Abstract
MicroRNAs (miRNAs), a class of small non-coding RNA molecules, are responsible for RNA silencing and post-transcriptional regulation of gene expression. They can mediate a fine-tuned crosstalk among coding and non-coding RNA molecules sharing miRNA response elements (MREs). In a suitable environment, both coding and non-coding RNA molecules can be targeted by the same miRNAs and can indirectly regulate each other by competing for them. These RNAs, otherwise known as competing endogenous RNAs (ceRNAs), lead to an additional post-transcriptional regulatory layer, where non-coding RNAs can find new significance. The miRNA-mediated interplay among different types of RNA molecules has been observed in many different contexts. The analyses of ceRNA networks in cancer and other pathologies, as well as in other physiological conditions, provide new opportunities for interpreting omics data for the field of personalized medicine. The development of novel computational tools, providing putative predictions of ceRNA interactions, is a rapidly growing field of interest. In this review, I discuss and present the current knowledge of the ceRNA mechanism and its implications in a broad spectrum of different pathologies, such as cardiovascular or autoimmune diseases, cancers and neurodegenerative disorders.
Keywords: competing endogenous RNAs, ceRNA mechanism, miRNA, non-coding RNAs, cancer, cardiovascular pathologies, neurodegenerative disorders
1. Introduction
MicroRNAs (miRNAs) are found in protozoa, plants and animals [1], and are known for their traditional role as post-transcriptional fine-tune regulators [2,3]; however, in the recent years, miRNAs have been investigated and associated with playing a new regulatory level: as an information medium, able to interact across the many different species of RNA molecules, establishing an elaborate dynamic balance among transcriptional products [4].
1.1. RNA Molecules Landscape and their Classical Roles
A variety of RNA molecules has been subjected to meticulous classifications [5] and their growing number subtypes, in particular, the class of coding and non-coding RNA with a potential regulatory effect have been the subject of in-depth studies [6,7,8], as well as being the topic of many functional annotation resources [9,10,11,12]. Beside the most famous RNA subtypes, such as coding messenger RNAs (mRNAs), transfer RNAs (tRNAs) and ribosomal RNAs (rRNAs), recent research has taken a particular interest in pseudo-genes (-genes), long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs) [6]. The non-coding elements have been shown to represent one of the largest portions of transcribed molecules [12,13,14,15], and to be involved in a very broad set of biological processes [16,17,18,19], cell-fate programming [20], aging [21,22] and diseases [23].
lncRNAs cooperate in gene regulation [17,24,25], from transcriptional and post-transcriptional levels [26,27] to translational and post-translational commitments [28,29], up to epigenetic [30,31] and cell signaling modulation [32]. -genes, once considered “genomic fossils”, play a fundamental role in the regulation of their cognate genes [33] and circRNAs, far from being considered experimental artifacts, participate in transcriptional and post-transcriptional gene regulation of their parental genes through interactions with specific spliceosomal components in the nucleus [34,35].
This list includes at least one other fundamental class: microRNAs (miRNAs). MicroRNAs are a subtype of small non-coding RNA of about 20–22 nucleotides in length [36] and are produced through an elaborate biogenesis process [3,37,38], starting from transcription in the nucleus until cytoplasmic processing by the RNase III enzyme Dicer [39]. Mature miRNAs are integrated into the RNA-induced silencing complex (RISC), a multiprotein complex, and guide it to target transcripts, usually interfering with their translation and sometimes even promoting their degradation [40,41]. Target recognition is driven by a partial sequence complementarity mechanism based on a short microRNA response element (MRE) sequence found on a transcript and the “seed” sequence on the miRNA, a 6 to 8-nt sequence highly conserved across species. Interestingly, this regulation mechanism is characterized by a high complexity for distinct miRNAs are able to modulate the expression of more than one target transcript, and conversely, each transcript, harboring different MREs, may be regulated by multiple miRNAs [2,42]. Even mutations that seem negligible can significantly affect this mechanism—a change in the seed sequence may alter a specific miRNA target set and a change in an MRE can free a target from the miRNA-modulation [40,43]. In particular, it was shown that non-coding RNA (ncRNA) molecules, such as pseudo-genes, lncRNA and circRNA, represent a large reservoir of putative miRNA targets, as they too harbor MREs and can thus be bound by mature miRNAs. This specific ability of miRNAs to regulate various types of RNAs represents one of the most intriguing discoveries and provides a possible explanation of many aspects of fine-tuned post-transcriptional gene regulation [2,44].
1.2. Competing Endogenous RNA (ceRNA) Hypothesis
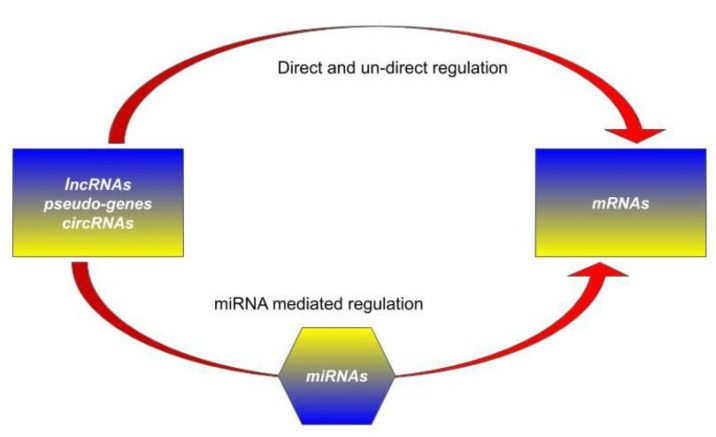
Since miRNAs can recognize their target sites on different RNA molecules, it was suggested that miRNAs could be capable of mediating a regulatory crosstalk between the various components of the transcriptome. This miRNA regulation should be modulated by other RNA molecules, as both mRNAs and non-coding RNAs are known to be bound by miRNA and to be significantly expressed in many different biological conditions. This mechanism offers an additional post-transcriptional gene regulation mechanism and a complementary point of view for the role of the large number of transcribed, but not translated, RNAs (see Figure 1) [4].
Figure 1.
Transcription and post-transcription regulation of messenger RNAs (mRNAs) can be affected by several direct and indirect mechanisms involving circular RNAs (circRNAs), pseudo-genes (-genes) and long non-coding RNAs (lncRNAs). Some of these processes act on the transcription rate in the nucleus through the specific RNA–RNA complex, some others help the stability of mRNA molecules in the cytoplasm. Alongside this, the competing endogenous RNA (ceRNA) mechanism offers a parallel and complementary way through the same actors, protein coding and non-coding RNAs, but instead mediated by microRNAs (miRNAs).
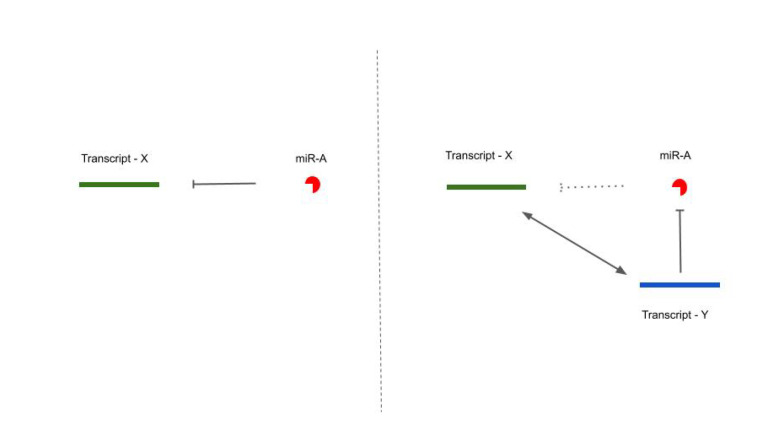
When two or more RNAs share common miRNA response elements (MREs) (mostly on their 3’ untranslated region (3’UTR), in the case of mRNAs) they can be targeted by the same microRNA(s), implying that they can cross-regulate each other indirectly because they compete to bind the same pool of sequences (see Figure 2) [4,45].
Figure 2.
Left panel: A naive situation with one miRNA: miR-A, and one target: transcript-X. Transcript-X harbors miR-A microRNA response elements (MREs) and can be post-transcriptionally regulated by miR-A. Right panel: In the same situation, a new miR-A target is added, transcript-Y. Transcript-Y harbors itself miR-A MREs and can sponge miR-A, thus leading to a reduced post-transcriptional regulation of transcript-X by miR-A through an indirect crosstalk.
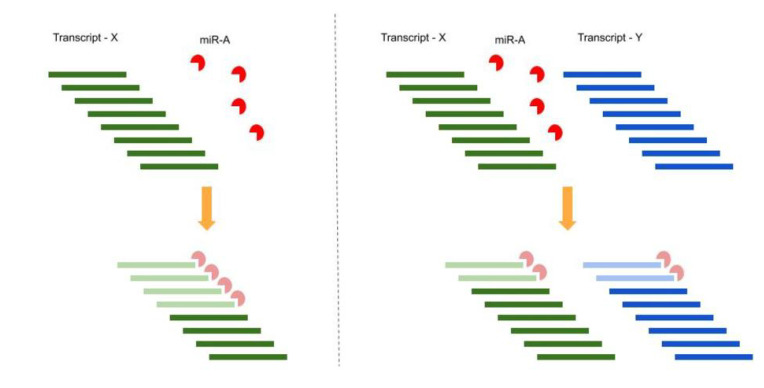
For instance, we can consider a very simple and naive situation composed of one miRNA: miR-A, and one target: transcript-X harboring one MRE per molecule. In a 1:2 proportion ratio at the steady state, 50% of the transcript-X molecules are not affected by miR-A control. If a new miR-A target (transcript-Y) is added, with the same MRE per molecule and in the same amount as transcript-X, the new ratio between miR-A and transcript-X will be 1:4, leading to an average of 75% of transcript-X molecules being free from miRNA regulation without any changes in the transcriptional rate of transcript-X (see Figure 3).
Figure 3.
Top left panel: Before interaction, a naive situation with one miRNA: miR-A, and one target: transcript-X, in a 1:2 proportion ratio. Transcript-X harbors one miRNA response element (MRE) per molecule. Bottom left panel: After interaction, at the steady state, 50% of transcript-X molecules are under the miRNA repressive action (whether post-transcriptional degradation or translational repression) and 50% of transcript-X molecules are not affected by miR-A control. Top right panel: Before interaction, a new miR-A target is added: transcript-Y, in the same amount of transcript-X. Transcript-Y also has the same MRE per molecule. The new ratio between miR-A and transcript-X is 1:4. Bottom right panel: After interaction, at the steady state, miRNAs are shared in the same proportion between the two transcripts’ molecules. On average, 75% of transcript-X molecules are free from miRNA regulation without any changes in the transcriptional rate of transcript-X.
In general, in this new approach, transcripts could actively communicate among them, regulating their respective expression levels through a specific language in which letters are coded into the MREs. This mechanism enlarges the number of 3’UTR regulatory possibilities—they regulate the expression of the encoded proteins acting in -cis and could modulate the abundance of other transcripts in -trans by sequestering miRNA molecules. The same mechanism extends to gene regulation network relationships, adding a novel layer of indirect interactions [42,46,47,48]. The competing endogenous RNAs (ceRNAs) mechanism provides a potential explanation of some of the unexpected effects elicited by highly up- or down-regulation [4,49,50,51]. Strong down-regulation of an miRNA-modulated transcript can release a large number of miRNA molecules, which would become free to bind to other target transcripts and hyper-repress them. Conversely, overexpression of an miRNA-modulated transcript can sequester a higher number of miRNA molecules, thus de-repressing other target transcripts.
Several in silico prediction strategies were devised as a corollary of the new ceRNA logic. Transcripts acting as ceRNA should show a correlated expression trend among themselves and an anticorrelated tendency with the miRNAs they compete with [49,52,53,54]. A fundamental step is represented by the predictions of miRNA-transcript interactions. In silico models rely mostly on the analysis of seed matching sequences and thermodynamic constraints (interacting free energy and RNAs secondary structures) [55,56] whereas other techniques, like those based on CLASH (crosslinking, ligation and sequencing of hybrids) or CLIP (crosslinking and immunoprecipitation) strategies, provide new evidence of non-canonical binding sites, enlarging and better specifying the miRNA-targets landscape [57,58,59,60]. ceRNA interaction databases combine differential expression and co-expression considerations of the putative long- and short-RNA players, and provide a scoring system based on the number of shared miRNAs, reflecting the assumption—the more common miRNAs transcripts share, the stronger their reciprocal modulation [52,61,62,63,64,65]. Alongside other ceRNA bioinformatic inferring packages [66,67,68], other in vitro and in vivo experimental evidence [50,69] was produced to support this additional layer of post-transcriptional regulation; this evidence simultaneously appeared both immediately captivating and highly disputed.
