Abstract
The aim of this study was to assess the annualized changes in body composition, including skeletal muscle, subcutaneous adipose tissue (SAT), and visceral adipose tissue (VAT) before, during, and after sorafenib treatment in patients with hepatocellular carcinoma (HCC). This retrospective study evaluated 61 HCC patients treated with sorafenib. Annualized changes (Δ; cm2/m2/year) in skeletal muscle index (SMI), SAT index (SATI), and VAT index (VATI), which were defined as the cross-sectional areas (cm2) of those areas on computed tomography normalized by the square of one’s height (m2), before (pre), during (during), and after (post) sorafenib treatment, were calculated. Patients within the 20th percentile cutoffs for these indices were classified into the rapid depletion group and the effects of these values on survival were analyzed using the Kaplan-Meier analysis and Cox proportional-hazards model. Annualized depletion rates of SMI (ΔSMIpre: −3.5, ΔSMIduring: −3.5, ΔSMIpost: −8.0) and VATI (ΔVATIpre: −3.2, ΔVATIduring: −2.8, ΔVATIpost: −15.1) accelerated after the cancellation of sorafenib, whereas that of SATI (ΔSATIpre: −4.8, ΔSATIduring; −7.6, ΔSATIpost; −8.0) had already accelerated during sorafenib treatment. Patients with rapid depletion of ΔSATIduring experienced significantly worse survival rates (p < 0.001), and it was an independent predictor of survival (p = 0.009), together with therapeutic effect (p < 0.001). Rapid depletion of SAT during sorafenib treatment can be used to predict survival in patients with HCC.
Keywords: body composition, hepatocellular carcinoma, sorafenib, prognostic factor, skeletal muscle, subcutaneous fat mass
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common global malignancies; more than half a million people are diagnosed with HCC annually [1]. The prognosis of HCC is notably poor due to difficulties in detecting this malignancy at an early stage [2,3]. Recently, oral molecular targeted drugs have played an important role in the treatment of advanced HCC [4,5]. Sorafenib is the first orally active multi-kinase inhibitor that has been confirmed to be efficacious against advanced HCC [6]. Therefore, sorafenib is now widely used for the treatment of patients with advanced HCC [4,5]; however, several adverse events, including hand–foot syndrome (HFS), fatigue, weight loss, and gastrointestinal events, such as anorexia, diarrhea, nausea, and vomiting often occur when using this agent [6]. Once these adverse gastrointestinal tract events occur, malnutrition can result, causing reductions in body weight and unfavorable changes in body composition, such as sarcopenia, depletion of adipose tissues, and cachexia.
Elevations of energy expenditure and catabolism due to tumor presence and tumor–host interactions are considered to be factors contributing to cancer-associated cachexia in several types of malignancies, including HCC [7]. Patients with advanced cancers frequently show losses in total body weight, skeletal muscle, and adipose tissue [8,9,10]. Therefore, it is easy to imagine that advanced HCC patients treated with sorafenib could be susceptible to decreases in skeletal muscle and adipose tissues, due to both enlarged tumor burden and decreased food intake, caused by digestive-system-related adverse events of the agent.
Several prognostic factors, such as clinical cancer stage, extrahepatic metastasis including bone lesions, and liver functional reserve, are reported in patients with HCC [11,12,13]. Recently, unfavorable changes in body composition, such as sarcopenia and cachexia, have attracted great attention as new prognostic factors for various malignancies, including HCC. For example, skeletal muscle depletion assessed by computed tomography (CT) can predict a poor prognosis in all cancer stages [14] and in sorafenib-treated patients with HCC [15]. Rapid depletions of skeletal muscle mass and subcutaneous adipose tissue (SAT) also predict worse survival rates in patients with HCC treated with sorafenib [16]. Moreover, sarcopenia and rapid skeletal muscle wasting are associated with worse survival rates in patients with liver cirrhosis, who are more susceptible to HCC [17,18].
Because sarcopenia and rapid depletions of skeletal muscle mass and SAT are involved in poor survival rates of HCC patients [14,15,16,17,18], correct assessment of body composition and nutritional therapy based on that assessment are required to improve the prognosis of such patients. However, there has been no study that evaluates changes in body composition in HCC patients treated with sorafenib from the initial stages to death using sequential CT images. The purpose of this study was to compare the annualized changes in body composition, including skeletal muscle, SAT, and visceral adipose tissue (VAT) before, during, and after sorafenib treatment in patients with advanced HCC. Impacts of those annualized changes on survival rates in sorafenib-treated HCC patients were also evaluated.
