Abstract
The vascular endothelial growth factor receptor 2 (VEGFR2) and c-mesenchymal epithelial transition factor (c-Met) are members of receptor tyrosine kinases which have a crucial role in the process of angiogenesis. Isatin moiety is a versatile group that is shared in many compounds targeting both c-Met and VEGFR2 kinases. In this study, we designed and synthesized different derivatives of substituted 3-(triazolo-thiadiazin-3-yl)indolin-2-one derivatives (6a–y) as dual inhibitors for c-Met and VEGFR2 enzymes. Eight compounds 6a, 6b, 6e, 6l, 6n, 6r, 6v, and 6y were assessed for their anticancer activities against a panel of 58 cancer cell lines according to the US-NCI protocol. Compound 6b revealed the most effective antiproliferative potency (GI %), with broad-spectrum activity against different subpanels of the most NCI 58 tumor cell lines. An in vivo hen’s egg-chorioallantoic membrane (HET-CAM) angiogenic study was carried out for 21 compounds 6a, b, d, f, h, i, k–o, t, and 6x to check their mortality and toxicity. At 100 μM concentration, all compounds produced 100% mortality of the chick embryos. At 40 μM concentration, 13 compounds did not exhibit any detectable mortality (nontoxic) and revealed a potent antiangiogenic effect. Seven compounds 6b, 6d, 6f, 6n, 6o, 6t, and 6x significantly decreased the number of blood vessels, and compound 6b was the most effective antiangiogenic agent comparable to dexamethasone. Molecular docking studies were conducted for compound 6b to investigate its mode of interaction within the binding site of both c-Met and VEGFR2 kinases.
1. Introduction
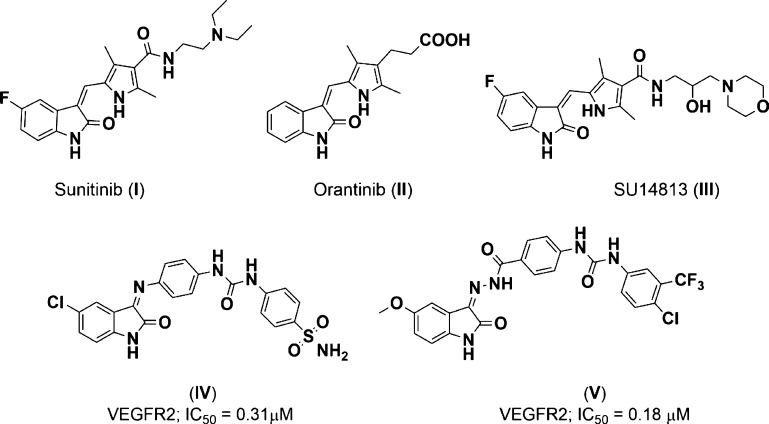
Receptor tyrosine kinases (RTKs) are a large family of kinase enzymes targeted by different chemotherapeutic medications. The main role of RTKs is to phosphorylate the tyrosine amino acid in several proteins aided by ATP (γ-phosphoryl group donor).1,2 This phosphorylation activates the signaling pathway at the cellular level, which is a crucial process for differentiation, proliferation, migration, and antiapoptotic pathway. However, dysregulation of RTK signaling at the cellular level leads to an assortment of human diseases, most notably cancers.3,4 The vascular endothelial growth factor 2 (VEGFR2) and c-mesenchymal epithelial transition factor (c-Met) are RTKs which are linked to the hepatocyte growth factor and endothelial cells, respectively. These RTKs activate the phosphorylation cascade of different tyrosines through the dimerization process aided by ATP to terminally induce cell growth.5−7 The role of c-Met and VEGFR2 is complementary in the process of induction of angiogenesis of human cancer cells.8,9 This significant role in tumorigenesis has received great attention in different research groups10−13 for dual targeting VEGFR2 and c-Met kinases by novel antiangiogenic agents, as represented in Figure 1.
Figure 1.
Isatin derivatives having a notable inhibitory activity against VEGFR2 kinase.
On the other hand, the isatin moiety was intensively reported as a key scaffold in several chemotherapeutic agents acting as VEGFR2 and/or c-Met inhibitors. Sunitinib (I; Ki = 9.0 nM), an isatin derivative targeting multiple tyrosine kinase, was clinically approved in 2006 as an angiogenic anticancer agent for the therapy of renal cell carcinoma.14 Orantinib (II, SU6668) is another isatin analogue in phase I for the treatment of solid tumors exhibiting a significant effect on multiple tumors in xenograft models. It reveals its effect through inhibition of multiple kinases including VEGFR2 (Ki = 9.0 nM).15 Likewise, SU14813 (III) is an orally active oxindole derivative, which inhibits multiple kinases including VEGFR2 (IC50 = 0.04 μM).16 The preclinical trials proposed its use in combination with docetaxel to significantly affect different tumor xenograft models.17 Other isatin derivatives such as compounds (IV) and (V) (Figure 1) were designed to target VEGFR2 kinase (IC50 = 0.31 and 0.18 μM, respectively).18,19 These analogues are indolinone-ureid hybrids that were designed to fit within the active site of the VEGFR2 enzyme targeting its key amino acids.
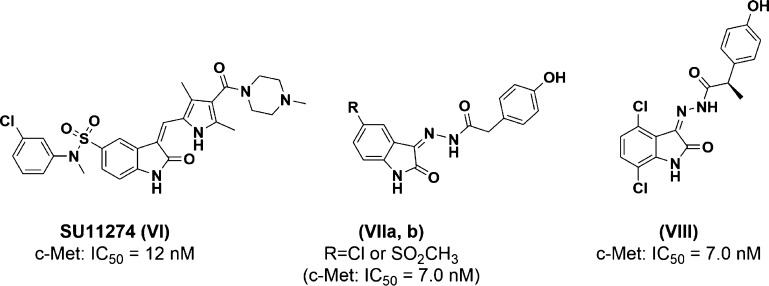
The c-Met kinase is the second target of interest to share VEGFR2 in the angiogenesis process within the cancer cells. Similar to VEGFR2, many isatin derivatives targeted c-Met kinase as inhibitors, for instance, an oxindole derivative (SU11274, VIFigure 2) that downregulates the viability of NSCLC cells via the inhibition of c-Met kinase with IC50 = 12 nM.20,21 3-Hydrazinoindolin-2-one derivatives (VIIa,b, and VIII; Figure 2) are equipotent inhibitors for c-Met kinase as well, with IC50 = 7 nM.22 Compound VIII was cocrystallized with c-Met kinase and submitted to the protein data bank (PDB code: 3ZZE).23
Figure 2.
Potent c-Met kinase inhibitors containing an isatin nucleus.
Many of the above-mentioned scaffolds with a triazole ring fused to aryl-substituted six-membered heterocycles and/or isatin functionalities were intensively reported to have potent c-Met kinase inhibitory activities.24−32 Moreover, different nitrogenous fused and hybrid heterocyclic compounds (pyrrolotriazine, benzimidazole, imidazopyridine, thienopyrimidine, and dioxoquinazoline) were reported to be promising dual-acting VEGFR2 and c-Met inhibitors.33−38 Besides, the compounds containing triazolothiadiazine fragments were reported to possess a broad spectrum of activities such as antitumor, antibacterial, antiviral, antifungal, and PDE4 inhibitory activities.39−42
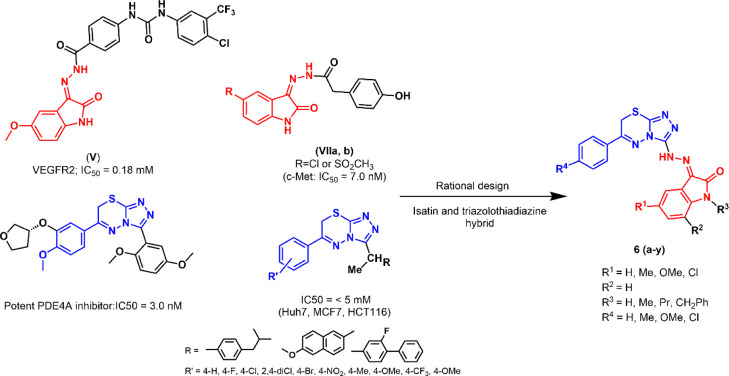
1.1. Rational Design
The rational design of our hybrid isatin and triazolothiadiazine compounds depends mainly on the aforementioned significant dual inhibitory activities of isatin and oxindole against VEGFR2 and c-Met-mediated tumorigenesis (Figure 3).
Figure 3.
Rational design of hybrid isatin and triazolothiadiazine compounds.
Such numerous reported studies for targeting VEGFR2 and c-Met-mediated tumorigenesis have drawn tremendous interest and prompt us to synthesize a series of hybrid 3-hydrazino-indolin-2-one derivatives (6a–y; Scheme 1). The synthesized compounds include two series of 3-hydrazino-indolin-2-one derivatives in which the terminal N is directly attached to the [1,2,4]triazolo[3,4-b][1,3,4]thiadiazine moiety. These compounds were investigated for their cytotoxic activity by the National Cancer Institute (NCI, USA) at 10 μM in a full NCI 58 cell panel. Then, the most active compound was screened for its inhibitory activities against VEGFR2 and c-Met kinases, and a molecular docking study was conducted to justify its mode of kinase inhibition. Additionally, the promising compounds were subjected to an in vivo hen’s egg-chorioallantoic membrane (HET-CAM) study to check their angiogenic effects.
Scheme 1. Synthesis of Compounds 5a–d and the Target Compounds 6a–y.
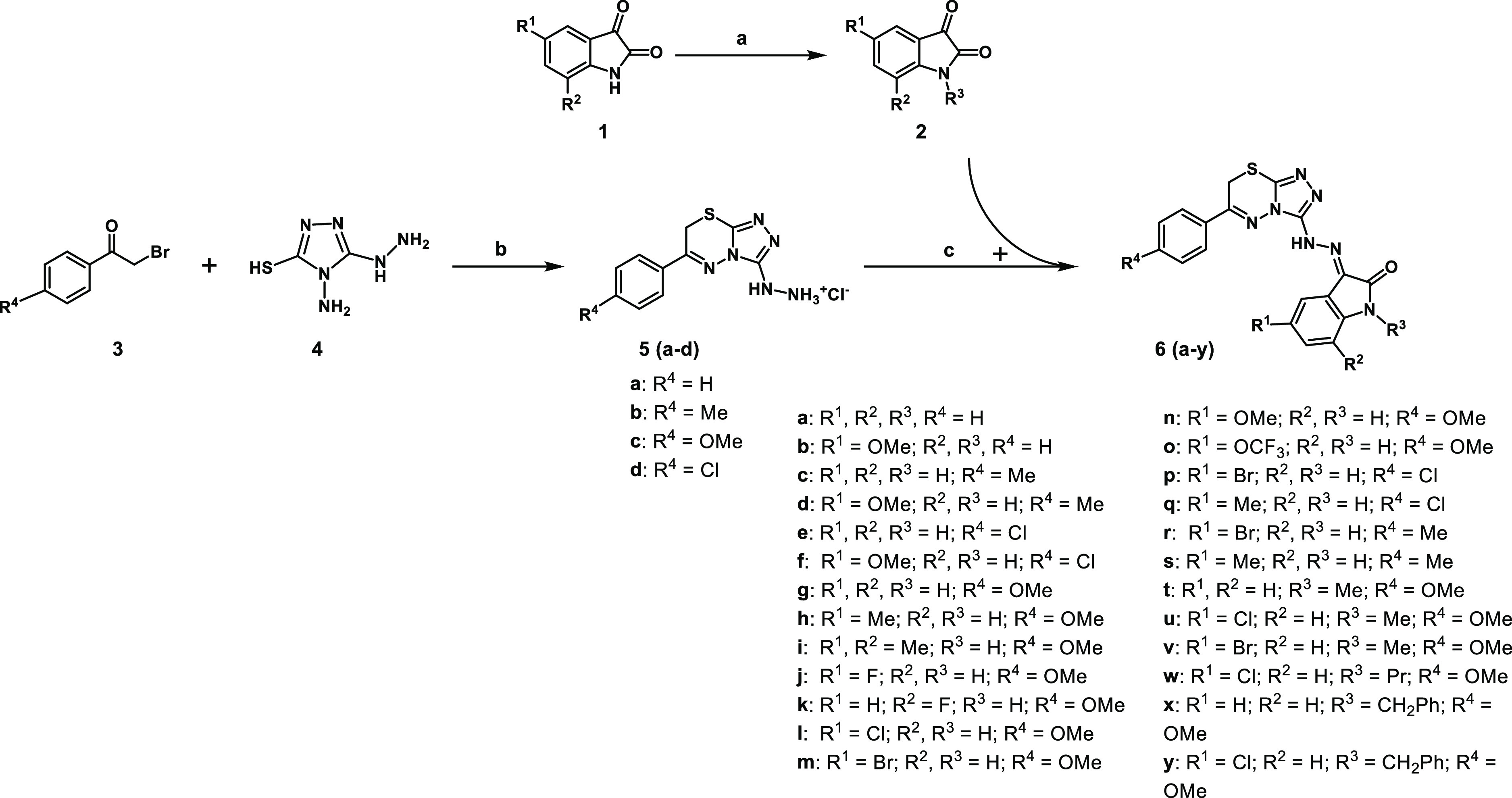
Reaction conditions: (a) Bromoalkanes, anhydrous DMF, K2CO3, rt, 24 h or reflux, 3 h, (b) 1 N HCl, reflux, 50 min, (c) EtOH, reflux, 1 h.
2. Results and Discussion
2.1. Chemistry
The synthesis of target compounds 6a–y was carried out, as shown in Scheme 1. The requisite N-substituted isatin 2 was prepared by alkylation from their N-unsubstituted analogues as reported43−45 with bromoalkanes under anhydrous condition via stirring in dimethylformamide (DMF) or acetonitrile in the presence of K2CO3 in room temperature overnight or under reflux. The synthesis of the requisite series of compounds 2-(6-aryl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydrazin-1-ium chloride (5a–d) was conducted by the reflux of purpald (4; 4-amino-5-hydrazino-1,2,4-triazole-3-thiol) and phenacyl bromides (3) in the presence of hydrochloric acid (1 N).46 The target compounds 6a–y were prepared by refluxing the intermediate 5 with isatin 2 in ethanol where the desired target product precipitates during the reaction, which is isolated by suction filtration, followed by recrystallization from aqueous DMF. As there is no literature precedence for the synthesis of intermediates 5a–d, a synthetic methodology was developed for these compounds taking into account the close nucleophilicity of sulfhydryl, amino, and hydrazino groups of purpald 4.46
Many attempts were made to react 2′-bromoacetophenone (3; R4 = H) with purpald (4) under reflux in ethanol in the absence or presence of a base (NaOH, Na2CO3) or in the presence of acetic acid. However, these attempts resulted in a mixture of products that were difficult to purify. This reaction was successful only by reflux in 1N hydrochloric acid for 50 min, as shown in Scheme 1.
In a related procedure,47 the benzaldehyde selectively reacted with the amino group of purpald (4) under reflux in aqueous ethanol in the presence of 1 N HCl to afford a very good yield. Upon application of the same method using phenacyl bromides instead of aldehydes, the desired intermediate compounds were isolated in good yields.
2.2. Biological Screening
2.2.1. In Vitro Primary Single-Dose (10 μM Concentration) Screening against NCI 58 Human Cell Panel
Eight representative compounds 6a, 6b, 6e, 6l, 6n, 6r, 6v, and 6y were investigated for their anticancer activities by single-dose in vitro screening at 10 μM concentration against NCI 58 human cell lines by the Developmental Therapeutics Program (DTP) in NCI (https://dtp.cancer.gov/).48 These cell lines were collected from nine different subpanels (hematopoietic system, lung cancer, colon, brain, skin melanoma, prostate, breast, kidney, and ovary). The antiproliferative data for each compound were reported as a mean percent cell growth of the treated cancer cells in comparison to the untreated negative control cells, and the.
