Abstract
Background: There are limited data on the epidemiology and timing of in-hospital death (IHD) in patients with acute myocardial infarction-cardiogenic shock (AMI-CS). Methods: Adult admissions with AMI-CS with IHDs were identified using the National Inpatient Sample (2000–2016) and were classified as early (≤2 days), mid-term (3–7 days), and late (>7 days). Inter-hospital transfers and those with do-not-resuscitate statuses were excluded. The outcomes of interest included the epidemiology, temporal trends and predictors for IHD timing. Results: IHD was noted in 113,349 AMI-CS admissions (median time to IHD 3 (interquartile range 1–7) days), with early, mid-term and late IHD in 44%, 32% and 24%, respectively. Compared to the mid-term and late groups, the early IHD group had higher rates of ST-segment-elevation AMI-CS (74%, 63%, 60%) and cardiac arrest (37%, 33%, 29%), but lower rates of acute organ failure (68%, 79%, 89%), use of coronary angiography (45%, 56%, 67%), percutaneous coronary intervention (33%, 36%, 42%), and mechanical circulatory support (31%, 39%, 50%) (all p < 0.001). There was a temporal increase in the early (adjusted odds ratio (aOR) for 2016 vs. 2000 2.50 (95% confidence interval (CI) 2.22–2.78)) and a decrease in mid-term (aOR 0.75 (95% CI 0.71–0.79)) and late (aOR 0.34 (95% CI 0.31–0.37)) IHD. ST-segment-elevation AMI-CS and cardiac arrest were associated with the increased risk of early IHD, whereas advanced comorbidity and acute organ failure were associated with late IHD. Conclusions: Early IHD after AMI-CS has increased between 2000 and 2016. The populations with early vs. late IHD were systematically different.
Keywords: in-hospital death, cardiogenic shock, acute myocardial infarction, cardiac intensive care unit, critical care cardiology, outcomes research
1. Introduction
Acute myocardial infarction (AMI) continues to be the leading cause of cardiogenic shock (CS) and is associated with poor outcomes [1,2,3,4]. With an increasing emphasis on early recognition, timely percutaneous coronary intervention (PCI) and protocoled AMI-CS care, there have been rapid improvements in the clinical outcomes of this population [5,6,7,8,9]. Several recent studies have detailed the changing demographics, comorbidities and clinical course for patients presenting with AMI-CS [1,2]. It is conceivable that in the last two decades, with early PCI and the use of newer mechanical circulatory support (MCS) devices, the landscape of AMI-CS may have evolved [1,2,5]. Though prior studies have highlighted short-term and long-term mortality in AMI-CS, there are very little published data on the timing of in-hospital death (IHD) in the AMI-CS population [10]. It is also conceivable that the populations with early IHD in AMI-CS may be systematically different from those with late mortality [11]. Therefore, this study sought to investigate the epidemiology of the timing of IHD in patients hospitalized with AMI-CS in the United States. We hypothesized that there would be a decrease in the risk of IHD within two days of admission. We also sought to systematically evaluate the differences in demographics, clinical course and management strategies of patients with IHD early, as compared to later, in hospitalization.
2. Material and Methods
The National (Nationwide) Inpatient Sample (NIS) is the largest all-payer database of hospital inpatient stays in the United States. NIS contains discharge data from a 20% stratified sample of community hospitals and is a part of the Healthcare Cost and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality. Using the HCUP-NIS data from 2000–2016, a retrospective cohort study of admissions with AMI in the primary diagnosis field (International Classification of Diseases 9.0 Clinical Modification (ICD-9CM) 410.x and ICD-10CM I21.x-22.x) and a secondary diagnosis of CS (ICD-9CM 785.51, ICD-10CM R57.0) with IHD were included. Admissions without IHD and length of stay data, those with a do-not-resuscitate status (ICD-9CM V49.86; ICD-10CM Z66.0) and inter-hospital transfers were excluded. Deyo’s modification of the Charlson Comorbidity Index was used to identify the burden of co-morbid diseases (Supplementary Table S1). Demographics, hospital characteristics, acute organ failure, coronary angiography, PCI, MCS and non-cardiac organ support use were identified for all admissions [1,3,4,6,8,12,13,14,15,16,17,18].
