Abstract
We described an indicator-free argentometric titration strategy using a microfluidic paper-based analytical device. This strategy was based on the formation of insoluble silver salts by reactions occurring between analytes and titrant (Ag+) on a paper channel. After the insoluble silver salts were formed and precipitated on the channel, the paper substrate modified with the surplus titrant on the channel turned reddish-brown by exposure of the devices to a simple and cheap UV light source for 5 min, generating a colored band on the channel. Distance-based detection of chloride was achieved by measuring the length of the colored band with a detection limit of 1.7 mg L–1 Cl–. This method was used to detect chlorides in tap water, with an analytical result (10.1 ± 1.2 mg L–1) agreeing well with that obtained by a classical conventional precipitation titration (9.8 mg L–1), which was based on the measurement of the consumed volume of titrant. This paper-based precipitation titration method is free of skilled personnel and has advantages of low reagent/sample consumption, disposability, portability, and simple operation over the conventional precipitation titration. More importantly, being free of any indicator, this method may be used to detect more species than the conventional precipitation titrations, which are limited by the indicator, for example, CO32– and SO42–, which could form insoluble silver salts in aqueous liquids. Additionally, comparing with most of those paper-based titrimetry reported previously, this presented precipitation titration is free of any indicator or ion selective electrode to detect the end point of titration.
Introduction
The past decade has witnessed the fast development of a microfluidic paper-based analytical device (μPAD) since this concept was introduced by the Whitesides group.1 Its advantages of simple fabrication, easy operation, low cost, and disposability are very promising and attractive for point-of-care analytical applications in clinical diagnostics,2,3 environmental testing4,5 and food safety monitoring.6−8 Various detection techniques have been coupled to paper-based devices to obtain instrumental signals for quantitative analysis, including fluorimetry,3,9 electrochemistry10,11 and chemiluminescence assay.12 These detection techniques, however, are usually limited by expensive instruments, time-consuming operations, and the requirement of trained operators. A cheap and convenient alternative is colorimetric assay, which is based on the chromogenic reactions occurring between the chromogenic reagents and analytes.13−17 However, this detection technique still requires a digital camera (or scanner) to take images of the detection zones and image processing software to extract the color intensity signals.
These abovementioned issues may be addressed by titrimetry on paper-based devices because titrimetry is free of any instrumental signals. In the conventional and classic titration analysis, the consumed volume of titrant at the end point is used to calculate the concentration of the analytes after the end point is detected by the color change with naked eyes. Karita and Kaneta18 demonstrated acid–base titration using a μPAD for analysis of acidic hot water in a natural hot spring. This method required neither any electronic instrument nor software, making it very suitable for on-site analysis. Unfortunately, the channel configuration of the paper device as well as the volumes of titrant, sample, and indicator solutions deposited onto the device should be calculated and strictly controlled. Furthermore, titrant solutions with varied concentrations are required for one assay, and the selection of proper concentrations of titrant deposited onto paper zones is challenging. These issues pose difficulties with real sample analysis with paper-based acid–base titration. Later, Nogueira et al.19 fabricated paper microzones to monitor acid–base titration through a free APP named Photometrix. The redox titrations have also been reported using μPADs.20,21 For example, Myers et al.20 fabricated a paper test card to perform iodometric titration of iodate and iodometric back-titration of amoxicillin. However, to accomplish iodometric titration and iodometric back-titration with a paper test card, a digital camera (or scanner) and image processing software are both required. More recently, Rahbar et al. developed argentometric back-titration of chlorides via distance-based μPADs.22 The principle of this method is very similar to the argentometric titration with a thread-based device reported previously.23 Although this method is free of any electronic instrument (digital camera or scanner) and image processing software, an indicator solution was required to be deposited onto the channel besides the titrant and sample solution to generate a colored band. Thus, tedious procedures are required for deposition and air drying. More importantly, only those species that could form silver salts with lower solubility than their chromate counterpart could produce a colored band and be detected, and thus the application of this method is limited by the selection of the indicator. Phoonsawat et al.24 described the distance-based detection of chloride ions by argentometric titration using μPADs. In their work, silver nanoparticles and hydrogen peroxide were mixed and deposited onto the paper channel; the analysis was based on the oxidative etching of silver nanoparticles to form AgCl precipitates in the presence of chloride ions and H2O2, forming a white-colored band on the paper channel.
