Abstract
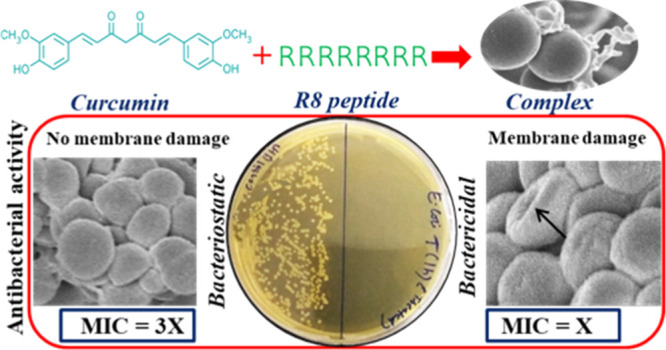
Bacterial resistance to antimicrobial drugs is one of the biggest threats to human health and novel drugs, and strategies are needed to obviate this resistance crisis. An innovative strategy for designing novel antimicrobial drugs is based on the hybridization of an antimicrobial agent with a second functional entity. Here, we use a cell-penetrating peptide–octaarginine (R8) as the second functional entity and develop a complex or hybrid of R8 and curcumin that possibly targets the bacterial cell membrane. Minimum inhibitory concentration assays show that the antibacterial activity of the complex is enhanced in a synergistic manner and rapid killing kinetics are obtained, emphasizing a bactericidal mode of action. In addition, electron microscopy images reveal bacterial membrane disruption by the complex. The R8–curcumin complex also displays activity against HeLa cells.
Introduction
Bacterial infections are ever-growing serious and global concern causing devastating health effects. Some of the infections caused by Escherichia coli (E. coli) are peritonitis, cholecystitis, appendicitis,1 life-threatening septicemia, and meningitis, while Staphylococcus aureus (S. aureus) is a causative agent of infections related to skin and soft tissue, osteoarticular, bacteremia, endocarditis, pleuropulmonary, and device-related infections.2 As we approach the post-antibiotic era where most of the existing antibiotics fail against drug-resistant bacteria, there is a high need for search and development of novel and alternative antimicrobials. The new drug discovery is a lengthy and costly process, and hence, novel strategies that improve the efficacy of presently available drugs may be more suitable. A combinatorial-based approach is now being increasingly applied to treat bacterial infections.3,4
Curcumin is a polyphenolic compound extracted from the rhizome of Curcuma longa, an ancient spice turmeric, and is one of the very few promising natural antimicrobials of plant origin.5 Curcumin possesses broad pharmacological activities such as anticancer,6,7 antioxidant, chemopreventive, antialzheimer, antiparasitic, anti-inflammatory, and antibacterial activities.8−10 The antibacterial activity of curcumin and its mode of action have been studied in different forms and formulations, and it has been seen that they depend on the delivery system, purity of curcumin, and bacterial strains used.11−14 Despite its multitude biological properties and safety, its clinical application is limited by its hydrophobic nature, leading to extremely low aqueous solubility and poor bioavailability.15 However, the versatility of curcumin deserves attempts to make it clinically viable.
Cell-penetrating peptides (CPPs) have been successfully used as vectors for efficient transportation of low bioavailable drugs into cells.16 CPPs are a class of small peptides with a few to 30 amino acids that show membrane translocation activities. Because of their intrinsic ability to gain access to cell interiors, they have been successfully used for intracellular delivery of various membrane-impermeable bioactive agents.17,18 Based on different physicochemical properties, there are many types of CPPs; however, amongst them, arginine-rich CPPs including the human immunodeficiency virus type 1 (HIV-1) TAT peptide and oligoarginines are extensively studied and employed.19 They have been used in conjugation with antimicrobial peptides,20 standard antibiotics,21 and nanoparticles22 for antimicrobial therapy. CPPs have also been used to deliver antibacterial agents to target intracellular bacteria which are otherwise inaccessible by well-known antibiotics such as Gentamicin and Paromomycin.23,24 They have found application in the inhibition of drug-resistant bacteria as well as persister cells.25−27 The current understanding is that CPP plays the role of cell translocation and takes the antibacterial drug to the cell interior to show the antibacterial action. The mechanism of action of combinatorial drug strategy with peptides is not fully understood. Chemical conjugation or complex formation of CPPs with biopharmaceuticals is the approach being used for the delivery of biofunctional molecules.28
In the present study, we have prepared a complex of curcumin with octaarginine (a CPP) and studied its antibacterial activity against both Gram-positive and Gram-negative bacteria. The results show that complexation enhances the antibacterial activity of curcumin. The complex appears to target bacterial cell membranes, leading to rupture. It is also known that an interconnection exists between anticancer and antibacterial activities of various drugs.29−31 Furthermore, curcumin has been explored for anticancer effects in combination with other drugs,32 nanoparticles,33,34 and polymeric carriers.35,36 On the other hand, CPPs have been extensively used for anticancer drug delivery;37 therefore, we also study the anticancer activity of the peptide-modified curcumin against HeLa cells. The results show that the attachment of water-soluble octaarginine peptides enhances the aqueous dispersibility and bioactivity of curcumin by enhanced interactions with the cellular target.
Results and Discussion
Curcumin is noncovalently conjugated with peptides by dropwise mixing and stirring the ethanolic curcumin and aqueous peptide solutions, in different mole ratios. All of the peptide–curcumin complexes have 1 mg of curcumin in it and varying amounts of the peptide, and the complexation method is described in detail in the materials and methods.
Physicochemical Characterization
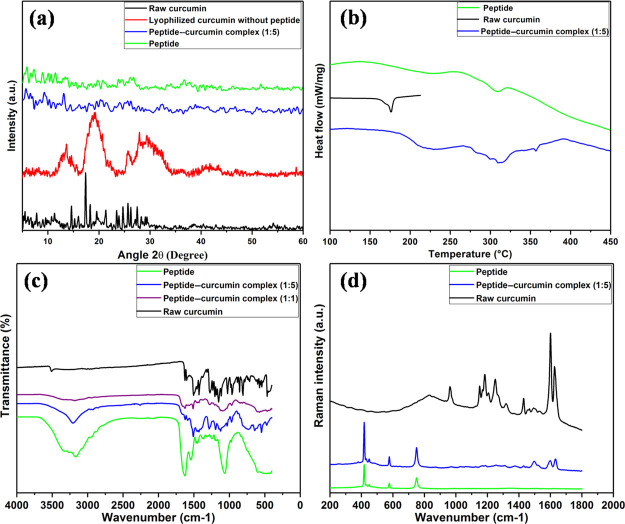
The crystallinity of curcumin in the peptide–curcumin complex is assessed by X-ray diffraction (XRD) analysis (Figure 1a). Raw curcumin exhibits characteristic crystalline XRD peaks consistent with earlier observations.38,39 Curcumin lyophilized from a water–ethanol system in the absence of octaarginine shows broad peaks; however, complexation with peptides results in the absence of peaks, indicating that the modification by the peptide inhibits the crystallization of curcumin. This suggests that the modification of curcumin by octaarginine peptide enhances the aqueous solubility as previous reports have shown that an amorphous nature leads to better solubility.9 The differential scanning calorimetry (DSC) data for raw curcumin show a sharp endothermic peak at 176 °C (Figure 1b) which corresponds to its melting point.40 The pure peptide shows a shallow peak around 300 °C owing to its thermal decomposition. In the DSC curve of the peptide–curcumin complex, there is no curcumin characteristic peak and it follows a trend similar to that of the pure peptide. This shows that complex formation with amorphous peptide results in complete disappearance of the endothermic peak of the unmodified curcumin, indicating that curcumin is dispersed in the matrix of peptide and is in an amorphous form which supports the observation from the powder XRD studies.