Percent growth inhibition (GI %) was calculated by the deduction of the percent cell growth from 100. As depicted in Table 1, compounds 6a, 6b, 6e, 6v, and 6y of the tested 3,5-substituted-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydrazineylidene)indolin-2-one derivatives exhibited low to moderate nonselective inhibition of different cancer cell lines.
Table 1. Growth Inhibition Percentage (GI %) of in Vitro Subpanel Tumor Cell Lines at Single-Dose (10 μM) Concentration for Compounds 6a, 6b, 6e, 6l, 6n, 6r, 6v, and 6y.
| subpanel/cell line | 6a | 6b | 6e | 6l | 6n | 6r | 6v | 6y | |
|---|---|---|---|---|---|---|---|---|---|
| leukemia | CCRF-CEM | a | 51 | 18 | 46 | ||||
| K-562 | 23 | 85 | 14 | 15 | 19 | 14 | 33 | 53 | |
| MOLT-4 | 13 | 51 | 17 | 12 | 18 | 30 | |||
| RPMI-8226 | 61 | 20 | 21 | 25 | |||||
| SR | 23 | 64 | 27 | 23 | 36 | 21 | 44 | 28 | |
| NSCLSb | A549/ATCC | 31 | 45 | 18 | 23 | 29 | 17 | 25 | 31 |
| EKVX | 13 | 12 | |||||||
| HOP-62 | 54 | 66 | 20 | 37 | |||||
| HOP-92 | 36 | 22 | 11 | ||||||
| NCI-H23 | 31 | 35 | 19 | 16 | |||||
| NCI-H322M | 22 | ||||||||
| NCI-H460 | 17 | 65 | 41 | 51 | |||||
| NCI-H522 | 57 | 60 | 39 | 34 | 25 | 41 | 41 | 35 | |
| colon cancer | COLO 205 | 17 | |||||||
| HCC-2998 | 19 | 12 | 13 | ||||||
| HCT-116 | 14 | 70 | 17 | 18 | 46 | ||||
| HCT-15 | 10 | 37 | 43 | ||||||
| HT29 | 28 | 57 | 27 | 27 | 24 | 24 | 15 | 16 | |
| KM12 | 40 | 24 | 18 | 22 | |||||
| SW-620 | 21 | 16 | |||||||
| CNS cancer | SF-268 | 31 | 12 | 16 | 25 | 26 | |||
| SF-295 | 12 | 10 | 30 | ||||||
| SF-539 | 18 | 15 | 13 | 12 | 18 | ||||
| SNB-19 | 17 | 37 | 10 | 18 | 26 | ||||
| SNB-75 | 35 | 17 | 12 | 16 | 18 | 17 | |||
| U251 | 50 | 37 | 15 | 33 | |||||
| melanoma | LOX IMVI | 38 | 27 | 27 | 37 | ||||
| MALME-3M | 25 | ||||||||
| M14 | 29 | 14 | 33 | 29 | |||||
| MDA-MB-435 | 22 | 19 | 52 | 14 | |||||
| SK-MEL-2 | 13 | 27 | 11 | 16 | 20 | ||||
| SK-MEL-28 | 15 | 15 | 12 | ||||||
| SK-MEL-5 | 20 | 16 | 17 | ||||||
| UACC-257 | 11 | 35 | 20 | 27 | 20 | 23 | 19 | 13 | |
| UACC-62 | 23 | 28 | 21 | ||||||
| ovarian cancer | IGROV1 | 17 | 22 | 33 | |||||
| OVCAR-3 | 12 | 17 | 22 | ||||||
| OVCAR-4 | 41 | 21 | 26 | 22 | 22 | ||||
| OVCAR-5 | 18 | ||||||||
| OVCAR-8 | 41 | 51 | 13 | 20 | |||||
| NCI/ADR-RES | 13 | ||||||||
| SK-OV-3 | 11 | ||||||||
| renal cancer | 786-0 | 19 | 20 | 12 | |||||
| A498 | 23 | 18 | 10 | 24 | |||||
| ACHN | 35 | 17 | 23 | ||||||
| CAKI-1 | 21 | 34 | 13 | 11 | 33 | 25 | |||
| RXF 393 | 18 | 45 | 30 | 12 | |||||
| SN12C | 21 | 12 | 25 | ||||||
| TK-10 | 29 | 17 | |||||||
| UO-31 | 27 | 23 | |||||||
| PCc | PC-3 | 20 | 44 | 10 | 11 | 25 | |||
| DU-145 | 16 | 50 | 23 | 19 | |||||
| breast cancer | MCF7 | 29 | 28 | 21 | 12 | 30 | 35 | ||
| MDA-MB-231 | 12 | 23 | 11 | 13 | |||||
| HS 578T | 28 | 14 | 27 | ||||||
| BT-549 | 28 | 41 | 29 | 24 | 24 | ||||
| T-47D | 36 | 47 | 29 | 15 | 12 | 18 | 29 | ||
| MDA-MB-468 | 33 | ||||||||
| mean growth, % | 16 | 31 | 10 | 2 | 18 | 21 | |||
| sensitive cell lines no. | 34 | 52 | 30 | 6 | 11 | 12 | 14 | 44 | |
Only GI % higher than 10% are shown.
Nonsmall cell lung cancer.
Prostate cancer.
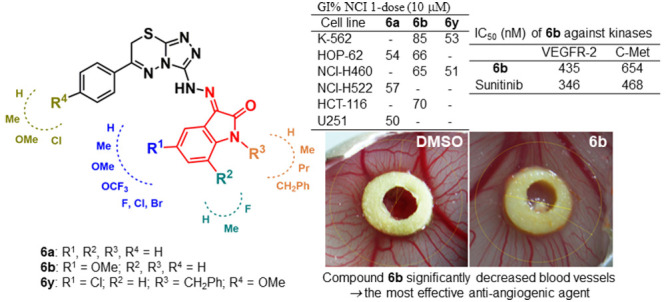
Compound 6b revealed the most efficient antiproliferative activity (GI %), with a broad-spectrum activity against numerous cell lines that belong to diverse cancer subpanels. In particular, compound 6b displayed a potent growth inhibitory impact against leukemia (CCRF-CEM, K-562, MOLT-4, RPMI-8226, and SR), nonsmall cell lung cancer (NCI-H522, NCI-H460, and HOP-62), colon cancer (HT29 and HCT-116), ovarian cancer (OVCAR-8), and prostate cancer (DU-145) cell lines with inhibition percents of 51, 85, 51, 61, 64, 66, 65, 60, 70, 57, 51, and 50%, respectively. In addition, compound 6b exerted cytotoxic activity with GI equal to or greater than 35% against nonsmall cell lung cancer (A549/ATCC and NCI-H23), colon cancer (KM12), CNS cancer (SNB-19 and U251), melanoma (LOX IMVI and UACC-257), renal cancer (ACHN and RXF 393), and breast cancer (BT-549 and T-47D) cell lines with inhibition percents of 45, 35, 40, 37, 37, 38, 35, 35, 45, 41, and 47%, respectively.
Moreover, compound 6y was found to be the second most potent analogue (mean % GI = 21) with broad-spectrum activity against different 44 cell lines. Compound 6y showed GI equal to or greater than 35% over leukemia (CCRF-CEM and K-562), nonsmall cell lung cancer (HOP-62, NCI-H460, and NCI-H522), colon cancer (HCT-116 and HCT-15), melanoma (LOX IMVI), and breast cancer (MCF7) cell lines with inhibition percents of 46, 53, 37, 51, 35, 46, 43, 37, and 35%, respectively.
Further investigation of the obtained results in Table 1 unveiled that leukemia (K-562 and SR), nonsmall cell lung cancer (A549/ATCC and NCI-H522), colon cancer (HT29), and melanoma (UACC-257) cell lines were susceptible to the cytotoxic effect of all tested compounds including 6a, 6b, 6e, 6l, 6n, 6r, 6v, and 6y. The susceptible cell lines to the effect of compounds 6a, 6v, and 6y with GI % ≥ 35 are listed in Table 1. The cell lines HOP-62, HOP-92, NCI-H522, SNB-75, U251, OVCAR-4, OVCAR-8, and T-47D are susceptible to compound 6a by GI of 35–57%. However, SR, NCI-H460, NCI-H522, HCT-15, and MDA-MB-435 cells are sensitive to compound 6v by a GI of 37–52%. Compound 6y exhibited a GI of 35–53% against CCRF-CEM, K-562, HOP-62, NCI-H460, HCT-116, HCT-15, LOX IMVI, and MCF7 cell lines.
2.2.2. Angiogenesis Study
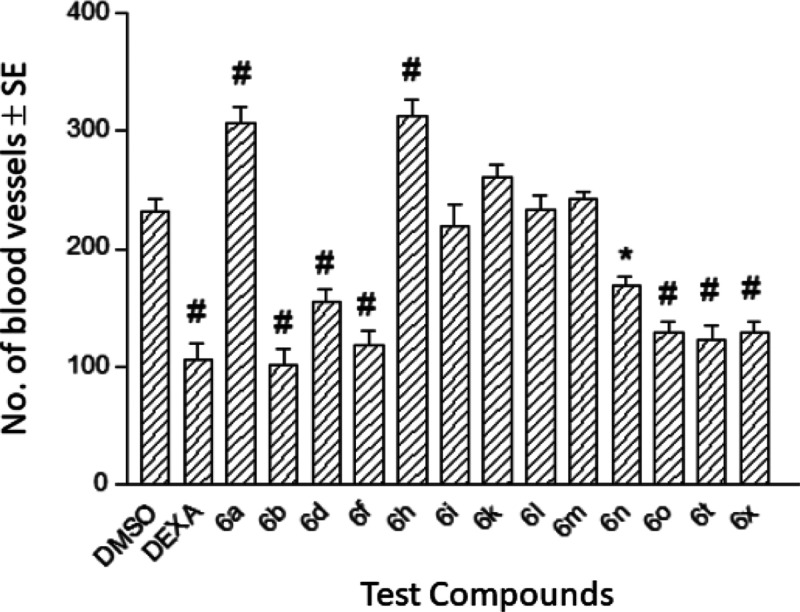
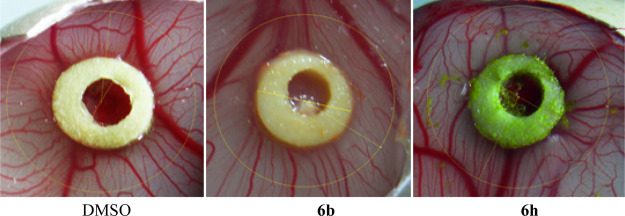
Twenty-one test compounds were subjected to an in vivo HET-CAM study to check their angiogenic effects. In this study, dexamethasone (DEXA) which was reported to demonstrate potent antiangiogenic activity49 was used as a positive control.50−54 This study was conducted in two phases to check the mortality and toxicity of the test compounds at 40 and 100 μM concentrations against HEM-CAM. In the first phase, 40 μM of each of the test compounds dissolved in 100% dimethyl sulfoxide (DMSO) equivalent was applied onto HET-CAM on the 9th day, and the embryos were examined on the 11th day. All compounds produced 100% mortality of the chick embryos. In the second phase, 40 μL of each compound dissolved in 40% DMSO in sterile saline equivalent 40 μM was applied onto HEM-CAM on the 9th day, and then, the embryos and CAM were examined on the 11th day. Yet, at lower concentration (40 μM), eight compounds, namely, 6c, 6e, 6g, 6j, 6u, 6v, 6w, and 6y, were toxic against the HEM-CAM and the chick embryos. The other 13 compounds did not exhibit any detectable mortality against the chick embryos. Figure 4 shows the number of newly formed blood capillaries in HET-CAM during exposure to 40 μM of the 13 nontoxic compounds in comparison to the formed ones by DEXA (0.02 μg) as a potent antiangiogenic agent and using 40% DMSO as a negative control. DEXA, the positive control, produced a 54% reduction in the number of newly formed blood capillaries (P < 0.001). Seven compounds 6b, 6d, 6f, 6n, 6o, 6t, and 6x showed a significant decrease in the number of blood vessels as well. Compound 6b is the most effective derivative producing 56% reduction of angiogenesis comparable to the effect of DEXA.
Figure 4.
Effects of 13 newly synthesized compounds 6a, b, d, f, h, i, k–o, t, and 6x at 40 μM concentration on the number of newly formed blood capillaries of HET-CAM.
Compound 6n was the least significantly effective (27%, P < 0.05). In contrast, two compounds 6a and 6h produced a significant increase in the number of newly formed blood capillaries (33 and 35%, respectively), whereas the other four compounds 6i, 6x, 6l, and 6m revealed a nonstatistically significant change.
Further validation was carried out by quantitative assessment of angiogenesis on HET-CAM using image analysis. Compound 6b (as the most effective antiangiogenic agent) and compound 6h (which increases angiogenesis) were tested. Their effects on the angiogenesis of CAM were examined on the 11th day using computerized image analysis to count newly formed small blood vessels in the circle of 100 mm2 area representing a standard area for all the angiographs. Figure 5 shows the development of new blood vessels in HEM-CAM, after the application of DMSO (vehicle), compound 6b, and compound 6h, respectively. The results are expressed as mean + standard error of three eggs using image analysis and compared with one-way ANOVA and Dunnett’s multiple comparison test.
Figure 5.
Quantitative assessment of angiogenesis on HET-CAM showing the rate of development of blood vessels and capillaries after application of DMSO (vehicle), compound 6b, and compound 6h, respectively.
2.3. VEGFR-2, PDGFR, and C-Met Kinase Inhibitory Activities
As aforementioned, compound 6b superiorly emerged as the most efficient antiangiogenic and antiproliferative agent; accordingly, 6b was selected to evaluate its inhibitory activity toward VEGFR-2, PDGFR, and C-Met kinases. Table 2 displays the inhibition data, as IC50 values, for compound 6b and the clinically used sunitinib as a standard reference. As shown in Table 2, 6b exhibited good VEGFR-2, PDGFR, and C-Met submicromolar inhibitory activities with IC50 values equal to 435, 371, and 654 nM, respectively.
Table 2. IC50 Values for the Inhibitory Activities of Compound 6b toward VEGFR-2, PDGFR, and C-Met Kinases.
| IC50 (nM) |
|||
|---|---|---|---|
| compound | VEGFR-2 | PDGFR | C-Met |
| 6b | 435 | 371 | 654 |
| sunitinib | 346 | 304 | 468 |
2.4. Molecular Docking Studies
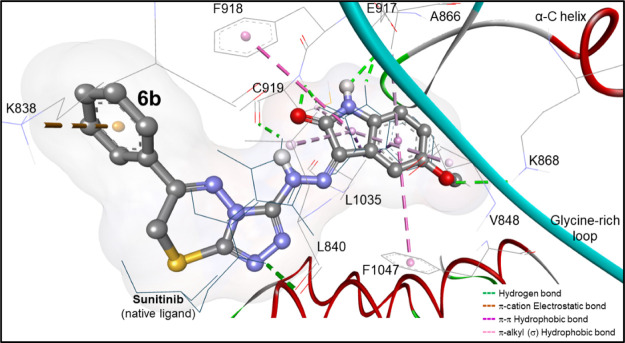
The significant inhibitory activity of compound 6b was deeply investigated through molecular docking simulations inside the active site of the VEGFR-2 crystal structure (PDB: 4agd).55 This crystal structure contains sunitinib as a cocrystallized ligand, which was useful to obtain the key amino acids responsible for the activity. This crystal structure showed that the oxindole moiety played an important role in the biological activity by forming two hydrogen bonds with Cys919 and Glu917 (CDOCKER energy = −27.56 kcal/mol). Docking simulations showed that the oxindole moiety of compound 6b played the same role as the H-bond with Cys919 was 2.03 Å, whereas the H-bond with Glu917 was 2.10 Å. The triazine ring and phenyl ring aided compound 6b to be embedded in the pocket by forming a π-hydrophobic interaction and π-cation interaction with Leu840 and Lys838, respectively (Figure 6).