The outcomes of interest were the temporal trends of IHD stratified into early (≤2 days), mid-term (3–7 days) and late (>7 days) from 2000–2016. Secondary outcomes included temporal trends stratified by patient and hospital characteristics, predictors of early and late IHD and temporal trends stratified by type of AMI (ST-segment myocardial infarction (STEMI) or non-ST-segment myocardial infarction (NSTEMI)), presence of cardiac arrest and performance of coronary angiography and PCI.
3. Statistical Analysis
As recommended by HCUP-NIS, survey procedures using discharge weights provided with the HCUP-NIS database were used to generate national estimates. Using the trend weights provided by the HCUP-NIS, samples from 2000–2011 were re-weighted to adjust for the 2012 HCUP-NIS re-design [19]. One-way analysis of variance and t-tests were used to compare categorical and continuous variables, respectively. Logistic regression was used to compare the risk of IHD in each year of the study using the year 2000 as the referent; results are reported as odds ratios (OR) with 95% confidence intervals (CI). A multivariable logistic regression analysis incorporating age, sex, race, primary payer status, socio-economic stratum, hospital characteristics, comorbidities, acute organ failure, AMI-type, cardiac procedures and non-cardiac procedures was performed for temporal trends analyses. For the multivariable modeling, regression analysis with a purposeful selection of statistically (liberal threshold of p < 0.20 in univariate analysis) and clinically relevant variables was conducted. Two-tailed p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS v25.0 (IBM Corp., Armonk, NY, USA).
4. Results
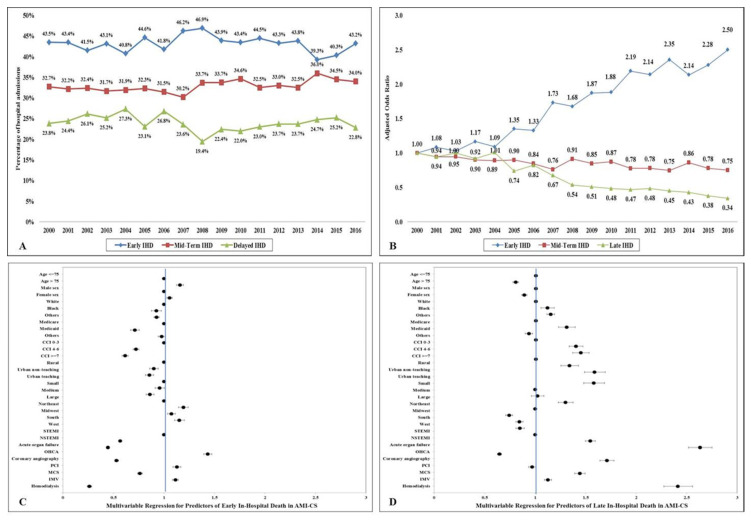
In the period between 1 January 2000 and 31 December 2016, there were 513,288 AMI-CS admissions, of which IHD was noted in 156,366 (38.8%) (Supplementary Figure S1). Of those, 113,349 admissions with a mean length of stay of 5.9 ± 8.9 days (median 3 (interquartile range 1–7) days) met our inclusion criteria and were included in the analysis (Supplementary Figure S1). Early (≤2 days), mid-term (3–7 days) and late (>7 days) IHD were noted in 50,235 (44.3%), 36,227 (32.0%) and 26,886 (23.7%) admissions. The group with early IHD was on average older, more often female, of white race and admitted to small and rural hospitals (Table 1). The 17-year trends showed a temporal increase in early (adjusted OR for 2016 vs. 2000 2.50 (95% CI 2.22–2.78)) and a decrease in mid-term (adjusted OR 0.75 (95% CI 0.71–0.79)) and late (adjusted OR 0.34 (95% CI 0.31–0.37)) IHD (Figure 1A,B).
Table 1.
