Abstract
Plasmid-mediated quinolone resistance (PMQR) remains one of the main mechanisms of bacterial quinolone resistance and plays an important role in the transmission of antibiotic resistance genes (ARGs). In this study, two novel plasmids, p3M-2A and p3M-2B, which mediate quinolone resistance in Proteus vulgaris strain 3M (P3M) were identified. Of these, only p3M-2B appeared to be a qnrD-carrying plasmid. Both p3M-2A and p3M-2B could be transferred into Escherichia coli, and the latter caused a twofold change in ciprofloxacin resistance, according to the measured minimum inhibitory concentration (MIC). Plasmid curing/complementation and qRT-PCR results showed that p3M-2A can directly regulate the expression of qnrD in p3M-2B under treatment with ciprofloxacin, in which process, ORF1 was found to play an important role. Sequence alignments and phylogenetic analysis revealed the evolutionary relationships of all reported qnrD-carrying plasmids and showed that ORF1–4 in p3M-2B is the most conserved backbone for the normal function of qnrD-carrying plasmids. The identified direct repeats (DR) suggested that, from an evolutionary perspective, p3M-2B may have originated from the 2683-bp qnrD-carrying plasmid and may increase the possibility of plasmid recombination and then of qnrD transfer. To the best of our knowledge, this is the first identification of a novel qnrD-carrying plasmid isolated from a P. vulgaris strain of shrimp origin and a plasmid that plays a regulatory role in qnrD expression. This study also sheds new light on plasmid evolution and on the mechanism of horizontal transfer of ARGs encoded by plasmids.
Keywords: quinolone resistance, Proteus vulgaris, qnrD-carrying plasmids, qnrD expression, direct repeats, homologous recombination
1. Introduction
The unregulated use of antibiotics has led to the emergence of resistant bacteria carrying different types of antibiotic resistance genes (ARGs), which spread in people, animals, and the environment (water, soil, air, etc.) by horizontal gene transfer (HGT), raising a health challenge [1,2,3,4]. Quinolones (ciprofloxacin, norfloxacin, ofloxacin, etc.) are among the most commonly prescribed antibiotics due to their broad-spectrum antibacterial activity, and the frequent use of quinolones has contributed to the emergence of quinolone resistance worldwide, posing a serious threat to public health [5,6,7,8]. The mechanisms of bacterial quinolone resistance are well understood. For instance, bacteria develop quinolone resistance mainly as a consequence of mutations of proteins such as topoisomerase IV and DNA gyrase [9,10,11]. Recent studies have shown that bacteria, especially clinically important bacteria, gain increased resistance to quinolones by the acquisition of plasmids harboring quinolone resistance genes [12,13,14]. Resistance plasmids play a crucial role in the horizontal transfer of quinolone resistance genes due to their good self-replication and transmission characteristics [15,16]. qnrD, a plasmid-mediated quinolone resistance (PMQR) determinant that encodes a pentapeptide repeat protein, induces low susceptibility to quinolone by binding to DNA–DNA gyrase complexes [17,18,19,20]. Unlike other qnr alleles (qnrA, qnrB, qnrC, qnrE, qnrS), qnrD is usually harbored by small, non-conjugative plasmids, is closely related to qnrB variants [17], and does not produce significant quinolone resistance by itself [18,21,22]. However, it is undeniable that qnrD is of vital importance in the transmission and development of quinolone resistance [19,23].
DNA recombination mediated by direct repeats (DR), a typical type of DNA repetition in which two copies of identical or highly similar sequences are arranged in the same orientation along a DNA strand, has been increasingly reported not only in prokaryotes but also in eukaryotes, making it clear that it may be a common genetic and evolutionary mechanism [24,25,26]. Several studies have shown that recombination may occur between DR regions in the chromosome or plasmids of Escherichia coli, further manifesting that the existence of DR might increase the possibility of the formation of various products of recombination [26,27,28,29]. Therefore, the role of DR-mediated recombination in the transmission of ARGs should not be neglected.
In this study, we identified two novel plasmids closely associated with enhanced quinolone resistance in a Proteus vulgaris strain of shrimp origin and preliminarily describe the possible formation process of qnrD-carrying plasmids of specific types via DR-mediated recombination, which might be a widespread and conserved mechanism of qnrD transfer from an evolutionary perspective.
2. Materials and Methods
2.1. Bacterial Strains and Growth Condition
The strains and plasmids used in this study are shown in Table 1. p3M-2A and p3M-2B plasmids were identified from P. vulgaris strain 3M (P3M), which was isolated from the intestines of shrimps in Tianjin, China. All strains were cultured in Luria–Bertani (LB) medium at 37 °C.
Table 1.
Strains and plasmids used in this study. P3M, Proteus vulgaris strain 3M.
| Strains or Plasmids a | Relevant Characteristics | Source or Reference |
|---|---|---|
| Strains | ||
| P3M | Wild-type strain containing p3M-2A and p3M-2B plasmids | This study |
| P3M-Δ2A | p3M-2A-deficient strain | This study |
| P3M-Δ2B | p3M-2B-deficient strain | This study |
| P3M-Δ2AΔ2B | p3M-2A- and p3M-2B-deficient strain | This study |
| P3M-Δ2A/2A* | p3M-2A-deficient strain complemented with p3M-2A* | This study |
| P3M-Δ2B/2B* | p3M-2B-deficient strain complemented with p3M-2B* | This study |
| Escherichia coli DH5α | Competent cell for cloning b | CWBIO Company (CW0808S) |
| DH5α-2A | DH5α complemented with p3M-2A | This study |
| DH5α-2B | DH5α complemented with p3M-2B | This study |
| DH5α-2A2B | DH5α complemented with p3M-2A and p3M-2B | This study |
| DH5α-2B/2A* | DH5α-2B complemented with p3M-2A* | This study |
| DH5α-2A/2B* | DH5α-2A complemented with p3M-2B* | This study |
| E. coli S17-1 | Mobilizing donor strain with streptomycin resistance | [30] |
| Plasmids | ||
| p3M-2A | 2656-bp plasmid isolated from P3M | This study |
| p3M-2B | 5903-bp plasmid carrying qnrD, isolated from P3M | This study |
| p3M-2A* | p3M-2A without ORF1 | This study |
| p3M-2B* | p3M-2B without ORF5 | This study |
a The asterisks (*) denote deletion of ORF1 from p3M-2A or deletion of ORF5 from p3M-2B. b The gyrA mutation in E. coli DH5α does not increase the minimum inhibitory concentration (MIC) for ciprofloxacin.