Figure 6.
Docking mode of compound 6b (in ball and stick view) into the binding site of VEGFR-2 (PDB: 4agd). It seems to be superimposed on the native ligand (B49; sunitinib in dark turquoise lines) within a root-mean-square deviation (rmsd) of 1.14 Å, revealing hydrogen, electrostatic, and hydrophobic binding interactions identical to sunitinib.
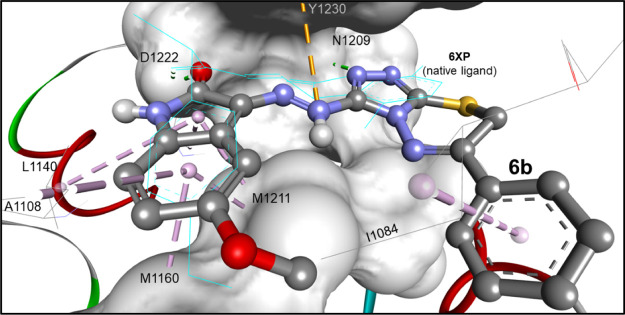
Compound 6b revealed additional inhibitory activity against c-Met kinase which can be illustrated by molecular docking studies using the crystal structure of c-Met kinase downloaded from protein databank (3ZZE) cocrystallized with an inhibitor with oxindole moiety.56
Compound 6b accepts a hydrogen bond with Met1160 (2.16 Å) by an oxindole ring, which is similar to the cocrystallized ligand (CDOCKER energy = −31.23 kcal/mol). This hydrogen bond was supported by another one between the triazine ring with Tyr1159 (2.69 Å). The oxindole moiety was embedded inside the hydrophobic region consisting of Ala1108, Val 1092, Met1211, and Tyr1230 (Figure 7).
Figure 7.
Docking mode of compound 6b into the binding site of the c-Met kinase (PDB: 3zze). It was docked within an rmsd of 2.0 Å from the cocrystallized ligand (6XP; cyan lines), revealing one hydrogen bond with D1222 in addition to electrostatic and multiple hydrophobic binding interactions.
3. Conclusions
Twenty-five 3-hydrazinoindolin-2-one derivatives directly attached to triazolo-thiadiazin-3-yl moiety (6a–y) were designed, synthesized, and investigated for their cytotoxicity. Eight compounds 6a, 6b, 6e, 6l, 6n, 6r, 6v, and 6y were assessed for their anticancer activity against a panel of NCI 58 tumor cell lines including nine different subpanels (hematopoietic system, lung cancer, colon, brain, skin melanoma, ovary, kidney, prostate, and breast). Five substituted 3-(triazolo-thiadiazin-3-yl)indolin-2-one derivatives revealed moderate to low broad-spectrum activity against most of NCI cancer cell lines. Among them, compound 6b was the most effective analogue revealing an average growth inhibition of 40%, specifically against leukemia, NSCLC, colon, ovarian, and prostate cancers within the range of 51–85%. Further angiogenesis assay was conducted using HET-CAM to check the mortality and toxicity of the test compounds at 100 and 40 μM concentrations. Thirteen out of 21 test compounds were nontoxic for the chick embryos, and 7 of these compounds revealed a remarkable reduction of the blood vessels developed including compound 6b as the most effective angiogenic agents. Therefore, compound 6b was subjected for VEGFR-2, PDGFR, and C-Met kinase inhibitory assays to exhibit submicromolar inhibitory activities of IC50 435, 371, and 654 nM, respectively. Docking of compound 6b inside the active site of both c-Met kinase and VEFGR2 showed the crucial role of the oxindole moiety as it revealed a hydrogen bond with Met1160 (2.16 Å) in c-Met kinase active site and hydrogen bond with D1222 in the VEGFR2 active site.
4. Experimental Section
4.1. Chemistry
4.1.1. General
The Bruker spectrometer was used to record the proton magnetic resonance 1H NMR, 13C NMR, and 13C DEPT135 at 300 MHz and 75 MHz. DMSO-d6 was used as a solvent to generate all NMR spectra. The strong peak of DMSO at δ 2.50 was used for spectral calibration. The coupling constants are expressed in Hz. Both protons of NH and OH protons were measured as D2O exchangeable. The chemical shifts were expressed in values (ppm). The coupling constant (J) is given in hertz (Hz). The abbreviations used are as follows: s, singlet; d, doublet; and m, multiplet. High-resolution mass spectrometry (HRMS) was performed at Campus Chemical Instrument Center (CCIC) Mass Spectrometry and Proteomics Facility at The Ohio State University, USA. Melting points (mp) were measured with a Stuart melting point apparatus and were uncorrected. Thin-layer chromatography using precoated glass plates silica gel plates of 60F254-Merck was used for monitoring the progress of reactions, and our fluorescent products were visualized by a UV lamp.
4.1.2. General Procedures for the Preparation of (6-Aryl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)-hydrazine Hydrochloride (5a–d)
Purpald (4; 2.92 g, 20 mmol, 1.1 equiv) was added to a stirred solution of 30 mL of 1 N HCl. The mixture was heated whereby a clear solution is obtained, and then a solution of 2′-bromoacetophenone (3; 18 mmol, 1.0 equiv) in 30 mL of ethanol was added dropwise over 10 min. Heating of the reaction mixture was continued for another 50 min. The reaction mixture was then left to cool unstirred for 2–3 h, whereby a precipitate of 5 is formed. The formed precipitate was suction filtered, washed with 10 mL of ice-cold water, and dried. The crude product 5 was used without further purification.
4.1.2.1. 2-(6-Phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydrazin-1-ium Chloride (5a)
Yield 3.87 g (76%) of yellowish-white powder; mp 205–206 °C; 1H NMR (300 MHz, DMSO-d6): δ 10.19 (2H, br s, D2O exchangeable, 3-NH), 8.11 (2H, br s, Ar-2′,6′-H), 7.61 (3H, br s, Ar-3′,4′,5′-H), 4.49 (2H, s, 7-CH2). 13C NMR (125 MHz, DMSO): δ 154.99 (1C, 6-C), 151.38 (1C, 8a-C), 137.56 (1C, 3-C), 132.25 (1C, Ar-1′-C), 129.97 (2C, Ar-2′,6′-CH), 129.58 (3C, Ar-3′,4′,5′-CH), 23.26 (1C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 129.97 (2C, Ar-2′,6′-CH), 129.58 (3C, Ar-3′,4′,5′-CH), 23.26 (1C, 7-CH2). HRMS (ES+): m/z calcd for C10H11N6S [M + H]+, 247.0766; found, 247.0763.
4.1.2.2. 2-(6-(p-Tolyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydrazin-1-ium Chloride (5b)
Yield 4.2 g (79%) of yellowish-white powder; mp 225–226 o; 1H NMR (300 MHz, DMSO-d6): δ 9.21 (2H, br s, D2O exchangeable, 3-NH-NH2), 9.21 (1H, br s, D2O exchangeable, 3-NH), 7.98 (2H, dd, J = 7.3 Hz, Ar-2′,6′-H), 7.39 (2H, br s, Ar-3′,5′-H), 4.43–4.50 (2H, br s, 7-CH2), 2.40 (3H, s, 5′-CH3). 13C NMR (125 MHz, DMSO): δ 154.99 (1C, 6-C), 151.38 (1C, 8a-C), 137.56 (1C, 3-C), 132.25 (1C, Ar-1′-C), 129.97 (2C, Ar-2′,6′-CH), 129.58 (3C, Ar-3′,4′,5′-CH), 23.26 (1C, 7-CH). DEPT C135 (125 MHz, DMSO): δ 129.97 (2C, Ar-2′,6′-CH), 129.58 (3C, Ar-3′,4′,5′-CH), 23.26 (1C, 7-CH2). HRMS (ES+): m/z calcd for C11H13N6S [M+1]+, 261.0922; found, 261.0918.
4.1.2.3. 2-(6-(4-Methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydrazin-1-ium Chloride (5c)
Yield 4.4 g (78%) of yellowish-white powder; mp 209–210 °C; 1H NMR (300 MHz, DMSO-d6): δ 9.17 (1H, br s, D2O exchangeable, 3-NH), 7.95–8.10 (2H, dd, J = 37.0, 8.8 Hz, Ar-2′,6′-H), 7.61 (3H, d, J = 8.8 Hz, Ar-3′,5′-H), 4.45 (2H, s, 7-CH2), 3.86 (3H, s, 5′-OCH3). 13C NMR (125 MHz, DMSO): δ 163.01 (1C, 6-C), 161.44 (1C, Ar-4′-C), 155.85 (1C, 8a-C), 151.15 (1C, 3-C), 130.15 (2C, Ar-2′,6′-CH), 125.35 (1C, Ar-1′-C), 114.90 (2C, Ar-3′, 5′-CH), 56.09 (1C, 5′-OCH3), 23.17 (1C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 130.15 (2C, Ar-2′,6′-CH), 114.90 (2C, Ar-3′, 5′-CH), 56.09 (1C, 5′-OCH3), 23.17 (C, 7-CH2). HRMS (ES+): m/z calcd for C11H13N6OS [M + H]+, 277.0872; found, 277.0865.
4.1.2.4. 2-(6-(4-Chloro-phenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)-hydrazine Hydrochloride (5d)
Yield 4.6 g (81%) of yellowish-white powder; mp 184–185 °C; 1H NMR (300 MHz, DMSO-d6): δ 9.18 (1H, br s, D2O exchangeable, 3-NH), 9.05 (1H, br s, D2O exchangeable, 3-NH-NH2), 8.13 (2H, d, J = 8.5 Hz, Ar-2′,6′-H), 7.68 (2H, d, J = 8.5 Hz, Ar-3′,5′-H), 4.47 (2H, s, 7-CH2). 13C NMR (125 MHz, DMSO): δ 154.99 (1C, 6-C), 151.38 (1C, 8a-C), 139.70 (1C, 3-C), 137.56 (2C, Ar-4′-C), 132.25 (1C, Ar-1′-C), 129.97 (2C, Ar-3,5′-CH), 129.58 (2C, Ar-2′,6′-CH), 23.26 (1C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 129.97 (2C, Ar-3,5′-CH), 129.58 (2C, Ar-2′,6′-CH), 23.26 (1C, 7-CH2). HRMS (ES+): m/z calcd for C10H10ClN6S [M+1]+, 281.0376; found, 281.0367.
4.1.3. General Procedure for the Synthesis of Compound 6a–y
A mixture of the appropriate isatin (2; 1.0 mmol, 1.0 equiv) and appropriate compound (5; 1.3 mmol, 1.3 equiv) in ethanol (5 mL) was heated under reflux for 1 h. The reaction mixture was cooled to room temperature, and the crude product was filtered off, washed, and dried. The crude product was recrystallized from the DMF/H2O mixture (5:1).
4.1.3.1. 3-(2-(6-Phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydrazineylidene)indolin-2-one (6a)
Yield 304 mg (81%) of yellowish white powder; mp 284–285 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.30 (1H, br s, D2O exchangeable, 3-NH), 11.26 (1H, br s, D2O exchangeable, 1″-NH), 8.05 (2H, d, J = 6.3 Hz, Ar-2′,6′-H), 7.71–7.59 (3H, m, Ar-3′,4′,5′-H), 7.56 (1H, d, J = 7.7 Hz, Ar-4″-H), 7.36 (1H, t, J = 7.7 Hz, Ar-6″-H), 7.11 (1H, t, J = 7.7 Hz, Ar-5″-H), 6.98 (1H, d, J = 7.7 Hz, Ar-7″-H), 4.48 (2H, s, 7-CH2). 13C NMR (125 MHz, DMSO): δ 163.88 (1C, 2″-C=O), 159.36 (1C, 6-C), 155.37 (1C, 8a-C), 149.48 (1C, 3-C), 141.74 (1C, 7″a-C), 134.32 (1C, Ar-1′-C), 133.61 (1C, 3″-C), 132.57 (1C, 6″-CH), 131.00 (1C, Ar-4′-CH), 129.60 (2C, Ar-3,5′-CH), 127.79 (2C, Ar-2′,6′-CH), 127.66 (1C, 4″-CH), 122.99 (1C, 5″-CH), 120.41 (1C, 3″a-C), 111.56 (1C, 7″-CH), 23.78 (2C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 132.57 (1C, 6″-CH), 131.01 (1C, Ar-4′-CH), 129.60 (2C, Ar-3,5′-CH), 127.79 (2C, Ar-2′,6′-CH), 127.66 (1C, 4″-CH), 122.99 (1C, 5″-CH), 120.50 ? 111.56 (1C, 7″-CH), 23.78 (1C, 7-CH2). HRMS (ES+): m/z calcd for C18H14N7OS [M + H]+, 376.0981; found, 376.0976.
4.1.3.2. 5-Methoxy-3-(2-(6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydraz-ineylidene)indolin-2-one (6b)
Yield 357 mg (88%) of yellowish-white powder; mp 223 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.34 (1H, br s, D2O exchangeable, 3-NH), 11.06 (1H, br s, D2O exchangeable, 1″-NH), 8.05 (2H, d, J = 6.3 Hz, Ar-2′,6′-H), 7.70–7.48 (3H, m, Ar-3′,4′,5′-H), 7.10 (1H, s, Ar-4″-H), 6.95–6.86 (1H, m Ar-7″-H), 6.83–6.66 (1H, m, Ar-6″-H), 4.47 (2H, s, 7-CH2), 3.78 (3H, s, 6″-OCH3). 13C NMR (125 MHz, DMSO): δ 164.00 (1C, 2″-C=O), 155.79 (1C, 6-C), 155.37 (1C, 8a-C), 154.72 (1C, 3-C), 149.46 (1C, 7″a-C), 135.42 (1C, Ar-1′-C), 134.60 (1C, 3″-C), 132.56 (1C, Ar-4′-CH), 129.59 (2C, Ar-3,5′-CH), 127.79 (2C, Ar-2′,6′-CH), 121.14 (1C, 3″a-C), 117.39 (1C, 7″-CH), 112.41 (1C, 4″-CH), 105.28 (1C, 6″-CH), 56.02 (1C, 5″-OCH3), 23.77 (1C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 132.57 (1C, Ar-4′-CH), 129.59 (2C, Ar-3,5′-CH), 127.79 (2C, Ar-2′,6′-CH), 117.39 (1C, 7″-CH), 112.41 (1C, 4″-CH), 105.28 (1C, 6″-CH), 56.02 (1C, 5″-OCH3), 23.76 (1C, 7-CH2). HRMS (ES+): m/z calcd for C19H16N7O2S [M + H]+, 406.1086; found, 406.1082.
4.1.3.3. 3-(2-(6-(p-Tolyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydrazineylidene)-indolin-2-one (6c)
Yield 362 mg (93%) of yellowish-white powder; mp 292–293 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.29 (1H, br s, D2O exchangeable, 3-NH), 11.25 (1H, br s, D2O exchangeable, 1″-NH), 7.95 (2H, d, J = 7.90 Hz, Ar-2′,6′-H), 7.56 (1H, d, J = 7.50 Hz, Ar-4″-H), 7.46–7.31 (3H, m, Ar-3′,5′, 6″-H), 7.11 (1H, t, J = 7.50 Hz, Ar-5″-H), 6.98 (1H, d, J = 7.50 Hz, Ar-7″-H), 4.44 (2H, s, 7-CH2), 2.40 (3H, s, 4′-CH3).
