Abstract
Gastric cancer is one of the most lethal cancers worldwide. FYN, a gene that is differentially expressed in gastric cancer, is considered a critical metastasis regulator in several solid tumors, but its role in gastric cancer is still unclear. This study aimed to evaluate the role of FYN and test whether FYN promotes migration and invasion of gastric cancer cells in vitro and in vivo via STAT3 signaling. FYN was overexpressed in gastric cancer and positively correlated with metastasis. FYN knockdown significantly decreased cancer cell migration and invasion, whereas FYN overexpression increased cancer migration and invasion. Genetic inhibition of FYN decreased the number of metastatic lung nodules in vivo. Several epithelial-mesenchymal transition markers were positively correlated with FYN expression, indicative of FYN involvement in this transition. Furthermore, gene set enrichment analysis of a Cancer Genome Atlas dataset revealed that the STAT3 signaling pathway was positively correlated with FYN expression. STAT3 inhibition reversed the FYN-mediated epithelial-mesenchymal transition and suppressed metastasis. In conclusion, FYN promotes gastric cancer metastasis possibly by activating STAT3-mediated epithelial mesenchymal transition and may be a novel therapeutic target for gastric cancer.
Keywords: FYN, Cell migration, Cell invasion, Therapeutic target
Abbreviations: GC, gastric cancer; GSEA, gene set enrichment analysis; siRNA, small interference RNA; OS, overall survival; DFS, disease free survival; EMT, epithelial mesenchymal transition; SFKs, Src family of protein tyrosine kinases
Introduction
Gastric cancer (GC) remains one of the most lethal cancers worldwide with over one million newly diagnosed cases and nearly 800,000 estimated deaths per year [1]. In China, GC is the second most common type of cancer and results in the death of approximately half of a million patients annually [2]. Surgery and chemotherapy remain the main treatment options for GC. In addition to surgery, targeted therapy and immunotherapy (i.e., trastuzumab, bevacizumab, and nivolumab) have been developed to tackle the disease. However, not all patients benefit from these new drugs [3]. Scientists concur that metastatic status is one of the most robust predictors of GC patient survival [4]. Therefore, assessing the mechanism underlying GC metastasis is essential for improving GC patient prognosis.
The Src kinase family is a family of non-receptor tyrosine kinases (SFKs) that consists of nine specific members: SRC, FGR, FYN, YES, BLK, HCK, LCK, LYN, and FRK. These kinases play pivotal roles in multiple signal transduction cascades that regulate cellular activities, such as proliferation, motility, and survival [5]. Aberrant activation of SFKs leading to an abnormal proliferation and malignant transformation of cells has been discovered in multiple cancers [6]. Findings of in-depth studies have sparked increased interest in FYN in the context of cancer. FYN is a 59-kDa protein-encoding gene that is implicated in cell growth, survival, cell motility, and adhesion [7,8]. Furthermore, FYN plays different roles in several cancers. Lee et al. have found that FYN establishes a positive feedback loop with STAT5 to promote breast cancer cell metastasis through NOTCH2 activation [9]. In other breast tumor xenograft models, researchers discovered that tumorigenesis induced by depletion of PTPN23 can be reversed by the suppression of FYN or through the Src inhibitor AZD0530 [10]. A study of pancreatic cancer also demonstrated that FYN inhibition promotes the phosphorylation and nuclear localization of hnRNP E1, which ultimately suppresses pancreatic cancer cell metastasis and invasion [11]. Research into glioblastoma has revealed that PIKE-A impairs the tumor suppressive actions of AMPK, which are mediated by FYN [12]. In hepatocellular carcinoma, FYN-mediated activation of the STAT3 pathway plays an important role in Fzd2-driven EMT and the migration of liver cancer cells [13]. Cumulatively, FYN clearly plays a part in cancer progression and metastasis, yet its role in GC remains unclear.
To investigate the mechanism of GC metastasis, we previously established a highly invasive GC cell line (BGC-823M3) using a repeated Transwell approach [14,15]. Afterward, control and BGC-823M3 cell lines were analyzed by gene expression microarray and several differentially expressed genes were identified. FYN was recognized as one of the most differentially expressed genes, which indicated that FYN might play a crucial role in GC metastasis. In the current study, we evaluated whether FYN could promote the migration and invasion of GC cells via the STAT3 signaling pathway. The current findings provide evidence for a potential new target for GC treatment.
Materials and methods
Patients and clinical specimens
One hundred clinical GC specimens were collected from the tissue bank of the First Affiliated Hospital of Sun Yat-sen University (China). All enrolled patients had been pathologically diagnosed as having GC and had undergone radical D2 lymphadenectomy between 2007 and 2012. None of the patients had undergone neoadjuvant chemotherapy prior to surgery, and all patients were diagnosed according to the 7th edition guidelines of the Union for International Cancer Control. All patients underwent a follow-up of at least 60 months. This study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University (Permit number: 2013-114). Written consent from all patients was obtained before the research began.
Cell lines and lentiviral transfection
Human GC cell lines (MGC-803, SGC7901, and SNU-216) were purchased from the Chinese Academy of Sciences (Shanghai, China) and were cultured in RPMI (Gibco™,Carlsbad, CA, USA) medium containing 10% fetal bovine serum (Gibco™, Carlsbad, CA, USA) at 37 °C in a humidified atmosphere of 5% CO2. Cell transfection was performed in a 6-well plate and Lipo 3000 (Invitrogen, Carlsbad, CA, USA) was used according to manufacturer's instructions. Specific siRNA against FYN was designed (RiboBio, Guangzhou, China) (FYN#1: 5′-GAGACCATGTCAAACATTA-3′; FYN#2: 5′-GTGAACTCTTCGTCTCATA-3′). The full-length cDNA of FYN was subcloned into a pcDNA3.1 vector (Vigene Biosciences, Rockville, MD, USA). In order to establish stable FYN-knockdown cells, the MGC-803 cell line was transfected with shRNA against FYN. Puromycin (2 μg/ml) was utilized to select stably transduced cells. The STAT3 selective inhibitor HO-3868 was purchased from Selleck (Sellcek, Houston, TX, USA).
Animal experiments
All animal experiments were performed according to the guidelines of First Affiliated Hospital of Sun Yat-sen University. BALB/c-nu mice (female, 4 weeks old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. and maintained in an SPF room. A pulmonary metastasis model was established to explore the role of FYN in GC metastasis. We injected MGC-803shFYN and MGC-803shCtrl (2.0 × 107 cells) into nude mice through the tail vein. All mice were euthanized after 4 weeks of feeding, and their lungs were harvested and studied through hematoxylin and eosin (H&E) staining. This study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University (No. [2018]087).
Immunoblot analysis
Cell lysates were harvested as previously described [16]. Cytoplasmic protein was extracted using the P0013G extraction kit (Beyotime, Shanghai, China), according to manufacturer's protocol. Protein extracts were isolated through SDS-PAGE gel electrophoresis and were then transferred to a PVDF membrane. Membranes were incubated with primary antibodies against FYN, CDH1, VIM, SNAI1, SNAI2, phosphorylated-STAT3, and STAT3 (Cell Signaling Technology, Danvers, MA; USA, 1:1000). Membranes were also incubated with a primary antibody against GAPDH (Sigma-Aldrich, St Louis, MO, USA, 1:2000). Images were obtained using the Image Lab Software (Bio-Rad, Hercules, CA, USA).