2.2. Plasmid Isolation and Sequencing
The plasmids p3M-2A and p3M-2B harbored by P3M were extracted using TIANprep Mini Plasmid Kit (DP103-03, TianGen, Beijing, China) and then purified with SPARKeasy Gel DNA Extraction Kit (AE0101-C, SparkJade, Qingdao, China). Six restriction endonucleases (Hind III, BamH I, Sma I, Xba I, Sac I, Sph I according to the multiple cloning sites of pUC19, Takara, Dalian, China) were used to digest these two plasmids at 37 °C for 1 h; the enzymes with only one restriction site on the plasmids were selected, which were Hind III for p3M-2B and Sac I for p3M-2A. The digested plasmid fragments and the pUC19 vector were ligated with T4 DNA ligase (Takara, Dalian, China) according to the manufacturer’s instructions. The ligation products were then transformed into E. coli DH5α competent cells (β-galactosidase-deficient strains), and blue colonies were selected. The correct colonies were cultured to extract the plasmids, which were sequenced later by Invitrogen, Beijing, China.
2.3. Construction of p3M-2A* and p3M-2B*
p3M-2A lacking ORF1 (p3M-2A*) and p3M-2B lacking ORF5 (p3M-2B*) were generated to verify the function of ORF1 and ORF5, respectively. Oligonucleotide primers with restriction sites were designed to generate linear fragments of p3M-2A and p3M-2B (Table S1). Amplified fragments were digested with the endonuclease BamHI and ligated with T4 DNA ligase to form the circular plasmids p3M-2A* and p3M-2B*.
2.4. Plasmid Transformation
The plasmids were transformed into E. coli DH5α competent cells by chemical transformation to yield different DH5α derivatives. For P3M derived strains, the plasmids were first transferred into E. coli S17-1 to form intermediate transformants and then transferred into the recipient strain by the parental conjugation method [31]. Transformants were selected on LB agar plates by colony PCR, using specific primers (Table S1).
2.5. Antimicrobial Susceptibility Testing
The MIC of both wild-type (WT) strains and transformants were determined by the broth microdilution method according to the Clinical & Laboratory Standards Institute (CLSI) recommendations [32].
2.6. Spot Growth Assays
P. vulgaris and E. coli strains were cultured overnight in LB medium at 37 °C. The cultures were transferred into fresh LB medium and grown up to OD600 of 0.6. After gradient dilution, 5 μL droplets of the cultures were dropped onto LB agar plates containing 0.25 mg/L or 0.05 mg/L ciprofloxacin, and the plates were incubated upside down at 37 °C for 24 h [33].
2.7. Phylogenetic Analysis and Sequence Alignment
Unrooted neighbor-joining trees of the qnrD-carrying plasmids were generated from the indicative aligned sequences using MEGA7 (Temple University, Philadelphia, Pennsylvania, USA) [34]. Linear comparison and map generation of the plasmids were performed and visualized using Easyfig 2.2.3 software (University of Queensland, Brisbane, Australia) [35].
2.8. Plasmid Curing
Overnight cultured P3M was transferred to fresh LB medium containing 0.25% of sodium dodecyl sulfonate (SDS) and cultured at 37 °C until reaching the late log phase. After that, the cultures were transferred again and cultured according to the method described by Verma et al. [36]. The cultures were diluted and transferred onto LB agar plates without antibiotics after several repetitions of SDS treatments and culturing, and the plasmid-deleted strains were screened by colony PCR using specific primers (Table S1).
2.9. Quantitative Real-Time PCR
Total bacterial RNA was isolated using an RNAprep Pure Cell/Bacteria Kit (DP430, TianGen, Beijing, China) and then reverse-transcribed into cDNA using the PrimeScript™ RT reagent Kit (RR047A, Takara, Dalian, China) according to the manufacturer’s instructions. The cDNAs of each sample were diluted to 100 ng/μL as templates for qRT-PCR experiments, which were repeated three times independently (three biological replicates). Specific primers for qnrD were designed based on the plasmid sequence of p3M-2B (Table S1). We used 16S rDNA as the endogenous reference gene to normalize the expression of the target genes in each cDNA template. The relative mRNA concentration was calculated by the comparative threshold cycle (2−∆∆CT) method.
2.10. Accession Number
The complete sequences of plasmids p3M-2A and p3M-2B were deposited in GenBank, with accession numbers JX514065 and JX514066, respectively.
3. Results
3.1. Effect of p3M-2A on the Expression of qnrD in p3M-2B
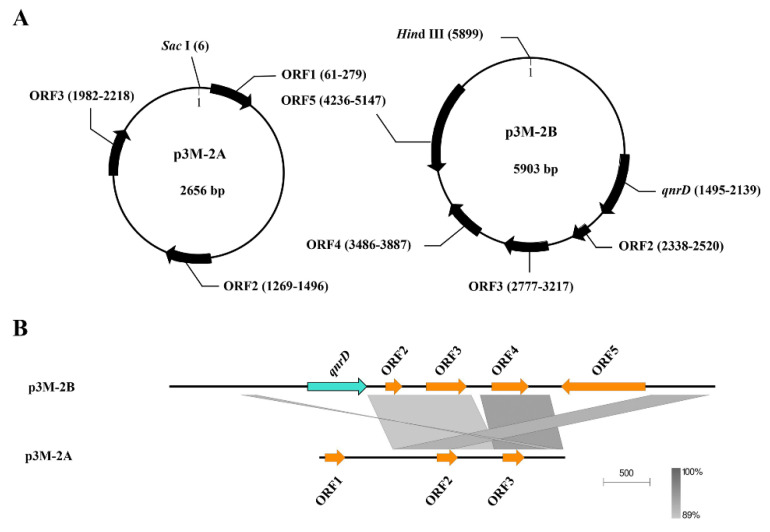
The plasmids p3M-2A and p3M-2B of P3M were sequenced, and their total length resulted to be 2656 bp and 5903 bp, respectively (Figure 1A). No resistance genes were present in p3M-2A, while p3M-2B carried qnrD. In this study, we found a certain correlation between these two plasmids—a large portion of the entire p3M-2A sequence shared a relatively high sequence identity (>89%) with ORF2–ORF4 and their intergenic regions of p3M-2B, implying that these two plasmids may originate from the same plasmid backbone (Figure 1B). Besides, we did not find any sequence with high identity with ORF1 of p3M-2A in the database, and the same was observed for ORF5 of p3M-2B.
