Abstract
Background and Aims
The Extra-Uterine Environment for Neonatal Development (EXTEND) aims to avoid the complications of prematurity, such as NEC. Our goal was to determine if bowel development occurs normally in EXTEND-supported lambs, with specific emphasis on markers of immaturity associated with NEC.
Methods
We compared terminal ileum from 17 pre-term lambs supported on EXTEND for 2- 4 weeks to bowel from age-matched fetal lambs that developed in utero. We evaluated morphology, markers of epithelial integrity and maturation, enteric nervous system structure, and bowel motility.
Results
EXTEND-supported lamb ileum had normal villus height, crypt depth, density of mucin-containing goblet cells, and enteric neuron density. Expression patterns for I-FABP, activated caspase-3 and EGFR were normal in bowel epithelium. Transmural resistance assessed in Ussing chambers was normal. Bowel motility was also normal as assessed by ex vivo organ bath and video imaging. However, Peyer’s patch organization did not occur normally in EXTEND ileum, resulting in fewer circulating B cells in experimental animals.
Conclusion
EXTEND supports normal ileal epithelial and enteric nervous system maturation in pre-term lambs. The classic morphologic changes and cellular expression profiles associated with NEC are not seen. However, immune development within the EXTEND supported lamb bowel does not progress normally.
Keywords: Extreme Prematurity, Necrotizing Enterocolitis, Bowel Development, Fetal Lamb
Abbreviations used in this paper: ENS, enteric nervous system; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; EXTEND, EXTrauterine Environment for Neonatal Development; I-FABP, intestinal fatty-acid binding protein; ICC, interstitial cells of Cajal; IQR, interquartile range; NEC, necrotizing enterocolitis; PP, Peyer’s patch; TTX, tetrodotoxin
Summary.
The EXTEND (EXTrauterine Environment for Neonatal Development) system aims to avoid complications of prematurity, including necrotizing enterocolitis. To determine if bowel development progresses in EXTEND-supported lambs, we compared terminal ileum from 17 premature EXTEND-supported lambs with age-matched in utero control animals. We found that EXTEND supports normal intestinal maturation.
Extreme prematurity is the leading cause of infant morbidity and mortality in the developed world.1,2 To reduce morbidity and mortality associated with prematurity, we developed the EXTEND (EXTra-uterine Environment for Neonatal Development) system, aimed at limiting iatrogenic injury to immature organ systems.3 The system incorporates a pumpless oxygenator circuit connected to the fetus via an umbilical cord interface that is maintained within a closed amniotic fluid environment that closely reproduces the environment of the womb. Fetal lambs maintained within this system for up to 4 weeks demonstrate stable hemodynamics, normal fetal blood gas and oxygenation parameters, normal fetal circulation, and normal lung maturation, brain growth, and myelination.3, 4, 5, 6 Effects of EXTEND therapy on bowel development are not yet reported.
Bowel development is of particular relevance because one of the most common and deadly problems for premature infants is necrotizing enterocolitis (NEC), for which mortality approaches 50%.7, 8, 9, 10 NEC is unique to preterm infants, with higher incidence and severity in extreme prematurity, and is characterized by severe damage to bowel epithelium, inflammation, hemorrhage, enteric nervous system (ENS) injury, bacterial invasion of the bowel wall, and sometimes full-thickness bowel necrosis. Mechanisms underlying NEC are incompletely understood but are related to bowel immaturity.11, 12, 13, 14, 15, 16 By allowing continued intestinal development within EXTEND, we hope to improve outcomes for these babies.
One concern is that human infants in utero continuously swallow growth factor and nutrient-rich amniotic fluid (up to 200 mL/kg/d at term).17 In contrast, the “physiologic saline solution” used for EXTEND therapy is an electrolyte solution lacking trophic factors. This is important because loss of swallowed trophic factors that support gut epithelium is hypothesized to predispose to villus atrophy, feeding intolerance, and NEC18 in preterm human infants. The fetus also receives 10%–15% of its nitrogen and energy requirements from swallowed amniotic fluid.19
To test the hypothesis that the absence of these trophic factors may negatively affect gut maturation in EXTEND fetal lambs, we compared gut maturation in EXTEND lambs with age-matched fetal lambs maintained in utero. Bowel morphology, epithelial integrity, selected epithelial cell gene expression patterns, ENS structure, and bowel motility appear normal in EXTEND-maintained lambs as compared with control animals. In contrast, the EXTEND-maintained lamb bowel did not form lymphoid aggregates known as Peyer’s patches (PP), suggesting either a swallowed amniotic fluid component or missing maternal contribution is needed to support PP formation in fetal lambs.
Results
For all analyses, we compared ileum from preterm lambs maintained in EXTEND for 14–28 days to ileum of lambs of matched gestational age maintained in utero. During normal late gestation, there is significant maturation of bowel epithelium and ENS. We therefore focused initial analyses on epithelial maturation, ENS structure, bowel motility, and bowel transmural electrical resistance as a measure of bowel permeability.
Bowel Epithelium Appears Normal in Lambs Maintained in EXTEND
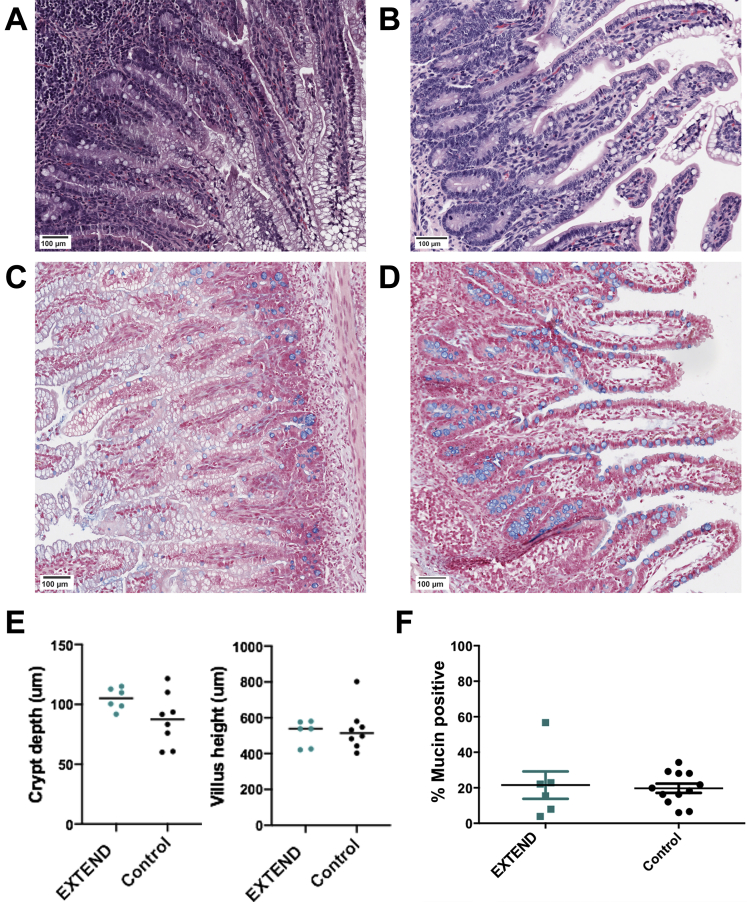
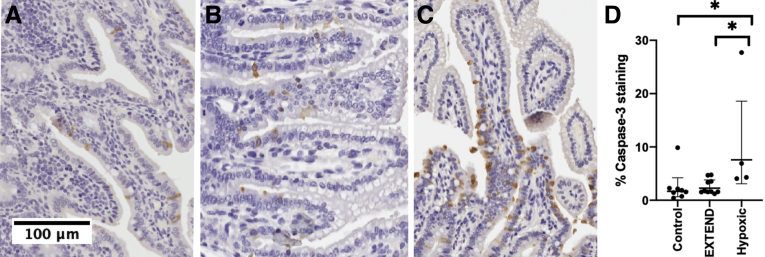
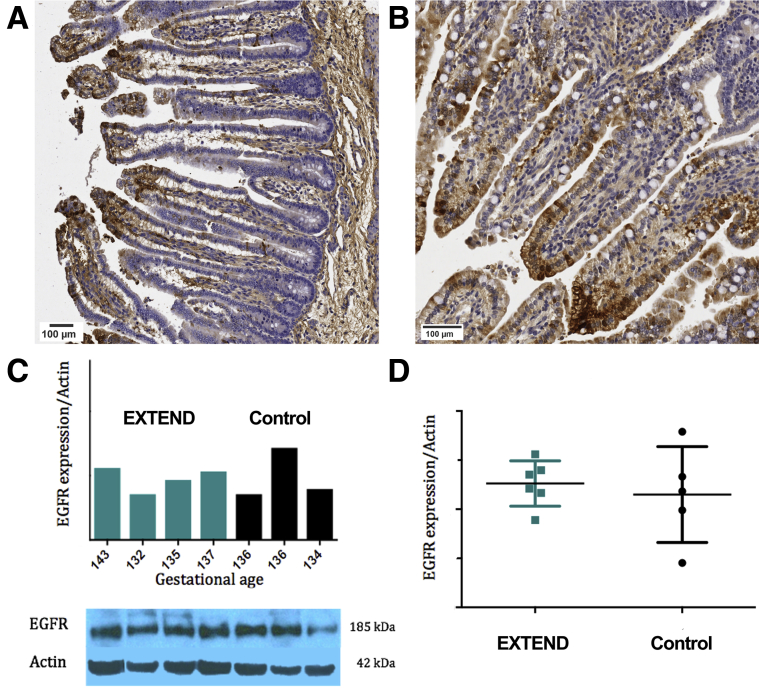
Ileum from EXTEND-maintained lambs did not have defects noted on gross anatomic analysis. Histology showed villus height (control group 536 ± 43 μm vs. EXTEND group 513.6 ± 29.3 μm; P = .631) and crypt depth (87.1 ± 7.7 μm vs. 104.8 ± 3.7 μm; P = .09) (Figure 1A–E) were not different between groups. Mucin-containing goblet cells, identified by Alcian blue staining, were present in comparable abundance (19.07% [interquartile range (IQR), 13.12%–28.13%] vs. 20.17% [IQR, 9.83%–22.7%]; P = .91) (Figure 1C, D, and F). Apoptotic cells identified by activated caspase-3 staining within the epithelial layer of the bowel appeared at normal frequencies (1.72% [IQR, 0.9%–2.2%] vs. 1.71% [IQR, 1.6%–3.8%]; P = .99). Animals maintained under hypoxic conditions within the EXTEND system were used as positive control animals for this experiment20 and had significantly more activated caspase-3 immunoreactive cells within terminal ileum sections (5.6% [IQR, 4.1–22.5]; P = .036 compared with control animals and P = .031 compared with normoxic EXTEND specimens) (Figure 2A–D). We also examined intestinal fatty-acid binding protein (I-FABP) expression, which tends to become more restricted to villus tips as lambs develop in utero and this progressive change in I-FABP staining pattern was observed in the EXTEND-maintained lamb ileum (Figure 3A and B). Finally, we evaluated levels of epidermal growth factor receptor (EGFR) by immunoblot.7,15 Ileal EGFR levels were similar in control and EXTEND-maintained terminal ileum (P = .73) (Figure 3C and D). These data suggest that maturation of lamb ileal epithelium proceeds normally in EXTEND.
