Highlights
-
•
Placebo analgesia and nocebo hyperalgesia can be induced by classical conditioning.
-
•
Oxytocin does not enhance placebo effect or decrease nocebo effect.
-
•
Oxytocin does not influence extinction of placebo and nocebo responses.
Key Words: Oxytocin, placebo effect, nocebo effect, analgesia, hyperalgesia
Abstract
Oxytocin has been shown to increase trust, decrease anxiety, and affect learning as has been observed in conditioning paradigms. Trust, anxiety, and learning are important factors that influence placebo effects. In this study, we investigated whether oxytocin can increase placebo analgesia, decrease nocebo hyperalgesia, and influence extinction processes of both. Eighty male volunteers were assigned to a 40 IU of oxytocin nasal spray group, or to a placebo control group. Placebo analgesia and nocebo hyperalgesia were induced by a conditioning procedure in combination with verbal suggestions. The results demonstrate that the conditioning procedure successfully elicited significant placebo analgesia and nocebo hyperalgesia responses (P < .001). Furthermore, extinction was observed (P < .001), although placebo and nocebo responses did not return to baseline and remained significant. Oxytocin did not influence placebo analgesia or nocebo hyperalgesia and had no effect on extinction. This study provides support against the placebo-boosting effects of oxytocin and was the first one to demonstrate that it also did not influence nocebo effects or extinction processes, however, these results pertain to only a male sample. As managing placebo and nocebo effects has widespread clinical implications, further research should investigate other neurobiological or behavioral pathways to boost placebo and decrease nocebo effects.
Perspective
The present study demonstrated that placebo analgesia and nocebo hyperalgesia can be successfully induced by conditioning and verbal suggestions. We could not confirm the hypothesis that oxytocin affects either of these phenomena. Other pharmacological agents and behavioral manipulations for increasing placebo and decreasing nocebo effects should be investigated.
Accumulating findings demonstrate the pain reducing effects of placebo analgesia21 and pain enhancing effects of nocebo hyperalgesia.31 Literature suggests that the placebo and nocebo effects are triggered by expectations which in turn can be induced by communication (verbal suggestions) or by classical conditioning.3,5,11 Positive expectations induced by verbal suggestions have been shown to induce robust reductions in pain in experimental and clinical settings,21 while expectations of negative treatment outcomes were associated with higher pain ratings.42 Classical conditioning, a presentation of an initially neutral stimulus (conditioned stimulus, CS) together with a physiologically relevant unconditioned stimulus, has been also frequently applied to elicit both placebo and nocebo effects for pain.10,12 Classical conditioning is furthermore characterized by the extinction of the conditioned response when contingencies between unconditioned stimulus and CS are changed.30 This extinction process is particularly found for placebo effects for pain.17,44 In contrast, a lack of extinction of nocebo effects for pain was repeatedly demonstrated.10,14 These negative learned associations are particularly relevant for clinical practice as these may be crucial processes underlying various chronic pain syndromes.
Despite the importance of managing placebo and nocebo effects for the treatment of pain, not much research has been done on the neurobiological pathways of these effects as a potential target to enhance placebo effects and decrease nocebo effects. So far, some evidence exists regarding possibilities to block nocebo effects by proglumide, the cholecystokinin agonist,4 but almost no evidence for the enhancement of placebo effects is available. A recent study aimed to boost placebo analgesia using dopaminergic agonist was not successful.46 Colloca et al, however, found that vasopressin enhances placebo effects in pain but only in women.13 Finding ways to influence placebo and nocebo effects is however crucial for application in clinical practice.
Oxytocin, a peptide hormone produced in the hypothalamus, was proposed as a possible mediator of the placebo effect18 due to its trust-inducing and stress-relieving properties. A few studies investigated the influence of oxytocin on placebo effects in experimental settings. Kessner et al27 demonstrated boosting effects of oxytocin on verbally induced placebo analgesia for experimentally induced pain. In contrast, Colloca et al13 and Skvortsova et al38 found no effects of oxytocin on verbally induced placebo analgesia. These 3 previous studies used verbal suggestions alone to induce placebo effects. However, it well known that the combination of verbal suggestions and conditioning induces the largest placebo and nocebo effects.1 No studies so far examined the effects of oxytocin on the placebo effect triggered by classical conditioning along with verbal suggestions and, furthermore, no studies looked at whether oxytocin is able to minimize nocebo effects. As oxytocin facilitates both learning performance during pain conditioning,16 it is possible that it would strengthen the development of both placebo and nocebo effects. On the other hand, it has been shown, that a reduction of anxiety does decrease the nocebo effect in pain,4 and therefore, oxytocin has a potential to decrease placebo hyperalgesia. Moreover, oxytocin might affect the extinction of placebo and nocebo responses as previous research showed that it speeds up fear extinction.15
In this study, we investigated the effects of oxytocin administration on placebo effects for pain induced by a combined conditioning and verbal suggestions approach. Moreover, we explored the effects of oxytocin on nocebo effects and on the extinction of placebo and nocebo effects. We hypothesized that oxytocin would boost the placebo effect due to its stress-reducing and learning enhancing properties, and speed up the extinction of nocebo effect as it facilitates fear extinction.15 We did not have a directional hypothesis regarding possible effects of oxytocin on the strength of the nocebo effect, as considering its stress-reducing properties,9 it could decrease the nocebo effect, but considering that it facilitates learning,16 it could increase it.
Methods
Study Design
A randomized placebo-controlled double-blind design was used for this experiment. Participants were randomly allocated to 1 of 2 groups: an oxytocin group, which received 40 IU of oxytocin nasal spray, and a control group, which received the same volume of a placebo nasal spray. The study protocol was approved by the Medical Ethical Committee of the Leiden University Medical Center (number NL60185.058.16), and the study was preregistered as a clinical trial on www.trialregister.nl (NTR6506). The randomization was performed by the Clinical Pharmacy of the Leiden University Medical Center using block randomization with the block size of 8.