Figure 1.
Graphical maps (A) and comparison of the structures (B) of p3M-2A and p3M-2B. The grey and dark shading in (b) indicates common regions between the plasmids.
In order to verify the function of p3M-2A and p3M-2B, different plasmid deletion strains of P3M were generated: P3M-∆2A (p3M-2A elimination), P3M-∆2B (p3M-2B elimination), and P3M-∆2A2B (p3M-2A and p3M-2B elimination). In addition, p3M-2A and p3M-2B were transferred into E. coli DH5α separately or simultaneously to generate transformants with different plasmids: E. coli DH5α-2A (transformant containing p3M-2A), E. coli DH5α-2B (transformant containing p3M-2B), and E. coli DH5α-2A2B (transformant containing p3M-2A and p3M-2B). The ciprofloxacin MIC of these strains were determined. As shown in Table 2, ciprofloxacin resistance was decreased in P3M-∆2A and P3M-∆2B compared with P3M. Likewise, the resistance to ciprofloxacin was also reduced in E. coli DH5αtransformants containing only p3M-2A or p3M-2B compared with E. coli DH5α-2A2B. These results showed that the presence of both plasmids caused an eight- or four-fold change in the ciprofloxacin resistance of P3M and E. coli DH5α-2A2B compared with the plasmid-free strains, respectively. In addition, we found that the ciprofloxacin resistance of P3M-∆2A and E. coli DH5α-2B strains containing only p3M-2B increased by four times or two times, respectively, while p3M-2A did not contribute to strain resistance. These results indicated that the two plasmids played synergistic roles in improving strain resistance to ciprofloxacin, and p3M-2B had a more obvious promoting effect. It is worth noting that, despite the absence of qnrD, p3M-2A still played a specific role in improving the quinolone resistance of the bacteria tested.
Table 2.
Determination of the MIC of ciprofloxacin in different transformants with respect to the parental strains.
| Strains | MIC to Ciprofloxacin (mg/L) a |
|---|---|
| P3M | 1 |
| P3M-Δ2A | 0.5 |
| P3M-Δ2B | 0.125 |
| P3M-Δ2AΔ2B | 0.125 |
| E. coli DH5α-2A2B | 0.125 |
| E. coli DH5α-2B | 0.06 |
| E. coli DH5α-2A | 0.03 |
| E. coli DH5α | 0.03 |
a All MIC determinations were performed by broth microdilution assays according to CLSI standards.
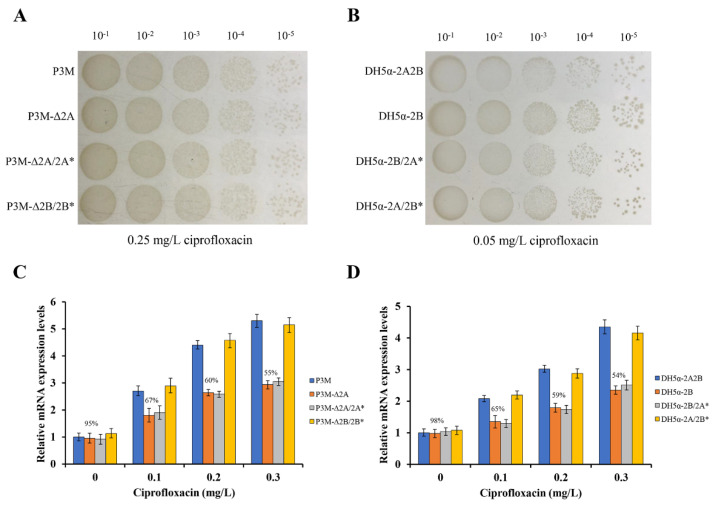
To confirm the function of p3M-2A, we carried out spot growth assays to test the effect of the absence of p3M-2A on the stability of p3M-2B. After subcultured for 100 generations, P3M-∆2A and DH5α-2B were able to preserve the ciprofloxacin-resistant phenotype in the presence of 0.25 mg/L and 0.05 mg/L of ciprofloxacin, respectively (Figure 2A,B), showing that p3M-2B could replicate and function independently and the absence of p3M-2A had no effect on the stability of p3M-2B in either P3M or E. coli DH5α strains. Considering that p3M-2A could improve the ciprofloxacin resistance of the strain even in the absence of qnrD, we speculated that this plasmid may have specific regulatory functions. Thus, we measured the expression level changes of qnrD in the presence or absence of p3M-2A using qRT-PCR (Figure 2C,D). The expression level of qnrD in P3M and P3M-∆2A showed little difference in the absence of ciprofloxacin (Figure 2C). As the concentration of ciprofloxacin increased, the expression level of qnrD in both strains increased to different degrees; qnrD expression in P3M-∆2A was apparently lower than that in the wild-type strain, and the difference became more obvious as the concentration increased. Similarly, the expression of qnrD showed the same changing trend in E. coli DH5α transformants containing p3M-2B and p3M-2A2B (Figure 2D). These results indicated that p3M-2A played a positive regulatory role in the expression of qnrD in p3M-2B, that is, the higher the ciprofloxacin concentration in the environment, the more obvious the regulatory effect of p3M-2A.
Figure 2.
(A) Spot growth assays of wild-type (WT) P3M, p3M-2A deleted strain (P3M-∆2A), p3M-2A* complemented strain (P3M-∆2A/2A*), and p3M-2B* complemented strain (P3M-∆2B/2B*) on LB agar in the presence of 0.25 mg/L of ciprofloxacin. (B) Spot growth assays of DH5α transformant containing both plasmids (DH5α-2A2B), DH5α transformant containing p3M-2B (DH5α-2B), DH5α-2B strain complemented with p3M-2A* (DH5α-2B/2A*), and DH5α-2A strain complemented with p3M-2B* (DH5α-2A/2B*) on LB agar in the presence of 0.05 mg/L of ciprofloxacin. (C) Expression of qnrD in P3M, P3M-∆2A, P3M-∆2A/2A*, and P3M-∆2B/2B* with different ciprofloxacin concentrations. (D) Expression of qnrD in DH5α-2A2B, DH5α-2B, DH5α-2B/2A*, and DH5α-2A/2B* with different ciprofloxacin concentrations.