2. Results
2.1. Baseline Characteristics and Laboratory Data of HCC Patients Treated with Sorafenib
The baseline characteristics and laboratory data of 61 patients (53 men and 8 women, average age 67.3 years) just before the introduction of sorafenib are shown in Table 1. All patients had good liver functional reserves (Child–Pugh score 5 or 6) and advanced stage HCC (cancer stage more than III), according to the Clinical Practice Guidelines for HCC issued by the Japan Society of Hepatology (JSH) [19]. The therapeutic effect of complete response (CR), partial response (PR), stable disease (SD), and progression disease (PD) was 3, 5, 19, and 34 cases, respectively. Sorafenib was discontinued because of PD (n = 22); severe adverse events, including HFS (n = 4); general fatigue (n = 4), ascites (n = 4); liver dysfunction (n = 4); appetite loss (n = 4); and other reasons. The median duration of sorafenib treatment was 15.7 months (95% confidence interval (CI) 12.3–19.0 months), and the median survival time after sorafenib introduction was 19.8 months (95% CI 15.1–26.1 months) and the 1-, 2-, and 3-year overall survival rates were 68.2%, 38.3%, and 17.2%, respectively. Female patients were significantly younger (p = 0.022), had more SATI (p < 0.001), and less hepatitis C virus (HCV)-related HCC (p = 0.042) when compared to male patients.
Table 1.
Baseline demographic and clinical characteristics of the enrolled patients just before the introduction of sorafenib.
| Variables | All (n = 61) | Male (n = 53) | Female (n = 8) | p-Value |
|---|---|---|---|---|
| Age (years) | 67.3 ± 11.5 | 68.9 ± 10.0 | 59.8 ± 12.4 | 0.022 |
| Etiology (HBV/HCV/others) | 13/28/20 | 9/27/17 | 4/1/3 | 0.042 |
| Child–Pugh score (5/6) | 43/18 | 39/14 | 3/5 | 0.094 |
| Cancer stage (III/IVA/IVB) | 20/13/28 | 16/13/24 | 4/0/4 | 0.262 |
| BMI (kg/m2) | 22.3 ± 3.0 | 22.1 ± 2.7 | 23.5 ± 4.7 | 0.218 |
| Extrahepatic metastasis (lung/bone/others) | 16/9/9 | 14/8/8 | 2/1/1 | 1.000 |
| SMI (cm2/m2) | 43.7 ± 7.4 | 44.2 ± 7.3 | 39.8 ± 6.8 | 0.118 |
| SATI (cm2/m2) | 34.9 ± 22.0 | 30.5 ± 17.7 | 64.2 ± 26.6 | <0.001 |
| VATI (cm2/m2) | 36.6 ± 20.9 | 37.4 ± 21.5 | 31.8 ± 16.8 | 0.485 |
| Therapeutic effect (CR/PR/SD/PD) | 3/5/19/34 | 3/3/16/31 | 0/2/3/3 | 0.203 |
| Sarcopenia (yes/no) | 25/36 | 22/31 | 3/5 | 1.000 |
| Median duration of sorafenib treatment (months) | 15.7 (12.3–19.0) | 13.3 (12.2–19.5) | 9.8 (6.3–22.3) | 0.754 |
| Median survival time after introducing sorafenib (months) | 19.8 (5.1–26.1) | 17.7 (5.5–35.6) | 15.8 (7.7–30.3) | 0.803 |
Values are presented as mean ± standard deviation. Sarcopenia was defined as an SMI value of ≤38.0 cm2/m2 for women and ≤42.0 cm2/m2 for men. HBV, hepatitis B virus; HCV, hepatitis C virus; BMI, body mass index; SMI, skeletal muscle index; SATI, subcutaneous adipose tissue index; VATI, visceral adipose tissue index; CR, complete response; PR, partial response; SD, stable disease; PD, progression disease.
2.2. Changes in Body Composition before, during, and after Sorafenib Treatment
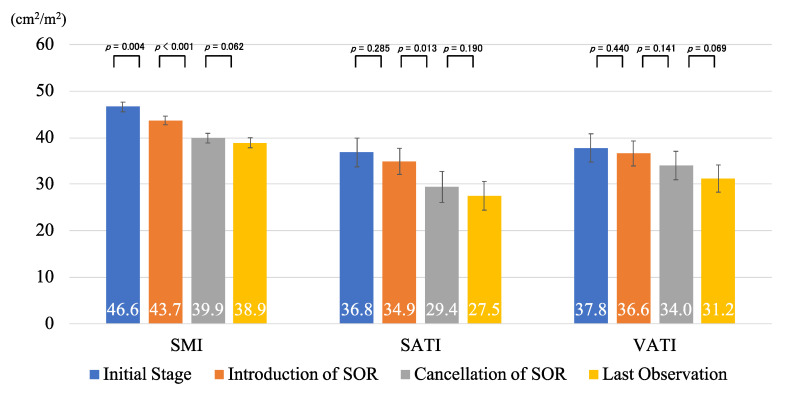
The values of skeletal muscle index (SMI), subcutaneous adipose tissue index (SATI), and visceral adipose tissue index (VATI) at the time of HCC diagnosis (initial stage), the introduction of sorafenib, the cancellation of sorafenib, and last observation were 46.6, 43.7, 39.9, and 38.9 (cm2/m2; SMI), 36.8, 34.9, 29.4, and 27.5 (cm2/m2; SATI), and 37.8, 36.6, 34.0, and 31.2 (cm2/m2; VATI), respectively. All of these tended to decrease over time, and some of these values showed significant differences (Figure 1).
