Abstract
Background
Computerized cognitive remediation therapy (CCRT) is generally effective for the cognitive deficits of schizophrenia. However, there is much uncertainty about what factors mediate or moderate effectiveness and are therefore important to personalize treatment and boost its effects.
Method
In total, 311 Chinese inpatients with Diagnostic and Statistical Manual of Mental Disorders-IV schizophrenia were randomized to receive CCRT or Active control for 12 weeks with four to five sessions per week. All participants were assessed at baseline, post-treatment and 3-month follow-up. The outcomes were cognition, clinical symptoms and functional outcomes.
Results
There was a significant benefit in the MATRICS Consensus Cognitive Battery (MCCB) total score for CCRT (F1,258 = 5.62; p = 0.02; effect size was 0.27, 95% confidence interval 0.04–0.49). There were no specific moderators of CCRT improvements. However, across both groups, Wisconsin Card Sort Test improvement mediated a positive effect on functional capacity and Digit Span benefit mediated decreases in positive symptoms. In exploratory analyses younger and older participants showed cognitive improvements but on different tests (younger on Symbol Coding Test, while older on the Spatial Span Test). Only the older age group showed MSCEIT benefits at post-treatment. In addition, cognition at baseline negatively correlated with cognitive improvement and those whose MCCB baseline total score was around 31 seem to derive the most benefit.
Conclusions
CCRT can improve the cognitive function of patients with schizophrenia. Changes in cognitive outcomes also contributed to improvements in functional outcomes either directly or solely in the context of CCRT. Age and the basic cognitive level of the participants seem to affect the cognitive benefits from CCRT.
Key words: Cognitive deficits, computerized cognitive remediation therapy, schizophrenia
Introduction
Cognitive impairment in schizophrenia is common and accounts for significant variation in real-world outcomes such as work performance even when supportive recovery programs are provided (Green et al., 2000; Bell et al., 2007). These impairments constitute a key component of recovery and are therefore logical treatment targets. They are resistant to current pharmacological treatments (Choi et al., 2013) and so a variety of cognitive remediation techniques have been developed to improve cognitive function in schizophrenia.
Cognitive remediation therapy (CRT) has moderate to large effects on cognitive outcomes (attention, memory, executive function, social cognition or metacognition) (Wykes et al., 2011) across different presentation modes e.g. paper and pencil (Wykes et al., 2007a, 2007b; Cella et al., 2014) or computer (Kurtz et al., 2015). The majority of reviews and meta-analyses have confirmed CRT benefits for both cognition and functioning in psychosis (Wykes et al., 2011; Tan and Liu, 2016). However, a few studies have found no benefits (Dickinson et al., 2010; Rass et al., 2012; Gomar et al., 2015). These rare instances might be explained by ineffective therapy or sampling differences, for instance including older participants as age has been shown to affect benefits (e.g. Wykes et al., 2009). As health resources are limited, it is crucial to identify whether some participants benefit more (or less) from CRT in order that scarce resources can be deployed efficiently. Previous research has suggested that age and baseline cognitive performance may moderate the impact of cognition (Wykes et al., 2009; Kontis et al., 2013; Ramsay et al., 2018). Previous studies feature small sample sizes that are insufficient for subgroup analysis. In the current study, a large sample of Chinese inpatients was recruited to investigate the efficacy of a new Chinese cognitive remediation computerized program, Computerized Cognitive Remediation Therapy (CCRT) for cognitive performance and then investigate whether these improvements contribute to improved functional capacity or symptoms. The size of the study allows the exploration of proposed moderators and mediators (age, cognition) of treatment benefit, as well as testing the CRT therapeutic model of improvements in cognition having an impact on functioning. Identifying predictors could guide personalization and tailored care and increase the benefits of cognitive remediation.
Methods
Design
This is a longitudinal, randomized, single blind multisite clinical trial which was part of a larger study. All participants who consented and fulfilled the inclusion criteria were randomly allocated to CCRT or Active control group in two hospitals (Beijing Anding Hospital and the Peking University Sixth Hospital) and at Beijing Huilongguan Hospital they were randomly allocated to three conditions (CCRT, Active control and Paper and pencil cognitive remediation). In this report only the comparisons between CCRT and Active control are presented. The primary cognitive outcome was MATRICS Consensus Cognitive Battery (MCCB) total score assessed at baseline (week 0), post-treatment (week 12) and follow-up (week 24). Secondary outcomes (cognition, functional capacity and symptoms) were assessed at the same times. All participants provided written informed consent, and the protocol was approved by the Beijing Huilongguan Hospital Ethics Committee (2006-3). The trial is registered at Chinese Clinical Trials Registry, identifier ChiCTR-TRC-08000249.
Participants
In total, 311 voluntarily admitted in-patients with schizophrenia were recruited from 14 May 2007 to 1 March 2009 from Beijing Huilongguan Hospital, Beijing Anding Hospital and the Peking University Sixth Hospital. The final follow-up visit was on 13 October 2009. They had chronic schizophrenia and symptoms that required prolonged hospitalization but were clinically stable during the study period. The inclusion criteria were: Diagnostic and Statistical Manual, 4th ed (DSM-IV) (American Psychiatric Association, 1994) schizophrenia diagnosis confirmed by two psychiatrists; age 20–60 years; cognitive impairment [<4 categories on the Wisconsin Card Sort Test (WCST), or <7 on the WAIS-R Digit Span Backward test], 6+ years full-education (ensured full understanding of the task instructions), a stable dose and type of medication (for at least 1 month prior to inclusion and no anticipated medication change over the course of the study). Exclusion criteria included a planned medication change, any difficulty in communicating effectively with therapists, diagnosis of substance abuse as defined by the DSM-IV and a history of organic brain disorder or other severe organic disorder.
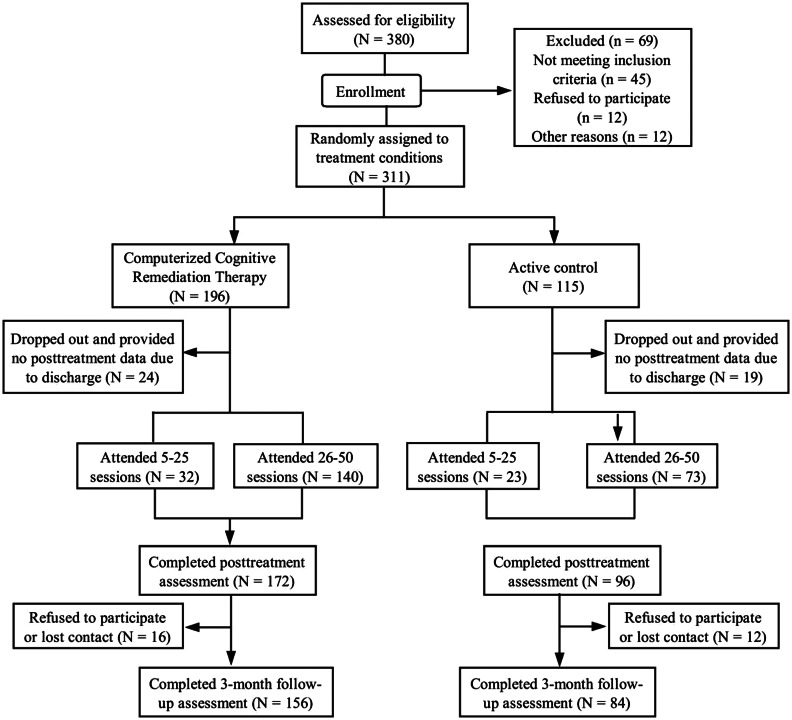
The CONSORT flow diagram in Fig. 1 shows 311 people were finally randomized (CCRT, N = 196; Active control, N = 115). There were 198 inpatients from Beijing Huilongguan Hospital, 72 from Beijing Anding Hospital and 41 from the Peking University Sixth Hospital. The participants from Beijing Huilongguan hospital were randomized by 3:1 (CCRT v. Active control) and from the other two hospitals were randomized 1:1 (CCRT v. Active control) due to the limited numbers. Randomization was independently conducted by a psychiatrist not otherwise involved in the study at the completion of all baseline assessments. A random number table was used to generate randomization which was provided in sealed envelopes. As expected from random allocation, the two groups were well balanced in age, gender, education, duration of illness, cognitive difficulties and social behavior deficits. The dropout rates from the study were 12.2% and 16.5% (CCRT v. Active control) at post-treatment, and 20.4% and 26.9% (CCRT v. Active control) at follow-up.
