Abstract
Purpose
To compare measurement of wall-to-lumen ratio (WLR) by means of high-resolution adaptive optics imaging (AO) with intuitive to use retinal vessel wall (VW) analysis (VWA). Moreover, to validate the techniques by comparing WLR of healthy young (HY) with healthy older patients.
Methods
Ten retinal VW images of 13 HY (24 ± 2 years) and 16 healthy older (60 ± 8 years) were obtained with AO and VWA. The average of five measurements of VW, retinal vessel lumen and WLR of a single vessel from AO and VWA were calculated and compared.
Results
WLR of AO and VWA images showed high correlations, r = 0.75, t(27) = 5.98, P < .001, but differed systematically (WLR: VWA, 40 ± 7% and AO, 35 ± 9%; P < .001). Comparable patterns were found for VW and vessel lumen. HY showed significantly lower WLR (AO, 31 ± 8% and VWA, 36 ± 8%) compared with healthy older (AO, 39 ± 9% [P = .012]; VWA, 42 ± 5% [P = .013]).
Conclusions
Assessment of WLR by VWA showed a good correlation with laborious analysis of the microstructure by high-resolution AO. Measurement of WLR in different age groups indicated good validity. Deviations in VW, vessel lumen, and WLR between AO and VWA can be explained by systematic differences in image scale and resolution. Future studies are needed to investigate the clinical relevance of microvascular WLR assessment by retinal VWA and its prognostic value.
Translational Relevance
Additional assessment of retinal WLR by use of digital VWA to evaluate microstructural remodeling may prove to be a valuable extension to the current use of retinal vessel diameters as biomarkers of cardiovascular risk.
Keywords: retinal vessel analysis, wall-to-lumen ratio, microcirculation, retina
Introduction
Retinal vessel diameters are valid biomarkers of cerebrovascular and cardiovascular risk.1 Narrower arteriolar and wider venular diameters have previously been associated with increased cardiovascular events such as stroke,2 coronary heart disease,3 and higher cardiovascular mortality.4 The application of retinal vessel diameters based on retinal vessel analysis on top of usual care can improve accuracy of cardiovascular risk stratification (net reclassification) by up to 21% and 10% for cardiovascular events5 and for risk calculation of stroke.6 Further systemic cardiovascular risk factors such as hypertension,7,8 diabetes, obesity, dyslipidemia, or inflammation have been associated with retinal vessel alterations.3,9 Retinal vessel diameters allow the evaluation of the structural and functional properties of the microvascular wall and determine retinal microvascular perfusion as the “gatekeeper” of flow to the capillaries.10 However, an analysis of retinal vessel diameters alone cannot differentiate between functional narrowing (autoregulation) or structural narrowing (remodeling). The assessment of retinal vessel wall (VW) and vessel lumen (VL) allow for the analysis of structural microvascular remodeling and may improve our understanding of the process of microvascular ageing.11
Adaptive optics (AO) scanning laser ophthalmoscopy or scanning laser Doppler flowmetry allow for a differentiation between VW and VL and, therefore, measurement of wall-to-lumen ratio (WLR).12–14 Previous studies have shown strong correlations of WLR with age12,15,16 and blood pressure or hypertension.12,15–17 Higher retinal WLR has been described in older participants12,15,16 or in patients with hypertension.12,15–17
However, these attempts to assess retinal WLR were based on complex technology and procedures, which restrict the use in daily clinical routine. Therefore, this study aimed to (a) compare measurement of WLR by means AO with intuitive to use retinal VW analysis (VWA) and (b) validate both techniques by comparing WLR of HY with healthy older (HO) participants.
Methods
Study Design
This study was approved by the Ethics Committee of Northwest and Central Switzerland (EKNZ 2015-351). It was planned and conducted in accordance with the protocol and principles stated in the Helsinki Declaration.18 Participants signed a written informed consent before the first measurement.
Participants were invited to the Department of Sport, Exercise and Health at the University of Basel, Switzerland, to obtain VWA images and to the Department of Ophthalmology at the University of Basel to assess AO images. Images were analyzed by one examiner blinded to the identity of participants and source of images based on a standard procedure as described elsewhere in this article. Participants were asked to refrain from any sport activity for 24 hours and to avoid any food intake for 2 hours before the examinations.
