elements of the SF-36 questionnaire (Figure 6). On the NRS scale, prior to implantation of an AUS, the mean score was 0.89 (SD = 0.80); after implantation, it increased slightly to 1.15 (SD = 0.96).
Discussion
In the current study, we report our mid-term experience in 86 patients with an implanted ZSI 375 device. During the median follow-up period of 21 months the overall success rate was 94.2%. Sixty-seven patients (77.9%) achieved total (n = 7; 8.1%) or social continence (n = 60; 69.8%), and 14 (16.3%) improved, leaving only 5 patients (5.8%) who failed with the treatment. The AUS AMS 800™ is currently regarded as the gold standard therapy of severe SUI in men. However, there are some serious problems connected with it, including complexity of the procedure, inability to adjust the pressure in the device, or to readjust the cuff in the case of postsurgical urethral atrophy [2, 17–21]. ZSI 375 is a relatively new device, with the first implantation carried out in 2009. To date, few studies have been published on the efficacy and safety of treatment of SUI in men by implantation of ZSI [4–8].
In our study, the mid-term complication rate was comparable to or better than that achieved with AMS 800™ [4, 12, 13, 14, 17–21]: the infection rate was nil in our series, and the infection rate for the AMS 800™ device is between 1 to 8% [17–21, 23, 28]. The complication most frequently reported in the present study was erosion, which affected 11 patients (12.8%). All urethral erosions occurred in patients post-prostatectomy in concordance with the aetiology of incontinence. Our urethral erosion rate is comparable to that of AMS 800™ [2, 3, 17–21]. Mechanical failure resulting in device re-implantation affected 4 patients (4.7%) at the early stage of this study. It likely reflects the inexperience of surgeons performing an implantation procedure, as there was no such complication observed after the first 4 cases performed by a given surgeon. The rate of mechanical failure of ZSI in our series is comparable to that of AMS 800™ in the contemporary series [2, 3, 17–21].
So far, no article has been published assessing the QoL of patients after implantation of ZSI. The QoL benefits of AUS implantation in patients with UI have been described in the literature, with most considering AMS devices as the gold standard AUS [1, 2, 3, 17–27]. In the current study, we report our mid-term experience in 86 patients with this ZSI device using ICIQ-UI SF questionnaire, SF-36 questionnaire, and NRS scale to assess QoL. The degree of UI is closely related to the QoL assessment. Our results are comparable to results obtained by Gnessin et al. who assessed the positive impact of AMS 800™ implantation on men’s QoL [28]. We compared the long-term efficacy and QoL of patients who underwent AUS placement and who completed the Incontinence Impact Questionnaire and the Urogenital Distress Inventory with our mid-term observations. The results are similar, and demonstrate the positive impact of the AUS on QoL [29]. Kaiho et al. also measured PPD use and the ICIQ-UI SF to estimate continence and QoL preoperatively and 1, 3, and 12 months postoperatively in 135 patients after AMS 800™ implantation [30]. Quality of life significantly improved after surgery, and this is similar to our results, but Kaiho et al. noted that 1 month after surgery the results were better than after 12 months. We did not reach such conclusions in our study. On the other hand, Imamoglu et al. [31] compared the scores of two groups of patients: after macroplastique injection and after AUS implantation, to assess the QoL score in the preoperative and in the postoperative period. We know that there were statistically significant differences between preoperative QoL scores, both in patients with minimal and total incontinence. In the group with minimal incontinence there was no statistically significant difference between macroplastique injection and AUS. However, the in group with total incontinence, AUS implantation offered better results. Fleshner [32] compared the health-related QoL and urinary symptoms in men with post-radical prostatectomy incontinence in 30 men with an AMS 800™ AUS, and 31 who did not require an AUS. No significant differences were noted with respect to QoL. Data confirmed that AMS 800™ and ZSI 375 are similar in terms of QoL. In a systematic review of the literature by Yafi et al. [33] looked at AUS for male SUI. Improvement in QoL indicators was reported in 90% of patients and all results confirmed improved QoL.
CONCLUSIONS
In conclusion, at the mid-term follow-up, the ZSI 375 AUS was successful in treating moderate and severe UI in men, achieving a high success rate and acceptably low complication rate. The QoL in study patients was assessed using ICIQ-UI SF and SF-36 questionnaires showed improvement. In our opinion, implantation of the ZSI 375 AUS is good option in the treatment of men with stress urinary incontinence.
CONFLICTS OF INTEREST
Ireneusz Ostrowski has once provided surgical support for Zephyr in Serbia.
