Abstract
Mesenchymal stem cells (MSCs) exhibit potent immunoregulatory abilities by interacting with cells of the adaptive and innate immune system. In vitro, MSCs inhibit the differentiation of T cells into T helper 17 (Th17) cells and repress their proliferation. In vivo, the administration of MSCs to treat various experimental inflammatory and autoimmune diseases, such as rheumatoid arthritis, type 1 diabetes, multiple sclerosis, systemic lupus erythematosus, and bowel disease showed promising therapeutic results. These therapeutic properties mediated by MSCs are associated with an attenuated immune response characterized by a reduced frequency of Th17 cells and the generation of regulatory T cells. In this manuscript, we review how MSC and Th17 cells interact, communicate, and exchange information through different ways such as cell-to-cell contact, secretion of soluble factors, and organelle transfer. Moreover, we discuss the consequences of this dynamic dialogue between MSC and Th17 well described by their phenotypic and functional plasticity.
Keywords: mesenchymal stem cells, Th17 cells, immunoregulation
1. Introduction
Mesenchymal stem cells (MSCs) are considered a self-renewing cell population present in a wide variety of tissues. Since the first reports by Friedenstein and coworkers during the 1960s and 1970s [1], describing cells with a fibroblast-like morphology and the capacity to proliferate rapidly in discrete colonies, many researchers delved deeper into the study of these cells, eventually establishing that they have the potential to differentiate into chondroblasts, osteoblasts, and adipocytes [2,3,4]. The name “mesenchymal stem cell” was termed in 1991 by Caplan due to their ability to differentiate into different cell lineages [5].
MSCs can be isolated from several adult biological sources including bone marrow, adipose tissue, dental tissue, synovial membrane, peripheral blood, and menstrual blood [6,7,8,9,10,11], as well as perinatal tissues such as umbilical cord, placenta, Wharton jelly, and amniotic fluid [12,13,14,15]. According to the International Society for Cellular Therapy (ISCT), there are three minimum criteria that allow these cells to be identified [16]. Firstly, they must be plastic-adherent when they are kept under standard growing conditions. Secondly, they must express the markers CD74, CD90, and CD105 with a lack of hematopoietic lineage markers such as CD11b, CD14, CD34, or CD45; in addition, they must have a low expression of HLA class II on their surface (<2%). Finally, they must be able to differentiate into cells of mesodermal lineage, more specifically osteoblasts, chondroblasts, and adipocytes, under standard in vitro tissue culture-differentiating conditions [16].
MSCs express a wide variety of chemokine receptors such as CXCR3, CXCR4, or CCR5, allowing them to be recruited from the bone marrow to the circulation and promoting their migration to damaged tissues in pathological conditions [17,18,19]. Additionally, MSCs are able to secrete chemokines such as CXCL12, CX2CL1, CXCL9, CXCL10, or CXCL11, which promote the recruitment of different circulating or resident cell types [19,20].
Independent of their biological sources, MSCs display anti-apoptotic properties which could determine the outcome of diseases and their therapeutic effects [21,22,23,24]. MSCs can also promote the formation of new blood vessels in vitro and in vivo through vasculogenesis or sprouting angiogenesis processes [25,26,27,28,29,30,31]. These cells are reportedly capable of secreting many factors which act in a paracrine way promoting angiogenesis in damaged tissues [32]. Despite this, the MSC secretome may vary depending on the cellular source, hypoxia, and the presence of growth factors and small molecules in the microenvironment [33,34,35]. Additionally, numerous reports suggest that MSCs and the MSC-derived secretome represent a promising alternative for the treatment of fibrosis and damaged tissue cytoprotection [36,37].
Due to their potent immunoregulatory and anti-inflammatory properties, MSCs represent one of the most promising cell products to treat autoimmune and inflammatory diseases. Up until 2020, 1042 clinical trials using MSCs were registered in the NIH clinical Trial Database (https://clinicaltrials.gov/), and about 31% (321 of all registered trials) were directed toward treating immune- or inflammation-associated disorders. Autoimmune diseases such as rheumatoid arthritis (RA) are characterized by a predominance of pathogenic immune cells releasing pro-inflammatory cytokines and an alteration of the peripheral immune tolerance due to immune response deficiencies. RA involves T cells and, in particular, CD4+ T cells [38,39]. CD4+ T cells of RA patients undergo a premature transition from a naïve to a memory phenotype. The resulting memory CD4+ T cells are hyper-proliferative and exhibit an enhanced capacity to differentiate into Th1 and Th17 pathogenic T cells [40,41]. In contrast to other T-cell subsets such as Th1 and Th2, Th17 cells and regulatory T cells (Treg) display a high plasticity degree. In RA individuals, the increased frequency of Th17 cells is mediated through either a reduction in the number of Treg or a qualitative defect in their function [42]. Thus, in this context, parallel to the biotherapies that are already widely used in the clinic, MSC-based therapy approaches were investigated for many years [43]. This attractiveness of MSCs to treat autoimmune and inflammatory disorders relies on their capacity to repress the function and the proliferation of pathogenic immune cells while educating regulatory immune cells. We and others demonstrated that MSCs prevent T-cell differentiation into Th17 and induce Treg cells in vitro and in vivo [44,45,46,47,48]. These potent MSC inhibitory/regulatory properties on Th17 cells are mediated through different mechanisms that are tackled in this review.
2. Immunoregulatory Properties of MSC
The therapeutic effect of MSCs in several diseases such as graft versus host disease (GvHD), arthritis, multiple sclerosis, fibrosis, systemic lupus erythematosus, or kidney injury, in which the immune system plays a key role, is widely reported [49]. This therapeutic effect is observed mainly due to the ability of MSCs to regulate the activation, proliferation, and function of several subsets of immune cells, from both innate and adaptive compartments (Table 1).
Table 1.
Mechanisms of mesenchymal stem cell (MSC) immunosuppression on immune cells. Recapitulation of recent reports describing the known mechanisms via which MSCs exert suppressive function to different immune populations.
| Target Cell | Involved Mechanism | Observed Effect | References |
|---|---|---|---|
| Innate Cells | |||
| DCs | TGF-β, HGF, EVs | ↓MHC-II, CD86, CD40; ↑phagocytic function; ↓IL-6, IL-12, TNF-α, IFN-α; ↑IL-10, TGF-β |
[50,51,52,53,54] |
| Macrophages | TGF-β, iNOS, mitochondrial transfer, EVs | ↓CD86; ↑phagocytic function, ↓IL-6, IL-8, TNF-α, IL-1β; ↑IL-10, TGF-β; ↑M2 type polarization |
[55,56,57,58,59,60,61,62] |
| NK cells | IDO, PGE2, EVs | ↓IFN-γ; ↓cytotoxic activity, proliferation | [63,64,65,66] |
| Neutrophils | TGF-β, EVs | ↓CRAMP and MPO messenger RNA (mRNA), ↓IL-17 | [52,57,61,67] |
| ILC | PGE2, IL-7 | ↑IL-22; ↑proliferation | [68] |
| Adaptive Cells | |||
| B cells | PD-1, IDO, TGF-β, EVs | ↓IgG production; ↓CD69, CD83, CD86; ↓IL-4 mRNA; ↓proliferation, plasmablast differentiation; ↑IL-10 | [66,69,70,71,72,73,74,75] |
| Th1 cells | PD-1, IDO, Fas, IL-6, TGF-β, IL-1Ra, EVs, mitochondrial transfer | ↓IFN-γ, IL-1β, TNF-α; ↑apoptosis; ↓proliferation; differentiation | [52,58,67,76,77,78,79,80,81,82,83,84,85] |
| Th2 cells | IL-6, EVs | ↑differentiation; ↑IL-4, IL5; ↓IL-4, IL-5, IL-13 | [67,76,86,87,88,89] |
| Th17 cells | PGE2, Fas, IDO, IL-6, TGF-β, IL-1Ra, EVs, mitochondrial transfer | ↓IL-17, IL-22; ↑apoptosis; ↓proliferation; differentiation; ↑interconversion to Treg cells. | [54,58,67,75,76,77,78,79,81,82,83,84,90,91,92,93,94,95,96,97,98,99] |
| Regulatory T (Treg) cells | PGE2, Fas, IDO, IL-6, iNOS, TGF-β, IL-1Ra, EVs, mitochondrial transfer | ↑PD-1, ↑IL-10, TGF-β; ↑proliferation; differentiation; ↑conversion from Th17 cells. | [54,58,59,66,67,75,76,77,78,79,80,81,82,85,90,91,92,93,94,96,97,98,99,100] |
CRAMP: cathelicidin-related antimicrobial peptide; DCs: dendritic cells; EVs: extracellular vesicles; HGF: hepatocyte growth factor; IDO: indoleamine 2,3-dioxygenase; IFN: interferon; IgG: immunoglobulin G; IL: interleukin; ILC: innate lymphoid cells; iNOS: inducible nitric oxide synthase; IL-1Ra: IL-1 receptor agonist; MHC-II: class II major histocompatibility complex; MPO: myeloperoxidase; PD-1: programmed cell death-1; PGE2: prostaglandin E2; TGF-β: transforming growth factor-β; Th: T helper; TNF-α: tumor necrosis factor-α. ↑: upregulation; ↓: downregulation.
2.1. Innate Cells
The differentiation of dendritic cells (DC) from monocyte precursors is affected by MSCs [101]. MSCs not only inhibit the acquisition of the DC mature phenotype, keeping them in an immature state [50,51], but also modulate their functional properties, inhibiting their ability to stimulate lymphocyte proliferation and skewing their cytokine secretion from a pro-inflammatory to an anti-inflammatory profile [52,53,102]. Under inflammatory conditions, MSCs can also modulate the macrophage response, resulting in their differentiation into the type 2 macrophage phenotype (M2) [56,103]. These MSC-modulated M2 macrophages are able to secrete several anti-inflammatory cytokines, possessing tissue remodeling and tolerogenic properties [46]. Moreover, MSCs can downregulate natural killer (NK) cell activation by IL-2 or IL-15, inhibiting NK cell proliferation, cytokine production, and cytotoxicity [63,64,65]. Regarding innate lymphoid cells (ILCs), MSCs stimulate their proliferation, as well as their capacity to release IL-22, which could contribute to immune homeostasis [68].
2.2. Adaptive Cells
Adaptive cells such as B and T cells are potently regulated by MSCs. MSCs can suppress B-cell activation by decreasing CD69 and CD86 expression, plasma cell differentiation, and immunoglobulin G (IgG) production [69,70,71]. Moreover, several reports consistently showed that MSCs convert B cells into IL-10-producing B regulatory (Breg) cells which exhibit inhibitory function [72,73,74,104].
Similarly, a compelling body of evidence supports the immunoregulatory effect of MSCs over CD4+ T-cell differentiation into the T helper subsets T helper 1 (Th1). MSCs suppress Th1 cell proliferation and the production of pro-inflammatory cytokines such as interferon (IFN)-γ and interleukin (IL)-12, among others [67,76,77]. MSCs can favor T-cell differentiation into the T helper 2 (Th2) subset, increasing IL-4 and IL-5 production [86]. However, MSCs were also shown to inhibit Th2-mediated inflammatory responses in experimental allergy models [87,88,89]. Finally, MSCs promote the differentiation or induction of T cells into Treg cells with immunosuppressive functions. Indeed, these Treg cells are characterized by their capacity to produce anti-inflammatory cytokines such as transforming growth factor-β (TGF-β) and IL-10, along with having tolerogenic properties [59,78,79,90,100].
3. Mechanisms behind the MSC Immunosuppressive Effect on Th17 Cells
Among the T-cell subsets that are involved in the immune response, Th17 cells represent a distinct lineage of T lymphocytes located in the lamina propria of the small intestine upon steady state [105]. These pro-inflammatory CD4+ T cells express the cytokines IL-17A, IL-17F, and IL-22, as well as the chemokine receptor CCR6 and the RAR-related orphan receptor γT (RORγT), the master transcription factor of both human and murine Th17 cell differentiation and a hallmark of a Th17-specific T-cell signature [41]. In response to an inadequate or deficient T-cell immune regulation, Th17 cells participate in the development of several autoimmune diseases, and several studies described Th17 cells as major players in the pathogenesis of many inflammatory and autoimmune diseases, such as rheumatoid arthritis, type 1 diabetes, multiple sclerosis, systemic lupus erythematosus, and bowel disease [106,107]. Th17 and Treg cells are highly plastic versatile cells that can adopt different phenotypes and functions when exposed to physiological or pathological conditions [108]. Hence, the balance between Treg cells and Th17 cells (Treg/Th17 ratio) often shapes the outcome of immune responses (immunosuppression versus inflammation) and, thus, represents a promising immune target to determine the effectiveness of immune therapy [108,109].