Baseline characteristics of AMI-CS stratified by the timing of IHD.
| Characteristic | Early IHD (n = 50,235) |
Mid-Term IHD (n = 36,227) |
Late IHD (n = 26,886) |
p | |
|---|---|---|---|---|---|
| Age (years) | 74.2 ± 12.5 | 73.5 ± 12.3 | 71.8 ± 11.8 | <0.001 | |
| Female sex | 45.4 | 43.5 | 39.1 | <0.001 | |
| Race | White | 63.9 | 63.6 | 60.9 | <0.001 |
| Black | 6.1 | 6.5 | 7.2 | ||
| Others a | 30.1 | 29.9 | 31.9 | ||
| Primary payer | Medicare | 73.7 | 72.6 | 70.9 | <0.001 |
| Medicaid | 4.3 | 5.3 | 7.0 | ||
| Private | 15.0 | 15.6 | 16.6 | ||
| Others b | 6.9 | 6.5 | 5.5 | ||
| Quartile of median household income for zip code | 0–25th | 22.1 | 22.2 | 22.1 | <0.001 |
| 26th–50th | 26.3 | 26.7 | 23.9 | ||
| 51st–75th | 25.6 | 24.2 | 25.4 | ||
| 75th–100th | 26.0 | 26.9 | 28.5 | ||
| Hospital teaching status and location | Rural | 10.9 | 8.6 | 4.9 | <0.001 |
| Urban non-teaching | 44.8 | 44.5 | 40.9 | ||
| Urban teaching | 44.3 | 47.0 | 54.2 | ||
| Hospital bed size | Small | 9.7 | 8.5 | 6.9 | <0.001 |
| Medium | 25.8 | 24.7 | 21.1 | ||
| Large | 64.5 | 66.9 | 72.0 | ||
| Hospital region | Northeast | 18.5 | 18.0 | 19.5 | <0.001 |
| Midwest | 22.4 | 21.8 | 20.0 | ||
| South | 38.3 | 40.1 | 39.0 | ||
| West | 20.7 | 20.1 | 21.5 | ||
| Charlson Comorbidity Index | 0–3 | 17.0 | 14.4 | 13.8 | <0.001 |
| 4–6 | 59.7 | 58.1 | 60.6 | ||
| ≥7 | 23.4 | 27.5 | 25.5 | ||
Legend: Represented as percentage or mean ± standard deviation; a Hispanic, Asian, Native American, others; b uninsured, no charge, others. Abbreviations: AMI: acute myocardial infarction; CS: cardiogenic shock; IHD: in-hospital death.
Figure 1.
Epidemiology and predictors of in-hospital death in AMI-CS. (A) 17-year unadjusted temporal trends of early, mid-term and late IHD in AMI-CS; all p < 0.001 for trend over time; (B): 17-year adjusted * temporal trends for early, mid-term and late IHD in AMI-CS (referent year 2000); all p < 0.001 for trend over time; (C,D) the adjusted odds ratios for early (C) and late (D) IHD in AMI-CS **. * Adjusted for age, sex, race, primary payer status, socio-economic stratum, hospital characteristics, comorbidities, acute organ failure, AMI-type, cardiac procedures, and non-cardiac procedures. ** For cohorts with multiple categories (i.e., age, sex, race, primary payer, Charlson Comorbidity Index, hospital location and teaching status, hospital bed size, hospital region and AMI type), the first category was used as reference category for calculating odds ratios. Abbreviations: AMI: acute myocardial infarction; CCI: Charlson Comorbidity Index; CS: cardiogenic shock; IHD: in-hospital death; IMV: invasive mechanical ventilation; MCS: mechanical circulatory support; NSTEMI: non-ST-segment elevation myocardial infarction; OHCA: out-of-hospital cardiac arrest; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction.
The cohort with early IHD had higher rates of STEMI-CS and out-of- and in-hospital cardiac arrests, but lower rates of acute organ failure and non-cardiac organ support (Table 2). There was a temporal increase in atrial and ventricular arrhythmias with an increase in timing of IHD. Compared to the admissions with mid-term and late IHD, the cohort with early IHD had a lower use of coronary angiography, PCI, invasive hemodynamic monitoring and MCS (all p < 0.001) (Table 2). There was a serial increase in the use of palliative care consultation and in-hospital complications with increasing hospital length of stay.