References
- 1.Milson I, Altman D, Cartwright R, Lapitan MC, Nelson R, Sillen U, Tikkinen K. Epidemiology of urinary incontinence (UI) and other urinary tract symptoms (LUTS), pelvic prolapse (POP) and anal incontinence (AI) In: Abrams P, Cardozo L, Khoury S, Wein AJ, editors. Incontinence. Paris: 5th International Consultation on Incontinence; 2013. pp. 43–47. [Google Scholar]
- 2.Bauer RM, Gozzi C, Hübner W, et al. Contemporary management of postprostatectomy incontinence. Eur Urol. 2011;59:985–996. doi: 10.1016/j.eururo.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Herschorn S, Bruschini H, Comiter C, et al. Committee of the International Consultation on Incontinence. Surgical treatment of stress incontinence in men. Neurourol Urodyn. 2010;29:179–190. doi: 10.1002/nau.20844. [DOI] [PubMed] [Google Scholar]
- 4.Staerman F, G-Llorens C, Leon P, Leclerc Y. ZSI 375 artificial urinary sphincter for male urinary incontinence: a preliminary study. BJU Int. 2013;111(4 Pt B):E202–206. doi: 10.1111/j.1464-410X.2012.11468.x. [DOI] [PubMed] [Google Scholar]
- 5.Ostrowski I, Blewniewski M, Neugart F, et al. Multicentre experience with ZSI 375 artificial urinary sphincter for the treatment of stress urinary incontinence in men. Urologia. 2017;84:148–152. doi: 10.5301/uj.5000246. [DOI] [PubMed] [Google Scholar]
- 6.Llorens C, Pottek T. Urinary artificial sphincter ZSI 375 for treatment of stress urinary incontinence in men: 5 and 7 years follow-up report. Urologia. 2017;84:263–266. doi: 10.5301/uj.5000243. [DOI] [PubMed] [Google Scholar]
- 7.Ostrowski I, Ciechan J, Śledź E, et al. Four-year follow-up on a Zephyr Surgical Implants 375 artificial urinary sphincter for male urinary incontinence from one urological centre in Poland. Cent European J Urol. 2018;71:320–325. doi: 10.5173/ceju.2018.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostrowski I, Golabek T, Ciechan J, et al. Preliminary outcomes of the European multicentre experience with the ZSI 375 artificial urinary sphincter for treatment of stress urinary incontinence in men. Cent European J Urol. 2019;72:263–269. doi: 10.5173/ceju.2019.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vakalopoulos I, Kampantais S, Laskaridis L, Chachopoulos V, Koptsis M, Toutziaris C. New artificial urinary sphincter devices in the treatment of male iatrogenic incontinence. Adv Urol. 2012;2012:439372. doi: 10.1155/2012/439372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reis R.B, et al. Lack of association between the ICIQ-SF questionnaire and the urodynamic diagnosis in men with post radical prostatectomy incontinence. Acta Cir Bras. 2013;28(Suppl 1):37. doi: 10.1590/s0102-86502013001300008. [DOI] [PubMed] [Google Scholar]
- 11.Tylka J, Piotrowicz R. Quality of life SF-36 questionnaire – the Polish version. Kardiol Pol. 2009;67:1166–1169. [PubMed] [Google Scholar]
- 12.Jenkinson C, Coulter A, Wright L. Short form 36 (SF-36) health survey questionnaire: normative data for adults of working age. BMJ. 1993;306:1437–1440. doi: 10.1136/bmj.306.6890.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sousa-Escandón A, Cabrera J, Mantovani F, et al. Adjustable suburethral sling (male remeex system) in the treatment of male stress urinary incontinence: a multicentric European study. Eur Urol. 2007;52:1473–1479. doi: 10.1016/j.eururo.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Leruth J, Waltregny D, de Leval J. The inside-out transobturator male sling for the surgical treatment of stress urinary incontinence after radical prostatectomy: midterm results of a single-center prospective study. Eur Urol. 2012;61:608–615. doi: 10.1016/j.eururo.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 15.Kim SW, Walsh R, Berger Y, Kim JH. Male Readjustable Sling (MRS) System for Postprostatectomy Incontinence: Experiences of 2 Centers. Urology. 2015;88:195–200. doi: 10.1016/j.urology.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Martens FM1, Lampe MI, Heesakkers JP. ProACT for stress urinary incontinence after radical prostatectomy. Urol Int. 2009;82:394–398. doi: 10.1159/000218526. [DOI] [PubMed] [Google Scholar]
- 17.Hajivassiliou CA. A review of the complications and results of implantation of the AMS artificial urinary sphincter. Eur Urol. 1999;35:36–44. doi: 10.1159/000019817. [DOI] [PubMed] [Google Scholar]
