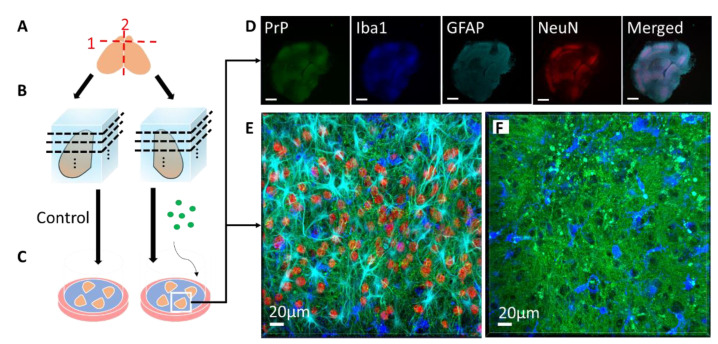
Figure 1.
Schematic of coronal slice culture preparation, infection, and confocal imaging. (A) The brain is removed from 8- or 9-day-old mouse pups, the olfactory bulbs are removed, and the brain is hemisectioned sagitally. (B) The hemispheres are embedded in low-melting-point agarose gel and sliced into 275 µm-thick coronal sections with a vibratome. About 40 slices anterior-posterior are obtained from each mouse, with 4 slices cultured per well. Each well is considered a “region”, from 1 (anterior) to 10 (posterior), each representing 1100 µm. (C) The slices are then placed on Milicell cell culture inserts with the culture media below the membrane and can be cultured for as long as three months. During the culture period, genetically identical, location-matched slices can be used as controls or subjected to different treatment conditions in the absence of the blood–brain barrier, such as infection with different prion strains or treatment with different drugs. At various time points, the slices can be immunostained for confocal imaging. (D) Low magnification confocal image of an RML-infected slice that was cultured for 56 days post-infection. PrP (Saf83) is shown in green, microglia (Iba1) are shown in blue, reactive astrocytes (GFAP) are shown in cyan, neuronal nuclei (NeuN) are shown in red. As seen in the NeuN channel, the boundaries of distinct brain regions are discernable (scale = 500 µm). (E) High magnification confocal image of a neocortical region from the RML-infected slice in E, showing the array of cell types and architecture. (F) High magnification confocal image of an RML-infected slice showing PrP aggregates (green) and activated microglia (blue).