Abstract
Background:
Children with inherited bone marrow failure syndromes (IBMFS) may be symptomatic in utero, resulting in maternal and fetal problems during the pregnancy. Subsequent pregnancies by their mothers should be considered “high risk”.
Methods:
We retrospectively analyzed outcomes of 575 pregnancies in 165 unaffected mothers of offspring with Fanconi anemia (FA), dyskeratosis congenita (DC), Diamond Blackfan anemia (DBA) and Shwachman Diamond syndrome (SDS) for events noted during pregnancy, labor and delivery. We compared outcomes of pregnancies with affected and unaffected offspring within each group of mothers and with the general population.
Results:
The rates of miscarriage (12-20%), elective abortion (5-10%) and live birth (68-78%) among mothers of all IBMFS groups were similar and comparable with general population rates but recurrent miscarriages (≥2) were significantly more common in mothers of offspring with DBA and SDS. Offspring with FA were more frequently born small for gestational age (SGA) than unaffected babies (39% versus 4%) and had fetal malformations (46%) with 18% having three or more, often necessitating early delivery and surgery; offspring with DC had higher rates of SGA (39% versus 8%) and fetal distress (26% versus 3%); and offspring with DBA had fetal hypoxia (19% versus 1%) leading to preterm and emergency cesarean deliveries (26% versus 6%). Offspring with early-onset severe phenotypes had the most prenatal and peripartum adverse events.
Conclusion:
We identified the high-risk nature of pregnancies in mothers with IBMFS-affected fetuses, suggesting the need for pre-pregnancy counseling and monitoring of subsequent pregnancies by high-risk fetal-maternal specialists.
Keywords: Inherited bone marrow failure syndromes, perinatal complications, fetal distress, small for gestation, preterm
INTRODUCTION
The four major inherited bone marrow failure syndromes (IBMFS), Fanconi anemia (FA), dyskeratosis congenita (DC), Diamond Blackfan anemia (DBA), and Shwachman Diamond syndrome (SDS), are rare cancer-prone disorders. FA and SDS are primarily autosomal recessive (AR); DC is autosomal dominant (AD), AR and X-linked recessive; and DBA is mostly AD.1,2 Individuals with DBA and SDS often present with symptoms early in infancy and those with FA and DC generally within the first two decades of life.1 However, children with severe features within each syndrome may be symptomatic at birth or in utero as documented by several case reports.3-6 Prenatal disease manifestations in fetuses affected with an IBMFS may lead to increased complications in mothers’ pregnancies and result in adverse pregnancy outcomes.
This is the first systematic study of pregnancy outcomes in mothers of offspring with an IBMFS. We hypothesized that unaffected mothers pregnant with fetuses affected with an IBMFS would have higher rates of miscarriages compared with the general population, due to reduced viability of severely affected fetuses, such as organ system malformations in FA,7 severe disease phenotype in DC,8 and hydrops fetalis in DBA.3 We postulated that there may be increased rates of elective abortions of affected fetuses,5 and greater frequency of adverse events in viable pregnancies (lasting for >20 weeks) with IBMFS-affected fetuses compared with unaffected fetuses. We studied the rates of miscarriages and elective abortions, and evaluated problems noted during pregnancy and delivery of live-born offspring with IBMFS versus their unaffected siblings, among mothers who themselves did not have an IBMFS.
METHODS
All patients with an IBMFS and their unaffected siblings and mothers were participants in the National Cancer Institute’s Institutional Review Board-approved Inherited Bone Marrow Failure Syndromes study (NCT-00027274), a longitudinal natural history cohort established in January 2002. A detailed description of the cohort was included in a previous publication.9 Written informed consent was obtained from all participants in accordance with Health and Human Services regulation 45 CFR 46. All participants completed comprehensive questionnaires at study entry and were followed prospectively with biennial follow-up forms. The diagnosis of FA, DC, DBA and SDS in patients with an IBMFS was based on specific diagnostic criteria and confirmed by identification of pathogenic variants in syndrome-specific genes when available.2, 9,10 Family members of each proband underwent disease-specific diagnostic tests and targeted testing of the pathogenic variant identified in the proband, and were classified as affected or unaffected with an IBMFS.
Unaffected mothers of offspring with FA, DC, DBA and SDS were included in this study of pregnancy outcomes. Any mother affected with an IBMFS (clinically or proven genetically) was excluded. The reproductive history of mothers included the total number of pregnancies, details about each pregnancy outcome (live birth, miscarriage, stillbirth, ectopic pregnancy, or elective abortion), gestational ages of pregnancies, maternal age and pregnancy order. Information regarding the use of assisted reproductive therapies and preimplantation genetic diagnosis was frequently provided by the participants. Details regarding complications noted during each pregnancy and delivery, mode of delivery, birth weight and congenital malformations in offspring were obtained from the questionnaires on mothers and each offspring (affected or unaffected), review of medical records of offspring with IBMFS, and in-person interviews of those who came to the National Institutes of Health Clinical Center.
Miscarriage was defined as spontaneous pregnancy loss through 20 weeks, excluding ectopic and molar pregnancy. Abortion included elective termination of pregnancy through 20 weeks. Stillbirth was fetal demise after 20 weeks. Live birth prior to 37 weeks of gestation was preterm. Small for gestational age (SGA) was weight below the 10th percentile for gestational age at birth, while weight below the 3rd percentile was severe SGA. Centers for Disease Control growth charts for term and preterm (Fenton) newborns were used to determine birth weight percentiles. The term intrauterine growth restriction (IUGR) was used if medical records indicated fetal growth restriction. A twin gestation was counted as one pregnancy and was excluded from the analyses of gestational and pregnancy complications.