Fig. 1.
Treatment study flowchart.
Outcome measures
Primary and secondary cognitive outcomes
Cognition was assessed using the Chinese version of the MCCB (Zou et al., 2009) which consists of 10 tests with seven domains and the alternate form was used at post-treatment. The primary outcome was the MCCB total score transformed to T scores (mean = 50, s.d. = 10) and the individual test scores were the secondary outcomes also transformed to T scores. The seven domains were:
-
(1)
Speed of processing: Category Fluency Test, Trail Making Test-A, Symbol Coding Test.
-
(2)
Attention/Vigilance: Continuous Performance Test (Continuous Performance Test-Identical Pair, CPT-IP).
-
(3)
Working memory: Spatial Span Test (Wechsler Memory Scale-Third Edition, WMS-III); Digit Sequencing Test.
-
(4)
Verbal learning: Hopkins Verbal Learning Test-Revised (HVLT, Chinese version).
-
(5)
Visual learning: Brief Visuospatial Memory Test-Revised (BVMT-R).
-
(6)
Reasoning and problem solving: Mazes Test (Neuropsychological Assessment Battery-Mazes, NAB-MAZES).
-
(7)
Social cognition: Mayer-Salovery-Caruso Emotional Intelligence Test (MSCEIT, Chinese version): Managing Emotions (Caruso et al., 2002).
Other secondary cognitive outcomes included: Verbal Working memory: Wechsler Adult Intelligence Scale-III Digit Span Test with the key measure being the total score. Executive function was measured with the WCST (Heaton et al., 1993), and the key score was the number of completed categories.
Secondary symptom and function outcomes
Functional capacity was measured with a Chinese version (Cui et al., 2012) of UCSD Performance-based Skills Assessment (UPSA), which uses structured role-play scenarios to measure ability in two everyday living domains (Finance, e.g. making change; Communication, e.g. using the telephone for emergencies). The Finance task consists of 10 items; the Communication task has nine items. These tasks require about 8 and 5 min, respectively, to complete with the highest scores being 10 and 9.
A Chinese version (Li, 1987) of Nurse's Observation Scale for Inpatient Evaluation (NOSIE)-30 (Honigfeld et al., 1966) was used to evaluate behavior and functional capacity. The outcomes included were the total score, positive factor score (Social Competence, Social Interest, Personal Neatness) and negative factor score (Irritability, Manifest Psychosis, Retardation and Depression).
The Rosenberg self-esteem scale (Rosenberg, 1965) which had been translated into Chinese (Wang Ping et al., 1998) was used to measure global feelings of self-worth or self-acceptance from 10 items. The key outcome is the overall score with higher scores reflecting higher levels of self-esteem.
Clinical assessment
The Chinese version of Positive and Negative Syndrome Scale (PANSS) (Yanlin and MingYuan, 2000), which has similar psychometric properties to those obtained in the original version (Kay, 1990; Tianmei et al., 2004), was used for the symptom assessment (PANSS total score, PANSS Positive score and PANSS Negative score).
Data collection
All cognitive assessments were completed by four trained clinical psychologists who had at least 5 years' experience of psychometric testing (before baseline, the raters received a high consistent κ of 0.90). The clinical symptom rating (PANSS) was conducted by eight attending psychiatrists (before the study start, they achieved a satisfactory κ of 0.80). The functional capacity assessment was completed by four senior nurses who had at least 5 years' experience of psychiatry nursing (before assessment, these raters were trained and achieved a κ value of 0.85). All raters were blind to group assignment.
Therapies
Both therapies were provided in groups and the duration and frequency of sessions were identical.
Cognitive remediation
Computer software (Computerized Cognitive Remediation Therapy System; CCRT) was developed from principles in Wykes and Reeder (Wykes and Reeder, 2005). It consists of 30 exercises that dynamically change in difficulty as accuracy reaches 80%. CCRT treatment was provided in four to five 45-min sessions per week over 12 weeks with a total of 50 sessions. It is supervised by experienced therapists at a ratio of 1 therapist to 4 participants. The therapist teaches participants to use CCRT in the first 2 weeks, and subsequent treatment is mainly carried out by the participant alone.
Active control
This therapy had the same number of sessions as CCRT but contained two different activities: learning to play a fairly easy instrument, usually the xylophone, and learning to dance, which were generally offered to almost every inpatient in these hospitals. The role of the therapist was to encourage these activities and take part actively in each session.
Statistical analyses
All data were analyzed using IBM SPSS 20.0 (IBM Corp, Armonk, NY, USA).
Efficacy analyses
First, a series of intent-to-treat analyses were used to examine all outcome variables. General linear mixed modeling (GLM) with model parameters estimated by maximum likelihood based on normality was used for both the efficacy analysis and to explore secondary outcomes. Models included fixed effects for the experimental factors [main effect of group (CCRT or Active control) and time (post-therapy or follow-up) and a group × time interaction] and baseline values of the outcome measure was a covariate. A main effect of group would be interpreted as an effect of CCRT consistent across both post-treatment and follow-up. A significant group × time interaction term implies a differential effect of treatment at the post-treatment and follow-up, and then further simple effect analysis for the significant group × time interaction was conducted with Bonferroni correction for multiple comparisons. In addition, random effects for participants and study sites were included. We will examine predictors of drop-out and if any are significant we will incorporate them into all analyses.