We herein developed a simple indicator-free precipitation titration strategy using a μPAD. Only one reagent (silver nitrate) was required to be deposited onto the paper channel; the analytes react with the titrant (silver nitrate) that modified the channel to form insoluble silver salts, which are precipitated onto the channel until all of the analytes were consumed. The channel modified with the surplus silver nitrate was then converted to reddish-brown by exposure to ultraviolet light, thus forming a clear boundary on the channel between the insoluble precipitates and the unreacted titrant. The length of the colored band formed by deposition of insoluble silver salts was measured to detect the analytes in sample solutions. This paper-based precipitation titration requires neither a digital camera (scanner) nor image processing software and has the advantages of low cost, easy operation, and reduced reagents/sample consumption.
Results and Discussion
Principle
The precipitation titration on μPADs was based on the formation of insoluble silver chlorides produced by the reaction occurring between silver ions and chlorides on the paper channel: Ag+ + Cl– = AgCl. Specifically, the paper channel was first modified with the titrant (silver nitrate solution) by adding titrant solution onto the channel with a micropipette (Figure 1B). As the sample or standard solution containing chlorides was added onto the circular zone of the device, the solution wicks on the channel owing to capillary action (Figure 1C). Thus, the silver ions that modified the channel react with chlorides that flowed along the channel, producing silver chlorides. Silver chlorides precipitated on the channel because of their insolubility in aqueous liquids until all chloride ions were consumed. More silver nitrate could be consumed to produce more silver chlorides by increasing the concentration of chlorides in solution, forming a precipitation zone with a longer distance on the channel. Although white silver chlorides were precipitated on the channel, the zone with precipitated silver chlorides could not be observed because the paper substrate modified with the surplus silver nitrate on the neighboring zone was white too. However, although the devices were exposed to UV light for several minutes, the color of the channel, wherein silver chlorides were not precipitated, turned reddish-brown, which may be due to the reaction that occurred between the paper cellulose and the surplus silver nitrate that modified the channel by exposure to UV light. Thus, white-colored bands were formed on the paper channels (Figure 1D). After the calibration curve between the length of the colored band and the concentration of chlorides was plotted, the length of the white-colored band formed by the sample solution was measured to quantify the chlorides in sample.
Figure 1.
Schematic diagrams illustrating the principle of paper-based precipitation titration. After the devices were sandwiched and fixed taut with glass slides (A), silver nitrate solution was deposited onto channels using a micropipette (B). Then Cl- solution was pipetted onto the circular zones of the devices to allow the solution to flow along the channel (C), forming white-colored bands after being exposed to UV light (D).