Figure 1.
Physicochemical characterizations of curcumin, peptide, and peptide–curcumin complexes. (a) XRD spectra, (b) DSC thermogram, (c) FTIR spectra, and (d) Raman spectra.
Figure 1c shows the Fourier transform infrared (FTIR) spectra for raw curcumin, bare peptide, and peptide–curcumin complex in ratios 1:1 and 1:5 (P/C). Curcumin shows a sharp FTIR peak at 3515 cm–1, indicating the presence of OH. The sharp band at 1628 cm–1 presents a predominantly mixed (C=C) and (C=O) character. The peak at 1603 cm–1 corresponds to the symmetric stretching vibrations of the aromatic ring (C=C ring). The 1506 cm–1 peak is assigned to the (C=O) whereas the 1276 cm–1 peak is assigned to the enol C–O. Bands at 1159, 857, and 810 cm–1 arise because of the bending of aromatic rings (CCH) and the inter-ring chain of curcumin. The band at 1027 cm–1 is assigned to the C–O–C peak, whereas trans-CH vibration and cis-CH vibration are obtained at 963 and 716 cm–1.41 For peptides, the band obtained at 1650 cm–1 accounts for amide I vibrations, amide II region corresponds to 1550 cm–1, and amide III vibrations produce a major peak at 1242 cm–1. N–H stretching vibrations (amide A and amide B) show a broad peak at 3270 cm–1. In the peptide–curcumin (pep–cur) complex, the sharp OH peak of curcumin disappears, and the amine peak of peptide gets broadened. This suggests the presence of hydrogen bonding between the alcohol group of the curcumin and the amine group of the peptide. The magnitude of peak broadening increases with a higher ratio of the peptide with respect to curcumin, indicating more of hydrogen bonding interactions, as visible in the spectrum for peptide–curcumin complex (1:1). Figure 1d represents the Raman intensities for raw curcumin, peptide, and peptide–curcumin complex of ratio 1:5. Curcumin presents significant Raman peaks at 1601 cm–1 corresponding to C–C ring stretching vibrations, 1630 cm–1 is due to carbonyl C=O stretch, 1429 cm–1 corresponds to phenol C–O vibrations, and 1250 cm–1 comes from enol C–O stretch.42 For octaarginine peptides, the CNH stretch peak comes at 752 cm–1. In the Raman spectrum of the peptide–curcumin complex, the intensity of the peaks at 1601, 1630, and 1429 cm–1 decreases and shifts indicating the involvement of the aromatic ring and phenolic OH with the peptide and supports the observation by FTIR study.
UV–Visible Spectroscopy and Aqueous Stability of the Peptide–Curcumin Complex
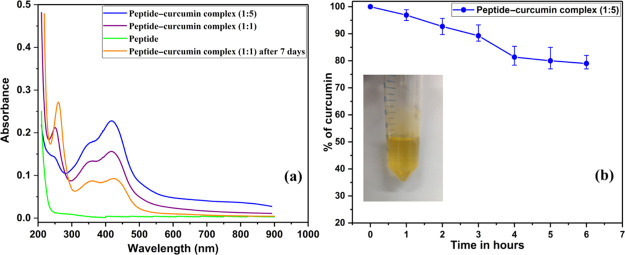
UV–visible spectroscopy has been very effectively utilized to characterize the interaction forces within curcumin–metal,43,44 curcumin–protein,45 and curcumin–nanoparticles33 complexes. Therefore, we measured the UV–vis absorption spectra of peptide–curcumin complexes by dissolving the lyophilized complex in Milli-Q water. Figure 2a shows the absorption spectra, where intensity at 425 nm, a curcumin characteristic band, increases with an increasing fraction of curcumin in the complex, consistent with previous reports.9,46 The absorbance of the peptide–curcumin complex of ratio 1:5 is higher than that of 1:1 complex, and absorbance decreases predictably on allowing intentional precipitation of curcumin from the solution, for a period of 7 days as seen in Figure 2a. Peptide-only solution does not show any absorption peak around 425 nm; it absorbs at 210 nm because of backbone peptide bonds. These changes, taken together, point to a strong association between peptide and curcumin molecules. The absorption spectral features of the peptide–curcumin complex indicate the presence of a monoanionic enol form of curcumin.47 This observation and the presence of a positively charged peptide support the role of electrostatic interaction between the peptide and curcumin along with hydrogen bonding. The role of enolization of curcumin and electrostatic interactions with different systems has been highlighted by Gupta et al.,48 and Banerjee and Chakravarty show that monoanionic curcumin retains its therapeutic potential even in its bound state.49
Figure 2.
UV–visible spectrophotometry of peptide–curcumin complexes (a) and aqueous stability of peptide–curcumin complex (1:5) in PBS (b); the inset shows the complex (1:5) dissolved in PBS.
