Abstract
Wolbachia, an obligate intracellular bacterium estimated to infect millions of arthropod species worldwide, is currently being utilized in novel control strategies to limit the transmission of Dengue and Zika viruses. A limitation for Wolbachia-based control approaches is the difficulty of transferring Wolbachia to novel hosts and the lack of tools for the genetic transformation of Wolbachia due to the inability to culture Wolbachia outside the insect host cell in an axenic media. Here, we applied extracellular Wolbachia to phenotypic microarrays to measure the metabolic response of Wolbachia in media formulations with different pH levels and supplementation with Casamino acids. Results suggested a pH of 6.5–6.8 and showed that the supplementation of 1 mg/mL casamino acids increased the survival and longevity of Wolbachia in an axenic medium. In addition, phenotypic microarrays are a useful tool to measure the phenotypic response of Wolbachia under different media conditions, as well as determine specific components that may be required for an axenic medium. This study is an initial step toward the development of a potential Wolbachia axenic culture system.
Keywords: axenic media, phenotypic microarray, Aa23, Wolbachia
1. Introduction
Wolbachia pipientis is an obligate intracellular maternally inherited bacterium found in >55% of insects, as well as filarial nematodes and terrestrial crustaceans [1,2]. In insects, Wolbachia alters host reproduction with several phenotypes, including feminization, parthenogenesis, male killing, and cytoplasmic incompatibility (CI) [3]. The discovery of these reproductive alterations, particularly CI, has caused Wolbachia to be a bacterium of interest for vector control. CI is used to drive desired phenotypes, such as refractoriness to disease transmission, with the goal of replacing natural infected populations [4,5,6,7,8]. In addition, Wolbachia-induced CI is also currently used as part of an incompatible insect technique (IIT) [9] strategy, where males harboring incompatible infections are released with the goal of suppressing natural populations by incompatible mating. An unexplored Wolbachia based approach centers around paratransgenesis, or the process of decreasing vector competence through genetically modifying an organism’s symbionts. Due to the maternal transmission of Wolbachia and the reproductive advantage afforded by Wolbachia, it may be possible to use paratransgenesis to introduce a transgene into a population, in turn producing an antipathogenic molecule to block the transmission of vectored pathogens [8,10,11,12]. However, the genetic transformation of Wolbachia has not been achieved, due to the difficulty of delivering genetic constructs into an intracellular symbiont.
Through the observed horizontal transmission events, it is reasoned that Wolbachia must have spent a portion of their life cycle outside of their host. Empirical studies subsequently demonstrated Wolbachia can live outside of its host and retain viability for up to seven days [13]. Studies in vivo demonstrated the presence of Wolbachia in the hemolymph of both larvae and adults of Drosophila and mosquitoes, which provides further evidence for the idea that Wolbachia can survive extracellularly [14,15,16,17]. It would be beneficial from a research perspective for Wolbachia to replicate outside of the host cell, so the bacterium can be better studied and made easily available for transfection strategies. However, cellular replication has never been observed outside of its host cells, suggesting metabolic limitations or replication factors are not available or produced outside of the insect cells to support Wolbachia replication.
Wolbachia has a reduced genome similar to other intracellular symbionts and pathogens, which is consistent with the hypothesis that Wolbachia relies on its host cell machinery for energy sources and other metabolic factors [18,19,20,21]. Genomic studies have suggested the metabolic properties of Wolbachia are similar to Rickettsia bacteria, meaning Wolbachia may have limited carbohydrate metabolism, participate in lipid synthesis, and depend on amino acid transport [18,19,20,21]. Recent studies have also suggested that Wolbachia titers in host tissue are dependent upon ubiquitin and endoplasmic reticulum associated protein degradation pathways (ERAD) to supply sufficient amino acids for growth and reproduction [22]. The results of these studies also suggest the absence of glycolytic and gluconeogenic enzymes, the reduced pathways for amino acid biosynthesis, and the presence of transporters for proline, aspartate/glutamate, and alanine, which again suggests a reliance on host amino acids as a major energy source [18,23].
The creation of a cell-free media to support intracellular endosymbiont growth has been accomplished before. Studies of the predicted metabolic capacities of bacteria from the genera Coxiella, Chlamydia, and Rickettsia and other obligate symbionts suggest a common nutritional deficiency of amino acids that is compensated for by the activities of amino acid and peptide permeates that scavenge amino acids from their hosts [24]. Particularly, the Q fever bacterium Coxiella burnetii was systematically evaluated for conditions to support extracellular growth [25], leading to the development of a complex nutrient medium that supported replication and growth of the bacteria. Another factor to consider when constructing a cell-free media is the pH required for growth. The pH level for insect cell culture growth is well defined depending on the insect cell type, but intracellular pH levels can vary depending upon the media type and media pH. Insect cell lines have been shown to have the highest growth rates and maximum densities at a pH of 6.2–6.8 [26]. In the presence of an acidic media, insect cells have been shown to have a capacity to buffer pH inside of cells and maintain a neutral pH level of 7.0 [27]. Previous attempts to isolate Wolbachia and examine replication were performed in Schneider’s media at a pH of 6.2–6.5, which may be optimal for insect cells that can buffer the pH level, but not extracellular Wolbachia, which may require pH levels in an axenic media to be similar to intracellular levels of 6.5–7.0. Axenic growth of C. burnetii has fueled important new areas of research to aid in understanding disease transmission, as well as a new set of genetic tools for functional genetic studies of the pathogen [28,29,30,31,32,33]. Similar to C. burnetii, a Wolbachia axenic culture and associated toolset have the potential to open similarly broad vistas of research.
Besides what is predicted from genomic data, little knowledge of host factors and metabolic requirements for Wolbachia survival and replication is available. Using a similar approach as that which was successful for the development of other axenic intracellular bacteria cultures [34,35,36,37,38,39], we attempted to determine general host factors and metabolic requirements involved in Wolbachia survival outside of its host cell. Here, we used a phenotypic microarray approach to examine the response of Wolbachia in an axenic culture. Specifically, we altered the pH and casamino acid concentration in an axenic medium and measured the metabolic response of extracellular Wolbachia using phenotypic microarrays. Results will be useful for the future testing and identification of the conditions required for Wolbachia survival and extracellular growth in an axenic media.
2. Materials and Methods
2.1. Cell Culture
The wAlbB infected Aa23 cell line used in experiments was obtained from Dr. Zhiyong Xi [40]. Cells were cultured in 25 cm2 or 75 cm2 cell culture flasks (Techno Plastic Products, Trasadingen, Switzerland) in Schneider’s insect medium (SM) (MilliporeSigma, St. Louis, MO, USA) (Table S1) supplemented with 10% fetal bovine serum (FBS). All cell cultures were incubated at 28 °C with a CO2 concentration of ~0.2% (ambient atmospheric concentration). Cells were passaged at a 1:4 cell to media ratio every seven days. Aposymbiotic Aa23-T cells were generated by treating Aa23 cells with 3 mg/mL tetracycline for three passages, and subsequently referred to as Aa23-T.