Figure 1.
The values of skeletal muscle index (SMI), subcutaneous adipose tissue index (SATI), and visceral adipose tissue index (VATI) at the initial stage, introduction of sorafenib (SOR), cancellation of SOR, and last observation time. Error bars represent standard errors.
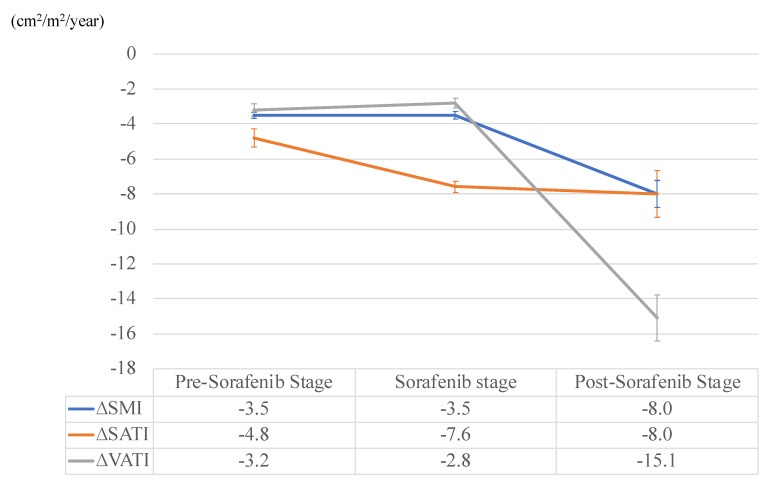
Mean intervals of pre-sorafenib, duration of sorafenib treatment, and post-sorafenib stages were 2.8, 1.3, and 0.7 years, respectively (Table 2). Annualized changes in ∆SMI, ∆SATI, and ∆VATI of each stage are shown in Figure 2. Annualized depletion rates of SMI (ΔSMIpre: −3.5, ΔSMIduring: −3.5, ΔSMIpost: −8.0) and VATI (ΔVATIpre: −3.2, ΔVATIduring: −2.8, ΔVATIpost: −15.1) accelerated at post-sorafenib stage for the first time, whereas that of SATI (ΔSATIpre: −4.8, ΔSATIduring; −7.6, ΔSATIpost; −8.0) had already accelerated at the sorafenib stage. During the administration of sorafenib (41 cases), and after cancellation of this agent (15 cases), other HCC treatments, such as radiofrequency ablation, transcatheter arterial chemoembolization, and radiation therapy, were conducted on the patients. Sorafenib was temporally stopped when other treatments were introduced, as the safety of combination therapies using sorafenib and any other treatments for HCC is not ensured. The detailed results of the treatments during each stage are shown in Table 2.
Table 2.
Detailed treatment results during the pre-sorafenib, sorafenib, and post-sorafenib stages.
| Variables | Pre-Sorafenib Stage | Sorafenib Stage | Post-Sorafenib Stage |
|---|---|---|---|
| Intervals (years) | 2.8 ± 2.5 | 1.3 ± 1.1 | 0.7 ± 0.5 |
| Treatment during each stage | |||
| Surgical resection (none/1/≥2 [times]) | 28/30/3 | 61/0/0 | 61/0/0 |
| RFA (none/1/2/3/≥4 [times]) | 38/4/11/3/5 | 58/2/0/1 | 51/9/0/0 |
| TACE (none/1/2/3/4/≥5 [times]) | 8/11/9/7/8/18 | 23/14/9/2/13 | 48/6/3/0/1/3 |
| Radiation therapy (none/1/≥2 [times]) | 45/13/3 | 53/6/2 | 51/9/1 |
| Other molecular target drugs (no/yes) | 61/0 | 61/0 | 56/5 * |
* Lenvatinib was used for 4 cases and regorafenib for 1 case. RFA, radiofrequency ablation; TACE, transcatheter arterial chemoembolization.
Figure 2.
Annualized changes in skeletal muscle index (SMI), subcutaneous adipose tissue index (SATI), and visceral adipose tissue index (VATI) at the pre-sorafenib, duration of sorafenib treatment, and post-sorafenib stages. Error bars represent standard errors.
