Abstract
Bilayer hydrogels with a soft cartilage-like layer and a stiff bone-like layer embedded with human mesenchymal stem cells (hMSCs) are promising for osteochondral tissue engineering. The goals of this work were to evaluate the effects of dynamic compressive loading (2.5% applied strain, 1 Hz) on osteogenesis in the stiff layer and spatially map local mechanical responses (strain, stress, hydrostatic pressure and fluid velocity). A bilayer hydrogel was fabricated from soft (24 kPa) and stiff (124 kPa) poly(ethylene glycol) hydrogels. With hMSCs embedded in the stiff layer, osteogenesis was delayed under loading evident by lower OSX and OPN expressions, alkaline phosphatase activity, and collagen content. At day 28, mineral deposits were present throughout the stiff layer without loading, but localized centrally and near the interface under loading. Local strains mapped by particle tracking showed substantial equivalent strain (~1.5%) transferring to the stiff layer. When hMSCs were cultured in stiff single-layer hydrogels subjected to similar strains, mineralization was inhibited. Finite element analysis revealed that hydrostatic pressures ≥~600 Pa correlated to regions lacking mineralization in both hydrogels. Fluid velocities were low (~1–10 nm/s) in the hydrogels with no apparent correlation to mineralization. Mineralization was recovered by inhibiting ERK1/2, indicating cell-mediated inhibition. These findings suggest that high strains (~1.5%) combined with higher hydrostatic pressures negatively impact osteogenesis, but in a manner that depends on the magnitude of each mechanical response. This work highlights the importance of local mechanical responses in mediating osteogenesis of hMSCs in bilayer hydrogels being studied for osteochondral tissue engineering.
Keywords: Osteochondral, bilayer hydrogel, mesenchymal stem cells, osteogenesis, dynamic loading, compressive strain, hydrostatic pressure
Graphical Abstract

1. INTRODUCTION
Osteochondral tissues in articulating joints are composed of soft articular cartilage that is connected to the stiff subchondral bone plate through a zone of calcified cartilage (Hoemann, Lafantaisie-Favreau, Lascau-Coman, Chen, & Guzman-Morales, 2012). During physical activity, the articular cartilage protects the underlying subchondral bone plate by absorbing the loads and undergoing high strains while minimizing strain transfer into the bone. Articular cartilage, however, is susceptible to injury and in severe cases can span into the subchondral bone (Hjelle, Solheim, Strand, Muri, & Brittberg, 2002). Therapies to treat osteochondral defects require simultaneous repair of the cartilage and bone accompanied by recapitulating the strain environment to restore joint function. Osteochondral autograft transfer system, or OATS, can achieve this repair, but is invasive. While other less invasive strategies (e.g., microfracture) can facilitate bone regeneration, they fail to produce hyaline cartilage (Makris, Gomoll, Malizos, Hu, & Athanasiou, 2015). Thus, new strategies are needed to regenerate both the articular cartilage and bone.
Tissue engineering that delivers autologous mesenchymal stem cells (MSCs) is one strategy that holds promise for regenerating osteochondral tissues (Yang, Zhang, Yue, & Khademhosseini, 2017). In particular, bilayer hydrogels have emerged as a promising platform for osteochondral tissue engineering due to their high water content, tunable biochemical cues and stiffness in each layer, and in situ forming capabilities to deliver cells minimally invasively. Examples of bilayer hydrogels include synthetic polymers that combine hyaluronic acid or chondroitin sulfate into the chondral layer and hydroxyapatite particles embedded into the bone layer (Radhakrishnan, Manigandan, Chinnaswamy, Subramanian, & Sethuraman, 2018; Schutz, Despang, Lode, & Gelinsky, 2014). Other studies have varied both the biochemical cues and the stiffness within each hydrogel layer (Steinmetz, Aisenbrey, Westbrook, Qi, & Bryant, 2015). Collectively, these and other studies demonstrate the potential of bilayer hydrogels to promote a specific differentiation fate and neo-tissue deposition within each layer by tuning the local biochemical cues and/or hydrogel stiffness.
The dynamic mechanical loading environment is also important to consider in osteochondral tissue engineering. Current efforts place a large emphasis on understanding the effects of dynamic loading on the chondral layer with a few studies investigating its effects on the bone layer (Goldman & Barabino, 2016; S. Lin et al., 2017; Scholtes et al., 2018; Steinmetz et al., 2015). However, the bone layer will also experience dynamic loads, which can result in a range of mechanical responses that include strain, stress, fluid flow, and hydrostatic pressure. For example, strain will be transferred from the chondral layer to the bone layer at a magnitude that will depend on the relative stiffness of the cartilage and bone layers. Studies have shown that dynamic microstrains when applied to MSCs in two-dimensional cultures have a positive effect, leading to increases in expression of early osteogenic genes like RUNX2 and alkaline phosphatase activity (Friedl et al., 2007; Guo et al., 2015). However, if the strains are too high, they can inhibit osteogenesis (Horner et al., 2018; Steinmetz & Bryant, 2011). Dynamic movement of the extravascular fluid in the bone matrix will also be induced under dynamic loading, producing interstitial fluid flow around the bone cells. A number of studies have reported positive effects on osteogenesis of MSCs subjected to fluid flow, upregulating osteogenic genes (e.g., osteopontin) and leading to increases in early osteogenic activity such as alkaline phosphatase activity (Fritton & Weinbaum, 2009; Kim et al., 2014; Yourek, McCormick, Mao, & Reilly, 2010). Several studies have reported a positive effect of cyclic hydrostatic pressure on osteogenesis and bone growth (Henstock, Rotherham, Rose, & El Haj, 2013; Huang & Ogawa, 2012). Understanding the complex loading environment within the bone layer and its effect on MSC differentiation are critical to developing a successful osteochondral tissue engineering strategy in articulating joints.
The overall goal of this study was to investigate the effects of dynamic compressive loading on the osteogenic capabilities of human MSCs (hMSCs) when embedded in the stiff layer of a bilayer hydrogel containing soft and stiff layers. The bilayer hydrogel was designed from crosslinked poly(ethylene glycol) (PEG) following methods previously developed (Aziz et al., 2016). The goals of this study were two-fold. The first goal was to investigate the osteogenic capacity of hMSCs when embedded in the stiff layer of a bilayer hydrogel containing an acellular soft layer, subjected to an applied dynamic compressive strain and cultured for four weeks. The second goal was to characterize the mechanical environment (i.e., matrix strain, equivalent stress, hydrostatic pressure, and fluid velocity) in a bilayer hydrogel under an applied dynamic compressive strain using a combination of experimental and finite element (FE) analyses. Follow-up studies were performed using single-layer stiff hydrogels to probe load-induced cell signaling response as a function of the applied dynamic compressive strain, with a focus on ERK1/2 signaling. ERK1/2 signaling was chosen for its known role in mediating osteogenesis (Schindeler & Little, 2006) and its known involvement in mechanotransduction (Iqbal & Zaidi, 2005). Findings from this study demonstrate that dynamic compressive loading of bilayer hydrogels with soft and stiff layers produces a highly complex loading environment that leads to spatial differences in matrix strain, equivalent stress, hydrostatic pressure, and fluid flow in the stiff layer, which are uniquely different from single-layer hydrogels and which differentially affect the osteogenic capacity of embedded hMSCs.
2. MATERIALS AND METHODS
2.1. Monomer Synthesis
An 8-arm PEG with terminal amines (20,000 g/mol; JenKem Technology USA, Plano, TX) was functionalized with norbornenes by reacting 5-norbornene-2-carboxylic acid with 2-(1H-7-azabenzotriazol-1-yl)-1,1,3,3-tetramethyl uranium hexafluorophosphate methanaminium (HATU) (Chem-Impex International, Inc., Wool Dale, IL), and N,N-diisopropylethylamine (DIPEA) (Chem-Impex) in dimethylformamide (DMF)/ dichloromethane (DCM). The reaction proceeded overnight at room temperature under argon. The product was precipitated in diethyl ether, filtered, dialyzed, and lyophilized. The extent of conjugation of norbornene to each arm of the 8-arm PEG-amine was determined to be 92% using 1H-NMR. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
2.2. Fabrication of Hydrogels
A precursor solution consisting of 8-arm PEG-norbornene monomer, PEG-dithiol crosslinker (1000 g/mol) and 0.05% (w/w) photoinitiator, 1-(4-(2- hydroxyethoxy)-phenyl)-2-hydroxy-2-methyl-1-propane-1-one (I2959; BASF, Tarrytown, NY), in phosphate-buffered saline (PBS) was photopolymerized by ultraviolet light (10 min, 5–10 mW/cm2, 352 nm). For the cell studies, CRGDS (2 mM) was added to the precursor solution for the stiff hydrogel. All experiments used a 1:1 thiol:ene ratio. The PEG-norbornene was 27% (w/w) for the ‘stiff’ layer and 6.0% (w/w) for the soft layer. Hydrogels were formed in a cylindrical geometry of 3 mm height and a diameter of 3 mm (single layer) from a single precursor solution. For bilayer cylindrical hydrogels, half-height (i.e., 2.5 mm) hydrogels with a diameter of 5 mm were fabricated from one precursor solution that was polymerized followed by a second precursor solution (equivalent to 2.5 mm height) that was carefully deposited on top of the first layer and immediately polymerized.
