Abstract
Colorectal cancer (CRC) is one of the most frequent and deadly forms of cancer. About half of patients are affected by metastasis, with the cancer spreading to e.g., liver, lungs or the peritoneum. The majority of these patients cannot be cured despite steady advances in treatment options. Immunotherapies are currently not widely applicable for this disease, yet show potential in preclinical models and clinical translation. The tumour microenvironment (TME) has emerged as a key factor in CRC metastasis, including by means of immune evasion—forming a major barrier to effective immuno-oncology. Several approaches are in development that aim to overcome the immunosuppressive environment and boost anti-tumour immunity. Among them are vaccination strategies, cellular transplantation therapies, and targeted treatments. Given the complexity of the system, we argue for rational design of combinatorial therapies and consider the implications of precision medicine in this context.
Keywords: metastasis, checkpoint blockade, TGF-β, immune suppression, solid tumour, tumor microenvironment
1. Introduction
The intestine is a vital organ for food digestion and has an important barrier function. This comes with an intricately balanced immune system that acts against pathogens, while tolerating beneficial microbiota as well as the foreign molecule antigens present in our food. Furthermore, immune cells can also detect and eliminate transformed cells to prevent the development of tumours, a process that is called immunosurveillance [1,2]. Successful cancers escape this mechanism in ways that are poorly understood [3]. Moreover, inflammatory signalling has also been linked to the formation and progression of tumours [4,5,6]. Although the underlying biology of cancer metastasis still has many aspects that remain incompletely explained, recent decades have seen a lot of progress—including in the field of cancer immunology. Unfortunately, colorectal cancer (CRC) has so far been mostly refractory to immunotherapies in the clinic. Nevertheless, accumulating evidence suggests that this will change. In this review, we give an immuno-oncological overview for this disease and describe immunotherapeutic strategies currently under development.
1.1. Tumorigenesis and Disease Progression
CRC is an adenocarcinoma that originates in epithelial cells of the large intestine. These epithelial cells are organized as a single-cell layer lining the inner surface of the intestinal tube, and are surrounded by a complex stroma that consists of supportive tissue, immune infiltrates, blood- and lymph vessels, neurons, and muscles. Different routes have been described that contribute to the transformation of normal intestinal epithelium into a tumour, reviewed in more detail elsewhere [7]. The majority of CRCs arise from spontaneous mutations, frequently leading to activation of the WNT/β-catenin pathway [8]. Resulting polyps or adenomas can accumulate further alterations that transform them into more aggressive adenocarcinomas. These events frequently include driver mutations in the mitogen-activated protein kinase (MAPK) and bone morphogenic protein (BMP)/transforming growth factor-β (TGF-β) pathways—as well as in tumour suppressor gene TP53 [9,10,11,12]. Besides this classical version of colorectal tumorigenesis there is also the serrated pathway, with precursor lesions differing on histological architecture as well as molecular characteristics [13]. Serrated tumours can become deficient in DNA mismatch repair, which can lead to hypermutated CRCs that also acquire atypical numbers of tandem repeats [14]. These cancers are also called microsatellite instable (MSI) tumours, a portion of which arise from hereditary mutations in DNA mismatch repair genes (Lynch syndrome) [15]. In contrast to hypermutated/MSI tumours, CRCs that are microsatellite stable (MSS) typically accumulate moderately low numbers of mutations [16].
As carcinomas become more invasive, they can migrate into the vasculature and spread to distant sites in the body. About half of the patients that are diagnosed with localized CRC already have cancer cells in one or more distant organs, albeit still undetectable [17]. Indeed, genetic evidence suggests that cancer dissemination may be an early event [18,19]. Months to years after surgical removal of the primary CRC, these cells can cause disease recurrence. Whereas primary CRC can often be completely removed by surgery, metastases are often more difficult to treat. Consequently, most deaths are due to (extensive) metastatic CRC (mCRC), the main focus of this review. Although multiple organs can be affected, including lungs, peritoneal cavity, bones, and brain; liver metastasis is the most common and best-studied form. Nevertheless, many questions about this process remain unanswered [20,21].
1.2. Tumour Heterogeneity
Besides the genetic background, many additional parameters are taken into consideration for disease prognosis. In current CRC staging practice, these include histopathological observations such as differentiation grades, cellular phenotypes, tumour budding, and lymph node involvement—many of which have been formalized in the TNM (tumour, lymph node, metastasis) classification. These parameters correlate with both disease outcome and metastatic patterns [22], indicating biological relevance. Another clinical parameter that is linked to disease outcome is the primary tumour location: ascending and transverse colon (right), versus descending and sigmoid colon (left) [23]. Despite all these factors, predicting a patient’s risk of metastasis is still a challenge.
To further dissect tumoral heterogeneity and explore new treatable targets, extensive molecular classification attempts have been made. Aside from the abovementioned frequent driver mutations, there is considerable genetic variation between tumours—without clearly ascribed prognostic value. This prompted a shift in focus, and technology, towards gene expression. A number of large transcriptomic stratification efforts have been reported, consolidated in a system with four consensus molecular subtypes (CMS), of which CMS4 has the worst prognosis [24]. Although this classification has not yet substantially impacted on clinical practice, it uncovered new biological aspects of CRC.
1.3. Focus on the Tumour Microenvironment
In parallel with transcriptomic studies that were mostly focused on epithelial cancer cells, an additional paradigm emerged in understanding disease progression: a complicit tumour microenvironment (TME), or tumour stroma. The TME consists of the cellular components surrounding the mutated cancer cells (i.e., tissue parenchymal cells, fibroblasts, immune infiltrates and vascular cells), as well as signalling molecules and metabolites, physical conditions (e.g., pH, oxygen, stiffness), and other factors such as the microbiota [4,25,26,27]. This marked complexity has long precluded in-depth analysis of the role of the TME in tumour progression and metastasis. However, specific and context-dependent roles of the TME in harbouring or advancing metastatic lesions have emerged.
For example, cancer-associated fibroblasts (CAFs) are recognized as a main constituent of tumours and have heterogeneous phenotypes, including paracrine functions that drive tumour progression [28,29]. Relatedly, TGF-β, a key activating growth factor for fibroblasts, was found to correlate with poor prognosis [30,31,32]. Specifically, levels of both ligand mRNA (TGFB1, −2 and −3) and downstream target gene expression in CAFs—and other cell types of the TME—carry robust prognostic value [31,33]. In fact, much of the predictive power that is captured in the CMS4 is associated with the TME [33,34]. Nevertheless, epithelial CRC cell-intrinsic signatures have also been found to facilitate patient stratification [35,36]. In about half of CRCs, epithelial TGF-β signalling is disrupted by mutations, suggesting that—in cancer progression—this pathway mainly acts on the TME. However, this may discount the role of TGF-β in CRCs that do not have such mutations. Interestingly, in serrated adenomas, TGF-β induces a mesenchymal phenotype—echoing with the well-described function of this cytokine in activating an epithelial-to-mesenchymal transition [37]—and contributing to the TGF-β-high, poor prognosis CMS4 CRCs [38,39].
Other influential components of the TME are cell types of the immune system, including antigen presenting cells (APCs) and cytotoxic immune cells. Of the first group, mainly macrophages and dendritic cells (DCs) have been studied in the context of CRC. In general, their presence is associated with anti-tumour immune responses, although their activation is commonly inhibited by the TME— reversing the balance towards immune suppression. This malignant polarization is best-described for tumour-associated macrophages (TAMs), which can use immunomodulatory cytokines, metabolism, and checkpoint molecules to regulate the anti-tumour immune response [40]; as well as for myeloid-derived suppressor cells (MDSCs), a heterogeneous collection of relatively immature immune cells with potent suppressive activities [41]. Conversely, cytotoxic cell types—T cells and natural killer (NK) cells—are important for immunosurveillance and may block metastasis initiation. Cytotoxic T lymphocytes (CTLs) can be powerful, long-lived cancer cell killers, yet are activated to a single specific peptide. NK cells are less specific, but they can still recognize cancer cells. Furthermore, NK cells also kill cancer cells through antibody-dependent cellular toxicity (ADCC), which is relevant for a number of targeted therapies.
Cytotoxic immune cells activation requires concerted action by other immune cell types and is very sensitive to negative regulation. Successful tumours exploit the richness of negative regulatory mechanisms of the immune system to thwart immunosurveillance [27]. Clearly, this immune contexture is relevant for cancer diagnosis, prognosis, and treatment decisions [42]. Importantly, the density and type of infiltrating T cells is a good prognostic marker [43], and has been formalized and validated in the Immunoscore [44,45]. The understanding of cancer as an ecosystem has amplified research into therapeutic strategies that target the whole tumour, rather than the currently predominant focus on the epithelial cancer cells [46].
2. Clinical Practice: Systemic Chemotherapy-Based Treatment of mCRC
Surgical removal of tumours remains the main treatment option for CRC, both in the setting of primary disease and, increasingly, also for patients with oligometastases—a clinical diagnosis that is generally defined as less than five detectable metastatic lesions in maximally two organs [47]. Improvements in the survival of patients undergoing resection of liver metastases from CRC have been reported in mostly observational studies [48,49,50]. In general, these reports indicate that curation can be obtained when complete resection is feasible. Alternative local treatment strategies are radiofrequency, microwave ablation or stereotactic ablative radiotherapy [51,52,53,54,55,56]. These may also improve survival as long as the disease is localized in liver or lungs. In patients with more extensive disease, local interventions have not provided survival benefit when only a part of the metastases can be removed [57,58]. Alternatively, complete debulking of multiple metastatic lesions may improve survival and is subject of an ongoing study when combined with systemic treatment [59] (NCT01792934)—results are expected in 2022/2023.
When local treatment alone fails or is not feasible, several systemic treatment options are available. Before the era of chemotherapy, patients with mCRC had a median overall survival of 6 months [60]. With the introduction of the chemotherapeutic agents fluoropyrimidines (5-FU or capecitabine), oxaliplatin and irinotecan—and later on the targeted agents against vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) in combination therapy and/or sequential administration—overall survival has improved to over 30 months in the most recent randomized phase III trials [61,62,63]. Multiple combination treatment strategies with these cytotoxic agents have been developed and studied, resulting in established combinations of 5-FU with oxaliplatin or irinotecan [64,65,66]. The antiangiogenic monoclonal antibody against VEGF, bevacizumab, is not active by itself but has some benefit in combination with chemotherapy added to either of the combinations mentioned above [67], and this may be related to a specific genetic alteration [68]. Recently, triple therapy with the drug regimen FOLFOXIRI (fluorouracil, leucovorin, oxaliplatin, and irinotecan) with bevacizumab has been evaluated in depth as first line therapy in relatively young patients with a good performance status [69]. Although some benefit in progression-free survival and potential overall survival was found compared to therapy with 5-FU, oxaliplatin and bevacizumab alone in the control group, this combination entailed increased toxicity.
The anti-EGFR monoclonal antibodies cetuximab and panitumumab are active by themselves, resulting in a survival benefit with adequate selection of patients with left-sided tumours with a RAS wild-type status [70]. Other targeted therapies that were developed for a specific subgroup of patients include treatment with trastuzumab/pertuzumab for HER2+ mCRCs [71], and the combination of encorafenib (BRAF inhibitor) and cetuximab—which was demonstrated to be efficacious and approved by regulatory agencies for BRAF V600E-mutant mCRC [72]. Additional trials investigating regorafenib (a multikinase inhibitor) and trifluridine/piperacil (a nucleoside analogue) have shown modest improvement compared to best supportive care [73,74]. Multiple other targeted agents, especially tyrosine kinase inhibitors (TKIs), have failed in mCRC for unknown reasons. Today, none of the systemic therapies provide a realistic chance for curation in patients with mCRC.
3. Immunotherapies
In recent decades, immunotherapies for cancer have seen a renaissance. A landmark development was the discovery and subsequent therapeutic blockade of immunological checkpoint molecules cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) and PD-L1, a ligand to programmed death receptor-1 (PD-1) [75]. Antibodies against these cell surface proteins, known as immune checkpoint inhibitors (ICIs), have been applied with remarkable responses in advanced melanoma. This outcome has been expanded to a number of other cancer types, including advanced non-small cell lung cancer, metastatic urothelial carcinoma, advanced renal cell carcinoma, hepatocellular carcinoma, head and neck squamous cell carcinoma, and recurrent lymphoma [76].
Antibodies against PD-1 were also tested in patients with mCRC [77]. Although promising responses were seen in MSI tumours, this was not the case for MSS cancers —the type that the vast majority of patients with mCRC have. This ostensible lack of success is likely associated with low tumour mutational burden and, therefore, with a low number of tumour-specific neo-antigens, which is also reflected in the observed scarcity of tumour-infiltrating lymphocytes (TILs) in many MSS cancers [78]. However, full immune activation is a complex process that involves both innate and adaptive immune systems [79], and may encounter TME interference on various levels in this multistep immune cycle. Consequently, disparate treatment types are being designed and investigated, each aiming to close the circle. These can be divided in three main topics: boosting immune recognition to stimulate effector responses; circumventing in situ immune regulation by using active immune cells, and overcoming immunosuppressive signalling. Subsequently, we will discuss immuno-oncology combinations that aim to overcome mono-therapeutic shortcomings.
3.1. Boosting Immune Recognition
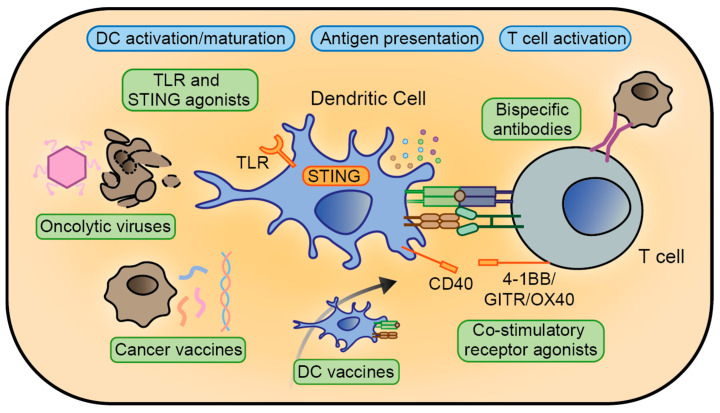
Already before much of the complex regulation of (anticancer) immunity was understood, Dr. William Coley found that intratumoral injection of bacterial extracts could induce curative effects—placing the first known instance of cancer immunotherapy at the end of the 19th century [80]. Modern knowledge of these signalling mechanisms offers the possibility of a more controlled approach to therapeutically boost cancer immunity. This can be achieved by stimulating pathogen- or damage-associated molecular pattern (PAMP/DAMP) recognition signalling, mediated by toll-like receptors (TLRs) and stimulator of interferon genes (STING) in innate immune cell types (Figure 1). A number of molecules have been designed to stimulate these mechanisms [81,82], which elicit the secretion of pro-inflammatory cytokines. This response aids the maturation of APCs such as DCs, as well as their ability to present tumour-associated antigens (TAAs) to T cells [83]. Although individual efforts have shown potential as monotherapies in mCRC [84,85] (Table 1), these approaches seem not be sufficient to elicit powerful immune responses that can eliminate tumours.
Figure 1.
Schematic overview of therapeutic strategies aimed at boosting immune recognition. This process involves dendritic cell (DC) activation and maturation, antigen processing and presentation to T cells, and the subsequent activation of T cells with cytokines and other co-stimulatory signals. Some of these steps can be circumvented using ex vivo-generated DC vaccines or by the application of bispecific antibody products. Cancer cells are depicted in grey.
Table 1.
Therapeutic approaches to boost immune recognition in clinical trials for mCRC.
| Type | Agent | Stage | Reference |
|---|---|---|---|
| Boosting Agents | |||
| Small molecules | TLR9 agonist (MGN1703) | Phase II | [84] |
| TLR9 agonist (MGN1703) | Phase III | NCT02077868 | |
| STING agonist (EE7766) | Phase I | NCT04144140 | |
| Antibodies/BiTEs | OX40 agonist (MEDI6469) + liver metastasis ablation | Phase I | NCT02559024 |
| Anti-4-11B agonist (urelumab) | Phase I/II | [101] | |
| Anti-GITR agonist (AMG228) | Phase I | [102] | |
| CEA-CD3 bispecific antibody (cibitasamab) | Phase I | NCT02324257 | |
| CEA-CD3 bispecific antibody (MEDI-565) | Phase I | NCT01284231 | |
| CEA-CD3 bispecific antibody (AMG 211) | Phase I | NCT02291614 | |
| EpCAM-CD3 bispecific antibody (solitomab) | Phase I | [103] | |
| Vaccination Approaches | |||
| Autologous tumour cells | Irradiated autologous tumour cells combined with BCG (OncoVAX®) (intradermal vaccine) | Phase III | [92] |
| Irradiated metastases-derived tumour cells incubated with Newcastle disease virus | Phase II/III | [93] | |
| OncoVAX® and surgery | Phase III | NCT02448173 | |
| Autologous or Allogeneic tumour cells | Phase I/II | NCT00722228 | |
| Oncolytic viruses | Genetically altered herpes simplex virus (NV1020) | Phase I/II | [97] |
| Enadenotucirev | Phase I/II | [98] | |
| Peptide vaccine | Ad5-GUCY2C-PADRE | Phase I | [104] |
| SART3 peptide vaccine | Phase I | [105] | |
| hCG peptide vaccine conjugated to diphtheria toxoid | Phase II | [106] | |
| 13-peptide cocktail vaccine | Phase I/II | [107] | |
| Personalized peptide vaccine | Phase II | [108] | |
| Personalized peptide vaccine | Phase I | NCT02600949 | |
| DC vaccine | CEA or Frameshifted peptide-loaded DCs (Lynch syndrome/MSI) |
Phase I/II | NCT01885702 |
| Ex vivo CD40L-activated DCs | Randomized | [109] | |
| CEA pulsed DC + tetanus toxoid and IL-2 | Phase I | [110] | |
| Autologous tumour antigens-loaded DC | Phase II | [111] | |
| Autologous tumour lysate activated DC | Phase II | NCT02919644 | |
Nevertheless, these agents also serve as immunoadjuvants in vaccination approaches [86,87], which can be applied either in early-stage disease—to prevent relapse—or in overt mCRC, as a method to boost immune responses that may control or reject advanced disease (Figure 1). One option is the use of autologous tumour cells, inactivated with e.g., radiation. Preclinical studies showed that such vaccinations can be effective in inhibiting tumour growth and extending mouse survival, linked to increased lymphocyte infiltration and responses [88,89,90,91]. Three phase III clinical trials of patients with stage II/III CRC investigated the intradermal use of irradiated autologous tumour cells, using Bacillus Calmette-Guerin (BCG) as an immunoadjuvant, demonstrating a decrease in risk of recurrence [92]. This result was not reached in a small randomized trial conducted after complete resection of metastases (stage IV), assessing the efficacy of irradiated metastases-derived CRC cells incubated with Newcastle disease virus—although some survival benefits were found [93].
