Abstract
Cortical gain regulation allows neurons to respond adaptively to changing inputs. Neural gain is modulated by internal and external influences, including attentional and arousal states, motor activity and neuromodulatory input. These influences converge to a common set of mechanisms for gain modulation, including GABAergic inhibition, synaptically driven fluctuations in membrane potential, changes in cellular conductance and changes in other biophysical neural properties. Recent work has identified GABAergic interneurons as targets of neuromodulatory input and mediators of state-dependent gain modulation. Here, we review the engagement and effects of gain modulation in the cortex. We highlight key recent findings that link phenomenological observations of gain modulation to underlying cellular and circuit-level mechanisms. Finally, we place these cellular and circuit interactions in the larger context of their impact on perception and cognition.
Patterns of neural activity in the cerebral cortex differ dramatically with changes in cognitive demand and in different behavioural states, such as during sleep or wakefulness, or under anaesthesia. Neural representations also rapidly adapt in response to changes in environmental context. Together, these flexible modes of operation in the cortex determine how we attend to different kinds of environmental input1, discriminate between these different inputs2 and integrate sensory stimuli3. Information from multiple input streams of cognitive, sensory or motor origin must be integrated and transformed to perform these complex tasks. Increasing evidence suggests that these diverse cortical functions are performed through a canonical neural computation called gain modulation4–6.
Neural gain is a metric describing the sensitivity of a neuron to changes in input and can be measured as the slope of the neural input–output (I/O) relationship. Gain modulation allows this input sensitivity to be actively regulated while maintaining the neuron’s selectivity for input features5. Regulation of neural gain thus provides an integration mechanism whereby information from multiple sources can be non-linearly combined via multiplicative modulation of the cell’s response to inputs (BOX 1).
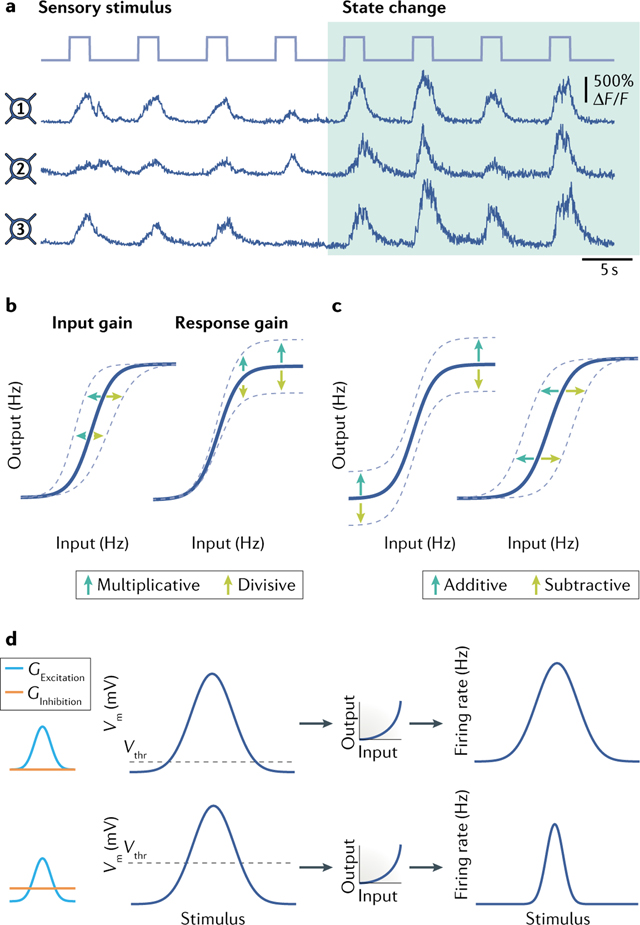
Box 1 |. Divisive versus additive modulation.
Neurons respond flexibly to changes in external and internal drives (such as changes in state) by transforming how they process and encode input (see hypothetical traces of neural responses in figure, part a). These transformations are captured by changes in the cell’s input–output (I/O) relationship and may comprise a complex combination of additive and multiplicative components, or even dynamically switch between the two. Varying modes of arithmetic transformation of the I/O relationship may arise naturally from a neural network owing to its connectivity, synaptic summation and overlap between the stimulus–response times of a cell and those of its target cells68.
Neural gain modulation occurs when a neuron’s I/O relationship is multiplied by a constant to produce a change in slope without a change in rheobase (the minimum current needed to generate an action potential)6,100. If the operation produces a gain increase, it is a multiplicative modulation (see blue arrows in the figure, part b), whereas a gain decrease is a divisive modulation (green arrows in figure, part b). The transformation may occur on the input (input gain) or on the output (response gain) and affects the sensitivity of the neuron to input without changing its selectivity. Response gain modulation, but not input gain modulation, alters the maximum possible neuronal output. Local inhibitory interactions may control the type of gain modulation that occurs (input gain or response gain)80. Normalization is a special case of gain modulation in which responses are adjusted to a ratio of the summed activity of a local population, widening the effective dynamic range5.
Alternatively, a transformation of I/O relationships may maintain the shape of the curve and shift the I/O relationship equally for all input values, performing an additive (blue arrows in the figure, part c) or subtractive operation (green arrows in the figure, part c). By uniformly modulating the I/O operation, additive or subtractive transformations maintain the sensitivity of a neuron to different inputs but alter the input required to reach the threshold for a response, thereby changing stimulus selectivity. These linear transformations may operate on either the input or the neuron’s output100.
Divisive input modulation may produce subtractive effects due to threshold non-linearities. Changes in spike threshold (Vthr) can create an iceberg effect on neural I/O responses227 whereby the tuning curve of firing-rate responses to stimulus features relative to underlying subthreshold membrane potential (Vm) responses is sharpened (see the figure, part d)228. Inhibitory synaptic input can produce these effects by changing the relationship between the Vm and spike threshold and by suppressing responses to non-preferred stimuli229. Ginhibition and Gexcitation represent inhibitory and excitatory synaptic input conductance, respectively.
Gain-modulated cells are ideally suited to perform multimodal computations, such as conversions from sensory-centred into motor-centred reference frames, and for the generation of invariant responses to input features despite contextual variability6–9. Contrast-invariant orientation tuning of cortical neurons for visual stimuli is a well-characterized example of stable encoding of one feature (in this case, orientation), regardless of changes in stimulus context (in this case, contrast)10–12.
Neurons in many cortical and subcortical brain areas exhibit robust gain modulation that may contribute to multiple cognitive functions, including attention, learning, sensory processing and multimodal integration1–3,7,8,13–17. As the encoding of sensory information by single neurons gives rise to the population-level representation of that information18,19, the sensitivity of individual neurons to changes in sensory input should correlate with psychophysical performance on sensory tasks. Indeed, several studies have found good agreement between the population-level responses of gain-modulated neurons (such as the population-average contrast response function) and psychophysical performance (for example, in visual contrast discrimination) in humans and non-human primates12,20–24. Correlations between the gain of neural responses and psychophysical performance support the hypothesis that gain modulation may mediate the trade-off between sensitivity to all salient signals and selectivity for specific signals25. However, a causal relationship remains to be fully established.
Here, we review how distinct environmental and internal sources of input modulate cortical gain. We examine how multiple influences on the gain of excitatory cortical neurons, including attention, locomotion, arousal and neuromodulation, converge to regulate common cellular mechanisms. We highlight the crucial role of GABAergic synaptic inhibition in these mechanisms and identify potential cell type-specific roles for diverse GABAergic populations in mediating gain modulation at the cellular and network levels. Last, we examine the intersection between behavioural state and neuromodulatory control of cortical gain.
Multiple modes of gain control
Neural gain is strongly regulated by both externally imposed and internally generated influences. Gain dynamically adapts in response to variations in the surrounding sensory environment and behavioural context26. In this way, neuronal responses are continuously rescaled to match the dynamically changing range of their inputs, and overall firing rates can be maintained across stimuli with different statistics.
Contrast-invariant tuning is an example of gain modulation that is induced by changes in sensory stimulation, in which neurons respond to stimuli of different levels of contrast while preserving their selectivity for other properties, such as orientation and spatial frequency27–29. Contrast invariance thus enables simultaneous overall signal amplification of visual responses and discrimination between stimulus features. Gain modulation has also been proposed to be crucial for multisensory integration3,30. In the high-order visual cortex of non-human primates, properties that must be decoded separately (for example, object identity and image attributes) are combined multiplicatively, whereas those that must be integrated (for example, parts of an object) are combined additively31. Importantly, subtle differences in the relative balance of additive versus multiplicative components of neural modulation at the single-neuron level may produce substantial differences in the downstream decoding of object properties at the population level31.
Gain modulation mechanisms are also robustly engaged by changes in internal state32–35. In the visual cortex, the onset of arousal (as measured by pupil diameter) and locomotion increase neuronal gain32,33,36,37. By contrast, locomotion is correlated with reductions in response gain in the primary auditory cortex (A1), suggesting that state-dependent gain modulation may vary between different brain areas38,39. Arousal and locomotion are associated with reduced and enhanced spontaneous firing rates in the mouse primary visual cortex (V1)33, respectively, indicating that mechanisms by which gain is enhanced during these two behavioural states may differ. In the primary somatosensory cortex (S1), whisker movement is correlated with gain changes that are heterogeneous across subpopulations of cells and across cortical layers40, further highlighting the complexity of gain regulation across circuits.
Enhancement of cortical responses during attention-demanding tasks is a prominent example of gain control by internally generated cognitive engagement41. Attentional modulation of neural gain has been particularly well studied in the non-human primate visual system1,42,43, in which visual spatial attention within the receptive field of a recorded neuron enhances both contrast gain (that is, scaling of that neuron’s stimulus-response relationship along the contrast axis)43 and response gain (that is, multiplicative transformation of responses to all contrasts)1,14,42,44–48 (BOX 1). This attentional mechanism for gain modulation enhances encoding of the salient (attended) signals, focusing perception on particular aspects of the incoming information49. Experiments and computational models further demonstrate that attention amplifies and stabilizes target cell responses, reducing their variance across trials50,51. Specific regimes of cell–network interactions, such as enhanced synchrony of inhibitory inputs, may be particularly permissive of attentional increases in contrast gain or response gain5,52,53.
Like attention, learning and plasticity also modulate neural gain and may be strongly tied to behavioural state. In non-human primates trained to identify the orientation of a visual stimulus, neural gain increases with learning specifically in cells tuned to the learned orientation54. In mice trained on an orientation-discrimination task, the learning phase immediately before attaining expert performance is associated with increased contrast gain in V1 neurons55. Locomotion paired with visual stimuli dramatically enhances the recovery of visual responses in mice with monocular deprivation in an NMDA receptor-dependent manner, whereas locomotion or visual stimuli alone are insufficient to rescue such responses56. As locomotion elicits gain increases in visually responsive cells32, these results point to a direct relationship between gain modulation and stimulus-specific synaptic plasticity.
Cellular mechanisms
Several lines of evidence link phenomenological observations of gain modulation to a common set of underlying cellular mechanisms. Variations in the statistics of synaptic input robustly modulate the gain of postsynaptic neurons, and GABAergic inhibition plays a crucial role in regulating neural gain.
Synaptic input regulation.
The sensitivity of individual neurons to input is regulated by several cellular mechanisms, including fluctuations in membrane potential (Vm) that are driven by temporally correlated synaptic inputs, changes in the conductance state of the cell and depolarization (FIG. 1). Each of these mechanisms is affected by both synaptic excitation and inhibition.
Fig. 1 |. Cellular and network-level mechanisms of gain modulation.

