Abstract
Background:
Ustekinumab is an effective therapy for Crohn disease currently approved for adults. Off-label use in the pediatric population is increasing, but its effectiveness in this age group has not been reported.
Aims:
The aim of the study was to describe real-world experience with ustekinumab at a tertiary care pediatric inflammatory bowel disease (IBD) center.
Methods:
As part of an ongoing observational cohort study of biologic-treated pediatric IBD patients initiated in October 2014, data on demographics, disease behavior, location and activity, treatment, and surgical history were collected for all patients receiving ustekinumab. Disease activity was assessed using the Harvey Bradshaw index or partial Mayo score. Primary outcome was steroid-free remission at 52 weeks. Descriptive statistics summarized the safety and efficacy outcomes, and univariate analyses were performed to examine associations of clinical characteristics with efficacy.
Results:
Fifty-two children and young adults initiating ustekinumab were analyzed; 81% Crohn Disease, 8% ulcerative colitis, and 11% IBD-unspecified. Median [IQR] age at induction was 16.8 [14–18] years. Patients were followed for a minimum of 12 months. Most patients (81%) failed >1 anti-TNF, and 37% failed anti-TNF and vedolizumab; 10 patients were biologic-naïve. At week 52, 75% were still on ustekinumab, and 50% (bio-exposed) and 90% (bio-naïve) were in steroid-free remission. Two infusion reactions and neither serious adverse events nor serious infections were observed.
Conclusions:
Our results suggest that ustekinumab is efficacious and safe in pediatric patients with IBD. Controlled clinical trial data are needed to confirm these observations.
Keywords: Crohn disease, inflammatory bowel disease, pediatrics, ulcerative colitis, ustekinumab
Ustekinumab is a first-in-class therapeutic human IgG1 kappa monoclonal antibody (mAb) that binds to interleukins (IL)-12 and IL-23, cytokines that modulate lymphocyte function, including T-helper (Th)-1 and Th17 cell subsets (1). Ustekinumab is approved for use in psoriatic arthritis and moderate-to-severe plaque psoriasis, and in September 2016, for the treatment of adult patients with moderate to severely active Crohn disease (CD) (2). The phase-3 registration trials for ustekinumab in adult CD patients naïve to anti-tumor necrosis factor (TNF) (UNITI-1) demonstrated week 6 remission and response rates of 28.7% and 51.7%, respectively. These rates were nearly 2-fold higher when compared with anti-TNF failure patients (UNITI-2) whose remission and response rates were 16.3% and 34.3%, respectively (3,4). The same effect of prior anti-TNF exposure with respect to maintenance of remission, however, was not observed (IM-UNITI) (5). The induction data in adults with ulcerative colitis (UC) met its primary outcome and the maintenance results are forthcoming (6).
As we have seen with prior biologics brought to market first in the adult IBD population, there is increasing off-label use of ustekinumab in children even in the absence of pediatric pharmacokinetic data. There remains limited data on the effectiveness of ustekinumab in the pediatric population. There are few published case reports showing clinical response in 50% of children (7–9) and Fusillo et al most recently described clinical response in 50%, 36%, and 35% of patients at weeks 6, 26, and 52, respectively, in a cohort of 28 pediatric patients (10). Similar 12-month outcomes were described in a cohort of 44 pediatric patients (11).
Pharmacokinetic trials for ustekinumab in pediatric CD are currently underway (Clinicaltrials.gov ID NCT02968108,); however, with increasing off-label use in children, more data are needed on the safety and effectiveness in pediatric IBD patients. Herein we report our experience on the clinical effectiveness and safety of ustekinumab in children and young adults with pediatric onset IBD from a single tertiary care IBD referral center.
METHODS
Study Design and Patient Population
As part of an ongoing observational cohort study of biologic treated pediatric patients with IBD ages 21 years and below, we conducted a retrospective review of all patients who initiated ustekinumab for the treatment of IBD between October 2014 and April 2018 at the Susan and Leonard Feinstein Inflammatory Bowel Disease Clinical Center at Mount Sinai in New York. Patients eligible for analysis included children and young adults ages 21 years and younger, diagnosed with IBD at or below the age of 18 years who received an induction dose of ustekinumab via intravenous (IV) or subcutaneous (SC) route, and were followed up for a minimum of 52 weeks. Patients who had received any IBD-related surgery (excluding peri-anal surgery alone) before ustekinumab start were excluded from analysis. All patients were cared for by a board-certified pediatric gastroenterologist and the decision to prescribe ustekinumab was at the discretion of the prescribing physician. The study was approved by our Institutional Review Board (IRB-18–00574).