Inclusion and Exclusion Criteria
We included healthy men and women between 20 and 30 years of age and between 50 and 80 years of age. Exclusion criteria were regular physical activity (more than twice per week), an active smoking status or positive smoking history, any cardiovascular medication or a blood pressure of 90/140 mm Hg or higher after 10 minutes of seated rest, pregnancy, an intraocular pressure of more than 20 mm Hg, or any ocular disease.
Assessment of the Retinal WLR
The ICare PRO (Tiolat Oy, Helsinki, Finland) rebound tonometry was used to evaluate the intraocular pressure. The right pupil was dilated with tropicamide (0.5%). A commercially available fundus camera (FF 450+; Carl Zeiss, Jena, Germany), equipped with a standard high capacity charge-coupled device camera, was used to obtain 10 consecutive high-quality fundus images, each with a resolution of 2.3 µm per pixel. Fundus images were taken at 20° using a green filter (red free). Image quality was rated by two independent graders. Quality criteria were defined as image centration on the optic disc, no detectable light or movement artifacts or overexpressed areas, and a high image sharpness. Corresponding imaging was performed using a commercial available AO camera system (rtx1TM-e, imagine eyes, Orsay, France). The AO images were obtained using the manufacturer's software.19 The software automatically corrected for potential artefacts. Two examiners rated the vessel segments with the best subjective contrast quality. The region of interest was determined in or directly around the optic nerve in almost all cases. The AO system provided 10 high-quality images from exactly the same region predefined by the previous assessment with the fundus camera. AO scans had a native resolution of 0.8 µm per pixel. Images were exported from the AO device and further processed using Visualis 3.00 (Visual Imaging System, Imedos, Jena, Germany) after adjusting image resolution and image scales.
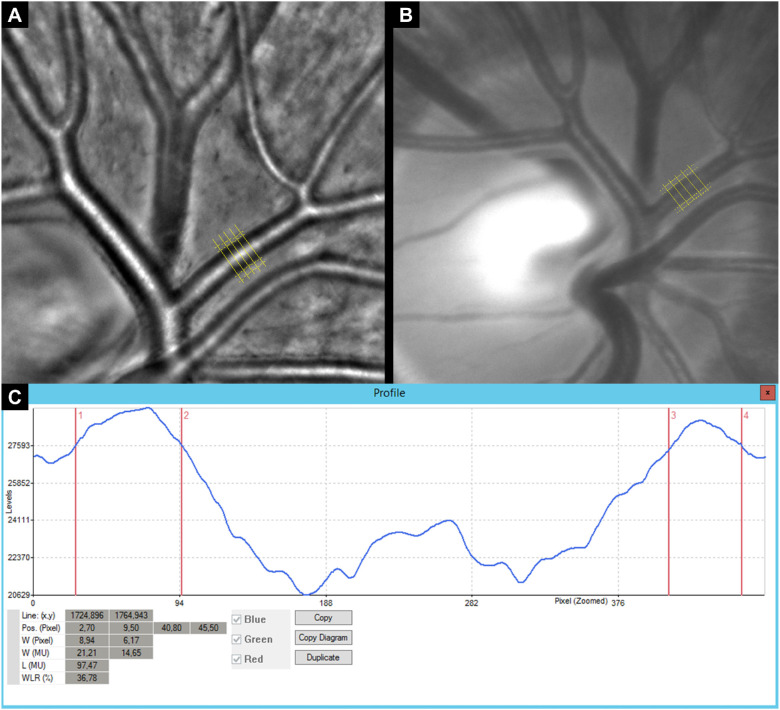
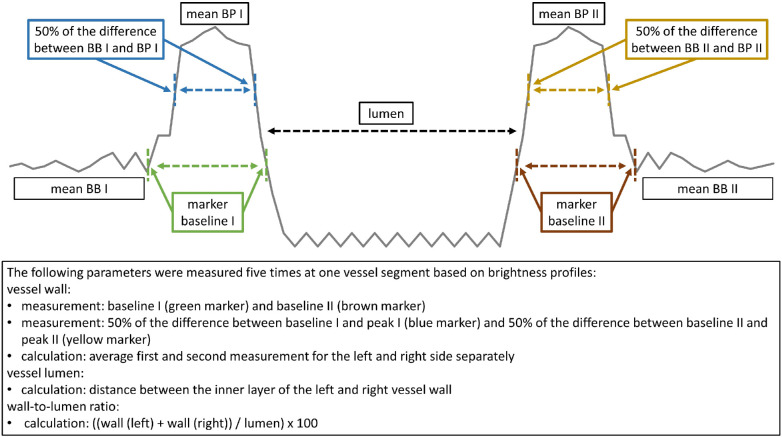
A single high-quality image was used to conduct the following steps. Vascular wall dimensions were measured on either side of one arteriole to calculate the VL and WLR. The WLR was analyzed four to five times at the same vessel segment of one AO and one VWA image by means of brightness profiles at a right angle to the surface (Fig. 1). All parameters described in Figure 1 were calculated in each brightness profile separately and averaged thereafter. VW was defined by the distance between the outer and inner boundaries of the VW on either side. VL was defined as the distance between the inner VW markers. Distance was reported as measuring units and the WLR as percentage. The methods to calculate the VW, VL, and WLR are described in detail in Figure 2.