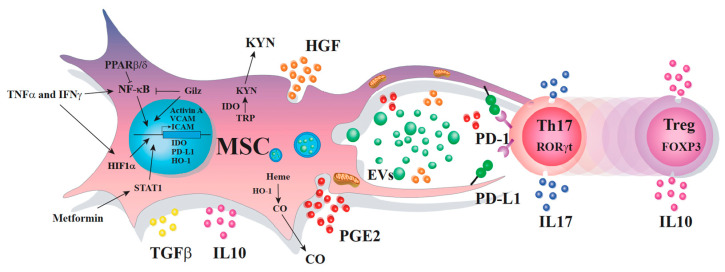
Due to their immunomodulatory and protective properties, MSCs are studied for their therapeutic potential in inflammatory and autoimmune diseases. Although the mechanisms via which MSCs inhibit the response of different immune cell populations, particularly the Th17 subsets, are not fully elucidated; to date, several processes were reported. Indeed, MSC immunoregulatory properties are mediated through the production of soluble factors, mechanisms dependent on cell-to-cell contact, mitochondria transfers, or the production of extracellular vesicles containing microRNAs (miRNAs) among other molecules (Figure 1).
Figure 1.
The multifaceted immunoregulatory dialogue between MSCs and Th17 cells. MSCs and Th17 cells communicate through many different ways that include the release of soluble factors, the transfer of mitochondria, the production of extracellular vesicles containing various molecules, and cell-to-cell contact. When MSCs and Th17 cells are near one another, they dialogue through the release of messengers including PD-L1, HGF, and PGE2 that are highly expressed when MSCs are co-cultured with Th17 cells. Moreover, MSCs (nearby Th17 cells) start to express high levels of IDO and HO-1, releasing some EV-containing immunoregulatory factors and transferring their mitochondrial via transient cell fusion and tunneling nanotubes. In return, Th17 cells start to express high levels of PD-1, exhibiting a reduced production of IL-17 and expression of RORγT, while increasing the capacity to release IL-10 and to express Foxp3. This type of signaling, in which MSCs and Th17 cells communicate over relatively short distances, regulates the phenotype and function of both cells.
3.1. Soluble Factors
The production of soluble factors by MSCs that mediate the immune response is widely studied.
Prostaglandin E2 (PGE2) is a lipid molecule capable of exerting its immunomodulatory effect in several immune cell populations, including Th1 and Th17 cell subsets [110]. However, depending on the maturation state of the different lymphocyte subsets, PGE2 can display a dual role. In mature Th1 cells, PGE2 inhibits their proliferation and the production of IFN-γ, whereas, during Th1 polarization, there is an increase in the secretion of IFN-γ [111]. In contrast, the opposite effect was observed in Th17 cells. During Th17 differentiation from naïve T cells in vitro, PGE2 increases the expression of IL-23R and IL-1R via the activation of PGE2 receptor 2 (EP2)- and receptor 4 (EP4)-dependent signaling pathways, along with the increase in cyclic AMP pathways [112]. However, when memory CD4+ T cells are exposed to Th17-skewing conditions, MSC-derived PGE2 inhibits IL-17A secretion via an EP4-mediated, contact-dependent mechanism [113,114]. This differential response to secreted PGE2 in Th1 and Th17 cell subsets might be mediated partially by the interaction with other cell types, such as myeloid cells, since selective removal of myeloid cells from a co-culture of MSCs and CD4+ T cells impairs Th17 immunosuppression mediated by PGE2 [48]. Nevertheless, this dual effect of PGE2 requires to be fully elucidated. Recent studies with PBMCs obtained from RA patients (co-cultivated with adipose tissue-derived MSCs) showed an improvement in the Treg/Th17 ratio. as well as an increase in the production of TGF-β and a decrease in the IL-17 levels [115]. The authors proposed that this effect was mediated by a series of factors secreted by MSCs, including PGE2 [48]. Injection of umbilical cord-derived MSCs in patients with active systemic lupus erythematosus (refractory to conventional therapies) was associated with an increased frequency of Foxp3+ Treg cells one week post MSC injection and up to three months later with an additional decrease in frequency of Th17 cells from three to 12 months post injection [96]. In this study, the authors evaluated the potential in vitro mechanisms behind this effect and concluded that the upregulation of Treg cells and downregulation of Th17 cells was mediated by PGE2 and TGF-β [96].
Several cytokines were shown to be involved in the immunosuppressive function of MSC. The anti-inflammatory cytokine IL-10 is secreted by MSCs, and previous studies reported that IL-10 secreted by MSCs inhibits the differentiation of naïve CD4+ T cells into Th17 cells in vitro, by downregulating RORγT and impairing the signaling pathways related to RORγT activation [116]. Despite this, more recent in vitro studies showed that MSC does not impair IL-23R expression on Th17 cells but strongly modulates the Th17 cell secretome [117]. MSCs themselves can secrete TGF-β and, thus, induce the differentiation of conventional CD4+ CD25− T cells into Foxp3+ Treg cells in vitro [118]. In addition to the canonical immunosuppressive pathways triggered by the binding of MSC-derived TGF-β on T cells, it was shown that the TGF-β produced by MSCs also modulates the function of some caspases such as the caspase 8, critical for T-cell activation in a renal xenograft mice model [119]. In addition, TGF-β, produced by MSC along with IL-10, suppresses Th17 differentiation mediated by dendritic cells [54,102]. Despite being described as a pro-inflammatory cytokine, IL-6 is massively produced by MSCs. This cytokine is essential for MSCs to produce PGE2 in vitro and to decrease inflammation in the murine model of collagen-induced arthritis (CIA) [120]. Accordingly, IL-6 released by MSCs contributes to decreased Th17 cell frequency and inhibits the production of IL-17 and IL-22 in vitro released by PBMCs from RA patients. This resulted in the increase of both Foxp3+ Treg cells and TGF-β produced by these PBMCs [115]. Moreover, MSCs secrete IL-1 receptor agonist (IL-1Ra) which inhibits IL-1α and IL-1β signaling and, thus, impacts the activation and function of several immune populations such as macrophages, DCs, and lymphocytes [121]. Recent studies showed that IL-1Ra produced by MSCs inhibits Th17 polarization in vitro. In vivo, in an IL-1ra knockout (KO) mice experimental arthritis model, MSC injection reduced the severity of arthritis while increasing the Treg/Th17 ratio as compared to the control group [77].
Another key mediator of MSC immunoregulatory properties, specific for human MSCs, is indoleamine 2, 3-dioxygenase (IDO), which promotes the degradation of tryptophan into kynurenine (a toxic metabolite), thus inhibiting T-cell proliferation or inducing apoptosis [122,123]. Kynurenine inhibits Th17 cells by suppressing the mammalian target of the rapamycin (mTOR) pathway and through tryptophan deprivation followed by the induction of general control non-depressible 2 (GCN2), a stress response kinase [124,125,126]. The infusion of MSC (or their conditioned media) in a model of carbon tetrahydrochloride-induced murine liver fibrosis attenuated fibrosis progression. These results were accompanied by higher serum levels of IDO, IL-10, and kynurenine and lower levels of IL-17. Moreover, the frequency of CD4+ IL-10+ was increased but the number of Il-17+ Th17 cells was reduced. These results were completely reversed in the presence of an IDO inhibitor [127].
Over the last few years, the enhancement of MSC immunoregulatory properties was intensively investigated through the development of different protocols of MSC pretreatment. For example, the pretreatment of adipose tissue-derived MSCs with metformin revealed an enhanced IDO expression in MSC in a STAT1-dependent manner [75]. The infusion of these cells in an experimental model of lupus significantly increased the Treg/Th17 ratio, as well as the production of anti-double stranded DNA (dsDNA) IgG antibodies, reducing inflammation [75].
Heme oxygenase-1 (HO-1) is an inducible enzyme with anti-inflammatory and immunoregulatory properties, which catalyzes the conversion of heme into carbon monoxide (CO), biliverdin, and Fe (2+) [128]. Biliverdin production protects cells against oxidative damage, while CO has a similar effect to nitric oxide (NO) by stimulating guanylyl cyclase and increasing intracellular levels of cGMP, reducing leukocyte adhesion, decreasing apoptosis, and downregulating the production of some pro-inflammatory cytokines [128]. The infusion of modified bone marrow-derived MSCs expressing higher levels of HO-1 ameliorated the severity of GvHD in a murine model by regulating the Treg/Th17 balance in lymph nodes and the spleen [97]. More recently, the overexpression of HO-1 by MSCs was shown to inhibit NK cells, reduce the Th1/Th2 balance, and induce Th17 into Treg in vitro [129]. When these modified MSC were tested in vivo in a reduced-size liver transplant rejection model, animals showed a lower transplant rejection rate and pro-inflammatory cytokine levels along with higher anti-inflammatory cytokines levels and number of peripheral Treg [129]. Together, these results suggest that the overexpression of HO-1 or activity in MSCs could be useful to improve MSC efficacy in pre-clinical and clinical applications [130].
Unlike IDO and HO-1, inducible nitric oxide synthase (iNOS) is an enzyme produced exclusively by murine MSC. iNOS was shown to exert an inhibitory effect on different subsets of T lymphocytes by suppressing their proliferation through the production of NO [131]. At high concentrations, NO inhibits TCR-induced T-cell proliferation and cytokine production [132]. NO produced by murine MSCs suppresses the phosphorylation of the signal transducer and activator of transcription 5 (STAT-5) and inhibits CD4+ T-cell proliferation [133]. MSC pretreatment with IFN-γ and other pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) or IL-1β, increases their capacity to produce NO and other chemokines, enhancing their immunomodulatory properties [133]. A recent study supported the importance of MSC licensing prior to their administration in vivo. Indeed, when MSCs were primed with TNF-⍺ and IL-1β, they suppressed lymphocyte proliferation through NO production. This effect was demonstrated in vivo in a rat cornea transplant model [59]. The treatment with licensed MSCs significantly improved graft survival in comparison to non-licensed MSCs, and this was evidenced by an increasing number of Treg cells in draining lymph nodes, spleen, and lungs, in addition to lower levels of pro-inflammatory cytokines [59]. In another recent study, the immunomodulatory effect of MSCs was evaluated in a model of facial nerve injury in rats [134]. When the facial nerve was harvested and co-cultured in vitro with bone marrow-derived MSCs, an upregulation of iNOS (among other immunosuppressive factors) was observed. The local infusion of MSC after nerve face injury led to a decrease in the frequency of Th17 cells in lymph nodes and an antiapoptotic effect in facial motor neurons [134].
Some studies showed that the production of hepatocyte growth factor (HGF) by MSCs can partially modulate the Treg/Th17 balance. An in vitro study using MSC co-cultured in transwell with CD4+ T cells activated with LPS proposed that HGF is necessary for an increased number of CD4+ Foxp3+ cells and a downregulated number of CD4+ RORγt+ cells [135]. In an in vivo model of bronchiolitis obliterans in mice, the infusion of umbilical cord-derived MSCs overexpressing HGF improved the Th1/Th2 ratio, increased the number of Treg cells, and decreased the number of Th17 cells in the spleen; these results were accompanied by decreased IFN-γ levels and increased IL-4 and IL-10 levels in serum [136]. While the expression of c-Met (HGF receptor) on Th17 is not yet reported, it is known that this receptor is expressed in immune cells with antigen-presenting capacities, such as DCs. Therefore, an indirect effect of HGF on Th17, with DCs as intermediaries, cannot be discarded [137].