The aqueous solubility of the peptide–curcumin complex is studied by a method described by Mohanty and Sahoo50 with some modifications. We dissolved the peptide–curcumin complex in 0.01 M, pH 7.4 phosphate buffer saline (PBS), at a concentration of 40 μg/mL and incubated at 37 °C, its UV absorbance at 425 nm at specific time points is taken, and percentage of curcumin in solution is represented in Figure 2b. It is notable that raw curcumin is practically insoluble in water, 11 ng/mL.51 For the peptide–curcumin complex, even after 6 h more than 75% of curcumin is stable in water. Similar observations are presented by Tapal’s and Dey’s reports in two different studies.52,53 As time passes, curcumin particles start precipitating out. Curcumin is also susceptible to degradation because of hydrolysis and photolysis which might be adding to the decrease in absorbance.50
Scanning Electron Microscopy Study
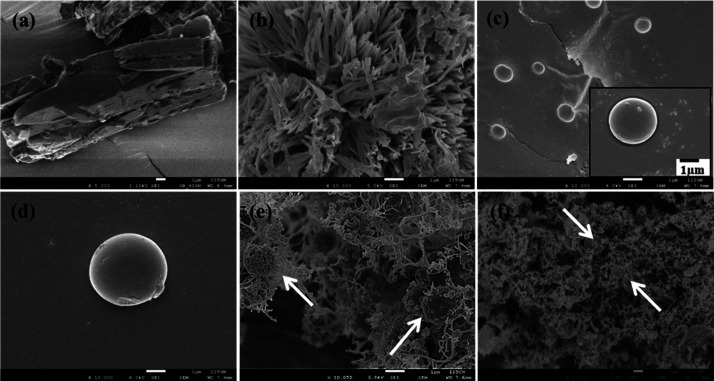
Figure 3 shows the scanning electron microscopy (SEM) micrographs of curcumin, peptide, and peptide–curcumin complexes in powder form. The raw curcumin presents a typical plate-like morphology, (a) which changes to a shorter and sharper needle-like morphology (b) when lyophilized from an ethanol–water system without the addition of peptide. The processing of curcumin alone, without peptide, is done with exactly similar procedure as employed for complexation and the morphological features are not much varying (b). In a similar way, the morphology of bare peptide is seen as spherical (c) and that of peptides lyophilized from the ethanol–water system without the addition of curcumin is also seen to be spherical (d). Peptide–curcumin complexes of 1:1 mol ratio (e) and 1:5 mol ratio (f) display spherical microparticles decorated with fibers of the size of few nanometers. We had also visualized the morphology of peptide–curcumin complexes with a higher amount of peptide which are 5:1 and 10:1 peptide–curcumin ratios (Supporting Information) and found the spherical shape dominating the morphology, as the amount of peptide is more. In agreement with this observation on decreasing the amount of peptide and increasing that of curcumin, we saw the morphology is dominated by the fibrous structure (f). This suggests that, in the peptide–curcumin complex, curcumin gets decorated in the form of nanosized fibers onto spherical peptide particles.
Figure 3.
SEM micrographs of (a) raw curcumin as obtained directly from the manufacturer; (b) SEM image of curcumin lyophilized without peptide using ethanol and water solvent; (c) bare peptide; the inset shows a magnified particle; (d) bare peptide lyophilized without curcumin using ethanol; (e) lyophilized peptide–curcumin complex in a ratio of 1:1; (f) lyophilized peptide–curcumin complex in a ratio of 1:5.
Minimum Inhibitory Concentration Assay
The peptide–curcumin complex showed good antibacterial activity against E. coli and S. aureus bacteria, as presented in Table 1. The amounts of peptide and curcumin, individually in the complex, are determined by standard calibration curves (Supporting Information) using a UV–vis spectrophotometer, and its corresponding survival rate is mentioned. The antibacterial activity of the individual partners separately was also studied (Supporting Information). The results show that the inhibitory values of curcumin in the complex form are significantly lower than those of free curcumin. Some inhibitory effect of the peptide in its bare form was also noticed. The experiments suggest that a certain minimum amount of peptide is needed to impart almost 100% antibacterial activity to the complex. In order to check whether the enhanced antibacterial activity is solely due to enhancement in aqueous solubility of curcumin, a complex of polyvinylpyrrolidone (PVP) and curcumin in 1:5 mole ratio was prepared, and its antibacterial activity was tested in a similar way. Interestingly, the water-soluble PVP–cur complex did not display significant inhibitory effects with the same amount of curcumin as in the peptide–curcumin complex (refer to the Supporting Information).
Table 1. Antibacterial Activity of the Pep–Cur Complex, Bare Peptide, and Raw Curcumin.
| bare
R8 (P) |
raw
cur (C) |
pep–cur complex |
||||
|---|---|---|---|---|---|---|
| conc. (nmol) | survival (%) | conc. (nmol) | survival (%) | conc. (nmol) | survival (%) | |
| E. coli | 7.9 | 60 ± 4 | 17 | 50 ± 2 | P = 9.2 ± 0.2, C = 6.8 ± 0.1 | 5 ± 2 |
| S. aureus | 3.9 | 40 ± 2 | 17 | 10 ± 3 | P = 5.2 ± 0.1, C = 3.0 ± 0.2 | 4 ± 1 |
Morphological Changes in the Bacterial Cell Membrane
The effect of the octaarginine–curcumin complex on the bacterial cell morphology was tested using SEM. SEM images of the bacteria (Figure 4) treated with the peptide–curcumin complex show punctures and dents on the membrane, likely mediated by the peptide component in the complex. The pores visible on the membrane suggest the membrane activity of the complex and indicate the bactericidal mode of action of the complex.
Figure 4.
SEM micrographs of bacteria, the top panel shows images of S. aureus while the bottom one shows images of E. coli. (a,e) Control and (b,f) treated with 17 nmol raw curcumin; (c,g) treated with 7.9 nmol peptide; (d,h) treated with MIC90 concentration of the peptide–curcumin complex (1:5), as mentioned in Table 1.
Time-Kill Assay
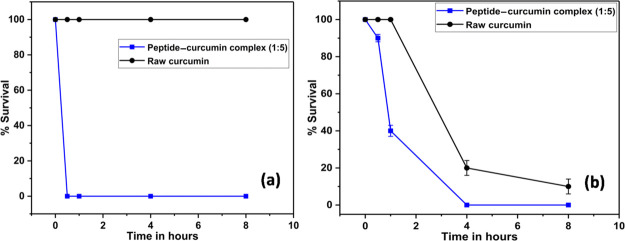
Time-kill assay or killing kinetics was performed to gauge the rate of killing of bacteria by the peptide–curcumin complex. Figure 5 shows the results of the time-kill assay, where the peptide–curcumin complex (1:5) kills 100% E. coli bacteria within half an hour (a) and 100% S. aureus cells within 4 h (b). On the contrary, raw curcumin shows no inhibition of E. coli cells within the tested period, whereas it shows a sluggish response toward S. aureus cells when compared with the complex form. The higher sensitivity and susceptibility of Gram-positive S. aureus than Gram-negative E. coli toward curcumin is clearly outlined in the literature.54 The different killing efficiencies and kinetics of raw curcumin against a set of bacteria reported by Tyagi and co-authors is worth mentioning here, where they obtain a faster and greater killing activity for S. aureus cells than that for E. coli.13 We do not observe any decrease in cell density of curcumin-treated E. coli cells with exposure time, which could be due to the limited inhibitory effect of raw curcumin as shown in Table 1. With this response of raw curcumin, a faster and complete killing of cells by the peptide complexed form is very interesting and distinctive.
Figure 5.
Time-kill assay for E. coli (a) and S. aureus (b). Black is raw curcumin and blue is peptide–curcumin complex (1:5).
