Abstract
Objective:
To investigate whether intrauterine exposure to maternal asthma or asthma exacerbations increases the risk of attention-deficit/hyperactivity disorder (ADHD).
Methods:
Using Danish register data, this cohort study comprised of 961,202 live singletons born in Denmark during 1997–2012. Children were followed to a maximum of 20.0 years from birth until the first of ADHD-diagnosis/prescription, emigration, death, or 31 December 2016. Cox regression models were used to evaluate the association between maternal or paternal asthma, asthma exacerbations and offspring ADHD.
Results:
During 11.4 million person-years of follow-up, 27,780 (2.9%) children were identified as having ADHD. ADHD risk was increased among offspring born to asthmatic mothers (hazard ratio (HR) 1.41, 95% CI: 1.36–1.46) or asthmatic fathers (HR 1.13, 95% CI: 1.08–1.18). Antenatal antiasthma medication treatment did not increase offspring ADHD. However, higher risks were observed among offspring of mothers with asthma exacerbations compared with children of asthmatic mothers with no exacerbations: HR 1.12 (95% CI: 1.00–1.25) for pre-pregnancy exacerbations; 1.21 (95% CI: 1.00–1.47) for exacerbations during pregnancy; and 1.25 (95% CI: 1.08–1.44) for exacerbations after delivery.
Conclusions:
These results support theories regarding shared genetic and environmental risk factors having a role in the development of ADHD.
Keywords: Asthma, attention-deficit/hyperactivity disorder, cohort studies, registries
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a highly heritable, neurodevelopmental condition characterized by inattention, hyperactivity, and impulsivity, with a worldwide prevalence of around 3.4% in children and adolescents (Polanczyk et al., 2015). ADHD often persists into adulthood (Riglin et al., 2016), and is associated with increased mortality rates for individuals with ADHD (Dalsgaard et al., 2015) and impairments across the lifespan both for affected individuals and their families (Harpin, 2005).
The etiology of ADHD has not been fully elucidated but is considered multifactorial. Genetic risk factors are important; twin studies demonstrate the heritability of ADHD and molecular genetic studies implicate several candidate genes in its development (Demontis et al., 2017; Faraone et al., 2005). In addition, associations with birth outcomes suggest prenatal exposures could play a role (Class et al., 2014; D'Onofrio et al., 2013; Gustafsson and Kallen, 2011; Halmoy et al., 2012; Indredavik et al., 2005; Indredavik et al., 2004; Lindstrom et al., 2011; Linnet et al., 2006; Nigg and Breslau, 2007; Pettersson et al., 2015; Sucksdorff et al., 2018; Sucksdorff et al., 2015). An association between maternal asthma and increased ADHD risk in offspring has been reported in three studies (Instanes et al., 2017; Liang et al., 2017; Cowell et al., 2019). One underlying assumption for the association with ADHD is that maternal asthma may impact fetal development via altered fetal immune responses in the central nervous system resulting in neuronal injury, thus predisposing children to ADHD (Strickland, 2014). If the immune dysfunction assumption holds true, we should expect that maternal asthma exacerbations during pregnancy, as a proxy of severity or poor control, are associated with higher risk of ADHD in offspring, compared to asthma with no exacerbations. Only one study examined maternal active asthma and risk of ADHD-like symptoms in the offspring (Cowell et al., 2019). However, this study was based on small sample size and reliance on maternal report of ADHD-like symptoms which is not able to explore the impact of medication use and vulnerable to information bias.
To address this important question, we carried out a longitudinal cohort study by defining ADHD cases according to hospital contact and medication treatment. We hypothesized that, when looking at parental asthma and ADHD risk in offspring, if an observed association between maternal asthma and ADHD was mainly due to exposure to intrauterine immune dysregulation, an elevated risk would be seen among offspring of women with asthma exacerbations during pregnancy. Furthermore, the risk would be more pronounced with maternal than paternal asthma exposure. Alternatively, if an observed association was also due to shared genetic and environmental risk factors, we would expect that asthma exacerbations before pregnancy or after delivery in both mothers and fathers would be associated with offspring ADHD.
2. Materials and methods
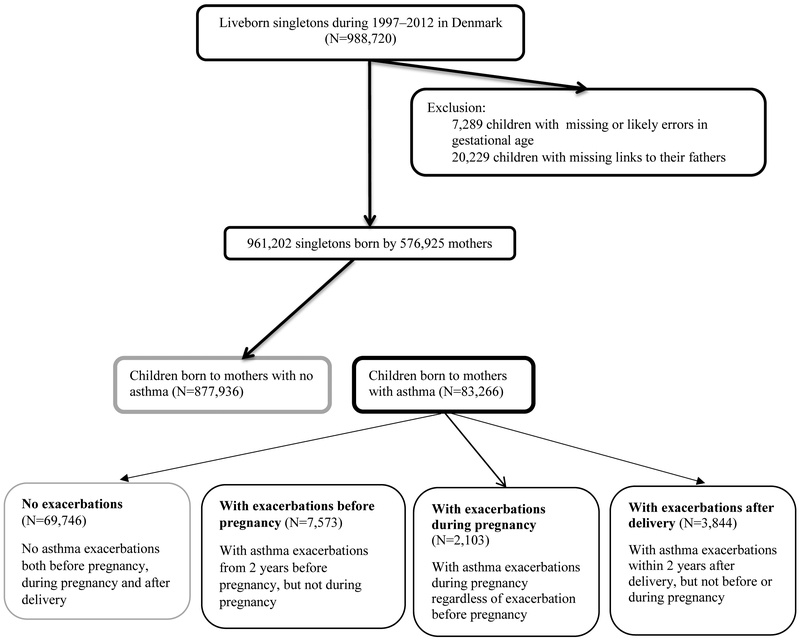
We conducted a population-based cohort study, linking data from Danish population registers. All children and residents in Denmark are assigned a unique personal identity number, which enables individual-level data linkage between registers. Using the Danish Medical Birth Registry (Bliddal et al., 2018), we identified all liveborn singletons in Denmark during 1997–2012 (N=988,720). We excluded 7,289 children who had missing or extreme gestational age (<154 or >315 days) and 20,229 (2.1%) children missing linkage to their fathers (Figure 1).
