Abstract
Natural killer (NK) cells represent one of the first lines of defense against malignant cells. NK cell activation and recognition are regulated by a balance between activating and inhibitory receptors, whose specific ligands can be upregulated on tumor cells surface and tumor microenvironment (TME). Hematological malignancies set up an extensive network of suppressive factors with the purpose to induce NK cell dysfunction and impaired immune-surveillance ability. Over the years, several strategies have been developed to enhance NK cells-mediated anti-tumor killing, while other approaches have arisen to restore the NK cell recognition impaired by tumor cells and other cellular components of the TME. In this review, we summarize and discuss the strategies applied in hematological malignancies to block the immune check-points and trigger NK cells anti-tumor effects through engineered chimeric antigen receptors.
Keywords: NK cells, hematological malignancies, check-point inhibitors, CAR NK cells, antibodies, immunotherapy
1. Tumor-Mediated NK Cell Exhaustion in Hematological Malignancies
NK cells represent one of the first lines of defense against malignant cells. Their immune-surveillance ability is mediated by the expression of activating receptors such as the natural killer group (NKG)2D, DNAX accessory molecule (DNAM)-1, and the natural cytotoxic receptors (NCRs) such as natural killer protein (NKp)30, NKp44, and NKp46 [1,2,3,4,5]. NK cell recognition is also dependent by an array of inhibitory receptors, such as killer inhibitory receptors (KIRs) and NKG2A molecule [6]. Once the target is acquired in the viewfinder, NK cells secrete cytotoxic granules (as granzymes and perforin) and cytokines (as tumor necrosis factor (TNF)-α and interferon (IFN)-γ), which lead to the killing of the target.
Several molecular mechanisms have been developed in hematological malignancies to allow tumor cells to elude and escape from NK cell-mediated recognition. Some of these mechanisms are represented by the inhibition of tumor antigen presentation, expression of immune checkpoint ligands as programmed death ligand-1 (PD-L1), secretion of suppressive factors like interleukin (IL)-10, soluble human leukocyte antigen (HLA)-G, transforming growth factor (TGF)-β, indoleamine 2,3-dioxygenase (IDO), recruitment and polarization of immunosuppressive cells as macrophages, regulatory T cells (Tregs), myeloid derived suppressor cells (MDSC), and mesenchymal stromal cells (MSC). Altogether these cells, with the tumor cells themselves, are present inthe tumor microenvironment (TME) [7,8,9,10,11] (Figure 1). Another major molecular mechanism used by tumor cells to impair NK cell recognition and activation is based on the expression of inhibitory (as major histocompatibility complex (MHC) class I molecules) and the release in a soluble form of ligands (such as MHC class I polypeptide–related sequence (MIC) A/B and UL16 binding protein (ULBP1-6) for NK cell-activating receptors [12,13]. The TME is insomuch efficient that NK cells isolated from patients with hematological malignancies display multiple abnormalities. In particular, NK cells isolated from chronic myeloid leukemia (CML), acute lymphocytic leukemia (ALL), myelodysplastic syndromes (MDS), and chronic lymphocytic leukemia (CLL) patients suffer a decreased cell number, activating receptors expression, and cytokines secretion [14,15,16,17,18,19]. Similar tumor-mediated impairment of NK cell functions has been also described in acute myeloid leukemia (AML) and multiple myeloma (MM) patients [15,16,17,20,21,22,23,24]. This is associated with an impaired polarization of cytolytic granules toward the immunological synapse against tumor cells [16,23,25,26] and increased expression of inhibitory receptors like NKG2A, programmed death receptor (PD)-1 and KIRs [27,28,29,30]. Interestingly, it has been shown in CLL patients that NK cells losing NKp30 on their surface acquire the inhibitory receptor T-cell immunoglobulin and mucin domain (TIM)-3, which was correlated with poor prognostic factors [31].
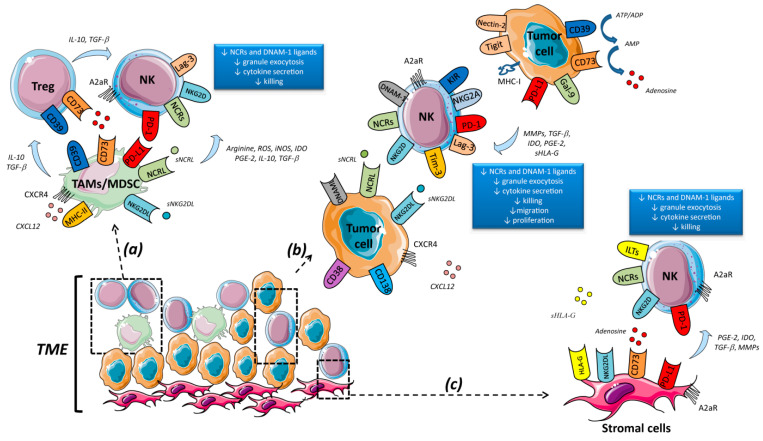
Figure 1.
Strategies used by the tumor microenvironment (TME) to impair natural killer (NK) cell immuno-surveillance in hematological malignancies. (a) Tumor cells secrete several chemokines as CXCL12 to recruit suppressive cells such as myeloid derived suppressors cells (MDSCs) and tumor–associated macrophages (TAMs). These cells inhibit NK cell functions by secreting soluble factors such as interleukin (IL)-10, transforming growth factor (TGF)-β, reactive oxygen species (ROS), arginine and nitric oxide synthase (NOS), or through the expression of inhibitory receptors as programmed death-ligand (PD-L)1 or release of ligands for NK activating receptors. In addition, MDSCs and TAMs can recruit other suppressive cells like regulatory T cells (Tregs), which indirectly contribute to induce an exhausted and dysfunctional profile in NK cells. (b) Tumor cells secrete immunosuppressive molecules whose impair NK cell proliferation, activation and cytotoxicity, such as TGF-β, prostaglandin (PG)E-2, indoleamine 2,3-dioxygenase (IDO) and soluble human leukocyte antigen (HLA)-G. A mechanism used by hematological malignancies to avoid NK cell-mediated recognition is the expression of inhibitory receptors as PD-L1 Also, tumor cells can secrete natural killer group (NKG)2DLs, which impair the interaction between tumor and NK cells affecting the positive signal induced by NKG2D. (c) Mesenchymal stromal cells (MSC) decrease granule exocytosis, cytokines secretion and cytotoxicity of NK cells through the secretion of soluble factors as PGE-2, TGF- β and soluble HLA-G and through the expression of PD-L1 and HLA-G.
2. Current Advanced Therapy in Hematological Malignancies
Once effectors cells infiltrate the tumor site, they have to fight both tumor cells and the other components of the TME. To that end, the purpose of recent therapeutic strategies is to improve NK cell survival, proliferation, activation, and cytotoxic functions in a hostile and immune-suppressive environment. Over the years, several approaches have been developed in hematological malignancies, like monoclonal antibodies (mAbs) and engineered NK cells.
2.1. Monoclonal Antibodies (mAbs)
In hematological malignancies, tumor cells and tumor-associated cells express activating and inhibitory receptors thataffect anti-tumor response. The monoclonal antibody-based therapy is an approach aimed to block the triggering of these receptors, with the purpose to target the entire TME and restore NK cell functions [32,33,34,35,36] (Figure 2). Herein, a list of mAbs and target molecules are analyzed in this context.
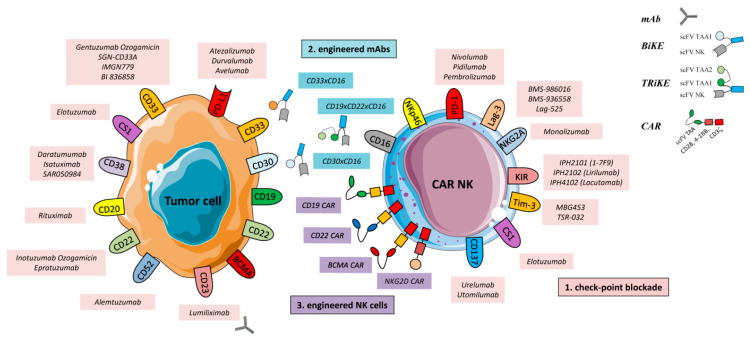
Figure 2.
Overview of emerging strategies to boost or restore NK cell-based anti-tumor response in hematological malignancies. (1) Checkpoint blockade. MAb-based therapy is an approach aimed to block the triggering of inhibitory receptors (as PD-1, NKG2A, and KIRs) expressed on NK cells and avoid tumor escape. Importantly, checkpoint inhibition can be also used to impair tumor cell functions through specific mAbs as Durvalumab (anti-PD-L1), Daratumumab (anti-CD38), or Elotuzumab (anti-CS1). (2) Engineered mAbs. BiKEs and TRiKEs bind to activating receptors (e.g., CD16) expressed on NK cells and several antigens (e.g., CD19, CD22, CD33, CD38, and CD123) expressed on tumor cells. Engineered mAbs facilitate the formation of an immunological synapse (IS) and improve ADCC activity by redirecting NK cells to tumor cells.(3) CAR NK cells are genetically modified to recognize specific antigens expressed on tumor cells. The consequence of the CAR activation is the formation of a strong IS, followed by the release of cytotoxic granules as perforin and granzymes and eventually the target cell killing. Abbreviations used: ADCC, antibody-dependent cell-mediated cytotoxicity; mAbs, monoclonal antibodies; BiKEs, bi-specific killing cell engagers; TRiKEs, tri-specific killer cell engagers; CARs, chimeric antigen receptors.
Rituximab and other more recent anti-CD20 mAbs, such as Ocaratuzumab and Ublituximab, have been shown to improve the clinical prognosis in B-cell hematologic malignancies, such as diffuse large B-cell lymphoma (DLBCL), CLL, and follicular lymphoma (FL) [37,38,39]. Interestingly, it has been shown that NK cells isolated from CLL, lymphomas, and Waldenström Macroglobulinemia (WM) patients treated with Rituximab, Ocaratuzumab, or Ublituximab displayed an increased antibody-dependent cellular cytotoxicity (ADCC) and degranulation function [40,41,42,43,44]. Moreover, the combined blockade of KIRs and CD20 with specific mAbs enhanced NK cell cytotoxicity against lymphoma cells in vitro and in murine lymphoma models [41]. By contrast, it has been also observed that Rituximab and Ofatumumab promoted the release of reactive oxygen species from monocytes, which impaired the NK cell-mediated ADCC against CLL cells [45].
Elotuzumab is a mAb interacting with the glycoprotein signaling lymphocytic activation molecule F7 (SLAM-F7, also named CS1 or CD319) expressed on malignant plasma cells.Notably, SLAM-F7 is also expressed on NK cells, and it has been reported that Elotuzumab enhances NK cell-mediated anti-myeloma activity by directly activating NK cells, inducing ADCC and disrupting the stromal/MM cell interaction [46,47,48,49,50,51]. In addition, Elotuzumab has been shown to improve the overall response rate in patients with refractory/relapsed (r/r) MM [52,53,54], and its efficacy can be enhanced by simultaneous treatment with drugs as Carfilzomib and Panobinostat [46,55], Bortezomib [56,57], or immunomodulatory drugs (IMiDs) [46,53,58,59,60]. Importantly, Elotuzumab action might be also enhanced when used combined with other mAbs. A phase I open-label study of the safety and tolerability of Elotuzumab (BMS-901608) administered in combination with either Lirilumab (BMS-986015, anti-KIR) or Urelumab (BMS-663513, anti-CD137) (NCT02252263) is under investigation in MM patients. In addition, three distinct clinical trials are investigating the efficacy between Elotuzumab and Nivolumab [an anti-Programmed Death-1 (PD-1) mAb] in r/r MM patients [NCT02726581 (CheckMate-602, phase III)], (NCT02612779, phase II), and (NCT03227432, phase II) (ClinicalTrials.gov) (Table 1).
Table 1.
Summary of selected either completed or ongoing clinical trials using mAbs in hematological cancers.
| Target | mAb | Drug Combination | Disease | Trial Number (NCT) | Phase |
|---|---|---|---|---|---|
| CD38 | Daratumumab | Lenalidomide + Dexamethasone | MM | 02076009 | III |
| Bortezomib + Dexamethasone | MM (CASTOR) | 02136134 | III | ||
| MM (SIRIUS) | 01985126 | II | |||
| Carfilzomib, Lenalidomide, Dexamethasone | newly diagnosed MM | 03290950 | II | ||
| Bortezomib, Lenalidomide, Dexamethasone | untreated MM (PERSEUS) | 03710603 | III | ||
| Lenalidomide, Dexamethasone | MM r/r (POLLUX) | 02076009 | III | ||
| Bortezomib, Cyclophosphamide, Dexamethasone | MM r/r (LYRA) | 01951819 | II | ||
| Prednisone, Bortezomib, Melphalan vs Daratumumab alone | MM r/r (ALCYONE) | 02195479 | III | ||
| Lenalidomide, Dexamethasone | untreated MM (MAIA) | 02252172 | III | ||
| Bortezomib, Thalidomide, Dexamethasone | untreated MM (CASSEOPEIA) | 02541383 | III | ||
| Isatuximab (SAR650984) | Lenalidomide, Dexamethasone | MM | 01749969 | Ib | |
| Pomalidomide, Dexamethasone | MM r/r (ICARIA) | 02990338 | III | ||
| CD137 | Urelumab | Nivolumab | B-cell NHL | 02253992 | I/II |
| Elozutumab | MM | 02252263 | I | ||
| SLAM-F7 (CS1) | Elotuzumab | MM | 03003728 | II | |
| Nivolumab | MM r/r (Checkmate-602) | 02726581 | III | ||
| Lenalidomide, Dexamethasone | MM (ELOQUENT-2) | 01239797 | III | ||
| KIR2DL1/2/3 | IPH2102 (Lirilumab) | Nivolumab, 5-Azacytidine | Leukemia | 02599649 | II |
| Rituximab (anti-CD20) | High-risk Untreated and r/r CLL | 02481297 | II | ||
| Elotuzumab, Urelumab | MM | 02252263 | I | ||
| Lenalidomide | MM | 01217203 | I | ||
| IPH2101 (1-7F9) | SMM | 01222286 (KIRMONO) | II | ||
| MM | 00999830 (REMYKIR) | II | |||
| AML | 01256073 | I | |||
| MM | 00552396 | I | |||
| Lenalidomide | MM | 01217203 (KIRIMID) | I | ||
| MEDI4736 | 5-Azacytidine | Leukemia | 02399917 | II | |
| MEDI6469 | Tremelimumab (anti-CTLA4) or Rituximab or MEDI4736 | B-cell lymphoma, MDS | 02205333 | I/II | |
| KIR3DL2 | IPH4102 (Lacutamab) | Cutaneous T-cell lymphoma | 02593045 | I | |
| NKG2A | IPH2201 (Monalizumab) | Hematological cancers | 02921685 | I | |
| CLL | 03088059 | II | |||
| CLL | 02557516 | I/II | |||
| Sym-021 (anti-PD-1), Sym-022 (anti-Lag-3) | Lymphoma | 03311412 | I | ||
| MBG453 | Decitabine (hypomethylating agent) | AML, high risk MDS | 03066648 | I | |
| Lag-3 | Sym-022 | Lymphoma | 03489369 | I | |
| Sym-021 (anti-PD-1), Sym-023 (anti-Tim-3) | Lymphoma | 03311412 | I | ||
| BMS-986016 | Nivolumab (BMS-936558) | DLBCL r/r, HL r/r | 02061761 | I/II | |
| PD-1 | Pembrolizumab | cHL r/r | 01953692 (Keynote-013) | Ib | |
| cHL r/r post ASCT | 02458594 (Keynote-087) | II | |||
| Ibrutinib | NHL r/r | 02950220 | I | ||
| Brutoximab Vedotin (anti-CD30 mAb) | cHL r/r | 02684292 (Keynote-024) | III | ||
| Lenalidomide, Dexamethasone | MM | 02036502 (Keynote-023) | I | ||
| Pomalidomide, Dexamethasone | MM r/r | 02576977 (Keynote-183) | III | ||
| Nivolumab | cHL r/r (Checkmate-205) | 01592370 | II | ||
| Epacadostat (anti-IDO1 mAb) | DLBCL, HL | 02327078 | I/II | ||
| Lenalidomide | NHL, cHL r/r | 03015896 | I/II | ||
| FL r/r (Checkmate-140) | 02038946 | II | |||
| Lenalidomide, Rituximab | DLBCL | 03558750 | I | ||
| DLBCL r/r (CheckMate-139) | 02038933 | II | |||
| Cyclophosphamide, Prednisone, Doxorubicin Hydrochloride | DLCBL | 03704714 | I/II | ||
| Urelumab (anti-CD137 mAb) | NHL | 02253992 | I/II | ||
| Varlilumab (CDX-1127) (anti-CD27 mAb) | DLCBL | 03038672 | II | ||
| HL r/r (ANIMATE) | 03337919 | II | |||
| Lenalidomide, Dexamethasone | high risk SMM | 02903381 | II | ||
| Lenalidomide | MM r/r | 03333746 | II | ||
| Daratumumab with or without Cyclophosphamide | MM r/r | 03184194 | II | ||
| Daratumumab or Pomalidomide and Dexamethasone | Hematological cancers | 01592370 | I | ||
| Ipilimumab (anti-CTLA4) | high risk MM | 02681302 | I/II | ||
| Elotuzumab with or without Pomalidomide and Dexamethasone | MM r/r | 03227432 | II | ||
| Elotuzumab, Pomalidomide, Dexamethasone | MM r/r | 02726581 | III | ||
| AML | 02275533 | II | |||
| Dasatinib (tyrosine kinase receptor inhibitor) | CML | 02011945 | I | ||
| HL | 02181738 | II | |||
| Rituximab, Gemcitabine, Bendamustine (alkylating agent) | DLBCL r/r | 03259529 | I/II | ||
| Pidilizumab | Rituximab | FL r/r | 00904722 | II | |
| Lenalidomide | MM | 02077959 | I/II | ||
| PD-L1 | Atezolizumab | Obinutuzumab (anti-CD20) | DLBCL, FL r/r | 02220842 | I |
| Obinutuzumab, Polatuzumab vedotin (anti-CD79b mAb) | DLBCL, FL r/r | 02729896 | I | ||
| Obinutuzumab, Lenalidomide | FL r/r | 02631577 | I | ||
| Obinutuzumab, Ibrutinib | untreated, high risk or r/r CLL | 02846623 | II | ||
| Guadecitabine (hypomethylating agent) | AML, MDS, CML r/r | 02935361 | I/II | ||
| Daratumumab vs Daratumumab, IMiDs | MM | 02431208 | I | ||
| Avelumab | cHL r/r | 02603419 | Ib | ||
| Itolizumab (anti-CD6) vs Itolizumab, 5-Azacytidine vs Bendamustine, Rituximab | DLBCL r/r | 02951156 | Ib/II | ||
| 5-Azacytidine | AML r/r | 02953561 | I | ||
| Cetrelimab (JNJ-63723283) | Daratumumab | MM r/r | 03357952 | II/III | |
| Durvalumab | Daratumumab | MM r/r | 03000452 | II | |
| Lenalidomide, Dexamethasone | newly diagnosed MM | 02685826 | I | ||
| Pomalidomide, Dexamethasone | MM r/r | 02616640 | I | ||
| Rituximab, Lenalidomide with or without Ibrutinib | NHL, CLL | 02733042 | I/II |
Abbreviations used: ALL, Acute Lymphocytic Leukemia; AML, Acute Myeloid Leukemia; CLL, Chronic Lymphocytic Leukemia; CML, Chronic Myeloid Leukemia; MM, Multiple Myeloma; MDS, Myelodysplastic Syndromes; NHL, Non-Hodgkin Lymphoma; FL, Follicular Lymphoma; DLBCL, Diffuse Large B-Cell Lymphoma; SMM, Smoldering MM; r/r, refractory/relapsed; IMiDs, immunomodulatory drugs.