Immunohistochemistry and immunofluorescence analysis
All 100 paraffin-embedded GC tissue samples were examined by the streptavidin-peroxidase method, as previously described [16]. Tissue sections were incubated with anti-FYN primary antibody (1:100, Abcam, Cambridge, United Kingdom) overnight at 4 °C. The scoring system was established as previously described [15]. High FYN expression and low FYN expression was determined according to the scoring system. For immunofluorescence staining, GC cells were incubated with primary antibodies against CDH1, VIM and phosphorylated-STAT3 at 4 °C overnight. Culture dishes were washed with PBS and then incubated with secondary antibodies (1:500) for 1 h. Nuclei were stained with DAPI (KeyGEN BioTECH, Nanjing, China) for 5 min. Images were acquired using a BX61 fluorescence microscope (Olympus, Shinjuku, Tokyo, Japan).
Migration and invasion assay
The cell migration assay was performed in a 24-well plate with an 8.0-μm pore polycarbonate filter (Corning, Corning, MA, USA). The upper chamber was seeded with serum-starved MGC-803, SNU-216, and SGC-7901 cells (5 × 104 cells/well), pretreated with siRNA or FYN plasmid, overnight. The lower chamber was covered with 10% FBS RPMI-1640. After 24 h of incubation, non-metastatic cells were gently removed by a cotton swab. Migrated cells were fixed using methanol and stained with crystal violet (Beyotime, Nantong, China). Migrated cells were counted, and images were captured by BX61 fluorescence microscope (Olympus, Shinjuku, Tokyo, Japan). The invasion assay was performed in a similar manner, except the filters were pre-coated with Matrigel (Corning, Corning, MA, USA).
Wound scratch assay
Cells were seeded into a 6-well plate, and a wound was scratched in the middle using a 100 μl pipette tip, at a confluency of 90%. Afterwards, the chamber was washed three times with PBS to remove non-adherent cells. After 24 h of culture, the scratch wound was captured at three randomly selected fields. Photoshop (Adobe, San Jose, CA, USA) was used to quantify the open area of the wound.
Analysis of the prognostic value of FYN
We utilized the K-M Plotter database to determine the prognostic value of FYN [17]. OS and DFS curves were generated based on the differential expression of FYN. High FYN expression was defined as mRNA expression higher than the median. Low FYN expression was defined as mRNA expression lower than the median. P < 0.05 was considered statistically significant.
Gene set enrichment analysis
Normalized TCGA-STAD RNA-seq data (level 3) and clinical data were downloaded from the GDC Data Portal of National Cancer Institute, NIH (https://portal.gdc.cancer.gov/). Maximally Selected Rank Statistics was applied to determine the cutoff expression value of FYN. The expression data of FYN was transformed by log2 and analyzed by maxstat package in R. The cutoff value is 10.18747. Samples with values larger than 10.18747 were defined as FYN-high, and those less than 10.18747 were defined as FYN-low. All analyses were performed in R (v3.6.3). Patients were stratified into a FYN-low and FYN-high group. Gene set enrichment analysis (GSEA) was conducted using the GSEA software (v4.0.1). Gene sets were downloaded from the MSigDB database and analyzed according to the user guide (https://www.gsea-msigdb.org/gsea/index.jsp).
Statistical analysis
All data were analyzed by SPSS 22.0 (SPSS Inc., Chicago, IL, USA) and the GraphPad Prism software 5.0 (GraphPad Software, CA, USA). The Student's t-test was used to assess differences between groups. The chi-squared test was applied to analyze the relationship between FYN expression and clinicopathological characteristics. The Kaplan-Meier method was adopted to generate overall survival curves, and the log-rank test was applied to assess the difference between different groups. Univariate and multivariate analyses were based on a COX proportional hazards model. P < 0.05 was considered statistically significant.
Results
Higher FYN expression is correlated with lymph node metastasis and poorer GC patient prognosis
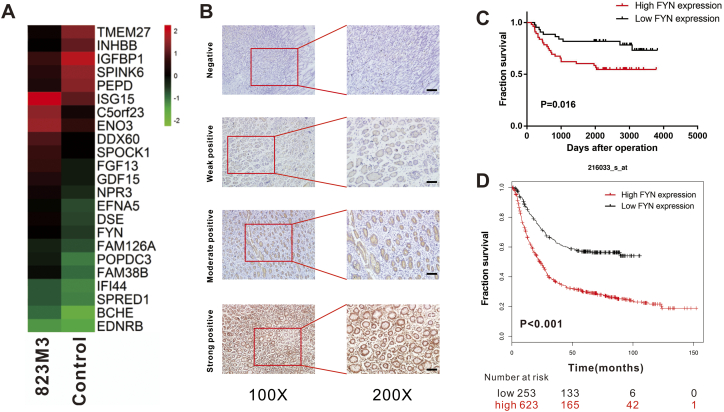
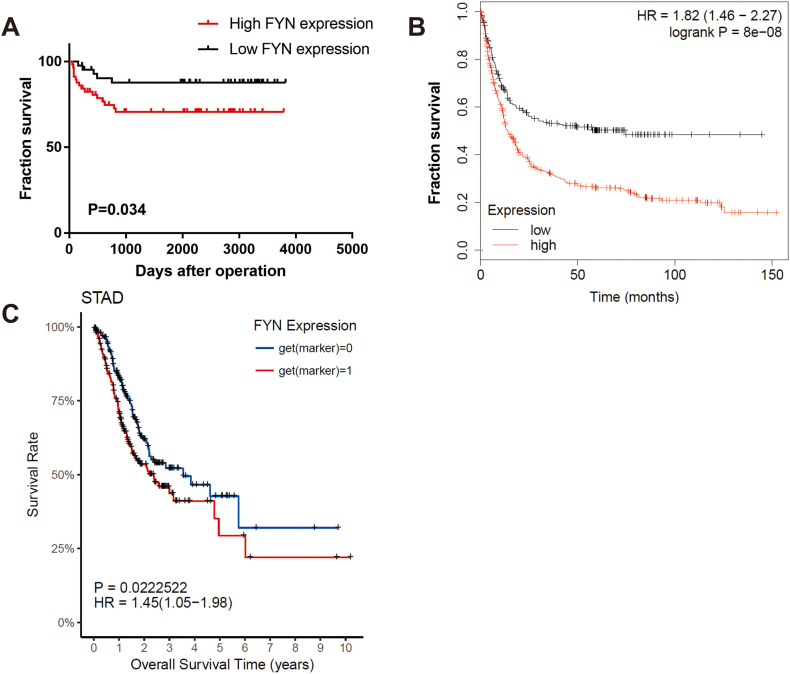
To investigate the mechanism of GC metastasis, we used a microarray assay to compare differentially expressed genes between control cell line and the highly invasive GC cell line, which was previously established [15]. The results revealed that FYN was one of the differentially expressed genes between the two groups (Fig. 1A). Although FYN displayed moderate increase, but our preliminary data showed that FYN did play a vital role in gastric cancer metastasis and we decided to take FYN forward for further investigation. To validate the importance of FYN in GC development, we evaluated FYN expression in clinical GC samples (Fig. 1B). The data from 100 GC patient samples collected, between January 2009 and December 2012 with at least 5 years of follow-up, are presented in Table 1. Immunohistochemistry staining indicated elevated FYN expression in 56% of patients and high FYN expression was correlated with increased lymph node metastasis (P = 0.022) (Table 2). We found that high FYN expression was associated with shorter overall survival (Fig. 1C and Fig. S1C). Meanwhile, the K-M Plotter database also demonstrated that FYN was positively correlated with a poorer prognosis in GC (Fig. 1D). Kaplan-Meier analysis confirmed the observation that high FYN expression was associated with shorter disease-free survival (Fig. S1A and B). Both univariate and multivariate analysis indicated that FYN expression was an independent risk factor for GC (HR: 2.153, 95% CI: 1.018–4.558, P = 0.045; Table 3, Table 4). These data indicated that FYN could serve as a prognostic biomarker in GC patients.
