Abstract
Neuroendocrine tumors (NETs) belong to a heterogeneous group of neoplasms arising from hormone secreting cells. These tumors are often associated with a dysfunction of their secretory activity. Neuroendocrine secretion occurs through calcium-regulated exocytosis, a process that is tightly controlled by Rho GTPases family members. In this review, we compiled the numerous mutations and modification of expression levels of Rho GTPases or their regulators (Rho guanine nucleotide-exchange factors and Rho GTPase-activating proteins) that have been identified in NETs. We discussed how they might regulate neuroendocrine secretion.
Keywords: neuroendocrine tumors, Rho GTPases, hormone secretion, vesicular trafficking, mutations, expression changes
1. Introduction
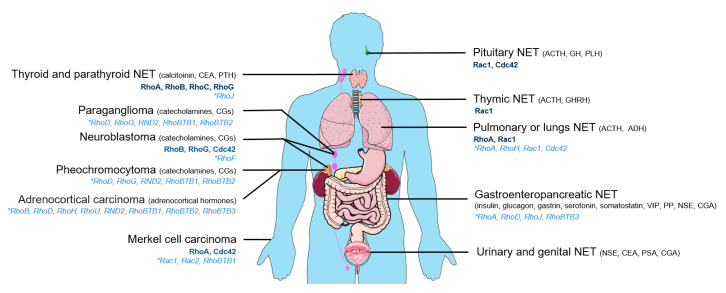
Neuroendocrine tumors (NETs) constitute a group of neoplasms that arise from cells secreting hormones, amines, or peptides. This family of tumors is highly heterogeneous in terms of morphology and function mainly because neuroendocrine cells are spread all over the body (Figure 1). The diffuse neuroendocrine system includes the neuroendocrine cells dispersed in various organs such as the thyroid (C cells), gastrointestinal tract, gallbladder, pancreas (islet cells), the respiratory tract, lungs, thymus, kidneys, liver, prostate, skin, cervix, ovaries, and the testicles. Few types of neuroendocrine cells actually constitute full organs such as the pituitary, paraganglia, parathyroid, and adrenal gland. From a cell biology perspective, the main common features of all these specialized cells are their ability to synthetize, stock in vesicles, and secrete, through calcium-regulated exocytosis, hormones, peptides, or amines. NETs are often associated with a deregulation of hormones secretion mainly leading to hypersecretion [1,2]. NETs with initial low secretory activity can evolve to high secreting lesions having a negative impact on prognosis simply by progressively becoming hormonally active [3,4,5,6,7,8]. Hence, cellular secretory activity appears to be a key controller of tumor behavior. However, how secretion becomes uncontrolled in NETs remains poorly understood.
Figure 1.
Main types of neuroendocrine tumors (NETs). Mutated Rho GTPases (light blue) or Rho GTPases for which expression level varies (dark blue) are indicated for each NET along with main secreted hormones. Hormone abbreviations: ACTH: adrenocorticotropic hormone, ADH: antidiuretic hormone, CEA: carcinoembryonic antigen, CGA: chromogranin A, CGs: chromogranins, GH: growth hormone, GHRH: growth hormone releasing-hormone, NSE: neuron specific enolase, PLH: prolactin luteinizing hormone, PP: pancreatic polypeptide, PSA: prostate specific antigen, PTH: parathyroid hormone, VIP: vasoactive intestinal peptide.
Belonging to the Ras GTPase superfamily, the monomeric Rho (Ras homologous) GTPase family contains 20 highly conserved members divided into eight subfamilies (Rho, Rac, Cdc42, RhoD/F, Rnd, RhoU/V, RhoH, and RhoBTB) classified into two major groups. These include the canonical (Rho, Rac, Cdc42, and RhoD/F) and the atypical members (Rnd, RhoU/V, RhoH, and RhoBTB) [9,10]. In the last 10 years, many comprehensive reviews described Rho GTPases signaling as affecting a large array of cancer biology aspects through the control of important cellular processes including polarity, cell cycle progression, cytoskeleton organization, motility, and intracellular membrane trafficking [11,12,13,14,15,16,17,18,19]. Dysfunction of these crucial processes through aberrant Rho GTPases signaling can favor distinct steps of cancer progression from tumor initiation to tumor cell proliferation, invasion, and metastasis. Although such altered Rho GTPase signaling is linked to many types of cancer, to what extent pathways controlled by Rho GTPases are involved in NETs is still an open question. Seminal works from our team and others demonstrated that monomeric G proteins, including members of the Rho GTPases family, tightly control neuroendocrine secretion. However, the link between Rho GTPases and the NETs-associated deregulation of secretion remains largely unexplored. Here, we review the literature supporting the implication of Rho GTPases in NETs and discuss the possible links between Rho GTPases signaling and the regulation of neuroendocrine secretion.
2. Neuroendocrine Tumors and Rho GTPases
The first idea that usually comes to mind regarding the origin of tumors is genetic mutations. In contrast to other members of the Ras superfamily, Rho sub-family members were initially thought to be rarely mutated in cancer [20]. However, progress in advanced sequencing and better access to human samples allowed, in the last decade, the uncovering of many mutations in Rho GTPases (for review, see [12,17,20,21,22,23,24,25]). By searching the literature, we inventoried about 30 mutations or polymorphisms directly affecting Rho GTPases in NETs essentially in pheochromocytoma, paraganglioma, adrenocortical adenoma, small cell lung cancer, and Merkel cell carcinoma (Table 1, Figure 1, and Table S1). Surprisingly, besides the mutants Y42C-RhoA and P29S-Rac1, the impact of the other mutations on Rho activity and function remains unknown. P29S-Rac1 is a fast cycling mutant with spontaneous activation and therefore acts as a gain-of-function mutation [26,27]. The Y42C mutation reduces both intrinsic- and GAP-stimulated GTP hydrolysis of RhoA, thereby enhancing the active GTP-bound form [28]. On the contrary, Wang et al. proposed that the Y42C mutation decreased the level of the activated GTP-associated form of RhoA [29].
Table 1.
Mutations and polymorphisms of Rho GTPases in NETs.
| Gene/Tumor | ACC | GCC-AC | MCC (−) | NBL | PLCNEC | PNET | PPGL | PTC | SCLC | SINET | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RHOA | Y42C | E40K | [30,31] | ||||||||
| D49H | |||||||||||
| RHOB | D28H | [32] | |||||||||
| RHOD | R174Q | R110Q | E149K | [32,33,34,35] | |||||||
| A165T | A173P | ||||||||||
| RHOF | K112 (fs) | [32] | |||||||||
| RHOG | P139L | [32,34] | |||||||||
| RHOH | A76E | P110A | S90 * | [30,32,36] | |||||||
| RHOJ | D8N | V25V | [32,37] | ||||||||
| RHOU | S249Y | [32] | |||||||||
| RND2 | E168K | R169S | [32] | ||||||||
| R137T | |||||||||||
| RAC1 | P29S | P92P | [36,38] | ||||||||
| RAC2 | R68W | [38] | |||||||||
| CDC42 | K184N | [30] | |||||||||
| RHOBTB1 | R670 * | P366S | A575C (fs) | [32,38,39] | |||||||
| RHOBTB2 | Q12H | D461G | [32,39] | ||||||||
| RHOBTB3 | R607H | S50T | [32,35] |
Note: fs: frameshift, * STOP codon. Tumor abbreviations: ACC: adrenocortical carcinoma, GCC-AC: goblet cell carcinoid—adenocarcinoma, MCC (−) Merkel cell carcinoma-MCPyV negative, NBL: neuroblastoma, PLCNEC: pulmonary large-cell neuroendocrine carcinoma, PNET: pancreatic neuroendocrine tumor, PPGL: pheochromocytoma and paraganglioma, PTC: parathyroid carcinoma, SCLC: small cell lung cancer, SINET: small intestine neuroendocrine tumor.