Figure 1.
Bowel morphology appears normal in EXTEND-maintained lambs. (A, C) Control ileum (134 days gestational age) and (B) EXTEND-maintained ileum—141 days’ gestation after 27 days in EXTEND; (D) 135 days’ gestation after 28 days in EXTEND—stained with (A, B) hematoxylin and eosin or (C, D) Alcian blue and show similar morphology. (E) Villus height and crypt depth were similar in the control and EXTEND-maintained ileums. Each dot represents mean length for a single animal (10 villi and 10 crypts measured per lamb). (F) Goblet cell numbers, as a percent of epithelial cells counted, were similar in control and EXTEND-maintained lambs. Scale bars = 100 μm.
Figure 2.
Activated caspase-3 staining is normal in EXTEND-maintained lambs. (A) Control lamb (135 days in utero), (B) EXTEND-maintained lamb (139 days’ gestation after 28 days in EXTEND), and (C) hypoxic EXTEND-maintained lamb (131 days’ gestation after 23 days in hypoxic conditions within EXTEND) ileum were stained for activated caspase-3 (brown cells) and counterstained with hematoxylin. (D) The percentage of epithelial cells that were activated caspase-3 immunoreactive were similar in control and normoxic EXTEND-maintained lamb ileum. There was significantly more staining in the hypoxic group compared with the other 2 groups. Each dot indicates mean percent activated caspase-3 positive cells in bowel epithelium of 1 animal (n = 22,528 ± 9599 cells evaluated). Cells sloughed into the lumen of the bowel were not included in these counts. Scale bar = 100 μm.
Figure 3.
I-FABP and EGFR expression is normal in EXTEND-maintained lambs. (A, B) Experimental ileum from fetal lamb supported in EXTEND, stained for I-FABP; (A) 124 days (14 days in EXTEND) and (B) 135 days (23 days in EXTEND), showing restriction of I-FABP (brown staining in epithelium) to villus tips as evidence of epithelial cell maturation. Scale bar = 100 μm. (C) Representative immunoblot from EXTEND-maintained or control lamb ileum showing EGFR expression. Bar graph above the immunoblot shows EGFR abundance normalized to actin with the EXTEND maintained ileum in green and the control ileum in black bars. (D) EGFR abundance relative to actin for all evaluated animals shows no difference in expression between groups (P = .73).
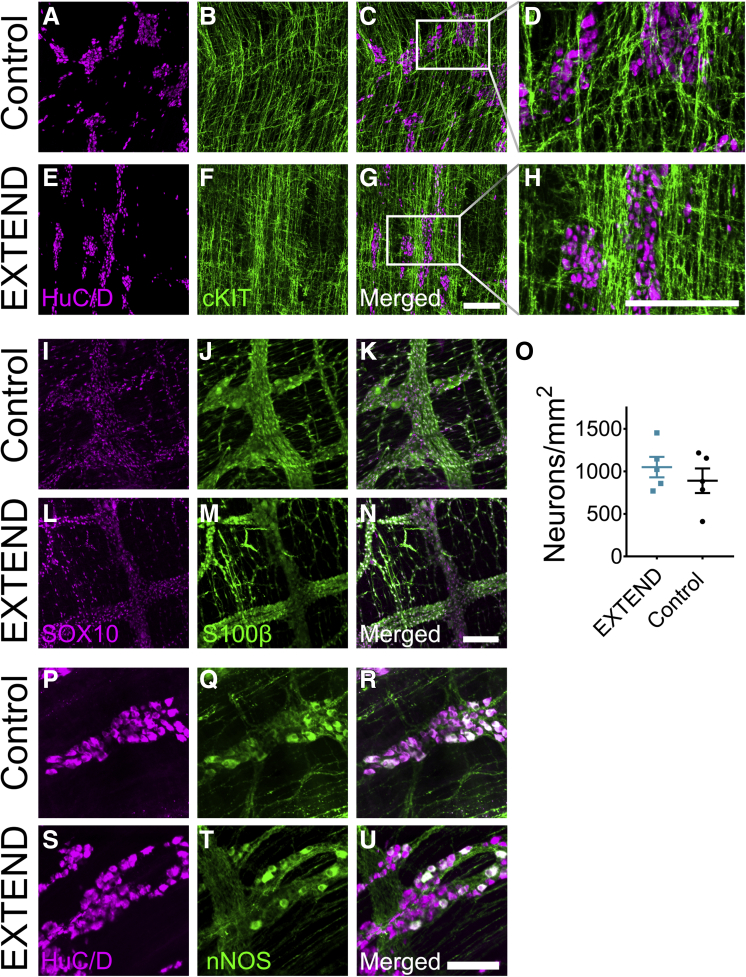
ENS and Interstitial Cells of Cajal Morphology Appears Normal in EXTEND-Maintained Lambs
The ENS, interstitial cells of Cajal (ICC), and bowel motility continue to mature before and after birth, but to our knowledge, bowel motility in fetal lambs has not been studied. To evaluate ENS and ICC structure in the EXTEND-maintained lamb ileum, we isolated muscle layers containing the myenteric plexus and performed confocal whole mount imaging after staining with antibodies against HuC/D (an RNA-binding protein specific to neurons), S100β (a mature glial marker), cKIT (a marker of ICC), SOX10 (an ENS precursor and glial marker), and nitric oxide synthase (the enzyme that makes nitric oxide to relax smooth muscle) (Figure 4A–N, P–U). Imaging showed similar organization of neurons, glia, ICC, and similar appearing nitric oxide synthase–expressing cells in both groups. Quantitative analysis demonstrated an equivalent density of enteric neurons (171.3 ± 43.8 vs. 131.2 ± 65.8, P = .33) (Figure 4O) in EXTEND lambs compared with control animals. This suggests that the ENS is structurally normal in the ileum of lambs maintained in EXTEND.
Figure 4.
Myenteric plexus appears normal in EXTEND-maintained lambs. (A–H) Representative confocal z-stacks of ileal muscle layers stained with antibodies against neuronal cell bodies (HuC/D) (magenta) and ICC (cKIT) (green) for (A–D) control and (E–H) EXTEND-maintained lambs. (I–N) Representative confocal z-stacks of ileal muscle stained with antibodies against glial cell nuclei (SOX10) (magenta) and glial cytoplasm (S100β) (green) for (I–K) control and (L–N) EXTEND-maintained lambs. (O) Myenteric neuron density was equivalent in control and EXTEND-maintained lambs. (P-U) Representative confocal z-stacks of ileal muscle stained with antibodies against neuronal cell bodies (HuC/D) (magenta) and neuronal nitric oxide synthase (nNOS) (green) for (P–R) control and (S–U) EXTEND-maintained lambs. All control images were taken from a control at 134-day gestational age. EXTEND images were taken from a lamb maintained for 27 days within the system to achieve 141 days’ gestational age. (U) Quantitative analysis of myenteric neuron density. Scale bars = 200 μm (A–H), 100 μm (I–T).
EXTEND Lambs Have Normal Bowel Contractility
To evaluate intestinal motility in EXTEND-maintained lambs and control animals, we generated spatiotemporal maps21 of bowel contractility over time (Figure 5A and D) using videos of ileal tissue maintained in a continuously perfused oxygenated organ bath. The spatiotemporal maps depict bowel diameter using a color code, location along the bowel on the x-axis, and time on the y-axis. Regular, fast (4.6–11.1 minutes–1) repetitive movements were observed in terminal ileum preparations from fetal lambs. Contractions with directional propagation accounted for some of the movement, while other movement appears to be due to longitudinal muscle contraction that shortens fetal bowel. Patterns of motility appeared similar in control and EXTEND-maintained ileum.
Figure 5.
