Abstract
Cognitive models of pain propose that catastrophic thinking is negatively associated with chronic pain. However, pain catastrophizing is a complex phenomenon requiring a multivariate examination. This study estimates the effects of mood variables (anxiety and depression) on pain catastrophizing in older adults with chronic pain. A postal survey addressing pain aspects was sent to 6611 people ≥ 65 years old living in south-eastern Sweden. Pain catastrophizing was measured using the pain catastrophizing scale. Anxiety and depression were assessed using two subscales of the general well-being schedule. Data were analysed using a path analysis approach. A total of 2790 respondents (76.2 ± 7.4 years old) reported chronic pain (≥three months). The mediation model accounted for 16.3% of anxiety, 17.1% of depression, and 30.9% of pain catastrophizing variances. Pain intensity, insomnia, number of comorbidities, and lifestyle factors (smoking, alcohol consumption, and weight) significantly affected both pain catastrophizing and mood. Anxiety (standardized path coefficient (bstd) = 0.324, p < 0.001) in comparison to depression (bstd = 0.125, p < 0.001) had a greater effect on pain catastrophizing. Mood mediated the relationship between pain catastrophizing and pain-related factors accounting for lifestyle and sociodemographic factors.
Keywords: pain catastrophizing, anxiety, depression, mediate, older people
1. Introduction
Although pain is a universal experience throughout the life course, chronic pain in later life is an increasing global health problem considering the rapid growth of older populations. The prevalence of chronic pain is known to increase with age, ranging between 25% and 76% in the general elderly population and up to 93% of elderly in residential care [1,2]. In Sweden, more than 50% of people aged 65 and over report chronic pain (irrespective of intensity) [3].
Chronic pain is generally not a symptom or complaint that exists in isolation. For older people, considerable evidence connects chronic pain with comorbidities, including mood disorders (anxiety and/or depression) [4,5,6,7] and sleep disturbance [8,9]. In this context, one’s psychological state is an important determinant of pain experiences as well as mental health [10,11,12]. Brain images indicate that cognitive and emotional modulations of pain are associated with alterations in specific brain regions [13,14]. Clinically, psychological factors such as anxiety, catastrophizing, and depressive symptoms seem to be important features of patients with chronic pain [11,15]. Pain catastrophizing—a persistently negative cognitive affective style characterized by helplessness, magnification, and ruminative thoughts regarding one’s pain—is a potent predictor of negative pain-related outcomes in general [16]. Vlaeyen and Lintons’ fear-avoidance model, perhaps the most known framework, highlighted pain catastrophizing as a cognitive precursor of fear and avoidance and a possible misinterpretation of pain [17]. The fear-avoidance model illustrated the transition from a common pain episode toward persistent pain and disability via pain catastrophizing [18]. In brief, pain catastrophizing is associated with pain severity, disability, and poor outcomes for patients with chronic pain [14,19,20,21]. Evidence suggests that pain catastrophizing in older adults is associated with sedentary behaviour which in in turn, contributed to greater pain catastrophizing [22]. Catastrophizing significantly predicts the development of chronic pain in pain free individuals as well as the chronification of acute pain [19]. Treatment studies show that initial decreases in pain catastrophizing can predict subsequent changes in pain intensity and/or pain interference [20,21]. In addition, reductions in pain intensity and/or interference early in treatment are associated with subsequent reductions in catastrophizing in patients with neuropathic pain [20]. Together, these results indicate complex interrelationships between pain and catastrophizing. A recent systematic review and meta-analysis concerning pain catastrophizing in chronic pain found weak associations with pain intensity and disability from both cross-sectional and longitudinal perspectives [23]. The meta-analyses indicated prominent heterogeneity. The authors suggest that moderators influence the strength of the associations between pain catastrophizing and pain intensity/disability and warrant research including large cohorts and adjusting for all covariates.
The relationship between pain catastrophizing and emotional processing (i.e., worry, anxiety, and depression) is not clear. Some studies suggest that pain catastrophizing mediates the relationship between pain and depressed mood [24,25,26,27,28]. One study highlights that depressive and anxiety symptoms mediate the relationship between pain catastrophizing and pain [29]. One disadvantage of these studies is that they do not always consider lifestyle and sociodemographic factors, which have been found to have significant effects on pain aspects and mood disorders [30,31,32]. Furthermore, sleep disturbance, especially insomnia, should also be considered due to its complex associations with pain and mood disorders [33,34,35].
In pain rehabilitation, catastrophizing is described as a maladaptive coping strategy [36,37]. Improvement of maladaptive coping strategies is an important goal of comprehensive pain rehabilitation programs. To achieve good rehabilitation results, the comprehensive rehabilitation process (i.e., interdisciplinary pain treatment according toInternational Association for the Study of Pain; IASP) considers many factors, focusing on the whole person rather than biomedical factors [36].
Catastrophizing is more strongly associated with pain intensity among older people, whereas pain intensity among younger people is more strongly associated with emotional responses to pain [38]. In addition, older people with chronic pain show less passive coping and higher life control than younger people with chronic pain [39]. Longitudinal data reveal that the predictive effect of catastrophizing on worse pain and disability disappeared when adjusting for age, gender, and mood disorders [29]. Hence, it is reasonable to expect that older people will exhibit other patterns associated with pain, mood, and pain catastrophizing. To efficiently treat patients who catastrophize chronic pain, it is necessary to discover the factors, particularly modifiable factors, that affect catastrophizing. Based on the fear avoidance model cognitive-behavioural risk factors play a crucial role in the development and persistence of pain. However, there is ongoing evidence supporting the necessity for modifications on the model to address how multi-dimensional factors may interact [40,41]. Therefore, an examination of all causal relationships is needed to better understand the key factors that influence pain catastrophizing in older adults.
This study estimates the effects of mood variables (anxiety and depression) on pain catastrophizing in older adults with chronic pain. We also explore the impact of pain intensity and insomnia on mood variables controlled for lifestyle and sociodemographic factors. We hypothesize that these relationships are mediated by mood variables (i.e., anxiety and depression).
2. Materials and Methods
2.1. Study Population
Cohort PainS65+, a Swedish population-based study, focuses on pain aspects and health experiences in the elderly. Data collection for Cohort PainS65+ was a cross-sectional design based on the Swedish Total Population Register (STPR) for the two large cities (Linköping and Norrköping) in a south-eastern county (Östergötland) of Sweden [11,30,42,43]. The STPR consists of ~49,320 older adults and a stratified random sample of 10,000 older adults (≥65 years old) in five age strata (65–69 years, 70–74 years, 75–79 years, 80–84 years, and 85 years and older) was selected by Statistics Sweden (SCB). A postal survey was conducted between October 2012 and January 2013. Two reminders at two-week intervals were mailed if necessary. The study was approved by the Regional Ethics Research Committee in Linköping, Sweden (Dnr: 2012/154-31).
2.2. Measurements
An overview of the validated instruments/scales of the survey has been presented elsewhere [11,30,42,43]. The relevant instruments for this study are described below.
2.2.1. Sociodemographic Data
Age (years), sex (men/women), marital status (married /not married), and highest educational level (university/not) were recorded from the respondents’ answers in the postal survey.
2.2.2. Chronic Pain
We began by asking the following question: Do you usually have pain, either all the time or occasionally? Three answer alternatives were provided: no; yes, with less duration than three months; and yes, with a duration of more than three months. Respondents who selected the third alternative answer were identified as participants with chronic pain. This study presents all other characteristics for this sample.