Since the sequence of ORF2–ORF3 in p3M-2A shares relatively high identity with that of p3M-2B, while ORF1 does not (Figure 1B), we speculated that ORF1 might play a certain regulatory role in qnrD expression. Hence, we constructed a new p3M-2A plasmid deleting ORF1 (p3M-2A*) and transferred it into P3M-∆2A and DH5α-2B to verify the effect of ORF1. As shown in Figure 2A and 2B, the new plasmid p3M-2A* did not affect the normal replication of plasmid 2B in P3M-∆2A and DH5α-2B, and qnrD expression in the transformants was subsequently detected. As expected, p3M-2A* failed to restore the expression of qnrD to the wild-type level, but the expression was similar to that of the p3M-2A-deficient strain (Figure 2C,D). These results indicated that ORF1 in P3M-2A was key to regulate qnrD expression, as p3M-2A without ORF1 could not play its important regulatory function.
3.2. Phylogenetic Analysis of qnrD-Carrying Plasmids
By analyzing all the qnrD-carrying plasmids reported so far, we found that almost all of them were harbored by bacteria isolated from common environmental “reservoirs” like human and animal intestine, urinary tract, feces, water body, etc. As shown in Table S2, 47 qnrD-carrying plasmids have been reported to date, of which most of them were isolated from Enterobacteriaceae, with the genus Proteus accounting for a relatively large proportion.
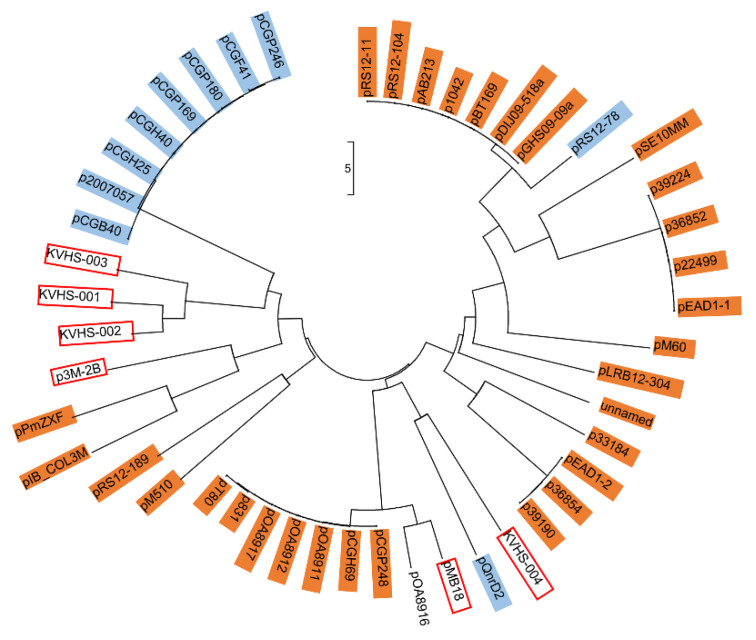
Almost all qnrD-carrying plasmids reported to date can be roughly divided into 2.7-kb and 4.3-kb categories according to their size [17,18,37]. Interestingly, the p3M-2A plasmid in this study was about 2.7 kb in size, although it did not carry qnrD. The size of the p3M-2B carrying qnrD was 5.9 kb, and thus it did not comply with the above classification characteristics. In addition, we also found other six special cases that did not conform to the size rule: KVHS-001 (6.9 kb), KVHS-002 (8.1 kb), KVHS-003 (9.4 kb), KVHS-004 (6.2 kb), pOA8916 (2.0 kb), and pMB18 (5.2 kb) (Table S2). In order to further understand the genetic relationship between these qnrD-carrying plasmids, a neighbor-joining phylogenetic tree was constructed. As shown in Figure 3, plasmids of the same category tend to be more closely related to each other, while plasmids isolated from Proteus were dispersed and could be found in each clade, further illustrating the important function of Proteus species as disseminators in the phylogenetic evolution of qnrD-carrying plasmids.
Figure 3.
Neighbor-joining phylogenetic tree of p3M-2B with other reported qnrD-carrying plasmids. Plasmids of 2.7 kb or 4.3 kb are highlighted in orange or blue, respectively. Plasmids larger than 4.3 kb are marked with red boxes. The evolutionary history was inferred using the neighbor-joining method [38]. The optimal tree with the sum of branch length of 102.98104110 is shown. The tree is drawn to scale, with branch lengths measured in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method [39] and are expressed as the number of base substitutions per site. The analysis involved 49 nucleotide sequences. The codon positions included were 1st + 2nd + 3rd + Noncoding. All positions containing gaps and missing data were eliminated. There were a total of 645 positions in the final dataset.
3.3. Possible Formation Process of p3M-2B
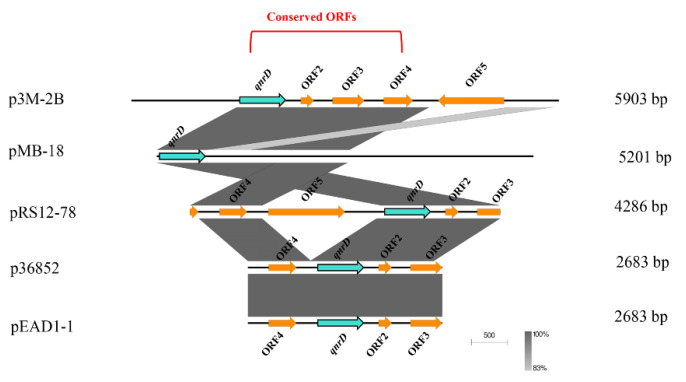
Due to the relatively large size of p3M-2B compared with the 2.7 and 4.3 kb qnrD-carrying plasmids, we hypothesized that it may have a specific formation process. We first focused on the other four plasmids isolated from P. vulgaris strains which were different in size and made sequence alignments. As shown in Figure 4, ORF1 (qnrD)-4 of p3M-2B was completely conserved in these four qnrD-carrying plasmids, with sequence identity up to 100%. Besides, the 2683-bp plasmids p36852 and pEAD1-1 appeared to be exactly composed of ORF1–4, implying that these four conserved genes of p3M-2B are the most conserved genes of the qnrD-carrying plasmids, necessary for their normal function.
Figure 4.
Linear comparison of p3M-2B with other closely related qnrD-carrying plasmids isolated from P. vulgaris strains.