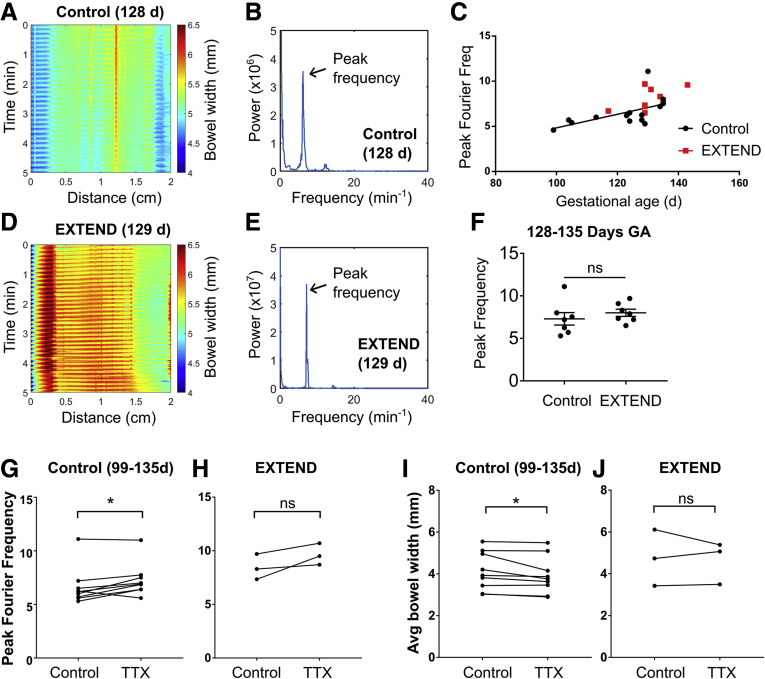
Bowel contractility is normal in control and EXTEND lambs. (A) Representative spatiotemporal map from a 128 days’ gestation control fetus. (B) Fourier transform of contraction data from the same fetus illustrating a peak frequency ∼6.4 minutes–1. (C) Control lambs (black circles) show increasing contraction frequency with age; contraction frequency in EXTEND lambs (red squares) is consistent with that of control animals. (D) Representative spatiotemporal map from 129 days’ gestation for an EXTEND fetus and (E) Fourier transform of same fetus with peak frequency ∼7.2 minutes–1. (F) EXTEND lambs had normal contraction frequency relative to age-matched controls (Student’s t test, P = .4197, n = 7 per group). (G) TTX mildly increased contraction frequency in control lambs at 99–135 days’ gestation (n = 9) and (I) decreased bowel width (n = 9). (H, J) These changes were not seen in EXTEND lambs, but the study likely had insufficient samples (n = 3) to detect these differences if these were present. ∗P < .05.
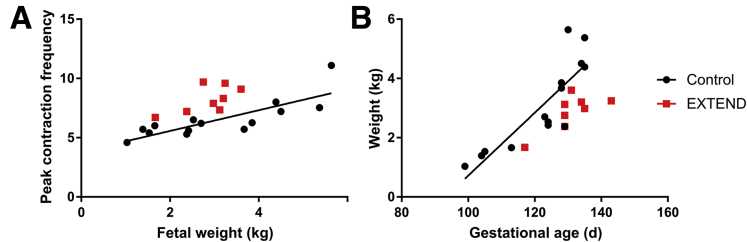
For more quantitative analysis of bowel motility, we used Fourier transforms to determine the peak oscillation frequencies during a 10-minute video (representative traces shown in Figure 5B and E; Supplementary Videos 1 and 2). We hypothesized that motility patterns might change with gestational age. We therefore assessed contraction rates in control lambs at ages ranging from 99 to 135 days’ gestation. We found a significant correlation between gestational age and contraction frequency in the control bowel (linear regression, P = .035; R2 = 0.3176) (Figure 5C, black line). Fetal weight also correlated with and may be a superior predictor of contraction frequency (linear regression, P = .0004; R2 = 0.665) (Figure 6). Ileum from EXTEND lambs had contractions similar to ileum of control lambs (Figure 5B and E). We did not perform linear regression on EXTEND lambs (red squares, Figure 5C) as there were insufficient age-variant data in this group. However, we found no difference between peak contraction frequencies in 128- to 135-day-old EXTEND and age-matched control animals using Student’s t test (P = .4197) (Figure 5F).
Figure 6.
Fetal weight and gestational age correlate with contraction frequency. (A) Fetal weight is positively correlated with contraction frequency in control lambs. Control animals represented by black circles (P = .0004, R2 = 0.665). (B) Lamb weights as a function of gestational age: control (black circles; P = .0001, R2 = 0.7294) and EXTEND (red squares). EXTEND lambs are known to weigh slightly less than age-matched control fetuses.
To determine if the rapid fetal bowel contractions had a neuronal origin, we applied the voltage-gated sodium-channel blocker tetrodotoxin (TTX), which blocks neural activity, to a subset of samples. Fast contractions still occurred in the presence of TTX, suggesting that the origin of this rhythm is probably non-neuronal (ie, owing to ICC or smooth muscle cell activity) (Figure 5G and H) as previously reported in other animals before birth).22 Notably, in control lambs at 99–135 days’ gestation, contraction frequencies were slightly (∼0.6175 ± 0.6598 Hz) but significantly higher in the presence of TTX (paired t test, P = .0229) (Figure 5G), indicating possible modulation of contraction frequency by neural inputs. TTX also slightly but significantly reduced average bowel width in control lambs, suggesting increased contractility as a result of blocking inhibitory motor neuron activity (signed rank test, P = .012) (Figure 5I). No difference in contraction frequency (paired t test, P = .148) (Figure 5H) or average bowel width (paired t test, P = .766) (Figure 5J) was found in EXTEND lambs, although only a few samples (n = 3) were analyzed compared with the analyses of samples from control animals (n = 9). Taken together, our results suggest that high-frequency contractions of non-neuronal origin are common in developing lamb bowel, and that these contractions may increase in frequency as the lamb matures. Bowel contractility in fetal lambs on EXTEND closely resembled contractility patterns in control animals, suggesting normal development of intestinal musculature.
Transileal Electrical Resistance Is Normal in EXTEND-Maintained Lambs
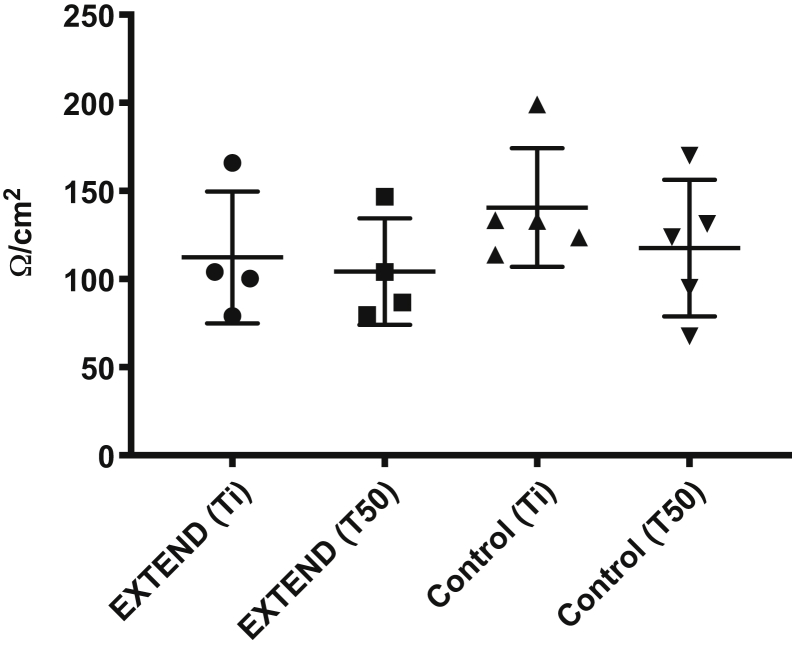
To test bowel barrier integrity, we measured transmural electrical resistance of ileum. There were no significant differences in transmural resistance measured across EXTEND ileum compared with the control ileum. Furthermore, the tissue did not exhibit a decrease in electrical resistance during a 50-minute interval in the Ussing chamber (unpaired t tests for each time point were all not significant, P > .1; 2-way analysis of variance, P = .38) (Figure 7).
Figure 7.
Transmural electrical resistance is normal in EXTEND-maintained lambs. Transmural electrical resistance was measured in terminal ileum from EXTEND-maintained (n = 4) and control lambs (n = 5; triangles) shortly after mounting tissue in an Ussing chamber (Ti) and after 50 minutes in the chamber (T50). Each point represents data from 1 lamb. There was no significant difference in transmural electrical resistance between the control and EXTEND-maintained bowels.
