Abstract
Background
South Asians are a high-risk ethnic group for cardiovascular disease despite having lower levels of conventional cardiovascular risk factors such as obesity and smoking. Ethnic differences in pulse wave reflections, arterial stiffness, and subclinical atherosclerosis as measured using augmentation index (AIX), pulse wave velocity (PWV), and carotid intima-media thickness (CIMT) may reflect some of this excess risk.
Methods
We conducted a cross-sectional analysis of pooled data from three community-based sources in Atlanta, Georgia, USA. Data on 530 South Asians collected from local health fairs was compared with data on 507 White and 192 African Americans from the Emory Predictive Health Initiative and 351 White and 382 African Americans from the Morehouse and Emory Team up to Eliminate Health Disparities Study.
Results
Linear regression models adjusted for age, sex, smoking, MAP, fasting glucose, TC, HDL-C, creatinine, and body mass index were used to assess the relationship between ethnicity and vascular function measures. In fully adjusted models, South Asians had higher heart rate corrected AIX as compared with Whites and African Americans (by 5.47%, p < 0.01 and 3.50%, p < 0.01; respectively), but lower PWV (by 0.51 m/s, p < 0.01 and 0.72 m/s, p < 0.01; respectively) and lower CIMT (by 0.02 mm p = 0.03 and 0.04 mm p < 0.01; respectively).
Conclusions
Systemic pulse wave reflections, independent of other risk factors, are higher in South Asians as compared with Whites and African Americans. Future research is needed to determine whether higher AIX explains the increased cardiovascular risk among South Asians.
Keywords: Cardiovascular disease, Arterial stiffness, Augmentation index, Pulse wave velocity, Ethnicity
1. Introduction
South Asians, or people tracing their ancestry to India, Pakistan, Bangladesh, or Sri Lanka, are a rapidly growing population in the United States. Between the years 2000 and 2010 the U.S. South Asian population increased by 68% and currently totals 3.2 million [1]. South Asians are a high risk special population for cardiovascular diseases (CVD) in that they have a higher CVD related morbidity and mortality compared with Whites and African Americans, despite having lower levels of traditional risk factors such as body mass index (BMI) and smoking [2], [3], [4], [5]. Furthermore, differences in conventional risk factors, such as diabetes mellitus, obesity, and hypertension, fail to completely account for this excess risk [4].
Pulse wave reflections estimated as augmentation index (AIX) [6], arterial stiffness measured as pulse wave velocity (PWV), and subclinical atherosclerosis measured as carotid intima-media thickness (CIMT) are markers of subclinical vascular disease and are important predictors of cardiovascular morbidity and mortality [7], [8], [9]; and abnormalities in these measures usually precede the development of overt symptomatic CVD [10]. However, to date, little comparative data exists on the independent relationship between ethnicity and subclinical vascular disease measures among asymptomatic South Asians, Whites, and African Americans living in the United States. We therefore determined the association of ethnicity (South Asian, White, and African American) with vascular function measures (AIX, PWV, and CIMT) among individuals living in the greater Atlanta metropolitan area.
2. Methods
We conducted a cross-sectional analysis of pooled data from three community-based sources. Data on 530 South Asian individuals were recruited from community health fairs in which participants provided demographic and clinical data. This was compared with 507 White and 192 African American individuals from the Emory Predictive Health Institute Center for Health Discovery and Well Being cohort [11], and 351 White and 382 African Americans from the Morehouse and Emory Team up to Eliminate Health Disparities (META-Health) Study [12]. Individuals who were pregnant and those with acute illnesses were excluded. All studies were approved by the Emory University Institutional Review Board and informed consent was obtained from all individuals prior to participation in the study.
2.1. Community health fair
A total of 530 participants who identified as South Asian were recruited at bi-annual community health fairs in Atlanta, Georgia from 2009 to 2014. Health fairs were often conducted in a clinic setting and were open to all individuals regardless of income or immigration status. The overall intent of the health fairs was to provide medical education and to address prevalent health concerns in an underserved South Asian population. Prior to study enrollment, community health fair attendees were informed about the study. Those individuals who were aged 18 and older and who expressed interest were enrolled by an authorized member of the research team. While individuals of any race/ethnic group were permitted to enroll in the study, for the purposes of analysis, we analyzed data only from individuals who self-identified as South Asian. After an overnight fast, venous blood was collected and pulse wave reflection testing with AIX was performed in 462 participants and arterial stiffness testing with PWV measurement was performed in 105 participants. In a subgroup of 138 individuals, ultrasound imaging was used to measure CIMT.
2.2. The Emory Predictive Health Initiative
Recruitment and data collection methods of the Emory Predictive Health Initiative have been previously described [13]. In short, healthy individuals aged between 20 and 79 years who were employees of Emory University or Georgia Institute of Technology were recruited by advertisement or invitation. All participants gave informed consent that was approved by the Emory and Georgia Tech Institutional Review Boards. Participants were given comprehensive clinical evaluations at baseline as well as at 1-year and 2-year visits. For the purposes of this analysis, only data from the baseline visit on 507 White individuals and 192 African American individuals was analyzed. Individuals enrolled in the study received vascular function testing, using AIX, PWV, and CIMT.