2.2.3. Pain Intensity
Participants were asked to rate their pain intensity over the previous seven days using an 11-point numeric rating scale (NRS-7d), ranging from 0 (no pain) to 10 (worst imaginable pain) [44].
2.2.4. Pain Catastrophizing
The pain catastrophizing scale (PCS) was used to quantify the catastrophic thinking related to pain [45]. Participants were asked to assess the degree to which they experience certain thoughts or feelings during pain. The PCS consists of 13 items and each item has five answer alternatives on a five-point scale, ranging from 0 (not at all) to 4 (always). A total score (0–52) was determined along with three subscale scores assessing rumination, magnification, and helplessness. We used the total score. However, due to a printing issue, the most negative alternative (4: all the time) was not included in the questionnaire. Therefore, the PCS resulted in a total possible score of 39. We also estimated the reliability of the instrument by calculating the Cronbach alpha (a), and reliability was good (a = 0.75) [11].
2.2.5. Mood (Anxiety and Depression)
We selected the items from the general well-being schedule (GWBS) to assess the mood state of the participants. As developed by Fazio, the GWBS measures psychological well-being and distress over a month [46]. The instrument provided good internal consistency, test-retest reliability, and validity [47,48]. The GWBS can be divided into six subscales. In this study, two subscales, namely anxiety (4 items; range: 0–25) and depression (3 items; range: 0–20), were used and treated as continuous variables. For the subscales of anxiety and depression, low values indicate higher anxiety and depression. These items were reverse scored, so high values indicate higher symptoms of anxiety and depression.
2.2.6. Insomnia
The insomnia severity index (ISI) was used to assess sleep problems. ISI is a reliable and valid instrument for detecting cases of insomnia and has excellent internal consistency [49,50]. Each item is rated on a five-point Likert scale (0–4). A sum of the seven items generates a score between 0 and 28.
2.2.7. Lifestyle Factors
Smoking: We used the instrument Health Curve (Hälsokurvan) [51] to gather data on health behaviours. Participants were asked about smoking habits including frequency (from never to daily). The variable is denoted as current smokers, ex-smokers, or never smokers.
2.2.8. Alcohol Consumption
Participants were asked about their alcohol consumption: Do you drink alcohol regularly? The response was yes or no. For those who replied with yes, we added the four CAGE (Cut-down, Annoy, Guilty, and Eye-opener) questions to evaluate the possible addiction problems [52]. A score between 0 and 1 was defined as low consumption and a score ≥ 2 was regarded as high consumption, indicating potential problems with alcohol addiction.
2.2.9. Weight Status
Body mass index (BMI = weight (kg)/height (m)2) was calculated based on self-reported body height and weight. BMI was classified according to the criteria developed by the World Health Organization (WHO): <18.5 = underweight; 18.5–24.9 = normal range; 25.0–29.9 = overweight; 30.0–34.9 = obesity I; and ≥35.0 = obesity II and III (severe obesity).
2.2.10. Comorbidity
The presence of comorbidity was also captured in the survey. We used a self-reported questionnaire (yes or no responses) covering 12 physical and psychological disorders/conditions over the previous two weeks: traumatic injuries, rheumatoid arthritis and osteoarthritis, cardiovascular disorders, pulmonary disorders, depressive disorders, anxiety disorders, gastrointestinal disorders, central nervous system disorders, urogenital disorders, skin disorders, tumours and cancers, and metabolic disorders [30]. The sum of all confirmed comorbidities for each participant was denoted as number of comorbidities, ranging from 0–12.
2.3. Statistical Analysis
The statistics were performed using the statistical package IBM SPSS Statistics (version 25.0; IBM Inc., New York, USA) and R statistical language and environment (version 3.6.1) using the lavaan package [53]. R is available as free software under the terms of the Free Software Foundation’s GNU General Public License in source code form. All tests with p < 0.05 were considered statistically significant. Descriptive statistics were calculated for all variables. Pearson’s r was used to examine the bivariate relationships among the psychometric scales. Analysis of covariance (ANCOVA) was used to examine the influence of lifestyle and sociodemographic factors on anxiety, depression, and pain catastrophizing. In the next step, the subscales anxiety and depression were examined for their influence on the pain catastrophizing using a path analysis model. Path analysis is a form of multiple regression analysis used to evaluate causal models by examining the relationships between a dependent variable and two or more independent variables. This method estimates both the magnitude and significance of causal links between variables. We used standardized path coefficients (bstd) to estimate effect size [54]: absolute values less than 0.10 indicate a small effect, values around 0.30 indicate a medium effect, and values greater than 0.50 indicate a large effect.
In the path model, the independent variables were pain intensity, insomnia, and lifestyle and sociodemographic characteristics, the dependent variable was pain catastrophizing, and the mediators were anxiety and depression. The basic rules as described in the literature were considered when defining the theoretical model [55,56,57]. To find possible multivariate outliers, we computed the mahalanobis distance (MD) for all cases. The MD measures distance relative to the centroid—a base or central point that can be thought of as an overall mean for multivariate data. The larger the MD, the further away the data point is from the centroid [58].
We tested the path model using the maximum likelihood estimation using the fit indices proposed by Hu and Bentler [59] as well as Barrett [60]. More analytically, we used the Chi-Square (χ2) value, which is the traditional measure for evaluating overall model fit and ‘assesses the magnitude of discrepancy between the sample and fitted covariances matrices [59]. A good model fit should provide an insignificant result at a 0.05 threshold [60]. Other indicators were the Tucker Lewis index (TLI), the normed fit index (NFI), the non-normed fit index (NNFI), the comparative fit index (CFI), the goodness-of-fit index (GFI), and the adjusted goodness-of-fit index (AGFI), which shows the model fit relative to the null model. Typically, all indices are considered acceptable when estimates ≥ 0.90 [59]. We also included the root mean square error of approximation (RMSEA) and the standardized root mean square residual (SRMSR). For both indices, estimates ≤ 0.05 were considered a good fit.
3. Results
The postal survey was completed and returned by 6,611 older adults, corresponding to a response rate of 66.1%, of which 42% reported chronic pain. Data on the survey characteristics for both the whole sample population and respondents without chronic pain have been described in detail elsewhere [11,30,42]. Therefore, our study sample consisted of 2,790 older adults (61.1% women) with chronic pain with partial missingness for 117 (4.2%), 89 (3.2%), 45 (1.6%), and 32 (1.1%) for smoking, education, alcohol, and BMI, respectively. The mean age was 76.2 years (SD: 7.4), 55.8% were married (Table 1), 8.1% smoked, and 5.9% had high alcohol consumption. Details of the demographic characteristics in this study sample (n = 2,790) are also described elsewhere [61,62]. Approximately 40% were overweight and over 20% were classified as obese. The median number of comorbidities was 2.0 (interquartile range (IQR): 1.9–3), ranging from 0 to 11 (Table 1). All the psychometric scales were significantly positively correlated (Supplementary Table S1). Τhe analysis of covariance (ANCOVA) showed that all independent variables were associated with at least one dependent or moderator variable, so all variables were selected for the path analysis (Table 2).
Table 1.
