Abstract
Background: Neoadjuvant therapy (NAT) represents a paradigm shift in the management of patients with pancreatic ductal adenocarcinoma (PDAC) with perceived benefits including a higher R0 rate. However, it is unclear whether NAT affects the sites and patterns of recurrence after surgery. This review seeks to compare sites and patterns of recurrence after resection between patients undergoing upfront surgery (US) or after NAT. Methods: The EMBASE, SCOPUS, PubMed, and Cochrane library databases were systematically searched to identify eligible studies that compare recurrence patterns between patients who had NAT (followed by resection) with those that had US. The primary outcome included site-specific recurrence. Results: 26 articles were identified including 4986 patients who underwent resection. Borderline resectable pancreatic cancer (BRPC, 47% 1074/2264) was the most common, followed by resectable pancreatic cancer (RPC 42%, 949/2264). The weighted overall recurrence rates were lower among the NAT group, 63.4% vs. 74% (US) (OR 0.67 (CI 0.52–0.87), p = 0.006). The overall weighted locoregional recurrence rate was lower amongst patients who received NAT when compared to US (12% vs. 27% OR 0.39 (CI 0.22–0.70), p = 0.004). In BRPC, locoregional recurrence rates improved with NAT (NAT 25.8% US 37.7% OR 0.62 (CI 0.44–0.87), p = 0.007). NAT was associated with a lower weighted liver recurrence rate (NAT 19.4% US 30.1% OR 0.55 (CI 0.34–0.89), p = 0.023). Lung and peritoneal recurrence rates did not differ between NAT and US cohorts (p = 0.705 and p = 0.549 respectively). NAT was associated with a significantly longer weighted mean time to first recurrence 18.8 months compared to US (15.7 months) (OR 0.18 (CI 0.05–0.32), p = 0.015). Conclusion: NAT was associated with lower overall recurrence rate and improved locoregional disease control particularly for those with BRPC. Although the burden of liver metastases was less, there was no overall effect upon distant metastatic disease.
Keywords: recurrence, neoadjuvant chemotherapy, pancreatic ductal adenocarcinoma, pancreatic surgery
1. Introduction
Patients who have undergone potentially curative treatment for pancreatic ductal adenocarcinoma (PDAC) typically suffer treatment failure with cancer recurrence [1], frequently within two years of resection [2]. Recurrence, including multi-site recurrence and liver metastases, are key prognosticators of overall survival [3]. Several risk factors for specific recurrence patterns have been identified. Positive resection margin and poor tumour differentiation [2,4] are risk factors for locoregional recurrence. Tumour size, elevated preoperative Ca19-9 serum level, and perineural invasion are risk factors for distant recurrence [5].
While upfront surgery followed by adjuvant therapy has been the standard of care for PDAC, this is being challenged by recent evidence with use of neoadjuvant chemotherapy (NAT) for borderline resectable pancreatic cancer (BRPC) [6] and resectable (RPC) [7] pancreatic cancer (LAPC) [8]. It is important to determine the impact of different treatment strategies on pattern of disease recurrence [9,10]. NAT is associated with a higher rate of achieving R0 margins [11,12] and may control micrometastatic disease; thus, it is important to determine whether NAT influences patterns of treatment failure in terms of the frequency and site of recurrent cancer.
While here have been meta-analyses examining the impact of NAT on overall survival [13,14], there is paucity of evidence examining the impact of NAT and upfront surgery (US) on disease recurrence and recurrence patterns with and without neoadjuvant chemotherapy.
This present study aimed to undertake a meta-analysis of available evidence to compare patients with PDAC undergoing upfront surgery versus neoadjuvant therapy followed by surgery to review sites of recurrence, recurrence-free survival patterns, overall recurrence rate, and time to recurrence in patients with resectable, borderline resectable and locally advanced pancreatic cancer.
2. Materials and Methods
The protocol of this review was registered on the Prospero database prior to inception (CRD42019159031).
2.1. Literature Search
The EMBASE, SCOPUS, PubMed and Cochrane library databases were systematically searched with the aid of a pre-devised literature search strategy that followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15]. Details of the query string with accompanying Boolean operators are reported in Appendix A. Limits were set to English studies reported from January 2000 to February 2020 in human patients. Reference lists of included studies were then examined by two authors (C.B.B.R. and A.Y.S.) to identify eligible studies missed by the original search strategy.
2.2. Data Selection, Extraction, Risk of Bias Assessment and Publication Bias Assessment
Studies were included in the quantitative meta-analysis if they reported comparative recurrence outcomes specific to patients who underwent NAT compared to those undergoing US for PDAC. More specifically, if they reported overall recurrence, time to first recurrence, sites of recurrence and/or recurrence free survival (RFS). Studies were excluded if they were case reports/series, conference abstracts, study protocols, non-comparative observational cohorts, did not report recurrence or RFS data for either NAT or upfront surgery patients, did not employ NAT, and articles published prior to 2000. Title, abstract and full text screening were performed by two investigators (C.B.B.R. and A.Y.S.). Consistency of data extraction was compared, and disputes were adjudicated by a third author (S.P.). Baseline characteristic data including: age; gender; location of tumour; preoperative tumour stage and resectability; details of NAT data; preoperative radiotherapy; adjuvant therapy; pathological outcome data including; nodal status; tumour differentiation; tumour size; perineural/lymphovascular invasion; R0 resection; and patterns of recurrence including overall recurrence rate, time to first recurrence, site of recurrence, and RFS. The primary outcome was site-specific recurrence. The secondary outcomes were overall recurrence, time to first recurrence, and recurrence-free survival.
2.3. Assessment of Study Quality
The risk of bias and study quality assessment was performed through use of the methodological index for non-randomized studies (MINORS) grading criteria [16] for non-randomised studies. The Cochrane’s risk of bias tool [17] was used for randomised controlled trials. Publication bias was identified through visual inspection of funnel plots whereby bias existed if individual studies lay beyond the funnel limits.
2.4. Terminology and Definitions
NAT included preoperative chemotherapy with or without radiotherapy. Sole radiotherapy-based NAT regimens were not assessed in this meta-analysis. Fluoropyrimidine-based chemotherapies included S-1 (5-fluorouracil prodrug tegafur with oteracil, and gimeracil) [18], capecitabine and 5-fluorouracil; platinum-based chemotherapies included cisplatin and oxaliplatin; and plant alkaloids included docetaxel and paclitaxel. FOLFIRINOX was a combination regime of 5-fluorouracil, leucovorin, oxaliplatin, and irinotecan. If an agent was used in combination with another, this was considered combination chemotherapy. Upfront surgery (US) included all forms of pancreatic resection (including pancreatoduodenectomy, distal pancreatectomy and total pancreatectomy) performed shortly after the diagnosis of a pancreatic cancer without preoperative NAT. The authors identified resectable (RPC), borderline resectable (BRPC), and locally advanced (LAPC) when reported as such. The definitions of these categories were variably reported and have changed over the course of the study with the publication of multiple definitions [19,20,21]. Nodal status was considered N0 when there were no positive nodes at the time of surgery. Tumour differentiation was based on histology of the specimen following resection [22] and tumour size by the largest diameter reported by the pathologist. Presence of malignant cells in neural tissue or lymphovascular tissue denoted perineural or lymphovascular invasion [23]. R0 resection was defined when there was no microscopic tumour present within 1 mm of the resection margin [24]. Recurrence patterns included overall recurrence rates, time to first recurrence and recurrence-free survival rates. Overall recurrence was defined by the first presentation of previously unknown disease activity during postoperative follow-up [25]. Locoregional recurrence was the presence of tumour recurrence at the site of previous resection or in surrounding lymph nodes (LN). Distant recurrence referred to recurrence present in distant organs. RFS rate was defined as the proportion of patients who had no confirmed recurrence of cancer and included the terms progression-free survival and disease-free survival [26]. Time to first recurrence represented the interval following resection that the first recurrence of disease was observed and was calculated by the mean RFS [27]. Disease-specific survival [28,29] or cancer-specific survival [8] were not included in this analysis as these terms focused solely on survival secondary to the cancer.
2.5. Statistical Analysis
The meta-analysis was conducted in its entirety with the packages: tidyverse [30], meta [31], metaphor [32], and MetaAnalyser (Jack Bowden and Christopher Jackson, UK) [33] in R project (R Foundation for Statistical Computing, Austria 2014). A Mantel-Haenszel model was utilised to perform the pairwise meta-analysis. The output was reported as odds ratios (OR) or standard mean difference (SMD) with 95% confidence intervals (CI). I2 test allowed the authors to determine statistical heterogeneity whereby a threshold of 50% suggested moderate heterogeneity and 75% indicative of substantial heterogeneity [34]. Weighted means and recurrence rate estimates with their corresponding 95% CI were calculated for age, tumour diameter, follow-up interval, and recurrence outcomes. Weighted means were calculated by the inverse variance method. Weighted recurrence rate estimates were determined by a random intercept logistic regression model [31]. Weighted estimates were only calculated when three or more datapoints were available. WebPlotDigitizer (Ankit Rohatgi, CA, USA, 2019) [35] was employed to extract raw data from reported Kaplan Meier survival curves if articles failed to provide raw numbers for RFS or time to first recurrence. The median (1st and 3rd quartile) time to first recurrence was only calculable from a Kaplein Meier curve if both survival curves crossed the 75% and 25% survival threshold. Mean estimates were then derived from median and ranges or quartiles through Wan et al. [36].
Propensity score matched data was used in preference to raw unmatched data to improve homogeneity of the datasets in relevant studies [8,27,37,38]. However, if baseline characteristic data or outcome data were not reported for propensity score matching, the unmatched data was used [28]. Historic [25] or unpublished [39] US cohorts were utilised if baseline characteristics were reported. Data for baseline characteristics was commonly reported for the entire study population irrespective of those who subsequently underwent resection. However, the remaining outcomes including pathological, resectability and recurrence data are reported for the population who ultimately underwent surgery and were subsequently followed-up. When calculating NAT agent-specific time to first recurrence, the authors included situations where the agent was used as the base NAT, used in combination with another agent or used in more than 30% of the cohort [40]. Recurrence outcomes were reported with significantly fewer patients than in the pathological and resectability outcomes in another study [41] and this is reflected in the meta-analysis.