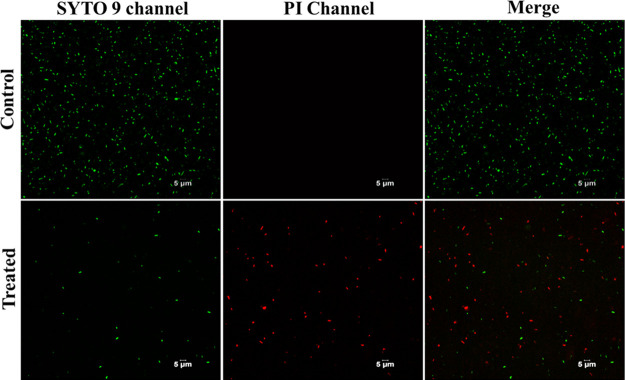
Bacterial Viability Staining
Figure 6 shows the confocal microscopy images of the control and treated bacteria with the peptide–curcumin complex and stained with the BacLight assay kit. SYTO 9 dye of the kit labels the live cells green, while propidium iodide (PI) dye labels the dead cells red. The peptide–curcumin complex causes death of bacteria, evident by the red dots seen in the treated group; however, the presence of only green dots in the control group indicates live cells and no bacterial cell death.
Figure 6.
Confocal microscopy images for E. coli cells control (top panel) and treated with the peptide–curcumin complex (bottom panel).
Membrane Permeabilization Assay
The effect of the peptide–curcumin complex on bacterial membrane integrity was further studied by using PI dye to stain the curcumin, peptide, and peptide–curcumin complex-treated bacteria and imaging them under the confocal laser scanning microscope in Figure 7. The PI dye can enter cells only if their membranes are damaged or punctured.55 The R8-treated cells show red fluorescence in the PI channel, whereas curcumin-treated cells do not display any signal in the PI channel; however, it shows green fluorescence in the curcumin channel. Intrinsic green fluorescence of curcumin has been utilized by previous reports to microscopically visualize its cellular uptake.56,57 The peptide–curcumin complex-treated cells also display signals in the PI channel and indicate that the complex causes membrane damage of the cells. This observation supports the SEM images where the complex-treated cells show membrane damage similar to that of peptide R8 while curcumin does not. It is likely that membrane damage is the mode of action for the peptide–curcumin complex, imparting it a bactericidal activity, unlike raw curcumin.
Figure 7.
Confocal microscopy images of curcumin-treated E. coli (a,b) and curcumin-treated S. aureus (d,e). Peptide-treated E. coli (c) and that of S. aureus (f). Peptide–curcumin complex-treated S. aureus (g,h) and their spatial merge (g)+(h).
Anticancer Activity, Cellular Internalization, and Cytocompatibility against Mammalian Cells
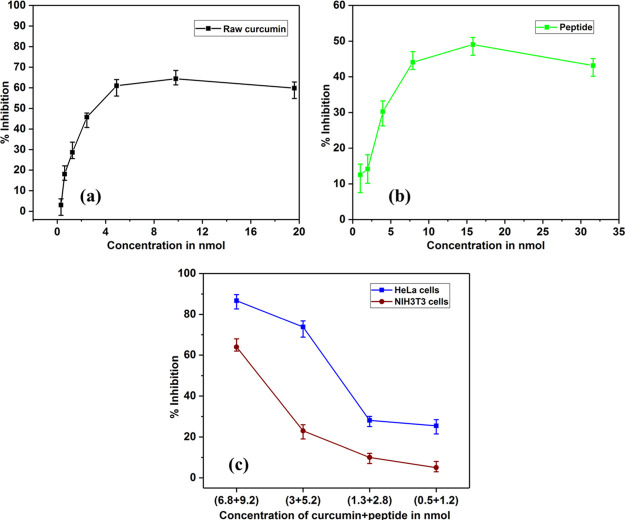
The anticancer activity of the peptide–curcumin complex was assessed by the MTT assay. As seen in Figure 8, the peptide–curcumin complex shows enhanced inhibitory effects with lower concentration of curcumin within the complex as compared to raw curcumin.
Figure 8.
Anticancer activity of (a) raw curcumin in DMSO, (b) bare peptide in water, and (c) blue—aqueous peptide–curcumin complex and crimson—cytocompatibility against NIH3T3 cells.
The ability of cellular internalization of the peptide–curcumin complex is studied using confocal microscopy. The addition of octaarginine enables curcumin to enter into fibroblasts cells NIH3T3 and HeLa cells (Figure 9).
Figure 9.
Cellular internalization of the peptide–curcumin complex (1:5) into HeLa cells (a,b) and into NIH3T3 (c); (d–f) images for DAPI costaining after fixing the cells. (d) DAPI channel, (e) curcumin channel, and (f) merged image.
The above results indicate that complexing curcumin with peptide not only enhances aqueous solubility of curcumin but also enhances its antibacterial and anticancer properties while also enabling cellular internalization.
Conclusions
The complex of curcumin and octaarginine is successfully prepared, as indicated by the physicochemical characterization techniques. The complex shows synergistically enhanced antibacterial activity against the Gram-positive and Gram-negative bacterial cells. A faster killing kinetics of the complex and the presence of synergism in the growth inhibitory effect strongly suggest that there is a unique mode of antibacterial action of the complex. The use of a cationic peptide in the complex further makes it interesting to investigate the detailed mode of activity in order to develop new antibacterial agents with a similar scaffold. Both the SEM and confocal microscopy images indicate that the peptide–curcumin complex causes damage to cell membranes of E. coli and S. aureus, suggesting it to be the main target of the complex. The complex also shows the anticancer activity against HeLa cells and internalizes into mammalian cells. These properties indicate that the octaarginine–curcumin complexes have potential use as antibiotic and anticancer agents. Further, the cellular internalization capability suggests that it may also be used for targeting intracellular bacteria.
Materials and Methods
Materials, Cells, and Media
Curcumin ≥65% pure (Cur), fmoc-Arg(Pbf)-OH, diisopropylcarbodiimide (DIC), piperidine, ethylcyano(hydroxyimino)acetate (Oxyma), N,N-dimethylformamide anhydrous (DMF), dichloromethane, trifluoroacetic acid (TFA), triisopropylsilane (TIS), and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich. PI P1304MP and LIVE/DEAD Baclight assay kit (L7007) were purchased from Invitrogen (Carlsbad, CA, USA). Methylthiazolyldiphenyl-tetrazolium bromide (MTT) reagent (M2128) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Bacterial cells E. coli (ATCC 25922) and S. aureus (ATCC 25923) were procured from Microbial Type Culture Collection and Gene Bank (MTCC), Chandigarh, India. NIH3T3 mouse fibroblast cells were obtained from National Centre for Cell Science (NCCS) Pune, India. Dulbecco’s modified Eagle’s medium, trypsin, was obtained from Invitrogen. Mueller Hinton (MH) agar and broth were obtained from HiMedia (Mumbai, India).