Beside mutations, variation in the expression levels of Rho GTPases has been described in many different types of tumors and at various stages of tumorigenesis (for previous key review articles, see [12,13,15,20,40,41,42,43]). However, only a few studies were performed in NETs, mainly in pituitary adenoma and neuroblastoma, as well as in tumors from the thyroid, parathyroid, and small cell lung. For instance, in pituitary adenoma, Rac1 overexpression and Cdc42 down-regulation may affect pathways controlling tumorigenesis such as mTOR- and Wnt-signalling pathways [44]. Arising from primitive cells of the sympathetic nervous systems, neuroblastoma is a common childhood extracranial solid tumor with neuroendocrine properties [45]. Although a large amount of molecular data were obtained from neuroblastoma, the situation appears complex as this tumor displays heterogeneous clinical behavior depending on multiple factors including tumor stage, patient age, and MYCN oncogene amplification. When these stratification parameters were used in these common childhood malignant tumors, different studies revealed modifications in the expression of proteins involved in Rho GTPases pathways (Cdc42, RhoG, RhoB, etc.) [46,47,48]. Most of these studies reported that deregulation of Rho GTPases pathways contributes to disease progression. Conversely, the most aggressive neuroblastoma presenting MYCN amplification also displayed down-regulation of Cdc42 expression through the control of N-myc, indicating that Rho GTPases overexpression is not always correlated with poor prognosis. Regarding thyroid or parathyroid tumors, elevated RhoA activity was correlated to the loss of proto-oncogene N-Ras and malignancy progression using the Rb1-deficient mice model of medullary thyroid (C-cell) adenomas [49]. A comprehensive proteomic study revealed differences in the expression levels of various Rho GTPases (mainly RhoA, B, C, and G) between medullary, anaplastic, and epithelium-derived differentiated thyroid cancers (for details see Supplementary Data in [50]). NETs represent around 25% of lung neoplasms with small cell lung cancer (SCLC), the most common and aggressive cancer [51]. In high-grade SCLC, RhoA is highly expressed [52,53], whereas Rac1 seems to be more abundantly expressed in low-grade pulmonary carcinoid tumors [54]. Finally, few studies reported the involvement of Rho GTPases in cervical, thymic, or skin (Merkel cells) tumors, most likely due to their low frequency. To the best of our knowledge, only one study performed on thymic carcinoid tissue reported Rac1 overexpression [55]. As Merkel cell carcinoma can be the consequence of oncogenic polyomavirus infection, the implication of RhoA and Cdc42 in the pathway by which virus small T antigen controls Merkel cells motility was proposed [56].
Overall, only few studies reported significant expression level modifications for Rho GTPases family members in NETs. It is, however, important to remember that Rho GTPases expression levels are not necessarily correlated with their activation levels. This balance has been largely overlooked.
3. Control of Rho Activity in NETs: Important Role of Rho GEFs and GAPs
The activity of most Rho GTPases is under the control of their regulators. Modulating the expression of guanine nucleotide-exchange factors (GEFs), which stimulate the exchange of GDP for GTP, as well as that of GTPases-activating proteins (GAPs) that catalyze GTP hydrolysis, are expected to alter Rho GTPases activity. For example, in pheochromocytoma, a NET arising from chromaffin cells of the adrenal medulla, we observed that the activity of Rac1 and Cdc42 was inhibited while their relative expression remained unchanged compared to non-tumor tissue [57]. In this study, we further showed that the inhibition of Rac1 and Cdc42 activities in human pheochromocytomas was directly correlated to reduced expression of the GEFs ARHGEF1 and FARP1, respectively [57,58]. In the very aggressive SDHB-pheochromocytoma, microRNAs controlling the Rho GAP ARHGAP18 expression are specifically overexpressed [59]. Expression level changes of Rho GEFs and Rho GAPs were reported in other different NETs from pancreas, lung, thyroid, prostate, and the pituitary gland (Table 2). For example, expression of Frabin (FGD4), a GEF specific for Cdc42, positively correlates with the aggressive phenotype of prostate cancer and the tumor grade of pancreatic neuroendocrine neoplasms, most likely by maintaining abnormal activation of Cdc42 [60,61]. Knock-down of FGD4 in PC-3 and LNCaP-104S prostate cell lines inhibited cell proliferation, cell cycle progression, and cell migration [60]. In pituitary adenoma, ARHGAP18 and ARHGEF17 are both upregulated, suggesting a modulation of the activity of the target Rho GTPases, most likely RhoA [44]. Variation in VAV isoforms (GEFs for Rho and Rac GTPases) expression levels was reported in small cell lung carcinoma [62,63].
Table 2.
Expression level changes of Rho GEFs and Rho GAPs in NETs.
| GEFs/GAPs | Protein/Gene | Expression Variation | Tumors with Expression Modifications | Preferential Targets of the GEFs and GAPs | References |
|---|---|---|---|---|---|
| GEFs | ARHGEF1 | PCC vs. non-tumor | RhoA | [57,58] | |
| ARHGEF10L | NBL MYCN− vs. MYCN+ short survivors (gene) | RhoA, B, C | [48] | ||
| ARHGEF17 | NFPA vs. non-tumor | RhoA | [44] | ||
| FARP1 | PCC vs. non-tumor | Rac1 | [57,58] | ||
| FGD4 | PNET grade 2, 3 vs. 1 | Cdc42 | [61] | ||
| NEPC (gene) | [60] | ||||
| RCC2 | SCLC | Rac1 | [54] | ||
| VAV1 | uSCLC | RhoA, Rac1 | [62] | ||
| SCLC cell lines vs. non-SCLC cell lines | [63] | ||||
| VAV3 | CRPC-NEPC | RhoA, RhoG, Rac1 | [64] | ||
| GAPs | ARHGAP6 | PHPT vs. non-tumor | RhoA | [65] | |
| NEPC (gene) | [66] | ||||
| ARHGAP11A | NEPC (gene) | RhoA | [66] | ||
| ARHGAP11B | NEPC (gene) | RhoA, Cdc42 | [66] | ||
| ARHGAP18 | NFPA vs. non-tumor | RhoA, B, C | [44] | ||
| PCC (miRNA) | [59] |
Up () or down () expression variations concerning proteins except when indicated (gene, miRNA). When available, the control (vs.) is indicated, as well as the main targeted-Rho GTPases. Tumor abbreviations: CRPC-NEPC: castrate-resistant prostate cancer-neuroendocrine prostate cancer, NBL: neuroblastoma, NEPC: neuroendocrine prostate cancer, NFPA: non-functional pituitary adenoma, PCC: pheochromocytoma, PHPT: primary hyperparathyroidism parathyroid adenoma, PNET: pancreatic neuroendocrine tumor, SCLC: small cell lung carcinoma, uSCLC: undifferentiated small cell lung carcinoma.
By searching the literature, we found that Rho GEFs and Rho GAPs seem to be more affected than the Rho GTPases in another aspect. Strikingly, we found a tremendous amount of mutations and polymorphisms for Rho GEFs and GAPs in NETs, which seem to exceed those found for Rho GTPase genes by far (Table S2). However, most of the time, how these mutations and polymorphisms affect Rho GTPases activity, their consequences on Rho GTPases signaling, and their impact on tumorigenesis remain completely unknown and will require further investigations.
4. Rho GTPases and Hormones Secretion
One common aspect of NETs is the perturbation of hormone secretion, a cellular process regulated by Rho GTPases pathways [11,67,68,69,70,71]. Regulation of hormone secretion in neuroendocrine cells has been mainly studied in two in vitro models: the chromaffin cells from the adrenal medulla (primary culture of mice and bovine chromaffin cells or the rat pheochromocytoma cell line PC12) and the pancreatic beta cells (primary culture or the mouse insulinoma cell line MIN6) [72,73,74]. These two models are particularly relevant to further understanding the mechanisms of NET-associated hypersecretion. Human pheochromocytoma is characterized by catecholamine hypersecretion, leading to severe hypertension, cardiopathy, and high risk of stroke. In the pancreatic islet cells adenoma (insulinoma), insulin secretion is dysregulated with a persistent hypersecretion that may lead to severe hypoglycemia with associated-neuroglycopenic symptoms [75,76].