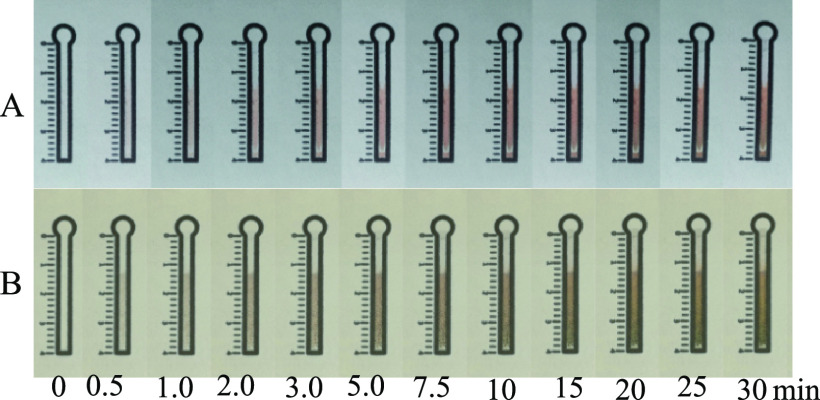
Effect of Exposure Time
We employed several exposure light sources for this paper-based precipitation titration, including a portable LED torch, sunlight, and a cheap ultraviolet sterilizing lamp. When the device was exposed to the light emitted from the LED torch, the color of the channel modified with silver nitrate was not changed even when the device was exposed for 60 min. However, when the device was exposed to sunlight on a sunny day for 3 min, the channel modified with silver nitrate turned reddish-brown (Figure 2A). However, the sunlight is limited by the weather, posing difficulties in performing paper-based precipitation titration on rainy/cloudy days and nights. To address this issue, a cheap and portable ultraviolet sterilizing lamp of 6 W was used as the exposure light source. Reddish-brown color was observed on the channel modified with silver nitrate after the device was exposed to the light emitted from this sterilizing lamp for only 2 min, producing a white-colored band on the device (Figure 2B). Thus, we used the sterilizing lamp (Philips TUV) as the exposure light source to form a colored band on the paper device.
Figure 2.
Images showing the effect of exposure time in a range of 0–30 min on the formation of the white-colored band with (A) sunlight and a (B) sterilizing lamp. Concentration of Cl–: 25 mg L–1; concentration of AgNO3: 0.1 mol L–1; volume of Cl– solution: 35 μL; volume of AgNO3: 7.5 μL.
Effect of AgNO3 Concentration
To study the effect of AgNO3 concentration on the formation of the white-colored band, AgNO3 solutions in a range of 0.025–0.20 mol L–1 were prepared and deposited onto the paper channel. After 100 mg L–1 Cl– solution was added onto the circular zone followed by exposure to UV light, the white-colored band was observed. Figure 3B shows that the length of the white-colored band decreased with AgNO3 concentration, indicating that higher sensitivity may be obtained with AgNO3 solution at lower concentration. However, when the concentration of AgNO3 solution was lower than 0.1 mol L–1, even an aqueous blank solution in the absence of chloride ions could produce a white-colored band on the paper channel (Figure 3A). This may be due to the dissolution of AgNO3 that modified the paper channel by the flowing aqueous liquid. Thus, 0.1 mol L–1 AgNO3 was used to modify the paper channel for analytical applications.
Figure 3.
(A) Image of μPADs captured after adding 35 μL of water onto the circular zones of devices with channels modified with AgNO3 solutions of varied concentrations. (B) Image of μPADs captured after adding 35 μL of solution containing 100 mg L–1 Cl– onto the circular zones of the devices with channels modified with AgNO3 solution of varied concentrations. Exposure light source: Philips TUV light source; exposure time: 5 min. Other conditions are the same as those in Figure 2.
Effect of Volume of Chloride Solution
Chloride solution (100 mg L–1) was used to study the effect of volume of chloride solution on the length of the white-colored band by keeping the volume and concentration of silver nitrate constant at 7.5 μL and 0.1 mol L–1, respectively. Figure 4 shows that the length of the white-colored band increased with volume in a range of 15–45 μL. Although the detection sensitivity may be enhanced by increasing the volume of chloride solution, the time required for air drying the device also increased, which may reduce the analysis speed. A total of 35 μL of chloride solution was used for analytical applications involving analysis speed and detection sensitivity.
Figure 4.
Effect of volume of chloride solution on the length of the white-colored band in a range of 15–45 μL. Concentration of Cl–: 100 mg L–1; exposure light source: Philips TUV light source; exposure time: 5 min. Other conditions are the same as those in Figure 2.