2.3. Impact of Annualized Changes in Body Composition on Survival in the Patients with HCC Treated with Sorafenib
In order to determine which parameters of annualized changes in body composition can predict prognosis of HCC patients treated with sorafenib, we divided the enrolled patients into two groups based on the 20th percentile cutoffs for each gender, because adipose tissue distribution is known to differ by gender [20]. The patients within the two groups were classified into a rapid depletion (RD) group and a non-rapid depletion (non-RD) group, and compared using the Cox proportional-hazards model. As shown in Table 3, five factors were identified as significant by univariate analysis, including therapeutic effect (PD vs. CR/PR/SD, p < 0.001), the presence of sarcopenia (yes vs. no, p = 0.032), ΔSMIduring (RD vs. non-RD groups, p = 0.004), ΔSATIduring (RD vs. non-RD groups, p = 0.002), and ΔVATIduring (RD vs. non-RD groups, p = 0.010). Among these factors, therapeutic effect (hazard ratio [HR]: 3.633, 95% confidence interval (CI): 1.811–7.286, p < 0.001) and ΔSATIduring (HR: 3.366, 95% CI: 1.362–8.321, p = 0.009) were independent predictors of survival in HCC patients treated with sorafenib.
Table 3.
Univariate and multivariate analyses of possible prognostic factors in the patients, according to the Cox proportional-hazards model.
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Sex (male vs. female) | 0.872 (0.365–2.082) | 0.758 | ||
| Age (years) | 0.980 (0.956–1.006) | 0.127 | ||
| Etiology (HCV vs. HBV) | 0.519 (0.245–1.101) | 0.088 | ||
| Etiology (others vs. HBV) | 0.904 (0.423–1.931) | 0.795 | ||
| Child–Pugh score (6 vs. 5) | 1.630 (0.850–3.154) | 0.141 | ||
| Bone metastasis (yes vs. no) | 1.077 (0.475–2.445) | 0.859 | ||
| Cancer stage (III vs. IV) | 1.009 (0.526–1.935) | 0.979 | ||
| Combination therapy (yes vs. no) | 1.007 (0.508–1.995) | 0.984 | ||
| Therapeutic effect (PD vs. CR/PR/SD) | 3.044 (1.624–5.705) | <0.001 | 3.633 (1.811–7.286) | <0.001 |
| BMI (kg/m2) | 0.954 (0.846–1.075) | 0.440 | ||
| Sarcopenia (yes vs. no) | 1.903 (1.057–3.428) | 0.032 | 1.330 (0.653–2.709) | 0.432 |
| ∆SMIpre (RD vs. non-RD groups) | 0.835 (0.394–1.770) | 0.639 | ||
| ∆SATIpre (RD vs. non-RD groups) | 0.797 (0.390–1.628) | 0.533 | ||
| ∆VATIpre (RD vs. non-RD groups) | 1.001 (0.490–2.046) | 0.998 | ||
| ∆SMIduring (RD vs. non-RD groups) | 3.190 (1.460–6.967) | 0.004 | 1.515 (0.609–3.772) | 0.372 |
| ∆SATIduring (RD vs. non-RD groups) | 3.061 (1.530–6.122) | 0.002 | 3.366 (1.362–8.321) | 0.009 |
| ∆VATIduring (RD vs. non-RD groups) | 2.455 (1.241–4.857) | 0.010 | 1.477 (0.786–2.774) | 0.226 |
| ∆SMIpost (RD vs. non-RD groups) | 2.239 (0.798–6.286) | 0.126 | ||
| ∆SATIpost (RD vs. non-RD groups) | 2.239 (0.798–6.286) | 0.126 | ||
| ∆VATIpost (RD vs. non-RD groups) | 1.280 (0.463–3.535) | 0.634 | ||
HR, hazard ratio; HBV, hepatitis B virus; HCV, hepatitis C virus; CI, confidence interval; PD, progressive disease; CR, complete response; PR, partial response; SD, stable disease; BMI, body mass index; RD, rapid depletion; MD, mild depletion; SMI, skeletal muscle index; SATI, subcutaneous adipose tissue index; VATI, visceral adipose tissue index.
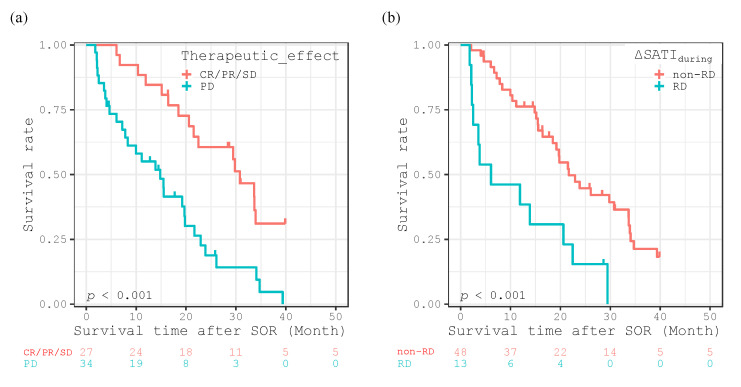
The Kaplan-Meier method revealed significantly poorer survival rates according to the log-lank test for patients with a worse therapeutic effect (PD; p < 0.001, Figure 3a), and for those in the RD group with ΔSATIduring (<−32.51 for women and <−15.49 for men, Figure 3b).
Figure 3.
Kaplan-Meier curves for overall survival time after introducing sorafenib (SOR) divided into therapeutic effect (CR/PR/SD vs. PD) (a), and the rapid depletion (RD) or non-rapid depletion (non-RD) groups in ΔSATIduring (b).