Descriptive characteristics of the sample.
| Characteristic | N (%) |
|---|---|
| Age, mean (SD) | 76.2 (7.4) |
| Sex | |
| Men | 1085 (38.9) |
| Women | 1705 (61.1) |
| Married | |
| Yes | 1557 (55.8) |
| No | 1233 (44.2) |
| University education | |
| Yes | 1213 (44.9) |
| No | 1488 (55.1) |
| Smoking | |
| Current smokers | 226 (8.5) |
| Ex-smokers | 1041(38.9) |
| Never smokers | 1406 (52.6.) |
| BMI | |
| Low/Normal weight (<25) | 1134 (43.1) |
| Overweight | 1017(38.6) |
| Obesity I | 387 (17.4) |
| Obesity II and III (≥35) | 95 (3.6) |
| Alcohol consumption | |
| Low | 1703 (62.0) |
| High | 161 (5.9) |
| No | 881 (32.1) |
| Number of comorbidities, median (IQR) | 2.0 (1.9-3.0) |
| ANX, mean (SD) | 6.2 (4.6) |
| DEP, mean (SD) | 5.5 (3.6) |
| PNI, mean (SD) | 4.9 (2.0) |
| ISI mean, (SD) | 10.2 (3.5) |
| PCS, mean (SD) | 13.3 (7.4) |
PCS: Pain Catastrophizing Scale; PNI: Pain Intensity as measured by numeric rating scale for the previous seven days; ANX: anxiety as measured by General Well-being Schedule; DEP: depression as measured by General Well-being Schedule; ISI: Insomnia Severity Index; BMI: Body Mass Index; IQR: Interquartile range SD: Standard Deviation.
Table 2.
Effect of sociodemographic factors on anxiety, depression subscales, and pain catastrophizing scale §.
| Factor/Scale | ANX | DEP | PCS |
|---|---|---|---|
| Age (AGE) | <0.001 | <0.001 | ns |
| Women (WOM) | <0.001 | 0.027 | 0.005 |
| Married (MRD) | 0.041 | ns | ns |
| University education (EDU) | ns | 0.005 | 0.037 |
| Smoking (SMK) | 0.001 | <0.001 | <0.001 |
| Body Mass Index (BMI) | 0.005 | 0.002 | 0.023 |
| Alcohol consumption (ALC) | <0.001 | <0.001 | <0.001 |
| Number of comorbidities (COM) | <0.001 | <0.001 | <0.001 |
ns: not significant. § Results are derived from analysis of covariance; ns: not significant; ANX: anxiety as measured by General Well-being Schedule; DEP: depression as measured by General Well-being Schedule; PCS: Pain Catastrophizing Scale.
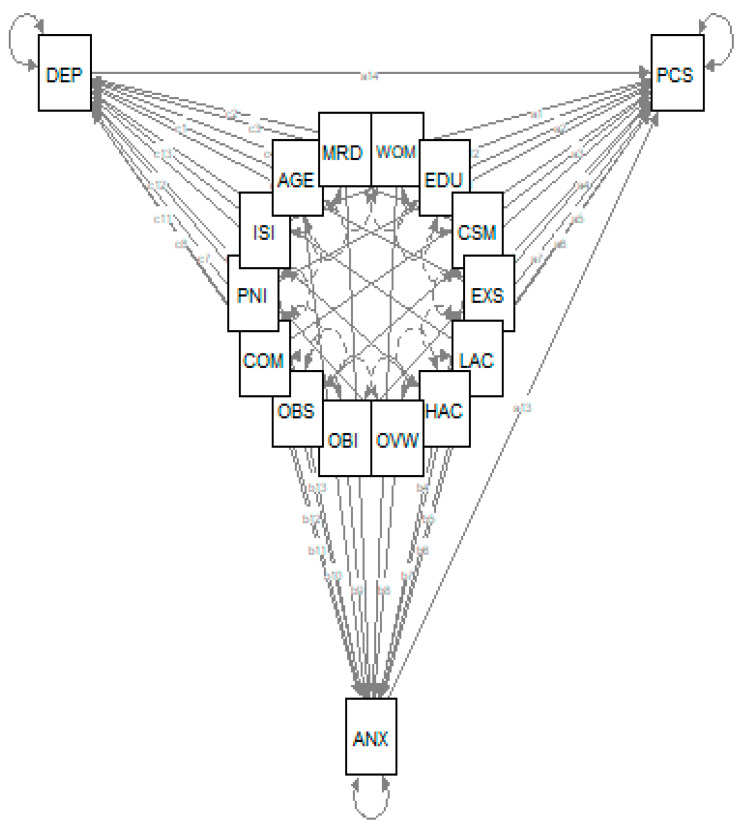
The MD calculation found 205 outliers (Supplementary Figure S1), which were excluded. Hence, the path analysis included 2229 individuals with chronic pain. We formulated and tested the theoretical model as presented in Figure 1. The model offers the possibility of examining all possible interrelations among dependent variable, independent variables, and moderator variables (i.e., anxiety and depression).
Figure 1.
Theoretical model. PCS: Pain Catastrophizing Scale; ANX: anxiety as measured by General Well-being Schedule; DEP: depression as measured by General Well-being Schedule; PNI: Pain Intensity as measured by numeric rating scale for the previous seven days; ISI: Insomnia Severity Index; WOM: Women; MRD: Married; EDU: University education; CSM: Current smokers; EXS: Ex-smokers; OVW: Overweight; OBI: Obesity I; OBS: Obesity II and III; LAC: Low alcohol consumption; HAC: High alcohol consumption; COM: Number of comorbidities.
The model had a very good fit with a non-significant Chi-square (χ2 (4) = 6.212, p = 0.184), indicating that the assumed path model is adequate for the data (i.e., the model and the data are not statistically significantly different). The output of the model indicated a very good fit to the data: χ2 (45) = 3490.184 (p < 0.001); Tucker Lewis index (TLI) = 0.992; normed fit index (NFI) = 0.998; non-normed fit index (NNFI) = 0.992; comparative fit index (CFI) = 0.999; goodness-of-fit index (GFI) = 0.998; adjusted goodness-of-fit index (AGFI) = 0.931; standardized root mean square residual (SRMR) = 0.005; and root mean square error of approximation (RMSEA) = 0.016 (95% C.I. 0.000–0.038). The model accounted for 16.3% of anxiety, 17.1% of depression, and 30.9% of pain catastrophizing variances (Table 3).
Table 3.
Path model’s parameters (1).