We compared the rates of miscarriage and elective abortion among the mothers of offspring with FA, DC, DBA and SDS with the United States (US) general population rates from the 2012 National Vital Statistics Report.11 We analyzed the occurrence of pregnancies and elective abortions prior to and following the birth of the first affected child in order to determine mothers’ reproductive choices. We compared the frequency and types of adverse events noted during pregnancy and delivery of offspring affected with an IBMFS with their unaffected siblings within each group. To determine the association of fetal problems with adverse outcomes at birth such as SGA, preterm birth and cesarean delivery, we compared the outcomes of offspring with severe syndromic features at birth against those with mild or no features within each group.
Statistical analyses were conducted using Microsoft Excel 12.0 (Microsoft, Redmond, WA, USA) and Stata 13 (StataCorp, College Station, TX, USA). Kruskal-Wallis rank sum test was used to compare continuous measures within and across multiple syndromes. Fisher’s exact or Pearson’s chi-square tests were used to analyze categorical variables, and binomial tests to compare observed versus expected distributions; P value <0.05 was statistically significant.
RESULTS
There were 56 unaffected mothers of offspring with FA, 42 of DC, 47 of DBA, and 20 of SDS (Table 1). Their ages at first and last pregnancy as well as gravidity and parity are shown in Table 1.
TABLE 1.
Obstetric data in unaffected mothers of offspring with inherited bone marrow failure syndromes
| Fanconi anemia |
Dyskeratosis congenita |
Diamond Blackfan anemia |
Shwachman Diamond syndrome |
P value across Syndromes |
|
|---|---|---|---|---|---|
| Number of mothers | 56 | 42 | 47 | 20 | |
| Pregnancy data | |||||
| Age at first pregnancy, median (range) | 25 (15-35) | 22 (17-34) | 23 (15-34) | 22.5 (16-36) | 0.3 |
| Age at last pregnancy, median (range) | 33 (22-47) | 31 (22-43) | 33 (24-47) | 33 (22-42) | 0.1 |
| Gravidity (≤20 weeks), median (range) | 3 (1-8) | 3 (1-10) | 3 (1-8) | 3 (2-6) | 1.0 |
| Parity (>20-week gestation), median (range) | 2 (1-7) | 2.5 (1-10) | 2 (1-6) | 2 (1-5) | 0.4 |
| Pregnancy outcomes | |||||
| Number of pregnancies | 196 | 152 | 161 | 66 | |
| Number of pregnancies with live births (%) | 151 (77%) | 118 (78%) | 122 (76%) | 45 (68%) | 0.5 |
| Number of twin pregnancies (%) | 2 (1%) | 3 (2%) | 5 (3%) | 1 (1.5%) | 0.5 |
| Number of ectopic pregnancies (%) | 4 (2%) | 0 | 4 (2%) | 2 (3%) | 0.3 |
| Number of stillbirths | 1 | 2 | 0 | 0 | 0.4 |
| Number of miscarriages (%) | 24 (12%) | 24 (16%) | 27 (17%) | 13 (20%) | 0.5 |
| Gestational age of miscarriages in weeks, median (range) | 8 (5-12) | 8 (4-19) | 12 (4-20) | 8 (6-15) | 0.1 |
| Number of mothers with miscarriages (%)a | 18 (32%) | 13 (31%) | 13 (28%) | 8 (40%) | 0.8 |
| Number of mothers with recurrent miscarriages (≥2) | 4 | 6 | 9 | 4 | |
| Number of elective abortions (%) | 16 (8%) | 8 (5%) | 8 (5%) | 6 (9%) | 0.5 |
| Number of mothers with elective abortions (%)a | 13 (23%) | 6 (14%) | 7 (15%) | 6 (30%) | 0.2 |
| Obstetric data in relation to affected and unaffected live births | |||||
| Number of pregnancies with affected offspring (%)b | 71 (47%) | 54 (46%) | 53 (43%) | 22 (49%) | 0.9 |
| Number of pregnancies with unaffected offspring (%)b | 80 (53%) | 64 (54%) | 69 (57%) | 23 (51%) | 0.9 |
| Number of pregnancies before 1st affected birth (%)c | 62 (32%) | 53 (35%) | 57 (35%) | 29 (44%) | 0.3 |
| Number of pregnancies after 1st affected birth (%)c | 78 (40%) | 57 (37.5%) | 57 (35%) | 17 (26%) | 0.2 |
| P value for pregnancy before/after 1st affected birth | 0.1 | 0.7 | 1.0 | 0.04 | - |
| Number of abortions before 1st affected birth (%)d | 8 (13%) | 6 (10%) | 7 (12%) | 6 (21%) | 0.7 |
| Number of abortions after 1st affected birth (%)e | 8 (10%) | 2 (4%) | 1 (2%) | 0 | 0.09 |
| P value for abortion before/after 1st affected birth | 0.79 | 0.15 | 0.06 | 0.07 | - |
| Complications in pregnancies resulting in live births | |||||
| Number of pregnancies with complications (%)b | 30 (20%) | 34 (29%) | 34 (28%) | 15 (33%) | 0.2 |
P values across syndromes are global comparing obstetric events among mothers of offspring with the four syndromes.
Denominator is the total number of mothers in each group.
Denominator is the total number of pregnancies with live births in each group.
Denominator is the total number of pregnancies in each group of mothers.
Denominator is the number of pregnancies before the 1st affected birth in each group.
Denominator is the number of pregnancies after the 1st affected birth in each group.
Pregnancy outcomes
The proportion of pregnancies resulting in live births ranged from 68% to 78% among all groups of mothers (P=0.5; Table 1) similar to the US population live birth estimates of 68% to 70%.11 The numbers of twin pregnancies, ectopic pregnancies and stillbirths were five or less per group (Table 1).