Participants
Eighty healthy male volunteers between 18 and 36 years old participated in this study. Male participants were recruited since previous studies reported on the oxytocin boosting placebo analgesia in men27 and not in women38 and oxytocin fluctuations during the menstrual cycle can be an interfering factor in studying oxytocin effects in women.33 Exclusion criteria were heart and lung diseases, high or low blood pressure, chronic or acute pain complains, heavy use of alcohol or other drugs, current diagnosis of psychiatric disorders, and current use of analgesic medications. Participants were asked to refrain from drinking alcohol and intense physical exercises up to 12 hours prior to the experiment and from drinking caffeinated drinks and smoking up to 2 hours before the experiment. Adherence to these rules was verbally asked, and no additional physical checks were done.
Procedure
Prior to being invited for the experiment, prospective participants were asked to give their informed consent and to complete an online questionnaire on Qualtrics (Provo, UT) to screen for exclusion criteria. All eligible candidates were invited to the laboratory for a 2-hour session.
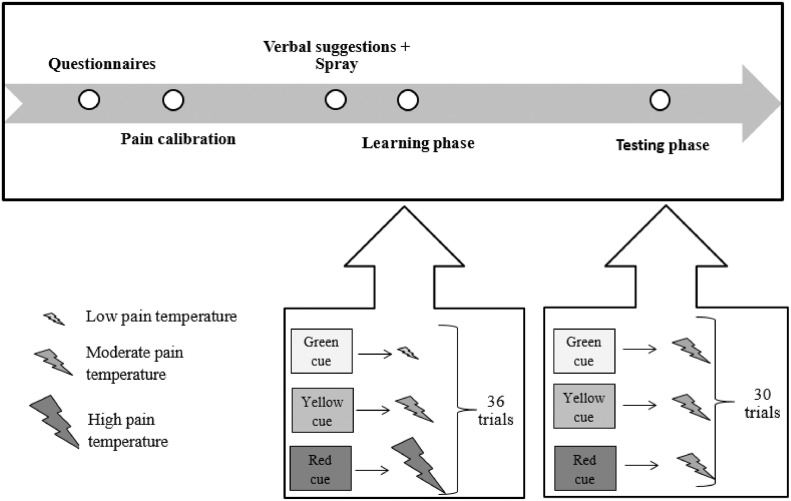
The experimental timeline is presented in a Fig 1. Upon arrival to the laboratory, participants were given the information about the experiment and were asked to sign a written informed consent form. The experiment was conducted by 2 experimenters: one gave instructions and the other controlled the equipment. First, participants were asked to fill in several short questionnaires on the computer. Then the pain calibration took place. After determining individual temperatures that elicited low-, moderate-, and high pain levels, participants were administered oxytocin or placebo nasal spray, depending on their group allocation, in a double-blind randomized manner. After the spray administration, participants were provided with verbal suggestions regarding the subsequent conditioning task and a 30-minute waiting time followed to allow oxytocin to reach its peak effects.40 During this break, participants were given magazines with a neutral (design and nature) content to read. After the break, the conditioning task took place. After the end of the task, participants filled in a closing questionnaire were debriefed and paid for their participation.
Figure 1.
The experimental timeline.
The data were collected at the laboratory of the Faculty of Social Sciences of Leiden University between March and November 2017.
Experimental Manipulations
Nasal Spray
Participants received 40 IU of oxytocin (Syntocinon) or placebo nasal spray depending on group allocation. This higher than standard 24 IU dose was chosen as 24 IU dose did not affect the placebo effect in previous research13,38 while a higher dose increased placebo effect in men.27 The spray was administered by the experimenter with 4 puffs (2 puffs per nostril) using a MAD Nasal Mucosal Atomization Device (Teleflex, Inc, Research Triangle Park). The placebo spray looked and tasted identically to oxytocin. The medication was prepared by Clinical Pharmacy of the Leiden University Medical Center that also was responsible for the randomization. The pharmacy assigned participants numbers and assigned participants to oxytocin or control groups. The researchers received the randomization list with the group assignments only after the end of the study.
Verbal Suggestions
After the nasal spray administration, participants received verbal instructions about the experiment. They were told that the aim of the experiment was to investigate how the oxytocin spray would influence a transcutaneous electrical nerve stimulation (TENS) device. It was explained to them that during the next task, a TENS electrode would be placed on their arm and that this electrode was able to regulate their pain sensitivity levels as it acted on the pain processing pathways. They were told that when they would see a green cue on a computer screen, the TENS would decrease their pain sensitivity, when they would see a yellow cue this would indicate that the TENS would be inactive, and when they would see a red cue this would indicate that the TENS would increase their pain sensitivity. However, the TENS remained inactive during the experiment; the information was used as a cover story for inducing expectations about the influence of TENS on pain sensitivity and thereby possibly strengthening the conditioning procedure.
Pain Calibration
Pain stimuli were delivered with a standardized heat pain application device (ATS-II, Medoc Advanced Medical Systems, Ramat Yishai, Israel). The stimuli were applied to the dorsal site of the not dominant arm. During the calibration procedure, 3 levels of heat stimulation were determined for each participant individually: 1) a temperature that elicited low pain (pain detection threshold, equal to around 1 on a 0–10 numeric rating scale [NRS] with 0 = no pain at all, 10 = worst pain ever experienced); 2) a temperature that elicited moderate levels of pain (equal to 4 on the 0–10 NRS); 3) a temperature that elicited high but bearable levels of pain (equal to 7 on the 0–10 NRS). In order to determine these 3 levels, we applied one sequence of ascending temperatures to participants’ arms with a peak temperature lasting for 4 seconds and a between-stimulus interval of 15 seconds. Participants were asked to rate each stimulus on an NRS ranging from 0 to 10. After the pain of 7 was reached, the calibration procedure was stopped.
Pain Conditioning Task
The pain conditioning task consisted of 2 phases: a learning phase and a testing phase. In the learning phase, a green cue on a computer screen was coupled to a low pain stimulus, a yellow cue to a moderate pain and a red cue to a high pain stimulus. The learning phase consisted of 36 stimuli (12 of each color/intensity).
