Abstract
Introduction
The aim of this study was to determine and quantify the mechanisms responsible for the delays in bladder cancer diagnosis and initial treatment.
Material and methods
Patients referred to two academic hospitals in Poland with a primary bladder tumor were prospectively identified and structurally interviewed. For all patients, time intervals between symptom onset, diagnostic and therapeutic interventions were assessed.
Results
A total of 144 patients diagnosed with bladder cancer were included in the analysis. The median time from symptom onset to treatment was 112 days. This comprised of the following median waiting times: 1) patient waiting time of 13 days, 2) assessment waiting time of 14 days and 3) treatment waiting time of 42 days. In the multivariate analysis, large city residence (OR 0.2, 95% CI 0.1–0.6) and comorbidity (OR 0.3, 95% CI 0.1–0.8) reduced the risk of delay, whereas medium-sized city residence (OR 1.4, 95% CI 0.4–5.1) and general practitioner as the first medical professional contact (OR 5.3, 95% CI 0.6–50.0) increased the risk of delay.
Conclusions
Diagnostic and treatment waiting times for bladder cancer in Poland are unsatisfactory. Potential solutions for shortening these delays include healthcare policy changes such as utilization of the oncological priority programs, primary care education and public health campaigns.
Keywords: bladder cancer, urinary bladder neoplasms, hematuria, diagnosis, quality of care
INTRODUCTION
Bladder cancer (BC) is a common malignancy occurring within the urinary tract and the 12th most common cancer. In a 2018 GLOBOCAN report, the incidence of BC in Eastern Europe was 16.1 and 3.2 new cases per 100 000 persons per year among men and women, respectively [1]. BC has typical symptoms, yet survival rate for patients with BC in Poland is significantly lower than in other European countries. In 2005–2008, the bladder cancer mortality rate in men in Poland was the highest in Europe, reaching 8.14 per 100 000 as opposed to an average of 5.52 per 100 000 in the European Union [2].
Increased delays in BC diagnosis correlate strongly with reduced survival. According to a retrospective analysis of 29 740 American patients with BC, those with increased delay between first episode of hematuria and the diagnosis of BC were at 34% higher risk of dying from the disease and each extra day of delay was associated with a 1% increase in risk of BC-related death [3]. Other studies addressing this topic have focused on the time from initial diagnosis of muscle-invasive tumors to radical cystectomy, and most have reported an association between longer delays and shorter survival [4–7]. Since the waiting time from establishing the indications for radical cystectomy to surgery for most cases in Poland is adequate and does not exceed 90 days as recommended in the European Association of Urology Muscle-invasive and Metastatic Bladder Cancer (MIBC) guidelines [8], worse outcome in BC treatment might be attributed to pre-treatment delays. Understanding the causes of these delays and identifying potential factors may improve outcomes among patients with BC.
The aim of this study was to determine and quantify the mechanisms responsible for the delays in the BC diagnosis and initial treatment.
MATERIAL AND METHODS
Patients referred to two academic hospitals in Poland in the period from November 2017 to October 2019 with primary bladder tumors were prospectively identified. Patients were interviewed using a 24-question structured survey. The interview concerned demographic data, symptoms on presentation, investigation, time intervals under study, patient knowledge and attitude. Additional data was collected from hospital charts. Basic study group characteristics are presented in Table 1. All participants in the study were included in the basic statistical analysis. Waiting times were calculated as follows: 1) from the onset of symptoms to the first medical consultation (patient waiting time); 2) from the first medical consultation to the first tumor-detecting imaging (assessment waiting time); 3) from the first tumor-detecting imaging to hospital admission for transurethral resection of bladder tumor (TURBT) (treatment waiting time) and 4) from the onset of symptoms to hospital admission for TURBT (total waiting time).
Table 1.