Figure 1.
Flow chart illustrating the identification of the study population
2.1. Maternal asthma history.
Mothers were considered to have asthma if they received asthma treatment before the index delivery. Asthma treatment was defined as ever (from mother’s birth until the birth of the index child) having had a hospital contact for asthma, or the redemption of two or more prescriptions for antiasthma medications, less than 12 months apart. Information on hospital contact for asthma was recorded in the National Patient Register (Andersen et al., 1999), which contains data on inpatient contacts since 1977, and also emergency room and outpatient treatments from 1995 onwards. The International Classification of Diseases (ICD), 8th Revision (ICD-8) codes were used before 1994 and ICD-10 codes from 1994 onwards. Information on asthma was identified based on ICD-8 code 493 and ICD-10 codes J45 or J46. Information on prescriptions of antiasthma medications was obtained from the National Prescription Registry (Kildemoes et al., 2011), which contains information on the Anatomical Therapeutic Chemical (ATC) classification codes and the dispensing date of all prescriptions dispensed in community pharmacies in Denmark since 1995. The ATC codes for antiasthma medications are: inhaled β2-agonists (R03AC02–04, −12, and −13), inhaled glucocorticoids/corticosteroids (ICS; R03BA01, −02, and −05), fixed-dose combination of inhaled β2-agonists and glucocorticoids (R03AK06 and −07), leukotriene receptor antagonists (R03DC03), and anti-IgE treatment (R03DX05) (Liu et al., 2017). We defined paternal asthma using the same exposure window. Mothers who did not have documented antenatal asthma were classified as “non-asthmatic”.
2.2. Asthma exacerbations
Mothers were considered to have an exacerbation if they received inpatient treatment, had an emergency room visit for asthma or filled a prescription for an oral corticosteroid. We defined three periods for exacerbations: a) during the index pregnancy, b) the two years period leading up to the index pregnancy or c) the two years period after the index delivery. Pregnancy was defined as the first day of the last menstrual period up until delivery. Paternal exacerbations were defined using the same window.
Mothers with asthma were further categorized hierarchically, according to asthma exacerbations and the timing of these: a) exacerbations during pregnancy, regardless of previous or later exacerbations; b) exacerbations before pregnancy, regardless of exacerbations after delivery; c) exacerbations after delivery only; and d) no exacerbations before, during pregnancy or after delivery. The number of exacerbations before, during pregnancy and after delivery was calculated by adding all the exacerbations and was categorized into 1, 2, 3, or 4 or more exacerbations.
2.3. Identification of ADHD.
ADHD cases were defined as having either a hospital diagnosis of ADHD or redemption of at least one ADHD medication prescription. Information on hospital contact for ADHD was retrieved from the Danish Psychiatric Central Research Register (Mors et al., 2011) and the National Patient Register. The ADHD diagnosis identified from the National Patient Register was included if the diagnosis was made by pediatricians or neurologists. The ICD-codes for ADHD were 308.01 in ICD-8 and F90 and F98.8 in ICD-10. Data on medications used to treat ADHD dispensed from pharmacies were extracted from the National Prescription Register. The ATC codes for ADHD medications were: N06BA01 (amfetamine), N06BA02 (dexamphetamine), N06BA04 (methylphenidate), N06BA09 (atomoxetine), N06BA11 (dexmethylphenidate), and N06BA12 (lisdexamfetamine). The date of the first diagnosis of ADHD was defined as the first day of hospital contact or first date of dispensation for ADHD medication, whichever occurred first.
2.4. Covariates
The following covariates were identified a priori based on causal diagrams using directed acyclic graphs and were included in the models: sex, maternal age at delivery (<25, 25–34, or ≥35 years), parity (1st, 2nd or above), smoking during pregnancy (yes, no), mental disorders before conception (no mental disorders, ADHD, or other mental disorders excluding ADHD), cohabiting status at delivery (yes, no), low social class (yes, no), large family size (yes, no), place of residence at delivery (capital or capital suburb, provincial city or town, or rural areas), paternal asthma before delivery (yes, no), and calendar year of birth (1997–2000, 2001–2005, 2006–2012).
Mothers were considered to have mental disorders if they had hospital contact for mental disorders (ICD-10 F codes) identified from the Danish Psychiatric Central Research Register, or they redeemed at least one prescription for psychotropic medications (ATC codes N05 and N06), retrieved from the Danish National Prescription Registry. Maternal ADHD was defined as hospital treatment or filling a prescription for ADHD medications. Maternal smoking during pregnancy was self-reported at the first midwife visit. Parity refers to the number of births the mother has had, including the index delivery. Large family size was defined as a household with ≥4 children including the index child. Low social class was defined as both parents being classified as “low” on at least one of the following variables: education, occupation, or income. Within this, lower education was defined as having completed compulsory schooling as a maximum; lower occupation was defined as receiving disability pension; and lower income as income level in the lowest quintile of the general population by sex and calendar year. A more detailed description of low social class can be found elsewhere (Ostergaard et al., 2016).
2.5. Statistical analysis
Analyses were performed with Stata 15.0 (StataCorp, College Station, TX, USA). Offspring were followed from birth until the date of first ADHD-diagnosis or -prescription, emigration, death, or 31 December 2016, whichever came first. Cox proportional hazards regression was used to test the associations. The proportional hazards assumption was tested using log-log plots. In those instances where hazards are not proportional over time, the Cox proportional hazards model estimates can be interpreted as an average hazard ratio over the entire follow-up period (Xu and O'Quigley, 2000). About 4.2% of the values were missing for any of the potential confounders, and we applied 20 imputations using the Markov Chain Monte Carlo technique for imputing missing values.
To examine whether a possible association between maternal asthma exacerbations and offspring ADHD was affected by the timing of asthma exacerbations, we categorized mothers with asthma exacerbations into two groups: exacerbations in the first trimester or exacerbations in late pregnancy (second or third trimester) only. To test whether in-utero exposure to antiasthma medication excluding oral corticosteroids itself increased offspring ADHD risk, we examined the association between antiasthma medication during pregnancy and offspring ADHD among children of asthmatic mothers with no exacerbations during pregnancy. We considered mothers as receiving antiasthma medication treatment if they redeemed at least one prescription for an antiasthma medication defined above during the index pregnancy.