Data Collection
Electronic medical records were reviewed for demographics, medical and surgical treatment history, including steroid use, previous biologics and immunomodulator exposure, disease location and behavior (Paris classification), and disease activity (Harvey Bradshaw index [HBI] (12) for CD and partial Mayo score [pMS] (13) for UC). The baseline demographics and disease characteristics are outlined in Table 1. Ustekinumab treatment information included dose and method of delivery at induction, as well as frequency and duration of maintenance therapy. Clinical activity scores, as well as pertinent laboratory tests were recorded at baseline (time of ustekinumab initiation) and at week 52. We also collected data on adverse events, including infusion and injection site reactions, as well as severe adverse events (SAE). Laboratory data reviewed included complete blood count, albumin, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP).
TABLE 1.
Baseline demographics and clinical characteristics
| CD, n = 42 | UC/IBDU, n = 10 | |
|---|---|---|
| Male sex, No. (%) | 21 (50) | 2 (20) |
| Age at diagnosis, median [IQR] years | 10.3 [7.7–13.3] | 13.9 [9.9–14.8] |
| Age at UST initiation, median [IQR], years | 16.9 [13.9–18.2] | 15.2 [11.1–17] |
| Duration of disease, median [IQR], years | 4.9 [2.6–8.3] | 1.8 [0.7–3.3] |
| Disease behavior, No. (%) | ||
| Non-stricturing (B1) | 37 (88.1) | – |
| Stricturing (B2) | 3 (7.1) | – |
| Penetrating (B3) | 2 (4.8) | – |
| Perianal perforating | 5 (11.9) | – |
| CD Disease location, No. (%) | ||
| Ileal (L1) | 8 (19) | – |
| Colonic (L2) | 13 (31) | – |
| Ileocolonic (L3) | 19 (45.2) | – |
| Upper tract (L4) | 22 (52.4) | – |
| Perianal (p) | 5 (11.9) | – |
| UC/IBDU disease location, No. (%) | ||
| Proctitis (E1) | – | 1 (10) |
| Left-sided (E2) | – | 1 (10) |
| Extensive (E3) | – | 4 (40) |
| Pancolitits (E4) | – | 4 (40) |
| Concomitant IMM at UST start, No. (%) | 10 (23.8) | 2 (20) |
| Concomitant Steroids at UST start, No. (%) | 21 (50) | 7 (70) |
| Prior anti-TNF agents, No. (%) | ||
| 0 | 9 (21.4) | 1 (10) |
| 1 | 33 (79) | 9 (90) |
| 2 | 15 (35.7) | 3 (30) |
| Time from last anti-TNF to UST, median [IQR], weeks | 10.4 [6.1–97.6] | 11 [3.7–34] |
| <30 days | 6 | 3 |
| 30 to 60 days | 9 | 1 |
| 61 to 90 days | 2 | 1 |
| >90 days | 16 | 4 |
| Prior vedolizumab, No. (%) | 12 (28.6) | 7 (70) |
| Time from VDZ to UST, median [IQR], weeks | 6 [5.2–6.9] | 3 [2.5–3.7] |
| Harvey Bradshaw Index, median [IQR] | 2 [0.25–6] | – |
| Partial Mayo Score, median [IQR] | – | 8 [6.9–9] |
| CRP abnormal No. (%) | 16 (38) | 2 (20) |
| CRP (xULN), median [IQR] | 0.6 [0.2–1.7] | 0.3 [0.2–0.5] |
| Albumin, median [IQR], g/L | 38 [35–40] | 37 [35–39] |
| Haematocrit, median [IQR], L/L | 0.38 [0.35–0.41] | 0.37 [0.36–0.38] |
| Psoriasiform rash | 12 (28.6) | 1 (10) |
CD = Crohn disease; CRP = C-reactive protein; IBDU = IBD-unclassified; IMM = immunomodulator; IQR = interquartile range; TNF = tumor necrosis factor; UC = ulcerative colitis; ULN = upper limit of normal; UST = ustekinumab; VDZ = vedolizumab.
Outcomes Measures
The primary outcome was steroid-free remission at week 52, defined as HBI ≤4 (12) or pMS <2 (13), and off any form of corticosteroids for at least 4 weeks before the 52-week endpoint. Secondary outcomes included clinical remission, defined as HBI ≤4, or pMS <2, and biomarker remission, defined as a normal CRP at week 52. Drug safety as of last follow-up and therapeutic drug-monitoring outcomes, whenever available, were also described.