Mediation and moderator analyses
CCRT improves functional outcome mainly by changing cognitive function and then this benefits functioning. CCRT can also promote functional improvement by enhancing the relationship between cognitive change and functional improvement. Cognitive improvement is therefore the mediator which is moderated by CCRT. For example, cognitive change was predictive of functional change only in the CR group (Reeder et al., 2004; Spaulding et al., 1999a, 1999b). In order to investigate these putative effects, analyses of covariance (ANCOVAs) were carried out with the follow-up measure of functional capacity as the dependent measure, cognitive change (post-treatment – baseline), group and an interaction between cognitive change and group as explanatory variables, and initial baseline level of functional capacity as a covariate. The interaction indicates that cognitive improvements could affect functional outcome if they were achieved in the context of CCRT. The model was re-fitted excluding the interaction term if it was not significant. A statistically significant and positive main effect of cognitive change on functional capacity and evidence of cognitive change improved by CCRT indicate that cognition could mediate functional improvement in CCRT.
In order to investigate the putative effects of MCCB baseline total score on cognitive benefits we performed a Pearson or partial correlation analysis controlling for age separately for the CCRT and the Active control groups, locally weighted scatterplot smoothing (LOESS) and polynomial fitting (quadratic polynomial). The MCCB difference (post-treatment – baseline) was considered as the cognitive benefit. The LOESS method provided an exploratory insight between two variables and help us choose parametric models (Cleveland and Devlin, 1988), and further polynomial fitting was used to clarify the model (Johnson, 1991).
In order to explore the influence of age on cognitive benefits, we divided the participants into two age groups (younger group = < or =39 years; older group >39 years). This cut-off was chosen because previous studies have shown differences for under and over 40 years (McGurk and Mueser, 2008; Wykes et al., 2009; Kontis et al., 2013). The GLM analyses were also used but these models included additional fixed effects for age group and two-way or three-way interaction for the moderator analyses. The model was re-fitted excluding the interaction term if it was not significant. Further simple effect analysis for the significant group × age group interaction was conducted.
Statistical thresholds for GLM analysis used p < 0.05. Because we are investigating potential moderators (age and MCCB baseline score) of cognitive benefits after CCRT treatment, we will adopt a broad view of significance with any group by X interaction below 0.1 as an effect to be investigated. In addition to the effects on the main cognitive outcome we will also carry out exploratory analyses on individual tests as others have indicated differential improvements by age (Thomas et al., 2017).
Sample size and power of the study
The sample calculation assumes a standardized benefit of 0.49 (Twamley et al., 2003). Assuming a CCRT group of 108 people and an Active control group of 54 people the study would have 90% power to detect this difference based on an independent samples t test for unequal groups at the 5% significance level. This was increased to more than 130 people for the CCRT group and more than 65 people for the Active control group in order to account for possible dropout.
Results
Table 1 shows the baseline characteristics of the randomized groups. There were no significant differences between the groups on demographics, antipsychotic type or chlorpromazine equivalents. Mean baseline scores of cognitive functions, clinical symptoms and functional outcomes were also similar between the two groups (see Table 2). No baseline characteristics predicted dropout and so none were added to our analyses (see online Supplementary Table S1 for details).
Table 1.
Demographic, clinical and cognitive variables of the two groups
| Characteristics | CCRT group (n = 196) | Active control group (n = 115) | t/χ2 | p value |
|---|---|---|---|---|
| Sex (male/female) | 122:74 | 69:46 | 0.154 | 0.695a |
| Mean age (s.d.), year | 45.60 ± 8.68 | 44.24 ± 8.31 | 1.354 | 0.177b |
| Mean education years (s.d.), year | 11.74 ± 2.82 | 11.86 ± 2.72 | −0.370 | 0.711b |
| Marriage (yes/no) | 48:148 | 28:87 | 0.001 | 0.978a |
| Duration of illness (s.d.), year | 21.16 ± 9.77 | 19.18 ± 9.44 | 1.659 | 0.098b |
| Mean onset age (s.d.), year | 24.44 ± 6.99 | 24.55 ± 7.10 | −0.124 | 0.902b |
| MCCB total score | 40.19 ± 10.62 | 40.36 ± 10.09 | −0.140 | 0.889b |
| UPSA score | 41.39 ± 9.41 | 38.36 ± 9.02 | 1.688 | 0.094b |
| Mean (s.d.) PANSS score | ||||
| Total score | 59.77 ± 12.44 | 61.62 ± 13.43 | −1.166 | 0.245b |
| Positive score | 12.12 ± 4.76 | 12.39 ± 5.08 | −0.454 | 0.650b |
| Negative score | 17.02 ± 5.17 | 17.72 ± 6.10 | −1.020 | 0.309b |
| Dose of chlorpromazine equivalents | 407.39 ± 260.44 | 363.91 ± 221.15 | 1.408 | 0.160b |
| Psychiatric medication (typical/atypical and both) | 31:147 | 16:85 | 0.114 | 0.736a |
CCRT, Computerized Cognitive Remediation Therapy; MCCB, MATRICS Consensus Cognitive Battery; UPSA, UCSD Performance-Based Skills Assessment; PANSS, Positive and Negative Syndrome Scale.
χ2 tests.
Independent samples t test.
Table 2.
Linear model for mean (s.d.) scores on cognitive function, clinical symptom and functional outcome by group (CCRT and Active control) for baseline, posttreatment and the 3 months follow-up
| CCRT group (n = 196/172/156) | Active control group (n = 115/96/84) | |||||
|---|---|---|---|---|---|---|
| Cognitive outcome | Baseline | Posttreatment | Follow-up | Baseline | Posttreatment | Follow-up |
| MCCB | ||||||
| Speed of processing | ||||||
| Category Fluency Test (animal) | 47.27 (11.09) | 49.31 (10.67) | 49.28 (11) | 46.39 (12.21) | 47.44 (12.61) | 48.47 (12.95) |
| Trail Making Test, Part A | 41.28 (13.50) | 42.79 (12.61) | 43.60 (13.27) | 42.81 (12.70) | 44.22 (13.47) | 47.38 (14.47) |
| Symbol Coding Test | 40.93 (9.28) | 41.75 (8.81) | 43.43 (9.03) | 40.35 (8.95) | 40.04 (9.96) | 43.1 (10.16) |
| Attention/Vigilance | ||||||
| Continuous Performance Test | 44.37 (9.57) | 45.49 (10.36) | 46.96 (10.73) | 42.12 (9.28) | 43.67 (9.37) | 44.86 (11.12) |
| Working memory | ||||||