2.3. Mechanical Testing of PEG Hydrogels
Cylindrical acellular single-layer PEG hydrogels (3 mm height and 3 mm diameter) were formed (see Section 2.2) and subjected to compressive testing conducted on a Mechanical Testing System (MTS Insight II; Eden Prairie, MN) equipped with a 2 N load cell. Equilibrium swollen hydrogels (n=3/group) were submerged in PBS and compressed to 15% strain in unconfined compression at a constant strain rate of 2 mm/s and then held for three hours. The instantaneous elastic modulus for the hydrogel was calculated from the slope of the true stress-strain curve, assuming an instantaneous Poisson’s ratio, = 0.5 (Bryant & Anseth, 2005). The true modulus is an intrinsic mechanical response and appropriate for large (i.e., > 5%) strains. Permeability (k) was determined by fitting the force relaxation response to an analytical solution for poroelastic unconfined compression (Armstrong, Lai, & Mow, 1984). Time-dependent effects were assumed to be due to poroelasticity. The polymer solid (s) skeleton’s Poisson’s ratio was assumed as = 0.0, which is supported by the observation that the long-term force dissipated to nearly two-thirds of the instantaneous force (Armstrong et al., 1984). The polymer solid skeleton elastic modulus () was determined to be two-thirds of the instantaneous modulus. The crosslinked polymer solid is assumed to be elastic and incompressible in the unconfined compression studies. The fluid, which is water, is assumed incompressible.
2.4. MSC Culture and Encapsulation
Primary human MSCs from one donor were obtained from Texas A&M University Health Science Center and College of Medicine, Institute for Regenerative Medicine. MSCs were cultured in growth media consisting of Modified Essential Medium (MEM) α (Gibco) supplemented with 10% FBS (Atlanta Biologicals), 5 ng/mL recombinant human basic-FGF (Peprotech), and penicillin/streptomycin/amphotericin B (PSF, Invitrogen). The cells were grown under standard cell culture conditions in a regulated incubator at 37°C with 5% CO2. Cells were plated at 3000 cells/cm2 in tissue culture polystyrene flasks and expanded to ~80–90% confluency until passage 4. Medium was changed thrice weekly.
hMSCs were encapsulated into the first stiff layer of bilayer hydrogels or into the single layer hydrogels at 20×106 cells/mL. Hydrogels were cultured in osteogenic differentiation media (ODM) consisting of α-Modified Essential Medium (α-MEM) (Gibco) supplemented with 10% FBS (Atlanta Biologicals), 100 nM dexamethasone (Sigma-Aldrich), 50 μg/mL ascorbate-2-phosphate, 1x MEM non-essential amino acids (Gibco), 10 mM β-glycerophosphate and penicillin/streptomycin/amphotericin B (PSF, Invitrogen). Hydrogels were cultured under standard cell culture conditions in unloaded or loaded environments with differentiation media changed thrice weekly. Unloaded and loaded hydrogels were removed at 1, 7, 14 and 28 days for analysis. Cell viability was assessed on day 1 by live/dead assay based on Calcein AM and ethidium homodimer. In the single layer experiments, medium for the high strain (i.e., 1.5%) condition was also supplemented with 10 μM PD98059, a commercially available small molecule inhibitor of MEK-1 activation (Pang, Sawada, Decker, & Saltiel, 1995), which is involved in the MAP kinase cascade.
2.5. Dynamic Compressive Loading
Bilayer cylindrical hydrogels were placed individually in 24-well plates and cultured under free-swelling for 24 hours. One set of bilayer hydrogels was placed in a custom-built bioreactor system (Villanueva, Hauschulz, Mejic, & Bryant, 2008). Each well of the bioreactor is equipped with porous platens and a porous base made from Porex®, high density polyethylene containing 40–70 μm pores per manufacturer (Nicodemus, Shiplet, Kaltz, & Bryant, 2009). The resultant loads imparted by the weight of the platen were removed prior to the start of the experiment. Constructs were subjected to an intermittent dynamic loading regime applied from 0 to 2.5% strain in a sinusoidal waveform at a frequency of 1 Hz for 1 hr/day for 28 days. This amplitude strain was selected based on previous findings, which found that 5% amplitude strains at 1 Hz stimulates chondrogenesis of hMSCs (Aisenbrey & Bryant, 2016). The other set of bilayer hydrogels were cultured under free-swelling conditions separate from the bioreactor and not subjected to strain. Single layer cylindrical hydrogels were cultured individually in 24-well plates and under free-swelling conditions for 24 hours and then placed in the bioreactor and subjected to either 0.15% or 1.5% amplitude strains at 1 Hz for 1 hr/day.
2.6. Gene Expression
Pre-encapsulated MSCs and hydrogel specimens were removed from culture at predetermined time points were placed in TRK lysis buffer (Omega), frozen in liquid nitrogen and stored at −80°C. RNA was isolated using E.Z.N.A. microelute kit (Omega) per the manufacturer instructions. The amount of pure RNA was quantified using a Nanodrop instrument (ND-1000, Thermo Scientific) and A260/280 was greater than 1.90. Purified RNA was reverse transcribed into cDNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) per manufacturer. qPCR with Fast SYBR Green Master Mix (Applied Biosystems) on a 7500 Fast system (Applied Biosystems) was performed. Custom primers were designed using Primer Express 3.0 software (Applied Biosystems) and evaluated for efficiency (E). The primers used for OSX were 5’-CATCTGCCTGGCTCCTTG-3’ (forward) and 5’-CAGGGGACTGGAGCCATA-3’ (reverse) (E= 2.0). The primers used for ALPL were 5’-GGGTCAGCTCCACCACAA-3’ (forward) and 5’-GGCATTGGTGTTGTACGTCTT-3’ (reverse) (E= 2.1). The primers used for OPN were 5’-GGGTCAGCTCCACCACAA-3’ (forward) and 5’- GGCATTGGTGTTGTACGTCTT-3’ (reverse) (E= 1.9). Data are presented as normalized expression defined by
where GOI is the gene of interest, HKG is the housekeeping gene, and E is the primer efficiency. Control is the pre-encapsulated MSCs.
2.7. Biochemical Assays
Hydrogel samples were removed from culture at predetermined time points and rinsed in PBS for 1 hr and then lysed in deionized water (diH2O), frozen in liquid nitrogen and stored at −80°C. Samples were homogenized using a tissue lyser (Qiagen), then further prepared through freeze-thaw-sonicate cycles to lyse the cells and a known amount of the sample was removed for each subsequent assay. Alkaline phosphatase activity was determined as the number of moles of p-nitrophenol phosphate catalyzed to p-nitrophenol with absorbance measured at 405 nm using a spectrophotometer. Total calcium was measured using the Calcium (CPC) Liquicolor® Assay (Stanbio) according to manufacturer specifications, with absorbance measured at 540 nm. For total collagen content, samples were digested in 1 mg/ml pepsin (Worthington) in 0.5 M acetic acid overnight prior to being incubated with 5 mg/ml Sirius Red (Sigma) for 30 min. Absorbance was measured at 540 nm using a spectrophotometer.
2.8. Histochemistry
Hydrogel specimens were removed from culture at day 28 and fixed in 4% paraformaldehyde at 4°C for 24 hr. The specimens were rinsed with PBS and stored in a 30% sucrose solution at 4°C until dehydration. Dehydration and paraffin embedding followed standard protocols. Sections, 10 μm in thickness, were stained with von Kossa and nuclear red counter stain, following standard protocols. Tissue sections were imaged using light microscopy (Zeiss Axiovert microscope) and a digital camera (Diagnostic Instruments, MN 14.2 Color Mosaic). For bilayer hydrogels, line plots were generated along the center line using NIH Image J and the plot profile analysis. To generate relative mineral intensity, gray values (0 to 255) were converted to a relative intensity with a 0 gray value = 1 for mineral (black) and 255 gray value = 0 for no mineral (white).
2.9. Statistics
Statistical analysis was performed using Real Statistics add-in for Excel. Two-way ANOVA (α = 0.05) was performed with time and culture condition as factors. If the interaction between factors was significant, follow-up tests were performed to evaluate the simple main effects. Post-hoc analyses were performed using Tukey’s HSD with α = 0.05. Significance was considered when p < 0.05. All numerical results are presented in the text as the mean with standard deviation reported parenthetically. Data were confirmed normally distributed and exhibited homogeneous variance. All graphical results are presented as the mean with standard deviation as error bars. All experiments had a sample size of 3 independent constructs from one donor.
2.10. Particle Tracking and Strain Mapping
Silicon dioxide microparticles (5 μm) were entrapped into cylindrical bilayer hydrogels, as described above, at 0.3% weight of solids per volume precursor solution. The density of microparticles did not alter the mechanical properties of the hydrogel. To minimize microparticle settling, the precursor solution was vortexed immediately before polymerization. The specimen were hemi-sectioned by cutting in half with a razor blade and placed cut side down on a windowed compression rig that sits on a bright field microscope (Zeiss Axiovert 40C). The compression rig is equipped with solid, polished, stainless steel platens. Images were acquired at 1% strain increments up to 25% strain. Images were acquired at equilibrium, which was confirmed by analyzing embedded particles over the course of several hours, which did not show any further movement. A MATLAB based Digital Image Correlation routine (Jones, Silberstein, White, & Sottos, 2014) was used to quantify the displacement of the microparticles in the x-y direction and represented as heat maps.