13C NMR (125 MHz, DMSO): δ 163.88 (1C, 2″-C=O), 155.27 (1C, 6-C), 154.74 (1C, 8a-C), 149.42 (1C, 3-C), 142.88 (1C, 7″a-C), 141.72 (1C, Ar-4′-C), 138.37 (1C, 3″-C), 134.24 (1C, Ar-1′-C), 130.97 (1C, 6″-CH), 130.75 (1C, 4″-CH), 130.18 (2C, Ar-3,5′-CH), 127.75 (2C, Ar-2′,6′-CH), 127.62 (1C, 4″-CH), 122.99 (1C, 5″-CH), 120.43 (1C, 3″a-C), 111.55 (1C, 7″-CH), 23.64 (1C, 7-CH2), 21.53 (1C, 4′-CH3). DEPT C135 (125 MHz, DMSO): δ 130.97 (1C, 6″-CH), 130.18 (2C, Ar-3,5′-CH), 127.75 (2C, Ar-2′,6′-CH), 127.62 (1C, 4″-CH), 122.98 (1C, 5″-CH), 120.48 (1C, 3″a-C), 111.55 (1C, 7″-CH), 23.64 (1C, 7-CH2), 21.53 (1C, 4′-CH3). HRMS (ES+): m/z calcd for C19H16N7OS [M + H]+, 390.1137; found, 390.1130.
4.1.3.4. 5-Methoxy-3-(2-(6-(p-tolyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydraz-ylidene)indolin-2-one (6d)
Yield 356 mg (85%) of yellowish-white powder; mp 282 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.33 (1H, br s, D2O exchangeable, 3-NH), 11.06 (1H, br s, D2O exchangeable, 1″-NH), 7.94 (2H, d, J = 7.70 Hz, Ar-2′,6′-H), 7.44–7.32 (2H, m, Ar-3′,5′-H), 7.09 (1H, s, Ar-4″-H), 6.77 (1H, dd, J = 19.0, 9.01 Hz, 6″-H), 6.97–6.85 (1H, m, Ar-7″-H), 4.44 (2H, s, 7-CH2), 3.78 (3H, s, 5″-OCH3), 2.40 (3H, s, 4′-CH3). 13C NMR (125 MHz, DMSO): δ 164.01 (1C, 2″-C=O), 155.78 (1C, 6-C), 155.26 (1C, 5″-C), 154.74 (1C, 8a-C), 149.40 (1C, 3-C), 142.87 (1C, 7″a-C), 141.25 (1C, Ar-4′-C), 138.88 (1C, 3″-C), 135.41 (1C, Ar-1′-C), 130.17 (2C, Ar-3,5′-CH), 127.75 (2C, Ar-2′,6′-CH), 123.90 (1C, 3″a-C), 117.35 (1C, 7″-CH), 112.39 (1C, 6″-CH), 105.28 (1C, 4″-CH), 56.02 (1C, 5″-OCH3), 23.64 (1C, 7-CH2), 21.53 (1C, 4′-CH3). DEPT C135 (125 MHz, DMSO): δ 130.17 (2C, Ar-3,5′-CH), 127.75 (2C, Ar-2′,6′-CH), 117.34 (1C, 7″-CH), 112.38 (1C, 6″-CH), 105.27 (1C, 4″-CH), 56.01 (1C, 5″-OCH3), 23.63 (1C, 7-CH2), 21.53 (1C, 4′-CH3). HRMS (ES+): m/z calcd for C20H18N7O2S [M + H]+, 420.1243; found, 420.1241.
4.1.3.5. 3-(2-(6-(4-Chlorophenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydraz-ineylidene)indolin-2-one (6e)
Yield 377 mg (92%) of yellowish-white powder; mp 295 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.28 (1H, br s, D2O exchangeable, 3-NH), 11.24 (1H, br s, D2O exchangeable, 1″-NH), 8.06 (2H, br s, Ar-2′,6′-H), 7.72 (3H, m, Ar-3′,5′-H), 7.36 (1H, br s, Ar-4″-H), 7.28–7.07 (1H, m, Ar-7″-H), 7.00 (1H, br s, Ar-6″-H), 6.84 (1H, br s, Ar-5″-H), 4.45 (2H, s, 7-CH2). 13C NMR (125 MHz, DMSO): δ 164.00 (1C, 2″-C=O), 155.79 (1C, 6-C), 155.37 (1C, 8a-C), 154.72 (1C, 3-C), 149.46 (1C, 7″a-C), 135.42 (1C, Ar-1′-C), 134.60 (1C, 3″-C), 132.56 (1C, Ar-4′-CH), 129.59 (2C, Ar-3,5′-CH), 127.79 (2C, Ar-2′,6′-CH), 121.14 (1C, 3″a-C), 117.39 (1C, 7″-CH), 112.41 (1C, 4″-CH), 105.28 (1C, 6″-CH), 56.02 (1C, 5″-OCH3), 23.77 (1C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 132.57 (1C, Ar-4′-CH), 129.59 (2C, Ar-3,5′-CH), 127.79 (2C, Ar-2′,6′-CH), 117.39 (1C, 7″-CH), 112.41 (1C, 4″-CH), 105.28 (1C, 6″-CH), 56.02 (1C, 5″-OCH3), 23.76 (1C, 7-CH2). HRMS (ES+): m/z calcd for C18H13ClN7OS [M + H]+, 410.0591; found, 410.0593.
4.1.3.6. 3-(2-(6-(4-Chlorophenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydrazine-eylidene)-5-methoxyindolin-2-one (6f)
Yield 396 mg (90%) of yellowish-white powder; mp 292 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.31 (1H, br s, D2O exchangeable, 3-NH), 11.04 (1H, br s, D2O exchangeable, 1″-NH), 8.23 (2H, br s, Ar-2′,6′-H), 8.02 (2H, br s, Ar-3′,5′-H), 7.07 (1H, s, Ar-4″-H), 6.98–6.20 (2H, m, 6″ and 7″-H), 4.44 (2H, s, 7-CH2), 3.75 (3H, s, 5″-OCH3). 13C NMR (125 MHz, DMSO): δ 163.97 (1C, 2″-C=O), 161.40 (1C, 5″-C), 155.77 (1C, 6-C), 154.31 (1C, 8a-C), 149.42 (1C, 3-C), 137.41 (1C, 7″a-C), 135.42 (1C, Ar-4′-C), 133.02 (1C, 3″-C), 132.46 (1C, Ar-1′-C), 129.68 (2C, Ar-3,5′-CH), 129.50 (2C, Ar-2′,6′-CH), 124.46 (1C, 3″a-C), 117.39 (1C, 7″-CH), 112.38 (1C, 6″-CH), 105.29 (1C, 4″-CH), 56.01 (1C, 5″-OCH3), 23.68 (1C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 129.70 (2C, Ar-3,5′-CH), 129.51 (2C, Ar-2′,6′-CH), 117.39 (1C, 7″-CH), 112.39 (1C, 6″-CH), 105.29 (1C, 4″-CH), 56.01 (1C, 5″-OCH3), 23.68 (1C, 7-CH2). HRMS (ES+): m/z calcd for C19H15ClN7O2S [M + H]+, 440.0696; found, 440.0691.
4.1.3.7. 3-(2-(6-(4-Methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydraz-ineylidene)indolin-2-one (6g)
Yield 361 mg (88%) of yellowish-white powder; mp 293 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.28 (1H, br s, D2O exchangeable, 3-NH), 11.23 (1H, br s, D2O exchangeable, 1″-NH), 8.02 (2H, d, J = 8.7 Hz, Ar-2′,6′-H), 7.56 (1H, d, J = 7.5 Hz, Ar-4″-H), 7.35 (1H, t, J = 7.7 Hz, Ar-6″-H), 7.20–7.08 (3H, m, Ar-3′,5′,5″-H), 6.98 (1H, d, J = 7.7 Hz, Ar-5″-H), 6.98 (1H, d, J = 7.7 Hz, Ar-7″-H), 4.43 (2H, s, 7-CH2), 3.86 (3H, s, 4′-OCH3). 13C NMR (125 MHz, DMSO): δ 163.87 (1C, 2″-C=O), 162.86 (1C, 6-C), 154.93 (1C, 8a-C), 149.36 (1C, 3-C), 141.69 (1C, 7″a-C), 138.33 (1C, Ar-4′-C), 134.15 (1C, 3″-C), 130.93 (1C, 6″-CH), 129.67 (2C, Ar-2′,6′-CH), 129.49 (1C, Ar-1′-C), 125.64 (1C, 3″a-C), 122.97 (1C, 4″-CH), 120.45 (1C, 5″-CH), 115.04 (2C, Ar-3′,5′-CH), 111.54 (1C, 7″-CH), 56.05 (1C, 4′-OCH3), 23.56 (1C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 130.93 (1C, 6″-CH), 129.67 (2C, Ar-2′,6′-CH), 122.97 (1C, 4″-CH), 120.45 (1C, 5″-CH), 115.04 (2C, Ar-3,5′-CH), 111.54 (1C, 7″-CH), 56.06 (1C, 4′-OCH3), 23.55 (1C, 7-CH2). HRMS (ES+): m/z calcd for C19H16N7O2S [M + H]+, 406.1086; found, 406.1082.
4.1.3.8. 3-(2-(6-(4-Methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydraz-ineylidene)-5-methylindolin-2-one (6h)
Yield 364 mg (87%) of yellowish-white powder; mp 283–284 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.26 (1H, br s, D2O exchangeable, 3-NH), 11.12 (1H, br s, D2O exchangeable, 1″-NH), 8.01 (2H, d, J = 8.4 Hz, Ar-2′,6′-H), 7.37 (1H, s, Ar-4″-H), 7.24–7.05 (3H, m, Ar-3′,5′,6″-H), 6.86 (1H, d, J = 7.9 Hz, Ar-7″-H), 4.42 (2H, s, 7-CH2), 3.86 (3H, s, 4′-OCH3), 2.31 (3H, s, 5″-CH3). 13C NMR (125 MHz, DMSO): δ 163.94 (1C, 2″-C=O), 162.84 (1C, 6-C), 154.87 (1C, 8a-C), 149.38 (1C, 3-C), 139.45 (1C, 7″a-C), 138.27 (1C, Ar-4′-C), 134.26 (1C, 3″-C), 131.37 (1C, 6″-CH), 129.66 (2C, Ar-2′,6′-CH), 129.37 (1C, Ar-1′-C), 125.64 (1C, 3″a-C), 120.83 (1C, 4″-CH), 115.02 (2C, Ar-3,5′-CH), 111.29 (1C, 7″-CH), 56.04 (1C, 4′-OCH3), 23.54 (1C, 7-CH2), 21.08 (1C, 5″-CH3). DEPT C135 (125 MHz, DMSO): δ 131.37 (1C, 6″-CH), 129.66 (2C, Ar-2′,6′-CH), 120.82 (1C, 4″-CH), 115.02 (2C, Ar-3,5′-CH), 111.29 (1C, 7″-CH), 56.04 (1C, 4′-OCH3), 23.53 (1C, 7-CH2), 21.08 (1C, 5″-CH3). HRMS (ES+): m/z calcd for C20H18N7O2S [M + H]+, 420.1243; found, 420.1238.
4.1.3.9. 3-(2-(6-(4-Methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydraz-ineyl-idene)-5,7-dimethylindolin-2-one (6i)
Yield 381 mg (88%) of yellowish-white powder; mp 233 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.46 (1H, br s, D2O exchangeable, 3-NH), 11.24 (1H, br s, D2O exchangeable, 1″-NH), 8.02 (2H, d, J = 8.7 Hz, Ar-2′,6′-H), 7.19–7.08 (3H, m, Ar-3′,5′,4″-H), 6.98 (1H, s, Ar-6″-H), 4.46 (2H, s, 7-CH2), 3.86 (3H, s, 4′-OCH3), 2.26 (3H, s, 5″-CH3), 2.19 (3H, s, 7″-CH3). 13C NMR (125 MHz, DMSO): δ 163.03 (1C, 2″-C=O), 162.61 (1C, 6-C), 155.59 (1C, 8a-C), 147.57 (1C, 3-C), 140.52 (1C, 7″a-C), 138.42 (1C, Ar-4′-C), 135.90 (1C, 3″-C), 133.28 (1C, 7″-C), 132.02 (1C, 5″-C), 130.19 (1C, 6″-CH), 129.78 (2C, Ar-2′,6′-CH), 129.39 (1C, Ar-1′-C), 125.41 (1C, 4″-CH), 124.25 (1C, 3″a-C), 114.91 (2C, Ar-3,5′-CH), 56.09 (1C, 4′-OCH3), 23.54 (1C, 7-CH2), 20.98 (1C, 5″-CH3), 16.37 (1C, 7″-CH3). DEPT C135 (125 MHz, DMSO): δ 129.78 (2C, Ar-2′,6′-CH), 125.48 (1C, 4″-CH), 130.17 (1C, 6″-CH), 114.91 (2C, Ar-3,5′-CH), 56.08 (1C, 4′-OCH3), 23.54 (1C, 7-CH2), 20.98 (1C, 5″-CH3), 16.37 (1C, 7″-CH3). HRMS (ES+): m/z calcd for C21H20N7O2S [M + H]+, 434.1399; found, 434.1396.
4.1.3.10. 5-Fluoro-3-(2-(6-(4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl) hydrazineylidene)indolin-2-one (6j)
Yield 360 mg (85%) of yellowish-white powder; mp 288–289 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.32 (1H, br s, D2O exchangeable, 3-NH), 11.24 (1H, br s, D2O exchangeable, 1″-NH), 8.09–7.98 (2H, m, Ar-2′,6′-H), 7.35 (1H, d, J = 8.0 Hz, Ar-7″-H), 7.24–7.03 (3H, m, Ar-3′,5′,4″-H), 7.02–6.93 (1H, d, J = 7.9 Hz, Ar-6″-H), 4.43 (2H, s, 7-CH2), 3.87 (3H, s, 4′-OCH3). 13C NMR (125 MHz, DMSO): δ 164.03 (1C, 2″-C=O), 162.88 (1C, 6-C), 155.08 (1C, 8a-C), 149.16 (1C, 3-C), 139.32 (1C, 7″a-C), 137.90 (1C, Ar-4′-C), 133.69 (1C, 3″-C), 129.68 (2C, Ar-2′,6′-CH), 126.18 (1C, Ar-1′-C), 125.59 (1C, 3″a-C), 129.68 (2C, Ar-2′,6′-CH), 115.03 (2C, Ar-3,5′-CH), 112.65 (1C, 7″-CH), 110.33 (1C, 4″-CH), 107.63 (1C, 6″-CH), 56.05 (1C, 4′-OCH3), 23.55 (1C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 129.68 (2C, Ar-2′,6′-CH), 115.03 (2C, Ar-3,5′-CH), 112.55 (1C, 7″-CH), 110.32 (1C, 4″-CH), 107.63 (1C, 6″-CH), 56.05 (1C, 4′-OCH3), 23.54 (1C, 7-CH2). HRMS (ES+): m/z calcd for C19H15FN7O2S [M + H]+, 424.0992; found, 424.0989.