CD137/4-1BB is a co-stimulatory molecule expressed on T and NK cells, whose triggering efficiently improved CTL-mediated tumor killing [61,62,63]. Recent clinical trials have demonstrated the promising effect of anti-CD137 agonistic mAbs as Urelumab (BMS-663513) and Utomilumab in hematological malignancies, alone or combined to other mAbs as Lirilumab, Elotuzumab, Rituximab, or Nivolumab [34,64,65,66,67].
- Adenosine, CD39, and CD73
The adenosine, generated by the ectonucleotidases CD39 and CD73, has been recently proposed as a novel target since it plays a key role in the inhibition of anti-tumor response through the activation of adenosine receptor (A2AR) expressed on effectors cells [68,69]. Interestingly, CD73 and CD39 are not only expressed on tumor cells, but also other components of the TME as tumor-associated macrophages (TAMs), MDSC, Tregs, and MSC [70,71]. Noteworthy, the adenosine secreted by the TME impairs NK cell proliferation, activation, and killing abilities [72,73,74] (Figure 1). CD73 is usually not expressed in healthy NK cells. Nonetheless, it has been reported that CD73 expression is up-regulated in tumor-infiltrating NK cells [75]. Further, these CD73+ NK cells also express other inhibitory checkpoints and they can suppress CD4+T-cell proliferation and IFN-γ production, thus promoting tumor growth. Notably, CD73 and CD39 are not only expressed on solid tumors but also in hematological malignancies [69,76,77,78,79]. More interestingly, it has been reported that the expression of CD39 on MSC increases significantly after co-culture with activated lymphocytes [80]. Along with this, Chatterjee et al. found that NK cells co-cultured with MSC displayed a significant up-regulation of CD73 expression, suggesting the possibility that CD73+ NK cells could convert AMP into adenosine upon exposure to MSC, hence maintaining the immune-suppressive milieu promoted by other cellular components of the TME [81]. Thus, MSC display a strong talent to suppress the immune response by converting NK cells in inhibitory partners; altogether these results highlight the importance to consider MSC a suitable target in immunotherapy [9,10,11]. In the past decade, several pharmacological inhibitors and specific mAbs have been evaluated in pre-clinical studies. Although the blockade of CD73 and/or A2AR signaling has been shown to restore effectors cell functions and inhibits tumor growth in solid tumors [70,71,82,83], there are no ongoing studies in hematological malignancies.
- Other Molecular Targets for NK Cell Immunotherapy
Other promising strategies under investigation include the targeting of the CD38, which is highly expressed on both normal and malignant plasma cells. Indeed, it has been shown that the anti-CD38 mAb Daratumumab enhances effectors cell-mediated lysis, degranulation, and ADCC against CD38+ tumor cells [84,85,86,87] improving the overall response rate in MM patients [88,89,90,91]. Interestingly, the efficacy of Daratumumab can be enhanced when combined with drugs or other mAbs [85,90,92]. Other anti-CD38 mAbs under investigation in hematological malignancies are Isatuximab and MOR-202 [93,94,95]. A detailed summary of ongoing and completed clinical trials using anti-CD38 mAbs are listed recently [85,90,91] (Table 1). Other promising targets of mAbs are CD52 (Alemtuzumab, in B-ALL precursors), CD23 (Lumiliximab, in CLL), CD22 (Inotuzumab Ozogamicin and Epratuzumab, in precursors and mature B-ALL), and CD33 (Gemtuzumab ozogamicin, in AML) [39,96,97]. Remarkably, it has been recently shown that an Fc-engineered CD33 mAb, BI-836858, promotes NK cell-mediated ADCC with in vitro activity against both AML cell lines and primary AML blasts [98]. Several findings suggest that CD47 could be another target for cancer immunotherapy in hematological malignancies. CD47 is principally expressed on myeloid cells and it is exploited by tumor cells to evade immune response [99,100,101]. Drugs targeting the CD47 signaling are currently evaluated in clinical studies and are represented by humanized antibodies including Hu5F9-G4 [(in AML, MDS and r/r B-cell Non-Hodgkin Lymphoma (NHL)] and CC-90002 (in AML, MDS and CD20+ NHL patients), respectively.
2.2. Checkpoint Inhibitors
- Anti-KIRs and Anti-NKG2A mAbs
As postulated by the “missing-self hypothesis,” the absence (or low expression) of MHC class I molecules on tumor cells trigger NK cells and leads to the tumor cell killing [2]. By contrast, the NK cell cytotoxicity is impaired when the tumor target expresses appropriate MHC class I alleles interacting with the KIRs expressed on NK cells. To improve NK cell functions, several strategies to block these KIRs have been developed. The blockade of KIRs with IPH2101 (formerly 1-7F9), an anti-pan-KIR antibody which interacts with KIR2DL1, KIR2DL2, and KIR2DL3 expressed on NK cells, strongly increases NK cell-mediated killing of tumor cells in AML, lymphoma, and MM patients [41,102,103,104,105,106,107]. IPH2101 efficacy has been tested in a phase I study in MM combined with the IMiD Lenalidomide (NCT01217203) [103]. Other anti-KIR mAbs are represented by Lirilumab (IPH2102/BMS-986015) and IPH4102, which interacts with KIR2DL1, KIR2DL2 and KIR2DL3, and KIR3DL2, respectively. Interestingly, IPH2102 has been shown to increase NK cell lysis against lymphoma cells [41] and synergistically acts with Lenalidomide to improve Daratumumab-treated MM cells lysis mediated by NK cells [86]. The effect of Lirilumab has been investigated in several hematological malignancies such as in a phase Ib/II study of relapsed AML in association with 5-Azacytidine (NCT02399917), for r/r or high-risk untreated CLL, treated with Rituximab (NCT02481297) in MM patients with Elotuzumab (BMS-901608) or Urelumab (BMS-663513) (NCT02252263, phase I), in MM and r/r lymphoma patients with Nivolumab (NCT01592370, phase II) [108], in MDS patients with Nivolumab and 5-Azacitidine (NCT02599649, phase II) and elderly AML patients in first complete remission as maintenance treatment (NCT01687387, phase II) [35,109]. The NKG2A ligand HLA-E is strongly expressed on malignant plasma cells [110]. Monalizumab (formerly IPH2201) blocks the inhibitory signaling induced by NKG2A/CD94 expressed on NK cells, restoring the anti-tumor response mediated by NK cells in hematological malignancies [30,61,111,112,113]. Also, Monalizumab is currently tested in a phase Ib/IIa study combined with Ibrutinib in patients with r/r CLL patients (NCT02557516) and a phase I study combined with Durvalumab (MEDI4736, an anti-PD-L1 antibody) in solid tumors (NCT02671435) (Table 1). Finally, the simultaneous blockade of NKG2A, the leukocyte-associated Ig-like receptor-1 (LAIR-1), and KIRs have been shown to strongly increase the NK cell-mediated killing of AML and ALL blasts [114].
- HLA-G
HLA-G binds to immunoglobulin-like transcripts (ILT)-2, ILT-4 and KIR2DL4 [115]. ILTs are expressed by most of the immune cells, including NK cells. ILT-2/HLA-G interaction impairs several functions on NK cells, such as cytokine secretion, chemotaxis, and the immunological synapse formation between NK cells and their target [115]. Of note, HLA-G belongs to the immunosuppressive factors secreted by the TME components in hematological malignancies, which contributes to the immune evasion of tumor cells [7,8,9,10,11,115,116]. Although it has been reported that Lenalidomide decreases the expression of ILT-2 on CLL cells, thus promoting NK cell proliferation and activation [117], today, there are no clinical studies evaluating the possible inhibition of HLA-G (or its receptors) in NK cell-based immunotherapy in hematological malignancies.
- Lymphocyte-activation gene-3 (LAG-3), TIM-3, PD-1, and T cell immunoglobulin and ITIM domain (TIGIT)
Associated to the increased expression of KIRs and NKG2A, exhausted effectors cells can exhibit elevated levels of inhibitory receptors such as lymphocyte activation gene-3 (LAG-3), TIM-3, and PD-1. These inhibitory receptors are currently under clinical investigation as potential therapeutic targets.
LAG-3 is expressed on B, T, and NK cells and binds to MHC class II molecules and L-SECtin bearing tumor cells [34,35,118]. However, LAG-3 is also expressed on PD-1+ tumor-infiltrating effectors cells (TILs) found in both pre-clinical models and patients, where they promote tumor escape. These PD-1+LAG-3+TILs exhibited an exhausted profile, characterized by reduced cytokines and cytotoxic granules secretion [119,120,121,122]. LAG-3high expression is associated with poor outcome in several hematological malignancies; ongoing clinical studies are evaluating the effect of anti-LAG-3 mAbs administered alone or in combination with other mAbs as Nivolumab [34,35,118,123].
TIM-3 is expressed by Tregs, DC, and T cells. TIM-3 interacts with Galectin-9, carcinoembryonic antigen-related cell adhesion molecule (CEACAM)-1, high-mobility group box (HMGB)-1, and phosphatidyl serine; these TIM-3 ligands are expressed not only by other immune cells but also by B-cell malignancies [34,35,118,119,124]. Similarly to LAG-3, the expression levels of TIM-3 on TILs correlates with cell dysfunction or exhaustion and poor prognostic factors, especially when TIM-3 is associated with other inhibitory receptors as PD-1 [35,125]. Interestingly, mature CD56dimCD16+ NK cells express TIM-3, and its expression can be induced upon activation. Intriguingly, compared with T cells, TIM-3high NK cells are fully activated and able to secrete cytokines and kill their targets. Thus, TIM-3 blockade may suppress NK cell-mediated cytotoxicity [124,126,127,128]. Notably, in hematologic malignancies TIM-3 (or its specific ligand Galectin-9) blockade restores immune response in AML, follicular lymphoma (FL), and lymphoma [124,126,129]. Interestingly, it has been recently shown that TIM-3 is also expressed on MDS blasts, and this expression is further enhanced in the presence of the cell culture supernatant of human stromal cell lines [130]. Based on these findings, the anti-TIM-3 mAb MBG453 is currently evaluated in a phase I clinical trial for r/r AML and high-risk MDS, combined with Decitabine, a nucleic acid synthesis inhibitor (NCT03066648).
PD-1 (or CD279) is expressed on both tumor and activated immune cells, and when it interacts with its specific ligands, it induces effectors cells exhaustion with consequent tumor progression [36,131,132]. This mechanism has been observed in most of hematological malignancies, PD-1 being expressed on AML [133,134], MM [27,135,136,137], NHL [138,139], DLBCL [140,141], and CLL cells [142]. In addition, TILs exhibiting PD-1 have been observed in FL [143,144,145] and NHL [146]. Of note, PD-1 is expressed on CD56dim/NKG2A-/KIR+/CD57+ NK cells, which correspond to a terminally differentiated and exhausted status, characterized by a decreased proliferation, cytokine secretion, and degranulation [147,148]. Interestingly, it has been recently observed in FL the presence of two different T-cell subsets displaying opposite localization and functions, based on the expression of PD-1 [146]. Whereas PD-1highT cells, which predominantly reside in the lymph node follicles, are TIM-3-, secrete IL-21 and support B-cell growth, PD-1lowT cells (mainly located in an inter-follicular pattern), have an exhausted phenotype, express TIM-3, and predict a poor outcome in FL patients. Noteworthy, recent evidence supports the fact that expression of PD-1 on lymphocytes in myeloma patients is lowered during Lenalidomide maintenance [149]. Interestingly, in several studies anti-PD-1 mAbs were combined with drugs as IMiDs [27,55,150,151,152], 5-azacytidine [153,154], Rituximab [155], or others checkpoint inhibitors as anti-LAG-3 [121] or anti-CTLA-4 antibodies [154,156].
The ligands for PD-1 are PD-L1 (CD274 or B7-H1) and PD-L2 (CD273 or B7-DC). PD-L1 expression can be modulated by epigenetic and post-transcriptional modifications, Toll-like receptor-mediated signaling, and the surrounding TME [27,137,157,158]. Paradoxically, PD-L1 expression is also up-regulated by the IFN-γ secreted by anti-tumor effectors cells; this may amplify PD-L1-mediated immunosuppressive effects [159]. Recent evidence has also shown that PD-L1 is expressed in different hematological malignancies [140,141,160,161,162,163]. There are several groups of mAbs used to disrupt the PD-1/PD-L1 axis, aimed to restore anti-tumor response. Whereas Nivolumab (MDX1106, BMS-936558), Pembrolizumab (MK-3475), and Pidilizumab (CT-011) block the PD-1-induced inhibitory signaling, BMS935559 (MDX-1105), MPDL3280A, and MEDI4736 (Atezolizumab, Durvalumab and Avelumab, respectively) affect the inhibitory signaling induced by PD-L1. Remarkably, it has been shown that the anti-PD-1/PD-L1 blockade restored NK cell cytotoxicity against MM cells [27,136,164,165,166,167]. Interestingly, it has been recently reported that combining a selective HDAC6 inhibitor (ACY-241) with an anti-PD-L1 mAb triggers the effector cell-mediated MM cell killing, supporting their utilization in the clinical studies aimed to restore immune response [137,157,168]. Based on these findings, several clinical studies are evaluating the therapeutic efficacy of both anti-PD-1 and anti-PD-L1 mAbs in most of hematological malignancies [35,109,139,150,169,170]. Another ligand of PD-1 is PD-L2. This molecule is also expressed in hematological cancers, where it participates inthe immune-tolerance [171,172,173,174,175]. Although further investigations are needed, altogether this evidence suggests that a possible strategy to induce the full restoration of the anti-tumor response could be the use of anti-PD-1/PD-L1 and anti-PD-L2 antibodies.
TIGIT is expressed on both activated T and NK cells and interacts with two specific DNAM-1 (CD226) ligands, CD155 (PVR) and CD112 (nectin-2), which are expressed on both immune and tumor cells [66,119,176,177]. Importantly, TIGIT binds CD155 with stronger affinity than DNAM-1; TIGIT interacting with both CD155 and CD112 promotes the decrease of IFN-γ production and NK cell-mediated cytotoxicity [178,179,180,181]. Importantly, both TIGIT and CD155 must form homodimers in cis to interact as heterotetramers in trans. This molecular mechanism is also used by DNAM-1, but it is inhibited by TIGIT, allowing an impaired anti-tumor response mediated by effectors cells (reviewed in [182,183]). Interestingly, TIGIT expressed on tumor-infiltrating effector cells synergizes with other co-inhibitory molecules to dampen the immune response and promote effector cells dysfunction [184,185], so that the co-blockade of TIGIT/PD-1/TIM-3 restored exhausted CD8+ T cells and induced complete tumor rejection [116,176,186,187]. Noteworthy, TIGIT ligands are also expressed in hematological malignancies, where they induce T-cell dysfunction associated with a poor clinical prognosis [188,189,190]. The nuisance is that TIGIT+PD-1+TIM-3+ [190] or TIGIT+PD-1+DNAM-1- [189] T cells exhibit strongly impaired cytokines secretion ability, which can be restored by blocking TIGIT, PD-1, and TIM-3 altogether [190]. Furthermore, the expression of DNAM-1 ligands on malignant plasma cells triggers human NK cell-mediated cytotoxicity against MM cells [20,187]. Noteworthy, TIGIT ligands CD112 and CD155 are not only highly expressed on AML cells, but the blockade of the TIGIT/CD112/CD155 axis augments T cell-mediated lysis of AML cells and enhances the cytotoxic effects of the CD33/CD3 bi-specific T cell engager (BiTE)® antibody construct AMG-330 [191,192]. Although evaluated only in solid tumors, this evidence indicates that TIGIT could represent a potentially promising target also for the treatment of hematological malignancies [34,116].