Fig. 1.
FYN was upregulated in a highly invasive gastric cancer (GC) cell line and was correlated with poor GC patient prognosis. A. Microarray analysis of the MGC-823M3 and control cell lines. B. Representative images of immunohistochemistry analysis of FYN in 100 gastric cancer tissue samples (scale bars, 50 μm). C. Kaplan-Meier overall survival curves for GC patients with different FYN expression from the First Affiliated Hospital of Sun Yat-sen University database. D. Overall survival data for GC patients with different FYN expression in the K-M Plotter database.
Table 1.
Baseline characteristics in gastric cancer patients.
| Characteristics | Total, n(%) |
|---|---|
| Age | |
| <60 years | 50(50) |
| ≥60 | 50(50) |
| Gender | |
| Male | 67(67) |
| Female | 33(33) |
| Location | |
| Upper third | 28(28) |
| Middle third | 33(33) |
| Lower third | 36(36) |
| Whole | 3(3) |
| Maximal tumor diameter | |
| <5 cm | 65(65) |
| ≥5 cm | 35(35) |
| pT | |
| T1–2 | 32(32) |
| T3–4 | 68(68) |
| pN | |
| No | 44(44) |
| Yes | 56(56) |
| TNM staging | |
| I/II | 54(54) |
| III/IV | 46(46) |
| Distant metastasis | |
| Negative | 92(92) |
| Positive | 8(8) |
| Histology type | |
| Well | 5(5) |
| Moderately | 36(36) |
| Poorly | 59(59) |
| Borrmann | |
| I | 6(6) |
| II | 27(27) |
| III | 57(57) |
| IV | 7(7) |
| V | 3(3) |
TNM, tumor-node-metastasis stage; pT, depth of invasion; pN, lymph node status.
Table 2.
The correlation between FYN and clinicopathologic characteristics.
| FYN expression |
|||
|---|---|---|---|
| Characteristics | Low | High | P-value |
| Age | |||
| <60 years | 23 | 27 | 0.687 |
| ≥60 | 21 | 29 | |
| Gender | |||
| Male | 30 | 37 | 0.824 |
| Female | 14 | 19 | |
| Location | |||
| Upper third | 12 | 16 | 0.862 |
| Middle third | 15 | 18 | |
| Lower third | 15 | 21 | |
| Whole | 2 | 1 | |
| Maximal tumor diameter | |||
| <5 cm | 31 | 34 | 0.311 |
| ≥5 cm | 13 | 22 | |
| pT | |||
| T1–2 | 18 | 14 | 0.090 |
| T3–4 | 26 | 42 | |
| pN | |||
| N0 + N1 | 25 | 19 | 0.022 |
| N2 + N3 | 18 | 37 | |
| TNM staging | |||
| I/II | 27 | 27 | 0.190 |
| III/IV | 17 | 29 | |
| Distant metastasis | |||
| Negative | 39 | 53 | 0.272 |
| Positive | 5 | 3 | |
| Histology type | |||
| Well | 3 | 2 | 0.626 |
| Moderately | 17 | 19 | |
| Poorly | 24 | 35 | |
| Borrmann | |||
| I | 3 | 3 | 0.552 |
| II | 13 | 15 | |
| III | 24 | 32 | |
| IV | 4 | 3 | |
| V | 0 | 3 | |
The P-value in bold font means P-value <0.05.
Fig. S1.
FYN was correlated with poorer gastric cancer (GC) patient outcomes in different databases. A. Kaplan-Meier disease-free survival curves for GC patients with different FYN expression in the First Affiliated Hospital of Sun Yat-sen University database. The P-value was determined by the log-rank test. B. DFS data for GC patients with different FYN expression from the K-M Plotter database. C. Overall survival data for GC patients with different FYN expression from the TCGA database.
Table 3.
Univariate survival analysis.
| Univariate analysis | |||
|---|---|---|---|
| Overall survival |
|||
| Variables | HR | 95% CI | P-value |
| Age | |||
| <60 years vs. ≥60 years | 0.471–1.773 | 0.914 | 0.789 |
| Gender | |||
| Male vs. female | 0.553–2.236 | 1.112 | 0.766 |
| Tumor size | |||
| <5 cm vs. ≥5 cm | 1.009–3812 | 1.961 | 0.047 |
| T classification | |||
| T1.2 vs. T3.4 | 1.893–20.216 | 6.186 | 0.003 |
| TNM | |||
| I/II vs. III/IV | 1.375–5.573 | 1.375 | 0.004 |
| pN | |||
| N0.1 vs. N2.3 | 2.550–9.984 | 5.046 | <0.001 |
| Distant metastasis | |||
| Yes vs. no | 0.154–2.677 | 0.642 | 0.543 |
| FYN expression | |||
| High vs. low | 1.148–5.011 | 2.398 | 0.020 |
HR, hazard ratio; CI, confidence interval; TNM, tumor-node-metastasis stage; T classification, depth of tumor invasion.
Table 4.
Multivariate survival analysis.
| Multivariate analysis | |||
|---|---|---|---|
| Overall survival |
|||
| Variables | HR | 95% CI | P-value |
| Tumor size | |||
| <5 cm vs. ≥5 cm | 1.155 | 0.582–2.289 | 0.680 |
| T classification | |||
| T1.2 vs. T3.4 | 3.653 | 0.970–13.752 | 0.055 |
| TNM | |||
| I/II vs. III/IV | 0.795 | 0.331–1.913 | 0.609 |
| pN | |||
| N0.1 vs. N2.3 | 4.100 | 1.815–9.262 | 0.001 |
| FYN expression | |||
| High vs. low | 2.153 | 1.018–4.558 | 0.045 |
HR, hazard ratio; CI, confidence interval.