2.2. Trypan Blue Staining and Hemocytometer Aa23 Cell Counts
To determine cell counts prior to Wolbachia isolations, 200 µL of infected Aa23 cells were subjected to a centrifugation step at 200× g for 10 min. The pellet was then resuspended in 200 µL of 1× phosphate-buffered saline (PBS) solution. The cellular resuspension was then diluted in a 1:1 ratio with 0.4% Trypan Blue stain (Gibco, Carlsbad, CA, USA). Approximately 200 µL of isolated Wolbachia was centrifuged for five minutes at 16,000× g and the pellet was also resuspended in 1× PBS solution and diluted with Trypan blue in an identical 1:1 ratio. After five minutes, 10 µL of the two suspensions were placed in a hemocytometer and cells counted using a DMIL LED inverted microscope (Leica biosystems, Wetzlar, Germany) at 20× magnification. The hemocytometer grid images were captured using a 3 MP USB 2.0 Color CMOST Camera and software (v3.2.0000) (Amscope, Irvine, CA, USA).
2.3. Confirmation of Wolbachia Infection Status Using PCR
A DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany) was used to extract DNA from cell lines and isolated extracellular Wolbachia following manufacturer’s instructions. Wolbachia-specific primers were used to confirm the presence of Wolbachia in both intracellular and extracellular conditions [41,42,43,44,45]. Polymerase chain reaction (PCR) was also used to check for contamination of Acinetobacter spp., Cardinium spp., and Asaia spp. All primer sets to confirm Wolbachia infections and other bacterial contamination are listed in Supplementary Table S2. For all reactions, 1 µL of isolated DNA was amplified in 25 mM of KCL, 25 mM of Tris-HCL (pH 9.0), 20 mM of (NH4)2SO4, and 0.025% Triton X-100, 0.25 mM of MgCl2, 0.25 mM of dNTPs, 0.5 mM of primers, and 1 µL of Taq DNA polymerase in a total volume of 25 µL. The PCR amplification protocol was 10 min at 95 °C, 35 cycles of 30 s at 95 °C, 30 s at 55 °C, and 1 min at 72 °C, followed by a 10-min extension step at 72 °C. The PCR reaction was run on a T100 Thermocycler (Bio-Rad laboratories, Hercules, CA, USA). All reactions products were visualized on a 1.52–% agarose gel, stained with GelRed (Biotium, Fremont, CA, USA), and visualized under ultraviolet illumination.
2.4. Fluorescent In-Situ Hybridization
Fluorescent in-situ hybridization (FISH) was performed on the Aa23 and Aa23-T cell lines to confirm the presence and absence of Wolbachia, respectively. Cells were grown to 90% confluency at 28 °C and 300 µL of the cells were added to four wells of an eight-well Nunc Lab-Tek™ Chamber slide system (ThermoFisher Scientific, Waltham, MA, USA). At 24 h post-extraction, isolated Wolbachia cells were added to the two of the other wells and Aa23-T cells were added to the two remaining wells. The cells were incubated in the chambered wells overnight for 15 h at 28 °C. Cells were taken out the next day and fixed in 4% formaldehyde (in 1× PBS) for 40 min at room temperature (RT). This was followed by two washes with 1× PBS. Next, the cells were prehybridized for ~ 2 h at RT. The prehybridization buffer consisted of 50% deionized formamide, 20% 20× sodium saline citrate (SSC) solution, 1% 50× Denhardt’s Reagent, 10% 1 mol of dithriothreitol (DTT), 0.25 of mg/mL t-RNA, and 0.25 mg/mL of poly(A). The prehybridization step was followed with overnight hybridization (~ 18 h) at 37 °C with gentle shaking. The prehybridization buffer was supplemented with 200 mg/mL of dextran sulfate, 250 mg/liter of salmon sperm DNA, and Wolbachia-specific probes (5′-/56-FAM/AATCCGGCCGARCCGACCC-3′); 5′-/56-FAM/CTTCTGTGAGTACCGTCATTATC-3′) [13]. After hybridization, the cells were washed with denatured SSC solution in the following order: Wash buffer 1 (1× SSC augmented with 10 mmol/liter DTT) at RT with gentle shaking, wash buffer 1 at 55 °C with gentle shaking, and two washes at 55 °C with buffer 2 (0.5× SSC augmented with 10 mmol/liter DTT) with gentle shaking. Following the wash steps, cells were stained with DAPI at room temperature for 5 min followed by three washes with 1× PBS for 5 min each. The cells were then observed under a BX-41 epifluorescence microscope (Nikon, Tokyo, Japan) with appropriate filters at 60x magnification. Images were captured and processed with the help of QCapture™ software (v2.0.12) (Teledyne Qimaging, Surry, British Columbia, Canada).
2.5. Isolation of Extracellular Wolbachia
To isolate the wAlbB infection, a mean of 9.59 × 105 ± 1.97 × 104 cells was normalized during the log growth phase and used for each isolation procedure (Figure 1a). Extracellular Wolbachia was isolated using a modified procedure previously described [13]. Before the final centrifugation step, 200 µL of a 250-mM sucrose solution was added to each milliliter of isolated Wolbachia solution. The final centrifugation step was performed at 17,000× g for 10 min. The resulting pellet contained extracellular Wolbachia and after discarding the supernatant, the pellet was resuspended in SM + 10% fetal bovine solution (FBS) and passed through a 2.7-µM filter. To examine for potential environmental contamination, 20 µL of the isolation solution was streaked on LB agar and incubated for 48 h at 28 °C.
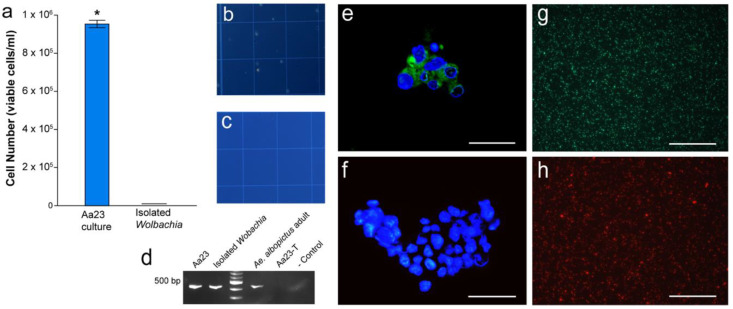
Figure 1.
(a) Aa23 cell counts prior to Wolbachia isolation procedures. * above the bars represents a significant difference (t-test, p < 0.05), (b) pre- and (c) post-Wolbachia isolation using Trypan Blue stain, (d) Polymerase chain reaction (PCR) confirmation of Wolbachia infections in Aa23 cells and isolations, (e) Fluorescent in-situ hybridization (FISH) staining of Aa23 cells confirming Wolbachia infection, (f) FISH staining of Aa23-T aposymbiotic cells showing the absence of a Wolbachia infection, and (g) Baclight LIVE/DEAD™ staining of Wolbachia immediately after isolation procedures, and (h) Baclight LIVE/DEAD™ staining of heat killed Wolbachia. White scale bars represent 50 µM.
2.6. Epifluorescence Microscopy
To confirm the isolation of extracellular Wolbachia, a Baclight LIVE/DEAD™ bacterial viability kit was used to visualize the bacterium (ThermoFisher Scientific, Waltham, MA, USA). SYTO9 and propidium iodide (PI) were used to differentiate the live and dead bacterial cells following manufacturer’s instructions. Briefly, 100 µL of each of SYTO9 and PI were pre-mixed to prepare the stain mix and 10 µL of the extracellular Wolbachia was added to the stain mix in a 1:1 ratio. This mixture was incubated in the dark for 10 min. The bacteria-stain mix (20 µL) was then placed on a glass slide, covered with a coverslip, and viewed using a Nikon BX41 inverted microscope at 100× magnification. To confirm staining efficacy, extracellular Wolbachia were heat-killed at 95 °C for 15 min and subsequently stained. Images were processed with QCapture™ software (v2.0.12) (Teledyne Qimaging, Surry, British Columbia, Canada).