GABAergic inhibition is a key mediator of gain modulation at both the cellular and network levels. a | Changes in external and internal influences converge to modulate neural gain at the single-cell level via a common set of mechanisms. The mechanisms include changes in the relative positions and amplitudes of active excitatory and inhibitory synaptic inputs to the dendrites, shunting inhibition at the soma and the overall conductance and depolarization state of the neuron10,57,59–61,64,83,84. Gain is also affected by the statistics of synaptic input, including short-term synaptic dynamics and the relative timing of inhibitory and excitatory inputs that give rise to synaptically driven fluctuations in the membrane potential (Vm)10,57,58,61. b | Cellular mechanisms converge to produce multiplicative gain modulation. As highlighted by computational models57,58 and experimental data59–61, divisive gain modulation of pyramidal neuron (PYR) responses can arise from a combination of increased shunting conductance and increased synaptically driven Vm fluctuations, both of which are driven by GABAergic inhibition. c | GABAergic inhibition can regulate gain at the network level flexibly over time, as different sources of synaptic inhibition are recruited into circuit activity105,106. Over time, or over repeated sensory stimulation, some GABAergic populations maintain or increase their responses (darker shading signifies more activity), whereas others show adaptation (that is, reduce their responses to repeated stimulation), altering the relative amount of inhibition from each population onto postsynaptic PYRs. d | Schematic of hypothetical Ca+ fluorescence traces from somatostatin-positive (SST+) interneurons (blue) and parvalbumin-positive (PV+) interneurons (orange) in the primary visual cortex in response to repeated visual stimulation. e | Gain modulation of different neural populations may change independently over time. Schematic shows one possible trajectory of the relative visual response gain of a PV+ interneuron–SST+ interneuron pair (upper panel) or a vasoactive intestinal peptide-expressing (VIP+) interneuron–PYR pair (lower panel) over time.
Changes in the level of synaptically driven fluctuations in Vm (also called synaptic fluctuations or synaptic noise) may regulate the gain of the I/O curve for individual neurons57–60 (but see REF.61). Under conditions of a tight balance between excitatory and inhibitory input, synaptically driven Vm fluctuations alone can produce gain control57, and this interaction is stable even across conditions in which synaptic inputs have different temporal statistics62. Synaptically driven fluctuations in the Vm smooth the transformation from Vm depolarization to spike output, creating a power-law relationship between the mean Vm and the mean firing rate13,63. These fluctuations may contribute to contrast-invariant tuning in neurons of the visual cortex of cats and rodents10,13,63,64. Importantly, Vm fluctuations are regulated by environmental factors and stimulus conditions, as well as by several neuromodulatory influences61.
Noisy background synaptic input can multiplicatively regulate tuned neural responses under different environmental conditions. Stochastic resonance can increase the sensitivity of sensory detectors, including neurons, enhancing the detection of weak signals65,66. Similarly, background synaptic noise that is uncorrelated with ongoing visual stimulation contributes to maintaining stable orientation tuning in visual cortex neurons across varying levels of visual contrast13. Computational and in vitro dynamic clamp studies demonstrate that, under in vivo-like conditions with noisy background synaptic inputs, pyramidal neurons can exhibit a broad dynamic range of firing rates67. In turn, this dynamic range can be adjusted through gain control that is mediated by the overall level of synaptic inhibition4,59,68,69.
The degree to which the synaptic input underlying Vm fluctuations is temporally correlated can vary with overall drive to a network or with firing rates70 and is regulated by behavioural state transitions during wakefulness29,71–74. Neuromodulatory inputs, such as acetylcholine (ACh), also alter the correlation statistics of synaptic activity in the cortex73,75. Correlations between pairs of excitatory inputs or pairs of inhibitory inputs increase fluctuations in synaptic drive, whereas excitatory–inhibitory correlations decrease fluctuations9.
However, the effects of synaptic input patterns on cellular I/O gain are not limited to independent changes in excitation or inhibition. Balanced changes in excitatory and inhibitory synaptic plasticity may further enhance the amplitude of membrane fluctuations to regulate the I/O gain of individual neurons76. Moreover, shunting inhibition combined with excitatory drive can modulate gain77, and excitatory or inhibitory synaptic input can modulate the gain of a larger, non-linear driving input to a cell78. Balanced synaptic input thus provides a potential mechanism for gain modulation57,79–82.
Unlike synaptically driven Vm fluctuations, changes in membrane conductance and depolarization cause lateral, or additive, shifts in the I/O transfer function of a neuron without changes in neuronal gain57,58,60,61,83,84. Together, temporally coincident changes in conductance state and synaptic fluctuations exert a powerful effect on neuronal gain at the single-cell level57,58,60,61,83,84. In addition to regulating gain at the level of individual cells, synaptic fluctuations also decrease pairwise correlations in output from neurons with shared inputs85–88. Furthermore, the sensitivity of individual neurons to synaptic fluctuations can vary with neural subtype or cortical area and with biophysical cellular properties, such as membrane capacitance and conductance89–93. Some subpopulations of neurons may be relatively insensitive or sensitive to synaptic fluctuations, biasing them towards encoding the mean or the variance, respectively, of stimulus-driven synaptic input.
Previous work has also suggested that different cellular mechanisms of gain regulation may be spatially segregated within individual neurons. For example, inputs to dendrites non-linearly engage active dendritic processes, increasing I/O gain in pyramidal neurons94. In turn, the gain enhancement conferred by dendritic action potentials may be counterbalanced in part by the distinct multiplicative effects and subtractive effects of dendritic inhibition and somatic inhibition, respectively95. Dendritic saturation, in combination with noisy shunting somatic inhibition, may further contribute to gain control59. In addition, morphological features of the dendrites regulate the extent to which gain control of individual neurons is possible, with moderate branching in pyramidal neurons potentially promoting the greatest possible range of gain modulation96.
Inhibitory regulation of neural sensitivity.
Although recent work has identified some potential mechanisms for excitatory regulation of cortical gain97,98, several lines of evidence suggest that cortical GABAergic inhibition has a crucial role in regulating the gain of sensory responses5,99,100 (FIG. 1). Local application of gabazine, a GABAA receptor antagonist, enhances responsiveness of cat V1 to visual stimuli69. GABAergic inhibition controls the sensitivity of V1 neurons in rodents and cats by specifically adjusting their response gain without altering local selectivity or input gain2,68,69,101–104.
Experiments in which GABAergic interneurons have been activated or suppressed have provided insight into the various ways in which interneuron activity can modulate the gain of their postsynaptic targets2,68,103,105–107. However, the diversity of GABAergic interneurons presents a major challenge to identifying their role in regulating the gain of excitatory cells. Cortical inhibitory cells exhibit varied morphology, physiological properties, connectivity patterns and biochemical composition, suggesting that they may contribute to distinct computational functions108,109. Most recent work has focused on three major GABAergic cell groups: fast-spiking cells that express the calcium-binding protein parvalbumin and target the perisomatic and axonic regions of excitatory neurons (PV+ interneurons); low-threshold spiking cells that express somatostatin and target dendrites (SST+ interneurons); and sparse, dendrite-targeting cells that express vasoactive intestinal peptide (VIP+ interneurons).
Optogenetic manipulations of interneurons provide evidence of cell type-specific effects of inhibitory interneurons on gain regulation2,68,104,105,107. However, initial studies of the roles of PV+ interneurons and SST+ interneurons in modulating visual response gain and tuning were conflicting, probably owing to differences in stimulation conditions2,68,104,107 (BOX 2). These discrepancies highlight the difficulty in identifying and controlling for numerous influences on gain modulation. Indeed, interneuron contributions to gain control are likely to be dynamic and affected by several factors, including behavioural state, cellular responses, sensory stimulation regime and manipulation parameters, such as optogenetic control103,106,107,110,111. Furthermore, synaptic interactions between different interneuron populations provide additional potential circuit-level mechanisms for gain regulation112 (BOX 2). Under the active neural network conditions observed in vivo, the spatiotemporal patterns of excitation, inhibition and neuromodulation vary with context, such that different interneuron populations may contribute in distinct ways to cortical gain110 and ultimately to perceptual and cognitive processes such as visual contrast perception113.
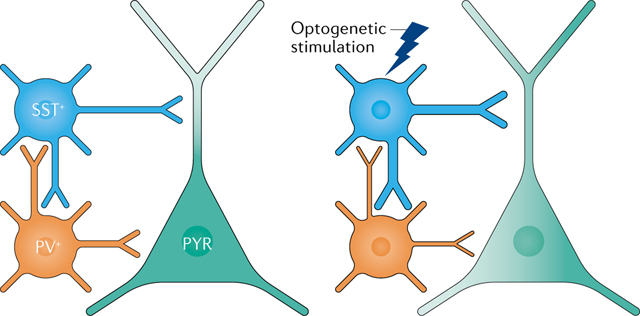
Box 2 |. Causal manipulations and cortical gain modulation.
The design and interpretation of causal manipulation experiments to probe neural gain control is hampered by the fact that external manipulations cause a cascade of interacting changes in the activity of the local circuit that may obscure the mechanisms of gain modulation106,116. The examples below are drawn from experiments using optogenetic tools, but apply to all techniques for causal manipulation.
Bidirectional optogenetic manipulations are widely used to infer the role of distinct cell populations in gain modulation2,68,103,104,107. However, the seemingly symmetrical optogenetic activation and inhibition of such populations may produce paradoxical results. For example, two inhibitory cell populations (parvalbumin-positive (PV+) interneurons and somatostatin-positive (SST+) interneurons; see figure) may perform opposing operations when inhibited and the same operation when activated106. Relatively small changes in baseline inhibitory activity, neural spiking threshold or the strength of manipulation may elicit an iceberg effect on tuned neural spiking responses (see BOX 1).
Likewise, short-term synaptic dynamics constrain the neural response to exogenous stimulation. Inhibitory postsynaptic responses at synapses from PV+ interneurons onto excitatory pyramidal neurons (PYRs) depress rapidly with repeated activation, whereas those elicited by SST+ interneurons are more sustained with repetition108. These short-term synaptic dynamics may be engaged differentially by repeated or sustained stimulation in experimental manipulations than by endogenous activity patterns. Furthermore, the precise impact of this short-term synaptic plasticity on neural gain may be difficult to determine in vivo, as presynaptic spiking may remain unchanged even when plasticity is engaged at the presynaptic or postsynaptic side of the synapse.
Owing to the highly non-linear connectivity of neural networks, experimental perturbation of a neuron or neural population may affect downstream targets in an undesirable or non-physiological manner. Experimental manipulations that activate neurons may increase synaptic efficacy between a subset of populations230, either by driving or depriving another cell population of activity in a non-physiological manner or by transiently shifting the balance of excitation and inhibition in the network231.
In the example in the figure, activation of SST+ interneurons may increase inhibition on the dendrites of the PYR while simultaneously inhibiting PV+ interneurons and thus reducing inhibition of the soma of the PYR232,233. The precise balance between dendritic and somatic inhibition may thus be affected by several factors associated with artificial manipulations (including stimulation power, frequency and duration of optogenetic stimulation, among others.).
Most research investigating the roles of distinct interneuron populations in shaping cortical gain has used transient stimulation2,26,68,104,105,107,110,114. However, recent work has demonstrated that acute manipulations (such as optogenetic silencing) and chronic manipulations (for example, ablation) have considerably different effects on downstream neural targets115–117. For example, learned motor skills are unaffected by motor cortex lesions but severely affected by transient inactivation of the motor cortex116. In addition, relatively small changes in key parameters, such as the spontaneous firing rate or strength of optogenetic manipulation, may produce inconsistency in responses to transient manipulations106. Transiently activating or silencing a specific cell type, such as GABAergic interneurons, may have unanticipated indirect effects on cortical circuit dynamics, yielding mixed effects on the gain of individual neurons (BOX 2).
