Abstract
Mussel-inspired chemistry has been embodied as a method for acquiring multifunctional nanostructures. In this research, a novel mussel-inspired magnetic nanoflower was prepared through a mussel-inspired approach. Herein, magnetic PDA–Cu nanoflowers (NFs) were assembled via incorporating magnetic Fe3O4@SiO2–NH2 core/shell nanoparticles (NPs) into mussel-inspired polydopamine (PDA) and copper phosphate as the organic and inorganic portions, respectively. Accordingly, the flower-like morphology of MNPs PDA–Cu NFs was characterized by scanning electron microscopy (SEM) images. X-ray diffraction (XRD) analysis confirmed the crystalline structure of magnetic nanoparticles (MNPs) and copper phosphate. Vibrating sample magnetometer (VSM) data revealed the superparamagnetic behavior of MNPs (40.5 emu/g) and MNPs PDA–Cu NFs (35.4 emu/g). Catalytic reduction of MNPs PDA–Cu NFs was evaluated through degradation of methylene blue (MB). The reduction of MB pursued the Langmuir–Hinshelwood mechanism and first-order kinetics, in which the apparent reduction rate Kapp of MB was higher than 1.44 min–1 and the dye degradation ability was 100%. MNPs PDA–Cu NFs also showed outstanding recyclability and reduction efficiency, for at least six cycles. Furthermore, the prepared MNPs PDA–Cu NFs demonstrated a peroxidase-like catalytic activity for catalyzing 3,3′,5,5′-tetramethylbenzidine (TMB) to a blue oxidized TMB (oxTMB) solution in the presence of H2O2. Antimicrobial assays for MNPs PDA–Cu and PDA–Cu NFs were conducted on both Gram-negative and Gram-positive bacteria. Moreover, we demonstrated how the existence of magnetic nanoparticles in PDA–Cu NFs influences the inhibition of an increasing zone. Based on the results, mussel-inspired magnetic nanoflowers appear to have great potential applications, including those relevant to biological, catalysis, and environmental research.
Introduction
Mussel-inspired chemistry has earned significant interest in the manufacturing of unique functional nanomaterials owing to its powerful functionalization, self-assembly, and adhesion properties. In this regard, the attempt to achieve mussel-inspired nanoparticles with constrained structures resulted in the synthesis of nanomaterials with unique structures and characteristics.1 Organic–inorganic hybrid nanostructures with a flower-like shape called nanoflowers (NFs), known as three-dimensional hierarchical structures, are prepared using a bio-inspired approach and provides a high surface area, reproducibility, excellent stability, great mechanical strength, and a porous structure. The nanoflowers with these unique properties can be used in various fields such as biosensing, drug delivery, bioremediation, biocatalysis, bioseparation, and cancer therapy. Biomolecules including proteins (such as enzymes, antibodies, and estrovidins), peptides, amino acids, DNA, and RNA are used as organic components whose amine or amide functional groups interact with the inorganic parts of the structure. Coordination interactions in formation of crystalline frameworks are achieved using inorganic metal compounds such as copper, iron, silver, gold, manganese, zinc, and cobalt.2,3
Mussel-inspired proteins (MIPs) are one of the biological adhesives that are secreted during adhesive formation in the adhesive plaque of mussel byssus. Dopamine is a form of MIPs known as a catecholamine neurotransmitter molecule that can be found in mammalian body tissues.4,5 Under alkaline conditions, this biomolecule can undergo self-polymerization to form mussel-inspired polydopamine (PDA).6 PDA is a nitrogen-doped carbon source that has two functional groups: ethylamine and catechol. It is also used as a biomimetic polymer for surface modification of various materials.6 Having a great potential in high-performance nanomaterial fabrication, PDA and its derivatives are ideal for blooming of mussel-inspired nanoflowers. Zare et al.7 were the pioneers in the field of using proteins to create nanoflowers that were synthesized by an ecofriendly self-assembly approach that resulted in stabilization of the biomolecule and increase in activity. Li et al.8 reported the ability to form a flower-like structure of phosphotungstic acid, dopamine, and its application in oral drug delivery. Zhang et al.9 evaluated the high catalytic activity of a hybrid nanoflower composed of dopamine and polyoxometalate in dye adsorption. Zhang et al.10 used copper and silver as inorganic compounds to form a flower-like structure, for which they reported a superb antimicrobial activity of Escherichia coli (Gram-negative bacteria) and the mentioned property can lead the compound to be used in clinical studies.
The enzymatic behavior of some nanomaterials leading to the creation of a new concept in enzyme mimetics called nanozymes that integrate the functions of both enzymes and nanomaterials due to their stability in harsh conditions, secure synthetic protocol, catalytic activity, and reduced cost. The activity of oxidase, catalase, peroxidase, and superperoxidase dismutase have been reported mimicking many nanostructured materials.11−16 According to previous studies, some nanoflowers are in the nanozyme classification; for example, Lee et al. investigated a peroxidase-like activity in Cu hydroxy double salt (HDS) nanoflowers. In light of the above considerations, it would be expected that this synthesis strategy for mussel-inspired nanoflowers could be used to construct multiapplication nanostructures by selecting an appropriate inorganic component.
One of the problems in using nanoflowers is their separation from the solution. Because of their separation, this measure requires using centrifuges that result in high energy consumption. Magnetic nanoparticles have extremely been used in the areas of biotechnology and medicine. They could solve this problem efficiently and make the separation mechanism simple only by an external magnetic field.12,17,18
In the present study, we have reported an approach for the preparation of novel multifunctional mussel-inspired nanoflowers through incorporation of superparamagnetic Fe3O4@SiO2−NH2 core/shell (MNPs) into polydopamine–Cu3(PO4)2·3H2O hybrid nanoflowers (MNPs PDA–Cu NFs). Initially, superparamagnetic Fe3O4@SiO2 core/shell with narrow size distribution have been prepared by a coprecipitation procedure and tetraethyl orthosilicate (TEOS) hydrolysis. Afterward, amine functionalization was performed using 3-aminopropyl-triethoxysilane (APTES). Subsequently, a simple, cost-efficient, facile, and green one-step procedure was used to shape the crystalline structure of the mussel-inspired magnetic nanoflowers without interference of any toxic chemicals, special equipment, and harsh operating conditions. To the best of our knowledge, no study has been conducted and published on this type of mussel-inspired magnetic nanoflowers. The structure, crystallization, morphology, and activity of the mussel-inspired magnetic nanoflowers have also been investigated. The interaction between the hierarchical structure and enzyme activity of the biometric MNPs PDA–Cu NFs has been studied thoroughly. Finally, the catalytic reduction, antimicrobial property, and peroxidase-like activity of MNPs PDA–Cu NFs for their biological and medical use as well as their environmental applications were investigated.
Materials
Dopamine hydrochloride, 3,3′,5,5′-tetramethylbenzidine (TMB), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), and aminoproyl-triethoxysilane (APTES) were obtained from Sigma-Aldrich. Iron(III) chloride hexahydrate (FeCl3·6H2O), iron(II) chloride tetrahydrate (FeCl2·4H2O), ammonium hydroxide solution (32%), oleic acid (99% purity), anhydrous toluene (99.9%), tetraethyl orthosilicate (TEOS, 99%), ethanol, copper sulfate pentahydrate (CuSO4.5H2O), sodium chloride, potassium chloride, disodium hydrogen phosphate (Na2HOP4), sodium acetate trihydrate (NaAc.3H2O), potassium dihydrogen phosphate (KH2PO4), acetic acid, dimethyl sulfoxide (DMSO), hydrogen peroxide, methylene blue (MB), and sodium borohydride (NaBH4) were purchased from Merck.
Antimicrobial strains of Gram-positive Staphylococcus aureus (S. aureus IBRC-M10917) and Gram-negative Pseudomonas aeruginosa (P. aeruginosa ATCC-27853) and Escherichia coli (E. coli IBRC-M11074) were grown in Mueller Hinton agar (MHA) and nutrient broth (NB) purchased from Merck.