Table 2.
Clinical characteristics of AMI-CS stratified by the timing of IHD.
| Characteristic | Early IHD (n = 50,235) |
Mid-Term IHD (n = 36,227) |
Late IHD (n = 26,886) |
p | |
|---|---|---|---|---|---|
| AMI type | STEMI-CS | 73.7 | 63.1 | 60.1 | <0.001 |
| NSTEMI-CS | 26.3 | 36.9 | 39.9 | ||
| Acute organ failure | Respiratory | 48.0 | 55.6 | 65.2 | <0.001 |
| Renal | 36.2 | 51.2 | 60.0 | <0.001 | |
| Hepatic | 8.4 | 13.1 | 14.3 | <0.001 | |
| Hematologic | 6.4 | 12.2 | 20.3 | <0.001 | |
| Neurologic | 18.3 | 21.1 | 21.7 | <0.001 | |
| Cardiac arrhythmias | VT | 15.2 | 18.2 | 22.3 | <0.001 |
| VF | 15.7 | 15.4 | 13.8 | <0.001 | |
| AFib | 20.3 | 27.4 | 32.9 | <0.001 | |
| AFlut | 1.3 | 2.7 | 6.0 | <0.001 | |
| SVT | 0.7 | 1.1 | 1.6 | <0.001 | |
| Out-of-hospital cardiac arrest | 36.9 | 32.5 | 28.8 | <0.001 | |
| In-hospital cardiac arrest | 22.1 | 15.9 | 12.5 | <0.001 | |
| Coronary angiography | 45.1 | 56.2 | 66.9 | <0.001 | |
| Percutaneous coronary intervention | 32.7 | 36.3 | 42.0 | <0.001 | |
| Coronary artery bypass grafting | 3.6 | 9.3 | 20.3 | <0.001 | |
| Invasive hemodynamic monitoring a | 13.5 | 20.0 | 27.0 | <0.001 | |
| Mechanical circulatory support | Total | 31.1 | 38.8 | 50.4 | <0.001 |
| IABP | 29.2 | 37.0 | 48.3 | <0.001 | |
| pLVAD | 2.2 | 2.0 | 2.2 | 0.06 | |
| ECMO | 0.4 | 0.7 | 1.3 | <0.001 | |
| Invasive mechanical ventilation | 53.8 | 53.0 | 61.6 | <0.001 | |
| Non-invasive ventilation | 2.2 | 3.4 | 4.1 | <0.001 | |
| Hemodialysis | 1.4 | 5.2 | 9.7 | <0.001 | |
| Palliative care consultation | 6.2 | 8.8 | 8.9 | <0.001 | |
| Complications | Vascular | 0.7 | 1.2 | 2.0 | <0.001 |
| Lower limb | 0.0 | 0.1 | 0.6 | <0.001 | |
| VSD | 1.3 | 1.6 | 1.9 | <0.001 | |
| Papillary muscle rupture | 0.5 | 0.3 | 0.5 | <0.001 | |
| Hemopericardium | 0.2 | 0.2 | 0.5 | <0.001 | |
| Cardiac tamponade | 0.5 | 0.6 | 0.7 | 0.001 | |
| Coronary dissection | 0.6 | 0.6 | 0.6 | 0.79 | |
| Ischemic CVA | 1.4 | 2.6 | 6.2 | <0.001 | |
| Hemorrhagic CVA | 0.6 | 0.8 | 1.1 | <0.001 | |
Legend: Represented as percentage; a pulmonary artery/right heart catheterization. Abbreviations: AFib: atrial fibrillation; AFlut: atrial flutter; AMI: acute myocardial infarction; CS: cardiogenic shock; CVA: cerebrovascular accident; ECMO: extracorporeal membrane oxygenation; IABP: intra-aortic balloon pump; IHD: in-hospital death; NSTEMI: non-ST-segment elevation myocardial infarction; pLVAD: percutaneous left ventricular assist device; STEMI: ST-segment elevation myocardial infarction; SVT: supraventricular tachycardia; VF: ventricular fibrillation; VSD: ventricular septal defect; VT: ventricular tachycardia.