Recent work using parallel optogenetic and computational approaches has provided support for a more nuanced and internally consistent model for the role of GABAergic inhibition in gain control. In the auditory cortex, optogenetically activating PV+ interneurons or SST+ interneurons evokes a mixture of divisive and subtractive modulation of postsynaptic excitatory neurons107. Variations in spike threshold and the strength of inhibitory suppression of individual excitatory neurons can also translate subtractive modulation of the individual neurons into divisive modulation at the population level, or vice versa107. The gain effects of a specific interneuron population may thus be altered by the cellular and synaptic properties of the surrounding network.
Furthermore, distinct interneuron populations may have unique roles in context-dependent modes of gain modulation such as adaptation105 and forward suppression118. A network model incorporating a biologically realistic mix of neuronal populations can account for these additional modes through short-term dynamic adjustments of synaptic inputs onto distinct interneuron populations106.
The effect of synaptic inhibition on neural gain may be strongly regulated by behavioural state. Recent work has highlighted state-dependent modulation of the activity of PV+ interneurons, SST+ interneurons and VIP+ interneurons, but the circuit-level effects of such modulation are probably complex. VIP+ interneurons are important regulators of cortical function119–123 and are activated by arousal and locomotion119,124. The increased firing of VIP+ interneurons in vivo during locomotion suppresses SST+ interneurons40, potentially leading to an overall decrease in the response gain of downstream excitatory cells. Indeed, optogenetic activation of VIP+ interneurons increases the response gain of local excitatory cells, mimicking some of the effects of locomotion16. However, locomotion may also increase the response gain of SST+ interneurons, which may potentially decrease the response gain of downstream excitatory neurons36,125. Given these complex and heterogeneous interactions between distinct interneuron populations112, the respective roles of these cell types in state-dependent and context-dependent gain modulation at the single-neuron and network levels remain to be fully explored.
Theoretical work suggests that, in an inhibition-stabilized network, feedback inhibition can balance excitatory recurrent activity, thereby maintaining stability during stimulus-evoked activity126,127. Experimental evidence further suggests that visual and auditory cortices operate like inhibition-stabilized networks126,128. Although few computational models of cortical networks take into account the extensive diversity of interneuron populations, some have extended inhibition-stabilized network regimes to include multiple distinct interneuron subpopulations129,130. Cell type-specific non-linear I/O relationships and diverse synaptic interactions between populations may each contribute to the influence of different interneurons on gain modulation. For example, regulation of the shape of the tuning curves of excitatory neurons may depend on both the I/O non-linearity and tuning of PV+ interneurons129,130.
Although inhibition may stabilize cortical networks, inhibitory gain regulation can be heterogeneous even within a local circuit. Indeed, functionally distinct subtypes of SST+ interneurons have been found in different layers of S140,119,122. In S1, VIP+ interneurons preferentially inhibit layer 2/3 (L2/3) SST+ interneurons and only weakly inhibit many L5 SST+ interneurons. This selective VIP+ inhibition causes state-dependent differential modulation of SST+ interneurons in L2/3 and L5, leading to distinct modes of gain regulation in superficial and deep cortical layers during whisking behaviour40. Laminar differences in gain modulation have also been observed in A1. Locomotion decreases the gain of responses in L2/3 of A1 but not in L4, potentially as a result of enhanced inhibition in the superficial cortical layers131.
Arousal and neuromodulation
Behavioural state.
In humans, fluctuations in behavioural state during wakefulness, such as from quiescence to arousal, potently regulate patterns of brain activity132–136 and perceptual and cognitive performance137,138. Brain states, which are typically distinguished by canonical patterns of rhythmic activity (such as alpha oscillations or gamma oscillations) or by arousal level (measured by pupillometry), strongly regulate the expression of task-evoked neural activity139. In humans, spontaneous changes in brain state are correlated with alterations in electroencephalography signals140,141 and functional magnetic resonance imaging signals132,134,135, and with changes in cognitive and perceptual task performance133–136,142 and sensory detection132. Thus, behavioural or arousal states may be associated with different widespread modulations of neural gain. However, a detailed mechanistic understanding of these interactions remains incomplete.
Work in rodents has largely examined this state-dependent modulation of cortical processing during wakefulness by measuring spontaneous motor activity (such as locomotion or whisking) as a proxy for arousal, as locomotion correlates with pupil diameter (an indicator of arousal)28,33 and changes in cortical electroencephalography and local field potential signals33,73. Although the precise pathways that link changes in pupil diameter to arousal remain poorly understood, locus coeruleus firing is highly correlated with pupil dynamics143,144, and locus coeruleus stimulation causes pupil dilation145. Cortical imaging of cholinergic and noradrenergic axons from the basal forebrain and locus coeruleus, respectively, found high correlations between the activity of these afferents and sustained pupil dilation and locomotion146. Pupil size itself does not regulate neural sensitivity to inputs, as atropine-induced pupil dilation does not affect the tuning of thalamic neurons147 or visual perceptual behaviour148 in mice.
During running, neurons in mouse V1 exhibit increased synaptic input, elevated firing rates and enhanced visual-response amplitudes28,29,32,33,111,147,149, resulting in a higher signal-to-noise ratio for visually evoked activity. Comparison of tuning curves during quiescence and locomotion reveals an increase in gain during running27,32,73,80,148. Locomotion increases the amplitude of visual responses in both excitatory and inhibitory neurons in mouse V1, suggesting a circuit-wide modulation of sensitivity to inputs, although this modulation varies across layers and depends on the sensory context in some cell classes27,33,36,125,147,148.
One prediction arising from studies in rodent V1 is that the increase in visual response gain associated with an enhanced arousal state (indexed by pupil diameter and locomotion) strengthens cortical visual encoding. Indeed, mice exhibit increased visual perceptual performance during locomotion29. However, visual response gain may not be monotonically correlated with visual perceptual performance. Some recent observations from V1 of mice performing a visual detection task suggest that visual response gain modulation increases at both moderate and high arousal levels and may be partly dissociable from behavioural performance, which peaks at moderate arousal levels150. Interestingly, in humans, locomotion may enhance visually evoked neural responses140,151 but not visual psychophysical performance152, further suggesting a dissociation between modulation of neural response gain and perceptual ability.
Substantial evidence suggests behavioural state-dependent regulation of sensory responses, but the direction of the resulting gain modulation may vary across cortical areas. In contrast to visual response gain in V1, A1 auditory response gain is reduced during locomotion, owing to increases in the activity of inhibitory interneurons38. Measures of auditory task performance suggest complex relationships between arousal, gain control in A1 and perception, with neuronal gain potentially decreasing with high arousal and psychophysical performance adversely affected by locomotion39,153. State-dependent regulation of neuronal activity levels and response gain in different brain areas may thus be mediated by different local circuit mechanisms and differentially relate to perceptual performance. In support of this idea, large-scale imaging approaches find varying effects of arousal on activity patterns across cortical areas. These approaches also reveal heterogeneity of the effects of arousal on the long-range functional connectivity of neighbouring individual neurons that reside within a cortical area154–156.
The complex interactions between motor and sensory areas that occur during motor action may cause simultaneous arousal-related and motor-related signals to arise in primary sensory cortical areas, potentially confounding the use of locomotion or whisking as indicators of arousal155,157. However, experiments in which motor activity and arousal are dissociated suggest that they regulate cortical gain independently. During locomotion, excitatory neurons in mouse V1 exhibit increased excitatory and inhibitory conductance associated with Vm depolarization and synaptic fluctuations, along with increased spontaneous and visually evoked firing29,32,33,73,149. By contrast, arousal induced by an air puff in the absence of locomotion causes a decrease, rather than an increase, in spontaneous firing without reducing visually evoked responses, thereby increasing the signal-to-noise ratio and enhancing stimulus sensitivity33. Locomotion and arousal thus seem to engage distinct cellular and network mechanisms for modulating cortical sensory response gain (FIG. 2).
Fig. 2 |. Multiple modes of state-dependent cortical gain modulation.
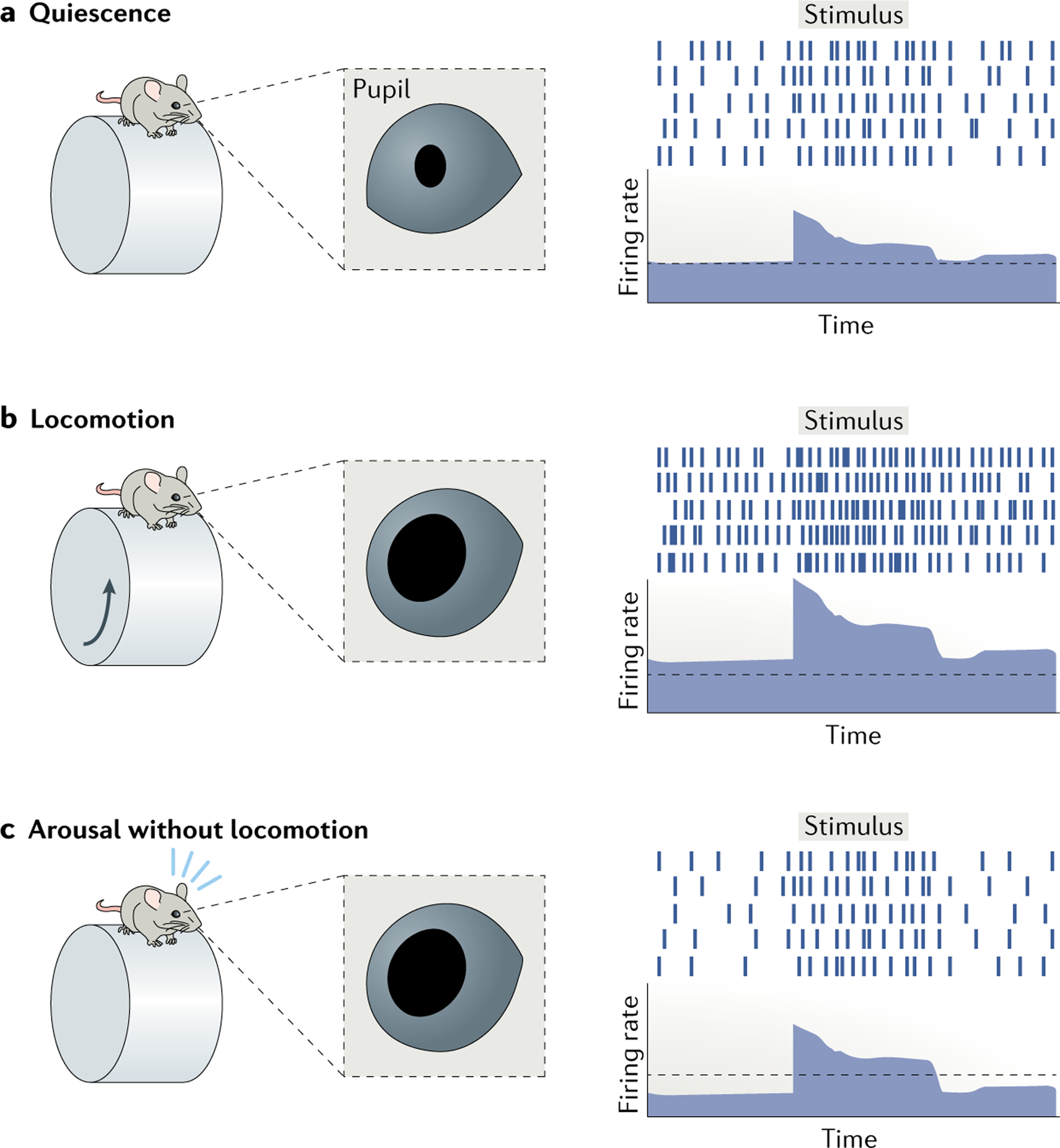
Different behavioural states during wakefulness are associated with discrete modes of gain modulation226. Arousal and locomotion increase the gain of visually evoked responses in the rodent primary visual cortex through different mechanisms33. a | During quiescence, arousal is low, as denoted by a constricted pupil, and cortical neurons typically show moderate spontaneous firing and moderate firing in response to a visual stimulus. b | During periods of locomotion, arousal increases, as denoted by pupil dilation. In association with locomotion cortical neurons depolarize and exhibit enhanced spontaneous and visually evoked firing28,32,33,73. c | By contrast, during periods of high arousal without motor activity, spontaneous firing decreases whereas sensory-evoked responses increase33.
