Abstract
Background
Alcohol abuse is a common and costly practice. Individuals high in negative urgency, the tendency to act rashly when experiencing negative emotions, are at particular risk for abusing alcohol. Alcohol abuse among individuals high in negative urgency may be due to (a) increased activity in the brain’s striatum, (b) decreased activity in brain regions associated with self-control, or (c) a combination of the two.
Methods
Thirty eight non-alcohol-dependent participants completed a measure of negative urgency and then underwent functional magnetic resonance imaging (fMRI) while passively viewing pleasant and alcohol images.
Results
Alcohol images (as compared to pleasant images) were associated with activation in the caudate nucleus, a brain region associated with linking reward to external stimuli. Negative urgency (above and beyond other facets of impulsivity) correlated positively with this caudate activation in response to alcohol images. Alcohol images and negative urgency were unassociated with activity in the lateral prefrontal cortex, a self-regulatory brain region.
Conclusions
These findings provide initial support that the alcohol abuse observed among individuals high in negative urgency may be due, in part, to heightened reactivity in the striatum to alcohol. Investigating such neural contributors to self-regulation failure is crucial to reducing substance abuse.
Keywords: negative urgency, alcohol, caudate nucleus, self-regulation, fMRI
Introduction
One facet of impulsivity, negative urgency, is characterized by rash behavior during negative affect, is robustly associated with alcohol abuse (Magid and Colder, 2007; Settles, Cyders, and Smith, 2010; Whiteside and Lynam, 2001). The literature is largely silent as to the mechanisms through which negative urgency promotes alcohol abuse. Using functional neuroimaging, we sought to fill this gap.
1.1. The UPPS model of Impulsivity
Whiteside and Lynam (2001) identified four distinct factors of impulsivity that include negative urgency (U), lack of premeditation (P), lack of perseverance (P), and sensation-seeking (S). Several studies have confirmed a five-factor structure of the UPPS-P, including positive urgency, which is impulsive reactivity to positive affect (-P; Lynam and Miller, 2004; Smith, Fischer, Cyders, Annus, and Spillane, 2007).
1.2. Negative Urgency and Alcohol Abuse
Negative urgency correlates with increased alcohol abuse above and beyond other facets of impulsivity (Magid and Colder, 2007; Settles et al., 2010; Whiteside and Lynam, 2001). But why do individuals high in negative urgency succumb to alcohol abuse? Self-regulation failures in the context of negative urgency are often explained as due to insufficient inhibition (e.g., Wilbertz et al., 2014). This reflects a more general trend to focus on inhibitory failure (e.g., Muraven and Baumeister, 2000). This emphasis on ‘top-down’ restraint ignores a significant ‘bottom-up’ process: craving (Hoffmann and Van Dillen, 2012). Malfunctions of self-regulation, such as alcohol abuse, could be due to a lack of self-regulatory restraint, heightened craving, or the simultaneous presence of the two (Hofmann and Van Dillen, 2012; Inzlicht and Schmeichel, 2012). These two processes arise in different regions of the brain.
1.3. Neural Correlates of Alcohol Cue Reactivity
1.3.1. Caudate Nucleus
The caudate nucleus is a subcortical structure in the dorsal striatum. This neural region links anticipated reward with behavior intended to achieve that reward (Knutson and Cooper, 2005). Activation of the caudate nucleus is associated with experimental inductions of alcohol craving, (Modell and Mountz, 1995), and is also positively correlated with self-reported alcohol cravings (Hommer, 1999). Alcohol-dependent individuals demonstrate increased activation of the left caudate nucleus in response to alcohol images, as compared to healthy controls (Heinz et al., 2004). Although these findings were observed among alcohol dependent individuals, the caudate is a crucial neural substrate of the formation of associations between novel stimuli (e.g., alcohol) and the experience of reward (Yin and Knowlton, 2006). Taken together, these findings suggest that the caudate plays a unique and reliable role in the desire to consume alcohol. Negative urgency has been previously linked to greater alcohol reactivity in other brain regions that are part of the same dopaminergic reward circuitry that includes the caudate (i.e., the ventromedial prefrontal cortex; Cyders et al., 2014; for a review of neural systems involved in negative urgency see Smith & Cyders, under review).
1.3.2. DLPFC
The dorsolateral prefrontal cortex (DLPFC) is a brain area regarded as the biological substrate of psychological processes necessary for successful self-regulatory restraint (see Banfield, Wyland, Macrae, Munte, and Heatherton, 2004). DLPFC stimulation reduced self-reported alcohol craving (Boggio et al., 2008). Thus, the DLPFC appears to regulate alcohol cravings.