Another receptor expressed on NK cells showing great interest is the T-cell activation increased late expression (TACTILE) molecule or CD96. TACTILE is constitutively expressed on resting NK cells; it can interact with CD155 and it appears to inhibit NK cell-mediated IFN-γ production in mice, while it may enhance NK cell-mediated cytotoxicity in humans. These contrasting effects make unclear the clinical significance of TACTILE targeting [119,177,180,187]. Interestingly, DNAM-1 and TACTILE induce two opposite signals when they interact with CD155. Whereas the complex DNAM-1/CD155 activates NK cells, the interaction TACTILE/CD155 leads to a strong reduction of cytotoxicity, granule polarization, and cytokine secretion in NK cells [116,180,184,185]. Moreover, TACTILE can be expressed by malignant plasma cells in AML, T-cell acute lymphoblastic leukemia (T-ALL), and myelodysplastic syndromes [184]. Despite a possible interest as a potential target for the treatment of hematological malignancies, in humans, the role of TACTILE in NK cells functions is not completely understood, because of the presence of both activating and inhibitory motifs.
- Other molecular Targets for NK Cell-Mediated Immunotherapy
An inhibitory receptor expressed on NK cells under investigation is sialic acid-binding Ig-like lectin-7 (Siglec-7) which dampens NK cell surveillance and lead to tumor cells escape [7,193,194,195]. Interestingly, Siglec-7+ NK cells strongly express CD16, DNAM-1, NKp30, and NKp46, and exhibit a strong CD107a degranulation and IFN-γ production [195]. Of note, several Siglec-7 ligands have been detected on NK cells including the ganglioside disialosyl globopentaosylceramide (DSGb5) [196] and the ganglioside GD3 [197]; the interaction of Siglec-7 with these two gangliosides can modulate NK cell-mediated cytotoxicity against kidney carcinoma cells and P815 mouse mastocytoma cell line. Importantly, Siglec ligands are expressed at tumor cell surface and they seem to play an important role in the tumor escape from NK cell-mediated immunosurveillance [193]. An exhaustive summary of Siglec ligands has been reported by [193,198]. In hematological malignancies, Siglec-7 ligands have been observed in CML, CLL, AML [199], and MM [193,194] cells.
Another attractive target for cancer immunotherapy is B7-H3 (CD276); this molecule plays a key role in the inhibition of T-cell function [34,200,201,202,203,204] and it is highly expressed on a wide range of human solid cancers; Its expression often correlates with both negative prognosis and poor clinical outcome of patients [202,203]. The B7-H3-mediated functions remain poorly investigated in hematological malignancies. To our knowledge, B7-H3 has been reported expressed only by AML cella [205,206] and mantle cell lymphomas (MCL) [207]. Interestingly, a bi-specific antibody CD3/B7-H3 (B7-H3Bi-Ab) has been reported to enhance the ability of T cells to secrete cytotoxic granules and cytokines, associated with the killing of hematological tumor cells [208]. Another inhibitory receptor expressed on NK cells is CD161 (NKR-P1A). CD161 can bind to C-type lectin-like transcript-1 (LLT-1) expressed by several hematological malignancies, including Burkitt lymphoma, FL, and DLBCL [209,210]. It is of note that the CD161/LLT1 interaction in NK cells impairs cytokines secretion and cytotoxic activity, thus decreasing tumor susceptibility to NK cells [209,210,211]. The negative role of LLT-1 on NK cell functions is confirmed by the fact that the blockade of CD161/LLT-1 axis increases the NK cell-mediated secretion of IFN-γ and the killing of tumor cells [210,211].
Finally, Polatuzumab vedotin is amAb recognizing the B-cell receptor component CD79b. This antibody is currently under investigation in hematological malignancies [212]. In r/r DLBCL patients, it has been used combined with bendamustine (an alkylant agent) and Obinutuzumab (an anti-CD20 mAb) (NCT02257567, phase Ib/II) [213], or in combination with Rituximab or Obinutuzumab and Cyclophosphamide, Doxorubicin, and Prednisone (NCT01992653, phase I/II) [214]. Also, in r/r NHL patients, Polatuzumab has been used in combination with Rituximab (NCT01691898, phase II) [215] (Table 1).
2.3. Engineered mAbs
2.3.1. Bi-specific T cell Engagers, Bi-Specific Killer Engagers, and Tri-Specific Killer Engagers (BITEs, BIKEs, and TRIKEs)
As discussed above, mAb-based therapy represents an important tool to promote an efficient anti-tumor immune response. At present, this therapeutic tool has been “further improved” with the development of bi-specific antibodies. These antibodies are engineered proteins recognizing simultaneously two different antigens: one target antigen is expressed on tumor cells and the second one is an activating receptor expressed on immune effector cells. Thus, bi-specific T-cell engagers (BiTEs) represent a promising approach, since effector cells stimulated with BiTEs display an increased expression of CD69 and CD25, with consequent effector cell proliferation, cytokine and cytotoxic granules secretion, leading to a strong anti-tumor response [216,217,218,219,220,221]. Noteworthy, several BiTEs are currently investigated in clinical trials in hematological malignancies for their safety and efficacy. Based on the BiTE’s philosophy, Bi-specific killing cell engagers (BiKEs) have been developed to improve NK cell functions by facilitating their interaction with the target, principally through the CD16 activation [222,223] (Figure 2). In vitro studies have demonstrated that the BiKE CD16 × CD33 increases NK cell cytotoxicity and cytokine production in AML [224] and MDS, respectively [224,225]. Other BiKEs are represented by CD16 × CD19 and CD16 × CD133, whose engagement promote NK cell activation against CD19+ and CD133+ tumor cells, respectively [61,226]. Based on results showing that the bi-specific antibody CD30/CD16A (AFM13) can enhance NK cell cytotoxicity against CD30+ HL cells, this BiKE is currently under evaluation in a clinical study (NCT01221571, phase I) to assess its safety in HL patients [225,227,228,229,230]. Other clinical studies have been performed, or are ongoing analyzing, the effect of CD19/CD16 [15,40,224,226,229], CD123/CD3 [15], and CD20 × CD16 [231] in hematological malignancies [220,232,233,234,235,236].
Recently, several tri-specific killer cell engagers (TriKEs) have been also developed to boost NK cell functions [222,223,224,225,226,227,228,229,230,231,232,233,234].TriKEs work similarly to BiKEs, indeed they bind to an activating receptor expressed on NK cells (e.g., CD16) and to two different antigens expressed on tumor cells; this can lead to the generation of a very strong immunological synapse between tumor and effector cells. For example, the CD16 × CD19 × CD22 or CD16 × CD33 × IL-15 TriKEs trigger NK cell activation, ADCC and cytokine secretion leading to the release of lytic granules against B-cell leukemia and AML cells [15,233,237,238]. Interestingly, the TriKE CD16 × CD33 × IL-15, in which one of the antibodies has been substituted with an immunostimulating cytokine such as IL-15, has been also reported to stimulate NK-cell function to overcome immune suppression mediated by MDSCs in MDS [239]. Also, Glorius et al. have demonstrated that “tri-body” engagers CD20 × CD20 × CD16 efficiently trigger effectors cell-mediated lysis of malignant B cells [231]. Remarkably, it has been recently reported by Gauthier and colleagues the possibility to generate tri-functional NK cell engagers (NKCEs), which can target NKp46 and CD16 on NK cells and a tumor antigen on cancer cells [210,240]. Noteworthy, in the attempt to improve the anti-leukemic specificity of activated NK cells, others have investigated ULBP2 (an NKG2D ligand) × CD19 × CD33 engagers [triple-bodies (TBs)] [241].
2.3.2. Dual-Affinity Re-Targeting T cells (DARTs)
Although today this strategy is not currently investigated in NK cells, the dual-affinity re-targeting T cells (DARTs®) merit to be mentioned. Indeed, it has emerged as a promising tool in the treatment of hematological malignancies. Similarly to BiTEs, DARTs trigger CD3 on T cells and a specific tumor-associated antigen (TAA) on malignant cells (e.g., CD19). Stimulated T cells are then able to kill tumor cells in vitroand to suppress tumor growth and induce tumor regression [96,242,243].
3. Engineered Effector Cells
3.1. Chimeric Antigen Receptor (CAR) NK Cells
In the past decade, besides the generation of several kinds of mAbs used in immunotherapy, also some engineered anti-tumor immune cells have been developed. Indeed, the chimeric antigen receptors (CARs), have been transduced in effector T lymphocytes of tumor bearing patients to improve their anti-tumor response. These CARs were composed of an extracellular domain able to recognize the tumor and an intracellular portion that delivers an activating signal to T lymphocytes. Thus, CAR T cells are ready-to-kill effector cells, equipped to migrate at the tumor site, circumvent TME traps, and then attack tumor cells [140,244,245,246,247,248,249]. While the 1st generation of CARs contained only one intracellular co-stimulatory domain (e.g., CD3ζ), the recent generations contain several co-stimulatory domains (e.g., CD3ζ and CD28 and CD137/4-1BB), which enhance long-term T-cell activation and are used in most recent clinical trials. To interact with a specific tumor-associated antigen(TAA), such as CD19 on neoplastic B cells, CAR-T cells express an extracellular domain with a single-chain fragment variable (scFv) derived from an anti-CD19 antibody. This scFv promotes the interaction with CD19 on tumor B cells and through the intracellular domains of CAR molecule, the effector cell activation, cytotoxic granules secretion leading to the tumor B cell killing.
Recently a similar approach has been proposed for NK cells as well. Indeed, it has been planned the introduction of CARs into NK cells to boost their potent killing activity to tumor cells. These CARs recognize specific antigens on target cells and help the natural propensity of NK cells to kill tumor cells based on their expression of activating receptors [250]. Preclinical studies have shown that CAR.NK cells expressing SLAM-F7 displayed enhanced cytotoxicity, cytokine secretion, and anti-tumor activity [251,252,253]. Similar results have been observed in CAR-NK cells expressing CD19, CD20, or TRAIL [254,255,256]. Interesting results have been also obtained using NK cells genetically modified with a CD138-CAR. CD138 is a member of the syndecan family of type I transmembrane proteoglycans and is highly expressed on MM cells, where it plays an important role in their adhesion, proliferation, and angiogenesis [257,258,259,260]. These CD138-CAR NK cells displayed considerably enhanced cytotoxicity against CD138+ MM cell lines and primary MM cells, compared to untransduced NK cells. Additionally, this enhanced CD138-CAR NK cell-mediated killing was associated with increased secretion of Granzyme B and IFN-γ [253,259,261]. Based on these results, several clinical trials are ongoing [258,259,260].
Based on the fact that tumor cells can increase the expression of NKG2D ligands on their surface upon stress signals but TME can simultaneously decrease the expression of NKG2D on NK cells, NKG2D-CARs have been recently developed in the attempt to further increase NK cell activation. These CAR NK cells express NKG2D combined with several co-stimulatory domains such as DNAX-activation protein (DAP)-10, 4-1BB, CD3ζ and CD28. Of note, these NK cells displayed an up-regulated expression of NKG2D and strong cytotoxicity abilities against malignant cells [247,262,263,264,265,266]. Importantly, NKG2D-CAR NK cells not only can recognize the NKG2D ligands expressed on tumor cells, but also on the other immunosuppressive cells within the TME. An interesting study reported that NK92 cells genetically modified with an extracellular domain of TGF-βRII and an intracellular domain of NKG2D were resistant to the TGF-β produced by the TME, secreted high amounts of IFN-γ, and exhibited strong killing capacity. In addition, these TGF-βRII+NKG2D+ NK cells impaired the generation of Treg populations and inhibited tumor growth [267]. Although these results have been obtained in solid tumors, this approach could also be investigated in hematological malignancies, to help infiltrated NK cells to move aside the TME’s traps. To avoid possible toxicities in patients, several groups recently started to introduce also suicide genes in engineered cells [268,269]. Interestingly, it has been recently shown that cord blood-derived NK cells expressing CD19 and the suicide gene inducible Caspase-9 (iC9) and producing IL-15 (CAR CD19/IL-15/iC9) exhibited an efficient killing of CD19+ tumor B cells both in vitro and in vivo [270]. To strengthen NK cell-mediated anti-tumor response and improve patient’s survival, the use of several therapeutic combinations have been evaluated. For example, CAR NK cell-based therapy in hematological malignancies has been associated with drugs as Lenalidomide [271,272] and mAbs as Elotuzumab [46,273], Nivolumab [274,275] or Pembrolizumab [276]. CAR NK cells represent a very exciting approach for cancer immunotherapy. Importantly, the advantage of the utilization of allogeneic CAR-NK cells is their “off-the-shelf” manufacturing, limited life-span, no induction of cytokine release syndrome (CRS) and do not cause graft versus host disease (GVHD) [18,210,219,247,249,250,277,278,279] (Figure 3). In addition, compared to CAR T cells, CAR NK cells will retain their ability to search and destroy targets through their natural arsenal. These features could allow allogeneic CAR NK cells to circumvent the traps found into the TME and promote their anti-tumor immune-surveillance, for an efficient treatment of hematological malignancies (Table 2). Despite all these strategies and weaponry, there are however some points that must be considered, like the optimal activating cocktail, drug combination strategies, the best source and subset population to generate ready-to-kill NK cells and the best way to enhance NK cells homing and survival at the tumor site [40,61,210,250,263,280,281,282,283,284].
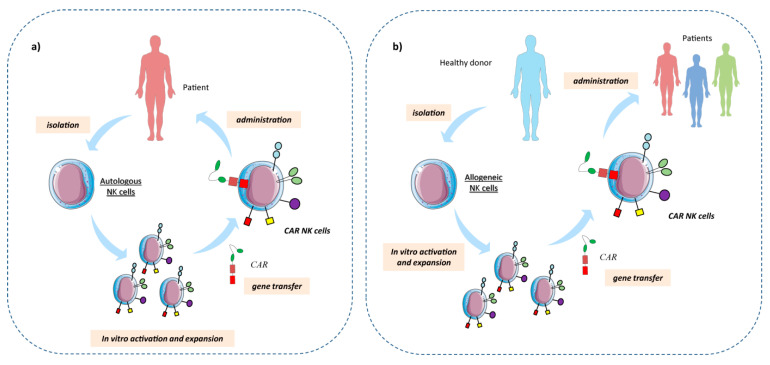
Figure 3.
Schematic chimeric antigen receptor (CAR) NK cells therapy. (a)NK cells are isolated from patients (autologous), activated, expanded, and then genetically modified to express specific CARs. Therefore, autologous CAR NK cells are administered to the patient. (b) NK cells isolated from healthy donors (allogeneic) are activated and then genetically modified to express specific CARs and consequently expanded. Allogeneic NK cells are then administered to several patients. Allogeneic CAR NK cells can be obtained from different sources, including peripheral blood mononuclear cells (PBMC), NK cell lines, umbilical cord blood (UBC), embryonic cells (ES) or induced pluripotent stem cells (iPSC).
Table 2.
Summary of current clinical trials using CAR NK cells in hematological cancers.
| Sources of NK Cells | Disease | Receptor Target | Trial Number (NCT) | Phase |
|---|---|---|---|---|
| NK92 | Lymphoma and Leukemia | CD7 | 02742727 | I/II |
| Lymphoma and Leukemia | CD19 | 02892695 | I/II | |
| AML r/r | CD33 | 02944162 | I/II | |
| MM r/r | BCMA | 03940833 | I/II | |
| B-cell lymphoma r/r | CD19 | 03690310 | I | |
| CB-derived NK cells | ALL, CLL and NHL r/r | CD19 | 03056339 | I/II |
| B-cell lymphoma | CD19 | 03579927 | I/II | |
| unknown | B-cell lymphoma r/r | CD19+CD22 | 03824964 | I |
| PB NK cells | ALL | CD19 | 01974479 | I |
| ALL | CD19 | 00995137 | I | |
| unknown | B-cell lymphoma r/r | CD22 | 03692767 | I |
| iPSC-derived NK cells | B-cell lymphoma r/r | CD19 | 03824951 | I |
Abbreviations used: ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; r/r, refractory/relapsed; CB, cord blood; iPSC, induced pluripotent stem cells; PB, peripheral blood; BCMA, B cell maturation antigen.
3.2. T-Cell Redirected for Universal Cytokine-Mediated Killing (TRUCKs)
Another strategy causing great enthusiasm is represented by T-cell redirected for universal cytokine-mediated killing (TRUCK), the fourth generation of CAR-T cells developed to affect not only the tumor cells themselves but the entire TME [285,286]. Although promising results on T cells have been reported, today there are no current studies in NK cells.
