Abstract
During the 2015 Korea Middle East Respiratory Syndrome coronavirus (MERS-CoV) outbreak, a lymphoma patient developed MERS pneumonia. His pneumonia improved by 45 days after illness onset, but the polymerase chain reaction tests remained (+) for 6 months. However, replication-competent virus was detected by 60 days after illness onset.
Keywords: coronavirus, lymphoma, MERS-CoV, patient isolation, real-time PCR
During the 2015 Middle East respiratory syndrome coronavirus (MERS-CoV) outbreak in South Korea, a 34-year-old man was diagnosed with MERS pneumonia and was isolated for 6 months, because the cycle threshold (Ct) values for MERS-CoV real-time reverse transcriptase polymerase chain reaction (rRT-PCR) assay had never become negative consecutively. The Ct values had been alternating between (+) and (-) near the cutoff value until the patient died of the progression of his underlying condition, peripheral T-cell lymphoma (PTCL). Because of a possibe spread of MERS-CoV from the patient, the Korean Government could not declare an official elimination of MERS-CoV until the patient died [1]. Here, we report the clinical course of the patient, virological investigation, and possible explanations of the prolonged period of (+) rRT-PCR tests.
CASE REPORT
On May 27, 2015, a 34-year-old man visited an emergency room (ER) because of 5-day dyspnea. One year before, he had received hematopoietic stem cell transplantation for his PTCL. The laboratory studies revealed autoimmune hemolytic anemia, suggesting recurrence of his PTCL. During the ER stay, he was unknowingly exposed to a patient with MERS pneumonia [2]. Four days later, he developed a fever and worsening dyspnea. On June 1, he was admitted to the hospital to evaluate his lymphoma status. Physical examination revealed a temperature of 38.8°C, a respiratory rate of 22 breaths per minute, a pulse of 78 per minute, and a blood pressure of 118/71 mmHg. Positron emission tomography–computed tomography and bone marrow examination confirmed the recurrence of PTCL. A dose of 125 mg/d of methylprednisolone was given for 3 days, and his fever subsided. After cessation of steroid therapy, his symptoms worsened. On June 5, he was diagnosed with MERS pneumonia as a sputum sample was positive on rRT-PCR assay, and chest X-ray revealed infiltrates on the upper lobes of both lungs. Beginning June 6, 70 mg/d of prednisolone was re-administered for 1 week to control autoimmune anemia and then tapered. The combination antiviral agents of ribavirin, lopinavir/ritonavir, and interferon-alpha 2a were started on June 7 and maintained for 20 days. On June 14, a computed tomography scan of the chest showed diffuse ground-glass opacities on both lungs (Figure 1A). His dyspnea was aggravated during the first 2 weeks but improved thereafter. Despite the improvement in his pneumonia, the results of rRT-PCR assays for his sputum samples did not become negative.
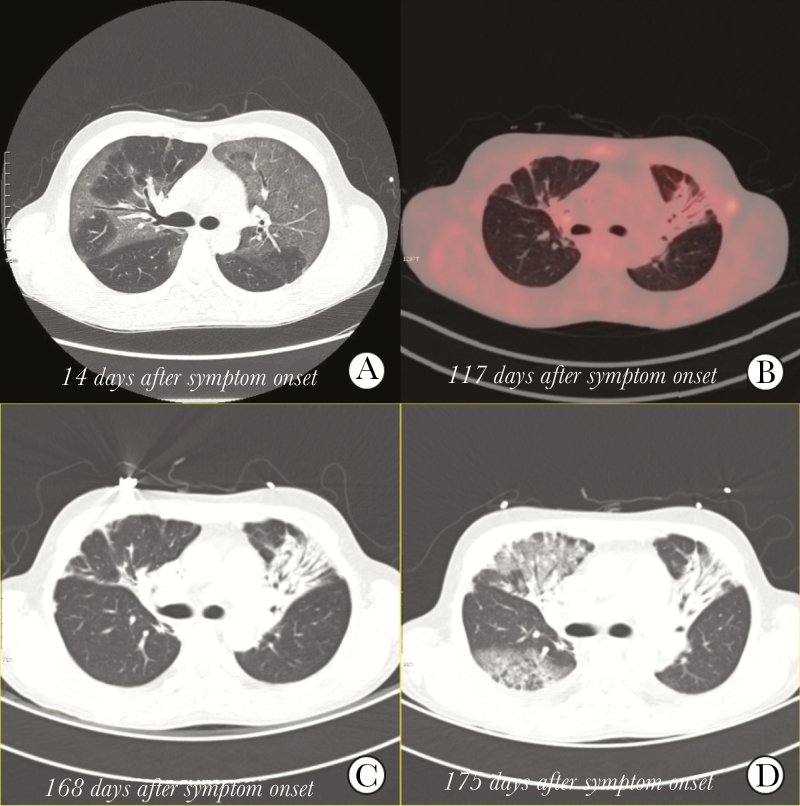
Figure 1.