2.11. Axisymmetric Finite Element Modeling
The bilayer and single layer hydrogels were modeled using an axisymmetric finite element (FE) model of unconfined compression developed in Abaqus v6.16 (Simulia) to visualize stress, pore pressure, and fluid velocity profiles. The model first simulated the swelling of the bilayer hydrogels using the moisture swelling model in Abaqus and by prescribing geometry changes that were measured experimentally. After reaching equilibrium, each model was compressed between two platens with frictionless contact. The platens were considered rigid and permeable and prescribed with a zero pore pressure boundary condition to allow fluid flow. The top platen moved until full contact with the gel surface was made to simulate initial conditions in the experimental bioreactor. After reaching equilibrium in this initial contact, the top platen was then used to apply a dynamic loading regime at 2.5%, 1.5% or 0.15% amplitude strain, depending on the experiment, in a sinusoidal waveform at a frequency of 1 Hz for 20 cycles. A total of 775 hybrid quadratic, pore fluid/stress elements with full integration (CAX8PH) were used. Poroelasticity and nonlinear geometric effects were considered, using the Soils solver with transient fluid response in Abaqus. Material properties, including the solid skeleton elastic modulus and permeability, k, were determined from mechanical testing (see section 2.3). A strain-dependent permeability function was employed following methods described in (Ficklin, Davol, & Klisch, 2009) and adapted from (Lai, Mow, & Roth, 1981). In brief, the permeability is assumed to be an exponential function that depends on the experimentally determined permeability, void ratio (i.e., volume fraction of fluid to solid), which is related to volumetric strain, and a nondimensional material constant, which was assumed to have a value of 10.4 similar to cartilage (Ficklin et al., 2009). Based on our previous work (Aziz et al., 2016), a 125 μm thick transition region with properties that were prescribed as an average between the soft and stiff layers was assumed to separate the top and bottom layers. These regions were joined to each other with tied contact constraints. The material model and axisymmetric mesh formulation was verified against an analytical solution for unconfined compression at small strain (1%) (Armstrong et al., 1984).
3. RESULTS
3.1. Bilayer Hydrogel Formation and Hydrogel Layer Characterization
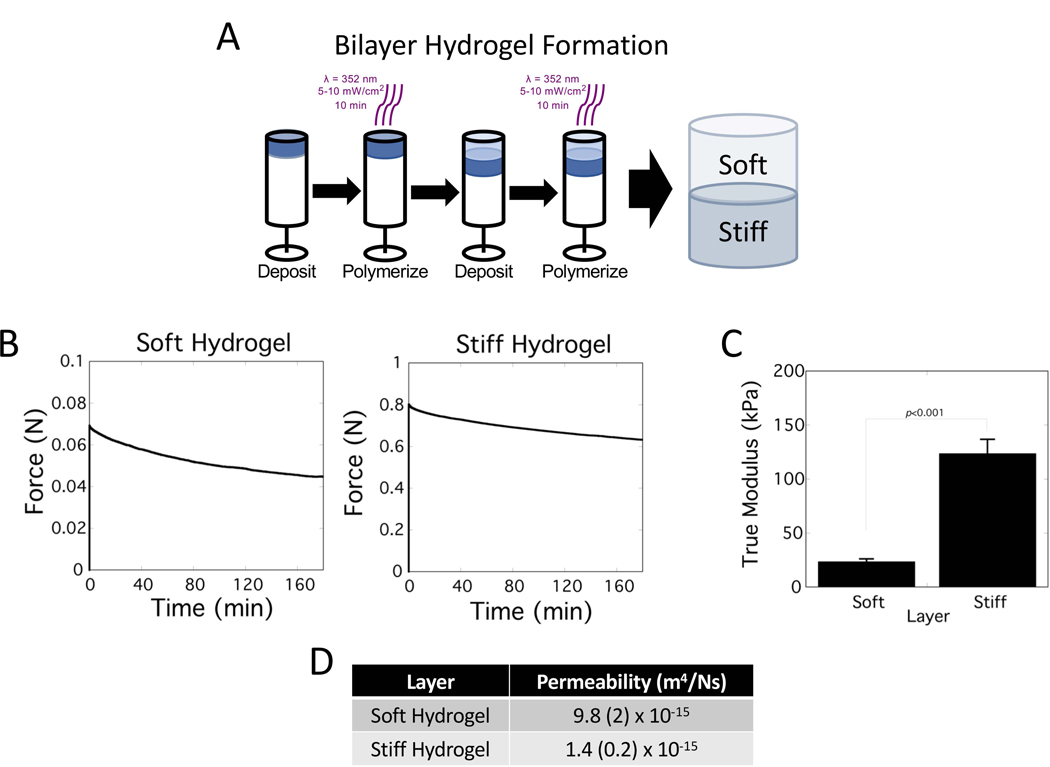
Bilayer hydrogels were formed by sequentially depositing and then immediately polymerizing each layer (Figure 1A). Each layer was formed from a soft and a stiff PEG hydrogel. Force relaxation tests performed on single layer hydrogels (Figure 1B). The force decreased over time by ~35% and ~20% after three hours for the soft and stiff hydrogels, respectively. From these results, the true modulus for the soft hydrogel was determined to be 24 (2) kPa and for the stiff hydrogel was ~5x stiffer with a modulus of 124 (13) kPa (Figure 1C). Permeability for each hydrogel layer was estimated from the relaxation tests to be 9.8 ×10−15 m4/N-s for the soft hydrogel and 86% lower for the stiff hydrogel (Figure 1C).
Figure 1.
Bilayer hydrogel formation and characterization. A) Schematic of fabrication method for forming the bilayer hydrogel through a sequential and repeating process involving deposition of the precursor solution and polymerization using light to form each hydrogel layer. B) Relaxation behavior of soft and stiff hydrogels after being subjected to a 15% compressive strains applied at 2 mm/s. Force was recorded as a function of time. C) The true modulus for poly(ethylene glycol) hydrogels used to form the soft and stiff layers of the bilayer hydrogel. D) Permeability determined from the force relaxation experiments for the soft and stiff hydrogel. Data are reported as mean with standard deviation as error bars or listed parenthetically (n=3).
3.2. Gene expression assessment of osteogenically differentiating hMSCs in the bilayer hydrogel
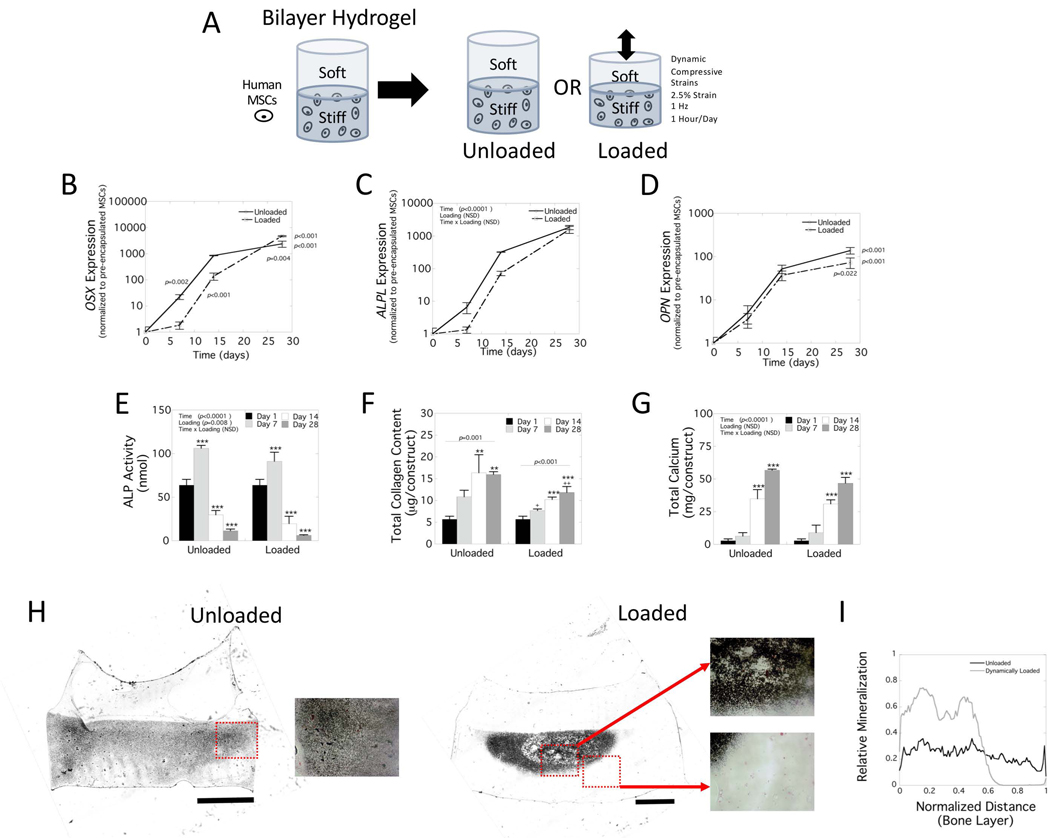
Human MSCs were encapsulated into the stiff layer of the bilayer hydrogels and subjected to dynamic compressive loading (2.5% amplitude strain and 1 Hz) (Figure 2A). Viability was greater than ~95% viability (data not shown). The cell-laden bilayer hydrogels were cultured for 28 days in the absence (unloaded) or presence of dynamic compressive loading. Gene expression for osterix (OSX) (Figure 2B), alkaline phosphatase (ALPL) (Figure 2C), and osteopontin (OPN) (Figure 2D) were normalized to pre-encapsulated MSCs.
Figure 2.