| Digit Sequencing Test | 45.17 (10.05) | 46.83 (10.39) | 47.26 (9.71) | 43.42 (10.39) | 45.86 (9.73) | 45.42 (11.37) |
| Spatial Span Test | 43.37 (11.63) | 46.81 (10.70) | 46.28 (12.67) | 41.80 (12.70) | 42.27 (11.11) | 43.24 (12.55) |
| Verbal learning | ||||||
| HVLT-R | 42.93 (11.78) | 43.60 (12.07) | 46.95 (12.41) | 42.71 (11.45) | 41.22 (12.61) | 44.9 (13.41) |
| Visual learning | ||||||
| BVMT-R | 43.22 (11.02) | 46.82 (11.44) | 47.40 (11.52) | 44.33 (11.30) | 46.99 (10.87) | 46.35 (11.53) |
| Reasoning and problem solving | ||||||
| Mazes Test | 40.47 (13.15) | 44.05 (11.61) | 43.95 (11.20) | 44.62 (12.65) | 44.78 (12.50) | 45.04 (11.53) |
| Social cognition | ||||||
| MSCEIT | 45.17 (10.79) | 46.8 (10.61) | 44.51 (10.34) | 45.62 (10.82) | 44.14 (10.56) | 43.59 (10.27) |
| MCCB total score | 40.19 (10.62) | 43.20 (10.99) | 43.94 (11.59) | 40.36 (10.09) | 41.36 (10.25) | 43.13 (11.92) |
| Digit Span Testa | 16.29 (4.27) | 17.71 (4.89) | 17.06 (4.35) | 15.36 (5.00) | 15.90 (4.66) | 15.4 (6.06) |
| WCST no. of categoriesa | 2.09 (2.11) | 2.72 (2.20) | 2.49 (2.14) | 2.19 (2.03) | 1.86 (1.98) | 1.85 (1.97) |
| Clinical Symptoms | ||||||
| PANSS total score | 59.77 (12.44) | 57.96 (13.23) | 58.47 (15.08) | 61.62 (13.43) | 60.92 (14.97) | 59.54 (14.73) |
| PANSS positive score | 12.12 (4.76) | 11.86 (4.92) | 11.85 (5.19) | 12.39 (5.08) | 12.51 (5.26) | 11.83 (5.00) |
| PANSS negative score | 17.02 (5.17) | 16.31 (5.22) | 16.94 (6.09) | 17.72 (6.10) | 17.83 (6.34) | 18.02 (6.42) |
| Functional Outcome | ||||||
| UPSA | 41.39 (9.41) | 42.56 (10.84) | 42.80 (10.31) | 38.36 (9.02) | 39.74 (10.78) | 41.84 (10.91) |
| NOSIE | ||||||
| Total positive factor | 78.38 (12.16) | 79.31 (12.81) | 78.40 (11.79) | 73.42 (14.46) | 74.94 (14.39) | 74.77 (12.90) |
| Total negative factor | 24.13 (14.26) | 20.48 (14.01) | 20.66 (14.67) | 27.43 (14.16) | 26.00 (15.78) | 22.24 (13.83) |
| Total score | 182.26 (23.21) | 187.21 (23.55) | 186.22 (23.25) | 173.39 (25.17) | 177.17 (26.69) | 180.48 (24.11) |
| Self-esteem | 27.01 (4.78) | 28.78 (3.71) | 28.64 (3.66) | 26.79 (5.26) | 28.34 (4.12) | 28.51 (3.70) |
CCRT, Computerized Cognitive Remediation Therapy; MCCB, MATRICS Consensus Cognitive Battery; HVLT-R, Hopkins Verbal Learning Test-Revised; BVMT-R, Brief Visuospatial Memory Test-Revised; MSCEIT, Mayer-Salovery-Caruso Emotional Intelligence Test; WCST, Wisconsin Card Sorting Test; PANSS, Positive and Negative Syndrome Scale; UPSA, UCSD Performance-Based Skills Assessment; NOSIE, Nurse's Observation Scale for Inpatient Evaluation.
The scales were not included in MCCB.
Is there a treatment effect on the primary and secondary outcomes?
Table 3 shows the results of the formal analyses of the cognitive outcomes, clinical symptoms and functional outcomes. The primary outcome, MCCB total score, showed a significant advantage to CCRT. In addition, five of the secondary cognitive outcomes (Spatial Span, HVLT-R, MSCEIT, Digit Span Test and WCST) also showed a CCRT group advantage.
Table 3.
Results of the mixed models analyses
| Cognitive outcome | Interaction test | Group effect (excluding non-significant interaction) | Estimated advantage to CCRT (no. of points on the scale)/95% CI | Effect size (95% CI) |
|---|---|---|---|---|
| MCCB | ||||
| Speed of processing | ||||
| Category Fluency Test (animal) | F(1,228) = 0.08; p = 0.77 | F(1,272) = 1.25; p = 0.26 | 1.09 (−0.82 to 3.00) | 0.13 (−0.09 to 0.34) |
| Trail Making Test, Part A | F(1,210) = 0.07; p = 0.79 | F(1,258) = 0.84; p = 0.36 | −1.04 (−3.25 to 1.18) | −0.11 (−0.33 to 0.12) |
| Symbol Coding Test | F(1,215) = 0.81; p = 0.37 | F(1,263) = 1.72; p = 0.19 | 0.92 (−0.46 to 2.31) | 0.15 (−0.07 to 0.37) |
| Attention/Vigilance | ||||
| Continuous Performance Test | F(1,205) = 1.20; p = 0.27 | F(1,266) = 0.35; p = 0.55 | 0.56 (−1.29 to 2.41) | 0.07 (−0.16 to 0.31) |
| Working memory | ||||
| Digit Sequencing Test | F(1,218) = 2.94; p = 0.09 | F(1,266) = 0.57; p = 0.45 | 0.64 (−1.03 to 2.30) | 0.08 (−0.13 to 0.30) |
| Spatial Span Test | F(1,211) = 0.32; p = 0.57 | F(1,268) = 9.36; p = 0.00 | 3.34 (1.19–5.50) | 0.32 (0.11–0.53) |
| Verbal learning | ||||
| HVLT-R | F(1,217) = 0.00; p = 0.98 | F(1,245) = 4.13; p = 0.04 | 2.17 (0.07–4.28) | 0.22 (0.01–0.43) |
| Visual learning | ||||
| BVMT-R | F(1,211) = 1.80; p = 0.18 | F(1,261) = 1.84; p = 0.18 | 1.25 (−0.56 to 3.06) | 0.15 (−0.07 to 0.38) |
| Reasoning and problem solving | ||||
| Mazes Test | F(1,222) = 0.02; p = 0.89 | F(1,252) = 1.51; p = 0.22 | 1.28 (−0.77 to 3.34) | 0.14 (−0.08 to 0.36) |
| Social cognition | ||||
| MSCEIT | F(1,218) = 2.01; p = 0.16 | F(1,262) = 4.55; p = 0.03 | 2.19 (0.17–4.20) | 0.24 (0.02–0.47) |
| MCCB total score | F(1,212) = 0.04; p = 0.84 | F(1,258) = 5.62; p = 0.02 | 1.73 (0.29–3.17) | 0.27 (0.04–0.49) |
| Digit Span Testa | F(1,165) = 0.18; p = 0.68 | F(1,212) = 8.84; p = 0.00 | 1.33 (0.45–2.21) | 0.38 (0.13–0.63) |
| WCST no. of categoriesa | F(1,212) = 1.05; p = 0.31 | F(1,257) = 13.5; p = 0.00 | 0.73 (0.34–1.12) | 0.42 (0.20–0.65) |
| Clinical Symptoms | ||||
| PANSS total score | F(1,235) = 0.39; p = 0.53 | F(1,258) = 1.25; p = 0.26 | −1.25 (−3.46 to 0.95) | −0.12 (−0.32 to 0.09) |
| PANSS positive score | F(1,243) = 1.07; p = 0.30 | F(1,262) = 0.55; p = 0.46 | −0.28 (−1.03 to 0.46) | −0.08 (−0.28 to 0.13) |
| PANSS negative score | F(1,233) = 0.09; p = 0.77 | F(1,258) = 4.82; p = 0.03 | −0.88 (−1.67 to −0.09) | −0.22 (−0.42 to −0.02) |
| Functional Outcome | ||||
| UPSA | F(1,111) = 0.29; p = 0.59 | F(1,118) = 0.05; p = 0.83 | 0.29 (−2.36 to 2.94) | 0.04 (−0.32 to 0.40) |
| NOSIE | ||||
| Total positive factor | F(1,212) = 0.15; p = 0.70 | F(1,241) = 0.11; p = 0.74 | 0.36 (−1.81 to 2.54) | 0.04 (−0.19 to 0.27) |
| Total negative factor | F(1,202) = 4.21; p = 0.04 | F(1,230) = 1.15; p = 0.29 | −1.42 (−4.02 to 1.19) | −0.13 (−0.36 to 0.11) |
| Total score | F(1,200) = 2.30; p = 0.13 | F(1,233) = 1.57; p = 0.21 | 2.77 (−1.59 to 7.12) | 0.15 (−0.09 to 0.38) |
| Self-esteem | F(1,247) = 0.51; p = 0.48 | F(1,262) = 0.28; p = 0.59 | 0.22 (−0.60 to 1.04) | 0.06 (−0.16 to 0.28) |
HVLT-R, Hopkins Verbal Learning Test-Revised; BVMT-R, Brief Visuospatial Memory Test-Revised; MSCEIT, Mayer-Salovery-Caruso Emotional Intelligence Test; WCST, Wisconsin Card Sorting Test; PANSS, Positive and Negative Syndrome Scale; UPSA, UCSD Performance-Based Skills Assessment; NOSIS, Nurse's Observation Scale for Inpatient Evaluation.
