Abstract

A series of NiMoP(x)-Al catalysts with different phosphorus contents were prepared by the incipient wetness co-impregnation method. The effects of phosphorus modification on the acidity, active phase nanostructure, and catalytic properties of the residue hydrodenitrogenation catalysts were investigated to find the role of phosphorus in the catalytic mechanism. The results of temperature-programmed desorption of NH3 and pyridine IR spectroscopy of the catalysts indicate that phosphorus modification can increase the total acid and Brønsted acid. Transmission electron microscopy analysis shows that phosphorus modification increases the stacking number NA, reduces the slab length LA of the active MoS2 phase, and increases the Mo dispersion fMo, leading to the promotion of the sulfidation degree of the active Mo phase and thus increasing the denitrification rate. The catalyst with a 3.4 wt % P2O5 loading shows the highest Brønsted/Lewis acid ratio, the largest amount of three-layer active phases, the smallest LA, the highest fMo, the optimal sulfurization degree, and the highest denitrification rate, 63.6%, indicating the correlation between the nanostructure of the active phase and its catalytic property because of the addition of phosphorus.
1. Introduction
Environmental regulations have become increasingly stringent so that petroleum products with high quality are required to meet the ecological standard. At the same time, with the trend of the crude oil becoming heavier and deteriorated, the interest in the preparation of highly active hydrotreating catalysts has increased rapidly.1 As a result, lighter and cleaner products from heavy oil have become the future research trend, and the key is to develop highly active catalysts.
A residue hydrodenitrogenation (HDN) catalyst requires both hydrogenation activity and hydrogenolysis activity.2 Compared with hydrodemetalation (HDM) and hydrodesulfurization (HDS) catalysts, HDN catalysts require not only high hydrogenation activity but also proper acid strength to facilitate the breakage of the C–N bond.3,4 Various modification methods of (Co)NiMo/Al2O3 catalysts have emerged to improve the performance of HDN catalysts. At present, three main modification methods have been reported. (1) Introducing a second promoter: one is to add an inorganic promoter such as phosphorus (P) and boron (B);5−8 the other is to add organic chelating agents such as citric acid (CA),9 ethylene glycol (EG),10 nitrotriacetic acid (NTA),1111 and ethylenediamine tetraacetic acid (EDTA);12 (2) Using new supports or modifying the supports; and (3) Optimizing the impregnation process.
Among the above-mentioned methods, phosphorus modification has attracted plenty of attention. A great deal of research has been done concerning the effect of phosphorus on the HDN activity of (Co)NiMo/Al2O3 catalysts.13−20 Ferdous et al.17 adopted NaH2PO4 as the precursor of phosphorus to prepare NiMoP catalysts by two different impregnation methods: step impregnation and modified co-impregnation. The results show that the acid amount of the catalyst prepared by modified co-impregnation increased significantly, while the increase of the acid amount of the catalyst prepared by step impregnation was not obvious, and the former catalyst showed higher activity. Vatutina et al.18 adopted different modification methods to modify the CoMo hydrotreating catalyst using H3PO4, and found that the direct impregnation method improved the HDS performance more obviously. At the same time, it was found that the addition of phosphorus increased the stacking number of the active phase of the catalyst. The sequence of adding phosphorus also has an influence on the performance of catalysts.19 NiMo/PAl2O3 (Al2O3 support was impregnated with phosphoric acid first) shows superior performance over NiMo/Al2O3 and NiMoP/Al2O3 (Al2O3 support was impregnated with a solution containing the precursors of Mo, Ni, and P) because NiMo/PAl2O3 shows the combination of acidity, porosity, and distribution of the Ni–Mo–S phases.
Shi et al.20 found that there is a competitive adsorption between phosphorus species and molybdenum species with the hydroxyl (−OH) groups on the alumina surface. In the impregnation process of adding H3PO4, H2PO4– ions from the dissociation of H3PO4 react with the Al–OH of the support to generate Al–O–P–OH species.21,22 As a result, the addition of phosphorus reduces the amount of basic hydroxyl groups adsorbed with molybdenum oxide species, which weakens the interaction between the active phase and the support, and finally leads to the formation of more type II Ni–Mo–S active phases.20 The addition of phosphorus can also optimize the average stacking number, average length of the slabs, and dispersion degree (fMo) of effective Mo atoms at the edges and corners of MoS2 particles, among which the increase of the stacking number is helpful for the hydrogenation reaction. Compared with the HDS reaction, the addition of phosphorus is more helpful for the HDN reaction.23
de Mello et al.24 found that phosphorus modification improved the dispersion of active metals, which formed more active sites and increased the Brønsted acidity of the catalyst. Zhou et al.25 also found that phosphorus modification could change the acidity of a Ni–Mo–S catalyst supported on USY zeolite, reduce the interaction between the active phase and the support, and increase the sulfidation degree of Mo species. Sundaramurthy et al.26 added phosphorus into NiMo/γ-Al2O3, which accelerated the breakage of the C–N bond and enhanced the HDN activity of the catalyst. The results show that the way of adding phosphorus can change the acid distribution of the catalysts. MoNiP/Al2O3 is the optimized catalyst for the HDN reaction.
The pore structure is also found to be important for the HDN catalyst. For the effect of pore structure on the performance of the HDN catalyst, if the pore size is too small, the diffusion of reactants will be hindered during the reaction, while a too large pore size tends to decrease the dispersion of the active phase. For the HDN of heavy fractions such as the residue, the pore size in the range of 10–20 nm is good for the reaction, particularly pores of 7–13 nm are effective for the HDN of heavy fractions.27,28 If the ratio of pores at 7–13 nm can be increased, the interaction between the active phase and the support can be weakened so as to improve the HDN performance of the catalysts. To conclude, the beneficial effect of phosphorus on HDN catalysts can be attributed to the modulation of the acidity (acid strength, acid amount, and acid distribution) and the pore structure of the catalysts, adjusting the interaction between the active phase (Mo, Ni) and the support, and facilitation of the formation of the type II Ni–Mo–S phase.