Synthesis of Fe3O4 and Fe3O4@SiO2-NH2 MNPs
Magnetic nanoparticles coated with oleic acid were prepared through the coprecipitation procedure.19 First, FeCl2·4H2O (1 mmol) and FeCl3·6H2O (2 mmol) were dissolved under the Argon protection via intense mechanical stirring in deionized water. Subsequently, oleic acid (100 μL) and ammonia solution (7 mL, 32%) were added to the mixture, respectively. Subsequently, the temperature of 70 °C for the reaction was then achieved, four times with 5 min intervals of oleic acid intermingled into the reaction mixture. The reaction was authorized to process to reaction condition for 30 min. The resultant dark brown suspension was collected with a magnetic field and washed repeatedly with water.
The Stober procedure was applied to synthesize Fe3O4@SiO2 core/shell with TEOS hydrolysis.20 Briefly, the as-synthesized magnetic nanoparticles were dispersed in anhydrous toluene and then added dropwise in the reaction mixture of ammonia solution (2 mL, 32%), deionized water (20 mL), and ethanol (80% w/w). The reaction mixture was homogenized through vigorous mechanical stirring for 20 min at room temperature. After that, 1 mL of TEOS was added to the mixture for 30 min dropwise. The resultant sample was isolated with a magnet and treated several times with ethanol and water. Then, Fe3O4@SiO2 core/shell nanoparticles were functionalized with APTES.21 First, Fe3O4@SiO2 (400 mg) was dispersed into a three-necked bottom flask containing anhydrous toluene (50 mL) via ultrasonication. Then, 200 μL of APTES was applied to the solution reaction and allowed to refluxed at 120 °C for about 12 h. After that, MNPs were separated and washed several with toluene and ethanol.
Preparation of Mussel-Inspired Nanoflowers (PDA–Cu NFs) and Mussel-Inspired Magnetic Nanoflowers (MNPs PDA–Cu FNs)
The PDA–Cu NFs and MNPs PDA–Cu NFs were prepared according to the reported methods, with some modifications.7 To synthesize PDA–Cu NFs, briefly, 50 mL of phosphate-buffered saline (PBS, 10 mM, pH 7.4) containing 0.003% dopamine was mixed with different concentrations of the CuSO4 aqueous solution (250 and 500 μL, 120 mM). The reaction solution was incubated at ambient temperature for about 72 h. Then, the dark brown precipitates were collected by centrifugation (5000 rpm, 7 min), washed several times with deionized water, and lyophilized.
To synthesize MNPs PDA–Cu NFs, first, 50 mg of MNPs (1 mg/mL) was dispersed in 50 mL of PBS containing dopamine and then the CuSO4 aqueous solution (250 and 500 μL, 120 mM) was added to it. After 72 h, the dark brown precipitates were magnetically separated from the solution, washed three times with water, and dried.
Characterization
The ultraviolet–visible (UV–vis) absorption spectra were recorded using a PerkinElmer Lambda35. The crystal characteristics of the synthesized nanoflowers were exanimated via X-ray diffraction (XRD) (Xpert PRO XRD, Panalytical, Poland). The scanning angles were in the range of 2θ = 10–80° at a rate of 0.02° using Cu Kα radiation (λ = 1.5405 Å) at 40 kV and 40 mA current strength with a step width of 2θ = 0.02°. To confirm the formation of functional groups Fourier transform infrared spectroscopy (Spectrum 100, PerkinElmer) was used. The spectra were measured in the 400–4000 cm–1 range using potassium bromide (KBr) pellets. Field-emission scanning electron microscopy (FESEM) was performed using a MIRA2, TESCAN, Czech Republic. FESEM and energy-dispersive X-ray spectroscopy (EDS) were used to obtain the morphology and structure of nanoflowers. A vibrating sample magnetometer (MDKFT, Danesh Pajouh Kashan Co., Iran) was used to evaluate the magnetic properties of samples. The ζ-potential was determined by Particle Metrix GmbH (Germany).
Assessment of Catalytic Reduction
The catalytic reduction ability of MNPs PDA–Cu NFs was evaluated by analyzing the catalytic reduction performance of organic dyes in the presence of NaBH4. Methylene blue (MB) was selected as a model of organic dyes. In general, 100 μL of MB (200 mg/L) and 660 μL of NaBH4 (3 M) aqueous solution were combined with 1240 μL of water and then 400 μg of MNPs PDA–Cu NFs was added. The concentration of the MB adsorption reaction solution was determined by UV–visible spectroscopy at varying times. The standard calibration curve of MB is demonstrated in Figure S1. In the following, the concentrations of MNPs PDA–Cu NFs and MB were investigated as the main factors in catalytic reduction. Accordingly, the concentrations of MNPs PDA–Cu NFs and MB were measured in the 50–300 and 100–400 mg/L ranges, respectively. The reduction catalytic rate constant (Kapp, min–1) of MB was determined by matching the experimental data with pseudo-first-order kinetics equation (eq 1)22
| 1 |
where C0 and Ct denote the MB concentrations at the initial step and time t, respectively. The percent degradation efficiency (Ed) of MB at any moment can be calculated by eq 2(9)
| 2 |
where C0 is the initial step and Ct belongs to time t.
Recyclability Test
The recyclability of MNPs PDA–Cu NFs was investigated for the reduction of MB through six cycles. In the first cycle, 2 mL of reaction solution including 200 mg/L MB, 1 M NaBH4, and 400 μg of MNPs PDA–Cu NFs was prepared and the catalytic reduction rate constant (Kapp, min–1) of MB was analyzed. After one reduction cycle, MNPs PDA–Cu NFs were isolated by the magnetic field and then were used for the next reduction reaction cycle under the above conditions. The percent catalytic reduction efficiency of MB (Edye) was calculated according to eq 3
| 3 |
where Ct and C0 are the equilibrium MB concentrations at time t and initial step, respectively.
Peroxidase-like Catalytic Activity of MNPs PDA–Cu NFs
The peroxidase-like activity of MNPs PDA–Cu NFs was examined using 3,3′,5,5′-tetramethylbenzidine (TMB) and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS). The substrate TMB could be catalyzed to produce a blue oxidized TMB (oxTMB) solution, which was appraised by absorbance at 652 nm using UV–vis spectroscopy. The potential of MNPs PDA–Cu NFs as a catalyst for TMB (or ABTS) oxidation was evaluated by 2 mL of solution containing different concentrations of MNPs PDA–Cu NFs (50, 10, 5, and 3 μg) in NaAc–HAc buffer (200 mM, pH 3.6) and mixed with 100 μL of TMB (or ABTS) solution (40 mM in DMSO). Then, H2O2 added to the reaction mixture. The solution was incubated about 10 min at 37 °C; then, magnetic nanoflowers were collected using a magnet, and so, the peroxidase-like activity was observed at 652 nm (TMB) and 420 nm (ABTS) absorbance.23
Antimicrobial Activity Assay
In this study, the antimicrobial activities of PDA–Cu NFs and MNPs PDA–Cu NFs were determined in close proximity to Gram-positive bacteria (S. aureus) and Gram-negative bacteria (E. coli and P. aeruginosa) as a reference organism by the Kirby–Bauer method for the disk diffusion assay. Herein, sterile disks with a diameter of 6 mm were used. Bacterial turbidity suspension equal to 0.5 McFarland (1.5 × 108 CFU mL–1) was distributed on MHA plates aseptically followed by application of a sterile disk stored for 10 min in the stock solution of PDA–Cu NFs and MNPs PDA–Cu NFs at 500 μg/mL concentration and placed on MHA plates. For the quantitative analysis, all bacteria cultured on MHA plates were evaluated. For 24 h, the plates were incubated at 37 °C and then investigated. Antibiotics ampicillin at a concentration of 10 μg per disk and ciprofloxacin at a concentration of 5 μg per disk were used as positive control. The periphery of the bright inhibition zone around the disk was determined, and the product sensitivities were evaluated from the inhibition zone diameter (mm).