3. Results
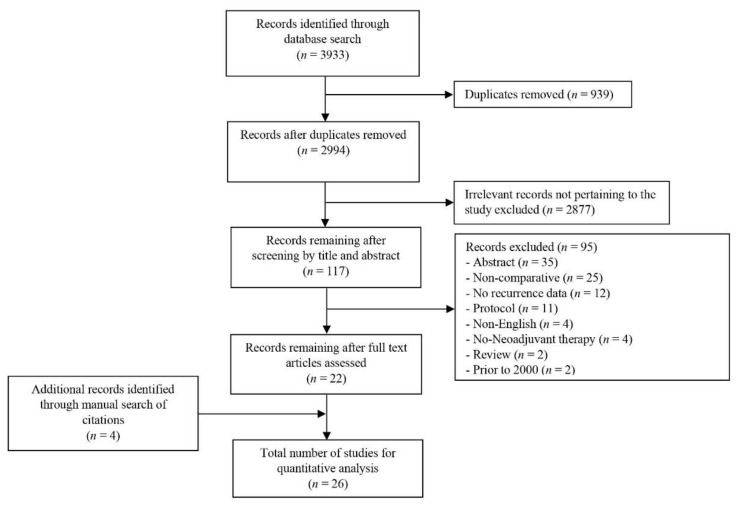
From 3933 potentially relevant studies, 22 were included in the quantitative analysis. Four additional studies [8,40,42,43] were recruited into the meta-analysis following a review of the reference lists of the 22 studies (Figure 1).
Figure 1.
PRISMA flow chart of literature search strategy.
Among 4986 patients, 1699 received NAT followed by pancreatic resection (34%, 1699/4986) and 3287 patients US (66%, 3287/4986). The studies were from predominantly single centers (81%, 21/26) in Asia (42%, 11/26) and retrospective in design (81%, 21/26) (characteristics of all studies are summarised in Table 1).
Table 1.
Characteristics of all included studies and patients recruited into the meta-analysis for quantitative analysis.
| Author | Publication Year | Country | Study Type (n) | Recruitment Dates | Centres (n) | Total Study Population (n) | Cohorts Undergoing Surgery for Comparison | Total in Quantitative Analysis (n) |
|
|---|---|---|---|---|---|---|---|---|---|
| NAT (n) | US (n) | ||||||||
| Versteijne et al. [44] | 2020 | Netherlands | Trial | 2013–2017 | 16 | 246 | 72 | 92 | 164 |
| Lof et al. [37] | 2019 | Europe | Retrospective | 2007–2015 | Multiple | 1236 | 94 * | 94 ± | 188 |
| Yoshiya et al. [26] | 2019 | Japan | Retrospective | 2008–2018 | 1 | 20 | 11 | 9 | 20 |
| Groot et al. [25] | 2019 | USA | Retrospective | 2007–2015 | 1 | 1188 | 231 | 95 * | 1188 |
| Nagakawa et al. [38] | 2019 | Japan | Prospective | 2011–2013 | 63 | 884 | 223 | 27 * | 500 |
| Nurmi et al. [27] | 2018 | Finland | Retrospective | 2000–2015 | 1 | 225 | 75 | 150 * | 225 |
| Jang et al. [7] | 2018 | Korea | Trial | 2012–2014 | 4 | 40 | 17 | 18 | 35 |
| Chen et al. [8] | 2017 | China | Retrospective | 2004–2013 | Multiple | 196 | 98 | 98 * | 196 |
| Masui et al. [45] | 2016 | Japan | Prospective | 2005–2010 | 1 | 37 | 15 | 19 | 34 |
| Ferrone et al. [43] | 2015 | USA | Retrospective | 2011–2014 | 1 | 127 | 40 | 87 | 127 |
| Fujii et al. [28] | 2015 | Japan | Retrospective | 2002–2014 | 1 | 92 | 18 | 50 | 68 |
| Ishikawa et al. [46] | 2015 | Japan | Retrospective | Until 2011 | 1 | 506 | 112 | 238 | 350 |
| Golcher et al. [47] | 2014 | Germany | Trial | 2003–2009 | 1 | 66 | 33 | 33 | 66 |
| Papavasiliou et al. [41] | 2014 | USA | Retrospective | 1990–2009 | 1 | 309 | 108 | 201 | 309 |
| Cho et al. [48] | 2013 | Korea | Retrospective | 2002–2011 | 1 | 51 | 30 | 21 | 51 |
| Jiang et al. [40] | 2013 | China | Retrospective | 2004–2010 | 1 | 232 | 11 β | 120 | 232 |
| Kang et al. [6] | 2012 | Korea | Retrospective | 1999–2010 | 1 | 136 | 32 | 104 | 136 |
| Tajima et al. [49] | 2012 | Japan | Retrospective | 2006–2009 | 1 | 34 | 13 | 21 | 34 |
| Barugola et al. [29] | 2012 | Italy | Retrospective | 2001–2008 | 1 | 403 | 41 | 362 | 403 |
| Katz et al. [50] | 2012 | USA | Retrospective | 2004–2008 | 1 | 147 | 147 | 47 | 194 |
| Barbier et al. [51] | 2011 | France | Retrospective | 1997–2006 | 1 | 173 | 38 | 67 | 105 |
| Sahora et al. [39] | 2011 | Asutria | Retrospective | 2003–2006 | 1 | 106 | 13 | 73 ¥ | 86 |
| Greer et al. [52] | 2008 | Lebanon | Retrospective | 1993–2005 | 1 | 102 | 42 | 41 € | 83 |
| Massucco et al. [42] | 2006 | Italy | Retrospective | 1999–2003 | 1 | 72 | 8 | 44 | 52 |
| Moutardier et al. [53] | 2004 | France | Retrospective | 1997–2002 | 1 | 87 | 23 | 17 | 40 |
| Pingpank et al. [54] | 2001 | USA | Retrospective | 1987–2000 | 1 | 100 | 53 | 47 | 100 |
NAT Neoadjuvant therapy; US Upfront surgery; * Propensity score matched data; € Did not include operation alone as specific outcomes were not reported for this; ± Historical cohort; ¥ Unpublished cohort; β Included both neoadjuvant chemotherapy (n = 81, 72%)and neoadjuvant radiotherapy alone (n = 31, 28%) as outcomes were reported for overall NAT cohort.
Baseline characteristic data was reported for all patients initially recruited into the studies before NAT was administered (n = 5328). Patients were commonly female (54%, 2885/5328) in their seventh decade of life (weighted mean age 62 years, CI 60.4–63.6). Tumour location was reported in 20 studies [6,7,8,26,29,37,38,39,40,41,42,44,45,47,48,49,50,51,53,54] and was predominantly the pancreatic head or neck (79%, 2478/3147) (Table S1).
3.1. Neoadjuvant and Adjuvant Therapy
Most patients received Gemcitabine-based NAT (24 studies [6,7,8,25,26,27,28,29,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54], 62%, 851/1382). Fluoropyrimidines (20%, 282/1382) and FOLFIRINOX (13%, 182/1382) based regimens were not uncommon, however, platinum-based and plant alkaloid regimens were rare (7% 97/1382 and 2% 23/1382 respectively) (Table S3). Combination therapy was also observed in 23% (312/1382). Preoperative radiotherapy was a component of NAT in 51% (868/1699) patients. A minority of patients in a single study undergoing solely radiotherapy as the NAT regime (28%, 31/112) [40] was included under NAT cohort as outcomes were reported for all patients.
Adjuvant therapy was reported in 14 studies [7,25,26,27,28,37,41,42,44,45,47,48,51,52] and was received by 66% of patients (1649/2505). This included either chemotherapy (61%, 1011/1649) or chemoradiotherapy (39%, 638/1649). In one study [52], only those with adjuvant therapy vs. neoadjuvant was compared and those with US without adjuvant therapy were excluded as outcomes were not reported.