2.7. Phenotypic Microarray Assays
Two categories of phenotypic microarray (PM) plates, PM1 carbon source plates and PM3B nitrogen source plates (Biolog, Hayward, CA, USA), were used to assess the metabolic activity of extracellular Wolbachia [46]. Biological replicates of each plate type and treatment were completed in duplicate or triplicate. For treatment plates, 100 µL of isolated Wolbachia in post-isolation media (SM supplemented with 10% FBS with either an adjusted pH or the addition of casamino acids) was added to each well with 110 µL of Redox Mix G (Biolog, Hayward, CA, USA). Control plates for the normalization of background absorbance consisted of post-isolation media at a pH of 6.8 for both PM1 and PM3B microarray plate assays. PM1 and PM3B microarray plates were incubated at 28 °C with a CO2 concentration of ~0.2% for four days. To examine for an effect of pH on the survival of the extracellular Wolbachia in an axenic medium, the unbuffered pH of the post-isolation media (~ 6.8) was adjusted to 6.5, 7, and 7.5, respectively. The resulting media was filter sterilized using a 0.2-µm filter before being used for the isolation of Wolbachia. To examine for an effect of casamino acids on extracellular Wolbachia, the post-isolation media was supplemented with 1 mg/mL, 2 mg/mL, and 4 mg/mL of casamino acids (VWR chemicals, Solon, OH, USA) and filter sterilized. The absorbance measured for each well on the PM plates was at an O.D. of 600 in a Biotek Synergy H1 Microplate Reader (Biotek, Winooski, VT, USA). Absorbance measurements were analyzed using a Biotek Gen 5 Microplate Reader and imager software v.3.02 (Biotek, Winooski, VT, USA). All absorbance measurements were taken every 24 h over a period of four days. To normalize absorbance values and to account for background noise from the isolation medium, absorbance values from control plates were subtracted from the treatment plate absorbance values for each plate type and replicate.
2.8. Quantitative Real-Time PCR Analysis
Extracellular Wolbachia was quantified using quantitative polymerase chain reaction (qPCR). DNA was isolated using a Qiagen DNeasy kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. qPCR was performed using PowerUp™ SYBR™ Green Master Mix (Applied Biosystems, Foster City, CA, USA) following manufacturer instructions with an ABI 7300 real-time qPCR system (Applied Biosystems, Foster City, CA, USA). Primers used for reactions are listed in Table S2. Wolbachia absolute cell counts were quantified by performing a serial dilution of an Aa23 cell culture sample and the generation of a standard curve. Three biological replicate samples were amplified in triplicate and the average Ct values were used to quantify Wolbachia.
2.9. Wolbachia Cell Counts
To confirm the isolation of extracellular Wolbachia a Baclight LIVE/DEAD™ bacterial viability kit (ThermoFisher Scientific, Waltham, MA, USA) was used to differentiate live and dead bacterial cells following manufacturer’s instructions. Ten µL of the stained solution was placed on a slide with a cover slip and imaged at 100× using a Nikon BX41 inverted microscope with epifluorescence. Viable Wolbachia cells stained green (SYTO9) and dead cells stained red (PI). Viable cells were counted using three representative images using Image-J.
2.10. Statistical Analyses
All statistical analyses were performed using JMP software (SAS, Cary, NC, USA). Statistical difference of Wolbachia counts using qPCR at each timepoint were determined using t-tests, with a significance level of p < 0.05. Heatmaps were generated using mean absorbance values from replicate phenotypic microarray assays. All data from phenotypic microarrays for the different treatment levels of casamino acids and pH were combined into a single data matrix. Differences between phenotypic microarray treatment groups within each time point were determined using a one-way analysis of variance (ANOVA). Differences in cell counts were determined using Kruskall–Wallis and pairwise Mann–Whitney tests. Difference between treatment levels were considered to be significant when the p-value was < 0.05.
3. Results
3.1. Confirmation of Wolbachia Infections and Isolation of Wolbachia
To determine that only Wolbachia was in the isolate and there were no residual cells, Aa23 cells isolates were stained with trypan blue. An analysis of the ratio of white (living) to blue (dead) Aa23 cells from three independent observations showed no Aa23 cells were observed in the Wolbachia isolate (Figure 1b,c). Wolbachia infections were confirmed in Aa23 cells and isolates using PCR and FISH (Figure 1d–f). Live Wolbachia was observed immediately after isolation using an SYTO9 stain as shown by green cells (Figure 1g). In addition, a heat-killed isolated Wolbachia sample showed only PI red-stained cells (Figure 1h). Aedes albopictus is known to harbor other bacterial symbionts, including Cardinium spp., Asaia spp., and Acinetobacter spp. [42,47], which could confound metabolic assays. Results from PCR assays suggest that isolates contained no Asaia spp., Cardinium spp., or Acinetobacter DNA (Table S3). Streaking of the Wolbachia isolate on LB plates also showed no evidence of contamination resulting from the isolation procedure. Plates remained colony-free after the 48-h incubation period.