There have been plenty of studies on the modification of phosphorus on HDN catalysts, and the promotion effect of phosphorus on HDN catalysts has been recognized. However, the role of phosphorus on the microstructure of the catalysts, such as the acidity and nanostructures of the active phase of residue HDN catalysts, and the effects of acidity and nanostructures of the active phase on the performance of residue HDN catalysts have not been fully studied. In this article, a co-impregnation method was used to modify residue HDN catalysts with phosphorus to obtain NiMoP catalysts. The effects of phosphorus modification on the acidity, nanostructure of the active phase, and HDN activity of the catalysts were studied. The study aims to achieve a better understanding of the effect of phosphorus on the microstructure of the residue HDN catalysts.
2. Results and Discussion
2.1. Effects of Phosphorus Modification on the Acidity of the Catalysts
2.1.1. NH3-Temperature-Programmed Desorption (TPD) Results
The oxidized NiMoP(x)-Al catalysts were characterized by NH3-TPD, and the results are shown in Figure 1a. The desorption peak at a low temperature (T ≤ 300 °C) corresponds to weak acid sites, the desorption peak at a medium temperature (T = 300–450 °C) corresponds to medium–strong acid sites, and the desorption peak at a high temperature (T = 450–550 °C) corresponds to strong acid sites.20Figure 1a shows that the amount of acid of the oxidized catalyst is obviously higher than that of the support and that of phosphorus-modified catalysts is obviously higher than that of the phosphorus-free catalyst. With the increase of phosphorus content, the NH3 desorption peak of the catalysts shifts slightly to the direction of high temperature, indicating that the addition of phosphorus can improve the strength of the acid on the catalyst. The reason is the strong interaction between phosphate anions and the acidic sites on the surface of the alumina support after the addition of phosphorus. The strong polarization of P5+ results in stronger acidic sites.
Figure 1.

NH3-TPD (a) and the distribution of acids (b) of oxidized NiMoP(x)-Al catalysts.
The distribution of the amount of acid with different strengths of the catalysts was obtained by integrating the peak area of the catalysts at the corresponding temperatures in Figure 1a, and the results are listed in Figure 1b. The total amount of acid of the P = 0 catalyst is regarded as 100, and the total acidity of other catalysts was calculated according to the ratio of peak areas to that of P = 0 (the same calculation for that of sulfided catalysts). From Figure 1b, it can be seen that the total amount of acid of NiMoP(x)-Al catalysts increases first and then decreases with the increase of phosphorus content in the oxidized state. Phosphorus modification obviously increases the total amount of acid of the catalyst. However, it is not the case that the higher the phosphorus content, the greater the total amount of acid. When the phosphorus content P = 3.0, the maximum amount of the total acid of the catalyst is 187.3 (Table S1 in the Supporting Information). The trend in the change in different strengths of acids (weak acid, medium–strong acid, and strong acid) is almost consistent with that of the total amount of acid. Among them, when the phosphorus content P = 3.4, the amount of weak acid of the catalyst is the highest, and when the phosphorus content P = 3.0, the amounts of medium–strong acid and strong acid are the largest. For the catalysts with different phosphorus contents, the acid is mainly weak acid and medium–strong acid.
The catalysts were in the sulfided state in HDN reactions, and the sulfided NiMoP(x)-Al catalysts were also characterized by NH3-TPD. The results are shown in Figure 2a. Table S2 shows the amount of acid distribution of the sulfided NiMoP(x)-Al catalysts. Figure 2b shows the amount of acid distribution of the sulfided NiMoP(x)-Al catalysts. Figure 2b and Table S2 show that the total acidity of the sulfided NiMoP(x)-Al catalysts decreases significantly compared with that of oxidized catalysts, while the overall trend is almost the same: the total amount of acid increases first and then decreases with the increase of phosphorus content, the total acid and weak acid content are the highest when the phosphorus content P = 3.4, the medium–strong acid is the highest when the phosphorus content P = 3.8, and the strong acid is the highest when the phosphorus content P = 3. For the catalysts with different phosphorus contents, the acid is still dominated by weak and medium–strong acids.
Figure 2.

NH3-TPD (a) and the distribution of acids (b) of sulfided NiMoP(x)-Al catalysts.
2.1.2. Pyridine Fourier Transform Infrared (Py-IR) Results of the Catalysts
Figure 3 shows Py-IR spectra of the support and oxidized
NiMoP(x)-Al catalysts. For the support, bands at
1450 and 1490 cm–1 can be observed, indicating that
only Lewis acids exist on the support, while for the catalysts with
different phosphorus contents, a new IR absorption peak appears at
1540 cm–1, indicating that Brønsted acids form
on the NiMoP(x)-Al catalysts. After calcination at
450 °C, the hydroxyl groups on the surface of the alumina support
dehydrate and condense to form only Lewis acid sites and no Brønsted
acid sites form. After the impregnation of the active components,
Al–OH groups on the surface of the alumina support interact
with the active component Mo species to form a  structure, which increases the
number of protons H+ on the surface of the support, resulting
in the formation of Brønsted acid sites on the surface of catalysts.
structure, which increases the
number of protons H+ on the surface of the support, resulting
in the formation of Brønsted acid sites on the surface of catalysts.