Basic characteristics of the study group
| Patient characteristics | ||
|---|---|---|
| Gender: | ||
| Female | 46 | 31.9% |
| Male | 98 | 68.1% |
| Age, years: | ||
| Mean | 66.5 | |
| <50 | 9 | 6.3% |
| 51-70 | 88 | 61.1% |
| >71 | 47 | 32.6% |
| Place of residence: | ||
| Town or village (<50,000 inhabitants) | 43 | 29.8% |
| Medium city (50-500,000 inhabitants) | 22 | 15.3% |
| Large city (>500,000 inhabitants) | 79 | 54.9% |
| Education: | ||
| Basic | 15 | 10.4% |
| Vocational | 37 | 25.7% |
| Secondary | 38 | 26.4% |
| Higher | 54 | 37.5% |
| Regularly visits a general practitioner: | ||
| Yes | 120 | 83.3% |
| No | 24 | 16.7% |
| Anticoagulant status*: | ||
| Yes | 51 | 35.4% |
| No | 93 | 64.6% |
| Private medical consultation in the course of diagnosis: | ||
| Yes | 69 | 47.9% |
| No | 75 | 52.1% |
| Comorbidity: | ||
| Hypertension | 60 | 41.7% |
| Diabetes | 21 | 14.6% |
| Kidney diseases | 14 | 9.7% |
| Other | 26 | 18.0% |
| None | 44 | 30.6% |
| Knew that hematuria can be a symptom of bladder cancer: | ||
| Yes | 36 | 25% |
| No | 108 | 75% |
| Evaluation of the diagnostic process: | ||
| Good | 98 | 68.0% |
| Bad | 25 | 17.4% |
| No opinion | 21 | 14.6% |
| Presentation, referral and diagnosis | ||
| First symptom: | ||
| Visible hematuria | 97 | 67.3% |
| Pain | 28 | 19.4% |
| Urinary frequency | 21 | 14.6% |
| Tumor found incidentally on imaging | 32 | 22.2% |
| Urinary retention | 2 | 1.4% |
| Microscopic hematuria | 8 | 5.6% |
| First medical professional contact: | ||
| Urologist | 32 | 22.2% |
| Emergency physician | 16 | 11.1% |
| General practitioner | 51 | 35.4% |
| Other | 8 | 5.6% |
| Missing | 37 | 25.7% |
| First technique visualizing the tumor: | ||
| Ultrasound | 123 | 85.4% |
| Computed tomography | 12 | 8.3% |
| Cystoscopy | 7 | 4.9% |
| MRI | 2 | 1.4% |
Considered as taking anticoagulant drugs at the onset of the first symptom MRI – magnetic resonance imaging
Continuous variables are presented as median or mean values accompanied by interquartile ranges (IQR). Baseline differences in waiting times were evaluated with Mann-Whitney U test. For identifying factors that predict a longer delay univariable and multivariable logistic regression was utilized. For all statistical analyses, a two-sided p value <0.05 was considered statistically significant. Statistical analyses were performed with the SAS System (version 9.4).
RESULTS
One hundred forty-four patients diagnosed with BC were suitable and agreed to participate in the study. The median total waiting time was 112 (IQR 51–238) days. This was comprised of patient waiting time of 13 (IQR 2–92) days, assessment waiting time of 14 (IQR 0–33) days and treatment waiting time of 42 (IQR 23–79) days. Table 2 presents reasons for prolonged patient waiting times as reported by patients.
Table 2.