Intestinal Immune Development Does Not Progress Normally Within EXTEND
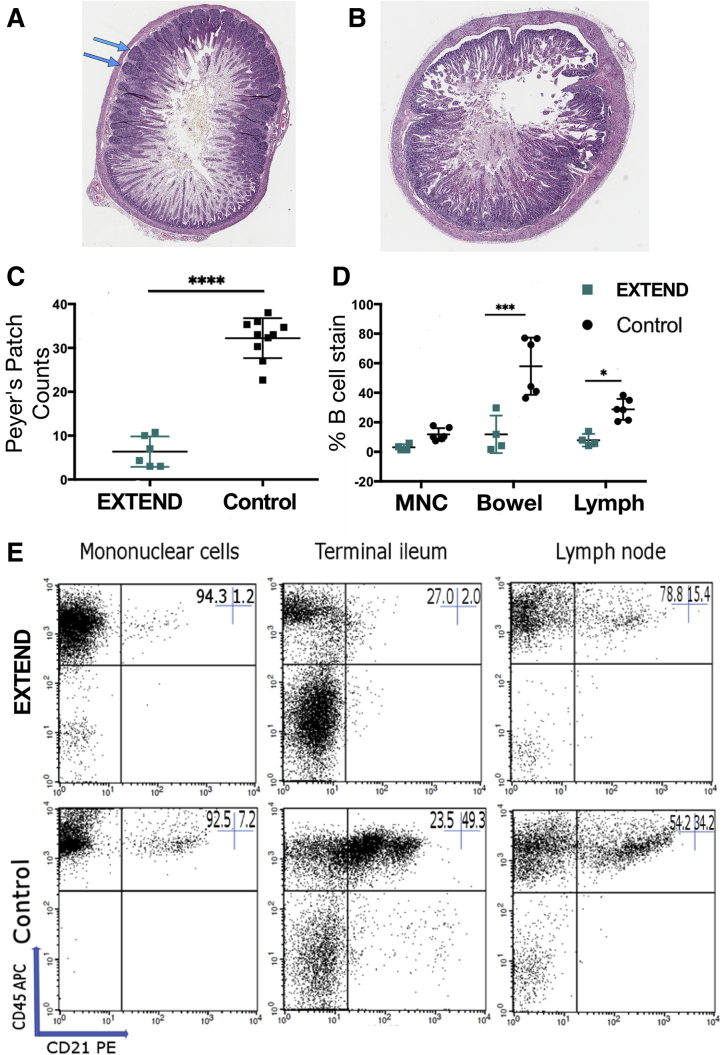
One difference we found between groups is a reduction in PP formation in the ileum of EXTEND bowel, compared with control animals (P < .00001) (Figure 8A–C). The absence of these primary lymphoid follicles correlated with reduced B cells in the ileum (P < .0001) and in lymph nodes (P = .02), as well as a trend toward reduced circulating mononuclear cells (P = .08) as measured by flow cytometry (Figure 8D and E). B cell populations in the other lymphoid organs, including the thymus (P = .61), spleen (P = .56), and liver (P = .63), were normal compared with control animals.
Figure 8.
PPs were rare in the EXTEND-maintained ileum, resulting in reduced B cell abundance in EXTEND-maintained lambs. (A) Control terminal ileum from 128 days’ gestational age has many organized lymphoid follicles. (B) Terminal ileum from a lamb that spent 111 days in utero and 28 days in EXTEND (total gestation 139 days) has few PPs (none visible in this section). (C) Quantitative analysis confirms dramatic reduction in PPs in the EXTEND-maintained terminal ileum. Each dot indicates mean PP counts from 1 lamb (3 cross-sections analyzed for each). (D) Cells staining positive for the B cell marker CD21 as a percentage of total cells staining for CD45 within peripheral blood mononuclear cells (MNC), terminal ileum, and prescapular lymph node cells. (E) Representative FACS plots showing decreased B cell populations in EXTEND-maintained MNC, terminal ileum, and lymph nodes. Percentages for these representative plots are recorded in the upper right-hand corner of each. ∗P < .05, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Discussion
The EXTEND system supports continued fetal development, allowing the transition to postnatal life at a later developmental stage. EXTEND lambs also avoid NEC risk factors like formula feeding, intermittent hypoxia, cold stress, and lipopolysaccharide exposure during that extremely premature period. Therefore, if gut maturation continues normally within EXTEND, it is possible that EXTEND will also reduce the frequency of NEC in premature mammals. However, to assume this, we need to know if gut maturation continues normally within the system. Because there are no established NEC models in lamb, and susceptibility to NEC-like disease in premature lambs is not known, these studies do not test if EXTEND prevents NEC. Our interest in NEC-associated changes is academic, providing a framework for the analysis, which is focused on bowel maturation. The absence of swallowed gut trophic factors in EXTEND raises concern that bowel in EXTEND-maintained fetal animals (or human children) might not mature normally. In this study, we demonstrate that important elements of barrier function, contractility, enteric nervous system development, and enterocyte maturation are equivalent in EXTEND-maintained lambs to age-matched control animals, and that the classic morphologic changes and cellular expression profiles associated with NEC are not seen. We did, however, see arrest of lymphoid follicle aggregation within the bowel in lambs developing within the EXTEND system. The cellular signaling error underlying this arrest in PP formation and the implications within the human fetus are unknown. Nevertheless, our data suggest that continued maturation of the intestine is supported within the EXTEND system.
The Intestinal Barrier
In premature humans, intestinal barrier immaturity results in increased intestinal permeability, enhanced bacterial adherence,7 intestinal injury, and bacterial translocation.11,12 The thick glycoprotein mucin layer, secreted by goblet cells, reinforces the cellular integrity of the intestinal barrier.11 In addition to mechanical protection, this layer facilitates aggregation and removal of adherent bacteria, and traps enzymes near the epithelial surface to aid digestion.7 During NEC, production of mucin is impaired, and a pathologic increase in epithelial apoptosis leads to compromised bowel integrity.23, 24, 25 In our experimental lambs, intestinal epithelium showed no difference in villus height, crypt depth, mucin-positive goblet cells, or epithelial apoptosis. These immunohistochemistry findings are supported by our evaluation of transmural resistance. The formation, quality, and permeability of tight junctions largely determines the resistance and integrity of the epithelial tissue.26 Furthermore, Ussing chamber studies have suggested that bowel transmural resistance correlates with tissue permeability and is significantly decreased in human tissue affected by NEC.27 Our results show no significant difference in transmural resistance in ileal samples. These findings suggest that bowel permeability after development within EXTEND is not significantly different than permeability of bowel from age-matched lambs developing in utero.
Gut Motility
In humans, gastrointestinal motility begins to develop in the 2nd trimester and continues to mature into the third trimester, with motor complexes appearing at ∼34 weeks’ gestation.7 Early delivery compromises normal gastrointestinal motility, and poor motility may increase vulnerability to NEC by permitting bacterial overgrowth, adherence, and increased endotoxin exposure.7,12,28 We have shown normal contractile function of the EXTEND lamb bowel in an in vitro organ bath. Because the contractions we observed were not blocked by TTX, they likely originated from a non-neuronal source (ie, ICC or smooth muscle cells). Developmental increases in slow wave frequency of bowel contraction have been previously described,22 and we saw a linear increase in frequency among control and experimental specimens with increasing gestational age and fetal weight. We also evaluated the ENS and found normal myenteric neuron density, suggesting that ENS morphology may develop normally in EXTEND-maintained lambs.
Epithelial Cell Differentiation
Many of our studies indicate normal epithelial maturation in the EXTEND environment. In addition to anatomic studies, we found normal expression patterns for I-FABP and EGFR. EGFR is especially important because EGF is a major trophic factor for the bowel epithelium found in amniotic fluid. EGFR levels are higher in healthy human fetal intestine than in intestine of premature infants.7,15 Exogenous infusion of EGF in utero can accelerate maturation of intestinal enzyme activity and stimulate intestinal growth in animal models.23,29,30 Our staining for caspase-3, which is activated within apoptotic epithelial cells, also showed equivalent rates of apoptosis in our experimental and control bowel, further supporting unperturbed balance within the experimental bowel epithelium.
Lamb Immune Development
An intriguing finding of our study was the decreased number of PP in the EXTEND-maintained intestine. Consistent with this observation, EXTEND-maintained lambs had fewer B cells in intestine and lymph nodes. PP in lamb are a primary germinal center for B lymphocytes.31,32 The lamb terminal ileum begins to organize PP from 100 to 110 days’ gestational age,33,34 the time frame during which we begin EXTEND support. In humans, B cells are produced in bone marrow and populate PPs, which are a secondary lymphoid organ, postnatally. We do not yet understand why PPs are missing from the ileum of the EXTEND lamb. One possibility is that our synthetic amniotic fluid lacks a swallowed ligand that is integral to PP organization. However, the absent factors might be contributed by maternal hematologic or placental contributions that are also missing in EXTEND. The importance of this finding for human applications is unclear, as PP organization in humans occurs predominantly after birth.
Why Don’t We See Impaired Bowel Maturation?
Trophic components of natural amniotic fluid thought to be beneficial for bowel maturation include EGF,18,35, 36, 37 heparin-binding EGF,38, 39, 40 granulocyte colony-stimulating factor,41,42 erythropoietin,43,44 tumor necrosis factor β, various interleukins, insulin-like growth factor-1, and glutamine,45,46 as well as other potentially beneficial factors. These factors are also found in colostrum and breast milk at increasing concentrations in the mothers of the most premature infants.47 Many hypothesize the increased susceptibility to developing NEC in preterm infants is related to the absence of these swallowed proteins.15,16,29 Given the significant body of evidence linking natural amniotic fluid proteins to bowel development,17,36, 37, 38 we were surprised that intestinal development appeared normal in EXTEND lambs. One possible explanation is that natural growth factors are not as critical for intestinal development as previously believed. There are contradictory data published in rabbit47 and sheep48,49 regarding bowel epithelial growth after fetal esophageal ligation. Some studies suggest that amniotic fluid factors may not be required for normal fetal intestinal development, while others report the opposite.49 Notably, within EXTEND, swallowing occurs in all experimental animals. The volume of fluid swallowed as gestation progresses is substantial and not replicated in the neonatal intensive care unit environment. Perhaps this mechanical influence on the intestine is sufficient to achieve normal intestinal maturation.50, 51, 52 Additionally, swallowed growth factors produced in the salivary gland (eg, EGF)53 may be sufficient to support gut development.