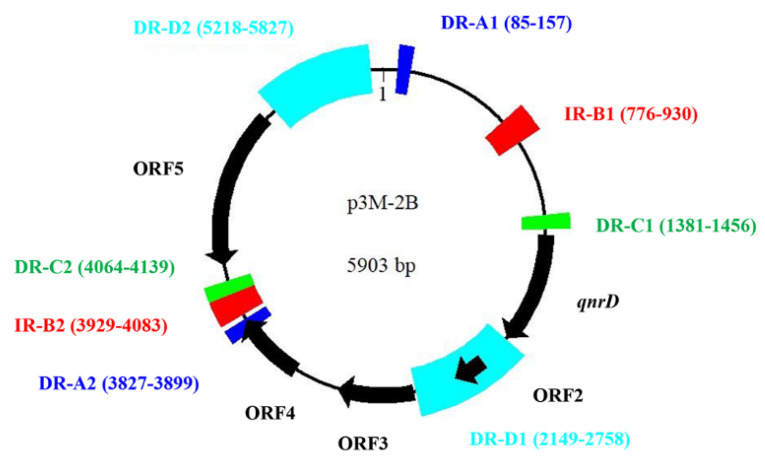
Subsequently, the p3M-2B plasmid sequence was analyzed with RepeatAround, a Windows-based software tool designed to find repeats from 3 bp to 64 bp of length in circular genomes [40]. We found that there were four pairs of DR or inverted repeats (IR) in p3M-2B (Figure 5), and it was notable that the sequence length between DR-C1 and -C2 was precisely 2683 bp, which fitted into the category of 2683-bp qnrD-carrying plasmids. In particular, the sequence between C1 and C2 corresponded exactly to ORF1–4, which appeared to be the most conserved and essential genetic component of qnrD-carrying plasmids for their proper function (Figure 4). It has been reported that DNA recombination mediated by DR regions is a major cause of genome plasticity [36,41], so it is conceivable that the DR present in p3M-2B would have a similar function. We hereby supposed that the 5903-bp p3M-2B may be originally derived from a 2683-bp plasmid, and DR-C1 and C2 play an important role in this process.
Figure 5.
Graphical map of p3M-2B including direct repeats (DR) and inverted repeats (IR). Motifs of the same color represent a pair of DR or IR regions.
To test the above hypothesis, a new 2683-bp p3M-2B plasmid without ORF5 (p3M-2B*) was constructed, and its stability and qnrD expression in the transformants P3M-∆2B/2B* and DH5α-2A/2B* (Figure 2) were tested. Just as we expected, p3M-2B* was stable in the transformed strains (Figure 2A,B), and the expression of qnrD at different ciprofloxacin concentrations was not affected by ORF5 deficiency (Figure 2C,D). These results further indicated that ORF1–4 in qnrD-carrying plasmids is a very conserved backbone, and ORF5 in p3M-2B, which appears to be an acquired exogenous sequence, does not affect the normal replication process and further quinolone resistance,.
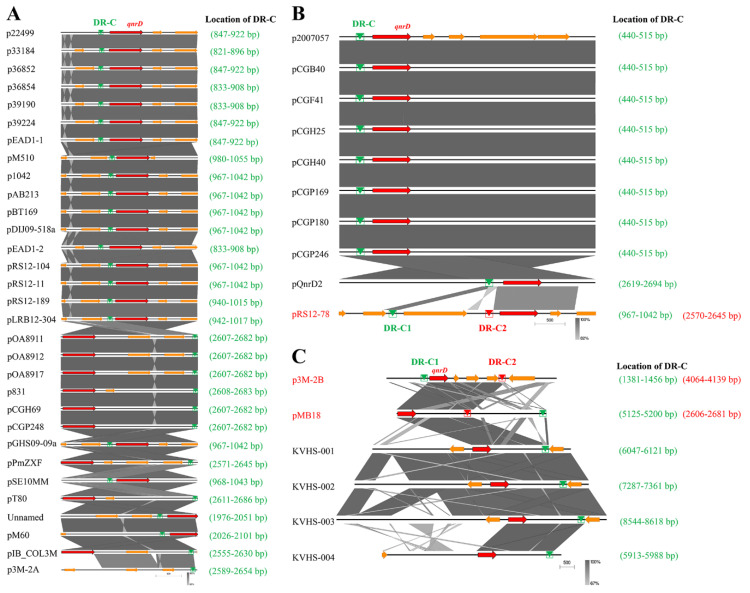
To further explore whether this is applicable to all larger qnrD-carrying plasmids, we made sequence alignments of the DR-C region in p3M-2B with similar regions in other reported qnrD-carrying plasmids. As shown in Figure 6, we found that although all plasmids contained DR regions sharing high sequence identity with DR-C, sequences varied slightly among different plasmids. The sequence with high identity to DR-C was present only in one site in the 2.7-kb plasmids, located upstream of qnrD (Figure 6A). It should be noted that although p3M-2A does not carry qnrD, it was also included in Figure 6A because it appeared to be in the 2.7-kb plasmid category and share high sequence identity with DR-C. Likewise, all 4.3-kb plasmids contained only one site identical with DR-C, with the exception of plasmid pRS12-78, containing two DR-C regions at different positions (Figure 6B). As shown in Figure 6C, among all plasmids larger than 4.3 kb, only pMB18 appeared to be consistent with p3M-2B, with two different DR regions (DR-C1 and C2), while only one DR-C was found in the four larger qnrD-carrying fragments isolated from Salmonella, which was unexpected. Plasmid pOA8916, smaller than 2.7 kb, contained one spot of DR-C upstream of qnrD as well (not shown). In summary, among all the qnrD-carrying plasmids analyzed, only p3M-2B, pMB18, and pRS12-78 presented two different DR regions, and the sequence lengths between them resulted to be exactly 2683 bp, containing ORF1–4. Notably, these three plasmids were isolated from P. vulgaris strains, which hints at the important host role of P. vulgaris in plasmid propagation.
Figure 6.
Comparative linear maps of qnrD-carrying plasmids of (A) 2.7 kb, (B) 4.3 kb, and (C) larger than 4.3 kb; qnrD and other predicted coding sequences (CDS) are denoted by red and orange arrows, respectively. The size of the arrows is to scale. The green and red triangles represent the regions sharing high sequence identity with DR-C in p3M-2B.
