Abstract
Background
We recently reported that the levels of activation, exhaustion, and terminal differentiation within the peripheral T-cell compartment were increased in men who have sex with men (MSM) compared with blood bank donors. During activation and differentiation, T cells undergo metabolic changes to maintain their energy demand.
Methods
The effect of cytomeglovirus (CMV) infection and risk behavior on the immune phenotype of peripheral T cells and the immune bioenergy metabolism profile in human immunodeficiency virus-negative MSM (with high or low sexual risk behavior) and blood bank donors was evaluated.
Results
Men who have sex with men exhibited increased levels of T-cell activation and terminal differentiation and an impairment of the bioenergy metabolism (mitochondrial respiration and glycolysis) compared with blood bank donors. Cytomeglovirus infection was associated with increased terminal differentiation of CD4+ (B = 3.41; 95% confidence interval [CI], 1.98–4.85; P < .0001) and CD8+ T cells (CD57+: B = 1.21, 95% CI = 0.41–2.02, P = .004; CD27−CD28−: B = 2.20, 95% CI = 1.21–3.18, P < .0001; and CD57+ of CD28−: B = 1.02, 95% CI = 0.38–1.66, P = .002) and increased glycolysis (B = 0.97; 95% CI, 0.27–1.67; P = .007). Risk behavior was associated with increase activation of CD4+ T cells (B = 0.22; 95% CI, 0.07–0.37; P = .005), increased terminal differentiation of CD4+ (B = 0.82; 95% CI, 0.44–1.20; P < .0001) and CD8+ T cells (B = 1.55; 95% CI, 0.58–2.51; P = .002), and decreased glycolysis (glycolysis: B = −0.40, 95% CI = −0.68 to 0.12, P = .006; and glycolytic capacity: B = −0.54, 95% CI = −0.91 to 0.16, P = .005).
Conclusions
Men who have sex with men show an increased prevalence of bloodborne and sexually transmitted infection, indicating that immunological changes in the T-cell population and the bioenergy metabolism observed in MSM can most likely be attributed to chronic antigen exposure.
Keywords: bioenergy, CMV, MSM, risk behavior, T cells
MSM exhibited increased levels of T-cell activation and terminal differentiation and an impairment of the bioenergy metabolism. Immunological and bioenergy metabolic changes can most likely be attributed to the overall higher infection pressure in MSM.
CD8+ T-cell responses play a crucial role in the defense against viral infections. However, in chronic infections such as cytomeglovirus (CMV), hepatitis B virus, hepatitis C virus, and human immunodeficiency virus infection (HIV), virus-specific CD8+ T cells are unable to clear the infection, which results in continuous stimulation of the immune system by persistent exposure to viral antigen. This chronic antigen stimulation has been shown to induce T-cell exhaustion and senescence by increasing expression of multiple inhibitory receptors (eg, programmed cell death 1 [PD-1], cytotoxic T lymphocyte-associated protein 4 and T-cell immunoglobulin [Ig], and mucin-domain containing-3), thereby affecting T-cell functionality [1]. Moreover, it has been demonstrated that memory CD8+ T cells exhibit a distinct virus-specific activation and differentiation profile, suggesting that the CD8+ T-cell functionality can be affected in a virus-specific manner [2, 3].
It has recently become clear that metabolic changes are required to maintain the energy demands of T cells upon antigen stimulation, supporting cellular activation, proliferation, differentiation, and functionality. Quiescent naive T cells depend on catabolic metabolism to generate adenosine triphosphate (ATP) for their energy requirement, relying predominantly on mitochondrial respiration [4, 5]. When T cells encounter antigen, a metabolic switch towards glycolysis is required to meet the increased energetic demands necessary for growth, proliferation, and biosynthesis of mediators of effector functions. The metabolic reprogramming required for differentiation into effector T cells relies on CD28 costimulatory signals, which is crucial for activating glycolysis [5–8]. In chronic infection, virus-specific T cells displayed an altered expression of genes involved in the bioenergy metabolism, implicating that metabolic changes may play an important role in T-cell exhaustion and dysfunction observed during chronic viral infection [9, 10].
We recently reported that the levels of activation, exhaustion, and terminal differentiation within the T-cell population were increased in HIV-negative men who have sex with men (MSM) from the COBRA study compared with blood bank donors (BBD), which could at least in part be attributed to the high prevalence of CMV infection in this population [11–15]. Moreover, MSM with high-risk behavior show an increased prevalence of bloodborne and sexually transmitted infections (STIs) [16], which are likely to contribute to the observed immunological changes. In the current study, we evaluated whether changes in immunological markers of T-cell activation, exhaustion, and (terminal) differentiation and the bioenergy metabolism profile of peripheral blood mononuclear cells (PBMCs) are associated with CMV infection and risk behavior in MSM.
MATERIALS AND METHODS
Study Subjects
The Amsterdam Cohort studies (ACS) among MSM is a prospective cohort study that started in 1984 to investigate the prevalence, incidence, and risk factors of HIV and other bloodborne infections and STIs [17, 18]. Questionnaires about sexual behavior and blood samples were collected during 6 monthly study visits at the Public Health Service of Amsterdam. Fifty-seven HIV-1-seronegative MSM participating in the ACS with available cryopreserved PBMC samples were included in this study, 35 of whom with high-risk sexual behavior (hr-MSM). High-risk was determined as having at least 1 reported episode of a sexually transmitted infection (syphilis, gonorrhea, chlamydia) and having reported unprotected receptive anal intercourse with a partner with unknown or seropositive HIV status, and 22 did not report high-risk behavior (lr-MSM). Twenty-four of the 35 hr-MSM reported pre-exposure prophylaxis (PrEP) use at the time of blood sampling. An additional control group comprised 31 BBD from the Dutch national blood bank in Amsterdam, the Netherlands (www.sanquin.nl). This study has been conducted in accordance with the ethical principles set out in the declaration of Helsinki and was approved by the institutional review board of the Academic Medical Center and the Ethics Advisory Body of the Sanquin Blood Supply Foundation in Amsterdam. Written informed consent was obtained from all participants.