FYN enhances GC cell migration and invasion
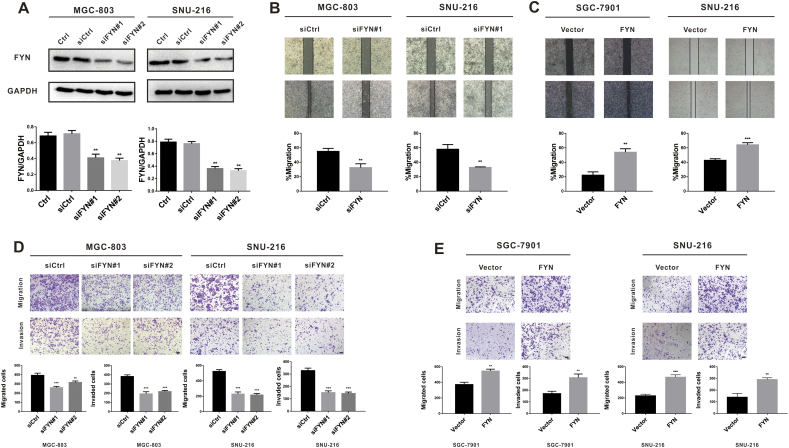
To further examine the role of FYN in GC cell migration and invasion, we performed the Western-blot to detect the basal expression of FYN in several gastric cancer cell lines and discovered that FYN was highly expressed in MGC-803 cell line. The SNU-216 cell line has moderate expression of FYN and SGC-7901 has the lowest expression of FYN. So we chose to knock down the expression of FYN in MGC-803 and SNU-216 cell lines and overexpressed FYN in SGC-7901 and SNU-216 cell lines. The expression of FYN was knocked down in both MGC-803 and SNU-216 cell lines. (Fig. 2A). The wound scratch assay indicated that the FYN knockdown significantly impaired the wound healing ability of both MGC-803 and SNU-216 cells (Fig. 2B). Furthermore, FYN overexpression enhanced wound healing capacity compared to the control groups in both SGC-7901 and SNU-216 cells (Fig. 2C). The Transwell assay results (performed to further examine the role of FYN in the promotion of migration and invasion in GC cell lines) demonstrated that knocking down FYN expression remarkably inhibited GC cell migration and invasion to the lower chamber (Fig. 2D). Similarly, FYN overexpression enhanced the migration and invasion of both GC cell lines (Fig. 2E). Taken together, these data indicated that FYN enhanced GC migration and invasion.
Fig. 2.
FYN was correlated with gastric cancer cell migration and invasion. A. Western blot analysis of FYN after siRNA knockdown. B. After transfection with siRNA against FYN, wound width was greater than in the controls. C. After FYN overexpression, wound width was smaller compared to controls. D. After transfection with siRNA targeting FYN, migration and invasion were significantly inhibited in both MGC-803 and SNU-216 cell lines. E. After FYN overexpression, cell migration and invasion were significantly enhanced in both SGC-7901 and SNU-216 cell lines. The scale bars indicate 100 μm. *P < 0.05, **P < 0.01, ***P < 0.001. The data are represented as mean ± SD.
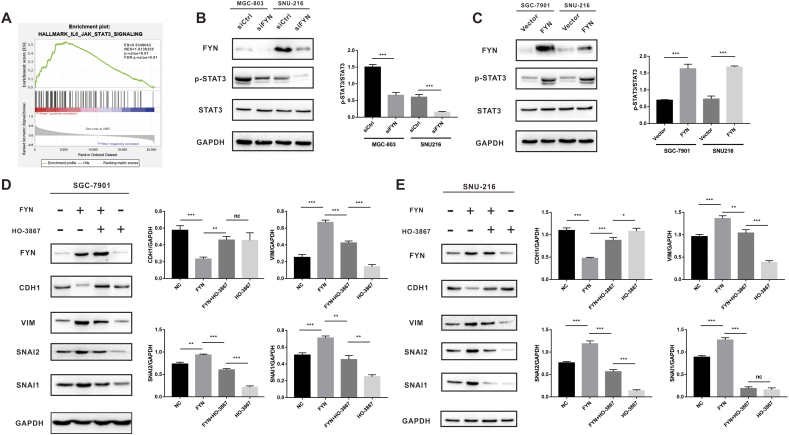
FYN promotes GC cell line migration by inducing EMT
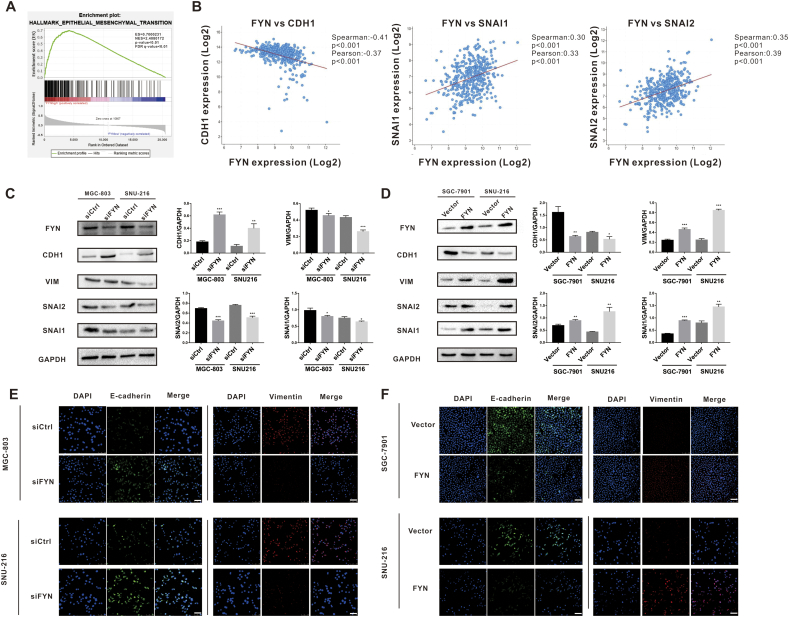
EMT plays a vital role in cancer cell metastasis. Therefore, we explored the possibility of EMT involvement in FYN-mediated metastasis. GSEA analysis of The Cancer Genome Atlas (TCGA) data indicated that FYN was indeed correlated with EMT markers such as SNAI1 and SNAI2 (Fig. 3A). Furthermore, the correlation analysis revealed that FYN was positively correlated with SNAI1 and SNAI2 and negatively correlated with CDH1 (Fig. 3B). After knocking down FYN expression, we found that the expression of CDH1 was upregulated, whereas VIM, SNAI1, and SNAI2 were down-regulated (Fig. 3C). Furthermore, FYN overexpression induced EMT transition (Fig. 3D). Immunofluorescence staining further confirmed that FYN was positively correlated with VIM expression and negatively correlated with CDH1 expression in all three GC cell lines (Fig. 3E and F). These results showed that FYN promotes the migration of GC cell lines by inducing EMT.
Fig. 3.
FYN was associated with EMT markers. A. GSEA analysis of the TCGA database indicated that FYN was associated with EMT markers. B. FYN was negatively correlated with CDH1 and positively correlated with SNAI1 and SNAI2. C. Western blot analysis of EMT marker protein levels after FYN knockdown in both MGC-803 and SNU-216 cell lines. D. Western blot analysis of EMT marker protein levels after FYN overexpression. E. Immunofluorescence staining of CDH1 and VIM after FYN knockdown in both MGC-803 and SNU-216 cell lines. F. Immunofluorescence staining of CDH1 and VIM after FYN overexpression. *P < 0.05, **P < 0.01, ***P < 0.001.