Miscarriage
The miscarriage rates ranged from 12% to 20% (Table 1) and were similar to the 15% to 25% rates in US women aged 15-44 years.11 Eighteen mothers of offspring with FA, 13 of DC, 13 of DBA, and eight of SDS reported at least one miscarriage. Four mothers of offspring with FA reported recurrent miscarriages (≥2) with two miscarriages in two and three in two. Six mothers of DC reported two (n=3), three (n=1) or four (n=2) miscarriages. Nine mothers of DBA reported two (n=5), three (n=3) or four (n=1) miscarriages, and four mothers of SDS reported two (n=3) or three (n=1) miscarriages. Recurrent miscarriage rates among the mothers of offspring with FA (7%) and DC (14%) were similar to the general population rates of 5% while the frequency of recurrent miscarriages was significantly higher among mothers of offspring with DBA (19%) and SDS (20%) (P=0.02 and 0.04, respectively).12
Mothers’ reproductive choices
The numbers of pregnancies prior to and following the birth of the first affected child were similar among mothers of offspring with FA, DC and DBA while mothers of offspring with SDS reported more pregnancies before than after the birth of the first affected child (P=0.04; Table 1).
The rates of elective abortions ranged from 5% to 9% among all groups of mothers (Table 1) similar to the 19% to 20% rates in the US women aged 15-44 years.11 Thirteen mothers of offspring with FA reported 16 elective abortions with equal numbers before and after the birth of the first affected child (Table 1). Only two mothers of offspring with FA reported terminating a subsequent pregnancy due to an affected fetus, while four others were unaware of the affected status of their previous live-born offspring at the time of elective abortion. Similarly, six of the eight elective abortions in mothers of offspring with DC, seven of eight in mothers of DBA and all six in mothers of SDS preceded the birth of an affected child, while all three elective abortions following an affected child’s birth (in mothers of DC and DBA) occurred prior to the diagnosis of the affected child. Thus, the rates of elective abortions did not increase after the birth of the first affected child (Table 1). Six mothers reported using assisted reproductive strategies to avoid having another affected child and to conceive a matched sibling donor for a stem cell transplant. These included three mothers of offspring with FA and one each of DC and DBA who had subsequent successful pregnancies through in vitro fertilization and preimplantation genetic diagnosis; one mother of offspring with FA used a sperm donor.
Complications in pregnancies resulting in live births
Twenty to 33% of viable pregnancies that ended in live births had complications (Table 1). Pregnancies with affected fetuses had significantly more problems than those with unaffected fetuses among mothers of offspring with FA, DC and DBA (28% vs 12.5%, P=0.02; 44% vs 16%, P=0.001; 49% vs 12%, P<0.001, respectively) (Table 2). The increased complication rates in pregnancies with affected fetuses were primarily related to fetal factors (discussed below). The overall predictive value of a fetus having an IBMFS was 76% in the presence of a complication among pregnancies resulting in live births. The frequency of preeclampsia, placenta previa, placenta abruption and gestational diabetes (gestational diabetes not shown) was not increased (Table 2).
TABLE 2.
Complications in pregnancies that resulted in live births of offspring with IBMFS and unaffected children
| Parameters | Fanconi anemia | Dyskeratosis congenita | Diamond Blackfan anemia | Shwachman Diamond | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pregnancies >20 weeks | Affected | Unaffected | P | Affected | Unaffected | P | Affected | Unaffected | P | Affected | Unaffected | P |
| Number | 71 | 80 | 54 | 64 | 53 | 69 | 22 | 23 | ||||
| N with complications (%) | 20 (28%) | 10 (12.5%) | 0.02 | 24 (44%) | 10 (16%) | 0.001 | 26 (49%) | 8 (12%) | <.0001 | 9 (41%) | 6 (26%) | 0.3 |
| Complication type | ||||||||||||
| Fetal distress | 11 (15%) | 2 (2.5%) | 0.007 | 14 (30%) | 2 (3%) | <0.0001 | 10 | 1 | 0.001 | 4 | 2 | 0.4 |
| Prolonged labor | 3a | 2 | 0.7 | 2 | 1 | 0.6 | 2 | 2 | 1.0 | 4 | 0 | 0.049 |
| Preterm labor | 0 | 5 | 0.06 | 4 | 6 | 0.7 | 5 | 4 | 0.5 | 1 | 0 | 0.5 |
| Preeclampsia | 1a | 0 | 0.5 | 1 | 0 | 0.5 | 1 | 1 | 1.0 | 0 | 1 | 1.0 |
| Placenta previa | 0 | 0 | - | 1 | 0 | 0.5 | 0 | 0 | - | 0 | 1 | 1.0 |
| Placenta abruption | 1a | 0 | 0.5 | 0 | 0 | - | 2 | 0 | 0.2 | 0 | 0 | - |
| Oligohydramnios | 3 | 1 | 0.3 | 1 | 0 | 0.4 | 0 | 0 | - | 0 | 0 | - |
| Polyhydramnios | 1 | 0 | 0.5 | 0 | 0 | - | 1 | 0 | 0.4 | 0 | 0 | - |
| Hematologic abnormality | 1b | 0 | 0.5 | 0 | 0 | - | 4c | 0 | 0.03 | 0 | 0 | - |
| Other complicationsd | 4 | 0 | 0.047 | 1 | 1 | 1.0 | 1 | 0 | 0.4 | 0 | 2 | 0.5 |
| Birth characteristics of offspring | ||||||||||||
| SGA (<10th percentile) | 28 (39%) | 3 (4%) | <0.0001 | 21 (39%) | 5 (8%) | <0.0001 | 8 (15%) | 2 (3%) | 0.02 | 6 (27%) | 1 (4%) | 0.1 |
| Preterm birth (<37weeks) | 8 (11%) | 3 (4%) | 0.2 | 15 (28%) | 8 (12.5%) | 0.06 | 14 (26%) | 4 (6%) | 0.002 | 4 (18%) | 1 (4%) | 0.2 |
| Cesarean delivery | 17 (24%) | 7 (9%) | 0.02 | 16 (30%) | 4 (6%) | 0.001 | 12 (23%) | 4 (6%) | 0.01 | 8 (36%) | 6 (26%) | 0.5 |
| Median gestation in weeks (range) | 40 (34-43) | 40 (34-47) | 0.03 | 38 (29-44) | 39 (30-42) | 0.1 | 40 (27-42) | 40 (32-42) | 0.09 | 39 (35-42) | 40 (35-42) | 0.5 |
| Median birthweight in Kg (range) | 2.5 (1.3-3.7) | 3.2 (1.8-4.5) | <0.0001 | 2.7 (0.48-4.1) | 3.2 (1.4-4.5) | <0.0001 | 2.7 (0.9-4.5) | 3.2 (1.8-4.2) | 0.0005 | 2.75 (1.8-3.6) | 3.2 (1.8-4.1) | 0.04 |
SGA, small for gestational age.