2.3. The Morehouse and Emory Team up to Eliminate Health Disparities (META-Health) study
Information regarding participant recruitment and data collection of the META-Health study has been previously published [14]. In brief, the META-Health study was designed as a two-stage cross-sectional community sample. The first stage consisted of a random digit dialing survey of White and African American individuals (N = 3,391) aged 30–65 years residing in the metropolitan Atlanta area. The second stage consisted of a subset of participants (N = 733) who consented to come to either Emory University or Morehouse School of Medicine for a detailed study visit. Vascular function testing and blood draws were performed after a 12 h fast. AIX was measured in 726 participants and PWV analysis was performed in 439 participants. However, CIMT was not measured in this study.
2.4. Vascular function testing
In all studies, vascular function testing was performed at rest in the supine position in a quiet, temperature‐controlled environment set at 22° Celsius after an overnight fast. These included measurements of AIX and PWV using the SphygmoCor device (AtCor Medical, Australia) [11], [15], [16].
2.4.1. Augmentation index
AIX was measured using a derived central aortic pressure waveform, which is a composite of the forward pressure wave from ventricular systole and a reflected wave from the periphery [17]. The SphygmoCor device then applies a transfer function to these peripheral measures to estimate aortic pressure parameters and the degree of pressure augmentation secondary to reflected waves from the periphery [18]. This is used to derive AIX (augmented pressure/total central pulse pressure), a ratio expressed as %, which is considered a complex composite marker of arterial stiffening. We performed pulse-wave analysis using the radial artery pressure waveforms and applied a generalized transfer function to derive the central aortic pressure waveform. AIX was standardized to a heart rate of 75 beats per minute yielding a heart rate-corrected AIX for each participant. Reproducibility studies in our laboratory on 9 Predictive Health Initiative subjects on consecutive days have demonstrated a coefficient of variation of 20.3% for AIX [11].
2.4.2. Pulse wave velocity
In brief, PWV measures the velocity at which pressure waveforms travel down the vasculature and serves as a measure of arterial stiffness [19]. PWV was assessed using the subtraction method [20]. Proximal and distal pulses (common carotid and femoral) were recorded using the SphygmoCor device to calculate transit time between the two arterial sites which was determined in relationship to the R-wave of the electrocardiogram. The “foot-to foot” method was used to calculate velocity (meters/second), measuring the interval between the R wave on the ECG and the foot of the recorded pressure waveform at each site. Distance between sites was measured manually by the operator, [6] and a coefficient of variation of 3.8% for PWV was observed on 9 Predictive Health Initiative subjects on consecutive days [11].
2.4.3. Carotid intima media thickness
CIMT was measured in millimeters (mm) with B-mode ultrasonography operating at 7 MHz using standardized techniques as described previously [21]. Longitudinal images of the distal common carotid arteries, proximal to the carotid bulb were obtained using multiple scanning angles at a standardized depth of 4 cm. Images were stored digitally, and measurements were made off-line using a semi-automated computerized analytical software (Carotid Tools, MIA Inc., Iowa City, Iowa) by two observers blinded to the test results. Average values of the CIMT of each of the four segments of the distal 1.0 cm of both common carotid arteries were used to define the CIMT values for each subject. The inter-observer variability for CIMT was 0.03 ± 0.02 mm.
2.4.4. Blood pressure measurement
In all three studies, blood pressure was measured three times while seated in the right arm using an automatic blood pressure monitor (A&D Medical, Model TM-2655). Blood pressure was measured after 5 min of rest and was based on the average of the final 2 of 3 readings measured 5 min apart. Mean arterial pressure (MAP) was calculated by adding the systolic blood pressure to two times the diastolic blood pressure reading and dividing this sum by three.
2.4.5. Cardiovascular risk factors
After the data was pooled, we used standard definitions for hypercholesterolemia, hypertension, type 2 diabetes, and obesity. Hypercholesterolemia was defined as LDL cholesterol >3.4 mmol/L, use of lipid lowering medication, or previous physician diagnosis [22]. Hypertension was defined as blood pressure ≥140/90 mm Hg, any use of antihypertensive medication, or previous physician diagnosis [22]. Type 2 diabetes was defined as fasting plasma glucose ≥7.0 mmol/L, any use of glucose lowering medication, or previous physician diagnosis [23]. Obesity was defined as a body mass index ≥30 kg/m2.
2.5. Statistical methods
Analyses were conducted using pooled data from the Community Health Fair, the Emory Predictive Health Initiative, and the META-Health cohorts. Participant characteristics were described as percentages, means, and geometric means by race/ethnicity. Differences in participant characteristics across race/ethnic groups were assessed using χ2 test, one-way analysis of variance, and Kruskal-Wallis analysis of variance (non-parametric continuous variables) as appropriate. Statistically significant differences in continuous variables between South Asians and Whites, and South Asians and African Americans were determined post-hoc.
Linear regression models were used to assess the unadjusted relationship between vascular function measures and a 3-level categorical race/ethnicity variable (Model 1). We subsequently adjusted the models for age and sex in Model 2; followed by further adjustment for current smoking, MAP, plasma glucose, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), serum creatinine, and body mass index (BMI) (Model 3). Models evaluating AIX were further adjusted for height (in meters) given its known confounding effect on ethnic differences in AIX [24]. Missing values for covariates were imputed using the Visualization and Imputation of Missing Values R package by employing the k-nearest neighbor approach [25]. Linear regression analyses in Model 4 were performed using covariates with imputed missing values.