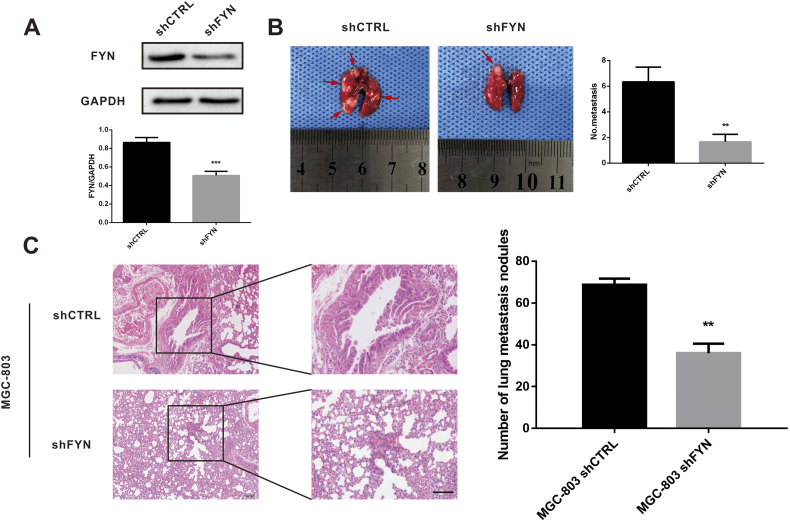
FYN promotes GC metastasis in vivo
To investigate the role of FYN in GC metastasis in vivo, we created the MGC803-shControl (shCTRL) and MGC803-shFYN (shFYN) stable cell lines through lentiviral transfection, and knockdown efficacy was confirmed by immunoblotting (Fig. 4A). The lungs of pulmonary metastasis model nude mice, injected with stable FYN-knockdown cells or control cells, were collected after 4 weeks and stained with H&E. The shFYN group had fewer lung metastatic nodules than the shCTRL group, as observed macroscopically (P < 0.001) (Fig. 4B) and microscopically (P < 0.001) (Fig. 4C). This confirmed that FYN promoted GC lung metastasis in vivo.
Fig. 4.
FYN promoted pulmonary gastric cancer metastasis in vivo. A. Successful FYN shRNA knockdown in MGC-803 cells was confirmed by western blot. B. Fewer lung metastatic nodules were found after FYN knockdown compared to the control group. C. Representative images of lung metastatic nodules. **P < 0.01, ***P < 0.001. The data are represented as mean ± SD.
FYN promotes GC cell EMT through STAT3 pathway activation
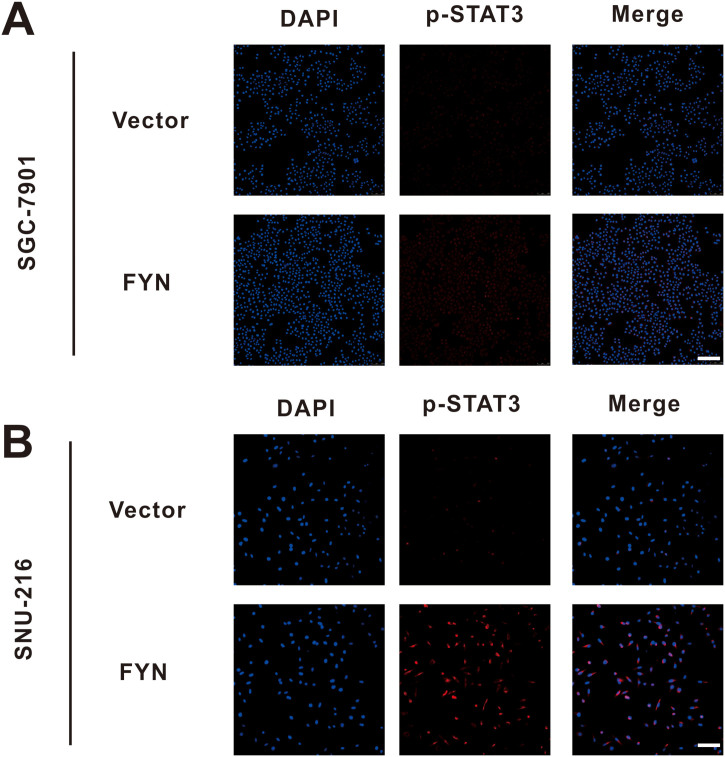
To explore the mechanism of FYN-mediated GC metastasis, we performed GSEA analysis with data from the TCGA database. The results revealed that FYN was positively correlated with STAT3 signaling pathway activation in GC. This result indicated that the STAT3 signaling pathway may play a role in FYN-mediated metastasis in GC (Fig. 5A). As shown in Fig. 5B, FYN knockdown significantly reduced the level of phospho-STAT3 (Fig. 5B), and FYN overexpression increased the level of phospho-STAT3 (Fig. 5C and Fig. S2). To further demonstrate FYN involvement in the EMT process via STAT3 pathway activation, we applied STAT3 selective inhibitor HO-3867. As previously demonstrated, FYN overexpression elevated the expression of mesenchymal markers and reduced the expression of the epithelial marker. The addition of HO-3867 significantly reduced the expression of VIM, SNAI2, and SNAI1 while upregulating CDH1 expression (Fig. 5D and E).
Fig. 5.
FYN promoted gastric cancer cell epithelial-mesenchymal transition through the STAT3 pathway. A. GSEA analysis indicated that FYN was correlated with the STAT3 signaling pathway. B. FYN knockdown inhibited p-STAT3 protein expression as revealed by western blot. C. FYN overexpression elevated p-STAT3 protein expression as revealed by western blot. HO-3867 effectively decreased the levels of VIM, SNAI2, and SNAI1 and increased the expression level of CDH1 as shown by western blot in both SGC-7901 (D) and SNU-216 (E) cell lines. *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. S2.
Immunofluorescence staining of p-STAT3 after FYN overexpression in both SGC-7901 and SNU-216 cell lines. A. After FYN overexpression, p-STAT3 levels were increased in the SGC-7901 cell line. B. After FYN overexpression, p-STAT3 levels were increased in the SNU-216 cell line.