Doubt was raised by considering the effective stoichiometric tolerance of RNA molecules and the extent of this mechanism in the cell life, from physiological, pathological and aberrant contexts. In this respect, different mathematical models were proposed in order to demonstrate, at least in principle, the feasibility of this mechanism [42,70,71]. The predictions offered by these mathematical models revealed that ceRNA-mediated cross-regulation depends on several factors. The absolute and relative abundance of RNA molecules, miRNAs-ceRNAs binding affinity and the number of MREs are among the most relevant. According to mathematical results, optimal conditions for ceRNA activity are reached when the number of seeds for an miR-family and its MREs are near equimolarity [42]. In such an environment, a small change in one or few transcript expression levels would greatly influence those of its or their ceRNAs by increasing or decreasing the number of free miRNA molecules.
To consider the possibility and extent of the proposed ceRNAs molecular permissive context in more depth, other biological models were paired with proper mathematical models [72,73,74,75,76]. This approach resulted in new criticisms and proposed new solutions. On the one hand, the abundance of targets seems to produce a conceptual obstacle—since each target is typically responsible only for a small fraction of the total MREs pool, it is unlikely that a variation in the expression of one of them can affect the others’ through a ceRNAs effect [72]. On the other hand, the emphasis on MREs’ hierarchical binding seems to open new perspectives: when miRNAs are expressed at a medium range and the miRNAs:targets ratio is low, we see that miRNAs are likely to bind to high-affinity sites (from 8mers to 6mers, according to TargetScan [55] seed classification). These “high-affinity” targets are shown to be more responsive to ceRNAs crosstalk and, whether the number of miRNA molecules and “high-affinity” targets verges on equimolarity, ceRNA crosstalk can be triggered by a relatively small number of additional targets [73]. It must however be stressed that non-canonical miRNA-target association sites, with their possible relevancy, function both in repression activity and competition. Previously described techniques [57,58,59,60] allow for the detection of non-canonical miRNA-target association sites even if the difficulty in knowing their precise extent only partially grants their use in mathematical models [73,74].
Alongside the first protein-coding genes ceRNA networks and lncRNA-ceRNA interactions, the growing availability of circRNAs sequences and expression data allows us to add them to the ceRNA regulatory molecules repertoire. This class of RNA has been identified since the mid-1980s, as a result of a specific type of exon scrambling, where a downstream splice donor site of an exon meets an upstream splice acceptor site [77], but it is the use of high-throughput sequencing techniques and specific alignment algorithms that have highlighted the widespread presence of these molecules in cell systems and made their systematic characterization possible [78,79]. circRNAs show cell- and tissue-specificity expression profiles, together with high expression levels, supporting their relevance in biological functions [35]. They are particularly effective as sponges because they harbor a high number of MREs [80] and exhibit an important characteristic as stable regulators—their enduring lifetime. circRNAs are less exposed to degradation driven by exonucleases because they lack a polyadenylated tail and show a median half-life 2.5 times longer than the median half-life of their linear counterparts [81]. In this respect, circRNAs are more likely to be useful as sponges and to enter into ceRNA circuitry than “linear” molecules [82].
Ever since this hypothesis was suggested, the number of papers containing references to competing endogenous RNAs is constantly growing, demonstrating that this new and indirect regulatory mechanism has made its way into the already rich panorama of biological schemes [45,83,84,85].
2. ceRNA and Diseases
Instances of ceRNA crosstalk were experimentally tested in a very large number of contexts and were observed in both normal and pathological backgrounds, demonstrating the wide spread proliferation of the mechanism. In particular, researchers collected evidence from normal physiology, for instance in brain architecture [86] and regeneration mechanisms [87], neuronal and muscle developmental processes [50,88], cellular differentiation [89] and reprogramming [90,91] and from the immense landscape of diseases, syndromes and disorders where highly complex gene regulation circuits are most affected by perturbations. Researchers work hard to model these regulatory networks in order to predict and understand how modifications could alter the dynamic balance among molecules, causing illness such as the onset of cancer and its progression, cardiovascular problems and neurodegenerative disorders and other pathologies, such as those relating to the immune and autoimmune response and to degenerative physical condition.
2.1. ceRNA and Cardiovascular Problems
Cardiovascular diseases are the leading causes of death worldwide. The aberrant balances of coding and non-coding RNA molecules are often a reflection of, or the cause of the high complexity of cardiovascular pathologies—from cardiac ischemia to cardiac fibrosis, from pathological cardiac hypertrophy to blood vessels deficiencies. Many miRNAs are related to cardiogenesis, heart development and heart normal functioning [92]: muscle-specific microRNAs (myomiRs) like miR-1 and miR-133a are involved in embryonic stem cell development and cardiac-specific muscle lineage commitment, whereas miR-208 and miR-499 collaborate to differentiate cardioblasts into cardiomyocytes and to properly specify fast and slow muscle fiber by regulating the expression of sarcomeric contractile proteins. miRNAs were proposed as potential therapeutic targets [93], but a global understanding of ncRNAs is necessary.
Some ncRNAs-miRNAs-mRNAs networks were analyzed, leading to the discovery of numerous lncRNAs functional modules in heart failure [94] and in cardiac hypertrophy (CH), involving important oncogenic and well-characterized disease-related lncRNAs, such as HOX transcript antisense intergenic RNA (HOTAIR) [95] or myocardial infarction and associated transcript (MIAT) [96,97]. Specifically in CH, the crucial role of three new characterized lncRNAs (SLC26A4-AS1, RP11-344E13.3 and MAGI1-IT) was proven [98] by combining miRNA-transcript interactions, expression data of cardiac hypertrophy from an expressly re-annotated gene expression dataset, publicly available on Gene Expression Omnibus (GEO, a public functional genomics data repository), and an analysis of the most important network topological features like the degree, betweenness and closeness. Studies on cardiomyocytes highlighted the role of miR-489 and of its target Myd88 (myeloid differentiation primary response gene 88). miR-489 was found down-modulated in a microarray study conducted to investigate miRNA differential expression in response to angiotensin II treatment. Further, in vitro studies revealed miR-489 involvement in cardiomyocyte hypertrophy—its knockdown by antagomiRs promoted cardiomyocyte hypertrophy and its overexpression resulted in the reduction of hypertrophic responses. Among the different miR-489 target genes, Myd88 was already involved in cardiomyocyte hypertrophy. In an experimental setting regarding angiotensin II treatment, miRNA expression changes were shown to impact the expression of the target gene and on the observable hypertrophic phenotype, revealing a functional relationship between miR-489 and Myd88 in hypertrophy. The same angiotensin II treatment perturbation shows a time-dependent up-regulation of cardiac hypertrophy related factor (CHRF) lncRNA levels. This lncRNA is able to directly bind to miR-489 and, under this pathological condition, regulates hypertrophy by impacting on miR-489 activity and, indirectly, on Myd88 expression (see Table 1) [99].
Table 1.
Table summarizing the miRNA-ceRNAs networks discussed in the review. Table fields are: mRNA, protein coding genes name; ncRNA, non-coding RNAs class and name; miRNA, microRNA involved; Disease; Reference. (a) HBMEC, Human Brain Microvascular Endothelial Cells; (b) SCA7, Spinocerebellar Ataxia Type 7; (c) ARDS, Acute Respiratory Distress Syndrome; (d) PCOS, Polycystic Ovary Syndrome; (e) CMEC, Cerebral Microvascular Endothelial Cell Injury.
| mRNA | ncRNA | miRNA | Disease | Reference |
|---|---|---|---|---|
| Myd88 | lncRNA—CHRF | miR-489 | Cardiac Hyperthrophy | [99] |
| Akt3 | lncRNA—CHRF | miR-93 | Cardiac Hyperthrophy | [100,101] |
| TRL4 | lncRNA—MIAT | miR-93 | Cardiac Hyperthrophy | [97] |
| PTEN | lncRNA—HOTAIR | miR-19 | Cardiac Hyperthrophy | [95] |
| ATG7 | lncRNA—APF | miR-188-3p | Cardiac Autophagy | [102] |
| PTAFR | lncRNA—PFL | let-7d | Cardiac Fibrosis | [103] |
| TGF-beta pathway | circ-0011565 | let-7d | Cardiac Fibrosis | [104] |
| TGF-beta pathway | circ-0010678 | let-7d | Cardiac Fibrosis | [104] |
| TGF-beta pathway | circ-0010219 | let-7d | Cardiac Fibrosis | [104] |
| TGF-beta1 | circRNA-010567 | miR-141 | Cardiac Fibrosis | [105] |
| Col1a2 | circRNA-000203 | miR-26b-5p | Cardiac Fibrosis | [106] |
| CTGF | circRNA-000203 | miR-26b-5p | Cardiac Fibrosis | [106] |
| COL1A1 | circHIPK3 | miR-29b-3p | Cardiac Fibrosis | [107] |
| COL1A3 | circHIPK3 | miR-29b-3p | Cardiac Fibrosis | [107] |
| Alpha-SMA | circHIPK3 | miR-29b-3p | Cardiac Fibrosis | [107] |
| DAPK2 | lncRNA—MIAT | miR-22-3p | Diabetic Cardiomyopathy | [108] |
| SOX7 | lncRNA—XIST | miR-485-3p | HBMEC (a) | [109] |
| Atxn7 | retro--gene—lnc-SCA7 | miR-124 | SCA7 (b) | [110] |
| BACE1 | lncRNA—BACE1-AS | miR-485-5p | Alzheimer’s Disease | [111,112] |
| VEGF | lncRNA—MIAT | miR-150-5p | Alzheimer’s Disease | [113] |
| HMGB1 | lncRNA—MIAT | miR-204-5p | CMEC (e) | [84] |
| IRF2 | lncRNA—XIST | miR-204 | ARDS (c) | [114] |
| TMEM120B | lncRNA—PWRN2 | miR-92b-3p | PCOS (d) | [115] |
| VEGF | lncRNA—MIAT | miR-150-5p | Diabetes Mellitus | [116] |
| BRAF | -gene—-BRAF | miR-134; miR-543; miR-653 | Diffuse Large B Cell Lymphoma | [69] |
| OCT4 | -gene—OCT4-pg4 | miR-145 | Hepatocellular Carcinoma | [117] |
| OCT4 | -gene—OCT4-pg5 | miR-145 | Endometrial Carcinoma | [118] |
| C-Myc pathway | lncRNA—HOTAIR | miR-130a | Gallbladder Cancer | [119] |
| HER2 | lncRNA—HOTAIR | miR-331-3p | Gastric Cancer | [120] |
| Derlin1 | lncRNA—MIAT | miR-132 | Colorectal Cancer | [121] |
| LASP1 | lncRNA—MIAT | miR-324-3p | Papillary Thyroid Cancer | [122] |
| PD-L1/CD274 | lncRNA—MIAT | miR-150-5p | Immunotherapy Involvement | [123] |
Interestingly, the same CHRF lncRNA was shown to act in cardiac hypertrophy through the axis miR-93-Akt [100]—the role of miR-93 (known to be involved in the progression of cardiac hypertrophy) was analyzed in combination with the behavior of its direct target, CHRF lncRNA. Experiments conducted in an isoproterenol induced-hypertrophy setting investigating cardiomyocytes showed the increased CHRF expression and, conversely, the decreased miR-93 expression and suggested the potential endogenous binding between miR-93 and its lncRNA target. miR-93 protein coding targets were analyzed in order to identify a possible specific gene responsible for cardiac hypertrophy. Akt3 was selected as it was found in the overlap among the protein-coding genes putative miR-93 targets and the PI3K/Akt signaling pathway—crucial regulators in the progression of cardiac hypertrophy. The authors were able to demonstrate that the high-expression of CHRF can sponge miR-93 expression and impact cardiac hypertrophy by altering Akt3 expression, even if pathway-specific regulatory effects remain to be further elucidated [100,101].