Figure 3.
Py-IR spectra of oxidized NiMoP(x)-Al catalysts.
Table S3 shows the amount of different acid sites of the support and the oxidized NiMoP(x)-Al catalyst. It can be seen that with the increase of phosphorus content, the Lewis acid content of the catalyst decreases gradually, while the Brønsted acid content increases first and then decreases, and the ratio of Brønsted acid to Lewis acid increases first and then decreases. When the phosphorus content P = 3.4, the ratio of B/L of the catalyst is the largest, which is 0.31. The reason is that after addition of phosphorus, the amount of −OH groups that can form Brønsted acid increases, causing the increase of the content of Brønsted acid. However, addition of too much phosphorus inhibits the increase of Brønsted acid. As a result, when the content of phosphorus P = 3.4, the content of Brønsted acid in the catalyst is the highest. A previous study31 has shown that the breakage of the C–N bond occurs on the Brønsted acid sites, so addition of an appropriate amount of phosphorus is beneficial for the HDN performance of the catalyst.
2.2. Effects of Modification of Phosphorus on the Reducibility of the Metal
Qu et al.32 show that the reduction of MoO3 is divided into two steps: MoO3 → MoO2 → Mo. The reduction peak at a low temperature (around 460 °C) is attributed to the reduction of Mo species with the coordination number 8 from Mo6+ to Mo4+, which is believed to be the precursor of type II Ni–Mo–S, the highly active phase nanostructures. The increase of the proportion of octahedral Mo species and the decrease of the proportion of tetrahedral Mo species will form more the type II Ni–Mo–S phase. Type II Ni–Mo–S will be formed when the interaction between the active phase and the support Al2O3 is weak; thus, the dispersion of fully reduced MoS2 will be high, together with the formation of the suitable stacking numbers and length of MoS2 slabs. Figure 4 shows the results of H2-TPR analysis of the catalysts. It is shown that the catalysts with different phosphorus contents have obvious low-temperature reduction peaks at about 460 °C, which is attributed to the reduction of Mo species with the coordination number 8 and the precursor of the type II Ni–Mo–S active phase. The strength of the reduction peak represents the number of Mo species with the coordination number 8. When the phosphorus content P = 3.4, the reduction peak of the catalyst at a low temperature is the strongest, indicating that it contains more highly active phases. In addition, the reduction temperature of the catalyst increased by about 10 °C with the addition of phosphorus, which is due to the formation of aluminum phosphate on the surface of the catalyst after the addition of phosphorus. Phosphorus weakens the interaction between the active phase and the support, leading to the increase of the particle size of the active phase, which requires a higher reduction temperature.
Figure 4.

H2-TPR and reduction temperature of NiMoP(x)-Al catalysts.
2.3. Effect of Phosphorus Modification on the Structure of the Active Phase of the Catalysts
Figure 5 shows the high-resolution transmission electron microscopy (HR-TEM) images of the catalysts. The black stripes in the images are the MoS2 active phase.30,32 MoS2 has a typical layered structure, and the distance between the parallel black lines is about 0.62–0.63 nm, which corresponds to the interplanar spacing of the (0 0 2) planes of MoS2 crystals. Besides, it can be seen that the distribution of MoS2 particles on each catalyst is relatively uniform.
Figure 5.
HR-TEM images of the NiMoP(x)-Al catalysts.
Table S4 shows the average stacking number NA, average slab length LA, and effective Mo atom ratio fMo of the active MoS2 phase of the catalysts with different phosphorus contents. For HDN catalysts with good performance, the stacking number of the active phase MoS2 needs to be compatible with the dispersion of active metals.33 The active phase MoS2 should have a certain value of the stacking number. Meanwhile, the average slab length should be smaller; thus, the active phase can have a larger effective Mo atom dispersion (fMo) so that the HDN activity of the catalyst can be enhanced. A too large stacking number and a too large average slab length will lead to the decrease of fMo, leading to low HDN activity. Table S4 shows that the average stacking number NA increases gradually with the increase of phosphorus content, the average slab length LA decreases first and then increases, and fMo increases first and then decreases. When the phosphorus content P = 3.4, the stacking number of the catalyst is 3.20 and the average slab length is 2.56 nm. At this time, the maximum effective Mo atom at the corner of the catalyst is 0.364, which accounts for the best HDN activity of the catalyst.
Figure 6 is the columnar distribution of the length and number of stacking layers of the active phase on NiMoP(x)-Al catalysts. Figure 6a shows that with the increase of phosphorus content, the proportion of one and two layers of the active phase decreases, the proportion of the four and five layers of the active phases increases, and the proportion of the three layers of the active phase increases first and then decreases. Usually, only two or more stacking layers of the active phase can show high HDN activity. Figure 6b shows that the addition of phosphorus increases the proportion of the active phase LA ≤ 3 nm, and decreases the proportion of the active phase LA > 3 nm. The LA of the active MoS2 phase should not be too long and 2–3 nm is optimal, which is responsible for the increase of activity.
Figure 6.