Effect of Common Foreign Species
To study the effect of some common foreign species on the determination of Cl–, 50 mg L–1 Cl– solution as well as the mixed solution containing 50 mg L–1 Cl– and a certain content of some foreign species were prepared. As the silver nitrate was added onto the channel and air dried, the two solutions were added onto the circular zones to produce white-colored bands. The variance in length value produced by the two solutions was measured to evaluate the tolerance level of foreign species for determination of 50 mg L–1 Cl–. A concentration of foreign species that produces a length difference less than 5% was defined as the tolerance level. The tolerance levels for the determination of 50 mg L–1 Cl– were 500, 50, 50, 50, 25, 25, 25, and 10 mg L–1 for SO42–, CO32–, SCN–, Pb2+, Br–, I–, H2PO4–, and SiO32–, respectively.
Sample Analysis
To plot a calibration curve and obtain
a linear correlation between the length of the white-colored band
and the concentration of Cl–, a series of Cl– standard solutions with varied concentrations in a
range of 5–200 mg L–1 were prepared. After
AgNO3 solutions were added onto the channels and air dried,
Cl– standard solutions were dropped onto the individual
circular zones to produce white-colored bands on the devices. Figure 5A shows that the
length of the white-colored band increased with the concentration
of Cl–. The linear correlation between the length
of the white-colored band (L, mm) and the concentration
of Cl– (C, mg L–1) is L (mm) = 16lgC (mg L–1) – 3.1 with a correlation coefficient of 0.995 (Figure 5B). To obtain the
detection limit of Cl– by this method, 2 mg L–1 Cl– was used as a model sample
solution and analyzed 11 times. The lengths of the white-colored band
from 11 repetitive assays were measured, and a standard deviation
of 1.3 mm was obtained. The detection limit for the determination
of Cl– (CL) was calculated
according to the equation  , where CL is
the detection limit of Cl–, S is
the standard deviation of lengths calculated from 11 repetitive runs,
and K is the slope of the calibration curve. Thus,
the detection limit of this method is 1.7 mg L–1 Cl–.
, where CL is
the detection limit of Cl–, S is
the standard deviation of lengths calculated from 11 repetitive runs,
and K is the slope of the calibration curve. Thus,
the detection limit of this method is 1.7 mg L–1 Cl–.
Figure 5.
(A) Image of μPADs with white-colored bands formed on the channels by adding Cl– solutions with varied concentrations onto circular zones (the image was adjusted using image J software). (B) Length of white-colored bands varying as a function of concentration of Cl–. Each data was obtained from three repetitive assays. Exposure light source: Philips TUV light source; exposure time: 5 min. Other conditions are the same as those in Figure 2.
This method was used to detect chlorides in tap water. Considering that the contents of common foreign species in tap water are lower than those of chlorides, the sample was not pretreated prior to analysis. The sample was analyzed as described in the procedure. Briefly, the sample solution was added onto the circular zone of the μPAD with a channel that was previously modified with silver nitrate. After the device was exposed to UV light, the length of the white-colored band was measured to calculate the content of chlorides in sample solution according to the abovementioned linear correlation. The average length of the white-colored band obtained from three repetitive runs is 13 ± 1 mm, and thus the content of Cl– in tap water was 10.1 ± 1.2 mg L–1. To evaluate the accuracy of this precipitation titration method using the μPAD, the sample was analyzed by a conventional standard titration method, and the contents of Cl– in sample were 9.8 mg L–1, which agreed well with that obtained by this paper-based precipitation titration strategy.
Conclusions
We developed precipitation titration using a simple paper-based microfluidic analytical device. This presented paper-based precipitation has the advantages of reduced sample/reagent consumption, disposability, portability, and simple operations over its conventional counterpart. Comparing with the paper-based acid–base and iodometric titration, this presented paper-based precipitation titration is free of a digital camera/scanner, image processing software, and an indicator. The paper channel modified with AgNO3 of the device was stable for 3 days by keeping in darkness at 4 °C. Furthermore, this indicator-free paper-based precipitation titration has some advantages over the paper-based argentometry reported previously, including simplified procedures and enlarged analytical applications. Additionally, this method may be used for determination of other species that could form white insoluble silver salts, for example, CO32– and SO42–. We believe that this paper-based precipitation titration may broaden the analytical applications of microfluidic paper-based analytical devices.