Statistical Analysis
Standard descriptive statistics, including frequency for categorical variables and median [interquartile range] (IQR) for continuous variables, were calculated. Univariate analyses were performed to examine associations of clinical and laboratory characteristics with ustekinumab effectiveness by chi-square analysis or the Fisher exact test whenever applicable. P value < 0.05 was considered for significance.
RESULTS
Patient Population
Of the 492 pediatric patients who initiated a biologic therapy between October 2014 and April 2018, 66 patients were started on ustekinumab for the treatment of IBD. Fourteen patients were excluded from analysis; 5 patients had not reached the 52-week endpoint, 7 patients started ustekinumab as postoperative maintenance therapy, and 2 patients were lost to follow-up.
A total of 52 patients were included for analysis: CD, n = 42 (81%); UC, n = 4 (8%); and IBD-unspecified (IBDU), n = 6 (11%). The median age at baseline was 16.8 [14–18] years, and median disease duration was 4 [1.8–7.2] years. Thirty-eight patients (73%) were below age 18 years at start of therapy. At baseline, 23% were on a concomitant immunomodulator (mercaptopurine [CD:3], azathioprine [CD:1], or methotrexate [CD:6, UC/IBDU:2)], and 54% were receiving corticosteroids [PO prednisone, median dose 0.7 [0.4–0.9] mg/kg (CD:6, UC/IBDU:3)], PO budesonide 9mg (CD:14, UC/IBDU:4), IV methylprednisolone 32mg (0.8 mg/kg) (CD:1)). Ten patients were biologic-naïve (CD:9, UC/IBDU:1), 81% had failed at least 1 anti-TNF agent (CD:33, UC/IBDU:9), and 35% had failed at least 2 (CD:15, UC/IBDU:3). A total of 37% of patients had failed a trial of vedolizumab (CD:12, UC/IBDU:7), with a median time on vedolizumab of 10.7 [7.1–15.4] months; all had also been exposed to anti-TNF.
The median CRP at baseline was 0.5 [0.17–1.7] × upper limit of normal, with 64% having a normal baseline CRP. Median HBI was 2 [0.25–6] and median partial Mayo score was 8 [6.9–9]. All UC/IBDU patients had active clinical disease at baseline but 23 of the 42 CD patients were in clinical remission (HBI ≤4,) with 10 still on corticosteroids. Of the 13 patients in remission without steroids, 8 were bio-exposed; 5 switched because of anti-TNF-induced psoriasiform dermatitis (2 with normal and 2 with abnormal endoscopy, and 1 without a scope,) 1 had endoscopic evidence of disease, 1 discontinued infliximab because of antidrug antibodies and infusion reaction and endoscopic disease, and 1 patient was switched to ustekinumab from a thiopurine, and had endoscopic inflammation. The remaining 5 of 13 were bio-naïve; 2 switched from a thiopurine in the absence of endoscopic activity and the remaining 3 started ustekinumab because of endoscopic disease per the treating physician on treat to target colonoscopy—(findings of erythema, edema, mucosal nodularity, aphthous ulcerations, and loss of normal vascular pattern, with corresponding cryptitis, crypt abscess, and crypt distortion). Regarding the 8 patients in steroid-free clinical remission with endoscopic disease activity at baseline, 4 patients were on infliximab before initiating ustekinumab, 1 patient was switched from mercaptopurine, 1 was on a 5-ASA and antibiotics, 1 was on cyclic enteral nutrition, and 1 patient had no previous treatment for IBD.
Ustekinumab Dosing
Forty-seven (90%) patients received ustekinumab via IV induction at 260 mg for patients less than 55 kg (n = 20, 38%), 390mg for patients between 55 and 85 kg (n = 25, 48%), and 520mg for patients >85kg (n = 2, 3.8%). Five CD patients were induced with ustekinumab via subcutaneous injection, all of whom initiated the drug before Food and Drug Administration (FDA) approval of the IV formulation. Maintenance dosing was then prescribed as 90mg subcutaneous injection every 8 weeks for all patients.
Ustekinumab Effectiveness Outcomes
Remission Rates at Last Follow-Up
Thirty-nine patients (75%) (CD:31, UC/IBDU:8) were still on ustekinumab at week 52. The median HBI and pMS were 1 [0–2] and 2 [0.8–3.3], respectively. Thirty patients (58%) (CD: 25 [60%], UC/IBDU: 5 [50%]) met the primary outcome of steroid-free remission at week 52. For the secondary efficacy endpoints, 34 patients (65%) achieved clinical remission (CD: 28 [67%], UC/IBDU: 6 [60%]; Fig. 1). In the subgroup of patients below 18 years of age at the start and at the week 52 endpoint (n = 38), 29 patients (76%) remained on ustekinumab, and 22 patients (58%) achieved steroid-free clinical remission. Thirty-three (64%) patients achieved biomarker remission (CD: 25 [60%], UC/IBDU: 8 [80%]). Median CRP decreased to 0.24 [0.06–0.51] and 0.18 [0.11–0.45] × upper limit of normal at week 52 for CD and UC, respectively. No significant associations were found with respect to disease type or location and any of the remission outcomes (Table 2).