The scales were not included in MCCB. Bold indicate statistically significant effects (p < 0.05).
For the secondary symptom and functional capacity outcomes far fewer advantages were found. There was a significant effect on the PANSS negative symptom score but none for other symptom measures or functional capacity. However, one significant interaction emerged from the NOSIE, which was opposite to our hypothesis – the Active control group decreased more on the negative factors at follow-up [estimated decrease of 4.63 points for Active control; 95% confidence interval (CI) 1.91–7.35; p = 0.001 after Bonferroni correction], while no change was found in the CCRT group (estimated increase of 0.66 points; 95% CI −1.68 to 3.00; p > 0.05).
Does cognitive change mediate functional outcome?
No significant interactions emerged in the ANCOVA analyses and, after removing them, there were significant overall effects of cognitive change on both functional capacity and symptoms. An improvement in the primary outcome (MCCB total score) was marginally associated with a significant improvement in UPSA (F1,108 = 3.01; p = 0.09). However, an improvement in the WCST was associated with a significant improvement in functional capacity as measured by UPSA (F1,108 = 4.15, p = 0.04; estimated improvement was 0.85 points, 95% CI 0.02–1.67) and an improvement in Digit Span was accompanied by a decrease in PANSS positive symptoms (F1,179 = 5.62, p = 0.02; estimated improvement was 0.21 points per extra unit decrease in Digit Span Test, 95% CI 0.03–0.38) (see online Supplementary Table S2 for details).
Moderators of CCRT benefits
Does MCCB baseline total score affect cognitive benefit?
For post-treatment outcome the MCCB baseline total score was negatively correlated with cognitive benefit in both groups (CCRT: r = −0.25, df = 170, p = 0.001; Active control: r = −0.28, df = 87, p = 0.009, respectively). After controlling for age, these correlations remained significant (CCRT group, r = −0.26, df = 167, p = 0.001; Active control group, r = −0.282, df = 84, p = 0.009). Although baseline cognition was related to sustained cognitive benefit of cognitive remediation (baseline – follow-up), it did not reach traditional statistical significance in the CCRT group (r = −0.14, df = 143, p = 0.088) and was not significant in the Active control group (r = −0.12, df = 61, p = 0.344), even after controlling for age (r = −0.15, df = 140, p = 0.075 and r = −0.13, df = 58, p = 0.322, respectively).
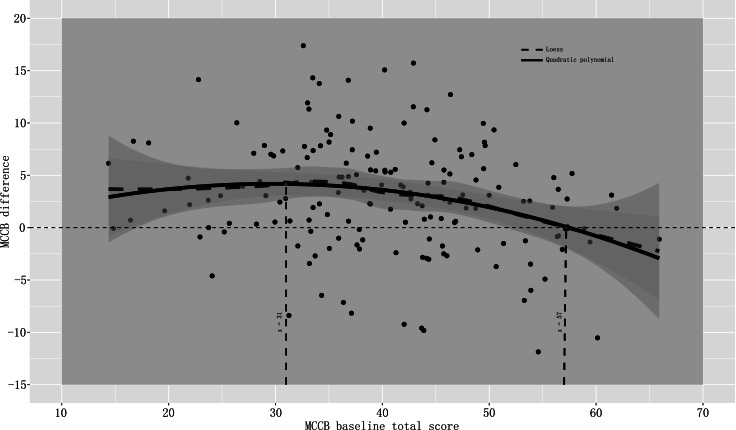
Figure 2 shows the inflection point of polynomial fitting for the baseline total score. Participants with an MCCB baseline total score around 31 seemed to receive the most cognitive improvement from CCRT; 57 was the intersection point with zero, indicating that the CCRT effect disappears when MCCB baseline total score was more than 57.
Fig. 2.
Effects of MCCB baseline total score on CCRT cognitive benefit. Note: The baseline total score 31 was the inflection point of polynomial fitting and represented the high point of effect; 57 was the intersection point with zero, and represented the no effect of CCRT on cognitive function.
Do younger and older participants show similar CCRT benefits?
There was no group by age interaction on age, education, antipsychotic agents or cognitive function at baseline among four age subgroups and no group by age interactions on the primary and secondary outcomes (online Supplementary Table S3). The GLM analyses assessed additional fixed effects for age group in exploratory analyses (see online Supplementary Table S4), and after removal of the non-significant interactions, we observed group by age interactions on Spatial Span (F1,271 = 8.46, p = 0.004), and Symbol Coding (F1,269 = 3.99, p = 0.047) with the relevant baseline scores as the covariates. Further analyses revealed that for the younger group, CCRT produced a significant benefit on Symbol Coding (F1,52 = 5.78, p = 0.020; estimated increase 3.71 points, 95% CI 0.61–6.81) at both post-treatment and follow-up but not for the older group (F1,208 = 0.07, p = 0.798; estimated increase 0.20 points, 95% CI −1.33 to 1.72). While, for the older group, CCRT produced a significant benefit on Spatial Span (F1,211 = 15.73, p < 0.001; estimated increase 5.04 points, 95% CI 2.53–7.54) at two time points but not for the younger group (F1,50 = 1.21, p = 0.277; estimated increase −2.03 points, 95% CI −5.74 to 1.68). In addition, there was a significant group by age by time interaction on MSCEIT (F1,219 = 5.42, p = 0.021). Simple effect analyses showed that for the older group CCRT produced a significant benefit on MSCEIT at post-treatment (F1,206 = 9.11, p = 0.003; estimated increase 3.6 points, 95% CI 1.28–6.1) but not at follow-up (F1,165 = 0.00, p = 0.989; estimated increase 0.02 points, 95% CI −2.90 to 2.94). However, for the younger group there was no effect of CCRT on MSCEIT at either time point (post-treatment F1,49 = 0.00, p = 0.947; estimated increase 0.2 points, 95% CI −5.76 to 6.15; follow-up F1,33 = 0.98, p = 0.330; estimated increase 3.67 points, 95% CI −3.88 to 11.22). There was no age effect on clinical symptoms or functional outcome benefits after CCRT treatment.