Admissions with STEMI-CS, concomitant out-of-hospital cardiac arrest and admissions without use of angiography and PCI had consistently higher rates of early IHD during this 17-year study period (Table 1). In a multivariable analysis, older age, white race, admission to a rural hospital, STEMI-CS presentation and out-of-hospital cardiac arrest were independent predictors of early IHD, whereas non-white race, advanced comorbidity, NSTEMI-CS presentation, acute organ failure and non-cardiac organ support were independent predictors of late IHD (Figure 1C,D).
5. Discussion
In the first nationally-representative study evaluating the timing of IHD in AMI-CS, early (≤2 days) IHD was noted in nearly half of all AMI-CS admissions, with an increase in prevalence during the 17-year study period. The population with early IHD was systematically different from the other two cohorts—they had lower comorbidity, received less frequent cardiac procedures, had higher rates of STEMI-CS, had higher rates of cardiac arrest, had lower rates of acute non-cardiac organ failure and non-cardiac organ support systems and had lower rates of complications.
The timing of in-hospital events, including IHD remains underexplored in critical illness [1,8,15]. In a nationally-representative population of STEMI admissions that received primary PCI, we previously demonstrated that ventricular arrhythmias were predominant in early in-hospital cardiac arrest, whereas non-shockable rhythms and multi-organ involvement were prevalent in the delayed group [13]. Similarly, Law et al. demonstrated a decline in all time periods of IHD in septic shock patients; however, when adjusted for acute respiratory failure, only the delayed IHD cohort had a temporal decrease [11]. The timing of in-hospital events remains extremely crucial in critical illness since many clinical interventions, such as fluid resuscitation, PCI, MCS, targeted temperature management, vasoactive medications and intensive care monitoring are geared towards the first 24–48 h of critical illness [11,13,16,20]. Therefore, studies evaluating the timing of events aid in prognostication, resource planning and advanced care planning [11,13,16,20]. In AMI-CS, most mortality analyses and predictive models have used IHD, 28-day or 30-day mortality as the end-points, with little additional granularity on the timing of IHD during the index hospitalization [10]. Previous work from our group has demonstrated that AMI-CS patients frequently transition to invasive mechanical ventilation in the first two days, as well as a temporal decrease in the use of prolonged mechanical ventilation and tracheostomy use in the United States [15,20]. Taken in aggregate, these data might suggest that there have been improvements in care delivery for this acutely ill population, decreasing the burden of chronic critical illness [11]. It is possible that patients in the early IHD group may have died before any intervention could be performed, which could potentially explain the lower use of coronary angiography, PCI and MCS in this cohort.
This study has several limitations, some of which are inherent to the analysis of a large administrative database. There is limited information on procedural details, intracoronary anatomy, echocardiographic features (including left ventricular ejection fraction and cardiac index), hemodynamic indices and physiological variables, limiting further patient-specific and disease-specific risk assessment. We are unable to comment on the severity of illness using standardized risk scores and are unable to ascertain the location of admissions within the hospital, which significantly impact these results. Despite these limitations, this study addressed an important knowledge gap, emphasizing the timing of IHD in AMI-CS admissions over the last 17 years.
In conclusion, this nationally-representative 17-year study of AMI-CS admissions noted a temporal increase in early IHD (≤2 days). The populations with early vs. late IHD were systematically different from each other, which suggests that these two populations constitute different phenotypes. Further studies are needed to understand potentially modifiable in-hospital factors that determine the course of AMI-CS with targeted interventions to improve survival in this critically ill population.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/7/2094/s1, Figure S1: Study cohort, Table S1: Administrative codes used for identification of diagnoses and procedures.
Author Contributions
Study design, literature review, data analysis, statistical analysis: S.V., P.E.M., W.C., P.R.S., Data management, data analysis, drafting manuscript: S.V., G.W.B., Access to data: S.V., S.M.D., M.R.B., P.E.M., W.C., P.R.S., K.K., B.J.G., A.S.J., D.R.H., G.W.B., Manuscript revision, intellectual revisions, mentorship: S.M.D., M.R.B., K.K., B.J.G., A.S.J., D.R.H., G.W.B., Final approval: S.V., S.M.D., M.R.B., P.E.M., W.C., P.R.S., K.K., B.J.G., A.S.J., D.R.H., G.W.B. All authors have read and agreed to the published version of the manuscript.