FIGURE 1.
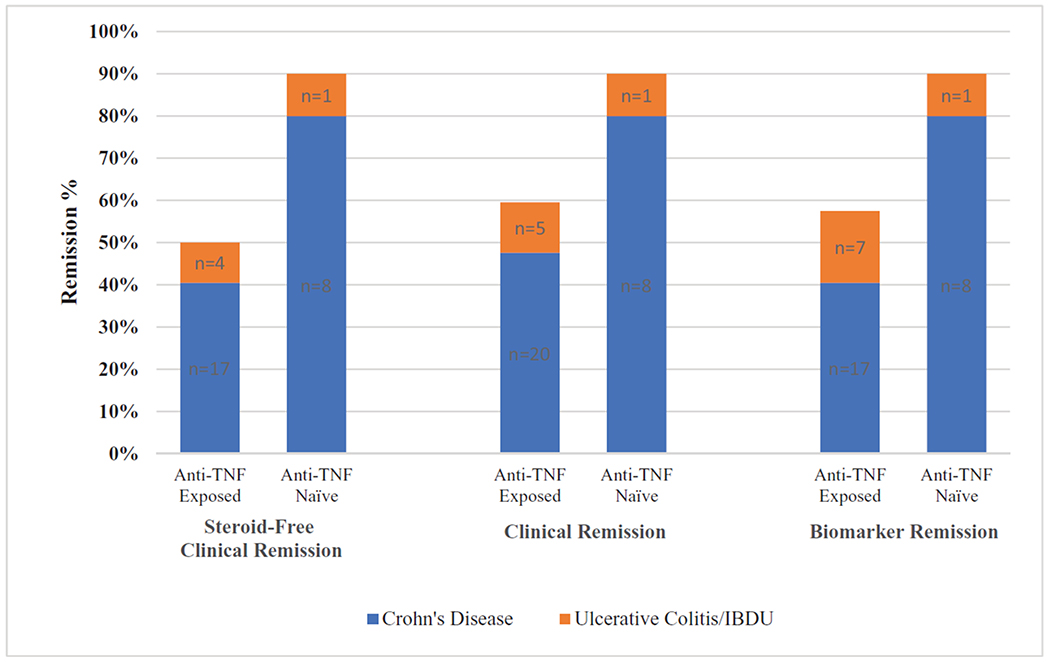
Ustekinumab remission rates at 1 year in biologic-exposed (n = 42) versus biologic-naïve (n = 10) Crohn disease and ulcerative colitis/inflammatory bowel disease-unspecified pediatric patients. Note overlap in patients achieving any of the 3 remission outcomes. TNF = tumor necrosis factor.
TABLE 2.
Predictors of response to ustekinumab in pediatric inflammatory bowel disease
| Clinical factor | Steroid-free remission at week 52, % n = 52 | P value | Clinical remission at week 52, % n = 52 | P value | Biomarker remission at week 52, % n = 52 | P value |
|---|---|---|---|---|---|---|
| Any large bowel disease | 55 | 0.488 | 64 | 1.000 | 62 | 0.729 |
| Large bowel disease only | 57 | 0.879 | 65 | 0.982 | 70 | 0.416 |
| Anti-TNF failure | 50 | 0.032 | 60 | 0.136 | 57 | 0.072 |
| Age <10 at diagnosis | 57 | 0.947 | 71 | 0.451 | 67 | 0.693 |
| Psoriasiform dermatitis | 62 | 0.746 | 62 | 0.736 | 62 | 0.868 |
| IMM at UST start | 54 | 0.746 | 69 | 1.000 | 69 | 0.746 |
| Steroids at UST start | 61 | 0.634 | 68 | 0.686 | 64 | 0.894 |
| CD | 62 | 0.500 | 67 | 0.723 | 62 | 0.729 |
| UC/IBDU | 50 | 0.500 | 60 | 0.723 | 70 | 0.729 |
| Vedolizumab failure | 47 | 0.253 | 68 | 0.727 | 63 | 0.457 |
| Dose escalation | 63 | 0.336 | 70 | 0.414 | 63 | 0.982 |
| Last Anti-TNF >30 d from UST start* | 52 | 1.000 | 64 | 0.446 | 61 | 0.462 |
| Last Anti-TNF >90 d from UST start* | 45 | 0.537 | 65 | 0.491 | 60 | 0.721 |
| Anti-TNF before Vedolizumab† | 44 | 0.582 | 69 | 1.000 | 69 | 1.000 |
CD = Crohn disease; d = days; IBD = inflammatory bowel disease; IBDU = inflammatory bowel disease unspecified; IMM = immunomodulator; TNF = tumor necrosis factor; UC = ulcerative colitis; UST = ustekinumab; VDZ = vedolizumab.