Neuromodulatory control.
Neuromodulators engage many cellular mechanisms of gain modulation and provide a crucial link between behavioural state and neuronal gain control. Although there are a large number of neuromodulatory systems that probably regulate cortical gain, only a few have been studied in detail. In particular, cholinergic modulation of cortical networks, mediated largely by widespread projections from cholinergic neurons in the basal forebrain, has been proposed to underlie state-dependent regulation of neuronal sensitivity to sensory inputs.
Cholinergic receptors are expressed by inhibitory and excitatory cortical neurons119,124,158–164, as well as on the terminals of thalamocortical neurons165–169. Stimulation of nicotinic ACh receptors (nAChRs) with nicotine in macaque V1 causes increases in firing rates and gain modulation of sensory-evoked activity in the thalamo-recipient cortical layers and has various effects in other layers165,170,171 (for further discussion, see REF.172). Similarly, systemically applied nicotine enhances response gain in mouse A1 (REF.173). Other work in non-human-primate cortex suggests that activation of muscarinic ACh receptors (mAChRs), but not nAChRs, may in part mediate the attentional modulation of neural response gain174. However, the effects of cholinergic modulation on neuronal response gain may vary across classes of excitatory neurons172. In contrast to the effects of pharmacological agents, optogenetic stimulation of endogenous ACh release in mouse V1 desynchronizes spiking and enhances visual perceptual performance, without altering overall firing rates175. Together, these findings suggest that the effects of cholinergic transmission on neural gain and stimulus encoding vary, potentially owing to the heterogeneity of cholinergic receptor expression across cortical populations.
Earlier work showed that cholinergic transmission acts on multiple cellular targets and functional pathways that may potentially contribute to gain modulation at the single-neuron level. Activation of mAChRs on cortical pyramidal neurons reduces the activity of multiple types of Ca2+ channels176, increases excitability via Ca2+-dependent potassium channels177–179 and can enhance bursting activity180, all potentially contributing to gain regulation at the single-neuron level. Furthermore, the effects of cholinergic signalling are heterogeneous across cortical layers: ACh suppresses excitatory neuron activity in L4, but increases the activity of excitatory neurons in L2/3 and L5 by promoting mAChR-mediated opening of GIRK channels (G protein-coupled inwardly-rectifying potassium channels)181. However, mAChR activation also suppresses L5 pyramidal neuron activity by triggering internal release of calcium, which activates inhibitory SK channels (small-conductance Ca2+-activated potassium channels)182,183. Optical stimulation of endogenous ACh release in S1 leads to activation of mAChRs on excitatory and inhibitory neurons in L4 and activation of nAChRs, presumably on inhibitory interneurons, in the superficial layers, causing overall suppression of cortical activity184.
In addition to these influences on overall neural activity, mAChRs at excitatory synapses may decrease presynaptic release185 and increase postsynaptic responses186 through independent mechanisms, further contributing to neural gain control. ACh release is likely to simultaneously affect excitatory and inhibitory cells and their synapses, and thus the cumulative impact of these various cellular mechanisms on response gain in vivo remains unclear.
In contrast to ACh, less is known about the impact of other neuromodulators on gain modulation in local cortical circuits. Noradrenaline increases the excitability of neurons that express β-adrenergic receptors187–189 and reduces excitatory synaptic transmission by acting on α-adrenergic receptors190,191, suggesting potential for noradrenergic regulation of neural gain. However, similar to ACh, the effects of noradrenaline on individual pyramidal neurons are heterogeneous192,193. Local application of noradrenaline or stimulation of noradrenergic afferents in vivo reduces spontaneous firing194,195, but enhances evoked responses196–198 (but see REF.199), potentially increasing the signal-to-noise ratio of sensory responses143. The depolarization and increased firing of mouse V1 neurons associated with locomotion require noradrenergic transmission, and blocking noradrenergic receptors results in hyperpolarization of pyramidal neurons, decreases their firing and prevents locomotion-induced increases in visual response gain73.
In contrast to the largely gain-enhancing effects of ACh and noradrenaline, serotonin seems to predominantly reduce neural gain. In macaque V1, locally applied serotonin reduces the gain of responses of excitatory neurons to visual stimuli200. However, cortical pyramidal neurons show heterogeneous expression of serotonin receptors and therefore may exhibit varied responses to activation of serotonergic afferents201. Nevertheless, consistent with the notion that serotonin reduces neural gain, increases in serotonin levels reduce behavioural sensitivity to mechanosensory stimuli202 and reduce startle responses203.
Evidence from non-human-primate studies suggests that dopamine may also regulate cortical response gain. Dopaminergic signalling has a role in the top-down regulation of spatial attention, and D1 dopaminergic receptors modulate response amplitude and selectivity in the dorsolateral prefrontal cortex for preferred spatial locations during working memory tasks204,205. Moreover, D1 dopaminergic receptor activation in the frontal eye fields enhances the amplitude and selectivity of neural responses in cortical area V4 (REF.206). However, little is known about the cell type-specific effects or underlying cellular and network mechanisms of dopaminergic regulation of cortical gain.
Overall, these findings suggest crucial roles for several neuromodulatory systems in regulating cortical gain. However, many of these effects have not yet been examined in detail, and the in vivo impacts of other potential neuromodulatory influences on gain, such as signalling through GABAB receptors, are poorly understood. In addition, the cellular mechanisms of potential interactions between neuromodulatory inputs, such as convergence to a small number of G protein-coupled receptor (GPCR) signalling pathways207, remain to be explored.
Interneurons as targets of neuromodulation.
A key portion of the impact of neuromodulation on neural response gain may occur via actions on inhibitory interneurons170. In particular, VIP+ interneurons express nAChRs and are strongly depolarized by nicotine or ACh119,124,163. In turn, SST+ interneurons receive strong GABAergic input from VIP+ interneurons and themselves exhibit mAChR-mediated depolarization, and connections from pyramidal neurons to SST+ interneurons are selectively enhanced by activation of presynaptic nAChRs164. Cholinergic input to the cortex increases SST+ interneuron activity, thus increasing inhibition of PV+ cells and pyramidal neurons and desynchronizing cortical networks75 and synaptic inputs to individual neurons. Cholinergic signalling can thus promote competing increases in the activity of presynaptic VIP+ cells and postsynaptic SST+ cells. The activity of interneurons in L1 is also enhanced by nAChR activation, potentially leading to reductions in the activity of postsynaptic PV+ interneurons in L2/3162.
L1 interneurons and VIP+ interneurons are characterized by robust expression of serotonin 3A receptors (5-HT3ARs)108, although the cellular actions of serotonin on these cells are not well understood. Recent work has also revealed that serotonin regulates the excitability of PV+ interneurons via 5-HT2ARs (REF.208). In addition to being influenced by cholinergic and serotonergic signalling, SST+ interneurons and a subset of PV+ interneurons are also depolarized by activation of α-adrenergic receptors209. Each population of inhibitory interneurons is thus subject to regulation by multiple streams of neuromodulatory input, potentially increasing the flexibility of their roles in modulating the gain of nearby pyramidal neurons.
Functions of gain modulation
The brain faces an ever-evolving challenge to support stable but flexible encoding of environmental information in the face of continually changing input regimes. Successfully meeting this challenge requires rapid adaptation to varying ranges of input and enhancing the salience of relevant information. Gain modulation serves both of these functions.
As individual neurons receive a broad range of inputs, neural encoding processes must be sensitive to weak inputs but not saturated in response to stronger ones. One mechanism by which this may occur is through adaptation-regulated changes in gain, whereby neurons dynamically maintain their firing rates to efficiently encode both weak and strong stimuli. Adaptation enables high sensitivity to small changes in stimulus features over a large range of intensities, and is found across sensory systems5,17,26,105,210–216. Moreover, within a cortical area, distinct cell populations may regulate gain modulation differently over time or as stimuli change. For example, in response to repeated auditory tones, excitatory neuron activity in mouse A1 adapts in a frequency-dependent manner, whereas the responses of PV+ interneurons are stable and those of SST+ interneurons increase. The increase in SST+ interneuron activity following adaptation results in enhanced gain modulation of excitatory neuron auditory responses, whereas PV+ interneurons do not affect the adaptation-induced response gain105. Increased inhibitory input reduces the sensitivity of excitatory neurons to changes in auditory input and expands the dynamic range of neural responses (BOX 1), and also potentially increases the efficiency of encoding217. SST+ interneurons may thus be key regulators of sensory adaptation, an important computation exhibited by many cortical regions that allows environmental changes to be detected across a wide range of backgrounds.
Gain modulation may also enhance encoding of relevant information during specific behavioural states. Decoders that predict the presence of visual stimuli on the basis of neural responses are more accurate when they use neural activity recorded in the mouse cortex during locomotion than that during quiescence27,37, suggesting that stimulus-invariant increases in neural gain produce more robust encoding during locomotion. Using two separate decoders during still and active periods does not improve stimulus prediction, indicating that a model with a single mode of gain modulation best reflects the improvements in encoding between the two states27,37. Gain increases mediated by spatial attention similarly contribute to improved neural encoding and perceptual performance18.
Gain increases may optimize signal discrimination in the presence of external noise by facilitating attractor dynamics218 and promoting a winner-take-all mechanism219,220. In network models of local cortical circuits, state-dependent increases in inhibitory and excitatory drive can cause convergence of competing, unstable patterns of activity to a single, stable representation221, prioritizing one stimulus over others. In the visual and auditory systems, neural representations of stimuli are relatively invariant to contrast or intensity changes but remain robustly responsive to the variance of other features, such as orientation or spatial frequency, allowing separation of distinct stimulus features. A cortical circuit model of decision-making indicates that dynamic co-modulation of both excitatory and inhibitory gain produces a more stable and robust network, allowing for more flexible and cognitively demanding decision-making, and that gain modulation can compensate for weaker recurrent excitation218.
Finally, a key role of gain modulation may be to increase information transmission and provide computational efficiency within a neural network222 under the constraint of limited resources. Gain regulation has been suggested to enable networks of neurons to produce distributed representations of stimulus features223, and to allow downstream decoders to be optimized for diverse stimuli by separating those features224. However, it remains unclear precisely how gain modulation at the single-cell level contributes to population coding that might be read out by a downstream target. In addition, given the diversity of cellular properties, receptor expression and connectivity, it is unclear whether cortical networks could exhibit uniform gain modulation, suggesting that a downstream decoder may receive a noisy population signal. Indeed, recent work suggests that spontaneous fluctuations in network activity modulate the gain of neuronal responses homogeneously across excitatory cells, whereas visual stimulus contrast modulates the gain of individual neurons independently225. Variability in stimulus-tuning preferences may further contribute to the heterogeneous distribution of gain modulation27, and different interneuron populations may also differentially influence this heterogeneity114. Similarly, whether individual neurons exhibit reliable gain modulation in response to repeated changes in internal or external influences, such as behavioural state or neuromodulatory input, is not clear. These complex relationships between different modes of gain modulation at the cellular and circuit levels remain to be fully explored.