Limitations
A caveat of this study is our inability to evaluate performance of the bowel once the fetus is delivered from the EXTEND system. We have pursued survival for very few experimental animals. In the animals that were delivered, ventilated, and formula fed, via orogastric and then bottle feeding, we did not see evidence of bowel necrosis or features of immaturity upon final histologic evaluation. However, we do not have enough experience with EXTEND survivors to draw scientific comparisons with control lambs. Further, we are unable to compare the extremely premature lambs who entered the EXTEND system to extremely premature lambs cared for with standard neonatal intensive care unit support, to understand if the natural incidence of NEC in the lamb is comparable to that in the human. The lamb is not an established animal model for NEC, and we do not know if NEC would develop in premature lambs as it does in humans. However, our study’s comparison with age-matched in utero control specimens does allow for evaluation of organ maturation as gestation progresses, and here we show no compromise as a result of support within EXTEND that might affect human fetal development, as compared with normal in utero development.
Conclusion
The EXTEND system supports continued development of the immature fetal intestine without significant adverse effects on ileal epithelial histology, transmural resistance, or bowel motility. This suggests that the clinical use of the EXTEND system as an alternate therapy for support of extremely premature infants may decrease the incidence of NEC by supporting continued organ maturation. The relevance of reduced PP formation in the EXTEND-maintained lamb compared with human infants is unknown because of differences in immune system development in the human and lamb.
Methods
Animals
The Institutional Animal Care and Use Committee of the Children’s Hospital of Philadelphia approved all procedures. Specimens were collected from 17 preterm lambs supported on EXTEND from 102–117 days (last in utero day) to 129–144 days (gestational age equivalent; term ∼145 days). Eighteen gestational age-matched lambs that developed in utero served as control animals. Four specimens from EXTEND animals maintained deliberately under hypoxic conditions were used as positive controls for the apoptosis analyses. Normoxia was defined as oxygen delivery of 20–25 mL/kg/min, and hypoxia was defined as oxygen delivery of 14–16 mL/kg/min to mimic physiologic and pathologic intrauterine conditions, respectively. Fetal oxygen delivery was continuously measured and recorded (LabChart 5; ADInstruments, Colorado Springs, CO) via measurement of weight-based umbilical blood flow (HXL Tubing Flowsensor; Transonic Systems Inc, Ithaca, NY), postmembrane saturation, and hematocrit concentration (M2-Sensor; Spectrum Medical, Gloucester, United Kingdom). When oxygen delivery fell outside target ranges, this triggered an adjustment in the oxygen tension of the sweep gas, a blended mixture of nitrogen, air, and oxygen. All animals were maintained for a period of 2–4 weeks in EXTEND. The terminal ileum was sectioned for analysis, as this is the area most susceptible to NEC in human neonates. Throughout EXTEND therapy, fetal lambs were immersed in “physiologic saline solution” (92-mEq NaCl, 19.4-mEq NaHCO3, 6.7-mEq KCl, 1.7-mEq CaCl2, pH 7.0) that was continuously exchanged at a rate of 40–80 mL/min. Nutrition was provided parenterally. Each analysis described subsequently includes a subset of the total tissue collected, with specimens chosen for each experiment based on appropriate age matching and tissue preparation for that particular assay (Table 1).
Table 1.
Experimental Animals
| Experiment | Experimental | Gestational age at removal (d) | Days on EXTEND | Control | Gestational age at delivery (d) |
|---|---|---|---|---|---|
| Histology | 6 | 135–144 (138.8 ± 3.9) | 19–28 (24.8 ± 4.2) | 8 | 135–141 (138.3 ± 2.4) |
| Immunohistochemistry | 10 | 135–144 (138.8 ± 3.9) | 17–28 (24.8 ± 4.2) | 12 | 135–141 (138.1 ± 3.9) |
| ENS evaluation | 5 | 128–135 (131.3 ± 3.0) | 14–27 (22.4 ± 5.1) | 5 | 129–135 (132.2 ± 2.5) |
| Contractility | 7 | 128–135 (130.7 ± 2.8) | 14–27 (21.7 ± 5.1) | 7 | 128–135 (131.3 ± 3.3) |
| EGFR expression | 6 | 130–143 (134.2 ± 4.9) | 18–27 (23.7 ± 3.7) | 5 | 134–137 (136 ± 1.2) |
| Permeability study | 4 | 128–134 (130 ± 2.7) | 21–27 (23.5 ± 2.6) | 5 | 128–134 (130 ± 2.6) |
| Immune development | 4 | 128–134 (130 ± 2.7) | 21–27 (23.5 ± 2.6) | 6 | 128–134 (130 ± 2.3) |
Values are n or range (mean ± SD). Within the immunohistochemistry section, 4 hypoxic-maintained EXTEND animals were included and used as positive controls.
EGFR, epidermal growth factor receptor; ENS, enteric nervous system; EXTEND, EXTrauterine Environment for Neonatal Development.
Histology
Three-centimeter sections from the final 10 cm of terminal ileum were formalin-fixed, paraffin-embedded, sectioned at 4 μm, and stained with hematoxylin and eosin for morphologic evaluation and Alcian blue for identification of mucin-containing goblet cells using established methods.54 Morphological analysis of villus height and crypt depth used 2 slides per animal, each with 2–3 transverse sections of ileum. A total of 8–12 fully intact villus/crypt pairs per animal were measured using the caliper tool within ImageScope Aperio eSlide Manager Version 12.2.1.5005 (Leica Biosystems, Buffalo Grove, IL). Lymphoid follicles were counted from 3 transverse sections per specimen.
Immunohistochemical Staining
Immunostaining for activated caspase-3 and I-FABP required heat-induced epitope retrieval, performed by microwave in target retrieval buffer at pH 6.0 (Cat#S699; Dako, Santa Clara, CA), followed by 30-minute cool down to room temperature. Slides were rinsed in Tris-buffered saline with Triton 0.1% (pH 7.6) and then incubated in primary antibody as follows: rabbit anti-activated caspase-3 (1:100, Cat #ab4051; Abcam, Cambridge, United Kingdom, RRID:AB_304243) and goat anti-I-FABP (1:400, Cat #ab60272; Abcam, RRID:AB_943726). Slides were then rinsed in Tris-buffered saline with Triton prior to incubation for 30 minutes with Super Picture HRP Polymer Conjugate Broad Spectrum (Invitrogen, Carlsbad, CA) at RT. Slides were developed with 3′3′-diaminobenzidene (Cat#SK4100; Vector Labs, Burlingame, CA) chromagen and counterstained with hematoxylin (Vector Labs).
Gut sections were analyzed at 20× magnification using image analysis software (ImageScope Aperio eSlide Manager Version 12.2.1.5005). Cell-counting macros, developed based on color thresholds, were used for quantification of mucin-containing goblet cells and for cells expressing caspase-3 within the epithelial layer of the terminal ileal sections (Figure 9).55,56 I-FABP staining was analyzed for previously described staining progression from the epithelial crypts to villous tips that correlates with advancing gestational age.22
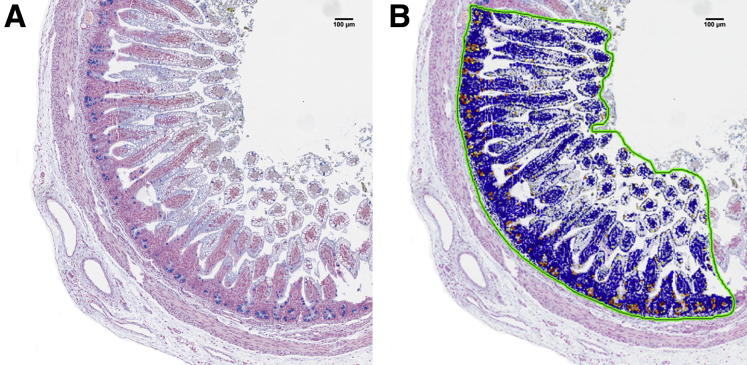
Figure 9.
Cell-counting macros. Macros were developed based on color thresholds and used for quantification of mucin-containing goblet cells and for cells expressing caspase-3 within the epithelial layer of the terminal ileal sections. (A) Goblet cells containing mucin stain blue; (B) Cell-counting macro shows epithelial cells in blue and mucin-containing goblet cells in yellow and orange.
Analysis of EGFR Expression
EGFR protein expression was assessed by immunoblot in full-thickness antimesenteric sections of fresh frozen terminal ileum. Tissue (30 mg) was homogenized in 600 μL of tissue lysis buffer (Sigma CelLytic MT; Sigma-Aldrich, St. Louis, MO) with a protease inhibitor and incubated at 4°C for 4 hours before centrifugation at 14,000 rpm for 10 minutes to pellet debris. Supernatant was loaded onto 7% Invitrogen Nu-Page Tris-acetate polyacrylamide gels at 25 μg of protein per well. Gels were run using Tris-acetate sodium dodecyl sulfate running buffer (180 volts, 1.5–2 hours) and transferred onto Novex Nitrocellulose membranes (0.2-μm pore size) (Thermo Fisher Scientific, Waltham, MA) using NuPAGE transfer buffer with 10% methanol for 5 hours at 35 volts. Membranes were blocked prior to incubating in primary antibody (rabbit anti-EGFR, 1:1000, Cat#ab52894; Abcam, RRID:AB_869579) at 4°C overnight. After washing in Tris-buffered saline with Triton, membranes were incubated in secondary antibody (horseradish peroxidase–conjugated anti-rabbit IgG, 1:10,000; Cell Signaling Technology, Danvers, MA) for 1 hour at 25°C. Thermo Scientific SuperSignal West Dura Extended Duration Substrate was used to generate signal prior to manual film development. Developed film was scanned and analyzed using ImageJ software (1.51n; National Institutes of Health, Bethesda, MD). Protein concentrations were normalized using an Actin probe as a separate incubation process. The membranes were stripped with Restore Western Blot Stripping Buffer Cat#21059; Thermo Fisher Scientific) and blocked prior to incubating in anti-Actin antibody produced in rabbit (Cat#A2066; Sigma-Aldrich).