Synthesis of Cell-Penetrating Peptide R8
RRRRRRRR (R8) peptides were synthesized on Rink amide MBHA resin using the standard fluorenylmethyloxycarbonyl (Fmoc) solid-phase peptide synthesis strategy.58 The Fmoc group was removed by 20% piperidine in DMF. Three equivalents of amino acid were used for coupling with DIC and Oxyma as coupling agents, and the coupling reaction was done for 8 h in a frit fitted syringe. The final deprotection was performed using a standard protocol (95% TFA: 2.5% TIS: 2.5% water) for 10 h at room temperature. The crude peptide was purified and analyzed using reverse-phase high-performance liquid chromatography (HPLC) on a C-18 preparative column with 5% acetonitrile in water as a mobile phase with the isocratic flow and characterized by mass spectrometry (Supporting Information).
Preparation of the Peptide–Curcumin Complex
The complex of peptide and curcumin was formed by mixing aqueous peptide and ethanolic curcumin in specific mole ratios. The solution of a peptide is added dropwise to the curcumin solution under stirring and allowed to stir for 48 h; after this, ethanol is removed in vacuo in a vacuum oven at 50 °C and 20 mmHg, and the remaining solution is frozen to lyophilize in order to obtain the powder form of the complex. The lyophilized complex is characterized by physicochemical tools and redispersed in water for studying the antibacterial activity. The concentration (conc.) of curcumin was kept constant as 2.7 μmol and that of the peptide is varied such that the mole ratios of peptide–curcumin (P/C) are 10:1, 5:1, 1:1, and 1:5. We also studied other ratios—P/C ranging from 1:1 to 1:10 in order to find the optimum ratio with a minimum amount of peptide required for forming a stable and bioactive complex. The mole ratio 1:5 (P/C) is chosen to study in detail, particularly for antibacterial assays as other ratios with peptide less than this amount caused precipitation of curcumin from the solution on standing. This was observed qualitatively before the lyophilization step. The possibility of a decrease in aqueous dispersibility of the complex due to reduction in the amount of peptide was also considered while fixing the mole ratio.
Characterization of the Peptide–Curcumin Complex
Powder XRD spectra were captured with a X-ray powder diffractometer (Discover 8.0-Bruker) in a small sample holder with 2θ range from 5 to 60° at a scan speed of 0.2 s per step. DSC analyses of solid samples were carried out on a NETZSCH STA 449F3 Jupiter (NETZSCH Geratebau GmbH; Selb, Germany) from 25 to 500 °C at a heating rate of 10 °C/min. FTIR spectra of the dried powders were recorded with attenuated total reflectance accessories in transmission mode using a PerkinElmer Spectrum Two spectrophotometer at 4 cm–1 resolution between 4000 and 400 cm–1. UV–vis absorption spectra were measured on a PerkinElmer LAMBDA 365 UV–vis spectrophotometer. The lyophilized dry powders were analyzed with Raman spectroscopic (Kaiser Optical Systems) analysis equipped with a 785 nm laser. SEM was conducted to observe morphological variations in the product. The SEM images were captured with a JEOL 7600F field-emission scanning electron microscope provided by the Central Instrumentation Facility of IIT Gandhinagar. Samples were coated with platinum inside a sputter coater with argon gas flow prior to the SEM analysis.
Preparation of Inoculum of Bacterial Cells and the Antibacterial Activity Assay
The bacterial culture grown to the stationary phase is diluted in MH broth (MHB) and allowed to grow till mid-log phase for around 2 h, and absorbance at 600 nm is read and 0.5 OD is adjusted.59 This is further diluted with broth to get 5 × 105 CFU/mL. For all antibacterial assays, the peptide–curcumin (pep–cur) complex of ratio 1:5 is studied. Lyophilized complex is redispersed in a specific volume of water, and twofold dilutions are prepared, of which 10 μL is added to 90 μL of bacterial suspension in quadruplets and incubated for 16 h at 37 °C and absorbance at 600 nm is read keeping negative and positive controls. Percentage of survival is calculated as shown below
Time-Kill Assay Protocol
Bacterial cells are prepared in a similar way as that for minimum inhibitory concentration (MIC) assay and 90 μL of suspensions is inoculated into a 96-well plate, and 10 μL of the aqueous peptide–curcumin complex is added. At specific time points, aliquots are taken from the treated as well as untreated wells, diluted with 10 mM sterile PBS by 50-fold, and 20 μL is plated onto the MH agar plates. Colony formation is observed in the plates after 24 h of incubation at 37 °C.
Study of the Morphology of Bacterial Cells by SEM
A fresh culture of bacteria was inoculated in MHB and was allowed to grow up to a mid-logarithmic phase. This is further diluted to OD 0.2 and incubated with the drug 1× MIC at 37 °C for 4 h. After the incubation, the treated and control cultures are centrifuged down at 6000 rpm for 2 min to obtain pellets. This is washed with PBS buffer and the pellet obtained is fixed with 2.5% glutaraldehyde in PBS overnight at 37 °C. After fixation, the cells were washed first with PBS and thrice with water. It is then lyophilized with water as a medium to obtain powder form of the cells which is visualized under a scanning electron microscope. The cells are sprayed onto the carbon tape glued over a metallic stub and coated with a thin layer of platinum for 120 s.
Live/Dead Assay and Membrane Permeabilization Assay by Confocal Microscopy
A diluted culture of bacteria, as prepared for the MIC assay, is used for confocal microscopy after staining with specific dyes, and appropriate filters are selected as per the excitation/emission values specified by the manufacturer. This 5 μL of cell suspension is sandwiched between the glass slide and cover-slip just before imaging.
Cell Viability/MTT Assay
Confluent cells maintained in complete cell culture media were trypsinized and counted, and 10,000 cells/well were treated in a 96-well plate with appropriate dilutions of the complex for 24 h, and the MTT reagent is added and incubated further for 4 h in dark. Absorbance at 570 nm is measured, and the survival percentage is calculated.
Acknowledgments
The authors would like to acknowledge the support from the Science and Engineering Research Board (SERB), Government of India (EMR/2016/000701), and the Indian Institute of Technology Gandhinagar.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02321.
Calibration curve of curcumin and peptide; mass spectrum and HPLC chromatogram of R8 peptides; SEM images for the pep–cur complex of ratios of 5:1 and 10:1; MIC plots for R8, curcumin, and the PVP–cur complex; PI uptake assay by E. coli cells; and confocal images of PVP–cur uptake by NIH3T3 cells (PDF)
Author Present Address
Indian Institute of Technology Gandhinagar, Palaj, Gandhinagar, 382355, Gujarat, India.