4.1. Control of Secretion through Actin Remodeling
In all kinds of tumors, Rho GTPases dysfunction is often linked to their role on actin cytoskeleton organization. Both in adrenal chromaffin and pancreatic beta cells, Rho GTPases were shown to play a key role in secretion by controlling actin remodeling. We demonstrated that the GTPases RhoA and Cdc42 play negative and positive roles on exocytosis, respectively, by differentially affecting actin organization [69,70,77]. Firstly, upon exocytosis, Cdc42 is activated at the plasma membrane of PC12 cells where RhoA is inhibited [67,78]. Following these early studies, RhoA was proposed to actively maintain the organization of the cortical actin network that controls granule positioning and likely limits their access to the plasma membrane in resting condition [77,79,80]. Consequently, inhibition of RhoA during exocytosis was postulated to be an essential step in promoting depolymerization of the cortical actin fence [78]. Conversely, once activated, Cdc42 recruits the neural Wiskott-Aldrich syndrome protein (N-WASP) at the exocytotic sites of the plasma membrane [78]. Subsequently, our observations allowed us to propose a model in which secretory granules tethering to the exocytotic sites allows the granule bound-actin-related protein-2/3 (Arp2/3) complex to interact with N-WASP and trigger actin nucleation and de novo polymerization of filaments that optimize the efficiency of the exocytotic process [69,78]. Accordingly, Rho GTPases-mediated actin organization tightly regulates insulin secretion in pancreatic cells islets according to a similar dual mechanism controlling actin polymerization: (i) F-actin network organized as a cortical negative barrier that restricts insulin-containing granule accumulation at the plasma membrane hence limiting basal insulin release and (ii) F-actin remodeling leading to a coordinated depolymerization of cortical actin and de novo polymerization or actin fiber assembly leading to positive effects on stimulus-induced insulin granule exocytosis [81]. Glucose-induced activation of Cdc42 was also shown to control insulin secretion in MIN6 pancreatic beta cells through the N-WASP-Arp2/3 or the PAK1-Rac1 signaling pathways, both leading to actin cytoskeleton remodeling [82,83,84].
How actin remodeling at the exocytotic sites controls hormone release is a key question that has attracted considerable attention, but that is not yet fully resolved. In BON cells, a pancreatic neuroendocrine cell line secreting serotonin, Cdc42, was shown to regulate fusion pore expansion through modulation of membrane tension [85]. As actin cytoskeleton is a known regulator of membrane tension, novel actin filaments generated by active Cdc42 may provide forces at the exocytotic sites to tense membrane and enhance fusion pore expansion and granule cargo release. The exact orientation of these novel actin filaments toward plasma and granule membranes has not been clearly established. Recently in bovine chromaffin cells, electron microscopy coupled to tomography revealed that actin bundles connected plasma and granule membranes of docked granules after exocytosis stimulation [78]. Accordingly, links between hormone secretion and coating of secretory granules with actin filaments or actin filaments anchoring secretory granules to the plasma membrane were described in chromaffin and insulinoma cells [86,87]. Usually, actin filaments need motors to provide forces to membranes. Rho GTPases were shown to regulate the activity of various myosins [88,89,90,91]. The involvement of myosin II and VI in endocrine secretion was described in adrenal chromaffin and PC12 cells, as well as in pancreatic BON and beta cells [85,92,93,94,95,96]. Together, these findings show that Rho GTPases may tightly regulate the polymerization status of F-actin in secreting cells, allowing for the close interplay of the negative control played by cortical actin and the positive action on exocytosis by de novo actin polymerization or bundling.
4.2. Control of Secretion through Lipids Action
Rho GTPases were also shown to control lipids metabolism pathways that are critical for neuroendocrine secretion [11,97]. In rat pheochromocytoma cells, we demonstrated that short interfering RNA (siRNA)-based knockdown of Rac1 inhibits hormone secretion by preventing the secretagogue-induced activation of phospholipase D1 (PLD1) [98]. PLD1 produces phosphatidic acid (PA), a coned-shape fusogenic lipid pivotal for efficient secretion in neuroendocrine cells including adrenal medulla and pancreatic islet cells [97,99,100,101]. Notably, PLD upregulation was shown to play various cellular and physiological roles in cancer [102,103]. Among the possible contributions of excessive PA synthesis in tumorigenesis, we mention the activation of the mTor pathway by PA that directly binds to mTor in a rapamycine-competitive manner and the increase in metalloprotease secretion triggered by PA [102,103].
A recent study highlighted the importance of the lipid transporter ABCA12 in insulin secretion. ABCA12 silencing in pancreatic β cells impaired secretory granule maturation and fusion, most likely through an altered cellular distribution of cholesterol between insulin granules and the plasma membrane lipid rafts required for secretion [104]. Remarkably, loss of ABCA12 expression also prevents Cdc42 activation and the associated actin remodeling [104].
Actin cytoskeleton remodeling and lipid organization are intimately linked during the process of hormone secretion [11]. For example, work from our laboratory demonstrated that formation of actin bundles connecting docked secretory granules to the plasma membrane contributes to the formation of GM1-enriched lipid microdomains at the exocytotic sites in chromaffin cells [86]. We showed that RhoA, which controls the organization of the cortical actin network at rest, can be recruited to the secretory granule membrane to regulate the phosphatidylinositol-4 kinase (PI 4-kinase) activity, hence modulating phosphatidylinositol 4-phosphate (PI4P) level [79]. How the level of PI4P on secretory granule membrane can impact secretion in currently unknown. One possible explanation is that PI4P is the precursor for phosphatidylinositol 4,5-bisphosphate (PIP2), a phosphoinositide that has been largely implicated in regulated secretion of hormones [105,106]. Coping with levels of phosphatidylinositol 4-phosphate 5-kinase (PI4P-5kinase), the enzyme that generates PIP2 from PI4P, dramatically affected exocytosis in chromaffin and beta pancreatic cells [105,106,107]. As RhoA activation diminished catecholamine secretion in chromaffin cells and since PIP2 controls many actin binding proteins, PIP2 might contribute to stabilizing secretory granules within the peripheral actin mesh.
4.3. Rho GEFs and Rho GAPs at the Commands
As mentioned above, one crucial checkpoint to insure physiological functioning of Rho GTPases is the tight regulation of their activation/inactivation cycle through the action of GEFs and GAPs proteins. Given uncovering the comprehensive mechanisms by which Rho GTPases regulate hormones secretion, we identified a set of different Rho regulators. In the chromaffin/PC12 cell models, stimulation of exocytosis triggers activation of Cdc42 and Rac1, associated with the inactivation of RhoA. We previously showed that the activation of Cdc42 is mediated by intersectin-1L, a member of the Dbl family of GEFs that also interacts with N-WASP and participates in actin organization [108,109,110]. In parallel, Rac1 is activated by β-PIX, a member of the Cool/Pix Rho GEFs family, which is recruited to the plasma membrane of stimulated-PC12 cells through its interaction with Scrib, the mammalian homologue of the Drosophila neoplasic tumor suppressor Scribble [98,111,112].
In pituitary and pancreatic cells, different GEFs have also been proposed to control hormone secretion. The transient activation of Rac1 required for glucose-induced insulin secretion was proposed to be under the control of VAV2, Tiam1, and Trio/Kalirin in pancreatic cells [113,114,115,116]. How these three GEFs coordinate spatially and temporally Rac activation needs further investigation. In the pituitary gland, the GEF trio has been also proposed to control hormone release [117].
Regarding RhoA, we proposed that Oligophrenin-1, a multi-domains GAP protein involved in various membrane trafficking events linked to synaptic functions (plasticity, post-synaptic receptor trafficking, and synaptic vesicle recycling [118,119,120,121,122]), might be responsible for the secretagogue-induced inactivation of RhoA [123]. Along the same line, inhibition of the RhoA/Rock pathway reduced neurotensin secretion in BON cells [124].
5. Conclusions
In comparison to other types of tumors, the role of Rho GTPases in NETs is not well documented. However, the high amount of genetic mutations and polymorphisms discovered in Rho GEFs and GAPs indicates that pathways controlled by Rho GTPases are likely affected in NETs. Today, a clear effort has to be directed toward understanding how mutations or variations in expression levels of Rho GTPases, GEFs, and GAPs identified in NETs favor tumorigenesis. Comparative genomic and proteomic analyses of human tumor samples remain among the most suitable general strategies to uncover new actors involved in Rho GTPases signaling.
Besides being a predictive factor for tumor occurrence or for its progression, whether Rho GTPases pathways could be used as therapeutic targets is clearly an aspect that needs to be developed in the near future. Several drugs directly targeting Rho GTPases have been recently designed and different strategies such as preventing Rho GEF interaction or inhibiting effectors have been proposed [125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141]. However, based on the complex involvement of Rho GTPases and their regulators in NETs hypersecretion, as reviewed here, the development of proper strategies to target each specific tumor will be critical and will require a perfect knowledge of the mechanisms leading to the deregulation of the Rho pathways, as well as their consequences on tumorigenesis.
Acknowledgments
Institut National de la Santé et de la Recherche Médicale (INSERM) is providing a salary to S.G. and N.V.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/7/1859/s1, Table S1: Mutations and polymorphisms of Rho GTPases in NETs, Table S2: Mutations and polymorphisms of Rho GTPases GEFs and GAPs in NETs.