3.2. Phenotypic Microarray Assays
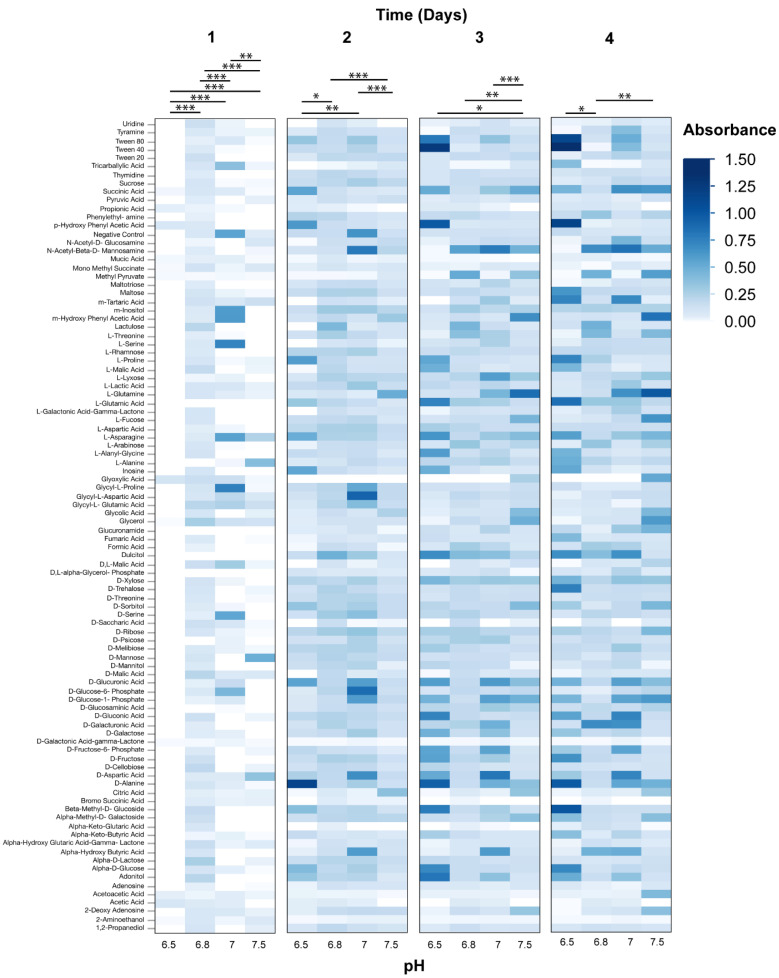
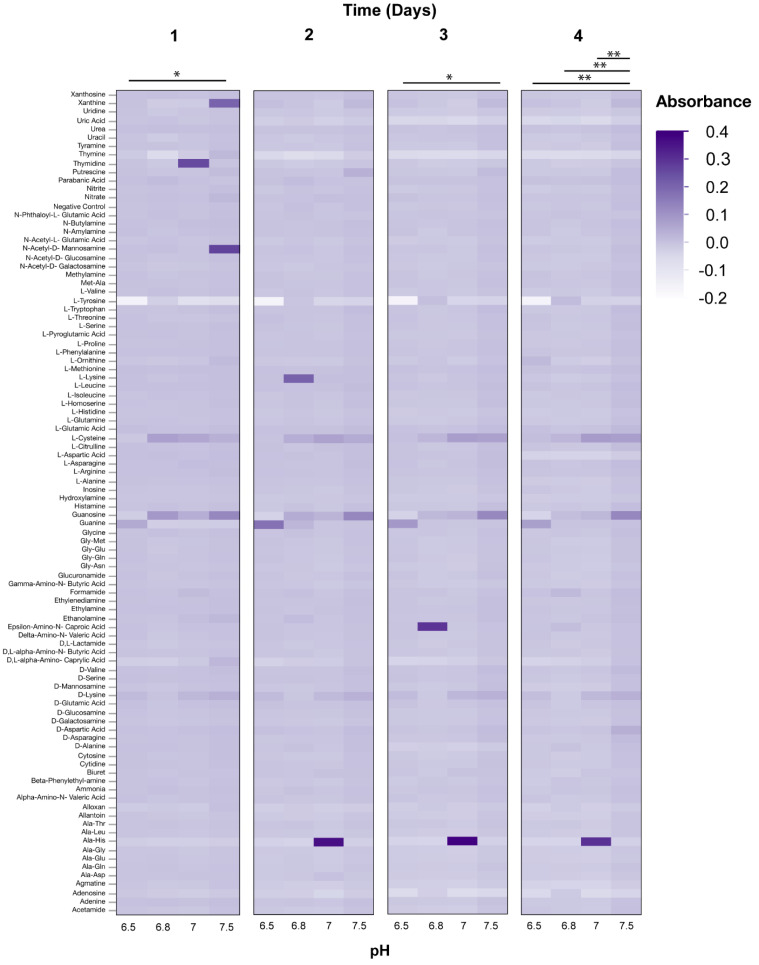
Phenotypic microarrays (PM) were used to measure the metabolic response of axenic Wolbachia to changes in pH over a four-day period. The range of normalized absorbance values (Au) of PM1 microarray plates were: pH 6.5—0.072–2.429, 6.8—0.073–1.32; 7.0—0.076–1.785; 7.5—0.065–1.779; Casamino acids 0 mg/mL—0.056–1.163, 1 mg/mL—0.061–1.221, 2 mg/mL—0.058–1.17, 4 mg/mL—0.054–1.992. The range of normalized absorbance values of PM3 microarray plates were: pH 6.5—0.051–0.451, 6.8—0.052–1.005, 7.0—0.05–1.402, 7.5—0.051–0.885; Casamino acids 0 mg/mL – 0.048–0.889, 1 mg/mL—0.05–1.477, 2 mg/mL—0.049–0.861, 4 mg/mL—0.051–1.168. Changes to media pH were demonstrated to impact the metabolic response of Wolbachia when added to PM1 plates (ANOVA, Day 1, F = 7.58, p < 0.0001; Day 2, F = 30.9, F < 0.0001; Day 3, F = 3.0, p = 0.0293; Day 4, F = 2.8, p = 0.0343). Media formulations of a pH of 6.5 and 6.8 typically had the highest metabolic response when examining absorbance values compared to media pH values of 7.0, and 7.5 (Figure 2). However, little differences in absorbance values were observed between the media formulations of different pH values when using PM3B plates (ANOVA, Day 1, F = 2.40, p = 0.0663; Day 2, F = 1.26, p = 0.2855; Day 3, F = 1.93, p = 0.1217; Day 4, F = 5.2135, p = 0.0014) (Figure 3). The metabolic response of axenic Wolbachia was also measured in response to changes in casamino acid concentrations in post-isolation media. Changes to media casamino acid concentration was demonstrated to impact the metabolic response of Wolbachia on PM1 plates (ANOVA, Day 1, F = 25.75, p < 0.0001; Day 2, F = 18.71, p = < 0.0001; Day 3, F = 7.05, p = 0.0001; Day 4, F = 5.10, p = 0.0016). When examining absorbance values, media formulations with a casamino acid concentration of 1 mg/mL had the highest metabolic response as compared to media formulations with no supplemented casamino acids, 2 mg/mL, and 4 mg/mL on PM1 plates (Figure 4). No difference in absorbance values were observed in media formulations with different casamino acid concentrations on PM3 plates (ANOVA, Day 1, F = 1.23, p = 0.30; Day 2, F = 2.39, p = 0.07; Day 3, F = 1.23, p = 0.30; Day 4, F = 0.66, p = 0.57) (Figure 5).
Figure 2.
Heat map of PM1 phenotypic microarray data from Wolbachia isolated and maintained in Schneider’s medium supplemented with 10% FBS at different pH levels (n = 2 for each treatment). All data were normalized by subtracting absorbance values of control plates containing Schneider’s medium only from absorbance readings of treatment plates. Significant differences between treatments are represented by horizontal bars above each column for each timepoint (t-tests, * p < 0.05, ** p < 0.005, *** p < 0.0005).
Figure 3.
Heat map of PM3 phenotypic microarray data from Wolbachia isolated and maintained in Schneider’s medium supplemented with 10% fetal bovine serum (FBS) at different pH levels (n = 3 for each treatment) All data were normalized by subtracting absorbance values of control plates containing Schneider’s medium only from absorbance readings of treatment plates. Significant differences between treatments are represented by horizontal bars above each column for each timepoint (t-tests, * p < 0.05, ** p < 0.005, *** p < 0.0005).
Figure 4.
Heat map of PM1 phenotypic microarray data from Wolbachia isolated and maintained in Schneider’s medium supplemented with 10% FBS and different concentrations of Casamino acids (n = 3 for each treatment). All data were normalized by subtracting absorbance values of control plates containing Schneider’s medium only from absorbance readings of treatment plates. Significant differences between treatments are represented by horizontal bars above each column for each timepoint (t-tests, * p < 0.05, ** p < 0.005, *** p < 0.0005).
Figure 5.
Heat map of PM3 phenotypic microarray data from Wolbachia isolated and maintained in Schneider’s medium supplemented with 10% FBS and different concentrations of Casamino acids (n = 3 for each treatment). All data were normalized by subtracting absorbance values of control plates containing Schneider’s medium only from absorbance readings of treatment plates. Significant differences between treatments are represented by horizontal bars above each column for each timepoint (t-tests, * p < 0.05, ** p < 0.005, *** p < 0.0005).