T-Cell Phenotyping and Flow Cytometry
Cryopreserved PBMCs were used for immune phenotyping. Peripheral blood mononuclear cells were thawed and stained with monoclonal antibodies (mAbs) (30 minutes at 4°C in the dark). The following directly conjugated mAbs were used for cell surface marker staining: CD3 V500, CD4 PE-Cy7, CD8 Pacific Blue, CD45RA PE-Cy7, CCR7 PE, HLA-DR FITC, CD38 PE, CD27 PerCP Cy5.5, CD28 PerCP Cy5.5, CD57 APC (BD Biosiences, San Jose, CA), CD4 APC eFluor780, CD27 APC eFluor780, and PD-1 PE (eBioscience, San Diego, CA). Fluorescence was measured with the FACS Canto II (BD Biosciences). The proportion of T cells expressing each marker and the mean fluorescence intensity were determined using FlowJo 7.6 (TreeStar, Ashland, OR).
Cytomeglovirus Antibody Titers
Cytomeglovirus total antibody titers were measured by ELISA-VIDITEST anti-CMV-IgG (VIDIA; Praha, Czech Republic) according to the manufacturer’s instruction.
Cellular Bioenergy Metabolism
Mitochondrial respiration and glycolysis were determined by measuring the oxygen consumption rate (OCR) and extracellular acidification rate (ECAR using the Mito Stress test and Glycolysis Stress test and analysis on the Seahorse XFe96 Extracellular Flux Analyzer according to manufacturer’s protocol (Agilent, Santa Clara, CA).
Seahorse XF RPMI Base Medium without phenol red (Agilent) was supplemented with L-glutamine (2 mM) for both tests and additionally with sodium pyruvate (1 mM) and glucose (25 mM) (Sigma-Aldrich) for the Mito Stress test. Peripheral blood mononuclear cells were plated in 4 replicate wells in poly-d-lysine-coated Seahorse XFe96 wells plate at a density of 200 000 cells/well. The culture plate was placed in a 37°C non-CO2 incubator to equilibrate.
After calibration with XF Calibrant, the culture plate was loaded into the Seahorse XFe96 analyzer to determine OCR and ECAR. Assay drugs were injected in the following order and with the following final concentrations: 1 µM oligomycin, 1.5 µM fluoro-carbonyl cynade phenylhydrazon, and 0.1 µM antimycin A + 1.0 µM rotenone for the Mito Stress Test; 10 mM glucose, 1 µM oligomycin, and 50 mM 2-deoxy-d-glucose (2-DG) for the Glycolysis Stress Test. Analyses were performed in the Seahorse Wave software (Agilent) to determine key parameters of mitochondrial respiration and glycolysis using OCR and ECAR.
Statistics
Differences in the immunological markers or cell bioenergy metabolism markers between the 3 study groups (BBDs, lr-MSM, and hr-MSM) were determined by linear regression adjusted for age. Multivariable linear regressions were performed to determine whether CMV seropositivity and risk behavior (high risk and low risk) were independently associated with the immunological markers or cell bioenergy metabolism markers. Some outcomes were transformed to obtain normality as indicated in the tables. Correction for multiple testing was performed using the Bonferroni procedure. Analyses were performed in IBM SPSS Statistics for Windows v.25 (IBM, Armonk, NY).
RESULTS
T-Cell Activation and Differentiation in Men Who Have Sex With Men and Blood Bank Donors
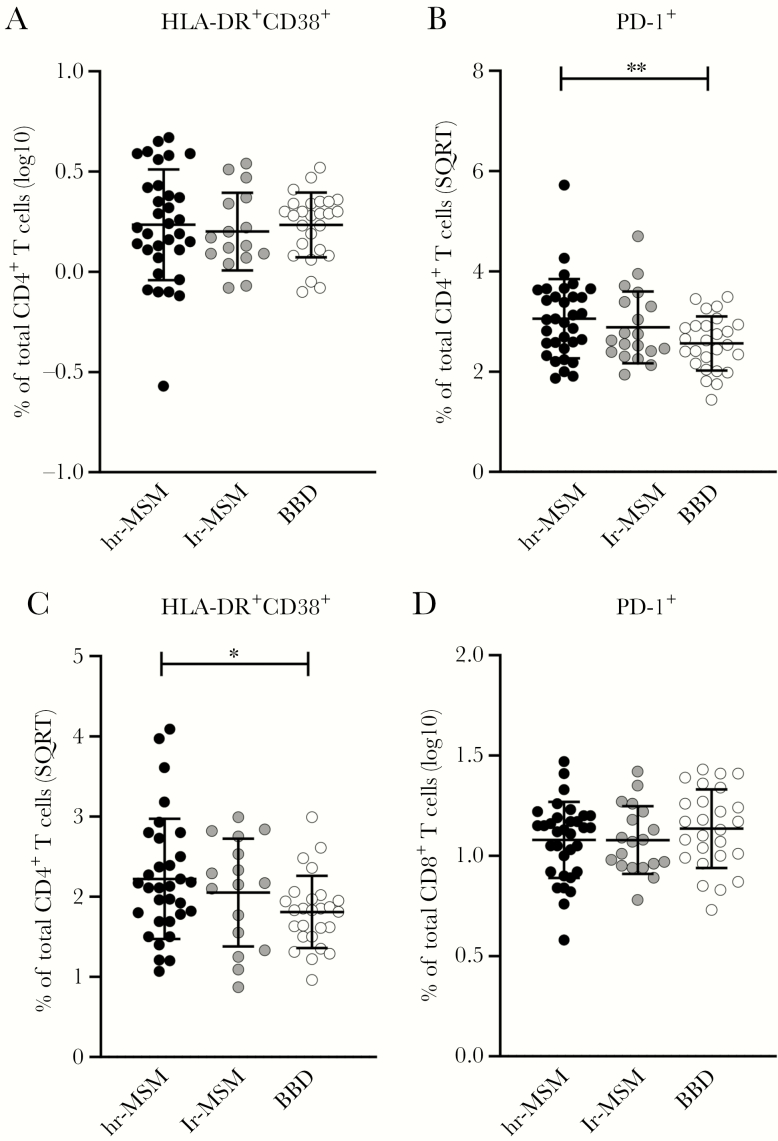
T-cell phenotype was compared between 3 study groups: BBDs (n = 31), hr-MSM (n = 35), and lr-MSM (n = 22). Activation and exhaustion of CD4+ and CD8+ T cells was determined by the percentage of HLA-DR+CD38+-expressing cells and the percentage of PD-1+-expressing cells. There were no differences in the percentage of activated CD4+ T cells between the study groups (Figure 1A and Supplementary Table S1), whereas the percentage of exhausted CD4+ T cells was higher in the hr-MSM compared with the BBD (Figure 1B and Supplementary Table S1). Within the CD8+ T-cell population, the percentage of activated cells was higher in hr-MSM compared with the BBD (Figure 1C and Supplementary Table S1), whereas no differences in the percentage of exhausted CD8+ T cells between the 3 groups were observed (Figure 1D and Supplementary Table S1). However, none of these differences were significant after Bonferroni correction (Supplementary Table S1).