Funding
Part of the author’s work discussed here was supported by grants from Ligue Contre le Cancer (CCIRGE) and from the ANR (SecretoNET) to SG.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kulke M.H., Shah M.H., Benson A.B., 3rd, Bergsland E., Berlin J.D., Blaszkowsky L.S., Emerson L., Engstrom P.F., Fanta P., Giordano T., et al. Neuroendocrine tumors, version 1.2015. J. Natl. Compr. Canc. Netw. 2015;13:78–108. doi: 10.6004/jnccn.2015.0011. [DOI] [PubMed] [Google Scholar]
- 2.Zandee W.T., Kamp K., van Adrichem R.C., Feelders R.A., de Herder W.W. Effect of hormone secretory syndromes on neuroendocrine tumor prognosis. Endocr. Relat. Cancer. 2017;24:R261–R274. doi: 10.1530/ERC-16-0538. [DOI] [PubMed] [Google Scholar]
- 3.Juhlin C.C., Skoglund S., Juntti-Berggren L., Karlberg M., Calissendorff J. Non-functioning neuroendocrine pancreatic tumors transforming to malignant insulinomas - four cases and review of the literature. Neuro. Endocrinol. Lett. 2019;40:175–183. [PubMed] [Google Scholar]
- 4.Song P., Sekhon H.S., Jia Y., Keller J.A., Blusztajn J.K., Mark G.P., Spindel E.R. Acetylcholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res. 2003;63:214–221. [PubMed] [Google Scholar]
- 5.Brown R.L., Muzzafar T., Wollman R., Weiss R.E. A pituitary carcinoma secreting TSH and prolactin: A non-secreting adenoma gone awry. Eur. J. Endocrinol. 2006;154:639–643. doi: 10.1530/eje.1.02141. [DOI] [PubMed] [Google Scholar]
- 6.Daems T., Verhelst J., Michotte A., Abrams P., De Ridder D., Abs R. Modification of hormonal secretion in clinically silent pituitary adenomas. Pituitary. 2009;12:80–86. doi: 10.1007/s11102-008-0085-7. [DOI] [PubMed] [Google Scholar]
- 7.Lenders N., McCormack A. Malignant transformation in non-functioning pituitary adenomas (pituitary carcinoma) Pituitary. 2018;21:217–229. doi: 10.1007/s11102-017-0857-z. [DOI] [PubMed] [Google Scholar]
- 8.Neumann H.P.H., Young W.F., Jr., Eng C. Pheochromocytoma and Paraganglioma. N. Engl. J. Med. 2019;381:552–565. doi: 10.1056/NEJMra1806651. [DOI] [PubMed] [Google Scholar]
- 9.Aspenstrom P., Ruusala A., Pacholsky D. Taking Rho GTPases to the next level: The cellular functions of atypical Rho GTPases. Exp. Cell Res. 2007;313:3673–3679. doi: 10.1016/j.yexcr.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Boureux A., Vignal E., Faure S., Fort P. Evolution of the Rho family of ras-like GTPases in eukaryotes. Mol. Biol. Evol. 2007;24:203–216. doi: 10.1093/molbev/msl145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croise P., Estay-Ahumada C., Gasman S., Ory S. Rho GTPases, phosphoinositides, and actin: A tripartite framework for efficient vesicular trafficking. Small GTPases. 2014;5:e29469. doi: 10.4161/sgtp.29469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haga R.B., Ridley A.J. Rho GTPases: Regulation and roles in cancer cell biology. Small GTPases. 2016;7:207–221. doi: 10.1080/21541248.2016.1232583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H., Peyrollier K., Kilic G., Brakebusch C. Rho GTPases and cancer. Biofactors. 2014;40:226–235. doi: 10.1002/biof.1155. [DOI] [PubMed] [Google Scholar]
- 14.Olayioye M.A., Noll B., Hausser A. Spatiotemporal Control of Intracellular Membrane Trafficking by Rho GTPases. Cells. 2019;8:1478. doi: 10.3390/cells8121478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orgaz J.L., Herraiz C., Sanz-Moreno V. Rho GTPases modulate malignant transformation of tumor cells. Small GTPases. 2014;5:e29019. doi: 10.4161/sgtp.29019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phuyal S., Farhan H. Multifaceted Rho GTPase Signaling at the Endomembranes. Front. Cell Dev. Biol. 2019;7:127. doi: 10.3389/fcell.2019.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter A.P., Papaioannou A., Malliri A. Deregulation of Rho GTPases in cancer. Small GTPases. 2016;7:123–138. doi: 10.1080/21541248.2016.1173767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warner H., Wilson B.J., Caswell P.T. Control of adhesion and protrusion in cell migration by Rho GTPases. Curr. Opin. Cell. Biol. 2019;56:64–70. doi: 10.1016/j.ceb.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson K.F., Erickson J.W., Antonyak M.A., Cerione R.A. Rho GTPases and their roles in cancer metabolism. Trends Mol. Med. 2013;19:74–82. doi: 10.1016/j.molmed.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alan J.K., Lundquist E.A. Mutationally activated Rho GTPases in cancer. Small GTPases. 2013;4:159–163. doi: 10.4161/sgtp.26530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bustelo X.R. RHO GTPases in cancer: Known facts, open questions, and therapeutic challenges. Biochem. Soc. Trans. 2018;46:741–760. doi: 10.1042/BST20170531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kataoka K., Ogawa S. Variegated RHOA mutations in human cancers. Exp. Hematol. 2016;44:1123–1129. doi: 10.1016/j.exphem.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Olson M.F. Rho GTPases, their post-translational modifications, disease-associated mutations and pharmacological inhibitors. Small GTPases. 2018;9:203–215. doi: 10.1080/21541248.2016.1218407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Svensmark J.H., Brakebusch C. Rho GTPases in cancer: Friend or foe? Oncogene. 2019;38:7447–7456. doi: 10.1038/s41388-019-0963-7. [DOI] [PubMed] [Google Scholar]
- 25.Zandvakili I., Lin Y., Morris J.C., Zheng Y. Rho GTPases: Anti- or pro-neoplastic targets? Oncogene. 2017;36:3213–3222. doi: 10.1038/onc.2016.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis M.J., Ha B.H., Holman E.C., Halaban R., Schlessinger J., Boggon T.J. RAC1P29S is a spontaneously activating cancer-associated GTPase. Proc. Natl. Acad. Sci. USA. 2013;110:912–917. doi: 10.1073/pnas.1220895110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawazu M., Ueno T., Kontani K., Ogita Y., Ando M., Fukumura K., Yamato A., Soda M., Takeuchi K., Miki Y., et al. Transforming mutations of RAC guanosine triphosphatases in human cancers. Proc. Natl. Acad. Sci. USA. 2013;110:3029–3034. doi: 10.1073/pnas.1216141110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H., Schaefer A., Wang Y., Hodge R.G., Blake D.R., Diehl J.N., Papageorge A.G., Stachler M.D., Liao J., Zhou J., et al. Gain-of-Function RHOA Mutations Promote Focal Adhesion Kinase Activation and Dependency in Diffuse Gastric Cancer. Cancer Discov. 2020;10:288–305. doi: 10.1158/2159-8290.CD-19-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang K., Yuen S.T., Xu J., Lee S.P., Yan H.H., Shi S.T., Siu H.C., Deng S., Chu K.M., Law S., et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat. Genet. 2014;46:573–582. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- 30.Augert A., Zhang Q., Bates B., Cui M., Wang X., Wildey G., Dowlati A., MacPherson D. Small Cell Lung Cancer Exhibits Frequent Inactivating Mutations in the Histone Methyltransferase KMT2D/MLL2: CALGB 151111 (Alliance) J. Thorac. Oncol. 2017;12:704–713. doi: 10.1016/j.jtho.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wen K.W., Grenert J.P., Joseph N.M., Shafizadeh N., Huang A., Hosseini M., Kakar S. Genomic profile of appendiceal goblet cell carcinoid is distinct compared to appendiceal neuroendocrine tumor and conventional adenocarcinoma. Hum. Pathol. 2018;77:166–174. doi: 10.1016/j.humpath.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Cao Y., Zhou W., Li L., Wang J., Gao Z., Jiang Y., Jiang X., Shan A., Bailey M.H., Huang K.L., et al. Pan-cancer analysis of somatic mutations across 21 neuroendocrine tumor types. Cell Res. 2018;28:601–604. doi: 10.1038/s41422-018-0019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilzen A., Rehammar A., Muth A., Nilsson O., Tesan Tomic T., Wangberg B., Kristiansson E., Abel F. Malignant pheochromocytomas/paragangliomas harbor mutations in transport and cell adhesion genes. Int. J. Cancer. 2016;138:2201–2211. doi: 10.1002/ijc.29957. [DOI] [PubMed] [Google Scholar]