Reasons for late presentation to a medical professional according to patients (number of patients)
| Underestimation of symptoms, lack of awareness (22/15.3%) |
| Antibiotic treatment / observation by a general practitioner (21/14.6%) |
| Waiting time for an appointment (7/4.9%) |
| Attributing symptoms to other diseases (4/2.8%) |
| Scared or anxious (4/2.8%) |
Open question answers as determined by the investigators based on interviews with the patients
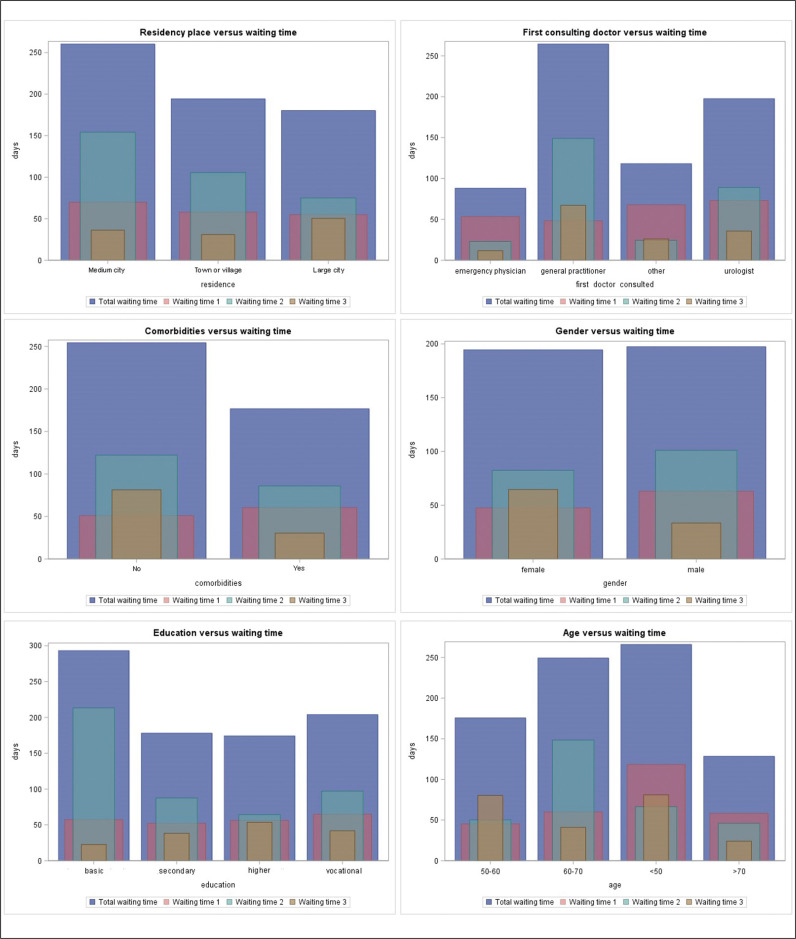
Waiting times adjusted to patient characteristics are shown in Figure 1. Table 3 presents results of univariate analyses of factors that predict longer waiting times. In the multivariate analysis, residence in a large city was significantly associated with a patient waiting time <30 days (OR 0.2, 95% CI 0.1–0.6), whereas residence in a medium city was associated with a patient waiting time >30 days (OR 1.4, 95% CI 0.4–5.1). Residence in a large city was also associated with a total waiting time <90 days (OR 0.4, 95% CI 0.1–1.0) and residence in a medium city predicted a total waiting time >90 days (OR 2.2, 95% CI 0.5–9.9). Presence of comorbidities (OR 0.3, 95% CI 0.1–0.8) as well as ultrasound as first technique visualizing the tumor (OR 0.1, 95% CI 0.01–0.4) were associated with an assessment waiting time <30 days. Simultaneously general practitioner (GP) as first medical professional contact predicted an assessment waiting time of >30 days (OR 5.3, 95% CI 0.6–50.0) (Table 4). Female sex has shown a trend towards a shorter treatment waiting time, with significantly lower risk of delay over 60 (OR 0.4, 95% CI 0.1–1.0) and 90 days (OR 0.3, 95% CI 0.1–1.0).
Figure 1.
Waiting times adjusted to patient characteristics.
Waiting time 1 – from the onset of symptoms to the first medical consultation; Waiting time 2 – from the first medical consultation to the first tumor-detecting imaging; Waiting time 3 – from the first tumor-detecting imaging to hospital admission for transurethral resection of bladder tumor (TURBT); Total waiting time – from the onset of symptoms to hospital admission for TURBT.
Table 3.