| 95% C.I. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | Standardized Regression Coefficients Bstd. (2) | Lower | Upper | Std. Err | z-Value | p-Value | Std.lv (3) | R2 | ||
| PCS ~ | 0.309 | |||||||||
| WOM | 0.305 | 0.021 | −0.222 | 0.832 | 0.269 | 1.134 | 0.257 | 0.305 | ||
| EDU | −0.493 | −0.035 | −1.001 | 0.015 | 0.259 | −1.903 | 0.057 | −0.493 | ||
| CSM | 1.025 | 0.040 | 0.007 | 2.044 | 0.520 | 1.973 | 0.049 | 1.025 | ||
| EXS | 0.599 | 0.041 | 0.060 | 1.137 | 0.275 | 2.180 | 0.029 | 0.599 | ||
| OVW | 0.080 | 0.005 | −0.461 | 0.621 | 0.276 | 0.289 | 0.773 | 0.080 | ||
| OBI | 0.090 | 0.004 | −0.708 | 0.888 | 0.407 | 0.220 | 0.826 | 0.090 | ||
| OBS | 2.002 | 0.051 | 0.437 | 3.567 | 0.799 | 2.507 | 0.012 | 2.002 | ||
| LAC | −1.134 | −0.077 | −1.754 | −0.515 | 0.316 | −3.589 | 0.000 | −1.134 | ||
| HAC | −0.951 | −0.032 | −2.095 | 0.193 | 0.584 | −1.629 | 0.103 | −0.951 | ||
| COM | 0.238 | 0.049 | 0.049 | 0.427 | 0.097 | 2.463 | 0.014 | 0.238 | ||
| PNI | 0.857 | 0.234 | 0.706 | 1.009 | 0.077 | 11.092 | 0.000 | 0.857 | ||
| ISI | 0.107 | 0.052 | 0.023 | 0.190 | 0.043 | 2.511 | 0.012 | 0.107 | ||
| ANX | 0.491 | 0.324 | 0.427 | 0.555 | 0.033 | 15.014 | 0.000 | 0.491 | ||
| DEP | 0.246 | 0.125 | 0.166 | 0.326 | 0.041 | 6.013 | 0.000 | 0.246 | ||
| ANX~ | 0.163 | |||||||||
| AGE | 0.046 | 0.073 | 0.018 | 0.074 | 0.014 | 3.199 | 0.001 | 0.046 | ||
| WOM | 0.592 | 0.062 | 0.209 | 0.975 | 0.196 | 3.028 | 0.002 | 0.592 | ||
| MRD | 0.113 | 0.012 | −0.268 | 0.493 | 0.194 | 0.580 | 0.562 | 0.113 | ||
| CSM | 0.643 | 0.038 | −0.114 | 1.401 | 0.387 | 1.664 | 0.096 | 0.643 | ||
| EXS | 0.060 | 0.006 | −0.329 | 0.449 | 0.198 | 0.303 | 0.762 | 0.060 | ||
| OVW | −0.458 | −0.047 | −0.851 | −0.065 | 0.201 | −2.283 | 0.022 | −0.458 | ||
| OBI | −0.323 | −0.025 | −0.889 | 0.242 | 0.288 | −1.121 | 0.262 | −0.323 | ||
| OBS | 0.124 | 0.005 | −0.907 | 1.156 | 0.526 | 0.236 | 0.813 | 0.124 | ||
| LAC | −0.773 | −0.079 | −1.198 | −0.348 | 0.217 | −3.562 | 0.000 | −0.773 | ||
| HAC | 0.826 | 0.042 | −0.092 | 1.744 | 0.468 | 1.763 | 0.078 | 0.826 | ||
| COM | 0.390 | 0.122 | 0.252 | 0.527 | 0.070 | 5.558 | 0.000 | 0.390 | ||
| PNI | 0.397 | 0.165 | 0.293 | 0.502 | 0.053 | 7.462 | 0.000 | 0.397 | ||
| ISI | 0.313 | 0.230 | 0.252 | 0.373 | 0.031 | 10.174 | 0.000 | 0.313 | ||
| DEP~ | 0.171 | |||||||||
| AGE | 0.082 | 0.169 | 0.061 | 0.103 | 0.011 | 7.751 | 0.000 | 0.082 | ||
| WOM | 0.298 | 0.040 | 0.003 | 0.594 | 0.151 | 1.977 | 0.048 | 0.298 | ||
| EDU | −0.392 | −0.054 | −0.677 | −0.108 | 0.145 | −2.704 | 0.007 | −0.392 | ||
| CSM | 0.821 | 0.063 | 0.266 | 1.376 | 0.283 | 2.901 | 0.004 | 0.821 | ||
| EXS | 0.226 | 0.030 | −0.072 | 0.523 | 0.152 | 1.486 | 0.137 | 0.226 | ||
| OVW | −0.356 | −0.048 | −0.660 | −0.052 | 0.155 | −2.297 | 0.022 | −0.356 | ||
| OBI | −0.091 | −0.009 | −0.525 | 0.344 | 0.222 | −0.408 | 0.683 | −0.091 | ||
| OBS | 0.448 | 0.022 | −0.407 | 1.304 | 0.436 | 1.027 | 0.304 | 0.448 | ||
| LAC | −0.759 | −0.101 | −1.097 | −0.422 | 0.172 | −4.405 | 0.000 | −0.759 | ||
| HAC | 0.630 | 0.042 | −0.087 | 1.346 | 0.366 | 1.722 | 0.085 | 0.630 | ||
| COM | 0.267 | 0.108 | 0.164 | 0.370 | 0.052 | 5.096 | 0.000 | 0.267 | ||
| PNI | 0.172 | 0.092 | 0.094 | 0.250 | 0.040 | 4.324 | 0.000 | 0.172 | ||
| ISI | 0.227 | 0.216 | 0.182 | 0.273 | 0.023 | 9.852 | 0.000 | 0.227 | ||
(1) TLI = 0.992, NFI = 0.998, NNFI = 0.992, CFI = 0.999, GFI = 0.998, SRMR = 0.005, RMSEA = 0.016. CI: Confidence Interval; PCS: Pain Catastrophizing Scale; ANX: anxiety as measured by General Well-being Schedule; DEP: depression as measured by General Well-being Schedule; PNI: Pain Intensity as measured by numeric rating scale for the previous seven days; ISI: Insomnia Severity Index; WOM: Women; MRD: Married; EDU: University education; CSM: Current smokers; EXS: Ex-smokers; OVW: Overweight; OBI: Obesity I; OBS: Obesity II and III; LAC: Low alcohol consumption; HAC: High alcohol consumption; COM: Number of comorbidities. (2) Completely standardized solution (estimates of parameters if the variances are unity). (3) Dependent Variable is standardized.
3.1. Direct Effects on Pain Catastrophizing
The overall findings from path analysis are illustrated in Table 4, and the standardized regression coefficients (bstd) are presented in Table 3. None of the sociodemographic factors (age, gender, marital status, and education) had a direct significant effect on pain catastrophizing. The lifestyle factors current smokers, ex-smokers, and obesity II and III had a significant direct positive effect on pain catastrophizing (bstd = 0.040, p = 0.048; bstd = 0.041, p = 0.029; and bstd = 0.051, p = 0.012, respectively), and low alcohol consumption (compared to non-drinkers) had a significant direct negative effect on pain catastrophizing (bstd = −0.077, p < 0.001). Number of comorbidities, pain intensity, and insomnia also had significant positive effects on pain catastrophizing (bstd = 0.049, p = 0.014; bstd = 0.234, p = 0.000; and bstd = 0.052, p = 0.012, respectively). Both anxiety and depression had a significant direct positive effect on pain catastrophizing (bstd = 0.324, p < 0.001; bstd = 0.125, p < 0.001), although anxiety had a greater effect than depression.
Table 4.
Results of path analysis (direct effects)-schematic presentation.
| Factor/Scale | ANX | DEP | PCS |
|---|---|---|---|
| Age (AGE) | ↑ | ↑ | ns |
| Women (WOM) | ↑ | ↑ | ns |
| Married (MRD) | ns | ns | ns |
| University education (EDU) | ns | ↓ | ns |
| Current smokers (CSM) | ns | ↑ | ↑ |
| Ex-smokers (EXS) | ns | ns | ↑ |
| Overweight (OVW) | ↓ | ↓ | ns |
| Obesity I (OBI) | ns | ns | ns |
| Obesity II and III (OBS) | ns | ns | ↑ |
| Low alcohol consumption (LAC) | ↓ | ↓ | ↓ |
| High alcohol consumption (HAC) | ns | ns | ns |
| Number of comorbidities (COM) | ↑ | ↑ | ↑ |
| PNI | ↑ | ↑ | ↑ |
| ISI | ↑ | ↑ | ↑ |
| ANX | ↑ | ||
| DEP | ↑ |
ns: not significant; ↑: positive relationship; ↓: negative relationship. PCS: Pain Catastrophizing Scale; PNI: Pain Intensity as measured by numeric rating scale for the previous seven days; ANX: anxiety as measured by General Well-being Schedule; DEP: depression as measured by General Well-being Schedule; ISI: Insomnia Severity Index.