In the test phase, only medium pain temperatures were presented coupled with green (placebo condition), yellow (control condition), and red (nocebo condition) cues. The test phase consisted of 30 stimuli (10 of each color). There was no break between the learning and the test phase. The peak temperature lasted 4 seconds with a ramp-up and ramp-down speed of 8 degrees per second and an interstimulus interval of 4 seconds. The stimuli were presented in a randomized fixed order: 4 random sequences of stimuli were created prior to the experiment and each participant was randomly allocated to 1 of the 4 sequences. To avoid habituation and sensitization to pain, the thermode was moved to another place on the forearm twice: after the twenty-second stimulus of the learning phase and eighth stimulus of the test phase (every 22 stimuli). After each pain stimulus, participants were asked to rate the pain intensity on a 0 (no pain) to 10 (most intense pain imaginable) NRS verbally.
Instruments and Materials
Visual Stimuli
E-Prime (version 2.0) software installed on a desktop computer was used to present the visual cues (green, yellow, and red) during the pain conditioning task. The resolution of the screen was 1,280 × 1,024 pixels, and participants were sitting approximately 60 cm from the screen. The visual cues were shown 2 seconds before the start of each heat stimulus. During the cues, the whole screen turned green-, yellow-, or red colored.
Questionnaires
A short version of the Positive and Negative Affect Schedule43 was used to measure the mood of participants at baseline. The questionnaire consisted of 10 items: 5 for measuring positive affect and 5 for measuring negative affect. Scores were obtained on a 5-point Likert scale and could range from 5 to 25. Higher scores indicated higher positive and higher negative affect, respectively. The Cronbach's alpha in our sample was .85 for positive affect and .80 for negative affect, indicating a good internal consistency.
The State Trait Anxiety Inventory – state version29 was used for measuring baseline state anxiety of the participants. This short version of the questionnaire was validated in relation to the longer version.29 The questionnaire consisted of 6 statements, and participants had to indicate on a scale from 1 (not at all) to 4 (very much) how much this statement applied to them at that specific moment. Scores could range from 6 to 24 with higher scores indicating higher state anxiety. In the present study, the Cronbach's alpha was .81 indicating a good internal consistency.
The revised Life Orientation Test36 was used to measure optimism. The revised Life Orientation Test consists of 3 positive, 3 negative, and 4 filler items and participants had to indicate whether they agree or disagree with each on a scale from 0 (strongly disagree) to 4 (strongly agree). Scores could range from 0 to 24 with higher scores indicating higher optimism. In the present study, the Cronbach's alpha was .75, indicating acceptable internal consistency.
The neuroticism and extraversion scales of the short version of the Eysenck Personality Questionnaire35 were used for measuring neuroticism and extraversion. The questionnaire consisted of 24 items: 12 items for measuring neuroticism and 12 for measuring extraversion. The participants were asked to use a dichotomous scale for giving the answers (“yes” or “no”). Scores could range from 0 to 12 with higher scores indicating higher neuroticism and higher extraversion, respectively. The Cronbach's alpha in our sample was .77 for neuroticism, indicating acceptable internal consistency, and .84 for extraversion, indicating a good internal consistency.
Finally, a closing questionnaire was designed to ask participants about their experiences. They were asked to answer the following questions: “Do you think you received oxytocin or placebo?”; “What do you think was the aim of this experiment?”; “Have you heard anything about this experiment from other people? If yes, what?”.
Data Analyses
The power calculation was performed with software G*Power 3.19 The input for the power calculation was derived from a study on the effect of oxytocin on placebo analgesia27 with an effect size of d = .495. A sample size calculation for a repeated measurements within-between analysis of variance (ANOVA) with 2 groups indicated that 48 participants in total (24 in each group) would be needed to obtain a power of .95 at an alpha level of α = .5. Taking into account the conflicting results of the previous studies and an additional 10% of possible technical failures, we adjusted the total sample size to the conservative number of 80 participants; 40 in each group.
The data analysis was performed using SPSS Statistics version 21 (IBM Corporation, Armonk, NY) with a 2-tailed significance level of α <.05. The data were screened for univariate outliers using z-scores. Z-scores above 3.29 or below −3.29, that correspond to ± 3 standard deviations (SDs), were considered to indicate univariate outliers. One outlier was found for the negative affect score and one outlier for the state anxiety score, with z-scores of 6.21 and 5.05, respectively. Nonparametric tests were applied for the analysis of the variables with outliers. Skewness, kurtosis, and Shapiro-Wilk tests indicated normality of distribution of all variables. Levene's tests indicated homogeneity of variances in the groups. Sphericity was measured with the Mauchly's test of sphericity and in case of the violation of this assumption, Greenhouse-Geisser corrections were used.
The heat stimuli directly following the repositioning of the thermode, elicited significantly higher pain ratings. For this reason, the 2 stimuli directly following the repositioning of the thermode were excluded from the analysis. In total, 35 learning trials and 29 test trials were included in the analysis.
Independent samples t-tests or nonparametric Mann-Whitney tests were used to compare the groups on the baseline and personality characteristics, as appropriate: age, heat pain detection threshold (temperature that was rated as 1 on the NRS pain scale), positive affect, anxiety, optimism, extraversion, and neuroticism.