3.2. Cell-to-Cell Contact
One molecule considered crucial for cell-to-cell contact-based inhibition is programed death 1 (PD-1). This molecule is necessary for the downregulation of immune responses by inducing apoptosis of target cells when it binds with its ligand (PD-L1) [138]. It is expressed in various cell types, including B and T lymphocytes [138]. When MSCs are co-cultured with mature Th17 lymphocytes, expression of PD-L1 is significantly increased on the surface of MSCs, promoting the inhibition of Th17 cell proliferation. This latter effect is reversed by using PD-L1-neutralizing antibodies [44]. It was shown that palatine tonsil-derived MSCs are capable of inhibiting Th17 differentiation in vitro through the PD-L1/PD-1 axis, and this effect is enhanced by the secretion of IFN-β by MSCs [139]. When these MSCs were locally administered in vivo in an imiquimod-induced psoriatic skin inflammation model, the disease symptoms were significantly decreased mainly by affecting Th17 response in a PD-L1-dependent manner [139].
Another important mechanism that depends on cell-to-cell contact relies on the Fas/FasL axis. The systemic infusion of bone marrow-derived MSC ameliorated the disease phenotypes in the in vivo models of fibrillin-1 mutated systemic sclerosis (SS) and dextran-sulfate-sodium-induced experimental colitis. This effect was mediated by T-cell recruitment through the secretion of monocyte chemotactic protein 1 (MCP-1) by MSC. In addition, their posterior apoptosis was triggered by the Fas/FasL union. This apoptotic effect promoted the recruitment of macrophages, which secreted high levels of TGF-β, promoting the upregulation of Foxp3+ regulatory T cells [58]. This same effect was also reported in Th17 cells, using gingiva-derived MSCs in a colitis mice model [98,99].
Adhesive molecules such as vascular cell adhesion protein (VCAM) and intercellular adhesion molecule (ICAM) were also described to play an important role in the immunosuppressive properties of MSC. These adhesive molecules promote proximity between MSCs and lymphocytes, improving the regulatory effect promoted by the secretion of soluble factors, such as NO, by MSCs [140,141].
3.3. Extracellular Vesicles
Most somatic cells release extracellular vesicles (EVs), which are membrane vesicles involved in cell-to-cell communication and several physiological processes that comprise different vesicle subsets which share features such as size and biochemical composition [142]. While exosomes (30–150 nm diameter) originate from endosomal compartments derived from the plasma membrane, microvesicles (100–1000 nm diameter) originate mainly from the plasma membrane while apoptotic bodies (50–4000 nm) emerge from the fragments of dying cells. The EV composition largely reflects that of the parenting cell and, along with EV-enriched proteins (e.g., Alix, Tsg101, CD63, HSP90), MSC-derived EVs also reportedly contain MSC markers such as CD44, CD90, and CD105 [143]. However, the immunosuppressive functions of MSC-derived EVs are predominantly attributed to other proteins, messenger RNAs (mRNAs), and non-coding RNAs, such as miRNAs that modulate gene expression by affecting the translation of other specific mRNAs [144,145]. Therefore, MSC-derived EVs are able to modulate the function of several immune populations such as inhibiting B-cell proliferation and plasmablast differentiation, modulating macrophage polarization, inhibiting DC maturation, impairing neutrophil mobilization, and suppressing NK cell and T lymphocyte proliferation, among others [54,60,61,66,81,82,146,147].
Although the mechanisms of MSC-derived EV regulation on Th17 cells are not yet fully understood, some reports indicated that these EVs downregulate the Th17 polarization of in vitro activated CD4+ T cells from PBMCs of healthy donors [94,95]. An additional study found that, among PBMCs from type 1 diabetes (T1D) patients, in vitro activation of islet antigen-specific T cells was inhibited by MSC EVs, decreasing the frequency of Th17 cells and IL-17 levels via a mechanism involving TGF-β and PGE2. These EVs also showed promising results as a treatment to control inflammatory immune responses in several animal models [147]. Studying the therapeutic potential of MSC EVs in Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease (a progressive model of multiple sclerosis), it was observed that MSC EV treatment decreased Th17-derived IL-17 serum levels and brain immune infiltration, which was associated with lower production of several microglial-derived pro-inflammatory cytokines [83]. Although EV treatment reduced brain atrophy and increased remyelination in TMEV-infected mice, the exact mechanism via which these MSC-EVs might contribute to ameliorate this disease remains to be elucidated [83,148].
However, other studies attributed to miR-21 the protective effect of MSC EVs inhibiting Th17 cell-driven inflammation, mainly by reducing renal DC maturation and cytokine secretion, using an ischemia/reperfusion injury model of acute kidney disease [149,150,151]. In addition, using the model of experimental autoimmune uveitis (EAU), it was shown that intravenous or periocular administration of MSC EVs decreased the number and frequency of IL-17-producing Th17 cells in immunized mouse’s eyes, which was confirmed using a T1D mice model showing decreased transcript levels of the DC-derived Th1- and Th17-related cytokines IL-1β, IL-6, and IL-12 [84]. Altogether, these reports implicate that EVs derived from MSCs exert a crucial role controlling inflammatory responses and they have significant potential for treating Th17-mediated autoimmune diseases. More research is needed to elucidate the participation of additional components and factors present in these EVs.
3.4. Transfer of Mitochondria
Mitochondrial function is necessary for oxidative phosphorylation, ATP generation, and several metabolic pathways in somatic cells, while its dysfunction leads to ROS overproduction and oxidative damage to cells [152]. Remarkably, it was observed that MSCs exert a cytoprotective function to damaged cells via mitochondrial transfer, leading to improved survival and modulation of cell proliferation, as well as signaling both in vitro and in vivo [153,154,155].
So far, MSCs were shown to transfer their mitochondrial load via contact-dependent mechanisms such as gap junctions, transient cell fusion, and tunneling nanotubes (TNT), in addition to contact-independent mechanisms such as mitochondria-containing EVs and isolated mitochondria transfer [57,156,157,158]. This mitochondrial transfer from MSCs exhibited cytoprotective effects in kidney injury, myocardial damage, injured retinal ganglion, pulmonary alveoli, and damaged cornea, among others [159,160,161]. Nevertheless, the understanding of the underlying molecular mechanisms of mitochondrial transfer and recipient cell repercussions remains unclear.
To date, there were very few studies focusing on the mitochondrial transfer from MSC to immune cells. As described by other cell types, MSCs were shown to transfer mitochondria to macrophages through both TNT- and EV-mediated mechanisms, which increased the phagocytic capacity of macrophages in a mouse model of Escherichia coli pneumonia, or modulated macrophages into an anti-inflammatory M2 phenotype, reducing lung inflammation and injury in mice [57,62]. Additionally, the artificial transfer of MSC-derived mitochondria reportedly induced Treg cell differentiation from activated human CD4+ T cells, and these pre-treated T cells with MSC mitochondria reduced leukocyte tissue infiltration and improved animal survival in a GvHD mouse model [85]. However, how naturally occurring mitochondrial transfer impacts T-cell activation and function still remains insufficiently described.
Previous reports from our group showed that MSCs exert immunosuppression to pathogenic Th17 cells in the context of rheumatoid arthritis (RA) [41,162]; thus, we aimed to investigate whether MSCs modulated the inflammatory environment in RA patient joints through mitochondrial transfer to T cells. When we cultured MSC with ex vivo expanded human Th17 cells, we observed a contact-dependent mitochondrial transfer that occurred as soon as four hours after co-culture [47]. We observed a decrease in IL-17 production of these modulated Th17 cells, and a portion of these cells interconverted into Foxp3+ Treg cells. Moreover, oxidative phosphorylation and oxygen consumption were increased in these MSC-treated Th17 cells, suggesting a metabolic switching associated with MSC immunomodulation and Th17–Treg interconversion [47]. Considering that MSCs are present in the synovium during RA onset, we wanted to reveal whether this mitochondrial transfer to CD4+ T cells was altered in MSCs from RA patients (RA-MSCs) compared to MSCs from healthy donors, eventually finding that mitochondrial transfer capacity of RA-MSCs was significantly lower compared to healthy MSCs [47]. Altogether, these results suggested that impaired mitochondrial transfer from MSC in the context of RA pathogenesis (and maybe in other autoimmune diseases) could contribute to inflammation and joint damage, worsening the outcome of the disease. However, additional studies are definitely needed to clarify the molecular mechanisms involved in this transfer and the contribution of metabolic switching in the immune function and phenotype of modulated T cells during RA.
4. MSC Enhancement to Improve Their Therapeutic Potential
Stimulating MSCs with biological, chemical, or physical factors was proven to be an efficient strategy to enhance their therapeutic function [163]. Several studies demonstrated that the activation of MSC with pro-inflammatory cytokines, as well as with growth factors, induces their multiple immunosuppressive mechanisms. For example, the pre-treatment of MSCs with IFN-γ prior to being co-cultured with activated lymphocytes enhanced their capacity to decrease the production of IFN-γ and TNF-α, increased the secretion of IL-6 and IL-10, increased the frequency of CD4+ CD25+ CD127dim/− regulatory T cells, and decreased the frequency of Th17 cells [164]. Moreover, IL-1β-primed MSCs were shown to upregulate the expression of genes related to several biological processes linked to the NF-κB pathway [165], and the infusion of these cells in a murine colitis model led to the polarization of peritoneal M2 macrophages, increased frequencies of Treg cells, and decreased the percentage of Th17 cells in the spleen and mesenteric lymph nodes [166]. Considering the interaction between Th17 and MSC, it was described that IL-17A, the main cytokine produced by Th17 cells, enhances the immunomodulatory properties of murine MSC, both in vitro and in vivo [167,168]. This effect depends on the expression of IL-17 receptor A (IL17RA) on the MSC surface, which is involved in the surface levels of VCAM1, ICAM1, and PD-L1, along with iNOS expression [167,168]. Moreover, one report showed that human MSCs treated with IL-17A exhibited a higher in vitro T-cell suppression of proliferation, a lower proinflammatory cytokine production, and a higher induction of Treg cells with no associated upregulation of major histocompatibility complex (MHC) class I and II compared to MSCs treated with IFN-γ [169]. However, some disadvantages were reported, including an increased immunogenicity of MSCs after IFN-γ stimulation, the elevated costs of recombinant cytokines, and variability in the response of MSCs from different sources [163].
Three-dimensional (3D) spheroid culture conditions were also shown to enhance MSC immunoregulatory functions. Indeed, human MSCs significantly increased their capacity to produce and release suppressive factors such as IDO when cultured as 3D aggregates [170]. Since oxygen availability in the BM compartment is quite limited, going as low as 1% [171], several studies already demonstrated that MSCs cultured under hypoxic conditions had increased production of soluble bioactive factors, higher angiogenic potential, and immunomodulatory activity [172]. MSCs cultured under hypoxic conditions induced the production of hypoxia-induced factor-1 alpha (HIF1α) expression, which is associated with the production of these multiple mechanisms mentioned, increasing the suppressive potential on Th1 and Th17 cells [173,174].
The peroxisome proliferator activator receptor β/δ (PPARβ/δ) was suggested to play a critical role in the control of the therapeutic potential of MSCs. PPARβ/δ is a transcription factor from the nuclear receptor superfamily that exhibits diverse biological functions like inflammation regulation through the inhibition of NF-κB signaling, which was described in adipocytes and macrophages [175,176]. As previously mentioned, the stimulation of NF-κB signaling with pro-inflammatory cytokines activates the immunosuppressive effect of MSCs. Since PPARβ/δ is a negative regulator of NF-κB activity, the inhibition of PPARβ/δ, either chemically or genetically on MSCs, enhanced their therapeutic potential both in vitro and in vivo in an experimental model of arthritis, diminishing the frequency of Th17 cells. This enhanced immunosuppressive potential of MSCs inhibited through PPARβ/δ was associated with a significant increase of NF-κB activity [177]. Several other strategies were proposed to enhance the anti-arthritic and immunoregulatory properties of MSCs on Th17 cells including the expression of glucocorticoid-induced leucine zipper (Gilz) by MSC, which induces Treg differentiation [45,178]. However, further research has to be completed in order to elucidate the molecular mechanisms associated and turn it into a future clinical tool.