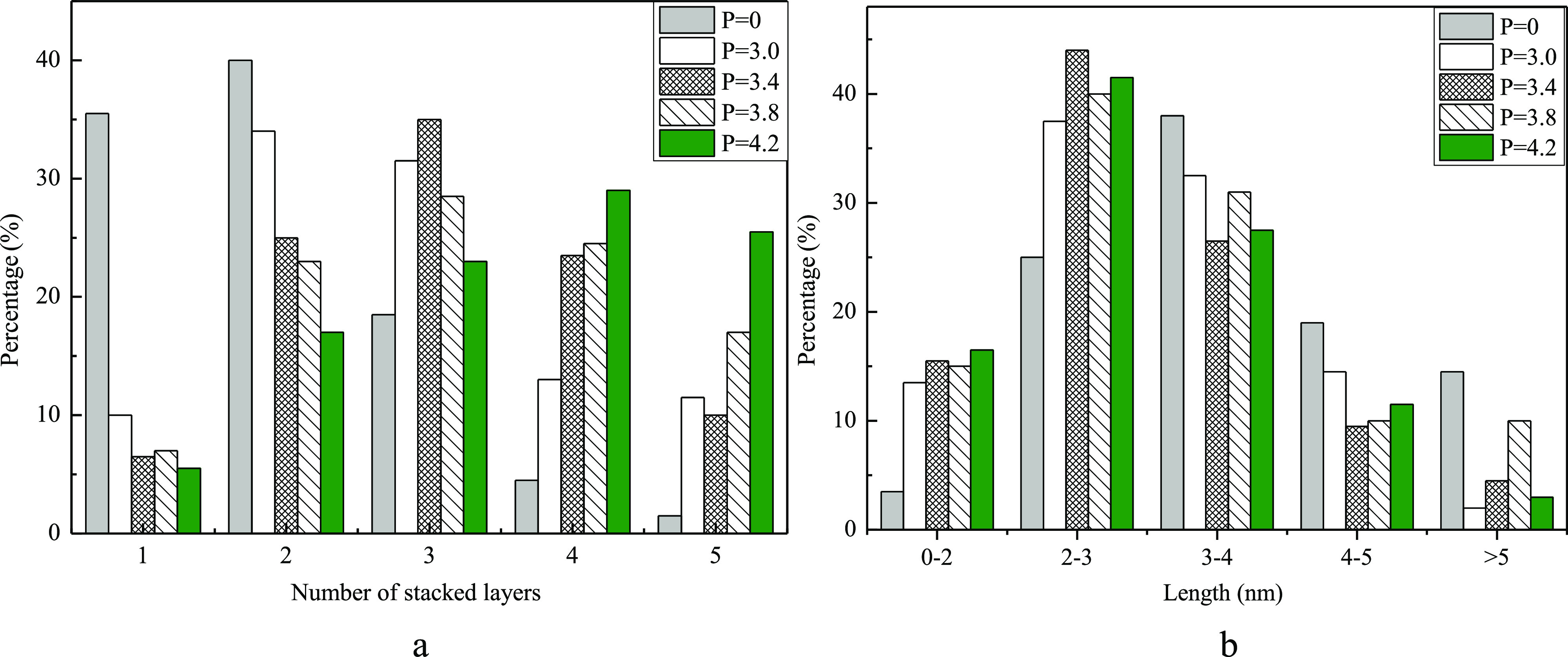
MoS2 stacking number (a) and slab length (b) distribution of NiMoP(x)-Al catalysts.
2.4. Effect of Phosphorus Modification on the Sulfidation Degree of Mo Species
Figure 7 shows X-ray photoelectron spectroscopy (XPS) specta of sulfided NiMoP(x)-Al catalysts. It is generally believed that the Mo 3d curve with different oxidation states is fitted with three sets of Mo 3d5/2 and Mo 3d3/2 double peaks:5 the first set of peaks are at 228.8 eV (3d5/2) and 231.9 eV (3d3/2), attributed to the binding energy of Mo4+; the second set of peaks are at 229.8 eV (3d5/2) and 232.9 eV (3d3/2), attributed to the binding energy of Mo5+; and the third set of peaks are at 233.0 eV (3d5/2) and 236.1 eV (3d3/2), attributed to the binding energy of Mo6+. The fitting of the Ni (2p3/2) curve also shows several Ni (2p3/2) peaks in different chemical environments: the peak at 853.1 eV is attributed to nickel sulfide, the peak at 856.1 eV is attributed to the Ni2+ species interacting with alumina, and the peak at 854.0 eV is attributed to the binding energy of Ni species in the “Ni–Mo–S” phase.
Figure 7.

XPS spectra of the sulfided NiMoP(x)-Al catalysts: (a) Mo 3d and (b) Ni 2p.
Figure 7 shows that all of the NiMoP(x)-Al catalysts manifest three sets of Mo 3d5/2 and Mo 3d3/2 double peaks, indicating the existence of Mo5+ and Mo6+. Mo6+ is mainly MoO3 that has not been sulfided yet. The Mo5+ is mainly Mo species that has not been completely sulfided, which forms coordination compounds with O and S atoms simultaneously, indicating that three catalysts exist incomplete sulfidation at different degrees.34 The reason is the strong interaction between Mo species and the alumina support during calcination at high temperature, leading to the formation of Mo–O–Al bonds.35 However, the Mo–O–Al bond is difficult to break during sulfidation at low temperatures (<500 °C), leading to incomplete converion of the Mo species to MoS2 with catalytic activity.36 When P = 0, the peak area of Mo4+ species is much smaller than that of phosphorus-modified catalysts, and the peak areas of Mo5+ and Mo6+ species are much larger than those of phosphorus-modified catalysts, which indicates that phosphorus modification promotes the sulfidation of Mo species into highly active Mo4+ species.