3.2. Resection and Resectability in the NAT Cohort
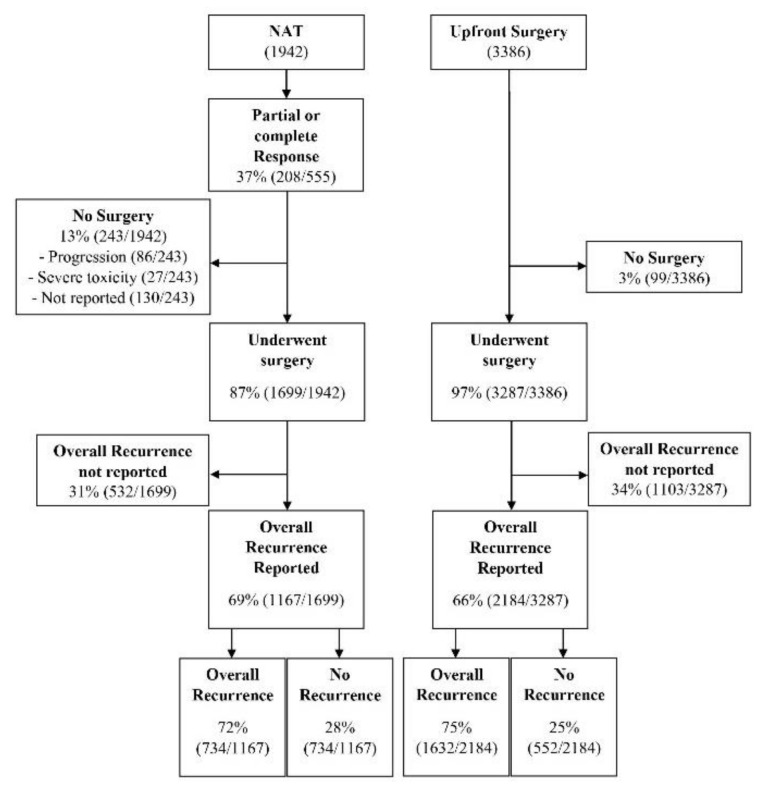
Following NAT, 1699 proceeded to resection (87%). Failure to proceed to resection was due to cancer progression during NAT (35%, 86/243) or severe NAT toxicity leading to disruption of therapy (11%, 27/243). However, the indication for non-operative management was not reported in 53% of patients (130/243, Figure 2). The indication for resection among BRPC and LAPC was reported in nine studies. BRPC was included for resection in patients with the superior mesenteric artery (SMA) or superior mesenteric vein (SMV) involvement not exceeding 180 degrees in six studies [28,38,43,45,51,52] and without tumour extension in the celiac axis unless considered safe for complete resection in two studies [28,38]. Those without SMA involvement and < 180-degree involvement of SMV was the criteria for resection in one study [29]. LAPC was considered resectable in patients with common hepatic artery, root of splenic artery or celiac axis involvement in one study [26], and SMA or coeliac axis (CA) involvement with stenosis or thrombosis of the vessel in another study [42]. Four studies [6,7,25,28] defined BRPC by the national cancer network guidelines [55]. The types of resection were reported in 17 studies [6,8,25,26,28,29,37,40,41,42,43,45,48,49,50,51,53]: pancreaticoduodenectomy (PD, including pylorus preserving variant, 79%, 2639/3344), distal pancreatectomy (17%, 564/3344), and total pancreatectomy (4%, 141/3344).
Figure 2.
Flow chart of patient management and overall recurrence for patients initially allocated to neoadjuvant therapy (NAT) and upfront surgery.
The preoperative tumour resectability for all patients undergoing resection was reported in 17 studies [6,7,8,26,27,28,29,38,41,43,45,47,48,49,50,52,53] for NAT and US groups. BRPC were the most prevalent (47%, 1074/2264), followed by RPC (42%, 949/2264) and locally advance pancreatic cancer (LAPC) (10%, 230/2264). In the NAT group, 15% (131/866) were LAPC, 23% (203/866) RPC, and 60% (522/866) were BRPC. In the US group, 7% (99/1398) were LAPC, 39% (552/1398) BRPC, and 53% were (746/1398) RPC. Eleven patients had unresectable tumours that subsequently underwent resection in three studies [26,42,52] (NAT 10/866, US 1/1398, Table S3).
3.3. Primary Outcome Measure
Sites of Recurrence
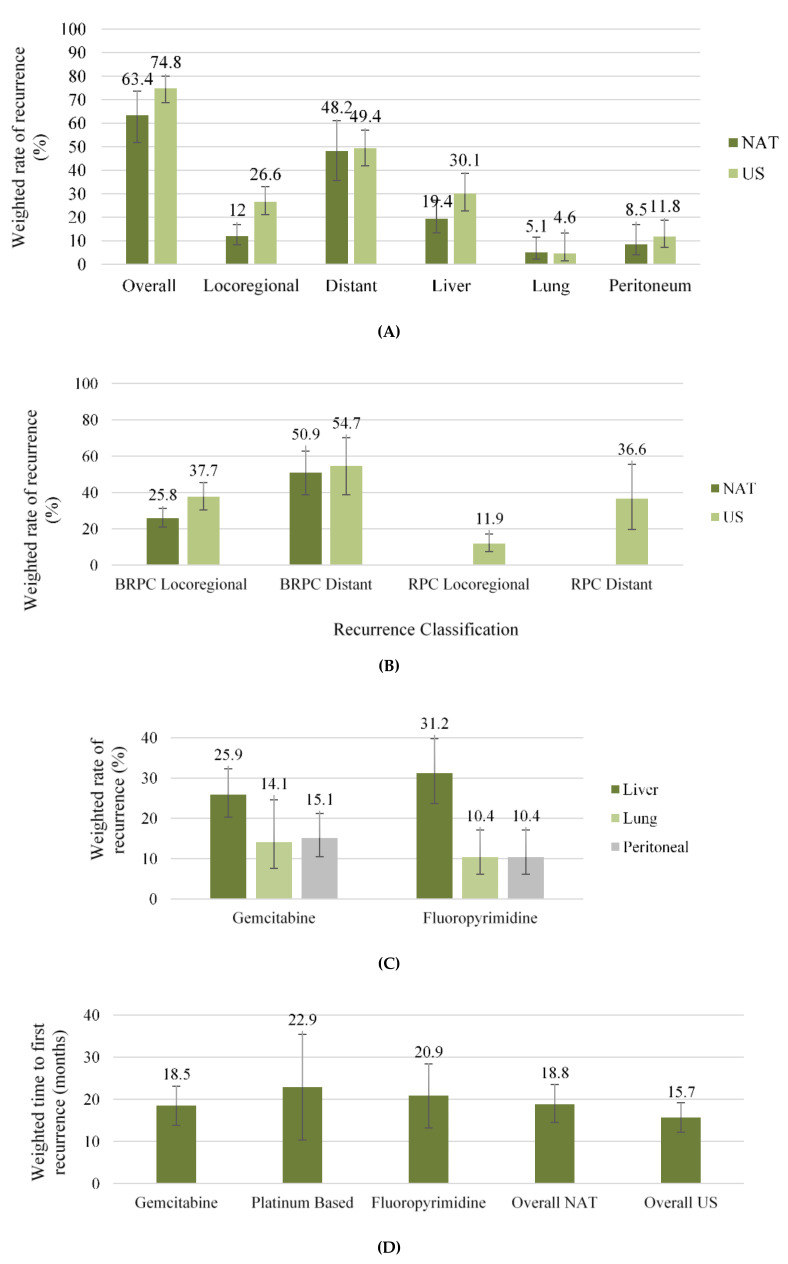
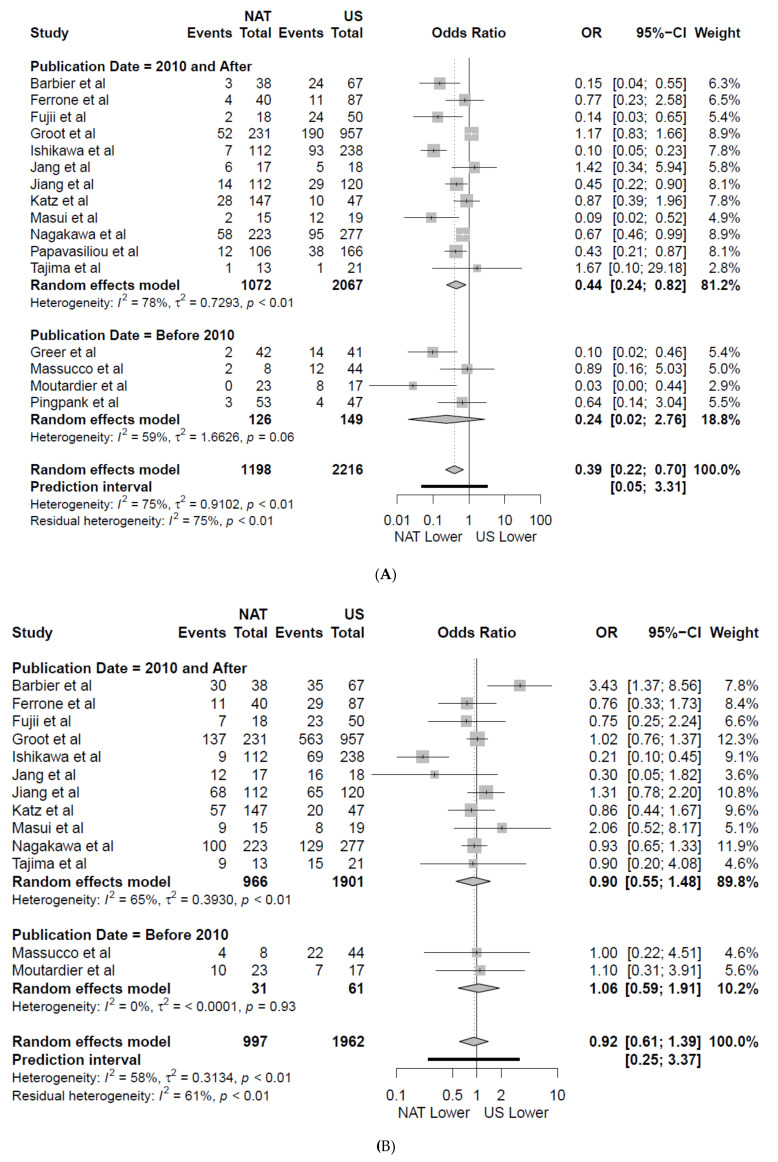
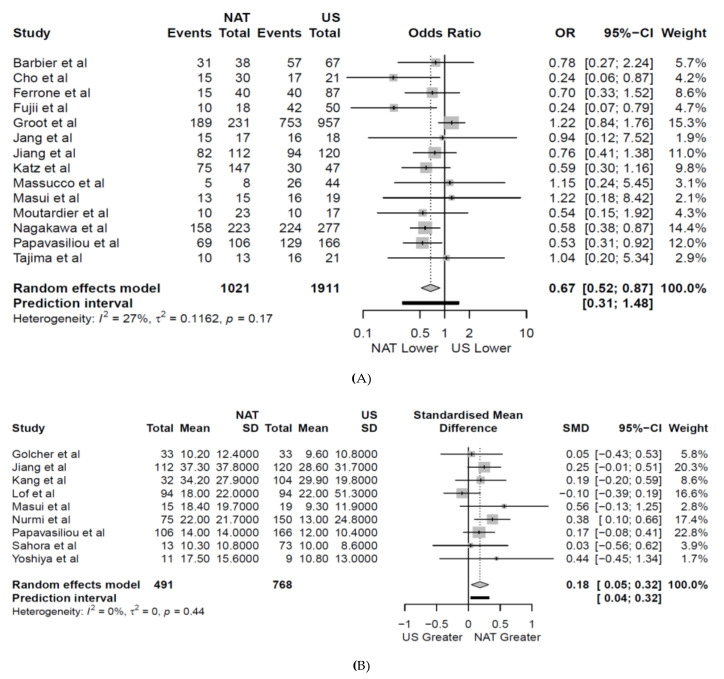
The weighted locoregional recurrence rate was lower amongst patients receiving NAT first, 12% and 27% (Figure 3A) in patients under NAT and US, respectively (OR 0.39 (CI 0.22–0.70); p = 0.004, Figure 4A) in 16 studies [7,25,28,38,40,41,42,43,45,46,49,50,51,52,53,54]. On subgroup analysis of all studies published after 2010, consistent improvement in locoregional recurrence with NAT was observed (p = 0.014, Figure 4A). The weighted locoregional recurrence rate in BRPC undergoing resection was also improved with NAT (NAT 25.8% (CI 20.9–31.2), Figure 3B) compared to US (US 37.7% (CI 30.4–45.4) OR 0.62 (CI 0.44–0.87), p = 0.007) in four studies [7,28,38,45]. The locoregional recurrence rate for RPC in the US was 11.9% (CI 7.5–17.1) in four studies [42,43,49,53]. This analysis could not be compared for RPC in the NAT group due to being only reported in two studies [49,53]. NAT was associated with a lower weighted liver recurrence rate (19.4% CI 13.4–27.2) compared to US (30.1% CI 22.7–38.7 Figure 3A) (OR 0.55 (CI 0.34–0.89), p = 0.023, Figure S4A) in eight studies [7,25,28,40,41,43,46,49]. Weighted lung and peritoneal recurrence rates did not differ between NAT and US cohorts (p = 0.705 and p = 0.549 respectively, Figure 3A and Figure S4B,C), and the overall rate of distant recurrence was similar between NAT and US in 13 studies [7,25,28,38,40,42,43,45,46,49,50,51,53] (NAT 46%, 463/997 US 51% 1003/1962, OR 0.92 (CI 0.61–1.39), p = 0.664, Figure 4B). Similarly, no difference was observed on subgroup analysis of studies published following 2010 (Figure 4B).