A 2 × 3 (group (oxytocin vs placebo) × cue color (green vs yellow vs red)) factorial ANOVA was used to compare the groups on the mean pain scores in response to green, yellow, and red cues in the learning phase of the conditioning task. To examine whether the placebo and nocebo effects were significant and differed between the groups, a 2 × 3 (group (oxytocin vs placebo) × cue color (green vs yellow vs red)) factorial ANOVA was used to compare the groups on their mean pain ratings in response to the 3 color cues in the test phase of the conditioning task. Furthermore, to examine the extinction process in more detail, yellow-versus-green and red-versus-yellow scores were calculated for each pair of green-yellow and red-yellow trials of the test phase. In total, 9 yellow-versus-green and 9 red-versus-yellow scores (corresponding to 9 red and yellow or green and yellow comparisons) were calculated. Repeated measures ANOVA were done with time (order number) as a within-subject factor, group as a between-subject factor and yellow-versus-green score as a dependent variable to evaluate the effects of the group and time on the yellow-versus-green scores. Bonferroni corrected post hoc tests were applied to investigate the difference between the time moments. The same analysis was run with red-versus-yellow scores as a dependent variable. To compare the size of yellow-versus-green and red-versus-yellow scores, the means of the scores were compared with a one-sample t-test. Finally, to check if the groups differed in their perceived group allocation, a chi-square test was performed.
To examine the effects of psychological and personality characteristics on the yellow-versus-green score, linear regression analyses were performed with positive affect, anxiety, optimism, extraversion, and neuroticism as independent variables and the yellow-versus-green score as a dependent variable. The same analysis was repeated with red-versus-yellow score as a dependent variable.
To examine whether the perceived group allocation has an effect on yellow-versus-green and red-versus-yellow scores, independent samples t-tests were used to compare people who thought they received placebo and people who thought they received oxytocin spray on their placebo and nocebo effects.
Partial eta squared was calculated for analyses as the indication of the effect sizes.
Results
Baseline Characteristics
Data of 76 participants were available for the analysis; data of 4 participants (1 from oxytocin group and 3 from control group) were excluded due to technical problems during the conditioning task. The baseline and personality characteristics are presented in Table 1. There were no differences between the groups on any of these variables, except for extraversion: participants randomized to the oxytocin group (M = 10.69, SD = 2.68) reported a higher extraversion in comparison to the control group (M = 9.11, SD = 3.43; t (74) = 2.25, P = .027).
Table 1.
Baseline and Personality Characteristics With Standard Deviations Across the Groups
| Oxytocin Group (n = 39) | Control Group (n = 37) | t/U | P | |
|---|---|---|---|---|
| Age | 23.30 (3.44) | 23.11(2.94) | .26 | .80 |
| State anxiety* | 8.99 (2.46) | 8.70 (2.23) | 682.00 | .68 |
| Positive mood | 31.90 (8.22) | 30.81 (7.07) | .62 | .54 |
| Negative mood* | 12.56 (2.58) | 13.28 (2.83) | 611.00 | .24 |
| Neuroticism | 2.59 (2.66) | 3.62 (2.78) | −1.65 | .10 |
| Extraversion | 10.69 (2.68) | 9.11 (3.43) | 2.25 | .027 |
| Optimism | 22.13 (3.43) | 22.27 (3.85) | −.17 | .87 |
| Pain detection threshold | 43.39 (1.72) | 43.87 (1.70) | −1.22 | .23 |
Mann-Whitney U test is presented instead of t-test, as nonparametric test was applied to these variables.
Learning Phase
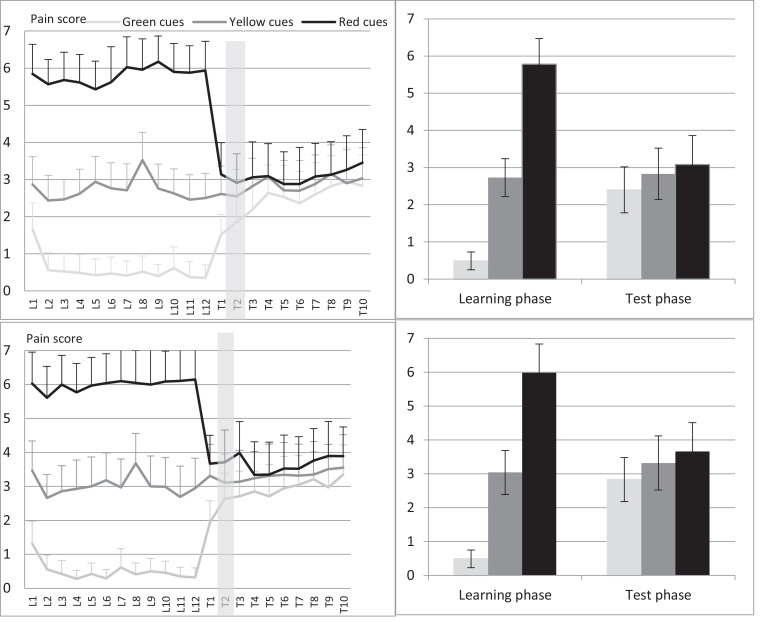
The mean levels of pain during each trial of the learning phase are presented in Fig 2. The temperatures that were calibrated to elicit the pain of 1, 4, and 7 on the 11-point NRS, caused lower pain during the learning phase of the conditioning task (pain of .49, 2.88, and 5.88, respectively). The 2 × 3 (group × cue color) factorial ANOVA with a Greenhouse-Geisser correction demonstrated that there was a significant main effect of the cue color on the pain ratings (F(1.65, 121.86) = 634.27, P < .001, ηp2 = .90), while the effect of the group (F(1, 74) = .69, P = .69, ηp2 = .01) and the group-cue color interaction (F(1.65, 121.86) = .55, P = .54, ηp2 = .01) were nonsignificant. Post hoc tests using Bonferroni corrections indicated that participants rated the stimuli following the red cues (M = 5.88, SD = .18) as significantly more painful than stimuli following yellow (M = 2.88, SD = .14) and green cues (M = .49, SD = .06); also the yellow cues stimuli were rated as significantly more painful than the green cues stimuli (all Ps < .001; Fig 2). Oxytocin did not influence the pain perception during the learning phase.
Figure 2.
Pain ratings and standard deviations during the learning and test trials in response to green, yellow, and red cues. Oxytocin group on the top panel, control group on the down panel, the first test trial is highlighted.