Figure 1.
Retinal vessel imaging using AO and corresponding standard color fundus imaging by intuitive to use retinal VWA. Five maker lines were applied to measure retinal vessel microstructure in the same eye imaged with AO (A) and VWA (B). The original brightness profile for one analysis of the retinal vessel microstructure is shown (C).
Figure 2.
Detailed description of retinal vessel microstructure assessment by means of brightness profiles. BB, mean brightness baseline, BP, mean brightness peak.
Statistical Analysis and Sample Size Calculation
The primary outcome was the difference in WLR between standard VWA and AO as well as between HY and HO. Descriptive statistics included means and standard deviations. Boxplots were used for descriptive analyses. Two-sided independent t-tests were used to analyze the differences between HY and HO. The differences between the AO and VWA in HY and HO were analyzed by paired sample t-tests separately. Two-tailed Pearson correlations were computed to investigate the association between wall dimensions of the AO and VWA. Moreover, the same test was applied to assess the association of the VW, VL, and WLR with age and body mass index (BMI) as well as systolic and diastolic blood pressure. R version 3.4.2 for Windows20 was used for data analysis, boxplots and scatterplots. Further graphs were generated in Excel 2016 (Microsoft, Redmond, Washington, WA). The level of significance was set at a P of less than .05.
Based on a previous study, we assumed a WLR difference between HY and HO of 5% with a standard deviation of 0.04%.12 With a two-sided significance level of .05 and a target power of 90%, we needed a total sample size of 30 participants. G*Power software 3.1.9.221 was used for sample size calculation.
Results
Images were taken from all participants, 13 HY with a mean age of 24 ± 2 years (62% female) and 16 HO participants with a mean age of 60 ± 8 years (43% female). High image quality was achieved with both devices. The clinical data are summarized in Table 1. Groups differed in their age profile but had no significantly differences in their gender distribution, weight, BMI, systolic or diastolic blood pressure or intraocular pressure.
Table 1.
Clinical Characteristics
| HY (n = 13), mean ± SD |
HO (n = 16), m ean ± SD |
P Valuea | |
|---|---|---|---|
| Age (years) | 24 ± 2 | 60 ± 8 | <.001 |
| Sex (female/male) | 8/5 | 7/9 | .358 |
| Weight (kg) | 64 ± 11 | 65 ± 8 | .713 |
| BMI (kg/m2) | 22 ± 2 | 23 ± 1 | .245 |
| Sys BP (mm Hg) | 118 ± 10 | 116 ± 11 | .465 |
| Dia BP (mm Hg) | 79 ± 3 | 77 ± 9 | .510 |
| IOP (mm Hg) | 14 ± 2 | 15 ± 2 | .633 |
P value, level of significance for two sample t-test. dia BP, diastolic blood pressure; IOP, intraocular pressure; SD, standard deviation; Sys BP, systolic blood pressure.