Figure 3.
Weighted estimates of: (A) Site-specific rates of recurrence; (B) Resectability-specific sites of recurrence; (C) NAT-specific rates of distant organ-specific recurrence; (D) NAT-specific time to first recurrence. Ninety-five percent confidence intervals are denoted by the central bar. Neoadjuvant therapy (NAT); Up-front Surgery (US); Borderline resectable pancreatic cancer (BRPC); resectable pancreatic cancer (RPC).
Figure 4.
Forest plot showing: (A) locoregional (n) and (B) distal recurrence rates (n) following neoadjuvant therapy (NAT) vs. up-front Surgery (US). The results of a subgroup analysis of all articles published after 2010 are also shown. A Manel–Haenszel random effects model with a Hartung–Knapp adjustment was used for the meta-analysis of all outcomes. A Sidik–Jonkman estimator was utilised for tau2. Odds ratios (OR) are shown with 95 percent confidence intervals (CI).
Similar weighted rates of distant recurrence were observed for BRPC in the NAT (NAT 50.9% CI 38.8–62.8) and US groups (US 54.7% CI 38.7–70.2), Figure 3B) in four studies [7,28,38,45]. The rate of distant recurrence for RPC in the US was 36.6% (CI 19.6–55.5) in four studies (Figure 3B). Again, this could not be compared to RPC in the NAT group as this was only reported in two studies [49,53]. The most common site of recurrence was the liver for BRPC and RPC in the US (21% 280/1342 and 53% 114/216 respectively) and NAT (9% 49/534 and 28% 65/231 respectively) groups in six [7,25,28,38,45,48] and five studies [42,43,49,50,53], respectively. The remaining distribution of resectability-specific organ sites were infrequently reported. On subgroup analysis of six studies [7,28,38,45,49,53] reporting cohorts comprised solely of BRPC or RPC, no significant differences were found for locoregional (p = 0.166) and distal recurrence rates (p = 0.786) (Figure S1A,B).
Site-specific recurrence was reported in at least three articles for both gemcitabine [6,40,45,49,50] and fluoropyrimidine [40,45,49,51], whereby liver recurrence was the most frequent and the distribution of organ-specific weighted recurrence rates were similar between both agents (Figure 3C). Comparisons of recurrence between different chemotherapeutic agents were not reported.
3.4. Secondary Outcome Measures
Overall Recurrence
The weighted mean follow-up interval for recurrence outcomes was 40.8 months (CI 33.4–48.1). During this interval, the absolute overall recurrence rate for the entire cohort was 70% (2345/3351, Figure 2) reported in 16 studies [7,25,28,38,40,41,42,43,45,46,49,50,51,52,53,54]. The weighted overall recurrence rate was 63.4% (CI 51.8–73%) for NAT, significantly lower than the 74% (CI 68.7–80%) weighted overall recurrence rate of the US cohort (OR 0.67 (CI 0.52–0.87), p = 0.006, Figure 5A). On subgroup analysis of RPC, consistent improvement in weighted overall recurrence rates with NAT was observed in five studies [42,43,49,50,53] (NAT 50% 115/231, US 56% 122/216, OR 0.68 (CI 0.50–0.94), p = 0.029). However, for BRPC, similar weighted overall recurrence rates between NAT and US were observed on subgroup analysis of six studies [7,25,28,38,45,48] (NAT 75% 400/534 US 80% 1068/1342, OR 0.61 (CI 0.28–1.31), p = 0.158, Figure S3A). The presence or absence of preoperative chemo radiotherapy in the NAT cohort did not impact the significant difference in weighted overall recurrence rate (with preoperative chemoradiotherapy 66% (CI 54.6–75.8) and without 68.9% (CI 53.4–81), Figure S3B). On meta-regression, borderline resectability, perineural invasion and N0 nodal status were independent predictors of overall recurrence (Table S4).
Figure 5.
Forest plot showing: (A) overall recurrence rates (n) and (B) time to first recurrence (months) following neoadjuvant therapy (NAT) vs. up-front Surgery (US). A Manel-Haenszel random effects model with a Hartung–Knapp adjustment was used for the meta-analysis of all outcomes. A Sidik–Jonkman estimator was utilised for tau2.
3.5. Time to First Recurrence
NAT was associated with a significantly longer weighted mean time to first recurrence (18.8 months, CI 14.5–23.5) compared to US (15.7months, CI 12.2–19.2) in nine studies [6,26,27,37,39,40,41,45,47] (OR 0.18 (CI 0.05–0.32), p = 0.015, Figure 5B). Fewer than three studies reported resectability-specific time to first recurrence in both the US and NAT groups and no study reported organ-specific time to first recurrence. The weighted time to first recurrence for each specific NAT agent ranged from 18.5–22.9 months (Figure 3D). The weighted time for each individual agent was calculated from studies using that specific NAT as the base or in combination with another agent. Two studies were excluded in the meta-analysis of time to first recurrence as outliers significantly skewed mean estimates for the US cohort [51] and the significant discrepancy in cohort numbers made the output uninterpretable [25].
3.6. Recurrence-Free Survival
The weighted RFS rate at 2 and 5 years was 40.5% (CI 29.8–52.2%) and 22.2% (CI 9.2–44.5%) in the NAT cohort and 24.9% (CI 17.7–33.9%) and 13.4% (CI 7.8–22.1%) in the US respectively. NAT was associated with a significantly higher RFS rate at both time points (Two year: OR 1.79 (CI 1.23–2.61), p = 0.005 and Five year: OR 1.95 (CI 1.03–3.69), p = 0.043 respectively) (Figure S2) in 15 [26,28,29,37,39,40,41,42,44,45,47,48,49,50,52] and 9 studies [8,29,40,41,45,47,48,50,52] respectively. One study [44] only reported 2-year RFS data for those who began NAT but not specific to those who ultimately underwent resection.
3.7. Meta-Analysis of Pathological Outcomes between NAT and Upfront Surgery
The post-resection pathology is summarised in Table S2. NAT was associated with a smaller tumour diameter (SMD-0.67 (CI 1.05–0.28), p = 0.002, Figure S5). This correlated with a lower rate of perineural invasion in 12 studies [6,25,27,28,37,41,42,43,44,45,49,51] (NAT 53%, 392/744 US 80% 1504/1886, OR 0.30 (CI 0.18–0.49), p < 0.001, Figure S6B), lymphovascular invasion in 14 studies [6,7,25,26,27,28,37,38,43,44,45,48,49,51] (NAT 43%, 394/909 US 52%, 1014/1966, OR 0.50 (CI 0.25–0.98), p = 0.044, Figure S6C), higher rates of N0 nodal status in 21 studies [6,7,8,25,26,27,28,29,37,38,41,42,43,44,45,46,47,48,49,50,53] (NAT 58%, 840/1441 US 29% 847/2939, OR 3.36 (CI 1.93–5.84), p < 0.001, Figure S7A) and higher rates of R0 resection in 22 studies [6,7,25,26,27,28,29,37,38,40,41,42,43,44,45,47,48,49,50,51,53,54] (NAT 78%, 1120/1434 US 67% 1909/2837, OR 1.86 (CI 1.27–2.71), p = 0.003, Figure S7B) compared to those undergoing US. However, comparable rates of poor tumour differentiation in 11 studies [6,8,25,27,28,29,40,41,42,45,50] (p = 0.494, Figure S6A) were observed.