Author Contributions
P.R. performed the experiments, analyzed results, and prepared the manuscript. S.V.D. provided suggestions and technical advices and reviewed the manuscript. A.M. conceived the idea and provided overall guidance and feedback and corrected the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Zhao L.; Gao S.; Huan H.; Xu X.; Zhu X.; Yang W.; Gao Q.; Liu X. Comparison of Virulence Factors and Expression of Specific Genes between Uropathogenic Escherichia Coli and Avian Pathogenic E. Coli in a Murine Urinary Tract Infection Model and a Chicken Challenge Model. Microbiology 2009, 155, 1634–1644. 10.1099/mic.0.024869-0. [DOI] [PubMed] [Google Scholar]
- Tong S. Y. C.; Davis J. S.; Eichenberger E.; Holland T. L.; Fowler V. G. Staphylococcus Aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R.; Patil S.; Singh N.; Gupta S. Dual Functionality Nanobioconjugates Targeting Intracellular Bacteria in Cancer Cells with Enhanced Antimicrobial Activity. Sci. Rep. 2017, 7, 1–10. 10.1038/s41598-017-06014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao W.; Liu X.; Min H.; Dong G.; Feng Q.; Zuo S. Preparation, Characterization, and Antibacterial Activity of Silver Nanoparticle-Decorated Graphene Oxide Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 6966–6973. 10.1021/acsami.5b00937. [DOI] [PubMed] [Google Scholar]
- Priyadarsini K. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. 10.3390/molecules191220091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwar R. K.; Tomar G. B.; Dhumale V. A.; Zinjarde S.; Sharma R. B.; Datar S. Curcumin Conjugated Silica Nanoparticles for Improving Bioavailability and Its Anticancer Applications. J. Agric. Food Chem. 2013, 61, 9632–9637. 10.1021/jf402894x. [DOI] [PubMed] [Google Scholar]
- Kumar S. S. D.; Mahesh A.; Mahadevan S.; Mandal A. B. Synthesis and Characterization of Curcumin Loaded Polymer/Lipid Based Nanoparticles and Evaluation of Their Antitumor Effects on MCF-7 Cells. Biochim. Biophys. Acta, Gen. Subj. 2014, 1840, 1913–1922. 10.1016/j.bbagen.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Mathew A.; Fukuda T.; Nagaoka Y.; Hasumura T.; Morimoto H.; Yoshida Y.; Maekawa T.; Venugopal K.; Kumar D. S. Curcumin Loaded-PLGA Nanoparticles Conjugated with Tet-1 Peptide for Potential Use in Alzheimer’s Disease. PLoS One 2012, 7, e32616 10.1371/journal.pone.0032616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P. K.; Wani K.; Kaul-Ghanekar R.; Prabhune A.; Ogale S. From Micron to Nano-Curcumin by Sophorolipid Co-Processing: Highly Enhanced Bioavailability, Fluorescence, and Anti-Cancer Efficacy. RSC Adv. 2014, 4, 60334–60341. 10.1039/C4RA07300B. [DOI] [Google Scholar]
- Singh A.; Singh J. V.; Rana A.; Bhagat K.; Gulati H. K.; Kumar R.; Salwan R.; Bhagat K.; Kaur G.; Singh N.; Kumar R.; Singh H.; Sharma S.; Bedi P. M. S. Monocarbonyl Curcumin Based Molecular Hybrids as Potent Antibacterial Agents. ACS Omega 2019, 4, 11673–11684. 10.1021/acsomega.9b01109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhawana; Basniwal R. K.; Buttar H. S.; Jain V. K.; Jain N. Curcumin Nanoparticles: Preparation, Characterization, and Antimicrobial Study. J. Agric. Food Chem. 2011, 59, 2056–2061. 10.1021/jf104402t. [DOI] [PubMed] [Google Scholar]
- Shlar I.; Droby S.; Choudhary R.; Rodov V. The Mode of Antimicrobial Action of Curcumin Depends on the Delivery System: Monolithic Nanoparticles: Vs. Supramolecular Inclusion Complex. RSC Adv. 2017, 7, 42559–42569. 10.1039/c7ra07303h. [DOI] [Google Scholar]
- Tyagi P.; Singh M.; Kumari H.; Kumari A.; Mukhopadhyay K. Bactericidal Activity of Curcumin I Is Associated with Damaging of Bacterial Membrane. PLoS One 2015, 10, e0121313 10.1371/journal.pone.0121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Ip M.; Leung A. W.; Xu C. Sonodynamic Inactivation of Methicillin-Resistant Staphylococcus Aureus in Planktonic Condition by Curcumin under Ultrasound Sonication. Ultrasonics 2014, 54, 2109–2114. 10.1016/j.ultras.2014.06.017. [DOI] [PubMed] [Google Scholar]
- Yang X.; Li Z.; Wang N.; Li L.; Song L.; He T.; Sun L.; Wang Z.; Wu Q.; Luo N.; Yi C.; Gong C. Curcumin-Encapsulated Polymeric Micelles Suppress the Development of Colon Cancer In Vitro and In Vivo. Sci. Rep. 2015, 5, 10322. 10.1038/srep10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalew L.; Acuna J.; Urfano S. F.; Morfin C.; Sablan A.; Oh M.; Gamboa A.; Slowinska K. Conjugation of Paclitaxel to Hybrid Peptide Carrier and Biological Evaluation in Jurkat and A549 Cancer Cell Lines. ACS Med. Chem. Lett. 2017, 8, 814–819. 10.1021/acsmedchemlett.7b00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterstam P.; Madani F.; Hirose H.; Takeuchi T.; Futaki S.; EL Andaloussi S.; Gräslund A.; Langel Ü. Elucidating Cell-Penetrating Peptide Mechanisms of Action for Membrane Interaction, Cellular Uptake, and Translocation Utilizing the Hydrophobic Counter-Anion Pyrenebutyrate. Biochim. Biophys. Acta, Biomembr. 2009, 1788, 2509–2517. 10.1016/j.bbamem.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Katayama S.; Nakase I.; Yano Y.; Murayama T.; Nakata Y.; Matsuzaki K.; Futaki S. Effects of Pyrenebutyrate on the Translocation of Arginine-Rich Cell-Penetrating Peptides through Artificial Membranes: Recruiting Peptides to the Membranes, Dissipating Liquid-Ordered Phases, and Inducing Curvature. Biochim. Biophys. Acta, Biomembr. 2013, 1828, 2134–2142. 10.1016/j.bbamem.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Schmidt N.; Mishra A.; Lai G. H.; Wong G. C. L. Arginine-Rich Cell-Penetrating Peptides. FEBS Lett. 2010, 584, 1806–1813. 10.1016/j.febslet.2009.11.046. [DOI] [PubMed] [Google Scholar]