4.1.3.11. 7-Fluoro-3-(2-(6-(4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydrazineylidene)indolin-2-one (6k)
Yield 381 g (90%) of yellowish-white powder; mp 288–290 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.32 (1H, br s, D2O exchangeable, 3-NH), 11.24 (1H, br s, D2O exchangeable, 1″-NH), 8.09–7.98 (2H, m, Ar-2′,6′-H), 7.35 (1H, d, J = 8.0 Hz, Ar-7″-H), 7.24–7.03 (3H, m, Ar-3′,5′,4″-H), 7.02–6.93 (1H, d, J = 7.9 Hz, Ar-6″-H), 4.43 (2H, s, 7-CH2), 3.87 (3H, s, 4′-OCH3). 13C NMR (125 MHz, DMSO): δ 164.03 (1C, 2″-C=O), 162.88 (1C, 6-C), 155.08 (1C, 8a-C), 149.16 (1C, 3-C), 139.32 (1C, 7″a-C), 137.90 (1C, Ar-4′-C), 133.69 (1C, 3″-C), 129.68 (2C, Ar-2′,6′-CH), 126.18 (1C, Ar-1′-C), 125.59 (1C, 3″a-C), 129.68 (2C, Ar-2′,6′-CH), 115.03 (2C, Ar-3,5′-CH), 112.65 (1C, 7″-CH), 110.33 (1C, 4″-CH), 107.63 (1C, 6″-CH), 56.05 (1C, 4′-OCH3), 23.55 (1C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 129.68 (2C, Ar-2′,6′-CH), 115.03 (2C, Ar-3,5′-CH), 112.55 (1C, 7″-CH), 110.32 (1C, 4″-CH), 107.63 (1C, 6″-CH), 56.05 (1C, 4′-OCH3), 23.54 (1C, 7-CH2). HRMS (ES+): m/z calcd for C19H15FN7O2S [M + H]+, 424.0992; found, 424.0989.
4.1.3.12. 5-Chloro-3-(2-(6-(4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydrazineylidene)indolin-2-one (6l)
Yield 440 mg (85%) of yellowish-white powder; mp 293–295 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.27 (1H, br s, D2O exchangeable, 3-NH), 11.35 (1H, br s, D2O exchangeable, 1″-NH), 8.72 (1H, s, Ar-4″-H), 8.06 (2H, d, J = 8.0 Hz, Ar-2′,6′-H), 7.25 (1H, d, J = 8.0 Hz, Ar-7″-H), 7.13–7.08 (2H, m, Ar-3′,5′-H), 6.84 (1H, d, J = 8.0 Hz, Ar-6″-H), 4.45 (2H, s, 7-CH2), 3.87 (3H, s, 4′-OCH3). 13C NMR (125 MHz, DMSO): δ 163.70 (1C, 2″-C=O), 161.93 (1C, 6-C), 154.61 (1C, 8a-C), 147.43 (1C, 3-C), 142.29 (1C, Ar-4′-C), 140.30 (1C, 7″a-C), 134.58 (1C, 3″-C), 132.90 (1C, 5″-C), 129.52 (2C, Ar-2′,6′-CH), 128.84 (1C, Ar-1′-C), 126.57 (1C, 6″-CH), 125.47 (1C, 3″a-C), 123.70 (1C, 4″-CH), 114.89 (2C, Ar-3,5′-CH), 111.02 (1C, 7″-CH), 56.05 (1C, 4′-OCH3), 22.61 (1C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 129.51 (2C, Ar-2′,6′-CH), 126.49 (1C, 6″-CH),123.59 (1C, 4″-CH), 114.87 (2C, Ar-3,5′-CH), 111.01 (1C, 7″-CH), 56.03 (1C, 4′-OCH3), 22.57 (1C, 7-CH2). HRMS (ES+): m/z calcd for C19H15ClN7O2S [M + H]+, 440.0696; found, 440.0698.
4.1.3.13. 5-Bromo-3-(2-(6-(4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydrazineylidene)indolin-2-one (6m)
Yield 416 mg (86%) of yellowish-white powder; mp 297–298 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.25 (1H, br s, D2O exchangeable, 3-NH), 11.34 (1H, br s, D2O exchangeable, 1″-NH), 8.87 (1H, s, Ar-4″-H), 8.12 (2H, d, J = 8.5 Hz, Ar-2′,6′-H), 7.38 (1H, d, J = 8.2 Hz, Ar-7″-H), 7.16–7.05 (2H, m, Ar-3′,5′-H), 6.80 (1H, d, J = 8.2 Hz, Ar-6″-H), 4.45 (2H, s, 7-CH2), 3.87 (3H, s, 4′-OCH3). 13C NMR (125 MHz, DMSO): δ 166.12 (1C, 2″-C=O), 162.74 (1C, 6-C), 154.85 (1C, 8a-C), 154.62 (1C, 3-C), 140.73 (1C, Ar-4′-C), 139.22 (1C, 7″a-C), 135.01 (1C, 3″-C), 131.62 (1C, 6″-CH), 129.55 (2C, Ar-2′,6′-CH), 129.36 (1C, Ar-1′-C), 125.97 (1C, 3″a-C), 123.54 (1C, 4″-CH), 114.96 (2C, Ar-3,5′-CH), 113.33 (1C, 5″-C), 111.55 (1C, 7″-CH), 56.06 (1C, 4′-OCH3), 22.57 (1C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 131.63 (1C, 6″-CH), 129.57 (2C, Ar-2′,6′-CH), 123.65 (1C, 4″-CH), 115.03 (2C, Ar-3,5′-CH), 111.58 (1C, 7″-CH), 56.07 (1C, 4′-OCH3), 22.59 (1C, 7-CH2). HRMS (ES+): m/z calcd for C19H15BrN7O2S [M + H]+, 484.0191; found, 484.0196.
4.1.3.14. 5-Methoxy-3-(2-(6-(4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydrazineylidene)indolin-2-one (6n)
Yield 370 mg (85%) of yellowish-white powder; mp 278–280 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.34 (1H, br s, D2O exchangeable, 3-NH), 11.05 (1H, br s, D2O exchangeable, 1″-NH), 8.02 (2H, d, J = 8.6 Hz, Ar-2′,6′-H), 7.21–7.03 (3H, m, Ar-3′,5′, 4″-H), 6.97–6.84 (1H, m, Ar-7″-H), 6.81–6.66 (1H, m, Ar-6″-H), 4.43 (2H, s, 7-CH2), 3.87 (3H, s, 4′-OCH3), 3.78 (3H, s, 6″-OCH3). 13C NMR (125 MHz, DMSO): δ 168.19 (1C, 2″-C=O), 163.88 (1C, 6-C), 157.54 (1C, 8a-C), 155.78 (1C, 3-C), 140.74 (1C, Ar-4′-C), 135.78 (1C, 7″a-C), 135.38 (1C, 3″-C), 130.63 (1C, 5″-C), 129.67 (2C, Ar-2′,6′-CH), 129.38 (1C, Ar-1′-C), 125.63 (1C, 3″a-C), 123.75 (1C, 7″-CH), 117.34 (1C, 6″-CH), 115.04 (2C, Ar-3,5′-CH), 114.82 (1C, 4″-CH), 56.03 (1C, 4′-OCH3), 55.65 (1C, 5″-OCH3), 23.55 (1C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 129.68 (2C, Ar-2′,6′-CH), 123.81 (1C, 7″-CH), 117.35 (1C, 6″-CH), 115.04 (2C, Ar-3,5′-CH), 114.82 (1C, 4″-CH), 56.02 (1C, 4′-OCH3), 55.64 (1C, 5″-OCH3), 23.54 (1C, 7-CH2). HRMS (ES+): m/z calcd for C20H18N7O3S [M + H]+, 436.1192; found, 436.1194.
4.1.3.15. 3-(2-(6-(4-Methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydraz-ineylidene)-5-(trifluoromethoxy)indolin-2-one (6o)
Yield 406 mg (83%) of yellowish-white powder; mp 284–285 °C (decompo.); 1H NMR (300 MHz, DMSO-d6): δ 13.28 (1H, br s, D2O exchangeable, 3-NH), 11.42 (1H, br s, D2O exchangeable, 1″-NH), 8.60 (1H, s, Ar-4″-H), 8.12–7.97 (2H, m, Ar-2′,6′-H), 7.21 (1H, d, J = 8.3 Hz, Ar-7″-H), 7.06 (2H, d, J = 8.3 Hz, Ar-3′,5′-H), 6.90 (1H, d, J = 8.3 Hz, Ar-6″-H), 4.45 (2H, s, 7-CH2), 3.86 (3H, s, 4′-OCH3). 13C NMR (125 MHz, DMSO): δ 168.46 (1C, 2″-C=O), 162.67 (1C, 6-C), 159.32 (1C, 5″-C), 155.10 (1C, 8a-C), 154.65 (1C, 3-C), 149.10 (1C, 5″-OCF3), 140.63 (1C, Ar-4′-C), 139.24 (1C, 3″-C), 135.27 (1C, 7″a-C), 129.41 (2C, Ar-2′,6′-CH), 124.14 (1C, Ar-1′-C), 123.36 (1C, 3″a-C), 122.53 (1C, 7″-CH), 119.76 (1C, 6″-CH), 114.65 (2C, Ar-3,5′-CH), 110.38 (1C, 4″-CH), 55.96 (1C, 4′-OCH3), 23.55 (1C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 129.68 (2C, Ar-2′,6′-CH), 123.81 (1C, 7″-CH), 117.35 (1C, 6″-CH), 115.04 (2C, Ar-3,5′-CH), 114.82 (1C, 4″-CH), 56.02 (1C, 4′-OCH3), 55.64 (1C, 5″-OCH3), 23.54 (1C, 7-CH2). HRMS (ES+): m/z calcd for C20H18N7O3S [M + H]+, 490.0909; found, 490.0916.
4.1.3.16. 5-Bromo-3-(2-(6-(4-chlorophenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydrazineylidene)indolin-2-one (6p)
Yield 420 mg (86%) of yellowish-white powder; mp 304–307 °C (decompo.); 1H NMR (300 MHz, DMSO-d6): δ 13.21 (1H, br s, D2O exchangeable, 3-NH), 11.34 (1H, br s, D2O exchangeable, 1″-NH), 8.83 (1H, s, Ar-4″-H), 8.13 (2H, d, J = 8.5 Hz, Ar-2′,6′-H), 7.65 (2H, d, J = 8.5 Hz, Ar-3′,5′-H), 7.37 (1H, d, J = 8.2 Hz, Ar-7″-H), 6.78 (1H, d, J = 8.2 Hz, Ar-6″-H), 4.46 (2H, s, 7-CH2). 13C NMR (125 MHz, DMSO): δ 166.08 (1C, 2″-C=O), 162.78 (1C, 6-C), 154.85 (1C, 8a-C), 153.81 (1C, 3-C), 140.83 (1C, 7″a-C), 137.25 (1C, Ar-4′-C), 133.05 (1C, 3″-C), 132.73 (1C, Ar-1′-C), 131.76 (1C, 6″-CH), 129.61 (2C, Ar-3,5′-CH), 129.34 (2C, Ar-2′,6′-CH), 123.99 (1C, 3″a-C), 122.66 (1C, 4″-CH), 113.42 (1C, 5″-C), 111.61 (1C, 7″-CH), 22.64 (1C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 131.76 (1C, 6″-CH), 129.62 (2C, Ar-3,5′-CH), 129.34 (2C, Ar-2′,6′-CH), 122.68 (1C, 4″-CH), 111.61 (1C, 7″-CH), 22.64 (1C, 7-CH2). HRMS (ES+): m/z calcd for C18H12BrClN7OS [M + 1]+, 487.9696; found, 487.9697.
4.1.3.17. 3-(2-(6-(4-Chlorophenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydraz-ineeylidene)-5-methylindolin-2-one (6q)
Yield 348 mg (82%) of yellow-powder; mp 303–306 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.26 (1H, br s, D2O exchangeable, 3-NH), 11.13 (1H, br s, D2O exchangeable, 1″-NH), 8.03 (2H, d, J = 8.4 Hz, Ar-2′,6′-H), 7.68 (2H, d, J = 8.4 Hz, Ar-3′,5′-H), 7.35 (1H, s, Ar-4″-H), 7.14 (1H, d, J = 8.0 Hz, Ar-6″-H), 6.85 (1H, d, J = 8.0 Hz, Ar-7″-H), 4.45 (2H, s, 7-CH2), 2.30 (3H, s, 5″-CH3). 13C NMR (125 MHz, DMSO): δ 164.78 (1C, 2″-C=O), 163.91 (1C, 6-C), 154.24 (1C, 8a-C), 153.21 (1C, 3-C), 139.51 (1C, 7″a-C), 138.16 (1C, Ar-4′-C), 132.46 (1C, 3″-C), 131.98 (1C, Ar-1′-C), 131.44 (1C, 6″-CH), 129.68 (2C, Ar-3,5′-CH), 129.50 (2C, Ar-2′,6′-CH), 120.86 (1C, 4″-CH), 120.39 (1C, 3″a-C), 111.30 (1C, 7″-CH), 23.66 (1C, 7-CH2), 21.08 (1C, 5″-CH3). DEPT C135 (125 MHz, DMSO): δ 131.44 (1C, 6″-CH), 129.69 (2C, Ar-3,5′-CH), 129.50 (2C, Ar-2′,6′-CH), 120.86 (1C, 4″-CH), 111.30 (1C, 7″-CH), 23.65 (1C, 7-CH2), 21.08 (1C, 5″-CH3). HRMS (ES+): m/z calcd for C19H15ClN7OS [M + H]+, 424.0747; found, 424.0747.
4.1.3.18. 5-Bromo-3-(2-(6-(p-tolyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydraz-ineylidene)indolin-2-one (6r)
Yield 379 mg (81%) of yellow-powder; mp 318–320 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.25 (1H, br s, D2O exchangeable, 3-NH), 11.37 (1H, br s, D2O exchangeable, 1″-NH), 8.06 (2H, d, J = 8.0 Hz, Ar-2′,6′-H), 7.94 (1H, d, J = 8.0 Hz, 7″-H, overlapped with CDCl3 peak), 7.41 (2H, J = 8.0 Hz, Ar-3′,5′-H), 7.37 (1H, s, Ar-4″-H), 6.80 (1H,d, J = 8.3 Hz, Ar-6″-H), 4.46 (2H, s, 7-CH2), 2.41 (3H, s, 4′-CH3). 13C NMR (125 MHz, DMSO): δ 166.18 (1C, 2″-C=O), 162.84 (1C, 6-C), 154.98 (2C, 8a-C and 3-C), 142.81 (1C, 7″a-C), 140.86 (1C, Ar-4′-C), 139.60 (1C, 3″-C), 131.75 (1C, 6″-CH), 131.04 (1C, Ar-1′-C), 130.25 (2C, Ar-3,5′-CH), 129.49 (1C, 4″-CH), 127.72 (2C, Ar-2′,6′-CH), 117.14 (1C, 3″a-C), 113.42 (1C, 5″-C), 111.67 (1C, 7″-CH), 22.66 (1C, 7-CH2), 21.57 (1C, 4′-CH3). DEPT C135 (125 MHz, DMSO): δ131.76 (1C, 6″-CH), 130.25 (2C, Ar-3,5′-CH), 129.49 (1C, 4″-CH), 127.71 (2C, Ar-2′,6′-CH), 111.67 (1C, 7″-CH), 22.66 (1C, 7-CH2), 21.57 (1C, 4′-CH3). HRMS (ES+): m/z calcd for C19H15BrN7OS [M + H]+, 468.0242; found, 468.0239.