3.2. Indirect Effects on Pain Catastrophizing
These results are presented in Supplementary Table S2. Female gender and number of comorbidities had a significant indirect positive effect on pain catastrophizing through pain intensity (p = 0.032 and p < 0.001). Low alcohol consumption had a significant indirect negative effect on pain catastrophizing through pain intensity (p = 0.003).
3.3. Direct Effects on Anxiety
These results are presented in Table 3. Age, being a woman, number of comorbidities, pain intensity, and insomnia had a direct significant positive effect on anxiety (bstd = 0.073, p =0.01; bstd = 0.062, p = 0.002; bstd = 0.122, p < 0.001; bstd = 0.165, p < 0.001; and bstd = 0.23, p < 0.001, respectively), while overweight and low alcohol consumption (compared to non-drinkers) had a direct negative effect on anxiety (bstd = −0.047, p = 0.022 and bstd = −0.079, p < 0.001, respectively).
3.4. Direct Effects on Depression
These results are presented in Table 3. Age, being a woman, current smoking, number of comorbidities, pain intensity, and insomnia had a direct significant positive effect on depression (bstd = 0.169, p < 0.001; bstd = 0.040, p = 0.048; bstd = 0.063, p = 0.004; bstd = 0.108, p < 0.001; bstd = 0.092, p < 0.001; and bstd = 0.216, p < 0.001, respectively). University education, overweight, and low alcohol consumption had a direct significant negative effect on depression (bstd = −0.054, p = 0.007; bstd = −0.048, p = 0.021; and bstd = −0.101, p < 0.001, respectively).
4. Discussion
The present study addresses the pain catastrophizing and mood (anxiety and depression) in older adults with chronic pain affected by pain intensity, insomnia, comorbidities, as well as lifestyle and sociodemographic factors. Using a mediation analysis to control for lifestyle and sociodemographic factors, we found direct and/or indirect effects on both pain catastrophizing and mood. Mood aspects mediate the relationships between pain catastrophizing and other factors. Anxiety had the largest effect on pain catastrophizing.
Pain catastrophizing was directly affected by pain intensity, insomnia, and number of comorbidities. In line with other studies, these variables also had direct effects on mood [63,64,65,66]. The significant contribution of pain intensity on pain catastrophizing is not only its direct influence but also its availability for other variables to affect pain catastrophizing indirectly, despite their relatively weaker effects in comparison with the direct contributions. Through pain intensity, the number of comorbidities also showed indirect effects on pain catastrophizing. This finding suggests some painful comorbidities influence pain catastrophizing. Additionally, as multimorbidity is more common in older people than in younger people, pain catastrophizing may be more preferentially related to pain intensity.
Lifestyle and sociodemographic factors (female gender and low alcohol consumption) via pain intensity also had indirect impacts on pain catastrophizing. It should be noted, sex difference or alcohol habits affect pain catastrophizing as well as pain intensity. Surprisingly, insomnia did not affect pain catastrophizing through pain intensity. In younger patients with chronic pain, insomnia had, via pain, indirect effects on pain catastrophizing [35]. Low correlation between insomnia and pain intensity has also been reported in the clinical patient cohorts [34]. As older people’s sleep problems are usually multifactorial rather than pain specific [67], sleep problems in older patients deserve more attention, regardless of whether pain intensity is affecting pain catastrophizing. That is, older patients need treatment strategies that younger patients may not.
Our model shows that mood aspects have strong direct effects on pain catastrophizing. A preclinical study suggested that mood had negative effects on cognition and pain [68]. Particularly, we found that anxiety had a larger effect than depression, which is partly reflected by the conceptual work of Flink and his colleges that pain catastrophizing is similar to worries and therefore overlaps with anxiety [69]. Notably, anxiety and depression had high correlation to each other, indicating non-trivial symptoms overlapping in older adults with chronic pain. Therefore, it is reasonable to expect that similar factors influenced both anxiety and depression.
Chronic pain often coexists with anxiety, depression, and pain catastrophizing [38,70,71]. Their concurrent associations as well as the predictive value of pain catastrophizing on poor outcomes for patients with chronic pain have been demonstrated in previous studies [14,19,20,21,23]. Changes in levels of pain catastrophizing by reducing emotional distress and maladaptive behaviours can result in better pain-related outcomes [72]. In this study, we also noted that several factors—including pain intensity, insomnia, comorbidities, and low alcohol consumption—directly affected pain catastrophizing and mood variables. Moreover, some lifestyle and sociodemographic factors significantly affected mood in different directions. To explore how mood mediates pain catastrophizing, our model controlled the parallel influences such as the effects of pain intensity and insomnia on mood and the effects of lifestyle and sociodemographic factors on mood as well as pain catastrophizing. We also quantified the magnitude and significance of links between variables. Clearly, the complex relationship between pain aspects, mood, and pain catastrophizing were influenced simultaneously by lifestyle and sociodemographic factors. Understanding these relationships provides us with the possibility of establishing prevention and management strategies for chronic pain in old age. These relationships include the following: never being a smoker associated with less pain catastrophizing in later life, smoking cessation is associated with decreased depressive symptoms, and weight control is associated with less pain catastrophizing and better mood. Interestingly, little but not too much alcohol (low alcohol consumption) is associated with better mood and less pain catastrophizing in this older population. The debate about pros and cons of alcohol consumption is long lasting [73,74,75]. A low dose of alcohol in contrast to a higher dose seems to be beneficial with respect to mood and pain catastrophizing, but it also might indicate a harmful effort to cope with emotional pain [76].
This study has several limitations. First, a cross-sectional study design does not allow for forming conclusions about causality. We could not know whether anxiety and depression developed after chronic pain or the mood disorder existed before pain debut. We did not know whether older people with chronic pain used alcohol as a strategy to cope with pain, pain catastrophizing or mood disorder. Thus, we assumed a linear association among the variables included in the path analysis model. Second, the non-response bias should be considered as frail older people often have severe impaired cognitive function. The study procedure might also have biased the voluntary sample, because the study design unintentionally selected participants who were strongly interested in the pain and pain-related issues. Therefore, the generalization of our results has its limitations. Third, although we measured most factors that negatively influence chronic pain, we did not measure some important psychological aspects, especially positive or protective factors (i.e., self-efficacy, locus of control, and cognitive coping and appraisal) [27,77]. Finally, the technical error in the response alternatives of the pain catastrophizing scale accompanied by the medium response rate may underestimate our results. In addition, different pain conditions could have distinct psychological profiles [37,78] so future research ought to consider these aspects too.