Assessment of Retinal Vessel Microstructure
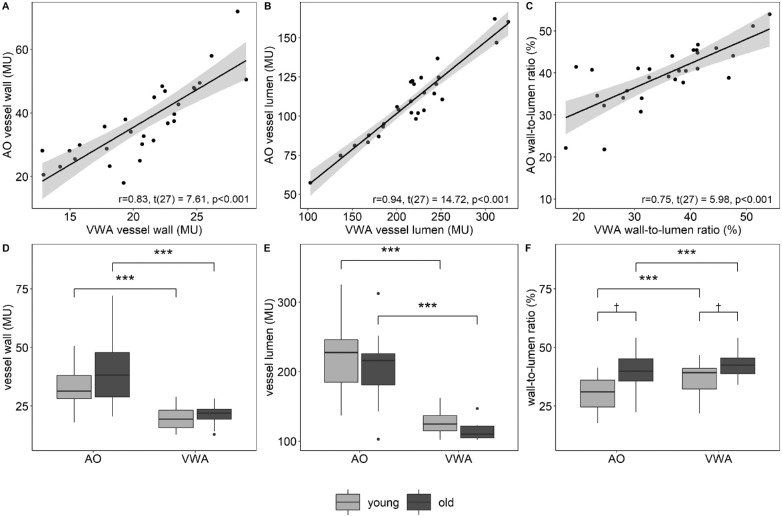
AO and VWA showed high correlations (Figs. 3A–C) for VW, r = 0.83, t(27) = 7.61, P < .001; VL, r = 0.94, t(27) = 14.72, P < .001; and WLR, r = 0.75, t(27) = 5.98, P < .001. A systematic difference for VW, VL, and WLR between the devices was found (Table 2, Figs. 3D–F).
Figure 3.
Two-tailed Pearson correlation of VW (A), VL (B), and WLR (C) measured by AO and VWA. Differences in VW (D), VL (E), and WLR (F) between AO and VWA as well as between HY and HO. ***P < .001 for two-sided paired sample t-tests; †P < .05 for two-sided independent t-tests.
Table 2.
Retinal VW, VL, and WLR in HY and HO Individuals Measured by AO and Retinal VWA
| HY | HO | |||||||
|---|---|---|---|---|---|---|---|---|
| AO | VWA | P Value | AO | VWA | P Valueb | P Valuec | P Valued | |
| VW (MU) | 34 ± 9 | 20 ± 5 | <.001 | 40 ± 14 | 21 ± 4 | <.001 | .201 | .381 |
| VL (MU) | 226 ± 53 | 115 ± 27 | <.001 | 206 ± 49 | 104 ± 20 | <.001 | .295 | .245 |
| WLR (%) | 31 ± 8 | 36 ± 8 | <.001 | 39 ± 9 | 42 ± 5 | <.001 | .012 | .013 |
P value for paired sample t tests AO vs. VWA in HY
P value for paired sample t-tests AO vs. VWA in HO.
P value for two-sample t-tests of AO parameter HY vs. HO.
P value for two-sample t-tests of VWA parameter HY vs. HO. MU, measuring units.
Validation of Retinal Vessel Microstructure
HY showed lower VW and higher VL values resulting in significantly lower WLR (AO, 31 ± 8%; VWA, 36 ± 8%) compared with HO (AO, 39 ± 9% [P = 0.012]; VWA, 42 ± 5% [P = .013]) (Table 2 and Fig. 3F). WLR measured with both devices was significantly correlated with age, r = 0.47, t(27) = 2.77, P = .010 and r = 0.43, t(27) = 2.49, P = .019, for AO and VWA respectively. VW, analyzed based on VWA images, was significantly correlated with BMI, r = 0.44, t(27) = 2.58, P = .016. Further correlations were not statistically significant (P > .05 for all; Table 3).
Table 3.
Correlation Coefficients of Retinal Vessel Microstructure with Age, BMI, and Systolic and Diastolic Blood Pressure
| AO | VWA | |||||
|---|---|---|---|---|---|---|
| VW | VL | WLR | VW | VL | WLR | |
| Age | 0.31 | −0.13 | 0.47 | 0.24 | −0.16 | 0.43b |
| BMI | 0.25 | 0.24 | 0.08 | 0.44b | 0.34 | 0.16 |
| Sys BP | −0.14 | 0.13 | −0.27 | −0.17 | 0.27 | −0.20 |
| Dia BP | 0.07 | 0.34 | −0.24 | 0.16 | 0.28 | −0.17 |
p > .01.
p < .05. Sys BP, systolic blood pressure; Dia BP, diastolic blood pressure; VWA, retinal vessel wall analysis.