Immunostaining of ENS and ICC
Immunofluorescent whole-mount staining of lamb ileum was performed to quantify neuronal cell bodies within a uniform area of terminal ileum. Segments of ileum (3–5 cm), obtained from 10 cm proximal to the ileocecal junction, were opened along the mesenteric border, pinned flat on Sylgard (Dow Corning, Midland, MI), fixed (4% paraformaldehyde, 25°C, 1–2 hours), and stored at –20°C in 50% glycerol/50% phosphate-buffered saline (PBS). Prior to immunostaining, bowel pieces were washed in PBS and further dissected to separate muscle layers from submucosa. Dissected smooth muscle was blocked overnight (5% normal donkey serum in PBS with 0.5% Triton X-100, 4°C), and subsequently incubated with primary antibody (7–10 days, 4°C). Primary antibodies used were mouse anti-HuC/D (1:400, Cat#A-21271; Invitrogen, RRID:AB_221448), goat anti-Sox10 (1:100, Cat#sc-17342; Santa Cruz Biotechnologies, Dallas, TX, RRID:AB_2195374), rabbit anti-c-Kit (1:100, Cat#ab-32363; Abcam, RRID:AB_713513), and rabbit anti-S100β (1:100, Cat#Z0311; Dako, Santa Clara, CA, RRID: AB_10013383). Samples washed overnight in phosphate-buffered saline, incubated with secondary antibodies (Alexa Fluor donkey anti-mouse, donkey anti-goat, donkey anti-rabbit 488, 594, or 647 nm; Invitrogen, Carlsbad, CA, 1:400) at 25°C for 1 hour, and washed overnight in phosphate-buffered saline before mounting (50% glycerol/50% phosphate-buffered saline). Z-stacks were obtained using a confocal microscope (Zeiss LSM 710 confocal; Zen Software, Oberkochen, Germany), and cells were counted using ImageJ software (1.51n). Neuron density was quantified by counting cells in a 100 μm2 area from 8 randomly selected 20× fields per lamb.
Contractility
To measure contractility, a 3-cm section of small bowel, taken 10 cm proximal to the ileocecal junction, was placed in warmed (37°C), oxygenated (95% O2, 5% CO2) Krebs-Ringer solution57 in a continuously circulating organ bath (Hugo Sachs Elektronik Harvard Apparatus D-79232; Harvard Apparatus, Holliston, MA). Bowel was equilibrated for 10 minutes, after which a 10-minute video was recorded using an E-PM1 Olympus camera (15 frames per second, 1920 × 1080 pixel resolution; Olympus, Tokyo, Japan). Tetrodotoxin (TTX) (Cat#4368-28-9; Abcam) reconstituted with sodium citrate buffer (pH 4.8) as a 1-mM stock was stored at –20°C until use. Stock solution was then diluted to 1 μM in Krebs-Ringer solution to evaluate TTX effects on the bowel.
Two-dimensional spatiotemporal maps21 were generated from video files using previously described MATLAB 2017 (The MathWorks, Natick, MA) scripts.57 Fourier transforms were performed on each cross-section of a given spatiotemporal map and averaged to determine peak contraction frequency.
Transmural Resistance
Ex vivo analysis of transmural electrical resistance as a measure of permeability58,59 was performed by mounting intestinal tissue in a vertical Ussing chamber and using Acquire & Analyze data acquisition software (Physiological Instruments, San Diego, CA). The antimesenteric portion of a segment of terminal ileum was pinned to sliders (Physiologic Instruments #P2305, 0.49-cm2 opening) between symmetric solutions of oxygenated Krebs-Ringer solution. Transmural resistance (Ω∗cm2) was monitored and calculated according to Ohm’s law under short circuit conditions by the application of 2-mV bidirectional pulses every 10 seconds. These resistance measurements were averaged over 5 minutes both initially upon tissue mounting, and after 50 minutes to test the stability of transmural resistance ex vivo.
Immune System Phenotyping
Sections of terminal ileum (250 mg, antimesenteric tissue, within 10 cm of the ileocecal junction) were cut into small pieces and incubated in digestion buffer (25-mL RPMI, 300-μL fetal bovine serum, 12.5-mg dispase, 37.5-mg collagenase II) for 1–2 hours at 37°C prior to mechanical cell straining through a 70-μm filter. Unweighed, undigested portions of spleen, thymus, prescapular lymph node, liver, and bone marrow were similarly mechanically disrupted and filtered. Ficoll (Sigma-Aldrich) was used to separate tissue cells from blood. Peripheral blood was lysed to isolate mononuclear cells. Terminal ileum cell isolates were counted. These preparations were stained with antibody (mouse anti-sheep CD45:Biotin, Cat#MCA2220B [Bio-Rad, Hercules, CA], RRID:AB_566761; mouse anti-sheep CD4:FITC, Cat#MCA2213F [Bio-Rad], RRID:AB_324690; mouse anti-sheep CD8:RPE, Cat#MCA2216PE [Bio-Rad], RRID:AB_566897; mouse anti-sheep CD14:FITC, Cat#MCA920GA [Bio-Rad], mouse anti-bovine CD21:RPE, Cat# MCA1424PE [Bio-Rad]). Flow cytometry was performed using BD FACSCalibur (BD Biosciences, Franklin Lakes, NJ) equipped with 2 lasers (blue laser, 488 nm; red diode laser, 635 nm) and compensated with APC, FITC, and PE single-stained controls before experimental sample acquisition. Percentage of CD4, CD8, CD14, and CD21 expression in CD45+ cell population were analyzed with CellQuest Pro software (BD Biosciences).
Statistical Analyses
Statistical analyses were performed using SigmaPlot 11 (Systat Software, San Jose, CA), Prism 8 (GraphPad Software, San Diego, CA), or MATLAB. Unpaired Student’s t tests compared normally distributed data, and Mann Whitney U tests were used for nonparametric data. Data are presented as mean ± SEM and median and IQR. A paired Student’s t test or signed rank test was used for comparing paired data. Two-way analysis of variance was used to evaluate transmural resistance between control and experimental tissue at different time points.
Footnotes
CRediT Authorship Contributions Heron Dawn Baumgarten, MD, MPH (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Project administration: Lead; Writing – original draft: Lead; Writing – review & editing: Lead) Christina M Wright, MD, PhD (Conceptualization: Supporting; Data curation: Supporting; Formal analysis: Equal; Investigation: Equal; Methodology: Equal; Project administration: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting) Avery C Rossidis, MD (Data curation: Supporting; Formal analysis: Supporting; Project administration: Supporting; Writing – review & editing: Supporting) Kendall M Lawrence, MD (Data curation: Supporting; Investigation: Supporting; Project administration: Supporting; Writing – review & editing: Supporting) Aimee G Kim, MD (Data curation: Supporting; Investigation: Supporting; Project administration: Supporting) Ali Y Mejaddam, MD (Data curation: Supporting; Investigation: Supporting; Project administration: Supporting) Patrick E McGovern, MD (Data curation: Supporting; Investigation: Supporting; Project administration: Supporting) Melissa N Orr, BS (Formal analysis: Supporting; Project administration: Supporting) Barbara E Coons, MD (Project administration: Supporting) Zoya Butt, MD (Data curation: Supporting; Investigation: Supporting) Haiying Li, BS (Data curation: Supporting; Formal analysis: Supporting; Methodology: Supporting; Validation: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting) Grace Hwang, BS (Project administration: Supporting) Antoneta Radu, MD (Methodology: Supporting; Project administration: Supporting) Lauren J Brown, BS (Project administration: Supporting) Ronald C Rubenstein, MD, PhD (Formal analysis: Supporting; Resources: Supporting;Writing – review & editing: Supporting) William H Peranteau, MD (Project administration: Supporting; Writing – review & editing: Supporting) Marcus G Davey, PhD (Investigation: Supporting; Project administration: Supporting; Supervision: Supporting; Writing – review & editing: Supporting) Robert O Heuckeroth, MD, PhD (Conceptualization: Supporting; Formal analysis: Supporting; Funding acquisition: Supporting; Investigation: Supporting; Methodology: Supporting; Resources: Supporting; Supervision: Equal; Writing – original draft: Supporting; Writing – review & editing: Equal) Alan W Flake, MD (Conceptualization: Equal; Formal analysis: Supporting; Funding acquisition: Lead; Resources: Equal; Supervision: Lead; Writing – review & editing: Supporting)
Conflicts of interest These authors disclose the following: Alan W. Flake and Marcus Davey are shareholders in corporate production of EXTEND. Robert O. Heuckeroth is a consultant for BlueRock Therapeutics and on a Scientific Advisory Board for Takeda. The remaining authors disclose no conflicts.
Funding This work was supported by the Canadian Institutes of Health Research (#377028) (to Robert O. Heuckeroth), the Irma and Norman Braman Endowment, the Suzi and Scott Lustgarten Center Endowment, the Children’s Hospital of Philadelphia Research Institute, and the Children’s Hospital of Philadelphia Institutional Development Fund (to Alan W. Flake).