- Li Z.; Teng D.; Mao R.; Wang X.; Hao Y.; Wang X.; Wang J. Improved Antibacterial Activity of the Marine Peptide N6 against Intracellular Salmonella Typhimurium by Conjugating with the Cell-Penetrating Peptide Tat11 via a Cleavable Linker. J. Med. Chem. 2018, 61, 7991–8000. 10.1021/acs.jmedchem.8b01079. [DOI] [PubMed] [Google Scholar]
- Ruczyński J.; Rusiecka I.; Turecka K.; Kozłowska A.; Alenowicz M.; Gągało I.; Kawiak A.; Rekowski P.; Waleron K.; Kocić I. Transportan 10 Improves the Pharmacokinetics and Pharmacodynamics of Vancomycin. Sci. Rep. 2019, 9, 3247. 10.1038/s41598-019-40103-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar M.; Cifuentes J.; Perez J.; Suarez-Arnedo A.; Serna J.; Groot H.; Muñoz-Camargo C.; Cruz J. Novel BUF2-Magnetite Nanobioconjugates with Cell-Penetrating Abilities. Int. J. Nanomed. 2018, 13, 8087–8094. 10.2147/IJN.S188074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomarasca M.; Martins T. F. C.; Greune L.; Hardwidge P. R.; Schmidt M. A. Bacterium-Derived Cell-Penetrating Peptides Deliver Gentamicin To Kill Intracellular Pathogens. Antimicrob. Agents Chemother. 2017, 61, e02545-16 10.1128/aac.02545-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparr C.; Purkayastha N.; Kolesinska B.; Gengenbacher M.; Amulic B.; Matuschewski K.; Seebach D.; Kamena F. Improved Efficacy of Fosmidomycin against Plasmodium and Mycobacterium Species by Combination with the Cell-Penetrating Peptide Octaarginine. Antimicrob. Agents Chemother. 2013, 57, 4689–4698. 10.1128/AAC.00427-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. L.; Maigre L.; Dorlet P.; Guillot R.; Pagès J.-M.; Crouse K. A.; Policar C.; Delsuc N. Conjugation of a New Series of Dithiocarbazate Schiff Base Copper(II) Complexes with Vectors Selected to Enhance Antibacterial Activity. Bioconjugate Chem. 2014, 25, 2269–2284. 10.1021/bc5004907. [DOI] [PubMed] [Google Scholar]
- Schmidt N. W.; Deshayes S.; Hawker S.; Blacker A.; Kasko A. M.; Wong G. C. L. Engineering Persister-Specific Antibiotics with Synergistic Antimicrobial Functions. ACS Nano 2014, 8, 8786–8793. 10.1021/nn502201a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P. L.; Ong H. K.; Teo J.; Ow D. S.-W.; Chao S.-H. HEXIM1 Peptide Exhibits Antimicrobial Activity against Antibiotic Resistant Bacteria through Guidance of Cell Penetrating Peptide. Front. Microbiol. 2019, 10, 203. 10.3389/fmicb.2019.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y.; Ise S.; Azuma Y.; Takeuchi T.; Kawano K.; Le T. K.; Ohkanda J.; Futaki S. Dipicolylamine/Metal Complexes That Promote Direct Cell-Membrane Penetration of Octaarginine. Bioconjugate Chem. 2019, 30, 454–460. 10.1021/acs.bioconjchem.8b00691. [DOI] [PubMed] [Google Scholar]
- Hoskin D. W.; Ramamoorthy A. Studies on Anticancer Activities of Antimicrobial Peptides. Biochim. Biophys. Acta, Biomembr. 2008, 1778, 357–375. 10.1016/j.bbamem.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heulot M.; Jacquier N.; Aeby S.; Le Roy D.; Roger T.; Trofimenko E.; Barras D.; Greub G.; Widmann C. The Anticancer Peptide TAT-RasGAP317-326 Exerts Broad Antimicrobial Activity. Front. Microbiol. 2017, 8, 994. 10.3389/fmicb.2017.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H.-L.; Yip B.-S.; Chen K.-H.; Yu H.-Y.; Chih Y.-H.; Cheng H.-T.; Chou Y.-T.; Cheng J.-W. Novel Antimicrobial Peptides with High Anticancer Activity and Selectivity. PLoS One 2015, 10, e0126390 10.1371/journal.pone.0126390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivanantham B.; Sethuraman S.; Krishnan U. M. Combinatorial Effects of Curcumin with an Anti-Neoplastic Agent on Head and Neck Squamous Cell Carcinoma Through the Regulation of EGFR-ERK1/2 and Apoptotic Signaling Pathways. ACS Comb. Sci. 2016, 18, 22–35. 10.1021/acscombsci.5b00043. [DOI] [PubMed] [Google Scholar]
- Khandelwal P.; Alam A.; Choksi A.; Chattopadhyay S.; Poddar P. Retention of Anticancer Activity of Curcumin after Conjugation with Fluorescent Gold Quantum Clusters: An in Vitro and in Vivo Xenograft Study. ACS Omega 2018, 3, 4776–4785. 10.1021/acsomega.8b00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R. K.; Kasoju N.; Bora U. Encapsulation of Curcumin in Alginate-Chitosan-Pluronic Composite Nanoparticles for Delivery to Cancer Cells. Nanomedicine 2010, 6, 153–160. 10.1016/j.nano.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Fatima M. T.; Chanchal A.; Yavvari P. S.; Bhagat S. D.; Gujrati M.; Mishra R. K.; Srivastava A. Cell Permeating Nano-Complexes of Amphiphilic Polyelectrolytes Enhance Solubility , Stability and Anti-Cancer Efficacy of Curcumin. Biomacromolecules 2016, 17, 2375–2383. 10.1021/acs.biomac.6b00417. [DOI] [PubMed] [Google Scholar]
- Boztas A. O.; Karakuzu O.; Galante G.; Ugur Z.; Kocabas F.; Altuntas C. Z.; Yazaydin A. O. Synergistic Interaction of Paclitaxel and Curcumin with Cyclodextrin Polymer Complexation in Human Cancer Cells. Mol. Pharm. 2013, 10, 2676–2683. 10.1021/mp400101k. [DOI] [PubMed] [Google Scholar]
- Nakase I.; Konishi Y.; Ueda M.; Saji H.; Futaki S. Accumulation of Arginine-Rich Cell-Penetrating Peptides in Tumors and the Potential for Anticancer Drug Delivery in Vivo. J. Controlled Release 2012, 159, 181–188. 10.1016/j.jconrel.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Thorat A. A.; Dalvi S. V. Particle Formation Pathways and Polymorphism of Curcumin Induced by Ultrasound and Additives during Liquid Antisolvent Precipitation. CrystEngComm 2014, 16, 11102–11114. 10.1039/c4ce02021a. [DOI] [Google Scholar]