Sensitivity analyses
First, to examine whether the association between maternal asthma exacerbations and offspring ADHD was confounded by shared environmental or genetic factors (Smith, 2008), we also examined whether paternal asthma exacerbations before, during the index pregnancy, or after the delivery were associated with offspring ADHD. If the association between maternal asthma and offspring ADHD was largely due to genetic predisposition or shared environmental factors, similar associations should be observed with paternal asthma exacerbations. Second, as sex differences have been observed with regards to the effects of maternal asthma during pregnancy (Murphy et al., 2003), we repeated analyses stratifying on the sex of the child. Third, as mothers with ADHD may have poor adherence to medication or may be less likely to redeem prescriptions, we repeated our analyses excluding 1,399 children born to mothers with pre-conception ADHD. Fourth, due to the pattern of inflammatory phenotypes differing between adults and children (Wang et al., 2011), we performed a sensitivity analysis defining maternal asthma as at least one hospital treatment for asthma or two redemptions of antiasthma prescriptions after age 15 years. Fifth, although oral prednisone and dexamethasone are the currently recommended systemic steroids for moderate to severe asthma exacerbations, we cannot be certain that they were always prescribed for this indication, we repeated our analysis defining asthma exacerbations only as receiving inpatient treatment or emergency visit for asthma.
Ethical approval
The study was approved by the Danish Data Protection Agency (Journal no: 2015-57-0002). No ethical approval is required for purely register-based studies on the basis of encrypted data in accordance with laws and regulations in Denmark.
3. Results
Among 961,202 children, 83,266 (8.7%) children were born to mothers with asthma. Table 1 shows the study population’s characteristics. In comparison to non-asthmatic mothers, asthmatic mothers were less likely to be married or cohabiting at the time of childbirth, more likely to have ADHD or other mental disorders, to be of low social class and more likely to smoke during pregnancy. Furthermore, children of asthmatic mothers were more likely to have an asthmatic father.
Table 1.
Characteristics of study population
| Characteristics | Non- asthmatic mothers (N=877,936) |
Asthmatic mothers | ||||
|---|---|---|---|---|---|---|
| All asthmatic mothers (N=83,266) |
No exacerbations (N=69,746) |
With exacerbations before pregnancy only (N=7,573) |
With exacerbations during pregnancy (N=2,103) |
With exacerbations after delivery (N=3,844) |
||
| Maternal age at delivery (years) | ||||||
| 25 | 111,758 (12.7) | 13,050 (15.7) | 11,076 (15.9) | 1,136 (15.0) | 305 (14.5) | 533 (13.9) |
| 25–34 | 614,502 (70.0) | 56,050 (67.3) | 47,012 (67.4) | 5,094 (67.3) | 1,302 (61.9) | 2,642 (68.7) |
| ≥>35 | 151,676 (17.3) | 14,166 (17.0) | 11,658 (16.7) | 1,343 (17.7) | 496 (23.6) | 669 (17.4) |
| Parity | ||||||
| 1 | 380,386 (43.3) | 36,530 (43.9) | 30,831 (44.2) | 3,392 (44.8) | 819 (38.9) | 1,488 (38.7) |
| ≥2 | 493,723 (56.2) | 46,296 (55.6) | 38,555 (55.3) | 4,136 (54.6) | 1,270 (60.4) | 2,335 (60.7) |
| Unknown | 3,827 (0.4) | 440 (0.5) | 360 (0.5) | 45 (0.6) | 14 (0.7) | 21 (0.6) |
| Maternal smoking during pregnancy | ||||||
| Yes | 146,268 (16.7) | 17,540 (21.1) | 14,573 (20.9) | 1,607 (21.2) | 506 (24.1) | 854 (22.2) |
| No | 705,865 (80.4) | 63,497 (76.3) | 53,359 (76.5) | 5,755 (76.0) | 1,522 (72.4) | 2,861 (74.4) |
| Unknown | 25,803 (2.9) | 2,229 (2.7) | 1,814 (2.6) | 211 (2.8) | 75 (3.6) | 129 (3.4) |
| Maternal mental disorder before pregnancy | ||||||
| No mental disorder | 731,199 (83.3) | 59,216 (71.1) | 50,287 (72.1) | 4,975 (65.7) | 1,339 (63.7) | 2,615 (68.0) |
| ADHD | 1,108 (0.1) | 367 (0.4) | 309 (0.4) | 28 (0.4) | 10 (0.5) | 20 (0.5) |
| Other mental disorders | 145,629 (16.6) | 23,683 (28.4) | 19,150 (27.5) | 2,570 (33.9) | 754 (35.9) | 1,209 (31.5) |
| Maternal cohabiting status | ||||||
| Married or cohabiting | 773,997 (88.2) | 71,590 (86.0) | 60,045 (86.1) | 6,465 (85.4) | 1,744 (82.9) | 3,336 (86.8) |
| Single, divorced, or widow | 95,348 (10.9) | 11,446 (13.7) | 9,498 (13.6) | 1,097 (14.5) | 351 (16.7) | 500 (13.0) |
| Unknown | 8,591 (1.0) | 230 (0.3) | 203 (0.3) | 11 (0.2) | 8 (0.4) | 8 (0.2) |
| Place of residence at delivery | ||||||
| Capital or capital suburb | 258,780 (29.5) | 22,844 (27.4) | 19,386 (27.8) | 1,864 (24.6) | 664 (31.6) | 930 (24.2) |
| Provincial city or town | 345,904 (39.4) | 33,107 (39.8) | 27,575 (39.5) | 3,129 (41.3) | 791 (37.6) | 1,612 (41.9) |
| Rural areas | 273,252 (31.1) | 27,315 (32.8) | 22,785 (32.7) | 2,580 (34.1) | 648 (30.8) | 1,302 (33.9) |
| Low social class at delivery | ||||||
| Yes | 85,382 (9.7) | 10,602 (12.7) | 8,687 (12.5) | 1,042 (13.8) | 353 (16.8) | 520 (13.5) |
| No | 764,450 (87.1) | 71,626 (86.0) | 60,243 (86.4) | 6,434 (85.0) | 1,695 (80.6) | 3,254 (84.7) |
