Abstract
Purpose
While using their amblyopic eye, individuals with strabismic amblyopia count inaccurately and underestimate the number of features. These deficits are attributed to limitations in high-level cortical functions and attention. In the current study, we examined whether feature counting is affected in strabismic and anisometropic amblyopia during dichoptic viewing, a setup that can better capture binocular function disruptions.
Methods
Through a mirror stereoscope, Gabor patches were presented for 200 msec (Experiment 1) or 350 msec (Experiment 2) in both the left eye and the right eye of observers, who were required to combine the percepts and report the total number of patches. Counting performance and errors were compared across amblyopic groups and normal-sighted observers. The contribution and relation of each eye to performance was also evaluated.
Results
Anisometropic and strabismic amblyopia groups counted inaccurately and underestimated the number of features compared to the normal-sighted group. In both amblyopic groups, the amblyopic eye contributed less in comparison to the fellow eye. The strabismic group exhibited worse performance, and a more pronounced difference in eye contribution, in comparison to the anisometropic group.
Conclusions
Overall, our results support the view of higher-level cortical and binocular function deficits in amblyopia.
Translational Relevance
The current study bridges the gap between research on high-cortical function deficits and clinical binocular function disruptions in amblyopia, which can help us better understand the neural mechanism of amblyopia and inform clinical therapeutic tasks and strategies.
Keywords: amblyopia, feature counting, binocular disfunction, strabismus, anisometropia
Introduction
Amblyopia, or “lazy eye,” is a neurodevelopmental abnormality that is commonly caused by a deviation of the eyes (strabismus), chronic optical blur due to unequal refractive error in the two eyes (anisometropia), or a mixture of strabismus and anisometropia during early childhood.
Amblyopia is classically defined on the basis of poor visual acuity, contrast sensitivity loss, and disruption of binocular function (e.g., loss of depth perception). These deficits are believed to be a result of changes in the properties of neurons in early visual cortical areas, V11–7 and V2.8 However, many behavioral studies suggest that the extrastriate cortex in amblyopia is also affected. These functional deficits include contour integration,9,10 global motion processing,11–13 and global form processing.14 Electrophysiological15,16 and neuroimaging17,18 studies have also found extrastriate abnormalities; For instance, direction discrimination of high-level random-dot kinematograms produce less activation in V3A, MT+, and posterior parietal cortex (PPC) in both eyes of children with amblyopia, in comparison to control subjects.17 These extrastriate deficits appear to be more severe in individuals with strabismic amblyopia than in individuals with anisometropic amblyopia.15,17,19, and cannot be explained by functional losses in V1 and V2, documented in physiological animal studies on amblyopia.7,20
High-level cortical functions, such as decision making21 and enumeration of features,22,23 are also affected in amblyopia. Sharma and colleagues,22 for instance, showed that during monocular viewing (the nontested eye is covered), the amblyopic eye of individuals with strabismic amblyopia significantly undercounts briefly presented visual features in comparison to normal observers, whereas performance in their fellow eye is normal. These findings have been replicated in another study using a similar monocular viewing paradigm23 and cannot not be explained by low-level deficits such as blur, visibility, crowding, undersampling, or topographical jitter, given that individuals with amblyopia also underestimate the number of features when such features were presented in a uniform grid.22 Instead, undercounting of features in amblyopia is believed to result from high-level cortical function deficits.22
Natural viewing conditions, however, are not monocular but instead binocular, in which both eyes with overlapping fields of view are used. Given the fact that individuals with strabismic amblyopia exhibit significant disruptions in binocular function and show strong visual suppression from the fellow eye onto the amblyopic eye,24–27 it is possible that those with amblyopia would show greater deficits in enumeration of features under binocular viewing conditions. To this end, in the current study, our first goal was to measure feature counting in adults with anisometropic and strabismic amblyopia using a dichoptic approach. We used a variant of the Sharma and colleagues22 paradigm, with modifications for dichoptic viewing. Our second goal was to compare feature counting performance between anisometropic and strabismic amblyopia, which has not been extensively examined before. Given that distinct mechanisms are believed to be involved in anisometropic and strabismic amblyopia,28 feature counting may be differentially affected across both types of amblyopia.
Materials and Methods
Participants
Ten normal-sighted observers and 17 patients with amblyopia (eight anisometropic amblyopes and nine strabismic or mixture of aniso- and strabismic amblyopia) participated in the study. For simplicity, we referred to the participants with strabismic amblyopia and with mixture of anisometropic and strabismic amblyopia as “participants with strabismic amblyopia” in the current study. The mean age for the normal-sighted observers (five males) was 45.3 years (range, 22 to 63 years) and 53 years (range, 28 to 68 years) for the participants with amblyopia (seven males). All participants were given eye examinations and were refracted under noncycloplegic conditions by a pediatric ophthalmologist (C.H.) before the experiment. Visual acuity was evaluated with a logMAR chart (Bailey-Lovie). Stereoacuity was evaluated with the random-dot stereo butterfly, with 2000 arcsec as the maximum measurable stereoacuity (Stereo Optical, Chicago, IL, USA). Visual acuity of the amblyopic eye was slightly worse in strabismic amblyopes (M = 0.57, SD = 0.23) than in anisometropic amblyopes (M = 0.50, SD = 0.16) (P < 0.001). Most of the participants with strabismic amblyopia had severe binocular function deficits with no measurable stereoacuity. Those with anisometropic amblyopia, on the other hand, had residual and measurable stereoacuities. General demographic and clinical information of the amblyopic participants are provided in Table 1. All normal-sighted observers had or corrected to 0 or better logMAR in each eye, and had stereoacuities of at least 40 arcsec.
Table 1.