Result and Discussion
Synthesis and Characterization of MNPs PDA–Cu NFs
For this research, the formation mechanism is demonstrated in Scheme 1. Initially, magnetite/silica core/shell nanoparticles (Fe3O4@SiO2) were synthesized and then amine functional magnetic nanoparticles (MNPs) were prepared by reaction with APTES. Subsequently, MNPs PDA–Cu nanoflowers were produced through the coprecipitation method. The structures of magnetic and nonmagnetic nanoflowers were investigated by FESEM. Figure 1 confirms the formation of the flower-like framework. This nanoflower has been prepared under mild reaction conditions including PBS as a reaction agent, ambient pressure, and temperature without using a toxic chemical agent. The process for nanoflower formation is composed of four phases involving coordination, coprecipitation, self-assembly, and size growth. The PDA imitates Mefp-5 action during development, where the copper ions attach to catechol strongly, promoting the creation and growth of the nanopetals (Cu3(PO4)2·3H2O). In other words, copper(II) ions (Cu2+) coordinate with the dopamine through its amine groups, leading to the formation of a primary complex. Another explanation to use amine magnetic nanoparticles was the tendency of the copper ion toward the amine groups; a few complexes began growing, and some small agglomerates generated, where the kinetically regulated growth of the complex of copper phosphates originated.2,24 Eventually, continuous growth led to the development of a final nanoflower structure.
Scheme 1. Mechanism of Formation of Mussel-Inspired Magnetic Nanoflowers (MNPs PDA–Cu NFs).
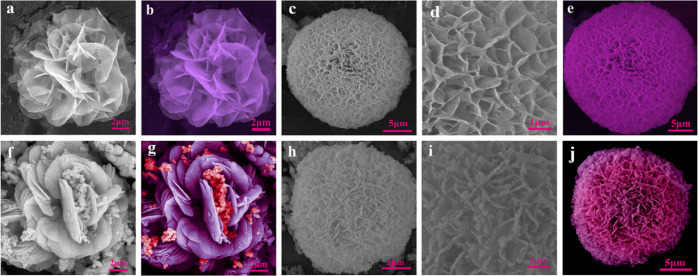
Figure 1.
Pseudocolored and SEM photographs of mussel-inspired magnetic PDA−Cu NFs with different amounts of copper sulfate: (a, b) 250 μL to 120 mM and (c–e) 500 μL to 120 mM. Pseudocolored and SEM images of magnetic mussel-inspired PDA–Cu NFs with different amounts of copper sulfate: (f, g) 250 μL to 120 mM and (h–j) 500 μL to 120 mM.
Furthermore, the use of amine magnetic nanoparticles in the nanoflower formulation process demonstrated that when assembled and deposited in structure, they were layered on nanopetals. The influence of nanoparticles on the nanoflower morphology is shown in Figure 1. The synthesis parameters, including the pH, time of reaction, temperature, and concentration of each material (dopamine, Cu2+), were effective for nanoflower synthesis to get the ideal size and morphology. According to the previous reports, Duan et al.,25 by investigating the influence of dopamine concentrations on the morphology and size of nanoflowers, revealed that when the amount of dopamine was higher than a certain amount, the formed nanoflower was smaller and more regular in size due to the effect of excess PDA. These results led to the growth restriction of the nanoflower at the nucleation stage. The initiation of dopamine polymerization requires an alkaline environment. Zhang et al.10 reported that increasing pH in the alkaline range did not affect the size of the nanoflower and nanopetal spacing because, in this range, dopamine was converted to PDA. Due to the lack of polymerization of dopamine in the acidic range, the formed structure did not resemble flowers. We evaluated the morphology of the mussel-inspired nanoflowers in the presence and absence of magnetic nanoparticles at two different concentrations of the copper sulfate precursor (Figure 1a–j). The incorporation of more condensed copper sulfate from 250 μL (120 mM) to 500 μL (120 mM) resulted in an increase in average diameter from 14 to 23 μm and a change in mussel-inspired nanoflower structure, as shown in Figure 1a,c,f,h. Hence, a mussel-inspired magnetic nanoflower with an average diameter of 23 μm was chosen for characterization and application experiments.
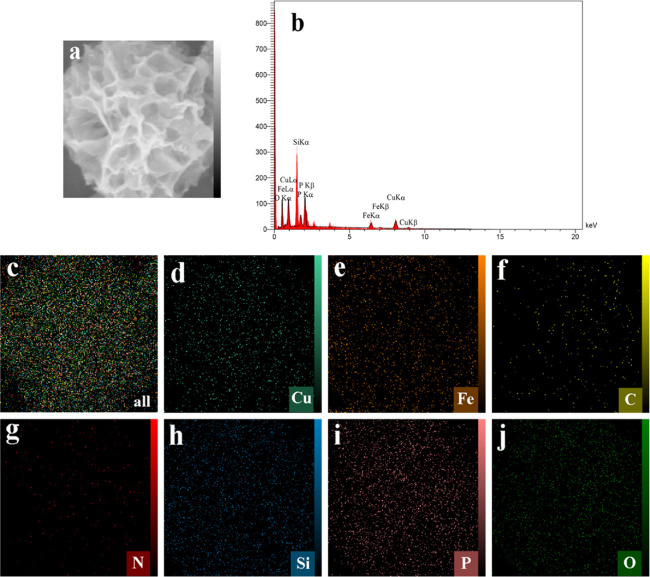
The composition of the MNPs PDA–Cu NFs was examined by EDS and mapping SEM analysis. Figure 2a–j demonstrates that elements Cu, P, C, O, N, Fe, and Si were distributed uniformly. The presence of Fe and Si elements in the EDS and their homogenous distribution in the mapping SEM indicate that MNPs were located and encapsulated in the structure of MNPs PDA–Cu NFs.
Figure 2.
(a, c–j) EDS element mapping images of MNPs PDA–Cu NFs: (d) Cu, (e) Fe, (f) C, (g) N, (h) Si, (i) P, and (j) O. (b) EDS spectra of MNPs PDA–Cu NFs.
XRD characterization was performed to confirm the crystalline structure and phase purity of PDA–Cu NFs and MNPs PDA–Cu NFs (Figure 3a). The XRD pattern of PDA–Cu NFs well aligned with the standard card data for Cu3(PO4)2·3H2O (JCPDS 022-0548), demonstrating that the PDA–Cu NFs have crystalline properties after integrating PDA. Furthermore, the XRD pattern of MNPs corresponded to the (111), (220), (311), (400), (422), (511), and (440) (hkl) planes (JCPDS 019-0629). The pattern of MNPs PDA–Cu NFs included all peaks belonging to MNPs and Cu3(PO4)2.3H2O.26,27
Figure 3.
(a) XRD patterns of MNPs, PDA–Cu NFs, and MNPs PDA–Cu NFs. (b) Fourier transform infrared (FTIR) spectra of MNPs, PDA–Cu NFs, and MNPs PDA–Cu NFs. (c) UV–vis absorbance of dopamine, PDA, CuSO4, MNPs, PDA–Cu NFs, and MNPs PDA–Cu NFs. (d) Vibrating sample magnetometer (VSM) curves for MNPs and MNPs PDA–Cu NFs. (e) ζ-Potentials of PDA, MNPs, PDA–Cu NFs, and MNPs PDA–Cu NFs.