Table S5 is the analysis of the degree of sulfurization and dispersion of catalysts with different phosphorus contents. It shows that the atomic ratio of Ni atoms to Mo atoms on the surface of catalysts modified by phosphorus is obviously increased. At P = 3.4, the atomic ratio of Ni atoms to Mo atoms on the surface is the largest, which is 0.24% and 0.46% higher than that of the non-modified catalyst, respectively. It can also be seen from Table S5 that phosphorus modification can significantly promote the sulfidation degree of the active components Ni and Mo. The sulfidation degree of Ni increased from 26.53% when the catalyst was not modified to 35.36% when P = 3.4, and the sulfidation degree of Mo increased from 10.41% when the catalyst was not modified to 48.07% when P = 4.2. However, the sulfidation degree of Mo when P = 4.2 was not significantly improved compared with that of when P = 3.4. To conclude, the catalyst shows the optimal surface atomic ratio and sulfidation degree when P = 3.4. The X-ray diffraction (XRD) results also show the same trend. Figure S1 shows the XRD traces of oxidized NiMoP(x)-Al catalysts with different phosphorus contents. The peaks are located at 2θ = 37.2, 39.7, 45.9, and 66.9°, which are attributed to the support, γ-Al2O3. When there is no phosphorus, a peak at 2θ = 27.4° can be attributed to the (0 2 1) plane of MoO3. However, the peak disappears when phosphorus is added to the catalysts, which proves that the addition of phosphorus promotes the dispersion of the metal component on the surface of the support, so MoO3 does not agglomerate on the surface of the support.
2.5. Effect of Phosphorus Modification on the Residue HDN Activity
Table 1 gives the H/C atomic ratio, desulfurization rate, and denitrification rate of oil products before and after hydrotreating. Compared with the feed oil, the sulfur and nitrogen content of the residual after hydrotreating decreases significantly, and the desulfurization rate increases with the increase of phosphorus content, while the denitrification rate increases first and then decreases with the increase of phosphorus content. Generally, the desulfurization rate of the catalysts is higher than the denitrification rate. The maximum desulfurization rate is 70.1% at P = 4.2, and the maximum denitrification rate is 65.6% at P = 3.4, which is consistent with the characterization results of the catalyst with suitable stacking layers: NA = 3.20, the shortest average slab length LA, and the largest effective Mo atom ratio fMo. The degree of sulfidation of the catalyst is the highest, and the sulfide catalyst also has the highest total acid, highest weak acid content, and the largest B/L ratio when P = 3.4. Before modification, the HDS rate and HDN rate of the catalyst were 56.9 and 47.1%, respectively. After phosphorus modification, the HDS rate and HDN rate increased by 13.2 and 18.5%, respectively, which fully shows that the addition of phosphorus is greatly beneficial to the improvement of HDN performance of the catalyst.
Table 1. Results of HDN Reaction Residue Oil on Catalysts.
| catalyst | H/C | S (%) | N (%) | HDS (%) | HDN (%) |
|---|---|---|---|---|---|
| crude oil | 1.64 | 1.40 | 0.34 | ||
| P = 0 | 1.68 | 0.604 | 0.180 | 56.9 | 47.1 |
| P = 3.0 | 1.73 | 0.471 | 0.133 | 66.4 | 60.9 |
| P = 3.4 | 1.71 | 0.440 | 0.117 | 68.6 | 65.6 |
| P = 3.8 | 1.69 | 0.439 | 0.128 | 68.6 | 62.4 |
| P = 4.2 | 1.68 | 0.418 | 0.140 | 70.1 | 58.8 |
3. Conclusions
-
(1)
The addition of phosphorus increases the total acid of the catalyst, especially the weak acid, and increases the B/L ratio. At the same time, the average stacking number of the active phase NA is increased, the average slab length LA is reduced, and the effective Mo atom ratio fMo is increased. The active atomic ratio on the surface and degree of sulfidation are optimized, leading to an increase of the denitrification rate.
-
(2)
With the increase of phosphorus content, the B/L ratio increases first and then decreases, the average stacking number NA increases accordingly, the average slab length LA decreases first and then increases, and fMo increases first and then decreases. With the increase of phosphorus content, the proportion of one and two layers of the active phase decreases, the proportion of the four and five layers of the active phases increases, and the proportion of the three layers of the active phase increases first and then decreases and reaches the largest value when P = 3.4. At the same time, the ratio of the active phase of LA ≤ 3 nm increases obviously and reaches the largest value when P = 3.4. The proportion of the active phase of LA > 3 nm decreases obviously, and decreases the most when P = 3.4.
-
(3)
With the increase of phosphorus content, the desulfurization rate increases, while the denitrification rate increases first and then decreases. The catalyst with P = 3.4 shows the highest B/L ratio, the largest amount of the three-layer active phase, the smallest LA, the largest fMo, the optimal sulfurization degree, and the highest denitrification rate.
4. Experimental Section
4.1. Materials
One molybdenum precursor was MoO3 (industrial grade) and was obtained from the Fushun Catalyst Plant. The nickel precursor, basic nickel carbonate (NiCO3·2Ni(OH)2·4H2O); the phosphorous precursor, H3PO4 (≥85%); another molybdenum precursor, (NH4)6Mo7O24·4H2O; Sesbania powder; HNO3 (65–68%); NH3·H2O (25–28%); and n-heptane were purchased from Sinopharm Chemical Reagent Co., Ltd. Aluminum hydrate xerogel was purchased from Shandong Alumina Plant. All of the materials were used as they were received without further purification. Deionized water was used in the experiments.
4.2. Preparation and Activity Measurement of the Catalysts
4.2.1. Preparation of the Support
Aluminum hydrate xerogel was used as the precursor of the support. Then, Sesbania powder and nitric acid were added into it, mixed, and extruded into strips. The weight ratio of xerogel to water is 0.9:1; the amount of Sesbania powder is 3 wt % of the xerogel, and the amount of nitric acid is 2.5 wt % of the xerogel. The precursor was dried at 120 °C for 4 h, followed by calcination at 450 °C for 4 h to obtain the support.