Another study suggests the important role of lncRNA autophagy-promoting factor (APF) in the molecular regulation of the autophagic program and myocardial infarction: in this biological context, the translation of autophagy related 7 gene (ATG7), involved in ischemia/reperfusion-induced myocardial injury, can be suppressed by miR-188-3p, an miRNA participating in autophagy inhibition and cell death. APF lncRNA directly binds to and competes for miR-188-3p regulating ATG7 expression and the consequent cardiac autophagy [102]. In specific myocardial infarction mouse models and in fibrotic cardiac fibroblasts, another lncRNA was found related to cardiac dysfunctions—pro-fibrotic (PFL) lncRNA inhibits the platelet-activating factor receptor (PTAFR) gene by competing for miR let-7d, and leads to fibrogenesis by increasing cell viability and promoting fibroblast-myofibroblast transition [103]. The same let-7d miR represents a hub node in a specific ceRNA network based on the high-throughput RNA sequencing data of cardiac fibroblasts from neonatal mice treated with cardiac fibrosis (CF) induced by TGF-beta1. This miRNA is a key component of a module characterized by cardiac fibrosis-related signaling pathways, like the transforming growth factor beta (TGF-beta) signaling pathway and AMPK signaling pathway and some putative ceRNAs of it (novel-circ-0011565, novel-circ-0010678 and novel-circ-0010219), found in the same module, may have a role in determining the progression of this pathology [104]. In a similar signaling pathway, TGF-beta1 transcript is also found to be regulated by miR-141 and, in a cardiac fibrosis context, this modulation is ceRNA-mediated by circRNA-010567 [105]; the same CF models show how several fibrosis-related genes could be under the regulatory effect of circ-ceRNA. In mouse CF models, circRNA-000203 was found to be up-regulated, it sponged miR-26b-5p and it was able to suppress the interaction of miR-26b-5p with Col1a2 and CTGF, to increase expression of Col1a2, Col3a1 and alpha-SMA genes and globally to eliminate the antifibrotic effect of miR-26b in this pathology [106]. The same genes, alpha-SMA and COL3A1, together with COL1A1, were found under the ceRNA regulation of circHIPK3 through the action on miR-29b-3p. When overexpressed in vitro, circHIPK3 sponged miR-29b-3p and reversed the miR-induced inhibition of cardiac fibroblasts proliferation and migration by altering the expression levels of miR-29b-3p targeting genes (COL1A1, COL3A1 and alpha-SMA) [107].
Several other specific conditions, such as coronary artery disease and nonvalvular persistent atrial fibrillation, have been associated with circRNAs, suggesting, respectively, that circ-YOD1 is a potential biomarker [124] and that circRNA002085 and circRNA001321 show evidence of circRNA-associated ceRNA mechanisms [125].
Furthermore, circRNAs and their paired miRNAs have been associated with atherosclerosis [126,127] and myocardial infarction [128,129], cardiac hypertrophy [130,131] and diabetic cardiomyopathy [106,132], aortic aneurysm [133] and ischemic heart disease [134,135] by allowing the expansion of the interconnection of RNA molecules and pathways affected by different pathologies [136].
2.2. ceRNA and Neurodegenerative Disorders
The understanding of ceRNA involvement in the broad spectrum of brain-related diseases has increased dramatically in recent years.
Notably, one of the first discoveries of circRNA putative ceRNA involvement was found in neuronal tissues. Characterized in a systematic screening of circRNAs in animals [80], the circRNA CDR1as, antisense to the cerebellar degeneration-related protein 1 transcript, showed 74 MREs for the highly conserved miR-7, 63 MREs, in particular, conserved in at least one species. CDR1as was characterized by a stable and well detectable expression in the cytoplasm; in several brain regions, as in mesencephalon, this circRNA showed significant co-expression with miR-7 and, moreover, was densely bound by miRNA effector Argonaute (AGO) proteins. To further prove this post-transcriptional regulation and support this competing balance, human and mouse CDR1as circular sequences were injected into Danio rerio animal model embryos and the specific brain phenotype obtained was similar to the phenotype caused by miR-7 knockdown.
Later on, ceRNAs crosstalk was associated with many facets of the biological processes involved in the formation and functioning of the central nervous system, such as nerve injury repair in axon regeneration through circ-Ankib1 action on different miRNAs in Schwann cells [137] or the proper activity of human brain microvascular endothelial cells (HBMEC) where lncRNAs X-inactive specific transcript (XIST), when down-modulated, impacts the vascular endothelial growth factor (VEGF) signaling pathway by impairing hypoxia-induced angiogenesis via the miR-485-3p/SOX7 axis [109].
lncRNAs and circRNAs are crucial in numerous neurodegenerative pathologies too [138] and evidence of RNAs balance disruption and ceRNA crosstalk was observed in Alzheimer’s and Parkinson’s disease [139,140] and Spinocerebellar Ataxia Type 7 (SCA7). In particular, several lncRNAs are known to exhibit abnormal expression in several types of cancers and in brain disorders, like lncRNA HOX transcript antisense intergenic RNA (HOTAIR) that exerts its regulatory roles in cell apoptosis by sponging miR-221 in specific Parkinson’s disease cell lines [141], or in the same context of Parkinson’s disease, like lncRNA MALAT1 (metastasis-associated lung adenocarcinoma transcript 1), through its involvement in dendritic and synapse development. An investigation into the role of MALAT1 in animal and in vitro models revealed its ceRNA function is linked to neuron apoptosis through the sponge effect on miR-124 [142]. The same miR-124 characterizes the SCA7 pathology by mediating the interaction of lnc-SCA7 and Spinocerebellar Ataxia Type 7 Protein (Atxn7) transcripts [110]. lnc-SCA7 is a retropseudogene highly conserved across mammals, its expression positively correlates with that of ATXN7 in human and mouse adult tissues as well as in several central nervous system areas. miR-124 MREs found both on the 3’UTRs of mouse lnc-SCA7 and Atxn7 suggests the possibility of competing mechanisms between the two transcripts. This ceRNA post-transcriptional regulation was proven by the observation that it is Dicer-dependent, among other evidence. lncRNA knockdown caused a significant reduction of Atxn7 only in wild-type embryonic stem (ES) cells whereas no significant reductions were observed in Dcr-deficient ES cells. This specific ceRNA network can partially explain the selective neurodegeneration observed in SCA7. Although ATXN7 is a ubiquitously expressed gene, miRNA-124 is most abundant in the retina and the cerebellum and lnc-SCA7 shows a stronger correlation with ATXN7 in these same regions where the tissue-specific pathology reveals itself.
An interesting example of a ceRNA complementary mechanism, which involves lncRNAs, mRNAs and miRNAs, is found in Alzheimer’s disease (AD). The physiological expression of beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) is fundamental for several aspects of nerve myelination [143] and synaptic functions [144]; however, elevated levels of the BACE1 protein are linked to the formation of plaques through the generation of beta-amyloid peptides by the cleavage of amyloid precursor protein [145]. lncRNA BACE1-AS, a conserved antisense transcript overlapping BACE1 locus exhibits a concordant expression with BACE1 and, through the formation of a stabilizing duplex with the BACE1 transcript, enhances the stability of BACE1 itself [146]. BACE1 harbor MREs for miR-485-5p, although the potential miRNA-induced translational repression is inhibited by the same transcripts duplex—BACE1-AS and miR-485-5p share a common binding site on the BACE1 transcript and the interaction of the two transcripts masks the MRE, thus preventing miR-485-5p action. Dysregulation of this pair of miRNA and lncRNA, both found over-expressed in different regions of AD brain tissues, may induce the up-regulation of BACE1 and consequently the onset of Alzheimer’s disease [111,112].
2.3. An Increasing Spectrum of Different Pathologies Involved
In vitro studies, in silico predictions and in vivo models have shown that the ceRNA mechanism is ubiquitous at a systemic level, as it could be detected in very different cellular phases and conditions. Derived from patients’ indications, specific examples on ceRNA interactions emerge from a very broad set of different syndromes.
lncRNA XIST, which is known to regulate X-chromosome inactivation by orchestrating the right gene expression on the X chromosome in female mammals [147], and miR-204, involved in arterial hypertension, diabetes, many cancer dysregulated pathways [148] but also fundamental in the development of eyes and adipogenesis [149], are found to show competing crosstalk with interferon regulatory factor 2 (IRF2) in lipo-polysaccharide-induced acute respiratory distress syndrome (ARDS) [114] and polycystic ovary syndrome (PCOS). The abnormal up-regulation of Prader–Willi region nonprotein coding RNA 2 (PWRN2) reduces the availability of hsa-miR-92b-3p and brings an up-regulation of hsa-miR-92b-3p direct target—transmembrane protein 120B (TMEM120B) protein. This up-regulation can promote adipocyte differentiation and, indirectly, cause spindle anomalies, leading to abnormal oocyte development [115].
Mutation of the LMNA gene can result in an accumulation in the nuclear membrane of progerin, a specific splicing isoform of Lamin-A, causing Hutchinson–Gilford progeria syndrome. In order to identify possible ceRNAs involved in this syndrome, several LMNA-predicted and validated miRNAs were used, such as miR-9—involved in neurogenesis [150] and protective against the effects of progeria [151], the tumor suppressor miR-34a [152] and miR-298 involved in Alzheimer’s disease [153]. In the top ranked list and based on the number of different shared miRNAs, key components of the RNA interference machinery, like Dicer1, Argonaute and Drosha, are found together with genes controlling the cell cycle, such as TP53 and CDKN1A, a result that enlarges the LMNA interactome and suggests new interactions that could impinge on key cellular pathways [154].
ceRNAs hypothesis offers a conceptual framework to explain part of certain biological responses in syndromes characterized by large chromosomal rearrangements [155], which is also observed in certain tumor conditions. In the 5q-syndrome, bone marrow hematopoietic cells undergo the loss of the 5q31.1 band, suffering a hematological disorder that could evolve into acute myeloid leukemia. The simultaneous loss of many genes can impact the availability and abundance of a specific set of microRNAs that subsequently may alter the activity of other target transcripts belonging to other non-altered and apparently unrelated genomic regions. By miRNA–mRNA interaction in silico analysis, nine miRNA (hsa-miR-3164, hsa-miR-513a-5p, hsa-miR-30c-1-3p, hsa-miR-1254, hsa-miR-3916, hsa-miR-27a-3p, hsa-miR-27b-3p, hsa-miR-4311 and hsa-miR-665) were identified and used to pinpoint possible common target genes. To increase sensitivity and specificity, miRNAs targets were crossed with the list of transcripts found dysregulated in a differential expression analysis based on a similar syndrome in vitro setting composed by 5q- CD34+ cells compared to control CD34+ cells. The filtered gene set was particularly interesting because it contained genes not yet associated with the syndrome and two of the differentially-expressed transcripts, GRAMD1B and HIPK2, both target of all nine miRNAs, were known to be already involved in other types of leukemia.
From inflammatory responses in diabetic nephropathy [156] and vascular endothelial cells (VECs) [157] to the regulation of osteoarthritis progression [158,159] or in periodontitis [160,161], many biological processes are found involved in the class of inflammatory mechanisms.
Several lncRNAs are found involved in miRNAs sponging activities as well in degenerative mechanisms, like in for instance lumbar intervertebral disc degeneration [162] or in age-related diseases (ARDs) where lncRNAs can affect many cellular homeostasis layers [163].
Interestingly, this competing mechanism also affects immune responses, such as in liver cirrhosis [164] or in early HIV infection (EHI) gene expression regulatory networks [165] and it participates in the regulation and evolution of deceitful autoimmune diseases like rheumatoid arthritis [166,167].
2.4. ceRNA and Cancer
Last but not least, cancer was the first, and is one of the most studied, set of abnormalities and dysfunction-causing disease where the ceRNA mechanism has been observed. From the very beginning, every long RNA class has been associated with the ceRNA mechanism: -genes, together with their mRNA related transcript [45,69]; lncRNAs with their pervasive presence [120,168,169]; circRNAs with their large number of MREs and longer lifetime [170,171]; protein-coding RNAs themselves exhibiting coding independent functions [49].
Several new ncRNAs (both lncRNA and circRNA) are constantly highlighted through tissue- and cell-specific next generation sequencing high-throughput experiments. Predictive gene regulatory frameworks are fundamental to obtaining a high-confidence functional characterization of these new molecules and to increasing the knowledge of the precise role of ncRNA. For instance, lncRNA HOTAIR was characterized as one of the most important regulatory ncRNAs in human cells because of its oncogenic role. It is located on chromosome 12q13.13 and transcribed from an antisense strand of the HoxC gene [172]. HOTAIR was used as a prognostic biomarker for its role in the initiation and progression of different tumor types and malignancies [119,173] and characterized for its ability to regulate gene expression, specifically to repress transcripts in the HOXD cluster, by binding the polycomb repressive complex (PRC)2 and by recruiting (PRC)2 itself to the locus [174], or to up-regulate SOX2 by epigenetically suppressing miR-34a and, in doing so, managing cell proliferation regulation [175]. Alongside this application, HOTAIR was associated with a competing mechanism in gallbladder cancer for its relationship with the c-Myc-activated pathway of malignancy and its negative regulation of miRNA-130a [119] and in gastric cancer for its regulation of HER2 expression by sponging miR-331-3p [120]. ceRNA crosstalk highlighted the regulatory role of HOTAIR on the ceRNA-characterized tumor suppressor protein coding gene (PTEN) [45,49] but in the different biological context of cardiac hypertrophy through its inhibitor activity of miR-19 [95].