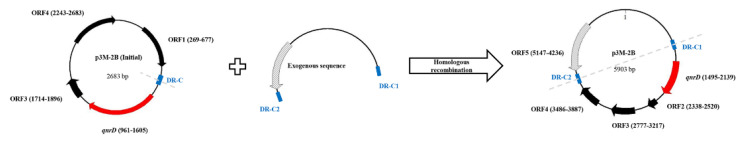
Based on the structural characteristics of the above three plasmids, we propose a potential model for the development of larger qnrD-carrying plasmids like p3M-2B (Figure 7). The p3M-2B backbone with an initial size of 2.7 kb contains DR-C region about 35–115 bp upstream of qnrD. The exogenous sequence containing DR-C at both ends in the adjacent environment may undergo homologous recombination with the DR-C region in p3M-2B (2.7 kb) and thus be integrated to turn into a new ORF. Ultimately, a novel plasmid p3M-2B of 5.9 kb is formed.
Figure 7.
Possible model for in vivo formation of 5903-bp p3M-2B.
4. Discussion
The Proteus genus consists of Gram-negative opportunistic pathogens ubiquitous in the intestine of humans and animals as well as in the natural environment, usually causing clinical infections in patients [42,43,44]. Quinolones are commonly used in the treatment of infections caused by Proteus, which, as a result, leads to an increase in the number of quinolone-resistant bacteria. As shown in many well-studied Proteus strains, qnr alleles are really important in reducing the bacterial sensitivity to quinolones, and, in particular, qnrD confers relatively low quinolone resistance and can be transferred via plasmid transformation. The data presented in this article further indicate that qnrD is present in small non-conjugative plasmids, and the species characteristics of the host strains of these qnrD-carrying plasmids indicate that these plasmids may have first originated from Enterobacteriaceae [19,23] and were then gradually transferred to other bacterial genera through HGT, in which process, Proteus spp. presumably played important roles as intermediate hosts. qnrD-carrying plasmids were classified in two major types according to their size [17,18,37]. Interestingly, plasmid p3M-2A without qnrD in P3M exhibited the size considered by this classification, while p3M-2B did not. Sequence alignment results showed that there was no sequence in the database with high identity with ORF1 of P3M-2A or ORF5 of P3M-2B, implying that the two ORFs were obtained by p3M-2A and p3M-2B in a specific way from the genome of P3M or unknown exogenous sources. The existence of DR-C in p3M-2A (Figure 6A) and the high sequence identity between p3M-2A and p3M-2B led us to speculate that these two plasmids may share a common ancestor, the 2.7-kb p3M-2B (Figure 7). Subsequently, with the occurrence of homologous recombination in the process of evolution, the qnrD gene on some copies of the 2.7-kb p3M-2B was excised, and a completely new sequence (ORF1) was obtained to form the p3M-2A plasmid. And as a result, the size of p3M-2A did not change too much, remaining about 2.7 kb. The other copies of the 2.7-kb p3M-2B directly obtained a new sequence (ORF5) and finally formed the novel p3M-2B of 5.9 kb.
p3M-2A promotes the expression of qnrD in p3M-2B to enhance the quinolone resistance of host bacteria, in which process ORF1 plays an important regulatory role, and this regulatory effect becomes more significant with increasing concentration of ciprofloxacin (Figure 2). We speculate here that ORF1 may code for a small RNA-binding protein and enhance the stability of qnrD mRNA by interacting with it, thus improving the expression of qnrD and further enhancing the quinolone resistance of P3M. We therefore conjectured that in the long evolutionary process, when the external environment became more and more hostile due to the presence of high concentrations of quinolones, the p3M-2A plasmid played a regulatory role, promoting P3M survival. The specific regulation mechanism remains to be fully elucidated in further studies.
DNA recombination is one of the most important factors that lead to changes of bacterial genetic information, during which DR play an important role [24,25,45]. In this study, we found that the existence of DR is a common feature of these plasmids containing qnrD, providing the potential to form a new plasmid when needed and showing that the conserved genes could be transferred and also substituted. This allows the recipient bacteria to obtain resistant genes like qnrD from the environment and to get a new resistant gene if the surroundings change. We speculate here that some qnrD-carrying plasmids larger than 4.3 kb in size, such as p3M-2B, may primordially have been plasmids of 2683-bp, which obtained new DNA sequence through recombination, which formed new ORFs and eventually formed plasmids of different sizes (Figure 7). The newly acquired sequences of novel plasmids probably derived from excised old plasmids, genomes, or unknown surroundings. Here, we only found three plasmids (p3M-2B, pRS12-78, and pMB18) which contained two different DR-C regions that were in line with the above hypothesis, demonstrating that each type of plasmid has its specific formation process and mode of action and the rules proposed may not be universal. We believe that with more research, more similar qnrD-carrying plasmids will be isolated, and our hypothesis will be further confirmed.
Together, three conclusions can be drawn based on our present research. First, the plasmids p3M-2A and p3M-2B function synergistically to improve the quinolone resistance of host strains; in this process, p3M-2A plays an important regulatory role through ORF1, promoting the expression of qnrD. Thorough studies should help to figure out the specific regulatory mechanisms of ORF1. Second, Proteus strains may play important roles as disseminators or intermediate hosts during the transmission of qnrD-carrying plasmids. Finally, DR regions in qnrD-carrying plasmids appear to be among the main elements increasing the possibility of homologous recombination during the formation of plasmids, further promoting the transfer and acquisition of ARGs from the environment [46,47]. Indeed, how repeat sequences are identified by specific targets and how resistance genes are excised and transferred should also be further investigated.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/7/1074/s1, Table S1: Primers used in this study, Table S2: qnrD-carrying plasmids isolated from different strains.