FTIR and UV–vis are vibrational spectroscopy techniques, which are useful for characterizing structural changes and component interactions. The FTIR spectra of MNPs, PDA–Cu NFs, and MNPs PDA–Cu NFs are shown in Figure 3b. The characteristic absorption peaks at 631 and 463 cm–1 in MNPs and MNPs PDA–Cu NFs corresponded to the Fe–O stretching vibrations. The peaks at 1075 and 802 cm–1 were assigned to the Si–O–Si vibrations.28,29 The peaks at around 1623 cm–1 in MNPs due to NH2 vibration and N–H stretching were representative of the free amino group in MNPs. The absorption peak at 2926 cm–1 was attributed to the −CH2– (asymmetric stretching) group as a result of APTES surface modification.30 Furthermore, the strong characteristic bond of stretching and vibration P–O was observed at 990, 1047, and 1149 cm–1 in PDA–Cu NFs and affirmed the existence of the phosphate group in them. Due to overlapping of phosphate and silica peaks in MNPs PDA–Cu NFs, only 990 cm–1 peak was observed.31 The peaks at 3600–3400 cm–1 might be associated with O–H stretching vibration in the hydroxyl functional group of crystal water and PDA in both PDA–Cu NFs and MNPs PDA–Cu NFs. Moreover, the aromatic ring and C=N vibration peak for PDA was registered at roughly 1598 cm–1.32 Accordingly, FTIR analysis verified that the MNPs PDA–Cu NFs were formed successfully. As shown in Figure 3c, the MNPs PDA–Cu NFs, PDA–Cu NFs, and MNPs UV–vis spectra demonstrated no adsorption peaks within the 800–200 nm range, but a peak was recorded for dopamine at 280 nm. As the MNPs PDA–Cu NFs and PDA–Cu NFs were formed with PDA participation, the characteristic peak of dopamine disappeared. Thus, these nanoflowers did not show any evidence of the dopamine peak in UV–vis spectra.
The magnetic properties of MNPs and MNPs PDA–Cu NFs were evaluated using the magnetization curve prepared by VSM at ambient temperature with the −10 000 ≤ H(Oe) ≤ 10 000 applied field. According to Figure 3d, both of the curves are S-shape and indicate no hysteresis with insignificant coercivity and remanence, illustrating the superparamagnetic behavior of MNPs and MNPs PDA–Cu NFs. The values of magnetization saturation were 40.5 and 35.4 emu/g for MNPs and MNPs PDA–Cu NFs, respectively. A decline in the saturation magnetization value of the MNPs PDA–Cu NFs, compared with that of MNPs, was due to the formation of the mussel-inspired nanoflower structure. This amount was adequate for rapid recovery, as demonstrated through the separation with the magnetic field in Figure 3d. Ultimately, ζ-potential analysis was conducted to emphasize further the participation of MNPs in the mechanism of mussel-inspired nanoflower formation (Figure 3e). After using MNPs, the positive charge of mussel-inspired nanoflowers increased because of the existing amine functional groups in MNPs.
Catalytic Reduction of MNPs PDA–Cu NFs
In recent years, polluted water containing organic dyes was identified as the key challenge in treating wastewater. Methylene blue (MB) is the most common organic dye used in the paper, textile, pharmaceutical, wood, and printing industries.33 Herein, owing to its resistance to degradation, which further induces mutation, deformity, cancer, and various diseases, MB was selected as the model dye.1
The catalytic reduction capability of MNPs PDA–Cu NFs was assessed by MB reduction in the presence of NaBH4. Figure 4a illustrates the MB reduction process through the UV–vis absorption spectra in the presence of NaBH4 + MNPs PDA–Cu NFs for 5 min. The aqueous solution of MB shows two UV–vis bands including π–π* transition at 665 nm and a shoulder peak at 614 nm. Diminishing the color of the MB solution by NaBH4 + MNPs PDA–Cu NFs leads to the reduction behavior of specific peak intensity of MB, which demonstrated the conversion of whole MB to leucomethylene blue (LMB) for less than 5 min by a catalytic reduction reaction.22,34,35 The essential catalytic reduction is the electron transfer from a donor to an acceptor.36 In the process, MNPs PDA–Cu NFs plays the role of an electron relay center so that MB and NaBH4 were absorbed on their surface. MB is instinctively electrophilic, while BH4– ions are nucleophilic. Thus, MNPs PDA–Cu NFs accepted electrons from BH4– (donor) and transferred to MB (acceptor), as a result of which MB converted to LMB (Figure 4b).37 Eventually, the cycle process continues again after the desorption of products from active sites. According to the results, the MNPs PDA–Cu NFs could function as an effective catalyst in the degradation of MB due to their composition of copper phosphate and PDA. Based on the previous studies, PDA is nontoxic, has excellent biocompatibility, and exhibits catalytic properties.1 Kim et al.38 reported that PDA is an effective redox mediator. Furthermore, PDA demonstrates the catalytic reduction of an organic dye. The explanation for reductive dye exhibit quinone moieties in PDA that facilitate electron transfer besides,39,40 based materials of copper and copper phosphate have been demonstrated a tremendous catalytic reduction as electron relay according to the literature reported.35,41Figure S2 illustrates the dye degradation through catalytic reduction of MB in the presence of NaBH4 by different components of MNPs PDA–Cu NFs, which confirmed the effect of the presence of PDA and copper phosphate.
Figure 4.
(a) UV–vis absorbance spectra of catalytic reduction of MB (200 mg/L) in the presence of NaBH4 by MNPs PDA–Cu NFs for 5 min. (b) Mechanism of catalytic reduction of MB by MNPs PDA–Cu NFs. Plots of (c) Ct/C0 with time and (d) ln(Ct/C0) with time for MB catalytic reduction. (Assay conditions: CMB = 200 mg/L, 400 μg of MNPs PDA–Cu NFs, presence of NaBH4, and ambient temperature.).
The catalytic reduction kinetics of MB with MNPs PDA–Cu NFs as a catalyst was recorded and reported through MB concentration versus time. Figure 4c indicates that an exponential relation exists between decreasing MB concentration (Ct/C0) and time, and Figure 4d reveals a decreasing linear relationship of ln(Ct/C0) to time. Hence, both of the results emphasize that catalytic reduction kinetics of MB follows the pseudo-first-order kinetics. Therefore, we determined the catalytic reduction rate constant Kapp (min–1) through the slope of ln(Ct/C0) against time, which show degradation of MB in each cycle (Figure 5a) and the apparent Kapp of MB appeared as 1.44 min–1 with 200 mg/mL MB for around 5 min. NP PDA–Cu NFs and the MB concentration were two critical factors in catalytic reduction, so their influence and dependence on the rate Kapp were evaluated. The results of changing MNPs PDA–Cu NF concentration demonstrated that Kapp of MB increased; then, by increasing the MNPs PDA–Cu NFs to 250 mg/L for insufficient NaBH4, Kapp did not significantly change (Figure 5b). Additionally, it was also found that increasing MB concentration resulted in a fast decreasing Kapp. The increasing Kapp in both conditions can be attributed to the increasing total available surface area of MNPs PDA–Cu NFs (Figure 5c). Wunder et al.42 studied the catalytic reduction mechanism of an organic dye by NaBH4 and reported that it followed the Langmuir–Hinshelwood mechanism. Hence, Kapp can be defined as the surface coverage degree of each reactant using eq 4.
| 4 |
where S is the total available surface area of MNPs PDA–Cu NFs, k is the molar rate constant per square meter of the catalyst, and θMB and θBH4– are surface coverage degrees by MB and BH4–. According to the mechanism, the competition between BH4– and MB on the surface of nanoflowers affects the Kapp. We observed that increasing MB concentration leads to decreasing Kapp because of enhanced MB and reduction of BH4– that decrease the rate of electron relay. Furthermore, Kapp’s behavior is justified by an increase in MNPs PDA–Cu NFs. With increasing MNPs PDA–Cu NFs, Kapp increased due to the increased surface area of MNPs PDA–Cu NFs against θMB and θBH4–. When the concentration of MNPs PDA–Cu NFs was increased by more than 250 mg/L, the change of Kapp decreased because of a lack of BH4 and MB in comparison to the available surface area of MNPs PDA–Cu NFs. Also, Xu et al.43 reported that the packing among the nanoflower nanopetals is important for the catalytic reduction ability, as demonstrated through the nanostructures. High surface area and active site due to no tightly packed nanopetals lead to a further effective catalytic reduction behavior. Eventually, the superior high catalytic reduction potential of MNPs PDA–Cu NFs can be attributed to two factors: (1) special properties of MNPs PDA–Cu NFs due to the presence of PDA and copper phosphate and (2) high surface area and good adsorption capability of the nanoflower structure.