For media formulations where pH or casamino acid concentration was altered, substrates on PM1 and PM3 plates associated with the TCA cycle and glycolysis were oxidized (i.e., Citric acid, Fructose-6-phosphate, Acetic acid), suggesting that these pathways are functional in extracellular Wolbachia. The presence of membrane transporters in Wolbachia genomes for proline, aspartate/glutamate, and alanine and pathways for metabolism of amino acids suggests that Wolbachia is dependent upon purine synthesis from amino acids. The oxidation of the amino acids (L-Alanine, L-Arabinose, L-Cysteine, L-Lyxose, L-Glutamine) and the purine guanine on PM3 plates support this hypothesis.
3.3. Quantitative Real-Time PCR
Phenotypic microarrays suggest that a pH of 6.5 is optimal for an axenic medium. To investigate Wolbachia survival in an axenic medium with a pH of 6.5, qPCR was used to quantify the number of Wolbachia cells post-isolation over a four-day period. A greater number of Wolbachia copies were observed in an axenic medium with a pH of 6.5 compared to compared to unaltered Schneider’s media + 10% FBS (pH 6.8) (Kruskal–Wallis, Chi-sq = 9.0, p = 0.01). Results suggest an increase in the survivorship and longevity of extracellular Wolbachia in an axenic medium supplemented with a pH of 6.5 (Figure 6). qPCR was also used to quantify the number of Wolbachia cells over a four-day period in an axenic Wolbachia medium supplemented with 1 mg/mL of casamino acids, which showed the greatest metabolic activity in the phenotypic microarray assays. A greater number of Wolbachia copies were observed in an axenic medium supplemented with 1 mg/mL of casamino acids compared to unaltered Schneider’s media + 10% FBS (Kruskal-Wallis, Chi-sq = 8.4, p = 0.2). Results suggest an increase in the survivorship and longevity of extracellular Wolbachia in an axenic medium supplemented with 1 mg/mL of casamino acids (Figure 6).
Figure 6.
Extracellular Wolbachia count using quantitative polymerase chain reaction (qPCR) in (A) Schneider’s medium with 10% FBS adjusted to a pH of 6.5, and (B) Schneider’s medium with 10% FBS and 1 mg/mL Casamino acids. Significant differences are indicated by different letters above each bar (Mann–Whitney, p < 0.05).
3.4. Wolbachia Cell Counts
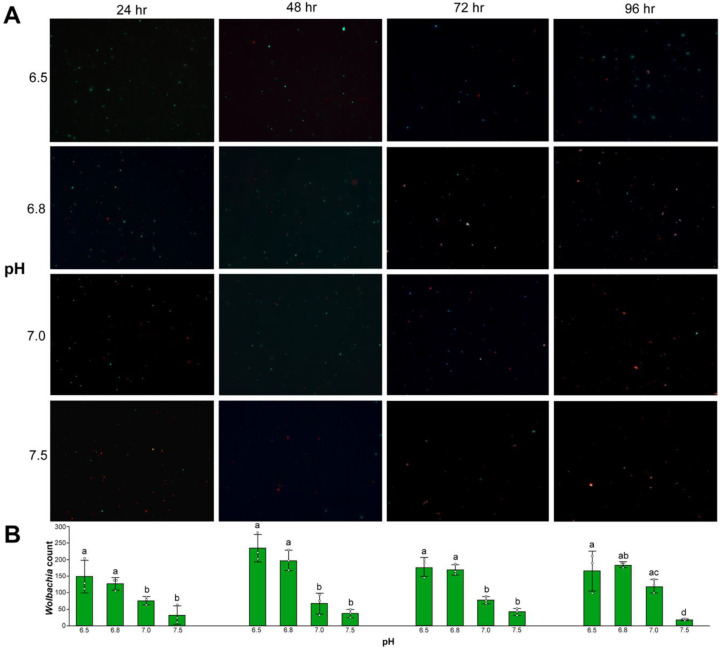
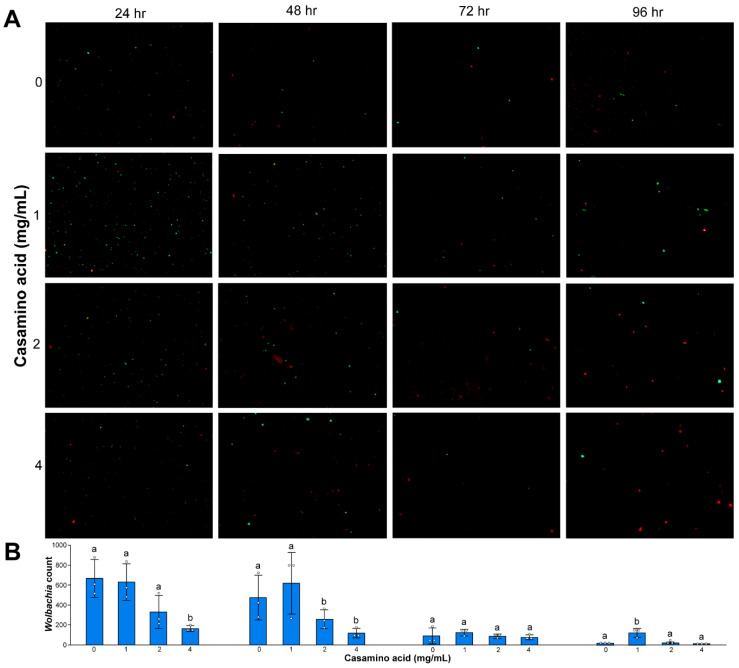
Baclight LIVE/DEAD™ images and cell counts were used as an additional metric to measure Wolbachia survivorship and longevity in axenic media formulations. Cell counts suggest a greater number of living Wolbachia cells in axenic media formulations with a pH of 6.5 and 6.8 at one, two, and three days post-Wolbachia isolation (Day 1, Kruskal–Wallis, Chi-sq = 8.94, p = 0.03; Day 2, Kruskal–Wallis, Chi-sq = 8.95, p = 0.03; Day 3, Kruskal–Wallis, Chi-sq = 9.46, p = 0.02; Day 4, Kruskal–Wallis, Chi-sq = 7.76, p = 0.05) (Figure 7). Cell counts also suggest a greater number of living Wolbachia cells in axenic media formulations when supplemented with 1 mg/mL of casamino acids (Day 1, Kruskal–Wallis, Chi-sq. = 8.23, p = 0.04; Day 2, Kruskal–Wallis, Chi-sq. = 8.3, p = 0.04; Day 3, Kruskal–Wallis, Chi-sq. = 2.3, p = 0.51; Day 4, Kruskal–Wallis, Chi-sq. = 9.4, p = 0.02) (Figure 8).
Figure 7.
(A) Isolated Wolbachia stained with Baclight LIVE/DEAD™ viability assays in Schneider’s medium supplemented with 10% FBS and an adjusted pH of 6.5, 6.8, 7.0, and 7.5. Live and dead Wolbachia are green and red, respectively. (B) Live Wolbachia was counted using Image-J from three representative fields of view at 100x. Significant differences are indicated by different letters above each bar (Mann–Whitney, p < 0.05).
Figure 8.
(A) Isolated Wolbachia stained with BacLight LIVE/DEAD™ viability assays in Schneider’s medium supplemented with 10% FBS and casamino acid concentrations of 0 mg/mL, 1 mg/mL, 2 mg/mL, and 4 mg/mL. Live and dead Wolbachia are green and red, respectively. (B) Live Wolbachia was counted using Image-J from three representative fields of view at 100x. Significant differences are indicated by different letters above each bar (Mann–Whitney, p < 0.05).