In a somewhat related approach, patients are injected with attenuated viruses that selectively infect and kill cancer cells. Besides increasing the detectability of tumour antigens upon cell rupture, the immunogenic cancer cell death induced by these oncolytic viruses releases PAMPs and DAMPs, boosting APC maturation and adaptive immune responses [94] (Figure 1). Studies in preclinical CRC models with a mutated herpes simplex virus (G207) and an adenovirus (Ad881) demonstrated cancer regression [95,96]. In the clinic, this strategy is met with early successes, but also with challenges such as optimal systemic delivery [97,98,99,100] (Table 1).
Viruses have also been used as vectors to express TAAs inside the patient. In fact, several vaccination strategies are being evaluated to elicit or boost specific immune responses in situ [112,113]. While MSI/hypermutated CRC is already associated with a high number of potential neo-antigens, vaccination is seen as an additional possibility in raising immunogenicity, especially in Lynch syndrome for some relatively frequent TAAs [114]. For MSS CRC, several specific antigens are under consideration, although none of these have a very high prevalence [115]. Preclinical studies vaccinating against the MYB proto-oncogene showed suppressed tumour growth through induction of T-cell-mediated anti-tumour immunity [116,117]. Similar results were obtained for viral vaccination strategies targeting GUCY2C [118,119], prompting a successful phase I clinical trial (Table 1). There has also been progress using mRNA-encoded vaccines [120]. A proof-of-concept study demonstrated the feasibility of harnessing cancer-genomics to synthesize personalized poly-neo-epitope mRNAs that conferred anti-tumour immunity in mouse models, including of CRC [121,122]. Clinical translation of this idea was recently reported for metastatic melanoma [123]. In addition, a number of generic or personalized peptide vaccines have shown potential in the preclinical setting [124,125,126,127] and in the clinic (Table 1).
Rather than activating APCs in situ, an alternative strategy is the administration of ex vivo stimulated and activated dendritic cells (Figure 1). For this approach, DCs are isolated from the patient, activated, pulsed with tumour lysates or specific TAAs, and then reinfused—often in combination with an adjuvant. DC vaccines have been studied in multiple clinical trials [109,110,111,128,129,130,131]. Although generally demonstrating safety—and in most cases increased tumour-specific T-cell concentrations [109,110,129,130,131]—therapeutic benefit is reported in only a subset of trials [104,110,111] (Table 1). Related to DC cell therapy is the concept of administrating synthetic immune niches; transplantable 3D-scaffolds that locally provide chemoattractants, tumour antigens, and adjuvants to recruit and activate DCs and T cells [132,133]. Although not implemented specifically for CRC, preclinical studies on lymphoma [134] and melanoma [135,136,137] show promising effects.
If priming tumour-reactive T cells is not the key problem, absent or inadequate stimulatory signalling cues from the TME can be counterbalanced therapeutically. Several antibodies have been designed to promote CTL activity (Figure 1). Agonistic targeting of the co-stimulatory receptors OX40 (CD134), 4-1BB (CD137), and glucocorticoid-induced tumour necrosis factor receptor-related protein (GITR) can increase infiltration and activity of effector T cells while decreasing infiltration of regulatory immune cells in preclinical CRC models [138,139,140,141,142]. These antibodies have entered clinical investigation (Table 1). Furthermore, a recent study reports the adaptation of an autoimmunity-directed CD40 inhibitor into an agonist by isotype switching, resulting in anti-cancer activity [143]. In an alternative strategy, bispecific T-cell engagers (BiTEs) have been designed to physically summon T lymphocytes to CRC cells; this proximity alone can in some cases be sufficient to elicit cytotoxic effects [144]. These agents are antibody derivatives with one arm binding CD3 on T cells and the other a cell surface protein on CRC cells, such as EpCAM [145,146], EGFR [147], CEA [148,149], and glycoprotein A33 [150]. Some of these therapies have entered clinical trials (Table 1).
3.2. Circumventing In Situ Immune Activation: Adoptive Cell Therapy
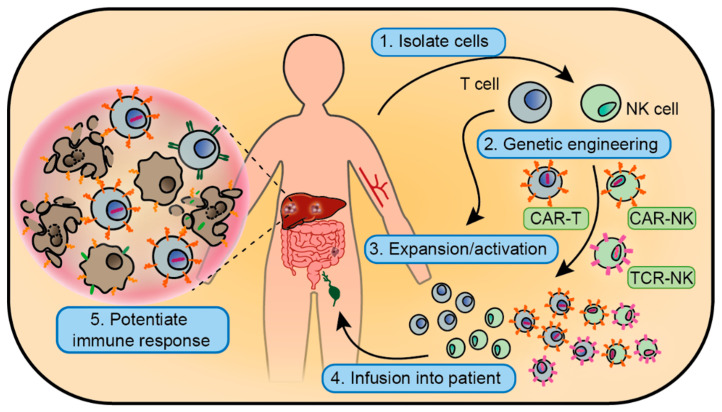
Beyond providing immune-boosting signals or administering pre-activated DCs, it is also possible to bypass all in situ immune priming and inject patients with activated cytotoxic immune cell products (Figure 2). In the 1960s and 70s, seminal studies with adoptive cell therapy (ACT) of autologous TILs helped establish the concept that T cells can recognize and kill tumour cells [151]. This strategy demonstrated encouraging clinical responses in patients with metastatic melanoma. Progress in other tumours including CRC has been complicated by the relative underrepresentation of tumour-reactive T cells in these cancers, although efforts remain ongoing (Table 2).
Figure 2.
Schematic overview of adoptive cell therapies. T cells or NK cells can be isolated from the patient, or from healthy donors, and expanded ex vivo. During this process, they can be genetically engineered to express a CAR or TCR variant. Upon infusion, these killer cells can reinforce the existing immune response or effect a new one.
Table 2.
Clinical trials investigating immune cell therapies in mCRC.
| Cell Therapy | Intervention/Target | Stage | Reference |
|---|---|---|---|
| TILs | Lymphodepletion + autologous TIL + IL-2 | Phase II | NCT03610490 |
| Lymphodepletion + autologous TIL + IL-2 | Phase II | NCT01174121 | |
| Lymphodepletion + autologous TIL + IL-2 | Phase II | NCT03935893 | |
| NK cells | Allogenic NK + cetuximab | Phase I | [158] |
| Adoptive transfer NK + trastuzumab/cetuximab-based chemotherapy | Phase I | [159] | |
| CAR-T cells | Anti-EGFR-IL12 CAR-T Cells | Phase I/II | NCT03542799 |
| Anti-EGFR CAR-T cells | Phase I/II | NCT03152435 | |
| Anti-MUC1 CAR-T cells | Phase I/II | NCT02617134 | |
| Anti-EpCAM CAR-T cells | Phase I/II | NCT03013712 | |
| Anti-CEA CAR-T cells | Phase I | [160] | |
| Anti-CEA CAR-T cells | Phase I | NCT02850536 | |
| Anti-CEA CAR-T cells | Phase I | NCT02349724 | |
| Anti-EGFR CAR-T cells | Phase I/II | NCT01869166 | |
| Anti-NKG2D CAR-T cells | Phase I | NCT03370198 | |
| Anti-NKG2DL CAR-(γδ) T cells | Phase I | NCT04107142 | |
| Anti-DR5 CAR-T cells | Phase I/II | NCT03638206 | |
| CAR-NK cells | Anti-MUC1 CAR-NK cells | Phase I/II | NCT02839954 |
T cells are highly dependent on human leukocyte antigen (HLA) matching—the variability of which precludes broad, off-the-shelf functionality. As an alternative to the need for autologous and antigen-specified immune cells, NKs have also been used as a therapeutic agent [152]. Interestingly, the use of allogenic NK products—sourced from healthy blood donors, umbilical cord blood or even cell lines—may have several benefits over autologous cells [153,154]. Moreover, preclinical efforts indicated that ACT of peripheral blood NK cells can synergize with antibody-based targeted therapy such as cetuximab via ADCC—independently of RAS/RAF mutation status [155,156,157]. Two phase I trials affirmed safety and suggested biological activity of this combination in patients with gastrointestinal cancers (Table 2).
The HLA-unrestricted killing ability that characterizes NK cells could be a great advantage when transferred to T cells, inspiring a number of engineered T-cell receptor (TCR) approaches (Figure 2). For instance, the chimeric antigen receptor (CAR)—a fusion of an immunoglobulin epitope-recognition domain with signalling regions of the TCR and co-stimulatory co-receptors—may be an auspicious therapeutic option for solid tumours with a moderate or low tumour mutational burden [161]. Although this treatment requires antigens to be expressed on the target surface, these antigens do not have to be presented by the HLA machinery, which is sometimes downregulated in tumour cells [162]. The most extensively studied CAR-target in CRC is CEA. This strategy showed anti-tumour responses in preclinical models [163,164,165] and is under evaluation in the clinic (Table 2). The targets of additional CARs that demonstrated promising in vivo results in CRC models—and that are now in clinical trials—include EpCAM [166,167], GUCY2C [168,169], NKG2D [170], and HER2 [171] (Table 2). Others such as doublecortin-like kinase 1 (DCLK1)—a proposed cancer stem cell marker—show preclinical potential [172]. Moreover, CAR-T cells might be exploited to target CAFs: T cells with a CAR against fibroblast activating protein (FAP) significantly inhibited subcutaneous growth of various solid tumours [173]. However, efficacy for CRC was not convincingly demonstrated, likely due to the strong immunosuppressive TME associated with CRC. Indeed, this problem may generally limit survival of (CAR) T cells [174,175]. Of note, significant toxicity has been linked to CAR-T-cell therapy [176]. Potential strategies relieving toxicity, and likely increasing efficacy, could involve localized administration of CAR-T cells [165,177,178].
Generally appreciated for their better safety profile, NK cells have also been genetically modified to express CARs. The NK-92 cell line was engineered to express a CAR targeting CEA [179] as well as EpCAM [180]. Adoptive transfer of either inhibited growth of subcutaneous human CRC xenografts, in mice. Furthermore, intraperitoneal infusion of allogeneic NKG2D CAR-NK cells reduced tumour burden in one patient with CRC liver metastasis [181]. In further attempts to pair the innate cytotoxic powers of NK cells with specific recognition ability, NK-92 cells have been equipped with a functional TCR; conferring phenotypic traits of T cells, while NK cell effector functions were retained [182]. An alternative approach that would be HLA-unrestricted is a recently cloned TCR, which—by binding the invariable HLA-relative MR1—can recognize various cancer cell types [183]. Approaches like this may be another step towards safe, efficacious, and off-the-shelf targeted immunotherapies. Nevertheless, clinical translation of ACT for solid tumours like CRC is still in early development.
3.3. Targeting Immunosuppressive Signalling
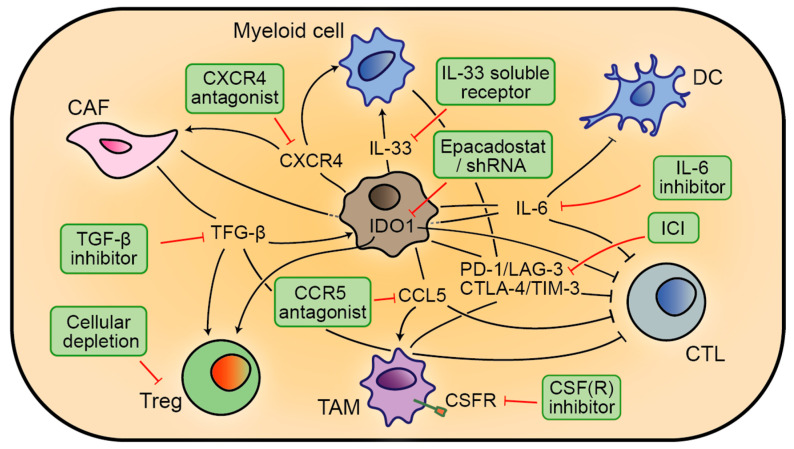
Similar to immune-boosting strategies, the efficacy of immune cell therapy is severely limited by the TME (Figure 3). Overcoming this immunosuppressive milieu could therefore be vital in allowing durable clinical responses in patients with advanced CRC [46]. This concept was recently illustrated with the inhibition of stromal TGF-β signalling [184,185]. TGF-β, besides activating CAFs, is a potent immune suppressor [186,187] and was therefore hypothesized to have an active role in immune evasion—putatively explaining its link to poor prognosis. Upon the generation of a genetic and transplantable, immunocompetent metastatic mouse model for CMS4-like MSS CRC, this hypothesis was tested. Indeed, the key role of TGF-β in metastatic initiation is the suppression of anti-tumour immune responses: inhibition of the pathway efficiently blocked metastatic liver colonization in a T-cell-dependent manner [184]. There are several other immunomodulatory cytokines that are associated with disease progression in CRC [188,189]; among them are IL-6 and IL-33 [190,191,192] (Figure 3). In mice, IL-6 deficiency reduced liver metastasis, concomitant with increased activity of DCs and cytotoxic T cells [193], and IL-33 facilitated metastasis by increasing myeloid cell infiltration and neo-angiogenesis [194]. Interestingly, administration of a soluble IL-33 receptor—which is downregulated in mCRC—normalized the TME and suppressed metastasis in mice [195].
Figure 3.
Schematic overview of some of the players, targets, and strategies that are involved in modulating and re-educating the immunosuppressive TME for mCRC, with the aim of promoting anti-tumour immunity. An IDO1-expressing epithelial CRC cell (grey) is depicted in the middle.
Furthermore, chemokines and their receptors regulate the recruitment of immune cells to the TME and thereby constitute interesting immunotherapeutic targets [196] (Figure 3). Preclinically, chemokine (receptor) inhibitors have been shown to suppress CRC liver metastasis formation and inhibit tumour growth by reducing the accumulation of immature myeloid cells and CAFs [197,198,199,200,201]. For instance, a CXCR4 antagonist suppressed metastasis by decreasing TME infiltration of CAFs and MDSCs [198]. Clinical data support the premise that chemokines help shape the immunosuppressive landscape, highlighting inflammatory crosstalk mechanisms between myeloid cells and T lymphocytes at the invasive margin of CRC liver metastases [201,202]. A pro-tumorigenic signalling cascade, mediated by CCL5, could be therapeutically interrupted by CCR5 antagonists. This therapy elicited therapeutic responses in a phase I study, including the repolarization and redistribution of TAMs, and the subsequent activation of T cells [201]. Regarding TAMs, a considerable fundamental and (pre-)clinical effort has been dedicated to targeting them [203,204], including a focus on inhibiting colony-stimulating factor 1 (CSF-1, also known as M-CSF) or its receptor CSF-1R [205]. An anti-CSF-R1 antibody was found to deplete TAMs in a murine CRC model as well as in patients, which associated with increased lymphocyte infiltration and slower tumour growth [206]. Despite this, as well as proving safe, no objective response was observed [207].
As metabolic reprogramming in the TME can also be a driver of immune evasion, immunometabolism has emerged as a new therapeutic frontier [208]. One example is the essential amino acid tryptophan: important for T-cell activation and catabolized by IDO1 (indoleamine 2,3-dioxygenase) into a suppressive immunomodulator (Figure 3). Expression of this enzyme is elevated in CRCs that have few TILs and in patients with poor prognosis [209,210,211]. Preclinical studies with IDO1 inhibitors in CRC models confirmed its role as an immune suppressor, but indicated that this strategy may fall short by itself [212,213]. Accordingly, early clinical trials could not show efficacy of a selective small-molecule IDO1 inhibitor (epacadostat) as monotherapy for solid malignancies [214]. Nevertheless, a recent study in mice targeting IDO1 with a short hairpin RNA found robust innate immune responses impeding tumour growth—outperforming epacadostat [215]. Furthermore, new ICI strategies have yielded good results in preclinical models of CRC (Figure 3), including the blockade of inhibitory receptors such as LAG-3 and TIM-3 [216,217,218,219,220]. Finally, the depletion of regulatory T cells may also contribute to negating tumour-induced immunosuppression [221].