Osteogenesis of hMSCs in stiff layer of bilayer hydrogel. A) Schematic of experiment. The hydrogel was either cultured under no loading (unloaded) or subjected to 2.5% amplitude strain at 1 Hz applied 1 hour per day for up to 28 days in osteogenic medium. B-D) Gene expression of hMSCs encapsulated in the stiff layer of the bilayer hydrogel for B) OSX (osterix), C) ALPL (alkaline phosphatase), and D) OPN (osteopontin). Relative gene expression for each gene was normalized to the pre-encapsulated hMSCs, denoted as day 0. E-G) Biochemical assessment of hMSCs encapsulated in the stiff layer of the bilayer hydrogel for E) alkaline phosphatase (ALP) activity, F) total collagen content, and G) total calcium content. Data in B-G are presented as mean with standard deviation as error bars (n=3). P-values are reported from either a two-way ANOVA for each factor of time and loading (ALPL, ALP activity, total calcium) or from simple main effects for time due to a significant two-way interaction (OSX, OPN, total collagen). In B-D, p-values denoted at a time point indicate the significance between unloaded and loaded from post-hoc analysis. In E-G, the * above a column indicates pairwise comparisons with the corresponding p-value from day 1 and the + indicates pairwise comparisons with the corresponding p-value from unloaded at the same time point (* is p<0.05, ** and ++ are p<0.01, and *** is p<0.001) from post-hoc analysis. H) Representative brightfield microscopy of the bilayer hydrogels stained for mineral deposits by von Kossa. Scale bar is 0.5 mm. Inset red boxes are magnified and shown to the right of each image. I) Line plots showing relative mineral intensity (0=white, 1 = black) from the center line of the unloaded (black) and loaded (gray) constructs normalized to distance in the bone layer only.
For OSX expression, there was a significant interaction (p<0.001) between factors. Follow-up analyses revealed a significant increase in expression with time for unloaded (p<0.001) and loaded (p<0.001) conditions. OSX expression was delayed under loading, but by day 28 was ~2-fold higher (p=0.004) under loading compared to no loading. ALPL expression was affected by time (p<0.001) not loading (p=0.08) with no significant interaction between the factors. ALPL expression increased significantly over the 28 day culture period for both culture conditions. There was an apparent delay under loading, but this was not statistically significant. For OPN expression, there was a significant interaction (p<0.001) between factors. Follow-up analyses revealed a significance increase in OPN expression with time for unloaded (p<0.001) and loaded (p<0.001) conditions. There was no effect due to loading until day 28, resulting a ~2-fold lower (p=0.02) OPN expression under loading.
3.3. Biochemical assessment of osteogenically differentiating hMSCs in the bilayer hydrogel
The cell-laden hydrogels were also evaluated for their biochemical contents for alkaline phosphatase (ALP) activity (Figure 2E), total collagen content (Figure 2F), total calcium content (Figure 2G) and spatial distribution of calcium deposits (Figure 2H). ALP activity was affected by time (p<0.0001) and loading (p=0.008), but there was no significant interaction between the two factors. Of the time points assessed, ALP activity was the greatest at day 7 in both culture conditions and then decreased to levels below values collected on day one. Overall, ALP activity was significantly lower under loading. For total collagen content, there was a significant interaction (p=0.04) between time and loading. Follow-up analyses revealed a significance increase in collagen content with time for unloaded (p=0.001) and loaded (p<0.001) conditions. Total collagen content increased by 2.8-fold and 2-fold in the unloaded and loaded conditions, respectively, from day 1 to 28. By day 28, total collagen content was significantly lower by 26% (p=0.009) under loading.
Total calcium content was affected by time (p<0.0001) not loading (p=0.09) with no significant interaction between the factors. Total calcium content increased by 20-fold and 17-fold in the unloaded and loaded conditions, respectively. The cell-laden hydrogels were collected at day 28 and stained for mineralization by von Kossa. The bilayer hydrogels were sectioned parallel to the loading direction to inspect visually for differences in the spatial distribution of mineral. In the unloaded hydrogels, there was mineralization present throughout the stiff layer with no presence of mineral in the soft layer. Under dynamic loading, there was an apparent more intense dark staining in the central region of the stiff layer near the interface with minimal staining elsewhere in the stiff layer. A line plot of relative mineral intensity as a function of height in the stiff layer and from a central region of each hydrogel construct (Figure 2I) confirms the spatial differences observed in mineralization as well as differences in intensity of mineralization between the unloaded and loaded conditions.
3.4. Characterization of local strains in the bilayer hydrogel: Experimental Analysis
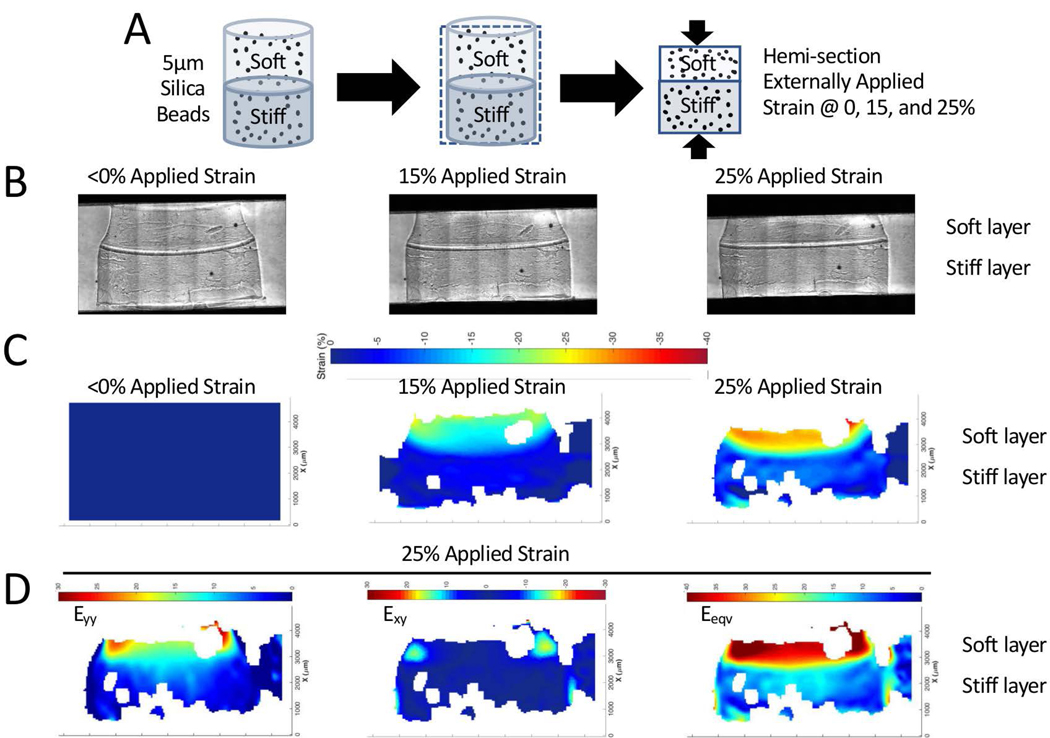
To understand the local mechanical responses in the stiff layer of the hydrogel, the strain profiles were mapped experimentally within the bilayer hydrogel by tracking silica microparticles that were encapsulated in each layer of the hydrogel during fabrication (Figure 3A). The particles were distributed throughout the bilayer hydrogel. The bilayer hydrogel was subjected to an externally applied strain and the hydrogels were imaged under bright field microscopy at each strain increment. Bright field images are shown before an external stain was applied (<0% strain) and at 15% and 25% stain (Figure 3B). Large strains were investigated to enable more reliable particle tracking due to large particle displacements. Strain heat maps were generated in the normal x-direction (Exx) direction, parallel to the direction of the external applied strain (Figure 3C). The Exx was highest in the soft layer and then decreased across the interface and was lowest in the stiff layer. The strains within each region increased within increasing amount of externally applied strain. For example, under the externally applied 25% strain, the Exx strains in the soft layer were ~30% strain (higher than the applied strain) while strains in the stiff layer were ~10% and less (lower than the applied strain).
Figure 3.
Strain heat maps in the bilayer hydrogel under an externally applied compressive strain. A) Schematic of experimental set-up showing silica beads encapsulated throughout the bilayer hydrogel, hemi-sectioned, and then placed into a custom straining rig that sits on the stage of a microscope. B) Brightfield microscopy images of the hemi-sectioned bilayer hydrogel prior to applying an external strain (<0% strain), 15% strain and 25% strain. C) Heat maps of strain prior to applying an external strain (<0% strain), 15% strain and 25% strain in the x-direction (Exx) parallel to the direction of the applied strain. D) Heat maps of strain at an 25% external applied strain in the normal y-direction (Eyy) perpendicular to the direction of the applied strain, shear xy-direction (Exy), and the equivalent strain (Eeqv).
At the externally applied 25% applied strain, the strain heat maps were also analyzed in the normal y-direction (Eyy), perpendicular to the applied strain and in the shear xy-direction (Exy = Eyx) (Figure 3D). Within the stiff layer, the Eyy was highest in the region near the interface. There was minimal Exy throughout the stiff layer with the exception of along the edges at the interface and along the edge of the stiff layer. From the individual normal and shear strains, the rationally invariant equivalent strain (Eeqv) was determined by
The Eeqv provides an indication of the state of strain independent of direction. Throughout the majority of the stiff layer (i.e., up to ~2 mm from the interface), the equivalent strain was ~15% under the applied 25% strain (Figure 3D). Extrapolating, the local strains in the stiff layer under a 2.5% applied strain are estimated to be ~1% for Exx, ~1% for Eyy, and ~1.5% for Eeqv with localized regions of strains for Exy of ~1.5% near the edge.