| Missing | 28,104 (3.2) | 1,038 (1.3) | 816 (1.2) | 97 (1.3) | 55 (2.6) | 70 (1.8) |
| Large family size at delivery | ||||||
| Yes | 40,573 (4.6) | 4,384 (5.3) | 3,493 (5.0) | 475 (6.3) | 175 (8.3) | 241 (6.3) |
| No | 837,363 (95.4) | 78,882 (94.7) | 66,253 (95.0) | 7,098 (93.7) | 1,928 (91.7) | 3,603 (93.7) |
| Calendar year of birth | ||||||
| 1997–2000 | 238,719 (27.2) | 14,008 (16.8) | 11,381 (16.3) | 1,432 (18.9) | 437 (20.8) | 758 (19.7) |
| 2001–2005 | 276,070 (31.4) | 23,709 (28.5) | 19,584 (28.1) | 2,279 (30.1) | 634 (30.2) | 1,212 (31.5) |
| 2006–2012 | 363,147 (41.4) | 45,549 (54.7) | 38,781 (55.6) | 3,862 (51.0) | 1,032 (49.1) | 1,874 (48.8) |
| Paternal asthma before delivery | 59,890 (6.8) | 7,274 (8.7) | 6,102 (8.8) | 647 (8.5) | 202 (9.6) | 323 (8.4) |
| Sex of the child | ||||||
| Boys | 427,372 (48.7) | 40,560 (48.7) | 33,972 (48.7) | 3,733 (49.3) | 1,021 (48.5) | 1,834 (47.7) |
| Girls | 450,564 (51.3) | 42,706 (51.3) | 35,774 (51.3) | 3,840 (50.7) | 1,082 (51.5) | 2,010 (52.3) |
| Preterm birth (gestational age <37 weeks) | ||||||
| Yes | 41,472 (4.7) | 4,725 (5.7) | 3,827 (5.5) | 481 (6.4) | 173 (8.2) | 244 (6.3) |
| No | 836,464 (95.3) | 78,541 (94.3) | 65,919 (94.5) | 7,092 (93.6) | 1,930 (91.8) | 3,600 (93.7) |
| Low birth weight (<2500 g) | ||||||
| Yes | 30,790 (3.5) | 3,462 (4.2) | 2,808 (4.0) | 355 (4.7) | 120 (5.7) | 179 (4.7) |
| No | 847,146 (96.5) | 79,804 (95.8) | 66,938 (96.0) | 7,218 (95.3) | 1,983 (94.3) | 3,665 (95.3) |
Figures are numbers (%).
During 11.4 million person-years of follow-up, 27,780 (2.9%) children were identified as having ADHD. The children were followed up to a maximum of 20.0 years of age, and the median age at ADHD treatment was 9.2 years (interquartile range: 7.3–11.9 years). Parental antenatal asthma was associated with increased risk of ADHD in offspring; the HR for maternal asthma (1.41, 95% CI: 1.36–1.46) was greater than that for paternal asthma (1.13, 95% CI: 1.08–1.18) (P-value <0.001) (Table 2). Altogether, 7,274 children were born to parents who both had asthma; their risk of ADHD was 1.56 (95% CI: 1.39–1.76), in comparison to children with two non-asthmatic parents.
Table 2.
Hazard ratios of attention-deficit/hyperactivity disorder according to maternal and paternal asthma
| Parental asthma | Offspring ADHD (n) |
N | Person- years at risk |
Crude HR | Adjusted HR (95% CI) |
|---|---|---|---|---|---|
| All children | |||||
| Non-asthmatic mothers | 24,619 | 877,936 | 10,456,945 | 1 (ref) | 1 (ref) |
| Asthmatic mothers | 3,161 | 83,266 | 880,402 | 1.67 (1.61 – 1.73) | 1.41 (1.36 – 1.46) |
| Non-asthmatic fathers | 25,879 | 894,038 | 10,625,998 | (ref) | 1 (ref) |
| Asthmatic fathers | 1,901 | 67,164 | 711,349 | 1.19 (1.14 – 1.25) | 1.13 (1.08 – 1.18) |
| Boys | |||||
| Non-asthmatic mothers | 17,607 | 450,564 | 5,334,356 | 1 (ref) | 1 (ref) |
| Asthmatic mothers | 2,242 | 42,706 | 448,652 | 1.63 (1.56 – 1.70) | 1.39 (1.33 – 1.45) |
| Non-asthmatic fathers | 18,465 | 458,980 | 5,422,165 | 1 (ref) | 1 (ref) |
| Asthmatic fathers | 1,384 | 34,290 | 360,843 | 1.20 (1.14 – 1.27) | 1.14 (1.08 – 1.21) |
| Girls | |||||
| Non-asthmatic mothers | 7,012 | 427,372 | 5,122,589 | 1 (ref) | 1 (ref) |
| Asthmatic mother | 919 | 40,560 | 431,750 | 1.79 (1.67 – 1.91) | 1.46 (1.36 – 1.57) |
| Non-asthmatic fathers | 7,414 | 435,058 | 5,203,833 | 1 (ref) | 1 (ref) |
| Asthmatic fathers | 517 | 32,874 | 350,506 | 1.18 (1.08 – 1.29) | 1.09 (0.99 – 1.19) |
Abbrevations: HR, hazard ratio; CI, confidence interval.
Adjusted for maternal age at delivery, parity, maternal smoking during pregnancy, maternal mental disorders before pregnancy, maternal cohabiting status, place of residence at delivery, low social class at delivery, large family size, sex of the child, and calendar year of birth. Maternal asthma and paternal asthma were mutually adjusted in the models.
Increased risks of ADHD were seen among those whose mothers experienced asthma exacerbations before pregnancy, during pregnancy, and after delivery, in comparison to children of asthmatic mothers with no exacerbations; HR 1.12 (95% CI: 1.00–1.25) , 1.21 (95% CI: 1.00– 1.47), and 1.25 (95% CI: 1.08–1.44), respectively. The association between maternal asthma exacerbations during pregnancy and offspring ADHD did not differ by the timing of first exacerbation during pregnancy, with HRs of 1.31 (95% CI: 0.99–1.74) for first-trimester exacerbations and 1.13 (95% CI: 0.87–1.47) for second or third-trimester exacerbations. Associations did not differ with the number of exacerbations. Paternal asthma exacerbations before, during pregnancy, or after delivery were also associated with enhanced offspring ADHD risk (Table 3). Of 69,746 mothers with asthma without exacerbations, 22,206 (31.8%) received antiasthma medications excluding oral corticosteroids. Among mothers with asthma, taking antiasthma medication during pregnancy did not increase ADHD in offspring (HR: 0.94, 95 CI: 0.86–1.02) compared to those who were not treated.