Although these approaches showed promising results in vitro and in pre-clinical animal studies using both murine and human MSCs, they are pending elucidation to see if these strategies are translatable to clinical studies. For example, the treatment of MSCs with GSK3787, a selective and irreversible antagonist of PPARβ/δ that provides MSCs with a potent immunosuppressive effect, seems promissory for the treatment of patients with autoimmune diseases such as RA [177]. However, despite this drug being described as non-toxic and non-bioaccumulative in tissues [179], it is not currently approved by the United States (US) Food and Drug Administration (FDA), which maintains PPARβ/δ as an attractive therapeutic target for MSC immunosuppressive function; however, it will require additional research to find new drugs. In 2007, a phase Ia clinical trial aimed to prove the safety of a PPARβ/δ agonist was successful and a phase Ib trial was started [180]; therefore, future studies cannot be discarded to test the safety of PPARβ/δ antagonists. This is one of the major aspects to consider in order to apply further studies for clinical therapies.
5. Conclusions
MSCs and Th17 cells are two plastic and versatile cells that interact both in physiological and in pathological conditions with different phenotype and functions. MSCs are not immunoregulatory per se. To acquire an immunoregulatory phenotype and function, and to regulate immune responses, MSCs need to be activated by factors released by pro-inflammatory immune cells such as Th17 cells. Once they acquire an immunoregulatory status, MSCs repress the differentiation program of Th17 cells, which is accompanied by T cells producing IL-10 and expressing Foxp3. This bi-directional immunoregulatory dialogue is mediated through different mechanisms that include the release of soluble factors and extracellular vesicles, a reservoir of proteins, lipids, mRNAs, and miRNAs, cell-to-cell contact, and the transfer of organelles (Figure 1). However, considering the mechanisms that govern the dialogue between MSCs and Th17 cells and the plasticity of the two cell types (in addition to the poor survival rate of MSCs upon in vivo injection), the immunoregulatory properties exerted by MSCs and Th17 cells can be effective only for a restricted period of time. Therefore, efforts to prolong MSC persistence in a pathological environment should be attempted in order to extend the exposure of Th17-infiltrated diseased tissue to MSC-derived immunoregulatory, cytoprotective, and therapeutic products.
Funding
This work was supported by Inserm, the University of Montpellier, and Fondation Arthritis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Friedenstein A.J., Petrakova K.V., Kurolesova A.I., Frolova G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. doi: 10.1097/00007890-196803000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Owen M., Friedenstein A.J. Stromal stem cells: Marrow-derived osteogenic precursors. Ciba Found. Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 3.Friedenstein A.J., Chailakhyan R.K., Gerasimov U.V. Bone marrow osteogenic stem cells: In vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 4.Piersma A.H., Brockbank K.G., Ploemacher R.E., van Vliet E., Brakel-van Peer K.M., Visser P.J. Characterization of fibroblastic stromal cells from murine bone marrow. Exp. Hematol. 1985;13:237–243. [PubMed] [Google Scholar]
- 5.Caplan A.I. Mesenchymal stem cells. J. Orthop. Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 6.Romanov Y.A., Darevskaya A.N., Merzlikina N.V., Buravkova L.B. Mesenchymal stem cells from human bone marrow and adipose tissue: Isolation, characterization, and differentiation potentialities. Bull. Exp. Biol. Med. 2005;140:138–143. doi: 10.1007/s10517-005-0430-z. [DOI] [PubMed] [Google Scholar]
- 7.Kassis I., Zangi L., Rivkin R., Levdansky L., Samuel S., Marx G., Gorodetsky R. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant. 2006;37:967–976. doi: 10.1038/sj.bmt.1705358. [DOI] [PubMed] [Google Scholar]
- 8.Alcayaga-Miranda F., Cuenca J., Luz-Crawford P., Aguila-Diaz C., Fernandez A., Figueroa F.E., Khoury M. Characterization of menstrual stem cells: Angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 2015;6:32. doi: 10.1186/s13287-015-0013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Djouad F., Bony C., Häupl T., Uzé G., Lahlou N., Louis-Plence P., Apparailly F., Canovas F., Rème T., Sany J., et al. Transcriptional Profiles Discriminate Bone Marrow-Derived and Synovium-Derived Mesenchymal Stem Cells. Arthritis Res. Ther. 2005;7:R1304. doi: 10.1186/ar1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bari C., Dell’Accio F., Tylzanowski P., Luyten F.P. Multipotent Mesenchymal Stem Cells from Adult Human Synovial Membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 11.Huang G.T., Yamaza T., Shea L.D., Djouad F., Kuhn N.Z., Tuan R.S., Shi S. Stem/Progenitor Cell-Mediated De Novo Regeneration of Dental Pulp with Newly Deposited Continuous Layer of Dentin in an In Vivo Model. Tissue Eng. Part A. 2010;16:605–615. doi: 10.1089/ten.tea.2009.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erices A.A., Allers C.I., Conget P.A., Rojas C.V., Minguell J.J. Human Cord Blood-Derived Mesenchymal Stem Cells Home and Survive in the Marrow of Immunodeficient Mice after Systemic Infusion. Cell Transplant. 2003;12:555–561. doi: 10.3727/000000003108747154. [DOI] [PubMed] [Google Scholar]
- 13.Miao Z., Jin J., Chen L., Zhu J., Huang W., Zhao J., Qian H., Zhang X. Isolation of mesenchymal stem cells from human placenta: Comparison with human bone marrow mesenchymal stem cells. Cell Biol. Int. 2006;30:681–687. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Weiss M.L., Anderson C., Medicetty S., Seshareddy K.B., Weiss R.J., VanderWerff I., Troyer D., McIntosh K.R. Immune Properties of Human Umbilical Cord Wharton’s Jelly-Derived Cells. Stem Cells. 2008;26:2865–2874. doi: 10.1634/stemcells.2007-1028. [DOI] [PubMed] [Google Scholar]
- 15.Marongiu F., Gramignoli R., Sun Q., Tahan V., Miki T., Dorko K., Ellis E., Strom S.C. Isolation of amniotic mesenchymal stem cells. Curr. Protoc. Stem Cell Biol. 2010;12:1E.5.1–1E.5.11. doi: 10.1002/9780470151808.sc01e05s12. [DOI] [PubMed] [Google Scholar]
- 16.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 17.Barhanpurkar-Naik A., Mhaske S.T., Pote S.T., Singh K., Wani M.R. Interleukin-3 enhances the migration of human mesenchymal stem cells by regulating expression of CXCR4. Stem Cell Res. Ther. 2017;8:168. doi: 10.1186/s13287-017-0618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishikawa G., Kawada K., Nakagawa J., Toda K., Ogawa R., Inamoto S., Mizuno R., Itatani Y., Sakai Y. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression via CCR5. Cell Death Dis. 2019;10:264. doi: 10.1038/s41419-019-1508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Croitoru-Lamoury J., Lamoury F.M., Zaunders J.J., Veas L.A., Brew B.J. Human mesenchymal stem cells constitutively express chemokines and chemokine receptors that can be upregulated by cytokines, IFN-beta, and Copaxone. J. Interferon Cytokine Res. 2007;27:53–64. doi: 10.1089/jir.2006.0037. [DOI] [PubMed] [Google Scholar]
- 20.Sordi V., Malosio M.L., Marchesi F., Mercalli A., Melzi R., Giordano T., Belmonte N., Ferrari G., Leone B.E., Bertuzzi F., et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106:419–427. doi: 10.1182/blood-2004-09-3507. [DOI] [PubMed] [Google Scholar]
- 21.Li B., Zhang H., Zeng M., He W., Li M., Huang X., Deng D.Y., Wu J. Bone Marrow Mesenchymal Stem Cells Protect Alveolar Macrophages from Lipopolysaccharide-Induced Apoptosis Partially by Inhibiting the Wnt/β-Catenin Pathway. Cell Biol. Int. 2015;39:192–200. doi: 10.1002/cbin.10359. [DOI] [PubMed] [Google Scholar]
- 22.Qu H., Guo Y.H., Zhu X.J., Gao W., Mao J.M. Anti-apoptotic Effects of Mesenchymal Stem Cells on Cardiac Myocytes: In Vitro Study with Rats. Zhonghua Yi Xue Za Zhi. 2007;87:271–274. [PubMed] [Google Scholar]
- 23.Huang W., Lv B., Zeng H., Shi D., Liu Y., Chen F., Li F., Liu X., Zhu R., Yu L., et al. Paracrine Factors Secreted by MSCs Promote Astrocyte Survival Associated with GFAP Downregulation after Ischemic Stroke via p38 MAPK and JNK. J. Cell. Physiol. 2015;230:2461–2475. doi: 10.1002/jcp.24981. [DOI] [PubMed] [Google Scholar]
- 24.Tang Y.L., Zhao Q., Qin X., Shen L., Cheng L., Ge J., Phillips M.I. Paracrine Action Enhances the Effects of Autologous Mesenchymal Stem Cell Transplantation on Vascular Regeneration in Rat Model of Myocardial Infarction. Ann. Thorac. Surg. 2005;80:229–237. doi: 10.1016/j.athoracsur.2005.02.072. [DOI] [PubMed] [Google Scholar]
- 25.Ucuzian A.A., Gassman A.A., East A.T., Greisler H.P. Molecular Mediators of Angiogenesis. J. Burn Care Res. 2010;31:158–175. doi: 10.1097/BCR.0b013e3181c7ed82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melero-Martin J.M., De Obaldia M.E., Kang S.Y., Khan Z.A., Yuan L., Oettgen P., Bischoff J. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ. Res. 2008;103:194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin R.Z., Moreno-Luna R., Zhou B., Pu W.T., Melero-Martin J.M. Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells. Angiogenesis. 2012;15:443–455. doi: 10.1007/s10456-012-9272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Traktuev D.O., Prater D.N., Merfeld-Clauss S., Sanjeevaiah A.R., Saadatzadeh M.R., Murphy M., Johnstone B.H., Ingram D.A., March K.L. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ. Res. 2009;104:1410–1420. doi: 10.1161/CIRCRESAHA.108.190926. [DOI] [PubMed] [Google Scholar]
- 29.Kachgal S., Putnam A.J. Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis. 2011;14:47–59. doi: 10.1007/s10456-010-9194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roubelakis M.G., Tsaknakis G., Pappa K.I., Anagnou N.P., Watt S.M. Spindle Shaped Human Mesenchymal Stem/Stromal Cells from Amniotic Fluid Promote Neovascularization. PLoS ONE. 2013;8:e54747. doi: 10.1371/journal.pone.0054747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merfeld-Clauss S., Gollahalli N., March K.L., Traktuev D.O. Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Eng. Part A. 2010;16:2953–2966. doi: 10.1089/ten.tea.2009.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon H.M., Hur S.M., Park K.Y., Kim C.K., Kim Y.M., Kim H.S., Shin H.C., Won M.H., Ha K.S., Kwon Y.G., et al. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vasc. Pharm. 2014;63:19–28. doi: 10.1016/j.vph.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Razban V., Lotfi A.S., Soleimani M., Ahmadi H., Massumi M., Khajeh S., Ghaedi M., Arjmand S., Najavand S., Khoshdel A. HIF-1α Overexpression Induces Angiogenesis in Mesenchymal Stem Cells. BioRes. Open Access. 2012;1:174–183. doi: 10.1089/biores.2012.9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranganath S.H., Levy O., Inamdar M.S., Karp J.M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu L., Gao J., Yuan Y., Chang Q., Liao Y., Lu F. Hypoxia preconditioned human adipose derived mesenchymal stem cells enhance angiogenic potential via secretion of increased VEGF and bFGF. Cell Biol. Int. 2013;37:551–560. doi: 10.1002/cbin.10097. [DOI] [PubMed] [Google Scholar]