Conclusions
Increasing evidence suggests that cortical gain is regulated by a wide range of influences, including attention, learning, locomotion, arousal and neuromodulatory activity, and that these may act through a common set of cellular and circuit mechanisms. Recent work highlights a key but complex role for GABAergic inhibition in gain modulation and suggests that the sensitivity of individual neurons to sensory stimuli is profoundly modulated by changes in arousal state and locomotion. Neuromodulatory inputs to the cortex have been implicated as linking behavioural state and the modulation of the I/O gain of principal neurons, in many cases by targeting inhibitory interneurons. Further elucidating how neuromodulators regulate specific inhibitory and excitatory cell types in the cortex during perceptual behaviour will be crucial for advancing our understanding of the mechanisms and functions of neuronal gain modulation.
The impact of the different mechanisms that underlie gain regulation at the network level remains unclear. In addition, the precise relationship between gain modulation of single neurons and the encoding and transmission of information at the population level is not well understood. Further complicating matters, the reliability and repeatability of gain modulation of single neurons and cortical networks is unknown. In particular, a more comprehensive understanding of the interactions between inhibitory interneuron populations may provide insight into the complex circuit-level mechanisms that link gain control at the single-cell and population levels. Finally, the contribution of neural gain control to perceptual and cognitive performance remains to be fully explored.
Understanding the complexity of gain modulation through modelling, dimensionality reduction and analyses of distributed variability in activity levels and encoding across cortical populations may provide additional insight into the contributions of different neuronal classes to population encoding of behaviourally relevant information and the behavioural consequences of gain modulation of neural sensory responses. Together with analyses of data from large-scale population recordings, such approaches should inform our understanding of the role of gain modulation in perception and cognition.
Gain modulation.
A phenomenon whereby the gain or sensitivity of a neuron to inputs, such as visual stimuli, is altered without changing selectivity.
Input–output (I/O) relationship.
The relationship between the inputs a neuron receives (such as synaptic inputs, direct currents or sensory stimulation) and the firing rate responses of that neuron.
Synaptic summation.
The summation of synaptic inputs to a neuron either spatially (when nearby synapses are coactive on a dendritic branch) or temporally (when synaptic inputs occur within a short time window mediated by the membrane time constant, τ).
Iceberg effect.
An effect whereby, if subthreshold responses to a stimulus are less selective than the neuron’s firing, a linear increase or decrease in activity may alter the neuron’s selectivity by raising or lowering the tuning curve of the neuron across the threshold.
Monocular deprivation.
An experimental paradigm in which an animal is deprived of vision from one eye during a critical developmental period. The mature binocular visual cortex then responds predominantly to inputs from the non-deprived eye.
Stochastic resonance.
A phenomenon in which the addition of noise non-linearly enhances the information content of a signal, by boosting resonant frequencies over a sensor’s detection threshold (such as a cell’s spike threshold).
Shunting inhibition.
A GABAergic synaptic input that minimally affects the membrane potential of a cell that is near the inhibitory synaptic reversal potential, but that leads to a reduction of nearby excitatory postsynaptic potential amplitudes.
Pairwise correlations.
A normalized measure of covariation between pairs of neurons that can give insight into their tuning similarity (signal correlations) or shared trial-to-trial variability (noise correlations).
Dendritic saturation.
A phenomenon in which an already depolarized dendritic branch shows reduced excitatory responses to temporally correlated excitatory inputs due to reduced driving force.
Synaptic efficacy.
The influence that a presynaptic input has on a postsynaptic cell’s probability of firing an action potential.
Adaptation.
A decrease in sensitivity to constant or repeated stimuli, leading to reduced stimulus-evoked neural responses over time.
Forward suppression.
A rapid form of sensory adaptation whereby the response to a stimulus is reduced when preceded by a stimulus with similar features.
Feedback inhibition.
A type of inhibition delivered through recurrent connections: that is, local inhibitory cells target the same population of excitatory cells that drive local inhibitory activity.
Brain states.
Spatiotemporal patterns of neural-network activity across the brain that are dynamically regulated by behaviour, the environment and the internal state.
Pupil diameter.
The diameter of the pupil of the eye. The diameter is tightly coupled to various emotional and cognitive factors, including global arousal and attention, even when controlling for changes in luminance and depth accommodation.
Attractor dynamics.
Temporal patterns that evolve towards a stable state from a large range of starting conditions. Attractor network characterization facilitates the identification of key network properties.
Winner-take-all mechanism.
A computational principle in which non-linearities in a recurrent neural network create strong competition between neurons. Only neurons (or sets thereof) with the strongest responses remain active, providing a mechanism for input selection or segregation.
Dimensionality reduction.
Reduction of the number of random variables of a system to a smaller set of principal variables to aid analysis.
Acknowledgements
This work was supported by US National Institutes of Health (NIH) R01 MH102365, NIH R01 EY022951, NIH R01 MH113852, a Simons Foundation Autism Research Initiative (SFARI) Research Grant, a Smith Family Award for Excellence in Biomedical Research, a Klingenstein Fellowship Award, an Alfred P. Sloan Fellowship, a US National Alliance for Research on Schizophrenia & Depression (NARSAD) Young Investigator Award, a McKnight Fellowship and a grant from the Ludwig Family Foundation to J.A.C.; and a Brown-Coxe fellowship and a NARSAD Young Investigator Award to K.A.F. The authors thank M. J. Higley and members of the Cardin and Higley laboratories for insightful discussions, and Q. Perrenoud for help with illustration.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Reynolds JH & Heeger DJ The normalization model of attention. Neuron 61, 168–185 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SH et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature 488, 379–383 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohshiro T, Angelaki DE & DeAngelis GC A normalization model of multisensory integration. Nat. Neurosci 14, 775–782 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carandini M & Heeger DJ Summation and division by neurons in primate visual cortex. Science 264, 1333–1336 (1994). [DOI] [PubMed] [Google Scholar]
- 5.Carandini M & Heeger DJ Normalization as a canonical neural computation. Nat. Rev. Neurosci 13, 51–62 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salinas E & Thier P Gain modulation: a major computational principle of the central nervous system. Neuron 27, 15–21 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Andersen RA, Snyder LH, Bradley DC & Xing J Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu. Rev. Neurosci 20, 303–330 (1997). [DOI] [PubMed] [Google Scholar]
- 8.Pouget A & Snyder LH Computational approaches to sensorimotor transformations. Nat. Neurosci 3, 1192–1198 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Salinas E & Sejnowski TJ Impact of correlated synaptic input on output firing rate and variability in simple neuronal models. J. Neurosci 20, 6193–6209 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finn IM, Priebe NJ & Ferster D The emergence of contrast-invariant orientation tuning in simple cells of cat visual cortex. Neuron 54, 137–152 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sclar G & Freeman RD Orientation selectivity in the cat’s striate cortex is invariant with stimulus contrast. Exp. Brain Res 46, 457–461 (1982). [DOI] [PubMed] [Google Scholar]
- 12.Skottun BC, Bradley A, Sclar G, Ohzawa I & Freeman RD The effects of contrast on visual orientation and spatial frequency discrimination: a comparison of single cells and behavior. J. Neurophysiol 57, 773–786 (1987). [DOI] [PubMed] [Google Scholar]
- 13.Anderson JS, Lampl I, Gillespie DC & Ferster D The contribution of noise to contrast invariance of orientation tuning in cat visual cortex. Science 290, 1968–1972 (2000). [DOI] [PubMed] [Google Scholar]
- 14.McAdams CJ & Maunsell JH Effects of attention on the reliability of individual neurons in monkey visual cortex. Neuron 23, 765–773 (1999). [DOI] [PubMed] [Google Scholar]
- 15.Somers DC, Nelson SB & Sur M An emergent model of orientation selectivity in cat visual cortical simple cells. J. Neurosci 15, 5448–5465 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treue S & Martinez Trujillo JC Feature-based attention influences motion processing gain in macaque visual cortex. Nature 399, 575–579 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Baccus SA & Meister M Fast and slow contrast adaptation in retinal circuitry. Neuron 36, 909–919 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Ruff DA, Ni AM & Cohen MR Cognition as a window into neuronal population space. Annu. Rev. Neurosci 41, 77–97 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shadlen MN, Britten KH, Newsome WT & Movshon JA A computational analysis of the relationship between neuronal and behavioral responses to visual motion. J. Neurosci 16, 1486–1510 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barlow HB, Kaushal TP, Hawken M & Parker AJ Human contrast discrimination and the threshold of cortical neurons. J. Opt. Soc. Am. A 4, 2366–2371 (1987). [DOI] [PubMed] [Google Scholar]
- 21.Boynton GM, Demb JB, Glover GH & Heeger DJ Neuronal basis of contrast discrimination. Vis. Res 39, 257–269 (1999). [DOI] [PubMed] [Google Scholar]
- 22.Clatworthy PL, Chirimuuta M, Lauritzen JS & Tolhurst DJ Coding of the contrasts in natural images by populations of neurons in primary visual cortex (V1). Vis. Res 43, 1983–2001 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Parker A & Hawken M Capabilities of monkey cortical cells in spatial-resolution tasks. J. Opt. Soc. Am. A 2, 1101–1114 (1985). [DOI] [PubMed] [Google Scholar]
- 24.Watson AB Gain, noise, and contrast sensitivity of linear visual neurons. Vis. Neurosci 4, 147–157 (1990). [DOI] [PubMed] [Google Scholar]
- 25.Eldar E, Cohen JD & Niv Y Amplified selectivity in cognitive processing implements the neural gain model of norepinephrine function. Behav. Brain Sci 39, e206 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Natan RG, Carruthers IM, Mwilambwe-Tshilobo L & Geffen MN Gain control in the auditory cortex evoked by changing temporal correlation of sounds. Cereb. Cortex 27, 2385–2402 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mineault PJ, Tring E, Trachtenberg JT & Ringach DL Enhanced spatial resolution during locomotion and heightened attention in mouse primary visual cortex. J. Neurosci 36, 6382–6392 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrates that the relative gain of visual responses between quiescence and locomotion is heterogeneous across cells and depends on the (spatial-frequency) tuning properties of the cell.
- 28.Reimer J et al. Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron 84, 355–362 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett C, Arroyo S & Hestrin S Subthreshold mechanisms underlying state-dependent modulation of visual responses. Neuron 80, 350–357 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohshiro T, Angelaki DE & DeAngelis GC A neural signature of divisive normalization at the level of multisensory integration in primate cortex. Neuron 95, 399–411.e8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ratan Murty NA & Arun SP Multiplicative mixing of object identity and image attributes in single inferior temporal neurons. Proc. Natl Acad. Sci. USA 115, E3276–E3285 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niell CM & Stryker MP Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinck M, Batista-Brito R, Knoblich U & Cardin JA Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 86, 740–754 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that arousal and locomotion differentially regulate neural activity and sensory response gain in mouse V1.
- 34.Destexhe A, Contreras D & Steriade M Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J. Neurosci 19, 4595–4608 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livingstone MS & Hubel DH Effects of sleep and arousal on the processing of visual information in the cat. Nature 291, 554–561 (1981). [DOI] [PubMed] [Google Scholar]
- 36.Pakan JM et al. Behavioral-state modulation of inhibition is context-dependent and cell type specific in mouse visual cortex. eLife 5, e14985 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that locomotion-induced gain modulation of neuronal activity is context dependent, and varies across light and dark conditions and across distinct cell populations.