As a sensitivity analysis, study participants were divided into two groups, ‘healthy’ (without diabetes, hypertension, hyperlipidemia, smoking, and BMI < 30 kg/m2) and ‘unhealthy’ (with any of the aforementioned risk factors). The mean (95% confidence interval) values of PWV, AIX, and CIMT for healthy and unhealthy individuals in each racial/ethnic group were plotted and compared. Furthermore, the association of race/ethnicity (South Asian vs White and South Asian vs African American) with indices of vascular function was assessed in linear regression models adjusted for age, sex, study site, and health status, following which the multiplicative interaction of ethnicity with healthy status was tested. Finally, the association of ethnicity with vascular function measures in both healthy and unhealthy participants was tested using linear regression models adjusted for age, sex, study site, current smoking, MAP, plasma glucose, TC, HDL-C, serum creatinine, and BMI. All analyses were performed using IBM SPSS Statistics Version 25 (Armonk, NY, US) and R version 3.3.3 (R Foundation for statistical computing, Vienna, Austria).
3. Results
On average, South Asian participants were younger than White participants, and had lower BMI and height than both White and African American participants. In addition, South Asian participants were more likely to be men, had higher mean systolic blood pressure, fasting glucose, triglycerides and prevalence of diabetes, and lower prevalence of hypertension, hyperlipidemia and obesity as well as lower mean total, low density lipoprotein and HDL-C levels compared to Whites and African Americans (Table 1).
Table 1.
Characteristics of participants by race/ethnicity.
| Characteristics | South Asian | White | African American | P-value |
|---|---|---|---|---|
| N | 530 | 858 | 574 | |
| Men (%) | 53.8 | 40.8 | 35.9 | <0.01 |
| Age (years) | 48.2 (12.3) | 50.5 (10.5) | 48.1 (9.4) | <0.01* |
| Systolic blood pressure (mm Hg) | 131.1 (19.4) | 119.6 (15.9) | 124.4 (18.4) | <0.01*† |
| Diastolic blood pressure (mm Hg) | 77.8 (13.5) | 76.3 (10.5) | 79.2 (11.7) | <0.01* |
| Mean arterial pressure (mm Hg) | 95.6 (14.0) | 90.8 (11.4) | 94.2 (13.1) | <0.01* |
| Hypertension (%) | 14.7 | 31.2 | 45.8 | <0.01 |
| Fasting glucose (mmol/L) | 5.9 (2.2) | 5.1 (1.0) | 5.2 (1.5) | <0.01*† |
| Diabetes (%) | 20.0 | 7.9 | 16.2 | <0.01 |
| Total cholesterol (mmol/L) | 4.9 (0.9) | 5.1 (1.0) | 5.1 (1.0) | <0.01*† |
| LDL-cholesterol (mmol/L) | 2.8 (0.7) | 2.9 (0.8) | 3.0 (0.9) | <0.01*† |
| HDL-cholesterol (mmol/L) | 1.3 (0.3) | 1.6 (0.5) | 1.5 (0.4) | <0.01*† |
| Triglycerides (mmol/L)¶ | 13.8 (1.3) | 11.6 (1.2) | 10.1 (1.0) | <0.01*† |
| Current Smoking (%) | 7.9 | 7.7 | 20.3 | <0.01 |
| Creatinine (mg/dL) | 0.9 (0.2) | 0.8 (0.2) | 0.9 (0.6) | <0.01† |
| BMI (kg/m2) | 26.0 (4.7) | 27.5 (5.8) | 31.2 (8.1) | <0.01*† |
| Obesity (%) | 18.0 | 26.4 | 49.0 | <0.01 |
| Height (m) | 1.6 (0.1) | 1.7 (0.1) | 1.7 (0.1) | <0.01*† |
| Pulse Wave Velocity (m/s) | 7.1 (2.0) | 7.2 (1.6) | 7.5 (1.7) | <0.01† |
| Augmentation Index (%) | 27.1 (10.7) | 20.5 (10.5) | 22.7 (11.5) | <0.01*† |
| Carotid Intima Media Thickness (mm) | 0.6 (0.1) | 0.6 (0.1) | 0.6 (0.1) | 0.63 |
P-Pvalues for chi-square test (categorical variables), one-way ANOVA (parametric continuous variables), and Kruskal-Wallis ANOVA (non-parametric continuous variables) across race/ethnic groups.
Abbreviations: LDL = low-density lipoprotein, HDL = high-density lipoprotein, BMI = body mass index.
Post-hoc p-value < 0.05 for South Asian vs White.
Post-hoc p-value < 0.05 for South Asian vs African American.
Geometric mean.