Figure 5.
(a) Dye degradation through catalytic reduction MB in existing NaBH4 by MNPs PDA–Cu NFs in one cycle. Influence of (b) MB concentration and (c) MNPs PDA–Cu NF concentration on Kapp. (d) Recyclability of MNPs PDA–Cu NFs in six cycles (CMB = 200 mg/L and 400 μg MNPs PDA–Cu NFs).
One of the important features of catalysts is recyclability, which affects their cost and efficiency. Magnetic strength is the favorable property of MNPs PDA–Cu NFs because they can be collected by a magnetic field after each cycle. Recyclability of MNPs PDA–Cu NFs evaluated the successive six catalytic reduction cycles and is illustrated in Figure 5d. After six cycles, a catalytic reduction efficiency higher than 96% was maintained. In other words, the catalyst indicates no severe loss in operation. Therefore, all of these advantages of MNPs PDA–Cu NFs demonstrate their high capacity in large-scale applications.
Peroxidase-Like Catalytic Activity of MNPs PDA–Cu NFs
The enzyme-like catalytic activity was assessed after the successful validation of the catalytic reduction ability of the MNPs PDA–Cu NFs. Nanoflowers provide high surface energy and large surface area, which leads to biosignal amplification in them.44 The chromogenic substrate TMB was assigned as a peroxidase substrate in the presence of H2O2 to evaluate peroxidase-like activity of MNPs PDA–Cu NFs.45 In this circumstance, TMB catalyzed and converted into blue oxidized TMB (oxTMB) solution, which was assessed through the UV–vis absorption spectra (Figure 6a). According to the results shown in Figure 6b, the blank solution comprising single TMB, TMB + H2O2 (1), and TMB + MNPs PDA–Cu NFs (2) did not indicate any evident absorption peaks within 200–800 nm. However, the sample comprising TMB + H2O2 + MNPs PDA–Cu NFs changed its color to blue (Figure 6b, inset), and the adsorption peak appeared at 652 nm, which verified the peroxidase-like activity of MNPs PDA–Cu NFs. The peroxidase-like activity was assessed with ABTS after the successful validation of peroxidase-like activity of the MNPs PDA–Cu NFs with the TMB substrate. As shown in Figure S3, MNPs PDA–Cu NFs catalyze ABTS one-electron oxidation into the radical cation ABTS•+, which showed a strong peak at 420 nm in UV–vis spectra in the presence of H2O2. These observations demonstrated that our mussel-inspired magnetic nanoflower possessed peroxidase-like catalytic activity in the presence of H2O2.
Figure 6.
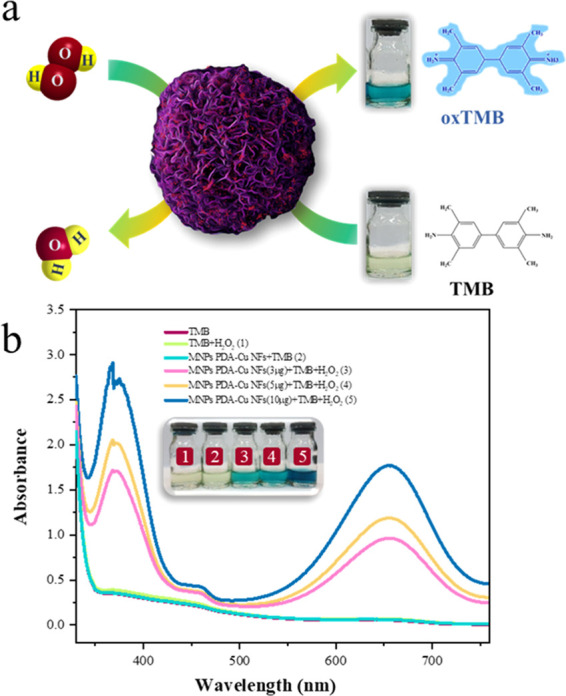
(a) Scheme of the mechanism of peroxidase-like activity of TMB in the existence of H2O2 by MNPs PDA–Cu NFs. (b) UV–vis absorbance of different solutions: TMB, TMB + H2O2, TMB + MNPs PDA–Cu NFs, and TMB + H2O2 + MNPs PDA–Cu NFs (3, 5, and 10 μg).
According to the previous research studies, the mechanism of peroxidase-like activity of nanoflowers could be attributed to the possibility that the nanoflowers promote electron transfer among H2O2 and TMB (eq 5).46
 |
5 |
Huang et al.47 investigated the peroxidase-like activity of the BSA Cu3(PO4)2·3H2O nanoflower and confirmed that the peroxidase-like activity was derived from the Cu3(PO4)2·3H2O crystal, whereas the BSA was used as an organic component to cause nucleation of Cu3(PO4)2·3H2O nanopetals. They also verified that the peroxidase-like activity was not due to free copper ion leaching during the reaction and relied on the nanoflower structure. Batule et al.48 also reported that the peroxidase-like activity of nanoflowers can be related to the inherent attributes of the three-dimensional hierarchical flower-like structure. Therefore, the presence of PDA, Cu3(PO4)2·3H2O, and nanoflower structure was essential for the peroxidase-like activity of MNPs PDA–Cu NFs. Figure S4 illustrates the peroxidase-like activity in the presence of H2O2 by different components of MNPs PDA–Cu NFs that confirmed the effect of the presence of PDA and copper phosphate. To emphasize the peroxidase-like activity of MNPs PDA–Cu NFs, various amounts of nanoflowers were catalyzed by constant amounts of TMB and H2O2. These results show that when the concentrations of nanoflowers increases, the adsorption intensity is also increased (Figure 6b).
Antimicrobial Activity
Gram-positive bacteria (S. aureus) and Gram-negative bacteria (P. aeruginosa and E. coli) are known as common pathogenic bacteria; thus, to quantitatively investigate the antimicrobial activity, the disk diffusion method was applied in this study. Besides the role of the catalytic reduction and peroxidase-like activity of MNPs PDA–Cu NFs, these nanoflowers can be investigated as antimicrobial materials. Figures 7a–k and S5 illustrate the growth of the inhibition zone to the bacteria models for comparison of the microbial activity of MNPs, PDA–Cu NFs, and MNPs PDA–Cu NFs. As shown in Figure 7i,j, the inhibition zones of P. aeruginosa treated with MNPs PDA–Cu NFs and PDA–Cu NFs were 12.7 ± 0.50 and 9.83 ± 0.67 mm, respectively. There was a significant increase in the antimicrobial activity between mussel-inspired nanoflower alone and mussel-inspired magnetic nanoflower (p < 0.05 by one-way ANOVA). Moreover, a similar effect was detected for S. aureus and E. coli (Figure 7l). Antimicrobial activity between MNPs PDA–Cu NFs and antibiotics (ampicillin and ciprofloxacin) was also significant (p < 0.05 by one-way ANOVA). According to the results, the region of inhibition in Gram-negative bacteria was longer than that in Gram-positive bacteria. The membrane of Gram-positive bacteria is more resistant to nanoparticles. In addition, the amine groups on the surface of magnetic nanoparticles in the modified mussel-inspired nanoflowers can be effective in their mechanism of action on the microorganism. Iqbal et al.49 reported that polydopamine has an intrinsic antimicrobial activity but is relatively weaker than metal compounds. Herein, metal compounds such as copper were used for the manufacturing of nanomaterials to improve the antimicrobial activity of polydopamine. Yeroslavsky et al.50 developed polydopamine capsules using a copper sulfate oxidizing agent that was highly efficient in killing bacteria. They showed that the simultaneous presence of polydopamine and copper increased the antimicrobial activity. Also, the membranes modified with copper nanoparticles and polydopamine, as demonstrated by Zhu et al.,51 showed biocidal behavior against E. coli.