Demographic and Clinical Information of Anisometropic and Strabismic Amblyopes
| Participant | Visual Acuity | Refractive Errors | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | Diagnosis | Age | Fellow Eye | Amblyopic Eye | Stereoacuity | Fellow Eye | Amblyopic Eye | Deviation | History | Experiment |
| A1 | A | 52 | 0.04 | 0.518 | 200″ | −1.75+1.00*10 | −5.00+0.75*160 | Ortho | Patching done | 1 & 2 |
| A2 | A | 22 | −0.097 | 0.341 | 200″ | +1.25+0.75*70 | +3.25+0.50*110 | Ortho | Patching done | 1 |
| A3 | A | 49 | 0 | 0.301 | 70″ | 0.50+0.50*90 | −1.50+2.25*130 | Ortho | Patching done | 1 |
| A4 | A | 63 | 0 | 0.377 | 70″ | +0.50+0.25*180 | +0.5+2.00*180 | Ortho | Patching done | 1 |
| A5 | A | 48 | −0.2 | 0.7189 | 1500″ | Plano | +3.00+2.25*110 | Ortho | No patching | 1 |
| A6 | A | 27 | 0.04 | 0.518 | 400″ | −7.00+0.50*90 | −8.00+1.25*40 | Ortho | No patching | 2 |
| A7 | A | 51 | 0 | 0.739 | 200″ | −0.25 | +3.00+0.50*90 | Ortho | Patching done | 2 |
| A8 | A | 50 | −0.2 | 0.498 | 800″ | −1.00+0.50*30 | +4.5+0.50*150 | Ortho | Patching done | 2 |
| S1 | S&A | 59 | −0.04 | 0.836 | n/a | +0.75 | −1.00+0.75*25 | XT 14, L/R 14, DVD | Surgery & patching | 1 |
| S2 | S | 38 | −0.097 | 0.341 | n/a | −1.50 | −1.50 | XT 12, R/L 4 | Patching done | 1 |
| S3 | S&A | 62 | 0 | 0.756 | 2000″ | +1.00/−0.50*130 | +5.00/−1.25*130 | XT 4, R/L 20, DVD | Patching done | 1 |
| S4 | S&A | 55 | 0 | 0.756 | n/a | −1.00/−1.25*180 | −3.75/−1.00*20 | XT 20 | No patching | 1 |
| S5 | S&A | 68 | −0.02 | 0.518 | 2000″ | +1.25/+1.00*105 | +3.50/+2.25*85 | XT 8 | Surgery & patching | 1 |
| S6 | S | 55 | −0.117 | 0.224 | 1500″ | +1.00+1.00*110 | +0.75+1.25*80 | ET 16, R/L 6 | Surgery & patching | 2 |
| S7 | S&A | 28 | −0.097 | 0.602 | 1500″ | Plano | +1.00+0.50*90 | ET 16 | No patching | 2 |
| S8 | S&A | 46 | 0 | 0.756 | n/a | −2.00 | −8.50+1.00*35 | ET 14 | Incomplete patching | 2 |
| S9 | S&A | 66 | 0 | 0.301 | n/a | −1.75/−0.75*65 | +1.00 | ET 16, R/L 8 | Surgery & patching | 2 |
Stereoacuity was measured at near distance. Stereoauity marked “n/a” indicates nonmeasurable stereoacuity. Ocular alignment at near with correction is shown in prism diopters. A, anisometropic amblyopia; S, strabismic amblyopia; S&A, mixed strabismus/anisometropia; DVD, disassociated vertical deviation; XT, exotropia; ET, esotropia; L/R, left-eye hypertropia; R/L, right-eye hypertropia.
One participant with anisometropic amblyopia (A1) participated in both Experiment 1 and Experiment 2. All other participants only participated in one experiment. Five normal-sighed observers, five participants with anisometropic amblyopia and five participants with strabismic amblyopia participated in Experiment 1. Five normal-sighted observers, four participants with anisometropic amblyopia and four participants with strabismic amblyopia participated in Experiment 2. The study was approved by The Smith-Kettlewell Institutional Review Board and conformed to the tenets of the Declaration of Helsinki. All participants provided written informed consent.
Stimuli and Procedures
Experiment 1
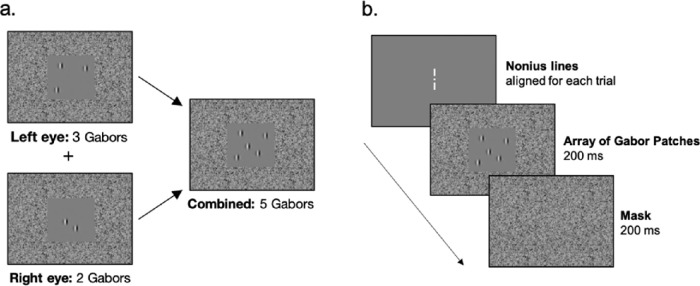
Figure 1 depicts example stimuli and the temporal sequence of the task. The experimental task was programmed in MATLAB (MathWorks, Natick, MA, USA) using the Psychophysics Toolbox.29–31 The stimuli were presented at a viewing distance of 85 cm on two separate Sony Trinitron Multiscan G400 CRT monitors (Sony, Tokyo, Japan) with a frame rate of 85 Hz. An adjustable mirror stereoscope was used to combine the left-eye and right-eye views into a single view. Randomly arrayed and numbered Gabor patches (20 to 35% contrast at 2 cpd) were presented for 200 ms in the left-eye and right-eye views, appearing in the central visual field (5.6° square frame) surrounded by noise in the periphery (21° × 18° in the visual field) when fused. The presentation of the Gabor patches was followed by a 200-ms noise mask. Gabor patches were highly visible, and the surrounding noise encouraged peripheral fusion. Observers were required to combine the percepts from both eyes and report the combined total number of Gabor patches (three to nine) by button press on a keyboard. Responses were required within two seconds, and no feedback was provided.
Figure 1.
An example of the visual counting task stimuli is depicted in (a). The temporal sequence of a given trial is depicted in (b).
All participants were tested under the conditions of best-corrected vision. Before the start of the task, participants were required to adjust the mirror stereoscope to align nonius lines presented at the center of each screen. For the participants with amblyopia the contrast of the Gabor patches across both eyes were matched for equal visibility if necessary. The combined total number of Gabor patches (fused percept from left-eye and right-eye views) was random across trials and interleaved within a block. A practice block was conducted to make sure the participant understood the task. Participants completed between one to three blocks of trials—each block included 90 to 120 trials. A central fixation point and nonius lines appeared before the initiation of all trials to ensure that the mirror stereoscope remained properly aligned. If the nonius lines were no longer aligned, participants were given the opportunity to readjust.
Experiment 2
Given the potential difficulty of counting features within a 200-ms timeframe, in Experiment 2 we increased the Gabor patch presentation time to 350 ms. All other the experimental stimuli and procedures were the same as in Experiment 1.
Equating Visibility Across the Fellow and Amblyopic Eye
Two horizontal sinusoidal gratings (35 cd/m2, 3 cpd, 2.5° in size) were presented in the upper visual field of one eye and the lower visual field of the other eye through a stereoscope. The participants were unaware of which eye was presented with the upper or lower gratings.