The independent predictors of vascular function are listed in Table 2. PWV was measured in 1230 participants (105 South Asian, 728 White, 397 African American), AIX in 1881 participants (462 South Asian, 851 White, 568 African American), and CIMT in 833 participants (138 South Asian, 506 White, 189 African American). Age, sex, MAP, fasting glucose and HDL-C were all independently associated with PWV, with men having greater PWV compared with women. Age, smoking, and MAP were all independently positively associated with AIX. Women had greater AIX compared to men. BMI was inversely associated with AIX, as was height (−2.34%; 95% CI −2.83%, −1.85%, p < 0.01 per 0.1-meter increase). Age, MAP, fasting glucose, and BMI were all independently associated with CIMT.
Table 2.
Independent predictors of vascular function.
| Augmentation Index (%) |
Pulse Wave Velocity (m/s) |
Carotid Intima-Media Thickness (mm) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | p-value | Estimate | 95% CI | p-value | Estimate | 95% CI | p-value | |
| South Asian vs White | 5.47 | (4.30, 6.65) | <0.01 | −0.51 | (−0.84, −0.17) | <0.01 | −0.02 | (−0.04, −0.003) | 0.03 |
| South Asian vs African American | 3.50 | (2.15, 4.84) | <0.01 | −0.72 | (−1.08, −0.36) | <0.01 | −0.04 | (−0.07, −0.02) | <0.01 |
| Age (per year) | 0.37 | (0.34, 0.41) | <0.01 | 0.04 | (0.03, 0.04) | <0.01 | 0.006 | (0.006, 0.007) | <0.01 |
| Sex | −6.72 | (−7.82, −5.62) | <0.01 | 0.31 | (0.09, 0.52) | <0.01 | 0.02 | (−0.001, 0.03) | 0.06 |
| Smoking Status | 3.27 | (2.05, 4.50) | <0.01 | 0.16 | (−0.13, 0.44) | 0.28 | 0.03 | (−0.001, 0.06) | 0.06 |
| MAP (per mm Hg) | 0.20 | (0.17, 0.23) | <0.01 | 0.03 | (0.03, 0.04) | <0.01 | 0.001 | (0.000, 0.002) | <0.01 |
| Fasting glucose (per mmol/L) | 0.04 | (−0.21, 0.30) | 0.75 | 0.12 | (0.06, 0.18) | <0.01 | 0.007 | (0.003, 0.012) | <0.01 |
| Total Cholesterol (per mmol/L) | 0.15 | (−0.26, 0.56) | 0.47 | −0.08 | (−0.17, 0.02) | 0.10 | −0.001 | (−0.08, 0.06) | 0.81 |
| HDL Cholesterol (per mmol/L) | 0.48 | (−1.00, 1.10) | 0.93 | 0.25 | (0.03, 0.47) | 0.03 | −0.07 | (−0.025, 0.011) | 0.45 |
| BMI (per kg/m2) | −0.17 | (−0.23, −0.10) | <0.01 | 0.08 | (−0.006, 0.02) | 0.26 | 0.003 | (0.002, 0.005) | <0.01 |
| Creatinine (per µmol/L) | −0.002 | (−0.01, 0.01) | 0.69 | 0.004 | (−0.03, 0.10) | 0.25 | 0.000 | (0.000, 0.0001) | 0.38 |
| Height (per 0.1 m) | −2.35 | (−2.84, −1.85) | <0.01 | – | – | – | – | – | – |
Abbreviations: MAP = mean arterial pressure, HDL = high-density lipoprotein, BMI = body mass index.
3.1. Ethnic differences in vascular function measures
In univariate analyses, South Asians had higher AIX as compared with both Whites and African Americans (Table 3, Model 1). However, CIMT was similar across ethnicities, and PWV was lower in South Asians as compared with African Americans (Table 3, Model 1). Ethnic differences in vascular function measures became apparent after controlling for cardiovascular risk factors (Table 3, model 4). After adjustment for age, sex, smoking status, MAP, fasting glucose, TC, HDL-C, creatinine, and BMI, South Asians continued to have higher AIX as compared with Whites and African Americans (by 5.47%; 95% CI: 4.30, 6.65, p < 0.01 and 3.50%, 95% CI: 2.15, 4.84, p < 0.01; respectively). In fully adjusted models, South Asians had lower PWV (by 0.51 m/s, 95% CI: −0.84, −0.17, p < 0.01 and 0.72 m/s, 95% CI: −1.08, −0.36, p < 0.01; respectively) and lower CIMT (by 0.02 mm, 95% CI: −0.04, −0.003), p = 0.04 and 0.04 mm, 95% CI: −0.07, −0.02, p < 0.01 respectively) as compared with Whites and African Americans.
Table 3.
Multivariable associations between race/ethnicity and vascular function.