4. Conclusions and Perspectives
NK cells are one of the most efficient immune cell killing machines available (cit. [219]) and play a major role in tumor surveillance. Unfortunately, tumor cells and the surrounding TME always develop new tricks to escape to their killing [7,10]. Decreased recognition and cytotoxic functions of NK cells have been described in hematologic malignancies, because of diminished expression of activating receptors, cytokine secretion, and granule exocytosis [16]. In an attempt to restore NK cell-mediated anti-tumor activities, several therapeutic strategies have been developed to treat hematological malignancies. The introduction of drugs as histone deacetylases inhibitors (HDACis) [287,288,289] and IMiDs [27,287,289,290,291,292] significantly improved NK cell recognition and negatively modulated the TME-induced inhibitory functions, thus promoting the killing of tumor cells. In the past decade, other therapeutic approaches have been developed, such as checkpoint inhibitors and engineered cells. The checkpoint inhibitors approach has been validated for the treatment of most of hematological malignancies. The blockade of KIRs and the PD-1/PD-L1 axis or other promising mAbs targeting specific antigens expressed on malignant plasma cells as CD22, CD38, and SLAM-F7 have been described to relieve the exhausted status of NK cells and to restore NK cell surveillance. On other hands, engineered mAbs as BiKEs/TRiKEs remarkably arisen as promising strategies for the treatment of hematological malignancies. Engineered mAbs improve NK cell activation through CD16 and, by facilitating the formation of an immunological synapse, increase NK cell cytolytic activity against tumor cells. These BiKEs/TRiKEs obtained to such an extent promising results that are currently investigated in preclinical and clinical studies [222,223,224,225,226,227,228,229,230,231,232,233,234,277]. In hematological cancers the adoptive cell therapy based on the utilization of CAR T cells has arisen as a promising strategy. However, qualities such as natural immune-surveillance, “off-the-shelf” manufacturing, limited life-span, no CRS or GVHD induction, render CAR NK cells a therapeutic tool in the treatment of hematological malignancies [18,210,219,247,249,250,277,278,279]. A consistent indication that CAR NK cells represent a safe alternative to CAR T cells has been recently provided by Tang and colleagues, whose reported that CD33-CAR NK cells administrated to r/r AML patients have not shown significant adverse effects [293]. Nonetheless, to generate the perfect functional ready-to-use CAR NK cell, there are still many questions and reefs to pass [40,61,210,250,263,280,281,282,294].To affect tumor escape and to restore an adequate anti-tumor response is needed. Although the strategies discussed in this review have shown brilliant results, robust evidence supports the fact that these approaches should be combined altogether to maximize the chances of NK cells to exhibit a complete immune-surveillance circumventing the immunosuppressive behavior of the TME.
Abbreviations
ALL: Acute Lymphocytic Leukemia; AML, Acute Myeloid Leukemia; CLL, Chronic Lymphocytic Leukemia; CML, Chronic Myeloid Leukemia; MM, Multiple Myeloma; MDS, Myelodysplastic Syndromes; NHL, Non-Hodgkin Lymphoma; FL, Follicular Lymphoma; DLBCL, Diffuse Large B-Cell Lymphoma; SMM, Smoldering MM; r/r, refractory/relapsed; IMiDs, immunomodulatory drugs
Author Contributions
M.G. planned the work, wrote and critically revised the paper; A.P. wrote and revised the paper. Figures were produced using Servier Medical Art: www.servier.com. All authors have read and agreed to the published version of the manuscript.
Funding
This work is partially supported by grants from the AIRC IG 21648 and 5xmille 2015 and 2016 and Ricerca Corrente from Italian Ministry of Health, to A.P.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lanier L.L. NK cell recognition. Annu. Rev. Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 2.Ljunggren H.G., Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-S. [DOI] [PubMed] [Google Scholar]
- 3.Moretta A., Bottino C., Vitale M., Pende D., Cantoni C., Mingari M.C., Biassoni R., Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 4.Moretta L., Moretta A. Unravelling natural killer cell function: Triggering and inhibitory human NK receptors. Embo. J. 2004;23:255–259. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 6.Pende D., Falco M., Vitale M., Cantoni C., Vitale C., Munari E., Bertaina A., Moretta F., Del Zotto G., Pietra G., et al. Killer Ig-Like Receptors (KIRs): Their Role in NK Cell Modulation and Developments Leading to Their Clinical Exploitation. Front. Immunol. 2019;10:1179. doi: 10.3389/fimmu.2019.01179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrow A.D., Colonna M. Tailoring Natural Killer cell immunotherapy to the tumour microenvironment. Semin. Immunol. 2017;31:30–36. doi: 10.1016/j.smim.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassani B., Baci D., Gallazzi M., Poggi A., Bruno A., Mortara L. Natural Killer Cells as Key Players of Tumor Progression and Angiogenesis: Old and Novel Tools to Divert Their Pro-Tumor Activities into Potent Anti-Tumor Effects. Cancers. 2019;11:461. doi: 10.3390/cancers11040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholas N.S., Apollonio B., Ramsay A.G. Tumor microenvironment (TME)-driven immune suppression in B cell malignancy. Biochim. Biophys. Acta. 2016;1863:471–482. doi: 10.1016/j.bbamcr.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Poggi A., Giuliani M. Mesenchymal Stromal Cells Can Regulate the Immune Response in the Tumor Microenvironment. Vaccines. 2016;4:41. doi: 10.3390/vaccines4040041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott D.W., Gascoyne R.D. The tumour microenvironment in B cell lymphomas. Nat. Rev. Cancer. 2014;14:517–534. doi: 10.1038/nrc3774. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Correa B., Gayoso I., Bergua J.M., Casado J.G., Morgado S., Solana R., Tarazona R. Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol. Cell Biol. 2012;90:109–115. doi: 10.1038/icb.2011.15. [DOI] [PubMed] [Google Scholar]
- 13.Spear P., Wu M.R., Sentman M.L., Sentman C.L. NKG2D ligands as therapeutic targets. Cancer Immun. 2013;13:8. [PMC free article] [PubMed] [Google Scholar]
- 14.Carlsten M., Jaras M. Natural Killer Cells in Myeloid Malignancies: Immune Surveillance, NK Cell Dysfunction, and Pharmacological Opportunities to Bolster the Endogenous NK Cells. Front. Immunol. 2019;10:2357. doi: 10.3389/fimmu.2019.02357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dulphy N., Chretien A.S., Khaznadar Z., Fauriat C., Nanbakhsh A., Caignard A., Chouaib S., Olive D., Toubert A. Underground Adaptation to a Hostile Environment: Acute Myeloid Leukemia vs. Natural Killer Cells. Front. Immunol. 2016;7:94. doi: 10.3389/fimmu.2016.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farnault L., Sanchez C., Baier C., Le Treut T., Costello R.T. Hematological malignancies escape from NK cell innate immune surveillance: Mechanisms and therapeutic implications. Clin. Dev. Immunol. 2012;2012:421702. doi: 10.1155/2012/421702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Rodriguez A.P., Villa-Alvarez M., Sordo-Bahamonde C., Lorenzo-Herrero S., Gonzalez S. NK Cells in the Treatment of Hematological Malignancies. J. Clin. Med. 2019;8:1557. doi: 10.3390/jcm8101557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofland T., Eldering E., Kater A.P., Tonino S.H. Engaging Cytotoxic T and NK Cells for Immunotherapy in Chronic Lymphocytic Leukemia. Int. J. Mol. Sci. 2019;20:4315. doi: 10.3390/ijms20174315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parry H.M., Stevens T., Oldreive C., Zadran B., McSkeane T., Rudzki Z., Paneesha S., Chadwick C., Stankovic T., Pratt G., et al. NK cell function is markedly impaired in patients with chronic lymphocytic leukaemia but is preserved in patients with small lymphocytic lymphoma. Oncotarget. 2016;7:68513–68526. doi: 10.18632/oncotarget.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Sherbiny Y.M., Meade J.L., Holmes T.D., McGonagle D., Mackie S.L., Morgan A.W., Cook G., Feyler S., Richards S.J., Davies F.E., et al. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 2007;67:8444–8449. doi: 10.1158/0008-5472.CAN-06-4230. [DOI] [PubMed] [Google Scholar]
- 21.Godfrey J., Benson D.M., Jr. The role of natural killer cells in immunity against multiple myeloma. Leuk. Lymphoma. 2012;53:1666–1676. doi: 10.3109/10428194.2012.676175. [DOI] [PubMed] [Google Scholar]
- 22.Khaznadar Z., Boissel N., Agaugue S., Henry G., Cheok M., Vignon M., Geromin D., Cayuela J.M., Castaigne S., Pautas C., et al. Defective NK Cells in Acute Myeloid Leukemia Patients at Diagnosis Are Associated with Blast Transcriptional Signatures of Immune Evasion. J. Immunol. 2015;195:2580–2590. doi: 10.4049/jimmunol.1500262. [DOI] [PubMed] [Google Scholar]
- 23.Lion E., Willemen Y., Berneman Z.N., Van Tendeloo V.F., Smits E.L. Natural killer cell immune escape in acute myeloid leukemia. Leukemia. 2012;26:2019–2026. doi: 10.1038/leu.2012.87. [DOI] [PubMed] [Google Scholar]
- 24.Stringaris K., Sekine T., Khoder A., Alsuliman A., Razzaghi B., Sargeant R., Pavlu J., Brisley G., de Lavallade H., Sarvaria A., et al. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica. 2014;99:836–847. doi: 10.3324/haematol.2013.087536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khaznadar Z., Henry G., Setterblad N., Agaugue S., Raffoux E., Boissel N., Dombret H., Toubert A., Dulphy N. Acute myeloid leukemia impairs natural killer cells through the formation of a deficient cytotoxic immunological synapse. Eur. J. Immunol. 2014;44:3068–3080. doi: 10.1002/eji.201444500. [DOI] [PubMed] [Google Scholar]
- 26.Ramsay A.G., Johnson A.J., Lee A.M., Gorgun G., Le Dieu R., Blum W., Byrd J.C., Gribben J.G. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J. Clin. Investig. 2008;118:2427–2437. doi: 10.1172/JCI35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giuliani M., Janji B., Berchem G. Activation of NK cells and disruption of PD-L1/PD-1 axis: Two different ways for lenalidomide to block myeloma progression. Oncotarget. 2017;8:24031–24044. doi: 10.18632/oncotarget.15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konjevic G., Vuletic A., Mirjacic Martinovic K., Colovic N., Colovic M., Jurisic V. Decreased CD161 activating and increased CD158a inhibitory receptor expression on NK cells underlies impaired NK cell cytotoxicity in patients with multiple myeloma. J. Clin. Pathol. 2016;69:1009–1016. doi: 10.1136/jclinpath-2016-203614. [DOI] [PubMed] [Google Scholar]
- 29.MacFarlane A.W.t., Jillab M., Smith M.R., Alpaugh R.K., Cole M.E., Litwin S., Millenson M.M., Al-Saleem T., Cohen A.D., Campbell K.S. NK cell dysfunction in chronic lymphocytic leukemia is associated with loss of the mature cells expressing inhibitory killer cell Ig-like receptors. Oncoimmunology. 2017;6:e1330235. doi: 10.1080/2162402X.2017.1330235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandoval-Borrego D., Moreno-Lafont M.C., Vazquez-Sanchez E.A., Gutierrez-Hoya A., Lopez-Santiago R., Montiel-Cervantes L.A., Ramirez-Saldana M., Vela-Ojeda J. Overexpression of CD158 and NKG2A Inhibitory Receptors and Underexpression of NKG2D and NKp46 Activating Receptors on NK Cells in Acute Myeloid Leukemia. Arch. Med. Res. 2016;47:55–64. doi: 10.1016/j.arcmed.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Hadadi L., Hafezi M., Amirzargar A.A., Sharifian R.A., Abediankenari S., Asgarian-Omran H. Dysregulated Expression of Tim-3 and NKp30 Receptors on NK Cells of Patients with Chronic Lymphocytic Leukemia. Oncol. Res. Treat. 2019;42:202–208. doi: 10.1159/000497208. [DOI] [PubMed] [Google Scholar]
- 32.Chiossone L., Vienne M., Kerdiles Y.M., Vivier E. Natural killer cell immunotherapies against cancer: Checkpoint inhibitors and more. Semin. Immunol. 2017;31:55–63. doi: 10.1016/j.smim.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Dempke W.C.M., Fenchel K., Uciechowski P., Dale S.P. Second- and third-generation drugs for immuno-oncology treatment-The more the better? Eur J. Cancer. 2017;74:55–72. doi: 10.1016/j.ejca.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Marin-Acevedo J.A., Dholaria B., Soyano A.E., Knutson K.L., Chumsri S., Lou Y. Next generation of immune checkpoint therapy in cancer: New developments and challenges. J. Hematol. Oncol. 2018;11:39. doi: 10.1186/s13045-018-0582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ok C.Y., Young K.H. Checkpoint inhibitors in hematological malignancies. J. Hematol. Oncol. 2017;10:103. doi: 10.1186/s13045-017-0474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall M.J.E., Stopforth R.J., Cragg M.S. Therapeutic Antibodies: What Have We Learnt from Targeting CD20 and Where Are We Going? Front. Immunol. 2017;8:1245. doi: 10.3389/fimmu.2017.01245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salles G., Barrett M., Foa R., Maurer J., O’Brien S., Valente N., Wenger M., Maloney D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017;34:2232–2273. doi: 10.1007/s12325-017-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei G., Wang J., Huang H., Zhao Y. Novel immunotherapies for adult patients with B-lineage acute lymphoblastic leukemia. J. Hematol. Oncol. 2017;10:150. doi: 10.1186/s13045-017-0516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dahlberg C.I., Sarhan D., Chrobok M., Duru A.D., Alici E. Natural Killer Cell-Based Therapies Targeting Cancer: Possible Strategies to Gain and Sustain Anti-Tumor Activity. Front. Immunol. 2015;6:605. doi: 10.3389/fimmu.2015.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohrt H.E., Thielens A., Marabelle A., Sagiv-Barfi I., Sola C., Chanuc F., Fuseri N., Bonnafous C., Czerwinski D., Rajapaksa A., et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood. 2014;123:678–686. doi: 10.1182/blood-2013-08-519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Garff-Tavernier M., Decocq J., de Romeuf C., Parizot C., Dutertre C.A., Chapiro E., Davi F., Debre P., Prost J.F., Teillaud J.L., et al. Analysis of CD16+CD56dim NK cells from CLL patients: Evidence supporting a therapeutic strategy with optimized anti-CD20 monoclonal antibodies. Leukemia. 2011;25:101–109. doi: 10.1038/leu.2010.240. [DOI] [PubMed] [Google Scholar]
- 43.Le Garff-Tavernier M., Herbi L., de Romeuf C., Azar N., Roos-Weil D., Bonnemye P., Urbain R., Leblond V., Merle-Beral H., Vieillard V. The optimized anti-CD20 monoclonal antibody ublituximab bypasses natural killer phenotypic features in Waldenstrom macroglobulinemia. Haematologica. 2015;100:e147–e151. doi: 10.3324/haematol.2014.118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Garff-Tavernier M., Herbi L., de Romeuf C., Prost J.-F., Debré P., Urbain R.M., Leblond V.R., Vieillard V., Merle-Béral H.L.N. Ublituximab, an Optimized Anti-CD20 Monoclonal Antibody, Demonstrates Greater NK-Mediated ADCC Than Rituximab in Waldenstrom’s Macroglobulinemia Patients Supporting a Therapeutic Strategy with Ublituximab. Blood. 2012;120:1654. doi: 10.1182/blood.V120.21.1654.1654. [DOI] [Google Scholar]