Figure 1.
CD4+ T-cell activation, exhaustion, differentiation, and terminal differentiation in high-risk and low-risk men who have sex with men (MSM) and blood bank donors (BBD). The normalized percentages of activated (HLA-DR+CD38+) CD4+ T-cells within the total CD4+ T-cell population (A) and activated/exhausted (programmed cell death 1 [PD-1]+) CD4+ T cells within the total CD4+ T-cell population (B). The normalized percentages of activated (HLA-DR+CD38+) CD8+ T cells within the total CD8+ T-cell population (C) and activated/exhausted (PD-1+) CD8+ T cells within the total CD8+ T-cell population (D). Men who have sex with men with high-risk sexual behavior (hr-MSM), n = 34; MSM without high-risk sexual behavior (lr-MSM), n = 19; blood bank donors (BBD), n = 26. Significance was assessed on normalized data with multivariable linear regression, corrected for age (*, P < .05; **, P < .01; significance after Bonferroni correction in red and bold). Data represent mean ± standard deviation. Some individuals were excluded from analysis due to insufficient cell numbers. SQRT, square root.
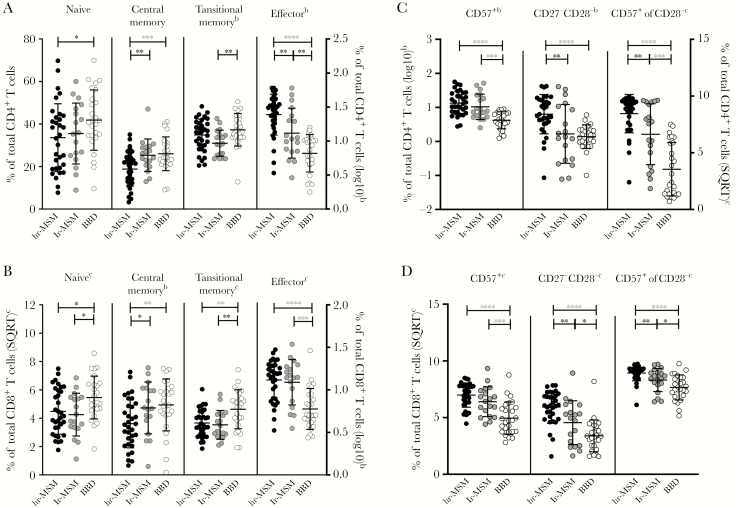
T-cell differentiation was assessed by analyzing the proportion of naive (CD45RA+CD27+CCR7+), central memory (CD45RA−CCR7+CD27+), transitional memory (CD45RA−CCR7−CD27+), and effector cells (CCR7−CD27−) within the total CD4+ or CD8+ T-cell population. Within total CD4+ T cells, lr-MSM had decreased percentages of transitional memory cells and increased percentages of effector T cells compared with BBD (Figure 2A and Supplementary Table S1). A decreased percentage of naive cells was observed in hr-MSM compared with BBD. Again, these difference were not significant after correction. Yet, a significant lower percentage of central memory cells and higher percentage of effector T cells was seen in hr-MSM compared with BBD (Figure 2A and Table S1). The lr-MSM had a higher percentage of central memory cells and lower percentage of effector T cells compared with hr-MSM albeit not significant of correction for multiple testing (Figure 2A and Supplementary Table S1).
Figure 2.
CD8+ T-cell activation, exhaustion, differentiation, and terminal differentiation in high-risk and low-risk men who have sex with men (MSM) and blood bank donors (BBD). The normalized percentages of T-cell subsets within the total CD4+ (A) and CD8+ T-cell population (B). The normalized percentages of CD57+, CD27−CD28−, and CD57+ expressing cells within CD28− cells within the total CD4+ (C) and CD8+ T-cell population (D). Men who have sex with men with high-risk sexual behavior (hr-MSM), n = 34; MSM without high-risk sexual behavior (lr-MSM), n = 19; blood bank donors (BBD), n = 26. Significance was assessed on normalized data with multivariable linear regression, corrected for age (*, P < .05; **, P < .01; ***, P < .001; ****, P < .0001; significance after Bonferroni correction in red and bold). Data represent mean ± standard deviation. Some individuals were excluded from analysis due to insufficient cell numbers. SQRT, square root.
Compared with BBD, lr-MSM had lower percentages of naive and transitional memory cells and a significantly higher percentage of effector T cells within the CD8+ T-cell population (Figure 2B and Supplementary Table S1). Lower percentages of naive and central memory T cells and a significantly lower percentage of transitional memory cells and higher percentage of effector cells were observed in hr-MSM compared with BBDs (Figure 2B and Supplementary Table S1). When comparing lr-MSM and hr-MSM, only a decreased percentage of central memory cells was observed in hr-MSM; however, this difference was not significant after correction (Figure 2B and Supplementary Table S1).