Univariate analyses of factors that predict longer waiting times
| Variables | Patient waiting time >30 days | Assessment waiting time >30 days | Treatment waiting time >30 days | Total waiting time >90 days | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Female sex | 1.0 (0.4–2.7) | 0.9959 | 0.9 (0.4–2.2) | 0.7993 | 0.3 (0.1–0.9) | 0.0251 | 0.9 (0.4–2.1) | 0.7869 |
| Age | 1.0 (0.97–1.05) | 0.6239 | 1.0 (0.9–1.0) | 0.1257 | 1.0 (0.9–1.0) | 0.9369 | 1.0 (0.9–1.0) | 0.1868 |
| Residence | ||||||||
| Large city | 0.4 (0.1–1.1) | 0.0023 | 0.7 (0.3–1.9) | 0.03082 | 0.6 (0.2–1.4) | 0.6632 | 0.3 (0.1–0.8) | 0.0011 |
| Medium city | 2.7 (0.7–10.0) | 0.0086 | 1.2 (0.4–4.0) | 0.4885 | 0.6 (0.2–2.3) | 0.7675 | 2.0 (0.5–9.2) | 0.0579 |
| Town or village | 1 | 1 | 1 | 1 | ||||
| Education | ||||||||
| Higher | 0.1 (0.04–0.8) | 0.3847 | 1.8 (0.3–9.9) | 0.8479 | 0.4 (0.1–1.5) | 0.3295 | 0.3 (0.1–1.5) | 0.1286 |
| Secondary | 0.1 (0.01–0.6) | 0.0699 | 2.7 (0.5–15.4) | 0.2463 | 0.4 (0.1–1.5) | 0.3149 | 0.5 (0.1–2.5) | 0.7829 |
| Vocational | 0.3 (0.06–1.1) | 0.8986 | 1.7 (0.3–9.4) | 0.9749 | 0.7 (0.2–2.5) | 0.6550 | 0.6 (0.1–2.8) | 0.8676 |
| Basic | 1 | 1 | 1 | |||||
| Regular visits to a GP | 0.4 (0.1–1.1) | 0.0792 | 1.0 (0.3–3.1) | 0.9958 | 0.6 (0.2–1.5) | 0.2569 | 0.9 (0.3–2.6) | 0.8167 |
| First technique visualizing the tumor: | ||||||||
| Ultrasound | 1.9 (0.4–9.1) | 0.4468 | 0.2 (0.1–0.7) | 0.0110 | 1.0 (0.3–3.4) | 0.9464 | 0.3 (0.1–1.3) | 0.1252 |
| CT/MRI | 0.4 (0.05–3.3) | 0.3860 | 2.6 (0.6–11.1) | 0.1962 | 1.2 (0.3–4.6) | 0.8144 | 2.8 (0.5–14.7) | 0.2216 |
| Cystoscopy | 1.0 (0.1–9.7) | 0.9715 | 10.9 (1.2–101.3) | 0.0364 | 0.6 (0.1–5.5) | 0.6757 | 2.7 (0.3–27.9) | 0.3983 |
| Private medical consultation in the course of diagnosis | 1.2 (0.5–3.0) | 0.6910 | 1.4 (0.6–3.2) | 0.4364 | 1.2 (0.5–2.8) | 0.6111 | 1.1 (0.5–2.5) | 0.7879 |
| First medical professional contact: | ||||||||
| Urologist | 3.0 (0.3–28.0) | 0.2666 | 1.3 (0.1–13.5) | 0.9241 | 1.0 (0.2–6.3) | 0.3918 | 2.2 (0.4–10.8) | 0.4742 |
| Emergency physician | 1.1 (0.1–14.1) | 0.4159 | 0.5 (0.02–9.2) | 0.2117 | 0.5 (0.1–4.1) | 0.4683 | 1.5 (0.3–8.4) | 0.7522 |
| GP | 3.4 (0.4–30.3) | 0.1469 | 6.0 (0.7–52.1) | 0.0012 | 0.6 (0.1–3.3) | 0.5676 | 2.5 (0.5–12.0) | 0.2448 |
| Other | 1 | 1 | 1 | |||||
| Presence of comorbidities | 1.0 (0.4–3) | 0.9205 | 0.4 (0.1–0.9) | 0.0241 | 1.6 (0.6–4.0) | 0.3593 | 1.2 (0.5–3.0) | 0.6830 |
| Knowledge that hematuria can be a symptom of BC | 0.3 (0.1–1.2) | 0.0980 | 0.7 (0.3–2.0) | 0.5042 | 1.0 (0.4–2.5) | 0.9237 | 0.5 (0.2–1.3) | 0.1796 |
BC – bladder cancer; CI – confidence interval; GP – general practitioner; OR – odds ratio
Table 4.