3.5. Characterization of local equivalent stress, hydrostatic pressure and fluid velocity in the bilayer hydrogel: FE Analysis
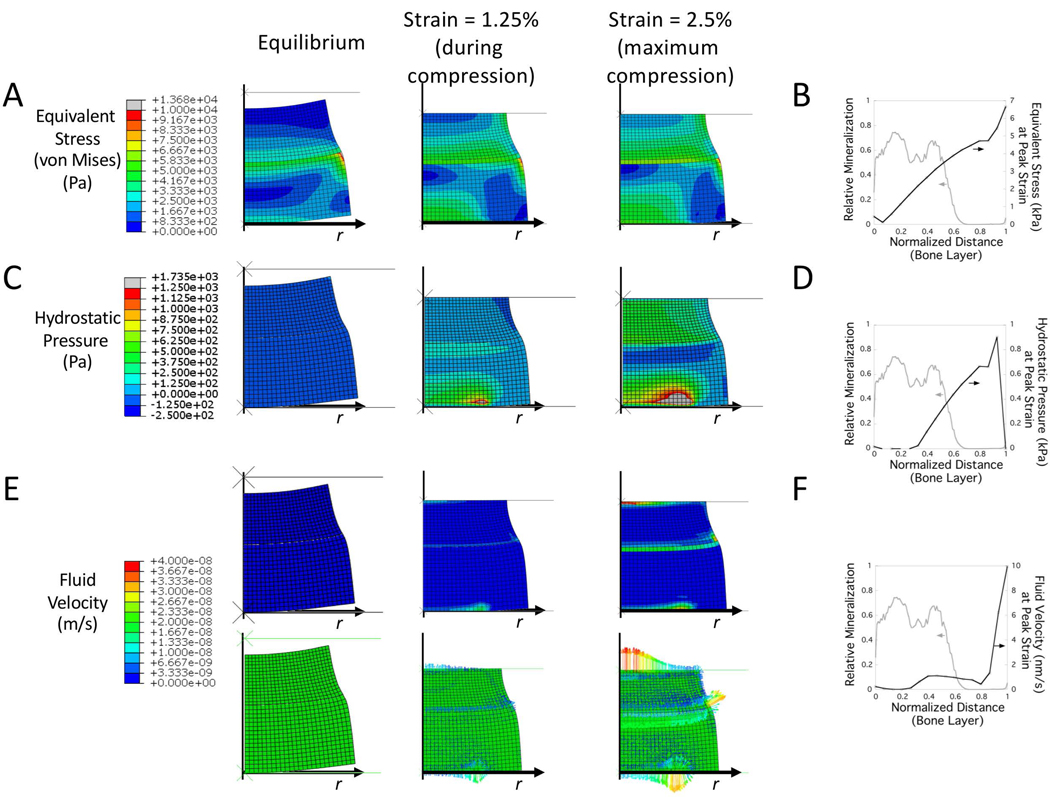
To investigate further the different mechanical responses, which are difficult to assess experimentally, finite element (FE) modelling was employed. Specifically, equivalent stress (i.e., von Mises stress), hydrostatic pressure (i.e., pore pressure), and fluid velocity were modeled in the bilayer hydrogels (Figure 4). The model assumed three distinct regions each with homogeneous properties. The moduli and permeabilities for the top and bottom layers were taken from experimental measurements for their respective single layers with an interfacial, connecting layer that was approximated to be an average in modulus of the soft and stiff layers. To capture the experimental system, hydrogel swelling was modeled from its initial state at the time of polymerization to its equilibrium swollen state. At equilibrium, residual equivalent stresses were present throughout the bilayer, most prominent near the interface, ranging in magnitude from ~2–5 in the central region to 10 kPa at the edge. At equilibrium, the hydrostatic pressure and fluid velocities were zero.
Figure 4.
Heat maps of equivalent stress (A,B), hydrostatic pressure (C,D), and fluid velocity (E,F) in the bilayer hydrogel under an externally applied dynamic compressive strain. Finite element analysis was performed on a bilayer hydrogel with soft, stiff and an interfacial layer under unconfined dynamic compression externally applied at an 2.5% amplitude strain at 1 Hz. Heat maps are shown at steady state (during the 18th cycle) for 1.25% strain during compression and at 2.5% maximum strain. Line plots from the center line of the bone layer for equivalent stress (B), hydrostatic pressure (D) and fluid velocity (F) are shown and overlaid with a representative line plot of mineralization from the hMSC experiment under loading (see Figure 2).
The equilibrium swollen bilayer hydrogel was then subjected to unconfined dynamic cyclic strains, following the same protocol used in the bioreactor, from 0 to 2.5% strain at a frequency of 1 Hz. Heat maps were generated after steady state was reached and shown for the18th cycle for equivalent stress (i.e., von Mises stress) (Figure 4A), hydrostatic pressure (Figure 4C) and fluid velocity (Figure 4D) across the bilayer hydrogel. In the stiff layer, the magnitude of the equivalent stress increased with increasing compressive strain during the cycle of compression. The stresses were highest (~2–10 kPa in magnitude) at the edge near the interface and in the central region of the layer that spanned from the bottom to õne-half to ~two-thirds the height. The region central and near the interface experienced low stresses (<1 kPa in magnitude). In the stiff layer, the hydrostatic pressure increased with increasing compressive strain during the cycle of compression and was highest (~0.5–3 kPa in magnitude) in the central region of the layer that spanned from the bottom to ~ one-half the height. The region central and near the interface experienced minimal hydrostatic pressure. In the stiff layer, fluid flow was extremely low (∼1 nm/s) throughout the layer with the exception of the interfacial region and the bottom of the layer, which exhibited velocities that reached ~20 nm/s in magnitude.
To compare the different mechanical responses to the spatial localization of mineralization under loading, line plots were generated along the center line of the hydrogel for the FE modeling results and the intensity of mineralization observed histologically. Line plots represent the stiff layer only with a normalized distance just below the interface (0) to the bottom of the stiff layer (1). The equivalent stress increased from near zero stress to 5 kPa throughout the mineralized regions (Figure 4B). In the region where mineralization was present, the equivalent stress increased from ~zero near the interface to ~4 Pa at the edge of the mineralization (Figure 4B). The hydrostatic pressures were low near the interface and then increased to ~500 Pa at the edge of this region (Figure 4D). Fluid velocities were minimal throughout this region (≤1 nm/s) and throughout the stiff layer and increased only until at the edge of the hydrogel (Figure 4F).
3.6. Assessment of hMSC osteogenesis and ERK1/2 signaling in single layer stiff hydrogels
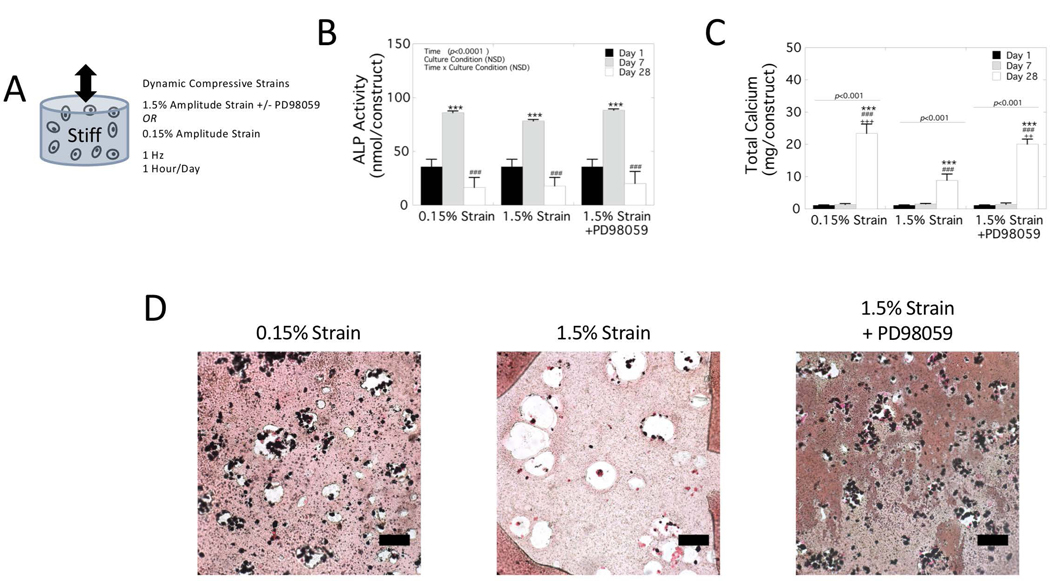
To investigate the effects of strain during dynamic compressive loading on the osteogenesis of encapsulated hMSCs and subsequent mineralization, hMSCs were encapsulated in a single layer stiff hydrogel and subjected to dynamic (1 Hz) compressive strains of 1.5%. This strain was chosen to mimic the equivalent strains that were achieved in the stiff layer of the bilayer hydrogel construct subjected to an 2.5% applied strain. Because the interface region in the bilayer hydrogel leads to higher strains in the y-direction (i.e., Eyy) and shear strains (i.e., Exy) than would be observed in a single layer hydrogel, we chose to match the applied compressive strain in the single layer hydrogel experiment (i.e., 1.5%) to that of the equivalent strain in the bilayer (i.e., 1.5%). A low strain that correlates to physiologically relevant strain levels in bone at 0.15% was also investigated. The high strain condition was also cultured in the presence of a MEK1 inhibitor, PD98059, which is upstream of the ERK1/2 signaling. The experimental design for the single layer stiff hydrogels is shown in Figure 5A.
Figure 5.