4.2.2. Preparation of the Catalyst
The impregnating solution was prepared using a condensation method.29 MoO3, NiCO3·2Ni(OH)2·4H2O, and H3PO4 were used as the precursors of Mo, Ni, and P, respectively, in the phosphorus-modified catalysts. When there is no phosphorus in the catalyst, (NH4)6Mo7O24·4H2O is adopted as the precursor of Mo because the solubility of MoO3 is too poor. Diluted NH3·H2O was also added to increase the solubility of the precursors of Mo and Ni. The loading of NiO was 3.6 wt %, and the loading of MoO3 was 16.0 wt %. The loading of phosphorus was calculated based on the mass ratio of P2O5 to the catalyst, and the contents of phosphorus were 0, 3.0, 3.4, 3.8 and 4.2 wt %. The catalysts were made by the incipient wetness impregnation method, followed by drying at 120 °C for 4 h and calcination at 450 °C for 4 h to obtain the oxidized NiMoP(x)-Al catalysts, which were labeled P = 0, P = 3.0, P = 3.4, P = 3.8, and P = 4.2, respectively. Then, the oxidized catalysts were presulfurized in a tubular furnace with a flow of 50 mL·min–1 10 vol % H2S/H2 at 400 °C for 4 h to sulfides.
4.2.3. Activity Measurement of the Catalysts
The HDN reactions were carried out in an autoclave using atmospheric residue as the feed. The reaction conditions are as follows: reaction temperature of 390 °C, reaction pressure of 11 MPa, stirring speed of 300 rpm, and reaction time of 2 h. The oil sample collected after HDN reactions was diluted 10 times with the oil sample/n-heptane volume ratio = 1:9, and the N element content was measured with a Multi EA3100 S/N microanalyzer. The N element content (C) of the oil sample was calculated by the following formula:
| 1 |
where C0 is the measured value by the microanalyzer and its unit is mg/L. C0 is multiplied by 10 because the oil sample was diluted 10 times and 0.686 is the density of n-heptane, which has the unit of kg/L. Then C will carry the unit of mg/kg.
4.3. Characterization of the Catalysts
The NH3-TPD was carried out with a chemisorption analyzer (CHEMBET-3000, Quantachrome). Samples were dried at 500 °C and then adsorbed with NH3 at 70 °C. After physical purging, the temperature was programmed to 700 °C to desorb NH3.
The H2-TPR was carried out with a chemisorption analyzer (CHEMBET-3000, Quantachrome) and the catalyst was heated to 800 °C at a rate of 10 °C min–1 in a mixture of 10 vol % H2–Ar.
Py-IR spectroscopy was conducted using a NEXUS FT-IR spectrometer (Thermo Nicolet Co.). The sample was dehydrated at 300 °C and then pyridine was adsorbed under vacuum conditions. After vacuum desorption at 150 °C, diffuse reflection infrared scanning was performed. The scanning range was 4000–400 cm–1.
HR-TEM was performed on a JEM-2100 UHR microscope (Hitachi, Japan) with an accelerating voltage of 200 kV, LaB6 filament, line resolution of 0.14, and point resolution of 0.23 nm.
XPS was conducted in a PHI 5300 spectrometer (Perkin-Elmer Physics Electronics); Mg Kα (1235.86 eV), power 250 W (12.5 kV × 20 mA), and fixed in Fixed Analyzer Transmission (FAT) mode). The pass selected was full scan 89.45 eV and narrow scan 44.75 eV, and the step size was 1.0 and 0.1 eV, respectively.
Average length of the MoS2 slabs (LA)25,30 was calculated with the following formula
| 2 |
where Ni is the number of layers with a length of the slab of Li (the unit of LA is Å).
The average stacking number of MoS2 layers (NA)25,30 was calculated with the following formula
| 3 |
where Bi is the number of active phase nanoclusters with a layer of Ni.
The dispersion degree of effective Mo atoms fMo(25,30)
| 4 |
where ni is the number of Mo atoms on an edge of a slab (L = 3.2(2ni – 1), the unit of L is Å) and t is the total number of layers of MoS2 nanoclusters in HR-TEM photographs.
Powder XRD patterns of the samples were measured on a PANalytical X′Pert PRO diffractometer with Cu Kα monochromatized radiation (λ = 1.54 Å) operated at 45 kV and 40 mA, with a scan rate of 8° min–1 and a 2θ scan range from 5 to 75°.
Acknowledgments
The authors acknowledge the financial support of the Petrochemical Research Institute, China National Petroleum Corporation (No. PRIKY17084). The authors also acknowledge the technicians of the State Key Laboratory of Heavy Oil Processing, China, for their help with the characterization of the catalysts.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02478.