3.4. Combinatorial Immuno-Oncology
It is becoming clear that few if any monotherapies will achieve broad, durable efficacy for advanced (solid) cancers, such as mCRC. Consequently, as seen in the development of chemotherapies, treatments like ICIs are increasingly used in pairs or together with other agents [222,223] (Table 3). For MSI mCRC, the combination of anti-CTLA-4 and anti-PD-1 proved quite successful in terms of response rate and survival outcomes [224]. Interestingly, a recent early stage neo-adjuvant trial with the same treatment combination demonstrated promising immune responses in primary tumours—even for MSS CRC—which might, in follow-up studies, translate to lower recurrence rates [225]. Furthermore, ongoing phase I trials for mCRC test the same two antibodies with a TLR9 agonist together with radiosurgery, or an anti-PD-1 antibody with a STING agonist (Table 3).
Table 3.
Active clinical trials investigating combinatorial immunotherapies in mCRC.
| Combinatorial Strategy | Stage | Ref. | ||||
|---|---|---|---|---|---|---|
| TLR9 agonist | + | radiosurgery | + | anti-PD1 & anti-CTLA4 | Phase I | NCT03507699 |
| STING agonist (MK-1454) | + | anti-PD1 | Phase I | NCT03010176 | ||
| MYB vaccine | + | anti-PD1 | Phase I | NCT03287427 | ||
| KRAS peptide vaccine | + | anti-PD1 & anti-CTLA4 | Phase I | NCT04117087 | ||
| Pexa-Vec oncolytic virus | + | anti-PD1 & anti-CTLA4 | Phase I/II | NCT03206073 | ||
| Oncolytic adenovirus (enadenotucirev) | + | anti-PD-1 | Phase I | NCT02636036 | ||
| Anti-CEA-CD3 bispecific antibody | + | anti-PD1 | Phase I | NCT02650713 | ||
| Anti-GITR | + | anti-PD1 & anti-CTLA4 | Phase I/II | NCT03126110 | ||
| Anti-CD27 | + | anti-PD1 | Phase I/II | NCT02335918 | ||
| Anti-HER2 CAR-T cells | + | oncolytic adenovirus | Phase I | NCT03740256 | ||
| Autologous TIL | + | anti-PD1 | Phase II | NCT01174121 | ||
| Autologous TIL | + | chemotherapy | Phase II | NCT03935893 | ||
| Anti-PD1 activated autologous TIL | + | chemotherapy | Phase I/II | NCT03904537 | ||
| Anti-NKG2D CAR-T cells | + | chemotherapy | Phase I | NCT03692429 | ||
| Anti-CEA CAR-T cells | + | chemotherapy | Phase I/II | NCT02959151 | ||
| Anti-NKG-2 CAR-T cells | + | chemotherapy | Phase I | NCT03310008 | ||
| Anti-CEA CAR-T cells | + | internal radiation therapy | Phase I | NCT02416466 | ||
| TGF-β inhibitor | + | anti-PD1 | Phase I/II | NCT03724851 | ||
| TGF-βRII (extracellular domain; ligand trap) | fused to | anti-PD-L1 | Phase I/II | NCT03436563 | ||
| CCR5 inhibitor | + | anti-PD1 | Phase I | NCT03274804 [231] | ||
| CCR2/CCR5 inhibitor | + | anti-PD1 or chemotherapy | Phase I/II | NCT03184870 | ||
| Fluoropyrimidine and bevacizumab | + | anti-PD-L1 | Phase III | NCT02291289 | ||
| CSF-1R inhibitor | + | anti-PD-L1 | Phase I | NCT02777710 | ||
| IDO1 inhibitor + azacytidine | + | anti-PD1 | Phase I/II | NCT02959437 | ||
| K-RAS(G12C) inhibitor | + | anti-PD1 | Phase I/II | NCT03600883 | ||
| Anti-TIM-3 antibody | + | anti-PD-1 | Phase I | NCT02817633 | ||
| Anti-TIM-3 antibody | + | anti-PD-1 | Phase I | NCT03099109 | ||
| Anti-LAG-3 | + | anti-PD-1 | Phase I/II | NCT01968109 | ||
| Anti-LAG-3 | + | anti-PD-1 | Phase I | NCT03250832 | ||
| Anti-LAG-3 | + | anti-PD-1 | Phase I | NCT03005782 | ||
| Anti-LAG-3 | + | anti-PD-1 | Phase I | NCT03219268 | ||
A strong dependency of effective immune checkpoint blockade on correctly functioning DCs has been reported [226,227], and a substantial replenishment of new tumour-reactive T-cell clones has been observed following PD-1 inhibition [228,229]. These findings indicate a potential synergy between ICI and vaccination therapy. This rationale is supported by preclinical studies, such as the combination of the MYB vaccine and anti-PD1 therapy [117], which has now entered a phase I trial (Table 3). Similarly, a KRAS peptide vaccine in combination with anti-PD-1 and anti-CTLA-4 is being investigated in patients with advanced CRC (Table 3). Somewhat relatedly, a specific KRAS G12C-mutant inhibitor was demonstrated to have a surprising immunotherapeutic effect by itself and to synergize with ICIs in a preclinical model [230], and has progressed to clinical testing (Table 3).
Some vaccination approaches have shown stronger efficiency when combined with chemotherapeutic agents [232,233,234], and the depletion of regulatory T cells by low-dose cyclophosphamide treatment offered a survival benefit to patients with mCRC that were treated with modified vaccinia Ankara-5T4 vaccination [235]. Also, there are clinical studies investigating oncolytic viruses in combination with ICI (Table 3). An added potential for oncolytic viruses is the engineered encoding of cytokines, antigens, inhibitors or other biological agents—rendering them into multiplexed immunomodulatory platforms [236]. Furthermore, combinations have been proposed of oncolytic viruses with TGF-β inhibition [237] or with CAR-T cells (Table 3). Interestingly, the latter may serve as viral carriers, addressing the currently limiting factor of oncolytic virus delivery [238].
Agonistic T-cell-directed antibodies are also being combined with other types of immunotherapy, e.g., CEA-CD3 BiTEs together with PD-1/PD-L1 blockade [239,240] (Table 3). Moreover, anti-OX40 treatment has been tested together with different agonistic agents [241] or with anti-PD-1 in preclinical models [242], and these strategies are being translated into the clinic. Following the observation of limited clinical therapeutic benefit of a GITR agonist as a monotherapy [100], a phase I/II study has been initiated to test this agent in combination with ICIs (Table 3). Similarly, the combination of antibodies stimulating CD27—another important co-stimulatory receptor of T cells [243]—with anti-PD-1 therapy is clinically evaluated (Table 3). Interestingly, agonistic anti-4-1BB antibodies were reported to increase the efficacy of CAR-T cells, not just by stimulating T-cell activity, but also by reducing the levels of infiltrating regulatory T cells and MDSCs [244]—a goal that could also be achieved by inhibiting IDO1 [245] or STAT3 [246]. Additionally, antibodies against CD25 (targeting regulatory T cells) and CSF-1R (targeting TAMs or MDSCs) have also been co-administered with ICIs in preclinical models to give promising results [247,248] (Table 3).
Regarding ACT, ICI combinations with TIL transfusion therapy are being tested in the clinic (Table 3). The anti-tumour activity of CEA CAR-T cells was increased by PD-L1 blockade in preclinical models; again involving MDSCs as a major source of checkpoint molecules [165]. Furthermore, a recent preclinical study demonstrated superior anti-tumour responses of anti-CD30/CEA bispecific CAR-T cells over CEA monospecific CAR-T cells. The additional anti-CD30 modality was proposed to antagonize negative regulation of effector T cells and might potentiate CAR-T-cell therapies in a broad manner [249]. Other combinations with CAR-T cell therapy include p38 and PKD inhibitors, which can stabilize interferon receptor IFNAR1 to increase T cell persistence in the TME—as observed with a FAP-CAR-T approach in preclinical models of CRC [174].
In the aforementioned metastatic mouse model for MSS mCRC, in which TGF-β inhibition could counteract the immunosuppressive TME and prevent metastatic initiation [184], this treatment was not effective for established liver metastases. Likewise, the monotherapy of with anti-PD-L1 antibodies resulted in minimal benefit. However, dual blockade led to potent, curative T-cell-mediated immune responses [184], confirming that immunotherapies can be effective upon overcoming (TGF-β-induced) immune evasion. Similar conclusions were drawn from other studies, including in MSI CRC or different cancer types, promulgating high levels of stromal TGF-β signalling as a therapeutic target as well as a prognostic and predictive biomarker in the context of (ICI) immunotherapy [250,251,252,253,254,255,256,257,258,259]. Not only T-cell-based approaches benefit from targeting the immunosuppressive TME, as TGF-β inhibition significantly increased infiltration and cytotoxicity of adoptively transferred NK cells [260]. Indeed, strategies inhibiting TGF-β signalling may play a key role in a range of effective immunotherapeutic combinations [184,186,250,251,252] and several inhibitors are in (early) clinical trials, including in combination with ICIs (Table 3). Other signalling inhibitor plus ICI combinations in clinical evaluation include a CCR2/CCR5 antagonist (Table 3). Interestingly, CCR2 antagonists [261], and other compounds targeting myeloid cells [262], might be particularly effective in combination with ICI for patients that underwent radiotherapy. Furthermore, epacadostat is being evaluated in combination with ICI as well as with the epigenetic agent azacytidine (Table 3), and a preclinical study reported a synergy of dual blockade of IDO1 and TLR7 [263].
Many of these studies illustrate the concept that clinically advanced immunotherapies are expected to be significantly potentiated by auxiliary treatments that overcome the immunosuppressive TME. The aim for these combinations is often the increase of immune cell infiltration and activation, or involves the depletion, re-education or repolarization of Tregs, CAFs, TAMs, or MDSCs [264]. New concepts and combinations that promise to meet this ambition continue to come out of preclinical research, such as ICI plus TNF blockade [265], ICI with BRAF and MEK inhibitors [266,267], or a combination of 4 agents designed to engage a variety of innate and adaptive immune cells [268]. Thus, with the expansion of both our understanding of cancer immunity and our therapeutic arsenal, concerted intervention at several critical points in the immune cycle is an emergent paradigm.
Although most studies for combinatorial immunotherapies described in this section are in early stages, some early results temper expectations. For example, the phase I study of the combination of anti-PD-1 with CCR5 inhibitor showed limited clinical activity, with an objective response rate (ORR) of only 5% [231]. Moreover, two clinical failures concerning patients with mCRC—one a phase II study of the addition of anti-PD-L1 to a combination of fluoropyrimidine with bevacizumab in maintenance after first line therapy [269], the other a phase III trial with a MEK inhibitor plus anti-PD-L1 in the third line [270]—indicate substantial challenges to translating promising preclinical activities [264]. As with some of the clinical disappointments in targeted therapies, the lack of predictive biomarkers for patient selection may contribute to negative trials.
4. Future Perspectives
Beyond the uncertainties involved in the clinical validation of new treatment strategies and combinations, determining the right combination among an ever-growing set of possibilities will become an even bigger challenge for immunotherapies than it already is for more conventional therapies. This is due to the heterogeneity between patients, as well as the high level of complexity within the tumours: to our limited understanding of the interactions between all components of the TME and of the underlying immunosuppressive signalling mechanisms. Improved insight could substantially prioritize approaches, reducing the number of relevant combinatorial possibilities. Recent and ongoing high-dimensional approaches on clinical samples with a strong focus on the TME, including single-cell techniques, are anticipated to help towards that goal. Moreover, these observations should be paired with improved experimental models. Until recently, preclinical models of CRC—typically involving heterotopic (i.e., subcutaneous) and/or xenotypic injections of (human) cell lines into mice—often recapitulated neither tumour heterogeneity nor the TME, likely contributing to failure in clinical translation. However, more sophisticated alternatives are being developed that allow the study of key effectors and molecular signalling networks in sufficient complexity, facilitate the design and validation of combinatorial treatments, and may enable the identification of biomarkers. In our view, suitable models would have to be either faithful, immunocompetent metastatic mouse models [184,271,272,273], or in vitro cultured TME models such as those based on patient-derived organoids or tumour explants [274,275]—potentially in combination with humanized mouse models.
Furthermore, adequate tools are needed to stratify patients based on their TME [276]. General progress is being made, such as a pan-cancer immunogenomics study that distinguished six immune subtypes [277]; and recent work on subtyping patient with CRC, based on TME [278,279,280], may make clinical testing of new therapies more efficient. For example, (combinatorial) treatment strategies designed for a given TME subtype—that potentially could be present in different cancer types—would benefit from basket trials [281]. Within this trend fits the tissue-agnostic FDA approval of anti-PD-1 antibodies in 2017. This ICI approval for patients with advanced mismatch-repair-deficient solid tumours, such as MSI-mCRC, was based on trials with in total 149 patients spanning 15 cancer types [282]. Also, further validation of immune subtypes—especially from primary CRC material—will improve prognostic resolution, and may even pave the way for a more individualized disease outlook [42,283]. In this scenario, an immunological balance, or set point, within the individual TME would be assessed to determine the best countermeasures [284]. Consequently, treatment combinations may increasingly become biomarker-driven and personalized, and their efficacy may be predicted by bespoke patient-derived TME culture models or humanized xenograft avatars.
An additional challenge for combinatorial immunotherapy lies in optimizing the timing and delivery sequence of different therapies, affecting both efficacy and toxicity. Preclinical studies addressing these factors indicate the significance of appropriate scheduling for combinations of immunotherapies, e.g., with radiotherapy [242,285,286,287]. Furthermore, as immunotherapies continue to gain ground for mCRC, the positioning of such treatment modalities in the context of disease management might need to be evaluated. Conventional wisdom would suggest second- or third-line implementation of experimental immuno-oncology strategies for patients with metastatic disease. However, perhaps presurgical, neo-adjuvant immunotherapy—with tumour and -draining lymph nodes still in place—may give a better result; as unleashing a strong immune response in the primary tumour could immunize the patient against future metastases [225,288,289]. A prudent approach would be to determine the long-term benefits of such therapies in high-risk patients first.
5. Conclusions
Despite the initial failure of immune checkpoint inhibitors in MSS mCRC, a host of promising immunotherapeutic options are under development that may be instrumental in extensively improving survival for patients with advanced disease. The realization that—like in many solid tumours—the CRC microenvironment strongly suppresses anti-tumour immune responses has prompted the development of strategies that include stromal modulation. As the complexity of interactions within the TME is steadily being dissected, we propose that an ecological type of cancer therapy will be attainable in the near future. This outlook promises precision immuno-oncology with individually optimized treatment combinations, and gives hope to more curative therapies for patients with metastatic CRC.
Acknowledgments
We would like to thank the members of the Tauriello and Verheul labs for their comments on this manuscript.
Author Contributions
E.J., B.S., F.d.l.J.O., and D.V.F.T. performed the literature search and made the figures and tables. All authors wrote the manuscript. D.V.F.T. coordinated the effort. All authors have read and agreed to the published version of the manuscript.