Experimental Section
Chemicals and Apparatuses
All reagents used were of analytical grade unless stated otherwise. Ultrapure water was produced with a water purification system (EPED-EQ-10T, Nanjing Eped Technology Development Co., Ltd., Nanjing, China) and used throughout. Silver nitrate was purchased from Zhujiang Chemical Co., Ltd. (Guangzhou, China). Sodium chloride, sodium metasilicate nonahydrate, and potassium chromate were purchased from Tianjin Yongda Chemical Reagent Development Center (Tianjin, China). Sodium sulfate anhydrous and lead nitrate were purchased from Sinopharm Chemical Reagent Co, Ltd. (Shanghai, China). Sodium carbonate anhydrous, sodium bromide, potassium iodide, sulfuric acid, and sodium hydroxide were purchased from Xilong Scientific Co., Ltd. (Shantou, China). Potassium thiocyanate and sodium dihydrogen phosphate were purchased from Tianjin Damao Chemical Reagent Factory (Tianjin, China) and Tianjin Zhiyuan Chemical Reagent Co. Ltd. (Tianjin, China), respectively. A cheap ultraviolet sterilizing lamp of 6 W (Philips TUV) and portable LED torch (Supfire A5) were purchased from a local commercial store.
Procedure for Precipitation Titration on μPADs
The configuration of the μPAD was designed with CorelDRAW X3 software. The designed configuration consists of a straight channel (3 × 40 mm), a circular zone (6 mm in diameter), and a ruler (40 mm). After the pattern was printed onto a filter paper sheet (102, Hangzhou Xinhua Paper Limited, Hangzhou, China) with a wax printer (Xerox ColorQube 8580), the paper was heated at 120 °C for 1.5 min on a heating plate, forming wax barriers owing to the penetration of melted wax into the thickness of the paper.
Prior to titration analysis, the μPADs were fixed taut by sandwiching with glass plates (Figure 1A). A total of 7.5 μL of 0.10 mol L–1 AgNO3 was deposited onto the channel with a micropipette. After the device was air dried for 10 min, 35 μL of standard or sample solutions containing chlorides was dropped onto the circular zone, allowing the liquid to flow along the channel. After being air dried for 10 min, the devices were then exposed to UV light for 5 min. The length of the white-colored band was measured for quantitative analysis of chlorides.
Procedure for Chloride Detection with a Standard Method25
After 100 mL of tap water was poured into a 250 mL Erlenmeyer flask, two drops (ca. 100 μL) of phenolphthalein (10 g L–1) were added onto the flask. NaOH (0.05 mol L–1) and HNO3 (0.05 mol L–1) were used to adjust the pH of solution until the color was converted from red to colorless. After 1.0 mL of 50 g L–1 K2CrO4 was added into the solution, 0.014 mol L–1 AgNO3 was loaded into a 25 mL burette and added dropwise into the mixed solutions until the color of the mixed solutions changed from yellow to orange-red. The consumed volume of titrant was then measured to calculate the contents of chlorides CCl- (g L–1) in the sample using the following equation:
where CAg and VAg are the concentration and consumed volume of silver nitrate solution, VCl- is the volume of the sample solution added into the Erlenmeyer flask, and 35.5 is the weight of a chlorine atom (g mol–1).
Acknowledgments
Financial support from the Guangdong Provincial Education Research Innovation Fund (no. 2018GXJK108), Guangdong Provincial Research and Reform Fund for Higher Education Learning and Teaching, Hanshan Normal University Construction Fund for Basic Education of Chemistry (no. Z18042), and the Learning and Teaching Reform Fund from Hanshan Normal University are gratefully acknowledged.
The authors declare no competing financial interest.