Figure 7.
Inhibition zone of S. aureus, P. aeruginosa, and E. coli by the disk diffusion method. Agar plate image of (a–d) S. aureus, (e–h) E. coli, and (i–k) P. aeruginosa for MNPs PDA–Cu NFs, PDA–Cu NFs, CIP, and AMP. (l) Comparison of the inhibition zone (mm) for MNPs PDA–Cu NFs, PDA–Cu NFs, CIP, and AMP. The data reveal the mean ± SD (n = 3) of the diameter inhibition zone. (p < 0.05 by one-way ANOVA.).
Some studies demonstrated that although copper compounds show considerable toxicity to different types of microorganisms, the leaching of Cu2+ ions is the contributing factor to the biocidal activity on the mode of contact killing. Antimicrobial activity of copper results in physical effects such as damage to the cell membrane, denaturation of its proteins, and loss of cytoplasmic content due to the release of copper ions and is exerted by stress, which subsequently produces of reactive oxygen species (ROS). ROS is an antimicrobial mechanism that resulted in a Fenton-type reaction with copper ions, which inhibits cell function by eventually destroying the DNA and mitochondria of the bacterium. There are various forms of ROS, including radical superoxide (•O2–), radical hydroxide (OH•), and singlet oxygen, which can all be produced by copper nanoparticles.51,52 Many studies show that direct interaction between the nanoparticles and the bacterial cell is due to the electrostatic forces originating by the negative carboxyl groups on the surface of the microbial cells and the positive groups of the nanoparticles. Hue et al.53 reported that the interaction among cationic amino groups and anions on the bacterial wall accelerates bacterial death. Similar research by Oliviera et al.54 analyzed the antimicrobial activity of silica nanoparticles functionalized with different amine percentages to measure their ζ-potential and reported that positive surface charge was sufficient for the antimicrobial properties of the nanoparticles. Rokicka-Konieczna et al.55 have done similar studies and Abbaszadegan et al.56 also confirmed the positive effect of nanoparticle surface charge; thereby, our findings are consistent with the previous reports, and the existence of amine groups is the main key to bacterial growth inhibition.
Conclusions
To date, most research studies have reported the comprehensive application of mussel-inspired chemistry to surface modification engineering; however, stupendous properties of mussel-inspired proteins, particularly dopamine, have provided opportunities for novel nanomaterial design. Herein, we proposed a novel facile mussel-inspired approach for the synthesis of mussel-inspired magnetic nanoflowers that contain superparamagnetic Fe3O4@SiO2−NH2 core/shell (MNPs), copper phosphate, and polydopamine. Comparative results on two different concentrations of the copper sulfate precursor revealed that increased concentration of copper sulfate leads to enhanced size, availability of surface, and change in the morphology of nanoflowers. The existence of MNPs in the MNPs PDA−Cu NFs structure also resulted in great separation performance with the magnetic field.
On the other hand, this work documented MNPs PDA–Cu NFs as an efficient catalyst for catalytic reduction of organic dyes with the ability of facile recyclability. Additionally, MNPs PDA–Cu NFs were recognized as one of the nanozymes owing to peroxidase-like activity. Polydopamine and copper nanoparticles in MNPs PDA–Cu NFs have shown antimicrobial behavior toward Gram-negative bacteria (P. aeruginosa and E. coli) and Gram-positive bacteria (S. aureus). Besides, MNPs PDA–Cu NFs have demonstrated higher antimicrobial activity in comparison to PDA–Cu NFs, which is due to positively charged magnetic nanoparticles. The MNPs PDA–Cu NFs have useful applications as a nanozyme, catalytic reduction, and an antimicrobial agent, which lead to biosignal amplification in them. We believe that the mussel-inspired magnetic nanoflowers as a new branch on multifunctional nanostructures hold great capacity in the design of biosensors, water remediation, treating wastewater, clinical application, and design of antimicrobial materials.
Acknowledgments
The authors acknowledge the financial support from Tarbiat Modares University.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01864.
Standard calibration curve for MB (Figure S1); catalytic reduction of MB in the presence of NaBH4 by different components of MNPs PDA–Cu NFs (Figure S2); UV–vis absorbance of different solutions: ABTS, ABTS + H2O2, ABTS + MNPs PDA–Cu NFs, and ABTS + H2O2 + MNPs PDA–Cu NFs (Figure S3); comparison of peroxidase-like activity of ABTS + H2O2, MNPs, Fe3O4@SiO2, PDA, Cu3(PO4)2.3H2O, PDA–Cu NFs, and MNPs PDA–Cu NFs with absorbance and relative activity (Figure S4), and inhibition zones of E. coli and P. aeruginosa for MNPs (Figure S5) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Wang Z.; Yang H.-C.; He F.; Peng S.; Li Y.; Shao L.; Darling S. B. Mussel-inspired surface engineering for water-remediation materials. Matter 2019, 1, 115–155. 10.1016/j.matt.2019.05.002. [DOI] [Google Scholar]
- Liu Y.; Ji X.; He Z. Organic–inorganic nanoflowers: from design strategy to biomedical applications. Nanoscale 2019, 11, 17179–17194. 10.1039/C9NR05446D. [DOI] [PubMed] [Google Scholar]
- Cheon H. J.; Adhikari M. D.; Chung M.; Tran T. D.; Kim J.; Kim M. I. Magnetic Nanoparticles-Embedded Enzyme-Inorganic Hybrid Nanoflowers with Enhanced Peroxidase-Like Activity and Substrate Channeling for Glucose Biosensing. Adv. Healthcare Mater. 2019, 8, 1801507 10.1002/adhm.201801507. [DOI] [PubMed] [Google Scholar]
- Wang H.; Lin C.; Zhang X.; Lin K.; Wang X.; Shen S. G. Mussel-inspired polydopamine coating: a general strategy to enhance osteogenic differentiation and osseointegration for diverse implants. ACS Appl. Mater. Interfaces 2019, 11, 7615–7625. 10.1021/acsami.8b21558. [DOI] [PubMed] [Google Scholar]
- Ryu J. H.; Messersmith P. B.; Lee H. Polydopamine surface chemistry: a decade of discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. 10.1021/acsami.7b19865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinard B.; Neo S. Z. Y.; Yeo E. L. L.; Heng H. P. S.; Neoh K. G.; Kah J. C. Y. Polydopamine nanoparticles enhance drug release for combined photodynamic and photothermal therapy. ACS Appl. Mater. Interfaces 2018, 10, 21125–21136. 10.1021/acsami.8b04799. [DOI] [PubMed] [Google Scholar]
- Ge J.; Lei J.; Zare R. N. Protein–inorganic hybrid nanoflowers. Nat. Nanotechnol. 2012, 7, 428–432. 10.1038/nnano.2012.80. [DOI] [PubMed] [Google Scholar]