4. Discussion
Understanding the phenotypic response of Wolbachia outside of its host cell is an important first step in the development of an axenic Wolbachia culture and may help lead to the development of a Wolbachia-based paratransgenic interventions for insect vector borne diseases. Wolbachia-based paratransgenic approaches have remained theoretical because Wolbachia has yet to be genetically transformed inside or outside the host cell [48,49,50]. Separation of Wolbachia from its host cell in an axenic culture will facilitate the genetic transformation of Wolbachia by providing an axenic system to select for transformed Wolbachia cells. Selected transformed cells can reintroduced back into aposymbiotic cell lines for propagation. Furthermore, an axenic culture system will support additional studies to determine which Wolbachia genes and proteins are involved in host reproductive modifications, as well as potentially direct Wolbachia effects on pathogen disruption.
The observed metabolic activity of Wolbachia in the phenotypic microarrays provides a valuable tool to measure metabolic response to different media formulations and growth conditions outlined. The results from the phenotypic microarrays have identified several key amino acids and metabolites that require testing as supplements to axenic media formulations. Several of the compounds shown to have higher metabolism by Wolbachia wAlbB are a potential for precursors in the Kreb’s cycle, as well as glycolysis. Also, since Wolbachia lacks any ATPase machinery and is known to rely on glycolysis to generate adenosine monophosphate (AMP), guanosine monophosphate (GMP), and xanthosine monophosphate (XMP), this suggests that Wolbachia may derive energy from amino acids [18,21]. Purine ring containing compounds like ribose, lyxose, and xylose could be potential precursors of adenosine triphosphate (ATP), guanosine triphosphate (GTP), and cyclic-AMP that may be beneficial for the Wolbachia maintenance and survival [18,51,52,53,54,55,56,57]. In addition, from studies on Wolbachia genomes, it is known that there are multiple proline, aspartate/glutamate, and alanine membrane transporters present in Wolbachia [18,54,55,58,59]. The known Wolbachia pathways of amino acid metabolism suggest that Wolbachia is dependent on purine synthesis for the amino acids. The oxidation of the above-mentioned amino acids on the PM3B plates also supports this hypothesis. Overall, this suggests a reliance of Wolbachia on host amino acids and less reliance on carbohydrates as a major source of its energy. Previous genomic studies have drawn similarities between Wolbachia and Rickettsia, where both have limited carbohydrate metabolism, lipid biosynthesis, and amino acid transport capabilities [18,19,20,21].
Changes in pH in an axenic medium may also play an important role in Wolbachia survival outside of its host cell. An observed increase in Wolbachia longevity and survivorship and increased metabolic activity on phenotypic microarrays suggests that an axenic medium consisting of a pH of 6.5–6.8 may be most appropriate for Wolbachia survival outside of its host cell. The observed increase in Wolbachia longevity and survivorship, and increased metabolic activity on phenotypic microarrays, suggest a 1 mg/mL of casamino acids may also be an important component in an axenic medium formulation by providing amino acid precursors for energy production in addition to the components and amino acids in Schneider’s media (Table S1). Collectively, the data suggests that a medium consisting of Schneider’s insect media, 10%FBS, 1 mg/mL Casamino acids, and a pH of 6.5-6.8 is the best suited for maintaining viable extracellular up to four days. Additional experiments with the aforementioned medium formulation are required to determine if the medium formulation identified in this work will sustain Wolbachia longer than previous reports of seven days [13].
The identified medium conditions, as well as other metabolites identified on phenotypic microarrays, suggest the potential to develop an axenic medium to support the survivorship and potential replication of Wolbachia outside of its host cell. Whereas replication of Wolbachia in an axenic medium would be a significant research achievement and would open multiple avenues for future research, this may not be possible due to the intracellular nature of Wolbachia and reliance of Wolbachia on its host cell for its machinery and nutrients. However, if Wolbachia could be maintained outside of the host cell for a period of time and remain viable, Wolbachia could be transformed, and the transformed cells could be selected and reintroduced back into a novel insect or host cell line. Future work will focus on increasing the longevity and survivorship outside of the host cell with the ultimate goal of Wolbachia replication in an axenic medium. The observed results from this study also provide insight into the development of methodology to culture intracellular pathogens, such as closely related Rickettsia species, as well as Chlamydiae species, which result in Rocky Mountain spotted fever (RMSF) and chlamydial infections, respectively.
5. Conclusions
In conclusion, we demonstrated that Wolbachia can be isolated and its metabolic activity can be measured using phenotypic microarrays. In addition, we demonstrated that amino acid supplementation, as well as adjusting pH similar to the internal environment of an insect cell, impacts the phenotypic response of Wolbachia and increases the survivorship of Wolbachia outside of its host cell. Future work will incorporate identified metabolites from phenotypic microarrays with different media formulations in an attempt to develop an axenic medium that will extend the survivorship and longevity of Wolbachia and/or examine for Wolbachia replication outside of the host cell.
Acknowledgments
We thank Catherine Wakeman (Texas Tech University) for allowing us to use the Biotek Synergy H1 Microplate Reader (Winooski, VT). We thank Zhiyong Xi (Michigan State University) for providing the Aa23 cell line.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/7/1060/s1, Table S1: Schneider’s media formulation (Sigma–S0146), Table S2: Primer sequences used to assay Wolbachia and other potential bacterial infections in cell cultures and Wolbachia extractions, Table S3: Wolbachia isolate bacterial infections status determined by PCR.