- 37.Dadarlat MC & Stryker MP Locomotion enhances neural encoding of visual stimuli in mouse V1 J. Neurosci 37, 3764–3775 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrates that locomotion enhances neural encoding of visual stimuli through increased firing rates and decreased noise correlations across the population.
- 38.Schneider DM, Nelson A & Mooney R A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature 513, 189–194 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article shows that locomotion decreases the response gain in the mouse primary auditory cortex via inhibitory interneuron actions in the local cortical circuit.
- 39.Schneider DM, Sundararajan J & Mooney R A cortical filter that learns to suppress the acoustic consequences of movement. Nature 561, 391–395 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munoz W, Tremblay R, Levenstein D & Rudy B Layer-specific modulation of neocortical dendritic inhibition during active wakefulness. Science 355, 954–959 (2017). [DOI] [PubMed] [Google Scholar]; This paper shows that distinct SST+ interneuron populations demonstrate lamina-dependent and state-dependent differences in gain modulation in S1.
- 41.Maunsell JHR Neuronal mechanisms of visual attention. Annu. Rev. Vis. Sci 1, 373–391 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McAdams CJ & Reid RC Attention modulates the responses of simple cells in monkey primary visual cortex. J. Neurosci 25, 11023–11033 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reynolds JH, Pasternak T & Desimone R Attention increases sensitivity of V4 neurons. Neuron 26, 703–714 (2000). [DOI] [PubMed] [Google Scholar]
- 44.Connor CE, Gallant JL, Preddie DC & Van Essen DC Responses in area V4 depend on the spatial relationship between stimulus and attention. J. Neurophysiol 75, 1306–1308 (1996). [DOI] [PubMed] [Google Scholar]
- 45.Connor CE, Preddie DC, Gallant JL & Van Essen DC Spatial attention effects in macaque area V4. J. Neurosci 17, 3201–3214 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J & Maunsell JH A normalization model of attentional modulation of single unit responses. PLOS ONE 4, e4651 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinez-Trujillo JC & Treue S Feature-based attention increases the selectivity of population responses in primate visual cortex. Curr. Biol 14, 744–751 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Williford T & Maunsell JH Effects of spatial attention on contrast response functions in macaque area V4. J. Neurophysiol 96, 40–54 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Reynolds JH, Chelazzi L & Desimone R Competitive mechanisms subserve attention in macaque areas V2 and V4. J. Neurosci 19, 1736–1753 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ecker AS, Denfield GH, Bethge M & Tolias AS On the structure of neuronal population activity under fluctuations in attentional state. J. Neurosci 36, 1775–1789 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rabinowitz NC, Goris RL, Cohen M & Simoncelli EP Attention stabilizes the shared gain of V4 populations. eLife 4, e08998 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tiesinga PH & Sejnowski TJ Rapid temporal modulation of synchrony by competition in cortical interneuron networks. Neural Comput 16, 251–275 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reynolds JH & Chelazzi L Attentional modulation of visual processing. Annu. Rev. Neurosci 27, 611–647 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Schoups A, Vogels R, Qian N & Orban G Practising orientation identification improves orientation coding in V1 neurons. Nature 412, 549–553 (2001). [DOI] [PubMed] [Google Scholar]
- 55.Jurjut O, Georgieva P, Busse L & Katzner S Learning enhances sensory processing in mouse V1 before improving behavior. J. Neurosci 37, 6460–6474 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaneko M & Stryker MP Sensory experience during locomotion promotes recovery of functionin adult visual cortex. eLife 3, e02798 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chance FS, Abbott LF & Reyes AD Gain modulation from background synaptic input. Neuron 35, 773–782 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Ho N & Destexhe A Synaptic background activity enhances the responsiveness of neocortical pyramidal neurons. J. Neurophysiol 84, 1488–1496 (2000). [DOI] [PubMed] [Google Scholar]
- 59.Prescott SA & De Koninck Y Gain control of firing rate by shunting inhibition: roles of synaptic noise and dendritic saturation. Proc. Natl Acad. Sci. USA 100, 2076–2081 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shu Y, Hasenstaub A, Badoual M, Bal T & McCormick DA Barrages of synaptic activity control the gain and sensitivity of cortical neurons. J. Neurosci 23, 10388–10401 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cardin JA, Palmer LA & Contreras D Cellular mechanisms underlying stimulus-dependent gain modulation in primary visual cortex neurons in vivo. Neuron 59, 150–160 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ly C & Doiron B Divisive gain modulation with dynamic stimuli in integrate-and-fire neurons. PLOS Comput. Biol 5, e1000365 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller KD & Troyer TW Neural noise can explain expansive, power-law nonlinearities in neural response functions. J. Neurophysiol 87, 653–659 (2002). [DOI] [PubMed] [Google Scholar]
- 64.Hansel D & van Vreeswijk C How noise contributes to contrast invariance of orientation tuning in cat visual cortex. J. Neurosci 22, 5118–5128 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bulsara A, Jacobs EW, Zhou T, Moss F & Kiss L Stochastic resonance in a single neuron model: theory and analog simulation. J. Theor. Biol 152, 531–555 (1991). [DOI] [PubMed] [Google Scholar]
- 66.Wiesenfeld K & Moss F Stochastic resonance and the benefits of noise: from ice ages to crayfish and SQUIDs. Nature 373, 33–36 (1995). [DOI] [PubMed] [Google Scholar]
- 67.Khubieh A, Ratte S, Lankarany M & Prescott SA Regulation of cortical dynamic range by background synaptic noise and feedforward inhibition. Cereb. Cortex 26, 3357–3369 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Atallah BV, Bruns W, Carandini M & Scanziani M Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron 73, 159–170 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Katzner S, Busse L & Carandini M GABAA inhibition controls response gain in visual cortex. J. Neurosci 31, 5931–5941 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de la Rocha J, Doiron B, Shea-Brown E, Josic K & Reyes A Correlation between neural spike trains increases with firing rate. Nature 448, 802–806 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Gentet LJ, Avermann M, Matyas F, Staiger JF & Petersen CC Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron 65, 422–435 (2010). [DOI] [PubMed] [Google Scholar]
- 72.Pala A & Petersen CC State-dependent cell-type-specific membrane potential dynamics and unitary synaptic inputs in awake mice. eLife 7, e35869 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Polack PO, Friedman J & Golshani P Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat. Neurosci 16, 1331–1339 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poulet JF & Petersen CC Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 454, 881–885 (2008). [DOI] [PubMed] [Google Scholar]
- 75.Chen N, Sugihara H & Sur M An acetylcholine-activated microcircuit drives temporal dynamics of cortical activity. Nat. Neurosci 18, 892–902 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carvalho TP & Buonomano DV Differential effects of excitatory and inhibitory plasticity on synaptically driven neuronal input–output functions. Neuron 61, 774–785 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitchell SJ & Silver RA Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron 38, 433–445 (2003). [DOI] [PubMed] [Google Scholar]
- 78.Murphy BK & Miller KD Multiplicative gain changes are induced by excitation or inhibition alone. J. Neurosci 23, 10040–10051 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abbott LF & Chance FS Drivers and modulators from push–pull and balanced synaptic input. Prog. Brain Res 149, 147–155 (2005). [DOI] [PubMed] [Google Scholar]
- 80.Ayaz A & Chance FS Gain modulation of neuronal responses by subtractive and divisive mechanisms of inhibition. J. Neurophysiol 101, 958–968 (2009). [DOI] [PubMed] [Google Scholar]
- 81.Brozovic M, Abbott LF & Andersen RA Mechanism of gain modulation at single neuron and network levels. J. Comput. Neurosci 25, 158–168 (2008). [DOI] [PubMed] [Google Scholar]
- 82.Vogels TP & Abbott LF Gating multiple signals through detailed balance of excitation and inhibition in spiking networks. Nat. Neurosci 12, 483–491 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fellous JM, Rudolph M, Destexhe A & Sejnowski TJ Synaptic background noise controls the input/output characteristics of single cells in an in vitro model of in vivo activity. Neuroscience 122, 811–829 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holt GR & Koch C Shunting inhibition does not have a divisive effect on firing rates. Neural Comput 9, 1001–1013 (1997). [DOI] [PubMed] [Google Scholar]
- 85.Litwin-Kumar A, Oswald AM, Urban NN & Doiron B Balanced synaptic input shapes the correlation between neural spike trains. PLOS Comput. Biol 7, e1002305 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosenbaum R & Josic K Membrane potential and spike train statistics depend distinctly on input statistics. Phys. Rev. E Stat. Nonlin. Soft Matter Phys 84, 051902 (2011). [DOI] [PubMed] [Google Scholar]
- 87.Shea-Brown E, Josic K, de la Rocha J & Doiron B Correlation and synchrony transfer in integrate-and-fire neurons: basic properties and consequences for coding. Phys. Rev. Lett 100, 108102 (2008). [DOI] [PubMed] [Google Scholar]
- 88.Tchumatchenko T & Wolf F Representation of dynamical stimuli in populations of threshold neurons. PLOS Comput. Biol 7, e1002239 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arsiero M, Luscher HR, Lundstrom BN & Giugliano M The impact of input fluctuations on the frequency–current relationships of layer 5 pyramidal neurons in the rat medial prefrontal cortex. J. Neurosci 27, 3274–3284 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Higgs MH, Slee SJ & Spain WJ Diversity of gain modulation by noise in neocortical neurons: regulation by the slow afterhyperpolarization conductance. J. Neurosci 26, 8787–8799 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hong S, Ratte S, Prescott SA & De Schutter E Single neuron firing properties impact correlation-based population coding. J. Neurosci 32, 1413–1428 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lundstrom BN, Famulare M, Sorensen LB, Spain WJ & Fairhall AL Sensitivity of firing rate to input fluctuations depends on time scale separation between fast and slow variables in single neurons. J. Comput. Neurosci 27, 277–290 (2009). [DOI] [PubMed] [Google Scholar]
- 93.Rauch A, La Camera G, Luscher HR, Senn W & Fusi S Neocortical pyramidal cells respond as integrate-and-fire neurons to in vivo-like input currents. J. Neurophysiol 90, 1598–1612 (2003). [DOI] [PubMed] [Google Scholar]
- 94.Larkum ME, Senn W & Luscher HR Top-down dendritic input increases the gain of layer 5 pyramidal neurons. Cereb. Cortex 14, 1059–1070 (2004). [DOI] [PubMed] [Google Scholar]
- 95.Mehaffey WH, Doiron B, Maler L & Turner RW Deterministic multiplicative gain control with active dendrites. J. Neurosci 25, 9968–9977 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jarvis S, Nikolic K & Schultz SR Neuronal gain modulability is determined by dendritic morphology: a computational optogenetic study. PLOS Comput. Biol 14, e1006027 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Quiquempoix M et al. Layer 2/3 pyramidal neurons control the gain of cortical output. Cell Rep 24, 2799–2807.e4 (2018). [DOI] [PubMed] [Google Scholar]
- 98.Sato TK, Haider B, Hausser M & Carandini M An excitatory basis for divisive normalization in visual cortex. Nat. Neurosci 19, 568–570 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haider B & McCormick DA Rapid neocortical dynamics: cellular and network mechanisms. Neuron 62, 171–189 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Silver RA Neuronal arithmetic. Nat. Rev. Neurosci 11, 474–489 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nelson S, Toth L, Sheth B & Sur M Orientation selectivity of cortical neurons during intracellular blockade of inhibition. Science 265, 774–777 (1994). [DOI] [PubMed] [Google Scholar]
- 102.Atallah BV, Scanziani M & Carandini M Atallah et al. reply. Nature 508, E3 (2014). [DOI] [PubMed] [Google Scholar]
- 103.El-Boustani S, Wilson NR, Runyan CA & Sur M El-Boustani et al. reply. Nature 508, E3–E4 (2014). [DOI] [PubMed] [Google Scholar]
- 104.Wilson NR, Runyan CA, Wang FL & Sur M Division and subtraction by distinct cortical inhibitory networks in vivo. Nature 488, 343–348 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Natan RG, Rao W & Geffen MN Cortical interneurons differentially shape frequency tuning following adaptation. Cell Rep 21, 878–890 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that distinct cortical interneuron populations differently modulate the gain of frequency-tuned excitatory responses during adaptation.