The procedure was as follows: (1) the participants were asked to match the contrast between the upper and the lower gratings, while the contrast in the non-amblyopic fellow eye remained fixed at around 20%, (2) the contrast of the amblyopic eye that participants deemed to match the fellow eye was considered as the increase in contrast of the amblyopic eye necessary to equate visibility in both eyes, (3) steps 1 and 2 were repeated three times, and the average of the contrast increases for the amblyopic eye across the three repetitions was computed and used throughout the experiment to equate visibility across both eyes. This procedure was done for Experiment 1 and Experiment 2.
Data Analysis
Feature Counting
A linear regression analysis was computed to examine the relationship between the reported number of features with the Gabor patch set-size and observer group. The normal-sighted observer group set as the reference group (intercept). All trials collected were included in the analysis. As a result, the data points were clustered within a given participant and therefore not independent. In order account for this dependency robust standard errors were used to get more accurate P values.32 All other regression assumptions were met. The Estimatr package33 was used in R34 to conduct the robust-clustered regression analysis. All subsequent analyses mentioned below were also conducted in R.
Counting Error
The feature counting error of each participant was computed as the difference between the total number of Gabor patches presented and the participants’ response. A feature counting error of 0 indicates an accurate response, whereby the reported total number of Gabor patches is equal to the actual number of patches presented. A positive feature counting error indicates that the participant overcounted and that the reported total number of Gabor patches exceeds the actual number of patches presented. Finally, a negative feature counting error indicates that the participant undercounted, where the reported total number of Gabor patches is lower than the actual number of patches presented. A similar linear regression analysis as done for the feature counting results was computed.
Contribution of the Fellow Eye/Dominant Eye Versus the Amblyopic/Non-Dominant Eye
A multiple linear regression analysis was computed to examine the relationship between the reported number of features, with the Gabor patch set-size presented in the observer group's dominant/fellow eye and nondominant/amblyopic eye. Given that we were interested in examining the contribution of each eye within each observer group only, separate analyses were computed for each observer group. All trials collected were included in the analysis.
Results
Experiment 1
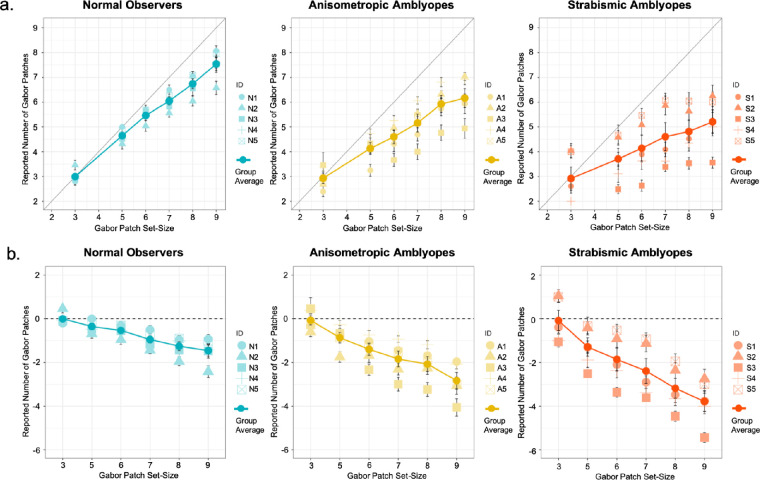
Feature Counting
The mean feature counting responses of all observer groups are plotted in Figure 2a. The results of our robust-clustered regression analysis are presented in Table 2. The regression model was significant F(5,14) = 95.02, P < 0.001, R2 = 0.44. The Gabor patch set-size coefficient was significant (P < 0.001), suggesting that it was linearly associated with the observers’ reported number of features. There was a significant interaction between the Gabor patch set-size and the observer group of strabismus (P < 0.001).
Figure 2.
Feature counting performance and error in Experiment 1. The mean feature counting performance across groups and individual mean performance is plotted in (a). The mean feature counting error across groups and individual mean errors is plotted in (b). Error bars: standard errors.
Table 2.
Summary Results of the Robust-Clustered Regression Analyses Examining Relationship Between the Feature Counting and Estimated Counting Error With the Gabor Patch Set-Size and Observer Group for Experiment 1
| Model | Coefficient | Estimate | t-statistic | P value | CI |
|---|---|---|---|---|---|
| Feature Counting | Intercept | 0.79 | 4.06 | 0.02 | 0.19, 1.39 |
| Set-size | 0.76 | 16.17 | 0.00 | 0.61, 0.90 | |
| Anisometropia group | 0.22 | 0.59 | 0.57 | −0.69, 1.14 | |
| Strabismus group | 0.71 | 1.73 | 0.13 | −0.27, 1.69 | |
| Set-size × Anisometropia group | −0.14 | −1.67 | 0.14 | −0.34, 0.06 | |
| Set-size × Strabismus group | −0.38 | −5.85 | 0.00 | −0.54, −0.23 | |
| Counting Error | Intercept | 0.788 | 4.0565 | 0.023966 | 0.19, 1.39 |
| Set-size | −0.2425 | −5.1774 | 0.011732 | −0.39, −0.10 | |
| Anisometropia group | 0.2243 | 0.5938 | 0.57351 | −0.69, 1.14 | |
| Strabismus group | 0.7096 | 1.7344 | 0.129845 | −0.27, 1.69 | |
| Set-size × Anisometropia group | −0.1372 | −1.6729 | 0.143323 | −0.34, 0.06 | |
| Set-size × Strabismus group | −0.383 | −5.8488 | 0.000822 | −0.54, −0.23 |
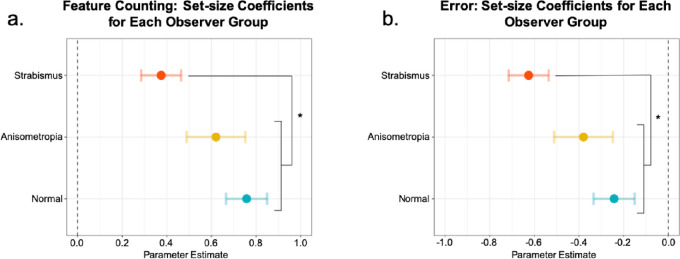
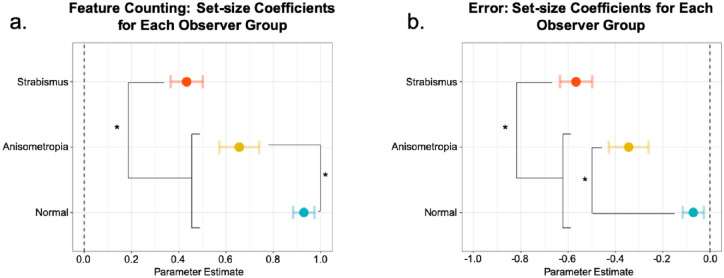
To compare the relationship between the reported number of features with the Gabor patch set-size separately across groups, the coefficients of set-size for each observer group were obtained from our regression model. The coefficients of set-size for each observer group are depicted in Figure 3. In all observer groups, coefficients of set-size were significantly greater than 0 (P < 0.05). Tukey adjusted comparisons revealed that the coefficient of set-size of the strabismic group (0.38 confidence interval [CI] [0.29, 0.46]) was significantly lower in comparison to the coefficient of set-size of the normal-sighted group (0.76 CI [0.66, 0.85]) (P < 0.001) and of the anisometropic group (0.62 CI [0.49, 0.75]) (P < 0.01). For the strabismic group, an increase in the Gabor patch set-size was only associated with an increase of 0.38 in their counting response, whereas for the anisometropic and the normal-sighted group, it was associated with an increase of 0.62 and 0.76, respectively.