- 34.Flynn A., Benn D., Clifton-Bligh R., Robinson B., Trainer A.H., James P., Hogg A., Waldeck K., George J., Li J., et al. The genomic landscape of phaeochromocytoma. J. Pathol. 2015;236:78–89. doi: 10.1002/path.4503. [DOI] [PubMed] [Google Scholar]
- 35.Scarpa A., Chang D.K., Nones K., Corbo V., Patch A.M., Bailey P., Lawlor R.T., Johns A.L., Miller D.K., Mafficini A., et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;543:65–71. doi: 10.1038/nature21063. [DOI] [PubMed] [Google Scholar]
- 36.George J., Walter V., Peifer M., Alexandrov L.B., Seidel D., Leenders F., Maas L., Muller C., Dahmen I., Delhomme T.M., et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nat. Commun. 2018;9:1048. doi: 10.1038/s41467-018-03099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Francis J.M., Kiezun A., Ramos A.H., Serra S., Pedamallu C.S., Qian Z.R., Banck M.S., Kanwar R., Kulkarni A.A., Karpathakis A., et al. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat. Genet. 2013;45:1483–1486. doi: 10.1038/ng.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harms P.W., Vats P., Verhaegen M.E., Robinson D.R., Wu Y.M., Dhanasekaran S.M., Palanisamy N., Siddiqui J., Cao X., Su F., et al. The Distinctive Mutational Spectra of Polyomavirus-Negative Merkel Cell Carcinoma. Cancer Res. 2015;75:3720–3727. doi: 10.1158/0008-5472.CAN-15-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castro-Vega L.J., Letouze E., Burnichon N., Buffet A., Disderot P.H., Khalifa E., Loriot C., Elarouci N., Morin A., Menara M., et al. Multi-omics analysis defines core genomic alterations in pheochromocytomas and paragangliomas. Nat. Commun. 2015;6:6044. doi: 10.1038/ncomms7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ellenbroek S.I., Collard J.G. Rho GTPases: Functions and association with cancer. Clin. Exp. Metastasis. 2007;24:657–672. doi: 10.1007/s10585-007-9119-1. [DOI] [PubMed] [Google Scholar]
- 41.Karlsson R., Pedersen E.D., Wang Z., Brakebusch C. Rho GTPase function in tumorigenesis. Biochim. Biophys. Acta. 2009;1796:91–98. doi: 10.1016/j.bbcan.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Gomez del Pulgar T., Benitah S.A., Valeron P.F., Espina C., Lacal J.C. Rho GTPase expression in tumourigenesis: Evidence for a significant link. Bioessays. 2005;27:602–613. doi: 10.1002/bies.20238. [DOI] [PubMed] [Google Scholar]
- 43.Sahai E., Marshall C.J. RHO-GTPases and cancer. Nat. Rev. Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 44.Long Y., Lu M., Cheng T., Zhan X., Zhan X. Multiomics-Based Signaling Pathway Network Alterations in Human Non-functional Pituitary Adenomas. Front. Endocrinol. (Lausanne) 2019;10:835. doi: 10.3389/fendo.2019.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veschi V., Verona F., Thiele C.J. Cancer Stem Cells and Neuroblastoma: Characteristics and Therapeutic Targeting Options. Front. Endocrinol. (Lausanne) 2019;10:782. doi: 10.3389/fendo.2019.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Q.R., Song Y.K., Yu L.R., Wei J.S., Chung J.Y., Hewitt S.M., Veenstra T.D., Khan J. Global genomic and proteomic analysis identifies biological pathways related to high-risk neuroblastoma. J. Proteome Res. 2010;9:373–382. doi: 10.1021/pr900701v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coco S., Theissen J., Scaruffi P., Stigliani S., Moretti S., Oberthuer A., Valdora F., Fischer M., Gallo F., Hero B., et al. Age-dependent accumulation of genomic aberrations and deregulation of cell cycle and telomerase genes in metastatic neuroblastoma. Int. J. Cancer. 2012;131:1591–1600. doi: 10.1002/ijc.27432. [DOI] [PubMed] [Google Scholar]
- 48.Stigliani S., Coco S., Moretti S., Oberthuer A., Fischer M., Theissen J., Gallo F., Garavent A., Berthold F., Bonassi S., et al. High genomic instability predicts survival in metastatic high-risk neuroblastoma. Neoplasia. 2012;14:823–832. doi: 10.1593/neo.121114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi C., Contreras B., Iwanaga T., Takegami Y., Bakker A., Bronson R.T., Noda M., Loda M., Hunt J.L., Ewen M.E. Nras loss induces metastatic conversion of Rb1-deficient neuroendocrine thyroid tumor. Nat. Genet. 2006;38:118–123. doi: 10.1038/ng1703. [DOI] [PubMed] [Google Scholar]
- 50.Gawin M., Wojakowska A., Pietrowska M., Marczak L., Chekan M., Jelonek K., Lange D., Jaksik R., Gruca A., Widlak P. Proteome profiles of different types of thyroid cancers. Mol. Cell. Endocrinol. 2018;472:68–79. doi: 10.1016/j.mce.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 51.Rekhtman N. Neuroendocrine tumors of the lung: An update. Arch. Pathol. Lab. Med. 2010;134:1628–1638. doi: 10.1043/2009-0583-RAR.1. [DOI] [PubMed] [Google Scholar]
- 52.Touge H., Chikumi H., Igishi T., Kurai J., Makino H., Tamura Y., Takata M., Yoneda K., Nakamoto M., Suyama H., et al. Diverse activation states of RhoA in human lung cancer cells: Contribution of G protein coupled receptors. Int. J. Oncol. 2007;30:709–715. doi: 10.3892/ijo.30.3.709. [DOI] [PubMed] [Google Scholar]
- 53.Varker K.A., Phelps S.H., King M.M., Williams C.L. The small GTPase RhoA has greater expression in small cell lung carcinoma than in non-small cell lung carcinoma and contributes to their unique morphologies. Int. J. Oncol. 2003;22:671–681. [PubMed] [Google Scholar]
- 54.Fujii K., Miyata Y., Takahashi I., Koizumi H., Saji H., Hoshikawa M., Takagi M., Nishimura T., Nakamura H. Differential Proteomic Analysis between Small Cell Lung Carcinoma (SCLC) and Pulmonary Carcinoid Tumors Reveals Molecular Signatures for Malignancy in Lung Cancer. Proteom. Clin. Appl. 2018;12:e1800015. doi: 10.1002/prca.201800015. [DOI] [PubMed] [Google Scholar]
- 55.Liu R.X., Wang W.Q., Ye L., Bi Y.F., Fang H., Cui B., Zhou W.W., Dai M., Zhang J., Li X.Y., et al. p21-activated kinase 3 is overexpressed in thymic neuroendocrine tumors (carcinoids) with ectopic ACTH syndrome and participates in cell migration. Endocrine. 2010;38:38–47. doi: 10.1007/s12020-010-9324-6. [DOI] [PubMed] [Google Scholar]
- 56.Stakaityte G., Nwogu N., Dobson S.J., Knight L.M., Wasson C.W., Salguero F.J., Blackbourn D.J., Blair G.E., Mankouri J., Macdonald A., et al. Merkel Cell Polyomavirus Small T Antigen Drives Cell Motility via Rho-GTPase-Induced Filopodium Formation. J. Virol. 2018;92 doi: 10.1128/JVI.00940-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Croise P., Houy S., Gand M., Lanoix J., Calco V., Toth P., Brunaud L., Lomazzi S., Paramithiotis E., Chelsky D., et al. Cdc42 and Rac1 activity is reduced in human pheochromocytoma and correlates with FARP1 and ARHGEF1 expression. Endocr. Relat. Cancer. 2016;23:281–293. doi: 10.1530/ERC-15-0502. [DOI] [PubMed] [Google Scholar]