5. Conclusions
This study highlights the importance of pain catastrophizing in older adults with chronic pain. Mood aspects play mediating roles in the relationship between pain catastrophizing and other pain-related factors (pain intensity, insomnia, and comorbidities), accounting for lifestyle and sociodemographic factors. Our findings also stress the importance of treating emotional distress in older adults with pain to increase awareness of potentially harmful self-coping strategies (e.g., alcohol) in this target group. Overall, these findings provide for the possibility of pain prevention and management strategies targeted for older patients.
Acknowledgments
The authors thank all the participants.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/7/2073/s1, Table S1: Correlation of psychometric scales, Table S2: Indirect effects upon pain catastrophizing (PCS), Figure S1: The mahalanobis distance (MD) for all cases.
Author Contributions
Conceptualization, H.-J.D., B.G., and E.D.; methodology, H.-J.D., B.G., L.B., L.-Å.L., and E.D.; data curation, H.-J.D., B.G., and E.D.; formal analysis, E.D.; Validation, H.-J.D. and B.G.; writing original draft, H.-J.D. and E.D.; Writing review and editing, H.-J.D., B.G., L.B., L-Å.L., and E.D. All authors commented on different versions of the article and all authors have approved the final version of the manuscript.
Funding
The present study was sponsored by a grant from Grünenthal Sweden AB. The sponsor of the study had no role in the study design, data collection, data analysis, data interpretation, writing ofthe report, or the decision to submit for publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Schofield P. The Assessment of Pain in Older People: UK National Guidelines. Age Ageing. 2018;47:i1–i22. doi: 10.1093/ageing/afx192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdulla A., Adams N., Bone M., Elliott A., Gaffin J., Jones D., Knaggs R., Martin D., Sampson L., Schofield P. Guidance on the management of pain in older people. Age Ageing. 2013;42:i1–i57. doi: 10.1093/ageing/afs199. [DOI] [PubMed] [Google Scholar]
- 3.Gerdle B., Björk J., Henriksson C., Bengtsson A. Prevalence of current and chronic pain and their influences upon work and healthcare-seeking: A population study. J. Rheumatol. 2004;31:1399–1406. [PubMed] [Google Scholar]
- 4.Aguera-Ortiz L., Failde I., Cervilla J., Mico J.A. Unexplained pain complaints and depression in older people in primary care. J. Nutr. Heal. Aging. 2013;17:574–577. doi: 10.1007/s12603-013-0012-0. [DOI] [PubMed] [Google Scholar]
- 5.López A.L., Montorio I., Izal M., Velasco L. The role of psychological variables in explaining depression in older people with chronic pain. Aging Ment. Heal. 2008;12:735–745. doi: 10.1080/13607860802154408. [DOI] [PubMed] [Google Scholar]
- 6.O’Regan C., Kearney P.M., Savva G.M., Cronin H., Kenny R.A. Age and sex differences in prevalence and clinical correlates of depression: First results from the Irish Longitudinal Study on Ageing. Int. J. Geriatr. Psychiatry. 2013;28:1280–1287. doi: 10.1002/gps.3955. [DOI] [PubMed] [Google Scholar]
- 7.El-Gabalawy R., MacKenzie C.S., Shooshtari S., Sareen J. Comorbid physical health conditions and anxiety disorders: A population-based exploration of prevalence and health outcomes among older adults. Gen. Hosp. Psychiatry. 2011;33:556–564. doi: 10.1016/j.genhosppsych.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Foley D., Ancoli-Israel S., Britz P., Walsh J. Sleep disturbances and chronic disease in older adults. J. Psychosom. Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Schechtman K.B., Kutner N.G., Wallace R.B., Buchner D.M., Ory M.G. Gender, self-reported depressive symptoms, and sleep disturbance among older community-dwelling persons. J. Psychosom. Res. 1997;43:513–527. doi: 10.1016/S0022-3999(97)00117-7. [DOI] [PubMed] [Google Scholar]
- 10.Kovacs F.M., Noguera J., Abraira V., Royuela A., Cano A., Gil Del Real M.T., Zamora J., Gestoso M., Muriel A., Mufraggi N., et al. The Influence of Psychological Factors on Low Back Pain-Related Disability in Community Dwelling Older Persons. Pain Med. 2008;9:871–880. doi: 10.1111/j.1526-4637.2008.00518.x. [DOI] [PubMed] [Google Scholar]
- 11.Larsson B., Gerdle B., Bernfort L., Levin L.-Å., Dragioti E. Distinctive subgroups derived by cluster analysis based on pain and psychological symptoms in Swedish older adults with chronic pain—A population study (PainS65+) BMC Geriatr. 2017;17:200. doi: 10.1186/s12877-017-0591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lumley M.A., Cohen J.L., Borszcz G.S., Cano A., Radcliffe A.M., Porter L.S., Schubiner H., Keefe F.J. Pain and emotion: A biopsychosocial review of recent research. J. Clin. Psychol. 2011;67:942–968. doi: 10.1002/jclp.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bushnell M.C., Ceko M., Low L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013;14:502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellingson L.D., Stegner A.J., Schwabacher I.J., Lindheimer J.B., Cook D.B. Catastrophizing Interferes with Cognitive Modulation of Pain in Women with Fibromyalgia. Pain Med. 2018;19:2408–2422. doi: 10.1093/pm/pny008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bäckryd E., Persson E.B., Larsson A.I., Fischer M.R., Gerdle B. Chronic pain patients can be classified into four groups: Clustering-based discriminant analysis of psychometric data from 4665 patients referred to a multidisciplinary pain centre (a SQRP study) PLoS ONE. 2018;13:e0192623. doi: 10.1371/journal.pone.0192623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell C.M., Buenaver L.F., Finan P., Bounds S.C., Redding M., McCauley L., Robinson M., Edwards R.R., Smith M.T. Sleep, Pain Catastrophizing, and Central Sensitization in Knee Osteoarthritis Patients With and Without Insomnia. Arthritis Rheum. 2015;67:1387–1396. doi: 10.1002/acr.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vlaeyen J.W., Linton S.J. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 18.Vlaeyen J.W.S., Crombez G. Behavioral Conceptualization and Treatment of Chronic Pain. Annu. Rev. Clin. Psychol. 2020;16:187–212. doi: 10.1146/annurev-clinpsy-050718-095744. [DOI] [PubMed] [Google Scholar]
- 19.Meints S., Mawla I., Napadow V., Kong J., Gerber J., Chan S.-T., Wasan A.D., Kaptchuk T.J., McDonnell C., Carriere J.S., et al. The relationship between catastrophizing and altered pain sensitivity in patients with chronic low-back pain. Pain. 2019;160:833–843. doi: 10.1097/j.pain.0000000000001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Racine M., Moulin D.E., Nielson W.R., Morley-Forster P.K., Lynch M., Clark A.J., Stitt L., Gordon A., Nathan H., Smyth C., et al. The reciprocal associations between catastrophizing and pain outcomes in patients being treated for neuropathic pain. Pain. 2016;157:1946–1953. doi: 10.1097/j.pain.0000000000000594. [DOI] [PubMed] [Google Scholar]