| Augmentation Index (%)* |
Pulse Wave Velocity (m/s) |
Carotid Intima-Media Thickness (mm) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | p-value | Estimate | 95% CI | p-value | Estimate | 95% CI | p-value | ||
| Model 1 | South Asian vs White | 6.55 | (5.32, 7.78) | <0.01 | −0.19 | (−0.52, 0.15) | 0.27 | −0.01 | (−0.03, 0.02) | 0.49 |
| South Asian vs African American | 4.37 | (3.03, 5.70) | <0.01 | −0.48 | (−0.84, −0.13) | 0.01 | −0.01 | (−0.04, 0.01) | 0.33 | |
| Model 2 | ||||||||||
| South Asian vs White | 8.85 | (7.70, 9.92) | <0.01 | −0.11 | (−0.44, 0.21) | 0.50 | −0.003 | (−0.02, 0.02) | 0.79 | |
| South Asian vs African American | 5.97 | (4.70, 7.23) | <0.01 | −0.55 | (−0.90, −0.19) | <0.01 | −0.04 | (−0.06, −0.02) | <0.01 | |
| Model 3 | ||||||||||
| South Asian vs White | 6.43 | (4.77, 8.10) | <0.01 | −0.58 | (−0.96, −0.20) | <0.01 | −0.02 | (−0.05, −0.000) | 0.05 | |
| South Asian vs African American | 4.62 | (2.83, 6.40) | <0.01 | −0.85 | (−1.25, −0.44) | <0.01 | −0.04 | (−0.07, −0.02) | 0.02 | |
| Model 4 | ||||||||||
| South Asian vs White | 5.47 | (4.30, 6.65) | <0.01 | −0.51 | (−0.84, −0.17) | <0.01 | −0.02 | (−0.04, −0.003) | 0.03 | |
| South Asian vs African American | 3.50 | (2.15, 4.84) | <0.01 | −0.72 | (−1.08, −0.36) | <0.01 | −0.04 | (−0.07, −0.02) | <0.01 | |
Model 1: Unadjusted.
Model 2: Adjusted for age, sex.
Model 3: Adjusted for age, sex, smoking status, mean arterial pressure, fasting glucose, total cholesterol, high density lipoprotein cholesterol, creatinine, and BMI.
Model 4: Imputed model.
Augmentation index models 3 and 4 further adjusted for height.
The association of ethnicity with AIX was modified by age (p-interaction < 0.01), however, such an interaction was not noted with PWV and CIMT (p-interactions > 0.15). Among younger participants (age < 50 years), South Asians had higher AIX (by 7.63%, 95% CI: 5.86, 9.40, p < 0.01 and by 5.67%, 95% CI: 3.85, 7.50, p < 0.01; respectively) as compared with Whites and African Americans. However, among those age ≥ 50 years, the magnitude of this difference was smaller (3.58%, 95% CI: 2.14, 5.03, p < 0.01 and 1.89%, 95% CI: 0.26, 3.52, p = 0.02; respectively).
3.2. Subgroup analyses in the ‘healthy’ cohort
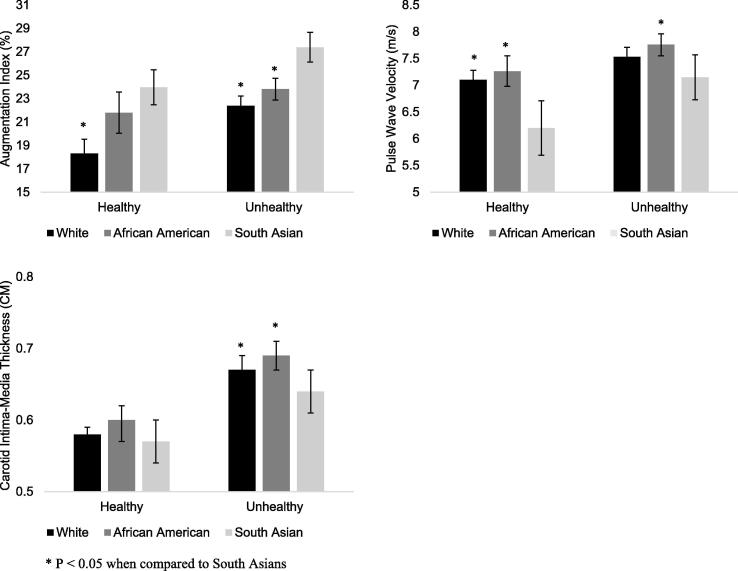
The study cohort was divided into healthy individuals without diabetes, hypertension, hyperlipidemia, smoking, and overweight/obesity (N = 602) and unhealthy individuals defined as those with any of the aforementioned factors (N = 1360). Health status is defined as ‘healthy’ or unhealthy individuals. The differences in vascular function measures by health status across ethnic groups are depicted in Fig. 1. After adjustment for age, sex, smoking status, MAP, fasting glucose, TC, HDL-C, creatinine, and BMI, healthy South Asians had higher AIX, lower PWV, but similar CIMT as compared with healthy White participants and similar AIX and CIMT but lower PWV compared to healthy African American participants (Fig. 1). Ethnicity based differences in AIX persisted among unhealthy participants. Interestingly, differences in PWV decreased in magnitude while differences in CIMT became evident in this subgroup as shown in Fig. 1. There was no interaction between ethnicity and health status in linear regression models adjusted for age, sex, and presence of risk factors (all p-interaction > 0.09).
Fig. 1.
Mean measures of vascular function among healthy and unhealthy participants by race ethnicity.
4. Discussion
In this cross-sectional analysis of three community-based samples including participants from three race/ethnic groups in the United States, we found differences in pulse wave reflections measured as AIX, arterial stiffness measured as PWV, as well as in arterial wall thickness as captured by CIMT in South Asian participants compared to White and African American participants. In particular, after adjustment for relevant covariates, South Asians had significantly higher AIX as compared with Whites and African Americans, while having relatively lower PWV and CIMT. The ethnicity-based differences in vascular function measures persisted even in a subset of ‘healthy’ individuals free of risk factors, particularly regarding PWV and AIX.