n = 42, accounting for only those patients exposed to anti-TNF.
n = 19, accounting for only those patients exposed to anti-TNF and VDZ.
Impact of Prior Biologic Exposure on Effectiveness
Forty-two patients (81%) (CD:33, UC/IBDU:9) had been exposed to anti-TNF therapy before starting ustekinumab. Eighteen of those patients had been on ≥2 anti-TNFs. Reasons for discontinuation of anti-TNF included secondary loss of response (n = 17), anti-TNF-mediated psoriasiform dermatitis (n = 10), antibodies to infliximab (n = 7), primary nonresponse to anti-TNF (n = 6), and infusion reactions without evidence of antidrug antibodies (n = 2). At week 52, bio-naïve patients were significantly more likely to achieve steroid-free remission than bio-exposed patients 90% (n = 9) versus 50% (n = 21), respectively (P = 0.03) (Fig. 1).
Nineteen patients (37%) had been previously treated with vedolizumab before initiating ustekinumab, and all 19 had been exposed to at least 1 anti-TNF. Sixteen patients received vedolizumab after anti-TNF and 3 received anti-TNF after vedolizumab. Nine of these 19 patients (47%) were in steroid-free remission at week 52. Order of biologic class before ustekinumab initiation was not associated with remission outcomes (Table 2).
Ustekinumab Durability
Of the 39 patients on ustekinumab at week 52, 38% remained on every 8-week SC dosing, and the remaining 62% had an interval shortening (CD:21, UC/IBDU:3); a re-induction IV dose was given to 3 patients because of persistence of symptoms (Fig. 2a). Thirteen patients (CD:11, UC:2,) were no longer on drug at week 52, and reasons for discontinuation are highlighted in Figure 2b. One CD patient discontinued ustekinumab because of nonspecific side effects including muscle pains, headaches, and fatigue and continued on dietary therapy alone, and another because of insurance denial. Nine of 13 patients who initiated ustekinumab because of psoriasiform dermatitis were still on drug at 1 year; 7/13 patients had documented improvement in their skin findings, but 3 of the 7 discontinued ustekinumab because of lack of clinical improvement.
FIGURE 2.
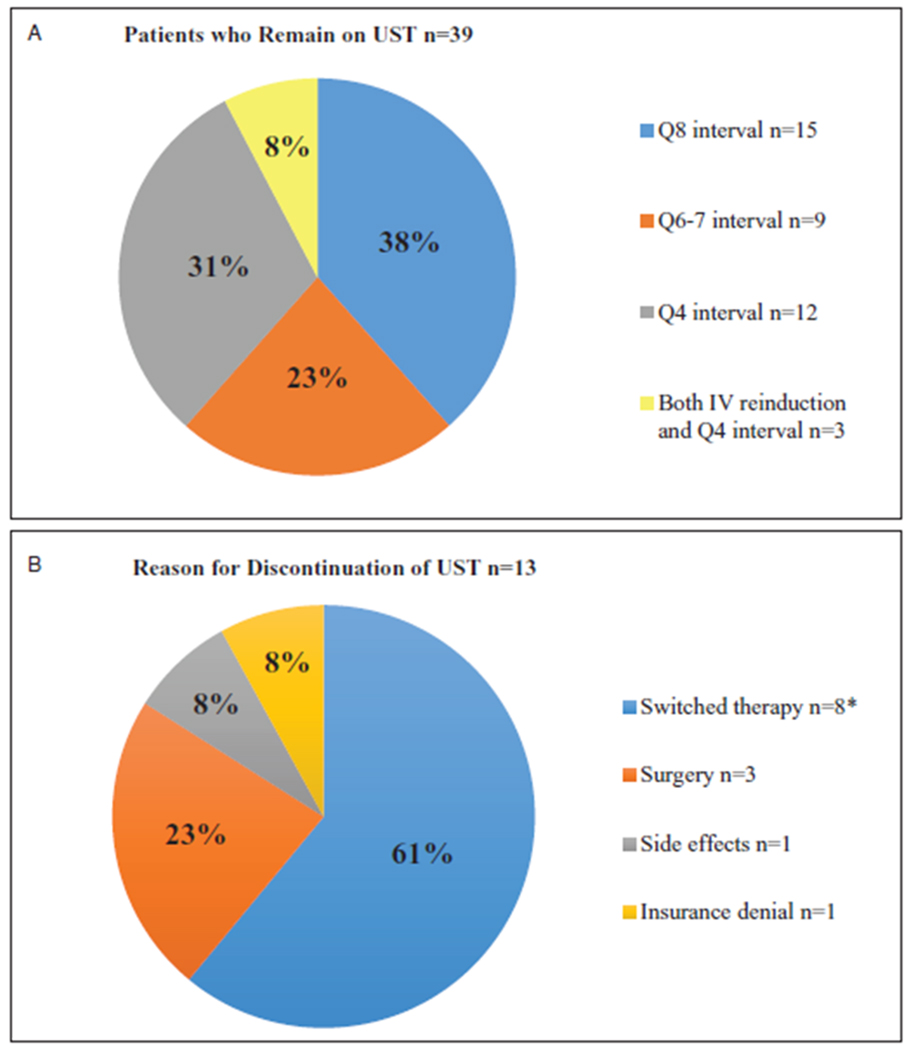
Dosing disposition for patients still on drug as of week 52 (A), and reasons for discontinuation in patients no longer on drug as of week 52 (B). *Two patients switched to vedolizumab, 1 patient switched back to infliximab, 1 patient switched to golimumab, and 2 patients switched to tofacitinib. UST = ustekinumab.
Drug Safety