Author Contributions
Conceptualization, C.L.B.; methodology, C.L.B., A.G., and A.M.K.; software, C.L.B.; validation, A.M.K., A.G.; formal analysis, C.L.B., A.G., A.M.K.; investigation, A.G. and A.M.K.; data curation, C.L.B. and A.K.; writing—original draft preparation, C.L.B., A.G., and A.M.K.; writing—review and editing, C.L.B and A.M.K.; visualization, C.L.B. and A.M.K.; supervision, C.L.B.; project administration, C.L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by start-up funds from Texas Tech University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Werren J.H., Windsor D.M. Wolbachia infection frequencies in insects: Evidence of a global equilibrium? Proc. Biol. Sci. 2000;267:1277–1285. doi: 10.1098/rspb.2000.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hilgenboecker K., Hammerstein P., Schlattmann P., Telschow A., Werren J.H. How many species are infected with Wolbachia?—A statistical analysis of current data. Fems. Microbiol. Lett. 2008;281:215–220. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werren J.H., Baldo L., Clark M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 4.Moreira L.A., Iturbe-Ormaetxe I., Jeffery J.A., Lu G., Pyke A.T., Hedges L.M., Rocha B.C., Hall-Mendelin S., Day A., Riegler M., et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 5.Hurk v.d.A.F., Hall-Mendelin S., Pyke A.T., Frentiu F.D., McElroy K., Day A., Higgs S., O’Neill S.L. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl. Trop. Dis. 2012;6:e1892. doi: 10.1371/journal.pntd.0001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caragata E.P., Dutra H.L.C., Moreira L.A. Inhibition of Zika virus by Wolbachia in Aedes aegypti. Microb. Cell. 2016;3:293–295. doi: 10.15698/mic2016.07.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan C.H., Wong P.J., Li M.I., Yang H.T., Ng L.C., O’Neill S.L. wMel limits zika and chikungunya virus infection in a Singapore Wolbachia-introgressed Ae. aegypti strain, wMel-Sg. PLoS. Negl. Trop. Dis. 2017;11:e0005496. doi: 10.1371/journal.pntd.0005496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flores H.A., O’Neill S.L. Controlling vector-borne diseases by releasing modified mosquitoes. Nat. Rev. Microbiol. 2018;16:508–518. doi: 10.1038/s41579-018-0025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mains J.W., Brelsfoard C.L., Rose R.I., Dobson S.L. Femal adult Aedes albopictus suppression by Wolbachia-infected male mosquitoes. Sci. Rep. 2016;6:33846. doi: 10.1038/srep33846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aultman K.S., Beaty B.J., Walker E.D. Genetically manipulated vectors of human disease: A practical overview. Trends Para. 2001;17:507–509. doi: 10.1016/S1471-4922(01)02094-3. [DOI] [PubMed] [Google Scholar]
- 11.Aksoy S., Weiss B., Attardo G. Transgenesis and the Management of Vector-Borne Disease. Volume 627. Springer; New York, NY, USA: 2008. Paratransgenesis applied for control of tsetse transmitted sleeping sickness; pp. 35–48. [DOI] [PubMed] [Google Scholar]
- 12.Bourtzis K. Wolbachia-based technologies for insect pest population control. Adv. Exp. Med. Biol. 2008;627:104–113. doi: 10.1007/978-0-387-78225-6_9. [DOI] [PubMed] [Google Scholar]
- 13.Rasgon J.L., Gamston C.E., Xiaoxia R. Survival of Wolbachia pipientis in cell-free medium. Appl. Env. Micro. 2006;72:6934–6937. doi: 10.1128/AEM.01673-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes G.L., Koga R., Xue P., Fukatsu T., Rasgon J.L. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 2011;7:e1002043. doi: 10.1371/journal.ppat.1002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobson S.L., Bourtzis K., Braig H.R., Jones B.F., Zhou W., Rousset F., O’Neill S.L. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem. Mol. Biol. 1999;29:153–160. doi: 10.1016/S0965-1748(98)00119-2. [DOI] [PubMed] [Google Scholar]
- 16.Frydman H.M., Li J.M., Robson D.N., Wieschaus E. Somatic stem cell niche tropism in Wolbachia. Nature. 2006;441:509–512. doi: 10.1038/nature04756. [DOI] [PubMed] [Google Scholar]
- 17.Gamston C., Rasgon J. Maintaining Wolbachia in cell-free medium. J. Vis. Exp. 2007:233. doi: 10.3791/223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu M., Sun L.V., Vamathevan J., Riegler M., Deboy R., Brownlie J.C., McGraw E.A., Martin W., Esser C., Ahmadinejad N., et al. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: A streamlined genome overrun by mobile genetic elements. PLoS Bio. 2004;2:E69. doi: 10.1371/journal.pbio.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brownlie J.C., O’Neill S.L. Wolbachia genomes: Insights into an intracellular lifestyle. Curr. Biol. 2005;15:R507–R509. doi: 10.1016/j.cub.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 20.Klasson L., Westberg J., Sapountzis P., Naslund K., Lutnaes Y., Darby A.C., Veneti Z., Chen L., Braig H.R., Garrett R., et al. The mosaic genome structure of the Wolbachia wRi strain infecting Drosophila simulans. Proc. Natl. Acad. Sci. USA. 2009;106:5725–5730. doi: 10.1073/pnas.0810753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maren Ellegaard K., Klasson L., Naslund K., Bourtzis K., Anderson S. Comparative genomics of Wolbachia and the bacterial species concept. PLoS Genet. 2013;9:e1003381. doi: 10.1371/journal.pgen.1003381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White P.M., Serbus L.R., Debec A., Codina A., Bray W., Guichet A., Lokey R.S., Sullivan W. Reliance of Wolbachia on High Rates of Host Proteolysis Revealed by a Genome-Wide RNAi Screen of Drosophila Cells. Genetics. 2017;205:1473–1488. doi: 10.1534/genetics.116.198903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster J., Ganatra M., Kamal I., Ware J., Makarova K., Ivanova N., Bhattacharyya A., Kapatral V., Kumar S., Posfai J., et al. The Wolbachia genome of Brugia malayi: Endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 2005;3:e121. doi: 10.1371/journal.pbio.0030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Driscoll T.P., Verhoeve V.I., Guillotte M.L., Lehman S.S., Rennoll S.A., Beier-Sexton M., Rahman M.S., Azad A.F., Gillespie J.J. Wholly Rickettsia! Reconstructed Metabolic Profile of the Quintessential Bacterial Parasite of Eukaryotic Cells. MBio. 2017;8:e00859-17. doi: 10.1128/mBio.00859-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marmion B.P., Storm P.A., Ayres J.G., Semendric L., Mathews L., Winslow W., Turra M., Harris R.J. Long-term persistence of Coxiella burnetii after acute primary Q fever. QJM. 2005;98:7–20. doi: 10.1093/qjmed/hci009. [DOI] [PubMed] [Google Scholar]
- 26.Vlak J.M., de Gooijer C.D., Tramper J., Miltenburger H.G. Insect Cell Cultures: Fundamental and Applied Aspects. Springer; Dordrecht, The Netherlands: 1996. [Google Scholar]
- 27.Medina M., Lopez-Rivas A., Zuidema D., Belsham G.J., Domingo E., Vlak J.M. Strong buffering capacity of insect cells. Implications for the baculovirus expression system. Cytotechnology. 1995;17:21–26. doi: 10.1007/BF00749217. [DOI] [PubMed] [Google Scholar]
- 28.Beare P.A., Jeffrey B.M., Long C.M., Martens C.M., Heinzen R.A. Genetic mechanisms of Coxiella burnetii lipopolysaccharide phase variation. PLoS Pathog. 2018;14:e1006922. doi: 10.1371/journal.ppat.1006922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beare P.A., Larson C.L., Gilk S.D., Heinzen R.A. Two systems for targeted gene deletion in Coxiella burnetii. Appl. Env. Microbiol. 2012;78:4580–4589. doi: 10.1128/AEM.00881-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clay K.A., Hartley M.G., Russell P., Norville I.H. Use of axenic media to determine antibiotic efficacy against Coxiella burnetii. Int. J. Antimicrob. Agents. 2018;51:806–808. doi: 10.1016/j.ijantimicag.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Omsland A., Cockrell D.C., Fischer E.R., Heinzen R.A. Sustained axenic metabolic activity by the obligate intracellular bacterium Coxiella burnetii. J. Bacteriol. 2008;190:3203–3212. doi: 10.1128/JB.01911-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez S.E., Vallejo-Esquerra E., Omsland A. Use of Axenic Culture Tools to Study Coxiella burnetii. Curr. Protoc. Microbiol. 2018;50:e52. doi: 10.1002/cpmc.52. [DOI] [PubMed] [Google Scholar]