- 45.Werlenius O., Aurelius J., Hallner A., Akhiani A.A., Simpanen M., Martner A., Andersson P.O., Hellstrand K., Thoren F.B. Reactive oxygen species induced by therapeutic CD20 antibodies inhibit natural killer cell-mediated antibody-dependent cellular cytotoxicity against primary CLL cells. Oncotarget. 2016;7:32046–32053. doi: 10.18632/oncotarget.8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen W.C., Kanate A.S., Craig M., Petros W.P., Hazlehurst L.A. Emerging combination therapies for the management of multiple myeloma: The role of elotuzumab. Cancer Manag Res. 2017;9:307–314. doi: 10.2147/CMAR.S117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collins S.M., Bakan C.E., Swartzel G.D., Hofmeister C.C., Efebera Y.A., Kwon H., Starling G.C., Ciarlariello D., Bhaskar S., Briercheck E.L., et al. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: Evidence for augmented NK cell function complementing ADCC. Cancer Immunol. Immunother. 2013;62:1841–1849. doi: 10.1007/s00262-013-1493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsi E.D., Steinle R., Balasa B., Szmania S., Draksharapu A., Shum B.P., Huseni M., Powers D., Nanisetti A., Zhang Y., et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin. Cancer Res. 2008;14:2775–2784. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y.C., Szmania S., van Rhee F. Profile of elotuzumab and its potential in the treatment of multiple myeloma. Blood Lymphat Cancer. 2014;2014:15–27. doi: 10.2147/BLCTT.S49780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pazina T., James A.M., MacFarlane A.W.t., Bezman N.A., Henning K.A., Bee C., Graziano R.F., Robbins M.D., Cohen A.D., Campbell K.S. The anti-SLAMF7 antibody elotuzumab mediates NK cell activation through both CD16-dependent and -independent mechanisms. Oncoimmunology. 2017;6:e1339853. doi: 10.1080/2162402X.2017.1339853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tai Y.T., Dillon M., Song W., Leiba M., Li X.F., Burger P., Lee A.I., Podar K., Hideshima T., Rice A.G., et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2008;112:1329–1337. doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Afifi S., Michael A., Lesokhin A. Immunotherapy: A New Approach to Treating Multiple Myeloma with Daratumumab and Elotuzumab. Ann. Pharm. 2016;50:555–568. doi: 10.1177/1060028016642786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lonial S., Dimopoulos M., Palumbo A., White D., Grosicki S., Spicka I., Walter-Croneck A., Moreau P., Mateos M.V., Magen H., et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2015;373:621–631. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 54.Sherbenou D.W., Mark T.M., Forsberg P. Monoclonal Antibodies in Multiple Myeloma: A New Wave of the Future. Clin. Lymphomamyeloma Leuk. 2017;17:545–554. doi: 10.1016/j.clml.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 55.Liu L., Zhao N., Xu W., Sheng Z., Wang L. Pooled analysis of the reports of carfilzomib, panobinostat, and elotuzumab combinations in patients with refractory/relapsed multiple myeloma. J. Hematol. Oncol. 2016;9:54. doi: 10.1186/s13045-016-0286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jakubowiak A., Offidani M., Pegourie B., De La Rubia J., Garderet L., Laribi K., Bosi A., Marasca R., Laubach J., Mohrbacher A., et al. Randomized phase 2 study: Elotuzumab plus bortezomib/dexamethasone vs bortezomib/dexamethasone for relapsed/refractory MM. Blood. 2016;127:2833–2840. doi: 10.1182/blood-2016-01-694604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Rhee F., Szmania S.M., Dillon M., van Abbema A.M., Li X., Stone M.K., Garg T.K., Shi J., Moreno-Bost A.M., Yun R., et al. Combinatorial efficacy of anti-CS1 monoclonal antibody elotuzumab (HuLuc63) and bortezomib against multiple myeloma. Mol. Cancer. 2009;8:2616–2624. doi: 10.1158/1535-7163.MCT-09-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gormley N.J., Ko C.W., Deisseroth A., Nie L., Kaminskas E., Kormanik N., Goldberg K.B., Farrell A.T., Pazdur R. FDA Drug Approval: Elotuzumab in Combination with Lenalidomide and Dexamethasone for the Treatment of Relapsed or Refractory Multiple Myeloma. Clin. Cancer Res. 2017;23:6759–6763. doi: 10.1158/1078-0432.CCR-16-2870. [DOI] [PubMed] [Google Scholar]
- 59.Mateos M.V., Granell M., Oriol A., Martinez-Lopez J., Blade J., Hernandez M.T., Martin J., Gironella M., Lynch M., Bleickardt E., et al. Elotuzumab in combination with thalidomide and low-dose dexamethasone: A phase 2 single-arm safety study in patients with relapsed/refractory multiple myeloma. Br. J. Haematol. 2016;175:448–456. doi: 10.1111/bjh.14263. [DOI] [PubMed] [Google Scholar]
- 60.Richardson P.G., Jagannath S., Moreau P., Jakubowiak A.J., Raab M.S., Facon T., Vij R., White D., Reece D.E., Benboubker L., et al. Elotuzumab in combination with lenalidomide and dexamethasone in patients with relapsed multiple myeloma: Final phase 2 results from the randomised, open-label, phase 1b-2 dose-escalation study. Lancet Haematol. 2015;2:e516–e527. doi: 10.1016/S2352-3026(15)00197-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fang F., Xiao W., Tian Z. NK cell-based immunotherapy for cancer. Semin Immunol. 2017;31:37–54. doi: 10.1016/j.smim.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 62.Makkouk A., Chester C., Kohrt H.E. Rationale for anti-CD137 cancer immunotherapy. Eur. J. Cancer. 2016;54:112–119. doi: 10.1016/j.ejca.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 63.Weigelin B., Bolanos E., Teijeira A., Martinez-Forero I., Labiano S., Azpilikueta A., Morales-Kastresana A., Quetglas J.I., Wagena E., Sanchez-Paulete A.R., et al. Focusing and sustaining the antitumor CTL effector killer response by agonist anti-CD137 mAb. Proc. Natl. Acad. Sci. USA. 2015;112:7551–7556. doi: 10.1073/pnas.1506357112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chester C., Sanmamed M.F., Wang J., Melero I. Immunotherapy targeting 4-1BB: Mechanistic rationale, clinical results, and future strategies. Blood. 2018;131:49–57. doi: 10.1182/blood-2017-06-741041. [DOI] [PubMed] [Google Scholar]
- 65.Mahoney K.M., Rennert P.D., Freeman G.J. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 2015;14:561–584. doi: 10.1038/nrd4591. [DOI] [PubMed] [Google Scholar]
- 66.Muntasell A., Ochoa M.C., Cordeiro L., Berraondo P., Lopez-Diaz de Cerio A., Cabo M., Lopez-Botet M., Melero I. Targeting NK-cell checkpoints for cancer immunotherapy. Curr. Opin. Immunol. 2017;45:73–81. doi: 10.1016/j.coi.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 67.Yonezawa A., Dutt S., Chester C., Kim J., Kohrt H.E. Boosting Cancer Immunotherapy with Anti-CD137 Antibody Therapy. Clin. Cancer Res. 2015;21:3113–3120. doi: 10.1158/1078-0432.CCR-15-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allard B., Longhi M.S., Robson S.C., Stagg J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol. Rev. 2017;276:121–144. doi: 10.1111/imr.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Whiteside T.L. Targeting adenosine in cancer immunotherapy: A review of recent progress. Expert Rev. Anticancer. 2017;17:527–535. doi: 10.1080/14737140.2017.1316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Antonioli L., Blandizzi C., Malavasi F., Ferrari D., Hasko G. Anti-CD73 immunotherapy: A viable way to reprogram the tumor microenvironment. Oncoimmunology. 2016;5:e1216292. doi: 10.1080/2162402X.2016.1216292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leone R.D., Lo Y.C., Powell J.D. A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy. Comput. Struct. Biotechnol. J. 2015;13:265–272. doi: 10.1016/j.csbj.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beavis P.A., Divisekera U., Paget C., Chow M.T., John L.B., Devaud C., Dwyer K., Stagg J., Smyth M.J., Darcy P.K. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc. Natl. Acad. Sci. USA. 2013;110:14711–14716. doi: 10.1073/pnas.1308209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chambers A.M., Matosevic S. Immunemetabolic Dysfunction of Natural Killer Cells Mediated by the Hypoxia-CD73 Axis in Solid Tumors. Front. Mol. Biosci. 2019;6:60. doi: 10.3389/fmolb.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vigano S., Alatzoglou D., Irving M., Menetrier-Caux C., Caux C., Romero P., Coukos G. Targeting Adenosine in Cancer Immunotherapy to Enhance T-Cell Function. Front. Immunol. 2019;10:925. doi: 10.3389/fimmu.2019.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neo S.Y., Yang Y., Record J., Ma R., Chen X., Chen Z., Tobin N.P., Blake E., Seitz C., Thomas R., et al. CD73 immune checkpoint defines regulatory NK cells within the tumor microenvironment. J. Clin. Investig. 2020;130:1185–1198. doi: 10.1172/JCI128895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang X., Zhang T., Song Z., Li L., Zhang X., Liu J., Liu X., Qiu L., Qian Z., Zhou S., et al. Tumor CD73/A2aR adenosine immunosuppressive axis and tumor-infiltrating lymphocytes in diffuse large B-cell lymphoma: Correlations with clinicopathological characteristics and clinical outcome. Int. J. Cancer. 2019;145:1414–1422. doi: 10.1002/ijc.32144. [DOI] [PubMed] [Google Scholar]
- 77.Cai Y., Feng L., Wang X. Targeting the tumor promoting effects of adenosine in chronic lymphocytic leukemia. Crit. Rev. Oncol. Hematol. 2018;126:24–31. doi: 10.1016/j.critrevonc.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 78.Kicova M., Michalova Z., Coma M., Gabzdilova J., Dedinska K., Guman T., Bernatova S., Hajikova M., Giertlova M., Veselinyova D., et al. The expression of CD73 on pathological B-cells is associated with shorter overall survival of patients with CLL. Neoplasma. 2020 doi: 10.4149/neo_2020_190826N822. [DOI] [PubMed] [Google Scholar]
- 79.Vaisitti T., Arruga F., Guerra G., Deaglio S. Ectonucleotidases in Blood Malignancies: A Tale of Surface Markers and Therapeutic Targets. Front. Immunol. 2019;10:2301. doi: 10.3389/fimmu.2019.02301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saldanha-Araujo F., Ferreira F.I., Palma P.V., Araujo A.G., Queiroz R.H., Covas D.T., Zago M.A., Panepucci R.A. Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Res. 2011;7:66–74. doi: 10.1016/j.scr.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 81.Chatterjee D., Tufa D.M., Baehre H., Hass R., Schmidt R.E., Jacobs R. Natural killer cells acquire CD73 expression upon exposure to mesenchymal stem cells. Blood. 2014;123:594–595. doi: 10.1182/blood-2013-09-524827. [DOI] [PubMed] [Google Scholar]
- 82.Vijayan D., Young A., Teng M.W.L., Smyth M.J. Targeting immunosuppressive adenosine in cancer. Nat. Rev. Cancer. 2017;17:709–724. doi: 10.1038/nrc.2017.86. [DOI] [PubMed] [Google Scholar]
- 83.Young A., Ngiow S.F., Gao Y., Patch A.M., Barkauskas D.S., Messaoudene M., Lin G., Coudert J.D., Stannard K.A., Zitvogel L., et al. A2AR Adenosine Signaling Suppresses Natural Killer Cell Maturation in the Tumor Microenvironment. Cancer Res. 2018;78:1003–1016. doi: 10.1158/0008-5472.CAN-17-2826. [DOI] [PubMed] [Google Scholar]
- 84.Atanackovic D., Steinbach M., Radhakrishnan S.V., Luetkens T. Immunotherapies targeting CD38 in Multiple Myeloma. Oncoimmunology. 2016;5:e1217374. doi: 10.1080/2162402X.2016.1217374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Costa F., Dalla Palma B., Giuliani N. CD38 Expression by Myeloma Cells and Its Role in the Context of Bone Marrow Microenvironment: Modulation by Therapeutic Agents. Cells. 2019;8:1632. doi: 10.3390/cells8121632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nijhof I.S., Lammerts van Bueren J.J., van Kessel B., Andre P., Morel Y., Lokhorst H.M., van de Donk N.W., Parren P.W., Mutis T. Daratumumab-mediated lysis of primary multiple myeloma cells is enhanced in combination with the human anti-KIR antibody IPH2102 and lenalidomide. Haematologica. 2015;100:263–268. doi: 10.3324/haematol.2014.117531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanchez L., Wang Y., Siegel D.S., Wang M.L. Daratumumab: A first-in-class CD38 monoclonal antibody for the treatment of multiple myeloma. J. Hematol. Oncol. 2016;9:51. doi: 10.1186/s13045-016-0283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chari A., Suvannasankha A., Fay J.W., Arnulf B., Kaufman J.L., Ifthikharuddin J.J., Weiss B.M., Krishnan A., Lentzsch S., Comenzo R., et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130:974–981. doi: 10.1182/blood-2017-05-785246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chung C. Role of Immunotherapy in Targeting the Bone Marrow Microenvironment in Multiple Myeloma: An Evolving Therapeutic Strategy. Pharmacotherapy. 2017;37:129–143. doi: 10.1002/phar.1871. [DOI] [PubMed] [Google Scholar]
- 90.Petrucci M.T., Vozella F. The Anti-CD38 Antibody Therapy in Multiple Myeloma. Cells. 2019;8:1629. doi: 10.3390/cells8121629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van de Donk N., Usmani S.Z. CD38 Antibodies in Multiple Myeloma: Mechanisms of Action and Modes of Resistance. Front. Immunol. 2018;9:2134. doi: 10.3389/fimmu.2018.02134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Soekojo C.Y., Ooi M., de Mel S., Chng W.J. Immunotherapy in Multiple Myeloma. Cells. 2020;9:601. doi: 10.3390/cells9030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martin T., Baz R., Benson D.M., Lendvai N., Wolf J., Munster P., Lesokhin A.M., Wack C., Charpentier E., Campana F., et al. A phase 1b study of isatuximab plus lenalidomide and dexamethasone for relapsed/refractory multiple myeloma. Blood. 2017;129:3294–3303. doi: 10.1182/blood-2016-09-740787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mikhael J., Richardson P., Usmani S.Z., Raje N., Bensinger W., Karanes C., Campana F., Kanagavel D., Dubin F., Liu Q., et al. A phase 1b study of isatuximab plus pomalidomide/dexamethasone in relapsed/refractory multiple myeloma. Blood. 2019;134:123–133. doi: 10.1182/blood-2019-02-895193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moreno L., Perez C., Zabaleta A., Manrique I., Alignani D., Ajona D., Blanco L., Lasa M., Maiso P., Rodriguez I., et al. The Mechanism of Action of the Anti-CD38 Monoclonal Antibody Isatuximab in Multiple Myeloma. Clin. Cancer Res. 2019;25:3176–3187. doi: 10.1158/1078-0432.CCR-18-1597. [DOI] [PubMed] [Google Scholar]
- 96.Assi R., Kantarjian H., Ravandi F., Daver N. Immune therapies in acute myeloid leukemia: A focus on monoclonal antibodies and immune checkpoint inhibitors. Curr. Opin. Hematol. 2018;25:136–145. doi: 10.1097/MOH.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 97.Daver N., Kantarjian H., Ravandi F., Estey E., Wang X., Garcia-Manero G., Jabbour E., Konopleva M., O’Brien S., Verstovsek S., et al. A phase II study of decitabine and gemtuzumab ozogamicin in newly diagnosed and relapsed acute myeloid leukemia and high-risk myelodysplastic syndrome. Leukemia. 2016;30:268–273. doi: 10.1038/leu.2015.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vasu S., He S., Cheney C., Gopalakrishnan B., Mani R., Lozanski G., Mo X., Groh V., Whitman S.P., Konopitzky R., et al. Decitabine enhances anti-CD33 monoclonal antibody BI 836858-mediated natural killer ADCC against AML blasts. Blood. 2016;127:2879–2889. doi: 10.1182/blood-2015-11-680546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chao M.P., Takimoto C.H., Feng D.D., McKenna K., Gip P., Liu J., Volkmer J.P., Weissman I.L., Majeti R. Therapeutic Targeting of the Macrophage Immune Checkpoint CD47 in Myeloid Malignancies. Front. Oncol. 2019;9:1380. doi: 10.3389/fonc.2019.01380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matlung H.L., Szilagyi K., Barclay N.A., van den Berg T.K. The CD47-SIRPalpha signaling axis as an innate immune checkpoint in cancer. Immunol. Rev. 2017;276:145–164. doi: 10.1111/imr.12527. [DOI] [PubMed] [Google Scholar]
- 101.Weiskopf K. Cancer immunotherapy targeting the CD47/SIRPalpha axis. Eur J. Cancer. 2017;76:100–109. doi: 10.1016/j.ejca.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 102.Benson D.M., Jr., Bakan C.E., Zhang S., Collins S.M., Liang J., Srivastava S., Hofmeister C.C., Efebera Y., Andre P., Romagne F., et al. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood. 2011;118:6387–6391. doi: 10.1182/blood-2011-06-360255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Benson D.M., Jr., Cohen A.D., Jagannath S., Munshi N.C., Spitzer G., Hofmeister C.C., Efebera Y.A., Andre P., Zerbib R., Caligiuri M.A. A Phase I Trial of the Anti-KIR Antibody IPH2101 and Lenalidomide in Patients with Relapsed/Refractory Multiple Myeloma. Clin. Cancer Res. 2015;21:4055–4061. doi: 10.1158/1078-0432.CCR-15-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Benson D.M., Jr., Hofmeister C.C., Padmanabhan S., Suvannasankha A., Jagannath S., Abonour R., Bakan C., Andre P., Efebera Y., Tiollier J., et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood. 2012;120:4324–4333. doi: 10.1182/blood-2012-06-438028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carlsten M., Korde N., Kotecha R., Reger R., Bor S., Kazandjian D., Landgren O., Childs R.W. Checkpoint Inhibition of KIR2D with the Monoclonal Antibody IPH2101 Induces Contraction and Hyporesponsiveness of NK Cells in Patients with Myeloma. Clin. Cancer Res. 2016;22:5211–5222. doi: 10.1158/1078-0432.CCR-16-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Romagne F., Andre P., Spee P., Zahn S., Anfossi N., Gauthier L., Capanni M., Ruggeri L., Benson D.M., Jr., Blaser B.W., et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114:2667–2677. doi: 10.1182/blood-2009-02-206532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vey N., Bourhis J.H., Boissel N., Bordessoule D., Prebet T., Charbonnier A., Etienne A., Andre P., Romagne F., Benson D., et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012;120:4317–4323. doi: 10.1182/blood-2012-06-437558. [DOI] [PubMed] [Google Scholar]