References
- Martinez A. W.; Phillips S. T.; Butte M. J.; Whitesides G. M. Patterned Paper as a Platform for Inexpensive, Low-Volume, Portable Bioassays. Angew. Chem., Int. Ed. 2007, 46, 1318–1320. 10.1002/anie.200603817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X.; Zhang L.; Chang S.; Cui W.; Zheng Z. Multiplex Microfluidic Paper-based Immunoassay for the Diagnosis of Hepatitis C Virus Infection. Anal. Chem. 2014, 86, 5338–5344. 10.1021/ac500247f. [DOI] [PubMed] [Google Scholar]
- Guo X.; Zong L.; Jiao Y.; Han Y.; Zhang X.; Xu J.; Li L.; Zhang C.-W.; Liu Z.; Ju Q.; Liu J.; Xu Z.; Yu H.; Huang W. Signal-Enhanced Detection of Multiplexed Cardiac Biomarkers by a Paper-Based Fluorogenic Immunodevice Integrated with Zinc Oxide Nanowires. Anal. Chem. 2019, 91, 9300–9307. 10.1021/acs.analchem.9b02557. [DOI] [PubMed] [Google Scholar]
- Sameenoi Y.; Panymeesamer P.; Supalakorn N.; Koehler K.; Chailapakul O.; Henry C. S.; Volckens J. Microfluidic Paper-Based Analytical Device for Aerosol Oxidative Activity. Environ. Sci. Technol. 2012, 47, 932–940. 10.1021/es304662w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y.; Dong H.; Zheng J.; Sun H. Portable detection of trace metals in airborne particulates and sediments via μPADs and smartphone. Biomicrofluidics 2017, 11, 064101 10.1063/1.5003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouanthavong S.; Nacapricha D.; Henry C. S.; Sameenoi Y. Pesticide analysis using nanoceria-coated paper-based devices as a detection platform. Analyst 2016, 141, 1837–1846. 10.1039/C5AN02403J. [DOI] [PubMed] [Google Scholar]
- Jokerst J. C.; Adkins J. A.; Bisha B.; Mentele M. M.; Goodridge L. D.; Henry C. S. Development of a Paper-Based Analytical Device for Colorimetric Detection of Select Foodborne Pathogens. Anal. Chem. 2012, 84, 2900–2907. 10.1021/ac203466y. [DOI] [PubMed] [Google Scholar]
- Jiang Q.; Wu J.; Yao K.; Yin Y.; Gong M. M.; Yang C.; Lin F. Paper-Based Microfluidic Device (DON-Chip) for Rapid and Low-Cost Deoxynivalenol Quantification in Food, Feed, and Feed Ingredients. ACS Sens. 2019, 4, 3072–3079. 10.1021/acssensors.9b01895. [DOI] [PubMed] [Google Scholar]
- Yamada K.; Takaki S.; Komuro N.; Suzuki K.; Citterio D. An antibody-free microfluidic paper-based analytical device for the determination of tear fluid lactoferrin by fluorescence sensitization of Tb3+. Analyst 2014, 139, 1637–1643. 10.1039/c3an01926h. [DOI] [PubMed] [Google Scholar]
- Dossi N.; Toniolo R.; Terzi F.; Piccin E.; Bontempelli G. Simple pencil-drawn paper-based devices for one-spot electrochemical detection of electroactive species in oil samples. Electrophoresis 2015, 36, 1830–1836. 10.1002/elps.201500083. [DOI] [PubMed] [Google Scholar]
- Ruecha N.; Chailapakul O.; Suzuki K.; Citterio D. Fully Inkjet-Printed Paper-Based Potentiometric Ion-Sensing Devices. Anal. Chem. 2017, 89, 10608–10616. 10.1021/acs.analchem.7b03177. [DOI] [PubMed] [Google Scholar]
- Yu J.; Ge L.; Huang J.; Wang S.; Ge S. Microfluidic paper-based chemiluminescence biosensor for simultaneous determination of glucose and uric acid. Lab Chip 2011, 11, 1286–1291. 10.1039/c0lc00524j. [DOI] [PubMed] [Google Scholar]