Multivariate analyses of factors that predict longer waiting times
| Variables | Patient waiting time >30 days | Assessment waiting time >30 days | Treatment waiting time >30 days | Total waiting time >90 days | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Female sex | 0.3 (0.1-1.0) | 0.0605 | ||||||
| Residence | ||||||||
| Large city | 0.2 (0.1–0.8) | 0.0007 | 0.4 (0.1–1.0) | 0.0028 | ||||
| Medium city | 3.4 (0.9–12.8) | 0.0019 | 2.2 (0.5–9.9) | 0.0614 | ||||
| Town or village | 1 | 1 | ||||||
| Regular visits to a GP | 0.1 (0.03–0.6) | 0.0063 | ||||||
| First technique visualizing the tumor: | ||||||||
| Ultrasound | 0.1 (0.01–0.4) | 0.0044 | ||||||
| CT/MRI | ||||||||
| Cystoscopy | ||||||||
| First medical professional contact: | ||||||||
| Urologist | 0.9 (0.1–9.9) | 0.7877 | ||||||
| Emergency physician | 0.2 (0.01–5.3) | 0.1052 | ||||||
| GP | 5.3 (0.6–50.0) | 0.0007 | ||||||
| Other | 1 | |||||||
| Presence of comorbidities | 0.3 (0.1-0.8) | 0.0171 | ||||||
CI – confidence interval; GP – general practitioner; OR – odds ratio
DISCUSSION
Despite its decreasing mortality in Europe over the last few decades, bladder cancer still causes significant morbidity and mortality, particularly in Poland [2]. The impact of increased delay from presentation to BC diagnosis (understood as TURBT) on reduced survival has been proven in several studies [3, 9]. While the waiting time from establishing the indications for radical cystectomy to surgery in Poland has already been assessed [8], the interval between first symptom and diagnosis has not been evaluated yet. This is the first Polish study that aimed at identifying factors that impact on the timeliness and adequacy of BC diagnosis and treatment. Our study identifies several factors influencing the risk of delay, as well as suggests potential areas for improvement.
The most common symptom of BC reported in the present study was visible hematuria, what is in agreement with the commonly cited value that the majority of patients with BC present with macroscopic hematuria [9]. However, other symptoms such as pain or urgency also occurred and should not be underestimated. It is especially important, as patients without hematuria typically have a longer time from onset of symptoms to diagnosis [10]. A surprisingly high 22.2% rate of tumors was found incidentally during imaging studies, a fact not evaluated before in contemporary studies. One reason for that may be low patient health awareness indicated in our study, which may lead to negligence of their symptoms or attributing them to other diseases. On the other hand, improper management and clinicians not asking questions about visible hematuria might also contribute to this number. The median total waiting time from onset of symptoms to BC diagnosis of 112 days improved compared to the 4.7 months reported in 1994-97 in our center [11] and is comparable to the 110 days presented by Wallace et al. [9] and longer than the 69.5 days reported by McCombie et al. [12]. There is still no consensus on the threshold of timely evaluation, referral and diagnosis [13], but concerningly 36% of patients in presented cohort experienced a significant delay of >90 days. The median total waiting time was much longer than the sum of the median constituent periods of time, which suggests that in most patients one or more of those waiting times was significantly prolonged.
Our study has underlined several factors that influence the risk of delay in BC diagnosis. Place of residence emerged as a significant factor, with patients from large cities having a shorter overall delay, as well as waiting shorter for first medical consultation and for first imaging study. Living in a large city was also associated with waiting time to first consultation of <30 days and total waiting time of <90 days. The relationship between place of residence and delay is complex, probably influenced by healthcare-related, as well as psychosociological factors. Similarly to our data, in a Polish study of 1373 cancer patients, those from bigger cities waited significantly shorter from suspicion to diagnosis than patients living in smaller cities [14].
The specialty of the first medical professional contact also seems to be a predictor for diagnostic delay. Patients who were first consulted by a GP had longer waiting time to imaging, waiting time to admission and total waiting time and were more likely to experience delay to imaging of >30 days. That fact may be due to lack of GP’s knowledge on the appropriate evaluation of patients with hematuria and other symptoms suggesting BC, as well as difficulties in access to urology specialists in Poland. Two North American studies have shown a reluctance amongst primary care physicians to refer patients with hematuria to urology for further investigation [15, 16]. Other studies underlined, that patients experienced shorter diagnostic intervals if they first presented to a urologist compared to other specialties. In a study by Garg et al. initial visit with a urologist was associated with reduced risk of delayed evaluation compared to primary care physician and gynecologist [17]. What is more, the present study showed several cases of hematuria being treated with antibiotics or observed by a GP. Proper education of primary care physicians about the significance of visible hematuria should be encouraged. Clinicians should include hematuria in their routine review of systems and refer for further urologic evaluation in all adults with visible hematuria [18].