Acid distribution of oxidized NiMoP(x)-Al catalysts (Table S1); acid distribution of sulfided NiMoP(x)-Al catalysts (Table S2); amounts of the different acid sites of oxidized NiMoP(x)-Al catalysts (Table S3); average stacking number (NA), average slab length (LA), and effective Mo ratio (fMo) (Table S4); analysis of the sulfidation degree and the dispersion of NiMoP(x)-Al catalysts (Table S5); XRD pattern of oxidized NiMoP(x)-Al catalysts (Figure S1) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- van Veen J. A. R. What’s new? On the development of sulphidic HT catalysts before the molecular aspects. Catal. Today 2017, 292, 2–25. 10.1016/j.cattod.2016.09.027. [DOI] [Google Scholar]
- Yang S. H.; Satterfield C. N. Catalytic hydrodenitrogenation of quinoline in a trickle-bed reaction effect hydrogen sulfide. Ind. Eng. Chem. Process Des. Dev. 1984, 23, 20–25. 10.1021/i200024a004. [DOI] [Google Scholar]
- Ferdous D.; Dalai A. K.; Adjaye J. A series of NiMo/Al2O3 catalysts containing boron and phosphorus Part I. Synthesis and characterization. Appl. Catal., A 2004, 260, 137–151. 10.1016/j.apcata.2003.10.010. [DOI] [Google Scholar]
- Ferdous D.; Dalai A. K.; Adjaye J. A series of NiMo/Al2O3 catalysts containing boron and phosphorus Part II. Hydrodenitrogenation and hydrodesulfurization using heavy gas oil derived from Athabasca bitumen. Appl. Catal., A 2004, 260, 153–162. 10.1016/j.apcata.2003.10.009. [DOI] [Google Scholar]
- Zhou T. N.; Yin H. L.; Liu Y. Q.; Han S. N.; Chai Y. M.; Liu C. G. Effect of phosphorus content on the active phase structure of NiMoP/Al2O3 catalyst. J. Fuel Chem. Technol. 2010, 38, 69–74. 10.1016/S1872-5813(10)60020-5. [DOI] [Google Scholar]
- Kaluža L.; Palcheva R.; Spojakina A.; Jirátová K.; Tyuliev G. Hydrodesulfurization NiMo catalysts supported on Co, Ni and B modified Al2O3 from Anderson heteropolymolybdates. Procedia Eng. 2012, 42, 873–884. 10.1016/j.proeng.2012.07.480. [DOI] [Google Scholar]
- Panwar O. S.; Khan M. A.; Satyanarayana B. S.; Kumar S.; Ishpal Properties of boron and phosphorous incorporated tetrahedral amorphous carbon films grown using filtered cathodic vacuum arc process. Appl. Surf. Sci. 2010, 256, 4383–4390. 10.1016/j.apsusc.2010.02.035. [DOI] [Google Scholar]
- Iwamoto R.; Grimblot J. Influence of Phosphorus on the Properties of Alumina-Based Hydrotreating Catalysts. Adv. Catal. 1999, 44, 417–503. 10.1016/S0360-0564(08)60516-7. [DOI] [Google Scholar]
- Yoshimura Y.; Matsubayashi N.; Sato T.; Shimada H.; Nishijima A. Molybdate catalysts prepared by a novel impregnation method: effect of citric acid as a ligand on the catalytic activities. Appl. Catal., A 1991, 79, 145–159. 10.1016/0926-860X(91)80001-F. [DOI] [Google Scholar]
- Nicosia D.; Prins R. The effect of glycol on phosphate-doped CoMo/Al2O3 hydrotreating catalysts. J. Catal. 2005, 229, 424–438. 10.1016/j.jcat.2004.11.014. [DOI] [Google Scholar]
- van Veen J. A. R.; Gerkema E.; van der Kraan A. M.; Knoester A. A real support effect on the activity of fully sulphided CoMoS for the hydrodesulphurization of thiophene. J. Chem. Soc., Chem. Commun. 1987, 22, 1684–1686. 10.1039/c39870001684. [DOI] [Google Scholar]
- Solís D.; Agudo A. L.; Ramírez J.; Klimova T. Hydrodesulfurization of hindered dibenzothiophenes on bifunctional NiMo catalysts supported on zeolite–alumina composites. Catal. Today 2006, 116, 469–477. 10.1016/j.cattod.2006.06.029. [DOI] [Google Scholar]
- Lewis J. M.; Kydd R. A.; Boorman P. M.; Rhyn P. H. V. Phosphorus promotion in nickel-molybdenum/alumina catalysts: model compound reactions and gas oil hydroprocessing. Appl. Catal., A 1992, 84, 103–121. 10.1016/0926-860X(92)80110-X. [DOI] [Google Scholar]
- Lewandowski M.; Sarbak Z. The effect of boron addition on texture and structure of NiMo/Al2O3 catalysts. Cryst. Res. Technol. 1997, 32, 499–508. 10.1002/crat.2170320403. [DOI] [Google Scholar]
- López Cordero R.; et al. Formation of Al2(MoO4)3 and MoO3 phases induced by phosphate in molybdena-phosphorus catalysts. J. Catal. 1990, 126, 8–12. 10.1016/0021-9517(90)90041-H. [DOI] [Google Scholar]
- Guichard B.; Roy-Auberger M.; Devers E.; Legens C.; Raybaud P. Aging of Co(Ni)MoP/Al2O3 catalysts in working state. Catal. Today 2008, 130, 97–108. 10.1016/j.cattod.2007.09.007. [DOI] [Google Scholar]
- Ferdous D.; Dalai A. K.; Adjaye J.; Kotlyar L. Surface morphology of NiMo/Al2O3 catalysts incorporated with boron and phosphorus: Experimental and simulation. Appl. Catal., A 2005, 294, 80–91. 10.1016/j.apcata.2005.07.025. [DOI] [Google Scholar]