3.8. Heterogeneity and Risk of Bias
Moderate heterogeneity was found in the outcomes to assess locoregional and distant recurrence rates. Moderate heterogeneity was further reported for liver recurrence and 2- and 5-year RFS. The pathological outcomes of tumour diameter, rates of lymphovascular involvement, and N0 nodal status also observed marked heterogeneity in the dataset. Overall, the non-randomised studies scored poorly in the MINORS criteria (Median 13 Range 11–19). Significant deficiency with regards to unbiased prospective data collection, blinded assessment of outcomes, and power calculations were seen globally (Table S5A). Many studies also failed in the domain of equivalent baseline characteristics (n = 12) [6,25,26,28,39,41,42,43,46,50,52,54]. Cochranes risk of bias tool also identified obvious deficits in the three randomized controlled trials (RCTS) [7,44,47] in the domains of blinding of participants and outcomes, which is an obvious consequence of the nature of the disease (Table S5B). Significant publication bias was observed on visual inspection of Forest plots for the outcomes of tumor diameter, N0 nodal status, and lymphovascular invasion (Figure S8).
4. Discussion
Recurrence after pancreatic resection for PDAC is frequent with 20% of patients developing recurrence within 6 months and a further 40% in the first year of resection in spite of a margin-free resection [56]. Even among those who complete adjuvant therapy, median overall survival remains less than 20 months [57,58]. Recent chemotherapeutic regimens such as FOLFORINOX and Gemcitabine plus Nab-paclitaxel have shown favourable results in improving resectability even in patients with LAPC with resection rates ranging between 0–40% [59]. However, there is paucity of level 1 evidence regarding the role of NAT on recurrence patterns for resectable and BRPC. Two recent RCTs explored the role of NAT in resectable and BRPC. Jang et al. in a recent phase 2/3 RCT comparing neoadjuvant chemo radiation with gemcitabine versus upfront resection in patients with BRPC showed comparable over all recurrence rates and recurrence patterns. On the contrary, the Dutch PREOPANC phase III randomised controlled trial comparing preoperative chemo radiotherapy versus upfront surgery for resectable and BRPC showed improved disease-free survival in the NAT group although the time to recurrence and recurrence patterns were not fully evaluated. The present review of 26 studies including 4986 patients has shown that NAT was associated with lower overall recurrence rates (63% vs. 74%), lower loco-regional recurrence rates (12 vs. 27%), and particularly in patients with BRPC. NAT was associated with a lower liver recurrence rate (19.4 vs. 30.1%) and improved recurrence-free survival at 2 years (40.5% vs. 24.9%) and 5 years (22.2% vs. 13.4%). Furthermore, on analysis of resection specimens, NAT appears to reduce rates lymphovascular (43% vs. 52%) and perineural invasion (53% vs. 80%) and increase the number of resections with N0 lymph node status (58% vs. 29%). These results suggest the superiority of neoadjuvant therapy over upfront resection in reducing loco-regional recurrences, overall recurrence, time to recurrence, and improving recurrence-free survival.
In the present review, subgroup analysis of recurrence rates in BRPC patients showed comparable overall recurrence rates for BPRC in NAT and US. This is despite improved locoregional recurrence with NAT in BRPC patients. This is in direct contrast to RPC where a significant overall recurrence benefit was observed. In theory, the effect on RPC is surprising, however, this finding is similar to the preliminary overall survival results from the Prep-02/JSAP-05 trial [60] in which NAT (gemcitabine and S-1) performed better than immediate surgery for resectable PDAC with no significant survival benefit in the BRPC group. Previous reports have highlighted significant discrepancies in the outcomes following NAT for BRPC, and many authors attribute this finding to the failure to reach a consensus with regards to a standardised definition [61,62]. The recently concluded PREOPANC trial showed superior overall survival (OS) after preoperative chemo radiotherapy for BRPC and no significant difference for resectable PDAC. Although multiple societies have endeavored to provide standardised and, more importantly, validated definitions for PDAC. Assifi et al. reported that 40% of patients diagnosed as BRPC using the AHPBA/SSO or SSAT definitions could be reclassified as RPC [63]. Indeed, the degree of vessel involvement [54] and the individual vessel involved [62] (portal vein, hepatic artery, and or superior mesenteric artery) are on their own predictors of disease-free survival and so variations in definitions will undoubtedly influence rates of recurrence. BRPC is known to have higher rates of failure to achieve R0 resection, higher rates of occult metastases and requires resection of more tissue [64]. BRPC may therefore represent a spectrum of disease rather than a single entity, which may account for the conflicting results noted in these groups of patients in the literature. The inclusion of this heterogeneity in the cohort may have contributed significantly to the lack of a survival advantage with NAT. NAT is generally tolerated much better and morbidity following NAT is considered independent of surgical morbidity [65]. Eighty-nine percent of patients successfully completed NAT and underwent surgery in the review cohort, significantly higher than previously reported, which may be contributed to by the high rates of RPC undergoing NAT. This is also far superior to the 66% completed rate for adjuvant therapy reported in the literature [66].
Although chemotherapeutic subgroup analysis was limited, time to first recurrence and the distribution of NAT-specific rates of organ-specific recurrence did not differ between agents. Gemcitabine was the most frequently utilised NAT regimen in this cohort employed in nearly 60% of patients. However, following the results of the PRODIGE-24 trial [67] that demonstrated superior disease free survival (DFS) in patients with FOLFIRINOX in comparison to Gemcitabine for adjuvant therapy, FOLFIRINOX has become common practice. Indeed, combined NAT regimens including Gemcitabine and FOLFIRINOX have shown a high response rate, and there is increasing evidence to suggest that multi-agent NAT may suppress the recurrence of distant metastases [68,69]; however, to date, no trial has been published comparing FOLFIRINOX to other options. In the current review, combination therapy was only employed in 23% of patients and 13% of patients were administered FOLFIRINOX, suggesting the outcomes of NAT in this review may be more reflective of other chemotherapeutic agents. The efficacy of FOLFIRINOX is the topic of current investigation. The interim results of ESPAC-5F (ISRCTN89500674), Phase II RCT comparing immediate surgery with neoadjuvant gemcitabine plus capecitabine or FOLFIRINOX or chemoradiotherapy in patients with borderline resectable pancreatic cancer showed a significant survival benefit with NAT. The currently ongoing PREOPANC2 trial (NTR7292, 2018-06-19), NorPACT-1 trial (NCT02919787) [70], SWOG S1505 and PANACHE01-PRODIGE48 trial (NCT02959879) [71] may provide further evidence to support NAT first strategy. Furthermore, additional radiotherapy to NAT remains uncertain [72,73]. Although some reports suggest a significantly reduced rate of lymph node metastases and local recurrence with neoadjuvant chemo-radiotherapy (NACRT) [38], the subgroup analysis from this review showed no significant advantage with NACRT. Radiotherapy also has the added risk of increased postoperative morbidity and may compromise surgical outcomes [38].
In the present review, NAT was also associated with reduced tumour diameter, lower rates of perineural invasion, and lymphovascular invasion, leading to higher rates of R0 resection. This may have contributed to the lower loco-regional recurrence rates in the NAT group. However, there were comparable rates of distant recurrence between NAT and upfront resection groups, and this may be contributed to by the lack of significant differences in lung or peritoneal recurrence rates between the two groups. Whether the improved pathological parameters would translate into long term reduction in recurrence rates and survival benefit is still a matter of debate. Even in patients achieving complete pathological response after NAT, recurrence often occurs in half the patients [74].
There remain several limitations to the methodology of this review. This is a review of largely retrospective articles with limited outcomes available for pairwise comparisons. There were obvious discrepancies in the definitions of BRPC and LAPC that would have added significant heterogeneity to the cohort. There also remains significant variability in the assessment of outcomes over time, including methods of assessment for liver metastases following the increasing availability of magnetic resonance imaging (MRI). This study also reported outcomes on solely patients who underwent resection following NAT, thereby excluding those who failed to complete NAT from the final analysis. An intention to treat analysis was abandoned due to the failure of most studies to report on the original cohort. Furthermore, there remains some selection bias in favor of NAT therapy for improving rates of liver metastases in view of the exclusion of NAT patients who ultimately failed to undergo resection. This may have been due to multiple factors including local disease progression or distant metastases. Patients were also recruited from various institutions around the world with differing perioperative care protocols. The preoperative functional status of patients in each arm were also poorly reported and were therefore underutilised to compare the cohorts at baseline. Propensity score matched data was used where reported, however, this represented less than 20% of the cohort. The study data was limited in its ability to report chemotherapy agent-specific outcomes, and FOLFIRINOX-based chemotherapy regimens were uncommon despite there being a current trend towards its use in the current day. Furthermore, the recurrence rate was reported with varying definitions from when it was calculated. Significant deficiency was also identified regarding prospective data collection, blinded assessment of outcomes, and power calculations. However, this is the first review to quantitatively report the comparative recurrence outcomes including patterns of recurrence for this complex cohort of patients.