Comorbidity presents a challenge in clinical practice, when a presenting symptom may be caused by cancer or concomitant benign disease. In our series, comorbidity was associated with a shorter waiting time to first consultation. Comorbidity is often associated with regular medical check-ups and such patients may have a higher chance of a proper evaluation of symptoms, quick referral and avoiding unnecessary investigations. Surprisingly, private medical consultation did not reduce the risk of a prolonged delay.
The relationship between gender and BC is complex, and is probably influenced by both biologic and epidemiologic factors [19]. In our study female sex has shown a trend towards a shorter waiting time to admission. However, large studies have underlined female sex as a significant risk factor in total waiting time, with reported delays of 73.6 vs. 85.4 days by Cohn et al. [20] and 58.9 vs. 72.2 days by Richards et al. [21] in men and women respectively. Moreover, in a study by Garg et al. women were more likely to undergo delayed (>30 days) hematuria evaluation [17]. Age also seems to be an important factor in prolonged delay, as found in two large US studies, where older bladder cancer patients with hematuria had longer delays to evaluation than younger patients [3, 17].
As longer delays in BC diagnosis correlate with reduced patient survival [3], various strategies that could lead to a reduction in pre-treatment delays should be discussed. Bladder cancer awareness among Polish patients remains relatively poor, as only 25% of patients in our series knew that hematuria could be a symptom of BC. That seems to be an international issue as shown in an Australian study, in which only 43% of patients with a bladder tumor knew hematuria may be due to BC [12]. Public health campaigns, such as establishing May as Bladder Cancer Awareness Month, Urology Week or the Be Clear on Cancer ‘blood in pee’ campaign, can lead to an improvement in this field. However the impact of such campaigns should be approached with caution, as an evaluation of the Be Clear on Cancer ‘blood in pee’ campaign from the UK indicated, that it significantly increased the number of new suspected cancer referrals, but showed no significant change in the diagnosis of target cancers [22].
Local actions by insurers, healthcare managers and scientific societies leading to the creation of models of comprehensive specialist care for patients with BC can lead to an improvement in the healthcare-related causes of pre-treatment delays. Examples of such policy changes in other countries are the introduction of ‘one-stop’ hematuria clinics in Australia [23] or the ‘2-week wait rule’ in the UK, which has successfully reduced the time from referral to first consultation with a specialist from 42.9 to 21.3 days [24]. In Sweden, the introduction of Red Phone initiative, a telephone hotline for patients with visible hematuria, reduced the time from hematuria to diagnosis from 50 to 29 days [25]. In the same country, the implementation of standardized care pathways had an effect on the lead time from hematuria to BC diagnosis, which was shortened by 10 days, but has not decreased times to treatment [26]. In Poland, the introduction of the ‘Diagnosis and Treatment of Cancer’ (DILO) program in 2015 intended to reduce waiting times for cancer patients. The statutory maximum time from suspicion to diagnosis is 28 days, while the time between diagnosis and starting treatment should not exceed 14 days [27]. Nevertheless, introduction of the oncological reform until now has not been proven to significantly shorten the waiting times in cancer patients for receiving oncological treatment [28]. As seen in our study, the median waiting time from imaging to TURBT of 42 days does not meet the assumptions of the DILO program. That said, this period of time was the largest constituent of the total waiting time and it seems, that quick hospitalization of patients with suspicion of BC would be the most effective way of shortening the total delay.
Our study is not free of limitations. Despite the prospective character of the study, which aimed at minimizing the recall bias, it was still present in some patients who had to recall events that took place sometimes more than a year before. Secondly, the study does not cover the whole population of patients diagnosed with bladder cancer in Poland. Just in 2016 over 5400 cases of bladder cancer were reported to the Polish National Cancer Registry [29]. However, this number covers primary, as well as recurrent tumors, while the exact number of new cases is unfortunately not known. The relatively small number of the study group may limit the power of obtained results. This study also failed to evaluate the relationship between the delay in the diagnosis and initial treatment of bladder cancer and cancer stage or survival.