Figure 3.
The coefficients of set-size for each observer group in Experiment 1. The coefficient of set-size depicts the relationship between the reported number of features with the Gabor patch set-size. The coefficients for each observer group for the (a) feature counting regression analysis and (b) estimated counting error regression analysis. Error bars: confidence intervals. * = P < 0.01.
Counting Error
The results of our robust-clustered regression analysis are presented in Table 2. The regression model was significant F(5,14) = 58.56, P < 0.001, R2 = 0.36. The Gabor patch set-size coefficient was significant (P < 0.001), suggesting that it was linearly associated with the observers’ reported number of features. There was a significant interaction between the Gabor patch set-size and the observer group of strabismus (P < 0.001).
The coefficients of set-size for each observer group are depicted in Figure 3. In all observer groups, coefficients of set-size were significantly smaller than 0 (P < 0.05), suggesting that all observer groups undercounted as the Gabor patches set-size increased. Tukey adjusted comparisons, however, revealed that as the Gabor patch set-size increased, the strabismic group (−0.63 CI [−0.72, −0.54]) significantly undercounted in comparison to the normal-sighted group (−0.24 CI [−0.33, −0.15]) and the anisometropic group (−0.38 CI [−0.51, −0.24]) (P < 0.01). For the strabismic group, an increase in the Gabor patch set-size was associated with undercounting by the amount of −0.63, whereas for the anisometropic and the normal-sighted group, it was associated with undercounting by −0.38 and −0.24, respectively.
Contribution of the Fellow Eye/Dominant Eye Versus the Amblyopic/Non-Dominant Eye
Normal-Sighted Group. For the normal-sighted observers, the regression model was significant F(2, 4) = 185.70, P < 0.001, R2 = 0.66. Both the coefficients of the dominant eye and nondominant eye were significant, suggesting that both were linearly associated with the normal observers’ feature counting response (P < 0.01). For the normal observers, an increase in the Gabor patch set-size in their dominant eye was associated with an increase of 0.77 in their response, whereas an increase in set-size in their nondominant eye was associated with an increase of 0.74 in their response.
Anisometropic Group. For the participants with anisometropic amblyopia, the regression model was significant F(2, 4) = 156.70, P < 0.001, R2 = 0.52. Both the coefficients of the fellow eye and amblyopic eye were significant, suggesting that both were linearly associated with the feature counting response of the participants with anisometropic amblyopia (P < 0.01). An increase in the Gabor patch set-size in their fellow eye was associated with an increase of 0.69 in their response, whereas an increase in set-size in their amblyopic eye was associated with an increase of 0.54 in their response.
Strabismic Group. For the participants with strabismic amblyopia, the regression model was significant F(2, 4) = 36.36, P < 0.001, R2 = 0.12. The coefficient of the fellow eye was significant (P < 0.05), suggesting that it was linearly associated with the feature counting response of the participants with strabismic amblyopia. The coefficient of the amblyopic eye was not significant (P > 0.05), indicating that it did not contribute anything to the regression model beyond the fellow eye as a predictor. An increase in the Gabor patch set-size in their fellow eye was associated with an increase of 0.42 in their response.
The results of the robust-clustered regression analyses are presented in Table 3.
Table 3.
Summary Results of the Multiple Robust-Clustered Regression Analyses Examining the Contribution of Fellow Eye/Dominant Eye Versus the Amblyopic/Nondominant Eye Across Groups for Experiment 1
| Observer Group | Coefficient | Estimate | t-statistic | P value | CI |
|---|---|---|---|---|---|
| Normal | Intercept | 0.78 | 4.16 | 0.02 | 0.20, 1.36 |
| Dominant eye | 0.77 | 18.26 | 0.0003 | 0.64, 0.91 | |
| Nondominant eye | 0.74 | 14.77 | 0.0004 | 0.59, 0.89 | |
| Anisometropic amblyopia | Intercept | 1.03 | 3.16 | 0.05 | 0.20, 1.36 |
| Fellow eye | 0.69 | 15.16 | 0.0005 | 0.64, 0.91 | |
| Amblyopic eye | 0.54 | 5.67 | 0.009 | 0.59, 0.89 | |
| Strabismic amblyopia | Intercept | 1.49 | 4.05 | 0.02 | 0.39, 2.60 |
| Fellow eye | 0.42 | 3.41 | 0.03 | 0.05, 0.77 | |
| Amblyopic eye | 0.34 | 3.04 | 0.05 | −0.002, 0.67 |
Experiment 2
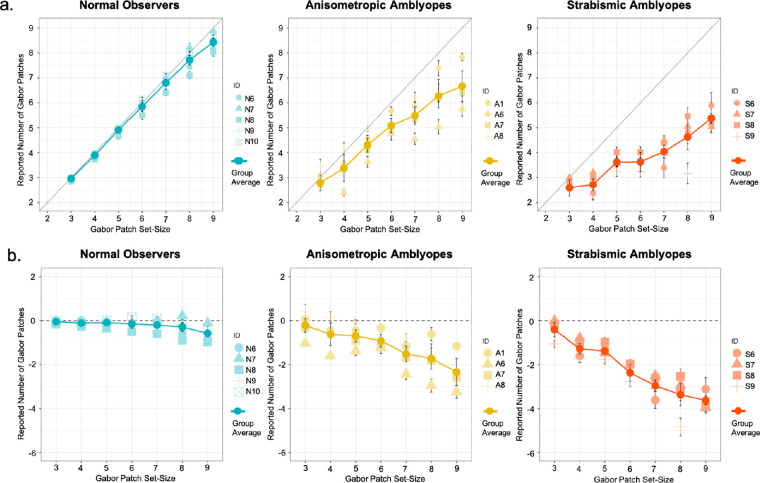
Feature Counting
The mean feature counting responses of all observer groups in Experiment 2 are plotted in Figure 4a. The results of our robust-clustered regression analysis are presented in Table 4. The regression model was significant F(5,12) = 625.10, P < 0.001, R2 = 0.69, and the Gabor patch set-size coefficient was significant (p < .001). The coefficient of the observer group of strabismus was also significant (P < 0.05). The interaction of Gabor patch set-size and the observer group of anisometropia, as well as Gabor patch set-size and the observer group of strabismus were also significant (P < 0.001).