- 18.Kim SP, Sarmast Z, Daignault S, Faerber GJ, McGuire EJ, Latini JM. Long-term durability and functional outcomes among patients with artificial urinary sphincters: a 10-year retrospective review from the University of Michigan. J Urol. 2008;179:1912–1916. doi: 10.1016/j.juro.2008.01.048. [DOI] [PubMed] [Google Scholar]
- 19.Elliott DS, Barrett DM. Mayo Clinic long-term analysis of the functional durability of the AMS 800 artificial urinary sphincter: a review of 323 cases. J Urol. 1998;159:1206–1208. [PubMed] [Google Scholar]
- 20.Clemens JQ, Schuster TG, Konnak JW, McGuire EJ, Faerber GJ. Revision rate after artificial urinary sphincter implantation for incontinence after radical prostatectomy: actuarial analysis. J Urol. 2001;166:1372–1375. [PubMed] [Google Scholar]
- 21.Lai HH, Hsu EI, Teh BS, Butler EB, Boone TB. 13 years of experience with artificial urinary sphincter implantation at Baylor College of Medicine. J Urol. 2007;177:1021–1025. doi: 10.1016/j.juro.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 22.Viers BR, Linder BJ, Rivera ME, Rangel LJ, Ziegelmann MJ, Elliott DS. Long-term quality of life and functional outcomes among primary and secondary artificial urinary sphincter implantations in men with stress urinary incontinence. J Urol. 2016;196:838–843. doi: 10.1016/j.juro.2016.03.076. [DOI] [PubMed] [Google Scholar]
- 23.Gousse AE, Madjar S, Lambert MM, Fishman IJ. Artificial urinary sphincter for post-radical prostatectomy urinary incontinence: long-term subjective results. J Urol. 2001;166:1755–1758. [PubMed] [Google Scholar]
- 24.Dalkin BL1, Wessells H, Cui H. A national survey of urinary and health related quality of life outcomes in men with an artificial urinary sphincter for post-radical prostatectomy incontinence. J Urol. 2003;169:237–239. doi: 10.1016/S0022-5347(05)64076-1. [DOI] [PubMed] [Google Scholar]
- 25.Wingate JT, Erickson BA, Murphy G, Smith TG, Breyer BN, Voelzke BB. Multicenter analysis of patient reported outcomes following artificial urinary sphincter placement for male stress urinary incontinence. J Urol. 2018;199:785–790. doi: 10.1016/j.juro.2017.09.089. [DOI] [PubMed] [Google Scholar]
- 26.ter Meulen PH, Zambon V, Kessels AG, van Kerrebroeck PE. Quality of life, functional outcome and durability of the AMS 800 artificial urinary sphincter in patients with intrinsic sphincter deficiency. Urol Int. 2003;71:55–60. doi: 10.1159/000071095. [DOI] [PubMed] [Google Scholar]
- 27.Bretterbauer KM, Huber ER, Remzi M, Huebner W. Telephone - delivered quality of life after 365 male stress urinary incontinence (SUI) operations. Int Braz J Urol. 2016;42:986–992. doi: 10.1590/S1677-5538.IBJU.2015.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gnessin E1, Livne PM, Baniel J, Gillon G. Continence and quality of life assessment after artificial urinary sphincter implantation. Isr Med Assoc J. 2004;6(10):592–594. [PubMed] [Google Scholar]
- 29.Haab F, Trockman BA, Zimmern PE, Leach GE. Quality of life and continence assessment of the artificial urinary sphincter in men with minimum 3.5 years of follow up. J Urol. 1997;158:435–439. [PubMed] [Google Scholar]
- 30.Kaiho Y, Masuda H, Takei M, et al. Surgical and patient reported outcomes of artificial urinary sphincter implantation: A multicenter, prospective, observational study. J Urol. 2018;199:245–250. doi: 10.1016/j.juro.2017.08.077. [DOI] [PubMed] [Google Scholar]
- 31.Imamoglu MA, Tuygun C, Bakirtas H, Yiğitbasi O, Kiper A. The comparison of artificial urinary sphincter implantation and endourethral macroplastique injection for the treatment of postprostatectomy incontinence. Eur Urol. 2005;47:209–213. doi: 10.1016/j.eururo.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 32.Fleshner N, Herschorn S. The artificial urinary sphincter for post-radical prostatectomy incontinence: impact on urinary symptoms and quality of life. J Urol. 1996;155:1260–1264. [PubMed] [Google Scholar]
- 33.Yafi FA, Powers MK, Zurawin J, Hellstrom WJ. Contemporary Review of Artificial Urinary Sphincters for Male Stress Urinary Incontinence. Sex Med Rev. 2016;4:157–166. doi: 10.1016/j.sxmr.2015.11.004. [DOI] [PubMed] [Google Scholar]