Funding
Saraschandra Vallabhajosyula is supported by the Clinical and Translational Science Award (CTSA) Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Conflicts of Interest
A.S.J. has been a consultant for Beckman, Abbott, Siemens, Roche, ET Healthcare, Sphingotoec, Quidel, Brava, Blade and Novartis. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Vallabhajosyula S., Dunlay S.M., Prasad A., Kashani K., Sakhuja A., Gersh B.J., Jaffe A.S., Holmes D.R., Jr., Barsness G.W. Acute noncardiac organ failure in acute myocardial infarction with cardiogenic shock. J. Am. Coll. Cardiol. 2019;73:1781–1791. doi: 10.1016/j.jacc.2019.01.053. [DOI] [PubMed] [Google Scholar]
- 2.Berg D.D., Bohula E.A., van Diepen S., Katz J.N., Alviar C.L., Baird-Zars V.M., Barnett C.F., Barsness G.W., Burke J.A., Cremer P.C., et al. Epidemiology of shock in contemporary cardiac intensive care units. Circ. Cardiovasc. Qual. Outcomes. 2019;12:e005618. doi: 10.1161/CIRCOUTCOMES.119.005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vallabhajosyula S., Zack C.J., Jentzer J.C. Cardiac arrest definition using administrative coding and outcomes in acute myocardial infarction. Mayo Clin. Proc. 2020;95:611–613. doi: 10.1016/j.mayocp.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Vallabhajosyula S., Vallabhajosyula S., Burstein B., Ternus B.W., Sundaragiri P.R., White R.D., Barsness G.W., Jentzer J.C. Epidemiology of in-hospital cardiac arrest complicating non-ST-segment elevation myocardial infarction receiving early coronary angiography. Am. Heart J. 2020;223:59–64. doi: 10.1016/j.ahj.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Tehrani B.N., Truesdell A.G., Sherwood M.W., Desai S., Tran H.A., Epps K.C., Singh R., Psotka M., Shah P., Cooper L.B., et al. Standardized team-based care for cardiogenic shock. J. Am. Coll. Cardiol. 2019;73:1659–1669. doi: 10.1016/j.jacc.2018.12.084. [DOI] [PubMed] [Google Scholar]
- 6.Vallabhajosyula S., Prasad A., Sandhu G.S., Bell M.R., Gulati R., Eleid M.F., Best P.J.M., Gersh B.J., Singh M., Lerman A., et al. Mechanical circulatory support-assisted early percutaneous coronary intervention in acute myocardial infarction with cardiogenic shock: 10-year national temporal trends, predictors and outcomes. EuroIntervention. 2019 doi: 10.4244/EIJ-D-19-00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vallabhajosyula S., Barsness G.W., Vallabhajosyula S. Multidisciplinary teams for cardiogenic shock. Aging (Albany NY) 2019;11:4774–4776. doi: 10.18632/aging.102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallabhajosyula S., Dunlay S.M., Barsness G.W., Rihal C.S., Holmes D.R., Jr., Prasad A. Hospital-level disparities in the outcomes of acute myocardial infarction with cardiogenic shock. Am. J. Cardiol. 2019;124:491–498. doi: 10.1016/j.amjcard.2019.05.038. [DOI] [PubMed] [Google Scholar]
- 9.Vallabhajosyula S., O′Horo J.C., Antharam P., Ananthaneni S., Vallabhajosyula S., Stulak J.M., Eleid M.F., Dunlay S.M., Gersh B.J., Rihal C.S., et al. Concomitant intra-aortic balloon pump use in cardiogenic shock requiring veno-arterial extracorporeal membrane oxygenation. Circ. Cardiovasc. Interv. 2018;11:e006930. doi: 10.1161/CIRCINTERVENTIONS.118.006930. [DOI] [PubMed] [Google Scholar]