Test Phase
The 2 × 3 (group × cue color) factorial ANOVA with a Greenhouse-Geisser correction demonstrated that there was a significant main effect of the cue color on the pain ratings (F(1.41, 104.61) = 61.71, P < .001, ηp2 = .46), while the main effect of the group (F(1, 74) = 2.31, P = .13, ηp2 = .01) and the group × cue color interaction (F(1.41, 104.61) = .63, P = .48, ηp2 = .01) were nonsignificant. Post hoc tests using Bonferroni corrections indicated that participants rated the stimuli following the red cues (M = 3.37, SD = .19) as significantly more painful than stimuli following yellow (M = 3.07, SD = .17) and green cues (M = 2.62, SD = .15), and green cues stimuli as significantly less painful than yellow and red cues stimuli (all Ps < .001).
The difference scores between the yellow and green trials (yellow-vs-green score) and red and yellow trials (red-vs-yellow score) of the test phase are presented in Table 2. The repeated measures ANOVA with a Greenhouse-Geisser correction for the yellow-versus-green score showed that the main effect of the group (F(1, 73) = .06, P = .82, ηp2 = .001) and time × group interaction (F(6.34, 456.80) = .80, P = .58, ηp2 = .01) were nonsignificant; however, there was a significant main effect of time on the yellow-versus-green score (F(6.34, 458.6) = 5.07, P < .001, ηp2 = .07). Post hoc tests using Bonferroni corrections indicated that the first yellow-versus-green score was significantly larger than the following scores.
Table 2.
Yellow-Versus-Green and Red-Versus-Yellow Scores With Standard Deviations Per Test Trial Across the Groups
| Yellow-Versus-Green Score |
Red-Versus-Yellow Score |
|||
|---|---|---|---|---|
| Oxytocin Group (n = 39) | Control Group (n = 37) | Oxytocin Group (n = 39) | Control Group (n = 37) | |
| Test trial 1 | 1.15 (.20) | 1.27 (.21) | .58 (.21) | .35 (.21) |
| Test trial 2 | .75 (.19) | .44 (.19) | .35 (.16) | .60 (.16) |
| Test trial 3 | .50 (.23) | .21 (.23) | .51 (.26) | .88 (.26) |
| Test trial 4 | .57 (.23) | .53 (.23) | −.25 (.212) | −.07 (.22) |
| Test trial 5 | .13 (.18) | .61 (.18) | .17 (.15) | .08 (.15) |
| Test trial 6 | .34 (.17) | .46 (.17) | .29 (.14) | .23 (.15) |
| Test trial 7 | .29 (.18) | .26 (.18) | .01 (.1) | .31 (.16) |
| Test trial 8 | .25 (.17) | .31 (.18) | .23 (.19) | .39 (.19) |
| Test trial 9 | .20 (.18) | .31 (.19) | .42 (.20) | .40 (.20) |
The repeated measures ANOVA with a Greenhouse-Geisser correction on the red-versus-yellow score showed that the main effect of the group (F(1, 73) = .67, P = .41, ηp2 = .01) and the time × group interaction (F(6.14, 448.19) = .61, P = .77, ηp2 = .01) were nonsignificant; however, there was a significant main effect of time on the red-versus-yellow score (F(6.14, 448,9) = 3.67, P = .001, ηp2 = .05). Post hoc tests using the Bonferroni corrections indicated that only one score in the middle of the test phase was significantly smaller than all other scores. No other significant differences over time were found.
Secondary Outcomes
It was demonstrated that the mean yellow-versus-green score across all test trials (M = .47, SD = .50) was larger than the mean red-versus-yellow score (M = .30, SD = .51; t (75) = 2.40, P = .019). None of the personality characteristics significantly predicted yellow-versus-green (all P > .08) or red-versus-yellow (all P > .19) scores.
Participants in the 2 groups did not differ in their perceived group allocation (chi-square (1, N = 76) = 1.88, P = .171). The majority of participants thought that they received a placebo spray: 71.8% in the oxytocin group and 56.8% in the control group. There was no difference between people who thought they received oxytocin (yellow-vs-green score: M = .56, SD = .71; red-vs-yellow score: M = .48, SD = .74) and who thought they received placebo (yellow-vs-green score: M = .64, SD = .68; red-vs-yellow score: M = .23, SD = .60) in their yellow-versus-green (t (74) = −.51, P = .61), and red-versus-yellow (t (74) = 1.64, P = .11) scores.
Finally, to explore whether the baseline personality characteristic of extraversion that differed between the groups, influenced the results of the analyses, the same analyses were performed with extraversion as a covariate. As the analyses did not change the results, these results are not reported further.
Discussion
The results of this study demonstrate that conditioning can successfully induce placebo analgesia and nocebo hyperalgesia. Importantly, we showed for the first time that intranasal oxytocin administration did not influence analgesia and hyperalgesia induced by conditioning combined with verbal suggestions or the extinction of these effects.
Our findings regarding on the induction of placebo analgesia and nocebo hyperalgesia by conditioning with verbal suggestions are in line with previous research.21,31 We used conditioning together with verbal suggestions as it was previously demonstrated that the combination of these 2 methods is more effective than applying them separately.1 The placebo effect of the first test trial was larger than 1 on the 11-point scale, what is consistent with previous literature12,26 and decreased during the test phase showing an extinction pattern. Interestingly, nocebo effects at the first test trial were smaller (.5 on the 11-point scale), however, they seemed to be affected by extinction less than placebo effects: the greatest extinction of the placebo effect occurred after the first test trial, while the nocebo effect remained stable with only one trial in the middle of the sequence that was significantly lower than others. These results might possibly indicate that the nocebo effect in the present study did not extinguish over time, however, it is important to consider that the effect was very small in general. These findings are in line with previous studies that found significant extinction of placebo17,44 but not of nocebo effects.10 This phenomenon can be explained by the fact that threatening information is more relevant for an organism's survival from an evolutionary point of view and that is why it should be stored in memory longer than positive information.2 Moreover, Colagiuri and Quinn9 showed that anticipatory anxiety and elevated autonomic arousal contribute to the persistence of nocebo effects in comparison to placebo effects.