5. Conclusions
This is the first quantitative analysis of recurrence patterns following NAT for PDAC. The review found NAT improved rates of overall recurrence in RPC with lower locoregional and liver recurrence. However, NAT did not appear to impact peritoneal or lung recurrence. NAT was also associated with a reduced time to recurrence and recurrence free survival at both 2 and 5 years. The review is limited by its largely retrospective dataset, and FOLFIRINOX-based chemotherapy regimens are poorly reflected in the included studies. We await the results of currently ongoing RCTs and large prospective cohorts to confirm the findings of this review and compare the relative efficacy of various NAT regimens.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/7/2132/s1, Figure S1. Forest plot showing rate of locoregional (A) and distant (B) recurrence in studies reporting solely either borderline resectable (BRPC) or resectable (RPC) pancreatic cancer for Neoadjuvant therapy (NAT) vs. Up-front Surgery (US). A Manel-Haenszel random effects model with a Hartung-Knapp adjustment was used for the meta-analysis of all outcomes. A Sidik-Jonkman estimator was utilised for tau2. Odds ratios (OR) are shown with 95 percent confidence intervals (CI). Figure S2. Forest plot showing recurrence-free survival at (A) two-years and (B) five-years postoperatively following Neoadjuvant therapy (NAT) vs. Up-front Surgery (US). A Manel-Haenszel random effects model with a Hartung-Knapp adjustment was used for the meta-analysis of all outcomes. A Sidik-Jonkman estimator was utilised for tau2. Odds ratios (OR) are shown with 95 percent confidence intervals (CI). Figure S3. Forest plot showing overall recurrence rates in articles reporting (A) preoperative resectability and (B) preoperative chemoradiotherapy subgroups following Neoadjuvant therapy (NAT) vs. Up-front Surgery (US). A Manel-Haenszel random effects model with a Hartung- Knapp adjustment was used for the meta-analysis of all outcomes. A Sidik-Jonkman estimator was utilised for tau2. Odds ratios (OR) are shown with 95 percent confidence intervals (CI). Figure S4. Forest plot showing (A) Liver, (B) Lung and (B) Peritoneal recurrence rates following Neoadjuvant therapy (NAT) vs. Up-front Surgery (US). A Manel-Haenszel random effects model with a Hartung-Knapp adjustment was used for the meta-analysis of all outcomes. A Sidik-Jonkman estimator was utilised for tau2. Odds ratios (OR) are shown with 95 percent confidence intervals (CI). Figure S5. Forest plot showing mean tumor diameter in mm following Neoadjuvant therapy (NAT) vs. Up-front Surgery (US). A Manel-Haenszel random effects model with a Hartung-Knapp adjustment was used for the meta-analysis of all outcomes. A Sidik-Jonkman estimator was utilised for tau2. Standard Mean Differences (SMD) are shown with 95 percent confidence intervals. Figure S6. Forest plot showing (A) Poor Tumor differentiation at the time of surgery (B) perineural and (A) Lymphovascular involvement rates following Neoadjuvant therapy (NAT) vs. Up-front Surgery (US). A Manel-Haenszel random effects model with a Hartung-Knapp adjustment was used for the meta-analysis of all outcomes. A Sidik-Jonkman estimator was utilised for tau2. Odds ratios (OR) are shown with 95 percent confidence intervals (CI). Figure S7. Forest plot showing (A) N0 Status and (B) R0 Resection rates following Neoadjuvant therapy (NAT) vs. Up-front Surgery (US). A Manel-Haenszel random effects model with a Hartung-Knapp adjustment was used for the meta-analysis of all outcomes. A Sidik-Jonkman estimator was utilised for tau2. Odds ratios (OR) are shown with 95 percent confidence intervals (CI). Figure S8. Funnel plots for all meta-analysis outcomes including (A) Tumor Size, (B) N0 Nodal Status, (C) Poor tumor differentiation, (D) Perineural invasion, (E) Lymphovascular invasion, (F) R0 Resection, (G) Overall recurrence, and (H) Recurrence free survival. Table S1. Patient characteristics among all studies included within the meta-analysis. Table S2. Pathological outcomes in all studies within the meta-analysis. Table S3. Resectability and eoadjuvant therapy of all patients who underwent resection included in the quantitative analysis within this meta-analysis. Table S4. Results of the mixed-effects meta-regression to identify potential predictors of overall recurrence in the (A) neoadjuvant therapy (NAT) and (B) up-front surgery (US) groups. Variables were included in the mixed effects model if they were significantly different between NAT and US where differences were identified on meta-analysis and with an incidence of ≥ three for categorical variables. Table S5. (A) The MINORS criteria [27] scores for all non-randomised studies included in the review and (B) Cochranes Risk of bias tool for randomised trials.
Appendix A
Table A1.
Literature search strategy.
| 1 | Adjuvant. mp. or exp Chemotherapy, Adjuvant/or exp Chemoradiotherapy, Adjuvant/ |
|---|---|
| 2 | exp Neoadjuvant Therapy/or neoadjuvant.mp. |
| 3 | Chemo *.mp. |
| 4 | Pancreatic Cancer.mp. or exp Pancreatic Neoplasms/ |
| 5 | exp Carcinoma, Pancreatic Ductal/or Pancreatic adenocarcinoma.mp. |
| 6 | Pancreatectomy.mp. or exp Pancreatectomy/ |
| 7 | Pancreatectomy/or Pancreatic Resection.mp. |
| 8 | pancreaticoduodenectomy.mp. or exp Pancreaticoduodenectomy/ |
| 9 | Whippl *.mp. |
| 10 | exp Recurrence/or exp Neoplasm Recurrence, Local/or Recurrence.mp. |
| 11 | Relapse.mp. or exp Recurrence/ |
| 12 | Failure.mp. or exp Treatment Failure/ |
Strategy: (1 OR 2 OR 3) AND [(4 OR 5) AND (6 OR 7 OR 8 OR 9)] AND (11 OR 12). Explode (exp) and relevant medical subjecting headings (MeSH) were employed to further expand the results of each individual query in order to capture all potentially relevant articles. * Denotes a wildcard search for multiple search terms.
Author Contributions
Conceptualization, B.R., C.H.W., J.A.W., K.R., J.J.F. and S.P.; Data curation, B.R. and A.Y.S.; Formal analysis, B.R., A.Y.S., J.A.W. and S.P.; Investigation, A.Y.S. and S.P.; Methodology, B.R., A.Y.S., M.N., C.H.W. and K.R.; Project administration, J.J.F.; Software, B.R.; Supervision, M.N., C.H.W., J.A.W., K.R., J.J.F. and S.P.; Validation, M.N. and J.A.W.; Writing – original draft, B.R. and A.Y.S.; Writing – review & editing, M.N., C.H.W., J.A.W., K.R., J.J.F. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Garrido-Laguna I., Hidalgo M. Pancreatic cancer: From state-of-the-art treatments to promising novel therapies. Nat. Rev. Clin. Oncol. 2015;12:319–334. doi: 10.1038/nrclinonc.2015.53. [DOI] [PubMed] [Google Scholar]
- 2.Groot V.P., Rezaee N., Wu W., Cameron J.L., Fishman E.K., Hruban R.H., Weiss M.J. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2018;267:936–945. doi: 10.1097/SLA.0000000000002234. [DOI] [PubMed] [Google Scholar]
- 3.Groot V.P., Gemenetzis G., Blair A.B., Ding D., Javed A.A., Burkhart R.A. Implications of the Pattern of Disease Recurrence on Survival Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2018;25:2475–2483. doi: 10.1245/s10434-018-6558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sergeant G., Ectors N., Van Steenbergen W., Aerts R., Topal B. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur. J. Surg. Oncol. 2008;35:600–604. doi: 10.1016/j.ejso.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Paik K.Y., Choi S.H., Heo J.S., Choi D.W. Analysis of liver metastasis after resection for pancreatic ductal adenocarcinoma. World J. Gastrointest. Oncol. 2012;4:109–114. doi: 10.4251/wjgo.v4.i5.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang C.M., Chung Y.E., Park J.Y., Sung J.S., Hwang H.K., Choi H.J., Kim H., Song S.Y., Lee J. Potential Contribution of Preoperative Neoadjuvant Concurrent Chemoradiation Therapy on Margin-Negative Resection in Borderline Resectable Pancreatic Cancer. J. Gastrointest. Surg. 2012;16:509–517. doi: 10.1007/s11605-011-1784-3. [DOI] [PubMed] [Google Scholar]
- 7.Jang J.-Y., Han Y., Lee H., Kim S.-W., Kwon W., Lee K.-H., Oh D.-Y., Chie E.K., Lee J.M., Heo J.S., et al. Oncological Benefits of Neoadjuvant Chemoradiation with Gemcitabine Versus Upfront Surgery in Patients with Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann. Surg. 2018;268:215–222. doi: 10.1097/SLA.0000000000002705. [DOI] [PubMed] [Google Scholar]