In particular, specific in vitro and in vivo models were generated to experimentally validate the involvement of -genes in ceRNA crosstalk. After the pioneering work showing that PTEN and KRAS -genes are able to affect the levels of their cognate gene [45], the role of the BRAF -gene as ceRNA was studied in an ad hoc tumorigenic system [69]. Several human cancers, including B cell lymphomas, show aberrations of BRAFP1, both at genomic and transcriptional levels; -gene BRAFP1 acts as a ceRNA with BRAF in human cancer cell lines, where the silencing of BRAFP1 affects MAPK signaling and cells proliferation; BRAFP1 mouse ortholog, BRAF-rs1, shows similar oncogenic activity in in vitro settings. From these considerations, mouse models, able to mimic human diffuse large B cell lymphoma, were engineered to overexpress murine BRAF -gene BRAF-rs1 and to follow its putative activity as ceRNA. Three different and independent Dox-inducible settings were planned: the overexpression of full-length -gene, its coding sequence and its 3’UTR. The oncogenic potential of -BRAF was underlined by the need to have no supplementary engineered mutations to force the onset of the phenotype and by the possibility to completely regress the tumor upon Dox withdrawal. -BRAF molecules were sensitive to miRNA activity and able to sequester specific miRNA acting on both BRAF and -BRAF, like miR-134, miR-543 and miR-653, leading to increased levels of BRAF when -gene BRAF-rs1 overexpression was activated. Though with different severity, all three engineered systems displayed a similar tumor phenotype supporting the hypothesis of an in vivo partial ceRNA regulation of BRAF through BRAF-rs1.
In a large portion of tumor types, from the most diffuse types, such as breast, colorectal and lung cancer, to the most rare types, such as head and neck squamous cell carcinoma or clear-cell renal cell carcinoma, -genes have been found dysregulated and their aberrant expressions, up-regulation or down-modulation, have been related to both oncogenic and tumor suppressor activities, respectively. Although -genes and -gene-derived lncRNA can affect gene expression regulation through other regulatory mechanisms, such as binding to transcription factors, several other ceRNA associations were observed involving -genes and -gene-derived lncRNA, in human cancer, sharing MREs and competing for common miRNAs with cognate or non-cognate genes [176].
OCT4, a key regulatory gene in the maintenance of stem cell pluripotency and proliferation, was found overexpressed in multiple human tumors too. Interestingly, in these aberrant conditions, miR-145 has been seen as a common mediator between OCT4 and two of its -genes, OCT4-pg4 and OCT4-pg5. Specifically, in hepatocarcinogenesis, the oncogenic role of OCT4-pg4, as a ceRNA, emerges by preventing OCT4 transcript inhibition by decoying miR-145. In particular, OCT4-pg4 is located in chromosomal region 1q22, frequently amplified in hepatocellular carcinoma and this -gene isoform, lacking the 3’UTR original region, harbors seed matches for miR-145 in the portion of sequence deriving from the coding sequence of the parental OCT4. Its high expression in hepatocellular carcinoma is able to sequester miR-145 molecules and, therefore, to de-repress OCT4 leading to the coding gene aberrant high expression in this context [117]. Similarly, OCT4-pg5 is overexpressed in endometrial cancer and shows a positive correlation with OCT4 high expression in the same tumor. This -gene isoform can be directly targeted by miR-145 thanks to the MREs in the conserved 3’UTR region, and its role in miR-145-mediated endometrial carcinoma cell proliferation emerges in the regulation of OCT4 expression, by competing for miR-145, and of PI3K/AKT-cyclin D1 signaling pathway [118].
Tumors continue to highlight many new molecular mutations and cellular defects that affect complex transcriptional and post-transcriptional balance [155,177] in almost all tissue-specific gene regulation networks [178,179,180,181,182,183,184]. International consortia efforts, such as The Cancer Genome Atlas (TCGA) [185], represent a huge step forward, and have collected and publicly shared the data of transcripts and protein expression, sequences and genomic variants and other omics data. Altogether, these efforts have made possible the transition from highly specific and context-dependent ncRNA–miRNA–mRNA subsets to wide-ranging systems of biological studies characterized by a much more general, complete and detailed set of regulatory networks [186,187,188].
3. Conclusions
This review traces a brief summary of the evolution of the ceRNA hypothesis and how the scientific community has expanded its range of application. The ceRNA mechanism was found to operate in a very broad set of biological contexts and almost all families of RNA molecules can participate in these regulatory strategies.
Together with some experimental evidence in in vivo models, the vast majority of ceRNA crosstalk is still predicted through in silico strategies and are shown to be effective in in vitro settings. Bioinformatics predictions demand caution in the acceptance of these suggestions [114] deriving from indirect biological evidence and statistical methods, but nevertheless, they demonstrate that molecules can participate in this regulatory mechanism and, at the same time, offer a large number of relationships that can be tested experimentally. The same miRNA identifiers used reflect the usage of mixed experimental strategies, which require bioinformatics analysis and studies on animal models and human cell lines. miRNAs are often indicated through their family name without specifying the species prefix, reflecting their involvement in relationships that play an important role in different species, demonstrating further the broad conservation of the action of the miRNAs themselves.
In particular, the importance of the ceRNA mechanism is related to its capacity to offer new indications for diagnostic and prognostic putative biomarkers [94,189], targets for drugs such as in hypertrophic scars treatment [190] and predictions for therapeutic strategies, such as in the cell proliferation of complex mechanisms in regenerating livers [87,178], or as in the broad spectrum of tumors [191,192,193,194] or still as in the pathogenesis of early HIV infection and its related antiviral therapy [165].
Efforts spent in modeling ceRNA interactions and in exploring their most permissive molecular environments [195] had the advantage of having contributed to the study and knowledge of the molecules stoichiometry balance inside the cells and can further improve precision medicine associated with the other most well known complex regulatory circuits (proteomics, metabolomics and more general omics) [196,197].
At the same time, the organization of all the information already available, coming from thousands of studies and spanning protein and RNA expression and co-expression networks, miRNAs target prediction information, transcription factors activity, epigenetics and genomic topological knowledge should be organized in order to allow for an easy expansion and integration with future studies and to make immediately accessible the wealth of details and particularities that biological interconnections offer at the level of different cells, tissues and organs contexts, stages of development and aging. As well as dynamic and multipartite network approaches should be adopted to display information and to browse across interactors, cellular contexts and diseases.
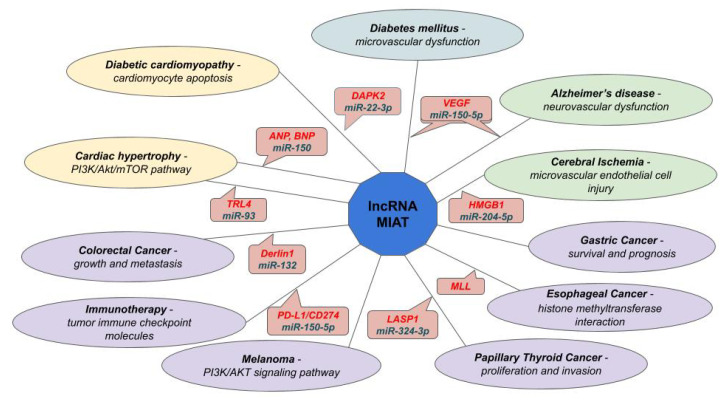
As a small and non-exhaustive example, lncRNA-myocardial infarction and associated transcript (MIAT) was characterized in a large variety of conditions [198] (see Figure 4). As already mentioned when discussing cardiac hypertrophy, MIAT has been found acting on at least two distinct miRNAs in this abnormal enlargement and thickening of the heart muscle. miR-150, an important miRNA involved in cardiac and cardiomyocyte hypertrophy, affects the development of cardiac hypertrophy as a downstream effector of MIAT [96]. miR-93 differential abundance, mediated by lncRNA-MIAT, influences the expression of target genes, as the highly conserved Toll-like receptor 4 (TLR4). In particular, MIAT knockdown can enhance miR-93 and inactivate the PI3K/Akt/mTOR pathway via regulating the TLR4 in angiotensin II-induced cardiac hypertrophy [97]. MIAT works as a ceRNA in pathological angiogenesis related to diabetes mellitus microvascular dysfunction, by competing with vascular endothelial growth factor (VEGF) for miR-150-5p [116] and, following the same pathway, in neurovascular dysfunction by inducing progressive neuronal loss and Alzheimer’s disease [113]. After cerebral ischemia, lncRNA-MIAT has proved to regulate the expression of HMGB1 (high-mobility group box 1) in cerebral microvascular endothelial cell (CMEC) injury by competing for miR-204-5p [84]. The same lncRNA has been associated with diabetic cardiomyopathy (DCM) for it affects the expression of death-associated protein kinase 2 (DAPK2) by sponging miR-22-3p: the resulting up-regulation of DAPK2 itself leads to cardiomyocyte apoptosis in DCM [108]. Its involvements in cancer are emerging too: in melanoma by acting on key master regulators of the signaling pathway [199]; in papillary thyroid cancer by sponging hsa-miR-324-3p and up-regulating LIM and SH3 domain protein 1 (LASP1) [122]; in colorectal cancer by regulating the miR-132/Derlin-1 pathway [121]. New observations have emerged in the control of immune checkpoint molecules: through hsa-miR-150-5p sponge interaction, lncRNA-MIAT, together with HLA complex P5 (HCP5), has been associated with the up-regulated expression of PD-L1/CD274, suggesting new involvements in the field of tumor immunity and immunotherapy [123]. Independently from the ceRNA mechanism, the same lnc-RNA MIAT molecules were characterized as oncogenic in esophageal cancer, as they promote cell invasion and migration by interacting with histone methyltransferase mixed-lineage leukemia (MLL) proteins [200] and, moreover, in gastric cancer, they have been linked to the prognosis and survival predictions: for instance, high MIAT level in serum exosomal characterizes patients as more prone to develop gastric cancer and its up-regulation is associated with shorter survival periods and represents an independent prognostic factor for gastric cancer [201].
Figure 4.
Partial representation of regulatory interaction of lnc-myocardial infarction and associated transcript (MIAT): in ellipses, diseases are reported in bold and dysfunctions in italics; in comics, interacting ceRNAs, or influenced transcripts, are reported in italics red and miRNAs in italics blue. Different colors of ellipses are linked to different types of pathologies.
ceRNA crosstalk is widespread in many contexts and the integration of this mechanism with all the other layers of gene regulation will guarantee new ways of increasing the understanding of molecular and cellular mechanisms and to intervene in increasingly punctual and specific ways in medicine.
Acknowledgments
The author is grateful to Paolo Provero and Ferdinando Di Cunto for their proof-readings and comments.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not for profit sectors.