- 58.Croise P., Brunaud L., Toth P., Gasman S., Ory S. Inhibition of Cdc42 and Rac1 activities in pheochromocytoma, the adrenal medulla tumor. Small GTPases. 2017;8:122–127. doi: 10.1080/21541248.2016.1202634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Cubas A.A., Leandro-Garcia L.J., Schiavi F., Mancikova V., Comino-Mendez I., Inglada-Perez L., Perez-Martinez M., Ibarz N., Ximenez-Embun P., Lopez-Jimenez E., et al. Integrative analysis of miRNA and mRNA expression profiles in pheochromocytoma and paraganglioma identifies genotype-specific markers and potentially regulated pathways. Endocr. Relat. Cancer. 2013;20:477–493. doi: 10.1530/ERC-12-0183. [DOI] [PubMed] [Google Scholar]
- 60.Bossan A., Ottman R., Andl T., Hasan M.F., Mahajan N., Coppola D., Chakrabarti R. Expression of FGD4 positively correlates with the aggressive phenotype of prostate cancer. BMC Cancer. 2018;18:1257. doi: 10.1186/s12885-018-5096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shahid M., George T.B., Saller J., Haija M., Sayegh Z., Boulware D., Strosberg J., Chakrabarti R., Coppola D. FGD4 (Frabin) Overexpression in Pancreatic Neuroendocrine Neoplasms. Pancreas. 2019;48:1307–1311. doi: 10.1097/MPA.0000000000001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lazer G., Idelchuk Y., Schapira V., Pikarsky E., Katzav S. The haematopoietic specific signal transducer Vav1 is aberrantly expressed in lung cancer and plays a role in tumourigenesis. J. Pathol. 2009;219:25–34. doi: 10.1002/path.2579. [DOI] [PubMed] [Google Scholar]
- 63.Ziv T., Barnea E., Segal H., Sharon R., Beer I., Admon A. Comparative proteomics of small cell lung carcinoma. Cancer Biomark. 2006;2:219–234. doi: 10.3233/CBM-2006-2601. [DOI] [PubMed] [Google Scholar]
- 64.Weissenrieder J.S., Reilly J.E., Neighbors J.D., Hohl R.J. Inhibiting geranylgeranyl diphosphate synthesis reduces nuclear androgen receptor signaling and neuroendocrine differentiation in prostate cancer cell models. Prostate. 2019;79:21–30. doi: 10.1002/pros.23707. [DOI] [PubMed] [Google Scholar]
- 65.Arya A.K., Bhadada S.K., Singh P., Dahiya D., Kaur G., Sharma S., Saikia U.N., Behera A., Rao S.D., Bhasin M. Quantitative proteomics analysis of sporadic parathyroid adenoma tissue samples. J. Endocrinol. Investig. 2019;42:577–590. doi: 10.1007/s40618-018-0958-1. [DOI] [PubMed] [Google Scholar]
- 66.Tsai H.K., Lehrer J., Alshalalfa M., Erho N., Davicioni E., Lotan T.L. Gene expression signatures of neuroendocrine prostate cancer and primary small cell prostatic carcinoma. BMC Cancer. 2017;17:759. doi: 10.1186/s12885-017-3729-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bader M.F., Doussau F., Chasserot-Golaz S., Vitale N., Gasman S. Coupling actin and membrane dynamics during calcium-regulated exocytosis: A role for Rho and ARF GTPases. Biochim. Biophys. Acta. 2004;1742:37–49. doi: 10.1016/j.bbamcr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 68.Gasman S., Chasserot-Golaz S., Bader M.F., Vitale N. Regulation of exocytosis in adrenal chromaffin cells: Focus on ARF and Rho GTPases. Cell Signal. 2003;15:893–899. doi: 10.1016/S0898-6568(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 69.Malacombe M., Bader M.F., Gasman S. Exocytosis in neuroendocrine cells: New tasks for actin. Biochim. Biophys. Acta. 2006;1763:1175–1183. doi: 10.1016/j.bbamcr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 70.Momboisse F., Houy S., Ory S., Calco V., Bader M.F., Gasman S. How important are Rho GTPases in neurosecretion? J. Neurochem. 2011;117:623–631. doi: 10.1111/j.1471-4159.2011.07241.x. [DOI] [PubMed] [Google Scholar]
- 71.Ory S., Gasman S. Rho GTPases and exocytosis: What are the molecular links? Semin. Cell. Dev. Biol. 2011;22:27–32. doi: 10.1016/j.semcdb.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 72.Bader M.F., Holz R.W., Kumakura K., Vitale N. Exocytosis: The chromaffin cell as a model system. Ann. N. Y. Acad. Sci. 2002;971:178–183. doi: 10.1111/j.1749-6632.2002.tb04461.x. [DOI] [PubMed] [Google Scholar]
- 73.Westerink R.H., Ewing A.G. The PC12 cell as model for neurosecretion. Acta. Physiol. (Oxf.) 2008;192:273–285. doi: 10.1111/j.1748-1716.2007.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miyazaki J., Araki K., Yamato E., Ikegami H., Asano T., Shibasaki Y., Oka Y., Yamamura K. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: Special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127:126–132. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- 75.Flatt P.R. Defective regulation of insulin secretion in diabetes and insulinoma. Biochem. Soc. Trans. 1990;18:124–127. doi: 10.1042/bst0180124. [DOI] [PubMed] [Google Scholar]
- 76.Okabayashi T., Shima Y., Sumiyoshi T., Kozuki A., Ito S., Ogawa Y., Kobayashi M., Hanazaki K. Diagnosis and management of insulinoma. World J. Gastroenterol. 2013;19:829–837. doi: 10.3748/wjg.v19.i6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gasman S., Chasserot-Golaz S., Popoff M.R., Aunis D., Bader M.F. Involvement of Rho GTPases in calcium-regulated exocytosis from adrenal chromaffin cells. J. Cell. Sci. 1999;112 Pt. 24:4763–4771. doi: 10.1242/jcs.112.24.4763. [DOI] [PubMed] [Google Scholar]
- 78.Gasman S., Chasserot-Golaz S., Malacombe M., Way M., Bader M.F. Regulated exocytosis in neuroendocrine cells: A role for subplasmalemmal Cdc42/N-WASP-induced actin filaments. Mol. Biol. Cell. 2004;15:520–531. doi: 10.1091/mbc.e03-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gasman S., Chasserot-Golaz S., Hubert P., Aunis D., Bader M.F. Identification of a potential effector pathway for the trimeric Go protein associated with secretory granules. Go stimulates a granule-bound phosphatidylinositol 4-kinase by activating RhoA in chromaffin cells. J. Biol. Chem. 1998;273:16913–16920. doi: 10.1074/jbc.273.27.16913. [DOI] [PubMed] [Google Scholar]
- 80.Gasman S., Chasserot-Golaz S., Popoff M.R., Aunis D., Bader M.F. Trimeric G proteins control exocytosis in chromaffin cells. Go regulates the peripheral actin network and catecholamine secretion by a mechanism involving the small GTP-binding protein Rho. J. Biol. Chem. 1997;272:20564–20571. doi: 10.1074/jbc.272.33.20564. [DOI] [PubMed] [Google Scholar]
- 81.Kalwat M.A., Thurmond D.C. Signaling mechanisms of glucose-induced F-actin remodeling in pancreatic islet beta cells. Exp. Mol. Med. 2013;45:e37. doi: 10.1038/emm.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Z., Oh E., Thurmond D.C. Glucose-stimulated Cdc42 signaling is essential for the second phase of insulin secretion. J. Biol. Chem. 2007;282:9536–9546. doi: 10.1074/jbc.M610553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Uenishi E., Shibasaki T., Takahashi H., Seki C., Hamaguchi H., Yasuda T., Tatebe M., Oiso Y., Takenawa T., Seino S. Actin dynamics regulated by the balance of neuronal Wiskott-Aldrich syndrome protein (N-WASP) and cofilin activities determines the biphasic response of glucose-induced insulin secretion. J. Biol. Chem. 2013;288:25851–25864. doi: 10.1074/jbc.M113.464420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kalwat M.A., Yoder S.M., Wang Z., Thurmond D.C. A p21-activated kinase (PAK1) signaling cascade coordinately regulates F-actin remodeling and insulin granule exocytosis in pancreatic beta cells. Biochem. Pharmacol. 2013;85:808–816. doi: 10.1016/j.bcp.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bretou M., Jouannot O., Fanget I., Pierobon P., Larochette N., Gestraud P., Guillon M., Emiliani V., Gasman S., Desnos C., et al. Cdc42 controls the dilation of the exocytotic fusion pore by regulating membrane tension. Mol. Biol. Cell. 2014;25:3195–3209. doi: 10.1091/mbc.e14-07-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gabel M., Delavoie F., Demais V., Royer C., Bailly Y., Vitale N., Bader M.F., Chasserot-Golaz S. Annexin A2-dependent actin bundling promotes secretory granule docking to the plasma membrane and exocytosis. J. Cell. Biol. 2015;210:785–800. doi: 10.1083/jcb.201412030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma W., Chang J., Tong J., Ho U., Yau B., Kebede M.A., Thorn P. Arp2/3 nucleates F-actin coating of fusing insulin granules in pancreatic beta cells to control insulin secretion. J. Cell. Sci. 2020;133 doi: 10.1242/jcs.236794. [DOI] [PubMed] [Google Scholar]