Terminal differentiation of CD4+ and CD8+ T cells was determined by the proportion of cells that expressed high levels of CD57 or loss of the expression of the costimulatory molecules CD27 and CD28. Within the CD4+ T cells, terminal differentiation as measured by the percentage of CD57+ cells and the percentage of CD57-expressing cells within the CD28−CD4+ cells was significantly higher in lr-MSM and hr-MSM compared with BBDs (Figure 2C and Supplementary Table S1). The percentage of CD27−CD28− cells within the CD4+ T-cell population was significantly higher in hr-MSM, but not in lr-MSM, compared with BBDs (Figure 2C and Supplementary Table S1). In the CD8+ T-cell compartment, terminal differentiation as measured by the percentage of CD57+ cells, CD27−CD28− cells, and CD57-expressing cells within the CD28−CD8+ cells was higher in lr-MSM and hr-MSM compared with BBD (Figure 2D and Supplementary Table S1), and these differences remained significant after Bonferroni correction when comparing hr-MSM and BBDs (Supplementary Table S1). Moreover, hr-MSM had higher percentages of CD27−CD28− cells and CD57-expressing cells within the CD28− cells in both CD4+ and CD8+ T cells compared with lr-MSM, albeit not significant after Bonferroni correction (Figure 2C and D and Supplementary Table S1).
Bioenergy Metabolism of Peripheral Blood Mononuclear Cell From Men Who Have Sex With Men and Blood Bank Donors
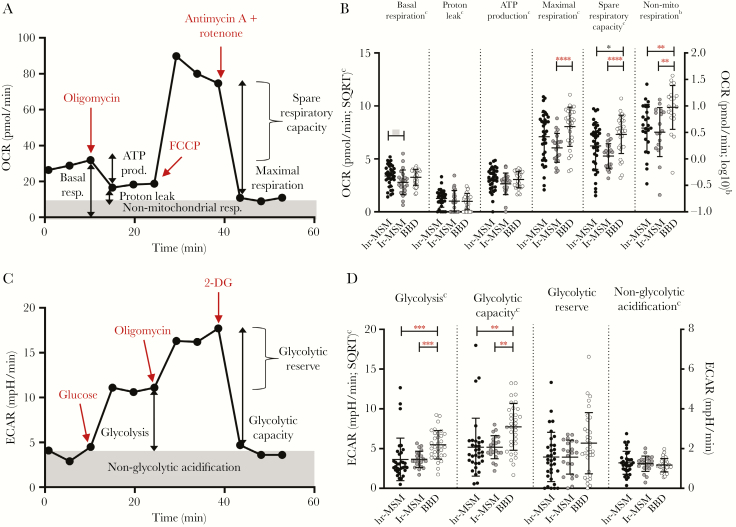
The bioenergetics profile of PBMCs from hr-MSM, lr-MSM, and BBD was assessed to evaluate mitochondrial respiratory function, by analyzing the OCR at different stages of the mitochondrial respiration, ie, basal respiration, proton leakage, ATP production, maximal respiration, spare respiratory capacity, and nonmitochondrial respiration (Figure 3A). Compared with BBD, lr-MSM had a significant decreased maximal respiration, spare respiratory capacity, and nonmitochondrial respiration (Figure 3B and Supplementary Table S2). A decreased spare respiratory capacity and nonmitochondrial respiration was also observed in hr-MSM compared with BBD, differences of which in nonmitochondrial respiration remain significant after multiple testing correction (Figure 3B and Supplementary Table S2). In hr-MSM, the basal respiration was higher compared with lr-MSM, but this difference was not significant after Bonferroni correction (Figure 3B and Supplementary Table S2).
Figure 3.
Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) of peripheral blood mononuclear cells (PBMCs) from high-risk and low-risk men who have sex with men (MSM) and blood bank donors (BBD). (A) Overview of the bioenergetics profile, of 1 representative BBD, used to evaluate mitochondrial respiratory function of PBMCs from MSM with high-risk sexual behavior (hr-MSM), MSM without high-risk sexual behavior (lr-MSM), and BBD. A sequential addition of specific inhibitors and uncouplers (in red) of the electron transport chain provides data of the OCR relative to the different components of mitochondrial respiratory function. (B) The normalized OCR measured at different stages of the mitochondrial respiration, ie, basal respiration, proton leakage, adenosine triphosphate (ATP) production, maximal respiration, spare respiratory capacity, and nonmitochondrial respiration in the different study groups. (C) Overview of the bioenergetics profile, of a representative BBD, used to evaluate glycolytic function of PBMCs from hr-MSM, lr-MSM and BBD. A sequential addition of specific stimulators and inhibitors (in red) of glycolysis provides data of the ECAR relative to the different components of glycolytic function. (D) The normalized ECAR measured at different stages of the glycolysis pathway, ie, glycolysis, glycolytic capacity, glycolytic reserve, and nonglycolytic acidification in the different study groups: hr-MSM, n = 34; lr-MSM, n = 22; BBD, n = 31. Significance was assessed on normalized data with multivariable linear regression, corrected for age (*, P < .05; **, P < .01; ***, P < .001; ****, P < .0001; significance after Bonferroni correction in red and bold). Data represent mean ± standard deviation. Some individuals were excluded from analysis due to insufficient cell numbers. SQRT, square root.
Glycolytic activity of PBMCs from hr-MSM, lr-MSM, and BBD was determined by the ECAR analyzed at different stages of the glycolysis pathway, ie, glycolysis, glycolytic capacity, glycolytic reserve, and nonglycolytic acidification (Figure 3C). Both lr-MSM and hr-MSM had significantly lower levels of glycolysis and glycolytic capacity compared with BBDs (Figure 3D and Supplementary Table S2). No other differences were observed in the glycolytic pathway between the 3 groups.