4.1.3.19. 5-Methyl-3-(2-(6-(p-tolyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydraz-ineylidene)indolin-2-one (6s)
Yield 327 mg (81%) of yellow powder; mp 295–297 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.27 (1H, br s, D2O exchangeable, 3-NH), 11.14 (1H, br s, D2O exchangeable, 1″-NH), 7.94 (2H, d, J = 8.1 Hz, Ar-3′,5′-H), 7.41 (2H, J = 8.1 Hz, Ar-2′,6′-H), 7.38 (1H, s, Ar-4″-H), 7.16 (1H, d, J = 8.0 Hz, 6″-H), 6.86 (1H,d, J = 7.9 Hz, Ar-7″-H), 4.44 (2H, s, 7-CH2), 2.40 (3H, s, 4′-CH3), 2.31 (3H, s, 5″-CH3). 13C NMR (125 MHz, DMSO): δ 166.33 (1C, 2″-C=O), 163.96 (1C, 6-C), 155.28 (1C, 8a-C), 149.45 (1C, 3-C), 142.88 (1C, Ar-4′-C), 139.51 (1C, 3″-C), 138.36 (1C, 7″a-C), 134.43 (1C, 5″-C), 132.02 (1C, Ar-1′-C), 131.45 (1C, 6″-CH), 130.77 (1C, 3″a-C), 130.18 (2C, Ar-3,5′-CH), 127.76 (2C, Ar-2′,6′-CH), 120.87 (1C, 4″-CH), 111.33 (1C, 7″-CH), 23.65 (1C, 7-CH2), 21.54 (1C, 4′-CH3), 21.09 (1C, 5″-CH3). DEPT C135 (125 MHz, DMSO): δ131.44 (1C, 6″-CH), 130.17 (2C, Ar-3,5′-CH), 127.75 (2C, Ar-2′,6′-CH), 120.86 (1C, 4″-CH), 111.33 (1C, 7″-CH), 23.64 (1C, 7-CH2), 21.53 (1C, 4′-CH3), 21.09 (1C, 5″-CH3). HRMS (ES+): m/z calcd for C20H18N7OS [M + H]+, 404.1294; found, 404.1295.
4.1.3.20. 3-(2-(6-(4-Methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydraz-ineylidene)-1-methylindolin-2-one (6t)
Yield 419 mg (84%) of yellowish-white powder; mp 253–255 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.13 (1H, br s, D2O exchangeable, 3-NH), 8.06–7.97 (2H, m, Ar-2′,6′-H), 7.58 (1H, d, J = 7.6 Hz, Ar-4″-H), 7.43 (1H, t, J = 7.6 Hz, Ar-6″-H), 7.21–7.13 (3H, m, Ar-3′,5′,7″-H), 7.08 (1H, t, J = 7.6 Hz, Ar-5″-H), 4.43 (2H, s, 7-CH2), 3.87 (3H, s, 4′-OCH3), 3.26 (3H, s, 1″-N-CH3). 13C NMR (125 MHz, DMSO): δ 162.89 (1C, 2″-C=O), 161.98 (1C, 6-C), 155.09 (1C, 8a-C), 152.90 (1C, 3-C), 143.17 (1C, 7″a-C), 136.95 (1C, Ar-4′-C), 133.53 (1C, 3″-C), 130.90 (1C, 6″-CH), 129.67 (2C, Ar-2′,6′-CH), 125.61 (1C, Ar-1′-C), 123.49 (1C, 3″a-C), 120.14 (1C, 4″-CH), 118.72 (1C, 5″-CH), 115.08 (2C, Ar-3,5′-CH), 110.28 (1C, 7″-CH), 56.07 (1C, 4′-OCH3), 26.20 (1C, 1″-N-CH3), 23.58 (1C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 131.37 (1C, 6″-CH), 129.66 (2C, Ar-2′,6′-CH), 120.82 (1C, 4″-CH), 115.02 (2C, Ar-3,5′-CH), 111.29 (1C, 7″-CH), 56.04 (1C, 4′-OCH3), 23.53 (1C, 7-CH2), 21.08 (1C, 5″-CH3). HRMS (ES+): m/z calcd for C20H18N7O2S [M + H]+, 420.1243; found, 420.1242.
4.1.3.21. 5-Chloro-3-(2-(6-(4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydrazineylidene)-1-methylindolin-2-one (6u)
Yield 417 mg (92%) of yellowish-white powder; mp 277–279 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.10 (1H, br s, D2O exchangeable, 3-NH), 8.74 (1H, s, Ar-4″-H), 8.10 (2H, d, J = 8.6 Hz, Ar-2′,6′-H), 7.34 (1H, d, J = 8.3 Hz, Ar-7″-H), 7.10 (2H, d, J = 8.6 Hz, Ar-3′,5′-H), 7.02 (1H, d, J = 8.3 Hz, Ar-6″-H), 4.45 (2H, s, 7-CH2), 3.87 (3H, s, 4′-OCH3), 3.19 (3H, s, 1″-N-CH3). 13C NMR (125 MHz, DMSO): δ 164.80 (1C, 2″-C=O), 162.73 (1C, 6-C), 155.08 (1C, 8a-C), 154.70 (1C, 3-C), 141.50 (1C, 7″a-C), 139.28 (1C, Ar-4′-C), 138.64 (1C, 3″-C), 129.68 (1C, 5″-C), 129.54 (2C, Ar-2′,6′-CH), 128.67 (1C, 6″-CH), 126.14 (1C, Ar-1′-C), 126.00 (1C, 3″a-C), 123.90 (1C, 4″-CH), 114.88 (2C, Ar-3,5′-CH), 109.73 (1C, 7″-CH), 56.04 (1C, 4′-OCH3), 26.27 (1C, 1″-N-CH3), 22.61 (1C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 129.54 (2C, Ar-2′,6′-CH), 128.67 (1C, 6″-CH), 123.86 (1C, 4″-CH), 114.88 (2C, Ar-3,5′-CH), 109.74 (1C, 7″-CH), 56.05 (1C, 4′-OCH3), 26.27 (1C, 1″-N-CH3), 22.61 (1C, 7-CH2). HRMS (ES+): m/z calcd for C20H17ClN7O2S [M + H]+, 454.0853; found, 454.0854.
4.1.3.22. 5-Bromo-3-(2-(6-(4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydra-zineeylidene)-1-methylindolin-2-one (6v)
Yield 418 mg (84%) of yellowish-white powder; mp 290–292 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.08 (1H, br s, D2O exchangeable, 3-NH), 8.90 (1H, s, Ar-4″-H), 8.11 (2H, d, J = 8.6 Hz, Ar-2′,6′-H), 7.47 (1H, d, J = 8.2 Hz, Ar-7″-H), 7.12 (2H, d, J = 8.6 Hz, Ar-3′,5′-H), 6.98 (1H, d, J = 8.3 Hz, Ar-6″-H), 4.45 (2H, s, 7-CH2), 3.87 (3H, s, 4′-OCH3), 3.19 (3H, s, 1″-N-CH3). 13C NMR (125 MHz, DMSO): δ 164.67 (1C, 2″-C=O), 162.76 (1C, 6-C), 154.88 (1C, 8a-C), 154.73 (1C, 3-C), 141.85 (1C, 7″a-C), 139.28 (1C, Ar-4′-C), 138.45 (1C, 3″-C), 131.47 (1C, 6″-CH), 129.57 (2C, Ar-2′,6′-CH), 129.04 (1C, 5″-C), 125.94 (1C, Ar-1′-C), 123.90 (1C, 4″-CH), 119.73 (1C, 3″a-C), 114.96 (2C, Ar-3,5′-CH), 110.27 (1C, 7″-CH), 56.06 (1C, 4′-OCH3), 26.25 (1C, 1″-N-CH3), 22.58 (1C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 131.47 (1C, 6″-CH), 129.58 (2C, Ar-2′,6′-CH), 123.91 (1C, 4″-CH), 114.97 (2C, Ar-3,5′-CH), 110.29 (1C, 7″-CH), 56.06 (1C, 4′-OCH3), 26.24 (1C, 1″-N-CH3), 22.57 (1C, 7-CH2). HRMS (ES+): m/z calcd for C20H17BrN7O2S [M + H]+, 498.0348; found, 498.0352.
4.1.3.23. 5-Chloro-3-(2-(6-(4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydrazineylidene)-1-propylindolin-2-one (6w)
Yield 448 mg (93%) of yellowish-white powder; mp 266–268 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.06 (1H, br s, D2O exchangeable, 3-NH), 8.75 (1H, s, Ar-4″-H), 8.09 (2H, d, J = 8.5 Hz, Ar-2′,6′-H), 7.31 (1H, d, J = 8.1 Hz, Ar-7″-H), 7.18–6.98 (3H, m, Ar-3′,5′-H and Ar-6″-H), 4.45 (2H, s, 7-CH2), 3.86 (3H, s, 4′-OCH3), 3.69 (2H, t, J = 7.0 Hz, 1″-N-CH2CH2CH3), 1.61 (2H, m, 1″-N-CH2CH2CH3), 0.88 (3H, t, J = 8.3 Hz, 1″-N-CH2CH2CH3). 13C NMR (125 MHz, DMSO): δ 164.80 (1C, 2″-C=O), 162.72 (1C, 6-C), 154.92 (1C, 8a-C), 154.67 (1C, 3-C), 141.83 (1C, 7″a-C), 139.29 (1C, Ar-4′-C), 138.77 (1C, 3″-C), 129.69 (1C, 5″-C), 129.54 (2C, Ar-2′,6′-CH), 128.61 (1C, 6″-CH), 126.36 (1C, 4″-CH), 126.00 (2C, Ar-1′-C and 3″a-C), 114.87 (2C, Ar-3,5′-CH), 109.91 (1C, 7″-CH), 56.04 (1C, 4′-OCH3), 23.63 (1C, 1″-N-CH2CH2CH3), 22.60 (1C, 7-CH2), 21.12 (1C, 1″-N-CH2CH2CH3), 11.63 (1C, 1″-N-CH2CH2CH3). DEPT C135 (125 MHz, DMSO): δ 129.54 (2C, Ar-2′,6′-CH), 128.63 (1C, 6″-CH), 126.36 (1C, 4″-CH), 114.87 (2C, Ar-3,5′-CH), 109.92 (1C, 7″-CH), 56.03 (1C, 4′-OCH3), 23.63 (1C, 1″-N-CH2CH2CH3), 22.59 (1C, 7-CH2), 21.13 (1C, 1″-N-CH2CH2CH3), 11.63 (1C, 1″-N-CH2CH2CH3). HRMS (ES+): m/z calcd for C22H21ClN7O2S [M + H]+, 482.1166; found, 482.1170.
4.1.3.24. 1-Benzyl-3-(2-(6-(4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)hydrazineyl idene)indolin-2-one (6x)
Yield 406 mg (82%) of yellowish-white powder; mp 242–244 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.12 (1H, br s, D2O exchangeable, 3-NH), 8.01 (2H, d, J = 8.5 Hz,, Ar-2′,6′-H), 7.61 (1H, d, J = 7.4 Hz, Ar-4″-H), 7.42–7.21 (6H, m, Ar-3′,5′,5″, 6″, 2‴,6‴-H), 7.20–7.09 (3H, m, Ar-3‴,4‴,5‴-H), 7.04 (1H, d, J = 7.5 Hz, Ar-7″-H), 5.05 (2H, s, 1″-N-CH2Ph), 4.44 (2H, s, 7-CH2), 3.85 (3H, s, 4′-OCH3). 13C NMR (125 MHz, DMSO): δ 162.87 (1C, 2″-C=O), 162.02 (1C, 6-C), 155.16 (1C, 8a-C), 154.74 (1C, 3-C), 141.96 (1C, 7″a-C), 138.59 (1C, Ar-4′-C), 133.16 (1C, 3″-C), 130.76 (1C, 6″-CH), 129.69 (2C, Ar-2′,6′-CH), 129.21 (2C, Ar-2‴,6‴-CH), 128.04 (1C, 4″-CH), 127.70 (2C, Ar-3‴,5‴-CH), 136.21 (1C, Ar-1‴-C), 124.38 (2C, Ar-1′-C and 3″a-C), 123.63 (1C, Ar-4‴-CH), 120.31 (1C, 5″-CH), 115.08 (2C, Ar-3,5′-CH), 110.82 (1C, 7″-CH), 56.05 (1C, 4′-OCH3), 42.91 (2H, s, 1″-N-CH2Ph), 23.66 (1C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 130.77 (1C, 6″-CH), 129.68 (2C, Ar-2′,6′-CH), 129.21 (2C, Ar-2‴,6‴-CH), 128.04 (1C, 4″-CH), 127.70 (2C, Ar-3‴, 5‴-CH), 123.63 (1C, Ar-4‴-CH), 120.32 (1C, 5″-CH), 115.08 (2C, Ar-3,5′-CH), 110.82 (1C, 7″-CH), 56.05 (1C, 4′-OCH3), 42.91 (2H, s, 1″-N-CH2Ph), 23.66 (1C, 7-CH2). HRMS (ES+): m/z calcd for C26H22N7O2S [M + H]+, 496.1556; found, 496.1563.
4.1.3.25. 1-Benzyl-5-chloro-3-(2-(6-(4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thia-diazin-3-yl)hydrazineylidene)indolin-2-one (6y)
Yield 477 mg (90%) of yellowish-white powder; mp 285–286 °C; 1H NMR (300 MHz, DMSO-d6): δ 13.10 (1H, br s, D2O exchangeable, 3-NH), 8.77 (1H, s, Ar-4″-H), 8.10 (2H, d, J = 8.5 Hz, Ar-2′,6′-H), 7.39–7.30 (4H, m, Ar-6″, 3‴,4‴,5‴-H), 7.26 (2H, d, J = 8.0 Hz,Ar-2‴,6‴-H), 7.10 (2H, d, J = 8.5 Hz, Ar-3′,5′-H), 6.93 (1H, d, J = 8.4 Hz, Ar-7″-H), 4.98 (2H, s, 1″-N-CH2Ph), 4.46 (2H, s, 7-CH2), 3.86 (3H, s, 4′-OCH3). 13C NMR (125 MHz, DMSO): δ 164.93 (1C, 2″-C=O), 162.77 (1C, 6-C), 154.92 (1C, 8a-C), 154.85 (1C, 3-C), 140.37 (1C, 7″a-C), 139.43 (1C, Ar-4′-C), 138.16 (1C, 3″-C), 137.23 (1C, Ar-1‴-C), 129.57 (2C, Ar-2′,6′-CH), 129.13 (2C, Ar-2‴,6‴-CH), 128.55 (1C, 4″-CH), 127.81 (1C, Ar-4‴-CH), 127.60 (2C, Ar-3‴,5‴-CH), 126.35 (2C, 6″-CH and 5″-C), 125.99 (1C, Ar-1′-C), 119.55 (1C, Ar-3″a-C), 114.91 (2C, Ar-3′,5′-CH), 110.30 (1C, 7″-CH), 56.05 (1C, 4′-OCH3), 42.89 (2H, s, 1″-N-CH2Ph), 22.62 (1C, 7-CH2). DEPT C135 (125 MHz, DMSO): δ 129.57 (2C, Ar-2′,6′-CH), 129.13 (2C, Ar-2‴,6‴-CH), 128.55 (1C, 4″-CH), 127.60 (2C, Ar-3‴,5‴-CH), 127.81 (1C, Ar-4‴-CH), 126.36 (1C, 6″-CH), 114.91 (2C, Ar-3,5′-CH), 110.31 (1C, 7″-CH), 56.05 (1C, 4′-OCH3), 42.89 (2H, s, 1″-N-CH2Ph), 22.62 (1C, 7-CH2). HRMS (ES+): m/z calcd for C26H21ClN7O2S [M + H]+, 530.1166; found, 530.1170.