Intranasal administration of 40 IU oxytocin did not influence pain-related placebo and nocebo effects and their extinction. These findings are in line with our earlier study in which we also did not find boosting effects of oxytocin on placebo effects for pain and itch induced by verbal suggestions in healthy females and with lower oxytocin doses.38 These findings further confirm previous findings from the study by Colloca et al13 where no effects of oxytocin on placebo analgesia in either men or women were found. The important addition of the current study is that we employed a conditioning procedure along with verbal suggestions to induce placebo effects and we furthermore explored the effects of oxytocin on nocebo effects. Previous research indicated that oxytocin facilitates learning during fear conditioning when given prior to the conditioning procedure16 and enhances neural indicators of fear extinction when given prior to the extinction phase.15 We hypothesized that since these effects of fear conditioning were induced by painful stimuli, they could possibly also be applied to the response to a pain conditioning paradigm which essentially represents associative learning. Several explanations can be provided for the lack of confirming these expectations. Placebo analgesia and hyperalgesia are not identical to psychophysiological and neurological responses to fear conditioning used as outcome measures in previous research, such as amygdala activation, skin conductance, and reaction times.15,16 Possibly oxytocin influences neurological conditioned responses but not the conditioned pain perception. By contrast, social aspects seem to play an important role in oxytocin effects. It has been demonstrated that oxytocin enhances learning with social reinforces (emotional faces) in comparison to nonsocial reinforces (color cues).25 As the conditioning procedure did not include any social stimuli, possibly nonsocial cue-based learning mechanisms remained unaffected by oxytocin. On the other hand, some studies15,16 found effects of oxytocin on fear conditioning regardless of any social aspect of the CS. Therefore, it remains unclear whether oxytocin influences only the perception of stimuli with a social component.
Another methodological difference of our study from previous research is the dose of oxytocin used. The only experiment that found boosting effects of oxytocin on the placebo effect27 used 36 IU of oxytocin while 2 other studies with null findings13,38 used a more standard dose of 24 IU. The evidence regarding the correlation between the dose and effectivity of oxytocin is mixed. For example, lower (8 IU) doses of oxytocin have been found to affect amygdala activation in men in response to emotional faces more than higher (24 IU) doses,32 while another study demonstrated that 24 IU had the largest effect on amygdala activation in men in comparison to 12 IU and 48 IU.39 Also, a 24 IU dose seems to have a stronger effect on cortisol levels than a dose of 48 IU.8 On the other hand, 48 IU of oxytocin has been found to improve the performance on a face emotion recognition task, while 24 IU did not.37 Furthermore, a U-shaped relationship between oxytocin dose, social reward, and neural activity has been recently proposed,6 indicating the dose-response relationship is initiated at lower doses in females than males. In the present study, we chose a dose that was higher than the standard 24 IU dose that did not affect the placebo effect in previous research.13,38 However, it remains unclear what the optimal dose is for these designs. Future research needs to determine the exact role of the dose of oxytocin for learning and extinction paradigms.
Additionally, we did not find effects of oxytocin on pain sensitivity. A lot of research focused on possible analgesic effects of oxytocin, but the results remain contradictory. A number of studies found pain reducing effects of oxytocin,7,33,45 however, others could not replicate these results.22,28,38,41
A few limitations of the current study should be mentioned. First of all, repeated administration of the heat stimuli leads to the habituation to pain: the temperatures that during the calibration caused low-, medium-, and high pain levels, during the pain conditioning task elicited lower levels of pain. As participants experienced smaller ranges of pain sensations, it could have decreased the magnitude of placebo and nocebo responses. Despite this fact, this procedure was successful in eliciting placebo analgesia and nocebo hyperalgesia. Additionally, many participants had relatively high pain thresholds, and it was therefore not possible to reach high levels of pain because of the protection mechanisms of the ATS termode that does not allow giving temperatures above certain thresholds.
Furthermore, the results regarding the lack of extinction of the nocebo effect should be interpreted carefully, because the nocebo effect was quite small in general, although significant. Possibly, larger effects might be affected by extinction processes more, however, this needs to be investigated in future studies in more detail.
Another limitation concerns the fact that the color cues and temperatures corresponding to them during the learning phase were not counterbalanced. In order to maximize placebo and nocebo effects, we choose colors that might be implicitly associated with low (green) and high (red) pain. However, within this study design, it is not possible to distinguish the effects of the colors and the effects of the conditioning procedure on the placebo and nocebo effects. Future research should investigate the role of the cue colors on pain conditioning.
Additional limitation of our study is that our design did not include a manipulation check: we have not measured endogenous oxytocin levels after administering a nasal spray, and therefore, we cannot be entirely sure, that intranasal oxytocin administration was successful. This is a common limitation of the intranasal oxytocin research which rarely measures endogenous oxytocin levels. On the other hand, several studies have measured oxytocin in saliva, blood, and cerebrospinal fluid after the intranasal administration,24,40,42 and all these studies have found that intranasal administration of oxytocin lead to the increase of endogenous levels.
Finally, a limitation of the current study is that we included only males. Sex differences may explain the mixed findings in oxytocin research. For example, it has been found that oxytocin dampens amygdala activation in response to emotional stimuli in males but enhances activation in females.20 Evidence for opposite effects of oxytocin on social processing in men and women has also been found.23,34 Since placebo-enhancing effects of oxytocin were previously found in males,27 we recruited a male sample in this experiment. However, we were unable to confirm these previous positive findings in a male sample. Our results are more in line with one of our previous studies which was performed in women38 and with a study by Colloca13 conducted in both sexes. As the effects of oxytocin on nocebo hyperalgesia have never been studied before, it remains unclear whether the effect would be found in women. To generalize the research on oxytocin and placebo effect to the clinical practice, it is necessary that future studies compare oxytocin effect in male and female samples within the same study design. Understanding sex differences in oxytocin effects would enable us to create personalized clinical interventions that take sex into account.