1.4. Overview
We predicted that negative urgency, independent of other facets of impulsivity, would be positively associated with alcohol-specific activation of the caudate nucleus. Conversely, we predicted that negative urgency, independent of other facets of impulsivity, would be negatively associated with alcohol-specific activation of the DLPFC. To test these hypotheses, participants completed a measure of impulsivity that included its five facets of negative urgency, lack of premeditation, lack of perseverance, sensation-seeking, and positive urgency. Then, participants viewed alcohol and pleasant images while undergoing fMRI.
2. Material and Methods
2.1. Participants
Participants were 40 healthy, right-handed undergraduates who received course credit and $65 (see Supplemental Materials for exclusion criteria). One participant was excluded from analyses because of distorted functional magnetic resonance imaging (fMRI) data. Another participant was deemed an outlier (see Results). Analyses were therefore performed on the 38 remaining participants (19 females; Age: M = 18.95, SD = 1.33).
2.2. Procedure
2.2.1. Pre-scan
Participants completed a demographics questionnaire, a screening form to ensure safety and comfort in the MRI environment, the Alcohol Use Disorders Identification Test (AUDIT; Barbor, La Fuente, Saunders, and Grant, 1992), a one-year Timeline Follow-Back Calendar of alcohol use (Sobell and Sobell, 1992), and the UPPS-P Impulsivity Scale (Lynam, Smith, Whiteside, and Cyders, 2006). The AUDIT and calendar measures of alcohol abuse were used to establish participants’ histories of drinking behavior. Participants then practiced the cue reactivity task and were placed in the scanner.
2.2.2. Cue reactivity task
While undergoing fMRI, participants completed a simple cue reactivity paradigm in which they passively viewed images depicting either alcohol, marijuana, polydrugs (e.g., cocaine), or control images that were neutral or pleasant. Alcohol, marijuana, and polydrug stimuli were acquired from published research on the appetitive nature of drugs and alcohol (Mun, von Eye, Bates, and Vaschillo, 2008; Buckman, White, and Bates, 2010; Ray, Hanson, Hanson, and Bates, 2010). Neutral and pleasant images were taken from the International Affective Picture Set (IAPS; Lang, Bradley, and Cuthbert, 2008). All images were pre-rated in the IAPS technical report along a 1 – 9 Likert scale on the dimensions of pleasantness (Lang et al., 2008).
Pleasant images included a diverse array of stimuli (e.g., appetizing food) and were rated as highly pleasant (M = 7.53, SD = 0.26). Each block of the task sequentially presented five images of a single condition (4 seconds per image, 20 seconds total). This was then followed by 10 seconds of a fixation crosshair. The task contained 21 blocks, which were divided into 3 alcohol, 3 marijuana, and 3 polydrug blocks that were matched with 9 pleasant blocks. Three neutral blocks were also included. The order of block-conditions was randomized but held constant across participants. Full details regarding MRI data acquisition, preprocessing, analysis, and region-of-interest construction are available in the Supplemental Materials.
3. Results
3.1. Descriptives
No participants exhibited alcohol dependence, as evidenced by AUDIT scores below the cutoff of 20 (range: 0 – 12, M = 3.50, SD = 3.33). For the year previous to the study, participants showed wide variability in the number of drinks they indicated on the alcohol calendar (range: 0 – 732 drinks; M = 92.94, SD = 156.87). All five subscales of the UPPS inventory were sufficiently reliable (αs = .74 - .84) and showed substantial variability (see Supplemental Table 1). Males reported greater negative urgency than females, t(36) = 2.16, p = .037.