- Li H.; Jia Y.; Wang A.; Cui W.; Ma H.; Feng X.; Li J. Self-Assembly of Hierarchical Nanostructures from Dopamine and Polyoxometalate for Oral Drug Delivery. Chem. - Eur. J. 2014, 20, 499–504. 10.1002/chem.201302660. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Guo L.-Y.; Jiao J.; Xin X.; Sun D.; Yuan S. Ionic self-assembly of polyoxometalate–dopamine hybrid nanoflowers with excellent catalytic activity for dyes. ACS Sustainable Chem. Eng. 2017, 5, 1358–1367. 10.1021/acssuschemeng.6b01805. [DOI] [Google Scholar]
- Zhang M.; Peltier R.; Zhang M.; Lu H.; Bian H.; Li Y.; Xu Z.; Shen Y.; Sun H.; Wang Z. In situ reduction of silver nanoparticles on hybrid polydopamine–copper phosphate nanoflowers with enhanced antimicrobial activity. J. Mater. Chem. B 2017, 5, 5311–5317. 10.1039/C7TB00610A. [DOI] [PubMed] [Google Scholar]
- Xu B.; Wang H.; Wang W.; Gao L.; Li S.; Pan X.; Wang H.; Yang H.; Meng X.; Wu Q. A Single-Atom Nanozyme for Wound Disinfection Applications. Angew. Chem., Int. Ed. 2019, 58, 4911–4916. 10.1002/anie.201813994. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Liu L.; Chen H.; Hu K.; Delahunty I.; Gao S.; Xie J. Surface impact on nanoparticle-based magnetic resonance imaging contrast agents. Theranostics 2018, 8, 2521–2548. 10.7150/thno.23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R.; Fan K.; Yan X. Nanozymes: created by learning from nature. Sci. China: Life Sci. 2020, 1–18. 10.1007/s11427-019-1570-7. [DOI] [PubMed] [Google Scholar]
- Fan K.; Xi J.; Fan L.; Wang P.; Zhu C.; Tang Y.; Xu X.; Liang M.; Jiang B.; Yan X. In vivo guiding nitrogen-doped carbon nanozyme for tumor catalytic therapy. Nat. Commun. 2018, 9, 1440 10.1038/s41467-018-03903-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan K.; Wang H.; Xi J.; Liu Q.; Meng X.; Duan D.; Gao L.; Yan X. Optimization of Fe3 O4 nanozyme activity via single amino acid modification mimicking an enzyme active site. Chem. Commun. 2017, 53, 424–427. 10.1039/C6CC08542C. [DOI] [PubMed] [Google Scholar]
- Meng X.; Fan K.; Yan X. Nanozymes: an emerging field bridging nanotechnology and enzymology. Sci. China: Life Sci. 2019, 62, 1543–1546. 10.1007/s11427-019-1557-8. [DOI] [PubMed] [Google Scholar]
- Ren W.; Li Y.; Wang J.; Li L.; Xu L.; Wu Y.; Wang Y.; Fei X.; Tian J. Synthesis of magnetic nanoflower immobilized lipase and its continuous catalytic application. New J. Chem. 2019, 43, 11082–11090. 10.1039/C8NJ06429F. [DOI] [Google Scholar]
- Cipolatti E. P.; Valerio A.; Henriques R. O.; Moritz D. E.; Ninow J. L.; Freire D. M.; Manoel E. A.; Fernandez-Lafuente R.; de Oliveira D. Nanomaterials for biocatalyst immobilization–state of the art and future trends. RSC Adv. 2016, 6, 104675–104692. 10.1039/C6RA22047A. [DOI] [Google Scholar]
- Maboudi S.; Shojaosadati S.; Arpanaei A. Synthesis and characterization of multilayered nanobiohybrid magnetic particles for biomedical applications. Mater. Des. 2017, 115, 317–324. 10.1016/j.matdes.2016.11.064. [DOI] [Google Scholar]
- Esmaeilnejad-Ahranjani P.; Kazemeini M.; Singh G.; Arpanaei A. Amine-functionalized magnetic nanocomposite particles for efficient immobilization of lipase: Effects of functional molecule size on properties of the immobilized lipase. RSC Adv. 2015, 5, 33313–33327. 10.1039/C5RA02471D. [DOI] [Google Scholar]
- Ahmadi F.; Mahmoudi-Yamchi T.; Azizian H. Super paramagnetic core–shells anchored onto silica grafted with C8/NH2 nano-particles for ultrasound-assisted magnetic solid phase extraction of imipramine and desipramine from plasma. J. Chromatogr. B 2018, 1077–1078, 52–59. 10.1016/j.jchromb.2018.01.033. [DOI] [PubMed] [Google Scholar]
- Wu M.; Li Y.; Yue R.; Zhang X.; Huang Y. Removal of silver nanoparticles by mussel-inspired Fe3O4@ polydopamine core-shell microspheres and its use as efficient catalyst for methylene blue reduction. Sci. Rep. 2017, 7, 42773 10.1038/srep42773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B.; Duan D.; Gao L.; Zhou M.; Fan K.; Tang Y.; Xi J.; Bi Y.; Tong Z.; Gao G. F.; et al. Standardized assays for determining the catalytic activity and kinetics of peroxidase-like nanozymes. Nat. Protoc. 2018, 13, 1506–1520. 10.1038/s41596-018-0001-1. [DOI] [PubMed] [Google Scholar]
- Bilal M.; Asgher M.; Shah S. Z. H.; Iqbal H. M. Engineering enzyme-coupled hybrid nanoflowers: The quest for optimum performance to meet biocatalytic challenges and opportunities. Int. J. Biol. Macromol. 2019, 135, 677–690. 10.1016/j.ijbiomac.2019.05.206. [DOI] [PubMed] [Google Scholar]
- Duan L.; Wang H.; Hou J.; Zhang Y.; Chen V. A facile, bio-inspired synthetic route toward flower-like copper phosphate crystals with high specific surface area. Mater. Lett. 2015, 161, 601–604. 10.1016/j.matlet.2015.09.041. [DOI] [Google Scholar]
- Han J.; Luo P.; Wang L.; Li C.; Mao Y.; Wang Y. Construction of magnetic nanoflower biocatalytic system with enhanced enzymatic performance by biomineralization and its application for bisphenol A removal. J. Hazard. Mater. 2019, 380, 120901 10.1016/j.jhazmat.2019.120901. [DOI] [PubMed] [Google Scholar]
- Ren W.; Fei X.; Tian J.; Li Y.; Jing M.; Fang H.; Xu L.; Wang Y. Multiscale immobilized lipase for rapid separation and continuous catalysis. New J. Chem. 2018, 42, 13471–13478. 10.1039/C8NJ01950A. [DOI] [Google Scholar]
- Fang Y.; Xing C.; Zhan S.; Zhao M.; Li M.; Liu H. A polyoxometalate-modified magnetic nanocomposite: a promising antibacterial material for water treatment. J. Mater. Chem. B. 2019, 7, 1933–1944. 10.1039/C8TB03331E. [DOI] [PubMed] [Google Scholar]
- Ahmadpoor F.; Shojaosadati S. A.; Delavari H.; Christiansen G.; Saber R. Synthesis of Fe5C2@SiO2 core@ shell nanoparticles as a potential candidate for biomedical application. Mater. Res. Express. 2018, 5, 055038 10.1088/2053-1591/aac3b7. [DOI] [Google Scholar]
- He J.; Zhang Y.; Yuan Q.; Liang H. Catalytic activity and application of immobilized chloroperoxidase by biometric magnetic nanoparticles. Ind. Eng. Chem. Res. 2019, 58, 3555–3560. 10.1021/acs.iecr.8b03910. [DOI] [Google Scholar]
- Sharma N.; Parhizkar M.; Cong W.; Mateti S.; Kirkland M.; Puri M.; Sutti A. Metal ion type significantly affects the morphology but not the activity of lipase–metal–phosphate nanoflowers. RSC Adv. 2017, 7, 25437–25443. 10.1039/C7RA00302A. [DOI] [Google Scholar]
- Wan D.; Yan C.; Zhang Q. Facile and Rapid Synthesis of Hollow Magnetic Mesoporous Polydopamine Nanoflowers with Tunable Pore Structures for Lipase Immobilization: Green Production of Biodiesel. Ind. Eng. Chem. Res. 2019, 58, 16358–16369. 10.1021/acs.iecr.9b02788. [DOI] [Google Scholar]