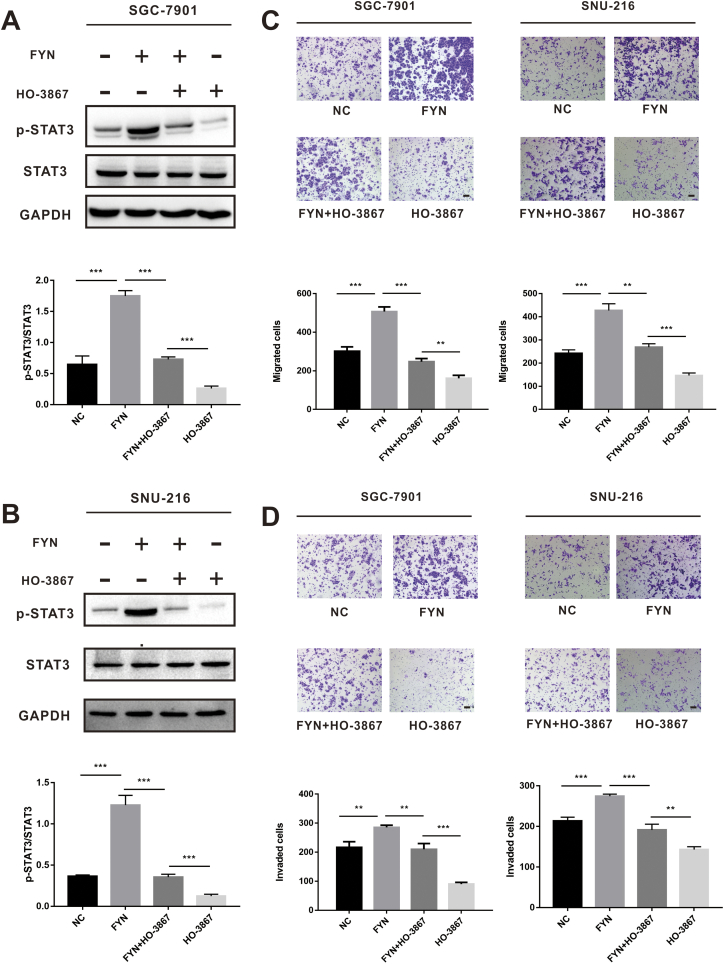
Inhibiting the STAT3 pathway could suppress FYN-mediated GC metastasis
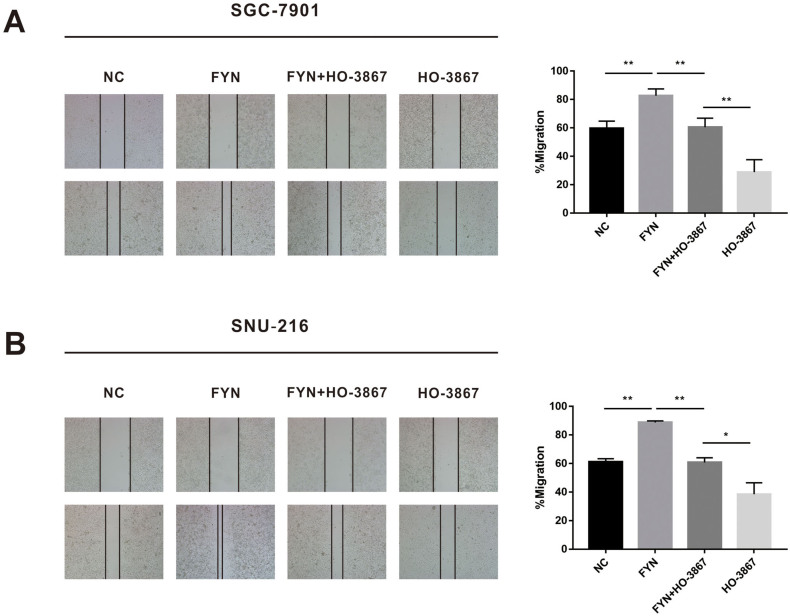
Given the capacity of the STAT3 inhibitor to suppress EMT transition, we speculated that STAT3 pathway inhibition could also limit FYN-mediated GC metastasis. SGC-7901 and SNU-216 cells, with or without FYN overexpression, were treated with or without HO-3867 before analysis by western blot. Results demonstrated that HO-3867 could reduce the expression of FYN-upregulated phospho-STAT3 (Fig. 6A and B). HO-3867 partially inhibited the migration and invasion capacity of FYN-overexpressing cells (Fig. 6C and D), and the wound healing assay demonstrated similar results (Fig. S3). Taken together, these data indicated that FYN promotes gastric cancer metastasis possibly by activating STAT3-mediated epithelial mesenchymal transition.
Fig. 6.
FYN promoted gastric cancer cell metastasis through the STAT3 pathway. A. HO-3867 effectively decreased the p-STAT3 levels in the SGC-7901 cell line. B. O-3867 effectively decreased the p-STAT3 levels in the SNU-216 cell line. C. With or without HO-3867 treatment, the number of migrating cells in the FYN overexpression group was increased compared to the control group. D. With or without HO-3867 treatment, the number of invading cells in the FYN overexpression group was increased compared to the control group. *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. S3.
FYN promoted gastric cancer cell migration through the STAT3 pathway. A. In SGC-7901 cells treated with/without HO-3867, the migration rate in the FYN overexpression group was increased compared to the control group. B. In SNU-216 cells treated with/without HO-3867, the migration rate in the FYN overexpression group was increased compared to the control group. *P < 0.05, **P < 0.01.
Discussion
It has been well established GC treatment is hampered by metastasis. However, the mechanism of GC metastasis remains unclear [18]. Previous research found that FYN was correlated with metastasis in several malignant cancers, including breast cancer, pancreatic cancer, cholangiocarcinoma, and esophageal squamous cell carcinoma [9,11,19,20]. In the current study, we found that FYN was essential for GC cell migration and invasion.
We showed that high FYN expression was associated with advanced N stage and correlated with poor prognosis in GC patients. In vitro experiments demonstrated that FYN knockdown significantly reduced the migration and invasion capacity of MGC-803 and SNU-216 cells, while FYN overexpression promoted the migration and invasion of SGC-7901 and SNU-216 cells. These observations suggested that FYN was correlated with metastasis in GC. EMT is a process during which cells lose their epithelial features and gain mesenchymal characteristics. The EMT is considered a central reason for cancer metastasis [21,22]. Previous research on colorectal cancer (CRC) has indicated that the STAT3 signaling pathway is involved in the EMT process [23]. Bioinformatics analysis of data from the TCGA database indicated that FYN was positively correlated with SNAI1 and SNAI2 and negatively correlated with CDH1. Further, immunoblot experiments revealed that elevated VIM, SNAI2, and SNAI1 expression was observed after FYN overexpression. This finding was in accordance with previous observations in breast cancer research [9]. In vivo experiments also confirmed that FYN could promote GC cell metastasis to the lungs. Our findings demonstrated that FYN played a vital role in GC metastasis.
To further explore the metastatic mechanism, we conducted bioinformatics analysis and found that the STAT3 signaling pathway might be involved in FYN-mediated metastasis (Fig. 5A). The STAT3 signaling pathway, which is involved in several cellular process including proliferation, survival, invasion, and metastasis, has been intensively researched [24]. Classical STAT3 pathway activation occurs by means of the JAK enzyme. Phosphorylation at Tyr705 of STAT3 leads to STAT3 protein dimerization and dimer translocation to the nucleus. Once in the nucleus, STAT3 promotes the expression of its target genes. SRC, a member of the Src kinase family, has been shown to directly activate STAT3 [25]. FYN (an SRK member) has been reported to interact with STAT3. Researchers have also reported that the FYN-STAT3 axis is crucial in renal and pulmonary fibrosis [[26], [27], [28]]. The current study confirmed that FYN influenced phospho-STAT3 expression using knockdown and overexpression models. Treatment with HO-3867 (a specific STAT3 inhibitor) further confirmed that FYN regulated cancer cell EMT through the STAT3 pathway in both SGC-7901 and SNU-215 cell lines. Transwell assays confirmed that HO-3867 partially counteracted the FYN-driven migratory and invasive capacity, indicating that FYN enhanced the metastatic ability of GC cells at least partially through the STAT3 pathway.
FYN is a member of the Src kinase family and is considered an essential factor for cancer progression and metastasis. Further, researchers have established the involvement of FYN in chemotherapy resistance. Mi et al. found that miR-381 directly targets and downregulates FYN, while also enhancing the chemosensitivity of breast cancer cells to doxorubicin [29]. In chronic myelogenous leukemia (CML), researchers identified FYN as a candidate biomarker for imatinib resistance through pan-genomic microarrays [30]. Several other Src kinase family members have also been associated with cancer progression, metastasis, and drug resistance. SRC is another extensively studied gene belonging to the SRC kinase family. Researchers found that hypoxia could induce prostate cancer cell metastasis in an SRC-dependent manner. Further, SRC knockdown successfully prevented the hypoxia-induced effects [31]. Another in vivo experiment supports evidence for the role of SRC in prostate cancer metastasis [32]. SRC has also been shown to meditate thyroid cancer metastasis. Chan et al. discovered that SRC is overexpressed in thyroid cancer, and the application of its inhibitor, dasatinib, significantly inhibits tumor growth and metastasis [33]. Previously, we found that CXCL1 and CXCL5 were closely correlated to cancer metastasis [15,34], and it has been reported that SRC is related to these chemokines. Lu et al. found that the CXCL1-LCN2 paracrine axis can activate SRC to promote prostate cancer progression [35]. Like SRC, LYN is a molecule that has drawn significant attention. Co-immunoprecipitation and immunofluorescence assays have revealed that LYN is involved in CD24-mediated ERK1/2 activation and tumor metastasis in CRC [36]. In head and neck squamous cell carcinomas (HNSCC), selective siRNA targeting of LYN inhibits the proliferation, migration, and invasion capacity of EGF receptor variant III-expressing HNSCC cells [37]. LYN involvement is not limited to cancer cells as LYN expression has been reported to be positively correlated with myeloid-derived suppressor cell (MDSC) markers, suggesting that LYN possibly participates in MDSC aggregation [38]. Lung cancer reports have described that genetic depletion of YES1 significantly inhibits tumor growth and metastasis, and high YES1 expression may predict a higher sensitivity to dasatinib [39]. Furthermore, a study of 1094 colorectal patients revealed that a combined FGR + HCK score could predict poor CRC patient outcome [40].