Table 3.
Hazard ratios of attention-deficit/hyperactivity disorder according to maternal and paternal asthma exacerbations
| Parental asthma exacerbations | Offspring ADHD (n) |
N | Person- years at risk |
Crude HR | Adjusted HR (95% CI) |
|---|---|---|---|---|---|
| Asthmatic mothers | |||||
| No exacerbations | 2,520 | 69,746 | 731,491 | 1 (ref) | 1 (ref) |
| Timing at exacerbations | |||||
| Exacerbations before pregnancy | 341 | 7,573 | 82.719 | 1.16 (1.04 – 1.30) | 1.12 (1.00 – 1.25) |
| Exacerbations during pregnancy | 106 | 2,103 | 23,158 | 1.28 (1.05 – 1.55) | 1.21 (1.00 – 1.47) |
| During early pregnancy | 49 | 891 | 9,758 | 1.41 (1.06 – 1.87) | 1.31 (0.99 – 1.74) |
| During late pregnancy | 57 | 1,212 | 13,400 | 1.18 (0.91 – 1.54) | 1.13 (0.87 – 1.47) |
| Exacerbations after delivery | 194 | 3,844 | 43,034 | 1.25 (1.08 – 1.45) | 1.25 (1.08 – 1.44) |
| Number of exacerbations before, during pregnancy and after delivery | |||||
| One | 331 | 7,176 | 78,830 | 1.18 (1.05–1.32) | 1.18 (1.05 – 1.32) |
| Two | 123 | 2,796 | 30,631 | 1.13 (0.94–1.35) | 1.12 (0.94 – 1.35) |
| Three | 71 | 1,407 | 15,169 | 1.33 (1.05–1.69) | 1.35 (1.07 – 1.71) |
| ≥four | 116 | 2,141 | 24,281 | 1.31 (1.09–1.58) | 1.28 (1.07 – 1.55) |
| Asthmatic fathers | |||||
| No exacerbations | 1,418 | 53,043 | 554,163 | 1 (ref) | 1 (ref) |
| Exacerbations before pregnancy | 182 | 5,765 | 63,433 | 1.08 (0.92 – 1.26) | 1.07 (0.92 – 1.25) |
| Exacerbations during pregnancy | 172 | 4,864 | 53,904 | 1.19 (1.02 – 1.40) | 1.17 (1.00 – 1.37) |
| Exacerbations after delivery | 129 | 3,492 | 39,849 | 1.18 (0.99 – 1.42) | 1.18 (0.98 – 1.41) |
Abbrevations: HR, hazard ratio; CI, confidence interval.
Adjusted for maternal age at delivery, parity, maternal smoking during pregnancy, maternal mental disorders before pregnancy, maternal cohabiting status, place of residence at delivery, low social class at delivery, large family size, sex of the child, and calendar year of birth. Maternal asthma exacerbations and paternal asthma exacerbations were mutually adjusted in the models.
Sex-specific associations between maternal asthma, asthma exacerbations and ADHD in offspring were similar (Table 2, and STable 1 and STable 2 in the supplement). The results remained unchanged after excluding children born to mothers with ADHD before delivery (data not shown). Approximately 77,919 (93.6%) out of 83,266 asthmatic mothers received treatment for asthma after age 15 years. Similar results were obtained when limiting the definition of maternal asthma to those who received asthma treatment after 15 years of age (data not shown). As oral corticosteroids may be prescribed for disorders other than asthma exacerbations, an additional sensitivity analysis was carried out, defining asthma exacerbations based only on inpatient or emergency visit. Associations observed were broadly comparable to analyses in which oral corticosteroid prescriptions were also included, although the point estimates of the associations decreased and were no longer statistically significant due to smaller sample size (STable 3 in the supplement).
4. Discussion
In this population-based cohort study, we found an increased risk of ADHD among children born to mothers and fathers with asthma, with a higher risk observed for maternal asthma. Additionally, maternal and paternal asthma exacerbations before, during pregnancy or after delivery were associated with a further enhanced risk of offspring ADHD. Maternal use of antiasthma medication during pregnancy did not itself increase the risk of ADHD in children.
Studies have demonstrated strong familial risk for ADHD (Chen et al., 2008; Faraone et al., 2000; Thapar and Stergiakouli, 2008). Prevalence of asthma is also higher among those with ADHD than the general population (Grizenko et al., 2015). In our results, associations were seen for both maternal and paternal asthma, with greatest strength seen for children of parents who both had asthma, consistent with findings from previous epidemiological studies (Instanes et al., 2017; Liang et al., 2017), and supporting the attribution of genetic predisposition and/or shared environmental factors to ADHD risk. Additionally, we observed a stronger association between offspring ADHD and maternal asthma than paternal asthma, suggesting intrauterine exposure could also play a role. Alternatively, this may be due to the fact that environmental factors are likely more closely tied to mothers than fathers. Furthermore, the risk was even higher among children of mothers with exacerbations both before, during pregnancy and after delivery compared to children born to asthmatic mothers with no exacerbations. The risk of ADHD did not change with the number of exacerbations. Similarly, we found an association between paternal asthma exacerbations and ADHD in the offspring. These findings do not provide any support for the hypothesis of inflammation during pregnancy being associated with offspring ADHD, and may indicate that asthma with exacerbations is a distinct and more severe asthma phenotype, conveying a greater risk to the offspring (Dougherty and Fahy, 2009).