Chest radiographs of the patients. A computed tomography scan of the chest at 14 days after symptom onset. A, revealed pneumonia in both lung fields. Positron emission tomography-computed tomography at 117 days after symptom onset. B, showed only scar lesions with no 18F-fluorodeoxyglucose activity in the lung parenchyma. A computed tomography scan of the chest at 168 days after symptom onset. C, Showed only scar lesions, but a computed tomography scan of the chest at 175 days after symptom onset. D, Showed newly developed ground-glass opacities in right lung filed.
On July 4, the patient was transferred to the isolation unit of the designated MERS treatment hospital by the government. On admission, he did not have cough, sputum, or dyspnea, and his chest x-ray showed no active pneumonia. However, the government decided to isolate him because his rRT-PCR test returned (+). When systemic steroid was tapered, his hemolytic anemia worsened. On June 17, cytoreductive chemotherapy was started. After 2 cycles of chemotherapy, there was a treatment response such as defervescence and reduction in transfusion requirements. On September 25, proton emission tomography showed no hypermetabolic lesions on lung parenchyma, only fibrotic changes suggesting healed pneumonia (Figure 1B). On October 3, the results of rRT-PCR assay for MERS-CoV turned negative twice at a 24-hour interval, and he was removed from the isolation unit.
On October 11, he visited the ER again with a fever and dyspnea. Laboratory study revealed worsening hemolytic anemia, suggesting the progression of his lymphoma. His sputum sample was positive again on rRT-PCR assay, so he was admitted to the isolation unit. On admission, chest radiography revealed no active pneumonic infiltration. He underwent chemotherapy for PTCL but did not respond; therefore, allogenic hematopoietic stem cell transplantation was planned. On November 15, computed tomography for staging workup before transplantation revealed only scar lesions on lung parenchyma (Figure 1C). On November 21, whole-body irradiation was started, and sudden dyspnea developed. On November 22, a computed tomography scan of the chest revealed newly developed ground-glass opacities on the right lung field (Figure 1D). He died on November 25.
METHOD
Remnant clinical specimens, including sputa, throat swabs, and sera, were collected every 3–4 days from the time of admission to the study hospitals and stored at –80°C until testing. The samples were tested by rRT-PCR and virus culture. Whole-genome sequences were obtained from a virus isolate. To investigate whether virus replication was occurring, we tested for subgenomic mRNA and evolutionary rate in protein S. Detailed experimental methods are described in the Supplementary Data.
The institutional review board at Seoul National University Hospital reviewed the study protocol and provided study approval. The board waived the requirement for written consent (IRB registration number 1506-093-681).
RESULTS
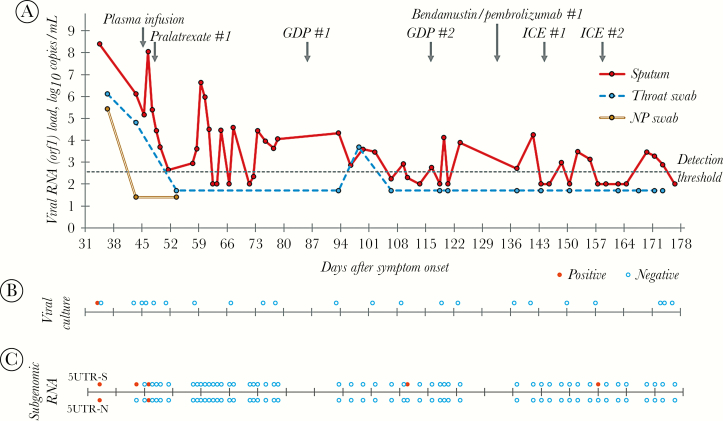
A total of 53 sputum samples, 15 oropharyngeal swab samples, 3 nasopharyngeal swab samples, 15 serum samples, 29 urine samples, and 39 environmental swab samples were tested. MERS-CoV RNA was detected in the sputum samples until 173 days after symptom onset. However, it was detected in the oropharyngeal swabs until 98 days after symptom onset and in the nasopharyngeal swabs until 36 days after symptom onset (Figure 2A). MERS-CoV RNA was not detected in any of the serum, urine, or environmental swab samples.