- 106.Phillips EA & Hasenstaub AR Asymmetric effects of activating and inactivating cortical interneurons. eLife 5, e18383 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that optogenetic activation of GABAergic interneurons in the cortex does not fully capture the impact of inhibition on excitatory neuron response gain.
- 107.Seybold BA, Phillips EAK, Schreiner CE & Hasenstaub AR Inhibitory actions unified by network integration. Neuron 87, 1181–1192 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fishell G & Rudy B Mechanisms of inhibition within the telencephalon: “where the wild things are”. Annu. Rev. Neurosci 34, 535–567 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Markram H et al. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci 5, 793–807 (2004). [DOI] [PubMed] [Google Scholar]
- 110.El-Boustani S & Sur M Response-dependent dynamics of cell-specific inhibition in cortical networks in vivo. Nat. Commun 5, 5689 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee AM et al. Identification of a brainstem circuit regulating visual cortical state in parallel with locomotion. Neuron 83, 455–466 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cardin JA Inhibitory interneurons regulate temporal precision and correlations in cortical circuits. Trends Neurosci 41, 689–700 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cone JJ, Scantlen MD, Histed MH & Maunsell JHR Different inhibitory interneuron cell classes make distinct contributions to visual contrast perception. eNeuro 10.1523/ENEURO.0337-18.2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ayzenshtat I, Karnani MM, Jackson J & Yuste R Cortical control of spatial resolution by VIP+ interneurons. J. Neurosci 36, 11498–11509 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hong YK, Lacefield CO, Rodgers CC & Bruno RM Sensation, movement and learning in the absence of barrel cortex. Nature 561, 542–546 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Otchy TM et al. Acute off-target effects of neural circuit manipulations. Nature 528, 358–363 (2015). [DOI] [PubMed] [Google Scholar]
- 117.Wolff SB & Olveczky BP The promise and perils of causal circuit manipulations. Curr. Opin. Neurobiol 49, 84–94 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Phillips EAK, Schreiner CE & Hasenstaub AR Cortical interneurons differentially regulate the effects of acoustic context. Cell Rep 20, 771–778 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fu Y et al. A cortical circuit for gain control by behavioral state. Cell 156, 1139–1152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides evidence that VIP+ interneurons are activated by locomotion and may contribute to state-dependent visual response gain modulation in mouse V1.
- 120.Karnani MM et al. Opening holes in the blanket of inhibition: localized lateral disinhibition by VIP interneurons. J. Neurosci 36, 3471–3480 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Karnani MM et al. Cooperative subnetworks of molecularly similar interneurons in mouse neocortex. Neuron 90, 86–100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee S, Kruglikov I, Huang ZJ, Fishell G & Rudy B A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat. Neurosci 16, 1662–1670 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pi HJ et al. Cortical interneurons that specialize in disinhibitory control. Nature 503, 521–524 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Batista-Brito R et al. Developmental dysfunction of VIP interneurons impairs cortical circuits. Neuron 95, 884–895.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dipoppa M et al. Vision and locomotion shape the interactions between neuron types in mouse visual cortex. Neuron 98, 602–615.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ozeki H, Finn IM, Schaffer ES, Miller KD & Ferster D Inhibitory stabilization of the cortical network underlies visual surround suppression. Neuron 62, 578–592 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tsodyks MV, Skaggs WE, Sejnowski TJ & McNaughton BL Paradoxical effects of external modulation of inhibitory interneurons. J. Neurosci 17, 4382–4388 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kato HK, Asinof SK & Isaacson JS Network-level control of frequency tuning in auditory cortex. Neuron 95, 412–423.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Garcia Del Molino LC, Yang GR, Mejias JF & Wang XJ Paradoxical response reversal of top-down modulation in cortical circuits with three interneuron types. eLife 6, e29742 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Litwin-Kumar A, Rosenbaum R & Doiron B Inhibitory stabilization and visual coding in cortical circuits with multiple interneuron subtypes. J. Neurophysiol 115, 1399–1409 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhou M et al. Scaling down of balanced excitation and inhibition by active behavioral states in auditory cortex. Nat. Neurosci 17, 841–850 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Boly M et al. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc. Natl Acad. Sci. USA 104, 12187–12192 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fox MD, Snyder AZ, Vincent JL & Raichle ME Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron 56, 171–184 (2007). [DOI] [PubMed] [Google Scholar]
- 134.Hesselmann G, Kell CA, Eger E & Kleinschmidt A Spontaneous local variations in ongoing neural activity bias perceptual decisions. Proc. Natl Acad. Sci. USA 105, 10984–10989 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hesselmann G, Kell CA & Kleinschmidt A Ongoing activity fluctuations in hMT+ bias the perception of coherent visual motion. J. Neurosci 28, 14481–14485 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Palva JM & Palva S Roles of multiscale brain activity fluctuations in shaping the variability and dynamics of psychophysical performance. Prog. Brain Res 193, 335–350 (2011). [DOI] [PubMed] [Google Scholar]
- 137.Diamond DM, Campbell AM, Park CR, Halonen J & Zoladz PR The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes–Dodson law. Neural. Plast 2007, 60803 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yerkes RM & Dodson JD The relation of strength of stimulus to rapidity of habit-formation. J. Comp. Neurol. Psychol 18, 459–482 (1908). [Google Scholar]
- 139.He BJ Spontaneous and task-evoked brain activity negatively interact. J. Neurosci 33, 4672–4682 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bullock T, Elliott JC, Serences JT & Giesbrecht B Acute exercise modulates feature-selective responses in human cortex. J. Cognit. Neurosci 29, 605–618 (2017). [DOI] [PubMed] [Google Scholar]
- 141.He BJ & Zempel JM Average is optimal: an inverted-U relationship between trial-to-trial brain activity and behavioral performance. PLOS Comput. Biol 9, e1003348 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Murphy PR, Vandekerckhove J & Nieuwenhuis S Pupil-linked arousal determines variability in perceptual decision making. PLOS Comput. Biol 10, e1003854 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aston-Jones G & Cohen JD An integrative theory of locus coeruleus–norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci 28, 403–450 (2005). [DOI] [PubMed] [Google Scholar]
- 144.Murphy PR, O’Connell RG, O’Sullivan M, Robertson IH & Balsters JH Pupil diameter covaries with BOLD activity in human locus coeruleus. Hum. Brain Mapp 35, 4140–4154 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Joshi S, Li Y, Kalwani RM & Gold JI Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron 89, 221–234 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Reimer J et al. Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat. Commun 7, 13289 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Erisken S et al. Effects of locomotion extend throughout the mouse early visual system. Curr. Biol 24, 2899–2907 (2014). [DOI] [PubMed] [Google Scholar]
- 148.Tang L & Higley MJ Layer 5 circuits in V1 differentially control visuomotor behavior. bioRxiv 10.1101/540807 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Saleem AB, Ayaz A, Jeffery KJ, Harris KD & Carandini M Integration of visual motion and locomotion in mouse visual cortex. Nat. Neurosci 16, 1864–1869 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Neske GT, Nestvogel D, Steffan PJ & McCormick DA Distinct waking states for strong evoked responses in primary visual cortex and optimal visual detection performance. J. Neurosci 39, 10044–10059 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bullock T, Cecotti H & Giesbrecht B Multiple stages of information processing are modulated during acute bouts of exercise. Neuroscience 307, 138–150 (2015). [DOI] [PubMed] [Google Scholar]
- 152.Benjamin AV, Wailes-Newson K, Ma-Wyatt A, Baker DH & Wade AR The effect of locomotion on early visual contrast processing in humans. J. Neurosci 38, 3050–3059 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McGinley MJ, David SV & McCormick DA Cortical membrane potential signature of optimal states for sensory signal detection. Neuron 87, 179–192 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Barson D et al. Simultaneous mesoscopic and two-photon imaging of neuronal activity in cortical circuits. Nat. Methods 10.1038/s41592-019-0625-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Clancy KB, Orsolic I & Mrsic-Flogel TD Locomotion-dependent remapping of distributed cortical networks. Nat. Neurosci 22, 778–786 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shimaoka D, Harris KD & Carandini M Effects of arousal on mouse sensory cortex depend on modality. Cell Rep 22, 3160–3167 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Musall S, Kaufman MT, Juavinett AL, Gluf S & Churchland AK Single-trial neural dynamics are dominated by richly varied movements. Nat. Neurosci 22, 1677–1686 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports that animal movements capture the majority of neural variability across the cortex, and those that are task-aligned account for features commonly attributed to cognitive task demands.