Biochemical assessment of hMSCs encapsulated in a stiff single layer hydrogel. The hydrogel was subjected to dynamic compressive strains applied at 1 Hz for up to 28 days in osteogenic medium under one of three experimental conditions: 0.15% amplitude strain, 1.5% amplitude strain, and 1.5% amplitude strain with 10 μM PD98059. A) Schematic of experiment. B-C) Biochemical assessments were made for B) alkaline phosphatase (ALP) activity and C) total calcium content. Data are presented as mean with standard deviation as error bars (n=3). P-values are reported from a two-way ANOVA for each factor of time and loading (ALP activity) or from simple main effects for time due to a significant two-way interaction (total calcium). P-values for pairwise comparisons from post-hoc analysis are denoted. The * and # symbols above a column indicate the significance level from day 1 and day 7, respectively and the + symbol above a column indicates the significance level from the 1.5% strain condition. Lines denote pairwise significance between culture condition. The ** and ++ denotes p<0.01, ***, ### and +++ denote p<0.001. E) Representative brightfield microscopy of the single layer hydrogels stained for mineral by von Kossa. Scale bar is 100 μm.
The cell-laden hydrogels were analyzed by biochemical assays for ALP activity (Figure 5B) and mineralization by total calcium content (Figure 5C) and spatial distribution of mineral (Figure 5D). ALP activity was affected by time (p<0.001) but not culture condition (0.15%, 1.5%%, and 1.5%+PD98059) and there was no statistically significant interaction between the factors. At the time points evaluated, ALP activity was the highest at day 7 in all three culture conditions and then decreased at day 28 to levels below values collected at day 1. For total calcium content, there was a statistically significant interaction (p<0.001) between the time and culture condition. Follow-up analyses of simple main effects revealed a significance increases in calcium content for 0.15% (p<0.001), 1.5% (p<0.001), and 1.5%+PD98059 (p<0.001) strain condition. At day 28 total calcium content was ~30% lower (p<0.001) under the 1.5% strain condition when compared to the 0.15% strain condition. The von Kossa stained sections for mineralization mirrored these findings with mineralization present in the 0.15% strain condition, but minimally present in the 1.5% strain condition. When PD98059 was added to the culture medium for the 1.5% strain condition, total calcium content reached levels similar to the 0.15% strain at day 28 and were significantly higher (p=0.009) than 1.5% strain at day 28. The von Kossa stained sections mirrored these findings as well resulting in restoration of mineralization under 1.5% strain.
Finite element (FE) modelling was employed to investigate the different mechanical responses in the single layer hydrogels subjected to unconfined dynamic compressive strains. Heat maps were generated after steady state was reached and shown for the 18th cycle of compression for equivalent stress (Figure 6A), hydrostatic pressure (Figure 6B), and fluid velocity (Figure 6C) for the low (0.15%) and high (1.5%) strain condition. The equivalent stresses at maximum strain reached ~0.15 kPa and 1.5 kPa in magnitude for the low and high strain condition, respectively, and were uniform throughout the bulk of the hydrogel. The hydrostatic pressures at maximum strain reached ~60 Pa and ~600 Pa in magnitude for the low and high strain condition, respectively, throughout the bulk of the hydrogel. Equivalent stress and hydrostatic pressures were higher along the edge of the construct. Fluid flow was limited to the edge of the hydrogel with magnitudes that were ~1 nm/s and ~10 nm/s in magnitude for the low and high strain condition, respectively.
Figure 6.
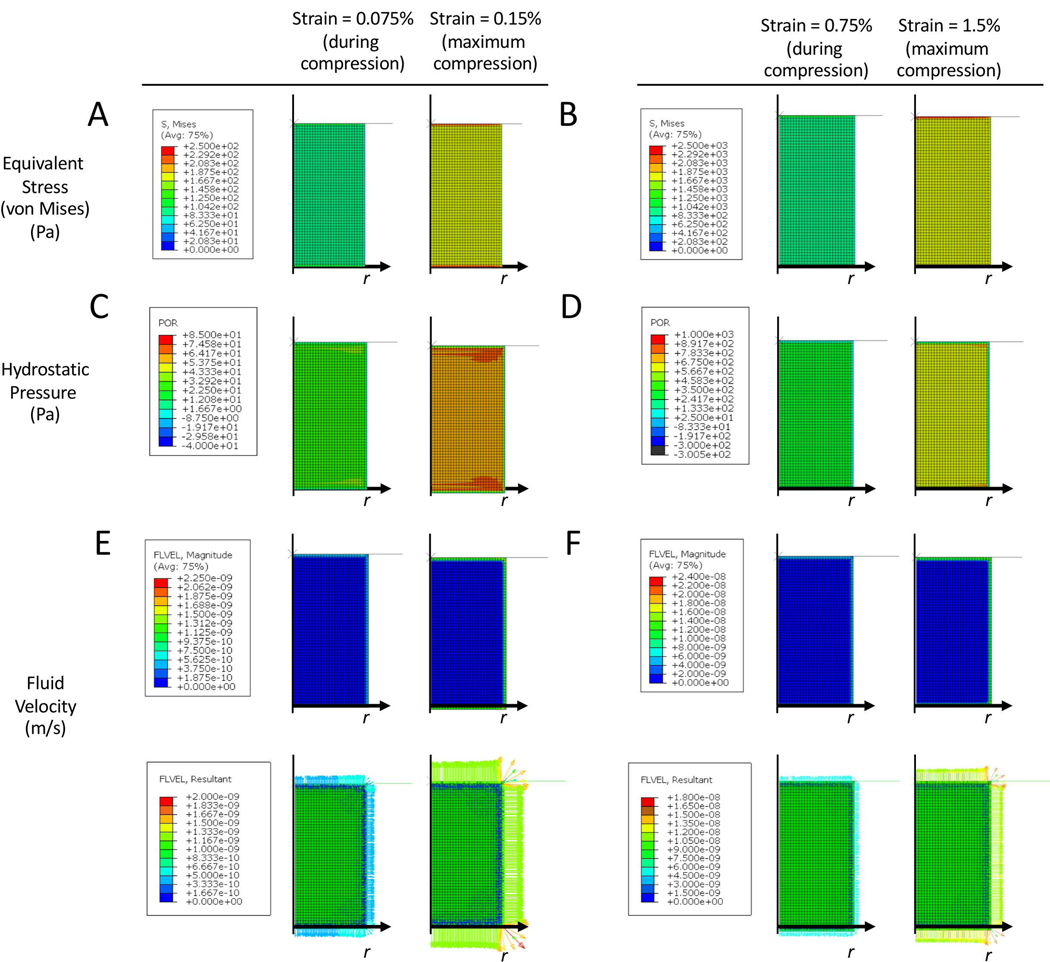
Heat maps of equivalent stress (A,B), hydrostatic pressure (C,D), and fluid velocity (E,F) in single layer hydrogels under an externally applied dynamic compressive strain at a maximum of 0.15% applied strain (A,C,E) or 1.5% applied strain (B,D,F) at 1 Hz. Heat maps are shown at steady state (during the 18th cycle) for the half-way point during compression and at the maximum strain.
DISCUSSION
This study describes a complex mechanical environment in a bilayer hydrogel under unconfined dynamic compressive loading and the impact it has on osteogenesis. The stiff layer containing cell adhesion ligands of RGD supported osteogenic differentiation concomitant with mineralization. However, dynamic loading delayed the onset of differentiation and affected late stage differentiation associated with mineralization, but in a manner that depended spatially on the local mechanical responses. Collectively, our data suggest an important role for combined mechanical responses in regulating osteogenesis.
The stiff layer of the bilayer hydrogel containing RGD supported osteogenesis of hMSCs. Differentiation was confirmed by upregulation of osteogenic genes of OSX, ALPL, and OPN, by elevated ALP activity, and increased deposition of collagen and calcium over the 28-day culture period. Dynamic loading, however, delayed osteogenesis. Most interesting was the spatial distribution of mineral deposits which was diffuse in the unloaded condition but concentrated and spatially restricted to the central region of the stiff layer near the interface. Mineralization was also maintained throughout a single layer hydrogel, but only when subjected to low (0.15%) and not high (1.5%) applied dynamic compressive strains. To identify potential mechanisms that led to the regulation of osteogenesis and specifically mineralization as a function of dynamic compressive loading, mechanical responses that emerge during dynamic compressive loading were investigated.
Strain mapping identified highly nonuniform strains across the bilayer hydrogel with divergent stiffness with the soft layer experiencing majority of the strain. However, relative large, finite strains were transferred to the stiff layer despite being five times stiffer. This observation is in large part attributed to the formation of an interfacial region that links the soft and the stiff layers through covalent bonds and polymer entanglements. Under compression, the soft layer undergoes lateral expansion (Kinneberg et al., 2015), which extends across the interface and into the stiff layer resulting in lateral strains and localized regions of shear strain. This results in relatively high equivalent strains that are distributed across the interface and into the stiff layer of a bilayer hydrogel with divergent properties.
Heat maps of equivalent stress revealed residual stresses in the equilibrium swollen bilayer hydrogels in the unloaded state. Because, the hydrogels are formed under non-equilibrium conditions, they undergo differential swelling. In this study, the stiff hydrogel exhibited a higher degree of swelling, a finding consistent with our previous study (Aziz et al., 2016). Residual stresses were visible around the interface and at the bottom of the stiff layer. In the FEM, it was not possible to differentiate residual strain from strain resulting from volumetric changes in hydrogel swelling. However, the residual stress is directly correlated to residual strain. We can therefore infer that residual strain was minimal throughout the central region of the stiff hydrogel, which is consistent with the hydrogel swelling isotropically in this region. Residual strains, however, will be concentrated at the interface and at the bottom of the layer. Under dynamic loading, the regions with high residual stress grew in size, but the magnitude of the stress (i.e., maximum stress) did not appear to increase. There was no obvious association between equivalent stress and the localized region of mineralization.