Liang et al’s (Liang et al., 2017) paper observed a positive association with ADHD among children whose mothers had been prescribed a β2-agonist not just during pregnancy, but also before pregnancy; leading them to conclude that the association was more likely due to the indication of treatment, rather than asthma medication. Our finding of no increased risk of ADHD among children of mothers who received antiasthma medication (excluding oral corticosteroids) during pregnancy corroborates this. One of the primary goals of asthma management during pregnancy is to prevent acute exacerbations. Although the safety of using asthma medications during pregnancy is reassuring (Murphy et al., 2007; National Heart et al., 2005), it has been estimated that approximately 29% of asthmatic women would discontinue asthma medication during pregnancy (Chambers, 2003), increasing their likelihood of having asthma exacerbations (Murphy et al., 2005). Results of the current study suggest that antenatal exacerbations of asthma in mothers may be associated with a higher risk of offspring ADHD and hence, our findings add to the evidence, that active pharmacological treatment of asthma using antiasthma medication should be continued during pregnancy to prevent exacerbations.
Methodological strengths of this study include its use of the Danish register data, which provide a large and representative sample, with sufficient power to investigate asthma treatment and exacerbations, to try to elucidate the findings. Register data are prospectively collected, so there is no risk of recall bias. The study has some limitations. First, we defined asthma cases as at least one hospital visit or two antiasthma medications. Parents with less severe asthma who have redeemed only one antiasthma medication prescription would not be included as having an asthma diagnosis. Correspondingly, not all patients with asthma exacerbations would receive inpatient treatment, visit an emergency ward or redeem a prescription of oral corticosteroids. We would have misclassified them as having had no exacerbations. These misclassifications will make our exposure and comparison group similar and bias our finding toward the null. Second, methylphenidate is also indicated for narcolepsy; the effect on the specificity of outcome ascertainment is expected to be minimal as narcolepsy is much less common than ADHD, especially during childhood (Ohayon et al., 2002), as the incidence of narcolepsy peaks at higher ages (Wijnans et al., 2013). Third, our findings may not be representative of the full spectrum of ADHD. The Danish health authorities stipulate that only specialists in child and adolescent psychiatry can diagnose ADHD and initiate treatment with ADHD-medications. It is likely that only moderate or severe cases of ADHD were identified. Several studies have examined the validity of register-based ADHD-diagnoses, all showing high positive predictive values (Dalsgaard et al., 2001; Linnet et al., 2009; Mohr-Jensen et al., 2016). However, clinical practice in Denmark regarding the diagnosis of ADHD may be more restrictive than in other countries, such as the United States (Dalsgaard et al., 2014; Dalsgaard et al., 2013), and the hospital register and prescription register may include only the more severe ADHD cases, limiting generalizability. Fourth, it is possible that compliance to antiasthmatic medication may be poorer among mothers with ADHD, meaning they are at higher risk of both asthma exacerbation and having a high genetic load for ADHD. The results were unaltered when we excluded mothers with pre-conception ADHD. Finally, we cannot exclude the possibility of chance findings and confounding by unknown/uncontrolled factors.
Taken together, these findings support theories on both shared genetic and common environmental risk factors having etiological roles in the development of ADHD. Moreover, our study’s finding of increased risk with exacerbations, but no obvious increased risk with maternal antiasthma medication treatment, supports the continued use of antiasthma medication during pregnancy.
Supplementary Material
Highlights.
Attention-deficit/hyperactivity disorder (ADHD) is a highly heritable condition. The etiology of ADHD has not been fully elucidated but is considered multifactorial; both genetic risk factors and prenatal exposures may play a role in its development. In this population-based cohort study comprising 961,202 live singletons, we found that both maternal and paternal asthma and asthma exacerbations during the pre- and perinatal period were associated with an increased risk of offspring ADHD, suggesting shared genetic and environmental risk factors having a role in the development of ADHD.
Acknowledgments
Funding: Liu X. is supported by the Danish Council for Independent Research (DFF-5053-00156B). Dalsgaard S. and Munk-Olsen T. are supported by iPSYCH, the Lundbeck Foundation Initiative for Integrative Psychiatric Research (R155-2014-1724). Dalsgaard S. is also supported by grants from Aarhus University Research Foundation (AUFF-E-2015-FLS-8-61), The Lundbeck Foundation (R102-A9118), National Institute of Health (R01, grant no ES026993), Novo Nordisk Foundation (grant no 22018) and the European Commission (Horizon 2020, grant no 667302). Momen N. and Li J. are supported by the Nordic Cancer Union (176673, 186200, R217-A13234-18-S65), the Danish Council for Independent Research (DFF-6110-00019B) and Karen Elise Jensens Fond (2016). Li J. is also supported by the National Natural Science Foundation of China (81530086). During the preparation of this manuscript, Wright R.J. was supported by the National Institutes of Health, UG3 OD23337, and R01 HD082078. The funders had no roles in study design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Potential Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
References
- Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH, 1999. The Danish National Hospital Register. A valuable source of data for modern health sciences. Danish medical bulletin 46, 263–268. [PubMed] [Google Scholar]
- Bliddal M, Broe A, Pottegard A, Olsen J, Langhoff-Roos J, 2018. The Danish Medical Birth Register. Eur J Epidemiol 33, 27–36. [DOI] [PubMed] [Google Scholar]