CONCLUSIONS
Waiting times to diagnosis and treatment of bladder cancer in Poland are unsatisfactory. Potential solutions to shorten these delays should be sought in order to improve outcome for patients. Steps towards meeting the assumptions of the Polish DILO program should be taken, prioritizing care for patients with hematuria and other symptoms suspicious of bladder cancer, especially those from smaller cities. Special attention should be given towards GP education in that matter. The use of public health campaigns, with regard to their limitations, may lead to the increase of the currently low bladder cancer awareness of patients.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Bosetti C, Bertuccio P, Chatenoud L, Negri E, La Vecchia C, Levi F. Trends in Mortality From Urologic Cancers in Europe, 1970-2008. Eur Urol. 2011;60:1–15. doi: 10.1016/j.eururo.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 3.Hollenbeck BK, Dunn RL, Ye Z, et al. Delays in diagnosis and bladder cancer mortality. Cancer. 2010;116:5235–5242. doi: 10.1002/cncr.25310. [DOI] [PubMed] [Google Scholar]
- 4.Lee CT, Madii R, Daignault S, et al. Cystectomy delay more than 3 months from initial bladder cancer diagnosis results in decreased disease specific and overall survival. J Urol. 2006;175:1262–1267. doi: 10.1016/S0022-5347(05)00644-0. [DOI] [PubMed] [Google Scholar]
- 5.Kulkarni GS, Urbach DR, Austin PC, Fleshner NE, Laupacis A. Longer wait times increase overall mortality in patients with bladder cancer. J Urol. 2009;182:1318–1324. doi: 10.1016/j.juro.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 6.Gore JL, Lai J, Setodji CM, Litwin MS, Saigal CS, Urologic Diseases in America P Mortality increases when radical cystectomy is delayed more than 12 weeks: results from a Surveillance, Epidemiology, and End Results-Medicare analysis. Cancer. 2009;115:988–996. doi: 10.1002/cncr.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahmy NM, Mahmud S, Aprikian AG. Delay in the surgical treatment of bladder cancer and survival: systematic review of the literature. Eur Urol. 2006;50:1176–1182. doi: 10.1016/j.eururo.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 8.Poletajew S, Lisiński J, Moskal K, et al. The time from diagnosis of bladder cancer to radical cystectomy in Polish urological centres - results of CysTiming Poland study. Cent European J Urol. 2014;67:329–332. doi: 10.5173/ceju.2014.04.art2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace DMA, Bryan RT, Dunn JA, Begum G, Bathers S, West Midlands Urological Research G Delay and survival in bladder cancer. BJU Int. 2002;89:868–878. doi: 10.1046/j.1464-410x.2002.02776.x. [DOI] [PubMed] [Google Scholar]
- 10.Månsson A, Anderson H, Colleen S. Time lag to diagnosis of bladder cancer--influence of psychosocial parameters and level of health-care provision. Scand J Urol Nephrol. 1993;27:363–369. doi: 10.3109/00365599309180448. [DOI] [PubMed] [Google Scholar]
- 11.Bęc L, Dybowski B, Marcheluk A, Piotrowicz G. Pierwotne guzy pęcherza moczowego - analiza kliniczna. Urol Pol. 1997;50(2A):18–19. [Google Scholar]
- 12.McCombie SP, Bangash HK, Kuan M, Thyer I, Lee F, Hayne D. Delays in the diagnosis and initial treatment of bladder cancer in Western Australia. BJU Int. 2017;120:28–34. doi: 10.1111/bju.13939. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y, Melle Mv, Singh H, Hamilton W, Lyratzopoulos G, Walter FM. Quality of the diagnostic process in patients presenting with symptoms suggestive of bladder or kidney cancer: a systematic review. BMJ Open. 2019;9:e029143. doi: 10.1136/bmjopen-2019-029143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osowiecka K, Rucinska M, Nowakowski JJ, Nawrocki S. How Long Are Cancer Patients Waiting for Oncological Therapy in Poland? Int J Environ Res Public Health. 