Discussion
This study, for the first time, used applicable digital VWA for the noninvasive assessment of the retinal microstructure. The results were quantified by use of AO and demonstrated a high correlation with digital VWA. Differences in the WLR between AO and VWA were systematic and a result of differences in image scale and resolution. Measurements with both techniques demonstrated a lower WLR in HY compared with HO.
Assessment of Retinal Vessel Microstructure
Retinal arterioles secure adequate blood flow to the retinal capillaries, protect against intravascular pressure peaks, and are, therefore, essential regulators of organ perfusion. A permanent decrease in arteriolar VL and an increase in arteriolar VW owing to, for example, proliferation of smooth muscle cells, resulting in a higher WLR, play a key role for microvascular remodeling in chronic disease states.22 Few studies have attempted to assess retinal vessel microstructure directly by use of high-resolution technical devices12–14,16,23,24 or indirectly by laser Doppler flowmetry.25 Spectral domain optical coherence tomography23,24 or AO12–14,16 as high-resolution imaging techniques used VW and VL measurements for calculation of WLR. Arichika et al.26 compared both methods and demonstrated a high correlation between optical coherence tomography and AO. Laser Doppler flowmetry demonstrated a good correlation between retinal WLR and invasive, subcutaneous measurement of media-to-lumen ratio.25
In our study, we were able to measure the retinal microvascular WLR by simple use of VWA. The reflected light from the optic nerve induces different scattering patterns of the VW as compared with the blood column and the surrounding perivascular tissue. This property explains why the retinal vessel microstructure is best analyzed in the optic nerve or in its direct vicinity as a weak, light area around the blood column. The VW, VL, and WLR measured by AO differed from the VWA, with significantly higher values for AO. The differences were systematic and likely to be explained by differences in image scale and resolution. Moreover, WLR measured by AO and VWA showed significant and high correlations, with correlation coefficients between 0.75 and 0.94. Arichika et al.26 showed similar correlation coefficients by comparing AO and optical coherence tomography. In this study, we used a standard fundus camera with a resolution of 2.3 µm per pixel without any image processing. AO is based on a confocal laser scanning system with higher contrast, higher resolution (0.8 µm per pixel) and a system for additional image correction. In addition to differences in image scale and resolution, the additional image correction might be a reason why the AO results showed significantly higher values. Although the resolution and image quality of AO images was higher compared with the standard fundus camera, taking a high-quality image without movement artefacts and with a high image definition was much more difficult with AO. Even an experienced examiner needed more time and more attempts to get high-quality images.
The additional measurement of WLR by use of VWA may prove to be a valuable extension to the currently used static27,28 and dynamic29 retinal vessel analyses as biomarkers for cerebrovascular and cardiovascular risk, which would add microstructural remodeling to the analysis of microvascular dysfunction.
Validation of Retinal Vessel Microstructure
Previous publications showed high correlations of retinal WLR with age.12,15,16 We measured retinal WLR in HY and HO to validate the retinal vessel microstructure by use of high-resolution AO and VWA. VW and VL were not significantly different between the groups. However, HY showed lower WLR compared with HO with both devices. Moreover, WLR was significantly correlated with age in both cases. These findings are comparable with previous reports. Arichika et al.12 compared retinal VW structure as measured by use of AO between healthy controls and patients with hypertension. They found that VW and VL were not significantly different between the groups. Only WLR was significantly higher in patients with hypertension compared with healthy controls.12 Baleanu et al.17 found higher WLR in patients with acute cerebrovascular events compared with hypertensive or normotensive controls. Meixner et al.16 reported correlations of WLR with high blood pressure and high BMI. In our study, we did not find statistically significant associations of WLR with blood pressure or BMI. Only VW thickness assessed by VWA was significantly correlated with BMI in our study. These results were to be expected, taking into account that we investigated a healthy population with normal blood pressure and BMI. In summary, assessment of WLR by VWA seems to be a valid new diagnostic tool to assess structural retinal microvascular remodeling.