Supplementary Material
References
- 1.Matthews T.J., MacDorman M.F. Infant mortality statistics from the 2010 period linked birth/infant death data set. Natl Vital Stat Rep. 2013;62:1–26. [PubMed] [Google Scholar]
- 2.Patel R.M., Kandefer S., Walsh M.C., Bell E.F., Carlo W.A., Laptook A.R., Sanchez P.J., Shankaran S., Van Meurs K.P., Ball M.B., Hale E.C., Newman N.S., Das A., Higgins R.D., Stoll B.J., Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372:331–340. doi: 10.1056/NEJMoa1403489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Partridge E.A., Davey M.G., Hornick M.A., McGovern P.E., Mejaddam A., Vrecenak J.D., Mesas-Burgos C., Olive A., Caskey R.C., Weiland T.R., Han J., Schupper A.J., Connelly J.T., Dysart K.C., Rychik J., Hedrick H.L., Peranteau W.H., Flake A.W. An extra-uterine system to physiologically support the extreme premature lamb. Nat Commu. 2017;8:15112. doi: 10.1038/ncomms15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossidis A.C., Angelin A., Lawrence K.M., Baumgarten H.D., Kim A.G., Mejaddam A.Y., Coons B.E., Hartman H.A., Hwang G., Monos S., Peranteau W.H., Davey M.G., Murdock D., Wallace D.C., Flake A.W. Premature Lambs exhibit normal mitochondrial respiration after long-term extrauterine support. Fetal Diagn Ther. 2019;46:306–312. doi: 10.1159/000496232. [DOI] [PubMed] [Google Scholar]
- 5.Mejaddam A.Y., Hornick M.A., McGovern P.E., Baumgarten H.D., Lawrence K.M., Rossidis A.C., Hwang G., Young K., Abdulmalik O., Partridge E.A., Peranteau W.H., Davey M.G., Flake A.W. Erythropoietin prevents anemia and transfusions in extremely premature lambs supported by an EXTrauterine Environment for Neonatal Development (EXTEND) Fetal Diagn Ther. 2019;46:231–237. doi: 10.1159/000493680. [DOI] [PubMed] [Google Scholar]
- 6.Hornick M., Mejaddam A., McGovern P., Hwang G., Han J., Peranteau W., Partridge E., Davey M., Flake A. Technical feasibility of umbilical cannulation in midgestation lambs supported by the EXTra-Uterine Environment for Neonatal Development (EXTEND) Artif Organs. 2019;43:1154–1161. doi: 10.1111/aor.13524. [DOI] [PubMed] [Google Scholar]
- 7.Lin P.W., Nasr T.R., Stoll B.J. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol. 2008;32:70–82. doi: 10.1053/j.semperi.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Nair R.R., Warner B.B., Warner B.W. Role of epidermal growth factor and other growth factors in the prevention of necrotizing enterocolitis. Semin Perinatol. 2008;32:107–113. doi: 10.1053/j.semperi.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Lee J.S., Polin R.A. Treatment and prevention of necrotizing enterocolitis. Semin Neonatol. 2003;8:449–459. doi: 10.1016/S1084-2756(03)00123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi Y.Y. Necrotizing enterocolitis in newborns: update in pathophysiology and newly emerging therapeutic strategies. Korean J Pediatr. 2014;57:505–513. doi: 10.3345/kjp.2014.57.12.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark J.A., Doelle S.M., Halpern M.D., Saunders T.A., Holubec H., Dvorak K., Boitano S.A., Dvorak B. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol. 2006;291:G938–G949. doi: 10.1152/ajpgi.00090.2006. [DOI] [PubMed] [Google Scholar]
- 12.Claud E.C. Probiotics and neonatal necrotizing enterocolitis. Anaerobe. 2011;17:180–185. doi: 10.1016/j.anaerobe.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Afrazi A., Branca M.F., Sodhi C.P., Good M., Yamaguchi Y., Egan C.E., Lu P., Jia H., Shaffiey S., Lin J., Ca C., Vincent G., Prindle T., Weyandt S., Neal M.D., Ozolek J.A., Wiersch J., Tschurtschenthaler M., Shiota C., Gittes G.K., Billiar T.R., Mollen K., Kaser A., Blumberg R., Hackam D.J. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. J Biol Chem. 2014;289:9584–9599. doi: 10.1074/jbc.M113.526517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edelson M.B., Bagwell C.E., Rozycki H.J. Circulating pro- and counterinflammatory cytokine levels and severity in necrotizing enterocolitis. Pediatrics. 1999;103:766–771. doi: 10.1542/peds.103.4.766. [DOI] [PubMed] [Google Scholar]
- 15.Good M., Siggers R.H., Sodhi C.P., Afrazi A., Alkhudari F., Egan C.E., Neal M.D., Yazji I., Jia H., Lin J., Branca M.F., Ma C., Prindle T., Grant Z., Shah S., Slagle D., Paredes J., Ozolek J., Gittes G.K., Hackam D.J. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc Natl Acad Sci U S A. 2012;109(no. 28):11330–11335. doi: 10.1073/pnas.1200856109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hackam D.J., Afrazi A., Good M., Sodhi C.P. Innate immune signaling in the pathogenesis of necrotizing enterocolitis. Clin Dev Immunol. 2013;2013:475415. doi: 10.1155/2013/475415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Ganzoury M.M., Awad H.A., El-Farrash, El-Gammasy T.M., Ismail E.A., Mohamed H.E., Suliman S.M. Enteral granulocyte-colony stimulating factor and erythropoietin early in life improves feeding tolerance in preterm infants: a randomized controlled trial. J Pediatr. 2014;165:1140–1145.e1. doi: 10.1016/j.jpeds.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 18.Jain S.K., Baggerman E.W., Mohankumar K., Namachivayam K., Jagadeeswaran R., Reyes V.E., Maheshwari A. Amniotic fluid-borne hepatocyte growth factor protects rat pups against experimental necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2014;306:G361–G369. doi: 10.1152/ajpgi.00272.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostergaard M.V., Shen R.L., Stoy A.C., Skovgaard K., Krych L., Leth S.S., Nielsen D.S., Hartmann B., Bering S.B., Schmidt M., Sangild P.T. Provision of amniotic fluid during parenteral nutrition increases weight gain with limited effects on gut structure, function, immunity, and microbiology in newborn preterm pigs. JPEN J Parenter Enteral Nutr. 2016;40:552–566. doi: 10.1177/0148607114566463. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence K.M., McGovern P.E., Mejaddam A., Rossidis A.C., Baumgarten H., Kim A., Grinspan J.B., Licht D.J., Didier R.A., Vossough A., Radaelli E., Rychik J., Song L., Peranteau W.H., Davey M.G., Flake A.W., Gaynor J.W. Ex utero extracorporeal support as a model for fetal hypoxia and brain dysmaturity. Ann Thorac Surg. 2020;109:810–819. doi: 10.1016/j.athoracsur.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Roberts R.R., Ellis M., Gwynne R.M., Bergner A.J., Lewis M.D., Beckett E.A., Bornstein J.C., Young H.M. The first intestinal motility patterns in fetal mice are not mediated by neurons or interstitial cells of Cajal. J Physiol. 2010;588:1153–1169. doi: 10.1113/jphysiol.2009.185421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bueno L., Ruckebusch Y. Perinatal development of intestinal myoelectrical activity in dogs and sheep. Am J Physiol. 1979;237:E61–E67. doi: 10.1152/ajpendo.1979.237.1.E61. [DOI] [PubMed] [Google Scholar]
- 23.Clark J.A., Lane R.H., Maclennan N.K., Holubec H., Dvorakova K., Halpern M.D., Williams C.S., Payne C.M., Dvorak B. Epidermal growth factor reduces intestinal apoptosis in an experimental model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G755–G762. doi: 10.1152/ajpgi.00172.2004. [DOI] [PubMed] [Google Scholar]
- 24.Zhang C., Xu Y.G., Duan X.N., Liu Y.H., Zhao J.X., Xu L., Ye J.M. Role of granulocyte colony-stimulating factor in paclitaxel-induced intestinal barrier breakdown and bacterial translocation in rats. Chinese Med J (Engl) 2011;124:1870–1875. [PubMed] [Google Scholar]
- 25.Rowland K.J., Choi P.M., Warner B.W. The role of growth factors in intestinal regeneration and repair in necrotizing enterocolitis. Semin Pediatr Surg. 2013;22:101–111. doi: 10.1053/j.sempedsurg.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vidyasagar S., MacGregor G. Ussing chamber technique to measure intestinal epithelial permeability. Methods Mol Biol. 2016;1422:49–61. doi: 10.1007/978-1-4939-3603-8_6. [DOI] [PubMed] [Google Scholar]