Cytomeglovirus Infection and Risk Behavior Are Independently Associated With Terminal Differentiation of T Cells and Glycolysis
Previous studies have shown that CMV infection is associated with increased expansion of terminally differentiated and senescent T cells [11–15]. Indeed, we observed higher frequencies of CMV infection in both lr-MSM (77.3%) and hr-MSM (91.4%) compared with BBD (29.0%) as determined by the presence of CMV-specific IgG in plasma. Moreover, risk behavior in MSM has been associated with an increased prevalence of bloodborne infections and STIs [16]. We analyzed whether CMV infection and risk behavior were associated with changes in the T-cell compartment and the bioenergy metabolism. Multivariable linear regression analysis showed that CMV serostatus was significantly associated with decreased transitional memory T cells, and increased effector cells and terminal differentiation (%CD57+, %CD27−CD28−, %CD57+ of CD28−) within CD8+ T cells and terminal differentiation (%CD57+ of CD28−) within CD4+ T cells after correction for multiple testing (Table 1). High-risk behavior was independently associated with increased CD4+ T-cell activation (HLA-DR+CD38+) and terminal differentiation (CD27−CD28−) of CD4+ and CD8+ T cells after Bonferroni correction (Table 1).
Table 1.
Multivariable Analyses of Associations Between Sexual Risk Behavior, CMV Serostatus, and T-Cell Differentiation, Activation, Exhaustion, and Terminal Differentiation
| Markers | Variable | B (95% CI) | P Valuea | B (95% CI) | P Valuea | |
|---|---|---|---|---|---|---|
| Activation and Exhaustion | CD4+ T Cells | CD8+ T Cells | ||||
| %HLA-DR+CD38+ | Group | BBD (ref) | ||||
| lr-MSM | −0.20 (−0.39 to 0.02)b | .03 | 0.00 (−0.59 to 0.59)c | 1.00 | ||
| hr-MSM | 0.22 (0.07–0.37)b | .005 | 0.53 (0.06–1.00)c | .03 | ||
| CMV | 0.06 (−0.09 to 0.20)b | .45 | 0.14 (−0.32 to 0.60)c | .55 | ||
| %PD-1+ | Group | BBD (ref) | ||||
| lr-MSM | 0.29 (−0.37 to 0.95)c | .39 | −0.01 (−0.18 to 0.17)b | .95 | ||
| hr-MSM | 0.36 (−0.15 to 0.86)c | .16 | 0.06 (−0.08 to 0.19)b | .41 | ||
| CMV | 0.15 (−0.37 to 0.67)c | .56 | −0.09 (−0.23 to 0.05)b | .21 | ||
| T-Cell Differentiation | ||||||
| %Naive | Group | BBD (ref) | ||||
| lr-MSM | −4.00 (−17.22 to 9.21) | .55 | 0.44 (−0.75 to 1.62)c | .47 | ||
| hr-MSM | −9.85 (−20.17 to 0.46) | .06 | −0.94 (−1.87 to 0.02)c | .05 | ||
| CMV | −0.21 (−10.56 to 10.13) | .97 | −1.03 (−1.96 to 0.10)c | .03 | ||
| %Central memory | Group | BBD (ref) | ||||
| lr-MSM | 7.17 (0.03–14.31) | .05 | 0.26 (−0.01 to 0.52)b | .06 | ||
| hr-MSM | −4.53 (−10.11 to 1.04) | .11 | −0.24 (−0.45 to 0.04)b | .02 | ||
| CMV | −6.56 (−12.15 to 0.97) | .02 | −0.16 (−0.37 to 0.04)b | .12 | ||
| %Transitional memory | Group | BBD (ref) | ||||
| lr-MSM | −0.15 (−0.36 to 0.06)b | .16 | −0.01 (−1.03 to 1.01)c | .98 | ||
| hr-MSM | 0.14 (−0.03 to 0.30)b | .10 | 0.65 (−0.14 to 1.45)c | .11 | ||
| CMV | −0.09 (−0.25 to 0.08)b | .28 | −1.23 (−2.02 to 0.43)c | .003 | ||
| %Effector | Group | BBD (ref) | ||||
| lr-MSM | 0.00 (−0.28 to 0.28)b | .98 | −0.32 (−1.48 to 0.85)b | .59 | ||
| hr-MSM | 0.30 (0.08–0.52)b | .008 | 0.57 (−0.34 to 1.47)b | .22 | ||
| CMV | 0.31 (0.09–0.53)b | .007 | 1.97 (1.05–2.88)b | 5.7e-05 | ||
| Terminal Differentiation | ||||||
| %CD57+ | Group | BBD (ref) | ||||
| lr-MSM | 0.13 (−0.16 to 0.42)b | .37 | −0.33 (−1.36 to 0.69)c | .52 | ||
| hr-MSM | 0.24 (0.02–0.46)b | .03 | 1.00 (0.22–1.79)c | .01 | ||
| CMV | 0.19 (−0.04 to 0.41)b | .11 | 1.21 (0.41–2.02)c | .004 | ||
| %CD27−CD28− | Group | BBD (ref) | ||||
| lr-MSM | −0.46 (−0.96 to 0.05)b | .08 | −1.10 (−2.36 to 0.16)c | .09 | ||
| 0.82 (0.44–1.20)b | 6.4e-05 | 1.55 (0.58–2.51)c | .002 | |||
| CMV | hr-MSM | 0.55 (0.13–0.97)b | .01 | 2.20 (1.21–3.18)c | 3.2e-05 | |
| %CD57+ of CD28− | Group | BBD (ref) | ||||
| lr-MSM | 0.23 (−1.61 to 2.06)c | .81 | −0.58 (−1.40 to 0.23)c | .16 | ||
| hr-MSM | 1.50 (0.10–2.91)c | .04 | 0.53 (−0.10 to 1.15)c | .10 | ||
| CMV | 3.41 (1.98–4.85)c | 1.1e-05 | 1.02 (0.38–1.66)c | .002 |
Abbreviations: B, unstandardized regression coefficient; BBD, blood bank donors; CI, confidence interval; CMV, cytomegalovirus; hr-MSM, men who have sex with men with high-risk sexual behavior; lr-MSM, MSM without high-risk sexual behavior; MSM, men who have sex with men; PD-1, programmed cell death 1; ref, reference group.
a P value of multivariable linear regression corrected for age. Unadjusted P values are given. Bonferroni correction for multiple testing required a P < .006 for statistical significance (bold).
bLog transformed to obtain normality.
cSquare root transformed to obtain normality.