- 8.Chen X., Liu G., Wang K., Chen G., Sun J. Neoadjuvant radiation followed by resection versus upfront resection for locally advanced pancreatic cancer patients: A propensity score matched analysis. Oncotarget. 2017;8:47831–47840. doi: 10.18632/oncotarget.18091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley A., Van Der Meer R. Upfront Surgery versus Neoadjuvant Therapy for Resectable Pancreatic Cancer: Systematic Review and Bayesian Network Meta-analysis. Sci. Rep. 2019;9:4354–4357. doi: 10.1038/s41598-019-40951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillen S., Schuster T., Meyer Zum Büschenfelde C., Friess H., Kleeff J. Preoperative/Neoadjuvant Therapy in Pancreatic Cancer: A Systematic Review and Meta-analysis of Response and Resection Percentages. PLoS Med. 2010;7:e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Embuscado E.E., Laheru D., Ricci F., Yun K.J., de Boom Witzel S., Seigel A., Flickinger K., Hidalgo M., Bova G.S., Iacobuzio-Donahue C.A. Immortalizing the complexity of cancer metastasis: Genetic features of lethal metastatic pancreatic cancer obtained from rapid autopsy. Cancer Biol. 2005;4:548–554. doi: 10.4161/cbt.4.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dholakia A.S., Kumar R., Raman S.P., Moore J.A., Ellsworth S., McNutt T., Laheru D.A., Jaffee E., Cameron J.L., Tran P.T., et al. Mapping Patterns of Local Recurrence After Pancreaticoduodenectomy for Pancreatic Adenocarcinoma: A New Approach to Adjuvant Radiation Field Design. Int. J. Radiat. Oncol. Biol. Phys. 2013;87:1007–1015. doi: 10.1016/j.ijrobp.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rangarajan K., Pucher P., Armstrong T., Bateman A., Hamady Z. Systemic neoadjuvant chemotherapy in modern pancreatic cancer treatment: A systematic review and meta-analysis. Ann. R. Coll. Surg. Engl. 2019;101:453–462. doi: 10.1308/rcsann.2019.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma G., Whang E.E., Ruan D.T., Ito H. Efficacy of Neoadjuvant Versus Adjuvant Therapy for Resectable Pancreatic Adenocarcinoma: A Decision Analysis. Ann. Surg. Oncol. 2015;22(Suppl. 3):1229. doi: 10.1245/s10434-015-4711-0. [DOI] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda M., Okusaka T., Ito Y., Ueno H., Morizane C., Furuse J., Ishii H., Kawashima M., Kagami Y., Ikeda H. A phase I trial of S-1 with concurrent radiotherapy for locally advanced pancreatic cancer. Br. J. Cancer. 2007;96:1650–1655. doi: 10.1038/sj.bjc.6603788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isaji S., Mizuno S., Windsor J.A., Bassi C., Fernández-del Castillo C., Hackert T., Hayasaki A., Katz M.H.G., Kim S., Kishiwada M., et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18:2–11. doi: 10.1016/j.pan.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Lu D.S., Reber H.A., Krasny R.M., Kadell B.M., Sayre J. Local staging of pancreatic cancer: Criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. Am. J. Roentgenol. 1997;168:1439–1443. doi: 10.2214/ajr.168.6.9168704. [DOI] [PubMed] [Google Scholar]
- 21.Soweid A. The borderline resectable and locally advanced pancreatic ductal adenocarcinoma: Definition. Endosc. Ultrasound. 2017;6:76. doi: 10.4103/eus.eus_66_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chatterjee D., Katz M.H., Rashid A., Varadhachary G.R., Wolff R.A., Wang H., Lee J.E., Pisters P.W.T., Vauthey J., Crane C., et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma. Cancer. 2012;118:3182–3190. doi: 10.1002/cncr.26651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haeberle L., Esposito I. Pathology of pancreatic cancer. Transl. Gastroenterol. Hepatol. 2019;4:50. doi: 10.21037/tgh.2019.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlitter A.M., Esposito I. Definition of Microscopic Tumor Clearance (R0) in Pancreatic Cancer Resections. Cancers. 2010;2:2001–2010. doi: 10.3390/cancers2042001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groot V.P., Blair A.B., Gemenetzis G., Ding D., Burkhart R.A., Borel Rinkes I.H.M., Yu J., Molenaar I.Q., Cameron J.L., Weiss M.J., et al. Recurrence after neoadjuvant therapy and resection of borderline resectable and locally advanced pancreatic cancer. Eur. J. Surg. Oncol. 2019;45:1674–1683. doi: 10.1016/j.ejso.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Yoshiya S., Fukuzawa K., Inokuchi S., Kosai-Fujimoto Y., Sanefuji K., Iwaki K., Motohiro A., Itoh S., Harada N., Ikegami T., et al. Efficacy of Neoadjuvant Chemotherapy in Distal Pancreatectomy with En Bloc Celiac Axis Resection (DP-CAR) for Locally Advanced Pancreatic Cancer. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract. 2019:1–7. doi: 10.1007/s11605-019-04324-8. [DOI] [PubMed] [Google Scholar]
- 27.Nurmi A., Mustonen H., Parviainen H., Peltola K., Haglund C., Seppänen H. Neoadjuvant therapy offers longer survival than upfront surgery for poorly differentiated and higher stage pancreatic cancer. Acta Oncol. 2018;57:799–806. doi: 10.1080/0284186X.2017.1415458. [DOI] [PubMed] [Google Scholar]
- 28.Fujii T., Yamada S., Murotani K., Kanda M., Sugimoto H., Nakao A., Kodera Y. Inverse probability of treatment weighting analysis of upfront surgery versus neoadjuvant chemoradiotherapy followed by surgery for pancreatic adenocarcinoma with arterial abutment. Medicine (Baltimore) 2015;94:e1647. doi: 10.1097/MD.0000000000001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barugola G., Partelli S., Crippa S., Capelli P., D’Onofrio M., Pederzoli P., Falconi M. Outcomes after resection of locally advanced or borderline resectable pancreatic cancer after neoadjuvant therapy. Am. J. Surg. 2012;203:132–139. doi: 10.1016/j.amjsurg.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Wickham H. Tidyverse: Easily Install and Load the ‘Tidyverse’. [(accessed on 15 January 2020)]; Available online: https://cran.r-project.org/web/packages/tidyverse/index.html.
- 31.Guido S. Meta: An R package for meta-analysis. R News. 2007;7:40–45. [Google Scholar]
- 32.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 33.Bowden J., Jackson C. MetaAnalyser: An Interactive Visualisation of Meta-Analysis as a Physical Weighing Machine. [(accessed on 15 January 2020)];2016 Available online: https://cran.r-project.org/web/packages/MetaAnalyser/index.html.
- 34.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ Br. Med. J. 2003;327:557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rohatgi A. WebPlotDigitizer 4.0. [(accessed on 15 January 2020)]; Available online: https://automeris.io/WebPlotDigitizer.
- 36.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lof S., Korrel M., van Hilst J., Alseidi A., Balzano G., Boggi U., Butturini G., Casadei R., Dokmak S., Edwin B., et al. Impact of Neoadjuvant Therapy in Resected Pancreatic Ductal Adenocarcinoma of the Pancreatic Body or Tail on Surgical and Oncological Outcome: A Propensity-Score Matched Multicenter Study. Ann. Surg. Oncol. 2019;27:1986–1996. doi: 10.1245/s10434-019-08137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagakawa Y., Sahara Y., Hosokawa Y., Murakami Y., Yamaue H., Satoi S., Unno M., Isaji S., Endo I., Sho M., et al. Clinical Impact of Neoadjuvant Chemotherapy and Chemoradiotherapy in Borderline Resectable Pancreatic Cancer: Analysis of 884 Patients at Facilities Specializing in Pancreatic Surgery. Ann. Surg. Oncol. 2019;26:1629–1636. doi: 10.1245/s10434-018-07131-8. [DOI] [PubMed] [Google Scholar]
- 39.Sahora K., Kuehrer I., Eisenhut A., Akan B., Koellblinger C., Goetzinger P., Teleky B., Jakesz R., Peck-Radosavljevic M., Ba’ssalamah A., et al. NeoGemOx: Gemcitabine and oxaliplatin as neoadjuvant treatment for locally advanced, nonmetastasized pancreatic cancer. Surgery. 2011;149:311–320. doi: 10.1016/j.surg.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 40.Jiang H., Du C., Cai M., He H., Chen C., Qiu J., Wu H. An Evaluation of Neoadjuvant Chemoradiotherapy for Patients with Resectable Pancreatic Ductal Adenocarcinoma. Hpb Surg. A World J. Hepatic Pancreat. Biliary Surg. 2013;2013:298726. doi: 10.1155/2013/298726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papavasiliou P., Hoffman J.P., Cohen S.J., Meyer J.E., Watson J.C., Chun Y.S. Impact of preoperative therapy on patterns of recurrence in pancreatic cancer. HPB. 2014;16:34–39. doi: 10.1111/hpb.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Massucco P., Capussotti L., Magnino A., Sperti E., Gatti M., Muratore A., Sgotto E., Gabriele P., Aglietta M. Pancreatic Resections after Chemoradiotherapy for Locally Advanced Ductal Adenocarcinoma: Analysis of Perioperative Outcome and Survival. Ann. Surg. Oncol. 2006;13:1201–1208. doi: 10.1245/s10434-006-9032-x. [DOI] [PubMed] [Google Scholar]
- 43.Ferrone C.R., Marchegiani G., Hong T.S., Ryan D.P., Deshpande V., McDonnell E.I., Sabbatino F., Santos D.D., Allen J.N., Blaszkowsky L.S., et al. Radiological and Surgical Implications of Neoadjuvant Treatment with FOLFIRINOX for Locally Advanced and Borderline Resectable Pancreatic Cancer. Ann. Surg. 2015;261:12–17. doi: 10.1097/SLA.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Versteijne E., Suker M., Groothuis K., Akkermans-Vogelaar J.M., Besselink M.G., Bonsing B.A., Buijsen J., Busch O.R. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 2020;38:1763–1773. doi: 10.1200/JCO.19.02274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masui T., Doi R., Kawaguchi Y., Sato A., Nakano K., Ito T., Anazawa T., Takaori K., Uemoto S. Concurrent gemcitabine+S-1 neoadjuvant chemotherapy contributes to the improved survival of patients with small borderline-resectable pancreatic cancer tumors. Surg. Today. 2016;46:1282–1289. doi: 10.1007/s00595-016-1310-z. [DOI] [PubMed] [Google Scholar]
- 46.Ishikawa O., Ohhigashi H., Takahashi H., Ito Y. Survival 3, 5, and 10 years after adjuvant regional and neoadjuvant chemotherapy in resectable pancreatic cancer patients: An institutional experience. Pancreat. CancerCyst. Neoplasms Endocr. Tumors Diagn. Manag. 2015;132:132–137. [Google Scholar]