Five out of 11 cases of fetal distress among FA had additional factors such as prolonged labor (n = 3), preeclampsia (n = 1) and placenta abruption (n = 1) that could have contributed to fetal distress.
Low cord blood platelet count.
Severe anemia necessitating red blood cell transfusion at birth.
Other complications within each group were fetal hydrocephalus with aqueduct stenosis (n = 2), cessation of fetal growth (n = 2) in Fanconi anemia; incompetent cervix in 1 mother with 1 affected and 1 unaffected pregnancy in dyskeratosis congenita; high grade lymphoma in one unaffected mother of an offspring with genetically proven Diamond Blackfan anemia; and, nuchal cord in 1 and infection in 1 pregnancy each in 2 mothers of offspring with Shwachman Diamond syndrome.
Fanconi anemia:
Eleven out of 71 (15%) offspring with FA developed fetal distress at 34–41 weeks of gestation compared with two out of 80 (2.5%) unaffected offspring (P=0.007; Table 2). Six affected cases with fetal distress had severe IUGR and were born SGA, while in three cases, fetal distress developed in association with prolonged labor, and one each occurred in the setting of preeclampsia or placental abruption. Ultrasound findings identified important aspects of pregnancies with affected fetuses, such as oligohydramnios in three with fetal renal malformations, polyhydramnios in one with fetal duodenal atresia and hydrocephalus with aqueductal stenosis in three.
Thirty-nine percent of affected fetuses with FA were reported to have IUGR and were born SGA compared with 4% of unaffected fetuses (P<0.0001; Table 2). The gestational ages as well as the birth weights of the affected offspring were lower than those of their unaffected siblings (P=0.03, P<0.0001, respectively; Table 2). Cesarean delivery for fetal problems was more frequent for offspring with FA than their unaffected siblings (P=0.02), and included nine emergency cesareans for fetal distress (two of the 11 with fetal distress were delivered by forceps) and eight planned cesareans for oligohydramnios (n=2), hydrocephalus (n=2), cessation of fetal growth (n=2), polyhydramnios (n=1), and low fetal platelet count (n=1).
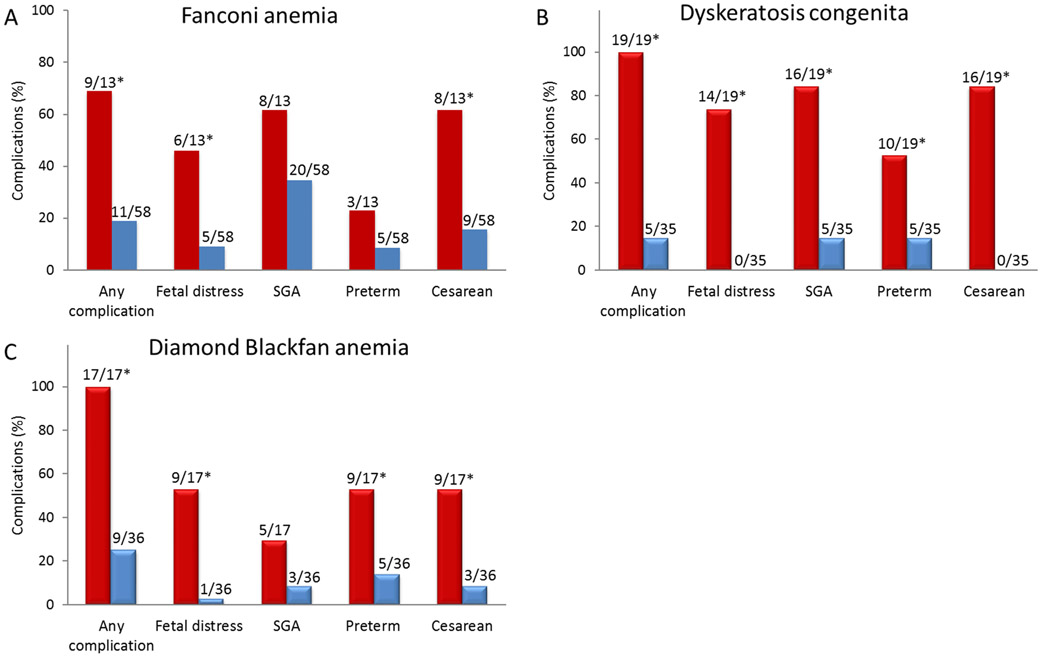
Fetal malformations involving radial ray, kidneys, gastrointestinal tract, cardiac defects, and hydrocephalus were noted at birth in 33 of 71 (46%) offspring with FA (Table 3). Thirteen had three or more malformations consistent with VACTERL-H phenotype (vertebral anomaly, anal atresia, congenital heart disease, tracheo-esophageal fistula, renal, limb anomalies, and hydrocephalus).13 FA was suspected and diagnosed in seven neonates with multiple physical abnormalities. Newborns with FA with three or more VACTERL-H type malformations were more likely to have fetal distress (46% vs 9%; P=0.003) and be delivered by cesarean (62% vs 16%; P=0.001) than those with none or fewer than three malformations (Fig. 1A). Ten of the 13 offspring (77%) with VACTERL-H malformations required surgical intervention and neonatal intensive care support at birth compared with two out of 20 (10%) with one or two malformations (P<0.0001; Table 3).