Discussion
Is CCRT effective?
To the best of our knowledge, this is the first randomized controlled study to explore the efficacy of CCRT treatment and the potential mechanism of improvement in a large sample of chronic and stable inpatients with a diagnosis of schizophrenia in China. As hypothesized, participants demonstrate a benefit of CCRT in the overall MCCB total score and specifically in four cognitive domains: working memory (Spatial Span Test, Digit Span Test), Verbal learning (HVLT-R), social cognition (MSCEIT) and executive function (WCST test). These results replicate other studies and meta-analyses and further suggest that CCRT can provide benefits to patients with cognitive difficulties (Grynszpan et al., 2011; Wykes et al., 2011). Unlike other studies there was no significant direct effect of cognitive remediation on any functional outcome (Wykes et al., 2007a, 2007b, 2011). There are several potential reasons for this lack of direct effect. We compared CCRT to a group who were receiving high levels of psychosocial activities and rehabilitation programs and these potentially have a positive effect on both cognition and functioning making it harder to see a signal, especially over the relatively short follow-up (Glicksohn and Cohen, 2000; Chen et al., 2016). A recent study (Buonocore et al., 2018) found that although cognition was stable over time following CR, sustained functional improvements required an additional 12 months of standard rehabilitation. A further explanation is that transfer to sustained functional outcome may require other supportive rehabilitation. We did, however, observe a significant direct effect of cognitive remediation on negative symptoms, which replicates previous studies (Bellucci et al., 2003; Twamley et al., 2012; Cella et al., 2017). CCRT has a relatively lack of face to face interaction with the therapist who can explicitly encourage ‘bridging’ strategies and provide social cues to improve participant's self-esteem, whereas the control group had a lot more group activity with interaction actually encouraged. In contrast to the benefit on negative symptoms, the Active control group improved more on the negative NOSIE factor. This is an informant measure of overt negative behaviors, rather than the, mostly, subjective report from the PANSS. The intensity of therapist contact and group interaction in the Active control group may have contributed to these improved negative behaviors. These are important results because a potential limitation of any computerized cognitive remediation is the lack of social and therapeutic contact. Other studies have highlighted the importance of the therapist and the therapeutic alliance drivers of cognitive and functional improvement (e.g. Rose et al., 2008; Huddy et al., 2012; Reeder et al., 2017; Cella and Wykes, 2019). In this study the CCRT therapist did not support the use of strategies, motivation or reinforcement. They also did not help to develop metacognition which is thought to be a key component for improving transfer from cognitive change to functional development (Wykes and Reeder, 2005; Cella et al., 2015). It is possible that limited opportunities to practice skills in the three inpatient settings were also a barrier to functional improvement.
Does cognitive improvement mediate functional benefit?
Previous reviews linked cognitive ability and functional outcome (Green et al., 2000; Reeder et al., 2004), so we hypothesized that CCRT might increase cognition and that these improvements contribute to improved functional capacity or symptoms. Consistent with this hypothesis, two cognitive change scores (working memory and executive functioning) respectively show a positive effect on symptoms (PANSS positive score) and functional capacity (UPSA), but these relationships were not specific to CCRT. This replicates a previous finding that executive functioning training rather than perceptual training led to improved functioning (Best et al., 2019). Our results are also consistent with findings from both Spaulding and Wykes who showed that executive tasks predicted improved social functioning and symptoms, regardless of whether or not participants had received cognitive remediation (Spaulding et al., 1999a, 1999b; Wykes et al., 1999, 2007a, 2007b; Reeder et al., 2006). The subtle effects of cognitive remediation therapeutic models have not been surfaced by this study despite larger numbers.
Does baseline cognition affect treatment benefit?
One of the important findings of this large study was the ability to assess whether and how baseline cognition might have an impact on treatment outcome. Participants with lower MCCB total score (<57 scores) at baseline are likely to benefit more from CCRT at post-treatment. This result is similar to a previous study in which poorer initial memory performances seemed to predict larger CR improvements (Pillet et al., 2015). Similarly Bell et al. discovered sustained effects of CR on work outcomes only in those individuals who entered the study with poorer levels of functioning (Bell et al., 2014). Although our result needs replication, it does suggest that with scarce health service resources, targeting those with lower baseline scores with the current version of CCRT would achieve the most benefit (see clinical implications).
Does age affects the cognitive benefits from CCRT?
The overall CCRT benefit (MCCB total score) was independent of age. But for some cognitive domains, the benefits varied between younger and older participants as in other studies. Younger participants improved on verbal processing speed, whereas older participants showed reduced positive symptoms and less decline in self-reported daily functioning. A similar differential pattern of improvements was found in another smaller study (Thomas et al., 2017). The advantage shown in the younger participants may be due to increased cognitive plasticity, especially in a basic cognitive function such as speed (Bürki et al., 2014). The larger cognitive effects on Spatial Span in the older group may be due to poor spatial working memory at baseline (as shown in online Supplementary Table S3), so the older participants have more room for improvement (Kern et al., 2008). The effects on emotion processing in the older group may be because, unlike basic cognitive functions, social cognitions such as emotional management in older patients improve with age (Kern et al., 2008).
There is evidence that CCRT facilitates benefits in both younger and older participants but in different domains. This makes the assessment of both moderators and mediators complex when samples have a wide age range and will require an even larger sample size to assess effects.
Clinical and development implications
The program improves cognition and negative symptoms but has limitations on functioning improvement. Currently the program does not offer many cognitive benefits if the baseline score is more than 57. With limited resources focusing on patients with an MCCB baseline total score around 31 who showed the most cognitive improvement from CCRT might be the most efficient way to use those resources. It is still unclear, however, whether the treatment should only be recommended if some cognitive areas show deterioration (despite the total score being above a T-score of 40). The study took part in hospitals with relatively little opportunity for transfer of cognitive benefit to functioning and the therapists were also not as involved throughout therapy. The next step may be to add transfer tasks to CCRT, to involve therapists in emphasizing metacognitive processes and considering providing further opportunities for developing transferable skills (see Reeder et al., 2018). In addition, CCRT could add more challenging tasks that might support cognitive benefit in those with better baseline cognitive function which might then boost cognition.
Strengths and limitations
This is the largest study of cognitive remediation. It replicated the beneficial cognitive effects which was particularly impressive as we included only those individuals who had a chronic course and experienced some cognitive deficits. But there was only a short follow-up so we may have underestimated the longer-term functioning benefits. Those improvements may develop slowly as opportunities to learn or relearn new skills are offered in the relatively restricted environment in the hospital. Further studies should include environmental supports that would allow the exploration of real life functioning. Age moderates CCRT benefit but only for individual cognitive tests rather than general cognitive function. Although this differential pattern has been found in other studies, the results need replication. Our sample did not include individuals living in the community which limits generalization. However, even in those with longer illness and poorer recovery trajectories, CCRT was beneficial and age was not a barrier to improvement.