3. Discussion
We have previously indicated that skeletal muscle decreases according to worsening liver functional reserve and larger tumor size in HCC [21]. Furthermore, accelerated skeletal muscle and adipose tissue depletions are reported to be associated with poor survival rates in patients with advanced cancers, including HCC [14,15,16,17,18,22,23]. However, evaluation of body composition using CT images was performed only once or twice in these previous studies. Therefore, this is the first study using sequential CT examinations to elucidate how body composition changes from the initial stage to the terminal stage of HCC patients treated with sorafenib, and to evaluate whether these rapid annualized changes lead to poor survival rates. Diagnosing HCC with sequential CT examinations is extremely useful for detailing changes in body composition because it can avoid the effect of weight gain related to edema, ascites, and pleural effusion, which are often seen in patients with advanced HCC.
The results of the present study showed that skeletal muscle mass, SAT, and VAT decreased over time in HCC patients treated with sorafenib. However, depletion patterns of these body compositions were different from each other. After cancellation of sorafenib, most patients could not be treated with any other active treatments for HCC and instead received only palliative care. Higher priority was given to alleviating pain than to providing sufficient nutritional treatment. Because the patients who could not continue sorafenib treatment suffered from both increased tumor burden and decreased food intake, annualized depletion rates of SMI and VATI were the greatest after cancellation of sorafenib. On the other hand, accelerated depletion of SATI had already occurred during sorafenib treatment and occurred earlier than in SMI and VATI. Furthermore, the annualized depletion rates of SATI during sorafenib treatment were an independent predictor, together with therapeutic effect. These findings, together with those of the previous reports [14,15,16,17,18,22,23], may suggest that evaluation of SAT is a useful strategy for the screening of high-risk patients who show poor prognosis in the early phases of HCC therapy.
The definite reason SAT alone rapidly decreased just after introducing sorafenib in advance of skeletal muscle and VAT remains unclear. SAT is known to act as metabolic storage that can accumulate superfluous energy [24]. Therefore, we consider that SAT might first be used as an energy source when a negative energy balance is induced by sorafenib. Sorafenib frequently causes strong adverse effects on the gastrointestinal tract [6]. Indeed, 44%, 21%, and 25% of patients treated with sorafenib experienced anorexia, diarrhea, and general fatigue, respectively, in the present study. If appetite loss or signs of malnutrition associated with sorafenib treatment were observed, appropriate nutrition therapy and the administration of antiemetics should be started as needed. Furthermore, in order to correct the negative energy balance caused by the adverse effects of sorafenib, it may be effective to reduce the dose of sorafenib or discontinue it temporarily without hesitation.
The results of the present study may suggest that rapid depletion of SAT during sorafenib treatment (∆SATI: <−32.51 for women and <−15.49 for men) implies malnutrition associated with poor survival rates in HCC patients. However, in addition to malnutrition, overnutrition must also be avoided, because obesity and related metabolic disorders can promote liver carcinogenesis [25,26,27]. A recent study revealed that patients with higher than 47.2 cm2/m2 of VATI had a significantly higher risk of recurrence of HCC after curative treatment [28]. Sarcopenia, which was defined as an SMI value ≤38.0 cm2/m2 for women and ≤42.0 cm2/m2 for men in Japan [29], is also a significant prognostic factor of HCC. These cutoff values of body composition may be useful in screening HCC patients showing a poor prognosis.
This study has several limitations. First, it was a retrospective, single-center study, and the sample size, particularly the number of female patients, was comparatively small. Second, 67% of the enrolled patients in this study received other HCC therapies in addition to sorafenib and, therefore, the pure effects of sorafenib on changes in body composition were not evaluated. Third, this retrospective study was unable to assess body weight over time. Body weight is the most convenient and non-invasive indicator of nutritional assessment, and loss of body weight is closely associated with cancer-associated cachexia. Therefore, the body composition data obtained by CT imaging should be compared with changes in body weight in future studies. Fourth, muscle radiodensity was not assessed because it cannot be measured with the image analysis software used in this study. Low muscle attenuation was reported to be a prognostic factor for patients with HCC [30]. Moreover, this study did not assess muscle function parameters such as hand grip strength, which is generally considered a diagnostic criterion for sarcopenia [29] and reported to be a prognostic factor for patients with liver cirrhosis [31]. Therefore, it is quite important to evaluate not only the amount of skeletal muscle, but also its quality. The relationship between skeletal muscle quality and changes in body composition over time is also unclear. In order to solve these limitations and to validate the findings of this study, a prospective study involving a larger number of patients enrolled from several centers should be performed.
4. Materials and Methods
4.1. Patients, Treatment, and Follow-Up Strategy
This retrospective study enrolled 76 patients treated with sorafenib for advanced HCC between May 2009 and December 2017 at Gifu University Hospital. Among the patients, 11 were excluded because they did not take the agent for more than 1 month, and 4 were excluded because they did not undergo sequential CT examination. The remaining 61 patients were analyzed in this study.