- Liu C.; Gomez F. A.; Miao Y.; Cui P.; Lee W. A colorimetric assay system for dopamine using microfluidic paper-based analytical devices. Talanta 2019, 194, 171–176. 10.1016/j.talanta.2018.10.039. [DOI] [PubMed] [Google Scholar]
- dos Santos G. P.; Corrêa C. C.; Kubota L. T. A simple, sensitive and reduced cost paper-based device with low quantity of chemicals for the early diagnosis of Plasmodium falciparum malaria using an enzyme-based colorimetric assay. Sens. Actuators, B 2018, 255, 2113–2120. 10.1016/j.snb.2017.09.005. [DOI] [Google Scholar]
- Worramongkona P.; Seeda K.; Phansomboon P.; Ratnarathorn N.; Chailapakul O.; Dungchai W. A Simple Paper-based Colorimetric Device for Rapid and Sensitive Urinary Oxalate Determinations. Anal. Sci. 2018, 34, 103–108. 10.2116/analsci.34.103. [DOI] [PubMed] [Google Scholar]
- Jayawardane B. M.; Wei S.; McKelvie I. D.; Kolev S. D. Microfluidic Paper-Based Analytical Device for the Determination of Nitrite and Nitrate. Anal. Chem. 2014, 86, 7274–7279. 10.1021/ac5013249. [DOI] [PubMed] [Google Scholar]
- Puangbanlang C.; Sirivibulkovit K.; Nacapricha D.; Sameenoi Y. A paper-based device for simultaneous determination of antioxidant activity and total phenolic content in food samples. Talanta 2019, 198, 542–549. 10.1016/j.talanta.2019.02.048. [DOI] [PubMed] [Google Scholar]
- Karita S.; Kaneta T. Acid-Base Titrations Using Microfluidic Paper-Based Analytical Devices. Anal. Chem. 2014, 86, 12108–12114. 10.1021/ac5039384. [DOI] [PubMed] [Google Scholar]
- Nogueira S. A.; Sousa L. R.; Silva N. K. L.; Rodrigues P. H. F.; Coltro W. K. T. Monitoring Acid–Base Titrations on Wax Printed Paper Microzones Using a Smartphone. Micromachines 2017, 8, 139. 10.3390/mi8050139. [DOI] [Google Scholar]
- Myers N. M.; Kernisan E. N.; Lieberman M. Lab on Paper: Iodometric Titration on a Printed Card. Anal. Chem. 2015, 87, 3764–3770. 10.1021/ac504269q. [DOI] [PubMed] [Google Scholar]
- Nogueira S. A.; Lemes A. D.; Chagas A. C.; Vieira M. L.; Talhavini M.; Morais P. A. O.; Coltro W. K. T. Redox titration on foldable paper-based analytical devices for the visual determination of alcohol content in whiskey samples. Talanta 2019, 194, 363–369. 10.1016/j.talanta.2018.10.036. [DOI] [PubMed] [Google Scholar]
- Rahbar M.; Paull B.; Macka M. Instrument-free argentometric determination of chloride via trapezoidal distance-based microfluidic paper devices. Anal. Chim. Acta 2019, 1063, 1–8. 10.1016/j.aca.2019.02.048. [DOI] [PubMed] [Google Scholar]
- Jarujamrus P.; Malahom N.; Puchum S.; Meelapsom R.; Amatatongchai M.; Siripinyanond A.; Chairam S.; Kulsing C. Complexometric and argentometric titrations using thread-based analytical devices. Talanta 2018, 183, 228–236. 10.1016/j.talanta.2018.02.058. [DOI] [PubMed] [Google Scholar]
- Phoonsawat K.; Ratnarathorn N.; Henry C. S.; Dungchai W. A distance-based paper sensor for the determination of chloride ions using silver nanoparticles. Analyst 2018, 143, 3867–3873. 10.1039/C8AN00670A. [DOI] [PubMed] [Google Scholar]
- Zhou W. S.; Zhu C. J.; Zhang Y. L.; Shao H. Q.; Lan C. J. Determination of Chloride in Industrial Circulating Cooling Water with Silver Nitrate Titration method. National Standard of the People’s Republic of China (GB/T 15453–1995).