Conflicts of Interest
The author declares no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Bartel D.P., Chen C.Z. Micromanagers of gene expression: The potentially widespread influence of metazoan microRNAs. Nat. Rev. Genet. 2004;5:396–400. doi: 10.1038/nrg1328. [DOI] [PubMed] [Google Scholar]
- 2.Bartel D.P. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 4.Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA hypothesis: The Rosetta Stone of a hid RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brosius J., Raabe C.A. What is an RNA? A top layer for RNA classification. RNA Biol. 2016;13:140–144. doi: 10.1080/15476286.2015.1128064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.St Laurent G., Wahlestedt C., Kapranov P. The Landscape of long noncoding RNA classification. Trends Genet. 2015;31:239–251. doi: 10.1016/j.tig.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahariya S., Paddibhatla I., Kumar S., Raghuwanshi S., Pallepati A., Gutti R.K. Long non-coding RNA: Classification, biogenesis and functions in blood cells. Mol. Immunol. 2019;112:82–92. doi: 10.1016/j.molimm.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Kopp F., Mendell J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell. 2018;172:393–407. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalvari I., Argasinska J., Quinones-Olvera N., Nawrocki E.P., Rivas E., Eddy S.R., Bateman A., Finn R.D., Petrov A.I. Rfam 13.0: Shifting to a genome-centric resource for non-coding RNA families. Nucleic Acids Res. 2018;46:D335–D342. doi: 10.1093/nar/gkx1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalvari I., Nawrocki E.P., Argasinska J., Quinones-Olvera N., Finn R.D., Bateman A., Petrov A.I. Non-Coding RNA Analysis Using the Rfam Database. Curr. Protoc. Bioinform. 2018;62:e51. doi: 10.1002/cpbi.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C.C., Qian X., Yoon B.J. RNAdetect: Efficient computational detection of novel non-coding RNAs. Bioinformatics. 2019;35:1133–1141. doi: 10.1093/bioinformatics/bty765. [DOI] [PubMed] [Google Scholar]
- 12.Uszczynska-Ratajczak B., Lagarde J., Frankish A., Guig R., Johnson R. Towards a complete map of the human long non-coding RNA transcriptome. Nat. Rev. Genet. 2018;19:535–548. doi: 10.1038/s41576-018-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frankish A., Diekhans M., Ferreira A.M., Johnson R., Jungreis I., Loveland J., Mudge J.M., Sisu C., Wright J., Armstrong J., et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019;47:D766–D773. doi: 10.1093/nar/gky955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang S., Zhang L., Guo J., Niu Y., Wu Y., Li H., Zhao L., Li X., Teng X., Sun X., et al. NONCODEV5: A comprehensive annotation database for long non-coding RNAs. Nucleic Acids Res. 2018;46:D308–D314. doi: 10.1093/nar/gkx1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyer M.K., Niknafs Y.S., Malik R., Singhal U., Sahu A., Hosono Y., Barrette T.R., Prensner J.R., Evans J.R., Zhao S., et al. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 2015;47:199–208. doi: 10.1038/ng.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pal D., Rao M.R.S. Long Noncoding RNAs in Pluripotency of Stem Cells and Cell Fate Specification. Adv. Exp. Med. Biol. 2017;1008:223–252. doi: 10.1007/978-981-10-5203-3_8. [DOI] [PubMed] [Google Scholar]
- 17.Wang K.C., Chang H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fatica A., Bozzoni I. Long non-coding RNAs: New players in cell differentiation and development. Nat. Rev. Genet. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- 19.Zhang N., Meng X., Mei L., Hu J., Zhao C., Chen W. The Long Non-Coding RNA SNHG1 Attenuates Cell Apoptosis by Regulating miR-195 and BCL2-Like Protein 2 in Human Cardiomyocytes. Cell Physiol. Biochem. 2018;50:1029–1040. doi: 10.1159/000494514. [DOI] [PubMed] [Google Scholar]
- 20.Flynn R.A., Chang H.Y. Long noncoding RNAs in cell-fate programming and reprogramming. Cell Stem Cell. 2014;14:752–761. doi: 10.1016/j.stem.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Degirmenci U., Lei S. Role of lncRNAs in Cellular Aging. Front. Endocrinol. (Lausanne) 2016;7:151. doi: 10.3389/fendo.2016.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costa M.C., Leito A.L., Enguita F.J. Noncoding Transcriptional Landscape in Human Aging. Curr. Top. Microbiol. Immunol. 2016;394:177–202. doi: 10.1007/82_2015_460. [DOI] [PubMed] [Google Scholar]
- 23.Jain S., Thakkar N., Chhatai J., Pal Bhadra M., Bhadra U. Long non-coding RNA: Functional agent for disease traits. RNA Biol. 2017;14:522–535. doi: 10.1080/15476286.2016.1172756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mercer T.R., Dinger M.E., Mattick J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 25.Ulitsky I., Bartel D.P. lincRNAs: Genomics, evolution, and mechanisms. Cell. 2013;154:26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dykes I.M., Emanueli C. Transcriptional and Post-transcriptional Gene Regulation by Long Non-coding RNA. Genom. Proteom. Bioinform. 2017;15:177–186. doi: 10.1016/j.gpb.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang J., Zhang A., Ho T.T., Zhang Z., Zhou N., Ding X., Zhang X., Xu M., Mo Y.Y. Linc-RoR promotes c-Myc expression through hnRNP I and AUF1. Nucleic Acids Res. 2016;44:3059–3069. doi: 10.1093/nar/gkv1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon J.H., Abdelmohsen K., Srikantan S., Yang X., Martindale J.L., De S., Huarte M., Zhan M., Becker K.G., Gorospe M. LincRNA-p21 suppresses target mRNA translation. Mol. Cell. 2012;47:648–655. doi: 10.1016/j.molcel.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandes J.C.R., Acua S.M., Aoki J.I., Floeter-Winter L.M., Muxel S.M. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Noncoding RNA. 2019;5:17. doi: 10.3390/ncrna5010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu W., Gius D., Onyango P., Muldoon-Jacobs K., Karp J., Feinberg A.P., Cui H. Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA. Nature. 2008;451:202–206. doi: 10.1038/nature06468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meller V.H., Joshi S.S., Deshpande N. Modulation of Chromatin by Noncoding RNA. Annu. Rev. Genet. 2015;49:673–695. doi: 10.1146/annurev-genet-112414-055205. [DOI] [PubMed] [Google Scholar]
- 32.Peng W.X., Koirala P., Mo Y.Y. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36:5661–5667. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tutar Y. Pseudogenes. Comp. Funct. Genom. 2012;2012:424526. doi: 10.1155/2012/424526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z., Huang C., Bao C., Chen L., Lin M., Wang X., Zhong G., Yu B., Hu W., Dai L., et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015;22:256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 35.Barrett S.P., Salzman J. Circular RNAs: Analysis, expression and potential functions. Development. 2016;143:1838–1847. doi: 10.1242/dev.128074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pu M., Chen J., Tao Z., Miao L., Qi X., Wang Y., Ren J. Regulatory network of miRNA on its target: Coordination between transcriptional and post-transcriptional regulation of gene expression. Cell. Mol. Life Sci. 2019;76:441–451. doi: 10.1007/s00018-018-2940-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 38.Khan S., Ayub H., Khan T., Wahid F. MicroRNA biogenesis, gene silencing mechanisms and role in breast, ovarian and prostate cancer. Biochimie. 2019;167:12–24. doi: 10.1016/j.biochi.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Lund E., Dahlberg J.E. Substrate selectivity of exportin 5 and Dicer in the biogenesis of microRNAs. Cold Spring Harb. Symp. Quant. Biol. 2006;71:59–66. doi: 10.1101/sqb.2006.71.050. [DOI] [PubMed] [Google Scholar]
- 40.Cai Y., Yu X., Hu S., Yu J. A brief review on the mechanisms of miRNA regulation. Genom. Proteom. Bioinform. 2009;7:147–154. doi: 10.1016/S1672-0229(08)60044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Catalanotto C., Cogoni C., Zardo G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016;17:1712. doi: 10.3390/ijms17101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ala U., Karreth F.A., Bosia C., Pagnani A., Taulli R., Leopold V., Tay Y., Provero P., Zecchina R., Pandolfi P.P. Integrated transcriptional and competitive endogenous RNA networks are cross-regulated in permissive molecular environments. Proc. Natl. Acad. Sci. USA. 2013;110:7154–7159. doi: 10.1073/pnas.1222509110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedman R.C., Farh K.K., Burge C.B., Bartel D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Djuranovic S., Nahvi A., Green R. miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science. 2012;336:237–240. doi: 10.1126/science.1215691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poliseno L., Salmena L., Zhang J., Carver B., Haveman W.J., Pandolfi P.P. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465:1033–1038. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.An Y., Furber K.L., Ji S. Pseudogenes regulate parental gene expression via ceRNA network. J. Cell. Mol. Med. 2017;21:185–192. doi: 10.1111/jcmm.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Long J., Xiong J., Bai Y., Mao J., Lin J., Xu W., Zhang H., Chen S., Zhao H. Construction and Investigation of a lncRNA-Associated ceRNA Regulatory Network in Cholangiocarcinoma. Front. Oncol. 2019;9:649. doi: 10.3389/fonc.2019.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z., Qian W., Wang S., Ji D., Wang Q., Li J., Peng W., Gu J., Hu T., Ji B., et al. Analysis of lncRNA-Associated ceRNA Network Reveals Potential lncRNA Biomarkers in Human Colon Adenocarcinoma. Cell. Physiol. Biochem. 2018;49:1778–1791. doi: 10.1159/000493623. [DOI] [PubMed] [Google Scholar]
- 49.Tay Y., Kats L., Salmena L., Weiss D., Tan S.M., Ala U., Karreth F., Poliseno L., Provero P., Di Cunto F., et al. Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell. 2011;147:344–357. doi: 10.1016/j.cell.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cesana M., Cacchiarelli D., Legnini I., Santini T., Sthandier O., Chinappi M., Tramontano A., Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karreth F.A., Tay Y., Perna D., Ala U., Tan S.M., Rust A.G., DeNicola G., Webster K.A., Weiss D., Perez-Mancera P.A., et al. In vivo identification of tumor- suppressive PTEN ceRNAs in an oncogenic BRAF-induced mouse model of melanoma. Cell. 2011;147:382–395. doi: 10.1016/j.cell.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sumazin P., Yang X., Chiu H.S., Chung W.J., Iyer A., Llobet-Navas D., Rajbhandari P., Bansal M., Guarnieri P., Silva J., et al. An extensive microRNA-mediated network of RNA-RNA interactions regulates established oncogenic pathways in glioblastoma. Cell. 2011;147:370–381. doi: 10.1016/j.cell.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karreth F.A., Ala U., Provero P., Pandolfi P.P. Pseudogenes as competitive endogenous RNAs: Target prediction and validation. Methods Mol. Biol. 2014;1167:199–212. doi: 10.1007/978-1-4939-0835-6_13. [DOI] [PubMed] [Google Scholar]
- 54.Chiu H.S., Llobet-Navas D., Yang X., Chung W.J., Ambesi-Impiombato A., Iyer A., Kim H.R., Seviour E.G., Luo Z., Sehgal V., et al. Cupid: Simultaneous reconstruction of microRNA-target and ceRNA networks. Genome Res. 2015;25:257–267. doi: 10.1101/gr.178194.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agarwal V., Bell G.W., Nam J.W., Bartel D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4:e0500. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miranda K.C., Huynh T., Tay Y., Ang Y.S., Tam W.L., Thomson A.M., Lim B., Rigoutsos I. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126:1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 57.Helwak A., Kudla G., Dudnakova T., Tollervey D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell. 2013;153:654–665. doi: 10.1016/j.cell.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chi S.W., Zang J.B., Mele A., Darnell R.B. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460:479–486. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamilton M.P., Rajapakshe K.I., Bader D.A., Cerne J.Z., Smith E.A., Coarfa C., Hartig S.M., McGuire S.E. The Landscape of microRNA Targeting in Prostate Cancer Defined by AGO-PAR-CLIP. Neoplasia. 2016;18:356–370. doi: 10.1016/j.neo.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hafner M., Landthaler M., Burger L., Khorshid M., Hausser J., Berninger P., Rothballer A., Ascano M., Jungkamp A.C., Munschauer M., et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129–141. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sarver A.L., Subramanian S. Competing endogenous RNA database. Bioinformation. 2012;8:731–733. doi: 10.6026/97320630008731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu K., Yan Z., Li Y., Sun Z. Linc2GO: A human LincRNA function annotation resource based on ceRNA hypothesis. Bioinformatics. 2013;29:2221–2222. doi: 10.1093/bioinformatics/btt361. [DOI] [PubMed] [Google Scholar]
- 63.Li J.H., Liu S., Zhou H., Qu L.H., Yang J.H. starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–D97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang P., Zhi H., Zhang Y., Liu Y., Zhang J., Gao Y., Guo M., Ning S., Li X. miRSponge: A manually curated database for experimentally supported miRNA sponges and ceRNAs. Database (Oxford) 2015;2015 doi: 10.1093/database/bav098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang P., Li X., Gao Y., Guo Q., Wang Y., Fang Y., Ma X., Zhi H., Zhou D., Shen W., et al. LncACTdb 2.0: An updated database of experimentally supported ceRNA interactions curated from low- and high-throughput experiments. Nucleic Acids Res. 2019;47:D121–D127. doi: 10.1093/nar/gky1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang J., Liu L., Xu T., Xie Y., Zhao C., Li J., Le T.D. miRspongeR: An R-Bioconductor package for the identification and analysis of miRNA sponge interaction networks and modules. BMC Bioinform. 2019;20:235. doi: 10.1186/s12859-019-2861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mengying Z., Yongsheng L., Xu J., Li X. CeRNASeek: Identification and Analysis of ceRNA Regulation. [(accessed on 24 June 2020)];2020 Available online: https://cran.r-project.org/web/packages/CeRNASeek/CeRNASeek.pdf.
- 68.Junpeng Z. miRSM: Inferring miRNA Sponge Modules by Integrating Expression Data and miRNA-Target Binding Information. [(accessed on 24 June 2020)];2020 Available online: https://www.bioconductor.org/packages/devel/bioc/vignettes/miRSM/inst/doc/miRSM.html.