Figure 4.
Feature counting performance and error in Experiment 2. The mean feature counting performance across groups and individual mean performance is plotted in (a). The mean feature counting error across groups and individual mean errors is plotted in (b). Error bars: standard error.
Table 4.
Summary Results of the Robust-Clustered Regression Analyses Examining Relationship Between the Feature Counting and Estimated Counting Error With the Gabor Patch Set-Size And Observer Group for Experiment 2
| Model | Coefficient | Estimate | t-statistic | P value | CI |
|---|---|---|---|---|---|
| Feature counting | Intercept | 0.23 | 3.57 | 0.028 | 0.04, 0.42 |
| Set-size | 0.93 | 40.86 | 0.0000061 | 0.86, 1.00 | |
| Anisometropia group | 0.55 | 1.94 | 0.104 | −0.16, 1.26 | |
| Strabismus group | 1.05 | 2.76 | 0.038 | 0.08, 2.01 | |
| Set-size × Anisometropia group | −0.27 | −5.62 | 0.0019 | −0.40, −0.15 | |
| Set-size × Strabismus group | −0.50 | −11.99 | 0.000052 | −0.60, −0.39 | |
| Counting error | Intercept | 0.23 | 3.57 | 0.03 | 0.04, 0.42 |
| Set-size | −0.07 | −3.11 | 0.04 | −0.14, −0.005 | |
| Anisometropia group | 0.55 | 1.94 | 0.104 | −0.16, 1.26 | |
| Strabismus group | 1.04 | 2.76 | 0.038 | 0.08, 2.01 | |
| Set-size × Anisometropia group | −0.27 | −5.62 | 0.0019 | −0.40, −0.15 | |
| Set-size × Strabismus group | −0.50 | −11.99 | 0.000053 | −0.60, −0.39 |
The coefficients of set-size for each observer group were obtained from our regression model and are depicted in Figure 56. In all observer groups, coefficients of set-size were significantly greater than 0 (P < 0.05). Tukey adjusted comparisons revealed that the coefficient of set-size of the strabismic group (0.43 CI [0.36, 0.50]) was significantly lower in comparison to the coefficient of set-size of the normal-sighted group (0.93 CI [0.89, 0.97]) (P < 0.0001) and the anisometropic group (0.66 CI [0.57, 0.74]) (P < 0.001). The coefficient of set-size of the anisometropic group was also significantly lower than that of the normal-sighted group (P < 0.0001). For the strabismic group, an increase in the Gabor patch set-size was associated with an increase of only 0.43 in their counting response, whereas for the anisometropic and the normal group, it was associated with an increase of 0.66 and 0.93, respectively.
Figure 5.
The coefficients of set-size for each observer group in Experiment 2. The coefficient of set-size depicts the relationship between the reported number of features with the Gabor patch set-size. The coefficients for each observer group for the (a) feature counting regression analysis and (b) estimated counting error regression analysis. Error bars: confidence intervals. * = P < 0.01.
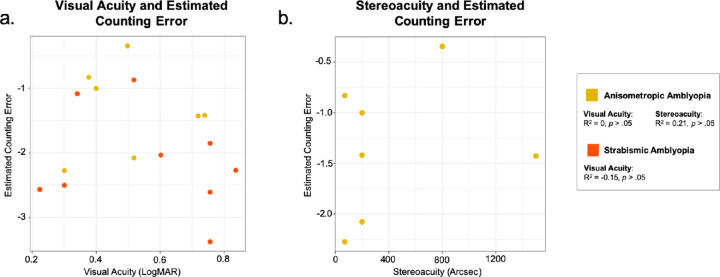
Figure 6.
Correlations between visual counting error and clinical characteristics. Associations between visual acuity (a) and stereoacuity (b) and the mean visual counting error in amblyopes were assessed using Spearman's rank correlation. The correlation between stereoacuity and visual counting errors in strabismic amblyopes was not computable, since majority of this group had no measurable stereoacuity.
Counting Error
The mean estimation counting error of all observer groups are plotted in Figure 4b. The results of our robust-clustered regression analysis are presented in Table 4. The regression model was significant F(5,12) = 454.50, P < 0.001, R2 = 0.49. The Gabor patch set-size coefficient was significant (P < 0.05. The coefficient of the observer group of strabismus was also significant (P < 0.05). The interaction of Gabor patch set-size and the observer group of anisometropia, as well as Gabor patch set-size and the observer group of strabismus were significant (P < 0.01).
The coefficients of set-size for each observer group were obtained from our regression model and are depicted in Figure 5. In all observer groups, coefficients of set-size were significantly smaller than 0 (P < 0.05), suggesting that all observer groups undercounted as the Gabor patches set-size increased. Tukey adjusted comparisons, however, revealed that as the Gabor patch set-size increased, the strabismic group (−0.57 CI [−0.63, −0.50]) significantly undercounted in comparison to the normal-sighted group (−0.07 CI [−0.12, −0.03]) and anisometropic group (−0.34 CI [−0.43, −0.26]) (P < 0.001). The anisometropic group also significantly undercounted in comparison to the normal-sighted group (P < 0.0001). For the strabismic group, an increase in the Gabor patch set-size was associated with undercounting by the amount of −0.57, whereas for the anisometropic and the normal-sighted group, it was associated with undercounting by −0.384 and only −0.07, respectively.
Contribution of the Fellow Eye/Dominant Eye Versus the Amblyopic/Non-Dominant Eye
Normal-Sighted Group. For the normal-sighted group, the regression model was significant F(2, 4) = 897.10, P < 0.001, R2 = 0.94. Both the coefficients of dominant eye and non-dominant eye were significant, suggesting that both were linearly associated with the normal-sighted observers’ counting response (P < 0.0001). For the normal-sighed observers, an increase in the Gabor patch set-size in their dominant eye was associated with an increase of 0.92 in their response, while an increase in set-size in their nondominant eye was associated with an increase of 0.94 in their response.