The median follow-up time for the entire cohort was 18 [14–24] months. Two patients (3.8%) experienced an anaphylactoid reaction during the initial IV induction of ustekinumab, which was treated with steroids and epinephrine, after which they were able to finish the induction infusion. One of the 2 patients experienced arthralgia, myalgia, fatigue, and headaches after the first maintenance injection and subsequently discontinued ustekinumab. One patient experienced an urticarial rash 1 day after the IV induction, and 1 patient experienced local irritation after the first maintenance injection, both of whom continued on drug. An additional patient complained of headaches, which resolved by 4 months from ustekinumab initiation; this same patient then developed intermittent, self-limited paresthesia of bilateral lower extremities at 16 months on therapy and remained on drug, off steroids and in remission at last follow-up. As of last follow-up, there have not been any serious adverse events or serious infections reported.
Drug Concentrations and Antidrug Antibodies
Of the 39 patients who remained on drug at week 52, 35 patients (90%) had ustekinumab drug concentrations and antidrug antibody levels tested (Prometheus Laboratories, San Diego, CA) at a median time of 47.5 [27.4–60.3] weeks from ustekinumab initiation at the discretion of the prescribing physician. Only 1 (4%) patient had detectable antibodies at a level of 2.2U/mL, with a corresponding drug concentration of 7.3 ug/mL; this patient remained on drug but was not in remission. The remaining 34 patients had no antibodies detected, and concentrations ranged from 2.4 to 24.9 μg/mL. Thirteen patients had concentrations drawn at or within 8 weeks of week 52— median trough concentration for those in steroid-free remission was 6.6 [5.2–9.6] μg/mL compared with 5.8 [5.7–9.5] μg/mL for those patients not in steroid-free remission at week 52 (NS).
DISCUSSION
This study reports on real-life experience using ustekinumab for the treatment of IBD in a cohort of pediatric and young-adult patients from a single IBD referral center. Our data demonstrate that pediatric and young adult patients experienced clinical benefit from ustekinumab as either a primary or secondary biologic therapy for the induction, or maintenance of remission of IBD, with 81% of our cohort having failed previous biological treatments. In our cohort, 75% remained on drug at 1 year, with clinical remission rates as high as 90% in biologic-naïve patients. Only a minority of our cohort (19%), however, were bio-naïve, and thus more data on exposure are needed to confirm this finding. The time from last anti-TNF exposure to IV induction of ustekinumab did not appear to influence effectiveness, which is in contrast to our previously published experience with vedolizumab (14).