3.2. Neuroimaging
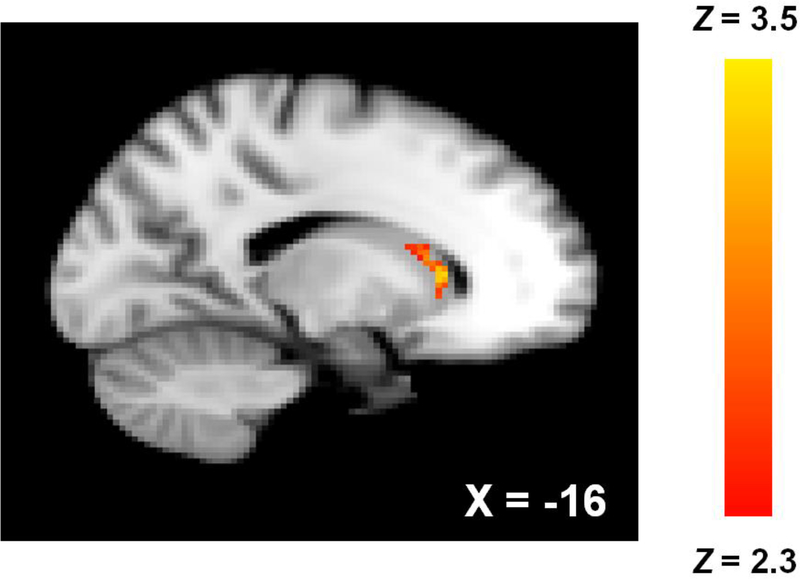
Alcohol images, compared with pleasant images, related to increased activity in the left head of the caudate nucleus [Figure 1; 60 contiguous voxels, maximum Z = 3.33, MNI peak coordinates (x, y, z) = −16, 22, 6]. This same contrast failed to elicit any voxels of significant activation in either hemisphere of the DLPFC and thus we were unable to assess urgency’s relation with activity in this region.
Figure 1.
Left caudate nucleus activation associated with alcohol > pleasant images. Coordinates are MNI.
Functional data from the activated main effect cluster in the caudate nucleus (alcohol > pleasant contrast) were converted to units of percent signal change and extracted (as outlined by Mumford, J. http://mumford.fmripower.org/perchange_guide.pdf). Males exhibited marginally greater caudate reactivity to alcohol images, t(36) = 1.85, p = .073. Whole brain analyses (alcohol > pleasant) revealed a large cluster of activity in the occipital cortex, potentially suggesting greater visual attention to alcohol stimuli (Supplemental Figure 1).
3.3. Multiple Regression Analysis
Four subscales of the UPPS-P Impulsivity Scale (excepting positive urgency) were included simultaneously as predictors in a multiple regression model with percent signal change units from the activated cluster of the left caudate (alcohol > pleasant contrast) as the dependent variable. For zero-order correlations between scores on each of the five UPPS facets and caudate activity see Table 1. Outlier detection was based on leverage (Cohen, Cohen, West, and Aiken, 2003). One case met this criterion and was removed.
Table 1.
Zero-order correlations between neural activity in units of percent signal change taken from the alcohol > pleasant contrast and UPPS-P subscales.
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| 1. left caudate | |||||
| 2. negative urgency | .34* | ||||
| 3. lack of perseverance | .14 | .34* | |||
| 4. positive urgency | .17 | .47** | .10 | ||
| 5. lack of premeditation | −.09 | .24 | .56*** | −.06 | |
| 6. sensation-seeking | .08 | −.02 | −.06 | −.01 | .20 |
p < .05
p < .01
p < .001
Results indicated that negative urgency was positively associated with greater alcohol-specific left caudate activation (Figure 2; β = .35, t(33) = 2.08, p = .045). Lack of premeditation (β = −.32, t(33) = −1.59, p = .12), lack of perseverance (β = .21, t(33) = 1.03, p = .31), and sensation-seeking (β = .16, t(33) = 0.97, p = .34) all simultaneously failed to significantly correlate with caudate activation. Removing participants who had not consumed alcohol in the past year, reduced the association between negative urgency and caudate reactivity to a statistical trend, β = .37, t(24) = 1.91, p = .068. Positive urgency, with identical covariates, was unassociated with alcohol-specific caudate activity, β = .13, t(34) = 0.77, p = .45.
Figure 2.
Positive correlation between left caudate nucleus activation (in percent signal change units; alcohol > pleasant contrast) and negative urgency.
4. Discussion
Previous research has shown that negative urgency is uniquely correlated with alcohol abuse. Yet the neural and psychological mechanisms underlying this relationship remain to be fully understood. The present study assessed whether negative urgency was associated with (A) increases in alcohol cue reactivity in the striatum, (B) decreases in prefrontal reactivity or (C) both. Supporting the first hypothesis, negative urgency (while controlling for other facets of impulsivity) was associated with increases in activation of the left caudate nucleus when individuals viewed images of alcohol (as compared to pleasant images). These findings suggest that negative urgency may predict alcohol abuse by amplifying caudate reactivity and not by reducing prefrontal responses. However, we were unable to assess whether negative urgency correlated with alcohol-specific activation of the DLPFC as this brain region was not reactive to our alcohol cues.