- 21.Campbell C., McCauley L., Bounds S.C., Mathur V.A., Conn L., Simango M., Edwards R., Fontaine K.R. Changes in pain catastrophizing predict later changes in fibromyalgia clinical and experimental pain report: Cross-lagged panel analyses of dispositional and situational catastrophizing. Arthritis Res. Ther. 2012;14:R231. doi: 10.1186/ar4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhaoyang R., Martire L.M., Darnall B.D. Daily pain catastrophizing predicts less physical activity and more sedentary behavior in older adults with osteoarthritis. Pain. 2020 doi: 10.1097/j.pain.0000000000001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Calderon J., Jensen M.P., Morales-Asencio J.M., Luque-Suarez A. Pain Catastrophizing and Function In Individuals With Chronic Musculoskeletal Pain. Clin. J. Pain. 2019;35:279–293. doi: 10.1097/AJP.0000000000000676. [DOI] [PubMed] [Google Scholar]
- 24.Hülsebusch J., Hasenbring M.I., Rusu A.C. Understanding Pain and Depression in Back Pain: The Role of Catastrophizing, Help-/Hopelessness, and Thought Suppression as Potential Mediators. Int. J. Behav. Med. 2015;23:251–259. doi: 10.1007/s12529-015-9522-y. [DOI] [PubMed] [Google Scholar]
- 25.Wood B.M., Nicholas M.K., Blyth F., Asghari A., Gibson S. Catastrophizing Mediates the Relationship Between Pain Intensity and Depressed Mood in Older Adults With Persistent Pain. J. Pain. 2013;14:149–157. doi: 10.1016/j.jpain.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Sturgeon J.A., Zautra A.J. Psychological Resilience, Pain Catastrophizing, and Positive Emotions: Perspectives on Comprehensive Modeling of Individual Pain Adaptation. Curr. Pain Headache Rep. 2013;17:317. doi: 10.1007/s11916-012-0317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng S.-T., Leung C.M.C., Chan K.L., Chen P.P., Chow Y.F., Chung J., Law A.C.B., Lee J.S.W., Leung E.M.F., Tam C.W.C. The relationship of self-efficacy to catastrophizing and depressive symptoms in community-dwelling older adults with chronic pain: A moderated mediation model. PLoS ONE. 2018;13:e0203964. doi: 10.1371/journal.pone.0203964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood B.M., Nicholas M.K., Blyth F., Asghari A., Gibson S. The mediating role of catastrophizing in the relationship between pain intensity and depressed mood in older adults with persistent pain: A longitudinal analysis. Scand. J. Pain. 2016;11:157–162. doi: 10.1016/j.sjpain.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Benyon K., Muller S., Hill S., Mallen C. Coping strategies as predictors of pain and disability in older people in primary care: A longitudinal study. BMC Fam. Pr. 2013;14:67. doi: 10.1186/1471-2296-14-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dragioti E., Larsson B., Bernfort L., Levin L., Gerdle B. Prevalence of different pain categories based on pain spreading on the bodies of older adults in Sweden: A descriptive-level and multilevel association with demographics, comorbidities, medications, and certain lifestyle factors (PainS65+) J. Pain Res. 2016;9:1131–1141. doi: 10.2147/JPR.S119845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Y., Hooten M.W., Roberts R.O., Warner D.O. Modifiable risk factors for incidence of pain in older adults. Pain. 2010;151:366–371. doi: 10.1016/j.pain.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 32.Van Hecke O., Torrance N., Smith B.H. Chronic pain epidemiology – where do lifestyle factors fit in? Br. J. Pain. 2013;7:209–217. doi: 10.1177/2049463713493264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dragioti E., Levin L.-Å., Bernfort L., Larsson B., Gerdle B. Insomnia severity and its relationship with demographics, pain features, anxiety, and depression in older adults with and without pain: Cross-sectional population-based results from the PainS65+ cohort. Ann. Gen. Psychiatry. 2017;16:15. doi: 10.1186/s12991-017-0137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alföldi P., Wiklund T., Gerdle B. Comorbid insomnia in patients with chronic pain: A study based on the Swedish quality registry for pain rehabilitation (SQRP) Disabil. Rehabilitation. 2013;36:1661–1669. doi: 10.3109/09638288.2013.864712. [DOI] [PubMed] [Google Scholar]
- 35.Wilt J.A., Davin S., Scheman J., Information P.E.K.F.C. A multilevel path model analysis of the relations between sleep, pain, and pain catastrophizing in chronic pain rehabilitation patients. Scand. J. Pain. 2016;10:122–129. doi: 10.1016/j.sjpain.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 36.Craner J.R., Sperry J.A., Evans M.M. The Relationship Between Pain Catastrophizing and Outcomes of a 3-Week Comprehensive Pain Rehabilitation Program. Pain Med. 2016;17:2026–2035. doi: 10.1093/pm/pnw070. [DOI] [PubMed] [Google Scholar]
- 37.Edwards R., Cahalan C., Mensing G., Smith M., Haythornthwaite J.A. Pain, catastrophizing, and depression in the rheumatic diseases. Nat. Rev. Rheumatol. 2011;7:216–224. doi: 10.1038/nrrheum.2011.2. [DOI] [PubMed] [Google Scholar]
- 38.Ruscheweyh R., Nees F., Marziniak M., Evers S., Flor H., Knecht S. Pain Catastrophizing and Pain-related Emotions. Clin. J. Pain. 2011;27:578–586. doi: 10.1097/AJP.0b013e31820fde1b. [DOI] [PubMed] [Google Scholar]
- 39.Wittink H., Rogers W., Lipman A., McCarberg W., Ashburn M., Oderda G., Carr D. Older and younger adults in pain management programs in the United States: Differences and similarities. J. Pain. 2005;6:S72. doi: 10.1016/j.jpain.2005.01.286. [DOI] [PubMed] [Google Scholar]
- 40.Wideman T.H., Asmundson G.G.J., Smeets R.J.E.M., Zautra A.J., Simmonds M.J., Sullivan M.J.L., Haythornthwaite J.A., Edwards R.R. Rethinking the fear avoidance model: Toward a multidimensional framework of pain-related disability. Pain. 2013;154:2262–2265. doi: 10.1016/j.pain.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wideman T.H., Adams H., Sullivan M.J. A prospective sequential analysis of the fear-avoidance model of pain. Pain. 2009;145:45–51. doi: 10.1016/j.pain.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 42.Bernfort L., Gerdle B., Rahmqvist M., Husberg M., Levin L.-Å. Severity of chronic pain in an elderly population in Sweden—impact on costs and quality of life. Pain. 2015;156:521–527. doi: 10.1097/01.j.pain.0000460336.31600.01. [DOI] [PubMed] [Google Scholar]
- 43.Dong H.-J., Larsson B., Levin L.-Å., Bernfort L., Gerdle B. Is excess weight a burden for older adults who suffer chronic pain? BMC Geriatr. 2018;18:270. doi: 10.1186/s12877-018-0963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farrar J.T., Young J.P., Lamoreaux L., Werth J.L., Poole M.R. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan M.J.L., Bishop S.R., Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol. Assess. 1995;7:524–532. doi: 10.1037/1040-3590.7.4.524. [DOI] [Google Scholar]
- 46.Fazio A.F. A concurrent validational study of the NCHS General Well-Being Schedule. Vital Health Stat 2. 1977;2:1–53. [PubMed] [Google Scholar]