- Choi J.-S. Development of Surface Curcumin Nanoparticles Modified with Biological Macromolecules for Anti-Tumor Effects. Int. J. Biol. Macromol. 2016, 92, 850–859. 10.1016/j.ijbiomac.2016.07.101. [DOI] [PubMed] [Google Scholar]
- Yallapu M. M.; Gupta B. K.; Jaggi M.; Chauhan S. C. Fabrication of Curcumin Encapsulated PLGA Nanoparticles for Improved Therapeutic Effects in Metastatic Cancer Cells. J. Colloid Interface Sci. 2010, 351, 19–29. 10.1016/j.jcis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Mandeville J.-S.; Froehlich E.; Tajmir-Riahi H. A. Study of Curcumin and Genistein Interactions with Human Serum Albumin. J. Pharm. Biomed. Anal. 2009, 49, 468–474. 10.1016/j.jpba.2008.11.035. [DOI] [PubMed] [Google Scholar]
- Mohan P. R. K.; Sreelakshmi G.; Muraleedharan C. V.; Joseph R. Water Soluble Complexes of Curcumin with Cyclodextrins: Characterization by FT-Raman Spectroscopy. Vib. Spectrosc. 2012, 62, 77–84. 10.1016/j.vibspec.2012.05.002. [DOI] [Google Scholar]
- Subhan M. A.; Alam K.; Rahaman M. S.; Rahman M. A.; Awal M. R. Synthesis and Characterization of Metal Complexes Containing Curcumin ( C 21 H 20 O 6 ) and Study of Their Anti-Microbial Activities and DNA Binding Properties. J. Sci. Res. 2014, 6, 97–109. 10.3329/jsr.v6i1.15381. [DOI] [Google Scholar]
- Mitra K.; Gautam S.; Kondaiah P.; Chakravarty A. R. Platinum ( II ) Complexes of Curcumin Showing Photocytotoxicity in Visible Light. Eur. J. Inorg. Chem. 2017, 2017, 1753–1763. 10.1002/ejic.201601078. [DOI] [Google Scholar]
- Hegde A. H.; Sandhya B.; Seetharamappa J. International Journal of Biological Macromolecules Investigations to Reveal the Nature of Interactions of Human Hemoglobin with Curcumin Using Optical Techniques. Int. J. Biol. Macromol. 2013, 52, 133–138. 10.1016/j.ijbiomac.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Huang F.; Gao Y.; Zhang Y.; Cheng T.; Ou H.; Yang L.; Liu J.; Shi L.; Liu J. Silver-Decorated Polymeric Micelles Combined with Curcumin for Enhanced Antibacterial Activity. ACS Appl. Mater. Interfaces 2017, 9, 16880–16889. 10.1021/acsami.7b03347. [DOI] [PubMed] [Google Scholar]
- Bhatia N. K.; Kishor S.; Katyal N.; Gogoi P.; Narang P.; Deep S. Effect of PH and Temperature on Conformational Equilibria and Aggregation Behaviour of Curcumin in Aqueous Binary Mixtures of Ethanol. RSC Adv. 2016, 6, 103275–103288. 10.1039/C6RA24256A. [DOI] [Google Scholar]
- Gupta S. C.; Prasad S.; Kim J. H.; Patchva S.; Webb L. J.; Priyadarsini I. K.; Aggarwal B. B. Multitargeting by Curcumin as Revealed by Molecular Interaction Studies. Nat. Prod. Rep. 2011, 28, 1937–1955. 10.1039/c1np00051a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S.; Chakravarty A. R. Metal Complexes of Curcumin for Cellular Imaging, Targeting, and Photoinduced Anticancer Activity. Acc. Chem. Res. 2015, 48, 2075–2083. 10.1021/acs.accounts.5b00127. [DOI] [PubMed] [Google Scholar]
- Mohanty C.; Sahoo S. K. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials 2010, 31, 6597–6611. 10.1016/j.biomaterials.2010.04.062. [DOI] [PubMed] [Google Scholar]
- Yu H.; Huang Q. Enhanced in Vitro Anti-Cancer Activity of Curcumin Encapsulated in Hydrophobically Modified Starch. Food Chem. 2010, 119, 669–674. 10.1016/j.foodchem.2009.07.018. [DOI] [Google Scholar]
- Tapal A.; Tiku P. K. Complexation of Curcumin with Soy Protein Isolate and Its Implications on Solubility and Stability of Curcumin. Food Chem. 2012, 130, 960–965. 10.1016/j.foodchem.2011.08.025. [DOI] [Google Scholar]
- Dey S.; Sreenivasan K. Conjugation of Curcumin onto Alginate Enhances Aqueous Solubility and Stability of Curcumin. Carbohydr. Polym. 2014, 99, 499–507. 10.1016/j.carbpol.2013.08.067. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Lu Z.; Wu H.; Lv F. Study on the Antibiotic Activity of Microcapsule Curcumin against Foodborne Pathogens. Int. J. Food Microbiol. 2009, 136, 71–74. 10.1016/j.ijfoodmicro.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Cauz A. C. G.; Carretero G. P. B.; Saraiva G. K. V.; Park P.; Mortara L.; Cuccovia I. M.; Brocchi M.; Gueiros-Filho F. J. Violacein Targets the Cytoplasmic Membrane of Bacteria. ACS Infect. Dis. 2019, 5, 539–549. 10.1021/acsinfecdis.8b00245. [DOI] [PubMed] [Google Scholar]
- Sahu A.; Kasoju N.; Bora U. Fluorescence Study of the Curcumin–Casein Micelle Complexation and Its Application as a Drug Nanocarrier to Cancer Cells. Biomacromolecules 2008, 9, 2905–2912. 10.1021/bm800683f. [DOI] [PubMed] [Google Scholar]
- Moustapha A.; Pérétout P.; Rainey N.; Sureau F.; Geze M.; Petit J.-M.; Dewailly E.; Slomianny C.; Petit P. Curcumin Induces Crosstalk between Autophagy and Apoptosis Mediated by Calcium Release from the Endoplasmic Reticulum , Lysosomal Destabilization and Mitochondrial Events. Cell Death Discovery 2015, 1, 15017. 10.1038/cddiscovery.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amblard M.; Fehrentz J.-A.; Martinez J.; Subra G. Methods and Protocols of Modern Solid Phase Peptide Synthesis. Mol. Biotechnol. 2006, 33, 239–254. 10.1385/mb:33:3:239. [DOI] [PubMed] [Google Scholar]
- Patel J. B.; Cockerill R. F.; Bradford A. P.; Eliopoulos M. G.; Hindler A. J.; Jenkins G. S.; Lewis S. J.; Limbago B.; Miller A. L.; Nicolau P. D.; Pwell M.; Swenson M. J.; Traczewski M. M.; Turnidge J. D.; Weinstein M. P.; Zimmer B. L.. M07-A10: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Tenth Edition; Clinical Laboratory Standards Institute, 2015; Vol. 35 ( (2), ).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.