- 10.Pöss J., Köster J., Fuernau G., Eitel I., de Waha S., Ouarrak T., Lassus J., Harjola V.P., Zeymer U., Thiele H., et al. Risk stratification for patients in cardiogenic shock after acute myocardial infarction. J. Am. Coll. Cardiol. 2017;69:1913–1920. doi: 10.1016/j.jacc.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 11.Law A.C., Stevens J.P., Walkey A.J. National trends in timing of death among patients with septic shock, 1994–2014. Crit. Care Med. 2019;47:1493–1496. doi: 10.1097/CCM.0000000000003956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallabhajosyula S., Prasad A., Bell M.R., Sandhu G.S., Eleid M.F., Dunlay S.M., Schears G.J., Stulak J.M., Singh M., Gersh B.J., et al. Extracorporeal membrane oxygenation use in acute myocardial infarction in the United States, 2000 to 2014. Circ. Heart Fail. 2019;12:e005929. doi: 10.1161/CIRCHEARTFAILURE.119.005929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallabhajosyula S., Vallabhajosyula S., Bell M.R., Prasad A., Singh M., White R.D., Jaffe A.S., Holmes D.R., Jr., Jentzer J.C. Early vs. delayed in-hospital cardiac arrest complicating ST-elevation myocardial infarction receiving primary percutaneous coronary intervention. Resuscitation. 2020;148:242–250. doi: 10.1016/j.resuscitation.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Vallabhajosyula S., Dunlay S.M., Barsness G.W., Vallabhajosyula S., Vallabhajosyula S., Sundaragiri P.R., Gersh B.J. Temporal trends, predictors, and outcomes of acute kidney injury and hemodialysis use in acute myocardial infarction-related cardiogenic shock. PLoS ONE. 2019;14:e0222894. doi: 10.1371/journal.pone.0222894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallabhajosyula S., Kashani K., Dunlay S.M., Vallabhajosyula S., Vallabhajosyula S., Sundaragiri P.R., Gersh B.J., Jaffe A.S., Barness G.W. Acute respiratory failure and mechanical ventilation in cardiogenic shock complicating acute myocardial infarction in the USA, 2000–2014. Ann. Intensive Care. 2019;9:96. doi: 10.1186/s13613-019-0571-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallabhajosyula S., Prasad A., Dunlay S.M., Murphree D.H., Jr., Ingram C., Mueller P.S., Gersh B.J., Barsness W.G. Utilization of palliative care for cardiogenic shock complicating acute myocardial infarction: A 15-year national perspective on trends, disparities, predictors, and outcomes. J. Am. Heart Assoc. 2019;8:e011954. doi: 10.1161/JAHA.119.011954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallabhajosyula S., Arora S., Sakhuja A., Lahewala S., Kumar V., Shantha G.P.S., Egbe A.C., Stulak J.M., Gersh B.J., Gulati R., et al. Trends, predictors, and outcomes of temporary mechanical circulatory support for postcardiac surgery cardiogenic shock. Am. J. Cardiol. 2019;123:489–497. doi: 10.1016/j.amjcard.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 18.Vallabhajosyula S., Deshmukh A.J., Kashani K., Prasad A., Sakhuja A. Tako-tsubo cardiomyopathy in severe sepsis: Nationwide trends, predictors, and outcomes. J. Am. Heart Assoc. 2018;7:e009160. doi: 10.1161/JAHA.118.009160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khera R., Krumholz H.M. With great power comes great responsibility: Big data research from the National Inpatient Sample. Circ. Cardiovasc. Qual. Outcomes. 2017;10:e003846. doi: 10.1161/CIRCOUTCOMES.117.003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallabhajosyula S., Dunlay S.M., Kashani K., Vallabhajosyula S., Vallabhajosyula S., Sundaragiri P.R., Jaffe A.S., Barness G.W. Temporal trends and outcomes of prolonged invasive mechanical ventilation and tracheostomy use in acute myocardial infarction with cardiogenic shock in the United States. Int. J. Cardiol. 2019;285:6–10. doi: 10.1016/j.ijcard.2019.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.