In our cohort, induction weight-based dosing was used, based on UNITI (3–5). In the absence of pharmacokinetic dosing studies in children, extrapolation of adult dosing is not unlike what pediatricians do with other biologics that are being used off-label. In our study, 57% of patients received a dose escalation with increasing frequency of maintenance injections because of inadequate clinical response from the standard dosing interval. This is in contrast to the report by Chavannes et al (11) in which only 30% of patients had a dose escalation; moreover, their patients were induced with various different subcutaneous dosing regimens. These differences in dosing may account for the higher remission rates seen in our cohort, although no association was found between dose escalation and steroid-free or clinical remission in our cohort, despite our median trough concentration being above those associated with higher response rates in the adult data (13,16). Although this was the first study to describe the pharmacokinetics of ustekinumab in pediatric IBD patients, there remains many unknown factors as it relates to exposure response with ustekinumab, especially in children. It remains unknown as to whether dose optimization based on a minimum trough concentration is the correct strategy and what that level should be (13–17).
In our cohort, the reason for treating with ustekinumab was different for CD as compared with UC/IBDU. Just over half of the CD patients were in clinical remission at the initiation of ustekinumab, albeit with nearly half on corticosteroids. Conversely, all of the UC/IBDU patients had moderately to severely active disease and 7 of 10 had prior anti-TNF and vedolizumab exposure. There was a 6-point median reduction in pMS from baseline as compared with 12 months, with 80% of UC/IBDU patients still on drug at 1 year, and 50% achieving steroid-free clinical remission. Although many of our patients had mild disease at baseline, we demonstrated that for those patients who started in clinical remission, all maintained their remission, and remained steroid-free at 1 year.
Only a small number of patients were on immunomodulators at baseline, which stems largely from the DEVELOP registry findings concluding that thiopurines are associated with malignancy, particularly when combined with anti-TNF therapy (18). Wils et al (19,18) reported that concomitant immunomodulator use at the start of ustekinumab was associated with increased odds of clinical benefit at 3 months, but these findings were not reproduced in his follow-up long-term efficacy study. Our study did not demonstrate any association between concomitant immunomodulators and remission. The low immunogenicity rate of ustekinumab (15), coupled with the lack of any consistent data showing the superiority of concomitant immunomodulator use (20), and the safety concerns associated with thiopurine combination therapy (18), suggest that ustekinumab monotherapy is possible and preferable for children.
With regard to safety, no serious infections, or other serious adverse events were reported in our cohort; however, larger studies with longitudinal exposure data are needed to confirm safety in children with IBD. Our findings are, however, similar to those of the other pediatric cohort who reported on a patient with migraines, and another with paresthesia (11). Adult studies have shown similar well-tolerance to ustekinumab with only isolated severe adverse events documented (20). In our cohort, only 1 patient discontinued drug because of side effects, which included muscle and joint pains as well as fatigue. There was no increased incidence of adverse events in patients on more frequent maintenance dosing.
There are several limitations to the present study, mainly attributed to its retrospective design and small sample size; however, this is the largest cohort with the longest median duration of follow-up in this age group reported to date. Another limitation is the heterogeneous population we examined, including patients with differing disease type, clinical severity, and previous medication exposures. Our results, however, are similar to the real-world postmarketing experience in adult patients with CD showing response rates of greater than 50% even in patients with difficult-to-treat, refractory disease. Additionally, 27% of our cohort was between 18 and 21 years of age, which could limit the generalizability to a purely pediatric population, but when we analyzed the subgroup of patients below age 18 years, the durability and steroid-free clinical remission rates were nearly identical to the total cohort. Another limitation is the lack of endoscopic and histologic data, and we plan to collect this data prospectively. Furthermore, although roughly half of our patients had drug concentrations measured, there was no consistency regarding timing of measurements. This, in turn, led to adjustments in dosing, which were not uniform throughout the cohort.
Our results suggest that ustekinumab is efficacious and safe in pediatric IBD patients, both in CD and UC/IBDU alike, with bio-naive patients experiencing higher rates of remission than bioexposed. Given the real world experience reported in our study and the various indications of use and phenotypes, these findings should be able to be generalized to pediatric IBD patients. Controlled clinical trial data are needed to confirm these observations.
What Is Known
Ustekinumab was approved for use in adults with moderate to severely active Crohn disease in 2016.
A subset of pediatric patients is refractory to currently available therapies for inflammatory bowel disease.
There is minimal data published on the use of ustekinumab in pediatric inflammatory bowel disease.
What Is New
Greater than 50% of pediatric patients achieved clinical remission on ustekinumab by week 52.
Higher remission rates were seen in bio-naïve versus bio-exposed patients.
Adverse events were uncommon, and included headache, myalgia, paresthesia, and infusion reaction.
Ustekinumab concentration was measured in 90% of patients still on drug at 52 weeks.
Acknowledgments
No specific funding from any agency in the public, commercial, or not-for-profit sectors has been provided for the research.