The objective of sorafenib introduction was determined according to the Clinical Practice Guidelines for HCC issued by JSH [19]. Each patient’s therapeutic response was judged using dynamic CT, magnetic resonance imaging, or ultrasound every 3 months according to the Response Evaluation Criteria in Cancer of the Liver [32], an appropriate system for the assessment of the post-therapeutic response of HCC to sorafenib [33]. CR, PR, SD, and PD are defined as a 100% tumor-necrotizing effect or a complete reduction in tumor size, a tumor-necrotizing effect or tumor size reduction rate between 50% and <100%, effects other than PR and PD, and tumor growth >25% regardless of the necrotizing effect or emergence of a new lesion, respectively [32]. We decided to discontinue this agent if PD was observed for a certain period of time or severe adverse events occurred. The survival time was defined as the interval from the date of sorafenib introduction to the date of death, or September 2019 for surviving patients. All study participants provided verbal informed consent, which was considered sufficient, as this study followed an observational research design that did not require new human biological specimens. The study design, including this consent procedure, was approved by the ethics committee of the Gifu University School of Medicine (ethical protocol code: 29–26).
4.2. Image Analysis of Skeletal Muscle Mass and Subcutaneous and Visceral Fat Mass
Skeletal muscle mass, SAT, and VAT were measured using an enhanced CT image (Discovery CT 750 HD, Revolution CT; GE Healthcare, Milwaukee, WI, USA) that had been taken solely for the purpose of diagnosing HCC. A transverse CT image at the third lumbar vertebra (L3) in the inferior direction was assessed. The muscles in the L3 region were analyzed using SYNAPSE VINCENT software (Fujifilm Medical, Tokyo, Japan), as described previously [34]. The cross-sectional areas of the muscle (cm2) at the L3 level computed from each image were normalized by the square of the height (m2) to obtain SMI (cm2/m2). Sarcopenia was defined as an SMI value ≤38.0 cm2/m2 for women and ≤42.0 cm2/m2 for men, according to JSH guidelines for sarcopenia [29]. In the same manner, the cross-sectional areas of SAT and VAT (cm2) at the umbilical point were measured using a built-in function in the SYNAPSE VINCENT software, which can automatically detect CT level at the umbilical point and also automatically measure the cross-sectional area of SAT and VAT at that CT level. These values were then normalized by the square of the height (m2) to obtain SATI (cm2/m2) and VATI (cm2/m2), respectively.
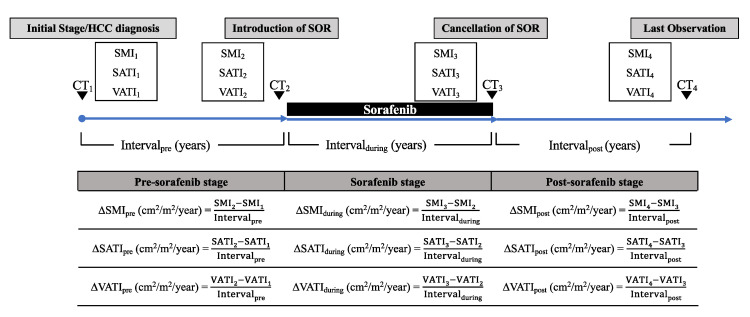
The progress of the patients was divided into the following three phases: pre-sorafenib stage, from the time of HCC diagnosis to sorafenib introduction (pre); sorafenib stage, from the introduction of sorafenib to the cancellation of the agent (during); and post-sorafenib stage, from the cancellation of sorafenib to the last observation (post). Annualized changes (Δ; cm2/m2/year) of SMI, SATI, and VATI in each phase of progression (ΔSMIpre, ΔSMIduring, ΔSMIpost, ΔSATIpre, ΔSATIduring, ΔSATIpost, ΔVATIpre, ΔVATIduring, and ΔVATIpost) were then analyzed. The outline and formula of ΔSMI, ΔSATI, and ΔVATI are shown in Figure 4.
Figure 4.
Outline and formula for ΔSMIpre, ΔSATIpre, ΔVATIpre, ΔSMIduring, ΔSATIduring, ΔVATIduring, ΔSMIpost, ΔSATIpost, and ΔVATIpost (cm2/m2/year). Skeletal muscle index (SMI) was defined as the cross-sectional area of the muscle (cm2) at the L3 level of the computed tomography image, normalized by the square of the height (m2). Subcutaneous adipose tissue index (SATI) and visceral adipose tissue index (VATI) were defined as the cross-sectional areas of the subcutaneous and visceral fat (cm2), respectively, at the umbilical point, normalized by the square of the height (m2).
4.3. Statistical Analysis
Overall survival time was estimated using the Kaplan-Meier method. Differences between curves were evaluated using the log-rank test. The Cox proportional-hazards model was used to analyze which factors affected overall survival. Statistical significance was defined as p < 0.05. All statistical analyses were performed using R ver. 3.3.1. (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/).