To summarize, although placebo analgesia and nocebo hyperalgesia could be successfully induced by conditioning and verbal suggestions, there was no evidence that 40 IU oxytocin given intranasally affected placebo analgesia and nocebo hyperalgesia, nor the extinction of both. Our study adds to the accumulating evidence toward the absence of the ability of oxytocin to influence placebo effects. Researchers interested in possibilities to strengthen placebo effects and reduce nocebo effects might benefit from focusing on other neurobiological and behavioral mechanisms that influence learning mechanisms of placebo and nocebo effects.
Acknowledgments
The authors would like to thank the bachelor students of Leiden University who assisted with the data collection.
Footnotes
Grant Support: This work was supported by the European Research Council consolidator grant [ERC-2013-CoG-617700, granted to A.E.]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to declare.
Trial Registration: The study protocol was preregistered on the website www.trialregister.nl under the number NTR6506.
References
- 1.Bartels DJ, van Laarhoven AI, Haverkamp EA, Wilder-Smith OH, Donders ART, van Middendorp H, van de Kerkhof PC, Evers AW. Role of conditioning and verbal suggestion in placebo and nocebo effects on itch. PLoS One. 2014;9:e91727. doi: 10.1371/journal.pone.0091727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Rev General Psychol. 2001;5:323. [Google Scholar]
- 3.Benedetti F. Mechanisms of placebo and placebo-related effects across diseases and treatments. Annu Rev Pharmacol Toxicol. 2008;48:33–60. doi: 10.1146/annurev.pharmtox.48.113006.094711. [DOI] [PubMed] [Google Scholar]
- 4.Benedetti F, Amanzio M, Casadio C, Oliaro A, Maggi G. Blockade of nocebo hyperalgesia by the cholecystokinin antagonist proglumide. Pain. 1997;71:135–140. doi: 10.1016/s0304-3959(97)03346-0. [DOI] [PubMed] [Google Scholar]
- 5.Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci. 2003;23:4315–4323. doi: 10.1523/JNEUROSCI.23-10-04315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borland JM, Rilling JK, Frantz KJ, Albers HE. Sex-dependent regulation of social reward by oxytocin: An inverted U hypothesis. Neuropsychopharmacology. 2018;44:97–110. doi: 10.1038/s41386-018-0129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouji M, Lecomte A, Gamez C, Blazy K, Villegier AS. Neurobiological effects of repeated radiofrequency exposures in male senescent rats. Biogerontology. 2016;17:841–857. doi: 10.1007/s10522-016-9654-8. [DOI] [PubMed] [Google Scholar]
- 8.Cardoso C, Cardoso M, Ellenbogen M, Orlando S, Bacon R, Joober Intranasal oxytocin attenuates the cortisol response to physical stress: A dose–response study. Psychoneuroendocrinology. 2013;38:399–407. doi: 10.1016/j.psyneuen.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Colagiuri B, Quinn VF. Autonomic arousal as a mechanism of the persistence of nocebo hyperalgesia. J Pain. 2018;19:476–486. doi: 10.1016/j.jpain.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Colagiuri B, Quinn VF, Colloca L. Nocebo hyperalgesia, partial reinforcement, and extinction. J Pain. 2015;16:995–1004. doi: 10.1016/j.jpain.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Colloca L, Miller FG. How placebo responses are formed: A learning perspective. Philos Trans R Soc B. 27; 2011;366:1859–1869. doi: 10.1098/rstb.2010.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colloca L, Petrovic P, Wager TD, Ingvar M, Benedetti F. How the number of learning trials affects placebo and nocebo responses. Pain. 2010;151:430–439. doi: 10.1016/j.pain.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colloca L, Pine DS, Ernst M, Miller FG, Grillon C. Vasopressin boosts placebo analgesic effects in women: A randomized trial. Biologic Psychiatry. 2016;79:794–802. doi: 10.1016/j.biopsych.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colloca L, Sigaudo M, Benedetti F. The role of learning in nocebo and placebo effects. Pain. 2008;136:211–218. doi: 10.1016/j.pain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Eckstein M, Becker B, Scheele D, Scholz C, Preckel K, Schlaepfer TE, Grinevich V, Kendrick KM, Maier W, Hurlemann R. Oxytocin facilitates the extinction of conditioned fear in humans. Biologic Psychiatry. 2015;78:194–202. doi: 10.1016/j.biopsych.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Eckstein M, Scheele D, Patin A, Preckel K, Becker B, Walter A, Domschke K, Grinevich V, Maier W, Hurlemann R. Oxytocin facilitates pavlovian fear learning in males. Neuropsychopharmacology. 2016;41:932–939. doi: 10.1038/npp.2015.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egorova N, Park J, Kong J. In the face of pain: The choice of visual cues in pain conditioning matters. Eur J Pain. 2017;21:1243–1251. doi: 10.1002/ejp.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enck P, Klosterhalfen S. The story of O–is oxytocin the mediator of the placebo response? Neurogastroenterol Motil. 2009;21:347–350. doi: 10.1111/j.1365-2982.2009.01285.x. [DOI] [PubMed] [Google Scholar]
- 19.Faul F, Erdfelder E, Lang A-G, Buchner A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 20.Feng C, Hackett PD, DeMarco AC, Chen X, Stair S, Haroon E, Ditzen B, Pagnoni G, Rilling JK. Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging Behavior. 2015;9:754–764. doi: 10.1007/s11682-014-9333-9. [DOI] [PubMed] [Google Scholar]
- 21.Forsberg JT, Martinussen M, Flaten MA. The placebo analgesic effect in healthy individuals and patients: A meta-analysis. Psychosom Med. 2017;79:388–394. doi: 10.1097/PSY.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 22.Goodin BR, Anderson AJ, Freeman EL, Bulls HW, Robbins MT, Ness TJ. Intranasal oxytocin administration is associated with enhanced endogenous pain inhibition and reduced negative mood states. Clin J Pain. 2014;31:757–767. doi: 10.1097/AJP.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoge EA, Anderson E, Lawson EA, Bui E, Fischer LE, Khadge SD, Barrett LF, Simon NM. Gender moderates the effect of oxytocin on social judgments. Hum Psychopharmacol. 2014;29:299–304. doi: 10.1002/hup.2402. [DOI] [PubMed] [Google Scholar]