There was an apparent association between mineral deposits and hydrostatic pressure in the bilayer hydrogel, which is further supported by the single layer hydrogels. The area with concentrated mineral deposits in the bilayer hydrogel experienced equivalent strains of ~1.5% and were accompanied by low hydrostatic pressures. In regions distant from the interface, hydrostatic pressures were much greater (i.e., ≥~600 Pa) and correlated to a lack of mineralization. The latter is consistent with single layer hydrogels subjected to 1.5% applied strain, which showed inhibited mineralization. The regions along the edge of the stiff layer also lacked mineralization. Interestingly, these regions had low hydrostatic pressures, but exhibited higher equivalent strains. On the contrary, single layer hydrogels subjected to low (0.15%) applied strain experienced low hydrostatic pressures (~60 Pa) and were able to maintain mineralization. These results suggest that the combination of strain and hydrostatic pressure can negatively affect mineralization, but the effect is dependent on the magnitude of each mechanical response.
Other studies have applied much higher hydrostatic pressures (e.g., ~300 kPa) and reported a positive effect on osteogenesis and mineralization (Henstock et al., 2013), but these studies were in the absence of any strain. We previously reported that high compressive strains (15%) applied dynamically to MSCs encapsulated in a PEG hydrogel inhibit mineralization (Steinmetz & Bryant, 2011), an environment that will also lead to relatively high hydrostatic pressures. Studies have reported positive effects of dynamic compressive strains on osteogenic markers and mineralization (Rath, Nam, Knobloch, Lannutti, & Agarwal, 2008). In these studies, however, cells were cultured on fibrous scaffolds where hydrostatic pressures will be minimal due to ease of fluid flow. We therefore postulate that when osteogenically differentiating stem cells experience moderate strains, the resultant hydrostatic pressure must be sufficiently low to maintain mineralization. Additional studies are needed to test this hypothesis.
The magnitude of the fluid flow generated in the stiff layer of the bilayer and in the single layer hydrogels was low (i.e., 1–10’s nm/s) due to unconfined compression and low permeabilities. Other studies have modeled fluid flow in the canaliculi surrounding bone cells and reported fluid velocities of ~2 μm/s under physiological stress (Goulet, Cooper, Coombe, & Zernicke, 2008) and up to ~60 μm/s in bone undergoing vigorous physical activity (Verbruggen, Vaughan, & McNamara, 2014). Thus, it is reasonable to postulate that fluid flow is unlikely affecting the osteogenic response in the bilayer and single-layer hydrogels. It is worth noting that we reported a region of reduced hydrogel crosslinking (and hence increased permeability) in regions immediately surrounding encapsulated cells (Schneider et al., 2017). Thus is it possible, that similar to bone, there are regions that could lead to fluid-induced effects on cells not accounted for in this study. However, further study is needed.
To determine whether the observed mineral inhibition was cell-mediated, ERK1/2 signaling was investigated. An inhibitor of MEK1, which is upstream of ERK1/2 signaling, was able to recover mineral deposition in the high strain single-layer stiff hydrogel, suggesting that ERK1/2 signaling is involved in the observed inhibition of mineralization. Although conflicting data have been reported for the role of ERK1/2 signaling in osteogenesis (Schindeler & Little, 2006), our findings are in accordance with the idea that ERK1/2 can act as an antagonist of osteogenesis. For example, the MEK1 inhibitor PD98059 enhanced mineralization of osteoblasts in 2D culture (F.-H. Lin, Chang, & Brigman, 2011) and mineralization of hMSCs in a collagen hydrogel (Lund, Stegemann, & Plopper, 2009). It is important to note that at the PD98059 concentrations used in this study, early osteogenesis as determined by ALP activity was not affected. It has been suggested that ERK1/2 signaling can modulate osteogenesis, but is not a critical regulator of osteogenesis (Schindeler & Little, 2006). In this work, the application of relatively large dynamic compressive strains (i.e., 1.5% applied strain) was shown to inhibit late stage osteogenesis. Studies have indicated that high strains favor chondrogenesis over osteogenesis (Dumas et al., 2010; Friedl et al., 2007; Horner et al., 2018; Li, Yao, Alini, & Stoddart, 2010) and ERK1/2 has been implicated in mediating chondrogenesis (Bobick, Matsche, Chen, & Tuan, 2010). Moreover, these observations are consistent with the fact that fracture healing occurs by endochondral ossification due to the high strains (Claes & Heigele, 1999). Studies have also demonstrated that high hydrostatic pressures applied intermittently favor chondrogenesis over osteogenesis (Wagner et al., 2008). Thus, it is reasonable to postulate that in this work higher compressive strains in combination with hydrostatic pressure are responsible for the delay in osteogenesis. Further investigation into the combined role of strain and hydrostatic pressure in mediating load-induced effects on osteogenesis is warranted.
Based on findings from this study, we put forth the following hypothesis. Our data suggest that supraphysical levels of strains (i.e., above 0.15% applied strain) when combined with relatively high hydrostatic pressures (i.e., ~600 Pa) are transferred to hMSCs encapsulated in a hydrogel delays osteogenesis shown by reduced expression of osteogenic genes and inhibition in mineralization at 28 days. However, if the hydrostatic pressure is sufficiently low, mineralization can be restored. Thus, our data strongly point towards the combination of strain and hydrostatic pressure as being a negative regulator, via ERK1/2 signaling, of late state osteogenesis, and specifically mineralization, but in a manner that depends on their relative magnitude. Further studies are needed to test this hypothesis.
This study has several limitations. The applied dynamic compressive loading was unconfined, which in part contributes to the complex mechanical responses across the bilayer hydrogels. Nonetheless, this study provides evidence for the differential effects of strain and hydrostatic pressure in mediating osteogenesis of hMSCs encapsulated in a PEG hydrogel. This study was limited by the inability to decouple the mechanical responses within the hydrogels. The absolute values of hydrostatic pressure and fluid velocities are dependent on the permeability of the hydrogel and are therefore limited by the assumptions made when analyzing the force relaxation experiments. Finally, we acknowledge that the FE model for the bilayer hydrogel was not explicitly validated with experiments or verified analytically due to a lack of currently available analytical solutions.
In conclusion, this work highlights that a bilayer hydrogel with soft and stiff layers produces highly complex mechanical responses under unconfined dynamic compressive strains. These divergent properties have a significant impact on osteogenesis of hMSCs. Our study suggests that the combination of high strain coupled with a high hydrostatic pressure is inhibitory of late-stage osteogenesis and is mediated by ERK1/2 signaling, but that if the hydrostatic pressure is reduced, mineralization is recovered. A better understanding of these local mechanical cues within a bilayer hydrogel will enable improved designs of hydrogel-based scaffolds for osteochondral tissue engineering where dynamic mechanical loads are prevalent.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institute of Health under Award Numbers 1R01AR069060 and 1R21AR069791 and by the National Institute of Child Health and Human Development of the NIH under Award Number 1R21HD090696. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest statement
The authors declare no conflict of interest.
References
- Aisenbrey EA, & Bryant SJ (2016). Mechanical loading inhibits hypertrophy in chondrogenically differentiating hMSCs within a biomimetic hydrogel. J. Mater. Chem. B, 4(20), 3562–3574. 10.1039/C6TB00006A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CG, Lai WM, & Mow VC (1984). An analysis of the unconfined compression of articular cartilage. J Biomech Eng, 106(2), 165–173. [DOI] [PubMed] [Google Scholar]
- Aziz AH, Wahlquist J, Sollner A, Ferguson V, DelRio FW, & Bryant SJ (2016). Mechanical characterization of sequentially layered photo-clickable thiol-ene hydrogels. Journal of the Mechanical Behavior of Biomedical Materials, 65, 454–465. 10.1016/j.jmbbm.2016.09.007 [DOI] [PubMed] [Google Scholar]
- Bobick BE, Matsche AI, Chen FH, & Tuan RS (2010). The ERK5 and ERK1/2 Signaling Pathways Play Opposing Regulatory Roles During Chondrogenesis of Adult Human Bone Marrow-Derived Multipotent Progenitor Cells. Journal of Cellular Physiology, 224(1), 178–186. 10.1002/jcp.22120 [DOI] [PubMed] [Google Scholar]
- Bryant SJ, & Anseth KS (2005). Photopolymerization of hydrogel scaffolds. CRC. [Google Scholar]
- Claes LE, & Heigele CA (1999). Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. Journal of Biomechanics, 32(3), 255–266. 10.1016/s0021-9290(98)00153-5 [DOI] [PubMed] [Google Scholar]
- Dumas V, Ducharne B, Perrier A, Fournier C, Guignandon A, Thomas M, … Rattner A (2010). Extracellular Matrix Produced by Osteoblasts Cultured Under Low-Magnitude, High-Frequency Stimulation is Favourable to Osteogenic Differentiation of Mesenchymal Stem Cells. Calcified Tissue International, 87(4), 351–364. 10.1007/s00223-010-9394-8 [DOI] [PubMed] [Google Scholar]