- 158.Disney AA, Alasady HA & Reynolds JH Muscarinic acetylcholine receptors are expressed by most parvalbumin-immunoreactive neurons in area MT of the macaque. Brain Behav 4, 431–445 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Disney AA & Aoki C Muscarinic acetylcholine receptors in macaque V1 are most frequently expressed by parvalbumin-immunoreactive neurons. J. Comp. Neurol 507, 1748–1762 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Disney AA, Domakonda KV & Aoki C Differential expression of muscarinic acetylcholine receptors across excitatory and inhibitory cells in visual cortical areas V1 and V2 of the macaque monkey. J. Comp. Neurol 499, 49–63 (2006). [DOI] [PubMed] [Google Scholar]
- 161.Disney AA & Reynolds JH Expression of m1-type muscarinic acetylcholine receptors by parvalbumin-immunoreactive neurons in the primary visual cortex: a comparative study of rat, guinea pig, ferret, macaque, and human. J. Comp. Neurol 522, 986–1003 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Letzkus JJ et al. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480, 331–335 (2011). [DOI] [PubMed] [Google Scholar]
- 163.Porter JT et al. Selective excitation of subtypes of neocortical interneurons by nicotinic receptors. J. Neurosci 19, 5228–5235 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Urban-Ciecko J, Jouhanneau JS, Myal SE, Poulet JFA & Barth AL Precisely timed nicotinic activation drives SST inhibition in neocortical circuits. Neuron 97, 611–625.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Disney AA, Aoki C & Hawken MJ Gain modulation by nicotine in macaque V1. Neuron 56, 701–713 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gil Z, Connors BW & Amitai Y Differential regulation of neocortical synapses by neuromodulators and activity. Neuron 19, 679–686 (1997). [DOI] [PubMed] [Google Scholar]
- 167.Hasselmo ME & Bower JM Cholinergic suppression specific to intrinsic not afferent fiber synapses in rat piriform (olfactory) cortex. J. Neurophysiol 67, 1222–1229 (1992). [DOI] [PubMed] [Google Scholar]
- 168.Kimura F Cholinergic modulation of cortical function: a hypothetical role in shifting the dynamics in cortical network. Neurosci. Res 38, 19–26 (2000). [DOI] [PubMed] [Google Scholar]
- 169.Kimura F, Fukuda M & Tsumoto T Acetylcholine suppresses the spread of excitation in the visual cortex revealed by optical recording: possible differential effect depending on the source of input. Eur. J. Neurosci 11, 3597–3609 (1999). [DOI] [PubMed] [Google Scholar]
- 170.Disney AA, Aoki C & Hawken MJ Cholinergic suppression of visual responses in primate V1 is mediated by GABAergic inhibition. J. Neurophysiol 108, 1907–1923 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Soma S, Shimegi S, Osaki H & Sato H Cholinergic modulation of response gain in the primary visual cortex of the macaque. J. Neurophysiol 107, 283–291 (2012). [DOI] [PubMed] [Google Scholar]
- 172.Herrero JL, Gieselmann MA & Thiele A Muscarinic and nicotinic contribution to contrast sensitivity of macaque area V1 neurons. Front. Neural Circuits 11, 106 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Askew C, Intskirveli I & Metherate R Systemic nicotine increases gain and narrows receptive fields in A1 via integrated cortical and subcortical actions. eNeuro 10.1523/ENEURO.0192-17.2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Herrero JL et al. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature 454, 1110–1114 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pinto L et al. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat. Neurosci 16, 1857–1863 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Stewart AE, Yan Z, Surmeier DJ & Foehring RC Muscarine modulates Ca2+ channel currents in rat sensorimotor pyramidal cells via two distinct pathways. J. Neurophysiol 81, 72–84 (1999). [DOI] [PubMed] [Google Scholar]
- 177.Lorenzon NM & Foehring RC Relationship between repetitive firing and afterhyperpolarizations in human neocortical neurons. J. Neurophysiol 67, 350–363 (1992). [DOI] [PubMed] [Google Scholar]
- 178.McCormick DA & Prince DA Mechanisms of action of acetylcholine in the guinea-pig cerebral cortex in vitro. J. Physiol 375, 169–194 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schwindt PC, Spain WJ & Crill WE Influence of anomalous rectifier activation on afterhyperpolarizations of neurons from cat sensorimotor cortex in vitro. J. Neurophysiol 59, 468–481 (1988). [DOI] [PubMed] [Google Scholar]
- 180.Wang Z & McCormick DA Control of firing mode of corticotectal and corticopontine layer V burst-generating neurons by norepinephrine, acetylcholine, and 1S,3R-ACPD. J. Neurosci 13, 2199–2216 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Eggermann E & Feldmeyer D Cholinergic filtering in the recurrent excitatory microcircuit of cortical layer 4. Proc. Natl Acad. Sci. USA 106, 11753–11758 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gulledge AT, Park SB, Kawaguchi Y & Stuart GJ Heterogeneity of phasic cholinergic signaling in neocortical neurons. J. Neurophysiol 97, 2215–2229 (2007). [DOI] [PubMed] [Google Scholar]
- 183.Gulledge AT & Stuart GJ Cholinergic inhibition of neocortical pyramidal neurons. J. Neurosci 25, 10308–10320 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dasgupta R, Seibt F & Beierlein M Synaptic release of acetylcholine rapidly suppresses cortical activity by recruiting muscarinic receptors in layer 4. J. Neurosci 38, 5338–5350 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Higley MJ, Soler-Llavina GJ & Sabatini BL Cholinergic modulation of multivesicular release regulates striatal synaptic potency and integration. Nat. Neurosci 12, 1121–1128 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Giessel AJ & Sabatini BL M1 muscarinic receptors boost synaptic potentials and calcium influx in dendritic spines by inhibiting postsynaptic SK channels. Neuron 68, 936–947 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Foehring RC, Schwindt PC & Crill WE Norepinephrine selectively reduces slow Ca2+- and Na+-mediated K+ currents in cat neocortical neurons. J. Neurophysiol 61, 245–256 (1989). [DOI] [PubMed] [Google Scholar]
- 188.Madison DV & Nicoll RA Actions of noradrenaline recorded intracellularly in rat hippocampal CA1 pyramidal neurones, in vitro. J. Physiol 372, 221–244 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mueller D, Porter JT & Quirk GJ Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J. Neurosci 28, 369–375 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dodt HU, Pawelzik H & Zieglgansberger W Actions of noradrenaline on neocortical neurons in vitro. Brain Res 545, 307–311 (1991). [DOI] [PubMed] [Google Scholar]
- 191.Mynlieff M & Dunwiddie TV Noradrenergic depression of synaptic responses in hippocampus of rat: evidence for mediation by α1-receptors. Neuropharmacology 27, 391–398 (1988). [DOI] [PubMed] [Google Scholar]
- 192.Guan D, Armstrong WE & Foehring RC Electrophysiological properties of genetically identified subtypes of layer 5 neocortical pyramidal neurons: Ca2+ dependence and differential modulation by norepinephrine. J. Neurophysiol 113, 2014–2032 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Waterhouse BD, Mouradian R, Sessler FM & Lin RC Differential modulatory effects of norepinephrine on synaptically driven responses of layer V barrel field cortical neurons. Brain Res 868, 39–47 (2000). [DOI] [PubMed] [Google Scholar]
- 194.Armstrong-James M & Fox K Effects of ionophoresed noradrenaline on the spontaneous activity of neurones in rat primary somatosensory cortex. J. Physiol 335, 427–447 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bassant MH, Ennouri K & Lamour Y Effects of iontophoretically applied monoamines on somatosensory cortical neurons of unanesthetized rats. Neuroscience 39, 431–439 (1990). [DOI] [PubMed] [Google Scholar]
- 196.Foote SL, Freedman R & Oliver AP Effects of putative neurotransmitters on neuronal activity in monkey auditory cortex. Brain Res 86, 229–242 (1975). [DOI] [PubMed] [Google Scholar]
- 197.Waterhouse BD, Moises HC & Woodward DJ Noradrenergic modulation of somatosensory cortical neuronal responses to iontophoretically applied putative neurotransmitters. Exp. Neurol 69, 30–49 (1980). [DOI] [PubMed] [Google Scholar]
- 198.Waterhouse BD, Moises HC & Woodward DJ Alpha-receptor-mediated facilitation of somatosensory cortical neuronal responses to excitatory synaptic inputs and iontophoretically applied acetylcholine. Neuropharmacology 20, 907–920 (1981). [DOI] [PubMed] [Google Scholar]
- 199.Ego-Stengel V, Bringuier V & Shulz DE Noradrenergic modulation of functional selectivity in the cat visual cortex: an in vivo extracellular and intracellular study. Neuroscience 111, 275–289 (2002). [DOI] [PubMed] [Google Scholar]
- 200.Seillier L et al. Serotonin decreases the gain of visual responses in awake macaque V1. J. Neurosci 37, 11390–11405 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Watakabe A et al. Enriched expression of serotonin 1B and 2A receptor genes in macaque visual cortex and their bidirectional modulatory effects on neuronal responses. Cereb. Cortex 19, 1915–1928 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dugue GP et al. Optogenetic recruitment of dorsal raphe serotonergic neurons acutely decreases mechanosensory responsivity in behaving mice. PLOS ONE 9, e105941 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Davis M, Strachan DI & Kass E Excitatory and inhibitory effects of serotonin on sensorimotor reactivity measured with acoustic startle. Science 209, 521–523 (1980). [DOI] [PubMed] [Google Scholar]
- 204.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV & Arnsten AF Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci 10, 376–384 (2007). [DOI] [PubMed] [Google Scholar]
- 205.Williams GV & Goldman-Rakic PS Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 376, 572–575 (1995). [DOI] [PubMed] [Google Scholar]
- 206.Noudoost B & Moore T Control of visual cortical signals by prefrontal dopamine. Nature 474, 372–375 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lur G & Higley MJ Glutamate receptor modulation is restricted to synaptic microdomains. Cell Rep 12, 326–334 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Athilingam JC, Ben-Shalom R, Keeshen CM, Sohal VS & Bender KJ Serotonin enhances excitability and gamma frequency temporal integration in mouse prefrontal fast-spiking interneurons. eLife 6, e31991 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kawaguchi Y & Shindou T Noradrenergic excitation and inhibition of GABAergic cell types in rat frontal cortex. J. Neurosci 18, 6963–6976 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Demb JB Multiple mechanisms for contrast adaptation in the retina. Neuron 36, 781–783 (2002). [DOI] [PubMed] [Google Scholar]
- 211.Farley BJ, Quirk MC, Doherty JJ & Christian EP Stimulus-specific adaptation in auditory cortex is an NMDA-independent process distinct from the sensory novelty encoded by the mismatch negativity. J. Neurosci 30, 16475–16484 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fishman YI & Steinschneider M Searching for the mismatch negativity in primary auditory cortex of the awake monkey: deviance detection or stimulus specific adaptation? J. Neurosci 32, 15747–15758 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kohn A & Movshon JA Neuronal adaptation to visual motion in area MT of the macaque. Neuron 39, 681–691 (2003). [DOI] [PubMed] [Google Scholar]
- 214.Szymanski FD, Garcia-Lazaro JA & Schnupp JW Current source density profiles of stimulus-specific adaptation in rat auditory cortex. J. Neurophysiol 102, 1483–1490 (2009). [DOI] [PubMed] [Google Scholar]
- 215.Ulanovsky N, Las L & Nelken I Processing of low-probability sounds by cortical neurons. Nat. Neurosci 6, 391–398 (2003). [DOI] [PubMed] [Google Scholar]
- 216.Dean I, Robinson BL, Harper NS & McAlpine D Rapid neural adaptation to sound level statistics. J. Neurosci 28, 6430–6438 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Barlow H in Sensory Communication (MIT Press, 1961). [Google Scholar]
- 218.Niyogi RK & Wong-Lin K Dynamic excitatory and inhibitory gain modulation can produce flexible, robust and optimal decision-making. PLOS Comput. Biol 9, e1003099 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Busse L, Wade AR & Carandini M Representation of concurrent stimuli by population activity in visual cortex. Neuron 64, 931–942 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hahnloser RH, Douglas RJ & Hepp K Attentional recruitment of inter-areal recurrent networks for selective gain control. Neural Comput 14, 1669–1689 (2002). [DOI] [PubMed] [Google Scholar]
- 221.Thiele A & Bellgrove MA Neuromodulation of attention. Neuron 97, 769–785 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Schwartz O & Simoncelli EP Natural signal statistics and sensory gain control. Nat. Neurosci 4, 819–825 (2001). [DOI] [PubMed] [Google Scholar]
- 223.Willmore BD, Bulstrode H & Tolhurst DJ Contrast normalization contributes to a biologically-plausible model of receptive-field development in primary visual cortex (V1). Vis. Res 54, 49–60 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ni AM, Ruff DA, Alberts JJ, Symmonds J & Cohen MR Learning and attention reveal a general relationship between population activity and behavior. Science 359, 463–465 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lee S, Park J & Smirnakis SM Internal gain modulations, but not changes in stimulus contrast, preserve the neural code. J. Neurosci 39, 1671–1687 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.McGinley MJ et al. Waking state: rapid variations modulate neural and behavioral responses. Neuron 87, 1143–1161 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Rose D & Blakemore C Effects of bicuculline on functions of inhibition in visual cortex. Nature 249, 375–377 (1974). [DOI] [PubMed] [Google Scholar]
- 228.Carandini M & Ferster D Membrane potential and firing rate in cat primary visual cortex. J. Neurosci 20, 470–484 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Isaacson JS & Scanziani M How inhibition shapes cortical activity. Neuron 72, 231–243 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhang YP & Oertner TG Optical induction of synaptic plasticity using a light-sensitive channel. Nat. Methods 4, 139–141 (2007). [DOI] [PubMed] [Google Scholar]
- 231.Allen BD, Singer AC & Boyden ES Principles of designing interpretable optogenetic behavior experiments. Learn. Mem 22, 232–238 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cottam JC, Smith SL & Hausser M Target-specific effects of somatostatin-expressing interneurons on neocortical visual processing. J. Neurosci 33, 19567–19578 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Pfeffer CK, Xue M, He M, Huang ZJ & Scanziani M Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat. Neurosci 16, 1068–1076 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]