Conclusions
To our knowledge, this is the largest cognitive remediation study which allowed us to explore mediation and moderation effects as well as efficacy. This form of cognitive remediation shows efficacy for cognition and negative symptoms, and evidence that those with poorer cognition show more cognitive improvement. Age was not a barrier to cognitive improvement although the cognitive domain benefits differed between age groups. Evidence that people with poorer cognition show more benefit needs further investigation as it is unclear whether achieving a large cognitive change is a barrier to functional improvement in this therapy. Identifying factors that influence cognitive benefit is the beginning of providing personalization and tailored care in clinical cognitive therapy applications.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31671145); Beijing Municipal Science & Technology Commission grant (Z141107002514016; D0906001000091; and D101107047810005); the Beijing Municipal Administration of Hospitals Clinical Medicine Development of special funding (XMLX201609 and zy201409); and Beijing Natural Science Foundation grant (No. 7162087). Professor Wykes acknowledges the support of the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London and support from her NIHR Senior Investigator Award. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Author ORCIDs
Til Wykes, 0000-0002-5881-8003
Conflict of interest
None.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291719001594.
click here to view supplementary material
References
- American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders, 4th Edn. Washington: American Psychiatric Association. [Google Scholar]
- Bell MD, Tsang HW, Greig T and Bryson G (2007) Cognitive predictors of symptom change for participants in vocational rehabilitation. Schizophrenia Research 96, 162–168. [DOI] [PubMed] [Google Scholar]
- Bell MD, Choi KH, Dyer C and Wexler BE (2014) Benefits of cognitive remediation and supported employment for schizophrenia patients with poor community functioning. Psychiatric Services 65, 469–475. [DOI] [PubMed] [Google Scholar]
- Bellucci DM, Glaberman K and Haslam N (2003) Computer-assisted cognitive rehabilitation reduces negative symptoms in the severely mentally ill. Schizophrenia Research 59, 225–232. [DOI] [PubMed] [Google Scholar]
- Best MW, Milanovic M, Iftene F and Bowie CR (2019) A randomized controlled trial of executive functioning training compared with perceptual training for schizophrenia Spectrum disorders: effects on neurophysiology, neurocognition, and functioning. American Journal of Psychiatry 176, 297–306. [DOI] [PubMed] [Google Scholar]
- Buonocore M, Spangaro M, Bechi M, Baraldi MA, Cocchi F, Guglielmino C, Bianchi L, Mastromatteo A, Bosia M and Cavallaro R (2018) Integrated cognitive remediation and standard rehabilitation therapy in patients of schizophrenia: persistence after 5 years. Schizophrenia Research 192, 335–339. [DOI] [PubMed] [Google Scholar]
- Bürki CN, Ludwig C, Chicherio C and de Ribaupierre A (2014) Individual differences in cognitive plasticity: an investigation of training curves in younger and older adults. Psychological Research 78, 821–835. [DOI] [PubMed] [Google Scholar]
- Caruso DR, Mayer JD and Salovey P (2002) Relation of an ability measure of emotional intelligence to personality. Journal of Personality Assessment 79, 306–320. [DOI] [PubMed] [Google Scholar]
- Cella M and Wykes T (2019) The nuts and bolts of cognitive remediation: exploring how different training components relate to cognitive and functional gains. Schizophrenia Research 203, 12–16. [DOI] [PubMed] [Google Scholar]
- Cella M, Reeder C and Wykes T (2014) It is all in the factors: effects of cognitive remediation on symptom dimensions. Schizophrenia Research 156, 60–62. [DOI] [PubMed] [Google Scholar]
- Cella M, Reeder C and Wykes T (2015) Lessons learnt? The importance of metacognition and its implications for Cognitive Remediation in schizophrenia. Frontiers in Psychology 6, 1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Preti A, Edwards C, Dow T and Wykes T (2017) Cognitive remediation for negative symptoms of schizophrenia: a network meta-analysis. Clinical Psychology Review 52, 43–51. [DOI] [PubMed] [Google Scholar]
- Chen MD, Kuo YH, Chang YC, Hsu ST, Kuo CC and Chang JJ (2016) Influences of aerobic dance on cognitive performance in adults with schizophrenia. Occupational Therapy International 23, 346–356. [DOI] [PubMed] [Google Scholar]
- Choi KH, Wykes T and Kurtz MM (2013) Adjunctive pharmacotherapy for cognitive deficits in schizophrenia: meta-analytical investigation of efficacy. The British Journal of Psychiatry 203, 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland WS and Devlin SJ (1988) Locally weighted regression: an approach to regression analysis by local fitting. Journal of the American Statistical Association 83, 596–610. [Google Scholar]
- Cui J-F, Zou Y-Z, Wang J, Chen N, Fan H-Z, Yao J and Duan J-H (2012) Reliability and validity of the UCSD Performance-based Skills Assessment-Brief. Chinese Mental Health Journal 26, 577–583. [Google Scholar]
- Dickinson D, Tenhula W, Morris S, Brown C, Peer J, Spencer K, Li L, Gold JM and Bellack AS (2010) A randomized, controlled trial of computer-assisted cognitive remediation for schizophrenia. American Journal of Psychiatry 167, 170–180. [DOI] [PubMed] [Google Scholar]
- Glicksohn J and Cohen Y (2000) Can music alleviate cognitive dysfunction in schizophrenia? Psychopathology 33, 43–47. [DOI] [PubMed] [Google Scholar]
- Gomar JJ, Valls E, Radua J, Mareca C, Tristany J, del Olmo F, Rebolleda-Gil C, Jañez-Álvarez M, de Álvaro FJ, Ovejero MR and Llorente A (2015) A multisite, randomized controlled clinical trial of computerized cognitive remediation therapy for schizophrenia. Schizophrenia Bulletin 41, 1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL and Mintz J (2000) Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the ‘right stuff’? Schizophrenia Bulletin 26, 119–136. [DOI] [PubMed] [Google Scholar]
- Grynszpan O, Perbal S, Pelissolo A, Fossati P, Jouvent R, Dubal S and Perez-Diaz F (2011) Efficacy and specificity of computer-assisted cognitive remediation in schizophrenia: a meta-analytical study. Psychological Medicine 41, 163–173. [DOI] [PubMed] [Google Scholar]
- Heaton R, Chelune G, Talley J, Kay G and Curtiss G (1993). Wisconsin Card Sorting Test Manual, Revised and Expanded. Florida: Psychological Resources. [Google Scholar]
- Honigfeld G, Gillis RD and Klett CJ (1966) NOSIE-30: a treatment-sensitive ward behavior scale. Psychological Reports 19, 180–182. [DOI] [PubMed] [Google Scholar]
- Huddy V, Reeder C, Kontis D, Wykes T and Stahl D (2012) The effect of working alliance on adherence and outcome in cognitive remediation therapy. The Journal of Nervous and Mental Disease 200, 614–619. [DOI] [PubMed] [Google Scholar]