Funding
D.V.F.T. is funded by a Hypatia Tenure Track Fellowship grant from the Radboudumc and by the Netherlands Organisation for Scientific Research (NWO/ZonMW VIDI grant number 91719371).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber R.D., Old L.J., Smyth M.J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 3.Vinay D.S., Ryan E.P., Pawelec G., Talib W.H., Stagg J., Elkord E., Lichtor T., Decker W.K., Whelan R.L., Kumara H.M.C.S., et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015;35:S185–S198. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D., Weinberg R.A., Adams J.M., Cory S., Aguirre-Ghiso J.A., Ahmed Z., Bicknell R., Al-Hajj M., Wicha M.S., Benito-Hernandez A., et al. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Ullman T.A., Itzkowitz S.H. Intestinal Inflammation and Cancer. Gastroenterology. 2011;140:1807–1816. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 6.Lasry A., Zinger A., Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat. Immunol. 2016;17:230–240. doi: 10.1038/ni.3384. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen L.H., Goel A., Chung D.C. Pathways of Colorectal Carcinogenesis. Gastroenterology. 2020;158:291–302. doi: 10.1053/j.gastro.2019.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Network Team Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fearon E.R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-I. [DOI] [PubMed] [Google Scholar]
- 10.Matano M., Date S., Shimokawa M., Takano A., Fujii M., Ohta Y., Watanabe T., Kanai T., Sato T. Modeling colorectal cancer using CRISPR-Cas9–mediated engineering of human intestinal organoids. Nat. Med. 2015;21:256–262. doi: 10.1038/nm.3802. [DOI] [PubMed] [Google Scholar]
- 11.Drost J., van Jaarsveld R.H., Ponsioen B., Zimberlin C., van Boxtel R., Buijs A., Sachs N., Overmeer R.M., Offerhaus G.J., Begthel H., et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature. 2015;521:43–47. doi: 10.1038/nature14415. [DOI] [PubMed] [Google Scholar]
- 12.Fujii M., Shimokawa M., Date S., Takano A., Matano M., Nanki K., Ohta Y., Toshimitsu K., Nakazato Y., Kawasaki K., et al. A Colorectal Tumor Organoid Library Demonstrates Progressive Loss of Niche Factor Requirements during Tumorigenesis. Cell Stem Cell. 2016;18:827–838. doi: 10.1016/j.stem.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Murcia O., Juárez M., Hernández-Illán E., Egoavil C., Giner-Calabuig M., Rodríguez-Soler M., Jover R. Serrated colorectal cancer: Molecular classification, prognosis, and response to chemotherapy. World J. Gastroenterol. 2016;22:3516. doi: 10.3748/wjg.v22.i13.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisenberger D.J., Siegmund K.D., Campan M., Young J., Long T.I., Faasse M.A., Kang G.H., Widschwendter M., Weener D., Buchanan D., et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat. Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 15.Boland C.R., Goel A. Microsatellite Instability in Colorectal Cancer. Gastroenterology. 2010;138:2073–2087. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexandrov L.B., Nik-Zainal S., Wedge D.C., Aparicio S.A.J.R., Behjati S., Biankin A.V., Bignell G.R., Bolli N., Borg A., Børresen-Dale A.-L., et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambert A.W., Pattabiraman D.R., Weinberg R.A. Emerging Biological Principles of Metastasis. Cell. 2017;168:670–691. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sottoriva A., Kang H., Ma Z., Graham T.A., Salomon M.P., Zhao J., Marjoram P., Siegmund K., Press M.F., Shibata D., et al. A Big Bang model of human colorectal tumor growth. Nat. Genet. 2015;47:209–216. doi: 10.1038/ng.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Z., Ding J., Ma Z., Sun R., Seoane J.A., Scott Shaffer J., Suarez C.J., Berghoff A.S., Cremolini C., Falcone A., et al. Quantitative evidence for early metastatic seeding in colorectal cancer. Nat. Genet. 2019;51:1113–1122. doi: 10.1038/s41588-019-0423-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romano G., Chagani S., Kwong L.N. The path to metastatic mouse models of colorectal cancer. Oncogene. 2018;37:2481–2489. doi: 10.1038/s41388-018-0155-x. [DOI] [PubMed] [Google Scholar]
- 21.Tauriello D.V.F., Calon A., Lonardo E., Batlle E. Determinants of metastatic competency in colorectal cancer. Mol. Oncol. 2017;11:97–119. doi: 10.1002/1878-0261.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hugen N., van de Velde C.J.H., de Wilt J.H.W., Nagtegaal I.D. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann. Oncol. 2014;25:651–657. doi: 10.1093/annonc/mdt591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrelli F., Tomasello G., Borgonovo K., Ghidini M., Turati L., Dallera P., Passalacqua R., Sgroi G., Barni S. Prognostic Survival Associated With Left-Sided vs Right-Sided Colon Cancer. JAMA Oncol. 2017;3:211. doi: 10.1001/jamaoncol.2016.4227. [DOI] [PubMed] [Google Scholar]
- 24.Guinney J., Dienstmann R., Wang X., de Reyniès A., Schlicker A., Soneson C., Marisa L., Roepman P., Nyamundanda G., Angelino P., et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pietras K., Östman A. Hallmarks of cancer: Interactions with the tumor stroma. Exp. Cell Res. 2010;316:1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 26.Hanahan D., Coussens L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 27.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostman A., Augsten M. Cancer-associated fibroblasts and tumor growth--bystanders turning into key players. Curr. Opin. Genet. Dev. 2009;19:67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Sahai E., Astsaturov I., Cukierman E., DeNardo D.G., Egeblad M., Evans R.M., Fearon D., Greten F.R., Hingorani S.R., Hunter T., et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsushima H., Ito N., Tamura S., Matsuda Y., Inada M., Yabuuchi I., Imai Y., Nagashima R., Misawa H., Takeda H., et al. Circulating transforming growth factor β1 as a predictor of liver metastasis after resection in colorectal cancer. Clin. Cancer Res. 2001;7:1258–1262. [PubMed] [Google Scholar]
- 31.Calon A., Espinet E., Palomo-Ponce S., Tauriello D.V.F., Iglesias M., Céspedes M.V., Sevillano M., Nadal C., Jung P., Zhang X.H.F., et al. Dependency of Colorectal Cancer on a TGF-β-Driven Program in Stromal Cells for Metastasis Initiation. Cancer Cell. 2012;22:571–584. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calon A., Tauriello D.V.F., Batlle E. TGF-beta in CAF-mediated tumor growth and metastasis. Semin. Cancer Biol. 2014;25:15–22. doi: 10.1016/j.semcancer.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Calon A., Lonardo E., Berenguer-llergo A., Espinet E., Hernando-momblona X., Iglesias M., Sevillano M., Palomo-ponce S., Tauriello D.V.F., Byrom D., et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Genet. 2015;47:320–329. doi: 10.1038/ng.3225. [DOI] [PubMed] [Google Scholar]
- 34.Isella C., Terrasi A., Bellomo S.E., Petti C., Galatola G., Muratore A., Mellano A., Senetta R., Cassenti A., Sonetto C., et al. Stromal contribution to the colorectal cancer transcriptome. Nat. Genet. 2015;47:312–319. doi: 10.1038/ng.3224. [DOI] [PubMed] [Google Scholar]
- 35.Isella C., Brundu F., Bellomo S.E., Galimi F., Zanella E., Porporato R., Petti C., Fiori A., Orzan F., Senetta R., et al. Selective analysis of cancer-cell intrinsic transcriptional traits defines novel clinically relevant subtypes of colorectal cancer. Nat. Commun. 2017;8:15107. doi: 10.1038/ncomms15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alderdice M., Richman S.D., Gollins S., Stewart J.P., Hurt C., Adams R., McCorry A.M., Roddy A.C., Vimalachandran D., Isella C., et al. Prospective patient stratification into robust cancer-cell intrinsic subtypes from colorectal cancer biopsies. J. Pathol. 2018;245:19–28. doi: 10.1002/path.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heldin C.-H., Vanlandewijck M., Moustakas A. Regulation of EMT by TGFβ in cancer. FEBS Lett. 2012;586:1959–1970. doi: 10.1016/j.febslet.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 38.Fessler E., Drost J., Hooff S.R., Linnekamp J.F., Wang X., Jansen M., De Sousa E Melo F., Prasetyanti P.R., IJspeert J.E., Franitza M., et al. TGFβ signaling directs serrated adenomas to the mesenchymal colorectal cancer subtype. EMBO Mol. Med. 2016;8:745–760. doi: 10.15252/emmm.201606184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakanishi Y., Diaz-Meco M.T., Moscat J. Serrated Colorectal Cancer: The Road Less Travelled? Trends Cancer. 2019;5:742–754. doi: 10.1016/j.trecan.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noy R., Pollard J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veglia F., Perego M., Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018;19:108–119. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fridman W.H., Zitvogel L., Sautès–Fridman C., Kroemer G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017;14:717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 43.Galon J., Costes A., Sanchez-Cabo F., Kirilovsky A., Mlecnik B., Lagorce-Pagès C., Tosolini M., Camus M., Berger A., Wind P., et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 44.Pagès F., Mlecnik B., Marliot F., Bindea G., Ou F.-S., Bifulco C., Lugli A., Zlobec I., Rau T.T., Berger M.D., et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet. 2018;391:2128–2139. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 45.Galon J., Mlecnik B., Bindea G., Angell H.K., Berger A., Lagorce C., Lugli A., Zlobec I., Hartmann A., Bifulco C., et al. Towards the introduction of the “Immunoscore” in the classification of malignant tumours. J. Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tauriello D.V.F., Batlle E. Targeting the Microenvironment in Advanced Colorectal Cancer. Trends Cancer. 2016;2:495–504. doi: 10.1016/j.trecan.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Hafez N., Gettinger S. Oligometastatic Disease and Local Therapies: A Medical Oncology Perspective. Cancer J. 2020;26:144–148. doi: 10.1097/PPO.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 48.Tomlinson J.S., Jarnagin W.R., DeMatteo R.P., Fong Y., Kornprat P., Gonen M., Kemeny N., Brennan M.F., Blumgart L.H., D’Angelica M. Actual 10-year survival after resection of colorectal liver metastases defines cure. J. Clin. Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 49.Primrose J.N. Surgery for colorectal liver metastases. Br. J. Cancer. 2010;102:1313–1318. doi: 10.1038/sj.bjc.6605659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanas G.P., Taylor A., Primrose J.N., Langeberg W.J., Kelsh M.A., Mowat F.S., Alexander D.D., Choti M.A., Poston G. Survival after liver resection in metastatic colorectal cancer: Review and meta-analysis of prognostic factors. Clin. Epidemiol. 2012;4:283–301. doi: 10.2147/CLEP.S34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cirocchi R., Trastulli S., Boselli C., Montedori A., Cavaliere D., Parisi A., Noya G., Abraha I. Radiofrequency ablation in the treatment of liver metastases from colorectal cancer. Cochrane Database Syst. Rev. 2012 doi: 10.1002/14651858.CD006317.pub3. [DOI] [PubMed] [Google Scholar]
- 52.Ruers T., Punt C., Van coevorden F., Pierie J.P.E.N., Borel-Rinkes I., Ledermann J.A., Poston G., Bechstein W., Lentz M.A., Mauer M., et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: A randomized eortc intergroup phase ii study (EORTC 40004) Ann. Oncol. 2012;23:2619–2626. doi: 10.1093/annonc/mds053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruers T., Van Coevorden F., Punt C.J.A., Pierie J.-P.E.N., Borel-Rinkes I., Ledermann J.A., Poston G., Bechstein W., Lentz M.-A., Mauer M., et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. JNCI J. Natl. Cancer Inst. 2017;109 doi: 10.1093/jnci/djx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olson R., Senan S., Harrow S., Gaede S., Louie A., Haasbeek C., Mulroy L., Lock M., Rodrigues G., Yaremko B., et al. Quality of Life Outcomes After Stereotactic Ablative Radiation Therapy (SABR) Versus Standard of Care Treatments in the Oligometastatic Setting: A Secondary Analysis of the SABR-COMET Randomized Trial. Int. J. Radiat. Oncol. Biol. Phys. 2019;105:943–947. doi: 10.1016/j.ijrobp.2019.08.041. [DOI] [PubMed] [Google Scholar]
- 55.Palma D.A., Olson R., Harrow S., Correa R.J.M., Schneiders F., Haasbeek C.J.A., Rodrigues G.B., Lock M., Yaremko B.P., Bauman G.S., et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 4-10 oligometastatic tumors (SABR-COMET-10): Study protocol for a randomized phase III trial. BMC Cancer. 2019;19:816. doi: 10.1186/s12885-019-5977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palma D.A., Olson R., Harrow S., Gaede S., Louie A.V., Haasbeek C., Mulroy L., Lock M., Rodrigues G.B., Yaremko B.P., et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet. 2019;393:2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 57.Adam R., De Haas R.J., Wicherts D.A., Vibert E., Salloum C., Azoulay D., Castaing D. Concomitant extrahepatic disease in patients with colorectal liver metastases: When is there a place for surgery? Ann. Surg. 2011;253:349–359. doi: 10.1097/SLA.0b013e318207bf2c. [DOI] [PubMed] [Google Scholar]
- 58.Gootjes E.C., Bakkerus L., ten Tije A.J., Witteveen P.O., Buffart T.E., Bridgewater J.A., Primrose J.N., Verhoef C., Verheul H.M.W. The value of tumour debulking for patients with extensive multi-organ metastatic colorectal cancer. Eur. J. Cancer. 2018;103:160–164. doi: 10.1016/j.ejca.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 59.Gootjes E.C., Stok E.P., Buffart T.E., Bakkerus L., Labots M., Zonderhuis B.M., Tuynman J.B., Meijerink M.R., Ven P.M., Haasbeek C.J.A., et al. Safety and Feasibility of Additional Tumor Debulking to First-Line Palliative Combination Chemotherapy for Patients with Multiorgan Metastatic Colorectal Cancer. Oncologist. 2020 doi: 10.1634/theoncologist.2019-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolpin B.M., Mayer R.J. Systemic Treatment of Colorectal Cancer. Gastroenterology. 2008;134:1296–1310. doi: 10.1053/j.gastro.2008.02.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Folprecht G., Seymour M.T., Saltz L., Douillard J.Y., Hecker H., Stephens R.J., Maughan T.S., Van Cutsem E., Rougier P., Mitry E., et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: Combined analysis of 2,691 patients in randomized controlled trials. J. Clin. Oncol. 2008;26:1443–1451. doi: 10.1200/JCO.2007.14.0509. [DOI] [PubMed] [Google Scholar]
- 62.Saltz L.B., Clarke S., Díaz-Rubio E., Scheithauer W., Figer A., Wong R., Koski S., Lichinitser M., Yang T.S., Rivera F., et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J. Clin. Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 63.Douillard J.Y., Siena S., Cassidy J., Tabernero J., Burkes R., Barugel M., Humblet Y., Bodoky G., Cunningham D., Jassem J., et al. Final results from PRIME: Randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann. Oncol. 2014;25:1346–1355. doi: 10.1093/annonc/mdu141. [DOI] [PubMed] [Google Scholar]
- 64.Tournigand C., André T., Achille E., Lledo G., Flesh M., Mery-Mignard D., Quinaux E., Couteau C., Buyse M., Ganem G., et al. FOLFIRI Followed by FOLFOX6 or the Reverse Sequence in Advanced Colorectal Cancer: A Randomized GERCOR Study. J. Clin. Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 65.Tournigand C., Cervantes A., Figer A., Lledo G., Flesch M., Buyse M., Mineur L., Carola E., Etienne P.-L., Rivera F., et al. OPTIMOX1: A Randomized Study of FOLFOX4 or FOLFOX7 With Oxaliplatin in a Stop-and-Go Fashion in Advanced Colorectal Cancer—A GERCOR Study. J. Clin. Oncol. 2006;24:394–400. doi: 10.1200/JCO.2005.03.0106. [DOI] [PubMed] [Google Scholar]
- 66.Cassidy J., Clarke S., Díaz-Rubio E., Scheithauer W., Figer A., Wong R., Koski S., Lichinitser M., Yang T.-S., Rivera F., et al. Randomized Phase III Study of Capecitabine Plus Oxaliplatin Compared With Fluorouracil/Folinic Acid Plus Oxaliplatin As First-Line Therapy for Metastatic Colorectal Cancer. J. Clin. Oncol. 2008;26:2006–2012. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 67.Baraniskin A., Buchberger B., Pox C., Graeven U., Holch J.W., Schmiegel W., Heinemann V. Efficacy of bevacizumab in first-line treatment of metastatic colorectal cancer: A systematic review and meta-analysis. Eur. J. Cancer. 2019;106:37–44. doi: 10.1016/j.ejca.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 68.van Dijk E., Biesma H.D., Cordes M., Smeets D., Neerincx M., Das S., Eijk P.P., Murphy V., Barat A., Bacon O., et al. Loss of Chromosome 18q11.2-q12.1 Is Predictive for Survival in Patients With Metastatic Colorectal Cancer Treated With Bevacizumab. J. Clin. Oncol. 2018;36:2052–2060. doi: 10.1200/JCO.2017.77.1782. [DOI] [PubMed] [Google Scholar]