Changes in the mitochondrial respiration could not be attributed to CMV serostatus or high-risk behavior; however, decreased mitochondrial respiration as measured by the maximal respiration and spare respiratory capacity was significantly associated with the lr-MSM group (Table 2). Cytomeglovirus serostatus did affect glycolysis, and being CMV positive was significantly associated with increased glycolytic reserve after Bonferroni correction (Table 2). In contrast, a significant decreased glycolysis and glycolytic capacity was associated with the lr-MSM group (Table 2).
Table 2.
Multivariable Analyses of Associations Between Sexual Risk Behavior, CMV Serostatus, and Markers of Mitochondrial Respiration and Glycolysis
| Markers | Variable | B (95% CI) | P Valuea | |
|---|---|---|---|---|
| Mitochondrial Respiration | ||||
| Basal respirationb | Group | BBD (ref) | ||
| lr-MSM | −0.20 (−0.88 to 0.48) | .56 | ||
| hr-MSM | 0.24 (−0.43 to 0.90) | .48 | ||
| CMV | −0.34 (−0.90 to 0.22) | .22 | ||
| Proton leakb | Group | BBD (ref) | ||
| lr-MSM | 0.32 (−0.29 to 0.93) | .30 | ||
| hr-MSM | 0.29 (−0.32 to 0.89) | .35 | ||
| CMV | −0.18 (−0.68 to 0.33) | .49 | ||
| Maximal respirationb | Group | BBD (ref) | ||
| lr-MSM | −2.06 (−3.42 to 0.69) | .004 | ||
| hr-MSM | 0.14 (−1.20 to 1.47) | .84 | ||
| CMV | 0.22 (−0.91 to 1.34) | .70 | ||
| Spare respiratory capacityb | Group | BBD (ref) | ||
| lr-MSM | −2.21 (−3.51 to 0.91) | .001 | ||
| hr-MSM | 0.06 (−1.21 to 1.34) | .91 | ||
| CMV | 0.37 (−0.70 to 1.44) | .49 | ||
| Nonmitochondrial respirationc | Group | BBD (ref) | ||
| lr-MSM | −0.36 (−0.74 to 0.02) | .06 | ||
| hr-MSM | 0.24 (−0.17 to 0.66) | .24 | ||
| CMV | −0.24 (−0.56 to 0.09) | .15 | ||
| ATP productionb | Group | BBD (ref) | ||
| lr-MSM | −0.20 (−0.87 to 0.46) | .54 | ||
| hr-MSM | 0.08 (−0.57 to 0.73) | .80 | ||
| CMV | −0.39 (−0.94 to 0.16) | .16 | ||
| Glycolysis | ||||
| Nonglycolytic acidificationb | Group | BBD (ref) | ||
| lr-MSM | 0.07 (−0.17 to 0.30) | .57 | ||
| hr-MSM | −0.03 (−0.28 to 0.22) | .81 | ||
| CMV | −0.04 (−0.24 to 0.15) | .66 | ||
| Glycolysisb | Group | BBD (ref) | ||
| lr-MSM | −0.40 (−0.68 to 0.12) | .006 | ||
| hr-MSM | −0.36 (−0.66 to 0.07) | .02 | ||
| CMV | 0.20 (−0.03 to 0.43) | .09 | ||
| Glycolytic capacityb | Group | BBD (ref) | ||
| lr-MSM | −0.54 (−0.91 to 0.16) | .005 | ||
| hr-MSM | −0.41 (−0.81 to 0.02) | .04 | ||
| CMV | 0.33 (0.02–0.63) | .04 | ||
| Glycolytic reserve | Group | BBD (ref) | ||
| lr-MSM | −1.02 (−1.87 to 0.17) | .02 | ||
| hr-MSM | −0.49 (−1.39 to 0.42) | .28 | ||
| CMV | 0.97 (0.27–1.67) | .007 |
Abbreviations: ATP, adenosine triphosphate; B, unstandardized regression coefficient; BBD, blood bank donors; CI, confidence interval; CMV, cytomegalovirus; hr-MSM, men who have sex with men with high-risk sexual behavior; lr-MSM, MSM without high-risk sexual behavior; MSM, men who have sex with men; ref, reference group.
a P of multivariable linear regression corrected for age. Unadjusted P values are given. Bonferroni correction for multiple testing required P < .008 or P < .013 for mitochondrial respiration and glycolysis, respectively (bold).
bLog transformed to obtain normality.
cSquare root transformed to obtain normality.
Pre-Exposure Prophylaxis Users Have Increased T-Cell Activation and Exhaustion
At the time of blood sampling, use of PrEP for HIV prevention had been reported by 24 of 35 individuals in the hr-MSM group (hr-MSM PrEP). This allowed us to compare T-cell activation, exhaustion, and (terminal) differentiation, mitochondrial respiration, and glycolysis between hr-MSM who did or did not use PrEP. We observed that hr-MSM who used PrEP had higher proportions of exhausted, central memory cells in the CD4+ T-cell compartment, CD8+ effector T cells, and lower percentage of CD8+ naive and central memory cells (Supplementary Figure 1A and B). However, these difference were not significant after Bonferroni correction. In contrast, hr-MSM who used PrEP had significantly higher proportions of activated CD4+ T cells compared with hr-MSM (Supplementary Figure 1A). Terminal differentiation markers were not significantly different between hr-MSM who did or did not use PrEP, with the exception of CD4+CD27−CD28− T cells, which were slightly increased in hr-MSM on PrEP (Supplementary Figure 1C). Mitochondrial respiration did not differ between the hr-MSM who did or did not use PrEP (Supplementary Figure 2A), whereas a slight decrease in glycolytic capacity and a significant decrease in glycolysis was observed in PrEP users (Supplementary Figure 2B).
DISCUSSION
In the present study, we evaluated the effect of risk behavior, by studying hr-MSM and lr-MSM, and CMV infection on the peripheral T-cell compartment and the immune bioenergy metabolism in MSM and BBDs. Our analysis showed that in agreement with previous observations [11], MSM had increased proportions of activated CD8+ T cells, exhausted CD4+ T cells, and terminal differentiation of CD4+ and CD8+ T cells compared with BBDs. The increased CD8+ T-cell activation, terminal differentiation of T cells, and proportion of CD4+ effector T cells were associated with high-risk behavior in the MSM. The CMV prevalence in HIV-1-seronegative MSM was significantly higher compared with BBD, confirming previous observations [11, 16]. Moreover, we observed that the frequency of CMV infection in hr-MSM was higher compared with lr-MSM. The CMV serostatus was strongly associated with increased CD4+ and CD8+ effector T-cell populations and terminal differentiation, as has been described previously [13, 19, 20].