Few studies have assessed differences in vascular function by race/ethnicity after taking into account differences in traditional cardiovascular risk factors [26]. The “DASH study” assessed differences in PWV in a multi-ethnic cohort of young adults aged 21–23 years and found that PWV was slightly lower in South Asians compared to Whites and Black Caribbean young adults [27]. The results of this study also reported higher AIX in South Asians compared to Whites, differences that persisted after adjustment for confounding variables [28]. Our results confirm the findings of lower PWV, a measure of arterial stiffness, and higher AIX, a measure of pulse wave reflection, in a middle-aged population of South Asian adults living in the United States compared to their White and African American counterparts. These associations remained significant after adjusting for demographics, cardiovascular risk factors, as well as height in case of AIX.
Two small studies from the United Kingdom found that South Asians had higher PWV compared to Europeans after adjustment for relevant risk factors [29], [30] which are contrary to our findings and could possibly be attributed to the relatively small sample sizes of the aforementioned studies, differences in socio-economic status, and other geographical factors. Another study in a multi-ethnic population-based sample from the Netherlands found that unadjusted PWV was higher in South Asians compared to individuals of European or African descent. However, these differences were no longer significant after adjustment for cardiovascular risk factors [24]. The pathophysiologic reason underlying these observations is unclear. However, it is well established that AIX and PWV capture different aspects of vascular function [31]. AIX is an index of pulse wave reflectance and PWV estimates aortic compliance/stiffness. There is also a weak or no correlation between these two indices [32]. Pulse wave reflections are also influenced by earlier changes in vessel size and branch points, that in turn are influenced by height and body size. Although we adjusted AIX for height, mean AIX was still significantly higher among SAs. Impaired glucose metabolism and insulin resistance are associated with higher AIX [33], this is particularly relevant among SA [10], an ethnic group with high risk of type 2 diabetes. Moreover, a higher AIX is an independent risk factor for cardiovascular disease and mortality [31], [32]. Whether this observed higher AIX among SA predisposes them to higher relative risk of CVD needs to be further investigated. Furthermore, in our study we found that the association of ethnicity and AIX was modified by age, such that the magnitude of ethnic differences in AIX was higher among younger participants (age < 50 years) as compared with those age ≥50 years. These observations are in line with an analysis of the Anglo-Cardiff Collaborative Trial which showed that AIX is a more sensitive marker of arterial stiffness up to the age of 50 years [34].
These results suggest that it is plausible that pulse wave reflectance, and not arterial stiffness, is responsible for the excess cardiovascular disease risk observed in South Asians, and studies have identified measures wave reflectance as independent risk factors for cardiovascular disease and mortality [35], [36]. These possibly divergent prognostic implications of PWV and AIX across racial groups should be evaluated in future studies.
Limitations of our study include its cross-sectional nature that precludes conclusions regarding temporal relationships between risk factors and vascular function. Furthermore, South Asians were older than Whites and African Americans even in the ‘healthy’ cohort. Therefore, despite adjusting for age in the models, age may remain a confounding factor. However, despite having a higher age, PWV and CIMT were lower in South Asians compared to Whites and African Americans. Furthermore, socio-economic data was not collected consistently across South Asian health fairs. Therefore, while socio-economic status may influence vascular function [37], [38], we were not able to adjust for this in our analysis. Future studies should consider the influence of socio-economic status on the differences in vascular function between race/ethnic groups. We also had limited data on CIMT and may have found different results in a larger sample. Furthermore, in our analysis we noted that despite having a lower prevalence of hypertension, SAs had higher mean SBP compared to Whites and African Americans. This may be due to a longer duration of anti-hypertemisive medication use in Whites and African Americans. However, we did not have the data available to assess this.
Additionally, while this is the first study to compare multiple measures of vascular function in South Asian, African American and White populations and includes both men and women in the analysis, the data is from three differing data sources that varied in recruitment methods. It is possible that individuals enrolled from an employment-based recruitment may have different health outcomes than those attending a community health fair. Furthermore, the sample size of South Asians included in the study was smaller than that of African Americans and Whites, which is in part a function of the differing data sources. Therefore, additional studies using a single data source to compare subclinical vascular health across multiple race/ethnic groups are needed to better assess this association.
In conclusion, we found that compared to Whites and African Americans, South Asian participants had a significantly higher AIX, indicating greater pulse wave reflections, even after adjustment for height and other relevant covariates, despite having lower arterial stiffness and lower carotid arterial wall thickness. These differences were also present in the ‘healthy’ subset of individuals free of conventional CVD risk factors. It remains to studied as to how increased pulse wave reflections in South Asians contribute to the increased cardiovascular risk in this population. Future research is needed to determine whether increased AIX in South Asians is associated with poorer cardiovascular disease incidence and mortality outcomes.