TABLE 3.
Malformations in offspring with Fanconi anemia and mothers’ pregnancy events
| Fetal malformations | Total number |
Pregnancy complication |
SGA | Preterm birth |
Cesarean | Surgery at birth |
|---|---|---|---|---|---|---|
| No malformations | 38 | 3 | 9 | 1 | 5 | 0 |
| Malformation affecting 1 organ | 7 | 1 | 2 | 1 | 1 | 0 |
| Radial raya (n=4); kidney (n=2); cardiac (n=1) | ||||||
| Malformations affecting 2 organs | 13 | 3 | 9 | 3 | 3 | 2 |
| Radial ray + kidney (n=9, or + cardiac (n=2), or + GI (n=1) | ||||||
| Cardiac + upper GI (n=1) | ||||||
| Malformations affecting ≥3 systemsb | 13 | |||||
| Radial ray + kidney + brain (n=5) | 3 | 3 | 1 | 3 | 2 | |
| Radial ray + kidney + GI (n=2) | 0 | 1 | 0 | 0 | 2 | |
| Radial ray + kidney + GI + brain (n=1) | 1 | 0 | 1 | 0 | 1 | |
| Radial ray + kidney + cardiac + GI (n=2) | 2 | 1 | 1 | 2 | 2 | |
| Radial ray + cardiac + GI + brain (n=1) | 1 | 1 | 0 | 1 | 1 | |
| Radial ray + kidney + cardiac + GI + brain (n=2) | 2 | 2 | 0 | 2 | 2 |
GI, gastrointestinal malformations including anal atresia (n = 4), duodenal atresia (n = 1), jejunal atresia (n = 1), tracheoesophageal fistula (n = 1), and pyloric stenosis (n = 1).
Radial ray refers to malformation affecting thumb with or without radial bone (8 patients also had radial bone hypoplasia).
Malformations affecting three or more organ systems consistent with VACTERL-H phenotype (vertebral anomalies, anal atresia, congenital heart disease, tracheo-esophageal fistula, renal, limb anomalies, and hydrocephalus).12
Figure 1.
Complications in pregnancies with affected offspring. *P<0.01 (A) Fanconi anemia: Children with ≥3 physical malformations consistent with VACTERL-H phenotype (Red bars) had significantly more perinatal complications (P=0.001), non-reassuring fetal status (fetal distress) (P=0.003) and cesarean delivery for complications (P=0.001) than in those with 0-2 malformations (Blue bars). SGA and preterm birth occurred with similar frequency in children with ≥3 or 0-2 malformations. B) Dyskeratosis congenita: Offspring with Hoyeraal-Hreidarsson syndrome or Revesz syndrome (Red bars) had significantly more complications (P<0.0001), fetal distress (P<0.0001), SGA (P<0.0001), preterm birth (P=0.004) and cesarean delivery for complications (P<0.0001) than those with other dyskeratosis congenita types (Blue bars). C) Diamond Blackfan anemia: Affected offspring with anemia at birth (Red bars) had significantly more complications (P<0.0001), fetal distress (P<0.0001), preterm birth (P=0.004) and cesarean delivery for complications (P=0.001) than those without anemia (Blue bars) while the frequency of SGA was similar.
Dyskeratosis congenita:
The increase in adverse pregnancy events among mothers of offspring with DC was primarily due to the higher frequency of fetal distress in affected than in unaffected fetuses (30% vs 3%, P<0.001; Table 2).
All 14 cases of fetal distress were noted in offspring who were later identified to have Hoyeraal-Hreidarsson (HH) or Revesz syndrome (RS) variants of DC (Fig. 1B), and all needed neonatal intensive care support at birth. HH is characterized by the presence of IUGR, microcephaly, cerebellar hypoplasia, bone marrow failure and immune deficiency in infancy and early childhood and RS by exudative retinopathy.8, 14 Sixteen of the 19 affected fetuses (84%) with HH (n=17) or RS (n=2) had IUGR and were born SGA compared with five out of 35 affected fetuses (14%) with other DC types (P<0.001; Fig. 1B). Offspring with HH or RS were also more likely to be born preterm (53% vs 14%; P=0.004) and by cesarean (84% vs 0; P<0.0001) than those with other DC types (Fig. 1B). Placental insufficiency was reported in all four cases of HH on whom we had records; two had small placentas (weight ≤200 grams) with multiple infarcts and increased syncytial knots.