Footnotes
M.C.D. has served as a consultant for Celgene, Boehringer Ingelheim, Genentech, Janssen, Pfizer, Takeda, and UCB, Abbvie, Protagonist, Valiant. The remaining authors have no conflict of interest.
This article has been developed as a Journal CME Activity by NASPGHAN. Visit http://www.naspghan.org/content/59/en/Continuing-Medical-Education-CME to view instructions, documentation, and the complete necessary steps to receive CME credit for reading this article.
REFERENCES
- 1.Benson J, Peritt D, Scallon BJ, et al. Discovery and mechanism of ustekinumab. mAbs 2011;36:535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansen T, Targownik LE. Ustekinumab for the treatment of Crohn’s disease. Expert Rev Gastroenterology Hepatology 2016;109:989–94. [DOI] [PubMed] [Google Scholar]
- 3.Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016; 37520:1646–60. [DOI] [PubMed] [Google Scholar]
- 4.Sandborn WJ, Gasink C, Gao LL, et al. , CERTIFI Study Group. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med 2012;36716:1519–28. [DOI] [PubMed] [Google Scholar]
- 5.Sandborn WJ, Rutgeerts P, Gasink C, et al. Long-term efficacy and safety of ustekinumab for Crohn’s disease through the second year of therapy. Aliment Pharmacol Ther 2018;48:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sands B, Sandborn WJ, Panaccione R, et al. Safety and efficacy of ustekinumab induction therapy in patients with moderate to severe ulcerative colitis; results from the phase 3 UNIFI study. United European Gastroenterol J 2018;6(Suppl 1)(in press). [Google Scholar]
- 7.Bishop C, Simon H, Suskind D, et al. Ustekinumab in pediatric Crohn’s disease patients. J Pediatr Gastroenterol Nutr 2016;63:348–51. [DOI] [PubMed] [Google Scholar]
- 8.Rinawi F, Rosenbach Y, Assa A, et al. Ustekinumab for resistant pediatric Crohn’s disease. J Pediatr Gastroenterol Nutr 2014;11:1111. [DOI] [PubMed] [Google Scholar]
- 9.Cameron FL, Garrick V, Russell RK. Ustekinumab in treatment of refractory Crohn disease. J Pediatr Gastroenterol Nutr 2016;62:e30. [DOI] [PubMed] [Google Scholar]
- 10.Fusillo S, Chang V, Stein RE, et al. Ustekinumab responders versus non-responders in refractory pediatric inflammatory bowel disease [Abstract 329]. Gastroenterology 2018;154:S-82. [Google Scholar]
- 11.Chavannes M, Martinez-Vinson C, Hart L, et al. Management of paediatric patients with medically-refractory Crohn’s disease using ustekinumab: a multi-centred cohort study. J Crohns Colitis 2018[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 13.Lewis JD, Chuai S, Nessel L. Use of the noninvasive components of the mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis 2008;1412:1660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh N, Rabizadeh S, Jossen J, et al. Multi-center experience of vedolizumab effectiveness in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2016;22:2121–6. [DOI] [PubMed] [Google Scholar]
- 15.Battat R, Kopylov U, Bessissow T, et al. Association between ustekinumab trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2017;15:1427–34. [DOI] [PubMed] [Google Scholar]
- 16.Adedokun O, Xu Z, Gasink C, et al. Pharmacokinetics and exposure response relationships of ustekinumab in patients with Crohn’s disease. Gastroenterology 2018;154:1660–71. [DOI] [PubMed] [Google Scholar]
- 17.Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008;371:1675–84. [DOI] [PubMed] [Google Scholar]
- 18.Hyams JS, Dubinsky MC, Baldassano RN, et al. Infliximab is not associated with increased risk of malignancy or hemophagocytic lymphohistiocytosis in pediatric patients with inflammatory bowel disease. Gastroenterology 2017;152:1901.e3–14.e3. [DOI] [PubMed] [Google Scholar]
- 19.Wils P, Bouhnik Y, Michetti P, et al. Subcutaneous ustekinumab provides clinical benefit for two-thirds of patients with Crohn’s disease refractory to anti-tumor necrosis factor agents (GETAID). Clin Gastroenterol Hepatol 2016;14:242–50. [DOI] [PubMed] [Google Scholar]
- 20.Wils P, Bouhnik Y, Michetti P, et al. Long-term efficacy and safety of ustekinumab in 122 refractory Crohn’s disease patients: a multicentre experience. Aliment Pharmacol Ther 2018;47:588–95. [DOI] [PubMed] [Google Scholar]