- Ficklin TP, Davol A, & Klisch SM (2009). Simulating the growth of articular cartilage explants in a permeation bioreactor to aid in experimental protocol design. J Biomech Eng, 131(4), 041008. 10.1115/1.3049856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl G, Schmidt H, Rehak I, Kostner G, Schauenstein K, & Windhager R (2007). Undifferentiated human mesenchymal stem cells (hMSCs) are highly sensitive to mechanical strain: transcriptionally controlled early osteo-chondrogenic response in vitro. Osteoarthritis and Cartilage, 15(11), 1293–1300. 10.1016/j.joca.2007.04.002 [DOI] [PubMed] [Google Scholar]
- Fritton SP, & Weinbaum S (2009). Fluid and Solute Transport in Bone: Flow-Induced Mechanotransduction. Annual Review of Fluid Mechanics, 41, 347–374. 10.1146/annurev.fluid.010908.165136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SM, & Barabino GA (2016). Hydrodynamic loading in concomitance with exogenous cytokine stimulation modulates differentiation of bovine mesenchymal stem cells towards osteochondral lineages. Bmc Biotechnology, 16, 10 10.1186/s12896-016-0240-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulet GC, Cooper DML, Coombe D, & Zernicke RF (2008). Influence of cortical canal architecture on lacunocanalicular pore pressure and fluid flow. Computer Methods in Biomechanics and Biomedical Engineering, 11(4), 379–387. 10.1080/10255840701814105 [DOI] [PubMed] [Google Scholar]
- Guo Y, Wang Y, Liu Y, Wang H, Guo C, & Zhang X (2015). Effect of the same mechanical loading on osteogenesis and osteoclastogenesis in vitro. Chinese Journal of Traumatology = Zhonghua Chuang Shang Za Zhi, 18(3), 150–156. [DOI] [PubMed] [Google Scholar]
- Henstock JR, Rotherham M, Rose JB, & El Haj AJ (2013). Cyclic hydrostatic pressure stimulates enhanced bone development in the foetal chick femur in vitro. Bone, 53(2), 468–477. 10.1016/j.bone.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Hjelle K, Solheim E, Strand T, Muri R, & Brittberg M (2002). Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy-the Journal of Arthroscopic and Related Surgery, 18(7), 730–734. 10.1053/jars.2002.32839 [DOI] [PubMed] [Google Scholar]
- Hoemann CD, Lafantaisie-Favreau C-H, Lascau-Coman V, Chen G, & Guzman-Morales J (2012). The cartilage-bone interface. The Journal of Knee Surgery, 25(2), 85–97. [DOI] [PubMed] [Google Scholar]
- Horner CB, Hirota K, Liu J, Maldonado M, Park BH, & Nam J (2018). Magnitude-dependent and inversely-related osteogenic/chondrogenic differentiation of human mesenchymal stem cells under dynamic compressive strain. Journal of Tissue Engineering and Regenerative Medicine, 12(2), E637–E647. 10.1002/term.2455 [DOI] [PubMed] [Google Scholar]
- Huang C, & Ogawa R (2012). Effect of Hydrostatic Pressure on Bone Regeneration Using Human Mesenchymal Stem Cells. Tissue Engineering Part A, 18(19–20), 2106–2113. 10.1089/ten.tea.2012.0064 [DOI] [PubMed] [Google Scholar]
- Iqbal J, & Zaidi M (2005). Molecular regulation of mechanotransduction. Biochemical and Biophysical Research Communications, 328(3), 751–755. 10.1016/j.bbrc.2004.12.087 [DOI] [PubMed] [Google Scholar]
- Jones EMC, Silberstein MN, White SR, & Sottos NR (2014). In Situ Measurements of Strains in Composite Battery Electrodes during Electrochemical Cycling. Experimental Mechanics, 54(6), 971–985. 10.1007/s11340-014-9873-3 [DOI] [Google Scholar]
- Kim H, Darwish I, Monroy M-F, Prockop DJ, Liles WC, & Kain KC (2014). Mesenchymal stromal (stem) cells suppress pro-inflammatory cytokine production but fail to improve survival in experimental staphylococcal toxic shock syndrome. Bmc Immunology, 15 10.1186/1471-2172-15-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinneberg KR, Nelson A, Stender ME, Aziz AH, Mozdzen LC, Harley BA, … Ferguson VL (2015). Reinforcement of Mono- and Bi-layer Poly(Ethylene Glycol) Hydrogels with a Fibrous Collagen Scaffold. Annals of Biomedical Engineering, 43(11), 2618–2629. 10.1007/s10439-015-1337-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai WM, Mow VC, & Roth V (1981). Effects of nonlinear strain-dependent permeability and rate of compression on the stress behavior of articular cartilage. J Biomech Eng, 103(2), 61–66. [DOI] [PubMed] [Google Scholar]
- Li Z, Yao SJ, Alini M, & Stoddart MJ (2010). Chondrogenesis of Human Bone Marrow Mesenchymal Stem Cells in Fibrin-Polyurethane Composites Is Modulated by Frequency and Amplitude of Dynamic Compression and Shear Stress. Tissue Engineering Part A, 16(2), 575–584. 10.1089/ten.tea.2009.0262 [DOI] [PubMed] [Google Scholar]
- Lin F-H, Chang JB, & Brigman BE (2011). Role of Mitogen-Activated Protein Kinase in Osteoblast Differentiation. Journal of Orthopaedic Research, 29(2), 204–210. 10.1002/jor.21222 [DOI] [PubMed] [Google Scholar]
- Lin S, Lee WYW, Feng Q, Xu L, Wang B, Man GCW, … Li G (2017). Synergistic effects on mesenchymal stem cell-based cartilage regeneration by chondrogenic preconditioning and mechanical stimulation. Stem Cell Research & Therapy, 8(1), 221 10.1186/s13287-017-0672-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund AW, Stegemann JP, & Plopper GE (2009). Inhibition of ERK promotes collagen gel compaction and fibrillogenesis to amplify the osteogenesis of human mesenchymal stem cells in three-dimensional collagen I culture. Stem Cells and Development, 18(2), 331–341. 10.1089/scd.2008.0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris EA, Gomoll AH, Malizos KN, Hu JC, & Athanasiou KA (2015). Repair and tissue engineering techniques for articular cartilage. Nature Reviews Rheumatology, 11(1), 21–34. 10.1038/nrrheum.2014.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodemus GD, Shiplet KA, Kaltz SR, & Bryant SJ (2009). Dynamic Compressive Loading Influences Degradation Behavior of PEG-PLA Hydrogels. Biotechnology And Bioengineering, 102(3), 948–959. 10.1002/bit.22105 [DOI] [PubMed] [Google Scholar]
- Radhakrishnan J, Manigandan A, Chinnaswamy P, Subramanian A, & Sethuraman S (2018). Gradient nano-engineered in situ forming composite hydrogel for osteochondral regeneration. Biomaterials, 162, 82–98. 10.1016/j.biomaterials.2018.01.056 [DOI] [PubMed] [Google Scholar]
- Rath B, Nam J, Knobloch TJ, Lannutti JJ, & Agarwal S (2008). Compressive forces induce osteogenic gene expression in calvarial osteoblasts. Journal Of Biomechanics, 41(5), 1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindeler A, & Little DG (2006). Ras-MAPK signaling in osteogenic differentiation: Friend or foe? Journal of Bone and Mineral Research, 21(9), 1331–1338. 10.1359/JBMR.060603 [DOI] [PubMed] [Google Scholar]
- Schneider MC, Chu S, Sridhar SL, de Roucy G, Vernerey FJ, & Bryant SJ (2017). Local Heterogeneities Improve Matrix Connectivity in Degradable and Photoclickable Poly(ethylene glycol) Hydrogels for Applications in Tissue Engineering. ACS Biomaterials Science & Engineering, 3(10), 2480–2492. 10.1021/acsbiomaterials.7b00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtes S, Kraemer E, Weisser M, Roth W, Luginbuehl R, Grossner T, & Richter W (2018). Global chondrocyte gene expression after a single anabolic loading period: Time evolution and re-inducibility of mechano-responses. Journal of Cellular Physiology, 233(1), 699–711. 10.1002/jcp.25933 [DOI] [PubMed] [Google Scholar]
- Schutz K, Despang F, Lode A, & Gelinsky M (2014). Cell-laden biphasic scaffolds with anisotropic structure for the regeneration of osteochondral tissue. J Tissue Eng Regen Med. 10.1002/term.1879 [DOI] [PubMed] [Google Scholar]
- Steinmetz NJ, Aisenbrey EA, Westbrook KK, Qi HJ, & Bryant SJ (2015). Mechanical loading regulates human MSC differentiation in a multi-layer hydrogel for osteochondral tissue engineering. Acta Biomater, 21, 142–153. 10.1016/j.actbio.2015.04.015 [DOI] [PubMed] [Google Scholar]
- Steinmetz NJ, & Bryant SJ (2011). The effects of intermittent dynamic loading on chondrogenic and osteogenic differentiation of human marrow stromal cells encapsulated in RGD-modified poly(ethylene glycol) hydrogels. Acta Biomaterialia, 7(11), 3829–3840. 10.1016/j.actbio.2011.06.031 [DOI] [PubMed] [Google Scholar]
- Verbruggen SW, Vaughan TJ, & McNamara LM (2014). Fluid flow in the osteocyte mechanical environment: a fluid–structure interaction approach. Biomechanics and Modeling in Mechanobiology, 13(1), 85–97. 10.1007/s10237-013-0487-y [DOI] [PubMed] [Google Scholar]
- Villanueva I, Hauschulz DS, Mejic D, & Bryant SJ (2008). Static and dynamic compressive strains influence nitric oxide production and chondrocyte bioactivity when encapsulated in PEG hydrogels of different crosslinking densities. Osteoarthritis and Cartilage, 16(8), 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DR, Lindsey DP, Li KW, Tummala P, Chandran SE, Smith RL, … Beaupre GS (2008). Hydrostatic pressure enhances chondrogenic differentiation of human bone marrow stromal cells in osteochondrogenic medium. Annals Of Biomedical Engineering, 36(5), 813–820. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang YS, Yue K, & Khademhosseini A (2017). Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomaterialia, 57, 1–25. 10.1016/j.actbio.2017.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourek G, McCormick SM, Mao JJ, & Reilly GC (2010). Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regenerative Medicine, 5(5), 713–724. 10.2217/rme.10.60 [DOI] [PMC free article] [PubMed] [Google Scholar]