- 88.Gomes E.R., Jani S., Gundersen G.G. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 89.Brooks A.B., Humphreys D., Singh V., Davidson A.C., Arden S.D., Buss F., Koronakis V. MYO6 is targeted by Salmonella virulence effectors to trigger PI3-kinase signaling and pathogen invasion into host cells. Proc. Natl. Acad. Sci. USA. 2017;114:3915–3920. doi: 10.1073/pnas.1616418114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kimura K., Ito M., Amano M., Chihara K., Fukata Y., Nakafuku M., Yamamori B., Feng J., Nakano T., Okawa K., et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 91.Wilkinson S., Paterson H.F., Marshall C.J. Cdc42-MRCK and Rho-ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nat. Cell. Biol. 2005;7:255–261. doi: 10.1038/ncb1230. [DOI] [PubMed] [Google Scholar]
- 92.Arous C., Rondas D., Halban P.A. Non-muscle myosin IIA is involved in focal adhesion and actin remodelling controlling glucose-stimulated insulin secretion. Diabetologia. 2013;56:792–802. doi: 10.1007/s00125-012-2800-1. [DOI] [PubMed] [Google Scholar]
- 93.Berberian K., Torres A.J., Fang Q., Kisler K., Lindau M. F-actin and myosin II accelerate catecholamine release from chromaffin granules. J. Neurosci. 2009;29:863–870. doi: 10.1523/JNEUROSCI.2818-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Neco P., Fernandez-Peruchena C., Navas S., Gutierrez L.M., de Toledo G.A., Ales E. Myosin II contributes to fusion pore expansion during exocytosis. J. Biol. Chem. 2008;283:10949–10957. doi: 10.1074/jbc.M709058200. [DOI] [PubMed] [Google Scholar]
- 95.Papadopulos A., Gomez G.A., Martin S., Jackson J., Gormal R.S., Keating D.J., Yap A.S., Meunier F.A. Activity-driven relaxation of the cortical actomyosin II network synchronizes Munc18-1-dependent neurosecretory vesicle docking. Nat. Commun. 2015;6:6297. doi: 10.1038/ncomms7297. [DOI] [PubMed] [Google Scholar]
- 96.Tomatis V.M., Josh P., Papadopulos A., Gormal R.S., Lanoue V., Martin S., Meunier F.A. ENA/VASP proteins regulate exocytosis by mediating myosin VI-dependent recruitment of secretory granules to the cortical actin network. Mol. Cell. Neurosci. 2017;84:100–111. doi: 10.1016/j.mcn.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 97.Gasman S., Vitale N. Lipid remodelling in neuroendocrine secretion. Biol. Cell. 2017 doi: 10.1111/boc.201700030. [DOI] [PubMed] [Google Scholar]
- 98.Momboisse F., Lonchamp E., Calco V., Ceridono M., Vitale N., Bader M.F., Gasman S. betaPIX-activated Rac1 stimulates the activation of phospholipase D, which is associated with exocytosis in neuroendocrine cells. J. Cell. Sci. 2009;122:798–806. doi: 10.1242/jcs.038109. [DOI] [PubMed] [Google Scholar]
- 99.Zeniou-Meyer M., Zabari N., Ashery U., Chasserot-Golaz S., Haeberle A.M., Demais V., Bailly Y., Gottfried I., Nakanishi H., Neiman A.M., et al. Phospholipase D1 production of phosphatidic acid at the plasma membrane promotes exocytosis of large dense-core granules at a late stage. J. Biol. Chem. 2007;282:21746–21757. doi: 10.1074/jbc.M702968200. [DOI] [PubMed] [Google Scholar]
- 100.Hughes W.E., Elgundi Z., Huang P., Frohman M.A., Biden T.J. Phospholipase D1 regulates secretagogue-stimulated insulin release in pancreatic beta-cells. J. Biol. Chem. 2004;279:27534–27541. doi: 10.1074/jbc.M403012200. [DOI] [PubMed] [Google Scholar]
- 101.Metz S.A., Dunlop M. Stimulation of insulin release by phospholipase D. A potential role for endogenous phosphatidic acid in pancreatic islet function. Biochem. J. 1990;270:427–435. doi: 10.1042/bj2700427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gomez-Cambronero J. Phospholipase D in cell signaling: From a myriad of cell functions to cancer growth and metastasis. J. Biol. Chem. 2014;289:22557–22566. doi: 10.1074/jbc.R114.574152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Y., Frohman M.A. Cellular and physiological roles for phospholipase D1 in cancer. J. Biol. Chem. 2014;289:22567–22574. doi: 10.1074/jbc.R114.576876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ursino G.M., Fu Y., Cottle D.L., Mukhamedova N., Jones L.K., Low H., Tham M.S., Gan W.J., Mellett N.A., Das P.P., et al. ABCA12 regulates insulin secretion from beta-cells. EMBO Rep. 2020;21:e48692. doi: 10.15252/embr.201948692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gong L.W., Di Paolo G., Diaz E., Cestra G., Diaz M.E., Lindau M., De Camilli P., Toomre D. Phosphatidylinositol phosphate kinase type I gamma regulates dynamics of large dense-core vesicle fusion. Proc. Natl. Acad. Sci. USA. 2005;102:5204–5209. doi: 10.1073/pnas.0501412102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Milosevic I., Sorensen J.B., Lang T., Krauss M., Nagy G., Haucke V., Jahn R., Neher E. Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. J. Neurosci. 2005;25:2557–2565. doi: 10.1523/JNEUROSCI.3761-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Waselle L., Gerona R.R., Vitale N., Martin T.F., Bader M.F., Regazzi R. Role of phosphoinositide signaling in the control of insulin exocytosis. Mol. Endocrinol. 2005;19:3097–3106. doi: 10.1210/me.2004-0530. [DOI] [PubMed] [Google Scholar]
- 108.Gubar O., Morderer D., Tsyba L., Croise P., Houy S., Ory S., Gasman S., Rynditch A. Intersectin: The Crossroad between Vesicle Exocytosis and Endocytosis. Front. Endocrinol. (Lausanne) 2013;4:109. doi: 10.3389/fendo.2013.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Malacombe M., Ceridono M., Calco V., Chasserot-Golaz S., McPherson P.S., Bader M.F., Gasman S. Intersectin-1L nucleotide exchange factor regulates secretory granule exocytosis by activating Cdc42. Embo. J. 2006;25:3494–3503. doi: 10.1038/sj.emboj.7601247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem. Sci. 2001;26:724–732. doi: 10.1016/S0968-0004(01)01973-9. [DOI] [PubMed] [Google Scholar]
- 111.Audebert S., Navarro C., Nourry C., Chasserot-Golaz S., Lecine P., Bellaiche Y., Dupont J.L., Premont R.T., Sempere C., Strub J.M., et al. Mammalian Scribble Forms a Tight Complex with the betaPIX Exchange Factor. Curr. Biol. 2004;14:987–995. doi: 10.1016/j.cub.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 112.Zhou W., Li X., Premont R.T. Expanding functions of GIT Arf GTPase-activating proteins, PIX Rho guanine nucleotide exchange factors and GIT-PIX complexes. J. Cell Sci. 2016;129:1963–1974. doi: 10.1242/jcs.179465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dufurrena Q., Back N., Mains R.E., Hodgson L., Tanowitz H., Mandela P., Eipper E., Kuliawat R. Kalirin/Trio Rho GDP/GTP exchange factors regulate proinsulin and insulin secretion. J. Mol. Endocrinol. 2018 doi: 10.1530/JME-18-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kowluru A. GPCRs, G Proteins, and Their Impact on beta-cell Function. Compr. Physiol. 2020;10:453–490. doi: 10.1002/cphy.c190028. [DOI] [PubMed] [Google Scholar]