Human immunodeficiency virus-1-negative MSM participating in the ACS, recruited at sexual health clinics, are generally at higher risk for sexually transmitted infections than the general population. Indeed, van Bilsen et al reported that self-reported risk behavior in HIV-1-seronegative MSM participating in the cohort is correlated with an overall higher infection pressure [16], and they demonstrated a high infection prevalence of CMV, syphilis, human herpesvirus type 8, and herpes simplex virus infection in MSM. This suggests that the infection pressure in MSM could be the underlying mechanism explaining the independent association between high-risk behavior and increased T-cell activation and (terminal) differentiation in our study.
Mitochondrial respiration (maximum respiration and spare respiration capacity) was lower in PBMCs obtained from MSM compared with BBDs, which is consistent with the observed increased immune activation and terminal differentiation in the T-cell compartment in MSM. Multivariable regression analysis demonstrated that the lower mitochondrial respiration observed in MSM was not associated with risk behavior and CMV infection. This suggests that ongoing antigen exposure as a result of the overall increased infection pressure [16], not specifically related to recent high-risk behavior, may lead to lower mitochondrial respiration and metabolic defects due to downregulation of the expression of genes involved in the bioenergy metabolism as previously reported [9, 10].
The PBMCs of MSM showed decreased glycolytic activity compared with BBDs, despite the increased T-cell immune activation and terminal differentiation observed in this group. Multivariable regression analysis demonstrated that the decreased glycolysis could in part be explained by high-risk behavior. Glucose uptake and glycolysis is mainly controlled by glucose transporters, expression levels of which can be regulated upon antigen stimulation. Indeed, glycolysis increases upon T-cell receptor activation and CD28 costimulation through the upregulation of the expression of glucose transporters [6]. We did not observe any differences in glucose uptake of the different T-cell subsets, as measured by 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose uptake (data not shown), between MSM and BBDs, indicating that the impaired glycolysis in MSM is not explained by lower expression of glucose transporters and suggests that chronic antigen exposure in MSM may also affect the expression of genes involved in glycolysis [9, 10]. Moreover, multivariable regression analysis also demonstrated that CMV infection was associated with increased glycolysis. Cytomeglovirus infection is characterized by the inflation of the memory T-cell population and the induction of terminal differentiation [13, 19, 20]. In agreement, we observed a strong association of CMV infection with increased proportion of terminally differentiated CD4+ and CD8+ T cells. The increased glycolysis associated with CMV is in line with the high energy demands essential for cell proliferation and effector function [5–8].
During the time of our study, PrEP for HIV prevention had been available for some hr-MSM participating in the Amsterdam PrEP project (AMPrEP) [21, 22]. We observed that hr-MSM who used PrEP had higher percentages of CD4+ central memory T cells, CD8+ effector T cells, and higher proportions of activated and exhausted CD4+ T cells compared with hr-MSM not on PrEP. Mitochondrial respiration did not differ between the hr-MSM who did or did not use PrEP, whereas a slight decrease in glycolysis was observed in PrEP users. Recent in vitro data showed that different nucleoside reverse-transcriptase inhibitors do not affect the mitochondrial respiration and glycolysis, indicating that PrEP use is not likely to interfere with the bioenergy profile in vivo [23]. The differences observed between PrEP-using and non-PrEP-using hr-MSM may be explained by an increase in risk behavior, such as condomless anal sex acts with casual partners, and occurrence of sexually transmitted infection reported in PrEP users [21, 24, 25].
In a recent study, Korencak et al [23] reported reduced mitochondrial respiration (basal and maximal) of immune cells from untreated HIV-1-infected individuals compared with BBDs. The reduced mitochondrial respiration was inversely correlated with the increased immune activation in CD8+ T cells and was restored after initiation of antiretroviral therapy. We observed that the bioenergy metabolism (mitochondrial respiration and glycolysis) was impaired in HIV-1-negative MSM compared with BBD. In our study, metabolic changes could not be attributed directly to changes in the levels of immune activation and terminal differentiation (data not shown), but they were more likely the result of overall higher infection pressure as has been reported for this group [16]. Human immunodeficiency virus-1-infected individuals studied by Korencak et al [23] were recruited at an outpatient HIV and sexually transmitted disease clinic, and no noninfected controls who have comparable sociodemographic and behavioral (risk) factors were included, and therefore no direct comparison with our study groups could be made.
Due to its observational nature, our study has several limitations. Immune phenotyping and the bioenergy metabolism were measured in a total of 88 individuals divided over 3 groups. Due to the limited sample size per group and the presumed low prevalence of infections (other than CMV), the effect of individual infections on the immune phenotype and bioenergy metabolism could not be determined. We also could not rule out that the effects observed in this study may be the result of other unmeasured differences between BBDs and MSM. Moreover, MSM included in this study participate in the ACS and may not be representative of the larger MSM community. Data on cigarette smoking, which is known to impact the immune system [26, 27], alcohol use, and recreational drug use are not available for most of the individuals included. However, our previous study in a comparable population of MSM did not show an association between smoking and T-cell activation, exhaustion, and terminal differentiation using similar assays [11].
CONCLUSIONS
In summary, HIV-1-negative MSM exhibited increased levels of T-cell activation and terminal differentiation and an impairment of the bioenergy metabolism. Immunological and metabolic changes could at least in part be explained by both high-risk behavior and CMV infection. The MSM included in our study were participating in the ACS and have been reported to have a higher infection burden and antigen exposure compared with BBDs [16]. High antigen exposure in this group of MSM is likely to induce the immunological changes in the T-cell population and the immune metabolic changes observed in this study.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank all participants of the Amsterdam Cohort studies for their contribution. The Amsterdam Cohort Studies on HIV infection and AIDS, a collaboration between the Public Health Service Amsterdam, the Amsterdam UMC of the University of Amsterdam, Sanquin Blood Supply Foundation, Medical Center Jan van Goyen, and the HIV Focus Center of the DC-Clinics, are part of the Netherlands HIV Monitoring Foundation and financially supported by the Center for Infectious Disease Control of the Netherlands National Institute for Public Health and the Environment.