Figure 2.
Changes in MERS-CoV concentrations in respiratory samples over time. A, Results of real-time reverse-transcriptase polymerase chain reactions; MERS-CoV RNA was detected in the sputum samples until 173 days after symptom onset. B, Results of viral culture; MERS-CoV was isolated from one sputum sample obtained at 34 days after symptom onset. C, Results of subgenomic mRNA assay; subgenomic mRNA was detected in the sputum collected at 34 days after symptom onset and 46 days after symptom onset. orf1, open reading frame 1; GDP, Gemcitabine/Cisplatin/Dexamethasone; ICE, Ifosfamide/carboplatin/etoposide.
A total of 23 sputum samples were tested for virus culture, and MERS-CoV was isolated from 1 sputum sample obtained at 34 days after symptom onset (Figure 2B). Among 53 sputum samples, subgenomic mRNA was detected in the sputum collected at 34 days after symptom onset and 46 days after symptom onset (Figure 2C).
In 2 of 53 sputum samples, PCR product for the S protein gene was obtained, and the sequence of the gene was analyzed. Compared with the NCBI reference sequence (NC_019843), 4 bases were different. When compared with the MERS-CoV/KOR/Seoul/080-3-2015 strain (GenBank accession No. KX034097), which was obtained from the same patient on day 17 after symptom onset, 1 base was different, and amino acid change was also observed. In 15 of 53 sputum samples, PCR products for the S protein gene were obtained partially, and the front part of the gene sequence through 1050 base pairs was compared. In the 901st spot, a T-C mutation resulting in amino acid change occurred between 59 days and 63 days after symptom onset (Supplementary Figure 1).
DISCUSSION
This case report demonstrated that MERS-CoV RNA could be detected in sputum samples as long as 6 months after symptom onset. MERS-CoV was isolated by cell culture at 34 days after illness onset, and subgenomic RNAs were detected as late as 46 days after illness onset. However, 60 days after illness onset, the Ct values of rRT-PCR assay began alternating between (+) and (-) near the cutoff value and never became (-) consistently.
There are 3 possible explanations for the prolonged period of (+) rRT-PCR tests in sputum samples: (1) continuous, low-grade replication of MERS-CoV, (2) chronic MERS-CoV infection with intermittent reactivation, (3) remnant RNA fragments in the respiratory epithelial cells. To evaluate the first possibility, we tried to isolate the virus from sputum samples by cell cuture technique, but we were not able to isolate the virus after day 34 of illness onset. Although cell culture technique is a sensitive method to detect a live virus, detection does not work when the virus titer in a sputum sample is low. Indeed, the Ct values were around the cutoff value, and the virus titers were <1000 copies per mL.
To overcome this possibility, we designed PCR primers targeting the subgenomic RNA (sgRNA) of MERS-CoV [3]. As MERS-CoV is a positive-strand, single-strand RNA virus, it has a unique transcription mechanism of sgRNAs systhesis that enables the expression of a subset of viral proteins [4]. The MERS-CoV has at least 7 distinct sgRNAs, and the detection of these indicates that the virus is replication-competent [5]. In the present study, we designed PCR primers targeting sgRNAs for spike protein and nucleocapsid protein. With the PCR assay, we were able to detect both of the sgRNAs for spike and nucleocapsid protein at 46 days after illness onset, although 1 of the 2 was also detected later than this time point. Previous studies showed that replication-competent viruses were detected up to 4 weeks after symptom onset [3, 6–8]. A longer duration of sgRNA detection in our patient might be attributable to his severe immunocompromised condition, such as lymphoma and hematopoietic stem cell transplantation.
We also did sequence analysis for microevolution of the gene encoding the spike protein. If the virus is replicating, nucleotide substitution in the RNA may occur during the replication process, and it accumulates over time [9, 10]. In the present study, a T to C substitution of nucleotide 901 of the S protein gene occurred between 59 days and 63 days after illness onset. This finding suggests that the virus was replicating up to 60 days after illness onset. However, the evidence of viral replication cannot explain the prolonged period of RNA detection beyond 60 days after illness onset.