4.2. Biological Screening
4.2.1. In Vitro Cytotoxic Assay (NCI Cell One-Dose Screening Assay)57
Eight compounds were submitted to the NCI Cell screen and tested at a single high dose (10–5 M) in the full NCI 58 cell panel including nine subpanels (hematopoietic system, lung cancer, colon, brain, skin melanoma, kidney, ovary, prostate, and breast cancers), as described in detail.57 Briefly, the human tumor cells were grown with 5% fetal bovine serum and 2 mM l-glutamine in the medium RPMI 1640. The cancer cells (100 μL) were inoculated in 96-well plates at densities from 5000 to 40,000 cells/well, and the microtiter plates were then incubated at 37 °C, 5% CO2, 95% air, and 100% relative humidity for 24 h. Within 24 h, two plates of each cell line were fixed in situ with trichloroacetic acid (TCA) to measure of the population of the cells at the time of addition of each drug (Tz). Compounds 6a, 6b, 6e, 6l, 6n, 6r, 6v, and 6y have been diluted in DMSO at 400 times the final concentration (10 μM) and frozen prior to use. During the addition of the test compound, an aliquot of frozen concentrate is thawed and diluted with a medium containing 50 μg/mL gentamicin to double the final concentration. Aliquots of 100 μL of different drug dilutions have been added to 100 μL of the medium in each well, resulting in the required final compound concentrations of 10–5 M. The plates were then incubated for an additional 48 h at 37 °C. The experiment was terminated by introducing cold TCA. Cold 50% TCA (50 μL) was gently added and incubated at 4 °C for 1.0 h. The plates were washed five times with H2O after discarding the supernatant and dried by air. To each well, 100 μL of 0.4% (w/v) sulforhodamine B in 1% acetic acid was added, and the plates were incubated for 10 min at ambient temperature. Following removing the unbound dye by washing 5× 1% acetic acid, the bound stain was solubilized with 10 mM trizma base, and the absorbance was read using a plate reader at L515 nm. The same protocol was applied with the suspension cells, except for terminating the experiment by adding 50 μL of 80% TCA to fix the settled cells at the bottom of the wells. % growth inhibition was calculated as
The one-dose data were used as a mean graph of the percent growth inhibition of treated cells relative to a negative control. This enables the detection of both the growth inhibition (between 0 and 100) and lethality (less than 0). In this assay, we calculated the growth inhibition by subtracting the % growth from 100, for example, a value of 100 growth means growth inhibition = 0, and a value of 40% growth percentage means 60% growth inhibition.
4.2.2. HET-CAM Angiogenesis Study49
4.2.2.1. Choosing an Optimum Nonembryotoxic Concentration of Each Compound
Fertilized hen eggs were incubated at 37 °C for 5 days with rotation for every 2 h. The viability of the incubated eggs was examined, and the air sac for every viable egg was determined by candling at the beginning of the 5th day. The dead ones were excluded from the test. An observatory window was opened at the contralateral location and covered with a sterilized adhesive tape. Eggs were returned to the incubator. On the 9th day, the adhesive tape was removed. The fewer blood vessel density area on CAM was determined, and then, 40 μL of the various concentrations of each compound dissolved in DMSO 40 or 100% in saline was added dropwise on a sterilized filter paper disc of 5 mm with an inner hole of 2.5 mm in diameter (3 eggs/concentration), and the exposed window was covered again by a sterile adhesive tape. The toxicity of each compound on the eggs was examined on the 11th day by candling (Galal et al., 2016).49
4.2.2.2. Testing the Effect of Synthesized compounds on HET-CAM Angiogenesis
The experiment was carried out as follows: the optimum nontoxic concentration of each compound (40 or 100 μM) was added on the filter paper disc. Fertilized eggs were incubated for 9 days at the same conditions and divided into the groups of three eggs. Each compound (40 μM) dissolved in 40 μL of 40% DMSO in saline was added dropwise on the filter paper disc. A control group was used where 40 μL of 40% DMSO in saline was used.
4.2.2.3. Quantitative Assessment of Angiogenesis on HET-CAM Using Image Analysis
The effect of the test materials on CAM angiogenesis was examined on the 11th day. An angiography was taken for every sampled egg. The examination was carried out using computerized image analysis to count newly formed small blood vessels in the circle of 100 mm2 area representing a standard area for all the angiographs where the blood vessels were analyzed (Galal et al., 2016).49 The procedures were carried out for each test group, and the data were collected, tabulated, and statistically analyzed using a one-way ANOVA test, followed by Dunnett’s multiple comparison test.
4.2.3. VEGFR-2, PDGFR, and C-Met Kinase Inhibitory Assays
The design and protocol for these assays for the selected compounds 6b were designed according to the reported procedures.18,19,58
4.3. Molecular Modeling Study
Molecular docking study was conducted using Accelrys software (Discovery Studio 4) for compound 6b against VEGFR-2 (PDB code: 4AGD) and c-MET kinase (PDB code: 3ZZE). The 3D protein structures were retrieved from PDB60 and then prepared for docking by adding missing loops, removing water molecules, and adding the missing hydrogens. The cocrystallized ligand and compound 6b were drawn and prepared by preparing ligand protocol to generate a 3D structure and refined using the CHARMM force field with full potential. Docking simulations were run using CDOCKER, and the top 10 docking poses would be finally saved. The docking process was verified by the alignment of the cocrystallized ligand and docked ligand to calculate rmsd values which were 0.95 and 0.87 Å, respectively. The docked poses were ranked according to their CDOCKER interaction energy, and the top pose was chosen for the analysis of interactions for each compound.
Acknowledgments
This work was supported in part by the Center of Drug Research and Development (CDRD), Faculty of Pharmacy, The British University in Egypt. In addition, it was supported in part by the start-up fund by Texas A&M Health Sciences Center (to H.I.A.; grant number: 121500-35558).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02038.
1H NMR, 13C DEPT, 13C NMR, and HRMS spectra of compounds 5a–6y and DTP-NCI 60 cell mean graphs for eight compounds (6a, 6b, 6e, 6l, 6n, 6r, 6v, and 6y) (PDF)
Author Contributions
Participated in research design: S.M., H.I.A., W.M.E., and M.M.E. Conducted experiments: S.M., M.G., W.M.E., D.C.G., and H.S.I. Performed data analysis: H.I.A. and S.M. Wrote or contributed to the writing of the manuscript: H.I.A., S.M., W.M.E., H.S.I., and M.M.E.
The authors declare no competing financial interest.
Supplementary Material
References
- Parang K.; Sun G.. Protein Kinase Inhibitors Drug Discovery. Drug Discovery; John Wiley & Sons, Inc., 2005. [Google Scholar]
- Broekman F.; Giovannetti E.; Peters G. J. Tyrosine kinase inhibitors: Multi-targeted or single-targeted?. World J. Clin. Oncol. 2011, 2, 80–93. 10.5306/wjco.v2.i2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson M.; García-Echeverría C.; Fabbro D.. Protein Tyrosine Kinases as Targets for Cancer and Other Indications. Protein Tyrosine Kinases; Springer, 2006; pp 1–29. [Google Scholar]
- Du Z.; Lovly C. M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Canc. 2018, 17, 58. 10.1186/s12943-018-0782-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier C.; Birchmeier W.; Gherardi E.; Vande Woude G. F. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003, 4, 915. 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- Christensen J. G.; Burrows J.; Salgia R. c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett. 2005, 225, 1–26. 10.1016/j.canlet.2004.09.044. [DOI] [PubMed] [Google Scholar]
- Folkman J.Role of angiogenesis in tumor growth and metastasis. Seminars in Oncology; Elsevier, 2002; Vol. 29, pp 15-18. [DOI] [PubMed] [Google Scholar]
- Ferrara N.; Gerber H.-P.; LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669. 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Mannion M.; Raeppel S.; Claridge S.; Zhou N.; Saavedra O.; Isakovic L.; Zhan L.; Gaudette F.; Raeppel F.; Déziel R.; Beaulieu N.; Nguyen H.; Chute I.; Beaulieu C.; Dupont I.; Robert M.-F.; Lefebvre S.; Dubay M.; Rahil J.; Wang J.; Ste-Croix H.; Robert Macleod A.; Besterman J. M.; Vaisburg A. N-(4-(6, 7-Disubstituted-quinolin-4-yloxy)-3-fluorophenyl)-2-oxo-3-phenylimidazolidine-1-carboxamides: A novel series of dual c-Met/VEGFR2 receptor tyrosine kinase inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 6552–6556. 10.1016/j.bmcl.2009.10.040. [DOI] [PubMed] [Google Scholar]
- Claridge S.; Raeppel F.; Granger M.-C.; Bernstein N.; Saavedra O.; Zhan L.; Llewellyn D.; Wahhab A.; Deziel R.; Rahil J.; Beaulieu N.; Nguyen H.; Dupont I.; Barsalou A.; Beaulieu C.; Chute I.; Gravel S.; Robert M.-F.; Lefebvre S.; Dubay M.; Pascal R.; Gillespie J.; Jin Z.; Wang J.; Besterman J. M.; MacLeod A. R.; Vaisburg A. Discovery of a novel and potent series of thieno [3, 2-b] pyridine-based inhibitors of c-Met and VEGFR2 tyrosine kinases. Bioorg. Med. Chem. Lett. 2008, 18, 2793–2798. 10.1016/j.bmcl.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Zhan Z.; Ai J.; Liu Q.; Ji Y.; Chen T.; Xu Y.; Geng M.; Duan W. Discovery of anilinopyrimidines as dual inhibitors of c-Met and VEGFR-2: synthesis, SAR, and cellular activity. ACS Med. Chem. Lett. 2014, 5, 673–678. 10.1021/ml500066m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Gu W.; Bi X.; Li H.; Liao C.; Liu C.; Huang W.; Qian H. Design, synthesis, and biological evaluation of thieno [2, 3-d] pyrimidine derivatives as novel dual c-Met and VEGFR-2 kinase inhibitors. Bioorg. Med. Chem. 2017, 25, 6674–6679. 10.1016/j.bmc.2017.11.010. [DOI] [PubMed] [Google Scholar]
- Gu W.; Dai Y.; Qiang H.; Shi W.; Liao C.; Zhao F.; Huang W.; Qian H. Discovery of novel 2-substituted-4-(2-fluorophenoxy) pyridine derivatives possessing pyrazolone and triazole moieties as dual c-Met/VEGFR-2 receptor tyrosine kinase inhibitors. Bioorg. Chem. 2017, 72, 116–122. 10.1016/j.bioorg.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Faivre S.; Demetri G.; Sargent W.; Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat. Rev. Drug Discovery 2007, 6, 734. 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- Laird A. D.; Vajkoczy P.; Shawver L. K.; Thurnher A.; Liang C.; Mohammadi M.; Schlessinger J.; Ullrich A.; Hubbard S. R.; Blake R. A.; Fong T. A.; Strawn L. M.; Sun L.; Tang C.; Hawtin R.; Tang F.; Shenoy N.; Hirth K. P.; McMahon G.; Cherrington J. M. SU6668 Is a Potent Antiangiogenic and Antitumor Agent That Induces Regression of Established Tumors. Cancer Res. 2000, 60, 4152–4160. [PubMed] [Google Scholar]
- Patyna S.; Laird A. D.; Mendel D. B.; O’Farrell A M.; Liang C.; Guan H.; Vojkovsky T.; Vasile S.; Wang X.; Chen J.; Grazzini M.; Yang C. Y.; Haznedar J. O.; Sukbuntherng J.; Zhong W. Z.; Cherrington J. M.; Hu-Lowe D. SU14813: a novel multiple receptor tyrosine kinase inhibitor with potent antiangiogenic and antitumor activity. Mol. Cancer Ther. 2006, 5, 1774–1782. 10.1158/1535-7163.mct-05-0333. [DOI] [PubMed] [Google Scholar]
- Patyna S.; Laird A. D.; Mendel D. B.; O’Farrell A.-M.; Liang C.; Guan H.; Vojkovsky T.; Vasile S.; Wang X.; Chen J.; Grazzini M.; Yang C. Y.; Haznedar J. Ö.; Sukbuntherng J.; Zhong W.-Z.; Cherrington J. M.; Hu-Lowe D. SU14813: a novel multiple receptor tyrosine kinase inhibitor with potent antiangiogenic and antitumor activity. Mol. Cancer Ther. 2006, 5, 1774–1782. 10.1158/1535-7163.mct-05-0333. [DOI] [PubMed] [Google Scholar]
- Eldehna W. M.; Fares M.; Ibrahim H. S.; Aly M. H.; Zada S.; Ali M. M.; Abou-Seri S. M.; Abdel-Aziz H. A.; Abou El Ella D. A. Indoline ureas as potential anti-hepatocellular carcinoma agents targeting VEGFR-2: Synthesis, in vitro biological evaluation and molecular docking. Eur. J. Med. Chem. 2015, 100, 89–97. 10.1016/j.ejmech.2015.05.040. [DOI] [PubMed] [Google Scholar]
- Eldehna W. M.; El Kerdawy A. M.; Al-Ansary G. H.; Al-Rashood S. T.; Ali M. M.; Mahmoud A. E. Type IIA - Type IIB protein tyrosine kinase inhibitors hybridization as an efficient approach for potent multikinase inhibitor development: Design, synthesis, anti-proliferative activity, multikinase inhibitory activity and molecular modeling of novel indolinone-based ureides and amides. Eur. J. Med. Chem. 2019, 163, 37–53. 10.1016/j.ejmech.2018.11.061. [DOI] [PubMed] [Google Scholar]
- Ma P. C.; Jagadeeswaran R.; Jagadeesh S.; Tretiakova M. S.; Nallasura V.; Fox E. A.; Hansen M.; Schaefer E.; Naoki K.; Lader A.; Richards W.; Sugarbaker D.; Husain A. N.; Christensen J. G.; Salgia R. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non–small cell lung cancer. Cancer Res. 2005, 65, 1479–1488. 10.1158/0008-5472.can-04-2650. [DOI] [PubMed] [Google Scholar]
- Sattler M.; Pride Y. B.; Ma P.; Gramlich J. L.; Chu S. C.; Quinnan L. A.; Shirazian S.; Liang C.; Podar K.; Christensen J. G.; Salgia R. A novel small molecule met inhibitor induces apoptosis in cells transformed by the oncogenic TPR-MET tyrosine kinase. Cancer Res. 2003, 63, 5462–5469. [PubMed] [Google Scholar]
- Ibrahim H. S.; Abou-Seri S. M.; Abdel-Aziz H. A. 3-Hydrazinoindolin-2-one derivatives: chemical classification and investigation of their targets as anticancer agents. Eur. J. Med. Chem. 2016, 122, 366–381. 10.1016/j.ejmech.2016.06.034. [DOI] [PubMed] [Google Scholar]