- 69.Karreth F.A., Reschke M., Ruocco A., Ng C., Chapuy B., Lopold V., Sjoberg M., Keane T.M., Verma A., Ala U., et al. The BRAF pseudogene functions as a competitive endogenous RNA and induces lymphoma in vivo. Cell. 2015;161:319–332. doi: 10.1016/j.cell.2015.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bosia C., Pagnani A., Zecchina R. Modelling Competing Endogenous RNA Networks. PLoS ONE. 2013;8:e66609. doi: 10.1371/journal.pone.0066609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Figliuzzi M., Marinari E., De Martino A. MicroRNAs as a selective channel of communication between competing RNAs: A steady-state theory. Biophys. J. 2013;104:1203–1213. doi: 10.1016/j.bpj.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Denzler R., Agarwal V., Stefano J., Bartel D.P., Stoffel M. Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol. Cell. 2014;54:766–776. doi: 10.1016/j.molcel.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bosson A.D., Zamudio J.R., Sharp P.A. Endogenous miRNA and target concentrations determine susceptibility to potential ceRNA competition. Mol. Cell. 2014;56:347–359. doi: 10.1016/j.molcel.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Denzler R., McGeary S.E., Title A.C., Agarwal V., Bartel D.P., Stoffel M. Impact of MicroRNA Levels, Target-Site Complementarity, and Cooperativity on Competing Endogenous RNA-Regulated Gene Expression. Mol. Cell. 2016;64:565–579. doi: 10.1016/j.molcel.2016.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bosia C., Sgro F., Conti L., Baldassi C., Brusa D., Cavallo F., Cunto F.D., Turco E., Pagnani A., Zecchina R. RNAs competing for microRNAs mutually influence their fluctuations in a highly non-linear microRNA-dependent manner in single cells. Genome Biol. 2017;18:37. doi: 10.1186/s13059-017-1162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martirosyan A., Del Giudice M., Bena C.E., Pagnani A., Bosia C., De Martino A. Kinetic Modelling of Competition and Depletion of Shared miRNAs by Competing Endogenous RNAs. Methods Mol. Biol. 2019;1912:367–409. doi: 10.1007/978-1-4939-8982-9_15. [DOI] [PubMed] [Google Scholar]
- 77.Lasda E., Parker R. Circular RNAs: Diversity of form and function. RNA. 2014;20:1829–1842. doi: 10.1261/rna.047126.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qu S., Liu Z., Yang X., Zhou J., Yu H., Zhang R., Li H. The emerging functions and roles of circular RNAs in cancer. Cancer Lett. 2018;414:301–309. doi: 10.1016/j.canlet.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 79.Salzman J., Gawad C., Wang P.L., Lacayo N., Brown P.O. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Memczak S., Jens M., Elefsinioti A., Torti F., Krueger J., Rybak A., Maier L., Mackowiak S.D., Gregersen L.H., Munschauer M., et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 81.Enuka Y., Lauriola M., Feldman M.E., Sas-Chen A., Ulitsky I., Yarden Y. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 2016;44:1370–1383. doi: 10.1093/nar/gkv1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilusz J.E., Sharp P.A. Molecular biology. A circuitous route to noncoding RNA. Science. 2013;340:440–441. doi: 10.1126/science.1238522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salzman J. Circular RNA Expression: Its Potential Regulation and Function. Trends Genet. 2016;32:309–316. doi: 10.1016/j.tig.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deng W., Fan C., Shen R., Wu Y., Du R., Teng J. Long noncoding MIAT acting as a ceRNA to sponge microRNA-204-5p to participate in cerebral microvascular endothelial cell injury after cerebral ischemia through regulating HMGB1. J. Cell. Physiol. 2020;235:4571–4586. doi: 10.1002/jcp.29334. [DOI] [PubMed] [Google Scholar]
- 85.Tay Y., Rinn J., Pandolfi P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cai Y., Sun Z., Jia H., Luo H., Ye X., Wu Q., Xiong Y., Zhang W., Wan J. Rpph1 Upregulates CDC42 Expression and Promotes Hippocampal Neuron Dendritic Spine Formation by Competing with miR-330-5p. Front. Mol. Neurosci. 2017;10:27. doi: 10.3389/fnmol.2017.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang G., Guo X., Cheng L., Chu P., Chen M., Chen Y., Chang C. An integrated analysis of the circRNA-miRNA-mRNA network reveals novel insights into potential mechanisms of cell proliferation during liver regeneration. Artif. Cells Nanomed. Biotechnol. 2019;47:3873–3884. doi: 10.1080/21691401.2019.1669623. [DOI] [PubMed] [Google Scholar]
- 88.Valluy J., Bicker S., Aksoy-Aksel A., Lackinger M., Sumer S., Fiore R., Wst T., Seffer D., Metge F., Dieterich C., et al. A coding-independent function of an alternative Ube3a transcript during neuronal development. Nat. Neurosci. 2015;18:666–673. doi: 10.1038/nn.3996. [DOI] [PubMed] [Google Scholar]
- 89.Yu Y., Chen Y., Zhang X., Lu X., Hong J., Guo X., Zhou D. Knockdown of lncRNA KCNQ1OT1 suppresses the adipogenic and osteogenic differentiation of tendon stem cell via downregulating miR-138 target genes PPARgamma and RUNX2. Cell Cycle. 2018;17:2374–2385. doi: 10.1080/15384101.2018.1534510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang X., Zhang J., Zheng K., Zhang H., Pei X., Yin Z., Wen D., Kong Q. Long noncoding RNAs sustain high expression levels of exogenous octamer-binding protein 4 by sponging regulatory microRNAs during cellular reprogramming. J. Biol. Chem. 2019;294:17863–17874. doi: 10.1074/jbc.RA119.010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen M.T., Lin H.S., Shen C., Ma Y.N., Wang F., Zhao H.L., Yu J., Zhang J.W.P.U. 1-Regulated Long Noncoding RNA lnc-MC Controls Human Monocyte/Macrophage Differentiation through Interaction with MicroRNA 199a-5p. Mol. Cell. Biol. 2015;35:3212–3224. doi: 10.1128/MCB.00429-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chistiakov D.A., Orekhov A.N., Bobryshev Y.V. Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction) J. Mol. Cell. Cardiol. 2016;94:107–121. doi: 10.1016/j.yjmcc.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 93.Bernardo B.C., Ooi J.Y., Lin R.C., McMullen J.R. miRNA therapeutics: A new class of drugs with potential therapeutic applications in the heart. Future Med. Chem. 2015;7:1771–1792. doi: 10.4155/fmc.15.107. [DOI] [PubMed] [Google Scholar]
- 94.Fan Z., Gao S., Chen Y., Xu B., Yu C., Yue M., Tan X. Integrative analysis of competing endogenous RNA networks reveals the functional lncRNAs in heart failure. J. Cell. Mol. Med. 2018;22:4818–4829. doi: 10.1111/jcmm.13739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lai Y., He S., Ma L., Lin H., Ren B., Ma J., Zhu X., Zhuang S. HOTAIR functions as a competing endogenous RNA to regulate PTEN expression by inhibiting miR-19 in cardiac hypertrophy. Mol. Cell. Biochem. 2017;432:179–187. doi: 10.1007/s11010-017-3008-y. [DOI] [PubMed] [Google Scholar]
- 96.Zhu X.H., Yuan Y.X., Rao S.L., Wang P. LncRNA MIAT enhances cardiac hypertrophy partly through sponging miR-150. Eur. Rev. Med. Pharmacol. Sci. 2016;20:3653–3660. [PubMed] [Google Scholar]
- 97.Li Y., Wang J., Sun L., Zhu S. LncRNA myocardial infarction-associated transcript (MIAT) contributed to cardiac hypertrophy by regulating TLR4 via miR-93. Eur. J. Pharmacol. 2018;818:508–517. doi: 10.1016/j.ejphar.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 98.Song C., Zhang J., Liu Y., Pan H., Qi H.P., Cao Y.G., Zhao J.M., Li S., Guo J., Sun H.L., et al. Construction and analysis of cardiac hypertrophy-associated lncRNA-mRNA network based on competitive endogenous RNA reveal functional lncRNAs in cardiac hypertrophy. Oncotarget. 2016;7:10827–10840. doi: 10.18632/oncotarget.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang K., Liu F., Zhou L.Y., Long B., Yuan S.M., Wang Y., Liu C.Y., Sun T., Zhang X.J., Li P.F. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ. Res. 2014;114:1377–1388. doi: 10.1161/CIRCRESAHA.114.302476. [DOI] [PubMed] [Google Scholar]
- 100.Wo Y., Guo J., Li P., Yang H., Wo J. Long non-coding RNA CHRF facilitates cardiac hypertrophy through regulating Akt3 via miR-93. Cardiovasc. Pathol. 2018;35:29–36. doi: 10.1016/j.carpath.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 101.Heineke J., Molkentin J.D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat. Rev. Mol. Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 102.Wang K., Liu C.Y., Zhou L.Y., Wang J.X., Wang M., Zhao B., Zhao W.K., Xu S.J., Fan L.H., Zhang X.J., et al. APF lncRNA regulates autophagy and myocardial infarction by targeting miR-188-3p. Nat. Commun. 2015;6:6779. doi: 10.1038/ncomms7779. [DOI] [PubMed] [Google Scholar]
- 103.Liang H., Pan Z., Zhao X., Liu L., Sun J., Su X., Xu C., Zhou Y., Zhao D., Xu B., et al. LncRNA PFL contributes to cardiac fibrosis by acting as a competing endogenous RNA of let-7d. Theranostics. 2018;8:1180–1194. doi: 10.7150/thno.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gu X., Jiang Y.N., Wang W.J., Zhang J., Shang D.S., Sun C.B., Tian J.T., Tian J.W., Yu B., Zhang Y. Comprehensive circRNA expression profile and construction of circRNA-related ceRNA network in cardiac fibrosis. Biomed. Pharmacother. 2020;125:109944. doi: 10.1016/j.biopha.2020.109944. [DOI] [PubMed] [Google Scholar]
- 105.Zhou B., Yu J.W. A novel identified circular RNA, circRNA-010567, promotes myocardial fibrosis via suppressing miR-141 by targeting TGF-beta1. Biochem. Biophys. Res. Commun. 2017;487:769–775. doi: 10.1016/j.bbrc.2017.04.044. [DOI] [PubMed] [Google Scholar]
- 106.Tang C.M., Zhang M., Huang L., Hu Z.Q., Zhu J.N., Xiao Z., Zhang Z., Lin Q.X., Zheng X.L., Yang M., et al. CircRNA-000203 enhances the expression of fibrosis-associated genes by derepressing targets of miR-26b-5p, Col1a2 and CTGF, in cardiac fibroblasts. Sci. Rep. 2017;7:40342. doi: 10.1038/srep40342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ni H., Li W., Zhuge Y., Xu S., Wang Y., Chen Y., Shen G., Wang F. Inhibition of circHIPK3 prevents angiotensin II-induced cardiac fibrosis by sponging miR-29b-3p. Int. J. Cardiol. 2019;292:188–196. doi: 10.1016/j.ijcard.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 108.Zhou X., Zhang W., Jin M., Chen J., Xu W., Kong X. lncRNA MIAT functions as a competing endogenous RNA to upregulate DAPK2 by sponging miR-22-3p in diabetic cardiomyopathy. Cell Death Dis. 2017;8:e2929. doi: 10.1038/cddis.2017.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hu C., Bai X., Liu C., Hu Z. Long noncoding RNA XIST participates hypoxia-induced angiogenesis in human brain microvascular endothelial cells through regulating miR-485/SOX7 axis. Am. J. Transl. Res. 2019;11:6487–6497. doi: 10.1111/micc.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tan J.Y., Vance K.W., Varela M.A., Sirey T., Watson L.M., Curtis H.J., Marinello M., Alves S., Steinkraus B., Cooper S., et al. Cross-talking noncoding RNAs contribute to cell-specific neurodegeneration in SCA7. Nat. Struct. Mol. Biol. 2014;21:955–961. doi: 10.1038/nsmb.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Faghihi M.A., Zhang M., Huang J., Modarresi F., Van der Brug M.P., Nalls M.A., Cookson M.R., St-Laurent G., Wahlestedt C. Evidence for natural antisense transcript-mediated inhibition of microRNA function. Genome Biol. 2010;11:R56. doi: 10.1186/gb-2010-11-5-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Roberts T.C., Morris K.V., Wood M.J. The role of long non-coding RNAs in neurodevelopment, brain function and neurological disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369 doi: 10.1098/rstb.2013.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiang Q., Shan K., Qun-Wang X., Zhou R.M., Yang H., Liu C., Li Y.J., Yao J., Li X.M., Shen Y., et al. Long non-coding RNA-MIAT promotes neurovascular remodeling in the eye and brain. Oncotarget. 2016;7:49688–49698. doi: 10.18632/oncotarget.10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang S., Cao F., Gu X., Chen J., Xu R., Huang Y., Ying L. LncRNA XIST, as a ceRNA of miR-204, aggravates lipopolysaccharide-induced acute respiratory distress syndrome in mice by upregulating IRF2. Int. J. Clin. Exp. Pathol. 2019;12:2425–2434. [PMC free article] [PubMed] [Google Scholar]