- Johnson AT (1991) Curvefitting In Weitkunat R (ed.), Digital Biosignal Processing. New York: Elsevier Science Ltd, pp. 309–336. [Google Scholar]
- Kay SR (1990) Positive-negative symptom assessment in schizophrenia: psychometric issues and scale comparison. Psychiatric Quarterly 61, 163–178. [DOI] [PubMed] [Google Scholar]
- Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, Keefe RS, Mesholam-Gately R, Mintz J, Seidman LJ and Stover E (2008) The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. American Journal of Psychiatry 165, 214–220. [DOI] [PubMed] [Google Scholar]
- Kontis D, Huddy V, Reeder C, Landau S and Wykes T (2013) Effects of age and cognitive reserve on cognitive remediation therapy outcome in patients with schizophrenia. The American Journal of Geriatric Psychiatry 21, 218–230. [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Mueser KT, Thime WR, Corbera S and Wexler BE (2015) Social skills training and computer-assisted cognitive remediation in schizophrenia. Schizophrenia Research 162, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y (1987) Application of NOSIE in the study of neuroleptic treatment. Zhonghua shen jing jing shen ke za zhi-Chinese Journal of Neurology and Psychiatry 20, 325–327. [PubMed] [Google Scholar]
- McGurk SR and Mueser KT (2008) Response to cognitive rehabilitation in older versus younger persons with severe mental illness. American Journal of Psychiatric Rehabilitation 11, 90–105. [Google Scholar]
- Pillet B, Morvan Y, Todd A, Franck N, Duboc C, Grosz A, Launay C, Demily C, Gaillard R, Krebs MO and Amado I (2015) Cognitive remediation therapy (CRT) benefits more to patients with schizophrenia with low initial memory performances. Disability and Rehabilitation 37, 846–853. [DOI] [PubMed] [Google Scholar]
- Ramsay IS, Ma S, Fisher M, Loewy RL, Ragland JD, Niendam T, Carter CS and Vinogradov S (2018) Model selection and prediction of outcomes in recent onset schizophrenia patients who undergo cognitive training. Schizophrenia Research: Cognition 11, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rass O, Forsyth JK, Bolbecker AR, Hetrick WP, Breier A, Lysaker PH and O'Donnell BF (2012) Computer-assisted cognitive remediation for schizophrenia: a randomized single-blind pilot study. Schizophrenia Research 139, 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder C, Newton E, Frangou S and Wykes T (2004) Which executive skills should we target to affect social functioning and symptom change? A study of a cognitive remediation therapy program. Schizophrenia Bulletin 30, 87–100. [DOI] [PubMed] [Google Scholar]
- Reeder C, Smedley N, Butt K, Bogner D and Wykes T (2006) Cognitive predictors of social functioning improvements following cognitive remediation for schizophrenia. Schizophrenia Bulletin 32(suppl. 1), S123–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder C, Huddy V, Cella M, Taylor R, Greenwood K, Landau S and Wykes T (2017) A new generation computerised metacognitive cognitive remediation programme for schizophrenia (CIRCuiTS): a randomised controlled trial. Psychological Medicine 47, 2720–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose D, Wykes T, Farrier D, Doran AM, Sporle T and Bogner D (2008) What do clients think of cognitive remediation therapy?: a consumer-led investigation of satisfaction and side effects. American Journal of Psychiatric Rehabilitation 11, 181–204. [Google Scholar]
- Rosenberg M (1965) Society and the Adolescent Self-Image. New Jersey: Princeton University Press. [Google Scholar]
- Spaulding WD, Fleming SK, Reed D, Sullivan M, Storzbach D and Lam M (1999a) Cognitive functioning in schizophrenia: implications for psychiatric rehabilitation. Schizophrenia Bulletin 25, 275–289. [DOI] [PubMed] [Google Scholar]
- Spaulding WD, Reed D, Sullivan M, Richardson C and Weiler M (1999b) Effects of cognitive treatment in psychiatric rehabilitation. Schizophrenia Bulletin 25, 657–676. [DOI] [PubMed] [Google Scholar]
- Tan S and Liu D (2016) A review of the Chinese literature on cognitive remediation in psychosis. Asian Journal of Psychiatry 22, 129–134. [DOI] [PubMed] [Google Scholar]
- Thomas KR, Puig O and Twamley EW (2017) Age as a moderator of change following compensatory cognitive training in individuals with severe mental illnesses. Psychiatric Rehabilitation Journal 40, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tianmei S, Jianzhong Y and Liang S (2004) The reliability, validity of PANSS and its implication. Chinese Journal of Mental Health 18, 45–47. [Google Scholar]
- Twamley EW, Jeste DV and Bellack AS (2003) A review of cognitive training in Schizophrenia. Schizophrenia Bulletin 29, 359–382. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Vella L, Burton CZ, Heaton RK and Jeste DV (2012) Compensatory cognitive training for psychosis: effects in a randomized controlled trial. The Journal of Clinical Psychiatry 73, 1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang-Ping GH, Xu-Jiayu HJ and Chengjiang W (1998) Reliability and validity of self-esteem scale. Shandong Journal of Psychiatry 4, 31–32. [Google Scholar]
- Wykes T and Reeder C (2005) Cognitive Remediation Therapy for Schizophrenia: Theory and Practice. London: Routledge. [Google Scholar]
- Wykes T, Reeder C, Corner J, Williams C and Everitt B (1999) The effects of neurocognitive remediation on executive processing in patients with schizophrenia. Schizophrenia Bulletin 25, 291–307. [DOI] [PubMed] [Google Scholar]
- Wykes T, Newton E, Landau S, Rice C, Thompson N and Frangou S (2007a) Cognitive remediation therapy (CRT) for young early onset patients with schizophrenia: an exploratory randomized controlled trial. Schizophrenia Research 94, 221–230. [DOI] [PubMed] [Google Scholar]
- Wykes T, Reeder C, Landau S, Everitt B, Knapp M, Patel A and Romeo R (2007b) Cognitive remediation therapy in schizophrenia: randomised controlled trial. The British Journal of Psychiatry 190, 421–427. [DOI] [PubMed] [Google Scholar]
- Wykes T, Reeder C, Landau S, Matthiasson P, Haworth E and Hutchinson C (2009) Does age matter? Effects of cognitive rehabilitation across the age span. Schizophrenia Research 113, 252–258. [DOI] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR and Czobor P (2011) A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. American Journal of Psychiatry 168, 472–485. [DOI] [PubMed] [Google Scholar]
- Yanlin H and MingYuan Z (2000) The Chinese norm and factors analysis of PANSS. Chinese Journal of Clinical Psychology 82, 65–69. [Google Scholar]
- Zou YZ, Cui JF, Wang J, Chen N, Tan SP, Zhang D, Xu Z, Song SG, Wang YH, Li Y and Gao W (2009) Clinical reliability and validity of the Chinese version of measurement and treatment research to improve cognition in schizophrenia consensus cognitive battery. Chinese Journal of Psychiatry 42, 29–33. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291719001594.
click here to view supplementary material