2018;15:577. doi: 10.3390/ijerph15040577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yafi FA, Aprikian AG, Tanguay S, Kassouf W. Patients with microscopic and gross hematuria: practice and referral patterns among primary care physicians in a universal health care system. Can Urol Assoc J. 2011;5:97–101. doi: 10.5489/cuaj.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nieder AM, Lotan Y, Nuss GR, Langston JP, Vyas S, Manoharan M, et al. Are patients with hematuria appropriately referred to Urology? A multi-institutional questionnaire based survey. Urol Oncol. 2010;28:500–503. doi: 10.1016/j.urolonc.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Garg T, Pinheiro Laura C, Atoria Coral L, et al. Gender Disparities in Hematuria Evaluation and Bladder Cancer Diagnosis: A Population Based Analysis. J Urol. 2014;192:1072–1077. doi: 10.1016/j.juro.2014.04.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen M, Qaseem A, High Value Care Task Force of the American College of P Hematuria as a Marker of Occult Urinary Tract Cancer: Advice for High-Value Care From the American College of Physicians. Ann Intern Med. 2016;164:488–497. doi: 10.7326/M15-1496. [DOI] [PubMed] [Google Scholar]
- 19.Dobruch J, Daneshmand S, Fisch M, et al. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur Urol. 2016;69:300–310. doi: 10.1016/j.eururo.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 20.Cohn JA, Vekhter B, Lyttle C, Steinberg GD, Large MC. Sex disparities in diagnosis of bladder cancer after initial presentation with hematuria: A nationwide claims-based investigation. Cancer. 2014;120:555–561. doi: 10.1002/cncr.28416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richards KA, Ham S, Cohn JA, Steinberg GD. Urinary tract infection-like symptom is associated with worse bladder cancer outcomes in the Medicare population: Implications for sex disparities. Int J Urol. 2016;23:42–47. doi: 10.1111/iju.12959. [DOI] [PubMed] [Google Scholar]
- 22.Hughes-Hallett A, Browne D, Mensah E, Vale J, Mayer E. Assessing the impact of mass media public health campaigns. Be Clear on Cancer ‘blood in pee’: a case in point. BJU Int. 2016;117:570–575. doi: 10.1111/bju.13205. [DOI] [PubMed] [Google Scholar]
- 23.Ooi WL, Lee F, Wallace DMA, Hayne D. 'One stop' haematuria clinic in Fremantle Hospital, Western Australia: a report of the first 500 patients. BJU Int. 2011;108(Suppl 2):62–66. doi: 10.1111/j.1464-410X.2011.10711.x. [DOI] [PubMed] [Google Scholar]
- 24.Blick C, Bailey D, Haldar N, Bdesha A, Kelleher J, Muneer A. The impact of the two-week wait rule on the diagnosis and management of bladder cancer in a single UK institution. Ann R Coll Surg Engl. 2010;92:46–50. doi: 10.1308/003588410X12518836440207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liedberg F, Gerdtham U, Gralén K, et al. Fast-track access to urologic care for patients with macroscopic haematuria is efficient and cost-effective: results from a prospective intervention study. Br J Cancer. 2016;115:770–775. doi: 10.1038/bjc.2016.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nilbert M, Bläckberg M, Ceberg J, Hagberg O, Stenhoff R, Liedberg F. Diagnostic pathway efficacy for urinary tract cancer: population-based outcome of standardized evaluation for macroscopic haematuria. Scand J Urol. 2018;52:237–243. doi: 10.1080/21681805.2018.1498124. [DOI] [PubMed] [Google Scholar]
- 27. http://www.nfz.gov.pl/dla-swiadczeniodawcy/pakiet-onkologiczny/.
- 28.Osowiecka K, Rucińska M, Nawrocki S. Have actual waiting times been reduced by introducing the DILO reform for cancer patients in Poland? Nowotwory. 2017;67:168–173. [Google Scholar]
- 29.Wojciechowska U, Czaderny K, Ciuba A, Olasek P, Didkowska J. Cancer in Poland in 2016. M. Skłodowska-Curie Memorial Cancer Center and Institute of Oncology. Warsaw: 2018. [Google Scholar]