Strength and Limitations
This study may be limited by the relatively small number of participants. Also, the inclusion criteria may represent a selection bias. However, the method showed its validity in HY and HO participants. There is a lack of comparability between our study and others because it is not possible to compare our absolute values with findings in previous reports owing to the differences in the location of the measurements, as well as differences in techniques and methods. One limitation of the study could be that only a 24-hour rest period was prescribed for sports. It is possible that the values would be different for athletes who regularly train under high intensity.30 In view of an initial feasibility study, however, this limitation is quite plausible and can be adjusted in future studies. As a strength of our study, all images of both devices were analyzed with the same software based on the same standardized procedures by a single examiner. Compared with AO, digital VWA seemed to be more feasible in its clinical application to achieve high image quality in a timely and patient-friendly manner.
Conclusions
Attempts to assess retinal WLR have so far been based on demanding complex high-resolution technology, partly based on indirect measures to assess WLR. As a new approach, this study applied standardized digital VWA to noninvasively assess retinal microvascular WLR. We have shown the assessment of WLR by VWA to be a valid new imaging tool to assess structural retinal microvascular alterations. The additional measurement of WLR by use of VWA may prove to be a valuable extension of the currently used analysis of retinal vessel diameters as biomarkers of cardiovascular risk. Future studies will have to investigate the clinical relevance of microvascular WLR assessment by VWA and its prognostic value.
Acknowledgments
We thank Imagine Eyes (Orsay, France) as well as Imedos (Jena, Germany) for providing technical specifications of their systems used in his study.
Supported by the Swiss National Science Foundation, SNSF [32003B_159518/1 to HH] and the Institute of Molecular and Clinical Ophthalmology Basel (IOB), Basel, Switzerland [to PMM].
Disclosure: L. Streese, None; L.Y. Brawand, None; K. Gugleta, None; P.M. Maloca, None; W. Vilser, Imedos Systems GmbH (I); H. Hanssen, None
References
- 1. Tapp RJ, Owen CG, Barman SA, et al.. Associations of retinal microvascular diameters and tortuosity with blood pressure and arterial stiffness: United Kingdom Biobank. Hypertension. 2019; 74: 1383–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ikram MK, de Jong FJ, Bos MJ, et al.. Retinal vessel diameters and risk of stroke: the Rotterdam Study. Neurology. 2006; 66: 1339–1343. [DOI] [PubMed] [Google Scholar]
- 3. Wong TY, Klein R, Sharrett AR, et al.. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA. 2002; 287: 1153–1159. [DOI] [PubMed] [Google Scholar]
- 4. Wang JJ, Liew G, Klein R, et al.. Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur Heart J. 2007; 28: 1984–1992. [DOI] [PubMed] [Google Scholar]
- 5. Seidelmann SB, Claggett B, Bravo PE, et al.. Retinal vessel calibers in predicting long-term cardiovascular outcomes: the Atherosclerosis Risk in Communities Study. Circulation. 2016; 134: 1328–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGeechan K, Liew G, Macaskill P, et al.. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. Am J Epidemiol. 2009; 170: 1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang JJ, Mitchell P, Leung H, Rochtchina E, Wong TY, Klein R. Hypertensive retinal vessel wall signs in a general older population: the Blue Mountains Eye Study. Hypertension. 2003; 42: 534–541. [DOI] [PubMed] [Google Scholar]
- 8. Wong TY, Klein R, Sharrett AR, et al.. Atherosclerosis Risk in Communities Study. Retinal arteriolar diameter and risk for hypertension. Ann Intern Med. 2004; 140: 248–255. [DOI] [PubMed] [Google Scholar]
- 9. Wong TY, Islam FM, Klein R, et al.. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA). Invest Ophthalmol Vis Sci. 2006; 47: 2341–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flammer J, Konieczka K, Bruno RM, Virdis A, Flammer AJ, Taddei S. The eye and the heart. Eur Heart J. 2013; 34: 1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005; 46: 454–462. [DOI] [PubMed] [Google Scholar]