CRediT authorship contribution statement
Unjali Gujral: Conceptualiztion, Formal analysis, Writing - original draft, Writing - review & editing. Anurag Mehta: Formal analysis, Writing - original draft, Writing - review & editing. Salman Sher: Formal analysis. Irina Uphoff: Data curation. Saket Kumar: Formal analysis, Data curation. Salim S. Hayek: Data curation, Formal analysis. Yi-An Ko: Data curation, Formal analysis. Greg S. Martin: Conceptualiztion, Data curation. Gary H. Gibbons: Data curation, Formal analysis. Arshed A. Quyyumi: Funding acquisition, Conceptualization, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We would like to thank Shafaat A. Khan for his coordination of the Community Health Fairs. We also thank the participants of the Community Health Fair Study, the Emory Predictive Health Initiative, and the META-Health Study for their important contributions.
Funding
UPG is supported by the NIDDK funded P30DK111024.
References
- 1.The Asian Population: 2010 - c2010br-11.pdf [Internet]. [cited 2016 Feb 2]. Available from: https://www.census.gov/prod/cen2010/briefs/c2010br-11.pdf.
- 2.Anand S.S., Yusuf S., Vuksan V., Devanesen S., Teo K.K., Montague P.A. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE) The Lancet. 2000;356(9226):279–284. doi: 10.1016/s0140-6736(00)02502-2. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi N. Ethnic differences in cardiovascular disease. Heart. 2003;89(6):681–686. doi: 10.1136/heart.89.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forouhi N.G., Sattar N., Tillin T., McKeigue P.M., Chaturvedi N. Do known risk factors explain the higher coronary heart disease mortality in South Asian compared with European men? Prospective follow-up of the Southall and Brent studies, UK. Diabetologia. 2006;49(11):2580–2588. doi: 10.1007/s00125-006-0393-2. [DOI] [PubMed] [Google Scholar]
- 5.Barnett A.H., Dixon A.N., Bellary S., Hanif M.W., O’Hare J.P., Raymond N.T. Type 2 diabetes and cardiovascular risk in the UK south Asian community. Diabetologia. 2006 Jul 18;49(10):2234–2246. doi: 10.1007/s00125-006-0325-1. [DOI] [PubMed] [Google Scholar]
- 6.Laurent S., Cockcroft J., Van Bortel L., Boutouyrie P., Giannattasio C., Hayoz D. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 7.Blacher J., Asmar R., Djane S., London G.M., Safar M.E. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33(5):1111–1117. doi: 10.1161/01.hyp.33.5.1111. [DOI] [PubMed] [Google Scholar]
- 8.Hansen T.W., Staessen J.A., Torp-Pedersen C., Rasmussen S., Thijs L., Ibsen H. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113(5):664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 9.Sutton-Tyrrell K., Najjar S.S., Boudreau R.M., Venkitachalam L., Kupelian V., Simonsick E.M. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111(25):3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 10.N.J. Din, O. Ashman, S. Aftab, A. Jubb, E.D. Newby, A. Flapan, Increased arterial stiffness in healthy young South Asian men, vol. 20, 2006, 163 p. [DOI] [PubMed]
- 11.Al Mheid I., Patel R., Murrow J., Morris A., Rahman A., Fike L. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J. Am .Coll. Cardiol. 2011;58(2):186–192. doi: 10.1016/j.jacc.2011.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris A., Zhao L., Ahmed Y., Stoyanova N., Hooper W.C., Gibbons G. Association between depression and inflammation – differences by race and sex: the META-health study. Psychosom. Med. 2011;73(6):462–468. doi: 10.1097/PSY.0b013e318222379c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Mheid I., Kelli H.M., Ko Y.-A., Hammadah M., Ahmed H., Hayek S. Effects of a health-partner intervention on cardiovascular risk. J. Am. Heart Assoc. 2016;5(10) doi: 10.1161/JAHA.116.004217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendley Y., Zhao L., Coverson D.L., Din-Dzietham R., Morris A., Quyyumi A.A. Differences in weight perception among blacks and whites. J. Womens Health. 2011;20(12):1805–1811. doi: 10.1089/jwh.2010.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel R.S., Al Mheid I., Morris A.A., Ahmed Y., Kavtaradze N., Ali S. Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis. 2011;218(1):90–95. doi: 10.1016/j.atherosclerosis.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris A.A., Patel R.S., Binongo J.N.G., Poole J., Al Mheid I., Ahmed Y. Racial differences in arterial stiffness and microcirculatory function between Black and White Americans. J. Am. Heart Assoc. 2013;2(2):e002154. doi: 10.1161/JAHA.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takazawa K. Augmentation index in heart disease. Am. J. Hypertens. 2005;18(S1):15S–18S. doi: 10.1016/j.amjhyper.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 18.Butlin M., Qasem A. Large artery stiffness assessment using SphygmoCor technology. Pulse. 2016;4(4):180–192. doi: 10.1159/000452448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirata K., Kawakami M., O’Rourke M.F. Pulse wave analysis and pulse wave velocity. Circ. J. 2006;70(10):1231–1239. doi: 10.1253/circj.70.1231. [DOI] [PubMed] [Google Scholar]