5. Conclusions
Skeletal muscle mass, SAT, and VAT decreased over time in HCC patients. Annualized depletion rates of SMI and VATI were greatest after withdrawal of sorafenib, but depletion of SATI had already accelerated during sorafenib treatment. Furthermore, together with the therapeutic effect, rapid depletion of SATI during sorafenib treatment (∆SATI: <−32.51 for women and <−15.49 for men) was an independent predictor of survival.
Author Contributions
K.I., K.T., T.M., D.T., T.H., A.S., M.S. (Makoto Shiraki) and M.S. (Masahito Shimizu) designed the study. K.I. analyzed the data and drafted the manuscript. K.T. supervised the treatment of the participants. K.T., T.M., D.T., T.H., A.S. and M.S. (Makoto Shiraki) contributed to the selection of the participants and collected the data. K.T., T.M., D.T., T.H., A.S. and M.S. (Makoto Shiraki) revised the manuscript, and M.S. (Masahito Shimizu) mainly reviewed and amended the manuscript. All authors read and approved the final manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.El-Serag H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 2.Njei B., Rotman Y., Ditah I., Lim J.K. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61:191–199. doi: 10.1002/hep.27388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singal A.G., El-Serag H.B. Hepatocellular Carcinoma From Epidemiology to Prevention: Translating Knowledge into Practice. Clin. Gastroenterol. Hepatol. 2015;13:2140–2151. doi: 10.1016/j.cgh.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galle P.R., Forner A., Llovet J.M., Mazzaferro V., Piscaglia F., Raoul J.L., Schirmacher P., Vilgrain V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J., Sherman M. American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.-F.F., de Oliveira A.C., Santoro A., Raoul J.-L.L., Forner A., et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Tisdale M.J. Cachexia in cancer patients. Nat. Rev. Cancer. 2002;2:862–871. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 8.Arends J., Bachmann P., Baracos V., Barthelemy N., Bertz H., Bozzetti F., Fearon K., Hütterer E., Isenring E., Kaasa S., et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017;36:11–48. doi: 10.1016/j.clnu.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Muscaritoli M., Anker S.D., Argilés J., Aversa Z., Bauer J.M., Biolo G., Boirie Y., Bosaeus I., Cederholm T., Costelli P., et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Fearon K., Strasser F., Anker S.D., Bosaeus I., Bruera E., Fainsinger R.L., Jatoi A., Loprinzi C., MacDonald N., Mantovani G., et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 11.Kudo M., Chung H., Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): Its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J. Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 12.Capuano G., Daniele B., GAETA G.B., GALLO C., Perrone F. A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 13.Uchino K., Tateishi R., Shiina S., Kanda M., Masuzaki R., Kondo Y., Goto T., Omata M., Yoshida H., Koike K. Hepatocellular carcinoma with extrahepatic metastasis: Clinical features and prognostic factors. Cancer. 2011;117:4475–4483. doi: 10.1002/cncr.25960. [DOI] [PubMed] [Google Scholar]
- 14.Iritani S., Imai K., Takai K., Hanai T., Ideta T., Miyazaki T., Suetsugu A., Shiraki M., Shimizu M., Moriwaki H. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J. Gastroenterol. 2015;50:323–332. doi: 10.1007/s00535-014-0964-9. [DOI] [PubMed] [Google Scholar]
- 15.Imai K., Takai K., Hanai T., Ideta T., Miyazaki T., Kochi T., Suetsugu A., Shiraki M., Shimizu M. Skeletal muscle depletion predicts the prognosis of patients with hepatocellular carcinoma treated with sorafenib. Int. J. Mol. Sci. 2015;16:9612–9624. doi: 10.3390/ijms16059612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imai K., Takai K., Miwa T., Taguchi D., Hanai T., Suetsugu A. Rapid Depletions of Subcutaneous Fat Mass and Skeletal Muscle Mass Predict Worse Survival in Patients with Hepatocellular Carcinoma Treated with Sorafenib. Cancers. 2019;11:1206. doi: 10.3390/cancers11081206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanai T., Shiraki M., Nishimura K., Ohnishi S., Imai K., Suetsugu A., Takai K., Shimizu M., Moriwaki H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31:193–199. doi: 10.1016/j.nut.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Hanai T., Shiraki M., Ohnishi S., Miyazaki T., Ideta T., Kochi T., Imai K., Suetsugu A., Takai K., Moriwaki H., et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol. Res. 2016;46:743–751. doi: 10.1111/hepr.12616. [DOI] [PubMed] [Google Scholar]