- Cui J. J.; McTigue M.; Nambu M.; Tran-Dubé M.; Pairish M.; Shen H.; Jia L.; Cheng H.; Hoffman J.; Le P.; Jalaie M.; Goetz G. H.; Ryan K.; Grodsky N.; Deng Y.-l.; Parker M.; Timofeevski S.; Murray B. W.; Yamazaki S.; Aguirre S.; Li Q.; Zou H.; Christensen J. Discovery of a Novel Class of Exquisitely Selective Mesenchymal-Epithelial Transition Factor (c-MET) Protein Kinase Inhibitors and Identification of the Clinical Candidate 2-(4-(1-(Quinolin-6-ylmethyl)-1 H-[1, 2, 3] triazolo [4, 5-b] pyrazin-6-yl)-1 H-pyrazol-1-yl) ethanol (PF-04217903) for the Treatment of Cancer. J. Med. Chem. 2012, 55, 8091–8109. 10.1021/jm300967g. [DOI] [PubMed] [Google Scholar]
- Zhan Z.; Peng X.; Liu Q.; Chen F.; Ji Y.; Yao S.; Xi Y.; Lin Y.; Chen T.; Xu Y.; Ai J.; Geng M.; Duan W. Discovery of 6-(difluoro(6-(4-fluorophenyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazin-3-yl)methyl)q uinoline as a highly potent and selective c-Met inhibitor. Eur. J. Med. Chem. 2016, 116, 239–251. 10.1016/j.ejmech.2016.03.076. [DOI] [PubMed] [Google Scholar]
- Peterson E. A.; Teffera Y.; Albrecht B. K.; Bauer D.; Bellon S. F.; Boezio A.; Boezio C.; Broome M. A.; Choquette D.; Copeland K. W.; Dussault I.; Lewis R.; Lin M.-H. J.; Lohman J.; Liu J.; Potashman M.; Rex K.; Shimanovich R.; Whittington D. A.; Vaida K. R.; Harmange J.-C. Discovery of potent and selective 8-fluorotriazolopyridine c-Met inhibitors. J. Med. Chem. 2015, 58, 2417–2430. 10.1021/jm501913a. [DOI] [PubMed] [Google Scholar]
- Jia H.; Dai G.; Weng J.; Zhang Z.; Wang Q.; Zhou F.; Jiao L.; Cui Y.; Ren Y.; Fan S.; Zhou J.; Qing W.; Gu Y.; Wang J.; Sai Y.; Su W. Discovery of (S)-1-(1-(Imidazo[1,2-a]pyridin-6-yl)ethyl)-6-(1-methyl-1H-pyrazol-4-yl)-1H-[1,2, 3]triazolo[4,5-b]pyrazine (volitinib) as a highly potent and selective mesenchymal-epithelial transition factor (c-Met) inhibitor in clinical development for treatment of cancer. J. Med. Chem. 2014, 57, 7577–7589. 10.1021/jm500510f. [DOI] [PubMed] [Google Scholar]
- Cui J. J.; Shen H.; Tran-Dubé M.; Nambu M.; McTigue M.; Grodsky N.; Ryan K.; Yamazaki S.; Aguirre S.; Parker M.; Li Q.; Zou H.; Christensen J. Lessons from (S)-6-(1-(6-(1-methyl-1H-pyrazol-4-yl)-[1,2,4]triazolo[4,3-b]pyridazin-3-yl)ethyl )quinoline (PF-04254644), an inhibitor of receptor tyrosine kinase c-Met with high protein kinase selectivity but broad phosphodiesterase family inhibition leading to myocardial degeneration in rats. J. Med. Chem. 2013, 56, 6651–6665. 10.1021/jm400926x. [DOI] [PubMed] [Google Scholar]
- Cui J. J.; McTigue M.; Nambu M.; Tran-Dubé M.; Pairish M.; Shen H.; Jia L.; Cheng H.; Hoffman J.; Le P.; Jalaie M.; Goetz G. H.; Ryan K.; Grodsky N.; Deng Y.-l.; Parker M.; Timofeevski S.; Murray B. W.; Yamazaki S.; Aguirre S.; Li Q.; Zou H.; Christensen J. Discovery of a novel class of exquisitely selective mesenchymal-epithelial transition factor (c-MET) protein kinase inhibitors and identification of the clinical candidate 2-(4-(1-(quinolin-6-ylmethyl)-1H-[1,2,3]triazolo[4,5-b]pyrazin-6-yl)-1H-pyrazol-1 -yl)ethanol (PF-04217903) for the treatment of cancer. J. Med. Chem. 2012, 55, 8091–8109. 10.1021/jm300967g. [DOI] [PubMed] [Google Scholar]
- Ryu J. W.; Han S.-Y.; Yun J. I.; Choi S.-U.; Jung H.; Ha J. D.; Cho S. Y.; Lee C. O.; Kang N. S.; Koh J. S.; Kim H. R.; Lee J. Design and synthesis of triazolopyridazines substituted with methylisoquinolinone as selective c-Met kinase inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 7185–7188. 10.1016/j.bmcl.2011.09.066. [DOI] [PubMed] [Google Scholar]
- Buchanan S. G.; Hendle J.; Lee P. S.; Smith C. R.; Bounaud P.-Y.; Jessen K. A.; Tang C. M.; Huser N. H.; Felce J. D.; Froning K. J.; Peterman M. C.; Aubol B. E.; Gessert S. F.; Sauder J. M.; Schwinn K. D.; Russell M.; Rooney I. A.; Adams J.; Leon B. C.; Do T. H.; Blaney J. M.; Sprengeler P. A.; Thompson D. A.; Smyth L.; Pelletier L. A.; Atwell S.; Holme K.; Wasserman S. R.; Emtage S.; Burley S. K.; Reich S. H. SGX523 is an exquisitely selective, ATP-competitive inhibitor of the MET receptor tyrosine kinase with antitumor activity in vivo. Mol. Cancer Ther. 2009, 8, 3181–3190. 10.1158/1535-7163.mct-09-0477. [DOI] [PubMed] [Google Scholar]
- Comoglio P. M.; Giordano S.; Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat. Rev. Drug Discovery 2008, 7, 504–516. 10.1038/nrd2530. [DOI] [PubMed] [Google Scholar]
- Albrecht B. K.; Harmange J.-C.; Bauer D.; Berry L.; Bode C.; Boezio A. A.; Chen A.; Choquette D.; Dussault I.; Fridrich C.; Hirai S.; Hoffman D.; Larrow J. F.; Kaplan-Lefko P.; Lin J.; Lohman J.; Long A. M.; Moriguchi J.; O’Connor A.; Potashman M. H.; Reese M.; Rex K.; Siegmund A.; Shah K.; Shimanovich R.; Springer S. K.; Teffera Y.; Yang Y.; Zhang Y.; Bellon S. F. Discovery and optimization of triazolopyridazines as potent and selective inhibitors of the c-Met kinase. J. Med. Chem. 2008, 51, 2879–2882. 10.1021/jm800043g. [DOI] [PubMed] [Google Scholar]
- Wei D.; Fan H.; Zheng K.; Qin X.; Yang L.; Yang Y.; Duan Y.; Zhang Q.; Zeng C.; Hu L. Synthesis and anti-tumor activity of [1,4] dioxino [2,3-f] quinazoline derivatives as dual inhibitors of c-Met and VEGFR-2. Bioorg. Chem. 2019, 88, 102916. 10.1016/j.bioorg.2019.04.010. [DOI] [PubMed] [Google Scholar]
- Shi W.; Qiang H.; Huang D.; Bi X.; Huang W.; Qian H. Exploration of novel pyrrolo[2,1-f][1,2,4]triazine derivatives with improved anticancer efficacy as dual inhibitors of c-Met/VEGFR-2. Eur. J. Med. Chem. 2018, 158, 814–831. 10.1016/j.ejmech.2018.09.050. [DOI] [PubMed] [Google Scholar]
- Ibrahim H. A.; Awadallah F. M.; Refaat H. M.; Amin K. M. Molecular docking simulation, synthesis and 3D pharmacophore studies of novel 2-substituted-5-nitro-benzimidazole derivatives as anticancer agents targeting VEGFR-2 and c-Met. Bioorg. Chem. 2018, 77, 457–470. 10.1016/j.bioorg.2018.01.014. [DOI] [PubMed] [Google Scholar]
- Li J.; Gu W.; Bi X.; Li H.; Liao C.; Liu C.; Huang W.; Qian H. Design, synthesis, and biological evaluation of thieno[2,3-d]pyrimidine derivatives as novel dual c-Met and VEGFR-2 kinase inhibitors. Bioorg. Med. Chem. 2017, 25, 6674–6679. 10.1016/j.bmc.2017.11.010. [DOI] [PubMed] [Google Scholar]
- Gu W.; Dai Y.; Qiang H.; Shi W.; Liao C.; Zhao F.; Huang W.; Qian H. Discovery of novel 2-substituted-4-(2-fluorophenoxy) pyridine derivatives possessing pyrazolone and triazole moieties as dual c-Met/VEGFR-2 receptor tyrosine kinase inhibitors. Bioorg. Chem. 2017, 72, 116–122. 10.1016/j.bioorg.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Jiang X.; Jiang Y.; Guo M.; Zhang S.; Li J.; He J.; Liu J.; Wang J.; Ouyang L. Recent advances in the development of dual VEGFR and c-Met small molecule inhibitors as anticancer drugs. Eur. J. Med. Chem. 2016, 108, 495–504. 10.1016/j.ejmech.2015.12.016. [DOI] [PubMed] [Google Scholar]
- Aytaç P. S.; Durmaz I.; Houston D. R.; Çetin-Atalay R.; Tozkoparan B. Novel triazolothiadiazines act as potent anticancer agents in liver cancer cells through Akt and ASK-1 proteins. Bioorg. Med. Chem. 2016, 24, 858–872. 10.1016/j.bmc.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Khan I.; Ibrar A.; Abbas N. Triazolothiadiazoles and triazolothiadiazines--biologically attractive scaffolds. Eur. J. Med. Chem. 2013, 63, 854–868. 10.1016/j.ejmech.2013.01.060. [DOI] [PubMed] [Google Scholar]
- Prakash O.; Aneja D. K.; Hussain K.; Lohan P.; Ranjan P.; Arora S.; Sharma C.; Aneja K. R. Synthesis and biological evaluation of dihydroindeno and indeno [1,2-e] [1,2,4]triazolo [3,4-b] [1,3,4]thiadiazines as antimicrobial agents. Eur. J. Med. Chem. 2011, 46, 5065–5073. 10.1016/j.ejmech.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Skoumbourdis A. P.; Leclair C. A.; Stefan E.; Turjanski A. G.; Maguire W.; Titus S. A.; Huang R.; Auld D. S.; Inglese J.; Austin C. P.; Michnick S. W.; Xia M.; Thomas C. J. Exploration and optimization of substituted triazolothiadiazines and triazolopyridazines as PDE4 inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 3686–3692. 10.1016/j.bmcl.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldehna W. M.; Abo-Ashour M. F.; Nocentini A.; El-Haggar R. S.; Bua S.; Bonardi A.; Al-Rashood S. T.; Hassan G. S.; Gratteri P.; Abdel-Aziz H. A.; Supuran C. T. Enhancement of the tail hydrophobic interactions within the carbonic anhydrase IX active site via structural extension: Design and synthesis of novel N-substituted isatins-SLC-0111 hybrids as carbonic anhydrase inhibitors and antitumor agents. Eur. J. Med. Chem. 2019, 162, 147–160. 10.1016/j.ejmech.2018.10.068. [DOI] [PubMed] [Google Scholar]
- Eldehna W. M.; Abo-Ashour M. F.; Ibrahim H. S.; Al-Ansary G. H.; Ghabbour H. A.; Elaasser M. M.; Ahmed H. Y. A.; Safwat N. A. Novel [(3-indolylmethylene)hydrazono]indolin-2-ones as apoptotic anti-proliferative agents: design, synthesis and in vitro biological evaluation. J. Enzyme Inhib. Med. Chem. 2018, 33, 686–700. 10.1080/14756366.2017.1421181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo-Ashour M. F.; Eldehna W. M.; George R. F.; Abdel-Aziz M. M.; Elaasser M. M.; Abdel Gawad N. M.; Gupta A.; Bhakta S.; Abou-Seri S. M. Novel indole-thiazolidinone conjugates: Design, synthesis and whole-cell phenotypic evaluation as a novel class of antimicrobial agents. Eur. J. Med. Chem. 2018, 160, 49–60. 10.1016/j.ejmech.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Mohamady S.; Gibriel A. A.; Ahmed M. S.; Hendy M. S.; Naguib B. H. Design and novel synthetic approach supported with molecular docking and biological evidence for naphthoquinone-hydrazinotriazolothiadiazine analogs as potential anticancer inhibiting topoisomerase-IIB. Bioorg. Chem. 2020, 96, 103641. 10.1016/j.bioorg.2020.103641. [DOI] [PubMed] [Google Scholar]
- Dickinson R. G.; Jacobsen N. W. 6-Substituted s-triazolo[4,3-b]-s-tetrazine-3-thiols: a sensitive and specific test for aldehydes. J. Chem. Soc., Perkin Trans. 1 1975, 975–979. 10.1039/p19750000975. [DOI] [Google Scholar]
- The Developmental Therapeutics Program (DTP). https://dtp.cancer.gov/.
- Galal M. A.; Abd M.; Mostafa A. M. A.; El-Khatib A. S. N.; Elmazar M. M. Assessment of Hen’s Egg Test Chorioallantoic Membrane (HET-CAM) for Screening of Anticancer Activities of Drugs. ALTEX—Alternatives Ani. Exp. 2016, 5, 156. [Google Scholar]
- Morgan R.; Keen J.; Halligan D.; O’Callaghan A.; Andrew R.; Livingstone D.; Abernethie A.; Maltese G.; Walker B.; Hadoke P. Species-specific regulation of angiogenesis by glucocorticoids reveals contrasting effects on inflammatory and angiogenic pathways. PLoS One 2018, 13, e0192746 10.1371/journal.pone.0192746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang F.; Liu M.; Li B.; Zhang X.; Sheng Y.; Liu S.; Han J.; Li H.; Xiu R. The anti-angiogenic effect of dexamethasone in a murine hepatocellular carcinoma model by augmentation of gluconeogenesis pathway in malignant cells. Cancer Chemother. Pharmacol. 2016, 77, 1087–1096. 10.1007/s00280-016-3030-x. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. C. M. L.; Vagnaldo Fechine F.; Ribeiro M. Z. M. L.; Barreiro E. J.; Moreira Lima L.; Ricardo N. M. P. S.; Amaral de Moraes M. E.; Odorico de Moraes M. Potential inhibitory effect of LASSBio-596, a new thalidomide hybrid, on inflammatory corneal angiogenesis in rabbits. Ophthalmic Res. 2012, 48, 177–185. 10.1159/000337137. [DOI] [PubMed] [Google Scholar]
- Nakao S.; Hata Y.; Miura M.; Noda K.; Kimura Y. N.; Kawahara S.; Kita T.; Hisatomi T.; Nakazawa T.; Jin Y.; Dana M. R.; Kuwano M.; Ono M.; Ishibashi T.; Hafezi-Moghadam A. Dexamethasone inhibits interleukin-1beta-induced corneal neovascularization: role of nuclear factor-kappaB-activated stromal cells in inflammatory angiogenesis. Am. J. Pathol. 2007, 171, 1058–1065. 10.2353/ajpath.2007.070172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori Y.; Hu D.-E.; Yasui K.; Smither R. L.; Gresham G. A.; Fan T.-P. D. Differential effects of angiostatic steroids and dexamethasone on angiogenesis and cytokine levels in rat sponge implants. Br. J. Pharmacol. 1996, 118, 1584–1591. 10.1111/j.1476-5381.1996.tb15578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue M.; Murray B. W.; Chen J. H.; Deng Y.-L.; Solowiej J.; Kania R. S. Molecular conformations, interactions, and properties associated with drug efficiency and clinical performance among VEGFR TK inhibitors. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 18281–18289. 10.1073/pnas.1207759109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J. J.; McTigue M.; Nambu M.; Tran-Dubé M.; Pairish M.; Shen H.; Jia L.; Cheng H.; Hoffman J.; Le P.; Jalaie M.; Goetz G. H.; Ryan K.; Grodsky N.; Deng Y.-l.; Parker M.; Timofeevski S.; Murray B. W.; Yamazaki S.; Aguirre S.; Li Q.; Zou H.; Christensen J. Discovery of a novel class of exquisitely selective mesenchymal-epithelial transition factor (c-Met) protein kinase inhibitors and identification of the clinical candidate 2-(4-(1-(quinolin-6-ylmethyl)-1H-[1, 2, 3] triazolo [4, 5-b] pyrazin-6-yl)-1H-pyrazol-1-yl) ethanol (PF-04217903) for the Treatment of Cancer. J. Med. Chem. 2012, 55, 8091–8109. 10.1021/jm300967g. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute, D. D. o. C. T. D. Discovery & Development Services|NCI-60 Human Cancer Cell Line Screen NCI-60 Screening Methodology, last updated: Aug 26, 2015.
- Jalaie H. S.; Albakri M. E.; Mahmoud W. R.; Allam H. A.; Reda A. M.; Abdel-Aziz H. A. Synthesis and biological evaluation of some novel thiobenzimidazole derivatives as anti-renal cancer agents through inhibition of c-MET kinase. Bioorg. Chem. 2019, 85, 337–348. 10.1016/j.bioorg.2019.01.006. [DOI] [PubMed] [Google Scholar]
- https://www.rcsb.org/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.