Diamond Blackfan anemia:
Pregnancies with fetuses affected with DBA were more likely to have complications than pregnancies with unaffected fetuses primarily due to fetal hypoxia (19% vs 1%; P=0.001; Table 2) from severe anemia (two had hydrops fetalis) in the affecteds that necessitated emergency preterm delivery and red blood cell transfusions at birth. An additional seven offspring with DBA were born anemic but without fetal distress and four were transfused at birth. Offspring with DBA were more likely to be SGA (15% vs 3%; P=0.02), preterm (26% vs 6%; P=0.002) and delivered by cesarean for fetal problems (23% vs 6%; P = 0.01) than unaffected offspring, and had lower median birth weight (2.7 kilograms vs 3.2 kilograms; P=0.0005) (Table 2).
Analyses of the outcomes of pregnancies with only affected offspring showed that those with anemia (n=17) were more likely to have experienced fetal distress (53% vs 3%; P<0.0001), preterm births (53% vs 14%; P 0.004) and cesarean delivery (53% vs 8%; P 0.001) compared with non-anemic (n=36) offspring with DBA (Fig. 1C).
Shwachman Diamond syndrome:
The frequency of complications among mothers of offspring with SDS was similar for pregnancies with affected and unaffected fetuses (P=0.3; Table 2). However, pregnancies with affected fetuses more commonly had prolonged labor associated with fetal distress (P=0.049; Table 2) and lower median birth weight than those with unaffected fetuses (P=0.04). No specific phenotype associated with pregnancy complications was identified in the offspring with SDS.
DISCUSSION
High frequencies of adverse obstetric events have been described in maternal pregnancies carrying fetuses with rare genetic diseases such as trichothiodystrophy or complex congenital anomalies.15,16 Higher than expected adverse obstetric events were also reported in women who themselves had FA, DBA or SDS.17-20 Here, we report the outcomes of pregnancies in the unaffected mothers of offspring with an IBMFS. We found increased rates of syndrome-specific complications in pregnancies carrying fetuses affected with an IBMFS compared with unaffected fetuses within each syndrome group. The complications were primarily due to fetal factors and resulted in high frequency of SGA, preterm and cesarean births, often with fetal distress. The adverse events were more common in pregnancies with severely affected fetuses; these included fetuses with VACTERL-H malformations in FA, HH or RS phenotypes in DC, and fetuses with severe anemia in DBA (Fig. 1).
The overall rates of miscarriage within each group of mothers were not higher than the general population rates of 15-25%.11 The miscarriage rates in our study may be an underestimate since early first trimester miscarriages may not be recognized clinically and thus underreported. The recurrent miscarriage rates among mothers of offspring with DC did not reach the level of significance possibly due to small sample size. The higher than expected frequency of recurrent miscarriages12 in mothers of offspring with DBA and SDS does suggest the loss of severely affected non-viable fetuses. We did not know the affected status of miscarried or stillbirths, but reports of embryonic lethality in some mouse models of FA, DBA and DC support our assumption.21-23 The numbers of stillbirths in our study were small; however, intrauterine death due to hydrops fetalis in DBA and perinatal death due to severely affected fetuses with FA and SDS has been reported by others.3,6,7
Our hypothesis that parents of children with an IBMFS may opt to terminate a subsequent affected pregnancy was not confirmed. While underreporting of elective abortions cannot be excluded, it is likely that many offspring with IBMFS were diagnosed only after they or their affected siblings developed characteristic symptoms and/or when specific diagnostic and confirmatory tests became available.1 More recently, next-generation sequencing technologies have facilitated a more rapid and earlier diagnosis of these syndromes.2 Parents may choose to abort an affected pregnancy or may choose assisted reproductive therapies to conceive an unaffected child who is also a tissue match and thus can be a hematopoietic stem cell donor for the affected child,24 as was noted in some families in our cohort.
We observed a high frequency of adverse events among viable pregnancies (>20-week gestation) with affected fetuses. Nearly 40% of offspring with FA had IUGR and were born SGA, although fetal growth restriction is known to affect only about 5% of newborns in the general population.25 Likewise, almost 50% of offspring with FA had physical abnormalities at birth, similar to those reported in the literature.1 The high frequency of complications in pregnancies with fetuses affected with FA are likely the consequence of genetic mutations in the FA DNA repair pathway that are antecedents of disordered developmental processes resulting in fetal malformations, placental insufficiency and IUGR. We did not have records of placental pathology, but previous publications have described smaller than normal placentas in pregnancies carrying fetuses with FA.26 Newborns in our FA cohort with three or more features of VACTERL-H13 were the ones with the most frequent adverse events and often required cesarean delivery and neonatal intensive care support (Fig. 1 and Table 3), consistent with a severe prenatal disease manifestation. Identification of an FA-type malformation in utero,1 particularly VACTERL-H,13 should alert the fetal-maternal specialist to consider FA testing to guide families in making informed decision regarding continuation or termination of an affected pregnancy.
DC is a disorder of telomere biology with a wide spectrum of clinical manifestations.1,14,27 HH and RS represent the severe end of this spectrum and are associated with the shortest telomeres compared with other DC types.28 All pregnancies with fetuses with HH and RS in our study were associated with complications with markedly high rates of IUGR, and fetal distress that often developed during early third trimester leading to emergency cesarean deliveries, frequently of severely preterm newborns. Previous studies have linked fetal growth restriction and placental pathology with reduced levels of telomerase activity in placentas.29 Placental insufficiency was noted in all four cases of HH on whom we had placental pathology reports. We postulate that extremely short telomeres in the most severely-affected fetuses would be accompanied by compromised placental telomere length, causing attenuated placental development with resultant IUGR and fetal distress.