- 20.Ellins E.A., Smith K.E., Lennon L.T., Papacosta O., Wannamethee S.G., Whincup P.H. Arterial pathophysiology and comparison of two devices for pulse wave velocity assessment in elderly men: the British regional heart study. Open Heart. 2017;4(2) doi: 10.1136/openhrt-2017-000645. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5761282/ [cited 2020 May 22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashfaq S., Abramson J.L., Jones D.P., Rhodes S.D., Weintraub W.S., Hooper W.C. The relationship between plasma levels of oxidized and reduced thiols and early atherosclerosis in healthy adults. J. Am. Coll. Cardiol. 2006;47(5):1005–1011. doi: 10.1016/j.jacc.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 22.Expert Panel on Detection E. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285(19) (2001) 2486–2497. [DOI] [PubMed]
- 23.American Diabetes Association, Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 33(Suppl. 1) (2010) S62–S69. [DOI] [PMC free article] [PubMed]
- 24.Snijder M.B., Stronks K., Agyemang C., Busschers W.B., Peters R.J., van den Born B.-J.H. Ethnic differences in arterial stiffness the Helius study. Int. J. Cardiol. 2015;15(191):28–33. doi: 10.1016/j.ijcard.2015.04.234. [DOI] [PubMed] [Google Scholar]
- 25.Kowarik A., Templ M. Imputation with the R Package VIM. J. Stat. Softw. 2016;74(1):1–16. [Google Scholar]
- 26.Faconti L., Nanino E., Mills C.E., Cruickshank K.J. Do arterial stiffness and wave reflection underlie cardiovascular risk in ethnic minorities? JRSM Cardiovasc. Dis. 2016;5(Dec) doi: 10.1177/2048004016661679. 2048004016661679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ethnic Differences in and Childhood Influences on Early Adult Pulse Wave Velocity, Hypertension [Internet] (cited 2019 Jul 2). Available from: https://www.ahajournals.org/doi/abs/10.1161/HYPERTENSIONAHA.115.07079. [DOI] [PMC free article] [PubMed]
- 28.Faconti L., Silva M.J., Molaodi O.R., Enayat Z.E., Cassidy A., Karamanos A. Can arterial wave augmentation in young adults help account for variability of cardiovascular risk in different British ethnic groups? J. Hypertens. 2016;34(11):2220–2226. doi: 10.1097/HJH.0000000000001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammad-Reza Rezai, Michael Wallace A., Naveed Sattar, Finn Joseph D., Wu Frederick C.W., Kennedy Cruickshank J. Ethnic differences in aortic pulse wave velocity occur in the descending aorta and may be related to Vitamin D. Hypertension. 2011;58(2):247–253. doi: 10.1161/HYPERTENSIONAHA.111.174425. [DOI] [PubMed] [Google Scholar]
- 30.Webb D.R., Khunti K., Lacy P., Gray L.J., Mostafa S., Talbot D. Conduit vessel stiffness in British south Asians of Indian descent relates to 25-hydroxyvitamin D status. J. Hypertens. 2012;30(8):1588–1596. doi: 10.1097/HJH.0b013e328354f385. [DOI] [PubMed] [Google Scholar]
- 31.Yasmin, Brown M.J. Similarities and differences between augmentation index and pulse wave velocity in the assessment of arterial stiffness. QJM Mon. J. Assoc. Phys. 1999;92(10):595–600. doi: 10.1093/qjmed/92.10.595. [DOI] [PubMed] [Google Scholar]
- 32.Weber T., O’Rourke M.F., Ammer M., Kvas E., Punzengruber C., Eber B. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. Am. J. Hypertens. 2008;21(11):1194–1202. doi: 10.1038/ajh.2008.277. [DOI] [PubMed] [Google Scholar]
- 33.Schram M.T., Henry R.M.A., van Dijk R.A.J.M., Kostense P.J., Dekker J.M., Nijpels G. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: the Hoorn Study. Hypertens Dallas Tex 1979. 2004;43(2):176–181. doi: 10.1161/01.HYP.0000111829.46090.92. [DOI] [PubMed] [Google Scholar]
- 34.McEniery C.M., Yasmin, Hall I.R., Qasem A., Wilkinson I.B., Cockcroft J.R. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT) J. Am. Coll. Cardiol. 2005;46(9):1753–1760. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 35.Chirinos J.A., Kips J.G., Jacobs D.R., Brumback L., Duprez D.A., Kronmal R. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis) J. Am. Coll. Cardiol. 2012;60(21):2170–2177. doi: 10.1016/j.jacc.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang K.-L., Cheng H.-M., Sung S.-H., Chuang S.-Y., Li C.-H., Spurgeon H.A. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertens Dallas Tex 1979. 2010;55(3):799–805. doi: 10.1161/HYPERTENSIONAHA.109.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saijo Y., Yoshioka E., Fukui T., Kawaharada M., Kishi R. Relationship of socioeconomic status to C-reactive protein and arterial stiffness in urban Japanese civil servants. Soc. Sci. Med. 2008;67(6):971–981. doi: 10.1016/j.socscimed.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Trudel X., Shipley M.J., McEniery C.M., Wilkinson I.B., Brunner E.J. Socioeconomic status, education, and aortic stiffness progression over 5 years: the Whitehall II prospective cohort study. J. Hypertens. 2016;34(10):2038–2044. doi: 10.1097/HJH.0000000000001057. [DOI] [PMC free article] [PubMed] [Google Scholar]