- 108.Ansell S.M., Lesokhin A.M., Borrello I., Halwani A., Scott E.C., Gutierrez M., Schuster S.J., Millenson M.M., Cattry D., Freeman G.J., et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pianko M.J., Liu Y., Bagchi S., Lesokhin A.M. Immune checkpoint blockade for hematologic malignancies: A review. Stem Cell Investig. 2017;4:32. doi: 10.21037/sci.2017.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sarkar S., van Gelder M., Noort W., Xu Y., Rouschop K.M., Groen R., Schouten H.C., Tilanus M.G., Germeraad W.T., Martens A.C., et al. Optimal selection of natural killer cells to kill myeloma: The role of HLA-E and NKG2A. Cancer Immunol. Immunother. 2015;64:951–963. doi: 10.1007/s00262-015-1694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mahaweni N.M., Ehlers F.A.I., Bos G.M.J., Wieten L. Tuning Natural Killer Cell Anti-multiple Myeloma Reactivity by Targeting Inhibitory Signaling via KIR and NKG2A. Front. Immunol. 2018;9:2848. doi: 10.3389/fimmu.2018.02848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McWilliams E.M., Mele J.M., Cheney C., Timmerman E.A., Fiazuddin F., Strattan E.J., Mo X., Byrd J.C., Muthusamy N., Awan F.T. Therapeutic CD94/NKG2A blockade improves natural killer cell dysfunction in chronic lymphocytic leukemia. Oncoimmunology. 2016;5:e1226720. doi: 10.1080/2162402X.2016.1226720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Hall T., Andre P., Horowitz A., Ruan D.F., Borst L., Zerbib R., Narni-Mancinelli E., van der Burg S.H., Vivier E. Monalizumab: Inhibiting the novel immune checkpoint NKG2A. J. Immunother. Cancer. 2019;7:263. doi: 10.1186/s40425-019-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Godal R., Bachanova V., Gleason M., McCullar V., Yun G.H., Cooley S., Verneris M.R., McGlave P.B., Miller J.S. Natural killer cell killing of acute myelogenous leukemia and acute lymphoblastic leukemia blasts by killer cell immunoglobulin-like receptor-negative natural killer cells after NKG2A and LIR-1 blockade. Biol Blood Marrow Transpl. 2010;16:612–621. doi: 10.1016/j.bbmt.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rouas-Freiss N., Moreau P., LeMaoult J., Carosella E.D. The dual role of HLA-G in cancer. J. Immunol. Res. 2014;2014:359748. doi: 10.1155/2014/359748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arruga F., Gyau B.B., Iannello A., Vitale N., Vaisitti T., Deaglio S. Immune Response Dysfunction in Chronic Lymphocytic Leukemia: Dissecting Molecular Mechanisms and Microenvironmental Conditions. Int. J. Mol. Sci. 2020;21:1825. doi: 10.3390/ijms21051825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Villa-Alvarez M., Sordo-Bahamonde C., Lorenzo-Herrero S., Gonzalez-Rodriguez A.P., Payer A.R., Gonzalez-Garcia E., Villa-Alvarez M.C., Lopez-Soto A., Gonzalez S. Ig-Like Transcript 2 (ILT2) Blockade and Lenalidomide Restore NK Cell Function in Chronic Lymphocytic Leukemia. Front. Immunol. 2018;9:2917. doi: 10.3389/fimmu.2018.02917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Long L., Zhang X., Chen F., Pan Q., Phiphatwatchara P., Zeng Y., Chen H. The promising immune checkpoint LAG-3: From tumor microenvironment to cancer immunotherapy. Genes Cancer. 2018;9:176–189. doi: 10.18632/genesandcancer.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Anderson A.C., Joller N., Kuchroo V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grosso J.F., Goldberg M.V., Getnet D., Bruno T.C., Yen H.R., Pyle K.J., Hipkiss E., Vignali D.A., Pardoll D.M., Drake C.G. Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J. Immunol. 2009;182:6659–6669. doi: 10.4049/jimmunol.0804211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Woo S.R., Turnis M.E., Goldberg M.V., Bankoti J., Selby M., Nirschl C.J., Bettini M.L., Gravano D.M., Vogel P., Liu C.L., et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang Z.Z., Kim H.J., Villasboas J.C., Chen Y.P., Price-Troska T., Jalali S., Wilson M., Novak A.J., Ansell S.M. Expression of LAG-3 defines exhaustion of intratumoral PD-1(+) T cells and correlates with poor outcome in follicular lymphoma. Oncotarget. 2017;8:61425–61439. doi: 10.18632/oncotarget.18251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Keane C., Law S.C., Gould C., Birch S., Sabdia M.B., Merida de Long L., Thillaiyampalam G., Abro E., Tobin J.W., Tan X., et al. LAG3: A novel immune checkpoint expressed by multiple lymphocyte subsets in diffuse large B-cell lymphoma. Blood Adv. 2020;4:1367–1377. doi: 10.1182/bloodadvances.2019001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gleason M.K., Lenvik T.R., McCullar V., Felices M., O’Brien M.S., Cooley S.A., Verneris M.R., Cichocki F., Holman C.J., Panoskaltsis-Mortari A., et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood. 2012;119:3064–3072. doi: 10.1182/blood-2011-06-360321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tang R., Rangachari M., Kuchroo V.K. Tim-3: A co-receptor with diverse roles in T cell exhaustion and tolerance. Semin. Immunol. 2019;42:101302. doi: 10.1016/j.smim.2019.101302. [DOI] [PubMed] [Google Scholar]
- 126.Folgiero V., Cifaldi L., Li Pira G., Goffredo B.M., Vinti L., Locatelli F. TIM-3/Gal-9 interaction induces IFNgamma-dependent IDO1 expression in acute myeloid leukemia blast cells. J. Hematol. Oncol. 2015;8:36. doi: 10.1186/s13045-015-0134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gallois A., Silva I., Osman I., Bhardwaj N. Reversal of natural killer cell exhaustion by TIM-3 blockade. Oncoimmunology. 2014;3:e946365. doi: 10.4161/21624011.2014.946365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ndhlovu L.C., Lopez-Verges S., Barbour J.D., Jones R.B., Jha A.R., Long B.R., Schoeffler E.C., Fujita T., Nixon D.F., Lanier L.L. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012;119:3734–3743. doi: 10.1182/blood-2011-11-392951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Przespolewski A., Szeles A., Wang E.S. Advances in immunotherapy for acute myeloid leukemia. Future Oncol. 2018;14:963–978. doi: 10.2217/fon-2017-0459. [DOI] [PubMed] [Google Scholar]
- 130.Asayama T., Tamura H., Ishibashi M., Kuribayashi-Hamada Y., Onodera-Kondo A., Okuyama N., Yamada A., Shimizu M., Moriya K., Takahashi H., et al. Functional expression of Tim-3 on blasts and clinical impact of its ligand galectin-9 in myelodysplastic syndromes. Oncotarget. 2017;8:88904–88917. doi: 10.18632/oncotarget.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B., Roche P.C., Lu J., Zhu G., Tamada K., et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 132.Francisco L.M., Sage P.T., Sharpe A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Daver N., Ravandi F. Enhancing cytotoxicity of immunotoxins in AML. Blood. 2016;127:2787–2788. doi: 10.1182/blood-2016-04-708214. [DOI] [PubMed] [Google Scholar]
- 134.Sehgal A., Whiteside T.L., Boyiadzis M. Programmed death-1 checkpoint blockade in acute myeloid leukemia. Expert Opin. Biol. 2015;15:1191–1203. doi: 10.1517/14712598.2015.1051028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Atanackovic D., Luetkens T., Radhakrishnan S., Kroger N. Coinhibitory Molecule PD-1 as a Therapeutic Target in the Microenvironment of Multiple Myeloma. Curr. Cancer Drug Targets. 2017;17:839–845. doi: 10.2174/1568009617666170906170348. [DOI] [PubMed] [Google Scholar]
- 136.Paiva B., Azpilikueta A., Puig N., Ocio E.M., Sharma R., Oyajobi B.O., Labiano S., San-Segundo L., Rodriguez A., Aires-Mejia I., et al. PD-L1/PD-1 presence in the tumor microenvironment and activity of PD-1 blockade in multiple myeloma. Leukemia. 2015;29:2110–2113. doi: 10.1038/leu.2015.79. [DOI] [PubMed] [Google Scholar]
- 137.Tremblay-LeMay R., Rastgoo N., Chang H. Modulating PD-L1 expression in multiple myeloma: An alternative strategy to target the PD-1/PD-L1 pathway. J. Hematol. Oncol. 2018;11:46. doi: 10.1186/s13045-018-0589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Andorsky D.J., Yamada R.E., Said J., Pinkus G.S., Betting D.J., Timmerman J.M. Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin. Cancer Res. 2011;17:4232–4244. doi: 10.1158/1078-0432.CCR-10-2660. [DOI] [PubMed] [Google Scholar]
- 139.Goodman A., Patel S.P., Kurzrock R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat. Rev. Clin. Oncol. 2017;14:203–220. doi: 10.1038/nrclinonc.2016.168. [DOI] [PubMed] [Google Scholar]
- 140.Batlevi C.L., Matsuki E., Brentjens R.J., Younes A. Novel immunotherapies in lymphoid malignancies. Nat. Rev. Clin. Oncol. 2016;13:25–40. doi: 10.1038/nrclinonc.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.de Charette M., Houot R. Hide or defend, the two strategies of lymphoma immune evasion: Potential implications for immunotherapy. Haematologica. 2018;103:1256–1268. doi: 10.3324/haematol.2017.184192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Grzywnowicz M., Zaleska J., Mertens D., Tomczak W., Wlasiuk P., Kosior K., Piechnik A., Bojarska-Junak A., Dmoszynska A., Giannopoulos K. Programmed death-1 and its ligand are novel immunotolerant molecules expressed on leukemic B cells in chronic lymphocytic leukemia. PLoS ONE. 2012;7:e35178. doi: 10.1371/journal.pone.0035178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Carreras J., Lopez-Guillermo A., Roncador G., Villamor N., Colomo L., Martinez A., Hamoudi R., Howat W.J., Montserrat E., Campo E. High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J. Clin. Oncol. 2009;27:1470–1476. doi: 10.1200/JCO.2008.18.0513. [DOI] [PubMed] [Google Scholar]
- 144.Myklebust J.H., Irish J.M., Brody J., Czerwinski D.K., Houot R., Kohrt H.E., Timmerman J., Said J., Green M.R., Delabie J., et al. High PD-1 expression and suppressed cytokine signaling distinguish T cells infiltrating follicular lymphoma tumors from peripheral T cells. Blood. 2013;121:1367–1376. doi: 10.1182/blood-2012-04-421826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xie M., Huang X., Ye X., Qian W. Prognostic and clinicopathological significance of PD-1/PD-L1 expression in the tumor microenvironment and neoplastic cells for lymphoma. Int. Immunopharmacol. 2019;77:105999. doi: 10.1016/j.intimp.2019.105999. [DOI] [PubMed] [Google Scholar]
- 146.Yang Z.Z., Grote D.M., Ziesmer S.C., Xiu B., Novak A.J., Ansell S.M. PD-1 expression defines two distinct T-cell sub-populations in follicular lymphoma that differentially impact patient survival. Blood Cancer J. 2015;5:e281. doi: 10.1038/bcj.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Della Chiesa M., Pesce S., Muccio L., Carlomagno S., Sivori S., Moretta A., Marcenaro E. Features of Memory-Like and PD-1(+) Human NK Cell Subsets. Front. Immunol. 2016;7:351. doi: 10.3389/fimmu.2016.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pesce S., Greppi M., Tabellini G., Rampinelli F., Parolini S., Olive D., Moretta L., Moretta A., Marcenaro E. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J. Allergy Clin. Immunol. 2017;139:335–346.e333. doi: 10.1016/j.jaci.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 149.Danhof S., Schreder M., Knop S., Rasche L., Strifler S., Loffler C., Gogishvili T., Einsele H., Hudecek M. Expression of programmed death-1 on lymphocytes in myeloma patients is lowered during lenalidomide maintenance. Haematologica. 2018;103:e126–e129. doi: 10.3324/haematol.2017.178947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cornell R.F., Kassim A.A. Evolving paradigms in the treatment of relapsed/refractory multiple myeloma: Increased options and increased complexity. Bone Marrow Transpl. 2016;51:479–491. doi: 10.1038/bmt.2015.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mateos M.V., Blacklock H., Schjesvold F., Oriol A., Simpson D., George A., Goldschmidt H., Larocca A., Chanan-Khan A., Sherbenou D., et al. Pembrolizumab plus pomalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma (KEYNOTE-183): A randomised, open-label, phase 3 trial. Lancet. Haematol. 2019;6:e459–e469. doi: 10.1016/S2352-3026(19)30110-3. [DOI] [PubMed] [Google Scholar]
- 152.San Miguel J., Mateos M.-V., Shah J.J., Ocio E.M., Rodriguez-Otero P., Reece D., Munshi N.C., Avigan D., Ge Y., Balakumaran A., et al. Pembrolizumab in Combination with Lenalidomide and Low-Dose Dexamethasone for Relapsed/Refractory Multiple Myeloma (RRMM): Keynote-023. Blood. 2015;126:505. doi: 10.1182/blood.V126.23.505.505. [DOI] [Google Scholar]
- 153.Daver N.G., Garcia-Manero G., Konopleva M.Y., Alfayez M., Pemmaraju N., Kadia T.M., DiNardo C.D., Cortes J.E., Ravandi F., Abbas H., et al. Azacitidine (AZA) with Nivolumab (Nivo), and AZA with Nivo + Ipilimumab (Ipi) in Relapsed/Refractory Acute Myeloid Leukemia: A Non-Randomized, Prospective, Phase 2 Study. Blood. 2019;134:830. doi: 10.1182/blood-2019-131494. [DOI] [Google Scholar]
- 154.Garcia-Manero G., Sasaki K., Montalban-Bravo G., Daver N.G., Jabbour E.J., Alvarado Y., DiNardo C.D., Ravandi F., Borthakur G., Bose P., et al. A Phase II Study of Nivolumab or Ipilimumab with or without Azacitidine for Patients with Myelodysplastic Syndrome (MDS) Blood. 2018;132:465. doi: 10.1182/blood-2018-99-119424. [DOI] [Google Scholar]
- 155.Westin J.R., Chu F., Zhang M., Fayad L.E., Kwak L.W., Fowler N., Romaguera J., Hagemeister F., Fanale M., Samaniego F., et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: A single group, open-label, phase 2 trial. Lancet Oncol. 2014;15:69–77. doi: 10.1016/S1470-2045(13)70551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ansell S., Gutierrez M.E., Shipp M.A., Gladstone D., Moskowitz A., Borello I., Popa-Mckiver M., Farsaci B., Zhu L., Lesokhin A.M., et al. A Phase 1 Study of Nivolumab in Combination with Ipilimumab for Relapsed or Refractory Hematologic Malignancies (CheckMate 039) Blood. 2016;128:183. doi: 10.1182/blood.V128.22.183.183. [DOI] [Google Scholar]
- 157.Ray A., Das D.S., Song Y., Hideshima T., Tai Y.T., Chauhan D., Anderson K.C. Combination of a novel HDAC6 inhibitor ACY-241 and anti-PD-L1 antibody enhances anti-tumor immunity and cytotoxicity in multiple myeloma. Leukemia. 2018;32:843–846. doi: 10.1038/leu.2017.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tamura H., Ishibashi M., Yamashita T., Tanosaki S., Okuyama N., Kondo A., Hyodo H., Shinya E., Takahashi H., Dong H., et al. Marrow stromal cells induce B7-H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia. 2013;27:464–472. doi: 10.1038/leu.2012.213. [DOI] [PubMed] [Google Scholar]
- 159.Chen L., Han X. Anti-PD-1/PD-L1 therapy of human cancer: Past, present, and future. J. Clin. Investig. 2015;125:3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Georgiou K., Chen L., Berglund M., Ren W., de Miranda N.F.C.C., Lisboa S., Fangazio M., Zhu S., Hou Y., Wu K., et al. Genetic basis of PD-L1 overexpression in diffuse large B-cell lymphomas. Blood. 2016;127:3026–3034. doi: 10.1182/blood-2015-12-686550. [DOI] [PubMed] [Google Scholar]
- 161.Laurent C., Charmpi K., Gravelle P., Tosolini M., Franchet C., Ysebaert L., Brousset P., Bidaut A., Ycart B., Fournie J.J. Several immune escape patterns in non-Hodgkin’s lymphomas. Oncoimmunology. 2015;4:e1026530. doi: 10.1080/2162402X.2015.1026530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Roemer M.G., Advani R.H., Ligon A.H., Natkunam Y., Redd R.A., Homer H., Connelly C.F., Sun H.H., Daadi S.E., Freeman G.J., et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J. Clin. Oncol. 2016;34:2690–2697. doi: 10.1200/JCO.2016.66.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yousef S., Marvin J., Steinbach M., Langemo A., Kovacsovics T., Binder M., Kroger N., Luetkens T., Atanackovic D. Immunomodulatory molecule PD-L1 is expressed on malignant plasma cells and myeloma-propagating pre-plasma cells in the bone marrow of multiple myeloma patients. Blood Cancer J. 2015;5:e285. doi: 10.1038/bcj.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Benson D.M., Jr., Bakan C.E., Mishra A., Hofmeister C.C., Efebera Y., Becknell B., Baiocchi R.A., Zhang J., Yu J., Smith M.K., et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: A therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116:2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Guo Y., Feng X., Jiang Y., Shi X., Xing X., Liu X., Li N., Fadeel B., Zheng C. PD1 blockade enhances cytotoxicity of in vitro expanded natural killer cells towards myeloma cells. Oncotarget. 2016;7:48360–48374. doi: 10.18632/oncotarget.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hallett W.H., Jing W., Drobyski W.R., Johnson B.D. Immunosuppressive effects of multiple myeloma are overcome by PD-L1 blockade. Biol. Blood Marrow Transpl. 2011;17:1133–1145. doi: 10.1016/j.bbmt.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 167.Rosenblatt J., Glotzbecker B., Mills H., Vasir B., Tzachanis D., Levine J.D., Joyce R.M., Wellenstein K., Keefe W., Schickler M., et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J. Immunother. 2011;34:409–418. doi: 10.1097/CJI.0b013e31821ca6ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Whitehill G.D., Chivers A., Danielson B., Tessier J.S., Hernandez Duran G., Bender G.L., Sborov D.W., Hofmeister C.C., Bradner J., Hideshima T., et al. Immunomodulatory Effects of HDACi in Combination with Checkpoint Blockade and Lenalidomide in the Immunosuppressive Multiple Myeloma Bone Marrow Microenvironment. Blood. 2017;130:4422. [Google Scholar]