These findings add nuance to mechanistic explanations of negative urgency’s association with substance abuse (e.g., Wilbertz et al., 2014). The association between urgency and the caudate imply that bottom-up impulses are crucial determinants of the impulsive behavior of individuals high in negative urgency. It is important to emphasize the correlational nature of these data and thus directionality of our effects is impossible to establish. It may be that greater caudate reactivity to alcohol cues is an underlying factor contributing to the development of negative urgency (as suggested by Cyders et al., 2014). Longitudinal and brain stimulation approaches may allow us to establish the directionality of this effect. Additionally, negative urgency reflects impulsive responses to negative affect and yet we did not induce negative affect in this study. As such, our findings may arise from a general, tonic experience of negative affect among this population and should be explored in the context of negative affect inductions.
This experiment was limited in that our alcohol stimuli were images and not actual objects that participants could consume. Future research could provide participants with alcohol while functional neural data were acquired, further elucidating whether negative urgency functions only on the anticipated reward of alcohol or the actual pleasure of consuming alcohol. Also, the three alcohol blocks were contrasted against the larger set of nine pleasant blocks, yielding a large power differential. Alcohol and pleasant images were also not equated on pleasantness or other criteria and thus our conditions may have differed along other criteria that may have affected our results. We also did not obtain subjective reports of craving for our alcohol stimuli and thus we are unable to determine the extent to which our sample perceived them as appetitive. Further, reverse inference issues prevent us from knowing with any certainty whether the caudate activity we observed represented craving for alcohol. In addition, our sample population consisted of undergraduate students who were not heavy drinkers. Thus, our sample may not have truly experienced alcohol ‘cravings.’ Further, approximately 24% of our sample were non-drinkers and it is thus difficult to interpret whether they might experience cravings for the alcohol stimuli and whether this was reflected in the caudate.
4.1. Conclusions
The association between negative urgency and alcohol abuse may arise from an increase in caudate reactivity to alcohol, not an insufficient prefrontal response. Our experiment represents a key step forward into understanding the mechanisms through which negative urgency promotes alcohol abuse. If individuals high in negative urgency exhibit greater caudate-mediated alcohol craving, this would allow for the development of interventions targeted specifically at this psychological process before it manifests as alcohol dependence.
Supplementary Material
Highlights.
Negative urgency is associated with alcohol abuse yet the mechanisms remain unknown.
Negative urgency correlated with greater caudate nucleus activity to alcohol cues.
Negative urgency’s relation to alcohol abuse may be due to greater alcohol craving.
Acknowledgments
This experiment was funded by a grant from the University of Kentucky’s Center for Drug Abuse Research and Translation (CDART; Sponsor: National Institute on Drug Abuse, Grant number: DA005312) to DRL, RM, and CND, and a grant from the National Science Foundation (Grant number: BCS1104118) to CND. The authors are grateful to Richard S. Pond Jr. for their assistance with data collection.
Role of Funding Source
Nothing declared.
Footnotes
Conflict of Interest
No conflict declared
References
- Banfield JF, Wyland CL, Neil C, Münte TF, Heatherton TF, 2004. The cognitive neuroscience of self-regulation, in: Baumeister RF Vohs KD (Eds.), Handbook of Self-Regulation: Research, Theory, and Applications. Guilford Press, New York, pp. 62–83. [Google Scholar]
- Barbor TE, La Fuente JR, Saunders J, Grant M, 1992. AUDIT–The alcohol use disorders identification test: guidelines for use in primary health care. Geneva: World Health Organization. [Google Scholar]
- Beckmann C, Jenkinson M, Smith SM, 2003. General multi-level linear modeling for group analysis in fMRI. NeuroImage. 20, 1052–1063. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual-Leone A, Basaglia A et al. , 2008. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: A double-blind, sham-controlled study. Drug. Alcohol. Depend 92, 55–60. [DOI] [PubMed] [Google Scholar]
- Buckman JF, White HR Bates ME, 2010. Psychophysiological reactivity to emotional picture cues two years after college students were mandated for alcohol interventions. Addict. Behav. 35, 786–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS, 2003. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences, third ed. Lawrence Erlbaum Associates, New York. [Google Scholar]