- 47.Golcher H., Brunner T.B., Witzigmann H., Marti L., Bechstein W.-O., Bruns C., Jungnickel H., Schreiber S., Grabenbauer G.G., Meyer T., et al. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: Results of the first prospective randomized phase II trial. Strahlenther. Onkol. 2014;191:7–16. doi: 10.1007/s00066-014-0737-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho I.R., Chung M.J., Bang S., Park S.W., Chung J.B., Song S.Y., Seong J., Hwang H.K., Kang C.M., Lee W.J., et al. Gemcitabine based neoadjuvant chemoradiotherapy therapy in patients with borderline resectable pancreatic cancer. Pancreatol. Off. J. Int. Assoc. Pancreatol. 2013;13:539–543. doi: 10.1016/j.pan.2013.07.064. [DOI] [PubMed] [Google Scholar]
- 49.Tajima H., Ohta T., Kitagawa H., Okamoto K., Sakai S., Makino I., Kinoshita J., Furukawa H., Nakamura K., Hayashi H., et al. Pilot study of neoadjuvant chemotherapy with gemcitabine and oral S-1 for resectable pancreatic cancer. Exp. Ther. Med. 2012;3:787–792. doi: 10.3892/etm.2012.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katz M., Wang H., Balachandran A., Bhosale P., Crane C.H., Wang X., Pisters P.W.T., Lee J.E., Vauthey J., Abdalla E.K., et al. Effect of Neoadjuvant Chemoradiation and Surgical Technique on Recurrence of Localized Pancreatic Cancer. J. Gastrointest. Surg. 2012;16:68–79. doi: 10.1007/s11605-011-1748-7. [DOI] [PubMed] [Google Scholar]
- 51.Barbier L., Turrini O., Gregoire E., Viret F., Le Treut Y., Delpero J. Pancreatic head resectable adenocarcinoma: Preoperative chemoradiation improves local control but does not affect survival. Hpb Off. J. Int. Hepato Pancreato Biliary Assoc. 2011;13:64–69. doi: 10.1111/j.1477-2574.2010.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greer S.E., Pipas J.M., Sutton J.E., Zaki B.I., Tsapakos M., Colacchio T.A., Gibson J.J., Wiener D.C., Ripple G.H., Barth R.J., Jr., et al. Effect of Neoadjuvant Therapy on Local Recurrence after Resection of Pancreatic Adenocarcinoma. J. Am. Coll. Surg. 2008;206:451–457. doi: 10.1016/j.jamcollsurg.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Moutardier V., Moutardier J.C., Turrini O., Huiart L., Viret F., Giovannini M., Giovannini M.H., Magnin V., Lelong B., Bories E., et al. A reappraisal of preoperative chemoradiation for localized pancreatic head ductal adenocarcinoma in a 5-year single-institution experience. J. Gastrointest. Surg. 2004;8:502–510. doi: 10.1016/j.gassur.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 54.Pingpank J.F., Hoffman J.P., Ross E.A., Cooper H.S., Meropol N.J., Freedman G., Pinover W.H., LeVoyer T.E., Sasson A.R., Eisenberg B.L. Effect of preoperative chemoradiotherapy on surgical margin status of resected adenocarcinoma of the head of the pancreas. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract. 2001;5:121–130. doi: 10.1016/S1091-255X(01)80023-8. [DOI] [PubMed] [Google Scholar]
- 55.Tempero M.A., Arnoletti J.P., Behrman S.W., Ben-Josef E., Benson A.B., Casper E.S., Cohen S.J., Czito B., Ellenhorn J.D.I., Hawkins W.G., et al. Pancreatic Adenocarcinoma, Version 2.2012. J. Natl. Compr. Cancer Netw. 2012;10:703–713. doi: 10.6004/jnccn.2012.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Groot V., Gemenetzis G., Blair A., Rivero-Soto R., Yu J., Javed A., Burkhart R., Rinkes I.H.M., Molenaar I., Cameron J., et al. Defining and Predicting Early Recurrence in 957 Patients with Resected Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2019;269:1154–1162. doi: 10.1097/SLA.0000000000002734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iacobuzio-Donahue C.A., Fu B., Yachida S., Luo M., Abe H., Henderson C.M., Vilardell F., Wang Z., Keller J.W., Banerjee P., et al. DPC4 Gene Status of the Primary Carcinoma Correlates with Patterns of Failure in Patients with Pancreatic Cancer. J. Clin. Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gnerlich J.L., Luka S.R., Deshpande A.D., Dubray B.J., Weir J.S., Carpenter D.H., Brunt E.M., Strasberg S.M., Hawkins W.G., Linehan D.C. Microscopic Margins and Patterns of Treatment Failure in Resected Pancreatic Adenocarcinoma. Arch. Surg. 2012;147:753–760. doi: 10.1001/archsurg.2012.1126. [DOI] [PubMed] [Google Scholar]
- 59.Suker M., Beumer B.R., Sadot E., Marthey L., Faris J.E., Mellon E.A., El-Rayes B.F., Wang-Gillam A., Lacy J., Hosein P.J., et al. FOLFIRINOX for locally advanced pancreatic cancer: A systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17:801–810. doi: 10.1016/S1470-2045(16)00172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Unno M., Motoi F., Matsuyama Y., Satoi S., Matsumoto I., Aosasa S., Shirakawa H., Wada K., Fujii T., Yoshitomi H., et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05) J. Clin. Oncol. 2019;37:189. doi: 10.1200/JCO.2019.37.4_suppl.189. [DOI] [PubMed] [Google Scholar]
- 61.Callery M.P., Chang K.J., Fishman E.K., Talamonti M.S., William Traverso L., Linehan D.C. Pretreatment Assessment of Resectable and Borderline Resectable Pancreatic Cancer: Expert Consensus Statement. Ann. Surg. Oncol. 2009;16:1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 62.Yamada S., Fujii T., Sugimoto H., Nomoto S., Takeda S., Kodera Y., Nakao A. Aggressive surgery for borderline resectable pancreatic cancer: Evaluation of National Comprehensive Cancer Network guidelines. Pancreas. 2013;42:1004–1010. doi: 10.1097/MPA.0b013e31827b2d7c. [DOI] [PubMed] [Google Scholar]
- 63.Assifi M.M., Lu X., Eibl G., Reber H.A., Li G., Hines O.J. Neoadjuvant therapy in pancreatic adenocarcinoma: A meta-analysis of phase II trials. Surg. Off. J. Soc. Univ. Surg. Cent. Surg. Assoc. Am. Assoc. Endocr. Surg. 2011;150:466–473. doi: 10.1016/j.surg.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Russo S., Ammori J., Eads J., Dorth J. The role of neoadjuvant therapy in pancreatic cancer: A review. Future Oncol. 2016;12:669–685. doi: 10.2217/fon.15.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heinrich S., Schäfer M., Weber A., Hany T.F., Bhure U., Pestalozzi B.C., Clavien P. Neoadjuvant chemotherapy generates a significant tumor response in resectable pancreatic cancer without increasing morbidity: Results of a prospective phase II trial. Ann. Surg. 2008;248:1014–1022. doi: 10.1097/SLA.0b013e318190a6da. [DOI] [PubMed] [Google Scholar]
- 66.Neoptolemos J.P., Palmer D.H., Ghaneh P., Psarelli E.E., Valle J.W., Halloran C.M., Faluyi O., O’Reilly D.A., Cunningham D., Wadsley J., et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 67.Edeline J., Bonnetain F., Phelip J.M., Watelet J., Hammel P., Joly J., Ben Abdelghani M., Rosmorduc O., Bouhier-Leporrier K., Jouve J., et al. Gemox versus surveillance following surgery of localized biliary tract cancer: Results of the PRODIGE 12-ACCORD 18 (UNICANCER GI) phase III trial. J. Clin. Oncol. 2017;35(Suppl. 4):225. doi: 10.1200/JCO.2017.35.4_suppl.225. [DOI] [Google Scholar]
- 68.Katz M.H.G., Shi Q., Ahmad S.A., Herman J.M., Marsh R.D.W., Collisson E., Schwartz L., Frankel W., Martin R., Conway W., et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer alliance for clinical trials in oncology trial A021101. JAMA Surg. 2016;151:e161137. doi: 10.1001/jamasurg.2016.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okada K., Hirono S., Kawai M., Miyazawa M., Shimizu A., Kitahata Y., Ueno M., Hayami S., Yamaue H. Phase I Study of Nab–Paclitaxel plus Gemcitabine as Neoadjuvant Therapy for Borderline Resectable Pancreatic Cancer. Anticancer Res. 2017;37:853–858. doi: 10.21873/anticanres.11389. [DOI] [PubMed] [Google Scholar]
- 70.Labori K.J., Lassen K., Hoem D., Grønbech J.E., Søreide J.A., Mortensen K., Smaaland R., Sorbye H., Verbeke C., Dueland S. Neoadjuvant chemotherapy versus surgery first for resectable pancreatic cancer (Norwegian Pancreatic Cancer Trial—1 (NorPACT-1))—Study protocol for a national multicentre randomized controlled trial. BMC Surg. 2017;17:94. doi: 10.1186/s12893-017-0291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwarz L., Vernerey D., Bachet J.-B., Tuech J.-J., Portales F., Michel P., Cunha A.S. Resectable pancreatic adenocarcinoma neo-adjuvant FOLF(IRIN)OX-based chemotherapy—A multicenter, non-comparative, randomized, phase II trial (PANACHE01-PRODIGE48 study) BMC Cancer. 2018;18:762. doi: 10.1186/s12885-018-4663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Satoi S., Toyokawa H., Yanagimoto H., Yamamoto T., Hirooka S., Yamaki S., Michiura T., Inoue K., Matsui Y., Kwon A.H. Neoadjuvant chemoradiation therapy using S-1 for patients with pancreatic cancer. Gastroenterology. 2012;142:S1097–S1098. doi: 10.1016/S0016-5085(12)64268-1. [DOI] [Google Scholar]
- 73.Murphy J.E., Wo J.Y., Ryan D.P., Jiang W., Yeap B.Y., Drapek L.C., Blaszkowsky L.S., Kwak E.L., Allen J.N., Clark J.W., et al. Total Neoadjuvant Therapy with FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4:963–969. doi: 10.1001/jamaoncol.2018.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He J., Blair A., Groot V., Javed A., Burkhart R., Gemenetzis G., Hruban R., Waters K., Poling J., Zheng L., et al. Is a Pathological Complete Response Following Neoadjuvant Chemoradiation Associated with Prolonged Survival in Patients with Pancreatic Cancer? Ann. Surg. 2018;268:1–8. doi: 10.1097/SLA.0000000000002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.