- 12. Arichika S, Uji A, Ooto S, Muraoka Y, Yoshimura N. Effects of age and blood pressure on the retinal arterial wall, analyzed using adaptive optics scanning laser ophthalmoscopy. Sci Rep. 2015; 5: 12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chui TY, Vannasdale DA, Burns SA. The use of forward scatter to improve retinal vascular imaging with an adaptive optics scanning laser ophthalmoscope. Biomed Opt Express. 2012; 3: 2537–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koch E, Rosenbaum D, Brolly A, et al.. Morphometric analysis of small arteries in the human retina using adaptive optics imaging: relationship with blood pressure and focal vascular changes. J Hypertens. 2014; 32: 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harazny JM, Ritt M, Baleanu D, et al.. Increased wall:lumen ratio of retinal arterioles in male patients with a history of a cerebrovascular event. Hypertension. 2007; 50: 623–629. [DOI] [PubMed] [Google Scholar]
- 16. Meixner E, Michelson G.. Measurement of retinal wall-to-lumen ratio by adaptive optics retinal camera: a clinical research. Graefes Arch Clin Exp Ophthalmol. 2015; 253: 1985–1995. [DOI] [PubMed] [Google Scholar]
- 17. Baleanu D, Ritt M, Harazny J, Heckmann J, Schmieder RE, Michelson G. Wall-to-lumen ratio of retinal arterioles and arteriole-to-venule ratio of retinal vessels in patients with cerebrovascular damage. Invest Ophthalmol Vis Sci. 2009; 50: 4351–4359. [DOI] [PubMed] [Google Scholar]
- 18. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 19. Besnerais GL, Ödlund E, Lévecq X. Robust processing of images sequences produced by an adaptive optics retinal camera. Adaptive Optics: Methods, Analysis and Applications. Optical Society of America. 2013. Paper no. S. OW3A. 3.2013. [Google Scholar]
- 20. Team RC. R: a language and environment for statistical computing. Vienna: The R Foundation; 2014. [Google Scholar]
- 21. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007; 39: 175–191. [DOI] [PubMed] [Google Scholar]
- 22. Kanbay M, Sanchez-Lozada LG, Franco M, et al.. Microvascular disease and its role in the brain and cardiovascular system: a potential role for uric acid as a cardiorenal toxin. Nephrol Dial Transplant. 2011; 26: 430–437. [DOI] [PubMed] [Google Scholar]
- 23. Rim TH, Choi YS, Kim SS, et al.. Retinal vessel structure measurement using spectral-domain optical coherence tomography. Eye. 2016; 30: 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu TP, Tong YH, Zhan HJ, Ma J. Update on retinal vessel structure measurement with spectral-domain optical coherence tomography. Microvasc Res. 2014; 95: 7–14. [DOI] [PubMed] [Google Scholar]
- 25. Rizzoni D, Porteri E, Duse S, et al.. Relationship between media-to-lumen ratio of subcutaneous small arteries and wall-to-lumen ratio of retinal arterioles evaluated noninvasively by scanning laser Doppler flowmetry. J Hypertens. 2012; 30: 1169–1175. [DOI] [PubMed] [Google Scholar]
- 26. Arichika S, Uji A, Ooto S, Muraoka Y, Yoshimura N. Comparison of retinal vessel measurements using adaptive optics scanning laser ophthalmoscopy and optical coherence tomography. Jpn J Ophthalmol. 2016; 60: 166–171. [DOI] [PubMed] [Google Scholar]
- 27. Streese L, Khan AW, Deiseroth A, et al.. Physical activity may drive healthy microvascular ageing via downregulation of p66(Shc). Eur J Prev Cardiol. 2019; 27: 168–176:2047487319880367. [DOI] [PubMed] [Google Scholar]
- 28. Streese L, Khan AW, Deiseroth A, et al.. High-intensity interval training modulates retinal microvascular phenotype and DNA methylation of p66Shc gene: a randomized controlled trial (EXAMIN AGE). Eur Heart J. 2019; 40: 3948–3949. [DOI] [PubMed] [Google Scholar]
- 29. Streese L, Kotliar K, Deiseroth A, et al.. Retinal endothelial function in cardiovascular risk patients: a randomized controlled exercise trial. Scand J Med Sci Sports. 2019 Oct 28. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30. Bonsignore A, Bredin SS, Wollmann H, et al.. The influence of race length on arterial compliance following an ultra-endurance marathon. Eur J Sport Sci. 2017; 17: 441–446. [DOI] [PubMed] [Google Scholar]