- 115.Huang X., Pan J., Wu B., Teng X. Construction and analysis of a lncRNA (PWRN2)-mediated ceRNA network reveal its potential roles in oocyte nuclear maturation of patients with PCOS. Reprod. Biol. Endocrinol. 2018;16:73. doi: 10.1186/s12958-018-0392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yan B., Yao J., Liu J.Y., Li X.M., Wang X.Q., Li Y.J., Tao Z.F., Song Y.C., Chen Q., Jiang Q. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ. Res. 2015;116:1143–1156. doi: 10.1161/CIRCRESAHA.116.305510. [DOI] [PubMed] [Google Scholar]
- 117.Wang L., Guo Z.Y., Zhang R., Xin B., Chen R., Zhao J., Wang T., Wen W.H., Jia L.T., Yao L.B., et al. Pseudogene OCT4-pg4 functions as a natural micro RNA sponge to regulate OCT4 expression by competing for miR-145 in hepatocellular carcinoma. Carcinogenesis. 2013;34:1773–1781. doi: 10.1093/carcin/bgt139. [DOI] [PubMed] [Google Scholar]
- 118.Bai M., Yuan M., Liao H., Chen J., Xie B., Yan D., Xi X., Xu X., Zhang Z., Feng Y. OCT4 pseudogene 5 upregulates OCT4 expression to promote proliferation by competing with miR-145 in endometrial carcinoma. Oncol. Rep. 2015;33:1745–1752. doi: 10.3892/or.2015.3763. [DOI] [PubMed] [Google Scholar]
- 119.Ma M.Z., Li C.X., Zhang Y., Weng M.Z., Zhang M.D., Qin Y.Y., Gong W., Quan Z.W. Long non-coding RNA HOTAIR, a c-Myc activated driver of malignancy, negatively regulates miRNA-130a in gallbladder cancer. Mol. Cancer. 2014;13:156. doi: 10.1186/1476-4598-13-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu X.H., Sun M., Nie F.Q., Ge Y.B., Zhang E.B., Yin D.D., Kong R., Xia R., Lu K.H., Li J.H., et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol. Cancer. 2014;13:92. doi: 10.1186/1476-4598-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu Z., Wang H., Cai H., Hong Y., Li Y., Su D., Fan Z. Long non-coding RNA MIAT promotes growth and metastasis of colorectal cancer cells through regulation of miR-132/Derlin-1 pathway. Cancer Cell Int. 2018;18:59. doi: 10.1186/s12935-017-0477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu W., Wang Z., Wang C., Ai Z. Long non-coding RNA MIAT promotes papillary thyroid cancer progression through upregulating LASP1. Cancer Cell Int. 2019;19:194. doi: 10.1186/s12935-019-0913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu S., Wang Q., Kang Y., Liu J., Yin Y., Liu L., Wu H., Li S., Sui S., Shen M., et al. Long noncoding RNAs control the modulation of immune checkpoint molecules in cancer. Cancer Immunol. Res. 2020 doi: 10.1158/2326-6066.CIR-19-0696. [DOI] [PubMed] [Google Scholar]
- 124.Miao L., Yin R.X., Zhang Q.H., Liao P.J., Wang Y., Nie R.J., Li H. A novel circRNA-miRNA-mRNA network identifies circ-YOD1 as a biomarker for coronary artery disease. Sci. Rep. 2019;9:18314. doi: 10.1038/s41598-019-54603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Y., Ke X., Liu J., Ma X., Liu Y., Liang D., Wang L., Guo C., Luo Y. Characterization of circRNA associated ceRNA networks in patients with nonvalvular persistent atrial fibrillation. Mol. Med. Rep. 2019;19:638–650. doi: 10.3892/mmr.2018.9695. [DOI] [PubMed] [Google Scholar]
- 126.Mao Y.Y., Wang J.Q., Guo X.X., Bi Y., Wang C.X. Circ-SATB2 upregulates STIM1 expression and regulates vascular smooth muscle cell proliferation and differentiation through miR-939. Biochem. Biophys. Res. Commun. 2018;505:119–125. doi: 10.1016/j.bbrc.2018.09.069. [DOI] [PubMed] [Google Scholar]
- 127.Sun J., Zhang Z., Yang S. Circ-RUSC2 upregulates the expression of miR-661 target gene SYK and regulates the function of vascular smooth muscle cells. Biochem. Cell Biol. 2019;97:709–714. doi: 10.1139/bcb-2019-0031. [DOI] [PubMed] [Google Scholar]
- 128.Wang K., Gan T.Y., Li N., Liu C.Y., Zhou L.Y., Gao J.N., Chen C., Yan K.W., Ponnusamy M., Zhang Y.H., et al. Circular RNA mediates cardiomyocyte death via miRNA-dependent upregulation of MTP18 expression. Cell Death Differ. 2017;24:1111–1120. doi: 10.1038/cdd.2017.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang S., Li X., Zheng H., Si X., Li B., Wei G., Li C., Chen Y., Chen Y., Liao W., et al. Loss of Super-Enhancer-Regulated circRNA Nfix Induces Cardiac Regeneration After Myocardial Infarction in Adult Mice. Circulation. 2019;139:2857–2876. doi: 10.1161/CIRCULATIONAHA.118.038361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li H., Xu J.D., Fang X.H., Zhu J.N., Yang J., Pan R., Yuan S.J., Zeng N., Yang Z.Z., Yang H., et al. Circular RNA circRNA-000203 aggravates cardiac hypertrophy via suppressing miR26b-5p and miR-140-3p binding to Gata4. Cardiovasc. Res. 2019;116:1323–1334. doi: 10.1093/cvr/cvz215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lim T.B., Aliwarga E., Luu T.D.A., Li Y.P., Ng S.L., Annadoray L., Sian S., Ackers-Johnson M.A., Foo R.S. Targeting the highly abundant circular RNA circSlc8a1 in cardiomyocytes attenuates pressure overload induced hypertrophy. Cardiovasc. Res. 2019;115:1998–2007. doi: 10.1093/cvr/cvz130. [DOI] [PubMed] [Google Scholar]
- 132.Yang F., Li A., Qin Y., Che H., Wang Y., Lv J., Li Y., Li H., Yue E., Ding X., et al. A Novel Circular RNA Mediates Pyroptosis of Diabetic Cardiomyopathy by Functioning as a Competing Endogenous RNA. Mol. Ther. Nucleic Acids. 2019;17:636–643. doi: 10.1016/j.omtn.2019.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zheng C., Niu H., Li M., Zhang H., Yang Z., Tian L., Wu Z., Li D., Chen X. Cyclic RNA hsa-circ-000595 regulates apoptosis of aortic smooth muscle cells. Mol. Med. Rep. 2015;12:6656–6662. doi: 10.3892/mmr.2015.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li M., Ding W., Tariq M.A., Chang W., Zhang X., Xu W., Hou L., Wang Y., Wang J. A circular transcript of ncx1 gene mediates ischemic myocardial injury by targeting miR-133a-3p. Theranostics. 2018;8:5855–5869. doi: 10.7150/thno.27285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou L.Y., Zhai M., Huang Y., Xu S., An T., Wang Y.H., Zhang R.C., Liu C.Y., Dong Y.H., Wang M., et al. The circular RNA ACR attenuates myocardial ischemia/reperfusion injury by suppressing autophagy via modulation of the Pink1/FAM65B pathway. Cell Death Differ. 2019;26:1299–1315. doi: 10.1038/s41418-018-0206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Su Q., Lv X. Revealing new landscape of cardiovascular disease through circular RNA-miRNA-mRNA axis. Genomics. 2020;112:1680–1685. doi: 10.1016/j.ygeno.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 137.Mao S., Zhang S., Zhou S., Huang T., Feng W., Gu X., Yu B. A Schwann cell-enriched circular RNA circ-Ankib1 regulates Schwann cell proliferation following peripheral nerve injury. FASEB J. 2019;33:12409–12424. doi: 10.1096/fj.201900965R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cai Y., Wan J. Competing Endogenous RNA Regulations in Neurodegenerative Disorders: Current Challenges and Emerging Insights. Front. Mol. Neurosci. 2018;11:370. doi: 10.3389/fnmol.2018.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang L.K., Chen X.F., He D.D., Li Y., Fu J. Dissection of functional lncRNAs in Alzheimer’s disease by construction and analysis of lncRNA-mRNA networks based on competitive endogenous RNAs. Biochem. Biophys. Res. Commun. 2017;485:569–576. doi: 10.1016/j.bbrc.2016.11.143. [DOI] [PubMed] [Google Scholar]
- 140.Zhang S., Zhu D., Li H., Li H., Feng C., Zhang W. Characterization of circRNA-Associated-ceRNA Networks in a Senescence-Accelerated Mouse Prone 8 Brain. Mol. Ther. 2017;25:2053–2061. doi: 10.1016/j.ymthe.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhou F., Xie S., Li J., Duan S. Long noncoding RNA HOTAIR promotes cell apoptosis by sponging miR-221 in Parkinson’s disease. RSC Adv. 2019;9:29502–29510. doi: 10.1039/C9RA06107J. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 142.Liu W., Zhang Q., Zhang J., Pan W., Zhao J., Xu Y. Long non-coding RNA MALAT1 contributes to cell apoptosis by sponging miR-124 in Parkinson disease. Cell Biosci. 2017;7:19. doi: 10.1186/s13578-017-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hu X., Hicks C.W., He W., Wong P., Macklin W.B., Trapp B.D., Yan R. Bace1 modulates myelination in the central and peripheral nervous system. Nat. Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 144.Laird F.M., Cai H., Savonenko A.V., Farah M.H., He K., Melnikova T., Wen H., Chiang H.C., Xu G., Koliatsos V.E., et al. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J. Neurosci. 2005;25:11693–11709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Querfurth H.W., LaFerla F.M. Alzheimer’s disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 146.Faghihi M.A., Modarresi F., Khalil A.M., Wood D.E., Sahagan B.G., Morgan T.E., Finch C.E., St Laurent G., Kenny P.J., Wahlestedt C. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat. Med. 2008;14:723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Da Rocha S.T., Heard E. Novel players in X inactivation: Insights into Xist-mediated gene silencing and chromosome conformation. Nat. Struct. Mol. Biol. 2017;24:197–204. doi: 10.1038/nsmb.3370. [DOI] [PubMed] [Google Scholar]
- 148.Yu Y., Wang Y., Xiao X., Cheng W., Hu L., Yao W., Qian Z., Wu W. MiR-204 inhibits hepatocellular cancer drug resistance and metastasis through targeting NUAK1. Biochem. Cell Biol. 2019;97:563–570. doi: 10.1139/bcb-2018-0354. [DOI] [PubMed] [Google Scholar]
- 149.Li T., Pan H., Li R. The dual regulatory role of miR-204 in cancer. Tumour Biol. 2016;37:11667–11677. doi: 10.1007/s13277-016-5144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Coolen M., Thieffry D., Drivenes Ø., Becker T.S., Bally-Cuif L. miR-9 controls the timing of neurogenesis through the direct inhibition of antagonistic factors. Dev. Cell. 2012;22:1052–1064. doi: 10.1016/j.devcel.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 151.Nissan X., Blondel S., Navarro C., Maury Y., Denis C., Girard M., Martinat C., De Sandre-Giovannoli A., Levy N., Peschanski M. Unique preservation of neural cells in Hutchinson- Gilford progeria syndrome is due to the expression of the neural-specific miR-9 microRNA. Cell Rep. 2012;2:1–9. doi: 10.1016/j.celrep.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 152.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17:193–199. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 153.Provost P. Interpretation and applicability of microRNA data to the context of Alzheimer’s and age-related diseases. Aging. 2010;2:166–169. doi: 10.18632/aging.100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Arancio W., Giordano C., Pizzolanti G. A ceRNA analysis on LMNA gene focusing on the Hutchinson-Gilford progeria syndrome. J. Clin. Bioinf. 2013;3:2. doi: 10.1186/2043-9113-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Arancio W., Genovese S.I., Bongiovanni L., Tripodo C. A ceRNA approach may unveil unexpected contributors to deletion syndromes, the model of 5q- syndrome. Oncoscience. 2015;2:872–879. doi: 10.18632/oncoscience.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zha F., Qu X., Tang B., Li J., Wang Y., Zheng P., Ji T., Zhu C., Bai S. Long non-coding RNA MEG3 promotes fibrosis and inflammatory response in diabetic nephropathy via miR-181a/Egr-1/TLR4 axis. Aging. 2019;11:3716–3730. doi: 10.18632/aging.102011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lin Z., Ge J., Wang Z., Ren J., Wang X., Xiong H., Gao J., Zhang Y., Zhang Q. Let-7e modulates the inflammatory response in vascular endothelial cells through ceRNA crosstalk. Sci. Rep. 2017;7:42498. doi: 10.1038/srep42498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Huang B., Yu H., Li Y., Zhang W., Liu X. Upregulation of long noncoding TNFSF10 contributes to osteoarthritis progression through the miR-376-3p/FGFR1 axis. J. Cell. Biochem. 2019;120:19610–19620. doi: 10.1002/jcb.29267. [DOI] [PubMed] [Google Scholar]