Anisometropic Group. For the participants with anisometropic amblyopia, the regression model was significant F(2, 3) = 168.80, P < 0.001, R2 = 0.53. Both the coefficients of fellow eye and amblyopic eye were significant, suggesting that both were linearly associated with the counting response of the participants with anisometropic amblyopia (P < 0.01). An increase in the Gabor patch set-size in their fellow eye was associated with an increase of 0.77 in their response, whereas an increase in set-size in their amblyopic eye was associated with an increase of 0.54 in their response.
Strabismic Group. For the participants with strabismic amblyopia, the regression model was significant F(2, 4) = 36.36, P < 0.001, R2 = 0.12. The coefficient of fellow eye (P < 0.05) and amblyopic eye (P < 0.05) was significant, suggesting that they were linearly associated with the counting response of the participants with strabismic amblyopia. An increase in the Gabor patch set-size in their fellow eye was associated with an increase of 0.71 in their response, whereas an increase in set-size in their amblyopic eye was associated with an increase of only 0.18 in their response.
The results of the robust-clustered regression analyses are presented in Table 5.
Table 5.
Summary Results of the Multiple Robust-Clustered Regression Analyses Examining the Contribution of Fellow Eye/Dominant Eye Versus the Amblyopic/Non-Dominant Eye Across Groups for Experiment 2
| Observer Group | Coefficient | Estimate | t-statistic | P value | CI |
|---|---|---|---|---|---|
| Normal | Intercept | 0.23 | 3.57 | 0.03 | 0.04, 0.41 |
| Dominant eye | 0.92 | 39.63 | 0.000008 | 0.86, 0.99 | |
| Nondominant eye | 0.94 | 42.13 | 0.000005 | 0.87, 0.99 | |
| Anisometropic amblyopia | Intercept | 0.79 | 2.97 | 0.07 | −0.16, 1.75 |
| Fellow eye | 0.77 | 10.10 | 0.005 | 0.48, 1.05 | |
| Amblyopic eye | 0.54 | 5.21 | 0.02 | 0.14, 0.94 | |
| Strabismic amblyopia | Intercept | 1.25 | 2.96 | 0.08 | −0.33, 2.85 |
| Fellow eye | 0.71 | 7.01 | 0.01 | 0.34, 1.08 | |
| Amblyopic eye | 0.18 | 6.00 | 0.02 | 0.06, 0.29 |
Correlation Between Feature Counting Error and Clinical Characteristics
The association between visual acuity and the mean feature counting error in all participants with amblyopia were assessed using Spearman's rank correlation. Data were collapsed across Experiment 1 and Experiment 2. Visual acuity in the amblyopic eye of the participants with anisometropic (R2 = 0, P > 0.05) and strabismic (R2 = −0.15, P > 0.05) amblyopia was not correlated with their mean estimating counting error, suggesting that in the current study, compromised performance on the feature counting task was not simply due to poorer visual acuity. This was expected given that the stimuli in our tasks were highly visible with low spatial frequency (2cpd) and high contrast (20% to 35%). The contrast of the Gabor patches across both eyes was also equated if necessary.
Stereoacuity and feature counting error was not correlated in the participants with anisometropic amblyopia (R2 = 0.21, P > 0.05). We were unable to compute the correlation between stereoacuity and feature counting errors for strabismic group, because the majority had no measurable stereoacuity.
Discussion
In the current study, both participants with anisometropic and strabismic amblyopia undercounted features in comparison to normal observers. Overall, feature counting performance in participants with strabismic amblyopia was worst in comparison to normal-sighted observers and participants with anisometropic amblyopia. Greater deficits in the strabismic group were likely driven by their more severe disruptions in binocular function compared to the anisometropic group. Indeed, as shown in Table 1, the majority of the participants with strabismic amblyopia had no measurable stereoacuity, whereas most of the participants with anisometropic amblyopia had residual stereoacuity. Our results also showed that in both amblyopic groups, their amblyopic eye contributed less to their feature counting response, in comparison to their fellow eye. However, this difference in eye contribution was greater in the participants with strabismic amblyopia. In Experiment 2, a different group of participants with amblyopia (at the exception of A1) undercounted, despite the longer feature presentation time. This replicates what was found of Experiment 1 and suggests good test/retest reliability for our findings, despite our small sample size, which is admittedly one limitation of the current study. We were unable to compute the correlation between counting errors and stereoacuity in the strabismic group, given that they had no measurable stereoacuity. However, in future studies the role of stereoacuity under dichoptic viewing could be further investigated by recruiting participants with strabismic amblyopia who have residual stereo acuities measurable through the random-dot stereotest card. We found no significant correlation between counting errors and stereoacuity in the anisometropic group. However, this null finding may be due to our small sample size. The correlation between counting errors and visual acuity in both the anisometropic and strabismic groups was not significant, which is consistent with previous feature counting studies featuring participants with strabismic amblyopia.22
Feature Counting Under Monocular and Dichoptic Viewing Conditions
During monocular viewing, where the untested eye is covered, participants with strabismic amblyopia have been found to accurately count features using their amblyopic eye when the set-size is small (n < 4) and perform like normal observers when using their fellow eye.22,23 In the current study, stimuli were presented dichoptically, and both participants with anisometropic and strabismic amblyopia exhibited deficits in feature counting. Our dichoptic task likely better captured binocular function disruptions in amblyopia, given that the processing of features in both eyes was needed to accurately count. Indeed, although the coefficients of both eyes were equally and linearly associated with the normal-sighted observers’ counting response, in some cases, such as strabismic group in Experiment 1, only the coefficient of fellow eye was significant, indicating that their amblyopic eye did not contribute anything to their response beyond their fellow eye. In anisometropic group, the coefficient for both eyes was significant, but the coefficient for fellow eye was greater than that of the amblyopic eye. Overall, these results demonstrate that under dichoptic viewing, participants with amblyopia primarily rely on their fellow eye and suppress input presented to their amblyopic eye, consistent with previous findings of visual suppression in amblyopia.24–27,35,36 However, the coefficient for the fellow eye of the participants with amblyopia was also smaller compared to normal-sighted observers, further providing evidence of higher-order dysfunction, in addition to adverse effects interocular suppression on visual counting in amblyopia.