- Chambers K, 2003. Asthma education and outcomes for women of childbearing age. The Case manager 14, 58–61. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhou K, Sham P, Franke B, Kuntsi J, Campbell D, Fleischman K, Knight J, Andreou P, Arnold R, Altink M, Boer F, Boholst MJ, Buschgens C, Butler L, Christiansen H, Fliers E, Howe-Forbes R, Gabriels I, Heise A, Korn-Lubetzki I, Marco R, Medad S, Minderaa R, Muller UC, Mulligan A, Psychogiou L, Rommelse N, Sethna V, Uebel H, McGuffin P, Plomin R, Banaschewski T, Buitelaar J, Ebstein R, Eisenberg J, Gill M, Manor I, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Sergeant J, Sonuga-Barke E, Steinhausen HC, Taylor E, Thompson M, Faraone SV, Asherson P, 2008. DSM-IV combined type ADHD shows familial association with sibling trait scores: a sampling strategy for QTL linkage. Am J Med Genet B Neuropsychiatr Genet 147B, 1450–1460. [DOI] [PubMed] [Google Scholar]
- Class QA, Rickert ME, Larsson H, Lichtenstein P, D'Onofrio BM, 2014. Fetal growth and psychiatric and socioeconomic problems: population-based sibling comparison. Br J Psychiatry 205, 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell WJ, Bellinger DC, Wright RO, Wright RJ, 2019. Antenatal active maternal asthma and other atopic disorders is associated with ADHD behaviors among school-aged children. Brain Behav Immun. Epub 2019 May 31; doi: 10.1016/j.bbi.2019.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Onofrio BM, Class QA, Rickert ME, Larsson H, Langstrom N, Lichtenstein P, 2013. Preterm birth and mortality and morbidity: a population-based quasi-experimental study. JAMA Psychiatry 70, 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard S, Hansen N, Mortensen PB, Damm D, Thomsen PH, 2001. Reassessment of ADHD in a historical cohort of children treated with stimulants in the period 1969-1989. Eur Child Adolesc Psychiatry 10, 230–239. [DOI] [PubMed] [Google Scholar]
- Dalsgaard S, Humlum MK, Nielsen HS, Simonsen M, 2014. Common Danish standards in prescribing medication for children and adolescents with ADHD. European child & adolescent psychiatry 23, 841–844. [DOI] [PubMed] [Google Scholar]
- Dalsgaard S, Nielsen HS, Simonsen M, 2013. Five-fold increase in national prevalence rates of attention-deficit/hyperactivity disorder medications for children and adolescents with autism spectrum disorder, attention-deficit/hyperactivity disorder, and other psychiatric disorders: a Danish register-based study. Journal of child and adolescent psychopharmacology 23, 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard S, Ostergaard SD, Leckman JF, Mortensen PB, Pedersen MG, 2015. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet (London, England) 385, 2190–2196. [DOI] [PubMed] [Google Scholar]
- Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, Belliveau R, Bybjerg-Grauholm J, Bækved-Hansen M, Cerrato F, Chambert K, Churchhouse C, Dumont A, Eriksson N, Gandal M, Goldstein J, Grove J, Hansen CS, Hauberg M, Hollegaard M, Howrigan DP, Huang H, Maller J, Martin AR, Moran J, Pallesen J, Palmer DS, Pedersen CB, Pedersen MG, Poterba T, Poulsen JB, Ripke S, Robinson EB, Satterstrom FK, Stevens C, Turley P, Won H, Andreassen OA, Burton C, Boomsma D, Cormand B, Dalsgaard S, Franke B, Gelernter J, Geschwind D, Hakonarson H, Haavik J, Kranzler H, Kuntsi J, Langley K, Lesch K-P, Middeldorp C, Reif A, Rohde LA, Roussos P, Schachar R, Sklar P, Sonuga-Barke D, Sullivan PF, Thapar A, Tung J, Waldman I, Nordentoft M, Hougaard DM, Werge T, Mors O, Mortensen PB, Daly MJ, Faraone SV, Børglum AD, Neale BM, 2017. Discovery Of The First Genome-Wide Significant Risk Loci For ADHD. bioRxiv. [Google Scholar]
- Dougherty RH, Fahy JV, 2009. Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 39, 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Friedman D, 2000. Validity of DSM-IV subtypes of attention-deficit/hyperactivity disorder: a family study perspective. J Am Acad Child Adolesc Psychiatry 39, 300–307. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P, 2005. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry 57,1313–1323. [DOI] [PubMed] [Google Scholar]
- Grizenko N, Osmanlliu E, Fortier ME, Joober R, 2015. Increased Risk of Asthma in Children with ADHD: Role of Prematurity and Maternal Stress during Pregnancy. J Can Acad Child Adolesc Psychiatry 24, 109–115. [PMC free article] [PubMed] [Google Scholar]
- Gustafsson P, Kallen K, 2011. Perinatal, maternal, and fetal characteristics of children diagnosed with attention-deficit-hyperactivity disorder: results from a population-based study utilizing the Swedish Medical Birth Register. Dev Med Child Neurol 53, 263–268. [DOI] [PubMed] [Google Scholar]
- Halmoy A, Klungsoyr K, Skjaerven R, Haavik J, 2012. Pre- and perinatal risk factors in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 71, 474–481. [DOI] [PubMed] [Google Scholar]
- Harpin VA, 2005. The effect of ADHD on the life of an individual, their family, and community from preschool to adult life. Archives of disease in childhood 90 Suppl 1, i2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indredavik MS, Vik T, Heyerdahl S, Kulseng S, Brubakk AM, 2005. Psychiatric symptoms in low birth weight adolescents, assessed by screening questionnaires. European Journal of Child and Adolescent Psychiatry 14, 226–236. [DOI] [PubMed] [Google Scholar]
- Indredavik MS, Vik T, Heyerdahl S, Kulseng S, Fayers P, Brubakk AM, 2004. Psychiatric symptoms and disorders in adolescents with low birth weight. Archives of Disease in Childhood. Fetal and Neonatal Edition 89, F445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instanes JT, Halmoy A, Engeland A, Haavik J, Furu K, Klungsoyr K, 2017. Attention-Deficit/Hyperactivity Disorder in Offspring of Mothers With Inflammatory and Immune System Diseases. Biological psychiatry 81, 452–459. [DOI] [PubMed] [Google Scholar]
- Kildemoes HW, Sorensen HT, Hallas J, 2011. The Danish National Prescription Registry. Scandinavian journal of public health 39, 38–41. [DOI] [PubMed] [Google Scholar]