The high complication rates in pregnancies with fetuses with DBA were a consequence of fetal hypoxia due to anemia (detected at birth) prompting emergency preterm cesarean deliveries. While severely anemic fetuses can end in first trimester miscarriages,3,4 pregnancies that continue with anemic fetuses are at increased risk of premature delivery and may require intrauterine fetal transfusions.30 None of the affected offspring in our study received intrauterine transfusions, perhaps because anemia was only recognized at delivery or prompted preterm delivery of a hypoxic fetus. Ultrasound measurement of increased Doppler blood flow velocity in the middle cerebral artery is a simple way to identify fetal anemia in suspected cases.31 Intrauterine transfusions and subsequent planned delivery has been used effectively in severely anemic fetuses with DBA.30
The lack of a specific SDS phenotype associated with pregnancy complications in our cohort may be explained by the small sample size of mothers with offspring with SDS. Published case reports of newborns with SDS suggest prenatal complications due to poor fetal growth, malformations and cytopenia, leading to fetal distress and preterm delivery of severely affected neonates.32,33
The high positive predictive value of identifying an IBMFS in a neonate with an adverse event in our study is biased by consideration of pregnancies in mothers of offspring with an IBMFS and might not be true in the general population where the prevalence of an IBMFS is extremely rare. However, it is important to note that affected fetuses give clues to their diagnoses. Other limitations include biased enrollment due to volunteerism, self-reported and retrospective data leading to potential inaccuracies in mothers’ pregnancy details. This limitation was mitigated in many cases by review of the child’s medical records from birth. Although we excluded the mothers with an AD disease-specific gene mutation or syndrome-specific positive diagnostic test, it is possible that some women labeled “unaffected” may be silent carriers of yet unidentified AD genes and may have been erroneously included in this group. The affected status of miscarried or aborted fetuses was not known.
The strengths of our study are the inclusion of unaffected mothers of offspring with all four major IBMFS and evaluation of comprehensive reproductive histories on affected as well as unaffected pregnancies. Our overall sample size was relatively large considering the rarity of these syndromes. The genetic diagnosis was established by syndrome-specific positive diagnostic test in all affected offspring9 and confirmed by identification of specific mutated genes in more than 90% of the offspring with recessive disorders and in over 60% with dominant inheritance.
Our study documents that pregnancies in unaffected mothers of offspring with an IBMFS have high rates of complications due to genetic abnormalities in affected fetuses that contribute to fetal growth restriction, malformations and anemia, with resultant fetal distress, cesarean and preterm deliveries. The more severely affected fetuses experience the worst outcomes. Pediatric hematologists involved in the care of patients with IBMFS should inform the parents of the increased risk of abnormal reproductive outcomes for future affected pregnancies. The parents need genetic counseling to help them make informed decisions. Subsequent pregnancies in mothers of offspring with an IBMFS require assessment for affected fetuses and management by maternal-fetal medicine specialists.
ACKNOWLEDGEMENTS
We are grateful to all the participants in the National Cancer Institute inherited bone marrow failure syndromes cohort and to the physicians who referred the patients. We thank Lisa Leathwood, RN, Maureen Risch, RN, and Ann Carr, MS, CGC from the Westat Inherited Bone Marrow Failure Syndromes team for their assistance in conducting this study. This research was funded by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute (N.G., B.P.A., S.A.S., H.D.R.) National Institutes of Health clinical trial NCT-00027274, Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development (P.S), and by contract HHSN261201100018C with Westat for data management. N.G and B.P.A have full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
ABBREVIATIONS KEY
- AD
Autosomal dominant
- AR
Autosomal recessive
- DBA
Diamond Blackfan anemia
- DC
Dyskeratosis congenita
- FA
Fanconi anemia
- HH
Hoyeraal-Hreidarsson syndrome
- IBMFS
Inherited bone marrow failure syndrome
- IUGR
Intrauterine growth restriction
- RS
Revesz syndrome
- SDS
Shwachman Diamond syndrome
- SGA
Small for gestational age
- VACTERL-H
Vertebral anomaly, Anal atresia, Congenital heart disease, Tracheo-esophageal fistula, Renal, Limb anomalies, and Hydrocephalus
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
REFERENCES
- (1).Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev 2010;24(3):101–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Wegman-Ostrosky T, Savage SA. The genomics of inherited bone marrow failure: from mechanism to the clinic. Br J Haematol 2017;177(4):526–542. [DOI] [PubMed] [Google Scholar]
- (3).Da CL, Chanoz-Poulard G, Simansour M et al. First de novo mutation in RPS19 gene as the cause of hydrops fetalis in Diamond-Blackfan anemia. Am J Hematol 2013;88(4):340–341. [DOI] [PubMed] [Google Scholar]
- (4).Souka AP, Bower S, Geerts L, Huggon I, Nicolaides KH. Blackfan-Diamond anemia and dyserythropoietic anemia presenting with increased nuchal translucency at 12 weeks of gestation. Ultrasound Obstet Gynecol 2002;20(2):197–199. [DOI] [PubMed] [Google Scholar]
- (5).Merrill A, Rosenblum-Vos L, Driscoll DA, Daley K, Treat K. Prenatal diagnosis of Fanconi anemia (Group C) subsequent to abnormal sonographic findings. Prenat Diagn 2005;25(1):20–22. [DOI] [PubMed] [Google Scholar]