- 36.El Agha E., Kramann R., Schneider R.K., Li X., Seeger W., Humphreys B.D., Bellusci S. Mesenchymal Stem Cells in Fibrotic Disease. Cell Stem Cell. 2017;21:166–177. doi: 10.1016/j.stem.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Rockel J.S., Rabani R., Viswanathan S. Anti-Fibrotic Mechanisms of Exogenously-Expanded Mesenchymal Stromal Cells for Fibrotic Diseases. Semin. Cell Dev. Biol. 2020;101:87–103. doi: 10.1016/j.semcdb.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Firestein G.S. Evolving Concepts of Rheumatoid Arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 39.Müller-Ladner U., Ospelt C., Gay S., Distler O., Pap T. Cells of the Synovium in Rheumatoid Arthritis. Synovial Fibroblasts. Arthritis Res. Ther. 2007;9:223. doi: 10.1186/ar2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Z., Shen Y., Oishi H., Matteson E.L., Tian L., Goronzy J.J., Weyand C.M. Restoring Oxidant Signaling Suppresses Proarthritogenic T Cell Effector Functions in Rheumatoid Arthritis. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aad7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luque-Campos N., Contreras-Lopez R.A., Jose Paredes-Martinez M., Torres M.J., Bahraoui S., Wei M., Espinoza F., Djouad F., Elizondo-Vega R.J., Luz-Crawford P. Mesenchymal Stem Cells Improve Rheumatoid Arthritis Progression by Controlling Memory T Cell Response. Front. Immunol. 2019;10:798. doi: 10.3389/fimmu.2019.00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehrenstein M.R., Evans J.G., Singh A., Moore S., Warnes G., Isenberg D.A., Mauri C. Compromised Function of Regulatory T Cells in Rheumatoid Arthritis and Reversal by Anti-TNFalpha Therapy. J. Exp. Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richardson S., Isaacs J. Novel Immunotherapies for Rheumatoid Arthritis. Clin. Med. 2013;13:391–394. doi: 10.7861/clinmedicine.13-4-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luz-Crawford P., Noël D., Fernandez X., Khoury M., Figueroa F., Carrión F., Jorgensen C., Djouad F. Mesenchymal Stem Cells Repress Th17 Molecular Program through the PD-1 Pathway. PLoS ONE. 2012;7:e45272. doi: 10.1371/journal.pone.0045272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luz-Crawford P., Tejedor G., Mausset-Bonnefont A.L., Beaulieu E., Morand E.F., Jorgensen C., Noel D., Djouad F. Glucocorticoid-induced leucine zipper governs the therapeutic potential of mesenchymal stem cells by inducing a switch from pathogenic to regulatory Th17 cells in a mouse model of collagen-induced arthritis. Arthritis Rheumatol. 2015;67:1514–1524. doi: 10.1002/art.39069. [DOI] [PubMed] [Google Scholar]
- 46.Luz-Crawford P., Jorgensen C., Djouad F. Macrophages. Vol. 62. Springer; Cham, Germany: 2017. Mesenchymal Stem Cells Direct the Immunological Fate of Macrophages; pp. 61–72. (Part of the Results and Problems in Cell Differentiation Book Series). [DOI] [PubMed] [Google Scholar]
- 47.Luz-Crawford P., Hernandez J., Djouad F., Luque-Campos N., Caicedo A., Carrère-Kremer S., Brondello J.M., Vignais M.L., Pène J., Jorgensen C. Mesenchymal Stem Cell Repression of Th17 Cells Is Triggered by Mitochondrial Transfer. Stem Cell Res. Ther. 2019;10:232. doi: 10.1186/s13287-019-1307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rozenberg A., Rezk A., Boivin M.N., Darlington P.J., Nyirenda M., Li R., Jalili F., Winer R., Artsy E.A., Uccelli A., et al. Human Mesenchymal Stem Cells Impact Th17 and Th1 Responses Through a Prostaglandin E2 and Myeloid-Dependent Mechanism. Stem Cells Transl. Med. 2016;5:1506–1514. doi: 10.5966/sctm.2015-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leyendecker A., Pinheiro C.C.G., Amano M.T., Bueno D.F. The Use of Human Mesenchymal Stem Cells as Therapeutic Agents for the In Vivo Treatment of Immune-Related Diseases: A Systematic Review. Front. Immunol. 2018;9:2056. doi: 10.3389/fimmu.2018.02056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X., Dong Y., Yin H., Qi Z., Wang D., Ren S. Mesenchymal Stem Cells Induced Regulatory Dendritic Cells from Hemopoietic Progenitor Cells through Notch Pathway and TGF-β Synergistically. Immunol. Lett. 2020;222:49–57. doi: 10.1016/j.imlet.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 51.Chen P., Huang Y., Womer K.L. Effects of Mesenchymal Stromal Cells on Human Myeloid Dendritic Cell Differentiation and Maturation in a Humanized Mouse Model. J. Immunol. Methods. 2015;427:100–104. doi: 10.1016/j.jim.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 52.Chen M., Peng J., Xie Q., Xiao N., Su X., Mei H., Lu Y., Zhou J., Dai Y., Wang S., et al. Mesenchymal Stem Cells Alleviate Moderate-to-Severe Psoriasis by Reducing the Production of Type I Interferon (IFN-I) by Plasmacytoid Dendritic Cells (pDCs) Stem Cells Int. 2019;2019:6961052. doi: 10.1155/2019/6961052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu Z., Chang W., Meng S., Xu X., Xie J., Guo F., Yang Y., Qiu H., Liu L. Mesenchymal Stem Cells Induce Dendritic Cell Immune Tolerance via Paracrine Hepatocyte Growth Factor to Alleviate Acute Lung Injury. Stem Cell Res. Ther. 2019;10:372. doi: 10.1186/s13287-019-1488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Favaro E., Carpanetto A., Caorsi C., Giovarelli M., Angelini C., Cavallo-Perin P., Tetta C., Camussi G., Zanone M.M. Human Mesenchymal Stem Cells and Derived Extracellular Vesicles Induce Regulatory Dendritic Cells in Type 1 Diabetic Patients. Diabetologia. 2016;59:325–333. doi: 10.1007/s00125-015-3808-0. [DOI] [PubMed] [Google Scholar]
- 55.He X., Dong Z., Cao Y., Wang H., Liu S., Liao L., Jin Y., Yuan L., Li B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019;2019:7132708. doi: 10.1155/2019/7132708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu F., Qiu H., Xue M., Zhang S., Zhang X., Xu J., Chen J., Yang Y., Xie J. MSC-secreted TGF-β Regulates Lipopolysaccharide-Stimulated Macrophage M2-like Polarization via the Akt/FoxO1 Pathway. Stem Cell Res. Ther. 2019;10:34. doi: 10.1186/s13287-019-1447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morrison T.J., Jackson M.V., Cunningham E.K., Kissenpfennig A., McAuley D.F., O’Kane C.M., Krasnodembskaya A.D. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am. J. Respir. Crit. Care Med. 2017;196:1275–1286. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akiyama K., Chen C., Wang D., Xu X., Qu C., Yamaza T., Cai T., Chen W., Sun L., Shi S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–555. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murphy N., Treacy O., Lynch K., Morcos M., Lohan P., Howard L., Fahy G., Griffin M.D., Ryan A.E., Ritter T. TNF-α/IL-1β-licensed Mesenchymal Stromal Cells Promote Corneal Allograft Survival via Myeloid Cell-Mediated Induction of Foxp3 + Regulatory T Cells in the Lung. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019;33:9404–9421. doi: 10.1096/fj.201900047R. [DOI] [PubMed] [Google Scholar]
- 60.Henao Agudelo J.S., Braga T.T., Amano M.T., Cenedeze M.A., Cavinato R.A., Peixoto-Santos A.R., Muscará M.N., Teixeira S.A., Cruz M.C., Castoldi A., et al. Mesenchymal Stromal Cell-Derived Microvesicles Regulate an Internal Pro-Inflammatory Program in Activated Macrophages. Front. Immunol. 2017;8:881. doi: 10.3389/fimmu.2017.00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu Y.G., Feng X.M., Abbott J., Fang X.H., Hao Q., Monsel A., Qu J.M., Matthay M.A., Lee J.W. Human Mesenchymal Stem Cell Microvesicles for Treatment of Escherichia Coli Endotoxin-Induced Acute Lung Injury in Mice. Stem Cells. 2014;32:116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jackson M.V., Morrison T.J., Doherty D.F., McAuley D.F., Matthay M.A., Kissenpfennig A., O’Kane C.M., Krasnodembskaya A.D. Mitochondrial Transfer via Tunneling Nanotubes Is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells. 2016;34:2210–2223. doi: 10.1002/stem.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu C.D., Kosaka Y., Marcus P., Rashedi I., Keating A. Differential Immunomodulatory Effects of Human Bone Marrow-Derived Mesenchymal Stromal Cells on Natural Killer Cells. Stem Cells Dev. 2019;28:933–943. doi: 10.1089/scd.2019.0059. [DOI] [PubMed] [Google Scholar]
- 64.Lu Y., Liu J., Liu Y., Qin Y., Luo Q., Wang Q., Duan H. TLR4 Plays a Crucial Role in MSC-induced Inhibition of NK Cell Function. Biochem. Biophys. Res. Commun. 2015;464:541–547. doi: 10.1016/j.bbrc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Blanco B., Herrero-Sánchez M.D., Rodríguez-Serrano C., García-Martínez M.L., Blanco J.F., Muntión S., García-Arranz M., Sánchez-Guijo F., Del Cañizo C. Immunomodulatory Effects of Bone Marrow versus Adipose Tissue-Derived Mesenchymal Stromal Cells on NK Cells: Implications in the Transplantation Setting. Eur. J. Haematol. 2016;97:528–537. doi: 10.1111/ejh.12765. [DOI] [PubMed] [Google Scholar]
- 66.Di Trapani M., Bassi G., Midolo M., Gatti A., Kamga P.T., Cassaro A., Carusone R., Adamo A., Krampera M. Differential and Transferable Modulatory Effects of Mesenchymal Stromal Cell-Derived Extracellular Vesicles on T, B and NK Cell Functions. Sci. Rep. 2016;6:24120. doi: 10.1038/srep24120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alves V.B.F., de Sousa B.C., Fonseca M.T.C., Ogata H., Caliári-Oliveira C., Yaochite J.N.U., Rodrigues Júnior V., Chica J.E.L., da Silva J.S., Malmegrim K.C.R., et al. A Single Administration of Human Adipose Tissue-Derived Mesenchymal Stromal Cells (MSC) Induces Durable and Sustained Long-Term Regulation of Inflammatory Response in Experimental Colitis. Clin. Exp. Immunol. 2019;196 doi: 10.1111/cei.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Hoeven V., Munneke J.M., Cornelissen A.S., Omar S.Z., Spruit M.J., Kleijer M., Bernink J.H., Blom B., Voermans C., Hazenberg M.D. Mesenchymal Stromal Cells Stimulate the Proliferation and IL-22 Production of Group 3 Innate Lymphoid Cells. J. Immunol. 2018;201:1165–1173. doi: 10.4049/jimmunol.1700901. [DOI] [PubMed] [Google Scholar]