- 24.Huffmeijer R, Alink LR, Tops M, Grewen KM, Light KC, Bakermans-Kranenburg MJ, Ijzendoorn MH. Salivary levels of oxytocin remain elevated for more than two hours after intranasal oxytocin administration. Neuro Endocrinol Lett. 2012;33:21–25. [PubMed] [Google Scholar]
- 25.Hurlemann R, Patin A, Onur O, Cohen M, Baumgartner T, Metzler S, Dziobek I, Gallinat J, Wagner M, Maier W, Kendrick K. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen KB, Kaptchuk TJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, Gollub RL, Ingvar M, Kong J. Nonconscious activation of placebo and nocebo pain responses. Proc Natl Acad Sci. 2012;109:15959–15964. doi: 10.1073/pnas.1202056109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kessner S, Sprenger C, Wrobel N, Wiech K, Bingel U. Effect of oxytocin on placebo analgesia: A randomized study. JAMA. 2013;310:1733–1735. doi: 10.1001/jama.2013.277446. [DOI] [PubMed] [Google Scholar]
- 28.Mameli S, Pisanu G, Sardo S, Marchi A, Pili A, Carboni M, Minerba L, Trincas G, Carta M, Melis MR. Oxytocin nasal spray in fibromyalgic patients. Rheumatol Int. 2014;34:1047–1052. doi: 10.1007/s00296-014-2953-y. [DOI] [PubMed] [Google Scholar]
- 29.Marteau TM, Bekker H. The development of a six‐item short‐form of the state scale of the Spielberger State—Trait Anxiety Inventory (STAI) Br J Clin Psychol. 1992;31:301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 30.Pavlov IP. 1927. Conditional Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. Dover. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen GL, Finnerup NB, Colloca L, Amanzio M, Price DD, Jensen TS, Vase L. The magnitude of nocebo effects in pain: A meta-analysis. PAIN. 2014;155:1426–1434. doi: 10.1016/j.pain.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quintana DS, Westlye LT, Alnæs D, Rustan ØG, Kaufmann T, Smerud KT, Mahmoud RA, Djupesland PG, Andreassen OA. Low dose intranasal oxytocin delivered with breath powered device dampens amygdala response to emotional stimuli: A peripheral effect-controlled within-subjects randomized dose-response fMRI trial. Psychoneuroendocrinology. 2016;69:180–188. doi: 10.1016/j.psyneuen.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Rash J, Campbell T. The effect of intranasal oxytocin administration on acute cold pressor pain: A placebo-controlled, double-blind, within-participants crossover investigation. Psychosom Med. 2014;76:422–429. doi: 10.1097/PSY.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 34.Rilling JK, DeMarco AC, Hackett PD, Chen X, Gautam P, Stair S, Haroon E, Thompson R, Ditzen B, Patel R, Pagnoni G. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology. 2014;39:237–248. doi: 10.1016/j.psyneuen.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanderman R, Arrindell WA, Ranchor AV. Eysenck Personality Questionnaire (EPQ) Groningen: Noordelijk Centrum voor Gezondheidsvraagstukken. 1991 [Google Scholar]
- 36.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the Life Orientation Test. J Personal Soc Psychol. 1994;67:1063. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 37.Shin EJ, Chae JS, Park SJ, Kim SC, Koo KH, Yamada K, Nabeshima T, Kim HC. Growth hormone-releaser diet attenuates beta-amyloid(1-42)-induced cognitive impairment via stimulation of the insulin-like growth factor (IGF)-1 receptor in mice. J Pharmacol Sci. 2009;109:139–143. doi: 10.1254/jphs.08145sc. [DOI] [PubMed] [Google Scholar]
- 38.Skvortsova A, Veldhuijzen DS, Van Middendorp H, Van den Bergh O, Evers AW. Enhancing placebo effects in somatic symptoms through oxytocin. Psychosom Med. 2018;80:353–360. doi: 10.1097/PSY.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spengler FB, Schultz J, Scheele D, Essel M, Maier W, Heinrichs M, Hurlemann R. Kinetics and dose dependency of intranasal oxytocin effects on amygdala reactivity. Biologic Psychiatry. 2017;82:885–894. doi: 10.1016/j.biopsych.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 40.Striepens N, Kendrick KM, Hanking V, Landgraf R, Wüllner U, Maier W, Hurlemann R. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep. 2013;3:3440. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tracy LM, Labuschagne I, Georgiou-Karistianis N, Gibson SJ, Giummarra MJ. Sex-specific effects of intranasal oxytocin on thermal pain perception: A randomised, double-blind, placebo-controlled cross-over study. Psychoneuroendocrinology. 2017;83:101–110. doi: 10.1016/j.psyneuen.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 42.Van IJzendoorn MH, Bhandari R, Van der Veen R, Grewen K, Bakermans-Kranenburg MJ. Elevated salivary levels of oxytocin persist more than 7 h after intranasal administration. Front Neurosci. 7; 2012;6:174. doi: 10.3389/fnins.2012.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Personal Soc Psychol. 1988;54:1063. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 44.Yeung STA, Colagiuri B, Lovibond PF, Colloca L. Partial reinforcement, extinction, and placebo analgesia. Pain. 2014;155:1110–1117. doi: 10.1016/j.pain.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zunhammer M, Geis S, Busch V, Eichhammer P, Greenlee MW. Pain modulation by intranasal oxytocin and emotional picture viewing—A randomized double-blind fMRI study. Sci Rep. 2016;6:31606. doi: 10.1038/srep31606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zunhammer M, Gerardi M, Bingel U. The effect of dopamine on conditioned placebo analgesia in healthy individuals: A double-blind randomized trial. Psychopharmacology. 2018;235:1–9. doi: 10.1007/s00213-018-4951-3. [DOI] [PubMed] [Google Scholar]