The current study provided a comprehensive analysis of how FYN regulates GC metastasis by STAT3 pathway activation. Through the use of clinical samples and in vitro and in vivo experiments, we revealed a novel role of FYN in tumor metastasis. Our study confirmed that FYN was an independent indicator of GC patients' prognosis. This means that the expression of FYN might be able to predict the survival outcome in clinical cases. And we also discovered that FYN promotes the metastasis of gastric cancer and this finding might be able to incentivize the development potential drugs that specifically target FYN. In further research, the relationship between STAT3 and FYN can be explored in greater detail, and the existence of a STAT3 and FYN positive feedback loop can be investigated.
The following are the supplementary data related to this article.
CRediT author statement
Jie Yu: Conceptualization, Methodology, Software, Investigation, Data Curation, Writing - Original Draft; Zhijun Zhou: Software, Investigation, Formal analysis, Data Curation, Visualization, Writing - Original Draft; Zhewei Wei: Investigation, Writing - Review & Editing; Jing Wu: Validation; Jun Ouyang: Validation; Weibin Huang: Validation; Yulong He: Supervision, Project administration, Funding acquisition; Changhua Zhang: Writing - Review & Editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors would like to acknowledge the assistance of Donglian Chen in maintaining our database and thank Dr. Sijia Liang for his guidance, as well as Dr. Xiaoshuang Xu for her support.
Funding
This study was supported by the National Natural Science Foundation of China [grant numbers 30700805, 81272637, 81272643, and 81702325], the ‘3 & 3’ project of The First Affiliated Hospital of Sun Yat-sen University, Natural Science Foundation of Guangdong Province [grant number 2017A030310565], and the Sanming Project of Medicine in Shenzhen [grant number SZSM201911010].
Contributor Information
YuLong He, Email: heyulong@mail.sysu.edu.cn.
ChangHua Zhang, Email: zhchangh@mail.sysu.edu.cn.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F., Jemal A., Yu X.Q., He J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Ding N., Zou Z., Sha H., Su S., Qian H., Meng F., Chen F., Du S., Zhou S., Chen H., Zhang L., Yang J., Wei J., Liu B. iRGD synergizes with PD-1 knockout immunotherapy by enhancing lymphocyte infiltration in gastric cancer. Nat. Commun. 2019;10:1336. doi: 10.1038/s41467-019-09296-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernards N., Creemers G.J., Nieuwenhuijzen G.A., Bosscha K., Pruijt J.F., Lemmens V.E. No improvement in median survival for patients with metastatic gastric cancer despite increased use of chemotherapy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013;24:3056–3060. doi: 10.1093/annonc/mdt401. [DOI] [PubMed] [Google Scholar]
- 5.Espada J., Martin-Perez J. An update on Src family of nonreceptor tyrosine kinases biology. Int. Rev. Cell Mol. Biol. 2017;331:83–122. doi: 10.1016/bs.ircmb.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Patel A., Sabbineni H., Clarke A., Somanath P.R. Novel roles of Src in cancer cell epithelial-to-mesenchymal transition, vascular permeability, microinvasion and metastasis. Life Sci. 2016;157:52–61. doi: 10.1016/j.lfs.2016.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito Y.D., Jensen A.R., Salgia R., Posadas E.M. FYN: a novel molecular target in cancer. Cancer. 2010;116:1629–1637. doi: 10.1002/cncr.24879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Semba K., Nishizawa M., Miyajima N., Yoshida M.C., Sukegawa J., Yamanashi Y., Sasaki M., Yamamoto T., Toyoshima K. yes-related protooncogene, syn, belongs to the protein-tyrosine kinase family. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:5459–5463. doi: 10.1073/pnas.83.15.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee G.H., Yoo K.C., An Y., Lee H.J., Lee M., Uddin N., Kim M.J., Kim I.G., Suh Y., Lee S.J. FYN promotes mesenchymal phenotypes of basal type breast cancer cells through STAT5/NOTCH2 signaling node. Oncogene. 2018;37:1857–1868. doi: 10.1038/s41388-017-0114-y. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S., Fan G., Hao Y., Hammell M., Wilkinson J.E., Tonks N.K. Suppression of protein tyrosine phosphatase N23 predisposes to breast tumorigenesis via activation of FYN kinase. Genes Dev. 2017;31:1939–1957. doi: 10.1101/gad.304261.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang P., Li Z., Tian F., Li X., Yang J. FYN/heterogeneous nuclear ribonucleoprotein E1 signaling regulates pancreatic cancer metastasis by affecting the alternative splicing of integrin beta1. Int. J. Oncol. 2017;51:169–183. doi: 10.3892/ijo.2017.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S., Qi Q., Chan C.B., Zhou W., Chen J., Luo H.R., Appin C., Brat D.J., Ye K. FYN-phosphorylated PIKE-A binds and inhibits AMPK signaling, blocking its tumor suppressive activity. Cell Death Differ. 2016;23:52–63. doi: 10.1038/cdd.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gujral T.S., Chan M., Peshkin L., Sorger P.K., Kirschner M.W., MacBeath G. A noncanonical Frizzled2 pathway regulates epithelial-mesenchymal transition and metastasis. Cell. 2014;159:844–856. doi: 10.1016/j.cell.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tie J., Pan Y., Zhao L., Wu K., Liu J., Sun S., Guo X., Wang B., Gang Y., Zhang Y., Li Q., Qiao T., Zhao Q., Nie Y., Fan D. MiR-218 inhibits invasion and metastasis of gastric cancer by targeting the Robo1 receptor. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiang Z., Zhou Z.J., Xia G.K., Zhang X.H., Wei Z.W., Zhu J.T., Yu J., Chen W., He Y., Schwarz R.E., Brekken R.A., Awasthi N., Zhang C.H. A positive crosstalk between CXCR4 and CXCR2 promotes gastric cancer metastasis. Oncogene. 2017;36:5122–5133. doi: 10.1038/onc.2017.108. [DOI] [PubMed] [Google Scholar]
- 16.Wei Z.-W., Xia G.-K., Wu Y., Chen W., Xiang Z., Schwarz R.E., Brekken R.A., Awasthi N., He Y.-L., Zhang C.-H. CXCL1 promotes tumor growth through VEGF pathway activation and is associated with inferior survival in gastric cancer. Cancer Lett. 2015;359:335–343. doi: 10.1016/j.canlet.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 17.Nagy Á., Lánczky A., Menyhárt O., Győrffy B. Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci. Rep. 2018;8:9227. doi: 10.1038/s41598-018-27521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willmer T., Contu L., Blatch G.L., Edkins A.L. Knockdown of Hop downregulates RhoC expression, and decreases pseudopodia formation and migration in cancer cell lines. Cancer Lett. 2013;328:252–260. doi: 10.1016/j.canlet.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Liu D., Gao M., Wu K., Zhu D., Yang Y., Zhao S. LINC00152 facilitates tumorigenesis in esophageal squamous cell carcinoma via miR-153-3p/FYN axis. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2019;112:108654. doi: 10.1016/j.biopha.2019.108654. [DOI] [PubMed] [Google Scholar]