- 47.Wang M., Wang S., Zhang X., Xia Q., Cai G., Yang X., Li X., Wang L., Xin L., Xu S., et al. Analysis on the situation of subjective well-being and its influencing factors in patients with ankylosing spondylitis. Heal. Qual. Life Outcomes. 2016;14:118. doi: 10.1186/s12955-016-0522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leonardson G.R., Daniels M.C., Ness F.K., Kemper E., Mihura J.L., Koplin B.A., Foreyt J.P. Validity and reliability of the general well-being schedule with northern plains American Indians diagnosed with type 2 diabetes mellitus. Psychol. Rep. 2003;93:49–58. doi: 10.2466/PR0.93.5.49-58. [DOI] [PubMed] [Google Scholar]
- 49.Morin C.M., Belleville G., Bélanger L., Ivers H. The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep. 2011;34:601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bastien C. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/S1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 51.Persson L.G., Lindström K., Lingfors H., Bengtsson C. A study of men aged 33-42 in Habo, Sweden with special reference to cardiovascular risk factors. Design, health profile and characteristics of participants and non-participants. Scand. J. Soc. Med. 1994;22:264–272. doi: 10.1177/140349489402200405. [DOI] [PubMed] [Google Scholar]
- 52.Persson L.G., Lindstrom K., Lingfors H., Bengtsson C., Lissner L. Cardiovascular risk during early adult life. Risk markers among participants in “Live for Life” health promotion programme in Sweden. J. Epidemiol. Community Heal. 1998;52:425–432. doi: 10.1136/jech.52.7.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosseel Y. Iavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012;48 doi: 10.18637/jss.v048.i02. [DOI] [Google Scholar]
- 54.Kline R.B. Principles and Practice of Structural Equation Modeling. 2nd ed. Guilford Press; New York, NY, USA: 2005. [Google Scholar]
- 55.Baron R.M., Kenny D. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 56.Judd C.M., Kenny D.A. Process Analysis: Estimating Mediation in Treatment Evaluations. Eval. Rev. 1981;5:602–619. doi: 10.1177/0193841X8100500502. [DOI] [Google Scholar]
- 57.James L.R., Brett J.M. Mediators, moderators, and tests for mediation. J. Appl. Psychol. 1984;69:307–321. doi: 10.1037/0021-9010.69.2.307. [DOI] [Google Scholar]
- 58.Brereton R.G. The chi squared and multinormal distributions. J. Chemom. 2014;29:9–12. doi: 10.1002/cem.2680. [DOI] [Google Scholar]
- 59.Hu L., Bentler P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. A Multidiscip. J. 1999;6:1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- 60.Barrett P. Structural equation modelling: Adjudging model fit. Pers. Individ. Differ. 2007;42:815–824. doi: 10.1016/j.paid.2006.09.018. [DOI] [Google Scholar]
- 61.Dong H.-J., Larsson B., Dragioti E., Bernfort L., Levin L.-Å., Gerdle B. Factors Associated with Life Satisfaction in Older Adults with Chronic Pain (PainS65+) J. Pain Res. 2020;13:475–489. doi: 10.2147/JPR.S234565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dragioti E., Bernfort L., Larsson B., Gerdle B., Levin L. Association of insomnia severity with well-being, quality of life and health care costs: A cross-sectional study in older adults with chronic pain (PainS65+) Eur. J. Pain. 2017;22:414–425. doi: 10.1002/ejp.1130. [DOI] [PubMed] [Google Scholar]
- 63.Li J.-X. Pain and depression comorbidity: A preclinical perspective. Behav. Brain Res. 2015;276:92–98. doi: 10.1016/j.bbr.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nicolson S.E., Caplan J.P., Williams D.E., Stern T.A. Comorbid Pain, Depression, and Anxiety. Harv. Rev. Psychiatry. 2009;17:407–420. doi: 10.3109/10673220903463226. [DOI] [PubMed] [Google Scholar]
- 65.Tsang A., Von Korff M., Lee S., Alonso J., Karam E., Angermeyer M.C., Borges G., Bromet E.J., De Girolamo G., De Graaf R., et al. Common Chronic Pain Conditions in Developed and Developing Countries: Gender and Age Differences and Comorbidity With Depression-Anxiety Disorders. J. Pain. 2008;9:883–891. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 66.Leong I., Farrell M.J., Helme R.D., Gibson S.J. The relationship between medical comorbidity and self-rated pain, mood disturbance, and function in older people with chronic pain. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007;62:550–555. doi: 10.1093/gerona/62.5.550. [DOI] [PubMed] [Google Scholar]
- 67.Wolkove N., Elkholy O., Baltzan M., Palayew M. Sleep and aging: 1. Sleep disorders commonly found in older people. Can. Med. Assoc. J. 2007;176:1299–1304. doi: 10.1503/cmaj.060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berna C., Leknes S., Holmes E.A., Edwards R.R., Goodwin G.M., Tracey I. Induction of Depressed Mood Disrupts Emotion Regulation Neurocircuitry and Enhances Pain Unpleasantness. Biol. Psychiatry. 2010;67:1083–1090. doi: 10.1016/j.biopsych.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 69.Flink I.K., Boersma K., Linton S.J. Pain Catastrophizing as Repetitive Negative Thinking: A Development of the Conceptualization. Cogn. Behav. Ther. 2013;42:215–223. doi: 10.1080/16506073.2013.769621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loevinger B.L., Shirtcliff E.A., Müller D., Alonso C., Coe C.L. Delineating psychological and biomedical profiles in a heterogeneous fibromyalgia population using cluster analysis. Clin. Rheumatol. 2011;31:677–685. doi: 10.1007/s10067-011-1912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirsch O., Strauch K., Held H., Redaelli M., Chenot J.-F., Leonhardt C., Keller S., Baum E., Pfingsten M., Hildebrandt J., et al. Low Back Pain Patient Subgroups in Primary Care. Clin. J. Pain. 2014;30:1023–1032. doi: 10.1097/AJP.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 72.Sullivan M.J., Stanish W.D. Psychologically Based Occupational Rehabilitation: The Pain-Disability Prevention Program. Clin. J. Pain. 2003;19:97–104. doi: 10.1097/00002508-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 73.Dufour M.C., Archer L., Gordis E. Alcohol and the elderly. Clin. Geriatr. Med. 1992;8:127–141. doi: 10.1016/S0749-0690(18)30502-0. [DOI] [PubMed] [Google Scholar]
- 74.Brennan P.L., Schutte K.K., Moos R.H. Pain and use of alcohol to manage pain: Prevalence and 3-year outcomes among older problem and non-problem drinkers. Addiction. 2005;100:777–786. doi: 10.1111/j.1360-0443.2005.01074.x. [DOI] [PubMed] [Google Scholar]
- 75.Yang H., Haldeman S. Behavior-Related Factors Associated With Low Back Pain in the US Adult Population. Spine. 2018;43:28–34. doi: 10.1097/BRS.0000000000001665. [DOI] [PubMed] [Google Scholar]
- 76.Thompson T., Oram C., Correll C.U., Tsermentseli S., Stubbs B. Analgesic Effects of Alcohol: A Systematic Review and Meta-Analysis of Controlled Experimental Studies in Healthy Participants. J. Pain. 2016;18:499–510. doi: 10.1016/j.jpain.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 77.Linton S.J., Shaw W.S. Impact of Psychological Factors in the Experience of Pain. Phys. Ther. 2011;91:700–711. doi: 10.2522/ptj.20100330. [DOI] [PubMed] [Google Scholar]
- 78.Morone N.E., Karp J.F., Lynch C.S., Bost J.E., El Khoudary S.R., Weiner D.K. Impact of chronic musculoskeletal pathology on older adults: A study of differences between knee OA and low back pain. Pain Med. 2009;10:693–701. doi: 10.1111/j.1526-4637.2009.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.