Author Contributions
Conceptualization, H.Z., M.Q.; methodology, H.Z., M.C., X.Z.; validation, P.C., Y.D.; investigation, X.Z., T.S.; writing—original draft preparation, H.Z., M.C.; writing—review and editing, Z.W., H.X., M.Q.; supervision, M.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Key Projects of the National Natural Science Foundation of China (grant number 41831287), Tianjin Postgraduate Scientific Research Innovation Project (grant number 2019YJSB042).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Kumburu H.H., Sonda T., van Zwetselaar M., Leekitcharoenphon P., Lukjancenko O., Mmbaga B.T., Alifrangis M., Lund O., Aarestrup F.M., Kibiki G.S. Using WGS to identify antibiotic resistance genes and predict antimicrobial resistance phenotypes in MDR Acinetobacter baumannii in Tanzania. J. Antimicrob. Chemother. 2019;74:1484–1493. doi: 10.1093/jac/dkz055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soucy S.M., Huang J., Gogarten J.P. Horizontal gene transfer: Building the web of life. Nat. Rev. Genet. 2015;16:472–482. doi: 10.1038/nrg3962. [DOI] [PubMed] [Google Scholar]
- 3.Mcewen S.A., Collignon P. Antimicrobial resistance: A one health perspective. Microbiol. Spectr. 2018;6:521–547. doi: 10.1128/microbiolspec.arba-0009-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferri M., Ranucci E., Romagnoli P., Giaccone V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. 2017;57:2857–2876. doi: 10.1080/10408398.2015.1077192. [DOI] [PubMed] [Google Scholar]
- 5.Tyson G.H., Tate H.P., Zhao S., Li C., Dessai U., Simmons M., McDermott P.F. Identification of plasmid-mediated quinolone resistance in Salmonella isolated from swine ceca and retail pork chops in the United States. Antimicrob. Agents Chemother. 2017;61:e01318-17. doi: 10.1128/AAC.01318-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adachi F., Yamamoto A., Takakura K.I., Kawahara R. Occurrence of fluoroquinolones and fluoroquinolone-resistance genes in the aquatic environment. Sci. Total. Environ. 2013;444:508–514. doi: 10.1016/j.scitotenv.2012.11.077. [DOI] [PubMed] [Google Scholar]
- 7.Hooper D.C. Emerging mechanisms of fluoroquinolone resistance. Emerg. Infect. Dis. 2001;7:337–341. doi: 10.3201/eid0702.010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lautenbach E., Strom B.L., Nachamkin I., Bilker W.B., Marr A.M., Larosa L.A., Fishman N.O. Longitudinal trends in fluoroquinolone resistance among Enterobacteriaceae isolates from inpatients and outpatients, 1989–2000: Differences in the emergence and epidemiology of resistance across organisms. Clin. Infect. Dis. 2004;38:655–662. doi: 10.1086/381549. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz J., Pons M.J., Gomes C. Transferable mechanisms of quinolone resistance. Int. J. Antimicrob. Agents. 2012;40:196–203. doi: 10.1016/j.ijantimicag.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Pathirana H., Shin G.W., Wimalasena S., Silva B.D., Hossain S., Heo G.J. Prevalence and characterization of quinolone resistance genes in Proteus species isolated from pet turtles. J. Exot. Pet Med. 2018;27:67–73. doi: 10.1053/j.jepm.2017.10.026. [DOI] [Google Scholar]
- 11.Weigel L.M., Anderson G.J., Tenover F.C. DNA gyrase and topoisomerase IV mutations associated with fluoroquinolone resistance in Proteus mirabilis. Antimicrob. Agents Chemother. 2002;46:2582–2587. doi: 10.1128/AAC.46.8.2582-2587.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klemm E.J., Shakoor S., Page A.J., Qamar F.N., Judge K., Saeed K.D., Wong V.K., Dallman T.J., Nair S., Baker S., et al. Emergence of an extensively drug-resistant Salmonella enterica serovar typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. mBio. 2018;9:e00105-18. doi: 10.1128/mBio.00105-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colobatiu L., Tabaran A., Flonta M., Oniga O., Mirel S., Mihaiu M. First description of plasmid-mediated quinolone resistance determinants and β-lactamase encoding genes in non-typhoidal Salmonella isolated from humans, one companion animal and food in Romania. Gut. Pathog. 2015;7:16. doi: 10.1186/s13099-015-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim D.W., Thawng C.N., Lee S.H., Cha C.J. Unique features of Aeromonas plasmid pAC3 and expression of the plasmid-mediated quinolone resistance genes. msphere. 2017;2:e00203-17. doi: 10.1128/mSphere.00203-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strahilevitz J., Jacoby G.A., Hooper D.C., Robicsek A. Plasmid-mediated quinolone resistance: A multifaceted threat. Clin. Microbiol. Rev. 2009;22:664–689. doi: 10.1128/CMR.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez-Martínez J.M., Machuca J., Cano M.E., Calvo J., Martínez-Martínez L., Pascual A. Plasmid-mediated quinolone resistance: Two decades on. Drug Resist. Updat. 2016;29:13–29. doi: 10.1016/j.drup.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Cavaco L.M., Hasman H., Xia S., Aarestrup F.M. qnrD, a novel gene conferring transferable quinolone resistance in Salmonella enterica serovar Kentucky and Bovismorbificans strains of human origin. Antimicrob. Agents Chemother. 2009;53:603–608. doi: 10.1128/AAC.00997-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillard T., Cambau E., Neuwirth C., Nenninger T., Mbadi A., Brasme L., Vernet-Garnier V., Bajolet O., De Champs C. Description of a 2683-base-pair plasmid containing qnrD in two Providencia rettgeri isolates. Antimicrob. Agents Chemother. 2012;56:565–568. doi: 10.1128/AAC.00081-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L., Zhang Y., Du J., Zhang X., Li M., Chen H., Yu X., Sun Y., Zhou T. Description and plasmid characterization of the qnrD determinant in Proteeae in Wenzhou, Southern China. J. Microbiol. Immunol. Infect. 2018;51:115–122. doi: 10.1016/j.jmii.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Tran J.H., Jacoby G.A., Hooper D.C. Interaction of the plasmid-encoded quinolone resistance protein QnrA with Escherichia coli topoisomerase IV. Antimicrob. Agents Chemother. 2005;49:3050–3052. doi: 10.1128/AAC.49.7.3050-3052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guillard T., Grillon A., De Champs C., Cartier C., Madoux J., Berçot B., Lebreil A.L., Lozniewski A., Riahi J., Vernet-Garnier V., et al. Mobile insertion cassette elements found in small non-transmissible plasmids in Proteeae may explain qnrD mobilization. PLoS ONE. 2014;9:e87801. doi: 10.1371/journal.pone.0087801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van der Putten B.C.L., Remondini D., Pasquini G., Janes V.A., Matamoros S., Schultsz C. Quantifying the contribution of four resistance mechanisms to ciprofloxacin MIC in Escherichia coli: A systematic review. J. Antimicrob. Chemother. 2019;74:298–310. doi: 10.1093/jac/dky417. [DOI] [PubMed] [Google Scholar]