Author contributions. Z. K. designed the study and the experiments, performed the experiments, interpreted data and wrote the manuscript. I. M. and K. A. v. D. performed experiments. M. A. M. v. d. E., E. H., and M. P. contributed to study design, sample collection, and data analyses. T. B. contributed to the study design and data analyses. N. A. K. designed the study and the experiments, interpreted data, and wrote the manuscript.
Financial support. This work was funded by Aids Fonds (Grant Number P-22301).
Potential conflicts of interest. M. P. reports grants and personal fees from Gilead Sciences, Roche, MSD and Abbvie, outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009; 10:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Appay V, Dunbar PR, Callan M, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med 2002; 8:379–85. [DOI] [PubMed] [Google Scholar]
- 3. van Aalderen MC, Remmerswaal EB, Verstegen NJ, et al. Infection history determines the differentiation state of human CD8+ T cells. J Virol 2015; 89:5110–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity 2013; 38:633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol 2005; 5:844–52. [DOI] [PubMed] [Google Scholar]
- 6. Frauwirth KA, Riley JL, Harris MH, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 2002; 16:769–77. [DOI] [PubMed] [Google Scholar]
- 7. Jacobs SR, Herman CE, Maciver NJ, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol 2008; 180:4476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Warburg O, Gawehn K, Geissler AW. [Metabolism of leukocytes]. Z Naturforsch B 1958; 13B:515–6. [PubMed] [Google Scholar]
- 9. Wherry EJ, Ha SJ, Kaech SM, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 2007; 27:670–84. [DOI] [PubMed] [Google Scholar]
- 10. McKinney EF, Lee JC, Jayne DR, et al. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 2015; 523:612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Booiman T, Wit FW, Girigorie AF, et al. ; Co-morBidity in Relation to Aids (COBRA) Collaboration Terminal differentiation of T cells is strongly associated with CMV infection and increased in HIV-positive individuals on ART and lifestyle matched controls. PLoS One 2017; 12:e0183357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gratama JW, Naipal AM, Oosterveer MA, et al. Effects of herpes virus carrier status on peripheral T lymphocyte subsets. Blood 1987; 70:516–23. [PubMed] [Google Scholar]
- 13. Lee SA, Sinclair E, Hatano H, et al. Impact of HIV on CD8+ T cell CD57 expression is distinct from that of CMV and aging. PLoS One 2014; 9:e89444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trzonkowski P, Myśliwska J, Szmit E, et al. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination–an impact of immunosenescence. Vaccine 2003; 21:3826–36. [DOI] [PubMed] [Google Scholar]
- 15. Wang EC, Moss PA, Frodsham P, et al. CD8highCD57+ T lymphocytes in normal, healthy individuals are oligoclonal and respond to human cytomegalovirus. J Immunol 1995; 155:5046–56. [PubMed] [Google Scholar]
- 16. van Bilsen WPH, Zaaijer HL, Matser A, et al. Infection pressure in men who have sex with men and their suitability to donate blood. Clin Infect Dis 2019; 68:1001–8. [DOI] [PubMed] [Google Scholar]
- 17. van Griensven GJ, de Vroome EM, Goudsmit J, Coutinho RA. Changes in sexual behaviour and the fall in incidence of HIV infection among homosexual men. BMJ 1989; 298:218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jansen IA, Geskus RB, Davidovich U, et al. Ongoing HIV-1 transmission among men who have sex with men in Amsterdam: a 25-year prospective cohort study. AIDS 2011; 25:493–501. [DOI] [PubMed] [Google Scholar]
- 19. Appay V, Fastenackels S, Katlama C, et al. Old age and anti-cytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected patients. AIDS 2011; 25:1813–22. [DOI] [PubMed] [Google Scholar]
- 20. Freeman ML, Mudd JC, Shive CL, et al. CD8 T-cell expansion and inflammation linked to CMV coinfection in ART-treated HIV infection. Clin Infect Dis 2016; 62:392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coyer L, van Bilsen W, Bil J, et al. Pre-exposure prophylaxis among men who have sex with men in the Amsterdam Cohort Studies: use, eligibility, and intention to use. PLoS One 2018; 13:e0205663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoornenborg E, Achterbergh RC, van der Loeff MFS, et al. ; Amsterdam PrEP Project team in the HIV Transmission Elimination AMsterdam Initiative Men who have sex with men more often chose daily than event-driven use of pre-exposure prophylaxis: baseline analysis of a demonstration study in Amsterdam. J Int AIDS Soc 2018; 21:e25105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Korencak M, Byrne M, Richter E, et al. Effect of HIV infection and antiretroviral therapy on immune cellular functions. JCI Insight 2019; 4:e126675. doi: 10.1172/jci.insight.126675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoornenborg E, Achterbergh RCA, Schim van der Loeff MF, et al. ; Amsterdam PrEP Project team in the HIV Transmission Elimination AMsterdam Initiative, MOSAIC study group MSM starting preexposure prophylaxis are at risk of hepatitis C virus infection. AIDS 2017; 31:1603–10. [DOI] [PubMed] [Google Scholar]
- 25. Hoornenborg E, Coyer L, Achterbergh RCA, et al. ; Amsterdam PrEP Project team in the HIV Transmission Elimination AMsterdam (H-TEAM) Initiative Sexual behaviour and incidence of HIV and sexually transmitted infections among men who have sex with men using daily and event-driven pre-exposure prophylaxis in AMPrEP: 2 year results from a demonstration study. Lancet HIV 2019; 6:e447–55. [DOI] [PubMed] [Google Scholar]
- 26. Holt PG. Immune and inflammatory function in cigarette smokers. Thorax 1987; 42:241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol 2009; 9: 377–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.