- (6).Nishimura G, Nakashima E, Hirose Y et al. The Shwachman-Bodian-Diamond syndrome gene mutations cause a neonatal form of spondylometaphysial dysplasia (SMD) resembling SMD Sedaghatian type. J Med Genet 2007;44(4):e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Vetro A, Iascone M, Limongelli I et al. Loss-of-Function FANCL Mutations Associate with Severe Fanconi Anemia Overlapping the VACTERL Association. Hum Mutat 2015;36(5):562–568. [DOI] [PubMed] [Google Scholar]
- (8).Glousker G, Touzot F, Revy P, Tzfati Y, Savage SA. Unraveling the pathogenesis of Hoyeraal-Hreidarsson syndrome, a complex telomere biology disorder. Br J Haematol 2015;170(4):457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Alter BP, Giri N, Savage SA et al. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol 2010;150(2):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Mirabello L, Khincha PP, Ellis SR et al. Novel and known ribosomal causes of Diamond-Blackfan anaemia identified through comprehensive genomic characterisation. J Med Genet 2017;54(6):417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ventura SJ, Curtin SC, Abma JC, Henshaw SK. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep 2012;60(7):1–21. [PubMed] [Google Scholar]
- (12).Ford HB, Schust DJ. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol 2009;2(2):76–83. [PMC free article] [PubMed] [Google Scholar]
- (13).Alter BP, Giri N. Thinking of VACTERL-H? Rule out Fanconi Anemia according to PHENOS. Am J Med Genet A 2016;170(6):1520–1524. [DOI] [PubMed] [Google Scholar]
- (14).Savage SA. Human telomeres and telomere biology disorders. Prog Mol Biol Transl Sci 2014;125:41–66. [DOI] [PubMed] [Google Scholar]
- (15).Tamura D, Merideth M, DiGiovanna JJ et al. High-risk pregnancy and neonatal complications in the DNA repair and transcription disorder trichothiodystrophy: report of 27 affected pregnancies. Prenat Diagn 2011;31(11):1046–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Witters I, Legius E, Devriendt K et al. Pregnancy outcome and long term prognosis in 868 children born after second trimester amniocentesis for maternal serum positive triple test screening and normal prenatal karyotype. J Med Genet 2001;38(5):336–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Alter BP, Frissora CL, Halperin DS et al. Fanconi's anaemia and pregnancy. Br J Haematol 1991;77(3):410–418. [DOI] [PubMed] [Google Scholar]
- (18).Nabhan SK, Bitencourt MA, Duval M et al. Fertility recovery and pregnancy after allogeneic hematopoietic stem cell transplantation in Fanconi anemia patients. Haematologica 2010;95(10):1783–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Faivre L, Meerpohl J, Da CL et al. High-risk pregnancies in Diamond-Blackfan anemia: a survey of 64 pregnancies from the French and German registries. Haematologica 2006;91(4):530–533. [PubMed] [Google Scholar]
- (20).Alter BP, Kumar M, Lockhart LL, Sprinz PG, Rowe TF. Pregnancy in bone marrow failure syndromes: Diamond-Blackfan anaemia and Shwachman-Diamond syndrome. Br J Haematol 1999;107(1):49–54. [DOI] [PubMed] [Google Scholar]
- (21).Kamimae-Lanning AN, Goloviznina NA, Kurre P. Fetal origins of hematopoietic failure in a murine model of Fanconi anemia. Blood 2013;121(11):2008–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Morgado-Palacin L, Varetti G, Llanos S, Gomez-Lopez G, Martinez D, Serrano M. Partial Loss of Rpl11 in Adult Mice Recapitulates Diamond-Blackfan Anemia and Promotes Lymphomagenesis. Cell Rep 2015;13(4):712–722. [DOI] [PubMed] [Google Scholar]
- (23).He J, Navarrete S, Jasinski M et al. Targeted disruption of Dkc1, the gene mutated in X-linked dyskeratosis congenita, causes embryonic lethality in mice. Oncogene 2002;21(50):7740–7744. [DOI] [PubMed] [Google Scholar]
- (24).Kahraman S, Beyazyurek C, Yesilipek MA et al. Successful haematopoietic stem cell transplantation in 44 children from healthy siblings conceived after preimplantation HLA matching. Reprod Biomed Online 2014;29(3):340–351. [DOI] [PubMed] [Google Scholar]
- (25).Chernausek SD. Mendelian genetic causes of the short child born small for gestational age. J Endocrinol Invest 2006;29(1 Suppl):16–20. [PubMed] [Google Scholar]
- (26).Wunder E, Burghardt U, Lang B, Hamilton L. Fanconi's anemia: anomaly of enzyme passage through the nuclear membrane? Anomalous intracellular distribution of topoisomerase activity in placental extracts in a case of Fanconi's anemia. Hum Genet 1981;58(2):149–155. [DOI] [PubMed] [Google Scholar]
- (27).Bertuch AA. The molecular genetics of the telomere biology disorders. RNA Biol 2016;13(8):696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Alter BP, Rosenberg PS, Giri N, Baerlocher GM, Lansdorp PM, Savage SA. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica 2012;97(3):353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Davy P, Nagata M, Bullard P, Fogelson NS, Allsopp R. Fetal growth restriction is associated with accelerated telomere shortening and increased expression of cell senescence markers in the placenta. Placenta 2009;30(6):539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Zhang EG, Regan F, Layton M et al. Managing the difficult case of fetal anemia. J Matern Fetal Neonatal Med 2011;24(12):1498–1503. [DOI] [PubMed] [Google Scholar]
- (31).Mari G, Deter RL, Carpenter RL et al. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative Group for Doppler Assessment of the Blood Velocity in Anemic Fetuses. N Engl J Med 2000;342(1):9–14. [DOI] [PubMed] [Google Scholar]
- (32).Black LV, Soltau T, Kelly DR, Berkow RL. Shwachman-Diamond syndrome presenting in a premature infant as pancytopenia. Pediatr Blood Cancer 2008;51(1):123–124. [DOI] [PubMed] [Google Scholar]
- (33).Saito-Benz M, Miller HE, Berry MJ. Shwachman-Diamond syndrome (SDS) in a preterm neonate. J Paediatr Child Health 2015;51(12):1228–1231. [DOI] [PubMed] [Google Scholar]