The Role of Attention in Visual Counting
Visual counting or the estimation of briefly presented features is believed to engage visual attention processes and require rapid shifts in attention.37,38 Indeed, neuroimaging research with humans and neurophysiological studies with monkeys have shown that visual counting of a large number of features (n > 4) engages higher visual pathways39 and the intraparietal sulcus (IPS).40,41 IPS is a region known to be involved in visual attention42,43; thus in the current study, the undercounting of features in the participants with anisometropic and strabismic amblyopia may also stem from difficulties in switching attention from one cluster of features to another when subitizing or counting features.22 Several behavioral studies have also reported deficits in the amblyopic eye for tasks demanding spatial attention or top-down mechanisms, such as object tracking18,44–47 attentional blink48 and decision making.21 These studies along with our results, suggest that deficits in selective visual attention may be an attribute of amblyopia. In fact, in a recent encephalography sourced-imaging study, degraded attentional modulation of neural populations in both V1 and the extrastriate cortex was found in participants with strabismic amblyopia,49 additionally lending support to the proposal of top-down processing deficits in amblyopia. More recently, numerosity, the ability to generate an estimate of a given quantity without serial counting, has been posited to engage a specific neural substrate for number processing.50 For instance, neuroimaging studies have revealed selectivity for numerosity in the human IPS and frontal cortex.51 Thus, undercounting of features in participants with amblyopia may be a result of numerosity processing impairments in regions beyond the visual cortex, further supporting the view of higher-level cortical function deficits in amblyopia.
Conclusion
In the current study, we examined whether feature counting is impaired in both strabismic and anisometropic amblyopia during dichoptic viewing. Our data show that both participants with anisometropic and strabismic amblyopia undercount features in comparison to normal-sighted observers. Participants with strabismic amblyopia exhibited greater deficits in feature counting in comparison to those with anisometropic amblyopia. Our results support the view of higher-level cortical function deficits22 in amblyopia. Future research featuring a larger sample of participants with amblyopia could potentially better capture the heterogeneity of amblyopic symptoms and allow for further examination of the consequences of visual deprivation on the development of high-level visual cortical functioning. Overall, the current study bridges the gap between research on high-cortical function deficits and clinical binocular function disruptions in amblyopia, which can help us better understand the neural mechanism of amblyopia and inform clinical therapeutic tasks and strategies.
Acknowledgments
The authors thank Spero C. Nicholas for programing the stimuli and Margaret Q. McGovern for assistance in recruiting the participants. The authors also thank Xie Jie Lai for contributing to the study design and data collection.
Supported by an NIH Grant R01- EY025018 and a Pacific Vision Foundation grant awarded to CH. AMBW was supported by the Rachel C. Atkinson Postdoctoral Fellowship.
Disclosure: A.M.B. Wong-Kee-You, None; H. Wei, None; C. Hou, None
References
- 1. Movshon JA, Eggers HM, Gizzi MS, Hendrickson AE, Kiorpes L, Boothe RG. Effects of early unilateral blur on the macaque's visual system. III. Physiological observations. J Neurosci. 1987; 7: 1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kiorpes L, Kiper DC, O'Keefe LP, Cavanaugh JR, Movshon JA. Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. J Neurosci. 1998; 18: 6411–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kiorpes L, Wallman J. Does experimentally-induced amblyopia cause hyperopia in monkeys? Vision Res. 1995; 35: 1289–1297. [DOI] [PubMed] [Google Scholar]
- 4. Chino YM, Bi H, Zhang B. The postnatal development of the neuronal response properties in primate visual cortex. In: Kaas J, Collins C, eds. Primate Vision. Boca Raton, FL: CRC Press; 2004;81–108. [Google Scholar]
- 5. Tychsen L, Wong AM, Burkhalter A. Paucity of horizontal connections for binocular vision in V1 of naturally strabismic macaques: Cytochrome oxidase compartment specificity. J Comp Neurol. 2004; 474: 261–275 [DOI] [PubMed] [Google Scholar]
- 6. Schmidt KF,, Lowel S. Strabismus modifies intrinsic and inter-areal connections in cat area 18. Neuroscience. 2008; 152: 128–137. [DOI] [PubMed] [Google Scholar]
- 7. Shooner C, Hallum LE, Kumbhani RD, et al.. Population representation of visual information in areas V1 and V2 of amblyopic macaques. Vision Res. 2015; 114: 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bi H, Zhang B, Tao X, Harwerth RS, Smith EL III, Chino YM. Neuronal responses in visual area V2 (V2) of macaque monkeys with strabismic amblyopia. Cerebral Cortex. 2011; 21: 2033–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hess RF, McIlhagga W, Field D. Contour integration in strabismic amblyopia: The sufficiency of explanation based on positional uncertainty. Vision Res. 1997; 37: 3145–3161. [DOI] [PubMed] [Google Scholar]
- 10. Chandna A, Pennefather PM, Kovacs I, Norcia AM. Contour integration deficits in anisometropic amblyopia. Invest Ophthalmol Vis Sci. 2001; 42: 875–878. [PubMed] [Google Scholar]
- 11. Simmers AJ, Ledgeway T, Hess RF, McGraw PV. Deficits to global motion processing in human amblyopia. Vision Res. 2003; 43: 729–738. [DOI] [PubMed] [Google Scholar]
- 12. Simmers AJ, Ledgeway T, Hess RF. The influences of visibility and anomalous integration processes on the perception of global spatial form versus motion in human amblyopia. Vision Res. 2005; 45: 449–460. [DOI] [PubMed] [Google Scholar]
- 13. Ho CS, Giaschi DE. Deficient maximum motion displacement in amblyopia. Vision Res. 2006; 46: 4595–4603. [DOI] [PubMed] [Google Scholar]
- 14. Simmers AJ, Ledgeway T, Hess RF. The influences of visibility and anomalous integration processes on the perception of global spatial form versus motion in human amblyopia. Vision Res. 2005; 45: 449–460. [DOI] [PubMed] [Google Scholar]