- 69.Lu D., Ma T., Zhou X., Jiang Y., Han Y., Li H. B Lymphocytes Are the Target of Mesenchymal Stem Cells Immunoregulatory Effect in a Murine Graft-versus-Host Disease Model. Cell Transplant. 2019;28:1279–1288. doi: 10.1177/0963689719860127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luk F., Carreras-Planella L., Korevaar S.S., de Witte S.F.H., Borràs F.E., Betjes M.G.H., Baan C.C., Hoogduijn M.J., Franquesa M. Inflammatory Conditions Dictate the Effect of Mesenchymal Stem or Stromal Cells on B Cell Function. Front. Immunol. 2017;8:1042. doi: 10.3389/fimmu.2017.01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang H., Qi F., Dai X., Tian W., Liu T., Han H., Zhang B., Li H., Zhang Z., Du C. Requirement of B7-H1 in Mesenchymal Stem Cells for Immune Tolerance to Cardiac Allografts in Combination Therapy with Rapamycin. Transpl. Immunol. 2014;31:65–74. doi: 10.1016/j.trim.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 72.Cho K.A., Lee J.K., Kim Y.H., Park M., Woo S.Y., Ryu K.H. Mesenchymal stem cells ameliorate B-cell-mediated immune responses and increase IL-10-expressing regulatory B cells in an EBI3-dependent manner. Cell. Mol. Immunol. 2017;14:895–908. doi: 10.1038/cmi.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gupte K.S., Vanikar A.V., Trivedi H.L., Patel C.N., Patel J.V. In-Vitro Generation of Interleukin-10 Secreting B-Regulatory Cells From Donor Adipose Tissue Derived Mesenchymal Stem Cells and Recipient Peripheral Blood Mononuclear Cells for Potential Cell Therapy. Biomed. J. 2017;40:49–54. doi: 10.1016/j.bj.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Franquesa M., Mensah F.K., Huizinga R., Strini T., Boon L., Lombardo E., DelaRosa O., Laman J.D., Grinyó J.M., Weimar W., et al. Human Adipose Tissue-Derived Mesenchymal Stem Cells Abrogate Plasmablast Formation and Induce Regulatory B Cells Independently of T Helper Cells. Stem Cells. 2015;33:880–891. doi: 10.1002/stem.1881. [DOI] [PubMed] [Google Scholar]
- 75.Jang S.G., Lee J., Hong S.M., Kwok S.K., Cho M.L., Park S.H. Metformin Enhances the Immunomodulatory Potential of Adipose-Derived Mesenchymal Stem Cells through STAT1 in an Animal Model of Lupus. Rheumatology. 2020;59:1426–1438. doi: 10.1093/rheumatology/kez631. [DOI] [PubMed] [Google Scholar]
- 76.Kiernan C.H., Asmawidjaja P.S., Fahy N., Witte-Bouma J., Wolvius E.B., Brama P.A.J., Lubberts E., Farrell E., Physiol M. Allogeneic Chondrogenic Mesenchymal Stromal Cells alter Helper T Cell Subsets in CD4+ Memory T Cells. Tissue Eng. Part A. 2020;26:490–502. doi: 10.1089/ten.tea.2019.0177. [DOI] [PubMed] [Google Scholar]
- 77.Lee K., Park N., Jung H., Rim Y.A., Nam Y., Lee J., Park S.H., Ju J.H. Mesenchymal Stem Cells Ameliorate Experimental Arthritis via Expression of interleukin-1 Receptor Antagonist. PLoS ONE. 2018;13:e0193086. doi: 10.1371/journal.pone.0193086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pianta S., Bonassi Signoroni P., Muradore I., Rodrigues M.F., Rossi D., Silini A., Parolini O. Amniotic Membrane Mesenchymal Cells-Derived Factors Skew T Cell Polarization Toward Treg and Downregulate Th1 and Th17 Cells Subsets. Stem Cell Rev. Rep. 2015;11:394–407. doi: 10.1007/s12015-014-9558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao J., Chen J., Huang F., Wang J., Su W., Zhou J., Qi Q., Cao F., Sun B., Liu Z., et al. Human Gingiva Tissue-Derived MSC Ameliorates Immune-Mediated Bone Marrow Failure of Aplastic Anemia via Suppression of Th1 and Th17 Cells and Enhancement of CD4+Foxp3+ Regulatory T Cells Differentiation. Am. J. Transl. Res. 2019;11:7627–7643. [PMC free article] [PubMed] [Google Scholar]
- 80.Ng J., Hynes K., White G., Sivanathan K.N., Vandyke K., Bartold P.M., Gronthos S. Immunomodulatory Properties of Induced Pluripotent Stem Cell-Derived Mesenchymal Cells. J. Cell. Biochem. 2016;117:2844–2853. doi: 10.1002/jcb.25596. [DOI] [PubMed] [Google Scholar]
- 81.Cosenza S., Toupet K., Maumus M., Luz-Crawford P., Blanc-Brude O., Jorgensen C., Noël D. Mesenchymal Stem Cells-Derived Exosomes Are More Immunosuppressive Than Microparticles in Inflammatory Arthritis. Theranostics. 2018;8:1399–1410. doi: 10.7150/thno.21072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Del Fattore A., Luciano R., Pascucci L., Goffredo B.M., Giorda E., Scapaticci M., Fierabracci A., Muraca M. Immunoregulatory Effects of Mesenchymal Stem Cell-Derived Extracellular Vesicles on T Lymphocytes. Cell Transplant. 2015;24:2615–2627. doi: 10.3727/096368915X687543. [DOI] [PubMed] [Google Scholar]
- 83.Laso-García F., Ramos-Cejudo J., Carrillo-Salinas F., Otero-Ortega L., Feliú A., Gómez-de Frutos M., Mecha M., Díez-Tejedor E., Guaza C., Gutiérrez-Fernández M. Therapeutic Potential of Extracellular Vesicles Derived from Human Mesenchymal Stem Cells in a Model of Progressive Multiple Sclerosis. PLoS ONE. 2018;13:e0202590. doi: 10.1371/journal.pone.0202590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shigemoto-Kuroda T., Oh J.Y., Kim D., Jeong H.J., Park S.Y., Lee H.J., Park J.W., Kim T.W., An S.Y., Prockop D.J., et al. MSC-Derived Extracellular Vesicles Attenuate Immune Responses in Two Autoimmune Murine Models: Type 1 Diabetes and Uveoretinitis. Stem Cell Rep. 2017;8:1214–1225. doi: 10.1016/j.stemcr.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Court A.C., Le-Gatt A., Luz-Crawford P., Parra E., Aliaga-Tobar V., Bátiz L.F., Contreras R.A., Ortúzar M.I., Kurte M., Elizondo-Vega R., et al. Mitochondrial Transfer from MSCs to T Cells Induces Treg Differentiation and Restricts Inflammatory Response. EMBO Rep. 2020;21:e48052. doi: 10.15252/embr.201948052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bai L., Lennon D.P., Eaton V., Maier K., Caplan A.I., Miller S.D., Miller R.H. Human Bone Marrow-Derived Mesenchymal Stem Cells Induce Th2-polarized Immune Response and Promote Endogenous Repair in Animal Models of Multiple Sclerosis. Glia. 2009;57:1192–1203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hur J., Kang J.Y., Kim Y.K., Lee S.Y., Jeon S., Kim Y., Jung C.K., Rhee C.K. Evaluation of Human MSCs Treatment Frequency on Airway Inflammation in a Mouse Model of Acute Asthma. J. Korean Med. Sci. 2020;35:e188. doi: 10.3346/jkms.2020.35.e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fang S.B., Zhang H.Y., Meng X.C., Wang C., He B.X., Peng Y.Q., Xu Z.B., Fan X.L., Wu Z.J., Wu Z.C., et al. Small Extracellular Vesicles Derived from Human MSCs Prevent Allergic Airway Inflammation via Immunomodulation on Pulmonary Macrophages. Cell Death Dis. 2020;11:409. doi: 10.1038/s41419-020-2606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chan C.K., Lin T.C., Huang Y.A., Chen Y.S., Wu C.L., Lo H.Y., Kuo M.L., Wu K.H., Huang J.L. The Modulation of Th2 Immune Pathway in the Immunosuppressive Effect of Human Umbilical Cord Mesenchymal Stem Cells in a Murine Asthmatic Model. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2016;65:795–801. doi: 10.1007/s00011-016-0961-y. [DOI] [PubMed] [Google Scholar]
- 90.Patel S.A., Dave M.A., Bliss S.A., Giec-Ujda A.B., Bryan M., Pliner L.F., Rameshwar P. Treg/Th17 Polarization by Distinct Subsets of Breast Cancer Cells Is Dictated by the Interaction with Mesenchymal Stem Cells. J. Cancer Stem Cell Res. 2014;2014:e1003. doi: 10.14343/JCSCR.2014.2e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Geng L., Tang X., Wang S., Sun Y., Wang D., Tsao B.P., Feng X., Sun L. Reduced Let-7f in Bone Marrow-Derived Mesenchymal Stem Cells Triggers Treg/Th17 Imbalance in Patients with Systemic Lupus Erythematosus. Front. Immunol. 2020;11:233. doi: 10.3389/fimmu.2020.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cao Y., Jin X., Sun Y., Wen W. Therapeutic Effect of Mesenchymal Stem Cell on Hashimoto’s Thyroiditis in a Rat Model by Modulating Th17/Treg Cell Balance. Autoimmunity. 2020;53:35–45. doi: 10.1080/08916934.2019.1697689. [DOI] [PubMed] [Google Scholar]
- 93.Wang M., Chen B., Sun X.X., Zhao X.D., Zhao Y.Y., Sun L., Xu C.G., Shen B., Su Z.L., Xu W.R., et al. Gastric Cancer Tissue-Derived Mesenchymal Stem Cells Impact Peripheral Blood Mononuclear Cells via Disruption of Treg/Th17 Balance to Promote Gastric Cancer Progression. Exp. Cell Res. 2017;361:19–29. doi: 10.1016/j.yexcr.2017.09.036. [DOI] [PubMed] [Google Scholar]
- 94.Chen W., Huang Y., Han J., Yu L., Li Y., Lu Z., Li H., Liu Z., Shi C., Duan F., et al. Immunomodulatory Effects of Mesenchymal Stromal Cells-Derived Exosome. Immunol. Res. 2016;64:831–840. doi: 10.1007/s12026-016-8798-6. [DOI] [PubMed] [Google Scholar]
- 95.Monguió-Tortajada M., Roura S., Gálvez-Montón C., Pujal J.M., Aran G., Sanjurjo L., Franquesa M., Sarrias M.R., Bayes-Genis A., Borràs F.E. Nanosized UCMSC-Derived Extracellular Vesicles but Not Conditioned Medium Exclusively Inhibit the Inflammatory Response of Stimulated T Cells: Implications for Nanomedicine. Theranostics. 2017;7:270–284. doi: 10.7150/thno.16154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang D., Huang S., Yuan X., Liang J., Xu R., Yao G., Feng X., Sun L. The Regulation of the Treg/Th17 Balance by Mesenchymal Stem Cells in Human Systemic Lupus Erythematosus. Cell. Mol. Immunol. 2017;14:423–431. doi: 10.1038/cmi.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu M., Wang J., Fang Q., Liu P., Chen S., Zhe N., Lin X., Zhang Y., Zhao J., Zhou Z. High expression of heme oxygenase-1 in target organs may attenuate acute graft-versus-host disease through regulation of immune balance of TH17/Treg. Transpl. Immunol. 2016;37:10–17. doi: 10.1016/j.trim.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 98.Yang R., Yu T., Liu D., Shi S., Zhou Y. Hydrogen sulfide promotes immunomodulation of gingiva-derived mesenchymal stem cells via the Fas/FasL coupling pathway. Stem Cell Res. Ther. 2018;9:62. doi: 10.1186/s13287-018-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu T., Yan B., Li J., Zhang T., Yang R., Wang X., Liu Y., Liu D. Acetylsalicylic acid rescues the immunomodulation of inflamed gingiva-derived mesenchymal stem cells via upregulating FasL in mice. Stem Cell Res. Ther. 2019;10:368. doi: 10.1186/s13287-019-1485-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Azevedo R.I., Minskaia E., Fernandes-Platzgummer A., Vieira A.I.S., da Silva C.L., Cabral J.M.S., Lacerda J.F. Mesenchymal Stromal Cells Induce Regulatory T Cells via Epigenetic Conversion of Human Conventional CD4 T Cells In Vitro. Stem Cells. 2020 doi: 10.1002/stem.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Djouad F., Charbonnier L.M., Bouffi C., Louis-Plence P., Bony C., Apparailly F., Cantos C., Jorgensen C., Noel D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 102.Gao W.X., Sun Y.Q., Shi J., Li C.L., Fang S.B., Wang D., Deng X.Q., Wen W., Fu Q.L. Effects of Mesenchymal Stem Cells from Human Induced Pluripotent Stem Cells on Differentiation, Maturation, and Function of Dendritic Cells. Stem Cell Res. Ther. 2017;8:48. doi: 10.1186/s13287-017-0499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abumaree M.H., Al Jumah M.A., Kalionis B., Jawdat D., Al Khaldi A., Abomaray F.M., Fatani A.S., Chamley L.W., Knawy B.A. Human Placental Mesenchymal Stem Cells (pMSCs) Play a Role as Immune Suppressive Cells by Shifting Macrophage Differentiation from Inflammatory M1 to Anti-Inflammatory M2 Macrophages. Stem Cell Rev. Rep. 2013;9:620–641. doi: 10.1007/s12015-013-9455-2. [DOI] [PubMed] [Google Scholar]