- Hu T.; Liu Q.; Gao T.; Dong K.; Wei G.; Yao J. Facile preparation of tannic acid–poly (vinyl alcohol)/sodium alginate hydrogel beads for methylene blue removal from simulated solution. ACS Omega 2018, 3, 7523–7531. 10.1021/acsomega.8b00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F.; Ren H.; Zhou H.; Wang H.; Wang N.; Pan D. Porous Montmorillonite@ Graphene Oxide@ Au Nanoparticle Composite Microspheres for Organic Dye Degradation. ACS Appl. Nano Mater. 2019, 2, 5420–5429. 10.1021/acsanm.9b01043. [DOI] [Google Scholar]
- Wang N.; Zhang Z.; Huang J.; Hu Y. Facile synthesis of copper ions chelated sand via dopamine chemistry for recyclable and sustainable catalysis. Chem. Eng. Sci. 2019, 203, 312–320. 10.1016/j.ces.2019.04.009. [DOI] [Google Scholar]
- Veerakumar P.; Chen S.-M.; Madhu R.; Veeramani V.; Hung C.-T.; Liu S.-B. Nickel nanoparticle-decorated porous carbons for highly active catalytic reduction of organic dyes and sensitive detection of Hg (II) ions. ACS Appl. Mater. Interfaces 2015, 7, 24810–24821. 10.1021/acsami.5b07900. [DOI] [PubMed] [Google Scholar]
- Zhang K.; Suh J. M.; Choi J.-W.; Jang H. W.; Shokouhimehr M.; Varma R. S. Recent advances in the nanocatalyst-assisted NaBH4 reduction of nitroaromatics in water. ACS Omega 2019, 4, 483–495. 10.1021/acsomega.8b03051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H.; Lee M.; Park C. B. Polydopamine as a biomimetic electron gate for artificial photosynthesis. Angew. Chem., Int. Ed. 2014, 53, 6364–6368. 10.1002/anie.201402608. [DOI] [PubMed] [Google Scholar]
- Du S.; Liao Z.; Qin Z.; Zuo F.; Li X. Polydopamine microparticles as redox mediators for catalytic reduction of methylene blue and rhodamine B. Catal. Commun. 2015, 72, 86–90. 10.1016/j.catcom.2015.09.020. [DOI] [Google Scholar]
- Xie Y.; Yan B.; Xu H.; Chen J.; Liu Q.; Deng Y.; Zeng H. Highly regenerable mussel-inspired Fe3O4@ polydopamine-Ag core–shell microspheres as catalyst and adsorbent for methylene blue removal. ACS Appl. Mater. Interfaces 2014, 6, 8845–8852. 10.1021/am501632f. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Zhu P.; Chen L.; Li G.; Zhou F.; Lu D. D.; Sun R.; Zhou F.; Wong C.-p. Hierarchical architectures of monodisperse porous Cu microspheres: synthesis, growth mechanism, high-efficiency and recyclable catalytic performance. J. Mater. Chem. A. 2014, 2, 11966–11973. 10.1039/C4TA01920B. [DOI] [Google Scholar]
- Wunder S.; Lu Y.; Albrecht M.; Ballauff M. Catalytic activity of faceted gold nanoparticles studied by a model reaction: evidence for substrate-induced surface restructuring. ACS Catal. 2011, 1, 908–916. 10.1021/cs200208a. [DOI] [Google Scholar]
- Xu M.; Zhang Y. Seed-mediated approach for the size-controlled synthesis of flower-like Ag mesostructures. Mater. Lett. 2014, 130, 9–13. 10.1016/j.matlet.2014.05.055. [DOI] [Google Scholar]
- Zhang Y.; Jin Y.; Cui H.; Yan X.; Fan K. Nanozyme-based catalytic theranostics. RSC Adv. 2020, 10, 10–20. 10.1039/C9RA09021E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D.; Jin R.; Zhao X.; Li H.; Yan X.; Liu F.; Sun P.; Gao Y.; Liang X.; Lin Y.; et al. Protein–Inorganic Hybrid Nanoflower-Rooted Agarose Hydrogel Platform for Point-of-Care Detection of Acetylcholine. ACS Appl. Mater. Interfaces 2019, 11, 11857–11864. 10.1021/acsami.8b21571. [DOI] [PubMed] [Google Scholar]
- Qiu N.; Liu Y.; Guo R. Electrodeposition-assisted Rapid Preparation of Pt Nanocluster/3D Graphene Hybrid Nanozyme with Outstanding Multiple Oxidase-Like Activity for Distinguishing Colorimetric Determination of Dihydroxybenzene Isomers. ACS Appl. Mater. Interfaces 2020, 12, 15553–15561. 10.1021/acsami.9b23546. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Ran X.; Lin Y.; Ren J.; Qu X. Self-assembly of an organic–inorganic hybrid nanoflower as an efficient biomimetic catalyst for self-activated tandem reactions. Chem. Commun. 2015, 51, 4386–4389. 10.1039/C5CC00040H. [DOI] [PubMed] [Google Scholar]
- Batule B. S.; Park K. S.; Gautam S.; Cheon H. J.; Kim M. I.; Park H. G. Intrinsic peroxidase-like activity of sonochemically synthesized protein copper nanoflowers and its application for the sensitive detection of glucose. Sens. Actuators, B 2019, 283, 749–754. 10.1016/j.snb.2018.12.028. [DOI] [Google Scholar]
- Iqbal Z.; Lai E. P.; Avis T. J. Antimicrobial effect of polydopamine coating on Escherichia coli. J. Mater. Chem. 2012, 22, 21608–21612. 10.1039/c2jm34825j. [DOI] [Google Scholar]
- Yeroslavsky G.; Richman M.; Dawidowicz L.-o.; Rahimipour S. Sonochemically produced polydopamine nanocapsules with selective antimicrobial activity. Chem. Commun. 2013, 49, 5721–5723. 10.1039/c3cc37762h. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Uliana A.; Wang J.; Yuan S.; Li J.; Tian M.; Simoens K.; Volodin A.; Lin J.; Bernaerts K.; et al. Elevated salt transport of antimicrobial loose nanofiltration membranes enabled by copper nanoparticles via fast bioinspired deposition. J. Mater. Chem. A. 2016, 4, 13211–13222. 10.1039/C6TA05661J. [DOI] [Google Scholar]
- Zou H. Y.; Lan J.; Huang C. Z. Dopamine derived copper nanocrystals used as an efficient sensing, catalysis and antibacterial agent. RSC Adv. 2015, 5, 55832–55838. 10.1039/C5RA06240C. [DOI] [Google Scholar]
- Hu S.-G.; Jou C.-H.; Yang M. Protein adsorption, fibroblast activity and antibacterial properties of poly (3-hydroxybutyric acid-co-3-hydroxyvaleric acid) grafted with chitosan and chitooligosaccharide after immobilized with hyaluronic acid. Biomaterials 2003, 24, 2685–2693. 10.1016/S0142-9612(03)00079-6. [DOI] [PubMed] [Google Scholar]
- de Oliveira L.; Bouchmella K.; Picco A.; Capeletti L.; Gonçalves K.; dos Santos J. H.; Kobarg J.; Cardoso M. Tailored silica nanoparticles surface to increase drug load and enhance bactericidal response. J. Braz. Chem. Soc. 2017, 28, 1715–1724. 10.21577/0103-5053.20170017. [DOI] [Google Scholar]
- Rokicka-Konieczna P.; Wanag A.; Sienkiewicz A.; Kusiak-Nejman E.; Morawski A. W. Antibacterial effect of TiO2 nanoparticles modified with APTES. Catal. Commun. 2020, 134, 105862 10.1016/j.catcom.2019.105862. [DOI] [Google Scholar]
- Abbaszadegan A.; Ghahramani Y.; Gholami A.; Hemmateenejad B.; Dorostkar S.; Nabavizadeh M.; Sharghi H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: a preliminary study. J. Nanomater 2015, 2015, 1–8. 10.1155/2015/720654. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.