- 33.Sandoz K.M., Popham D.L., Beare P.A., Sturdevant D.E., Hansen B., Nair V., Heinzen R.A. Transcriptional Profiling of Coxiella burnetii Reveals Extensive Cell Wall Remodeling in the Small Cell Variant Developmental Form. PLoS ONE. 2016;11:e0149957. doi: 10.1371/journal.pone.0149957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pontes M.H., Dale C. Culture and manipulation of insect facultative symbionts. Trends Microbiol. 2006;14:406–412. doi: 10.1016/j.tim.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Omsland A., Cockrell D.C., Howe D., Fischer E.R., Virtaneva K., Sturdevant D.E., Porcella S.F., Heinzen R.A. Host cell-free growth of the Q fever bacterium Coxiella burnetii. Proc. Natl. Acad. Sci. USA. 2009;106:4430–4434. doi: 10.1073/pnas.0812074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Omsland A., Hackstadt T., Heinzen R.A. Bringing culture to the uncultured: Coxiella burnetii and lessons for obligate intracellular bacterial pathogens. PLoS Pathog. 2013;9:e1003540. doi: 10.1371/journal.ppat.1003540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brandt J.W., Chevignon G., Oliver K.M., Strand M.R. Culture of an aphid heritable symbiont demonstrates its direct role in defence against parasitoids. Proc. Biol. Sci. 2017;284:20171925. doi: 10.1098/rspb.2017.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matthew C.Z., Darby A.C., Young S.A., Hume L.H., Welburn S.C. The rapid isolation and growth dynamics of the tsetse symbiont Sodalis glossinidius. Fems. Microbiol. Lett. 2005;248:69–74. doi: 10.1016/j.femsle.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 39.Masson F., Calderon Copete S., Schupfer F., Garcia-Arraez G., Lemaitre B. In Vitro Culture of the Insect Endosymbiont Spiroplasma poulsonii Highlights Bacterial Genes Involved in Host-Symbiont Interaction. MBio. 2018;9:e00024-18. doi: 10.1128/mBio.00024-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Neill S.L., Pettigrew M.M., Sinkins S.P., Braig H.R., Andreadis T.G., Tesh R.B. In vitro cultivation of Wolbachia pipientis in an Aedes albopictus cell line. Insect Mol. Biol. 1997;6:33–39. doi: 10.1046/j.1365-2583.1997.00157.x. [DOI] [PubMed] [Google Scholar]
- 41.McGraw E.A., Merritt D.J., Droller J.N., O’Neill S.L. Wolbachia-mediated sperm modification is dependent on the host genotype in Drosophila. Proc. Biol. Sci. 2001;268:2565–2570. doi: 10.1098/rspb.2001.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minard G., Tran F.H., Raharimalala F.N., Hellard E., Ravelonandro P., Mavingui P., Valiente Moro C. Prevalence, genomic and metabolic profiles of Acinetobacter and Asaia associated with field-caught Aedes albopictus from Madagascar. Fems. Microbiol. Ecol. 2013;83:63–73. doi: 10.1111/j.1574-6941.2012.01455.x. [DOI] [PubMed] [Google Scholar]
- 43.Mühling M., Woolven-Allen J., Murrell J.C., Joint I. Improved group-specific PCR primers for denaturing gradient gel electrophoresis analysis of the genetic diversity of complex microbial communities. Isme. J. 2008;2:379. doi: 10.1038/ismej.2007.97. [DOI] [PubMed] [Google Scholar]
- 44.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voronin D., Tran-Van V., Potier P., Mavingui P. Transinfection and growth discrepancy of Drosophila Wolbachia strain wMel in cell lines of the mosquito Aedes albopictus. J. Appl. Microbiol. 2010;108:2133–2141. doi: 10.1111/j.1365-2672.2009.04621.x. [DOI] [PubMed] [Google Scholar]
- 46.Mackie A.M., Hassan K.A., Paulsen I.T., Tetu S.G. Biolog Phenotype Microarrays for phenotypic characterization of microbial cells. Methods Mol. Biol. 2014;1096:123–130. doi: 10.1007/978-1-62703-712-9_10. [DOI] [PubMed] [Google Scholar]
- 47.Mee P.T., Weeks A.R., Walker P.J., Hoffmann A.A., Duchemin J.-B. Detection of Low-Level Cardinium and Wolbachia Infections in Culicoides. Appl. Env. Microbiol. 2015;81:6177–6188. doi: 10.1128/AEM.01239-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turelli M., Hoffman A.A. Microbe-induced cytoplasmic incompatibility as a mechanism for introducing transgenes into arthropod populations. Insect Mol. Biol. 1999;8:243–255. doi: 10.1046/j.1365-2583.1999.820243.x. [DOI] [PubMed] [Google Scholar]
- 49.Coutinho-Abreu I.V., Zhu K.Y., Ramalho-Ortigao M. Transgenesis and paratransgenesis to control insect-borne diseases: Current status and future challenges. Parasitol. Int. 2010;59:1–8. doi: 10.1016/j.parint.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kriesner P., Hoffmann A.A., Lee S.F., Turelli M., Weeks A.R. Rapid sequential spread of two Wolbachia variants in Drosophila simulans. PLoS Pathog. 2013;9:e1003607. doi: 10.1371/journal.ppat.1003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berg I.A., Kockelkorn D., Buckel W., Fuchs G. A 3-hydroxypropionate/4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in Archaea. Science. 2007;318:1782–1786. doi: 10.1126/science.1149976. [DOI] [PubMed] [Google Scholar]
- 52.Cho E.A., Lee D.W., Cha Y.H., Lee S.J., Jung H.C., Pan J.G., Pyun Y.R. Characterization of a novel D-lyxose isomerase from Cohnella laevoribosii RI-39 sp. nov. J. Bacteriol. 2007;189:1655–1663. doi: 10.1128/JB.01568-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Darby A.C., Armstrong S.D., Bah G.S., Kaur G., Hughes M.A., Kay S.M., Koldkjaer P., Rainbow L., Radford A.D., Blaxter M.L., et al. Analysis of gene expression from the Wolbachia genome of a filarial nematode supports both metabolic and defensive roles within the symbiosis. Genome Res. 2012;22:2467–2477. doi: 10.1101/gr.138420.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grote A., Voronin D., Ding T., Twaddle A., Unnasch T.R., Lustigman S., Ghedin E. Defining Brugia malayi and Wolbachia symbiosis by stage-specific dual RNA-seq. PLoS Negl. Trop. Dis. 2017;11:e0005357. doi: 10.1371/journal.pntd.0005357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreira L.A., Ye Y.H., Turner K., Eyles D.W., McGraw E.A., O’Neill S.L. The wMelPop strain of Wolbachia interferes with dopamine levels in Aedes aegypti. Parasit. Vectors. 2011;4:28. doi: 10.1186/1756-3305-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rohrscheib C.E., Bondy E., Josh P., Riegler M., Eyles D., van Swinderen B., Weible M.W., Brownlie J.C. Wolbachia Influences the Production of Octopamine and Affects Drosophila Male Aggression. Appl. Env. Microbiol. 2015;81:4573–4580. doi: 10.1128/AEM.00573-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valentine R.C., Bojanowski R., Gaudy E., Wolfe R.S. Mechanism of the allantoin fermentation. J. Biol. Chem. 1962;237:2271–2277. [PubMed] [Google Scholar]
- 58.Beckmann J.F., Ronau J.A., Hochstrasser M. A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat. Microbiol. 2017;2:17007. doi: 10.1038/nmicrobiol.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang G., Hussain M., Asgari S. Regulation of arginine methyltransferase 3 by a Wolbachia-induced microRNA in Aedes aegypti and its effect on Wolbachia and dengue virus replication. Insect Biochem. Mol. Biol. 2014;53:81–88. doi: 10.1016/j.ibmb.2014.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.