- 69.Cremolini C., Antoniotti C., Rossini D., Lonardi S., Loupakis F., Pietrantonio F., Bordonaro R., Latiano T.P., Tamburini E., Santini D., et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): A multicentre, open-label, phase 3, rand. Lancet Oncol. 2020;21:497–507. doi: 10.1016/S1470-2045(19)30862-9. [DOI] [PubMed] [Google Scholar]
- 70.van Helden E.J., Menke-van der Houven van Oordt C.W., Heymans M.W., Ket J.C.F., van den Oord R., Verheul H.M.W. Optimal use of anti-EGFR monoclonal antibodies for patients with advanced colorectal cancer: A meta-analysis. Cancer Metastasis Rev. 2017;36:395–406. doi: 10.1007/s10555-017-9668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lai E., Liscia N., Donisi C., Mariani S., Tolu S., Pretta A., Persano M., Pinna G., Balconi F., Pireddu A., et al. Molecular-Biology-Driven Treatment for Metastatic Colorectal Cancer. Cancers (Basel) 2020;12:1214. doi: 10.3390/cancers12051214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kopetz S., Grothey A., Yaeger R., Van Cutsem E., Desai J., Yoshino T., Wasan H., Ciardiello F., Loupakis F., Hong Y.S., et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E–mutated colorectal cancer. N. Engl. J. Med. 2019;381:1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 73.Grothey A., Van Cutsem E., Sobrero A., Siena S., Falcone A., Ychou M., Humblet Y., Bouché O., Mineur L., Barone C., et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 74.Mayer R.J., Van Cutsem E., Falcone A., Yoshino T., Garcia-Carbonero R., Mizunuma N., Yamazaki K., Shimada Y., Tabernero J., Komatsu Y., et al. Randomized Trial of TAS-102 for Refractory Metastatic Colorectal Cancer. N. Engl. J. Med. 2015;372:1909–1919. doi: 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
- 75.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zappasodi R., Merghoub T., Wolchok J.D. Emerging Concepts for Immune Checkpoint Blockade-Based Combination Therapies. Cancer Cell. 2018;33:581–598. doi: 10.1016/j.ccell.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D., Skora A.D., Luber B.S., Azad N.S., Laheru D., et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Joyce J.A., Fearon D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 79.Chen D.S., Mellman I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 80.Mccarthy E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006;26:154–158. [PMC free article] [PubMed] [Google Scholar]
- 81.Braunstein M.J., Kucharczyk J., Adams S. Targeting Toll-Like Receptors for Cancer Therapy. Target. Oncol. 2018;13:583–598. doi: 10.1007/s11523-018-0589-7. [DOI] [PubMed] [Google Scholar]
- 82.Kerr W.G., Chisholm J.D. The Next Generation of Immunotherapy for Cancer: Small Molecules Could Make Big Waves. J. Immunol. 2019;202:11–19. doi: 10.4049/jimmunol.1800991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Demaria O., Cornen S., Daëron M., Morel Y., Medzhitov R., Vivier E. Harnessing innate immunity in cancer therapy. Nature. 2019;574:45–56. doi: 10.1038/s41586-019-1593-5. [DOI] [PubMed] [Google Scholar]
- 84.Schmoll H.J., Wittig B., Arnold D., Riera-Knorrenschild J., Nitsche D., Kroening H., Mayer F., Andel J., Ziebermayr R., Scheithauer W. Maintenance treatment with the immunomodulator MGN1703, a Toll-like receptor 9 (TLR9) agonist, in patients with metastatic colorectal carcinoma and disease control after chemotherapy: A randomised, double-blind, placebo-controlled trial. J. Cancer Res. Clin. Oncol. 2014;140:1615–1624. doi: 10.1007/s00432-014-1682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chon H.J., Kim H., Noh J.H., Yang H., Lee W.S., Kong S.J., Lee S.J., Lee Y.S., Kim W.R., Kim J.H., et al. STING signaling is a potential immunotherapeutic target in colorectal cancer. J. Cancer. 2019;10:4932–4938. doi: 10.7150/jca.32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mocellin S., Rossi C.R., Lise M., Nitti D. Colorectal cancer vaccines: Principles, results, and perspectives. Gastroenterology. 2004;127:1821–1837. doi: 10.1053/j.gastro.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 87.Guo C., Manjili M.H., Subjeck J.R., Sarkar D., Fisher P.B., Wang X.Y. Therapeutic cancer vaccines. Past, present, and future. Adv. Cancer Res. 2013;119:421–475. doi: 10.1016/B978-0-12-407190-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suh K.W., Piantadosi S., Yazdi H.A., Pardoll D.M., Brem H., Choti M.A. Treament of liver metastases from colon carcinoma with autologous tumor vaccine expressing granulocyte-macrophage colony-stimulating factor. J. Surg. Oncol. 1999;72:218–224. doi: 10.1002/(SICI)1096-9098(199912)72:4<218::AID-JSO7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 89.Ju H., Xing W., Yang J., Zheng Y., Jia X., Zhang B., Ren H. An effective cytokine adjuvant vaccine induces autologous T-cell response against colon cancer in an animal model. BMC Immunol. 2016;17 doi: 10.1186/s12865-016-0172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dong T., Yi T., Yang M., Lin S., Li W., Xu X., Hu J., Jia L., Hong X., Niu W. Co-operation of α-galactosylceramide-loaded tumour cells and TLR9 agonists induce potent anti-Tumour responses in a murine colon cancer model. Biochem. J. 2016;473:7–19. doi: 10.1042/BJ20150129. [DOI] [PubMed] [Google Scholar]
- 91.Qin J., Kunda N.M., Qiao G., Tulla K., Prabhakar B.S., Maker A.V. Vaccination with mitoxantrone-treated primary colon cancer cells enhances tumor-infiltrating lymphocytes and clinical responses in colorectal liver metastases. J. Surg. Res. 2019;233:57–64. doi: 10.1016/j.jss.2018.07.068. [DOI] [PubMed] [Google Scholar]
- 92.Hanna M.G., Hoover H.C., Vermorken J.B., Harris J.E., Pinedo H.M. Adjuvant active specific immunotherapy of stage II and stage III colon cancer with an autologous tumor cell vaccine: First randomized phase III trials show promise. Vaccine. 2001;19:2576–2582. doi: 10.1016/S0264-410X(00)00485-0. [DOI] [PubMed] [Google Scholar]
- 93.Schulze T., Kemmner W., Weitz J., Wernecke K.-D., Schirrmacher V., Schlag P.M. Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: Results of a prospective randomized trial. Cancer Immunol. Immunother. 2009;58:61–69. doi: 10.1007/s00262-008-0526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raja J., Ludwig J.M., Gettinger S.N., Schalper K.A., Kim H.S. Oncolytic virus immunotherapy: Future prospects for oncology 11 Medical and Health Sciences 1107 Immunology 11 Medical and Health Sciences 1112 Oncology and Carcinogenesis. J. Immunother. Cancer. 2018;6:140. doi: 10.1186/s40425-018-0458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kooby D.A., Carew J.F., Halterman M.W., Mack J.E., Bertino J.R., Blumgart L.H., Federoff H.J., Fong Y. Oncolytic viral therapy for human colorectal cancer and liver metastases using a multi-mutated herpes simplex virus type-1 (G207) FASEB J. 1999;13:1325–1334. doi: 10.1096/fasebj.13.11.1325. [DOI] [PubMed] [Google Scholar]
- 96.Yamano T., Kubo S., Fukumoto M., Yano A., Mawatari-Furukawa Y., Okamura H., Tomita N. Whole cell vaccination using immunogenic cell death by an oncolytic adenovirus is effective against a colorectal cancer model. Mol. Ther.-Oncolytics. 2016;3:16031. doi: 10.1038/mto.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Geevarghese S.K., Geller D.A., De Haan H.A., Hörer M., Knoll A.E., Mescheder A., Nemunaitis J., Reid T.R., Sze D.Y., Tanabe K.K., et al. Phase I/II study of oncolytic herpes simplex virus NV1020 in patients with extensively pretreated refractory colorectal cancer metastatic to the liver. Hum. Gene Ther. 2010;21:1119–1128. doi: 10.1089/hum.2010.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Machiels J.-P., Salazar R., Rottey S., Duran I., Dirix L., Geboes K., Wilkinson-Blanc C., Pover G., Alvis S., Champion B., et al. A phase 1 dose escalation study of the oncolytic adenovirus enadenotucirev, administered intravenously to patients with epithelial solid tumors (EVOLVE) J. Immunother. Cancer. 2019;7:20. doi: 10.1186/s40425-019-0510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seymour L.W., Fisher K.D. Oncolytic viruses: Finally delivering. Br. J. Cancer. 2016;114:357–361. doi: 10.1038/bjc.2015.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chaurasiya S., Warner S. Viroimmunotherapy for Colorectal Cancer: Clinical Studies. Biomedicines. 2017;5:11. doi: 10.3390/biomedicines5010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Segal N.H., Logan T.F., Hodi F.S., McDermott D., Melero I., Hamid O., Schmidt H., Robert C., Chiarion-Sileni V., Ascierto P.A., et al. Results from an integrated safety analysis of urelumab, an agonist anti-CD137 monoclonal antibody. Clin. Cancer Res. 2017;23:1929–1936. doi: 10.1158/1078-0432.CCR-16-1272. [DOI] [PubMed] [Google Scholar]
- 102.Tran B., Carvajal R.D., Marabelle A., Patel S.P., LoRusso P.M., Rasmussen E., Juan G., Upreti V.V., Beers C., Ngarmchamnanrith G., et al. Dose escalation results from a first-in-human, phase 1 study of glucocorticoid-induced TNF receptor–related protein agonist AMG 228 in patients with advanced solid tumors. J. Immunother. Cancer. 2018;6:93. doi: 10.1186/s40425-018-0407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kebenko M., Goebeler M.-E., Wolf M., Hasenburg A., Seggewiss-Bernhardt R., Ritter B., Rautenberg B., Atanackovic D., Kratzer A., Rottman J.B., et al. A multicenter phase 1 study of solitomab (MT110, AMG 110), a bispecific EpCAM/CD3 T-cell engager (BiTE®) antibody construct, in patients with refractory solid tumors. Oncoimmunology. 2018;7:e1450710. doi: 10.1080/2162402X.2018.1450710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Snook A.E., Baybutt T.R., Xiang B., Abraham T.S., Flickinger J.C., Jr., Hyslop T., Zhan T., Kraft W.K., Sato T., Waldman S.A. Split tolerance permits safe Ad5-GUCY2C- PADRE vaccine-induced T-cell responses in colon cancer patients. J. Immunother. Cancer. 2019;2:1–12. doi: 10.1186/s40425-019-0576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miyagi Y., Imai N., Sasatomi T., Yamada A., Mine T., Katagiri K., Nakagawa M., Muto A., Okouchi S., Isomoto H., et al. Induction of Cellular Immune Responses to Tumor Cells and Peptides in Colorectal Cancer Patients by Vaccination with SART3 peptides. Clin. Cancer Res. 2001;7:3950–3962. [PubMed] [Google Scholar]
- 106.Moulton H.M., Yoshihara P.H., Mason D.H., Iversen P.L., Triozzi P.L. Active specific immunotherapy with a β-human chorionic gonadotropin peptide vaccine in patients with metastatic colorectal cancer: Antibody response is associated with improved survival. Clin. Cancer Res. 2002;8:2044–2051. [PubMed] [Google Scholar]
- 107.Mayer F., Mayer-Mokler A., Nowara E., Torday L., Ludwig J., Kuttruff S., Weinschenk T., Walter S., Singh H., Reinhardt C. A phase I/II trial of the multipeptide cancer vaccine IMA910 in patients with advanced colorectal cancer (CRC) J. Clin. Oncol. 2012;30:555. doi: 10.1200/jco.2012.30.4_suppl.555. [DOI] [Google Scholar]
- 108.Kibe S., Yutani S., Motoyama S., Nomura T., Tanaka N., Kawahara A., Yamaguchi T., Matsueda S., Komatsu N., Miura M., et al. Phase II Study of Personalized Peptide Vaccination for Previously Treated Advanced Colorectal Cancer. Cancer Immunol. Res. 2014;2:1154–1162. doi: 10.1158/2326-6066.CIR-14-0035. [DOI] [PubMed] [Google Scholar]
- 109.Barth R.J., Fisher D.A., Wallace P.K., Channon J.Y., Noelle R.J., Gui J., Ernstoff M.S. A randomized trial of ex vivo CD40L activation of a dendritic cell vaccine in colorectal cancer patients: Tumor-specific immune responses are associated with improved survival. Clin. Cancer Res. 2010;16:5548–5556. doi: 10.1158/1078-0432.CCR-10-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu K.J., Chao T.Y., Chang J.Y., Cheng A.L., Ch’Ang H.J., Kao W.Y., Wu Y.C., Yu W.L., Chung T.R., Whang-Peng J. A phase I clinical study of immunotherapy for advanced colorectal cancers using carcinoembryonic antigen-pulsed dendritic cells mixed with tetanus toxoid and subsequent IL-2 treatment. J. Biomed. Sci. 2016;23:1–11. doi: 10.1186/s12929-016-0279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rodriguez J., Castañón E., Perez-Gracia J.L., Rodriguez I., Viudez A., Alfaro C., Oñate C., Perez G., Rotellar F., Inogés S., et al. A randomized phase II clinical trial of dendritic cell vaccination following complete resection of colon cancer liver metastasis. J. Immunother. Cancer. 2018;6:1–7. doi: 10.1186/s40425-018-0405-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Berry J., Vreeland T., Trappey A., Hale D., Peace K., Tyler J., Walker A., Brown R., Herbert G., Yi F., et al. Cancer vaccines in colon and rectal cancer over the last decade: Lessons learned and future directions. Expert Rev. Clin. Immunol. 2017;13:235–245. doi: 10.1080/1744666X.2016.1226132. [DOI] [PubMed] [Google Scholar]
- 113.Li H., Yu J., Wu Y., Shao B., Wei X. In situ antitumor vaccination: Targeting the tumor microenvironment. J. Cell. Physiol. 2020;235:5490–5500. doi: 10.1002/jcp.29551. [DOI] [PubMed] [Google Scholar]
- 114.Westdorp H., Fennemann F.L., Weren R.D.A., Bisseling T.M., Ligtenberg M.J.L., Figdor C.G., Schreibelt G., Hoogerbrugge N., Wimmers F., de Vries I.J.M. Opportunities for immunotherapy in microsatellite instable colorectal cancer. Cancer Immunol. Immunother. 2016;65:1249–1259. doi: 10.1007/s00262-016-1832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li M., Yuan Y.H., Han Y., Liu Y.X., Yan L., Wang Y., Gu J. Expression profile of cancer-testis genes in 121 human colorectal cancer tissue and adjacent normal tissue. Clin. Cancer Res. 2005;11:1809–1814. doi: 10.1158/1078-0432.CCR-04-1365. [DOI] [PubMed] [Google Scholar]
- 116.Williams B.B., Wall M., Miao R.Y., Williams B., Bertoncello I., Kershaw M.H., Mantamadiotis T., Haber M., Norris M.D., Gautam A., et al. Induction of T cell-mediated immunity using a c-Myb DNA vaccine in a mouse model of colon cancer. Cancer Immunol. Immunother. 2008;57:1635–1645. doi: 10.1007/s00262-008-0497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cross R.S., Malaterre J., Davenport A.J., Carpinteri S., Anderson R.L., Darcy P.K., Ramsay R.G. Therapeutic DNA vaccination against colorectal cancer by targeting the MYB oncoprotein. Clin. Transl. Immunol. 2015;4:e30. doi: 10.1038/cti.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Snook A.E., Stafford B.J., Li P., Tan G., Huang L., Birbe R., Schulz S., Schnell M.J., Thakur M., Rothstein J.L., et al. Guanylyl cyclase C-induced immunotherapeutic responses opposing tumor metastases without autoimmunity. J. Natl. Cancer Inst. 2008;100:950–961. doi: 10.1093/jnci/djn178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xiang B., Baybutt T.R., Berman-Booty L., Magee M.S., Waldman S.A., Alexeev V.Y., Snook A.E. Prime-Boost Immunization Eliminates Metastatic Colorectal Cancer by Producing High-Avidity Effector CD8 + T Cells. J. Immunol. 2017;198:3507–3514. doi: 10.4049/jimmunol.1502672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grunwitz C., Kranz L.M. Current Topics in Microbiology and Immunology. Volume 405. Springer; Cham, Switzerland: 2017. mRNA Cancer Vaccines—Messages that Prevail; pp. 145–164. [DOI] [PubMed] [Google Scholar]
- 121.Castle J.C., Kreiter S., Diekmann J., Lower M., van de Roemer N., de Graaf J., Selmi A., Diken M., Boegel S., Paret C., et al. Exploiting the Mutanome for Tumor Vaccination. Cancer Res. 2012;72:1081–1091. doi: 10.1158/0008-5472.CAN-11-3722. [DOI] [PubMed] [Google Scholar]