- 159.Liu Y., Lin L., Zou R., Wen C., Wang Z., Lin F. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle. 2018;17:2411–2422. doi: 10.1080/15384101.2018.1526603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Han Y., Wang F., Shao L., Huang P., Xu Y. LncRNA TUG1 mediates lipopolysaccharide-induced proliferative inhibition and apoptosis of human periodontal ligament cells by sponging miR-132. Acta Biochim. Biophys. Sin. 2019;51:1208–1215. doi: 10.1093/abbs/gmz125. [DOI] [PubMed] [Google Scholar]
- 161.Li S., Liu X., Li H., Pan H., Acharya A., Deng Y., Yu Y., Haak R., Schmidt J., Schmalz G., et al. Integrated analysis of long noncoding RNA-associated competing endogenous RNA network in periodontitis. J. Periodont. Res. 2018;53:495–505. doi: 10.1111/jre.12539. [DOI] [PubMed] [Google Scholar]
- 162.Zhu J., Zhang X., Gao W., Hu H., Wang X., Hao D. lncRNA circRNA miRNA mRNA ceRNA network in lumbar intervertebral disc degeneration. Mol. Med. Rep. 2019;20:3160–3174. doi: 10.3892/mmr.2019.10569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nie L., Zhang P., Wang Q., Zhou X., Wang Q. lncRNA-Triggered Macrophage Inflammaging Deteriorates Age-Related Diseases. Mediat. Inflamm. 2019;2019:4260309. doi: 10.1155/2019/4260309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gao B., Zhang X., Huang Y., Yang Z., Zhang Y., Zhang W., Gao Z.H., Xue D. Coding and non-coding gene regulatory networks underlie the immune response in liver cirrhosis. PLoS ONE. 2017;12:e0174142. doi: 10.1371/journal.pone.0174142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang Y., Zhang H., An M., Zhao B., Ding H., Zhang Z., He Y., Shang H., Han X. Crosstalk in competing endogenous RNA networks reveals new circular RNAs involved in the pathogenesis of early HIV infection. J. Transl. Med. 2018;16:332. doi: 10.1186/s12967-018-1706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jiang H., Ma R., Zou S., Wang Y., Li Z., Li W. Reconstruction and analysis of the lncRNA-miRNA-mRNA network based on competitive endogenous RNA reveal functional lncRNAs in rheumatoid arthritis. Mol. Biosyst. 2017;13:1182–1192. doi: 10.1039/C7MB00094D. [DOI] [PubMed] [Google Scholar]
- 167.Yan S., Wang P., Wang J., Yang J., Lu H., Jin C., Cheng M., Xu D. Long Non-coding RNA HIX003209 Promotes Inflammation by Sponging miR-6089 via TLR4/NF-kB Signaling Pathway in Rheumatoid Arthritis. Front. Immunol. 2019;10:2218. doi: 10.3389/fimmu.2019.02218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Paci P., Colombo T., Farina L. Computational analysis identifies a sponge interaction network between long non-coding RNAs and messenger RNAs in human breast cancer. BMC Syst. Biol. 2014;8:83. doi: 10.1186/1752-0509-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tiansheng G., Junming H., Xiaoyun W., Peixi C., Shaoshan D., Qianping C. lncRNA Metastasis-Associated Lung Adenocarcinoma Transcript 1 Promotes Proliferation and Invasion of Non-Small Cell Lung Cancer Cells via Down-Regulating miR-202 Expression. Cell J. 2020;22:375–385. doi: 10.22074/cellj.2020.6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xu X.W., Zheng B.A., Hu Z.M., Qian Z.Y., Huang C.J., Liu X.Q., Wu W.D. Circular RNA hsa-circ-000984 promotes colon cancer growth and metastasis by sponging miR-106b. Oncotarget. 2017;8:91674–91683. doi: 10.18632/oncotarget.21748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhong Z., Huang M., Lv M., He Y., Duan C., Zhang L., Chen J. Circular RNA MYLK as a competing endogenous RNA promotes bladder cancer progression through modulating VEGFA/VEGFR2 signaling pathway. Cancer Lett. 2017;403:305–317. doi: 10.1016/j.canlet.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 172.Hajjari M., Salavaty A. HOTAIR: An oncogenic long non-coding RNA in different cancers. Cancer Biol. Med. 2015;12:1–9. doi: 10.7497/j.issn.2095-3941.2015.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nie Y., Liu X., Qu S., Song E., Zou H., Gong C. Long non-coding RNA HOTAIR is an independent prognostic marker for nasopharyngeal carcinoma progression and survival. Cancer Sci. 2013;104:458–464. doi: 10.1111/cas.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rinn J.L., Kertesz M., Wang J.K., Squazzo S.L., Xu X., Brugmann S.A., Goodnough L.H., Helms J.A., Farnham P.J., Segal E., et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Deng J., Yang M., Jiang R., An N., Wang X., Liu B. Long Non-Coding RNA HOTAIR Regulates the Proliferation, Self-Renewal Capacity, Tumor Formation and Migration of the Cancer Stem-Like Cell (CSC) Subpopulation Enriched from Breast Cancer Cells. PLoS ONE. 2017;12:e0170860. doi: 10.1371/journal.pone.0170860. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 176.Lou W., Ding B., Fu P. Pseudogene-Derived lncRNAs and Their miRNA Sponging Mechanism in Human Cancer. Front. Cell Dev. Biol. 2020;8:85. doi: 10.3389/fcell.2020.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li L., Wang D., Xue M., Mi X., Liang Y., Wang P. 3’UTR shortening identifies high-risk cancers with targeted dysregulation of the ceRNA network. Sci. Rep. 2014;4:5406. doi: 10.1038/srep05406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sanchez-Mejias A., Tay Y. Competing endogenous RNA networks: Tying the essential knots for cancer biology and therapeutics. J. Hematol. Oncol. 2015;8:30. doi: 10.1186/s13045-015-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Naorem L.D., Prakash V.S., Muthaiyan M., Venkatesan A. Comprehensive analysis of dysregulated lncRNAs and their competing endogenous RNA network in triple-negative breast cancer. Int. J. Biol. Macromol. 2020;145:429–436. doi: 10.1016/j.ijbiomac.2019.12.196. [DOI] [PubMed] [Google Scholar]
- 180.Yang R., Xing L., Wang M., Chi H., Zhang L., Chen J. Comprehensive Analysis of Differentially Expressed Profiles of lncRNAs/mRNAs and miRNAs with Associated ceRNA Networks in Triple-Negative Breast Cancer. Cell. Physiol. Biochem. 2018;50:473–488. doi: 10.1159/000494162. [DOI] [PubMed] [Google Scholar]
- 181.Tian W., Jiang C., Huang Z., Xu D., Zheng S. Comprehensive analysis of dysregulated lncRNAs, miRNAs and mRNAs with associated ceRNA network in esophageal squamous cell carcinoma. Gene. 2019;696:206–218. doi: 10.1016/j.gene.2019.02.051. [DOI] [PubMed] [Google Scholar]
- 182.Wang X., Hu K.B., Zhang Y.Q., Yang C.J., Yao H.H. Comprehensive analysis of aberrantly expressed profiles of lncRNAs, miRNAs and mRNAs with associated ceRNA network in cholangiocarcinoma. Cancer Biomark. 2018;23:549–559. doi: 10.3233/CBM-181684. [DOI] [PubMed] [Google Scholar]
- 183.Wang H., Niu L., Jiang S., Zhai J., Wang P., Kong F., Jin X. Comprehensive analysis of aberrantly expressed profiles of lncRNAs and miRNAs with associated ceRNA network in muscle-invasive bladder cancer. Oncotarget. 2016;7:86174–86185. doi: 10.18632/oncotarget.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Arun K., Arunkumar G., Bennet D., Chandramohan S.M., Murugan A.K., Munirajan A.K. Comprehensive analysis of aberrantly expressed lncRNAs and construction of ceRNA network in gastric cancer. Oncotarget. 2018;9:18386–18399. doi: 10.18632/oncotarget.24841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hoadley K.A., Yau C., Hinoue T., Wolf D.M., Lazar A.J., Drill E., Shen R., Taylor A.M., Cherniack A.D., Thorsson V., et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell. 2018;173:291–304. doi: 10.1016/j.cell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang X., Zhang W., Jiang Y., Liu K., Ran L., Song F. Identification of functional lncRNAs in gastric cancer by integrative analysis of GEO and TCGA data. J. Cell. Biochem. 2019;120:17898–17911. doi: 10.1002/jcb.29058. [DOI] [PubMed] [Google Scholar]
- 187.Li Z., Jiang C., Yuan Y. TCGA based integrated genomic analyses of ceRNA network and novel subtypes revealing potential biomarkers for the prognosis and target therapy of tongue squamous cell carcinoma. PLoS ONE. 2019;14:e0216834. doi: 10.1371/journal.pone.0216834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li Y., Gu J., Xu F., Zhu Q., Ge D., Lu C. Transcriptomic and functional network features of lung squamous cell carcinoma through integrative analysis of GEO and TCGA data. Sci. Rep. 2018;8:15834. doi: 10.1038/s41598-018-34160-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang W., Zhuang Q., Ji K., Wen B., Lin P., Zhao Y., Li W., Yan C. Identification of miRNA, lncRNA and mRNA-associated ceRNA networks and potential biomarker for MELAS with mitochondrial DNA A3243G mutation. Sci. Rep. 2017;7:41639. doi: 10.1038/srep41639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhu Z., Hou Q., Li M., Fu X. Molecular mechanism of myofibroblast formation and strategies for clinical drugs treatments in hypertrophic scars. J. Cell. Physiol. 2020;235:4109–4119. doi: 10.1002/jcp.29302. [DOI] [PubMed] [Google Scholar]
- 191.Fan C.N., Ma L., Liu N. Systematic analysis of lncRNA-miRNA-mRNA competing endogenous RNA network identifies four-lncRNA signature as a prognostic biomarker for breast cancer. J. Transl. Med. 2018;16:264. doi: 10.1186/s12967-018-1640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ye G., Guo L., Xing Y., Sun W., Yuan M. Identification of prognostic biomarkers of prostate cancer with long non-coding RNA-mediated competitive endogenous RNA network. Exp. Ther. Med. 2019;17:3035–3040. doi: 10.3892/etm.2019.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Xiong D.D., Dang Y.W., Lin P., Wen D.Y., He R.Q., Luo D.Z., Feng Z.B., Chen G. A circRNA-miRNA-mRNA network identification for exploring underlying pathogenesis and therapy strategy of hepatocellular carcinoma. J. Transl. Med. 2018;16:220. doi: 10.1186/s12967-018-1593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhou M., Diao Z., Yue X., Chen Y., Zhao H., Cheng L., Sun J. Construction and analysis of dysregulated lncRNA-associated ceRNA network identified novel lncRNA biomarkers for early diagnosis of human pancreatic cancer. Oncotarget. 2016;7:56383–56394. doi: 10.18632/oncotarget.10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Smillie C.L., Sirey T., Ponting C.P. Complexities of post-transcriptional regulation and the modeling of ceRNA crosstalk. Crit. Rev. Biochem. Mol. Biol. 2018;53:231–245. doi: 10.1080/10409238.2018.1447542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fiscon G., Conte F., Farina L., Paci P. Network-Based Approaches to Explore Complex Biological Systems towards Network Medicine. Genes. 2018;9:437. doi: 10.3390/genes9090437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang Z., He T., Huang L., Ouyang Y., Li J., Huang Y., Wang P., Ding J. Two precision medicine predictive tools for six malignant solid tumors: From gene-based research to clinical application. J. Transl. Med. 2019;17:405. doi: 10.1186/s12967-019-02151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sun C., Huang L., Li Z., Leng K., Xu Y., Jiang X., Cui Y. Long non-coding RNA MIAT in development and disease: A new player in an old game. J. Biomed. Sci. 2018;25:23. doi: 10.1186/s12929-018-0427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yang Y., Zhang Z., Wu Z., Lin W., Yu M. Downregulation of the expression of the lncRNA MIAT inhibits melanoma migration and invasion through the PI3K/AKT signaling pathway. Cancer Biomark. 2019;24:203–211. doi: 10.3233/CBM-181869. [DOI] [PubMed] [Google Scholar]
- 200.Zhang W., Chen Q., Lei C. lncRNA MIAT promotes cell invasion and migration in esophageal cancer. Exp. Ther. Med. 2020;19:3267–3274. doi: 10.3892/etm.2020.8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xu H., Zhou J., Tang J., Min X., Yi T., Zhao J., Ren Y. Identification of serum exosomal lncRNA MIAT as a novel diagnostic and prognostic biomarker for gastric cancer. J. Clin. Lab. Anal. 2020;2020:e23323. doi: 10.1002/jcla.23323. [DOI] [PMC free article] [PubMed] [Google Scholar]