- 23.Kraychete G.B., Campana E.H., Picão R., Bonelli R.R. qnrD-harboring plasmids in Providencia spp. recovered from food and environmental Brazilian sources. Sci. Total Environ. 2019;646:1290–1292. doi: 10.1016/j.scitotenv.2018.07.378. [DOI] [PubMed] [Google Scholar]
- 24.Prado F., Aguilera A. Role of reciprocal exchange, one-ended invasion crossover and single-strand annealing on inverted and direct repeat recombination in Yeast: Different requirements for the RAD1, RAD10, and RAD52 genes. Genetics. 1995;139:109–123. doi: 10.1093/genetics/139.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliveira P.H., Lemos F., Monteiro G.A., Prazeres D.M.F. Recombination frequency in plasmid DNA containing direct repeats--predictive correlation with repeat and intervening sequence length. Plasmid. 2005;60:159–165. doi: 10.1016/j.plasmid.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Dunn M.J., Anderson M.Z. To repeat or not to repeat: Repetitive sequences regulate genome stability in Candida albicans. Genes. 2019;10:866. doi: 10.3390/genes10110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matfield M., Badawi R., Brammar W.J. Rec-dependent and Rec-independent recombination of plasmid-borne duplications in Escherichia Coli K12. Mol. Gen. Genet. 1985;199:518–523. doi: 10.1007/BF00330768. [DOI] [PubMed] [Google Scholar]
- 28.Bi X., Liu L.F. recA-independent and recA-dependent intramolecular plasmid recombination. Differential homology requirement and distance effect. J. Mol. Biol. 1994;235:414–423. doi: 10.1006/jmbi.1994.1002. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro S.C., Oliveira P.H., Prazeres D.M.F., Monteiro G.A. High frequency plasmid recombination mediated by 28 bp direct repeats. Mol. Biotechnol. 2008;40:252–260. doi: 10.1007/s12033-008-9082-3. [DOI] [PubMed] [Google Scholar]
- 30.Simon R., Priefer U.B., Puhler A. A broad host range mobilization system for in vivo genetic engineering: Transposon mutagenesis in gram negative bacteria. Nat. Biotechnol. 1983;1:784–791. doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
- 31.Teng F., Murray B.E., Weinstock G.M. Conjugal transfer of plasmid DNA from Escherichia coli to Enterococci: A method to make insertion mutations. Plasmid. 1998;39:182–186. doi: 10.1006/plas.1998.1336. [DOI] [PubMed] [Google Scholar]
- 32.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing. CLSI Approved Standard M100-S15. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2018. [Google Scholar]
- 33.Zhang H., Zhan Y., Yan Y., Liu Y., Hu G., Wang S., Yang H., Qiu X., Liu Y., Li J., et al. The Pseudomonas stutzeri-specific regulatory noncoding RNA NfiS targets katB mRNA encoding a catalase essential for optimal oxidative resistance and nitrogenase activity. J. Bacteriol. 2019;201:e00334-19. doi: 10.1128/JB.00334-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sullivan M.J., Petty N.K., Beatson S.A. Easyfig: A genome comparison visualizer. Bioinformatics. 2011;27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma J., Bag S., Saha B., Kumar P., Ghosh T.S., Dayal M., Senapati T., Mehra S., Dey P., Desigamani A., et al. Genomic plasticity associated with antimicrobial resistance in Vibrio cholerae. Proc. Natl. Acad. Sci. USA. 2019;116:6226–6231. doi: 10.1073/pnas.1900141116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu Y., Cai J., Zhang R., Zhou H., Sun Q., Chen G. Emergence of Proteus mirabilis harboring blaKPC-2 and qnrD in a Chinese hospital. Antimicrob. Agents Chemother. 2012;56:2278–2282. doi: 10.1128/AAC.05519-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saitou N., Nei M. The neighbor-joining method, a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 39.Tamura K., Nei M., Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA. 2004;101:11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goios A., Meirinhos J., Rocha R., Lopes R., Amorim A., Pereira L. RepeatAround, A software tool for finding and visualizing repeats in circular genomes and its application to a human mtDNA database. Mitochondrion. 2006;6:218–224. doi: 10.1016/j.mito.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Bi X., Liu L.F. A replicational model for DNA recombination between direct repeats. J. Mol. Biol. 1996;256:849–858. doi: 10.1006/jmbi.1996.0131. [DOI] [PubMed] [Google Scholar]
- 42.Al-Qahtani M., Safan A., Jassim G., Abadla S. Efficacy of anti-microbial catheters in preventing catheter associated urinary tract infections in hospitalized patients: A review on recent updates. J. Infect. Public Health. 2019;12:760–766. doi: 10.1016/j.jiph.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Mokracka J., Gruszczyńska B., Kaznowski A. Integrons, β-lactamase and qnr genes in multidrug resistant clinical isolates of Proteus mirabilis and P. vulgaris. Apmis. 2012;120:950–958. doi: 10.1111/j.1600-0463.2012.02923.x. [DOI] [PubMed] [Google Scholar]
- 44.Ro’żalski A., Sidorczyk Z., Kotełko K. Potential virulence factors of Proteus bacilli. Microbiol. Mol. Biol. Rev. 1997;61:65–89. doi: 10.1128/.61.1.65-89.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu Y., Zepeda-Gurrola R.C., Aguilar-Gutiérrez G.R., Lara-Ramírez E.E., De Luna-Santillana E.J., Rodríguez-Luna I.C., Sánchez-Varela A., Carreño-López R., Moreno-Medina V.R., Rodríguez-Pérez M.A., et al. The detection of inherent homologous recombination between repeat sequences in H. pylori 26695 by the PCR-Based Method. Curr. Microbiol. 2014;68:211–219. doi: 10.1007/s00284-013-0466-7. [DOI] [PubMed] [Google Scholar]
- 46.He L., Partridge S.R., Yang X., Hou J., Deng Y., Yao Q., Zeng Z., Chen Z., Liu J. Complete nucleotide sequence of pHN7A8, an F33:A-:B-type epidemic plasmid carrying blaCTX-M-65, fosA3 and rmtB from China. J. Antimicrob. Chemother. 2013;68:46–50. doi: 10.1093/jac/dks369. [DOI] [PubMed] [Google Scholar]
- 47.Zhan Z., Hu L., Jiang X., Zeng L., Feng J., Wu W., Chen W., Yang H., Yang W., Gao B., et al. Plasmid and chromosomal integration of four novel blaIMP-carrying transposons from Pseudomonas aeruginosa, Klebsiella pneumoniae and an Enterobacter sp. J. Antimicrob. Chemother. 2018;73:3005–3015. doi: 10.1093/jac/dky288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.