- 122.Kreiter S., Vormehr M., van de Roemer N., Diken M., Löwer M., Diekmann J., Boegel S., Schrörs B., Vascotto F., Castle J.C., et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520:692–696. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sahin U., Derhovanessian E., Miller M., Kloke B.-P., Simon P., Löwer M., Bukur V., Tadmor A.D., Luxemburger U., Schrörs B., et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 124.Calvo Tardón M., Allard M., Dutoit V., Dietrich P.-Y., Walker P.R. Peptides as cancer vaccines. Curr. Opin. Pharmacol. 2019;47:20–26. doi: 10.1016/j.coph.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 125.Yamaguchi S., Tatsumi T., Takehara T., Sasakawa A., Yamamoto M., Kohga K., Miyagi T., Kanto T., Hiramastu N., Akagi T., et al. EphA2-derived peptide vaccine with amphiphilic poly(γ-glutamic acid) nanoparticles elicits an anti-tumor effect against mouse liver tumor. Cancer Immunol. Immunother. 2010;59:759–767. doi: 10.1007/s00262-009-0796-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kwon S., Kim Y.-E., Park J.-A., Kim D.-S., Kwon H.-J., Lee Y. Therapeutic effect of a TM4SF5-specific peptide vaccine against colon cancer in a mouse model. BMB Rep. 2014;47:215–220. doi: 10.5483/BMBRep.2014.47.4.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rioux C.R., Clapper M.L., Cooper H.S., Michaud J., St Amant N., Koohsari H., Workman L., Kaunga E., Hensley H., Pilorget A., et al. Self-antigen MASH2 combined with the AS15 immunostimulant induces tumor protection in colorectal cancer mouse models. PLoS ONE. 2019;14:e0210261. doi: 10.1371/journal.pone.0210261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ueda Y., Itoh T., Nukaya I., Kawashima I., Okugawa K., Yano Y., Yamamoto Y., Naitoh K., Shimizu K., Imura K., et al. Dendritic cell-based immunotherapy of cancer with carcinoembryonic antigen-derived, HLA-A24-restricted CTL epitope: Clinical outcomes of 18 patients with metastatic gastrointestinal or lung adenocarcinomas. Int. J. Oncol. 2004;24:909–917. doi: 10.3892/ijo.24.4.909. [DOI] [PubMed] [Google Scholar]
- 129.Kavanagh B., Ko A., Venook A., Margolin K., Zeh H., Lotze M., Schillinger B., Liu W., Lu Y., Mitsky P., et al. Vaccination of metastatic colorectal cancer patients with matured dendritic cells loaded with multiple major histocompatibility complex class I peptides. J. Immunother. 2007;30:762–772. doi: 10.1097/CJI.0b013e318133451c. [DOI] [PubMed] [Google Scholar]
- 130.Liu Y., Zhang W., Zhang B., Yin X., Pang Y. DC vaccine therapy combined concurrently with oral capecitabine in metastatic colorectal cancer patients. Hepatogastroenterology. 2013;60:23–27. doi: 10.5754/hge12522. [DOI] [PubMed] [Google Scholar]
- 131.Caballero-Baños M., Benitez-Ribas D., Tabera J., Varea S., Vilana R., Bianchi L., Ayuso J.R., Pagés M., Carrera G., Cuatrecasas M., et al. Phase II randomised trial of autologous tumour lysate dendritic cell plus best supportive care compared with best supportive care in pre-treated advanced colorectal cancer patients. Eur. J. Cancer. 2016;64:167–174. doi: 10.1016/j.ejca.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 132.Irvine D.J., Swartz M.A., Szeto G.L. Engineering synthetic vaccines using cues from natural immunity. Nat. Mater. 2013;12:978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Weiden J., Tel J., Figdor C.G. Synthetic immune niches for cancer immunotherapy. Nat. Rev. Immunol. 2018;18:212–219. doi: 10.1038/nri.2017.89. [DOI] [PubMed] [Google Scholar]
- 134.Kim J., Li W.A., Choi Y., Lewin S.A., Verbeke C.S., Dranoff G., Mooney D.J. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat. Biotechnol. 2015;33:64–72. doi: 10.1038/nbt.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ali O.A., Huebsch N., Cao L., Dranoff G., Mooney D.J. Infection-mimicking materials to program dendritic cells in situ. Nat. Mater. 2009;8:151–158. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bencherif S.A., Sands R.W., Ali O.A., Li W.A., Lewin S.A., Braschler T.M., Shih T.Y., Verbeke C.S., Bhatta D., Dranoff G., et al. Injectable cryogel-based whole-cell cancer vaccines. Nat. Commun. 2015;6 doi: 10.1038/ncomms8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ali O.A., Lewin S.A., Dranoff G., Mooney D.J. Vaccines combined with immune checkpoint antibodies promote cytotoxic T-cell activity and tumor eradication. Cancer Immunol. Res. 2016;4:95–100. doi: 10.1158/2326-6066.CIR-14-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Taraban V., Rowley T., O’Brien L., Chan H.T.C., Haswell L.E., Green M.H.A., Tutt A.L., Glennie M.J., Al-Shamkhani A. Expression and costimulatory effects of the TNF receptor superfamily members CD134 (OX40) and CD137 (4-1BB), and their role in the generation of anti-tumor immune responses. Eur. J. Immunol. 2002;32:3617–3627. doi: 10.1002/1521-4141(200212)32:12<3617::AID-IMMU3617>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 139.Gough M.J., Ruby C.E., Redmond W.L., Dhungel B., Brown A., Weinberg A.D. OX40 Agonist Therapy Enhances CD8 Infiltration and Decreases Immune Suppression in the Tumor. Cancer Res. 2008;68:5206–5215. doi: 10.1158/0008-5472.CAN-07-6484. [DOI] [PubMed] [Google Scholar]
- 140.Qi X., Li F., Wu Y., Cheng C., Han P., Wang J., Yang X. Optimization of 4-1BB antibody for cancer immunotherapy by balancing agonistic strength with FcγR affinity. Nat. Commun. 2019;10:1–11. doi: 10.1038/s41467-019-10088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zappasodi R., Sirard C., Li Y., Budhu S., Abu-Akeel M., Liu C., Yang X., Zhong H., Newman W., Qi J., et al. Rational design of anti-GITR-based combination immunotherapy. Nat. Med. 2019;25:759–766. doi: 10.1038/s41591-019-0420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Narumi K., Miyakawa R., Shibasaki C., Henmi M., Mizoguchi Y., Ueda R., Hashimoto H., Hiraoka N., Yoshida T., Aoki K. Local Administration of GITR Agonistic Antibody Induces a Stronger Antitumor Immunity than Systemic Delivery. Sci. Rep. 2019;9:5562. doi: 10.1038/s41598-019-41724-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yu X., Chan H.T.C., Fisher H., Penfold C.A., Kim J., Inzhelevskaya T., Mockridge C.I., French R.R., Duriez P.J., Douglas L.R., et al. Isotype Switching Converts Anti-CD40 Antagonism to Agonism to Elicit Potent Antitumor Activity. Cancer Cell. 2020;37:850–866. doi: 10.1016/j.ccell.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huehls A.M., Coupet T.A., Sentman C.L. Bispecific T-cell engagers for cancer immunotherapy. Immunol. Cell Biol. 2015;93:290–296. doi: 10.1038/icb.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brischwein K., Schlereth B., Guller B., Steiger C., Wolf A., Lutterbuese R., Offner S., Locher M., Urbig T., Raum T., et al. MT110: A novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol. Immunol. 2006;43:1129–1143. doi: 10.1016/j.molimm.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 146.Herrmann I., Baeuerle P.A., Friedrich M., Murr A., Filusch S., Rüttinger D., Majdoub M.W., Sharma S., Kufer P., Raum T., et al. Highly Efficient Elimination of Colorectal Tumor-Initiating Cells by an EpCAM/CD3-Bispecific Antibody Engaging Human T Cells. PLoS ONE. 2010;5:e13474. doi: 10.1371/journal.pone.0013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lutterbuese R., Raum T., Kischel R., Hoffmann P., Mangold S., Rattel B., Friedrich M., Thomas O., Lorenczewski G., Rau D., et al. T cell-engaging BiTE antibodies specific for EGFR potently eliminate KRAS- and BRAF-mutated colorectal cancer cells. Proc. Natl. Acad. Sci. USA. 2010;107:12605–12610. doi: 10.1073/pnas.1000976107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Osada T., Hsu D., Hammond S., Hobeika A., Devi G., Clay T.M., Lyerly H.K., Morse M.A. Metastatic colorectal cancer cells from patients previously treated with chemotherapy are sensitive to T-cell killing mediated by CEA/CD3-bispecific T-cell-engaging BiTE antibody. Br. J. Cancer. 2010;102:124–133. doi: 10.1038/sj.bjc.6605364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Oberst M.D., Fuhrmann S., Mulgrew K., Amann M., Cheng L., Lutterbuese P., Richman L., Coats S., Baeuerle P.A., Hammond S.A. CEA/CD3 bispecific antibody MEDI-565/AMG 211 activation of T cells and subsequent killing of human tumors is independent of mutations commonly found in colorectal adenocarcinomas. MAbs. 2014;6:1571–1584. doi: 10.4161/19420862.2014.975660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Moore P.A., Shah K., Yang Y., Alderson R., Roberts P., Long V., Liu D., Li J.C., Burke S., Ciccarone V., et al. Development of MGD007, a gpA33 x CD3-bispecific DART protein for T-cell immunotherapy of metastatic colorectal cancer. Mol. Cancer Ther. 2018;17:1761–1772. doi: 10.1158/1535-7163.MCT-17-1086. [DOI] [PubMed] [Google Scholar]
- 151.Rosenberg S.A., Restifo N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Terme M., Ullrich E., Delahaye N.F., Chaput N., Zitvogel L. Natural killer cell–directed therapies: Moving from unexpected results to successful strategies. Nat. Immunol. 2008;9:486–494. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- 153.Lim O., Jung M.Y., Hwang Y.K., Shin E.C. Present and future of allogeneic natural killer cell therapy. Front. Immunol. 2015;6:286. doi: 10.3389/fimmu.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Spanholtz J., Preijers F., Tordoir M., Trilsbeek C., Paardekooper J., de Witte T., Schaap N., Dolstra H. Clinical-Grade Generation of Active NK Cells from Cord Blood Hematopoietic Progenitor Cells for Immunotherapy Using a Closed-System Culture Process. PLoS ONE. 2011;6:e20740. doi: 10.1371/journal.pone.0020740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen S., Li X., Chen R., Yin M., Zheng Q. Cetuximab intensifies the ADCC activity of adoptive NK cells in a nude mouse colorectal cancer xenograft model. Oncol. Lett. 2016;12:1868–1876. doi: 10.3892/ol.2016.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Veluchamy J.P., Spanholtz J., Tordoir M., Thijssen V.L., Heideman D.A.M., Verheul H.M.W., De Gruijl T.D., Van Der Vliet H.J. Combination of NK cells and cetuximab to enhance anti-tumor responses in RAS mutant metastatic colorectal cancer. PLoS ONE. 2016;11:1–16. doi: 10.1371/journal.pone.0157830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Veluchamy J.P., Lopez-Lastra S., Spanholtz J., Bohme F., Kok N., Heideman D.A.M., Verheul H.M.W., Di Santo J.P., de Gruijl T.D., van der Vliet H.J. In Vivo Efficacy of Umbilical Cord Blood Stem Cell-Derived NK Cells in the Treatment of Metastatic Colorectal Cancer. Front. Immunol. 2017;8:1–11. doi: 10.3389/fimmu.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jackson H.J., Rafiq S., Brentjens R.J. Driving CAR T-cells forward. Nat. Rev. Clin. Oncol. 2016;13:370–383. doi: 10.1038/nrclinonc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Leone P., Shin E.C., Perosa F., Vacca A., Dammacco F., Racanelli V. MHC class i antigen processing and presenting machinery: Organization, function, and defects in tumor cells. J. Natl. Cancer Inst. 2013;105:1172–1187. doi: 10.1093/jnci/djt184. [DOI] [PubMed] [Google Scholar]
- 160.Darcy P.K., Kershaw M.H., Trapani J.A., Smyth M.J. Expression in cytotoxic T lymphocytes of a single-chain anti-carcinoembryonic antigen antibody. Redirected Fas ligand-mediated lysis of colon carcinoma. Eur. J. Immunol. 1998;28:1663–1672. doi: 10.1002/(SICI)1521-4141(199805)28:05<1663::AID-IMMU1663>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 161.Simmons A., Whitehead R.P., Kolokoltsov A.A., Davey R.A. Use of recombinant lentivirus pseudotyped with vesicular stomatitis virus glycoprotein G for efficient generation of human anti-cancer chimeric T cells by transduction of human peripheral blood lymphocytes in vitro. Virol. J. 2006;3:1–10. doi: 10.1186/1743-422X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Burga R.A., Thorn M., Point G.R., Guha P., Nguyen C.T., Licata L.A., DeMatteo R.P., Ayala A., Joseph Espat N., Junghans R.P., et al. Liver myeloid-derived suppressor cells expand in response to liver metastases in mice and inhibit the anti-tumor efficacy of anti-CEA CAR-T. Cancer Immunol. Immunother. 2015;64:817–829. doi: 10.1007/s00262-015-1692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ang W.X., Li Z., Chi Z., Du S.H., Chen C., Tay J.C.K., Toh H.C., Connolly J.E., Xu X.H., Wang S. Intraperitoneal immunotherapy with T cells stably and transiently expressing anti-EpCAM CAR in xenograft models of peritoneal carcinomatosis. Oncotarget. 2017;8:13545–13559. doi: 10.18632/oncotarget.14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang B.L., Li D., Gong Y.L., Huang Y., Qin D.Y., Jiang L., Liang X., Yang X., Gou H.F., Wang Y.S., et al. Preclinical Evaluation of Chimeric Antigen Receptor-Modified T Cells Specific to Epithelial Cell Adhesion Molecule for Treating Colorectal Cancer. Hum. Gene Ther. 2019;30:402–412. doi: 10.1089/hum.2018.229. [DOI] [PubMed] [Google Scholar]
- 165.Magee M.S., Kraft C.L., Abraham T.S., Baybutt T.R., Marszalowicz G.P., Li P., Waldman S.A., Snook A.E. GUCY2C-directed CAR-T cells oppose colorectal cancer metastases without autoimmunity. Oncoimmunology. 2016;5:1–10. doi: 10.1080/2162402X.2016.1227897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Magee M.S., Abraham T.S., Baybutt T.R., FlickingerJr J.C., Ridge N.A., Marszalowicz G.P., Prajapati P., Hersperger A.R., Waldman S.A., Snook A.E. Human GUCY2C-targeted chimeric antigen receptor (CAR)-expressing T cells eliminate colorectal cancer metastases. Cancer Immunol. Res. 2018;6:509–516. doi: 10.1158/2326-6066.CIR-16-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Deng X., Gao F., Li N., Li Q., Zhou Y., Yang T., Cai Z., Du P., Chen F., Cai J. Antitumor activity of NKG2D CAR-T cells against human colorectal cancer cells in vitro and in vivo. Am. J. Cancer Res. 2019;9:945–958. [PMC free article] [PubMed] [Google Scholar]
- 168.Teng R., Zhao J., Zhao Y., Gao J., Li H., Zhou S., Wang Y., Sun Q., Lin Z., Yang W., et al. Chimeric Antigen Receptor-modified T Cells Repressed Solid Tumors and Their Relapse in an Established Patient-derived Colon Carcinoma Xenograft Model. J. Immunother. 2019;42:33–42. doi: 10.1097/CJI.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sureban S.M., Berahovich R., Zhou H., Xu S., Wu L., Ding K., May R., Qu D., Bannerman-Menson E., Golubovskaya V., et al. DCLK1 Monoclonal Antibody-Based CAR-T Cells as a Novel Treatment Strategy against Human Colorectal Cancers. Cancers (Basel) 2019;12:54. doi: 10.3390/cancers12010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang L.C.S., Lo A., Scholler J., Sun J., Majumdar R.S., Kapoor V., Antzis M., Cotner C.E., Johnson L.A., Durham A.C., et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol. Res. 2014;2:154–166. doi: 10.1158/2326-6066.CIR-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Katlinski K.V., Gui J., Katlinskaya Y.V., Ortiz A., Chakraborty R., Bhattacharya S., Carbone C.J., Beiting D.P., Girondo M.A., Peck A.R., et al. Inactivation of Interferon Receptor Promotes the Establishment of Immune Privileged Tumor Microenvironment. Cancer Cell. 2017;31:194–207. doi: 10.1016/j.ccell.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Guedan S., Ruella M., June C.H. Emerging Cellular Therapies for Cancer. Annu. Rev. Immunol. 2019;37:145–171. doi: 10.1146/annurev-immunol-042718-041407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Brudno J.N., Kochenderfer J.N. Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood. 2016;127:3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Katz S.C., Burga R.A., McCormack E., Wang L.J., Mooring W., Point G.R., Khare P.D., Thorn M., Ma Q., Stainken B.F., et al. Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor-Modified T-cell Therapy for CEA+ Liver Metastases. Clin. Cancer Res. 2015;21:3149–3159. doi: 10.1158/1078-0432.CCR-14-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Katz S.C., Hardaway J., Prince E., Guha P., Cunetta M., Moody A., Wang L.J., Armenio V., Espat N.J., Junghans R.P. HITM-SIR: Phase Ib trial of intraarterial chimeric antigen receptor T-cell therapy and selective internal radiation therapy for CEA+ liver metastases. Cancer Gene Ther. 2020;27:341–355. doi: 10.1038/s41417-019-0104-z. [DOI] [PubMed] [Google Scholar]