- 169.Ilcus C., Bagacean C., Tempescul A., Popescu C., Parvu A., Cenariu M., Bocsan C., Zdrenghea M. Immune checkpoint blockade: The role of PD-1-PD-L axis in lymphoid malignancies. Onco. Targets. 2017;10:2349–2363. doi: 10.2147/OTT.S133385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xu-Monette Z.Y., Zhou J., Young K.H. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131:68–83. doi: 10.1182/blood-2017-07-740993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Armand P. Immune checkpoint blockade in hematologic malignancies. Blood. 2015;125:3393–3400. doi: 10.1182/blood-2015-02-567453. [DOI] [PubMed] [Google Scholar]
- 172.Korkmaz S., Erdem S., Akay E., Tasdemir E.A., Karaman H., Keklik M. Do PD-1 and PD-L2 expressions have prognostic impact in hematologic malignancies? Turk. J. Med. Sci. 2019;49:265–271. doi: 10.3906/sag-1706-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Panjwani P.K., Charu V., DeLisser M., Molina-Kirsch H., Natkunam Y., Zhao S. Programmed death-1 ligands PD-L1 and PD-L2 show distinctive and restricted patterns of expression in lymphoma subtypes. Hum. Pathol. 2018;71:91–99. doi: 10.1016/j.humpath.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 174.Rozali E.N., Hato S.V., Robinson B.W., Lake R.A., Lesterhuis W.J. Programmed death ligand 2 in cancer-induced immune suppression. Clin. Dev. Immunol. 2012;2012:656340. doi: 10.1155/2012/656340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tanaka Y., Maeshima A.M., Nomoto J., Makita S., Fukuhara S., Munakata W., Maruyama D., Tobinai K., Kobayashi Y. Expression pattern of PD-L1 and PD-L2 in classical Hodgkin lymphoma, primary mediastinal large B-cell lymphoma, and gray zone lymphoma. Eur. J. Haematol. 2018;100:511–517. doi: 10.1111/ejh.13033. [DOI] [PubMed] [Google Scholar]
- 176.Johnston R.J., Comps-Agrar L., Hackney J., Yu X., Huseni M., Yang Y., Park S., Javinal V., Chiu H., Irving B., et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 177.Khan M., Arooj S., Wang H. NK Cell-Based Immune Checkpoint Inhibition. Front. Immunol. 2020;11:167. doi: 10.3389/fimmu.2020.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chan C.J., Martinet L., Gilfillan S., Souza-Fonseca-Guimaraes F., Chow M.T., Town L., Ritchie D.S., Colonna M., Andrews D.M., Smyth M.J. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat. Immunol. 2014;15:431–438. doi: 10.1038/ni.2850. [DOI] [PubMed] [Google Scholar]
- 179.Gao J., Zheng Q., Xin N., Wang W., Zhao C. CD155, an onco-immunologic molecule in human tumors. Cancer Sci. 2017;108:1934–1938. doi: 10.1111/cas.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Martinet L., Smyth M.J. Balancing natural killer cell activation through paired receptors. Nat. Rev. Immunol. 2015;15:243–254. doi: 10.1038/nri3799. [DOI] [PubMed] [Google Scholar]
- 181.Stanietsky N., Simic H., Arapovic J., Toporik A., Levy O., Novik A., Levine Z., Beiman M., Dassa L., Achdout H., et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. USA. 2009;106:17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kucan Brlic P., Lenac Rovis T., Cinamon G., Tsukerman P., Mandelboim O., Jonjic S. Targeting PVR (CD155) and its receptors in anti-tumor therapy. Cell Mol. Immunol. 2019;16:40–52. doi: 10.1038/s41423-018-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stengel K.F., Harden-Bowles K., Yu X., Rouge L., Yin J., Comps-Agrar L., Wiesmann C., Bazan J.F., Eaton D.L., Grogan J.L. Structure of TIGIT immunoreceptor bound to poliovirus receptor reveals a cell-cell adhesion and signaling mechanism that requires cis-trans receptor clustering. Proc. Natl. Acad. Sci. USA. 2012;109:5399–5404. doi: 10.1073/pnas.1120606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Blake S.J., Dougall W.C., Miles J.J., Teng M.W., Smyth M.J. Molecular Pathways: Targeting CD96 and TIGIT for Cancer Immunotherapy. Clin. Cancer Res. 2016;22:5183–5188. doi: 10.1158/1078-0432.CCR-16-0933. [DOI] [PubMed] [Google Scholar]
- 185.Dougall W.C., Kurtulus S., Smyth M.J., Anderson A.C. TIGIT and CD96: New checkpoint receptor targets for cancer immunotherapy. Immunol. Rev. 2017;276:112–120. doi: 10.1111/imr.12518. [DOI] [PubMed] [Google Scholar]
- 186.Chauvin J.M., Pagliano O., Fourcade J., Sun Z., Wang H., Sander C., Kirkwood J.M., Chen T.H., Maurer M., Korman A.J., et al. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J. Clin. Investig. 2015;125:2046–2058. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sanchez-Correa B., Valhondo I., Hassouneh F., Lopez-Sejas N., Pera A., Bergua J.M., Arcos M.J., Banas H., Casas-Aviles I., Duran E., et al. DNAM-1 and the TIGIT/PVRIG/TACTILE Axis: Novel Immune Checkpoints for Natural Killer Cell-Based Cancer Immunotherapy. Cancers. 2019;11:877. doi: 10.3390/cancers11060877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Asimakopoulos F. TIGIT checkpoint inhibition for myeloma. Blood. 2018;132:1629–1630. doi: 10.1182/blood-2018-08-864231. [DOI] [PubMed] [Google Scholar]
- 189.Wang M., Bu J., Zhou M., Sido J., Lin Y., Liu G., Lin Q., Xu X., Leavenworth J.W., Shen E. CD8(+)T cells expressing both PD-1 and TIGIT but not CD226 are dysfunctional in acute myeloid leukemia (AML) patients. Clin. Immunol. 2018;190:64–73. doi: 10.1016/j.clim.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 190.Zhang X., Zhang H., Chen L., Feng Z., Gao L., Li Q. TIGIT expression is upregulated in T cells and causes T cell dysfunction independent of PD-1 and Tim-3 in adult B lineage acute lymphoblastic leukemia. Cell Immunol. 2019;344:103958. doi: 10.1016/j.cellimm.2019.103958. [DOI] [PubMed] [Google Scholar]
- 191.Friedrich M., Henn A., Raum T., Bajtus M., Matthes K., Hendrich L., Wahl J., Hoffmann P., Kischel R., Kvesic M., et al. Preclinical characterization of AMG 330, a CD3/CD33-bispecific T-cell-engaging antibody with potential for treatment of acute myelogenous leukemia. Mol. Cancer. 2014;13:1549–1557. doi: 10.1158/1535-7163.MCT-13-0956. [DOI] [PubMed] [Google Scholar]
- 192.Stamm H., Klingler F., Grossjohann E.M., Muschhammer J., Vettorazzi E., Heuser M., Mock U., Thol F., Vohwinkel G., Latuske E., et al. Immune checkpoints PVR and PVRL2 are prognostic markers in AML and their blockade represents a new therapeutic option. Oncogene. 2018;37:5269–5280. doi: 10.1038/s41388-018-0288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Daly J., Carlsten M., O’Dwyer M. Sugar Free: Novel Immunotherapeutic Approaches Targeting Siglecs and Sialic Acids to Enhance Natural Killer Cell Cytotoxicity Against Cancer. Front. Immunol. 2019;10:1047. doi: 10.3389/fimmu.2019.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Daly J., Duggan T., Hu J., Natoni A., Sarkar S., Kirkham-McCarthy L., McEllistrim C., Krawczyk J., O’Dwyer M. Targeting Siglec-7: A Novel Immunotherapeutic Approach to Potentiate the Cytotoxic Functions of Natural Killer Cells Against Multiple Myeloma. Blood. 2017;130:1799. [Google Scholar]
- 195.Shao J.Y., Yin W.W., Zhang Q.F., Liu Q., Peng M.L., Hu H.D., Hu P., Ren H., Zhang D.Z. Siglec-7 Defines a Highly Functional Natural Killer Cell Subset and Inhibits Cell-Mediated Activities. Scand. J. Immunol. 2016;84:182–190. doi: 10.1111/sji.12455. [DOI] [PubMed] [Google Scholar]
- 196.Kawasaki Y., Ito A., Withers D.A., Taima T., Kakoi N., Saito S., Arai Y. Ganglioside DSGb5, preferred ligand for Siglec-7, inhibits NK cell cytotoxicity against renal cell carcinoma cells. Glycobiology. 2010;20:1373–1379. doi: 10.1093/glycob/cwq116. [DOI] [PubMed] [Google Scholar]
- 197.Nicoll G., Avril T., Lock K., Furukawa K., Bovin N., Crocker P.R. Ganglioside GD3 expression on target cells can modulate NK cell cytotoxicity via siglec-7-dependent and -independent mechanisms. Eur J. Immunol. 2003;33:1642–1648. doi: 10.1002/eji.200323693. [DOI] [PubMed] [Google Scholar]
- 198.Crocker P.R., Paulson J.C., Varki A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 199.Jandus C., Boligan K.F., Chijioke O., Liu H., Dahlhaus M., Demoulins T., Schneider C., Wehrli M., Hunger R.E., Baerlocher G.M., et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J. Clin. Investig. 2014;124:1810–1820. doi: 10.1172/JCI65899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chapoval A.I., Ni J., Lau J.S., Wilcox R.A., Flies D.B., Liu D., Dong H., Sica G.L., Zhu G., Tamada K., et al. B7-H3: A costimulatory molecule for T cell activation and IFN-gamma production. Nat. Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 201.Loos M., Hedderich D.M., Friess H., Kleeff J. B7-h3 and its role in antitumor immunity. Clin. Dev. Immunol. 2010;2010:683875. doi: 10.1155/2010/683875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Picarda E., Ohaegbulam K.C., Zang X. Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin. Cancer Res. 2016;22:3425–3431. doi: 10.1158/1078-0432.CCR-15-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang L., Kang F.B., Shan B.E. B7-H3-mediated tumor immunology: Friend or foe? Int. J. Cancer. 2014;134:2764–2771. doi: 10.1002/ijc.28474. [DOI] [PubMed] [Google Scholar]
- 204.Yonesaka K., Haratani K., Takamura S., Sakai H., Kato R., Takegawa N., Takahama T., Tanaka K., Hayashi H., Takeda M., et al. B7-H3 Negatively Modulates CTL-Mediated Cancer Immunity. Clin. Cancer Res. 2018;24:2653–2664. doi: 10.1158/1078-0432.CCR-17-2852. [DOI] [PubMed] [Google Scholar]
- 205.Guery T., Roumier C., Berthon C., Renneville A., Preudhomme C., Quesnel B. B7-H3 protein expression in acute myeloid leukemia. Cancer Med. 2015;4:1879–1883. doi: 10.1002/cam4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hu Y., Lv X., Wu Y., Xu J., Wang L., Chen W., Zhang W., Li J., Zhang S., Qiu H. Expression of costimulatory molecule B7-H3 and its prognostic implications in human acute leukemia. Hematology. 2015;20:187–195. doi: 10.1179/1607845414Y.0000000186. [DOI] [PubMed] [Google Scholar]
- 207.Zhang W., Wang Y., Wang J., Dong F., Zhu M., Wan W., Li H., Wu F., Yan X., Ke X. B7-H3 silencing inhibits tumor progression of mantle cell lymphoma and enhances chemosensitivity. Int. J. Oncol. 2015;46:2562–2572. doi: 10.3892/ijo.2015.2962. [DOI] [PubMed] [Google Scholar]
- 208.Sun X., Yu Y., Ma L., Xue X., Gao Z., Ma J., Zhang M. T cell cytotoxicity toward hematologic malignancy via B7-H3 targeting. Invest. New Drugs. 2019;38:722–732. doi: 10.1007/s10637-019-00819-y. [DOI] [PubMed] [Google Scholar]
- 209.Germain C., Guillaudeux T., Galsgaard E.D., Hervouet C., Tekaya N., Gallouet A.S., Fassy J., Bihl F., Poupon G., Lazzari A., et al. Lectin-like transcript 1 is a marker of germinal center-derived B-cell non-Hodgkin’s lymphomas dampening natural killer cell functions. Oncoimmunology. 2015;4:e1026503. doi: 10.1080/2162402X.2015.1026503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sivori S., Meazza R., Quintarelli C., Carlomagno S., Della Chiesa M., Falco M., Moretta L., Locatelli F., Pende D. NK Cell-Based Immunotherapy for Hematological Malignancies. J. Clin. Med. 2019;8:1702. doi: 10.3390/jcm8101702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bialoszewska A., Malejczyk J. Biological and Clinical Significance of Human NKRP1A/LLT1 Receptor/Ligand Interactions. Crit Rev. Immunol. 2018;38:479–489. doi: 10.1615/CritRevImmunol.2019029559. [DOI] [PubMed] [Google Scholar]
- 212.Deeks E.D. Polatuzumab Vedotin: First Global Approval. Drugs. 2019;79:1467–1475. doi: 10.1007/s40265-019-01175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sehn L.H., Herrera A.F., Flowers C.R., Kamdar M.K., McMillan A., Hertzberg M., Assouline S., Kim T.M., Kim W.S., Ozcan M., et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2020;38:155–165. doi: 10.1200/JCO.19.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tilly H., Morschhauser F., Bartlett N.L., Mehta A., Salles G., Haioun C., Munoz J., Chen A.I., Kolibaba K., Lu D., et al. Polatuzumab vedotin in combination with immunochemotherapy in patients with previously untreated diffuse large B-cell lymphoma: An open-label, non-randomised, phase 1b-2 study. Lancet. Oncol. 2019;20:998–1010. doi: 10.1016/S1470-2045(19)30091-9. [DOI] [PubMed] [Google Scholar]
- 215.Morschhauser F., Flinn I.W., Advani R., Sehn L.H., Diefenbach C., Kolibaba K., Press O.W., Salles G., Tilly H., Chen A.I., et al. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: Final results from a phase 2 randomised study (ROMULUS) Lancet. Haematol. 2019;6:e254–e265. doi: 10.1016/S2352-3026(19)30026-2. [DOI] [PubMed] [Google Scholar]
- 216.Barrett D.M., Singh N., Porter D.L., Grupp S.A., June C.H. Chimeric antigen receptor therapy for cancer. Annu. Rev. Med. 2014;65:333–347. doi: 10.1146/annurev-med-060512-150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Goebeler M.E., Bargou R.C. T cell-engaging therapies—BiTEs and beyond. Nat. Rev. Clin. Oncol. 2020;17:418–434. doi: 10.1038/s41571-020-0347-5. [DOI] [PubMed] [Google Scholar]
- 218.Nelson M.H., Paulos C.M. Novel immunotherapies for hematologic malignancies. Immunol. Rev. 2015;263:90–105. doi: 10.1111/imr.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Strohl W.R., Naso M. Bispecific T-Cell Redirection versus Chimeric Antigen Receptor (CAR)-T Cells as Approaches to Kill Cancer Cells. Antibodies. 2019;8:41. doi: 10.3390/antib8030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Suryadevara C.M., Gedeon P.C., Sanchez-Perez L., Verla T., Alvarez-Breckenridge C., Choi B.D., Fecci P.E., Sampson J.H. Are BiTEs the “missing link” in cancer therapy? Oncoimmunology. 2015;4:e1008339. doi: 10.1080/2162402X.2015.1008339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Aldoss I., Bargou R.C., Nagorsen D., Friberg G.R., Baeuerle P.A., Forman S.J. Redirecting T cells to eradicate B-cell acute lymphoblastic leukemia: Bispecific T-cell engagers and chimeric antigen receptors. Leukemia. 2017;31:777–787. doi: 10.1038/leu.2016.391. [DOI] [PubMed] [Google Scholar]
- 222.Felices M., Lenvik T.R., Davis Z.B., Miller J.S., Vallera D.A. Generation of BiKEs and TriKEs to Improve NK Cell-Mediated Targeting of Tumor Cells. Methods Mol. Biol. 2016;1441:333–346. doi: 10.1007/978-1-4939-3684-7_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tay S.S., Carol H., Biro M. TriKEs and BiKEs join CARs on the cancer immunotherapy highway. Hum. Vaccines Immunother. 2016;12:2790–2796. doi: 10.1080/21645515.2016.1198455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gleason M.K., Verneris M.R., Todhunter D.A., Zhang B., McCullar V., Zhou S.X., Panoskaltsis-Mortari A., Weiner L.M., Vallera D.A., Miller J.S. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol. Cancer. 2012;11:2674–2684. doi: 10.1158/1535-7163.MCT-12-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gleason M.K., Ross J.A., Warlick E.D., Lund T.C., Verneris M.R., Wiernik A., Spellman S., Haagenson M.D., Lenvik A.J., Litzow M.R., et al. CD16 × CD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood. 2014;123:3016–3026. doi: 10.1182/blood-2013-10-533398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Portner L.M., Schonberg K., Hejazi M., Brunnert D., Neumann F., Galonska L., Reusch U., Little M., Haas R., Uhrberg M. T and NK cells of B cell NHL patients exert cytotoxicity against lymphoma cells following binding of bispecific tetravalent antibody CD19 × CD3 or CD19 × CD16. Cancer Immunol. Immunother. 2012;61:1869–1875. doi: 10.1007/s00262-012-1339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Reiners K.S., Kessler J., Sauer M., Rothe A., Hansen H.P., Reusch U., Hucke C., Kohl U., Durkop H., Engert A., et al. Rescue of impaired NK cell activity in hodgkin lymphoma with bispecific antibodies in vitro and in patients. Mol. Ther. 2013;21:895–903. doi: 10.1038/mt.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Reusch U., Burkhardt C., Fucek I., Le Gall F., Le Gall M., Hoffmann K., Knackmuss S.H., Kiprijanov S., Little M., Zhukovsky E.A. A novel tetravalent bispecific TandAb (CD30/CD16A) efficiently recruits NK cells for the lysis of CD30+ tumor cells. MAbs. 2014;6:728–739. doi: 10.4161/mabs.28591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wu J., Fu J., Zhang M., Liu D. AFM13: A first-in-class tetravalent bispecific anti-CD30/CD16A antibody for NK cell-mediated immunotherapy. J. Hematol. Oncol. 2015;8:96. doi: 10.1186/s13045-015-0188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pahl J.H.W., Koch J., Gotz J.J., Arnold A., Reusch U., Gantke T., Rajkovic E., Treder M., Cerwenka A. CD16A Activation of NK Cells Promotes NK Cell Proliferation and Memory-Like Cytotoxicity against Cancer Cells. Cancer Immunol. Res. 2018;6:517–527. doi: 10.1158/2326-6066.CIR-17-0550. [DOI] [PubMed] [Google Scholar]