- 19.Kudo M., Matsui O., Izumi N., Iijima H., Kadoya M., Imai Y., Okusaka T., Miyayama S., Tsuchiya K., Ueshima K., et al. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3:458–468. doi: 10.1159/000343875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wajchenberg B.L., Lé B., Wajchenberg O. Subcutaneous and Visceral Adipose Tissue: Their relation to the metabolic syndrome. Endocr. Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 21.Imai K., Takai K., Watanabe S., Hanai T., Suetsugu A., Shiraki M., Shimizu M. Sarcopenia Impairs Prognosis of Patients with Hepatocellular Carcinoma: The Role of Liver Functional Reserve and Tumor-Related Factors in Loss of Skeletal Muscle Volume. Nutrients. 2017;9:1054. doi: 10.3390/nu9101054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Sebastiano K.M., Yang L., Zbuk K., Wong R.K., Chow T., Koff D., Moran G.R., Mourtzakis M. Accelerated muscle and adipose tissue loss may predict survival in pancreatic cancer patients: The relationship with diabetes and anaemia. Br. J. Nutr. 2013;109:302–312. doi: 10.1017/S0007114512001067. [DOI] [PubMed] [Google Scholar]
- 23.Murphy R.A., Wilke M.S., Perrine M., Pawlowicz M., Mourtzakis M., Lieffers J.R., Maneshgar M., Bruera E., Clandinin M.T., Baracos V.E., et al. Loss of adipose tissue and plasma phospholipids: Relationship to survival in advanced cancer patients. Clin. Nutr. 2010;29:482–487. doi: 10.1016/j.clnu.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto A., Kikuchi Y., Kusakabe T., Takano H., Sakurai K., Furui S., Oba H. Imaging spectrum of abnormal subcutaneous and visceral fat distribution. Insights Imaging. 2020;11:1–17. doi: 10.1186/s13244-019-0833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki Y., Imai K., Takai K., Hanai T., Hayashi H., Naiki T., Nishigaki Y., Tomita E., Shimizu M., Moriwaki H. Hepatocellular carcinoma patients with increased oxidative stress levels are prone to recurrence after curative treatment: A prospective case series study using the d-ROM test. J. Cancer Res. Clin. Oncol. 2013;139:845–852. doi: 10.1007/s00432-013-1389-1. [DOI] [PubMed] [Google Scholar]
- 26.Imai K., Takai K., Hanai T., Suetsugu A., Shiraki M., Shimizu M. Homeostatic Model Assessment of Insulin Resistance for Predicting the Recurrence of Hepatocellular Carcinoma after Curative Treatment. Int. J. Mol. Sci. 2019;20:605. doi: 10.3390/ijms20030605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe N., Takai K., Imai K., Shimizu M., Naiki T., Nagaki M., Moriwaki H. Increased levels of serum leptin are a risk factor for the recurrence of stage I/II hepatocellular carcinoma after curative treatment. J. Clin. Biochem. Nutr. 2011;49:153–158. doi: 10.3164/jcbn.10-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imai K., Takai K., Maeda T., Watanabe S., Hanai T., Suetsugu A., Shiraki M., Shimizu M. Increased visceral fat volume raises the risk for recurrence of hepatocellular carcinoma after curative treatment. Oncotarget. 2018;9:14058–14067. doi: 10.18632/oncotarget.24500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishikawa H., Shiraki M., Hiramatsu A., Moriya K., Hino K., Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016;46:951–963. doi: 10.1111/hepr.12774. [DOI] [PubMed] [Google Scholar]
- 30.Martin L., Birdsell L., Macdonald N., Reiman T., Clandinin M.T., Mccargar L.J., Murphy R., Ghosh S., Sawyer M.B., Baracos V.E., et al. Cancer Cachexia in the Age of Obesity: Skeletal Muscle Depletion Is a Powerful Prognostic Factor, Independent of Body Mass Index. J. Clin. Oncol. 2020;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 31.Hanai T., Shiraki M., Imai K., Suetsugu A., Takai K., Moriwaki H., Shimizu M. Reduced handgrip strength is predictive of poor survival among patients with liver cirrhosis: A sex-stratified analysis. Hepatol. Res. 2019;49:1414–1426. doi: 10.1111/hepr.13420. [DOI] [PubMed] [Google Scholar]
- 32.Takayasu K., Furuse J., Nakamura K., Tanaka M., Kudo M., Ikai I., Kubo S., Sakamoto M., Makuuchi M. Response Evaluation Criteria in Cancer of the Liver (RECICL) proposed by the Liver Cancer Study Group of Japan (2009 Revised Version) Hepatol. Res. 2010;40:686–692. doi: 10.1111/j.1872-034x.2010.00674.x. [DOI] [PubMed] [Google Scholar]
- 33.Arizumi T., Ueshima K., Takeda H., Osaki Y., Takita M., Inoue T., Kitai S., Yada N., Hagiwara S., Minami Y., et al. Comparison of systems for assessment of post-therapeutic response to sorafenib for hepatocellular carcinoma. J. Gastroenterol. 2014;49:1578–1587. doi: 10.1007/s00535-014-0936-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitsiopoulos N., Ross R., Gallagher D., Lyons W., Baumgartner R.N., Heymsfield S.B. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J. Appl. Physiol. 2017;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]