- 176.Shiozawa M., Chang C.H., Huang Y.C., Chen Y.C., Chi M.S., Hao H.C., Chang Y.C., Takeda S., Chi K.H., Wang Y.S. Pharmacologically upregulated carcinoembryonic antigen-expression enhances the cytolytic activity of genetically-modified chimeric antigen receptor NK-92MI against colorectal cancer cells. BMC Immunol. 2018;19:1–13. doi: 10.1186/s12865-018-0262-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang Q., Zhang H., Ding J., Liu H., Li H., Li H., Lu M., Miao Y., Li L., Zheng J. Combination therapy with EpCAM-CAR-NK-92 cells and regorafenib against human colorectal cancer models. J. Immunol. Res. 2018;2018:1–11. doi: 10.1155/2018/4263520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xiao L., Cen D., Gan H., Sun Y., Huang N., Xiong H., Jin Q., Su L., Liu X., Wang K., et al. Adoptive Transfer of NKG2D CAR mRNA-Engineered Natural Killer Cells in Colorectal Cancer Patients. Mol. Ther. 2019;27:1114–1125. doi: 10.1016/j.ymthe.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mensali N., Dillard P., Hebeisen M., Lorenz S., Theodossiou T., Myhre M.R., Fåne A., Gaudernack G., Kvalheim G., Myklebust J.H., et al. NK cells specifically TCR-dressed to kill cancer cells. EBioMedicine. 2019;40:106–117. doi: 10.1016/j.ebiom.2019.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Crowther M.D., Dolton G., Legut M., Caillaud M.E., Lloyd A., Attaf M., Galloway S.A.E., Rius C., Farrell C.P., Szomolay B., et al. Genome-wide CRISPR–Cas9 screening reveals ubiquitous T cell cancer targeting via the monomorphic MHC class I-related protein MR1. Nat. Immunol. 2020;21:178–185. doi: 10.1038/s41590-019-0578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Adotevi O., Godet Y., Galaine J., Lakkis Z., Idirene I., Certoux J.M., Jary M., Loyon R., Laheurte C., Kim S., et al. In situ delivery of allogeneic natural killer cell (NK) combined with Cetuximab in liver metastases of gastrointestinal carcinoma: A phase I clinical trial. Oncoimmunology. 2018;7:e1424673. doi: 10.1080/2162402X.2018.1424673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ishikawa T., Okayama T., Sakamoto N., Ideno M., Oka K., Enoki T., Mineno J., Yoshida N., Katada K., Kamada K., et al. Phase I clinical trial of adoptive transfer of expanded natural killer cells in combination with IgG1 antibody in patients with gastric or colorectal cancer. Int. J. Cancer. 2018;142:2599–2609. doi: 10.1002/ijc.31285. [DOI] [PubMed] [Google Scholar]
- 183.Zhang C., Wang Z., Yang Z., Wang M., Li S., Li Y., Zhang R., Xiong Z., Wei Z., Shen J., et al. Phase I Escalating-Dose Trial of CAR-T Therapy Targeting CEA + Metastatic Colorectal Cancers. Mol. Ther. 2017;25:1248–1258. doi: 10.1016/j.ymthe.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tauriello D.V.F., Palomo-Ponce S., Stork D., Berenguer-Llergo A., Badia-Ramentol J., Iglesias M., Sevillano M., Ibiza S., Cañellas A., Hernando-Momblona X., et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538–543. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 185.Tauriello D.V.F. From poor prognosis to promising treatment. Sciencec. 2019;363:1051. doi: 10.1126/science.aaw3609. [DOI] [PubMed] [Google Scholar]
- 186.Batlle E., Massagué J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity. 2019;50:924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yang L., Pang Y., Moses H.L. TGF-β and immune cells: An important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220–227. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.West N.R., Mccuaig S., Franchini F., Powrie F. Emerging cytokine networks in colorectal cancer. Nat. Rev. Immunol. 2015;15:615–629. doi: 10.1038/nri3896. [DOI] [PubMed] [Google Scholar]
- 189.Mager L.F., Wasmer M.-H., Rau T.T., Krebs P. Cytokine-Induced Modulation of Colorectal Cancer. Front. Oncol. 2016;6:96. doi: 10.3389/fonc.2016.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Schetter A.J., Giang H.N., Bowman E.D., Mathé E.A., Siu T.Y., Hawkes J.E., Croce C.M., Suet Y.L., Harris C.C. Association of inflammation-related and microRNA gene expression with cancer-specific mortality of colon adenocarcinoma. Clin. Cancer Res. 2009;15:5878–5887. doi: 10.1158/1078-0432.CCR-09-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Waldner M.J., Foersch S., Neurath M.F. Interleukin-6—A key regulator of colorectal cancer development. Int. J. Biol. Sci. 2012;8:1248–1253. doi: 10.7150/ijbs.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liu X., Zhu L., Lu X., Bian H., Wu X., Yang W., Qin Q. IL-33/ST2 pathway contributes to metastasis of human colorectal cancer. Biochem. Biophys. Res. Commun. 2014;453:486–492. doi: 10.1016/j.bbrc.2014.09.106. [DOI] [PubMed] [Google Scholar]
- 193.Toyoshima Y., Kitamura H., Xiang H., Ohno Y., Homma S., Kawamura H., Takahashi N., Kamiyama T., Tanino M., Taketomi A. IL6 modulates the immune status of the tumor microenvironment to facilitate metastatic colonization of colorectal cancer cells. Cancer Immunol. Res. 2019;7:1944–1957. doi: 10.1158/2326-6066.CIR-18-0766. [DOI] [PubMed] [Google Scholar]
- 194.Zhang Y., Davis C., Shah S., Hughes D., Ryan J.C., Altomare D., Peña M.M.O. IL-33 promotes growth and liver metastasis of colorectal cancer in mice by remodeling the tumor microenvironment and inducing angiogenesis. Mol. Carcinog. 2017;56:272–287. doi: 10.1002/mc.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Akimoto M., Maruyama R., Takamaru H., Ochiya T., Takenaga K. Soluble IL-33 receptor sST2 inhibits colorectal cancer malignant growth by modifying the tumour microenvironment. Nat. Commun. 2016;7:13589. doi: 10.1038/ncomms13589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mollica Poeta V., Massara M., Capucetti A., Bonecchi R. Chemokines and Chemokine Receptors: New Targets for Cancer Immunotherapy. Front. Immunol. 2019;10:379. doi: 10.3389/fimmu.2019.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kitamura T., Fujishita T., Loetscher P., Revesz L., Hashida H., Kizaka-Kondoh S., Aoki M., Taketo M.M., Cavenee W.K. Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses colon cancer livermetastasis by blocking accumulation of immature myeloid cells in a mouse model. Proc. Natl. Acad. Sci. USA. 2010;107:13063–13068. doi: 10.1073/pnas.1002372107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Benedicto A., Romayor I., Arteta B. CXCR4 receptor blockage reduces the contribution of tumor and stromal cells to the metastatic growth in the liver. Oncol. Rep. 2018;39:2022–2030. doi: 10.3892/or.2018.6254. [DOI] [PubMed] [Google Scholar]
- 199.Sasaki S., Baba T., Shinagawa K., Matsushima K., Mukaida N. Crucial involvement of the CCL3-CCR5 axis-mediated fibroblast accumulation in colitis-associated carcinogenesis in mice. Int. J. Cancer. 2014;135:1297–1306. doi: 10.1002/ijc.28779. [DOI] [PubMed] [Google Scholar]
- 200.Peng S.B., Zhang X., Paul D., Kays L.M., Gough W., Stewart J., Uhlik M.T., Chen Q., Hui Y.H., Zamek-Gliszczynski M.J., et al. Identification of LY2510924, a novel cyclic peptide CXCR4 antagonist that exhibits antitumor activities in solid tumor and breast cancer metastatic models. Mol. Cancer Ther. 2015;14:480–490. doi: 10.1158/1535-7163.MCT-14-0850. [DOI] [PubMed] [Google Scholar]
- 201.Halama N., Zoernig I., Berthel A., Kahlert C., Klupp F., Suarez-Carmona M., Suetterlin T., Brand K., Krauss J., Lasitschka F., et al. Tumoral Immune Cell Exploitation in Colorectal Cancer Metastases Can Be Targeted Effectively by Anti-CCR5 Therapy in Cancer Patients. Cancer Cell. 2016;29:587–601. doi: 10.1016/j.ccell.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 202.Halama N., Michel S., Kloor M., Zoernig I., Benner A., Spille A., Pommerencke T., Von Knebel Doeberitz M., Folprecht G., Luber B., et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71:5670–5677. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- 203.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.van Dalen F., van Stevendaal M., Fennemann F., Verdoes M., Ilina O. Molecular Repolarisation of Tumour-Associated Macrophages. Molecules. 2018;24:9. doi: 10.3390/molecules24010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cannarile M.A., Weisser M., Jacob W., Jegg A.-M., Ries C.H., Rüttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J. Immunother. Cancer. 2017;5:53. doi: 10.1186/s40425-017-0257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ries C.H., Cannarile M.A., Hoves S., Benz J., Wartha K., Runza V., Rey-Giraud F., Pradel L.P., Feuerhake F., Klaman I., et al. Targeting Tumor-Associated Macrophages with Anti-CSF-1R Antibody Reveals a Strategy for Cancer Therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 207.Gomez-Roca C.A., Italiano A., Le Tourneau C., Cassier P.A., Toulmonde M., D’Angelo S.P., Campone M., Weber K.L., Loirat D., Cannarile M.A., et al. Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages. Ann. Oncol. 2019;30:1381–1392. doi: 10.1093/annonc/mdz163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.O’Neill L.A.J., Kishton R.J., Rathmell J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016;16:553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Munn D.H., Shafizadeh E., Attwood J.T., Bondarev I., Pashine A., Mellor A.L. Inhibition of T Cell Proliferation by Macrophage Tryptophan Catabolism. J. Exp. Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Brandacher G., Perathoner A., Ladurner R., Schneeberger S., Obrist P., Winkler C., Werner E.R., Werner-Felmayer G., Weiss H.G., Göbel G., et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: Effect on tumor-infiltrating T cells. Clin. Cancer Res. 2006;12:1144–1151. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 211.Mezrich J.D., Fechner J.H., Zhang X., Johnson B.P., Burlingham W.J., Bradfield C.A. An Interaction between Kynurenine and the Aryl Hydrocarbon Receptor Can Generate Regulatory T Cells. J. Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Koblish H.K., Hansbury M.J., Bowman K.J., Yang G., Neilan C.L., Haley P.J., Burn T.C., Waeltz P., Sparks R.B., Yue E.W., et al. Hydroxyamidine Inhibitors of Indoleamine-2,3-dioxygenase Potently Suppress Systemic Tryptophan Catabolism and the Growth of IDO-Expressing Tumors. Mol. Cancer Ther. 2010;9:489–498. doi: 10.1158/1535-7163.MCT-09-0628. [DOI] [PubMed] [Google Scholar]
- 213.Takamatsu M., Hirata A., Ohtaki H., Hoshi M., Ando T., Ito H., Hatano Y., Tomita H., Kuno T., Saito K., et al. Inhibition of indoleamine 2,3-dioxygenase 1 expression alters immune response in colon tumor microenvironment in mice. Cancer Sci. 2015;106:1008–1015. doi: 10.1111/cas.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Beatty G.L., O’Dwyer P.J., Clark J., Shi J.G., Bowman K.J., Scherle P.A., Newton R.C., Schaub R., Maleski J., Leopold L., et al. First-in-human phase I study of the oral inhibitor of indoleamine 2,3-dioxygenase-1 epacadostat (INCB024360) in patients with advanced solid malignancies. Clin. Cancer Res. 2017;23:3269–3276. doi: 10.1158/1078-0432.CCR-16-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Phan T., Nguyen V.H., D’Alincourt M.S., Manuel E.R., Kaltcheva T., Tsai W., Blazar B.R., Diamond D.J., Melstrom L.G. Salmonella-mediated therapy targeting indoleamine 2, 3-dioxygenase 1 (IDO) activates innate immunity and mitigates colorectal cancer growth. Cancer Gene Ther. 2020;27:235–245. doi: 10.1038/s41417-019-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Woo S.R., Turnis M.E., Goldberg M.V., Bankoti J., Selby M., Nirschl C.J., Bettini M.L., Gravano D.M., Vogel P., Liu C.L., et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ngiow S.F., von Scheidt B., Akiba H., Yagita H., Teng M.W.L., Smyth M.J. Anti-TIM3 Antibody Promotes T Cell IFN- -Mediated Antitumor Immunity and Suppresses Established Tumors. Cancer Res. 2011;71:3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 218.Sakuishi K., Apetoh L., Sullivan J.M., Blazar B.R., Kuchroo V.K., Anderson A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ganesh K., Stadler Z.K., Cercek A., Mendelsohn R.B., Shia J., Segal N.H., Diaz L.A. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019;16:361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ciardiello D., Vitiello P.P., Cardone C., Martini G., Troiani T., Martinelli E., Ciardiello F. Immunotherapy of colorectal cancer: Challenges for therapeutic efficacy. Cancer Treat. Rev. 2019;76:22–32. doi: 10.1016/j.ctrv.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 221.Onda M., Kobayashi K., Pastan I. Depletion of regulatory T cells in tumors with an anti-CD25 immunotoxin induces CD8 T cell-mediated systemic antitumor immunity. Proc. Natl. Acad. Sci. USA. 2019;116:4575–4582. doi: 10.1073/pnas.1820388116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sharma P., Allison J.P. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Signorini L., Delbue S., Ferrante P., Bregni M. Review on the immunotherapy strategies against metastatic colorectal carcinoma. Immunotherapy. 2016;8:1245–1261. doi: 10.2217/imt-2016-0045. [DOI] [PubMed] [Google Scholar]
- 224.Overman M.J., Lonardi S., Wong K.Y.M., Lenz H.J., Gelsomino F., Aglietta M., Morse M.A., Van Cutsem E., McDermott R., Hill A., et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J. Clin. Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 225.Chalabi M., Fanchi L.F., Dijkstra K.K., Van den Berg J.G., Aalbers A.G., Sikorska K., Lopez-Yurda M., Grootscholten C., Beets G.L., Snaebjornsson P., et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 2020:1–11. doi: 10.1038/s41591-020-0805-8. [DOI] [PubMed] [Google Scholar]
- 226.Haag G.M., Halama N., Springfeld C., Grün B., Apostolidis L., Zschaebitz S., Dietrich M., Berger A.-K., Weber T.F., Zoernig I., et al. Combined PD-1 inhibition (Pembrolizumab) and CCR5 inhibition (Maraviroc) for the treatment of refractory microsatellite stable (MSS) metastatic colorectal cancer (mCRC): First results of the PICCASSO phase I trial. J. Clin. Oncol. 2020;38:3010. doi: 10.1200/JCO.2020.38.15_suppl.3010. [DOI] [Google Scholar]
- 227.Garris C.S., Arlauckas S.P., Kohler R.H., Trefny M.P., Garren S., Piot C., Engblom C., Pfirschke C., Siwicki M., Gungabeesoon J., et al. Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-γ and IL-12. Immunity. 2018;49:1148–1161. doi: 10.1016/j.immuni.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sánchez-Paulete A.R., Cueto F.J., Martínez-López M., Labiano S., Morales-Kastresana A., Rodríguez-Ruiz M.E., Jure-Kunkel M., Azpilikueta A., Aznar M.A., Quetglas J.I., et al. Cancer immunotherapy with immunomodulatory anti-CD137 and anti–PD-1 monoclonal antibodies requires BATF3-dependent dendritic cells. Cancer Discov. 2016;6:71–79. doi: 10.1158/2159-8290.CD-15-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yost K.E., Satpathy A.T., Wells D.K., Qi Y., Wang C., Kageyama R., McNamara K.L., Granja J.M., Sarin K.Y., Brown R.A., et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 2019;25:1251–1259. doi: 10.1038/s41591-019-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wu T.D., Madireddi S., de Almeida P.E., Banchereau R., Chen Y.-J.J., Chitre A.S., Chiang E.Y., Iftikhar H., O’Gorman W.E., Au-Yeung A., et al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature. 2020;579:274–278. doi: 10.1038/s41586-020-2056-8. [DOI] [PubMed] [Google Scholar]
- 231.Canon J., Rex K., Saiki A.Y., Mohr C., Cooke K., Bagal D., Gaida K., Holt T., Knutson C.G., Koppada N., et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 232.Okuno K., Sugiura F., Inoue K., Sukegawa Y. Clinical trial of a 7-peptide cocktail vaccine with oral chemotherapy for patients with metastatic colorectal cancer. Anticancer Res. 2014;34:3045–3052. [PubMed] [Google Scholar]
- 233.Kawamura J., Sugiura F., Sukegawa Y., Yoshioka Y., Hida J.I., Hazama S., Okuno K. Cytotoxic T lymphocyte response to peptide vaccination predicts survival in stage III colorectal cancer. Cancer Sci. 2018;109:1545–1551. doi: 10.1111/cas.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Okuno K., Sugiura F., Hida J.I., Tokoro T., Ishimaru E., Sukegawa Y., Ueda K. Phase I clinical trial of a novel peptide vaccine in combination with UFT/LV for metastatic colorectal cancer. Exp. Ther. Med. 2011;2:73–79. doi: 10.3892/etm.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Scurr M., Pembroke T., Bloom A., Roberts D., Thomson A., Smart K., Bridgeman H., Adams R., Brewster A., Jones R., et al. Effect of Modified Vaccinia Ankara–5T4 and Low-Dose Cyclophosphamide on Antitumor Immunity in Metastatic Colorectal Cancer. JAMA Oncol. 2017;3:e172579. doi: 10.1001/jamaoncol.2017.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Twumasi-Boateng K., Pettigrew J.L., Kwok Y.Y.E., Bell J.C., Nelson B.H. Oncolytic viruses as engineering platforms for combination immunotherapy. Nat. Rev. Cancer. 2018;18:419–432. doi: 10.1038/s41568-018-0009-4. [DOI] [PubMed] [Google Scholar]