- 104.Fan L., Hu C., Chen J., Cen P., Wang J., Li L. Interaction between Mesenchymal Stem Cells and B-Cells. Int. J. Mol. Sci. 2016;17:650. doi: 10.3390/ijms17050650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miossec P., Korn T., Kuchroo V.K. Interleukin-17 and Type 17 Helper T Cells. N. Engl. J. Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 106.Yasuda K., Takeuchi Y., Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019;41:283–297. doi: 10.1007/s00281-019-00733-8. [DOI] [PubMed] [Google Scholar]
- 107.Lee G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018;19:730. doi: 10.3390/ijms19030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ueno A., Jeffery L., Kobayashi T., Hibi T., Ghosh S., Jijon H. Th17 Plasticity and Its Relevance to Inflammatory Bowel Disease. J. Autoimmun. 2018;87:38–49. doi: 10.1016/j.jaut.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 109.Ren J., Li B. The Functional Stability of FOXP3 and RORγt in Treg and Th17 and Their Therapeutic Applications. Adv. Protein Chem. Struct. Biol. 2017;107:155–189. doi: 10.1016/bs.apcsb.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 110.Harris S.G., Padilla J., Koumas L., Ray D., Phipps R.P. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/S1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 111.Yao C., Sakata D., Esaki Y., Li Y., Matsuoka T., Kuroiwa K., Sugimoto Y., Narumiya S. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat. Med. 2009;15:633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 112.Boniface K., Bak-Jensen K.S., Li Y., Blumenschein W.M., McGeachy M.J., McClanahan T.K., McKenzie B.S., Kastelein R.A., Cua D.J., de Waal Malefyt R. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J. Exp. Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Duffy M.M., Pindjakova J., Hanley S.A., McCarthy C., Weidhofer G.A., Sweeney E.M., English K., Shaw G., Murphy J.M., Barry F.P., et al. Mesenchymal stem cell inhibition of T-helper 17 cell-differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur. J. Immunol. 2011;41:2840–2851. doi: 10.1002/eji.201141499. [DOI] [PubMed] [Google Scholar]
- 114.Kalinski P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vasilev G., Ivanova M., Ivanova-Todorova E., Tumangelova-Yuzeir K., Krasimirova E., Stoilov R., Kyurkchiev D. Secretory Factors Produced by Adipose Mesenchymal Stem Cells Downregulate Th17 and Increase Treg Cells in Peripheral Blood Mononuclear Cells from Rheumatoid Arthritis Patients. Rheumatol. Int. 2019;39:819–826. doi: 10.1007/s00296-019-04296-7. [DOI] [PubMed] [Google Scholar]
- 116.Qu X., Liu X., Cheng K., Yang R., Zhao R.C. Mesenchymal Stem Cells Inhibit Th17 Cell Differentiation by IL-10 Secretion. Exp. Hematol. 2012;40:761–770. doi: 10.1016/j.exphem.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 117.Najar M., Lombard C.A., Fayyad-Kazan H., Faour W.H., Merimi M., Sokal E.M., Lagneaux L., Fahmi H. Th17 Immune Response to Adipose Tissue-Derived Mesenchymal Stromal Cells. J. Cell. Physiol. 2019;234:21145–21152. doi: 10.1002/jcp.28717. [DOI] [PubMed] [Google Scholar]
- 118.Svobodova E., Krulova M., Zajicova A., Pokorna K., Prochazkova J., Trosan P., Holan V. The Role of Mouse Mesenchymal Stem Cells in Differentiation of Naive T-cells into Anti-Inflammatory Regulatory T-cell or Proinflammatory Helper T-cell 17 Population. Stem Cells Dev. 2012;21:901–910. doi: 10.1089/scd.2011.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li C.L., Leng Y., Zhao B., Gao C., Du F.F., Jin N., Lian Q.Z., Xu S.Y., Yan G.L., Xia J.J., et al. Human iPSC-MSC-Derived Xenografts Modulate Immune Responses by Inhibiting the Cleavage of Caspases. Stem Cells. 2017;35:1719–1732. doi: 10.1002/stem.2638. [DOI] [PubMed] [Google Scholar]
- 120.Bouffi C., Bony C., Courties G., Jorgensen C., Noël D. IL-6-Dependent PGE2 Secretion by Mesenchymal Stem Cells Inhibits Local Inflammation in Experimental Arthritis. PLoS ONE. 2010;5:e14247. doi: 10.1371/journal.pone.0014247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Harrell C.R., Markovic B.S., Fellabaum C., Arsenijevic N., Djonov V., Volarevic V. The Role of Interleukin 1 Receptor Antagonist in Mesenchymal Stem Cell-Based Tissue Repair and Regeneration. BioFactors. 2020;46:263–275. doi: 10.1002/biof.1587. [DOI] [PubMed] [Google Scholar]
- 122.Ren G., Su J., Zhang L., Zhao X., Ling W., L’Huillie A., Zhang J., Lu Y., Roberts A.I., Ji W., et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 123.Su J., Chen X., Huang Y., Li W., Li J., Cao K., Cao G., Zhang L., Li F., Roberts A.I., et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 2014;21:388–396. doi: 10.1038/cdd.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Volarevic V., Markovic B.S., Jankovic M.G., Djokovic B., Jovicic N., Harrell C.R., Fellabaum C., Djonov V., Arsenijevic N., Lukic M.L. Galectin 3 Protects from Cisplatin-Induced Acute Kidney Injury by Promoting TLR-2-Dependent Activation of IDO1/Kynurenine Pathway in Renal DCs. Theranostics. 2019;9:5976–6001. doi: 10.7150/thno.33959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang Y., Liu K., Chen Y., Gong Y., Liang Y. Indoleamine 2,3-Dioxygenase (IDO) Regulates Th17/Treg Immunity in Experimental IgA Nephropathy. Folia Biol. 2019;65:101–108. [PubMed] [Google Scholar]
- 126.Routy J.P., Routy B., Graziani G.M., Mehraj V. The Kynurenine Pathway Is a Double-Edged Sword in Immune-Privileged Sites and in Cancer: Implications for Immunotherapy. Int. J. Tryptophan Res. 2016;9:67–77. doi: 10.4137/IJTR.S38355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Milosavljevic N., Gazdic M., Simovic Markovic B., Arsenijevic A., Nurkovic J., Dolicanin Z., Jovicic N., Jeftic I., Djonov V., Arsenijevic N., et al. Mesenchymal stem cells attenuate liver fibrosis by suppressing Th17 cells–An experimental study. Transpl. Int. 2018;31:102–115. doi: 10.1111/tri.13023. [DOI] [PubMed] [Google Scholar]
- 128.Grochot-Przeczek A., Dulak J., Jozkowicz A. Haem oxygenase-1: Non-canonical roles in physiology and pathology. Clin. Sci. 2012;122:93–103. doi: 10.1042/CS20110147. [DOI] [PubMed] [Google Scholar]
- 129.Shen Z.Y., Wu B., Liu T., Yang Y., Yin M.L., Zheng W.P., Zhang B.Y., Song H.L. Immunomodulatory Effects of Bone Marrow Mesenchymal Stem Cells Overexpressing Heme Oxygenase-1: Protective Effects on Acute Rejection Following Reduced-Size Liver Transplantation in a Rat Model. Cell. Immunol. 2017;313:10–24. doi: 10.1016/j.cellimm.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 130.Langrzyk A., Nowak W.N., Stępniewski J., Jaźwa A., Florczyk-Soluch U., Józkowicz A., Dulak J. Critical View on Mesenchymal Stromal Cells in Regenerative Medicine. Antioxid. Redox Signal. 2018;29:169–190. doi: 10.1089/ars.2017.7159. [DOI] [PubMed] [Google Scholar]
- 131.Ren G., Zhang L., Zhao X., Xu G., Zhang Y., Roberts A.I., Zhao R.C., Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 132.Niedbala W., Cai B., Liew F.Y. Role of Nitric Oxide in the Regulation of T Cell Functions. Ann. Rheum. Dis. 2006;65(Suppl. 3):iii37–iii40. doi: 10.1136/ard.2006.058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sato K., Ozaki K., Oh I., Meguro A., Hatanaka K., Nagai T., Muroi K., Ozawa K. Nitric Oxide Plays a Critical Role in Suppression of T-cell Proliferation by Mesenchymal Stem Cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 134.Ge Y., Zhang Y., Tang Q., Gao J., Yang H., Gao Z., Zhao R.C. Mechanisms of the Immunomodulation Effects of Bone Marrow-Derived Mesenchymal Stem Cells on Facial Nerve Injury in Sprague-Dawley Rats. Stem Cells Dev. 2019;28:489–496. doi: 10.1089/scd.2018.0104. [DOI] [PubMed] [Google Scholar]
- 135.Chen Q.H., Wu F., Liu L., Chen H.B., Zheng R.Q., Wang H.L., Yu L.N. Mesenchymal Stem Cells Regulate the Th17/Treg Cell Balance Partly through Hepatocyte Growth Factor In Vitro. Stem Cell Res. Ther. 2020;11:91. doi: 10.1186/s13287-020-01612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cao X.P., Han D.M., Zhao L., Guo Z.K., Xiao F.J., Zhang Y.K., Zhang X.Y., Wang L.S., Wang H.X., Wang H. Hepatocyte Growth Factor Enhances the Inflammation-Alleviating Effect of Umbilical Cord-Derived Mesenchymal Stromal Cells in a Bronchiolitis Obliterans Model. Cytotherapy. 2016;18:402–412. doi: 10.1016/j.jcyt.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 137.Hübel J., Hieronymus T. HGF/Met-Signaling Contributes to Immune Regulation by Modulating Tolerogenic and Motogenic Properties of Dendritic Cells. Biomedicines. 2015;3:138–148. doi: 10.3390/biomedicines3010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim J.Y., Park M., Kim Y.H., Ryu K.H., Lee K.H., Cho K.A., Woo S.Y. Tonsil-derived mesenchymal stem cells (T-MSCs) prevent Th17-mediated autoimmune response via regulation of the programmed death-1/programmed death ligand-1 (PD-1/PD-L1) pathway. J. Tissue Eng. Regen. Med. 2018;12:e1022–e1033. doi: 10.1002/term.2423. [DOI] [PubMed] [Google Scholar]
- 140.Luz-Crawford P., Djouad F., Toupet K., Bony C., Franquesa M., Hoogduijn M.J., Jorgensen C., Noël D. Mesenchymal Stem Cell-Derived Interleukin 1 Receptor Antagonist Promotes Macrophage Polarization and Inhibits B Cell Differentiation. Stem Cells. 2016;34:483–492. doi: 10.1002/stem.2254. [DOI] [PubMed] [Google Scholar]
- 141.Ren G., Zhao X., Zhang L., Zhang J., L’Huillier A., Ling W., Roberts A.I., Le A.D., Shi S., Shao C., et al. Inflammatory Cytokine-Induced Intercellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule-1 in Mesenchymal Stem Cells Are Critical for Immunosuppression. J. Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Colombo M., Raposo G., Théry C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 143.L Ramos T., Sánchez-Abarca L.I., Muntión S., Preciado S., Puig N., López-Ruano G., Hernández-Hernández Á., Redondo A., Ortega R., Rodríguez C., et al. MSC Surface Markers (CD44, CD73, and CD90) Can Identify Human MSC-derived Extracellular Vesicles by Conventional Flow Cytometry. Cell Commun. Signal. 2016;14:2. doi: 10.1186/s12964-015-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Quesenberry P.J., Goldberg L.R., Aliotta J.M., Dooner M.S., Pereira M.G., Wen S., Camussi G. Cellular Phenotype and Extracellular Vesicles: Basic and Clinical Considerations. Stem Cells Dev. 2014;23:1429–1436. doi: 10.1089/scd.2013.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Montecalvo A., Larregina A.T., Shufesky W.J., Stolz D.B., Sullivan M.L., Karlsson J.M., Baty C.J., Gibson G.A., Erdos G., Wang Z., et al. Mechanism of Transfer of Functional microRNAs between Mouse Dendritic Cells via Exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hidalgo-Garcia L., Galvez J., Rodriguez-Cabezas M.E., Anderson P.O. Can a Conversation between Mesenchymal Stromal Cells and Macrophages Solve the Crisis in the Inflamed Intestine? Front. Pharmacol. 2018;9:179. doi: 10.3389/fphar.2018.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rad F., Ghorbani M., Mohammadi Roushandeh A., Habibi Roudkenar M. Mesenchymal Stem Cell-Based Therapy for Autoimmune Diseases: Emerging Roles of Extracellular Vesicles. Mol. Biol. Rep. 2019;46:1533–1549. doi: 10.1007/s11033-019-04588-y. [DOI] [PubMed] [Google Scholar]