- Liang H, Chen J, Miao M, Christensen J, Dalsgaard S, Yuan W, Li J, 2017. In utero exposure to beta-2-adrenergic receptor agonist and attention-deficit/hyperactivity disorder in children. European child & adolescent psychiatry 26, 847–856. [DOI] [PubMed] [Google Scholar]
- Lindstrom K, Lindblad F, Hjern A, 2011. Preterm birth and attention-deficit/hyperactivity disorder in schoolchildren. Pediatrics 127, 858–865. [DOI] [PubMed] [Google Scholar]
- Linnet KM, Wisborg K, Agerbo E, Secher NJ, Thomsen PH, Henriksen TB, 2006. Gestational age, birth weight, and the risk of hyperkinetic disorder. Arch Dis Child 91, 655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnet KM, Wisborg K, Secher NJ, Thomsen PH, Obel C, Dalsgaard S, Henriksen TB, 2009. Coffee consumption during pregnancy and the risk of hyperkinetic disorder and ADHD: a prospective cohort study. Acta paediatrica (Oslo, Norway : 1992) 98, 173–179. [DOI] [PubMed] [Google Scholar]
- Liu X, Agerbo E, Schlunssen V, Wright RJ, Li J, Munk-Olsen T, 2017. Maternal asthma severity and control during pregnancy and risk of offspring asthma. The Journal of allergy and clinical immunology 141, 886–892. [DOI] [PubMed] [Google Scholar]
- Mohr-Jensen C, Vinkel Koch S, Briciet Lauritsen M, Steinhausen HC, 2016. The validity and reliability of the diagnosis of hyperkinetic disorders in the Danish Psychiatric Central Research Registry. European psychiatry : the journal of the Association of European Psychiatrists 35, 16–24. [DOI] [PubMed] [Google Scholar]
- Mors O, Perto GP, Mortensen PB, 2011. The Danish Psychiatric Central Research Register. Scandinavian Journal of Public Health 39, 54–57. [DOI] [PubMed] [Google Scholar]
- Murphy VE, Fittock RJ, Zarzycki PK, Delahunty MM, Smith R, Clifton VL, 2007. Metabolism of synthetic steroids by the human placenta. Placenta 28, 39–46. [DOI] [PubMed] [Google Scholar]
- Murphy VE, Gibson P, Talbot PI, Clifton VL, 2005. Severe asthma exacerbations during pregnancy. Obstetrics and gynecology 106, 1046–1054. [DOI] [PubMed] [Google Scholar]
- Murphy VE, Gibson PG, Giles WB, Zakar T, Smith R, Bisits AM, Kessell CG, Clifton VL, 2003. Maternal asthma is associated with reduced female fetal growth. Am J Respir Crit Care Med 168, 1317–1323. [DOI] [PubMed] [Google Scholar]
- National Heart L, Blood I, National Asthma E, Prevention Program A, Pregnancy Working G, 2005. NAEPP expert panel report. Managing asthma during pregnancy: recommendations for pharmacologic treatment-2004 update. J Allergy Clin Immunol 115, 34–46. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Breslau N, 2007. Prenatal smoking exposure, low birth weight, and disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry 46, 362–369. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Priest RG, Zulley J, Smirne S, Paiva T, 2002. Prevalence of narcolepsy symptomatology and diagnosis in the European general population. Neurology 58, 1826–1833. [DOI] [PubMed] [Google Scholar]
- Ostergaard SD, Larsen JT, Dalsgaard S, Wilens TE, Mortensen PB, Agerbo E, Mors O, Petersen L, 2016. Predicting ADHD by Assessment of Rutter's Indicators of Adversity in Infancy. PloS one 11, e0157352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson E, Sjolander A, Almqvist C, Anckarsater H, D'Onofrio BM, Lichtenstein P, Larsson H, 2015. Birth weight as an independent predictor of ADHD symptoms: a within-twin pair analysis. J Child Psychol Psychiatry 56, 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA, 2015. Annual research review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. Journal of child psychology and psychiatry, and allied disciplines 56, 345–365. [DOI] [PubMed] [Google Scholar]
- Riglin L, Collishaw S, Thapar AK, Dalsgaard S, Langley K, Smith GD, Stergiakouli E, Maughan B, O'Donovan MC, Thapar A, 2016. Association of Genetic Risk Variants With Attention-Deficit/Hyperactivity Disorder Trajectories in the General Population. JAMA Psychiatry 73, 1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, 2008. Assessing intrauterine influences on offspring health outcomes: can epidemiological studies yield robust findings? Basic & clinical pharmacology & toxicology 102, 245–256. [DOI] [PubMed] [Google Scholar]
- Strickland AD, 2014. Prevention of cerebral palsy, autism spectrum disorder, and attention deficit-hyperactivity disorder. Med Hypotheses 82, 522–528. [DOI] [PubMed] [Google Scholar]
- Sucksdorff M, Lehtonen L, Chudal R, Suominen A, Gissler M, Sourander A, 2018. Lower Apgar scores and Caesarean sections are related to attention-deficit/hyperactivity disorder. Acta Paediatr. [DOI] [PubMed] [Google Scholar]
- Sucksdorff M, Lehtonen L, Chudal R, Suominen A, Joelsson P, Gissler M, Sourander A, 2015. Preterm Birth and Poor Fetal Growth as Risk Factors of Attention-Deficit/ Hyperactivity Disorder. Pediatrics 136, e599–608. [DOI] [PubMed] [Google Scholar]
- Thapar A, Stergiakouli E, 2008. An Overview on the Genetics of ADHD. Xin Li Xue Bao 40, 1088–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, He XY, Baines KJ, Gunawardhana LP, Simpson JL, Li F, Gibson PG, 2011. Different inflammatory phenotypes in adults and children with acute asthma. The European respiratory journal 38, 567–574. [DOI] [PubMed] [Google Scholar]
- Wijnans L, Lecomte C, de Vries C, Weibel D, Sammon C, Hviid A, Svanstrom H, Molgaard-Nielsen D, Heijbel H, Dahlstrom LA, Hallgren J, Sparen P, Jennum P, Mosseveld M, Schuemie M, van der Maas N, Partinen M, Romio S, Trotta F, Santuccio C, Menna A, Plazzi G, Moghadam KK, Ferro S, Lammers GJ, Overeem S, Johansen K, Kramarz P, Bonhoeffer J, Sturkenboom MC, 2013. The incidence of narcolepsy in Europe: before, during, and after the influenza A(H1N1)pdm09 pandemic and vaccination campaigns. Vaccine 31, 1246–1254. [DOI] [PubMed] [Google Scholar]
- Vejledning om medikamentel behandling af børn og unge med psykiske lidelser]. VEJ nr 9194 af November/04/2013 Copenhagen: SUM, Sundhedsstyrelsen,2019. Available: https://www.retsinformation.dk/pdfPrint.aspx?id=146409 (Accessed March 21, 2019). [Google Scholar]
- Xu R, O'Quigley J, 2000. Estimating average regression effect under non-proportional hazards. Biostatistics (Oxford, England) 1, 423–439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.