- 27.Moore S.A., Nighot P., Reyes C., Rawat M., McKee J., Lemon D., Hanson J., Ma T.Y. Intestinal barrier dysfunction in human necrotizing enterocolitis. J Pediatr Surg. 2016;51:1907–1913. doi: 10.1016/j.jpedsurg.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei J., Zhou Y., Besner G.E. Heparin-binding EGF-like growth factor and enteric neural stem cell transplantation in the prevention of experimental necrotizing enterocolitis in mice. Pediatr Res. 2015;78:29–37. doi: 10.1038/pr.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Good M., Sodhi C.P., Egan C.E., Afrazi A., Jia H., Yamaguchi Y., Lu P., Branca M.F., Ma C., Prindle T., Jr., Mielo S., Pompa A., Hodzic Z., Ozolek J.A., Hackam D.J. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 2015;8:1166–1179. doi: 10.1038/mi.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buchmiller T.L., Shaw K.S., Copourian H.L., Lloyd K.C., Gregg J.P., Rivera A., Jr., Lam L., Diamond J.M., Fonkalsrud E.W. Effect of transamniotic administration of epidermal growth factor on fetal rabbit small intestinal nutrient transport and disaccharidase development. J Pediatr Surg. 1993;28:1239–1244. doi: 10.1016/s0022-3468(05)80305-7. [DOI] [PubMed] [Google Scholar]
- 31.Pearson L.D., Simpson-Morgan M.W., Morris B. Lymphopoiesis and lymphocyte recirculation in the sheep fetus. J Exp Med. 1976;143:167–186. doi: 10.1084/jem.143.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyasaka M., Trnka Z. Lymphocyte migration and differentiation in a large-animal model: the sheep. Immunol Rev. 1986;91:87–114. doi: 10.1111/j.1600-065x.1986.tb01485.x. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds J.D., Kennedy L., Peppard J., Pabst R. Ileal Peyer’s patch emigrants are predominantly B Cells and travel to all lymphoid tissues in sheep. Eur J Immunol. 1991;21:283–289. doi: 10.1002/eji.1830210207. [DOI] [PubMed] [Google Scholar]
- 34.Yasuda M., Jenne C.N., Kennedy L.J., Reynolds J.D. The sheep and cattle Peyer’s patch as a site of B-cell development. Vet Res. 2006;37:401–415. doi: 10.1051/vetres:2006008. [DOI] [PubMed] [Google Scholar]
- 35.Cellini C., Xu J., Arriaga A., Buchmiller-Crair T.L. Effect of epidermal growth factor infusion on fetal rabbit intrauterine growth retardation and small intestinal development. J Pediatr Surg. 2004;39:891–897. doi: 10.1016/j.jpedsurg.2004.02.008. discussion 891–897. [DOI] [PubMed] [Google Scholar]
- 36.Dvorak B., Halpern M.D., Holubec H., Williams C.S., McWilliam D.L., Dominguez J.A., Stepankova R., Payne C.M., McCuskey R.S. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol. 2002;282:G156–G164. doi: 10.1152/ajpgi.00196.2001. [DOI] [PubMed] [Google Scholar]
- 37.Halpern M.D., Dominguez J.A., Dvorakova K., Holubec H., Williams C.S., Meza Y.G., Ruth M.G., Dvorak B. Ileal cytokine dysregulation in experimental necrotizing enterocolitis is reduced by epidermal growth factor. J Pediatr Gastroenterol Nutr. 2003;36:126–133. doi: 10.1097/00005176-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 38.Feng J., El-Assal O.N., Besner G.E. Heparin-binding epidermal growth factor-like growth factor decreases the incidence of necrotizing enterocolitis in neonatal rats. J Pediatr Surg. 2006;41:144–149. doi: 10.1016/j.jpedsurg.2005.10.018. discussion 144–9. [DOI] [PubMed] [Google Scholar]
- 39.Su Y., Yang J., Besner G.E. HB-EGF promotes intestinal restitution by affecting integrin-extracellular matrix interactions and intercellular adhesions. Growth Factors. 2013;31:39–55. doi: 10.3109/08977194.2012.755966. [DOI] [PubMed] [Google Scholar]
- 40.Michalsky M.P., Lara-Marquez M., Chun L., Besner G.E. Heparin-binding EGF-like growth factor is present in human amniotic fluid and breast milk. J Pediatr Surg. 2002;37:1–6. doi: 10.1053/jpsu.2002.29415. [DOI] [PubMed] [Google Scholar]
- 41.Canpolat F.E., Yurdakok M., Ozsoy S., Haziroglu R., Korkmaz A. Protective effects of recombinant human granulocyte colony stimulating factor in a rat model of necrotizing enterocolitis. Pediatr Surg Int. 2006;22:719–723. doi: 10.1007/s00383-006-1728-2. [DOI] [PubMed] [Google Scholar]
- 42.Canpolat F.E., Yurdakok M., Korkmaz A., Yigit S., Tekinalp G. Enteral granulocyte colony-stimulating factor for the treatment of mild (stage i) necrotizing enterocolitis: a placebo-controlled pilot study. J Pediatr Surg. 2006;41:1134–1138. doi: 10.1016/j.jpedsurg.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Shiou S.R., Yu Y., Chen S., Ciancio M.J., Petrof E.O., Sun J., Claud E.C. Erythropoietin protects intestinal epithelial barrier function and lowers the incidence of experimental neonatal necrotizing enterocolitis. J Biol Chem. 2011;286:12123–12132. doi: 10.1074/jbc.M110.154625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu Y., Shiou S.R., Guo Y., Lu L., Westerhoff M., Sun J., Petrof E.O., Claud E.C. Erythropoietin protects epithelial cells from excessive autophagy and apoptosis in experimental neonatal necrotizing enterocolitis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Athanasiadis A.P., Michaelidou A.-M., Fotiou M., Menexes G., Theodoridis T.D., Ganidou M., Tzevelekis B., Assimakopoulos E., Tarlatzis B.C. Correlation of 2nd trimester amniotic fluid amino acid profile with gestational age and estimated fetal weight. J Matern Fetal Neonatal Med. 2011;24:1033–1038. doi: 10.3109/14767058.2010.545909. [DOI] [PubMed] [Google Scholar]
- 46.Beaulieu J.F., Calvert R. Permissive effect of glutamine on the differentiation of fetal mouse small intestine in organ culture. Differentiation. 1985;29:50–55. doi: 10.1111/j.1432-0436.1985.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 47.Dvorak B. Milk epidermal growth factor and gut protection. J Pediatr. 2010;156:S31–S35. doi: 10.1016/j.jpeds.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cellini C., Xu J., Buchmiller T.L. Effect of esophageal ligation on small intestinal development in normal and growth-retarded fetal rabbits. J Pediatr Gastroenterol Nutr. 2006;43:291–298. doi: 10.1097/01.mpg.0000231588.24491.bb. [DOI] [PubMed] [Google Scholar]
- 49.Avila C.G., Harding R. The development of the gastrointestinal system in fetal sheep in the absence of ingested fluid. J Pediatr Gastroenterol Nutr. 1991;12:96–104. doi: 10.1097/00005176-199101000-00019. [DOI] [PubMed] [Google Scholar]
- 50.Trahair J.F., Harding R. Ultrastructural anomalies in the fetal small intestine indicate that fetal swallowing is important for normal development: an experimental study. Virchows Arch A Pathol Anat Histopathol. 1992;420:305–312. doi: 10.1007/BF01600209. [DOI] [PubMed] [Google Scholar]
- 51.Mulvihill S.J., Stone M.M., Debas H.T., Fonkalsrud E.W. The role of amniotic fluid in fetal nutrition. J Pediatr Surg. 1985;20:668–672. doi: 10.1016/s0022-3468(85)80021-x. [DOI] [PubMed] [Google Scholar]
- 52.Christensen R.D., Havranek T., Gerstmann D.R., Calhoun D.A. Enteral administration of a simulated amniotic fluid to very low birth weight neonates. J Perinatol. 2005;25:380–385. doi: 10.1038/sj.jp.7211306. [DOI] [PubMed] [Google Scholar]
- 53.Warner B.B., Ryan A.L., Seeger K., Leonard A.C., Erwin C.R., Warner B.W. Ontogeny of salivary epidermal growth factor and necrotizing enterocolitis. J Pediatr. 2007;150:358–363. doi: 10.1016/j.jpeds.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 54.Prophet E.B., Mills B., Arrington J.B., Sobin L.H., editors. Laboratory methods in histotechnology. American Registry of Pathology; Washington, DC: 1994. [Google Scholar]
- 55.Gaudio F., Tamma R., Ingravallo G., Perrone T., Laddaga F.E., De Candia M., Maiorano E., Ribatti D., Specchia G. Computer-driven quantitative image analysis in the assessment of tumor cell and T cell features in diffuse large B cell lymphomas. Ann Hematol. 2018;97:663–668. doi: 10.1007/s00277-017-3212-6. [DOI] [PubMed] [Google Scholar]
- 56.Marinaccio C., Ribatti D. A simple method of image analysis to estimate CAM vascularization by APERIO ImageScope software. Int J Dev Biol. 2015;59:217–219. doi: 10.1387/ijdb.150025dr. [DOI] [PubMed] [Google Scholar]
- 57.Schill E.M., Wright C.M., Jamil A., LaCombe J.M., Roper R.J., Heuckeroth R.O. Down syndrome mouse models have an abnormal enteric nervous system. JCI Insight. 2019;5:124510. doi: 10.1172/jci.insight.124510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Brien D.P., Nelson L.A., Kemp C.J., Williams J.L., Wang Q., Erwin C.R., Hasselgren P.O., Warner B.W. Intestinal permeability and bacterial translocation are uncoupled after small bowel resection. J Pediatr Surg. 2002;37:390–394. doi: 10.1053/jpsu.2002.30807. [DOI] [PubMed] [Google Scholar]
- 59.Bzik V.A., Brayden D.J. An assessment of the permeation enhancer, 1-phenyl-piperazine (PPZ), on paracellular flux across rat intestinal mucosae in Ussing chambers. Pharm Res. 2016;33:2506–2516. doi: 10.1007/s11095-016-1975-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.