- 115.Veluthakal R., Madathilparambil S.V., McDonald P., Olson L.K., Kowluru A. Regulatory roles for Tiam1, a guanine nucleotide exchange factor for Rac1, in glucose-stimulated insulin secretion in pancreatic beta-cells. Biochem. Pharmacol. 2009;77:101–113. doi: 10.1016/j.bcp.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Veluthakal R., Tunduguru R., Arora D.K., Sidarala V., Syeda K., Vlaar C.P., Thurmond D.C., Kowluru A. VAV2, a guanine nucleotide exchange factor for Rac1, regulates glucose-stimulated insulin secretion in pancreatic beta cells. Diabetologia. 2015;58:2573–2581. doi: 10.1007/s00125-015-3707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xin X., Ferraro F., Back N., Eipper B.A., Mains R.E. Cdk5 and Trio modulate endocrine cell exocytosis. J. Cell Sci. 2004;117:4739–4748. doi: 10.1242/jcs.01333. [DOI] [PubMed] [Google Scholar]
- 118.Khelfaoui M., Gambino F., Houbaert X., Ragazzon B., Muller C., Carta M., Lanore F., Srikumar B.N., Gastrein P., Lepleux M., et al. Lack of the presynaptic RhoGAP protein oligophrenin1 leads to cognitive disabilities through dysregulation of the cAMP/PKA signalling pathway. Philos. Trans. R Soc. Lond. B Biol. Sci. 2014;369:20130160. doi: 10.1098/rstb.2013.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khelfaoui M., Pavlowsky A., Powell A.D., Valnegri P., Cheong K.W., Blandin Y., Passafaro M., Jefferys J.G., Chelly J., Billuart P. Inhibition of RhoA pathway rescues the endocytosis defects in Oligophrenin1 mouse model of mental retardation. Hum. Mol. Genet. 2009;18:2575–2583. doi: 10.1093/hmg/ddp189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nadif Kasri N., Nakano-Kobayashi A., Malinow R., Li B., Van Aelst L. The Rho-linked mental retardation protein oligophrenin-1 controls synapse maturation and plasticity by stabilizing AMPA receptors. Genes Dev. 2009;23:1289–1302. doi: 10.1101/gad.1783809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nakano-Kobayashi A., Kasri N.N., Newey S.E., Van Aelst L. The Rho-linked mental retardation protein OPHN1 controls synaptic vesicle endocytosis via endophilin A1. Curr. Biol. 2009;19:1133–1139. doi: 10.1016/j.cub.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Powell A.D., Gill K.K., Saintot P.P., Jiruska P., Chelly J., Billuart P., Jefferys J.G. Rapid reversal of impaired inhibitory and excitatory transmission but not spine dysgenesis in a mouse model of mental retardation. J. Physiol. 2012;590:763–776. doi: 10.1113/jphysiol.2011.219907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Houy S., Estay-Ahumada C., Croise P., Calco V., Haeberle A.M., Bailly Y., Billuart P., Vitale N., Bader M.F., Ory S., et al. Oligophrenin-1 Connects Exocytotic Fusion to Compensatory Endocytosis in Neuroendocrine Cells. J. Neurosci. 2015;35:11045–11055. doi: 10.1523/JNEUROSCI.4048-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li J., O’Connor K.L., Hellmich M.R., Greeley G.H., Jr., Townsend C.M., Jr., Evers B.M. The role of protein kinase D in neurotensin secretion mediated by protein kinase C-alpha/-delta and Rho/Rho kinase. J. Biol. Chem. 2004;279:28466–28474. doi: 10.1074/jbc.M314307200. [DOI] [PubMed] [Google Scholar]
- 125.Arias-Romero L.E., Chernoff J. Targeting Cdc42 in cancer. Expert. Opin. Ther. Targets. 2013;17:1263–1273. doi: 10.1517/14728222.2013.828037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cardama G.A., Gonzalez N., Maggio J., Menna P.L., Gomez D.E. Rho GTPases as therapeutic targets in cancer (Review) Int. J. Oncol. 2017;51:1025–1034. doi: 10.3892/ijo.2017.4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Evelyn C.R., Ferng T., Rojas R.J., Larsen M.J., Sondek J., Neubig R.R. High-throughput screening for small-molecule inhibitors of LARG-stimulated RhoA nucleotide binding via a novel fluorescence polarization assay. J. Biomol. Screen. 2009;14:161–172. doi: 10.1177/1087057108328761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Friesland A., Zhao Y., Chen Y.H., Wang L., Zhou H., Lu Q. Small molecule targeting Cdc42-intersectin interaction disrupts Golgi organization and suppresses cell motility. Proc. Natl. Acad. Sci. USA. 2013;110:1261–1266. doi: 10.1073/pnas.1116051110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gao Y., Dickerson J.B., Guo F., Zheng J., Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl. Acad. Sci. USA. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee M.H., Kundu J.K., Chae J.I., Shim J.H. Targeting ROCK/LIMK/cofilin signaling pathway in cancer. Arch. Pharm. Res. 2019;42:481–491. doi: 10.1007/s12272-019-01153-w. [DOI] [PubMed] [Google Scholar]
- 131.Lin Y., Zheng Y. Approaches of targeting Rho GTPases in cancer drug discovery. Expert Opin. Drug Discov. 2015;10:991–1010. doi: 10.1517/17460441.2015.1058775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu W., Du W., Shang X., Wang L., Evelyn C., Florian M.C., Ryan M.A., Rayes A., Zhao X., Setchell K., et al. Rational identification of a Cdc42 inhibitor presents a new regimen for long-term hematopoietic stem cell mobilization. Leukemia. 2019;33:749–761. doi: 10.1038/s41375-018-0251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mardilovich K., Olson M.F., Baugh M. Targeting Rho GTPase signaling for cancer therapy. Future Oncol. 2012;8:165–177. doi: 10.2217/fon.11.143. [DOI] [PubMed] [Google Scholar]
- 134.Montalvo-Ortiz B.L., Castillo-Pichardo L., Hernandez E., Humphries-Bickley T., De la Mota-Peynado A., Cubano L.A., Vlaar C.P., Dharmawardhane S. Characterization of EHop-016, novel small molecule inhibitor of Rac GTPase. J. Biol. Chem. 2012;287:13228–13238. doi: 10.1074/jbc.M111.334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nassar N., Cancelas J., Zheng J., Williams D.A., Zheng Y. Structure-function based design of small molecule inhibitors targeting Rho family GTPases. Curr. Top. Med. Chem. 2006;6:1109–1116. doi: 10.2174/156802606777812095. [DOI] [PubMed] [Google Scholar]
- 136.Pajic M., Herrmann D., Vennin C., Conway J.R., Chin V.T., Johnsson A.K., Welch H.C., Timpson P. The dynamics of Rho GTPase signaling and implications for targeting cancer and the tumor microenvironment. Small GTPases. 2015;6:123–133. doi: 10.4161/21541248.2014.973749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shang X., Marchioni F., Sipes N., Evelyn C.R., Jerabek-Willemsen M., Duhr S., Seibel W., Wortman M., Zheng Y. Rational design of small molecule inhibitors targeting RhoA subfamily Rho GTPases. Chem. Biol. 2012;19:699–710. doi: 10.1016/j.chembiol.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xiao X.H., Lv L.C., Duan J., Wu Y.M., He S.J., Hu Z.Z., Xiong L.X. Regulating Cdc42 and Its Signaling Pathways in Cancer: Small Molecules and MicroRNA as New Treatment Candidates. Molecules. 2018;23:787. doi: 10.3390/molecules23040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang Y., Li J., Lai X.N., Jiao X.Q., Xiong J.P., Xiong L.X. Focus on Cdc42 in Breast Cancer: New Insights, Target Therapy Development and Non-Coding RNAs. Cells. 2019;8:146. doi: 10.3390/cells8020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rath N., Olson M.F. Rho-associated kinases in tumorigenesis: Re-considering ROCK inhibition for cancer therapy. EMBO Rep. 2012;13:900–908. doi: 10.1038/embor.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang Y., Lei R., Zhuang X., Zhang N., Pan H., Li G., Hu J., Pan X., Tao Q., Fu D., et al. DLC1-dependent parathyroid hormone-like hormone inhibition suppresses breast cancer bone metastasis. J. Clin. Investig. 2014;124:1646–1659. doi: 10.1172/JCI71812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.