- 148.Zheng Y., Dong C., Yang J., Jin Y., Zheng W., Zhou Q., Liang Y., Bao L., Feng G., Ji J., et al. Exosomal microRNA-155-5p from PDLSCs Regulated Th17/Treg Balance by Targeting Sirtuin-1 in Chronic Periodontitis. J. Cell. Physiol. 2019;234:20662–20674. doi: 10.1002/jcp.28671. [DOI] [PubMed] [Google Scholar]
- 149.Song N., Zhang T., Xu X., Lu Z., Yu X., Fang Y., Hu J., Jia P., Teng J., Ding X. miR-21 Protects against Ischemia/Reperfusion-Induced Acute Kidney Injury by Preventing Epithelial Cell Apoptosis and Inhibiting Dendritic Cell Maturation. Front. Physiol. 2018;9:790. doi: 10.3389/fphys.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gu D., Zou X., Ju G., Zhang G., Bao E., Zhu Y. Mesenchymal Stromal Cells Derived Extracellular Vesicles Ameliorate Acute Renal Ischemia Reperfusion Injury by Inhibition of Mitochondrial Fission through miR-30. Stem Cells Int. 2016;2016:2093940. doi: 10.1155/2016/2093940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Harrell C.R., Fellabaum C., Jovicic N., Djonov V., Arsenijevic N., Volarevic V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells. 2019;8:467. doi: 10.3390/cells8050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kapnick S.M., Pacheco S.E., McGuire P.J. The Emerging Role of Immune Dysfunction in Mitochondrial Diseases as a Paradigm for Understanding Immunometabolism. Metab. Clin. Exp. 2018;81:97–112. doi: 10.1016/j.metabol.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Torralba D., Baixauli F., Sánchez-Madrid F. Mitochondria Know No Boundaries: Mechanisms and Functions of Intercellular Mitochondrial Transfer. Front. Cell Dev. Biol. 2016;4:107. doi: 10.3389/fcell.2016.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Berridge M.V., McConnell M.J., Grasso C., Bajzikova M., Kovarova J., Neuzil J. Horizontal Transfer of Mitochondria between Mammalian Cells: Beyond Co-Culture Approaches. Curr. Opin. Genet. Dev. 2016;38:75–82. doi: 10.1016/j.gde.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 155.Fan X.L., Zhang Y., Li X., Fu Q.L. Mechanisms Underlying the Protective Effects of Mesenchymal Stem Cell-Based Therapy. Cell. Mol. Life Sci. 2020;77:2771–2794. doi: 10.1007/s00018-020-03454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang J., Liu X., Qiu Y., Shi Y., Cai J., Wang B., Wei X., Ke Q., Sui X., Wang Y., et al. Cell Adhesion-Mediated Mitochondria Transfer Contributes to Mesenchymal Stem Cell-Induced Chemoresistance on T Cell Acute Lymphoblastic Leukemia Cells. J. Hematol. Oncol. 2018;11:11. doi: 10.1186/s13045-018-0554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ahmad T., Mukherjee S., Pattnaik B., Kumar M., Singh S., Kumar M., Rehman R., Tiwari B.K., Jha K.A., Barhanpurkar A.P., et al. Miro1 Regulates Intercellular Mitochondrial Transport & Enhances Mesenchymal Stem Cell Rescue Efficacy. EMBO J. 2014;33:994–1010. doi: 10.1002/embj.201386030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim M.J., Hwang J.W., Yun C.K., Lee Y., Choi Y.S. Delivery of Exogenous Mitochondria via Centrifugation Enhances Cellular Metabolic Function. Sci. Rep. 2018;8:3330. doi: 10.1038/s41598-018-21539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Konari N., Nagaishi K., Kikuchi S., Fujimiya M. Mitochondria Transfer from Mesenchymal Stem Cells Structurally and Functionally Repairs Renal Proximal Tubular Epithelial Cells in Diabetic Nephropathy In Vivo. Sci. Rep. 2019;9:5184. doi: 10.1038/s41598-019-40163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Islam M.N., Das S.R., Emin M.T., Wei M., Sun L., Westphalen K., Rowlands D.J., Quadri S.K., Bhattacharya S., Bhattacharya J. Mitochondrial Transfer from Bone-Marrow-Derived Stromal Cells to Pulmonary Alveoli Protects against Acute Lung Injury. Nat. Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jiang D., Gao F., Zhang Y., Wong D.S., Li Q., Tse H.F., Xu G., Yu Z., Lian Q. Mitochondrial Transfer of Mesenchymal Stem Cells Effectively Protects Corneal Epithelial Cells From Mitochondrial Damage. Cell Death Dis. 2016;7:e2467. doi: 10.1038/cddis.2016.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Luz-Crawford P., Torres M.J., Noël D., Fernandez A., Toupet K., Alcayaga-Miranda F., Tejedor G., Jorgensen C., Illanes S.E., Figueroa F.E., et al. The Immunosuppressive Signature of Menstrual Blood Mesenchymal Stem Cells Entails Opposite Effects on Experimental Arthritis and Graft versus Host Diseases. Stem Cells. 2016;34:456–469. doi: 10.1002/stem.2244. [DOI] [PubMed] [Google Scholar]
- 163.Noronha N.C., Mizukami A., Caliári-Oliveira C., Cominal J.G., Rocha J.L.M., Covas D.T., Swiech K., Malmegrim K.C.R. Priming Approaches to Improve the Efficacy of Mesenchymal Stromal Cell-Based Therapies. Stem Cell Res. Ther. 2019;10:131. doi: 10.1186/s13287-019-1224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang Q., Yang Q., Wang Z., Tong H., Ma L., Zhang Y., Shan F., Meng Y., Yuan Z. Comparative Analysis of Human Mesenchymal Stem Cells from Fetal-Bone Marrow, Adipose Tissue, and Warton’s Jelly as Sources of Cell Immunomodulatory Therapy. Hum. Vaccines Immunother. 2016;12:85–96. doi: 10.1080/21645515.2015.1030549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Carrero R., Cerrada I., Lledó E., Dopazo J., García-García F., Rubio M.P., Trigueros C., Dorronsoro A., Ruiz-Sauri A., Montero J.A., et al. IL1β Induces Mesenchymal Stem Cells Migration and Leucocyte Chemotaxis Through NF-κB. Stem Cell Rev. Rep. 2012;8:905–916. doi: 10.1007/s12015-012-9364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fan H., Zhao G., Liu L., Liu F., Gong W., Liu X., Yang L., Wang J., Hou Y. Pre-treatment with IL-1β Enhances the Efficacy of MSC Transplantation in DSS-induced Colitis. Cell. Mol. Immunol. 2012;9:473–481. doi: 10.1038/cmi.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Han X., Yang Q., Lin L., Xu C., Zheng C., Chen X., Han Y., Li M., Cao W., Cao K., et al. Interleukin-17 Enhances Immunosuppression by Mesenchymal Stem Cells. Cell Death Differ. 2014;21:1758–1768. doi: 10.1038/cdd.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kurte M., Luz-Crawford P., Vega-Letter A., Contreras R., Tejedor G., Elizondo-Vega R., Martinez-Viola L., Fernández-O’Ryan C., Figueroa F., Jorgensen C., et al. IL17/IL17RA As a Novel Signaling Axis Driving Mesenchymal Stem Cell Therapeutic Function in Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2018;9:802. doi: 10.3389/fimmu.2018.00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sivanathan K.N., Rojas-Canales D.M., Hope C.M., Krishnan R., Carroll R.P., Gronthos S., Grey S.T., Coates P.T. Interleukin-17A-Induced Human Mesenchymal Stem Cells Are Superior Modulators of Immunological Function. Stem Cells. 2015;33:2850–2863. doi: 10.1002/stem.2075. [DOI] [PubMed] [Google Scholar]
- 170.Zimmermann J.A., McDevitt T.C. Pre-Conditioning Mesenchymal Stromal Cell Spheroids for Immunomodulatory Paracrine Factor Secretion. Cytotherapy. 2014;16:331–345. doi: 10.1016/j.jcyt.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 171.Spencer J.A., Ferraro F., Roussakis E., Klein A., Wu J., Runnels J.M., Zaher W., Mortensen L.J., Alt C., Turcotte R., et al. Direct Measurement of Local Oxygen Concentration in the Bone Marrow of Live Animals. Nature. 2014;508:269–273. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Saparov A., Ogay V., Nurgozhin T., Jumabay M., Chen W.C. Preconditioning of Human Mesenchymal Stem Cells to Enhance Their Regulation of the Immune Response. Stem Cells Int. 2016;2016:3924858. doi: 10.1155/2016/3924858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Haque N., Rahman M.T., Abu Kasim N.H., Alabsi A.M. Hypoxic Culture Conditions As a Solution for Mesenchymal Stem Cell Based Regenerative Therapy. Sci. World J. 2013;2013:632972. doi: 10.1155/2013/632972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Contreras-Lopez R., Elizondo-Vega R., Paredes M.J., Luque-Campos N., Torres M.J., Tejedor G., Vega-Letter A.M., Figueroa-Valdés A., Pradenas C., Oyarce K., et al. HIF1α-Dependent Metabolic Reprogramming Governs Mesenchymal Stem/Stromal Cell Immunoregulatory Functions. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020;34:8250–8264. doi: 10.1096/fj.201902232R. [DOI] [PubMed] [Google Scholar]
- 175.Grimaldi P.A. Metabolic and Nonmetabolic Regulatory Functions of Peroxisome Proliferator-Activated Receptor Beta. Curr. Opin. Lipidol. 2010;21:186–191. doi: 10.1097/MOL.0b013e32833884a4. [DOI] [PubMed] [Google Scholar]
- 176.Palomer X., Barroso E., Pizarro-Delgado J., Peña L., Botteri G., Zarei M., Aguilar D., Montori-Grau M., Vázquez-Carrera M. PPARβ/δ: A Key Therapeutic Target in Metabolic Disorders. Int. J. Mol. Sci. 2018;19:913. doi: 10.3390/ijms19030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Luz-Crawford P., Ipseiz N., Espinosa-Carrasco G., Caicedo A., Tejedor G., Toupet K., Loriau J., Scholtysek C., Stoll C., Khoury M., et al. PPARβ/δ Directs the Therapeutic Potential of Mesenchymal Stem Cells in Arthritis. Ann. Rheum. Dis. 2016;75:2166–2174. doi: 10.1136/annrheumdis-2015-208696. [DOI] [PubMed] [Google Scholar]
- 178.Luz-Crawford P., Espinosa-Carrasco G., Ipseiz N., Contreras R., Tejedor G., Medina D.A., Vega-Letter A.M., Ngo D., Morand E.F., Pène J., et al. Gilz-Activin A As a Novel Signaling Axis Orchestrating Mesenchymal Stem Cell and Th17 Cell Interplay. Theranostics. 2018;8:846–859. doi: 10.7150/thno.21793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Palkar P.S., Borland M.G., Naruhn S., Ferry C.H., Lee C., Sk U.H., Sharma A.K., Amin S., Murray I.A., Anderson C.R., et al. Cellular and Pharmacological Selectivity of the Peroxisome Proliferator-Activated Receptor-β/δ Antagonist GSK3787. Mol. Pharmacol. 2010;78:419–430. doi: 10.1124/mol.110.065508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hong F., Xu P., Zhai Y. The Opportunities and Challenges of Peroxisome Proliferator-Activated Receptors Ligands in Clinical Drug Discovery and Development. Int. J. Mol. Sci. 2018;19:2189. doi: 10.3390/ijms19082189. [DOI] [PMC free article] [PubMed] [Google Scholar]