Another possible explanation for the prolonged viral RNA detection may include a chronic infection with intermittent reactivation. We consider this is unlikely, because (1) during the coronavirus replication life cycle, the viral RNA do not integrate into the host genome and (2) so far, no human coronaviruses have caused a chronic infection.
The third possibility is a remnant RNA in the respiratory epithelial cells. After the viral replication has completed, remnant RNA remains in the cytoplasm of the host cells, and it falls off when the host cells are shedding. A study using a mouse model suggested that the half-life of the respiratory ciliated cells was 6 months in the trachea and 17 months in the lung [11]. In our patient, MERS-CoV RNA was detected for 6 months in the sputum samples, but not in the throat swab samples. This difference in duration of RNA detection may be due to the different half-lives of the oral and respiratory epithelial cells. There is a case report of a nurse who excreted MERS-CoV for 42 days [12]. It is interesting to note that she did not develop any symptoms and her Ct values were above 30. Due to prolonged PCR (+) results, she was isolated at home for more than 6 weeks.
This study has a few limitations. First, we used sputum samples rather than respiratory tissues containing epithelial cells where the virus replication occurred. Although no studies have evaluated the stability of subgenomic mRNA in sputum samples from MERS-CoV patients, it has been reported that the concentration of subgenomic mRNA was significantly lower in the sputum than in the infected tissue in SARS patients [13]. Second, sequence analysis of the S protein gene was performed only in the beginning part of the S protein gene and not in the latter part, where the variation was known to occur relatively frequently.
In conclusion, our patient had been isolated for 173 days when he died of progression of his lymphoma. Although his rRT-PCR tests were alternating (+) and (-) for a prolonged period of time, evidence of viral replication was found until 60 days after symptom onset. This case highlights that rRT-PCR testing may detect non-replication-competent viral RNA, and we need better criteria for de-isolation of MERS patients.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors thank the staff at Macrogen Inc. (Seoul, Korea), who provided technical support in performing whole-genome sequencing and subgenomic mRNA assay.
Financial support. This work was supported by a grant from the Korean Healthcare Technology R&D Project, funded by the Korea Centers for Disease Control and Prevention (grant number: 2015ER480400)
Potential conflicts of interest. P.G.C. reports grants from the Korea Centers for Disease Control and Prevention during the conduct of the study. All other authors have no potential conflicts to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization (WHO). Middle East respiratory syndrome coronavirus (MERS-CoV)—Republic of Korea. 2015. Available at: https://www.who.int/csr/don/25-october-2015-mers-korea/en/. Accessed 1 December 2015.
- 2. Oh MD, Choe PG, Oh HS, et al. Middle East respiratory syndrome coronavirus superspreading event involving 81 persons, Korea 2015. J Korean Med Sci 2015; 30:1701–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Park WB, Poon LLM, Choi SJ, et al. Replicative virus shedding in the respiratory tract of patients with Middle East respiratory syndrome coronavirus infection. Int J Infect Dis 2018; 72:8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nicholson BL, White KA. Functional long-range RNA-RNA interactions in positive-strand RNA viruses. Nat Rev Microbiol 2014; 12:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Wit E, Rasmussen AL, Falzarano D, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc Natl Acad Sci U S A 2013; 110:16598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corman VM, Albarrak AM, Omrani AS, et al. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis 2016; 62:477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Memish ZA, Al-Tawfiq JA, Makhdoom HQ, et al. Respiratory tract samples, viral load, and genome fraction yield in patients with Middle East respiratory syndrome. J Infect Dis 2014; 210:1590–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Min CK, Cheon S, Ha NY, et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep 2016; 6:25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cotten M, Watson SJ, Zumla AI, et al. Spread, circulation, and evolution of the Middle East respiratory syndrome coronavirus. MBio 2014; 5:e01062-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seong MW, Kim SY, Corman VM, et al. Microevolution of outbreak-associated Middle East respiratory syndrome coronavirus, South Korea, 2015. Emerg Infect Dis 2016; 22:327–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rawlins EL, Hogan BL. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol 2008; 295:L231–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al-Gethamy M, Corman VM, Hussain R, et al. A case of long-term excretion and subclinical infection with Middle East respiratory syndrome coronavirus in a healthcare worker. Clin Infect Dis 2015; 60:973–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poon LL, Chan KH, Wong OK, et al. Detection of SARS coronavirus in patients with severe acute respiratory syndrome by conventional and real-time quantitative reverse transcription-PCR assays. Clin Chem 2004; 50:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.