- 231.Glorius P., Baerenwaldt A., Kellner C., Staudinger M., Dechant M., Stauch M., Beurskens F.J., Parren P.W., Winkel J.G., Valerius T., et al. The novel tribody [(CD20)(2) × CD16] efficiently triggers effector cell-mediated lysis of malignant B cells. Leukemia. 2013;27:190–201. doi: 10.1038/leu.2012.150. [DOI] [PubMed] [Google Scholar]
- 232.Berrien-Elliott M.M., Wagner J.A., Fehniger T.A. Human Cytokine-Induced Memory-Like Natural Killer Cells. J. Innate. Immun. 2015;7:563–571. doi: 10.1159/000382019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Childs R.W., Carlsten M. Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: The force awakens. Nat. Rev. Drug Discov. 2015;14:487–498. doi: 10.1038/nrd4506. [DOI] [PubMed] [Google Scholar]
- 234.Davis Z.B., Vallera D.A., Miller J.S., Felices M. Natural killer cells unleashed: Checkpoint receptor blockade and BiKE/TriKE utilization in NK-mediated anti-tumor immunotherapy. Semin. Immunol. 2017;31:64–75. doi: 10.1016/j.smim.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Del Bano J., Chames P., Baty D., Kerfelec B. Taking up Cancer Immunotherapy Challenges: Bispecific Antibodies, the Path Forward? Antibodies. 2015;5:1. doi: 10.3390/antib5010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Rothe A., Sasse S., Topp M.S., Eichenauer D.A., Hummel H., Reiners K.S., Dietlein M., Kuhnert G., Kessler J., Buerkle C., et al. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2015;125:4024–4031. doi: 10.1182/blood-2014-12-614636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Grandjenette C., Dicato M., Diederich M. Bispecific Antibodies: An Innovative Arsenal to Hunt, Grab and Destroy Cancer Cells. Curr. Pharm. Biotechnol. 2015;16:670–683. doi: 10.2174/1389201016666150505124037. [DOI] [PubMed] [Google Scholar]
- 238.Vallera D.A., Felices M., McElmurry R., McCullar V., Zhou X., Schmohl J.U., Zhang B., Lenvik A.J., Panoskaltsis-Mortari A., Verneris M.R., et al. IL15 Trispecific Killer Engagers (TriKE) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, In Vivo Expansion, and Enhanced Function. Clin. Cancer Res. 2016;22:3440–3450. doi: 10.1158/1078-0432.CCR-15-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Sarhan D., Brandt L., Felices M., Guldevall K., Lenvik T., Hinderlie P., Curtsinger J., Warlick E., Spellman S.R., Blazar B.R., et al. 161533 TriKE stimulates NK-cell function to overcome myeloid-derived suppressor cells in MDS. Blood Adv. 2018;2:1459–1469. doi: 10.1182/bloodadvances.2017012369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gauthier L., Morel A., Anceriz N., Rossi B., Blanchard-Alvarez A., Grondin G., Trichard S., Cesari C., Sapet M., Bosco F., et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell. 2019;177:1701–1713.e16. doi: 10.1016/j.cell.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 241.Kloess S., Ede Valverde da Silva A., Oberschmidt O., Gardlowski T., Matthies N., Vyas M., Arseniev L., Heuser M., Pogge von Strandmann E., Kohl U. Triplebody Mediates Increased Anti-Leukemic Reactivity of IL-2 Activated Donor Natural Killer (NK) Cells and Impairs Viability of Their CD33-Expressing NK Subset. Front. Immunol. 2017;8:1100. doi: 10.3389/fimmu.2017.01100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Duell J., Lammers P.E., Djuretic I., Chunyk A.G., Alekar S., Jacobs I., Gill S. Bispecific Antibodies in the Treatment of Hematologic Malignancies. Clin. Pharmacol. Ther. 2019;106:781–791. doi: 10.1002/cpt.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Guy D.G., Uy G.L. Bispecific Antibodies for the Treatment of Acute Myeloid Leukemia. Curr Hematol Malig Rep. 2018;13:417–425. doi: 10.1007/s11899-018-0472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Dai H., Wang Y., Lu X., Han W. Chimeric Antigen Receptors Modified T-Cells for Cancer Therapy. J. Natl. Cancer Inst. 2016;108:djv439. doi: 10.1093/jnci/djv439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Maude S.L., Teachey D.T., Porter D.L., Grupp S.A. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125:4017–4023. doi: 10.1182/blood-2014-12-580068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Maus M.V., June C.H. Making Better Chimeric Antigen Receptors for Adoptive T-cell Therapy. Clin. Cancer Res. 2016;22:1875–1884. doi: 10.1158/1078-0432.CCR-15-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Rezvani K., Rouce R., Liu E., Shpall E. Engineering Natural Killer Cells for Cancer Immunotherapy. Mol. Ther. 2017;25:1769–1781. doi: 10.1016/j.ymthe.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Weber E.W., Maus M.V., Mackall C.L. The Emerging Landscape of Immune Cell Therapies. Cell. 2020;181:46–62. doi: 10.1016/j.cell.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ye B., Stary C.M., Li X., Gao Q., Kang C., Xiong X. Engineering chimeric antigen receptor-T cells for cancer treatment. Mol. Cancer. 2018;17:32. doi: 10.1186/s12943-018-0814-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Oberschmidt O., Kloess S., Koehl U. Redirected Primary Human Chimeric Antigen Receptor Natural Killer Cells As an “Off-the-Shelf Immunotherapy” for Improvement in Cancer Treatment. Front. Immunol. 2017;8:654. doi: 10.3389/fimmu.2017.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Atanackovic D., Radhakrishnan S.V., Bhardwaj N., Luetkens T. Chimeric Antigen Receptor (CAR) therapy for multiple myeloma. Br. J. Haematol. 2016;172:685–698. doi: 10.1111/bjh.13889. [DOI] [PubMed] [Google Scholar]
- 252.Chu J., Deng Y., Benson D.M., He S., Hughes T., Zhang J., Peng Y., Mao H., Yi L., Ghoshal K., et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28:917–927. doi: 10.1038/leu.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Jiang H., Zhang W., Shang P., Zhang H., Fu W., Ye F., Zeng T., Huang H., Zhang X., Sun W., et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol. Oncol. 2014;8:297–310. doi: 10.1016/j.molonc.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Boissel L., Betancur-Boissel M., Lu W., Krause D.S., Van Etten R.A., Wels W.S., Klingemann H. Retargeting NK-92 cells by means of CD19- and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity. Oncoimmunology. 2013;2:e26527. doi: 10.4161/onci.26527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Chu Y., Hochberg J., Yahr A., Ayello J., van de Ven C., Barth M., Czuczman M., Cairo M.S. Targeting CD20+ Aggressive B-cell Non-Hodgkin Lymphoma by Anti-CD20 CAR mRNA-Modified Expanded Natural Killer Cells In Vitro and in NSG Mice. Cancer Immunol. Res. 2015;3:333–344. doi: 10.1158/2326-6066.CIR-14-0114. [DOI] [PubMed] [Google Scholar]
- 256.Kobayashi E., Kishi H., Ozawa T., Hamana H., Nakagawa H., Jin A., Lin Z., Muraguchi A. A chimeric antigen receptor for TRAIL-receptor 1 induces apoptosis in various types of tumor cells. Biochem Biophys Res. Commun. 2014;453:798–803. doi: 10.1016/j.bbrc.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 257.Caraccio C., Krishna S., Phillips D.J., Schurch C.M. Bispecific Antibodies for Multiple Myeloma: A Review of Targets, Drugs, Clinical Trials, and Future Directions. Front. Immunol. 2020;11:501. doi: 10.3389/fimmu.2020.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Lin Q., Zhao J., Song Y., Liu D. Recent updates on CAR T clinical trials for multiple myeloma. Mol. Cancer. 2019;18:154. doi: 10.1186/s12943-019-1092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Timmers M., Roex G., Wang Y., Campillo-Davo D., Van Tendeloo V.F.I., Chu Y., Berneman Z.N., Luo F., Van Acker H.H., Anguille S. Chimeric Antigen Receptor-Modified T Cell Therapy in Multiple Myeloma: Beyond B Cell Maturation Antigen. Front. Immunol. 2019;10:1613. doi: 10.3389/fimmu.2019.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wang H., Kaur G., Sankin A.I., Chen F., Guan F., Zang X. Immune checkpoint blockade and CAR-T cell therapy in hematologic malignancies. J. Hematol. Oncol. 2019;12:59. doi: 10.1186/s13045-019-0746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sun C., Mahendravada A., Ballard B., Kale B., Ramos C., West J., Maguire T., McKay K., Lichtman E., Tuchman S., et al. Safety and efficacy of targeting CD138 with a chimeric antigen receptor for the treatment of multiple myeloma. Oncotarget. 2019;10:2369–2383. doi: 10.18632/oncotarget.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Demoulin B., Cook W.J., Murad J., Graber D.J., Sentman M.L., Lonez C., Gilham D.E., Sentman C.L., Agaugue S. Exploiting natural killer group 2D receptors for CAR T-cell therapy. Future Oncol. 2017;13:1593–1605. doi: 10.2217/fon-2017-0102. [DOI] [PubMed] [Google Scholar]
- 263.Glienke W., Esser R., Priesner C., Suerth J.D., Schambach A., Wels W.S., Grez M., Kloess S., Arseniev L., Koehl U. Advantages and applications of CAR-expressing natural killer cells. Front. Pharm. 2015;6:21. doi: 10.3389/fphar.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sentman C.L., Meehan K.R. NKG2D CARs as cell therapy for cancer. Cancer J. 2014;20:156–159. doi: 10.1097/PPO.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Chang Y.H., Connolly J., Shimasaki N., Mimura K., Kono K., Campana D. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 2013;73:1777–1786. doi: 10.1158/0008-5472.CAN-12-3558. [DOI] [PubMed] [Google Scholar]
- 266.Leivas A., Rio P., Mateos R., Paciello M.L., Garcia-Ortiz A., Fernandez L., Perez-Martinez A., Lee D.A., Powell D.J., Jr., Valeri A., et al. NKG2D-CAR Transduced Primary Natural Killer Cells Efficiently Target Multiple Myeloma Cells. Blood. 2018;132:590. doi: 10.1182/blood-2018-99-114522. [DOI] [Google Scholar]
- 267.Wang Z., Guo L., Song Y., Zhang Y., Lin D., Hu B., Mei Y., Sandikin D., Liu H. Augmented anti-tumor activity of NK-92 cells expressing chimeric receptors of TGF-betaR II and NKG2D. Cancer Immunol. Immunother. 2017;66:537–548. doi: 10.1007/s00262-017-1959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Di Stasi A., Tey S.K., Dotti G., Fujita Y., Kennedy-Nasser A., Martinez C., Straathof K., Liu E., Durett A.G., Grilley B., et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Minagawa K., Al-Obaidi M., Di Stasi A. Generation of Suicide Gene-Modified Chimeric Antigen Receptor-Redirected T-Cells for Cancer Immunotherapy. Methods Mol. Biol. 2019;1895:57–73. doi: 10.1007/978-1-4939-8922-5_5. [DOI] [PubMed] [Google Scholar]
- 270.Liu E., Tong Y., Dotti G., Shaim H., Savoldo B., Mukherjee M., Orange J., Wan X., Lu X., Reynolds A., et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32:520–531. doi: 10.1038/leu.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Otahal P., Prukova D., Kral V., Fabry M., Vockova P., Lateckova L., Trneny M., Klener P. Lenalidomide enhances antitumor functions of chimeric antigen receptor modified T cells. Oncoimmunology. 2016;5:e1115940. doi: 10.1080/2162402X.2015.1115940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Wang X., Walter M., Urak R., Weng L., Huynh C., Lim L., Wong C.W., Chang W.C., Thomas S.H., Sanchez J.F., et al. Lenalidomide Enhances the Function of CS1 Chimeric Antigen Receptor-Redirected T Cells Against Multiple Myeloma. Clin. Cancer Res. 2018;24:106–119. doi: 10.1158/1078-0432.CCR-17-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Magen H., Muchtar E. Elotuzumab: The first approved monoclonal antibody for multiple myeloma treatment. Ther. Adv. Hematol. 2016;7:187–195. doi: 10.1177/2040620716652862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Cao Y., Lu W., Sun R., Jin X., Cheng L., He X., Wang L., Yuan T., Lyu C., Zhao M. Anti-CD19 Chimeric Antigen Receptor T Cells in Combination With Nivolumab Are Safe and Effective Against Relapsed/Refractory B-Cell Non-hodgkin Lymphoma. Front. Oncol. 2019;9:767. doi: 10.3389/fonc.2019.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Petty A.J., Heyman B., Yang Y. Chimeric Antigen Receptor Cell Therapy: Overcoming Obstacles to Battle Cancer. Cancers. 2020;12:842. doi: 10.3390/cancers12040842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Chong E.A., Melenhorst J.J., Svoboda J., Dwivedy Nasta S., Landsburg D.J., Mato A.R., Tian L., Parakandi H., Lacey S.F., June C.H., et al. Phase I/II Study of Pembrolizumab for Progressive Diffuse Large B Cell Lymphoma after Anti-CD19 Directed Chimeric Antigen Receptor Modified T Cell Therapy. Blood. 2017;130:4121. [Google Scholar]
- 277.Hodgins J.J., Khan S.T., Park M.M., Auer R.C., Ardolino M. Killers 2.0: NK cell therapies at the forefront of cancer control. J. Clin. Investig. 2019;129:3499–3510. doi: 10.1172/JCI129338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Klingemann H. Are natural killer cells superior CAR drivers? Oncoimmunology. 2014;3:e28147. doi: 10.4161/onci.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Kloess S., Kretschmer A., Stahl L., Fricke S., Koehl U. CAR-Expressing Natural Killer Cells for Cancer Retargeting. Transfus. Med. Hemother. 2019;46:4–13. doi: 10.1159/000495771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Cheng M., Chen Y., Xiao W., Sun R., Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol. Immunol. 2013;10:230–252. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hu W., Wang G., Huang D., Sui M., Xu Y. Cancer Immunotherapy Based on Natural Killer Cells: Current Progress and New Opportunities. Front. Immunol. 2019;10:1205. doi: 10.3389/fimmu.2019.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Saetersmoen M.L., Hammer Q., Valamehr B., Kaufman D.S., Malmberg K.J. Off-the-shelf cell therapy with induced pluripotent stem cell-derived natural killer cells. Semin. Immunopathol. 2019;41:59–68. doi: 10.1007/s00281-018-0721-x. [DOI] [PubMed] [Google Scholar]
- 283.Li Y., Hermanson D.L., Moriarity B.S., Kaufman D.S. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell. 2018;23:181–192.e185. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Wang W.N., Zhou G.Y., Zhang W.L. NK-92 cell, another ideal carrier for chimeric antigen receptor. Immunotherapy. 2017;9:753–765. doi: 10.2217/imt-2017-0022. [DOI] [PubMed] [Google Scholar]
- 285.Chmielewski M., Abken H. CAR T cells transform to trucks: Chimeric antigen receptor-redirected T cells engineered to deliver inducible IL-12 modulate the tumour stroma to combat cancer. Cancer Immunol. Immunother. 2012;61:1269–1277. doi: 10.1007/s00262-012-1202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Chmielewski M., Abken H. TRUCKs: The fourth generation of CARs. Expert Opin. Biol. 2015;15:1145–1154. doi: 10.1517/14712598.2015.1046430. [DOI] [PubMed] [Google Scholar]
- 287.Krieg S., Ullrich E. Novel immune modulators used in hematology: Impact on NK cells. Front. Immunol. 2012;3:388. doi: 10.3389/fimmu.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kroesen M., Gielen P., Brok I.C., Armandari I., Hoogerbrugge P.M., Adema G.J. HDAC inhibitors and immunotherapy; a double edged sword? Oncotarget. 2014;5:6558–6572. doi: 10.18632/oncotarget.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Nijhof I.S., van de Donk N., Zweegman S., Lokhorst H.M. Current and New Therapeutic Strategies for Relapsed and Refractory Multiple Myeloma: An Update. Drugs. 2018;78:19–37. doi: 10.1007/s40265-017-0841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Davies F., Baz R. Lenalidomide mode of action: Linking bench and clinical findings. Blood Rev. 2010;24(Suppl. 1):S13–S19. doi: 10.1016/S0268-960X(10)70004-7. [DOI] [PubMed] [Google Scholar]
- 291.Gribben J.G., Fowler N., Morschhauser F. Mechanisms of Action of Lenalidomide in B-Cell Non-Hodgkin Lymphoma. J. Clin. Oncol. 2015;33:2803–2811. doi: 10.1200/JCO.2014.59.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Quach H., Ritchie D., Stewart A.K., Neeson P., Harrison S., Smyth M.J., Prince H.M. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010;24:22–32. doi: 10.1038/leu.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Tang X., Yang L., Li Z., Nalin A.P., Dai H., Xu T., Yin J., You F., Zhu M., Shen W., et al. First-in-man clinical trial of CAR NK-92 cells: Safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am. J. Cancer Res. 2018;8:1083–1089. [PMC free article] [PubMed] [Google Scholar]
- 294.Pfefferle A., Huntington N.D. You Have Got a Fast CAR: Chimeric Antigen Receptor NK Cells in Cancer Therapy. Cancers. 2020;12:706. doi: 10.3390/cancers12030706. [DOI] [PMC free article] [PubMed] [Google Scholar]