- 237.Groeneveldt C., van Hall T., van der Burg S.H., ten Dijke P., van Montfoort N. Immunotherapeutic Potential of TGF-β Inhibition and Oncolytic Viruses. Trends Immunol. 2020;41:406–420. doi: 10.1016/j.it.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 238.VanSeggelen H., Tantalo D.G., Afsahi A., Hammill J.A., Bramson J.L. Chimeric antigen receptor–engineered T cells as oncolytic virus carriers. Mol. Ther.-Oncolytics. 2015;2:15014. doi: 10.1038/mto.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Osada T., Patel S.P., Hammond S.A., Osada K., Morse M.A., Lyerly H.K. CEA/CD3-bispecific T cell-engaging (BiTE) antibody-mediated T lymphocyte cytotoxicity maximized by inhibition of both PD1 and PD-L1. Cancer Immunol. Immunother. 2015;64:677–688. doi: 10.1007/s00262-015-1671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tabernero J., Melero I., Ros W., Argiles G., Marabelle A., Rodriguez-Ruiz M.E., Albanell J., Calvo E., Moreno V., Cleary J.M., et al. Phase Ia and Ib studies of the novel carcinoembryonic antigen (CEA) T-cell bispecific (CEA CD3 TCB) antibody as a single agent and in combination with atezolizumab: Preliminary efficacy and safety in patients with metastatic colorectal cancer (mCRC) J. Clin. Oncol. 2017;35:3002. doi: 10.1200/JCO.2017.35.15_suppl.3002. [DOI] [Google Scholar]
- 241.Pan P.-Y., Zang Y., Weber K., Meseck M.L., Chen S.-H. OX40 Ligation Enhances Primary and Memory Cytotoxic T Lymphocyte Responses in an Immunotherapy for Hepatic Colon Metastases. Mol. Ther. 2002;6:528–536. doi: 10.1006/mthe.2002.0699. [DOI] [PubMed] [Google Scholar]
- 242.Messenheimer D.J., Jensen S.M., Afentoulis M.E., Wegmann K.W., Feng Z., Friedman D.J., Gough M.J., Urba W.J., Fox B.A. Timing of PD-1 Blockade Is Critical to Effective Combination Immunotherapy with Anti-OX40. Clin. Cancer Res. 2017;23:6165–6177. doi: 10.1158/1078-0432.CCR-16-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Buchan S.L., Fallatah M., Thirdborough S.M., Taraban V.Y., Rogel A., Thomas L.J., Penfold C.A., He L.-Z., Curran M.A., Keler T., et al. PD-1 Blockade and CD27 Stimulation Activate Distinct Transcriptional Programs That Synergize for CD8+ T-Cell–Driven Antitumor Immunity. Clin. Cancer Res. 2018;24:2383–2394. doi: 10.1158/1078-0432.CCR-17-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Mardiana S., John L.B., Henderson M.A., Slaney C.Y., von Scheidt B., Giuffrida L., Davenport A.J., Trapani J.A., Neeson P.J., Loi S., et al. A Multifunctional Role for Adjuvant Anti-4-1BB Therapy in Augmenting Antitumor Response by Chimeric Antigen Receptor T Cells. Cancer Res. 2017;77:1296–1309. doi: 10.1158/0008-5472.CAN-16-1831. [DOI] [PubMed] [Google Scholar]
- 245.Huang Q., Xia J., Wang L., Wang X., Ma X., Deng Q., Lu Y., Kumar M., Zhou Z., Li L., et al. MiR-153 suppresses IDO1 expression and enhances CAR T cell immunotherapy. J. Hematol. Oncol. 2018;11:1–12. doi: 10.1186/s13045-018-0600-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Guha P., Gardell J., Darpolor J., Cunetta M., Lima M., Miller G., Espat N.J., Junghans R.P., Katz S.C. STAT3 inhibition induces Bax-dependent apoptosis in liver tumor myeloid-derived suppressor cells. Oncogene. 2019;38:533–548. doi: 10.1038/s41388-018-0449-z. [DOI] [PubMed] [Google Scholar]
- 247.Arce Vargas F., Furness A.J.S., Solomon I., Joshi K., Mekkaoui L., Lesko M.H., Miranda Rota E., Dahan R., Georgiou A., Sledzinska A., et al. Fc-Optimized Anti-CD25 Depletes Tumor-Infiltrating Regulatory T Cells and Synergizes with PD-1 Blockade to Eradicate Established Tumors. Immunity. 2017;46:577–586. doi: 10.1016/j.immuni.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Holmgaard R.B., Brachfeld A., Gasmi B., Jones D.R., Mattar M., Doman T., Murphy M., Schaer D., Wolchok J.D., Merghoub T. Timing of CSF-1/CSF-1R signaling blockade is critical to improving responses to CTLA-4 based immunotherapy. Oncoimmunology. 2016;5:e1151595. doi: 10.1080/2162402X.2016.1151595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hombach A.A., Rappl G., Abken H. Blocking CD30 on T Cells by a Dual Specific CAR for CD30 and Colon Cancer Antigens Improves the CAR T Cell Response against CD30− Tumors. Mol. Ther. 2019;27:1825–1835. doi: 10.1016/j.ymthe.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mariathasan S., Turley S.J., Nickles D., Castiglioni A., Yuen K., Wang Y., Kadel III E.E., Koeppen H., Astarita J.L., Cubas R., et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Holmgaard R.B., Schaer D.A., Li Y., Castaneda S.P., Murphy M.Y., Xu X., Inigo I., Dobkin J., Manro J.R., Iversen P.W., et al. Targeting the TGFβ pathway with galunisertib, a TGFβRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J. Immunother. Cancer. 2018;6:47. doi: 10.1186/s40425-018-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lan Y., Zhang D., Xu C., Hance K.W., Marelli B., Qi J., Yu H., Qin G., Sircar A., Hernández V.M., et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci. Transl. Med. 2018;10:eaan5488. doi: 10.1126/scitranslmed.aan5488. [DOI] [PubMed] [Google Scholar]
- 253.Nakanishi Y., Duran A., L’Hermitte A., Shelton P.M., Nakanishi N., Reina-Campos M., Huang J., Soldevila F., Baaten B.J.G., Tauriello D.V.F., et al. Simultaneous Loss of Both Atypical Protein Kinase C Genes in the Intestinal Epithelium Drives Serrated Intestinal Cancer by Impairing Immunosurveillance. Immunity. 2018;49:1132–1147. doi: 10.1016/j.immuni.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chakravarthy A., Khan L., Bensler N.P., Bose P., De Carvalho D.D. TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat. Commun. 2018;9:1–10. doi: 10.1038/s41467-018-06654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Endo E., Okayama H., Saito K., Nakajima S., Yamada L., Ujiie D., Kase K., Fujita S., Endo H., Sakamoto W., et al. A TGFβ-dependent stromal subset underlies immune checkpoint inhibitor efficacy in DNA mismatch repair-deficient/microsatellite instability-high colorectal cancer. Mol. Cancer Res. 2020 doi: 10.1158/1541-7786.MCR-20-0308. [DOI] [PubMed] [Google Scholar]
- 256.Rodríguez-Ruiz M.E., Rodríguez I., Mayorga L., Labiano T., Barbes B., Etxeberria I., Ponz-Sarvise M., Azpilikueta A., Bolaños E., Sanmamed M.F., et al. TGFβ Blockade Enhances Radiotherapy Abscopal Efficacy Effects in Combination with Anti-PD1 and Anti-CD137 Immunostimulatory Monoclonal Antibodies. Mol. Cancer Ther. 2019;18:621–631. doi: 10.1158/1535-7163.MCT-18-0558. [DOI] [PubMed] [Google Scholar]
- 257.Zhao F., Evans K., Xiao C., DeVito N., Theivanthiran B., Holtzhausen A., Siska P.J., Blobe G.C., Hanks B.A. Stromal Fibroblasts Mediate Anti–PD-1 Resistance via MMP-9 and Dictate TGFβ Inhibitor Sequencing in Melanoma. Cancer Immunol. Res. 2018;6:1459–1471. doi: 10.1158/2326-6066.CIR-18-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Dodagatta-Marri E., Meyer D.S., Reeves M.Q., Paniagua R., To M.D., Binnewies M., Broz M.L., Mori H., Wu D., Adoumie M., et al. α-PD-1 therapy elevates Treg/Th balance and increases tumor cell pSmad3 that are both targeted by α-TGFβ antibody to promote durable rejection and immunity in squamous cell carcinomas. J. Immunother. Cancer. 2019;7:62. doi: 10.1186/s40425-018-0493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Martin C.J., Datta A., Littlefield C., Kalra A., Chapron C., Wawersik S., Dagbay K.B., Brueckner C.T., Nikiforov A., Danehy F.T., et al. Selective inhibition of TGFβ1 activation overcomes primary resistance to checkpoint blockade therapy by altering tumor immune landscape. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.aay8456. [DOI] [PubMed] [Google Scholar]
- 260.Otegbeye F., Ojo E., Moreton S., Mackowski N., Lee D.A., De Lima M., Wald D.N. Inhibiting TGF-beta signaling preserves the function of highly activated, in vitro expanded natural killer cells in AML and colon cancer models. PLoS ONE. 2018;13:1–13. doi: 10.1371/journal.pone.0191358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Shi L., Wang J., Ding N., Zhang Y., Zhu Y., Dong S., Wang X., Peng C., Zhou C., Zhou L., et al. Inflammation induced by incomplete radiofrequency ablation accelerates tumor progression and hinders PD-1 immunotherapy. Nat. Commun. 2019;10:5421. doi: 10.1038/s41467-019-13204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Jones K.I., Tiersma J., Yuzhalin A.E., Gordon-Weeks A.N., Buzzelli J., Im J.H., Muschel R.J. Radiation combined with macrophage depletion promotes adaptive immunity and potentiates checkpoint blockade. EMBO Mol. Med. 2018;10:e9342. doi: 10.15252/emmm.201809342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ito H., Ando T., Arioka Y., Saito K., Seishima M. Inhibition of indoleamine 2,3-dioxygenase activity enhances the anti-tumour effects of a Toll-like receptor 7 agonist in an established cancer model. Immunology. 2015;144:621–630. doi: 10.1111/imm.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Galon J., Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 265.Perez-Ruiz E., Minute L., Otano I., Alvarez M., Ochoa M.C., Belsue V., de Andrea C., Rodriguez-Ruiz M.E., Perez-Gracia J.L., Marquez-Rodas I., et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature. 2019;569:428–432. doi: 10.1038/s41586-019-1162-y. [DOI] [PubMed] [Google Scholar]
- 266.Liu L., Mayes P.A., Eastman S., Shi H., Yadavilli S., Zhang T., Yang J., Seestaller-Wehr L., Zhang S.-Y., Hopson C., et al. The BRAF and MEK Inhibitors Dabrafenib and Trametinib: Effects on Immune Function and in Combination with Immunomodulatory Antibodies Targeting PD-1, PD-L1, and CTLA-4. Clin. Cancer Res. 2015;21:1639–1651. doi: 10.1158/1078-0432.CCR-14-2339. [DOI] [PubMed] [Google Scholar]
- 267.Ebert P.J.R., Cheung J., Yang Y., McNamara E., Hong R., Moskalenko M., Gould S.E., Maecker H., Irving B.A., Kim J.M., et al. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity. 2016;44:609–621. doi: 10.1016/j.immuni.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 268.Moynihan K.D., Opel C.F., Szeto G.L., Tzeng A., Zhu E.F., Engreitz J.M., Williams R.T., Rakhra K., Zhang M.H., Rothschilds A.M., et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat. Med. 2016;22:1402–1410. doi: 10.1038/nm.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Grothey A., Tabernero J., Arnold D., De Gramont A., Ducreux M.P., O’Dwyer P.J., Van Cutsem E., Bosanac I., Srock S., Mancao C., et al. Fluoropyrimidine (FP) + bevacizumab (BEV) + atezolizumab vs FP/BEV in BRAFwt metastatic colorectal cancer (mCRC): Findings from Cohort 2 of MODUL—A multicentre, randomized trial of biomarker-driven maintenance treatment following first-line induction th. Ann. Oncol. 2018;29:viii714–viii715. doi: 10.1093/annonc/mdy424.020. [DOI] [Google Scholar]
- 270.Eng C., Kim T.W., Bendell J., Argilés G., Tebbutt N.C., Di Bartolomeo M., Falcone A., Fakih M., Kozloff M., Segal N.H., et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019;20:849–861. doi: 10.1016/S1470-2045(19)30027-0. [DOI] [PubMed] [Google Scholar]
- 271.O’Rourke K.P., Loizou E., Livshits G., Schatoff E.M., Baslan T., Manchado E., Simon J., Romesser P.B., Leach B., Han T., et al. Transplantation of engineered organoids enables rapid generation of metastatic mouse models of colorectal cancer. Nat. Biotechnol. 2017;35:577–582. doi: 10.1038/nbt.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Roper J., Tammela T., Cetinbas N.M., Akkad A., Roghanian A., Rickelt S., Almeqdadi M., Wu K., Oberli M.A., Sánchez-Rivera F., et al. In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis. Nat. Biotechnol. 2017;35:569–576. doi: 10.1038/nbt.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Jackstadt R., van Hooff S.R., Leach J.D., Cortes-Lavaud X., Lohuis J.O., Ridgway R.A., Wouters V.M., Roper J., Kendall T.J., Roxburgh C.S., et al. Epithelial NOTCH Signaling Rewires the Tumor Microenvironment of Colorectal Cancer to Drive Poor-Prognosis Subtypes and Metastasis. Cancer Cell. 2019;36:319–336. doi: 10.1016/j.ccell.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Neal J.T., Li X., Zhu J., Giangarra V., Grzeskowiak C.L., Ju J., Liu I.H., Chiou S.-H., Salahudeen A.A., Smith A.R., et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell. 2018;175:1972–1988. doi: 10.1016/j.cell.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Dijkstra K.K., Cattaneo C.M., Weeber F., Chalabi M., van de Haar J., Fanchi L.F., Slagter M., van der Velden D.L., Kaing S., Kelderman S., et al. Generation of Tumor-Reactive T Cells by Co-culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell. 2018;174:1586–1598. doi: 10.1016/j.cell.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., Coussens L.M., Gabrilovich D.I., Ostrand-Rosenberg S., Hedrick C.C., et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Thorsson V., Gibbs D.L., Brown S.D., Wolf D., Bortone D.S., Ou Yang T.-H., Porta-Pardo E., Gao G.F., Plaisier C.L., Eddy J.A., et al. The Immune Landscape of Cancer. Immunity. 2018;48:812–830. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Soldevilla B., Carretero-Puche C., Gomez-Lopez G., Al-Shahrour F., Riesco M.C., Gil-Calderon B., Alvarez-Vallina L., Espinosa-Olarte P., Gomez-Esteves G., Rubio-Cuesta B., et al. The correlation between immune subtypes and consensus molecular subtypes in colorectal cancer identifies novel tumour microenvironment profiles, with prognostic and therapeutic implications. Eur. J. Cancer. 2019;123:118–129. doi: 10.1016/j.ejca.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 279.Roelands J., Kuppen P., Vermeulen L., Maccalli C., Decock J., Wang E., Marincola F., Bedognetti D., Hendrickx W. Immunogenomic Classification of Colorectal Cancer and Therapeutic Implications. Int. J. Mol. Sci. 2017;18:2229. doi: 10.3390/ijms18102229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Shen R., Li P., Li B., Zhang B., Feng L., Cheng S. Identification of Distinct Immune Subtypes in Colorectal Cancer Based on the Stromal Compartment. Front. Oncol. 2020;9:1–15. doi: 10.3389/fonc.2019.01497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Garralda E., Dienstmann R., Piris-Giménez A., Braña I., Rodon J., Tabernero J. New clinical trial designs in the era of precision medicine. Mol. Oncol. 2019;13:549–557. doi: 10.1002/1878-0261.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Lemery S., Keegan P., Pazdur R. First FDA Approval Agnostic of Cancer Site—When a Biomarker Defines the Indication. N. Engl. J. Med. 2017;377:1409–1412. doi: 10.1056/NEJMp1709968. [DOI] [PubMed] [Google Scholar]
- 283.Becht E., de Reyniès A., Giraldo N.A., Pilati C., Buttard B., Lacroix L., Selves J., Sautès-Fridman C., Laurent-Puig P., Fridman W.H. Immune and Stromal Classification of Colorectal Cancer Is Associated with Molecular Subtypes and Relevant for Precision Immunotherapy. Clin. Cancer Res. 2016;22:4057–4066. doi: 10.1158/1078-0432.CCR-15-2879. [DOI] [PubMed] [Google Scholar]
- 284.Chen D.S., Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 285.Young K., Cottam B., Baird J.R., Gough M.J., Crittenden M. Ideal Timing of Immunotherapy With Radiation in Murine Tumor Models. Int. J. Radiat. Oncol. 2014;90:S58. doi: 10.1016/j.ijrobp.2014.05.202. [DOI] [Google Scholar]
- 286.Young K.H., Baird J.R., Savage T., Cottam B., Friedman D., Bambina S., Messenheimer D.J., Fox B., Newel P., Bahjat K.S., et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS ONE. 2016;11:1–15. doi: 10.1371/journal.pone.0157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Sckisel G.D., Mirsoian A., Bouchlaka M.N., Tietze J.K., Chen M., Blazar B.R., Murphy W.J. Late administration of murine CTLA-4 blockade prolongs CD8-mediated anti-tumor effects following stimulatory cancer immunotherapy. Cancer Immunol. Immunother. 2015;64:1541–1552. doi: 10.1007/s00262-015-1759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Fransen M.F., Schoonderwoerd M., Knopf P., Camps M.G., Hawinkels L.J., Kneilling M., van Hall T., Ossendorp F. Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight. 2018;3:e124507. doi: 10.1172/jci.insight.124507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Topalian S.L., Taube J.M., Pardoll D.M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367:eaax0182. doi: 10.1126/science.aax0182. [DOI] [PMC free article] [PubMed] [Google Scholar]