- 20.Lyu S.-C., Han D.-D., Li X.-L., Ma J., Wu Q., Dong H.-M., Bai C., He Q. FYN knockdown inhibits migration and invasion in cholangiocarcinoma through the activated AMPK/mTOR signaling pathway. Oncol. Lett. 2018;15:2085–2090. doi: 10.3892/ol.2017.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pastushenko I., Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29:212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Mittal V. Epithelial Mesenchymal transition in tumor metastasis. Annu. Rev. Pathol. 2018;13:395–412. doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- 23.Rokavec M., Öner M.G., Li H., Jackstadt R., Jiang L., Lodygin D., Kaller M., Horst D., Ziegler P.K., Schwitalla S., Slotta-Huspenina J., Bader F.G., Greten F.R., Hermeking H. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J. Clin. Invest. 2014;124:1853–1867. doi: 10.1172/JCI73531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson D.E., O'Keefe R.A., Grandis J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018;15:234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benekli M., Baumann H., Wetzler M. Targeting signal transducer and activator of transcription signaling pathway in leukemias. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009;27:4422–4432. doi: 10.1200/JCO.2008.21.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Q., Liu Y., Pan H., Xu T., Li Y., Yuan J., Li P., Yao W., Yan W., Ni C. Aberrant expression of miR-125a-3p promotes fibroblast activation via FYN/STAT3 pathway during silica-induced pulmonary fibrosis. Toxicology. 2019;414:57–67. doi: 10.1016/j.tox.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Zhu X., Shi D., Cao K., Ru D., Ren J., Rao Z., Chen Y., You Q., Dai C., Liu L., Zhou H. Sphingosine kinase 2 cooperating with FYN promotes kidney fibroblast activation and fibrosis via STAT3 and AKT. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2018;1864:3824–3836. doi: 10.1016/j.bbadis.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Seo H.Y., Jeon J.H., Jung Y.A., Jung G.S., Lee E.J., Choi Y.K., Park K.G., Choe M.S., Jang B.K., Kim M.K., Lee I.K. FYN deficiency attenuates renal fibrosis by inhibition of phospho-STAT3. Kidney Int. 2016;90:1285–1297. doi: 10.1016/j.kint.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 29.Mi H., Wang X., Wang F., Li L., Zhu M., Wang N., Xiong Y., Gu Y. miR-381 induces sensitivity of breast cancer cells to doxorubicin by inactivation of MAPK signaling via FYN. Eur. J. Pharmacol. 2018;839:66–75. doi: 10.1016/j.ejphar.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Grosso S., Puissant A., Dufies M., Colosetti P., Jacquel A., Lebrigand K., Barbry P., Deckert M., Cassuto J.P., Mari B., Auberger P. Gene expression profiling of imatinib and PD166326-resistant CML cell lines identifies FYN as a gene associated with resistance to BCR-ABL inhibitors. Mol. Cancer Ther. 2009;8:1924–1933. doi: 10.1158/1535-7163.MCT-09-0168. [DOI] [PubMed] [Google Scholar]
- 31.Dai Y., Siemann D. C-Src is required for hypoxia-induced metastasis-associated functions in prostate cancer cells. OncoTargets Ther. 2019;12:3519–3529. doi: 10.2147/OTT.S201320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gelman I.H., Peresie J., Eng K.H., Foster B.A. Differential requirement for Src family tyrosine kinases in the initiation, progression, and metastasis of prostate cancer. Mol. Cancer Res. MCR. 2014;12:1470–1479. doi: 10.1158/1541-7786.MCR-13-0490-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan C.M., Jing X., Pike L.A., Zhou Q., Lim D.J., Sams S.B., Lund G.S., Sharma V., Haugen B.R., Schweppe R.E. Targeted inhibition of Src kinase with dasatinib blocks thyroid cancer growth and metastasis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012;18:3580–3591. doi: 10.1158/1078-0432.CCR-11-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Z., Xia G., Xiang Z., Liu M., Wei Z., Yan J., Chen W., Zhu J., Awasthi N., Sun X., Fung K.-M., He Y., Li M., Zhang C. A C-X-C chemokine receptor type 2-dominated cross-talk between tumor cells and macrophages drives gastric cancer metastasis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019;25:3317–3328. doi: 10.1158/1078-0432.CCR-18-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Y., Dong B., Xu F., Xu Y., Pan J., Song J., Zhang J., Huang Y., Xue W. CXCL1-LCN2 paracrine axis promotes progression of prostate cancer via the Src activation and epithelial-mesenchymal transition. Cell Commun. Signal. 2019;17:118. doi: 10.1186/s12964-019-0434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su N., Peng L., Xia B., Zhao Y., Xu A., Wang J., Wang X., Jiang B. Lyn is involved in CD24-induced ERK1/2 activation in colorectal cancer. Mol. Cancer. 2012;11:43. doi: 10.1186/1476-4598-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wheeler S.E., Morariu E.M., Bednash J.S., Otte C.G., Seethala R.R., Chiosea S.I., Grandis J.R. Lyn kinase mediates cell motility and tumor growth in EGFRvIII-expressing head and neck cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012;18:2850–2860. doi: 10.1158/1078-0432.CCR-11-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao L., Deng W.W., Yu G.T., Bu L.L., Liu J.F., Ma S.R., Wu L., Kulkarni A.B., Zhang W.F., Sun Z.J. Inhibition of SRC family kinases reduces myeloid-derived suppressor cells in head and neck cancer. Int. J. Cancer. 2017;140:1173–1185. doi: 10.1002/ijc.30493. [DOI] [PubMed] [Google Scholar]
- 39.Garmendia I., Pajares M.J., Hermida-Prado F., Ajona D., Bértolo C., Sainz C., Lavín A., Remírez A.B., Valencia K., Moreno H., Ferrer I., Behrens C., Cuadrado M., Paz-Ares L., Bustelo X.R., Gil-Bazo I., Alameda D., Lecanda F., Calvo A., Felip E., Sánchez-Céspedes M., Wistuba I.I., Granda-Diaz R., Rodrigo J.P., García-Pedrero J.M., Pio R., Montuenga L.M., Agorreta J. YES1 drives lung cancer growth and progression and predicts sensitivity to dasatinib. Am J Respir Crit Care Med. 2019;200:888–899. doi: 10.1164/rccm.201807-1292OC. [DOI] [PubMed] [Google Scholar]
- 40.Roseweir A.K., Powell A., Horstman S.L., Inthagard J., Park J.H., McMillan D.C., Horgan P.G., Edwards J. Src family kinases, HCK and FGR, associate with local inflammation and tumour progression in colorectal cancer. Cell Signal. 2019;56:15–22. doi: 10.1016/j.cellsig.2019.01.007. [DOI] [PubMed] [Google Scholar]