- Vatutina Y. V.; Klimov O. V.; Stolyarova E. A.; Nadeina K. A.; Danilova I. G.; Chesalov Y. A.; Gerasimov E. Y.; Prosirin I. P.; Noskov A. S. Influence of the phosphorus addition ways on properties of CoMo-catalysts of hydrotreating. Catal. Today 2019, 329, 13–23. 10.1016/j.cattod.2019.01.005. [DOI] [Google Scholar]
- Rayo P.; Ramírez J.; Torres-Mancera P.; Marroquín G.; Maity S. K.; Ancheyta J. Hydrodesulfurization and hydrocracking of Maya crude with P-modified NiMo/Al2O3 catalysts. Fuel 2012, 100, 34–42. 10.1016/j.fuel.2011.12.004. [DOI] [Google Scholar]
- Shi L.; Zhang Z. H.; Qiu Z. G.; Guo F.; Zhang W.; Zhao L. F. Effect of phosphorus modification on the catalytic properties of Mo-Ni/Al2O3 in the hydrodenitrogenation of coal tar. J. Fuel Chem. Technol. 2015, 43, 74–80. 10.1016/S1872-5813(15)60007-X. [DOI] [Google Scholar]
- Gishti K.; Iannibello A.; Marengo S.; Morellili G.; Tittarelli P. On the role of phosphate anion in the MoO3-Al2O3 based catalysts. Appl. Catal. 1984, 12, 381–393. 10.1016/S0166-9834(00)81675-9. [DOI] [Google Scholar]
- Morales A.; Ramirez M. M.; Hernandes F. Adsorption mechanism of phosphorus on alumina. Appl. Catal. 1988, 41, 261–271. 10.1016/S0166-9834(00)80397-8. [DOI] [Google Scholar]
- de Mello M. D.; de Almeida Braggio F.; da Costa Magalhães B.; Zotin J. L.; da Silva M. A. P. Effects of Phosphorus Content on Simultaneous Ultradeep HDS and HDN Reactions over NiMoP/Alumina Catalysts. Ind. Eng. Chem. Res. 2017, 56, 10287–10299. 10.1021/acs.iecr.7b02718. [DOI] [Google Scholar]
- de Mello M. D.; de Almeida Braggio F.; da Costa Magalhães B.; Zotin J. L.; da Silva M. A. P. Kinetic modeling of deep hydrodesulfurization of dibenzothiophenes on NiMo/alumina catalysts modified by phosphorus. Fuel Process. Technol. 2018, 177, 66–74. 10.1016/j.fuproc.2018.04.010. [DOI] [Google Scholar]
- Zhou W. W.; Zhang Q.; Zhou Y. S.; Wei Q.; Du L.; Ding S. J.; Jiang S. J.; Zhang Y. N. Effects of Ga- and P-modified USY-based NiMoS catalysts on ultra-deep hydrodesulfurization for FCC diesels. Catal. Today 2018, 305, 171–181. 10.1016/j.cattod.2017.07.006. [DOI] [Google Scholar]
- Sundaramurthy V.; Dalai A. K.; Adjaye J. The effect of phosphorus on hydrotreating property of NiMo/g-Al2O3 nitride catalyst. Appl. Catal., A 2008, 335, 204–210. 10.1016/j.apcata.2007.11.024. [DOI] [Google Scholar]
- Lewandowski M.; Sarbak Z. The effect of boron addition on hydrodesulfurization and hydrodenitrogenation activity of NiMo/Al2O3 catalysts. Fuel 2000, 79, 487–495. 10.1016/S0016-2361(99)00151-9. [DOI] [Google Scholar]
- Soltanali S.; Mashayekhi M.; Mohaddecy S. R. S. Comprehensive investigation of the effect of adding phosphorus and/or boron to NiMo/γ-Al2O3 catalyst in diesel fuel hydrotreating. Process Saf. Environ. Prot. 2020, 137, 273–281. 10.1016/j.psep.2020.02.033. [DOI] [Google Scholar]
- Lu X. H.; He J. H.. One Method of Impregnating Liquid and Catalyst Preparation. Chinese Patent CN1172692A1998.
- Hensen E. J. M.; Kooyman P. J.; van der Meer Y.; van der Kraan A. M.; de Beer V. H. J.; van Veen J. A. R.; van Santen R. A. The Relation between Morphology and Hydrotreating Activity for Supported MoS2 Particles. J. Catal. 2001, 199, 224–235. 10.1006/jcat.2000.3158. [DOI] [Google Scholar]
- Jian M.; Kapteijn F.; Prins R. Kinetics of the Hydrodenitrogenation of ortho-Propylaniline over NiMo(P)/Al2O3 Catalysts. J. Catal. 1997, 168, 491–500. 10.1006/jcat.1997.1650. [DOI] [Google Scholar]
- Qu L.; Zhang W.; Kooyman P. J.; Prins R. MAS NMR, TPR, and TEM studies of the interaction of NiMo with alumina and silica-alumina supports. J. Catal. 2003, 215, 7–13. 10.1016/S0021-9517(02)00181-1. [DOI] [Google Scholar]
- Payen E.; Hubaut R.; Kasztelan S.; Poulet O.; Grimblot J. Morphology Study of MoS2- and WS2-Based Hydrotreating Catalysts by High-Resolution Electron Microscopy. J. Catal. 1994, 147, 123–132. 10.1006/jcat.1994.1122. [DOI] [Google Scholar]
- Pawelec B.; Navarro R. M.; Campos-Martin J. M.; Agudo A. L.; Vasudevan P. T.; Fierro J. L. G. Silica-alumina-supported transition metal sulphide catalysts for deep hydrodesulphurization. Catal. Today 2003, 86, 73–85. 10.1016/S0920-5861(03)00405-X. [DOI] [Google Scholar]
- Wang X.; Ozkan U. S. Characterization of active sites over reduced Ni-Mo/Al2O3 catalysts for hydrogenation of linear aldehydes. J. Phys. Chem. B 2005, 109, 1882–1890. 10.1021/jp046489q. [DOI] [PubMed] [Google Scholar]
- Hayden T. F.; Dumesic J. A. Studies of the structure of molybdenum oxide and sulfide supported on thin films of alumina. J. Catal. 1987, 103, 366–384. 10.1016/0021-9517(87)90128-X. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