- Cyders MA, Dzemidzic M, Eiler WJ, Coskunpinar A, Karyadi K, Kareken DA, 2014. Negative urgency and ventromedial prefrontal cortex responses to alcohol cues: FMRI evidence of emotion-based impulsivity. Alcohol. Clin. Exp. Res 38, 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Fischl B, 2009. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 48, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grüsser-Sinopoli SM, Flor H et al. , 2004. Correlation between dopamine D2 receptors in the ventral striatum and central processing of alcohol cues and craving. Am. J. Psychiat 161, 1783–1789. [DOI] [PubMed] [Google Scholar]
- Heller R, Stanley D, Yekutieli D, Rubin N, Benjamini Y, 2006. Cluster-based analysis of fMRI data. NeuroImage. 33, 599–608. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Van Dillen L, 2012. Desire: The new hot spot in self-control research. Curr. Dir. Psychol. Sci 21, 317–322. [Google Scholar]
- Hommer DW, 1999. Functional imaging of craving. Alcohol. Res. Health 23, 187–196. [PMC free article] [PubMed] [Google Scholar]
- Inzlicht M, Schmeichel BJ, 2012. What is ego depletion? Toward a mechanistic revision of the resource model of self-control. Perspect. Psychol. Sci 7, 450–463. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SM, 2001. A global optimisation method for robust affine registration of brain images. Med. Image. Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 17, 825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM, 2012. FSL. NeuroImage. 62, 782–790. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC, 2005. Functional magnetic resonance imaging of reward prediction. Curr. Opin. Neurol 18, 411–417. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN, 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual Technical Report A-8. University of Florida, Gainesville, FL. [Google Scholar]
- Lynam DR Miller JD, 2004. Personality pathways to impulsive behavior and their relations to deviance: Results from three samples. J. Quant. Criminol. 20, 319–341. [Google Scholar]
- Lynam DR, Smith GT, Whiteside SP, Cyders MA, 2006. The UPPS-P: Assessing Five Personality Pathways to Impulsive Behavior (Technical Report). Purdue University, West Lafayette. [Google Scholar]
- Magid V, Colder CR, 2007. The UPPS Impulsive Behavior Scale: Factor structure and associations with college drinking. Pers. Individ. Diff 43, 1927–1937. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH, 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 19, 1233–1239. [DOI] [PubMed] [Google Scholar]
- Modell JG, Mountz JM, 1995. Focal cerebral blood flow change during craving for alcohol measured by SPECT. J. Neuropsychiatry Clin. Neurosci 7, 15–22. [DOI] [PubMed] [Google Scholar]
- Mun EY, von Eye A, Bates ME, Vaschillo EG, 2008. Finding groups using model-based cluster analysis: Heterogeneous emotional self-regulatory processes and heavy alcohol use risk. Dev. Psychol, 44, 481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraven M, Baumeister RF, 2000. Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychol. Bull 126, 247–259. [DOI] [PubMed] [Google Scholar]
- Ray S, Hanson S, Hanson C, Bates ME, 2010. fMRI bold response in high risk college students: During exposure to alcohol, marijuana, polydrug and emotional picture cues. Alcohol. Alcohol, 45, 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settles RF, Cyders M, Smith GT, 2010. Longitudinal validation of the acquired preparedness model of drinking risk. Psychol. Addict. Behav 24, 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GT & Cyders M, under review. Integrating affect and impulsivity: The role of positive and negative urgency in substance abuse risk. Drug and Alcohol Dependence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GT, Fischer S, Cyders MA, Annus AM, Spillane NS, McCarthy DM, 2007. On the validity and utility of discriminating among impulsivity-like traits. Assessment. 14, 155–170. [DOI] [PubMed] [Google Scholar]
- Smith SM, 2002. Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H et al. , 2004. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 23 S208–S219. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1992. Timeline Follow-Back, in: Litten RZ, Allen JP (Eds.), Measuring Alcohol Consumption. Humana Press, New York, pp. 41–72. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, et al. , 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 15, 273–289. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR, 2001. The Five Factor Model and impulsivity: Using a structural model of personality to understand impulsivity. Pers. Individ. Diff, 30, 669–689. [Google Scholar]
- Wilbertz T, Deserno L, Horstmann A, Neumann J, Villringer A, Heinze H-J, et al. , 2014. Response inhibition and its relation to multidimensional impulsivity. NeuroImage. 103, 241–248. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T et al. , 2009. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 45, S173–S186. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, 2001. Statistical analysis of activation images, in: Jezzard P. Matthews PM, Smith SM (Eds.) Functional MRI: An Introduction to Methods, Oxford University Press, Oxford, pp. 251–270. [Google Scholar]
- Yin HH, Knowlton BJ, 2006. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci 7, 464–476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.