- 15. Wang X, Cui D, Zheng L, Yang X, Yang H, Zeng J. Combination of blood oxygen level-dependent functional magnetic resonance imaging and visual evoked potential recordings for abnormal visual cortex in two types of amblyopia. Mol Vis. 2012; 18: 909–919. [PMC free article] [PubMed] [Google Scholar]
- 16. Hou C, Pettet MW, Norcia AM. Abnormalities of coherent motion processing in strabismic amblyopia: Visual-evoked potential measurements. J Vis. 2008; 8: 2–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho CS, Giaschi DE.. Low- and high-level motion perception deficits in anisometropic and strabismic amblyopia: Evidence from fMRI. Vis Res. 2009; 49: 2891–2901. [DOI] [PubMed] [Google Scholar]
- 18. Mendola JD, Conner IP, Roy A, et al.. Voxel‐based analysis of MRI detects abnormal visual cortex in children and adults with amblyopia. Hum Brain Mapp. 2005; 25: 222–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hou C, Pettet MW, Norcia AM. Acuity-independent effects of visual deprivation on human visual cortex. Proc Natl Acad Sci. 2014; 111: E3120–E3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kiorpes L. Visual processing in amblyopia: Animal studies. Strabismus. 2006; 14: 3–10. [DOI] [PubMed] [Google Scholar]
- 21. Farzin F, Norcia AM.. Impaired visual decision-making in individuals with amblyopia. J Vis. 2011; 11: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sharma V, Levi DM, Klein SA. Undercounting features and missing features: Evidence for a high-level deficit in strabismic amblyopia. Nat Neurosci. 2000; 3: 496–501. [DOI] [PubMed] [Google Scholar]
- 23. Li RW, Ngo C, Nguyen J,, Levi DM. Video-game play induces plasticity in the visual system of adults with amblyopia. PLoS Biol. 2011; 9: e1001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Travers TA. Suppression of vision in squint and its association with retinal correspondence and amblyopia. Br J Ophthalmol. 1938; 22: 577–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jampolsky A. Characteristics of suppression in strabismus. AMA Arch Ophthalmol. 1955:54: 683–696. [DOI] [PubMed] [Google Scholar]
- 26. Sireteanu R. Human amblyopia: consequence of chronic interocular suppression. Human neurobiology. 1982; 1: 31–33. [PubMed] [Google Scholar]
- 27. Holopigian K, Blake R, Greenwald MJ. Clinical suppression and amblyopia. Invest Ophthalmol Vis Sci. 1988; 29: 444–451. [PubMed] [Google Scholar]
- 28. Levi DM, Klein S. Hyperacuity and amblyopia. Nature. 1982; 298: 268. [DOI] [PubMed] [Google Scholar]
- 29. Brainard DH. The psychophysics toolbox. Spat Vis. 1997; 10: 433–6. [PubMed] [Google Scholar]
- 30. Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat Vis. 1997; 10: 437–42. [PubMed] [Google Scholar]
- 31. Kleiner M, Brainard D, Pelli D. What's new in Psychtoolbox-3?. Cognitive & Computational Psychophysics. 2007.
- 32. Bell RM, McCaffrey DF. Bias reduction in standard errors for linear regression with multi-stage samples. Survey Methodology. 2002; 28: 169–181. [Google Scholar]
- 33. Blair G, Cooper J, Coppock A, Humphreys M, Sonnet L. Estimatr: Fast Estimators for Design-Based Inference. R package version 0.14 2018. [Google Scholar]
- 34. Team RC. R: A language and environment for statistical computing. 2013: 201.
- 35. Li J, Hess RF, Chan LY, et al.. Quantitative measurement of interocular suppression in anisometropic amblyopia: a case-control study. Ophthalmology. 2013; 120: 1672–80. [DOI] [PubMed] [Google Scholar]
- 36. Travers TA. Suppression of vision in squint and its association with retinal correspondence and amblyopia. Br J Ophthalmol. 1938; 22: 577–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Egeth HE, Leonard CJ, Palomares M.. The role of attention in subitizing: Is the magical number 1? Vis Cogn. 2008; 16: 463–473. [Google Scholar]
- 38. Anobile G, Turi M, Cicchini GM, Burr DC. The effects of cross-sensory attentional demand on subitizing and on mapping number onto space. Vis Res. 2012; 74: 102–109. [DOI] [PubMed] [Google Scholar]
- 39. Sathian K, Simon TJ, Peterson S, Patel GA, Hoffman JM, Grafton ST. Neural evidence linking visual object enumeration and attention. J Cogn Neurosci. 1999; 11: 36–51. [DOI] [PubMed] [Google Scholar]
- 40. Nieder A, Diester I, Tudusciuc O. Temporal and spatial enumeration processes in the primate parietal cortex. Science. 2006; 313: 1431–5. [DOI] [PubMed] [Google Scholar]
- 41. Nieder A, Dehaene S.. Representation of number in the brain. Ann Rev Neurosci. 2009; 32: 185–208. [DOI] [PubMed] [Google Scholar]
- 42. Sylvester CM, Shulman GL, Jack AI, Corbetta M. Asymmetry of anticipatory activity in visual cortex predicts the locus of attention and perception. J Neurosci. 2007; 27: 14424–14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bressler SL, Tang W, Sylvester CM, Shulman GL, Corbetta M. Top-down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. J Neurosci. 2008; 28: 10056–10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ho CS, Paul PS, Asirvatham A, Cavanagh P, Cline R, Giaschi DE. Abnormal spatial selection and tracking in children with amblyopia. Vis Res. 2006; 46: 3274–3283. [DOI] [PubMed] [Google Scholar]
- 45. Secen J, Culham J, Ho C, Giaschi D. Neural correlates of the multiple-object tracking deficit in amblyopia. Vis Res. 2011; 51: 2517–27. [DOI] [PubMed] [Google Scholar]
- 46. Tripathy SP, Levi DM.. On the effective number of tracked trajectories in amblyopic human vision. J Vis. 2008; 8: 1–22. [DOI] [PubMed] [Google Scholar]
- 47. Chow A, Giaschi D,, Thompson B. Dichoptic attentive motion tracking is biased toward the nonamblyopic eye in strabismic amblyopia. Invest Ophthalmol Vis Sci. 2018; 59: 4572–4580. [DOI] [PubMed] [Google Scholar]
- 48. Popple AV, Levi DM. The attentional blink in amblyopia. J Vis. 2008; 8: 12.1–9. [DOI] [PubMed] [Google Scholar]
- 49. Hou C, Kim YJ, Lai XJ, Verghese P. Degraded attentional modulation of cortical neural populations in strabismic amblyopia. J Vis. 2016; 16: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Anobile G, Turi M, Cicchini GM, Burr DC. Mechanisms for perception of numerosity or texture-density are governed by crowding-like effects. J Vis. 2015; 15: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Piazza M, Pinel P, Le Bihan D, Dehaene S. A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron. 2007; 53: 293–305. [DOI] [PubMed] [Google Scholar]